Abstract
Objective
This study compared intermittent fasting and protein pacing (IF‐P) versus a heart‐healthy caloric restriction (CR) diet, matched for energy intake and physical activity energy expenditure, on body weight, total and visceral fat mass, and cardiometabolic health outcomes in adults with obesity.
Methods
IF‐P (n = 21) and CR (n = 20) were assessed pre‐ (week 0), mid‐ (week 5), and post‐ (week 9) intervention.
Results
Both groups reduced (p < 0.05) weight, total and visceral fat mass, blood pressure and lipids, and desire to eat food and increased proportion of fat‐free mass. IF‐P resulted in greater (p < 0.05) reductions in weight (−9% vs. −5%), total (−16% vs. −9%) and visceral (−33% vs. −14%) fat mass, and desire to eat (−17% vs. 1%) and increased fat‐free mass percent (6% vs. 3%) compared with CR. These improvements were despite similar weekly total energy intake (IF‐P, 9470 ± 550 vs. CR, 9095 ± 608 kcal/wk; p = 0.90) and physical activity energy expenditure (IF‐P, 300 ± 150 vs. CR, 350 ± 200 kcal/d; p = 0.79).
Conclusions
IF‐P and CR optimize weight loss, body composition, cardiometabolic health, and hunger management, with IF‐P providing greater benefits.
Study Importance.
What is already known?
Obesity continues to burden society by adversely promoting impaired cardiometabolic function and overall health.
Modifiable lifestyle factors such as diet and nutrition remain the preferred interventions.
Previous research has demonstrated that nutrient‐dense, calorie‐restricted, evenly spaced meals throughout the day (~4 meals/d) providing 20 to 40 g of protein (protein pacing) yield significant loss of weight, fat mass, and visceral fat while minimizing fat‐free mass loss.
What does this study add?
Daily caloric restriction (CR) and combined intermittent fasting and protein pacing both significantly reduce body weight, fat mass, visceral fat, and desire to eat and increase percentage of fat‐free mass.
Intermittent fasting and protein pacing result in greater reductions in all of these outcomes compared with CR despite similar energy intakes and unchanged levels of physical activity energy expenditure during an 8‐week weight loss period.
How might these results change the direction of research or the focus of clinical practice?
Previous findings show negligible benefit from intermittent fasting compared with daily CR because they have not emphasized intermittent fasting combined with protein intake and pacing nor matched groups for total energy intake and expenditure.
The current findings should emphasize the quality of nutrient intake (reduced sugar and sodium and increased protein and fiber) and quantity of food consumed to promote weight loss, body composition, and food‐intake behaviors.
INTRODUCTION
The primary defense against obesity continues to be modifiable lifestyle factors such as diet [1]. Our laboratory has consistently demonstrated that higher‐quality, nutrient‐dense meals, evenly spaced throughout the day and providing 20 to 40 g of protein (termed “protein pacing” [P]), combined with reduced highly processed foods, sugar, and fat as well as higher fiber, yield significant body weight (BW), fat mass (FM), and visceral fat (VF) loss while maintaining fat‐free mass (FFM) and enhancing cardiometabolic health [2]. Furthermore, combining P with caloric restriction (CR) augments these favorable changes [3]. Another targeted dietary option is intermittent fasting (IF), alone or in conjunction with CR. Several types of IF have varying degrees of efficacy concerning weight loss (WL) and cardiometabolic health improvement, including fasting for 1 (IF1) and 2 (IF2) d/wk [4, 5, 6, 7, 8, 9, 10, 11]. Please note, we have previously published a portion of this study comparing the acute phase response (weeks 0‐4) of IF1 versus IF2 of a subgroup (n = 20) of the participants in this study [17]. This preliminary subgroup analysis provided evidence‐based efficacy of both IF protocols (1 and 2 days) to serve as a comparison with daily CR.
IF is associated with improved body composition (reductions in total and VF mass) that may result in enhanced “metabolic switching” and cardiometabolic health outcomes. This switching is characterized by increased fat oxidation, ketone body synthesis, insulin sensitivity, and autophagy, as well as reduced inflammation, oxidative stress, and enhanced lean body mass [5, 12]. Most IF regimens focus on the timing and quantity of calories consumed and de‐emphasize the nutritional quality (higher protein and fiber, reduced intake of sugar and highly processed foods) of the overall diet. This omission is relevant considering the evidence supporting the efficacy of CR, which emphasizes diet quality, including P meals combined with IF to support WL and cardiometabolic health, which may augment metabolic switching [3, 13, 14].
Current United States dietary recommendations to improve cardiometabolic health and weight management emphasize a CR heart‐healthy diet of increased intake of fruits, vegetables, whole grains, and liquid plant oils and minimal intakes of processed foods, added sugars, salt, and alcohol (65% carbohydrate intake, 20% fat, and 15% protein) [15, 16]. In comparison, the IF approach used in our laboratory is a modified IF regimen, which allows consumption of 20% to 25% of energy needs on scheduled fasting days (1‐2/wk), combined with a P (IF‐P) meal plan consisting of 35% to 45% carbohydrate, 20% to 30% fat, and 30% to 35% protein for 5 or 6 days weekly [3]. In addition, to our knowledge, no research has directly compared the effects of extended (≥36 hours) IF‐P versus a heart‐healthy CR meal pattern, matched for total weekly energy intake (EI), meal frequency (4‐5 meals/d), and physical activity energy expenditure (PAEE) on cardiometabolic health, BW and composition, hormones, and hunger responses, in women and men with overweight and obesity.
Therefore, the purpose of this study was to compare the influence of IF (36‐60 hours) and P (35%; IF‐P) versus a heart‐healthy daily CR (protein, 15%) dietary regimen on total (lean mass and FM) and regional (abdominal/visceral) body composition, cardiometabolic, hormonal, and hunger responses for 8 weeks in women and men with overweight and obesity. We hypothesized that an IF‐P regimen would improve cardiometabolic health, body composition, and satiation compared with a CR regimen.
METHODS
Participants
This study included 200 individuals from the Saratoga Springs, New York, area who expressed interest. Potential participants responded to flyers, local newspapers, or emails advertising the study. The number of participants initially screened was 55, of which 41 were eligible for participation. Participants were healthy nonsmoking men and women with overweight and obesity. Their physicians performed a comprehensive medical examination/history assessment to rule out any current cardiovascular or metabolic disease prior to enrollment. For at least 6 months before the start of the study, all participants were either sedentary or lightly active (<30 minutes, 2 d/wk of organized physical activity), with overweight or obesity (body mass index [BMI] > 27.5 kg/m2; percent body fat >30%), weight stable (± 2 kg), and middle‐aged (30‐65 years). Every participant provided written informed consent in accordance with the Skidmore College Human Subjects Institutional Review Board before participation. The study was approved by the Human Subjects Institutional Review Board of Skidmore College (IRB#: 1911‐859). All experimental procedures were performed in adherence with related New York state regulations and the Federal Wide Assurance, consistent with the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, and in agreement with the Helsinki Declaration (revised in 1983). This trial was registered on March 6, 2020, at ClinicalTrials.gov as NCT04327141.
