Abstract
Background
The SARS‐CoV‐2 pandemic has strained health systems worldwide, and infection numbers continue to rise. While previous data have already shown that many patients suffer from symptoms for months after an acute infection, data on risk factors and long‐term outcomes are incomplete, particularly for the working population.
Objectives
We aimed to provide information on the prevalence of post‐COVID‐19 conditions in a subset of the German working‐age population (18–61 years old) and to analyze risk factors.
Methods
We conducted an online survey with a health questionnaire among registered potential stem cell donors with or without a self‐reported history of polymerase chain reaction (PCR)‐confirmed SARS‐CoV‐2 infection. Logistic regression models were used to examine the risks of severity of acute infection, sex, age, body mass index, diabetes mellitus, and arterial hypertension medication on post‐COVID‐19 symptoms.
Results
A total of 199,377 donors reported evaluable survey questionnaires—12,609 cases had a history of SARS‐CoV‐2 infection and 186,768 controls had none. Overall, cases reported physical, cognitive, and psychological complaints more frequently compared to controls. Increased rates of complaints persisted throughout 15 months postinfection, for example, 28.4%/19.3% of cases/controls reported fatigue (p <0.0001) and 9.5%/3.6% of cases/controls reported loss of concentration (p <0.0001). No significant differences were observed in the frequency of reported symptoms between 3 and 15 months postinfection. Multivariate analysis revealed a strong influence of the severity of the acute SARS‐CoV‐2 infection episode and age on the risk for post‐COVID‐19 conditions.
Conclusion
We report the prevalence of post‐COVID‐19 conditions in mainly unvaccinated individuals with SARS‐CoV‐2 infections between February 2020 and August 2021. The severity of the acute course and age were major risk factors. Vaccinations may reduce the risk of post‐COVID‐19 conditions by reducing the risk of severe infections.
Keywords: COVID‐19, long COVID, post‐COVID‐19 condition, SARS‐CoV‐2, survey
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has caused a pandemic that has strained health systems worldwide since early 2020 [1]. While the initial focus was only on the acute course of the resulting coronavirus disease 2019 (COVID‐19), many COVID‐19 patients struggle with symptoms that persist over several months [2, 3]. Common symptoms include fatigue, shortness of breath, and cognitive impairment, which in many patients lead to a decline in the quality of life [3, 4, 5, 6]. The term “long COVID” summarizes symptoms present later than 4 weeks after SARS‐CoV‐2 infection. The post‐COVID‐19 condition was defined by the WHO in October 2021 as typical symptoms present later than 12 weeks after infection, lasting for at least 2 months, not explained by an alternative diagnosis, “which generally have an impact on everyday functioning” [7].
Lingering symptoms following acute infections have been reported for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome [6, 8]. Patients with post‐COVID‐19 conditions have activated innate immune cells, lack naïve T and B cells, and show elevated expression of interferon (IFN)‐beta and IFN‐lambda‐1 and higher interleukin 6 plasma levels even months after SARS‐CoV‐2 infections, interpreted as an indication of a disrupted immune system [9, 10]. Whether these long‐lasting changes of parameters of the immune system are due to persisting viral material triggering chronic inflammation, autoimmunity, or both is the subject of ongoing research [11, 12, 13, 14, 15]. Chronic inflammation due to a disrupted immune system may affect many tissues directly or indirectly. For example, small fiber neuropathy as diagnosed in individuals with post‐COVID‐19 conditions may explain the dysautonomia of the circulatory and respiratory system [16].
Studies using data from US healthcare providers and electronic health records ascertained that the risk of nonhospitalized SARS‐CoV‐2‐infected individuals developing respiratory or neurocognitive disorders, mental health disorders, metabolic or cardiovascular disorders, and gastrointestinal disorders exceeded that of a 2020 historic control group by 5% [17] and was higher after COVID‐19 than after influenza [18, 19].
The impact of post‐COVID‐19 conditions on healthcare systems and economies worldwide will be substantial due to the high number of individuals infected with SARS‐CoV‐2 [2]. More so, previous studies revealed that even asymptomatic [20, 21] or mild acute infections can lead to the post‐COVID‐19 condition. A German study observed nonhospitalized COVID‐19 patients, of whom 13%–28% experienced post‐COVID‐19 conditions after 7 months [4]. A US study reported that 69% of nonhospitalized individuals suffered from at least one complaint 30 days after the infection, and the proportion increased to 73% after ≥60 days [22]. These studies lacked control groups and thus could not adjust for the impact of the pandemic situation on the physical [23] and psychological [24] well‐being of the general population.
Early in the pandemic, DKMS Germany—a major stem cell donor registry [25]—conducted a study aimed at identifying immunogenetic risk factors for severe courses of COVID‐19. More than 900,000 donors completed a brief health questionnaire in August 2020 [26]. One year later, in July 2021, DKMS conducted a follow‐up study. A total of 199,377 participating donors answered questions concerning persistent symptoms and long‐term consequences on the participants’ lives. All participants, regardless of whether they had contracted SARS‐CoV‐2, provided information about symptom occurrence and their current well‐being.
With this study, we aim to provide information on the presence of post‐COVID‐19 conditions in a rather young adult (18–61 years of age) subset of the German population representing mainly the working population. The resulting dataset may provide more in‐depth information on this additional burden on healthcare systems and economies worldwide.
Methods
Study design
The initial study [27] and the follow‐up study were designed as registry‐based cross‐sectional studies and were approved by the responsible institutional review board of the Technische Universität Dresden (IRB00001473). The study was registered with the trial registry of the German Center for Infection Research (https://clinicalsite.org/~dzif/de/cat/2099/trial/4361) and was conducted in compliance with the principles of the Declaration of Helsinki. All participants provided explicit consent to participate in the study. Data privacy was protected in accordance with the General Data Protection Regulation of the European Union.
Study population
Invitations to participate were sent by email to potential stem cell donors registered with DKMS Germany who had already consented to participate in the initial COVID‐19 survey [27]. All participants were between 18 and 61 years of age (median: 38 years) in July 2021.
