Abstract
The concept of phase-separation-mediated formation of biomolecular condensates provides a new framework to understand cellular organization and cooperativity-dependent cellular functions. With growing understanding of how biological systems drive phase separation and how cellular functions are encoded by biomolecular condensates, opportunities have emerged for cellular control through engineering of synthetic biomolecular condensates. In this Review, we discuss how to construct synthetic biomolecular condensates and how they can regulate cellular functions. We first describe the fundamental principles by which biomolecular components can drive phase separation. Next, we discuss the relationship between the properties of condensates and their cellular functions, which informs the design of components to create programmable synthetic condensates. Finally, we describe recent applications of synthetic biomolecular condensates for cellular control and discuss some of the design considerations and prospective applications.
Subject terms: Synthetic biology, Soft materials, Intrinsically disordered proteins, Biotechnology
Phase separation of biomolecular condensates provides a new framework to understand cellular organization and functions. In this Review, the authors discuss the applications and design considerations of synthetic biomolecular condensates to regulate cellular functions as a new paradigm for synthetic biology.
Key points
Molecular rules can be extracted for engineering synthetic condensates.
The properties of condensates and the benefits of compartmentalization dictate their functions.
Condensates encode spatial regulations for cellular functions.
Design considerations influence the function of synthetic condensation systems.
New cellular functions can be uniquely modulated by synthetic condensates.
Introduction
Cells control the compartmentalization of biomolecules to realize specific cellular functions and make regulatory decisions1. Biomolecular condensates are membraneless organelles that enable the spatial and temporal organization of specific biomolecules into distinct compartments2. These condensates form through spontaneous or driven phase transitions that result in the formation of two coexisting and distinct phases when the phase boundary — defined as the saturation concentration for the phase transition — of the system is crossed3. The partitioning of specific biomolecules into condensates is crucial for various cellular processes, including transcriptional regulation4, stress response5, molecular transport6 and cell division7. With a growing understanding of the fundamental mechanisms underlying condensate formation and the dependence of cellular functions on the properties of such condensates, it is now possible to use the principles of macromolecular phase separation and condensate biology to develop synthetic functional biomolecular condensates, by which the benefits of phase transition and membraneless compartments can be exploited for synthetic biology applications8,9. From the perspective of synthetic biology, an overlooked feature is how to regulate the spatial localization of biomolecules within cells, as most cellular reactions happen in a specific region inside the cell10. The ability to programme condensate formation in cells provides new capabilities to organize cellular processes with spatial precision, which is an additional step forward for synthetic biology.
In this Review, we first discuss the molecular principles of condensate formation underlying the engineering of synthetic condensates. Next, we discuss the relationship between condensates’ properties and their cellular functions, which provides insights into the design of components to create synthetic condensates with programmable properties. We then describe recent examples of synthetic condensates for cellular control to illustrate the current state-of-the-art of synthetic condensates. We finally discuss some design considerations and prospective applications of synthetic biomolecular condensates.
Principles of condensate formation
To design synthetic systems capable of phase separation, it is important to understand how to form a molecular network that enables concentration-dependent molecular demixing. We describe the molecular principles of phase separation using multivalent macromolecules and genetically encoded biopolymers as examples11–13 and discuss how these principles provide generalizable design strategies to engineer synthetic condensates.
Multivalent interactions
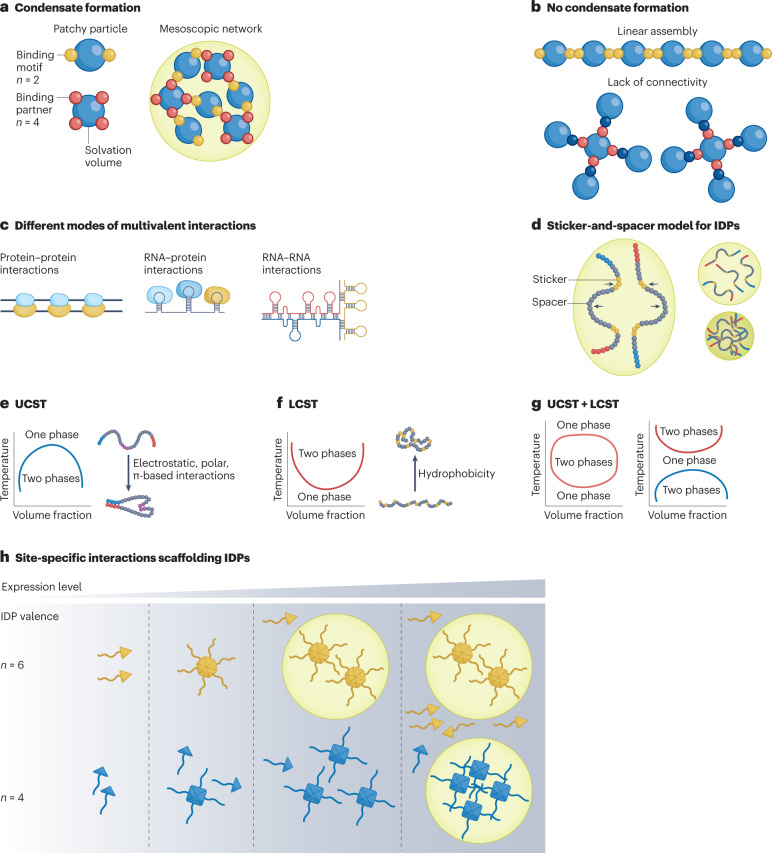
Multivalency — the interaction between molecules at multiple sites — is one of the primary molecular principles by which biomolecular condensates are formed11,14. To illustrate this concept, we use a simple patchy particle model, a classic colloid model that describes the self-assembly of a molecular network by multivalent interactions15–18. In a patchy particle, the node (the small sphere) represents a stereospecific interaction motif, and the building block (the blue sphere) represents the solvation volume (Fig. 1a). These particles can undergo valence-dependent phase separation by assembling into large oligomers upon mixing17. Percolation of these molecules into a network decreases the solubility of the molecules, thus promoting phase separation owing to entropy-driven effects19,20. As illustrated by this model (Fig. 1b), the key to forming a network is maintaining the high-order connectivity between the patches17. Therefore, it is not only the valence of the interactions that is important, but also whether the valence is ‘effective’, which suggests that the interaction should contribute to the expansion of the condensate network, instead of blocking network formation16. Examples of biomolecular condensates that form through multivalency include proteins that contain several copies of an interaction domain, multiple proteins scaffolded by a nucleic acid and repetitive RNA molecules19 (Fig. 1c).
Fig. 1. Principles of condensate formation.
a, Patchy particle model that explains the principle of multivalency mediated phase separation. b, Network formation is determined by the valency and the connectivity within the network. c, Different modes of multivalent interactions between different types of biomolecules. d, Sticker-and-spacer model represents how intrinsically disordered proteins (IDPs) undergo solvent-mediated phase transition. e, Phase diagram for upper-critical-solution-temperature (UCST) phase behaviour, in which a decrease in the system temperature results in strong intermolecular interactions and drives phase separation. f, Phase diagram for lower-critical-solution-temperature (LCST) phase behaviour, in which an increase in temperature increases the entropic penalty of water solvating the protein backbone and drives phase separation. g, Different double phase behaviours (UCST + LCST) are dictated by the differences in the transition temperatures of UCST and LCST systems. h, Effect of modulating the valency of site-specific interactions in an IDP-containing domain on its phase boundary.
The first demonstration of a multivalent phase-separation system was a tandem-array signalling protein consisting of the SRC homology 3 (SH3) domain and its proline-rich motif (PRM) ligands13. At a concentration at which (SH3)N and (PRM)N remain soluble, increasing the valency (N) of either SH3 or PRM or the affinity between the modules promotes phase separation. Nucleic acids can also serve as scaffolds to initiate phase separation12. Specific sequences that encode secondary structures in RNA can enable multivalent protein–RNA interactions and trigger phase separation12. For example, the SARS-CoV-2 nucleocapsid protein interacts with double-stranded RNA (dsRNA) in the SARS-CoV-2 RNA genome to initiate phase separation21,22, which has been implicated in viral replication and packaging23. Moreover, the eukaryotic genome uses a cluster of enhancer regions — DNA elements that bind transcription factors24 — to localize a high density of transcription factors. The transcription factors recruit transcriptional mediators and RNA polymerase, forming a condensate that activates transcription4. Condensates formed by such multivalent interactions between DNA and multiple coactivators are essential to maintain the expression of cell identity genes4.
Multivalent RNA–RNA interactions can also drive phase separation. For example, RNA multimerization can enable multivalent base pairing between repetitive cytosine–adenine–guanine (CAG) RNA molecules (for example, 47 CAG repeats) that drives the RNA to undergo phase separation and gelation25. Such repeats of CAG triplets in RNA are correlated with multiple neurological and neuromuscular disorders, strongly implicating the role of phase transitions in these diseases. Besides molecularly specific interactions to initiate phase separation, complex coacervation, which involves oppositely charged molecules, is another form of multivalent interaction that can result in the formation of condensates26,27. However, these charged molecules can also non-specifically interact with various molecules in living cells26, which can limit their utility for engineering synthetic condensates.