Experimental design
Participants were enrolled in two separate cohorts because of COVID‐19 restrictions regarding personnel laboratory access, such that half enrolled in fall (September through November) 2020 and the other half in spring (March through May) 2021. Participants were matched for BW, BMI, and body fat and randomly assigned in parallel to one of two CR groups: 1) IF‐P (n = 21); or 2) a heart‐healthy daily CR diet (n = 20) for 8 weeks. The IF‐P was further subdivided into two groups for the first 4 weeks only by randomly assigning participants to one of two groups: 1) IF 1 d/wk (36 hours total) and P regimen for the remaining 6 d/wk (IF1‐P); or 2) IF for two consecutive days (60 hours total) and P for the remaining 5 d/wk (IF2‐P) for 4 weeks. Both groups consumed similar total weekly calories throughout the 4 weeks. The comparison of the IF1‐P versus IF2‐P has been previously published [17]. Beginning at week 5, all participants in IF‐P (IF1‐P and IF2‐P) followed identical eating patterns of IF 1 d/wk (36 hours) and P the remaining 6 d/wk. Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) study flow chart.
FIGURE 1.
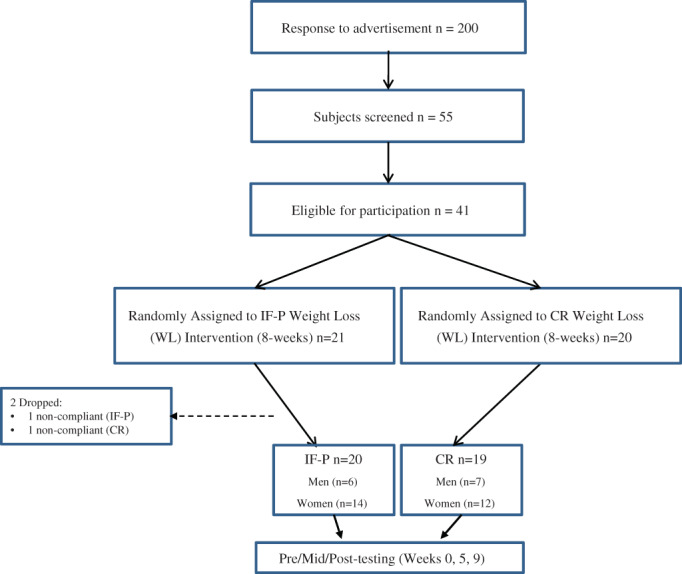
CONSORT (Consolidated Standards of Reporting Trials) flow diagram for the study. CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing [Color figure can be viewed at wileyonlinelibrary.com]
All laboratory testing procedures (see the Laboratory testing procedures section) were performed at weeks 0 (baseline control [CON]), 5 (mid), and 9 (post) (Figure 2), with the exception of abdominal fat (AF), VF, and subcutaneous AF mass measured using dual‐energy x‐ray absorptiometry (DXA) at weeks 0 (CON) and 9 (post) only. In addition, dietary intake and physical activity assessments were completed during weeks 0, 4, and 8, as noted.
FIGURE 2.
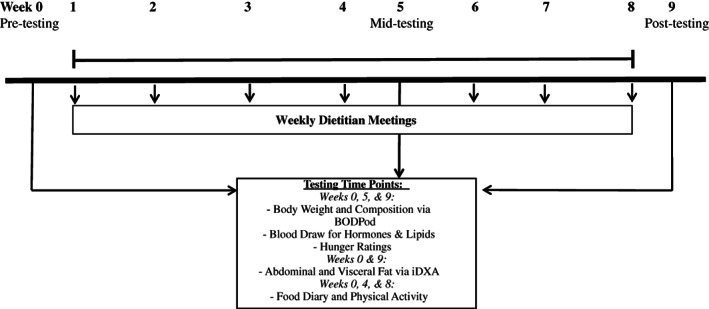
Study timeline for testing during IF‐P vs. CR study. Pre‐testing, week 0; mid‐testing, week 5; post‐testing, week 9. All participants were tested between 6:00 a.m. and 9:00 a.m. after an overnight fast for body weight and composition assessments (body weight and total body composition via BODPod), plasma biomarkers, and hunger ratings at weeks 0, 5, and 9. At weeks 0 and 9, participants were also assessed for abdominal and visceral fat mass via iDXA and, at weeks 0, 4, and 8, for dietary intake (2‐day food records) and physical activity (accelerometry via ActiGraph). CR, heart‐healthy daily caloric restriction; DXA, dual‐energy x‐ray absorptiometry; IF‐P, intermittent fasting and protein pacing
The WL intervention began with all participants following CR meal plans, as detailed in the following sections. During the 1‐week baseline CON, participants maintained a stable BW by consuming a similar caloric intake as their pre‐enrollment caloric intake while maintaining their sedentary lifestyle. Following CON baseline testing, participants were provided with detailed instructions on their WL dietary guidelines and scheduled weekly 1‐hour meetings with a registered dietitian.
Dietary interventions
IF‐P diet
IF day meals
IF1‐P consisted of ~400 kcal/d, in which participants were provided a variety of supplements and snacks (online Supporting Information Methods). IF2‐P followed an identical meal pattern for both IF days, except for consuming an additional 100 kcal from the “options” list to achieve ~500 kcal/d for each of the two consecutive fasting days during weeks 1 through 4, in order to achieve a similar weekly total EI as IF1‐P. Beginning in week 5, IF2‐P followed the identical meal pattern as IF1‐P, and the combined group is referred to as IF‐P for the week 8 (post) comparisons. Sample menus and meal timing for IF1‐P and IF2‐P fasting days are shown in Supporting Information Table S1.
P day diet
P days for IF1‐P consisted of four and five meals per day providing 1350 and 1700 kcal/d for women and men, respectively, and a macronutrient distribution targeting 35% protein, 35% carbohydrate, and 30% fat. IF2‐P followed a similar P meal protocol providing 1500 and 1850 kcal/d for women and men, respectively, and similar macronutrient distribution and total weekly calorie intakes (~8500 kcal/wk) as IF1‐1 for weeks 1 through 4. Thereafter, IF1‐P and IF2‐P followed identical meal plans.