Health questionnaire
On 12 and 14 July 2021, 894,263 emails were sent to potential study participants. Participation in the study was possible until 1 September 2021. Participants had to provide explicit informed consent before answering the health questionnaire via a web‐based, password‐protected login. All participants completed a standardized questionnaire to assess (1) risk factors for severe acute COVID‐19 courses, (2) their physical and psychological performance in comparison to the time before the COVID‐19 pandemic, and (3) the occurrence of 20 potential post‐COVID‐19 symptoms [28, 29] during the 2 weeks prior to the survey, and reported their (4) vaccination and (5) infection status. Participants who reported a positive SARS‐CoV‐2 polymerase chain reaction (PCR) test were asked about symptoms during the acute phase of their infection. All participants with COVID‐19 history were asked about reinfection and long‐term sequelae. The full questionnaire is listed in Fig. S1 (German version only).
Case and phenotype definitions
Participants who reported a positive SARS‐CoV‐2 PCR test were considered as cases and all other participants as COVID‐19 controls. Cases were grouped into four different phenotypes based on their self‐reported symptoms during the acute phase: (1) asymptomatic, (2) moderate, (3) severe respiratory tract infections (RTIs), and (4) respiratory hospitalization. Severe RTI was defined as a combination of at least fever and cough, dyspnea and fever, dyspnea and cough, or dyspnea and myalgia. These case and phenotype definitions have previously been described [27, 30].
Statistical analysis
We were primarily interested in daily symptoms experienced in July/August 2021. We compared outcomes between cases and controls and by the severity of the acute infection.
Binary logistic regression models were used to investigate the risk for long‐term COVID‐19 symptoms. Odds ratios (ORs) and their 95% confidence intervals (CIs) were used to describe associations. Statistical testing was based on two‐sided Wald tests for the regression coefficients. Weighted regressions were fitted in order to correct for the imbalance of age and sex among the participants. We weighted the participants’ age according to the general population but could not weigh by their health status. For example, 21.3% of participants were women aged between 30 and 39 years. This subgroup comprises only 12.0% of the German population between 18 and 61 years, resulting in a weight of 0.562. Table S1 summarizes the weighting factors.
All models were adjusted for sex, age (three groups: 18–24 years, i.e., adolescence [31]/youth [32]; 25–39 years; and 40–61 years), body mass index (BMI) (four groups: <18.5 kg/m2, 18.5 to <25 kg/m2, 25 to <30 kg/m2, and ≥30 kg/m2), diabetes mellitus medication, and arterial hypertension medication (yes/no). Likewise, significant interactions—for example, between age and sex and age and COVID‐19 cases (yes/no)—were included in the multivariable models.
To increase statistical power, we combined asymptomatic and moderate acute infections into one group and severe RTI along with respiratory hospitalization into a severe acute infection group. Complete observations were used in the analysis of the impact of acute infections because the average prevalence of daily symptoms among cases with available information on acute infections did not differ significantly from that of cases with missing information.
To exclude symptoms of the acute (0–4 weeks) and subacute (4–12 weeks) phases [6], only participants with the first positive PCR test at least 3 months before study participation were included. Symptom trajectories were based on observed frequencies reported by individual participants at defined time intervals since acute infection. This allowed investigation of post‐COVID‐19 conditions between 3 and 15 months after the acute infections. To test the effects of time since infection, a logistic model with linear, quadratic, and cubic terms was applied. As no statistically significant temporal differences in the frequency of daily symptoms were detected, we pooled data from all COVID‐19 cases from January 2020 to April 2021.
Symptom severity was measured using a three‐point scale (0 = never, 1 = occasionally, 2 = daily). Spearman correlation was used to evaluate the multicollinearity of the 20 symptoms. To identify symptom clusters, we conducted a maximum likelihood factor analysis with varimax rotation on symptom severity. Factor loadings greater than 0.3 were considered meaningful.
All analyses were carried out using R statistical software version 4.1.0.
Results
Description
A total of 893,101 emails were successfully sent to potential stem cell donors registered with DKMS in Germany (for detailed information, see Fig. S2). Of these, 199,380 donors consented to participate. The response rates for female and male recipients were 23.1% (139,478/602,670) and 20.6% (59,902/290,431), respectively. At the time of participation, 176,854 individuals (88.7%) had received a first vaccination against SARS‐CoV‐2, and 123,793 (62.1%) a second dose.
A total of 12,609 responders reported PCR‐confirmed infections accumulating to an incidence of 6.3% by August 2021, which is comparable to the age‐ and sex‐adjusted value of 5.6% reported by the Robert Koch Institute on 1 September 2021 [33, 34]. Overall, 12,491 participants reported a first infection with SARS‐CoV‐2 prior to vaccination, while 118 participants reported infections after having received at least one vaccination.
Among the 11,861 participants with a first positive PCR test before May 2021, 10,520 (88.7%) reported the course of their acute infection—830 (7.9%) had asymptomatic infections, 6782 (64.5%) described moderate symptoms, 2674 (25.4%) had symptoms of a severe RTI without hospitalization, and 234 (2.2%) reported respiratory hospitalization. We show demographic information of cases and controls in Table S2.
A second PCR‐confirmed infection at least 3 months after the first infection was reported by 92 out of 12,609 (0.7%) participants, of whom none required respiratory hospitalization for the first infection but four for the second.
Prevalence of post‐COVID‐19 symptoms since the time of infection
Among cases, we observed dynamic courses of daily symptoms, but did not detect statistically significant differences between reported symptom frequencies at 3, 6, 9, 12, and 15 months postinfection (Fig. 1 and Table S3).
Fig. 1.