Intrinsically disordered protein
From the perspective of engineering condensates, a programmable sole-driver of phase transition can be an ideal selection as a modular component. Intrinsically disordered proteins (IDPs), a class of proteins typically containing low-complexity sequences and lacking a fixed tertiary structure, can mediate condensate formation themselves28–33. IDPs can phase-separate through homotypic interactions, defined as interactions between the same molecules. Their phase behaviour can be illustrated by a linear sticker-and-spacer model3 (Fig. 1d), where a sticker represents specific amino acids or short linear motifs that exhibit short-range attractive interactions (for example, Arg–Tyr, Tyr–Tyr), and a spacer represents the sequences between the stickers and consists of amino acids that largely interact with the solvent (for example, Gly and Ser). When the net attraction (for example, electrostatic interactions)34 between polymer chains makes water a poor solvent for the polymer, the polymer undergoes chain compaction and phase separation to create two immiscible phases — a dilute phase and a dense phase35. Therefore, even though the spacers are not directly involved in inter/intrachain interactions, their role in interacting with solvents can be crucial to modulate the phase behaviour of an IDP36,37.
To illustrate the principles of IDP-mediated phase separation, we discuss synthetic IDPs (synIDPs)38–40 that are genetically encoded polymers of short peptide repeats. They have also been previously called intrinsically disordered protein polymers (IDPPs)39 or artificial IDPs (A-IDPs)38. These synIDPs exhibit upper-critical-solution-temperature (UCST) or lower-critical-solution-temperature (LCST)41 phase behaviour, similar to many naturally occurring IDPs (for example, low-complexity sequence of fused in sarcoma protein (FUS)42, heterogeneous nuclear ribonucleoproteins (hnRNP) A1 (ref. 43), Sup35 (ref. 44)). Because of their simplicity, their phase behaviour can be rationally tuned at the molecular level by control of their sequence, composition and chain length far more readily than natural IDPs, thereby being extremely useful for the design of synthetic biomolecular condensates.
For IDPs that exhibit UCST phase behaviour, at a fixed concentration (volume fraction), decreasing the temperature of the system below a threshold temperature (cloud point) triggers phase separation of the IDP (Fig. 1e). Many native IDPs, such as hnRNP A1 (ref. 43), FUS45 and Laf1 (ref. 46), exhibit UCST phase behaviour, indicating that intermolecular interactions in these IDPs are enhanced relative to entropic contributions at lower temperature41. To understand how such interactions can be programmed, we have systematically studied a synIDP — resilin-like polypeptide (RLP)38 — that exhibits UCST phase behaviour, to uncover the underlying molecular principles that drive UCST phase transition. Informed by sequence heuristics developed in previous studies38,39, a wild-type (WT) RLP with a [GRGDSPYS]N sequence was synthesized with a repeat number (N) ranging from 20–80.
Systematic mutational studies of the WT RLP sequence demonstrate that the phase diagram of RLPs can be programmed by control of three orthogonal variables38: first, the number of repeat units of the RLP; second, the frequency of aromatic residues, in the order of Trp (W) > Tyr (Y) > Phe (F) as drivers of phase separation; and third, the fraction of positively charged residues, with arginine (R) as a stronger driver of separation than lysine (K) under the condition that the RLP has a balanced distribution of oppositely charged residues and a fixed frequency of aromatic residues. Together, these variables can tune the saturation concentration at which phase separation occurs by over seven orders of magnitude. As a repetitive homopolymer, within a lattice model, a longer RLP chain correlates with a higher probability for chains to overlap in a fixed volume47, thereby increasing the propensity for phase transition. For N = 20, substituting Tyr with Val (V) eliminates the UCST phase behaviour, which is observed within 5–90 °C, indicating the importance of cation–π and π–π interactions on phase separation. Substituting Tyr with Trp, however, promotes π-based interactions and increases the cloud-point temperature, indicating the enhancement of phase transition by π-based interactions. Substituting Lys for Arg substantially decreases the propensity to phase separation, indicating that Arg is not only important to balance electrostatic interactions but is also crucial for cation–π interactions48. This systematic study of RLPs highlights the important contributions of cation–π and π–π interactions to phase separation. Simulations and experimental evaluations also show the importance of π-based interactions in stabilizing the dense phase49,50.
Another strategy to control phase behaviour is to modulate the pattern of sticker residues. For example, WT RLP is a polyampholyte with a uniform pattern of charged (R/D) and aromatic (Y) residues. By segregating the oppositely charged residues into long stretches of like charges, the strengthened electrostatic interactions result in a conformational change (that is, compaction between N-terminus and C-terminus of the protein) at the single-chain level51,52, which is directly correlated with their propensity for phase separation53. Similarly, by patterning the aromatic residues into patches, local π-based interactions are enhanced within the dense phase, thereby resulting in an increased concentration and decreased molecular mobility in the dense phase54. Theoretical studies provide quantitative parameters of the residue pattern, such as an aromatic residue pattern parameter (Ω)54, charge pattern parameter (κ)52 and sequence charge decoration pattern parameter51, which can be used directly to quantitatively guide the design of synIDP sequences to form condensates.
In contrast to many native IDPs that exhibit UCST phase behaviour, fewer native IDPs exhibit LCST phase behaviour. Examples include the poly (A)-binding protein in stress granules55, the nucleocapsid protein of SARS-CoV-2 (ref. 22) and ELF3 of Arabidopsis thaliana56. For IDPs that exhibit LCST phase behaviour, at a fixed concentration, increasing the temperature above the cloud-point temperature triggers phase separation41 (Fig. 1f). From the perspective of the temperature dependency of the free energy of solvation, an increase in temperature increases the entropic penalty incurred in organizing solvent molecules around the protein backbone, and the system gains entropy through the release of solvent molecules from the backbone, thus triggering LCST phase transition30.
Building on previous studies57–60, we have extensively investigated a class of synIDPs called elastin-like polypeptides (ELPs)39,40,61–64 and their fusions to soluble proteins65–68 to understand the underlying driving forces for LCST phase transition. ELPs are composed of a repetitive Val–Pro–Gly–Xaa–Gly sequence, where Xaa is any amino acid except proline69. The LCST phase behaviour of ELPs can be tuned by the number of repeats (N) and the identity of the guest residue, Xaa. The LCST phase transition of ELPs is a hydrophobicity-driven effect, such that increasing the hydrophobicity of Xaa enhances phase separation by lowering the cloud-point temperature. For example, the cloud-point temperature of [VPGVG]N is lower than that of [VPGSG]N because Ser is less hydrophobic than Val70. By using ELPs with different hydrophobicity, a multiphase condensate can be constructed71, suggesting that the dense-phase environment and surface properties such as surface tension of the condensate can also be modulated by choice of the guest residue.
In addition to the importance of hydrophobicity as a driving force for ELP phase transition72, another important question in the design of synthetic condensates is how this hydrophobic sequence can avoid the formation of fibrils, such as ordered amyloid beta-sheets73, which is an aggregation phenomenon74. To realize the amorphous internal structure of a condensate, we suggest that the existence of a proline residue may be necessary72,75, which can ensure the extended conformation of the polypeptide chain within the dense phase. For example, in a polypeptide, the hydrogen-bonded backbone turn content is inversely correlated with the backbone hydration76, which is important to maintain conformational disorder. Comparing sequences consisting of repeats of PGVGVA or GGVGVA77, the inclusion of proline is shown to enhance the backbone’s rigidity. A rigid backbone decreases the probability of the formation of intramolecular hydrogen bonds, thus increasing the hydration of the protein backbone. The increased hydration allows the polypeptide to maintain a disordered single-chain state, resulting in an amorphous structure within the condensate.
Theoretically, if a sequence that exhibits UCST phase behaviour is embedded within or appended to a sequence that exhibits LCST phase behaviour, this chimeric sequence could exhibit both UCST and LCST phase behaviours41,78 (Fig. 1g). For example, in synthetic polymers that exhibit phase transition behaviours, inserting a block of poly(ethylene glycol) (a polymer with LCST phase behaviour) into an acrylamide–acrylonitrile copolymer (a polymer with UCST phase behaviour)79 generates a block copolymer with double thermoresponsive behaviour, with an LCST transition at lower temperatures and a UCST transition at higher temperatures. Such dual phase behaviours have not been reported in any native proteins, although a synIDP of Rec1-resilin has been shown to exhibit such behaviour80. Using this approach in living cells would diversify the stimulus-responsive controls to modulate the formation and dissolution of condensates.