CR diet
Participants assigned to the CR diet followed specific guidelines of the National Cholesterol Education Program Therapeutic Lifestyle Changes (TLC) diet of the American Heart Association. The specific macronutrient distribution was <35% of kcalories as fat; 50% to 60% of kilocalories as carbohydrates; <200 mg/dL of dietary cholesterol; 20 to 30 g/d of fiber; and low sugar intake (<50 g/d). The total calorie intake was 1200 and 1500 kcal/d for women and men, respectively, during the 8‐week WL intervention. Sample menus and meal timing for IF‐P and CR days are shown in Supporting Information Table S1.
Compliance
Noncompliance was defined as being absent from more than two consecutive dietitian meetings and under‐ or overconsuming the prescribed EI amounts for ≥3 meal/supplement servings a week for ≥2 consecutive weeks.
Laboratory testing procedures
Body composition assessments
All participants were tested between 6:00 a.m. and 9:00 a.m. after an overnight fast for body composition assessments (height, BW, and total body composition) at weeks 0, 5, and 9. BW was obtained in spandex shorts and sports bra (women) with shoes/socks removed, using a calibrated standard digital scale (53080 model number FS0900, Befour Inc.), and height was obtained without shoes/socks using a stadiometer. Waist circumferences in centimeters were obtained with a standard tape measure placed around the waist 2 cm above the iliac crest by the same investigator (Karen M. Arciero) at each time point. Body composition was assessed by BODPod (Cosmed) at each testing period (weeks 0, 5, and 9) for the measurement of total FM, percent body fat, FFM, and percent FFM (BW/FFM) and by DXA (iDXA; Lunar iDXA, GE Healthcare) at weeks 0 and 9 only for the measurement of AF, VF, and subcutaneous AF mass to limit exposure to radiation.
Energy balance assessment
Energy balance was calculated for each individual by closely monitoring both PAEE (Actigraph LLC, Pensacola, Florida), as well as EI, using the Food Processor SQL Edition (version 11.6.522; ESHA Research, 2022) for 2 days during CON (week 0), week 4, and week 8 (Supporting Information Methods), as previously described [17]. To verify sedentary/low activity levels, all participants wore an Actigraph Data Analysis Software accelerometer (version 6.13.3; Actigraph) around their waist for 2 days during weeks 0, 4, and 8.
Cardiovascular and plasma biomarkers
Blood pressure and heart rate were obtained with an automated blood pressure monitor (Omron Healthcare Inc.) following >15 minutes of quiet sitting. Plasma hormone measurements (12‐hour fasted; ~20 mL) were collected into EDTA‐coated vacuum tubes and centrifuged (Hettich Rotina 46R5) for 15 minutes at 2500 rpm at −4 °C. After separation, plasma was collected in triplicate and stored at −80 °C until analysis.
Feelings of hunger and satiety
Visual analog scales were administered at weeks 0, 5, and 9 to evaluate the effects of the dietary protocols on hunger, satiation, the quantity of food to eat, and desire to eat, as previously described [17]. All visual analog scale scoring was measured by the same investigator (Michelle Poe).
Statistical analysis
Statistical analysis was performed using SPSS Statistics software (version 27; IBM Corp.). Sample size was determined through power analysis based on the primary outcome variables of BW and body composition to achieve an effect size of F = 0.21 with 80% power at α = 0.05 based on previous data [18]. This analysis determined that n = 38 total sample size was required to detect a significant mean difference of 1.4‐kg WL between the two diet intervention groups (IF‐P vs. CR) during WL. Two‐way factorial mixed model ANOVA was constructed using diet and time to determine the main and interaction effects. Post hoc comparisons (Bonferroni) were performed where appropriate. Data analysis was not performed blinded, but each intervention group was assigned a number code. A per‐protocol approach was used on data for all compliant study participants, and this is presented in the Results section. Two‐tailed tests were used for this study, and the significance was set at p < 0.05. To adjust for multiple hypothesis testing on the primary outcomes, a false discovery rate correction was used with a significance level of p.adj <0.05. Unless stated otherwise, all values are reported as means ± standard error (SE). Normality statistics were generated to test normality assumptions, and log transformations were performed as appropriate.
RESULTS
WL, weeks 0 through 9
The overall compliance rate in each group was high (>90%), defined as consuming more than 90% of the respective meals/supplemented feedings. One participant from each group (IF‐P and CR) was noncompliant with the dietary guidelines and was dropped from the analysis. Therefore, descriptive baseline characteristics of the 39 participants (26 women and 13 men) who completed WL are reported in Table 1. Both groups were similar for all variables at baseline.
TABLE 1.
Baseline (week 0) characteristics of participants for WL
| IF‐P (n = 20) | CR (n = 19) | |
|---|---|---|
| Age (y) | 49.7 ± 2.1 | 50.7 ± 2.4 |
| Height (cm) | 169.6 ± 2.6 | 166.8 ± 1.7 |
| Weight (kg) | 93.1 ± 5.1 | 92.6 ± 5.5 |
| Body fat (%) | 43.4 ± 1.5 | 44.1 ± 1.6 |
| BMI (kg/m2) | 32.4 ± 1.7 | 33.0 ± 1.6 |
| Waist circumference (cm) | 103.4 ± 3.4 | 105.4 ± 3.7 |
| Systolic blood pressure (mm Hg) | 123 ± 4 | 128 ± 3 |
| Diastolic blood pressure (mm Hg) | 85 ± 2 | 85 ± 2 |
| Resting heart rate (beats/min) | 72 ± 2 | 67 ± 1 |
Note: Values are means ± SE.
Abbreviations: CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing; WL, weight loss.
Dietary intake and physical activity during WL
The IF‐P and CR WL diet interventions significantly changed both groups' dietary intake (Table 2).
TABLE 2.