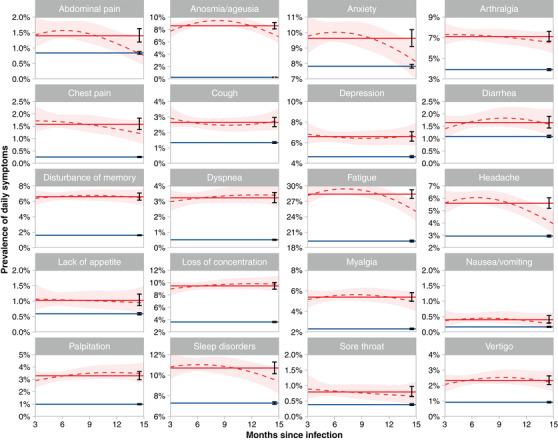
Daily occurrence of symptoms by interval between positive SARS‐CoV‐2 polymerase chain reaction (PCR) test and time of survey (July/August 2022). Blue line: average prevalence controls, dotted red line: smoothed (second order polynomial) prevalence among cases, light red area: 95% confidence interval of smoothed prevalence curve, solid red line: average prevalence of cases, black error bars: 95% confidence intervals of cases and controls.
Cases were significantly more likely to report daily symptoms than controls at all time points postinfection. For example, 19.3% (95% CI 19.1%–19.5%) of controls reported daily fatigue compared to 28.4% (27.6%–29.2%) of cases (p <0.0001)—ranging from 25.0% (23.1%–27.0%) at 15 months to 29.3% (28.4%–30.3%) at 6 months postinfection—and daily loss of concentration was reported by 3.6% (3.5%–3.7%) of controls and by 9.5% (8.9%–10.0%) of cases (p <0.0001)—ranging from 9.0% (7.9%–10.2%) at 3 months to 9.8% (8.9%–10.8%) at 12 months postinfection (for all symptoms, see Table S3).
Univariate analysis of risk factors for daily symptoms
The severity of the acute SARS‐CoV‐2 infection was the strongest predictor for daily symptoms 3 months and more thereafter. Cases with severe acute infections reported daily impairments significantly more often than those with asymptomatic infections. For all 20 symptoms, we observed a gradual increase in ORs with increasing severity of the acute infection episode compared with controls (Fig. 2a). ORs for the most prevalent symptom, fatigue, were 0.90 (95% CI 0.75–1.07) for asymptomatic infections, 1.27 (1.19–1.34) for moderate infections, 3.26 (3.00–3.53) for severe RTI, and 4.25 (3.34–5.40) for respiratory hospitalizations versus controls. The largest ORs were determined for anosmia/ageusia with 3.02 (95% CI: 1.41–6.48) for asymptomatic infections, 34.75 (30.71–39.33) for moderate infections, 54.31 (46.78–63.06) for severe RTI, and 35.03 (22.62–54.26) for respiratory hospitalization versus controls.
Fig. 2.
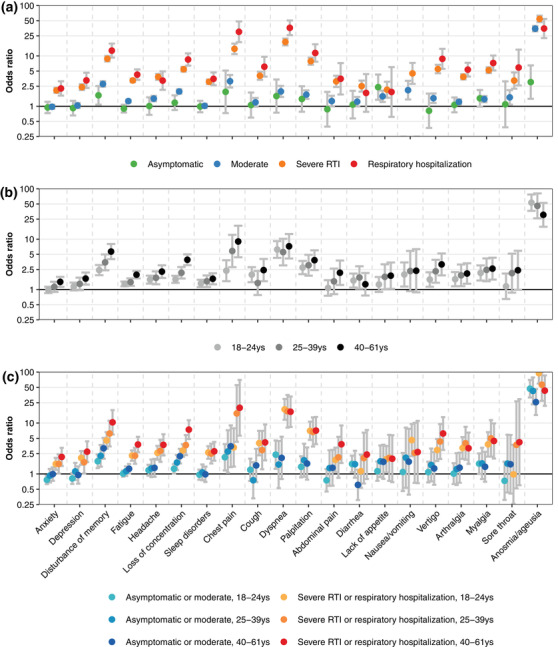
Odds ratios (ORs) for all 20 symptoms experienced by the participants daily by the severity of the acute infection and age compared to controls. The y‐axis is logarithmically scaled. The ORs are based on the univariate analysis of the severity of acute infection (a), on the univariate analysis of age (b), and on the multivariate analysis of both factors along with sex, diabetes mellitus, hypertension, and body weight (c). In panel (b), daily nausea/vomiting was not reported for asymptomatic infections and infections requiring respiratory hospitalization, leaving the estimates of the corresponding ORs undefined. In panel (c), severe acute infections combine severe respiratory tract infection (RTI) and respiratory hospitalization. Upper 95% confidence limits are 151.8 and 117.2 for anosmia/ageusia, severe, 18–24 years, and 25–39 years, respectively.
Besides the severity of the acute infection, age showed a strong effect on the prevalence of daily symptoms (Fig. 2b). COVID‐19 cases between 40 and 61 years of age had a significantly higher risk than cases between 18 and 24 years for 13 out of 20 symptoms. For 18 symptoms (exceptions were anosmia/ageusia and diarrhea), cases who were 40 years and older revealed the highest ORs among the three age groups, and for 16 symptoms (exceptions were anosmia/ageusia, diarrhea, cough, and dyspnea), the youngest age group revealed the lowest ORs. ORs for fatigue were 1.97 (95% CI: 1.67–2.33) in 40‐ to 61‐year olds, 1.40 (1.18–1.66) in 25‐ to 39‐year olds, and 1.29 (1.16–1.44) in the 18‐ to 24‐year‐old group. For dyspnea, ORs were 7.23 (4.12–12.66), 5.54 (3.02–10.16), and 6.23 (4.25–9.13) in the three age groups from the oldest to the youngest. Comprehensive information on the impact of the severity of the acute episode and age is provided in Table S4.
Chest pain was significantly more often reported (p <0.001) by participants with hypertension (OR: 12.90) than those without hypertension (OR: 5.37). Loss of concentration was significantly more often reported (p <0.001) by individuals with obese BMI (≥ 30 kg/m2) compared to normal‐weight participants (BMI between 18.5 to <25 kg/m2) (OR: 2.35). Palpitations were more often reported (p = 0.01) by participants with obese BMI (OR: 4.59) than those with normal weight (OR: 3.14). Also, lack of appetite was significantly more often reported (p = 0.01) by individuals with overweight BMI (25 to ≤30 kg/m2) (OR: 2.82) than with normal weight (OR: 1.48).