Combination of different interactions
Although an IDP alone is sufficient to drive condensate formation, in native condensates, site-specific interactions are often coupled with IDP–IDP interactions to dictate condensate assembly and functional specificity. The site-specific interactions mediate oligomerization to enrich the IDP-rich proteins to promote phase separation81 (Fig. 1h). For example, the proteins nucleophosmin 1 (NPM1) in the nucleolus82,83 and G3BP1 in stress granules84,85 contain a self-oligomerization domain that provides a homotypic driving force to promote phase separation. Such site-specific homotypic interactions have also been implemented in the design of a synthetic phase-separation system, OptoDroplets86. In this system, the light-activated oligomerization of the Cry2 domain increases the effective valence of IDP interactions, thereby promoting phase separation upon light activation. In contrast, a recent study shows the opposite behaviour of oligomerization on phase separation87, in which the enhanced helical propensity in the glutamine-rich domain of a fungal RNA-binding protein, Whi3, mediates dimer formation in the dilute phase, thereby suppressing phase separation.
Heterotypic site-specific interactions are also crucial to modulate phase separation. For example, Whi3, an RNA-binding protein responsible for spatial regulation of RNA transcripts88, can drive condensate formation through a polyglutamine-based disordered domain. In the presence of target mRNAs that interact with Whi3’s RNA-binding domain, the phase boundary of Whi3 is altered to a lower concentration88. Similarly, in the case of SARS-CoV-2 nucleocapsid condensate, the nucleocapsid protein undergoes LCST phase transition driven by its intrinsically disordered region (IDR) domain. The incorporation of favourable dsRNA interaction sites in the RNA-binding domain of nucleocapsid proteins increases the phase-separation propensity of the nucleocapsid protein22. These studies illustrate the importance of the quantity and the affinity of the valence on modulating phase separation. From an engineering perspective, modulating the effective valence of IDPs is therefore another useful tool to programme their phase behaviour.
In synthetic condensates, homotypic and heterotypic interactions are coupled together. Homotypic interactions driven by IDPs can mediate phase transition and modulate the physical properties of the condensate. Heterotypic interactions driven by site-specific interactions, instead, provide functional specificity. Therefore, understanding how the cooperativity between these interactions can tune the phase diagram is a crucial first step to engineer functional condensates.
Condensate properties and function
Increasing evidence suggests that the material properties of condensates are relevant to their function2,55,89,90, although much remains to be determined experimentally about their precise correlation. Experimentally, the material properties of condensates are commonly characterized by microrheology, fluorescence recovery after photobleaching (FRAP), and the partitioning of molecules of different sizes within condensates91. These techniques provide information about the viscosity, molecular diffusivity and pore size of condensates (Fig. 2a), which can serve as the basic design considerations for synthetic condensates. We herein discuss some examples that demonstrate how these properties can dictate cellular functions.
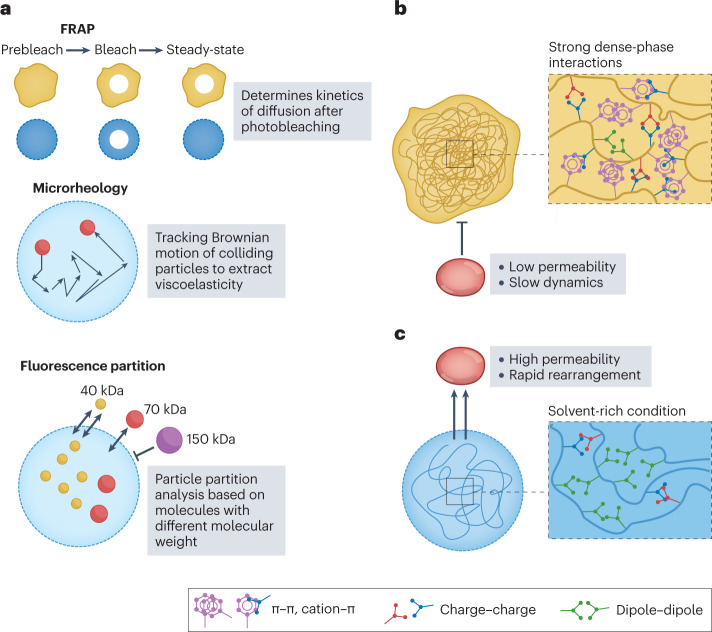
Fig. 2. Condensate material properties are a key determinant of condensate function.
a, Different experimental techniques to quantify the material properties of condensates. b, Condensates with strong dense-phase interactions result in low permeability and slow dynamics of the components in the condensate. c, Condensates with a solvent-rich dense phase result in high permeability and rapid rearrangement of molecules within that phase. FRAP, fluorescence recovery after photobleaching.
Phase separation can be coupled with percolation20 that leads to the formation of a network by the phase-separation drivers in the dense phase, which can alter their diffusive behaviour compared with molecules in the dilute phase (Fig. 2b). For example, stress granules, which are multicomponent condensates, protect mRNA when the cell encounters stress92. In mammalian cells, stress granules undergo fusion and fission, and most components exchange and diffuse rapidly with the dilute phase as evaluated by FRAP92. However, FRAP of the core of the stress granule, which is responsible for interacting with polysomes, reveals that there are immobile fractions within the granules93. This observation suggests that these static components selectively reduce the dynamics of molecules within the core, thereby mediating stress-dependent inhibition of protein translation. Another example of an arrested dense phase that dynamically regulates condensate function comes from SARS-CoV-2 (ref. 94). In condensates of the nucleocapsid protein of SARS-CoV-2 with viral genomic RNA, the protein and the RNA show slow recovery rates, as evaluated by FRAP, and the fusion of condensates is also slow. These data suggest that the long viral RNA is stably trapped inside the nucleocapsid condensate, which is consistent with the function of the nucleocapsid protein to package the virus before assembly of the viral particle94. In both examples, the phase-separation drivers of the condensates exhibit dynamically arrested behaviour, which prevents material exchange, and thereby serve as storage compartments for specific molecules.
Condensates can also serve as reaction crucibles to recruit and enrich specific molecules and accelerate the reactions in which they are involved95. The reaction products typically need to be able to diffuse out of the condensate. For this to happen, the condensates must be liquid-like or have large pores to allow the exchange of molecules in and out of the condensate (Fig. 2c). For example, transcriptional condensates, mediated by super-enhancer DNA elements and IDRs of transcriptional factors, drive robust gene expression that plays a prominent role in determining cell identity4,96. FRAP of the transcriptional factors within the transcriptional condensate show rapid recovery4,97, which indicates dynamic reorganization and rapid exchange of molecules within the condensate. This dynamic environment drives a transcriptional burst during RNA production98 and the produced RNAs do not change the material properties of the condensates. Once a certain level of RNA is transcribed, resulting in a charge imbalance in the condensate, the transcriptional condensate dissolves, thus terminating transcription. This self-regulating process demonstrates how initiation and termination of condensate function are modulated through a feedback loop determined by the condensate composition and its dynamics.
Another example is the P granules of Caenorhabditis elegans, which was one of the first systematically studied biomolecular condensates46,99. P granules contain a heterogeneous mixture of RNAs and proteins that are associated with RNA metabolism, which is essential to post-transcriptional regulation100. This function requires the condensates to be highly permeable and allow the free diffusion of molecules in and out. FRAP analysis demonstrates that the key component of P granules — the LAF1 protein — exhibits dynamic behaviour with more than 80% fluorescent recovery46. As evaluated by ultrafast-scanning fluorescence correlation spectroscopy and dextran partitioning101, the density of LAF1 in the dense phase of P granules is around two orders of magnitude lower than other known phase-separating proteins, with a mesh size of ∼3–8 nm, indicating a solvent-rich and permeable condensate. These properties could assist the function of P granule as intracellular RNA microreactors46,102, which require the RNA transcripts to be recruited, processed and released from the condensate.
These examples suggest that to design functional synthetic biomolecular condensates, their material properties must be carefully considered to realize a target function, which in turn informs the selection of the phase-separating macromolecules that will yield condensates with the desired material properties.
Synthetic biomolecular condensates
The goal of synthetic biology is to engineer biology through the synthesis of complex, biologically based (or inspired) systems that display functions that do not exist in nature, or to modulate biological functions by an orthogonal set of molecules103,104. To this end, the use of synthetic condensates offers a compartmentalization-dependent approach that is complementary to conventional methods to control cell function. In this section, we first highlight some recent examples of synthetic biomolecular condensates for synthetic biology to illustrate the rationale behind their application to regulate cellular processes, the methods used for the construction of synthetic condensates and the new cellular functions achieved by these synthetic systems. Next, we describe optogenetics-based synthetic condensates and underscore how the programmability and modularity of these engineered platforms enable spatiotemporal control of phase behaviour in cells.
Phase-separation-mediated compartmentalization can dictate cellular functions through three distinct attributes of condensates (Fig. 3a). First, the molecules undergoing phase separation can be fused to other molecules to endow the synthetic condensate with functional specificity. Second, phase separation results in a concentrated dense phase, in which the enrichment of molecules in condensates can boost kinetics of reactions in which these molecules participate. Third, forming a distinct compartment can serve as a protection strategy for molecules that are sequestered in the compartment and protect them from participating in biochemical reactions that occur in the cytosol. Examples of synthetic biology applications that exploit these distinct features of biomolecular condensates are described next.