Changes in dietary intake and physical activity during WL
| Variable | IF‐P (n = 20) | CR (n = 19) | |
|---|---|---|---|
| Energy intake (kcal/d) a | Pre | 2468 ± 109 | 2408 ± 146 |
| Mid | 1458 ± 69 | 1429 ± 42 | |
| Post | 1516 ± 84 | 1362 ± 68 | |
| Energy intake (kcal/wk) a | Pre | 17,275 ± 763 | 16,855 ± 1023 |
| Mid | 8723 ± 406 | 9896 ± 335 | |
| Post | 9470 ± 550 | 9095 ± 608 | |
| Protein (%) a , b | Pre | 16 ± 1 | 15 ± 1 |
| Mid | 35 ± 2 | 21 ± 1 | |
| Post | 35 ± 2 | 21 ± 1 | |
| Protein (g) b | Pre | 99 ± 7 | 94 ± 9 |
| Mid | 123 ± 8 | 74 ± 3 | |
| Post | 127 ± 7 | 70 ± 5 | |
| Fat (%) a | Pre | 39 ± 2 | 40 ± 2 |
| Mid | 31 ± 1 | 37 ± 2 | |
| Post | 31 ± 2 | 38 ± 3 | |
| Fat (g) a | Pre | 107 ± 6 | 107 ± 8 |
| Mid | 51 ± 4 | 60 ± 4 | |
| Post | 52 ± 5 | 57 ± 5 | |
| Carbohydrate (%) a | Pre | 42 ± 2 | 42 ± 2 |
| Mid | 35 ± 3 | 41 ± 2 | |
| Post | 33 ± 1 | 41 ± 2 | |
| Carbohydrates (g) a | Pre | 263 ± 17 | 258 ± 20 |
| Mid | 117 ± 10 | 148 ± 9 | |
| Post | 123 ± 11 | 137 ± 12 | |
| Sodium (mg) a | Pre | 3327 ± 274 | 4223 ± 646 |
| Mid | 1529 ± 147 | 1708 ± 117 | |
| Post | 1574 ± 156 | 1834 ± 198 | |
| Fiber (g) a , b | Pre | 20 ± 2 | 24 ± 3 |
| Mid | 29 ± 2 | 23 ± 2 | |
| Post | 26 ± 2 | 24 ± 2 | |
| Sugar (g) a , b | Pre | 103 ± 11 | 81 ± 9 |
| Mid | 36 ± 5 | 47 ± 6 | |
| Post | 37 ± 4 | 45 ± 6 | |
| Physical activity (kcal/d) | Pre | 287 ± 41 | 376 ± 50 |
| Mid | 253 ± 29 | 347 ± 45 | |
| Post | 346 ± 45 | 350 ± 53 |
Note: Values are means ± SE.
Abbreviations: CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing.
Significant time effect (pre vs. mid vs. post), p < 0.05.
Significant time × group effect (pre vs. mid vs. post; IF‐P vs. CR), p < 0.05.
Total EI was reduced by ~40%, or 1000 kcal/d (p < 0.001), in both groups over the 8‐week intervention (pre vs. post), with no differences between groups. This reduction was attributed to total gram and percentage decreases in dietary fat and carbohydrate intake, respectively (p < 0.001). In contrast, total protein grams and percentage of protein intake increased to a significantly greater magnitude in IF‐P compared with CR (p < 0.001). Moreover, dietary fiber (p < 0.005) increased and sugar (p < 0.035) decreased significantly in IF‐P compared with CR. Both groups maintained similar amounts of PAEE (~350 kcal/d; p > 0.260) throughout the WL period.
BW and composition during WL
BW and composition changes following IF‐P and CR are shown in Table 3.
TABLE 3.
Changes in BW and composition during WL
| Variable | IF‐P (n = 20) | CR (n = 19) | |
|---|---|---|---|
| BW (kg) a , b | Pre | 93.1 ± 5.1 | 92.6 ± 5.5 |
| Mid | 87.3 ± 4.5 | 89.3 ± 5.3 | |
| Post | 84.9 ± 4.2 | 87.6 ± 5.3 | |
| WC (cm) a , b | Pre | 103.4 ± 3.4 | 105.4 ± 3.7 |
| Mid | 97.0 ± 3.5 | 102.5 ± 3.6 | |
| Post | 93.6 ± 3.3 | 98.9 ± 3.6 | |
| BMI (kg/m2) a , b | Pre | 32.4 ± 1.7 | 33.0 ± 1.6 |
| Mid | 30.4 ± 1.5 | 31.9 ± 1.5 | |
| Post | 29.6 ± 1.5 | 31.2 ± 1.6 | |
| %BF (%) a , b | Pre | 40.1 ± 1.8 | 41.5 ± 1.9 |
| Mid | 38.1 ± 1.8 | 40.7 ± 1.9 | |
| Post | 36.7 ± 1.9 | 39.7 ± 1.9 | |
| FM (kg) a , b | Pre | 37.3 ± 3.1 | 38.7 ± 3.3 |
| Mid | 33.3 ± 2.7 | 36.7 ± 3.2 | |
| Post | 31.3 ± 2.6 | 35.2 ± 3.3 | |
| AF mass (kg) a , b | Pre | 3.9 ± 0.4 | 4.0 ± 0.4 |
| Post | 3.0 ± 0.3 | 3.5 ± 0.4 | |
| VF mass (kg) a , b | Pre | 1.8 ± 0.3 | 1.9 ± 0.3 |
| Post | 1.2 ± 0.2 | 1.6 ± 0.3 | |
| Subcutaneous AF (kg) a , b | Pre | 2.6 ± 0.3 | 2.8 ± 0.3 |
| Post | 2.0 ± 0.2 | 2.5 ± 0.2 | |
| FFM (kg) a | Pre | 54.8 ± 2.9 | 53.0 ± 2.9 |
| Mid | 53.2 ± 2.8 | 52.0 ± 2.8 | |
| Post | 53.0 ± 2.7 | 51.7 ± 2.7 | |
| FFM/BW (%) a , b | Pre | 59.9 ± 1.8 | 58.5 ± 1.9 |
| Mid | 61.9 ± 1.8 | 59.3 ± 1.9 | |
| Post | 63.3 ± 1.9 | 60.3 ± 2.0 |
Note: Values are means ± SE; AF, VF, and subcutaneous AF mass were measured using DXA at pre and post only.
Abbreviations: AF, abdominal fat; BF, body fat; BW, body weight; CR, heart‐healthy daily caloric restriction; FM, fat mass; FFM, fat‐free mass; IF‐P, intermittent fasting and protein pacing; VF, visceral fat; WC, waist circumference; WL, weight loss.
Significant time effect (pre vs. mid vs. post), p.adj <0.05.
Significant time × group effect (pre vs. mid vs. post; IF‐P vs. CR), p.adj <0.05.
Compared with baseline, both IF‐P and CR participants improved all body composition measures (p.adj <0.05), with IF‐P producing significantly greater improvements in all outcomes on an absolute and relative basis (Figure 3A ‐3F). Specifically, IF‐P lost significantly more total BW (−8.2 kg vs. −5.0 kg; p = 0.009, or − 9% vs. −5% of initial BW; p = 0.003), total body fat (−8.5% vs. −4.3%; p = 0.019), AF (−23% vs. −12.5%; p = 0.018), and VF (−33% vs. −15.8%; p = 0.030) mass. Most interestingly, whereas absolute FFM decreased in both groups (~1.5 kg), the proportion of FFM to total BW increased significantly following IF‐P compared with CR (5.7% vs. 3%, respectively; p = 0.030).