Neither sex nor diabetes mellitus had a significant effect on the risk of post‐COVID conditions.
Multivariate analysis of the impact of acute infection severity and age
The prevalence of long‐term symptoms was associated with age and the severity of the acute infection (Fig. 2c). Numerical values of the estimated OR are shown in Table S4. For example, 18‐ to 24‐year‐old individuals with asymptomatic or moderate SARS‐CoV‐2 infections had significantly lower risks of experiencing daily symptoms than cases with severe COVID‐19 (severe RTI and respiratory hospitalization).
Among cases with severe COVID‐19, the risk of daily impairments was higher for 40‐ to 61‐year‐old compared to 18‐ to 25‐year‐old participants. Only anosmia/ageusia presented a completely different picture. Here, older patients (40–61 years) had a significantly lower risk than younger patients (18–24 years), both with asymptomatic/moderate infections (p = 0.006) and with severe infections (p = 0.002).
Differences in prevalence of daily symptoms between cases and controls
While the relative risk for post‐COVID symptoms for cases versus controls did not differ depending on sex, statistically the prevalence was significantly different. For example, among women aged 40–61 years, 34.6% of cases and 21.9% of controls reported daily fatigue, that is, a difference of +12.7%. Men showed lower frequencies of daily fatigue compared to women, with 20.7% of cases and 12.1% of controls among 40‐ to 61‐year olds, resulting in a difference of +8.6%. The corresponding frequencies among young women aged between 18 and 24 years were 42.1% of cases and 36.1% of controls, resulting in a difference of +6.0%. Among men of the same age group, the difference was only +3.5% (23.3% daily fatigue among cases and 19.8% among controls). The severity of the acute infection also had a strong impact. Among female cases aged between 40 and 61 years, 14.2% with an asymptomatic or moderate infection reported daily fatigue, compared to 36.8% of cases with a severe RTI or respiratory hospitalization.
Figure 3 shows comprehensive data on the prevalence of all symptoms by sex, age group, and the severity of the acute infection episode (Tables S5 and S6 provide the corresponding prevalence in numbers). More aggregated information on risk factors and symptom frequencies can be requested from the authors directly.
Fig. 3.
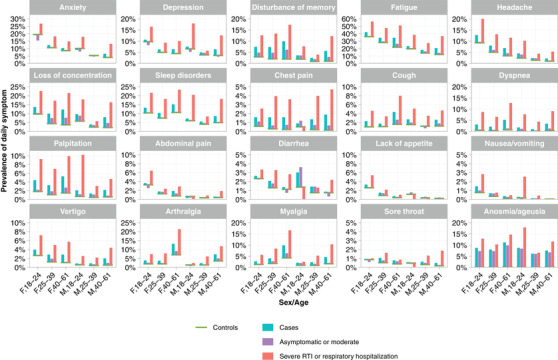
Daily prevalence of 20 different post‐COVID‐19 symptoms of controls (green line), cases (turquoise bar), cases with asymptomatic or moderate acute infection (violet bar), and cases with severe respiratory tract infection (RTI) or respiratory hospitalization (red bar) for three age groups and for female (F) and male (M) participants.
Identification of symptom clusters
Symptom clusters detected among controls (e.g., between depression and anxiety, or between loss of concentration and disturbance of memory) were also reported by cases. However, we identified pairwise correlations (>0.3) that were stronger among cases, for example, fatigue and disturbance of memory, chest pain and dyspnea, chest pain and palpitations, and disturbance of memory and dyspnea (Fig. 4).
Fig. 4.
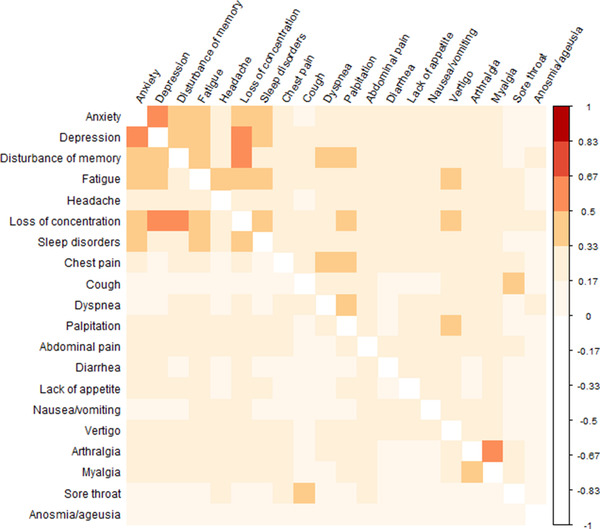
Pairwise correlation coefficients between the 20 self‐reported symptoms that participants experienced daily. SARS‐CoV‐2‐positive cases are in the upper triangular area and controls in the lower triangular area.
Using factor analysis, a total of six symptom clusters could be distinguished, with the first three main clusters comprising at least four symptoms:
neurological/psychiatric (anxiety, depression, disturbance of memory, headache, loss of concentration, sleep disorders),
pulmonary/cardiovascular (chest pain, cough, dyspnea, palpitations),
gastrointestinal (abdominal pain, diarrhea, loss of appetite, nausea/vomiting, vertigo),
joint/muscle (arthralgia, myalgia),
upper respiratory (sore throat), and
sensory (anosmia, ageusia) symptoms.
While only 1.3% of cases with asymptomatic infections reported daily occurrence of five or six neuropsychiatric symptoms, this rate increased to 7.0% of those with severe RTI and to 8.5% of COVID‐19 patients who required respiratory hospitalization. The number of symptoms experienced daily increased with the increasing severity of the acute infection (Fig. 5). Considering all symptoms, 4.4% of moderate cases and 22.6% of cases with respiratory hospitalization experienced more than five symptoms daily, compared to 2.7% among controls.