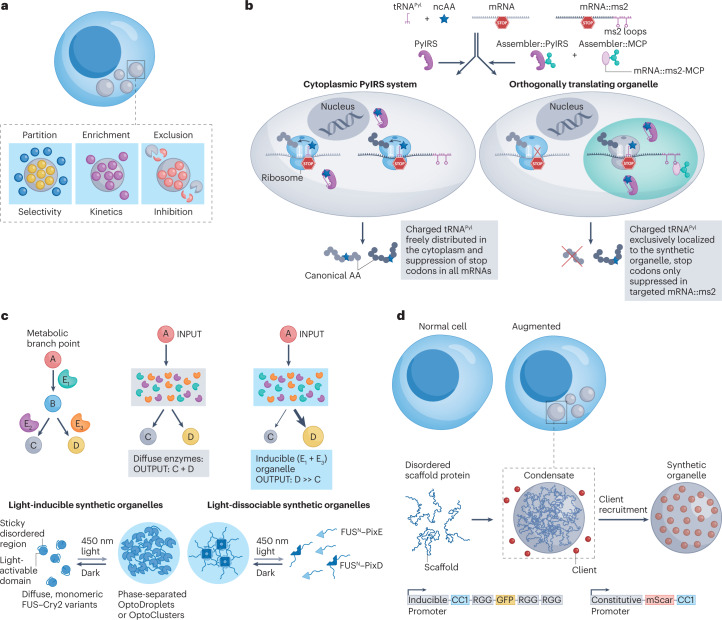
Fig. 3. Applications of synthetic biomolecular condensates for cellular control.
a, The mechanisms by which synthetic condensates can modulate cellular functions. b, Intrinsically disordered protein (IDP)-mediated synthetic condensates recruit specific transfer RNA (tRNA) synthetase and target mRNA, creating an orthogonal translational organelle107. c, Light-controlled assembly of synthetic condensates enriches enzymes of interest, improves product yields and minimizes production of side-products95. d, A synthetic condensate formed by an IDP disrupts cellular pathways by sequestering endogenous proteins of interest110. FUS, fused in sarcoma protein; GFP, green fluorescent protein; MCP, MS2 coat protein; ncAA, non-natural amino acid; PN, N peptide; PyIRS, pyrrolsyl tRNA synthetase. Panel b from Reinkemeier, C. D., Girona, G. E. & Lemke, E. A. Designer membraneless organelles enable codon reassignment of selected mRNAs in eukaryotes. Science 363, eaaw2644 (2019). Reprinted with permission from AAAS ref. 107, AAAS. Panel c reprinted from ref. 95, Springer Nature Limited. Panel d reprinted from ref. 110, Springer Nature Limited.
Regulation of cellular processes
Condensates can enhance reaction specificity
An approach to increase the chemical diversity of genetically encoded polypeptides is the site-specific incorporation of non-canonical amino acids during translation that contain functional groups not found in native proteins. The most common approach uses a transfer RNA (tRNA) synthetase and tRNA pair that are orthogonal to the native tRNA-synthetase–tRNA pairs in a cell105. The tRNA synthetase recognizes a non-canonical amino acid of interest and attaches it to its cognate tRNA. The charged tRNA then recognizes an internal stop codon — typically the amber UAG stop codon — in the target mRNA, leading to incorporation of the non-canonical amino acid at the stop codon in the target mRNA during its translation into a polypeptide within the cell. However, this approach suffers from the off-target (non-specific) incorporation of the non-canonical amino acid at stop codons that are present in many other mRNA transcripts in the cell106. Phase separation provides one approach to solve this problem in eukaryotes by spatial sequestration of the key components of the translational machinery in synthetic condensates. To do so, IDP domains of FUS and Ewing sarcoma RNA-binding protein 1 (EWSR1) can be fused to the pyrrolysyl tRNA synthetase and an RNA-binding protein, the MS2 coat protein (MCP) that binds a specific mRNA motif107 (Fig. 3b). The IDP–tRNA-synthetase–MCP fusion recruits a target mRNA transcript that has an MCP-binding RNA tag to the synthetic condensate. Translation of the target mRNA then occurs preferentially in the condensate compared with the cytosol, and it also decreases the off-target incorporation of the non-canonical amino acid at the many TAG stop codons that populate the genome of the cell. A follow-up study demonstrated the integration of a membrane-binding domain that spatially localizes the synthetic condensates next to different membrane-based organelles such as the Golgi body and mitochondria108.
Condensates can accelerate reaction kinetics
Phase separation results in a dense phase with a concentration that is higher than the total solution concentration of the molecules participating in phase separation3. Therefore, synthetic condensates are useful for applications in which a high local concentration of specific molecules is advantageous. In metabolic engineering, compartmentalization of specific molecules involved in an enzymatic pathway can maximize product yield and minimize side-product production109. For example, light-controlled phase separation can be used to mediate the assembly of synthetic condensates to regulate metabolic flux95 (Fig. 3c). To initiate phase separation, a light-activated oligomerization domain, Cry2, is fused to an IDP — the FUS N-terminal domain (FUSN) — and to the enzymes of interest, VioE and VioC, which are crucial to catalyse the production of deoxyviolacein. Light-induced oligomerization of Cry2 in the ternary fusion of VioC and VioE with Cry2–FUSN raises the local concentration of VioE and VioC in irradiated regions of the cell and triggers their phase separation into a condensate, which leads to a high local concentration of the two enzymes, shifting the flux of metabolic reactions to favour the production of the product of interest. This approach increases the product formation by 6-fold and the product specificity by 18-fold compared with the same reaction in the absence of condensate formation in the cytosol.
Similarly, a synIDP based on an RLP can be fused to the α-peptide (αp) of LacZ β-galactosidase to recruit the other inactive fragment of the enzyme (LacZΔM15), resulting in an active enzyme in a synthetic condensate38. This synthetic condensate catalyses the cleavage of a small molecule substrate, di-β-D-galactopyranoside (FDG), which fluoresces green upon cleavage. Increasing the number of the repeats of the synIDP decreases the saturation concentration (Csat) for condensate formation, which in turn leads to a four-fold increase in the enzymatic reaction efficiency. This study suggests that programming the Csat of condensates can be a powerful strategy to regulate the functional efficiency of the condensates in cells. Using the same principle to acceleration reaction, a synthetic condensate was designed to amplify transcription90. A synIDP–DNA-binding-protein fusion and a synIDP–transcription factor fusion collaboratively contribute to phase separation and recruit a target plasmid and RNA polymerase into the synthetic condensates formed in Escherichia coli, achieving transcription amplification. This study shows that condensate material properties, which dictate the free exchange of molecules based on the dynamics of phase-separating components and the pore size of the condensates, are crucial to the amplification function, establishing a direct correlation between the material properties and the function of the condensates.
Condensates can silence functional pathways
Condensates can also be used to downregulate or silence endogenous pathways in a cell by sequestering target molecules within a condensate. For example, a synthetic condensate was designed to sequester enzymes, thereby removing them from the endogenous pathway in the cytosol110 (Fig. 3d). The RGG domain of LAF1, which enables phase separation, was fused to different functional domains that target the enzymes of interest. This modular approach enables control of various cellular behaviours, including proliferation, division and cytoskeletal organization, by simply changing the functional domain to target a specific enzyme of interest.
With the same modular approach based on a phase separation driver and a functional domain, an RNA-based phase-separating system was created by fusing phase-separable RNA repeats of rCAG to an aptamer that binds to a target protein that specifically recruits the target protein into the condensate111. This approach enables the repression of the lycopene pathway by sequestering the CrtBI enzyme into the condensate and away from the subcellular location — the cell membrane — where it normally functions. Notably, when a phase-separating RNA element is transcriptionally fused with a ribosome-binding-site (RBS)/green-fluorescent-protein (GFP) gene, GFP expression is significantly repressed compared with untagged RBS–GFP RNA, indicating that the ability of synthetic condensates to repress a specific cellular function is a robust approach and is likely to be generalizable through such modular engineering strategies.
Studying intracellular phase behaviours
Synthetic systems that can undergo controllable phase transition in living cells can be used as model systems to test new hypotheses and expand our knowledge on the possible mechanisms of condensate formation in living cells. To enable a stepwise study of phase separation from subsaturated clusters to phase-separated condensates112 and map the intracellular phase diagram, the synthetic system should exhibit reversible phase behaviour in cells, and its local concentration should be tunable over a wide range. We next discuss examples of light-dependent intermolecular interactions that enable spatiotemporal control of phase transitions within cells.