FIGURE 3.
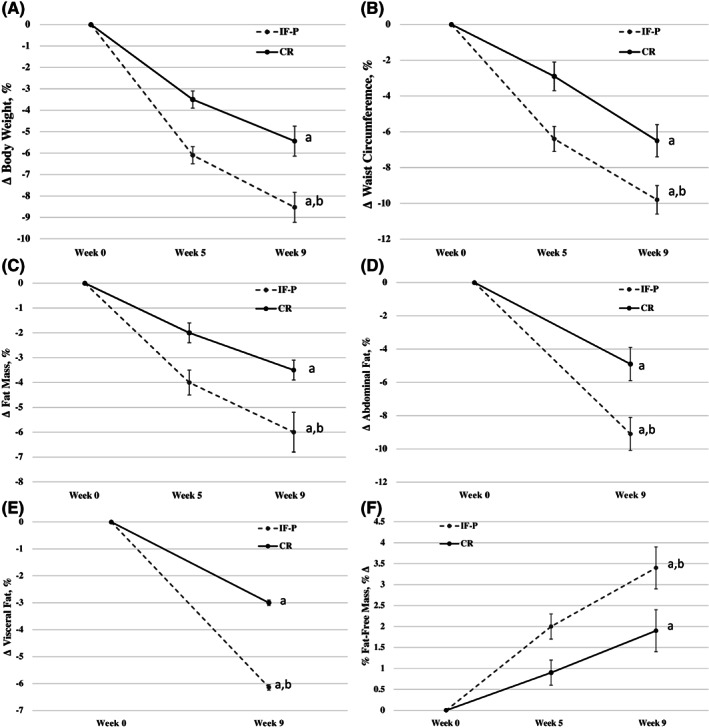
(A) Changes in body weight with IF‐P and CR over 8 weeks. (B) Changes in waist circumference with IF‐P and CR over 8 weeks. (C) Changes in total body fat mass with IF‐P and CR over 8 weeks. (D) Changes in abdominal fat mass with IF‐P and CR over 8 weeks. (E) Changes in visceral fat mass with IF‐P and CR over 8 weeks. (F) Changes in proportion of lean body mass with IF‐P and CR over 8 weeks. a p.adj <0.05, pre vs. post pairwise differences; b p.adj <0.05, group × time interaction. Data are means ± SE. CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing
Cardiometabolic and hormonal responses during WL
Both nutritional interventions resulted in significant (p < 0.05) improvements in cardiovascular outcomes (Table 4). For example, blood pressure (p = 0.001), total cholesterol (p = 0.001), low‐density lipoprotein cholesterol (p = 0.001), and triglycerides (p = 0.002) were reduced significantly in both IF‐P and CR groups at each time during the intervention, with no differences between groups. High‐density lipoprotein cholesterol (HDL‐C) decreased significantly in IF‐P compared with CR (p = 0.008); however, the Total Cholesterol:HDL‐C ratio was consistent throughout the intervention for both groups.
TABLE 4.
Changes in cardiometabolic and hormonal variables during WL
| Variable | IF‐P (n = 20) | CR (n = 19) | |
|---|---|---|---|
| SBP (mm Hg) a | Pre | 123 ± 3.5 | 128 ± 3.3 |
| Mid | 117 ± 3.4 | 123 ± 3.1 | |
| Post | 119 ± 3.2 | 121 ± 3.1 | |
| DBP (mm Hg) a | Pre | 85 ± 1.7 | 85 ± 1.9 |
| Mid | 81 ± 2.1 | 80 ± 2.4 | |
| Post | 78 ± 1.8 | 80 ± 2.3 | |
| Total cholesterol (mg/dl) a | Pre | 182.9 ± 7.5 | 184.0 ± 6.0 |
| Mid | 153.7 ± 8.2 | 166.3 ± 7.6 | |
| Post | 160.0 ± 7.2 | 170.0 ± 7.3 | |
| LDL‐C (mg/dL) a | Pre | 108.1 ± 7.1 | 102.2 ± 5.8 |
| Mid | 95.7 ± 7.6 | 91.2 ± 6.2 | |
| Post | 97.6 ± 7.6 | 93.5 ± 6.0 | |
| HDL‐C (mg/dL) a , b | Pre | 52.9 ± 3.4 | 55.1 ± 4.2 |
| Mid | 42.3 ± 2.6 | 53.8 ± 4.4 | |
| Post | 46.5 ± 3.3 | 53.3 ± 4.6 | |
| Triglycerides (mg/dL) a | Pre | 109.6 ± 17.5 | 133.9 ± 14.5 |
| Mid | 79.0 ± 7.1 | 106.4 ± 14.2 | |
| Post | 75.3 ± 7.8 | 114.0 ± 17.0 | |
| TC:HDL (mg/dL) | Pre | 3.9 ± 0.3 | 3.7 ± 0.3 |
| Mid | 3.9 ± 0.3 | 3.4 ± 0.3 | |
| Post | 3.8 ± 0.3 | 3.6 ± 0.3 | |
| Glucose (mg/dL) | Pre | 93.6 ± 3.7 | 95.4 ± 4.0 |
| Mid | 91.1 ± 2.2 | 96.0 ± 2.2 | |
| Post | 93.5 ± 2.8 | 94.8 ± 3.3 | |
| Insulin (μU/mL) | Pre | 10.4 ± 0.6 | 9.4 ± 0.9 |
| Mid | 9.9 ± 0.5 | 8.9 ± 0.9 | |
| Post | 10.5 ± 0.6 | 9.1 ± 0.9 | |
| Ghrelin (pg/mL) | Pre | 423.8 ± 43.1 | 405.1 ± 48.0 |
| Mid | 421.5 ± 25.1 | 423.7 ± 40.9 | |
| Post | 438.5 ± 37.0 | 433.4 ± 34.9 | |
| Glucagon (pg/mL) | Pre | 41.6 ± 4.5 | 53.9 ± 8.4 |
| Mid | 45.0 ± 3.4 | 54.6 ± 7.6 | |
| Post | 49.5 ± 9.2 | 49.3 ± 8.6 | |
| IGF‐1 (ng/mL) | Pre | 47.2 ± 3.7 | 37.2 ± 2.3 |
| Mid | 50.1 ± 3.8 | 38.5 ± 3.2 | |
| Post | 53.4 ± 4.2 | 37.7 ± 2.6 |
Note: Values are means ± SE.