Fig. 5.
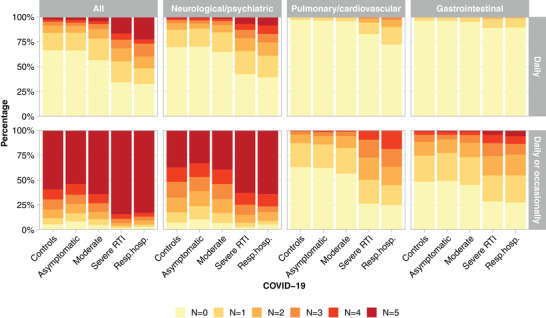
Percentage of participants in the different severity groups of the cases as well as the controls who experienced between N = 0 and N ≥5 symptoms daily out of all 20 symptoms and out of the three main symptom classes: neurological/psychiatric (anxiety, depression, disturbance of memory, headache, loss of concentration, sleep disorders), pulmonary/cardiovascular (chest pain, cough, dyspnea, palpitations), and gastrointestinal (abdominal pain, diarrhea, loss of appetite, nausea/vomiting, vertigo).
Physical and mental performance
Participants were asked to rate their physical and mental performance compared with their prepandemic level. The more severe the acute infection had been, the higher was the reported deterioration of mental and/or physical performance (Fig. 6).
Fig. 6.
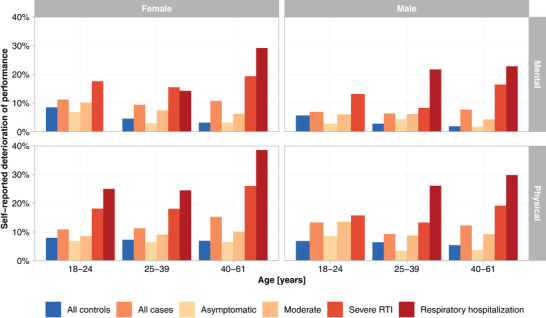
Self‐reported deterioration of mental and physical performance (“much worse” performance compared to the prepandemic level) depending on age, sex, and severity of the acute SARS‐CoV‐2 infection.
Female controls reported a deterioration significantly more often than male controls. Younger controls (18–24 years) reported a deterioration of their mental performance more often. A deterioration of physical performance was significantly more common among controls under 40 years of age (Table S7).
We identified strong correlations between low mental performance ratings and daily occurrence of symptoms of the neurological/psychiatric cluster, and between low physical performance and daily symptoms of the neurological/psychiatric and pulmonary/cardiovascular clusters (Fig. S3).
Incapacity to work and impairments in activities of daily living
The influence of the severity of the acute phase on the post‐COVID‐19 condition was also evident in the assessment of work incapacity and impairments in basic (e.g., washing, dressing alone) and more advanced (e.g., preparing meals, housework) activities of daily living (ADL).
A total of 745 (6.3%) participants reported being unable to work at the time of participation due to health problems related to COVID‐19. One year after infection, 3.4% (95% CI: 2.7%–4.1%) of cases with moderate acute infections were unable to work. In contrast, among cases with severe RTI or respiratory hospitalization, the rates were 12.7% (10.7%–15.0%) and 25.6% (18.5%–34.2%), respectively (Fig. 7).
Fig. 7.
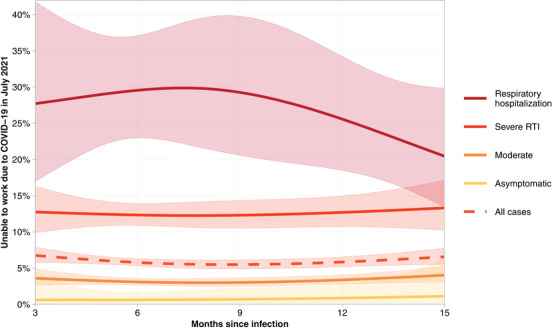
Proportion of cases reporting being unable to work in July 2021 by acute phase severity and time since infection.
A total of 173 out of 745 (23.2%) participants had been unable to work for more than 12 weeks. Among them were 59.1% (94/159, 14 with unknown severity) with severe acute infections, that is, severe RTI or respiratory hospitalization.
Notably, 28 out of 745 individuals who were unable to work reported impairments in basic ADL, 87.5% of them after a severe acute infection course. Impairments in more advanced ADL were reported by 112 individuals, of whom 77.3% reported a severe acute infection.
Discussion
In this study, we analyzed the long‐term impact of an infection with SARS‐CoV‐2 and quantified post‐COVID‐19 symptoms for the population aged between 18 and 61 years. Overall, 199,377 individuals were included in the study. Besides 12,609 COVID‐19 cases, 186,768 participants who had never tested positive for SARS‐CoV‐2 by PCR served as a control group. Social distancing, stay‐at‐home orders, job loss, extreme workload, economic loss, and fear of infecting oneself or others have been reported to have had a substantial influence on the whole population [23, 24, 35, 36]. The impact of these stressors for the general population needs to be considered when analyzing post‐COVID‐19 conditions. Because post‐COVID‐19 conditions are poorly distinguished from other stress‐related symptoms, it is important to have an appropriate control group that was exposed to the same stressors. Previous studies often focused solely on COVID‐19 patients [4, 5, 22, 37]. Others based their analysis on electronic health records and thus on individuals who sought professional medical help [17, 19], which may not fully represent population health. We analyzed the different acute courses of SARS‐CoV‐2 infections in detail, allowing for clearer risk estimates.