Light-dependent oligomerization of IDPs to control phase behaviour
Interactions between native IDPs can be modulated by self-oligomerization and heterotypic interactions19,113. External control of the timing and spatial location of phase separation provides a powerful tool to probe their intracellular phase behaviour. For example, a light-activated condensation strategy, OptoDroplets86 (Fig. 4a), has been designed in which an IDR is fused with a light-activated oligomerization domain, Cry2. In the presence of blue light, Cry2 mediates the formation of a multivalent IDR cluster, resulting in reversible condensate formation. Because the intensity of the blue light is positively correlated with the association strength between Cry2 domains, increasing the light intensity modulates the location of the OptoDroplets in the two-phase regime of the phase diagram. Based on this light-tunable interaction of Cry2 domains, a correlation between the location in the phase diagram and ageing of the condensate (for example, a liquid-to-solid transition of condensate material properties) could be established.
Fig. 4. Optogenetics-based synthetic phase-separation systems allow investigation of intracellular phase behaviours.
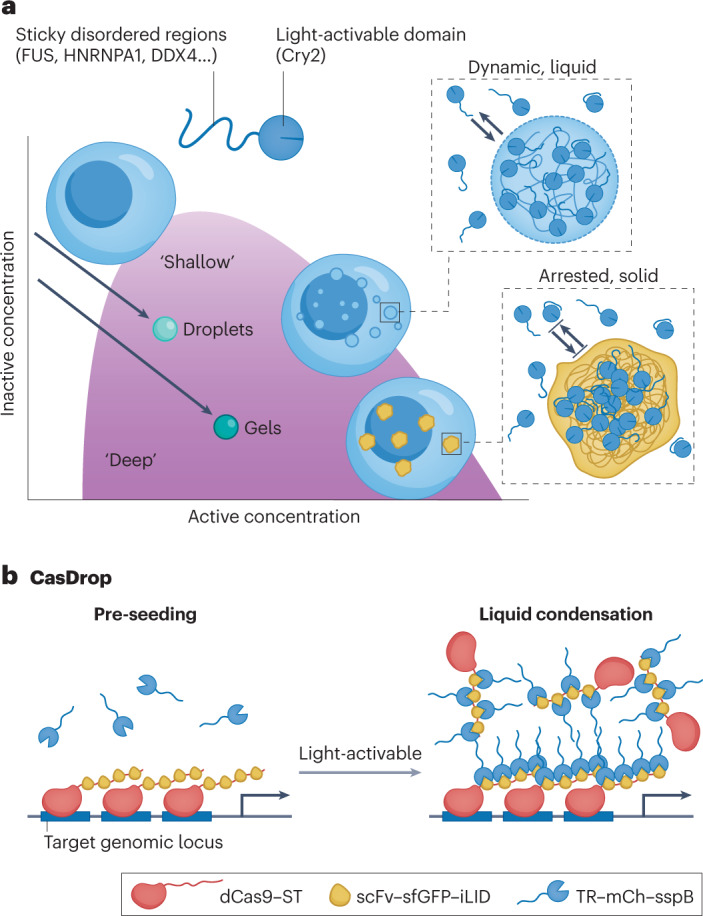
a, The OptoDroplet system consists of a light-activated Cry2 domain fused to an IDP. Activation of light intensity tunes the intermolecular interaction strength86. b, The CasDrop system mechanically restructures the genome by light-activated condensate formation on a targeted genomic locus114. Cry2, cryptochrome 2; dCAS9, enzymatically dead Cas9; iLID, improved light-induced dimer; mCh, mCherry; scFV, single-chain variable fragment; sfGFP, superfolder green fluorescence protein; sspB, stringent starvation protein B; ST, SunTag; TR, transcriptional regulator. Part a reprinted with permission from ref. 86, CellPress, Elsevier. Part b reprinted with permission from ref. 114, CellPress, Elsevier.
In contrast to the polydisperse oligomers created by light-triggered Cry2 oligomerization, another system enables precise control of the oligomerization state of IDPs81. To do so, a core of 24 human ferritin heavy chain subunits that self-assemble into a micelle was used as the high-valence scaffold to mediate association with an IDR-module through light-dependent heterodimerization between improved light-induced dimer domain (iLID) and stringent starvation protein B (SspB). This association results in a defined valency of the IDR particle. Based on the ratio between the IDR and the ferritin core, different regions of the phase diagram could be accessed in cells by control of the intracellular expression levels of the multivalent IDR particle, such that a higher IDR to ferritin core ratio results in lower phase boundary. The intracellular phase diagram shows typical features of phase separation, including spinodal decomposition, nucleation and growth, and Ostwald ripening. There is also a strong dependence of the phase behaviour on the sequence of the IDRs, such that condensates formed by C-terminal IDR of TAR DNA-binding protein 43 (TDP43) or N-terminal IDR of FUS show different morphology. These studies confirm that the concepts of phase separation are valid intracellularly and provide a modular synthetic system for the systematic study of intracellular phase behaviours.
Condensate formation on genomic loci can sense and restructure the genome
Besides optically triggered condensate formation by proteins, nucleic acids have also been used as a scaffold to achieve local enrichment of IDPs through light-regulated assembly. For example, an enzymatically dead Cas9 (dCas9) was used to guide IDP-mediated condensation at specific genomic loci through two interacting modular proteins114 (Fig. 4b). First, dCas9 was fused with SunTag (ST), a scaffold containing multiple repetitive epitopes115. The second construct is a single-chain variable fragment (scFv) antibody that binds ST, fused to superfolder GFP (sfGFP) and the optogenetic dimerization protein iLID. When co-expressed in cells, the IDP–mCherry–sspB fusion interacts with iLID upon light activation, thereby mediating the formation of condensates at the genomic loci targeted by the dCas9–gRNA complex. This synthetic condensate excludes bulk chromatin and senses the presence of mechanical stress. Furthermore, when two condensates seeded on different telomeres fuse to each other, these two distinct loci targeted by gRNA are pulled into proximity, thus restructuring a specific region of the genome. These results show how condensate–condensate interactions can mediate functional selectivity while mechanically excluding the non-targeted ‘stiff’ heterochromatin.
These examples illustrate how synthetic condensates can contribute to fundamental studies of phase separation and condensate behaviour in living systems. Such tunable condensation systems allow the understanding of phase behaviour in living cells and interface with endogenous environments to uncover unknown cellular principles such as capillary forces generated by condensates116. The integration of optogenetic control into condensate formation further diversifies the available tools to achieve in situ spatiotemporal regulation of cellular processes.
Functional synthetic condensates
Design considerations
Establishing the modularity and programmability of synthetic condensates is challenging. For example, synIDPs have a long history of in vitro applications, ranging from protein purification to drug delivery and regenerative medicine38–40,63,69–71,117,118. However, with only a few exceptions38,90, applications of synIDPs in living cells are rare, probably owing to an incomplete understanding of all the molecular features that drive phase separation, the material properties of synthetic condensates and how these properties can be exploited to control their function. Developing a molecular level understanding of the relationship between sequence, structural ensembles and the property of synthetic condensates is essential to provide robust, quantitative tools for de novo engineering of designer condensates for specific applications in cells. In this section, we discuss some of the considerations for engineering synthetic biomolecular condensates in living cells.
Phase transition and condensate formation
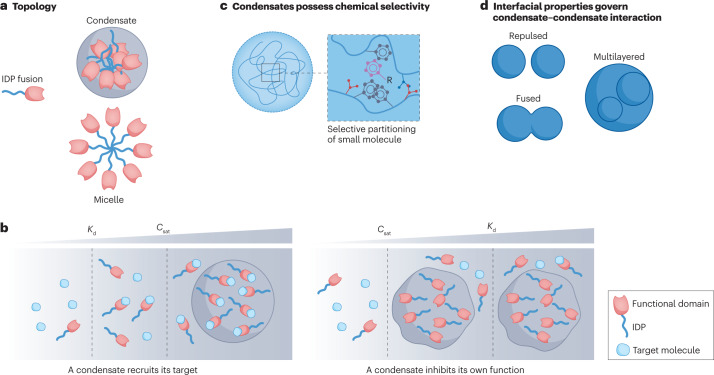
Cellular condensates are thought as an isotropic cluster of molecules forming a condensed network20,119, thereby enabling the partitioning and sequestration of selected molecules to regulate cellular functions as a membraneless compartment, which suggests that the molecules should be ‘included’ in the condensates. However, other types of topologies can also be achieved through phase transition (Fig. 5a), such as vesicles119 or micelles120. For example, a synIDP such as an ELP fused to a protein can be amphiphilic and undergo micelle formation121. The micelle displays multiple copies of the functional domain on its corona and exposes the fused protein to the dilute phase environment122, the opposite of the sequestration that condensates typically offer. From a design perspective for a modular phase-separation system, considering the relative hydrophobicity of the phase-separation module and the functional domain is therefore important to regulate the topology of the condensate and achieve the desired function.