Abbreviations: CR, heart‐healthy daily caloric restriction; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; IF‐P, intermittent fasting and protein pacing; IGF‐1, insulinlike growth factor; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; WL, weight loss.
Significant time effect (pre vs. mid vs. post), p < 0.05.
Significant time × group effect (IF‐P vs. CR; pre vs. mid vs. post), p < 0.05.
Plasma hormones are also shown in Table 4 and indicate no differences within and between groups.
Hunger ratings during WL
Self‐reported feelings of hunger, desire to eat, the quantity of food to eat, and fullness are shown in Table 5. The desire (p = 0.046) and quantity of food to eat (p = 0.015) decreased significantly (p < 0.05) in both IF‐P and CR. However, the absolute change in desire to eat decreased significantly in IF‐P compared with CR (−40% vs. 2.4%; p = 0.046).
TABLE 5.
Changes in hunger ratings during WL
| Variable | IF‐P (n = 20) | CR (n = 19) | |
|---|---|---|---|
| Hunger (mm) | Pre | 36.3 ± 4.7 | 32.4 ± 5.2 |
| Mid | 26.9 ± 3.9 | 31.8 ± 5.8 | |
| Post | 27.5 ± 3.8 | 28.4 ± 4.8 | |
| Desire to eat (mm) a , b | Pre | 42.9 ± 5.0 | 37.2 ± 5.4 |
| Mid | 24.7 ± 3.7 | 33.6 ± 6.8 | |
| Post | 25.6 ± 3.4 | 38.1 ± 5.4 | |
| Quantity to eat (mm) a | Pre | 46.8 ± 4.6 | 44.4 ± 4.9 |
| Mid | 33.3 ± 3.8 | 40.0 ± 5.6 | |
| Post | 33.6 ± 3.8 | 35.2 ± 4.5 | |
| Fullness (mm) | Pre | 50.6 ± 4.7 | 44.1 ± 4.8 |
| Mid | 48.8 ± 4.2 | 52.4 ± 5.0 | |
| Post | 46.3 ± 4.3 | 46.1 ± 3.1 |
Note: Values are means ± SE.
Significant time effect (pre vs. mid vs. post), p < 0.05.
Significant time × group effect (IF‐P vs. CR; pre vs. mid vs. post), p < 0.05.
Abbreviations: CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing; WL, weight loss.
DISCUSSION
This study shows that 8 weeks of IF‐P significantly enhances body composition and food‐intake behaviors more than a CR regimen in middle‐aged women and men with overweight and obesity. These favorable effects appear independent of alterations in circulating hormones and differences in energy balance. Furthermore, the current findings' public health impact and messaging support an emphasis on the quality of nutrient‐density intake, including reduced sugar and increased protein and fiber intake, instead of the total quantity of food consumption to augment WL [19], body composition, and food‐intake behaviors for adults with overweight. These findings corroborate previous data from our laboratory supporting enhanced body composition and food intake behaviors in women and men with obesity following IF‐P versus heart‐healthy daily CR during a 1‐year WL maintenance period [3, 13].
BW and body composition
To our knowledge, the current findings are the first to compare an IF‐P regimen directly versus a CR diet, both of which were low‐sugar, high nutrient‐density, and isocaloric, on WL and body composition. Most notable, the IF‐P group lost >3 kg of total BW and twice as much FM and VF while increasing the proportion of FFM (FFM/total body mass) by 100% compared with the CR group. The per‐week WL in IF‐P agree with governing bodies' “healthy weight loss” classification. In the current study, both groups had similar energy intakes (~1400 kcal/d) and PAEE (~350 kcal/d) over the entire 8‐week intervention; however, IF‐P lost >3 kg BW compared with CR. Recent work has challenged this [20], and previous work from our laboratory has refuted its viability by showing a metabolic advantage to CR with increased dietary protein and meal frequency compared with isocaloric, lower‐moderate protein intake and three meals per day [2].
Our finding of increased WL/fat loss with IF agrees with recent investigations and meta‐analyses [10] and contrasts with others [21]. We extend these previous studies by comparing IF‐P with a widely researched and implemented diet often recommended to support healthy weight management and enhance cardiometabolic health [15]. The P component of IF‐P played an instrumental role in the favorable body composition outcomes, particularly attenuation of loss of FFM [22]. Interestingly, the increased proportion of FFM in IF‐P compared with CR was associated with significantly lower sugar and increased fiber intakes in IF‐P, despite comparable weekly nutrition counseling by the registered dietitian. Whether the increased sugar and lower fiber intake induced an insulin‐mediated fat deposition or blunting of fat oxidation via the carbohydrate‐insulin model remains to be determined [20].
An improved body composition status with calorie‐restricted IF protocols compared with daily CR is less common [21, 23]. Previous research, including from our laboratory [3, 17, 22], shows that increased dietary protein consumption during calorie‐restricted IF protocols substantially enhances FFM preservation, suggesting enhanced protein synthesis [24, 25]. Given the well‐established greater amount of leucine present in animal protein sources, including whey protein, IF‐P improved FFM retention compared with CR [26]. In some cases, IF protocols induce losses of FFM up to 60% of the total weight lost [23]. However, the current study minimized losses of absolute FFM to 20% and increased relative amounts by more than double in IF‐P compared with CR (6% vs. 3%). Therefore, P should be considered in IF protocols to facilitate healthy WL among the adult population.
Plasma biomarkers
We observed little impact of IF‐P and CR interventions on plasma hormones throughout the intervention, suggesting other factors, including blood biomarkers not measured in the current study, may be responsible. This finding is in opposition to previous findings from our laboratory [3] and others [8]. The primary reason for this discrepancy is the previous study participants were more at risk of cardiometabolic disease, whereas the current participants were relatively healthy adults with overweight and obesity. Another plausible explanation for the lack of notable change in hormonal and metabolic biomarkers in the current study is that a longer nutritional intervention period may be necessary to influence these outcomes in a physiological meaningful way. However, others have noted significant changes may occur in 5 weeks or less [5, 27, 28].
Cardiovascular responses
IF‐P and CR interventions significantly improved cardiometabolic health, including reductions in blood pressure, total and low‐density lipoprotein cholesterol, and triglycerides, all of which support previous findings [9, 10, 13, 29]. HDL‐C also decreased in both groups and to a greater extent in IF‐P; however, this did not affect the cardiovascular disease risk profile (TC:HDL‐C). The overall reduction in cardiometabolic risk factors in the current study is particularly noteworthy, as the majority of participants were within normal healthy ranges for all variables (healthy adults with overweight and obesity) but experienced significant improvement during the 8‐week intervention. The beneficial effect of both nutrition interventions may be explained by reduced vascular oxidative stress and inflammation [30], as well as enhanced parasympathetic tone associated with neurotrophic factors and insulin sensitivity [31].