Direct comparisons of our data with other studies are hampered because post‐COVID‐19 conditions are difficult to diagnose and are measured by different approaches [3]. Published studies evaluate either the presence of one or several symptoms after a defined time [4, 22, 29, 37, 38], the presence of new symptoms that were absent in the acute phase of the infection [17], or long‐term reduction in quality of life [39]. Comparable with the descriptive results of a large German study [40], our data indicate that individuals suffering from post‐COVID‐19 conditions show a broad range of unspecific symptoms, many of which may also be identified in participants never infected by SARS‐CoV‐2. However, we found significant differences between cases and controls in both the frequency with which daily symptoms were reported and the number of daily symptoms experienced. We further observed that the frequency with which cases experienced daily symptoms did not differ between 3 and 15 months postinfection. A French survey recently demonstrated that 85% of patients symptomatic after 2 months still reported symptoms 1 year after symptom onset [41].
We identified the severity of acute infection as the main risk factor for developing post‐COVID‐19 condition, consistent with previously published data [18, 19, 22, 38]. This finding is in line with the observation that the level of SARS‐CoV‐2 RNA is a biomarker for COVID‐19 severity [15]. Age is a major risk factor for severe infections and has also been identified as a major risk factor for COVID‐19 sequelae by many others [26, 42, 43, 44, 45]. Diabetes was predictive for post‐COVID‐19 conditions in a recently published risk factor analysis, but not in our study [15]. Patients with severe forms of diabetes probably carry the greatest risk in this context but were under represented in our relatively young population. Women showed slightly higher frequencies in experiencing daily symptoms. Along with the age effect, this finding implies that young females carry an increased risk for post‐COVID condition as observed in the majority of long‐COVID studies [37, 45, 46]. Because men are more likely to develop severe COVID‐19 [47], higher rates of hospitalization in our study group were not unexpected [26].
The most prevalent symptom in our dataset was fatigue, which was experienced by 28.4% of cases as compared to 19.3% of controls. This rate is in line with previously published results, which, however, show a very wide variance in prevalence between studies. For example, Davis et al. [48] reported 80% fatigue at 6 months, Augustin et al. [4] reported 10% at 4 months, and Taquet et al. [19] 6% at 90–180 days postinfection. Fatigue was more frequent in women as reported by others [49]. Because of our large control group, the inclusion of all severity levels, and the random participant selection process, our values should be generalizable to those between 18 and 61 years of age.
Our study has limitations. First, this study utilizes self‐reported data and not objective medical assessments. Second, our results may not be generalizable to the general population because potential stem cell donors only represent the working age population (18–61 years of age) [50]. Third, we limited the list of symptoms to the 20 most common to allow for answering the questions in a short time frame, and thus possibly underestimated the prevalence of post‐COVID complaints. However, we included items to assess ADL, which provided an additional way to quantify the individual disease burden. Fourth, because of the timing of our study, our data only include individuals infected with SARS‐CoV‐2 variants that were present until July 2021. Thus, we cannot provide data on the prevalence of post‐COVID‐19 condition after infection with the Omicron variant [51]. Moreover, our data come from a largely unvaccinated population at the time of the first SARS‐CoV‐2 infection, as less than 1% of participants who reported a positive PCR test had received at least one vaccine prior to their infection. Data from a UK study showed a 41.1% decrease in the odds of experiencing post‐COVID‐19 condition if the infection occurred after full vaccination [52], and similar results were observed in a study from Israel [53]. However, a recent study analyzing US veterans' health care data found that in this cohort, the risk for postacute sequelae was reduced compared to unvaccinated participants (hazard ratio: 0.85) but was still elevated relative to uninfected controls (hazard ratio: 1.5) [54]. Fifth, a fraction of the controls may have undergone unrecognized SARS‐CoV‐2 infections. Differences between cases and controls would then be underestimated. Finally, a strength of this study is that our survey was not hospital based and thus may represent the general (working) population of the respective age groups. Nonetheless, motivation to participate may have been higher among individuals with more severe acute infections and more problematic post‐COVID‐19 conditions, resulting in overestimation of differences between cases and controls.
In summary, we report the prevalence of post‐COVID‐19 conditions in mainly unvaccinated individuals aged 18–61 years, with a history of PCR‐confirmed SARS‐CoV‐2 infections between February 2020 and August 2021. More severe acute infections and higher age were major risk factors in this population. With this perspective, preventing severe infections is the cornerstone for preventing post‐COVID‐19 conditions.
Vaccination programs and antiviral treatment for high‐risk patients with COVID‐19 infection may thus reduce the risk for post‐COVID‐19 conditions by reducing the severity of the acute course. Moreover, retrospective studies showed that SARS‐CoV‐2 vaccinations for individuals who already suffered from post‐COVID‐19 conditions were associated with decreasing symptoms during follow‐up [55]. Vaccinations may therefore directly and indirectly lower the population health burden of post‐COVID‐19 conditions.
Conflict of Interest
The authors declare no conflict of interest.
Author contributions
Stefanie N. Bernas: Conceptualization; Investigation; Project administration; Validation; Visualization; Writing – original draft; Writing – review and editing. Henning Baldauf: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review and editing. Ruben Real: Conceptualization; Methodology; Validation; Writing – review and editing. Jürgen Sauter: Conceptualization; Methodology; Resources; Software; Writing – review and editing. Jan Markert: Conceptualization; Resources; Writing – review and editing. Sarah Trost: Conceptualization; Writing – review and editing. Kristin Tausche: Validation; Writing – review and editing. Uta Behrends: Validation; Writing – review and editing. Alexander H. Schmidt: Conceptualization, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review and editing. Johannes Schetelig: Conceptualization; Formal analysis; Methodology; Project administration; Resources; Supervision; Validation; Writing – original draft; Writing – review and editing.
Supporting information
Figure S1: Questionnaire. All participants completed a standardized questionnaire to assess risk factors
Figure S2: 894,263 emails were sent to registered stem cell donors; 1,162 emails could not be delivered due to reasons such as server unavailability or full email inboxes. A total of 199,380 (22.2%) participants consented to study participation, 3 of whom had to be excluded due to a temporary problem with the database
Figure S3: Correlations between low mental and physical performance ratings and daily occurrence of symptoms of the neuropsychiatric and pulmonary/cardiovascular clusters
Table S1: Calculation of the weighting factor for different age groups and male and female participants to account for proportional differences in the study group compared to the German population.