Fig. 5. Design considerations for synthetic condensates.
a, Phase separation of intrinsically disordered protein (IDP) fusion can result in macromolecular complexes with different topology. b, The relationship between binding affinity Kd of a functional domain for a target molecule and the saturation concentration Csat of the IDP dictates whether the condensate can recruit the target molecule. c, Condensates formed by different molecular components possess distinct chemical environments. A condensate with a hydrophobic environment can attract hydrophobic-type interactions. d, Condensate–condensate interactions can be modulated by interfacial properties, which can be regulated by the molecular components within the condensate, solvent environment and biomolecular surfactants.
Interplay between sequestered components and condensates
The function of many biomolecular condensates is directed by the components sequestered in the condensate, such as an mRNA or protein8,110,111. However, the sequestered molecules in a condensate are not necessarily passive participants but can themselves modulate the formation and dissolution of the condensate17. For example, in condensates involved in transcriptional control, low abundance of RNA promotes condensate formation through electrostatic-interaction-mediated complex coacervation, whereas higher abundance of RNA will dissolve the condensate because of charge repulsion98. Therefore, when designing a synthetic condensate to recruit a target molecule, it is important to consider how the site-specific interactions of the target molecule at its physiological concentration affect the phase boundary of the phase-separation driving molecule (the scaffold). This scenario has been discussed in polyphasic linkage theory123, in which a ligand might be able to move the phase boundary of the scaffold depending on the relative affinity between scaffold–scaffold and scaffold–ligand, and the concentration of the ligand. Another consideration is how the target molecule modulates the physical properties of the condensate. For example, in the DEAD-box ATPase Dhh1 condensate, the presence of structured RNA results in dynamically arrested condensates, whereas unstructured poly(U) RNA maintains the fluidity of the Dhh1 condensate124. Therefore, from an experimental perspective, a systematic investigation of the effect of homotypic and heterotypic components on phase separation and the physical properties of the condensate are crucial to predict how the engineered condensates can dictate cellular functions.
Binding affinity of folded domains and saturation concentration of IDPs
A simple strategy to construct a functional synthetic condensate is to combine a phase-separation domain — an IDR — and a functional domain. The phase-separation domain will mediate the formation and modulate the physical properties of the condensates, whereas the functional domain can recruit the target molecule to dictate the roles of the condensate. Therefore, it is important to consider the relative concentration threshold for both domains to function. Considering a scenario in which the heterotypic interactions — those of the functional domain with its ligand — do not change the phase behaviour of the IDP, so that the phase diagram is not perturbed by ligand binding, if the binding affinity (Kd) of the functional domain for its ligand is substantially lower than Csat of the IDP, then most of the ligand-binding events will occur at concentrations lower than Csat. In this case, after the expression level of the IDP reaches Csat, the system would phase-separate and incorporate the bound ligand into the condensate (Fig. 5b). In contrast, if the Kd of the functional domain is considerably higher than Csat of the IDP, then phase separation will happen before the ligand-binding event (Fig. 5b). In this scenario, if the condensate is designed to sequester the target molecule, the physical properties of the condensate could possibly prevent interactions between the ligand and the functional domain, and the extent of this would depend on the physico-chemical properties of the condensate that control molecular transport in and out of it. Therefore, the condensate would not function as designed.
Another important aspect dictated by the binding affinity between the functional domain and the target molecule is the mobility of the target. Assuming that the phase-separation domain mediates the formation of a dynamically arrested network within the dense phase does not necessarily mean that the target molecule should also be dynamically arrested. It is possible that the dynamically arrested network would hinder molecular diffusion based on a condensed pore size of the condensate, whereas a biophysical parameter directly modulating the diffusive behaviours of the binding partner is the rate of dissociation (koff) between the binding domain and the target. For example, quantitative FRAP experiments have revealed strong correlation between the molecular mobility and its binding dynamics in vivo125,126.
Chemical environment of condensates dictates their selectivity
The partitioning of biomolecules into a condensate and their movement in and out of them can be dictated by the physico-chemical environments of the condensate. For example, in transcriptional initiation, RNA polymerase II partitions into the transcriptional condensate with the mediator complex subunit 1 (ref. 4). On hyperphosphorylation of RNA polymerase II, it exits the transcriptional condensate and moves into RNA-splicing condensates during the transcription elongation stage127, suggesting that the selectivity of a molecular component for different condensates can in turn be dictated by the physico-chemical properties of the condensate. Furthermore, small molecules (in this case, cancer drugs) with distinct chemical features can selectively partition into condensates, suggesting that the chemical environment of the latter can dictate its affinity for the former128 (Fig. 5c).
These examples suggest that the specific and often unique chemical environment of a condensate should be considered in its design for a specific function. For example, one application of synthetic condensates is to selectively enrich functional enzymes into the condensate, regulating metabolic flux95. An important assumption for this is that the substrate freely diffuses into and out of the condensate. However, an enzymatic condensate contains various molecular components129 that regulate its chemical environment130. Given the complexity of these environments and their diversity across condensate types, it might be inaccurate to assume that the small size of substrate molecules allows them to diffuse freely into and out of the condensates.
Condensate surface properties modulate condensate functions
Condensates can coalesce, repulse or engulf each other (Fig. 5d). These interactions are dictated by their surface properties131. Understanding how to modulate the interfacial properties of condensate at their liquid–liquid interface is therefore crucial in designing synthetic condensates that do not exhibit crosstalk with endogenous condensates in the cell. For example, P granules consist of RNA molecules and P granule abnormality protein 3 (PGL3) that form dispersed emulsion-like droplets in cells. A protein called maternal-effect germline defective 3 (MEG3) resides on the surface of the PGL3–RNA condensate, reducing the surface tension of the droplet and preventing the droplet from coarsening131. Such Pickering agents, like MEG3, maintain the structural integrity of the condensate. The molecules assembling the condensates can also modulate their surface properties, such as their viscoelasticity132,133. In a protocell, for example, the size of a condensate of a monoblock ELP can be tuned by the addition of a diblock ELP that acts as a temperature-sensitive amphiphile71,134. By controlling the molar ratio of the diblock to monoblock ELP, the size of the condensate droplets can be tuned by over three orders of magnitude from ~100 nm up to several tens of micrometres. Building on these insights to control the size and orthogonality of synthetic condensates in living cells is ripe for exploration.
Outlook
Prospects of condensate functions
Phase separation provides a new approach to regulate cellular processes compared with current approaches in synthetic biology. The main question to address is defining the kind of cellular function that synthetic biomolecular condensates are best suited to control. Existing examples suggest that cellular functions that depend on spatial localization of specific molecules within the cell, or that benefit from high local concentrations, are ideal candidates. However, whether synthetic biomolecular condensates are necessary for a specific function needs to be clarified. In other words, can the target function be realized by traditional single-molecule-based lock-and-key interactions instead of forming a distinct compartment? If the answer is yes, a follow-up question is whether there is an advantage in using condensates to achieve this function, or whether it provides features that are difficult to realize by molecular recognition. We next discuss some attributes of condensates that could help answer these questions.
Condensates enable reversible control of cellular function
An important feature of phase separation is its reversibility on a change in the environment. Condensates are extremely sensitive to post-translational modifications (for example, phosphorylation), and phase separation can be driven by changes in temperature or pH conditions19. Equilibrium phase behaviour is thermodynamically reversible simply by reversing the stimulus that drives condensate formation61, which suggests that functions encoded by biomolecular condensates can be designed a priori to be reversible. Such programmable reversibility is not always possible with simple lock-and-key interactions. For example, translational repression, which can be achieved by simple RNA interference135, can also be realized through biomolecular condensates, as shown in fragile X messenger ribonucleoprotein (FMRP)-based neuronal condensates136 and in protocells137. Phase-separation-mediated condensate formation represses translation by selectively sequestering mRNA in the condensate, thereby reducing its access to the translational machinery of the cell (Fig. 6a). Dissolving the condensate in response to an external stimulus then rescues translation of the sequestered mRNA. Such reversible control of RNA regulation is not possible with RNA interference techniques.