Hunger ratings
It is well known that both short‐ and long‐term calorie restriction increases appetite‐related changes [32, 33]. Moreover, previous research has shown decreased satiation (increased hunger) ratings in IF compared with CR [34], no difference [35], or an increase in satiation, similar to the current study [36]. These disparate findings suggest reduced feelings of hunger and/or increased satiation may be sustainable mechanisms for successful WL during IF or daily CR [37] and support cognitive control of eating restraint as an adaptive approach needed for optimal weight management [38]. IF‐P resulted in a 42% reduction in desire to eat compared with CR. It has been speculated that increased body fat lost during CR creates less satiation, leading to increased subsequent food intake, which may minimize additional reductions in FM and may even replace energy stores that have been depleted [21].
Strengths and limitations
Several strengths of the current study include the following: 1) direct comparison within and between IF‐P and daily CR interventions; 2) monitoring and counseling of the nutrition regimens with a registered dietitian weekly; 3) measuring PAEE with accelerometry; 4) standardization of all measurements and laboratory procedures; 5) excellent compliance (>97%); and 6) balancing the complexities of conducting the study during the COVID‐19 pandemic starting in fall of 2020 through the spring of 2021.
There are several limitations associated with the current study: 1) inclusion of more participants and extending the length of the intervention are necessary in future investigations; 2) resting energy expenditure was not assessed in the current study and may have contributed to the enhanced body composition (WL and fat loss, increased proportion of lean body mass) and satiation in IF‐P, although previous investigations have not supported this mechanism; 3) circulating ketone concentrations were not measured and are surrogate markers for fat oxidation but were not feasible in the current study due to scheduling conflicts and stringent COVID‐19 laboratory access restrictions; and 4) participants had weekly interaction with research team members, including a registered dietitian, that, although fostered compliance, may have increased the risk of investigator bias. The current findings should be extended to longer IF and daily CR interventions beyond a year in adults with overweight and obesity.
CONCLUSION
Our results provide compelling support for both IF‐P and heart‐healthy daily CR nutritional regimens to enhance all aspects of body composition (BW, waist circumference, total FM, percent FFM), cardiovascular (systolic and diastolic blood pressure, blood lipids), and satiety responses. IF‐P proved superior on BW, FM, and VF loss and increased proportion of FFM and satiety compared with heart‐healthy CR regimens in women and men with overweight and obesity.
AUTHOR CONTRIBUTIONS
Paul J. Arciero designed the study, supervised subject recruitment, completed data collection and analysis, completed manuscript preparation and publication, obtained the grant, and served as the study principal investigator; Karen M. Arciero, Michelle Poe, and Autumn Arciero assisted in the design of the study, subject recruitment, data collection and analysis, and manuscript preparation; Eric Gumpricht, Alex E. Mohr, and Karen L. Sweazea assisted with manuscript preparation and publication; Stephen J. Ives assisted with data analysis and manuscript preparation. All authors read and approved the final manuscript.
FUNDING INFORMATION
This study was supported by a grant (IRB#: 1911‐859) from Isagenix International LLC through an unrestricted research grant to Skidmore College and Paul J. Arciero. The funder had no roles in the study design, data collection and analysis, and decision to publish.
CONFLICT OF INTEREST
Paul J. Arciero is a scientific advisory board member and consultant for Isagenix International LLC, the study's sponsor, he is an advisory board member of the International Protein Board (iPB), and he receives financial compensation for books and keynote presentations on protein pacing (www.paularciero.com). Eric Gumpricht and Alex E. Mohr are employed by Isagenix International. No authors have financial interests regarding the outcomes of this investigation. The other authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier NCT04327141.
Supporting information
Appendix S1. Supporting Information.
Figure S1. Individual participant response to IF‐P and CR interventions over 8 weeks. p < 0.05 group × time interaction. CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing.
Table S1. Sample menus and meal timing for IF‐P days and CR study participants during 8‐week WL. CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing; WL, weight loss.
ACKNOWLEDGMENTS
We are thankful for the time and effort put forth by all study volunteers during both phases of the study. We are grateful for the assistance of Carmen Barrios Castellanos and Jamie Martin, RD, throughout the entire intervention.
Arciero PJ, Poe M, Mohr AE, et al. Intermittent fasting and protein pacing are superior to caloric restriction for weight and visceral fat loss. Obesity (Silver Spring). 2023;31(Suppl. 1):139‐149. doi: 10.1002/oby.23660
Funding information Isagenix International LLC, Grant/Award Number: 1911‐899
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet. 2016;387:1947‐1956. [DOI] [PubMed] [Google Scholar]
- 2. Arciero PJ, Ormsbee MJ, Gentile CL, Nindl BC, Brestoff JR, Ruby M. Increased protein intake and meal frequency reduces abdominal fat during energy balance and energy deficit. Obesity (Silver Spring). 2013;21:1357‐1366. [DOI] [PubMed] [Google Scholar]
- 3. Arciero PJ, Edmonds R, He F, et al. Protein‐pacing caloric‐restriction enhances body composition similarly in obese men and women during weight loss and sustains efficacy during long‐term weight maintenance. Nutrients. 2016;8:476. doi: 10.3390/nu8080476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anton SD, Moehl K, Donahoo WT, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring). 2018;26:254‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541‐2551. [DOI] [PubMed] [Google Scholar]
- 6. Cioffi I, Evangelista A, Ponzo V, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta‐analysis of randomized controlled trials. J Transl Med. 2018;16:371. doi: 10.1186/s12967-018-1748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris L, Hamilton S, Azevedo LB, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta‐analysis. JBI Database System Rev Implement Rep. 2018;16:507‐547. [DOI] [PubMed] [Google Scholar]
- 8. Allaf M, Elghazaly H, Mohamed OG, et al. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2021;1:CD013496. doi: 10.1002/14651858.CD013496.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu D, Huang Y, Huang C, et al. Calorie restriction with or without time‐restricted eating in weight loss. N Engl J Med. 2022;386:1495‐1504. [DOI] [PubMed] [Google Scholar]