Table S2: Characteristics of SARS‐CoV‐2 positive (cases) and negative (controls) participants.
Table S3: Frequency of daily symptom occurrence reported by controls and cases at three, six, nine, 12, and 15 months post‐infection at the time of survey participation.
Table S4: Odds ratios for cases to experience symptoms daily or daily and/or occasionally compared to controls. Univariate analysis of the impact of the severity of the acute infection and age, multivariate analysis of infection severity and age.
Table S5: Prevalence of daily experienced symptoms for controls and cases for different age groups and male and female participants.
Table S6: Prevalence of daily and/or occasionally experienced symptoms for controls and cases for different age groups and male and female participants.
Table S7: Ratings of physical and mental performance compared to their pre‐pandemic levels for different age groups and for male and female participants, for controls and for cases with infections before May 2021 (Pos before May 2021) or after May 2021 (Pos from May 2021).
Acknowledgments
We thank all donors registered with DKMS Germany and especially those who participated in this study.
Bernas SN, Baldauf H, Real R, Sauter J, Markert J, Trost S, et al. Post‐COVID‐19 condition in the German working population: A cross‐sectional study of 200,000 registered stem cell donors. J Intern Med. 2023;293:354–370.
Stefanie N. Bernas and Henning Baldauf contributed equally to this work.
Contributor Information
Stefanie N. Bernas, Email: bernas@dkms.de.
Henning Baldauf, Email: baldauf@dkms.de.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. El Bcheraoui C, Weishaar H, Pozo‐Martin F, Hanefeld J. Assessing COVID‐19 through the lens of health systems' preparedness: time for a change. Global Health. 2020;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phillips S, Williams MA. Confronting our next national health disaster—long‐haul covid. N Engl J Med. 2021;385:577–9. [DOI] [PubMed] [Google Scholar]
- 3. WHO . In the wake of the pandemic—preparing for long COVID. 2021. Policy Brief 39. [PubMed]
- 4. Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post‐COVID syndrome in non‐hospitalised patients with COVID‐19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in adults at 6 months after COVID‐19 infection. JAMA Netw Open. 2021;4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO . A clinical case definition of post COVID‐19 condition by a Delphi consensus. 2021. [DOI] [PMC free article] [PubMed]
- 8. Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long‐term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta‐analysis. J Rehabil Med. 2020;52:jrm00063. [DOI] [PubMed] [Google Scholar]
- 9. Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild‐to‐moderate SARS‐CoV‐2 infection. Nat Immunol. 2022;23:210–6. [DOI] [PubMed] [Google Scholar]
- 10. Peluso MJ, Lu S, Tang AF, Durstenfeld MS, Ho HE, Goldberg SA, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;224:1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chertow D, Stein S, Ramelli S, Grazioli A, Chung J‐Y, Singh M, et al. SARS‐CoV‐2 infection and persistence throughout the human body and brain. 2021. Research Square PREPRINT (Version 1).
- 12. Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Natarajan A, Zlitni S, Brooks EF, Vance SE, Dahlen A, Hedlin H, et al. Gastrointestinal symptoms and fecal shedding of SARS‐CoV‐2 RNA suggest prolonged gastrointestinal infection. Med (N Y). 2022;3:371–87.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Rossler A, et al. Post‐acute COVID‐19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology. 2022;28:S0016‐5085(22)00450‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post‐acute COVID‐19 sequelae. Cell. 2022;185:881–95.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Novak P, Mukerji SS, Alabsi HS, Systrom D, Marciano SP, Felsenstein D, et al. Multisystem involvement in post‐acute sequelae of coronavirus disease 19. Ann Neurol. 2022;91:367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daugherty SE, Guo Y, Heath K, Dasmarinas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS‐CoV‐2 infection: retrospective cohort study. BMJ. 2021;373:n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Aly Z, Xie Y, Bowe B. High‐dimensional characterization of post‐acute sequelae of COVID‐19. Nature. 2021;594:259–64. [DOI] [PubMed] [Google Scholar]
- 19. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co‐occurrence, and evolution of long‐COVID features: a 6‐month retrospective cohort study of 273,618 survivors of COVID‐19. PLoS Med. 2021;18:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang Y, Pinto MD, Borelli JL, Mehrabadi MA, Abrihim H, Dutt N, et al. COVID symptoms, symptom clusters, and predictors for becoming a long‐hauler: looking for clarity in the haze of the pandemic. medRxiv. 2021. 10.1101/2021.03.03.21252086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Ebinger JE, Mostafa R, Budde P, Gajewski J, Walker B, et al. Paradoxical sex‐specific patterns of autoantibody response to SARS‐CoV‐2 infection. J Transl Med. 2021;19:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell ML, Catalfamo CJ, Farland LV, Ernst KC, Jacobs ET, Klimentidis YC, et al. Post‐acute sequelae of COVID‐19 in a non‐hospitalized cohort: results from the Arizona CoVHORT. PLoS One. 2021;16:e0254347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muniz‐Pardos B, Shurlock J, Debruyne A, Steinacker JM, Borjesson M, Wolfarth B, et al. Collateral health issues derived from the covid‐19 pandemic. Sports Med Open. 2020;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daly M, Sutin AR, Robinson E. Longitudinal changes in mental health and the COVID‐19 pandemic: evidence from the UK Household Longitudinal Study. Psychol Med. 2022;52:2549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt AH, Sauter J, Baier DM, Daiss J, Keller A, Klussmeier A, et al. Immunogenetics in stem cell donor registry work: the DKMS example (Part 1). Int J Immunogenet. 2020;47:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schetelig J, Baldauf H, Wendler S, Heidenreich F, Real R, Kolditz M, et al. Risk factors for a severe course of COVID‐19 in persons aged 18 to 61. Dtsch Arztebl Int. 2021;118:288–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schetelig J, Baldauf H, Wendler S, Heidenreich F, Real R, Kolditz M, et al. Severity of respiratory infections due to SARS‐CoV‐2 in working population: age and body mass index outweigh ABO blood group. medRxiv. 2021. 10.1101/2020.11.05.20226100 [DOI] [Google Scholar]
- 28. Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post‐covid syndrome in individuals admitted to hospital with covid‐19: retrospective cohort study. BMJ. 2021;372:n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayoubkhani D, Pawelek P; Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID‐19) infection in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/7april2022 (2022). Accessed 30 May 2022.