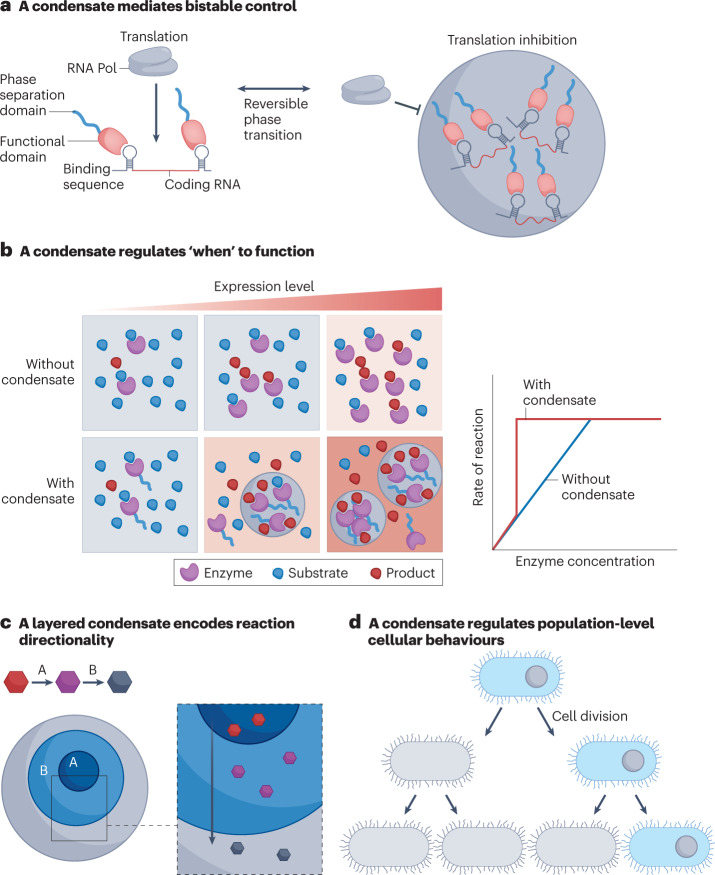
Fig. 6. Prospects of cellular functions modulated by synthetic condensates.
a, Reversible formation and dissolution of synthetic condensates can mediate bistable cellular functions. b, Phase separation is a concentration-dependent (and hence time-dependent) process to initiate cellular function, thereby regulating the ‘timing’ of and the efficiency of the synthetic function. c, A multilayered condensate can modulate reaction directionality. d, Control of the spatial location of a condensate in a cell can regulate the function of cells at the population level. RNA Pol, RNA polymerase.
Phase separation can regulate reaction kinetics
Phase separation drives condensate formation when the expression level of the phase-separable components in a cell rises above Csat. The Csat of condensates is a tunable parameter — for example, in RLPs, the Csat has been tuned by their repeat sequence and chain length over seven orders of magnitude — that can be exploited to control the kinetics of biochemical processes in a cell. For example, for an enzymatic reaction where the substrate concentration is saturated, the rate of the reaction is determined by the amount of the enzyme–substrate complex formed in the cell, which speeds up reaction kinetics by lowering the free energy of the reaction138. Therefore, from a cellular perspective, the expression level of the enzyme becomes the rate-determinant step by dictating the frequency of collisions between the enzyme and the substrate (Fig. 6b). If the enzyme is fused with a phase-separable domain capable of phase-separating at a concentration below the binding affinity of the enzyme and the substrate, the formation of a condensate with a higher concentration of enzyme in the dense phase (relative to its total concentration in the cell) will produce the enzymatic product in an earlier time point within a cell cycle than a cell containing the same amount of the enzyme but without a phase-separation domain38. This scenario suggests that regulating the saturation concentration of condensates can manipulate ‘when’ the designed cellular functions happen within a cell cycle90.
Condensate architecture can augment cellular function
The nucleolus provides a powerful demonstration of how physico-chemically distinct multilayers of a condensate can dictate different cellular functions based on the difference between the components in each layer139. A remarkable feature of the nucleolus is the coordination between multiple layers of the nucleolus and its function in ribosome biogenesis, in which the biogenesis process starts from the inner layer and ends at the outer layer of the condensate83. From an engineering perspective, the ability to create such multilayered condensates presents an opportunity to encode assembly line logic into biological systems. We have demonstrated the use of synIDPs to create such multilayer condensate in vitro71. The next, immediate challenge is to recapitulate multilayer condensates in vivo and to encode distinct — but connected — functions into each layer, such that the output of each layer becomes the input of an adjacent one, enabling directional biochemical or chemical transformations to be encoded (Fig. 6c). Creating directionally encoded multilayer functional condensates presents an exciting challenge and a new opportunity for synthetic condensates.
Besides layered structures, different condensates that do not interact with each other can also be useful for synthetic biology. For example, to develop artificial cellular systems, instead of engineering membrane-bound organelles, the use of biomolecular condensates can be a simple strategy to create distinct functional compartments140. For this purpose, the condensates must not interact or fuse with each other141. Decoding the molecular grammars that regulate the fusion and repulsion of condensates is an important next step to create synthetic condensates that talk to and work with each other to realize a specific function. With this in hand, a condensate network can be designed within cells and used to create multicompartment synthetic protocells that begin to mimic the sophisticated internal architecture of cells.
Condensate regulation acts beyond a single cell and not only in the dense phase
The fact that condensates are spatially localized compartments offers another opportunity to control cell function142, because the inhomogeneous spatial distribution of biomolecules can itself be a powerful strategy for biological control143–146. For example, a cytoplasmic pole-organizing protein (PopZ), containing an IDR, selectively enriches phospho-signalling proteins into the new pole of the bacterium Caulobacter crescentus, resulting in a signal propagation gradient during asymmetric cell division147. This observation suggests that if condensate formation can happen with spatial selectivity, such as at one pole of the cell, then on cell division, the daughter cell and the progenitor cell will have different components defined by the molecules sequestered by the condensates (Fig. 6d). For example, if that sequestered molecule is a plasmid, then the spatial condensation would result in asymmetric plasmid partitioning, which could regulate population-level cellular behaviours90,144. The same rationale can be applied to regulate molecules that are crucial to cellular identity or cell fate upon cell division. Furthermore, condensation-mediated asymmetric distribution of biomolecules could provide a control strategy to buffer intracellular concentration fluctuations111,148, because phase separation would result in a fixed concentration of the dilute phase. Further expression of biomolecules after phase separation would only change the volume fraction of the two phases, thereby maintaining a concentration homeostasis within the cytoplasm of the cell. For example, in the exponential growth stage of cells, the change of cellular volume would require an increased rate of production of biomolecules to maintain cellular fitness149. Therefore, if a synthetic condensate can maintain a stable concentration of molecules that are essential for cellular functions (for example, transcription), phase separation can be used as a generalizable strategy to modulate cellular fitness.
Crosstalk with endogenous systems
Another important aspect to consider is whether the introduction of new phase-separable components to create engineered condensates would interfere with endogenous condensates in cells. Phase separation is typically mediated by weak multivalent interactions, suggesting that the specificity of these interactions can be difficult to programme. For example, when cells are infected with SARS-CoV-2 virus, the nucleocapsid protein of SARS-CoV-2 also interacts with G3BP1, a component of endogenous stress granules, diminishing the innate immune response150. To circumvent this issue, two approaches can be considered: first, most IDP-mediated phase-separable systems are mediated by enthalpy-driven electrostatic interactions and π-based interactions151. Therefore, if phase separation by a synIDP is instead mediated by entropy-driven temperature-dependent dissolution of hydrophobic residues152, potential crosstalk between the synthetic condensate and native condensates could be reduced. Second, phase separation is a concentration-dependent process, suggesting that if the Csat of the synthetic condensate is considerably lower than that of endogenous condensates, the phase separation of the synIDP might not interfere with native condensates.
Another unresolved issue is the extent to which the introduction of a synthetic condensate will change cellular fitness, as endogenous condensates are known to do so153. For example, condensates can affect cellular fitness, depending on their material properties154. Other factors that require further investigation are the protein expression level of the IDPs used to create synthetic condensate, the copy number of the plasmids that encode the IDPs needed to create a synthetic condensate, the role of functional domains that are fused to an IDP to confer function to the synthetic condensates and their potential toxicity to the cells.
In summary, synthetic biomolecular condensates provide a new strategy for biological design. Interdisciplinary contributions from polymer physics, genetically encoded materials science, cell biology and biochemistry are beginning to converge on a new approach to engineer cells using phase separation. The development of new types of synthetic condensates will not only provide new tools to engineer biology and treat condensate-related diseases but will also provide biological insights into this fundamental mechanism by which cells regulate themselves.
Acknowledgements
The authors appreciate discussion with R. V. Pappu at Washington University in St Louis, C. P. Brangwynne and J. L. Avalos at Princeton University, A. Gladfelter at Duke University and members of their groups. This work was supported by the Air Force Office of Scientific Research through grant FA9550–20–1–0241 to A.C. and L.Y., and by the NIH through a MIRA grant GM127042 to A.C.
Author contributions
All authors drafted and revised the manuscript.
Peer review
Peer review information
Nature Reviews Bioengineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alberts, B. Molecular Biology of the Cell (Norton, 2017).