- 10. He S, Wang J, Zhang J, Xu J. Intermittent versus continuous energy restriction for weight loss and metabolic improvement: a meta‐analysis and systematic review. Obesity (Silver Spring). 2021;29:108‐115. [DOI] [PubMed] [Google Scholar]
- 11. Sundfør TM, Tonstad S, Svendsen M. Effects of intermittent versus continuous energy restriction for weight loss on diet quality and eating behavior. A randomized trial. Eur J Clin Nutr. 2019;73:1006‐1014. [DOI] [PubMed] [Google Scholar]
- 12. Mohr AE, McEvoy C, Sears DD, Arciero PJ, Sweazea KL. Impact of intermittent fasting regimens on circulating markers of oxidative stress in overweight and obese humans: a systematic review of randomized controlled trials. Adv Redox Res. 2021;3:100026. doi: 10.1016/j.arres.2021.100026 [DOI] [Google Scholar]
- 13. Zuo L, He F, Tinsley GM, Pannell BK, Ward E, Arciero PJ. Comparison of high‐protein, intermittent fasting low‐calorie diet and heart healthy diet for vascular health of the obese. Front Physiol. 2016;7:350. doi: 10.3389/fphys.2016.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He F, Zuo L, Ward E, Arciero PJ. Serum polychlorinated biphenyls increase and oxidative stress decreases with a protein‐pacing caloric restriction diet in obese men and women. Int J Environ Res Public Health. 2017;14:59. doi: 10.3390/ijerph14010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation. 2021;144:e472‐e487. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2020–2025 Dietary Guidelines for Americans. 9th ed. Published December 2020. Accessed October 19, 2022. https://www.dietaryguidelines.gov/
- 17. Arciero PJ, Arciero KM, Poe M, et al. Intermittent fasting two days versus one day per week, matched for total energy intake and expenditure, increases weight loss in overweight/obese men and women. Nutr J. 2022;21:36. doi: 10.1186/s12937-022-00790-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arciero PJ, Baur D, Connelly S, Ormsbee MJ. Timed‐daily ingestion of whey protein and exercise training reduces visceral adipose tissue mass and improves insulin resistance: the PRISE study. J Appl Physiol (1985). 2014;117:1‐10. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:267‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ludwig DS, Aronne LJ, Astrup A, et al. The carbohydrate‐insulin model: a physiological perspective on the obesity pandemic. Am J Clin Nutr. 2021;114:1873‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Templeman I, Smith HA, Chowdhury E, et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med. 2021;13:eabd8034. doi: 10.1126/scitranslmed.abd8034 [DOI] [PubMed] [Google Scholar]
- 22. Ogilvie AR, Schlussel Y, Sukumar D, Meng L, Shapses SA. Higher protein intake during caloric restriction improves diet quality and attenuates loss of lean body mass. Obesity (Silver Spring). 2022;30:1411‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laurens C, Grundler F, Damiot A, et al. Is muscle and protein loss relevant in long‐term fasting in healthy men? A prospective trial on physiological adaptations. J Cachexia Sarcopenia Muscle. 2021;12:1690‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen J, Ou Y, Luo R, et al. SAR1B senses leucine levels to regulate mTORC1 signaling. Nature. 2021;596:281‐284. [DOI] [PubMed] [Google Scholar]
- 25. Vellai T. How the amino acid leucine activates the key cell‐growth regulator mTOR. Nature. 2021;596:192‐194. [DOI] [PubMed] [Google Scholar]
- 26. Lim MT, Pan BJ, Toh DWK, Sutanto CN, Kim JE. Animal protein versus plant protein in supporting Lean mass and muscle strength: a systematic review and meta‐analysis of randomized controlled trials. Nutrients. 2021;13:661. doi: 10.3390/nu13020661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu T, Mills KT, Yao L, et al. Effects of low‐carbohydrate diets versus low‐fat diets on metabolic risk factors: a meta‐analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176(suppl 7):S44‐S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hutchison AT, Regmi P, Manoogian ENC, et al. Time‐restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27:724‐732. [DOI] [PubMed] [Google Scholar]
- 29. Jospe MR, Roy M, Brown RC, et al. Intermittent fasting, paleolithic, or Mediterranean diets in the real world: exploratory secondary analyses of a weight‐loss trial that included choice of diet and exercise. Am J Clin Nutr. 2020;111:503‐514. [DOI] [PubMed] [Google Scholar]
- 30. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660‐667. [DOI] [PubMed] [Google Scholar]
- 31. Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well‐being during a 4 to 21‐day fasting period in an observational study including 1422 subjects. PLoS One. 2019;14:e0209353. doi: 10.1371/journal.pone.0209353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deighton K, Batterham RL, Stensel DJ. Appetite and gut peptide responses to exercise and calorie restriction. The effect of modest energy deficits. Appetite. 2014;81:52‐59. [DOI] [PubMed] [Google Scholar]
- 33. Dorling JL, Das SK, Racette SB, et al. Changes in body weight, adherence, and appetite during 2 years of calorie restriction: the CALERIE 2 randomized clinical trial. Eur J Clin Nutr. 2020;74:1210‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1‐year trial. Nutr Metab Cardiovasc Dis. 2018;28:698‐706. [DOI] [PubMed] [Google Scholar]
- 35. Beaulieu K, Casanova N, Oustric P, et al. Matched weight loss through intermittent or continuous energy restriction does not Lead to compensatory increases in appetite and eating behavior in a randomized controlled trial in women with overweight and obesity. J Nutr. 2020;150:623‐633. [DOI] [PubMed] [Google Scholar]
- 36. Coutinho SR, Halset EH, Gasbakk S, et al. Compensatory mechanisms activated with intermittent energy restriction: a randomized control trial. Clin Nutr. 2018;37:815‐823. [DOI] [PubMed] [Google Scholar]
- 37. Hansen TT, Andersen SV, Astrup A, Blundell JE, Sjödin A. Is reducing appetite beneficial for body weight management in the context of overweight and obesity? A systematic review and meta‐analysis from clinical trials assessing body weight management after exposure to satiety enhancing and/or hunger reducing products. Obes Rev. 2019;20:983‐997. [DOI] [PubMed] [Google Scholar]
- 38. Urbanek JK, Metzgar CJ, Hsiao PY, Piehowski KE, Nickols‐Richardson SM. Increase in cognitive eating restraint predicts weight loss and change in other anthropometric measurements in overweight/obese premenopausal women. Appetite. 2015;87:244‐250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Figure S1. Individual participant response to IF‐P and CR interventions over 8 weeks. p < 0.05 group × time interaction. CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing.
Table S1. Sample menus and meal timing for IF‐P days and CR study participants during 8‐week WL. CR, heart‐healthy daily caloric restriction; IF‐P, intermittent fasting and protein pacing; WL, weight loss.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