- 30. Schetelig J, Heidenreich F, Baldauf H, Trost S, Falk B, Hossbach C, et al. Individual HLA‐A, ‐B, ‐C, and ‐DRB1 genotypes are no major factors which determine COVID‐19 severity. Front Immunol. 2021;12:698193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223–8. [DOI] [PubMed] [Google Scholar]
- 32. United Nations General Assembly . International youth year: participation, development, peace. https://undocs.org/en/A/36/215 (1981). Accessed 30 April 2022.
- 33. RKI COVID‐19 . CSV mit den aktuellen Covid‐19 Infektionen pro Tag (Zeitreihe). https://www.arcgis.com/home/item.html?id=f10774f1c63e40168479a1feb6c7ca74 (2022). Accessed 2 March 2022.
- 34. German Federal Statistical Office (Destatis) . https://www‐genesis.destatis.de/genesis/online (2022). Table 12411‐0006. Accessed 2 March 2022.
- 35. Rodriguez‐Rey R, Garrido‐Hernansaiz H, Collado S. Psychological impact and associated factors during the initial stage of the coronavirus (COVID‐19) pandemic among the general population in Spain. Front Psychol. 2020;11:1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saladino V, Algeri D, Auriemma V. The psychological and social impact of covid‐19: new perspectives of well‐being. Front Psychol. 2020;11:577684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME, Steig BA, Gaini S, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2021;73:e4058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Sar‐van der Brugge S, Talman S, Boonman‐de Winter L, de Mol M, Hoefman E, van Etten RW, et al. Pulmonary function and health‐related quality of life after COVID‐19 pneumonia. Respir Med. 2021;176:106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wild P, Beutel M, Lackner KJ, Münzel T, Pfeiffer N, Strauch K. Gutenberg COVID‐19 Studie. https://www.unimedizin‐mainz.de/GCS/dashboard/#/app/pages/AktuelleErgebnisse/ergebnisselc (2022). Accessed 30 Dec 2021.
- 41. Tran VT, Porcher R, Pane I, Ravaud P. Course of post COVID‐19 disease symptoms over time in the ComPaRe long COVID prospective e‐cohort. Nat Commun. 2022;13:1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugiyama A, Miwata K, Kitahara Y, Okimoto M, Abe K, Bunthen E, et al. Long COVID occurrence in COVID‐19 survivors. Sci Rep. 2022;12:6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whitaker M, Elliott J, Chadeau‐Hyam M, Riley S, Darzi A, Cooke G, et al. Persistent COVID‐19 symptoms in a community study of 606,434 people in England. Nat Commun. 2022;13:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mule G, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28:611.e9–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS‐CoV‐2 infection is common and independent of severity of initial infection. PLoS One. 2020;15:e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmidt AH, Mengling T, Hernandez‐Frederick CJ, Rall G, Pingel J, Schetelig J, et al. Retrospective analysis of 37,287 observation years after peripheral blood stem cell donation. Biol Blood Marrow Transplant. 2017;23:1011–20. [DOI] [PubMed] [Google Scholar]
- 51. Sigal A. Milder disease with Omicron: is it the virus or the pre‐existing immunity? Nat Rev Immunol. 2022;22:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ayoubkhani D, Bosworth M, King S; Office for National Statistics . Self‐reported long COVID after two doses of a coronavirus (COVID‐19) vaccine in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidaftertwodosesofacoronaviruscovid19vaccineintheuk/26january2022 (2022). Accessed 30 May 2022.
- 53. Kuodi P, Gorelik Y, Zayyad H, Wertheim O, Wiegler KB, Jabal KA, et al. Association between vaccination status and reported incidence of post‐acute COVID‐19 symptoms in Israel: a cross‐sectional study of patients tested between March 2020 and November 2021. medRxiv. 2022. 10.1101/2022.01.05.22268800 [DOI] [Google Scholar]
- 54. Al‐Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS‐CoV‐2 infection. Nat Med. 2022;28:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, et al. Trajectory of long covid symptoms after covid‐19 vaccination: community based cohort study. BMJ. 2022;377:e069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Questionnaire. All participants completed a standardized questionnaire to assess risk factors
Figure S2: 894,263 emails were sent to registered stem cell donors; 1,162 emails could not be delivered due to reasons such as server unavailability or full email inboxes. A total of 199,380 (22.2%) participants consented to study participation, 3 of whom had to be excluded due to a temporary problem with the database
Figure S3: Correlations between low mental and physical performance ratings and daily occurrence of symptoms of the neuropsychiatric and pulmonary/cardiovascular clusters
Table S1: Calculation of the weighting factor for different age groups and male and female participants to account for proportional differences in the study group compared to the German population.
Table S2: Characteristics of SARS‐CoV‐2 positive (cases) and negative (controls) participants.
Table S3: Frequency of daily symptom occurrence reported by controls and cases at three, six, nine, 12, and 15 months post‐infection at the time of survey participation.
Table S4: Odds ratios for cases to experience symptoms daily or daily and/or occasionally compared to controls. Univariate analysis of the impact of the severity of the acute infection and age, multivariate analysis of infection severity and age.
Table S5: Prevalence of daily experienced symptoms for controls and cases for different age groups and male and female participants.
Table S6: Prevalence of daily and/or occasionally experienced symptoms for controls and cases for different age groups and male and female participants.
Table S7: Ratings of physical and mental performance compared to their pre‐pandemic levels for different age groups and for male and female participants, for controls and for cases with infections before May 2021 (Pos before May 2021) or after May 2021 (Pos from May 2021).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