- 2.Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4382. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 3.Choi J-M, Holehouse AS, Pappu RV. Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys. 2020;49:107–133. doi: 10.1146/annurev-biophys-121219-081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabari BR, et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361:eaar3958. doi: 10.1126/science.aar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyon AS, Peeples WB, Rosen MK. A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol. 2021;22:215–235. doi: 10.1038/s41580-020-00303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Cai Q, Feng Z, Zhang M. Liquid–liquid phase separation in neuronal development and synaptic signaling. Dev. Cell. 2020;55:18–29. doi: 10.1016/j.devcel.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Ong JY, Torres JZ. Phase separation in cell division. Mol. Cell. 2020;80:9–20. doi: 10.1016/j.molcel.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastings RL, Boeynaems S. Designer condensates: a toolkit for the biomolecular architect. J. Mol. Biol. 2021;433:166837. doi: 10.1016/j.jmb.2021.166837. [DOI] [PubMed] [Google Scholar]
- 9.Reinkemeier CD, Lemke EA. Synthetic biomolecular condensates to engineer eukaryotic cells. Curr. Opin. Chem. Biol. 2021;64:174–181. doi: 10.1016/j.cbpa.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Stelling J, Sauer U, Szallasi Z, Doyle FJ, III, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Banani SF, et al. Compositional control of phase-separated cellular bodies. Cell. 2016;166:651–663. doi: 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden C, Gladfelter AS. RNA contributions to the form and function of biomolecular condensates. Nat. Rev. Mol. Cell Biol. 2021;22:183–195. doi: 10.1038/s41580-020-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshikawa M, Yoshii T, Ikuta M, Tsukiji S. Synthetic protein condensates that inducibly recruit and release protein activity in living cells. J. Am. Chem. Soc. 2021;143:6434–6446. doi: 10.1021/jacs.0c12375. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi E, Blaak R, Likos CN. Patchy colloids: state of the art and perspectives. Phys. Chem. Chem. Phys. 2011;13:6397–6410. doi: 10.1039/c0cp02296a. [DOI] [PubMed] [Google Scholar]
- 16.Sanders DW, et al. Competing protein–RNA interaction networks control multiphase intracellular organization. Cell. 2020;181:306–324.e28. doi: 10.1016/j.cell.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinosa JR, et al. Liquid network connectivity regulates the stability and composition of biomolecular condensates with many components. Proc. Natl Acad. Sci. USA. 2020;117:13238–13247. doi: 10.1073/pnas.1917569117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kastelic M, Kalyuzhnyi YV, Vlachy V. Modeling phase transitions in mixtures of β–γ lens crystallins. Soft Matter. 2016;12:7289–7298. doi: 10.1039/C6SM01513A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittag T, Pappu RV. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell. 2022;82:2201–2214. doi: 10.1016/j.molcel.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iserman C, et al. Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol. Cell. 2020;80:1078–1091.e6. doi: 10.1016/j.molcel.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roden CA, et al. Double-stranded RNA drives SARS-CoV-2 nucleocapsid protein to undergo phase separation at specific temperatures. Nucleic Acids Res. 2022;50:8168–8192. doi: 10.1093/nar/gkac596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cubuk J, et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun. 2021;12:1936. doi: 10.1038/s41467-021-21953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoury G, Gruss P. Enhancer elements. Cell. 1983;33:313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- 25.Jain A, Vale RD. RNA phase transitions in repeat expansion disorders. Nature. 2017;546:243–247. doi: 10.1038/nature22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeong V, Werth EG, Brown LM, Obermeyer AC. Formation of biomolecular condensates in bacteria by tuning protein electrostatics. ACS Cent. Sci. 2020;6:2301–2310. doi: 10.1021/acscentsci.0c01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu T, Spruijt E. Multiphase complex coacervate droplets. J. Am. Chem. Soc. 2020;142:2905–2914. doi: 10.1021/jacs.9b11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Der Lee R, et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014;114:6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia Quiroz F, et al. Intrinsically disordered proteins access a range of hysteretic phase separation behaviors. Sci. Adv. 2019;5:eaax5177. doi: 10.1126/sciadv.aax5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng X, et al. Design of intrinsically disordered proteins that undergo phase transitions with lower critical solution temperatures. APL Mater. 2021;9:021119. doi: 10.1063/5.0037438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fossat MJ, Zeng X, Pappu RV. Uncovering differences in hydration free energies and structures for model compound mimics of charged side chains of amino acids. J. Phys. Chem. B. 2021;125:4148–4161. doi: 10.1021/acs.jpcb.1c01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng X, Ruff KM, Pappu RV. Competing interactions give rise to two-state behavior and switch-like transitions in charge-rich intrinsically disordered proteins. Proc. Natl Acad. Sci. USA. 2022;119:e2200559119. doi: 10.1073/pnas.2200559119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nott TJ, et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y-H, Forman-Kay JD, Chan HS. Sequence-specific polyampholyte phase separation in membraneless organelles. Phys. Rev. Lett. 2016;117:178101. doi: 10.1103/PhysRevLett.117.178101. [DOI] [PubMed] [Google Scholar]
- 35.Brangwynne CP, Tompa P, Pappu RV. Polymer physics of intracellular phase transitions. Nat. Phys. 2015;11:899–904. doi: 10.1038/nphys3532. [DOI] [Google Scholar]
- 36.Harmon TS, Holehouse AS, Rosen MK, Pappu RV. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife. 2017;6:e30294. doi: 10.7554/eLife.30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harmon TS, Holehouse AS, Pappu RV. Differential solvation of intrinsically disordered linkers drives the formation of spatially organized droplets in ternary systems of linear multivalent proteins. N. J. Phys. 2018;20:045002. doi: 10.1088/1367-2630/aab8d9. [DOI] [Google Scholar]
- 38.Dzuricky M, Rogers BA, Shahid A, Cremer PS, Chilkoti A. De novo engineering of intracellular condensates using artificial disordered proteins. Nat. Chem. 2020;12:814–825. doi: 10.1038/s41557-020-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quiroz FG, Chilkoti A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 2015;14:1164–1171. doi: 10.1038/nmat4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quiroz FG, et al. Intrinsically disordered proteins access a range of hysteretic phase separation behaviors. Sci. Adv. 2019;5:eaax5177. doi: 10.1126/sciadv.aax5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruff KM, Roberts S, Chilkoti A, Pappu RV. Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. J. Mol. Biol. 2018;430:4619–4635. doi: 10.1016/j.jmb.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 42.Murakami T, et al. ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron. 2015;88:678–690. doi: 10.1016/j.neuron.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molliex A, et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163:123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franzmann TM, et al. Phase separation of a yeast prion protein promotes cellular fitness. Science. 2018;359:eaao5654. doi: 10.1126/science.aao5654. [DOI] [PubMed] [Google Scholar]
- 45.Kato M, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elbaum-Garfinkle S, et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl Acad. Sci. USA. 2015;112:7189–7194. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubinstein, M. & Colby, R. H. Polymer Physics Vol. 23 (Oxford Univ. Press, 2003).
- 48.Bremer A, et al. Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion-like domains. Nat. Chem. 2022;14:196–207. doi: 10.1038/s41557-021-00840-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krainer G, et al. Reentrant liquid condensate phase of proteins is stabilized by hydrophobic and non-ionic interactions. Nat. Commun. 2021;12:1085. doi: 10.1038/s41467-021-21181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vernon RM, et al. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. eLife. 2018;7:e31486. doi: 10.7554/eLife.31486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawle L, Ghosh K. A theoretical method to compute sequence dependent configurational properties in charged polymers and proteins. J. Chem. Phys. 2015;143:085101. doi: 10.1063/1.4929391. [DOI] [PubMed] [Google Scholar]
- 52.Das RK, Pappu RV. Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. Proc. Natl Acad. Sci. USA. 2013;110:13392–13397. doi: 10.1073/pnas.1304749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Y-H, Chan HS. Phase separation and single-chain compactness of charged disordered proteins are strongly correlated. Biophys. J. 2017;112:2043–2046. doi: 10.1016/j.bpj.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin EW, et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science. 2020;367:694–699. doi: 10.1126/science.aaw8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riback JA, et al. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell. 2017;168:1028–1040.e19. doi: 10.1016/j.cell.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung J-H, et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature. 2020;585:256–260. doi: 10.1038/s41586-020-2644-7. [DOI] [PubMed] [Google Scholar]
- 57.Reguera J, Urry DW, Parker TM, McPherson DT, Rodríguez-Cabello JC. Effect of NaCl on the exothermic and endothermic components of the inverse temperature transition of a model elastin-like polymer. Biomacromolecules. 2007;8:354–358. doi: 10.1021/bm060936l. [DOI] [PubMed] [Google Scholar]
- 58.Urry DW, et al. Hydrophobicity scale for proteins based on inverse temperature transitions. Biopolymers. 1992;32:1243–1250. doi: 10.1002/bip.360320913. [DOI] [PubMed] [Google Scholar]
- 59.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J. Phys. Chem. B. 1997;101:11007–11028. doi: 10.1021/jp972167t. [DOI] [Google Scholar]
- 60.Urry D, Okamoto K, Harris R, Hendrix C, Long M. Synthetic, crosslinked polypentapeptide of tropoelastin: an anisotropic, fibrillar elastomer. Biochemistry. 1976;15:4083–4089. doi: 10.1021/bi00663a026. [DOI] [PubMed] [Google Scholar]
- 61.Kurzbach D, et al. Hydration layer coupling and cooperativity in phase behavior of stimulus responsive peptide polymers. J. Am. Chem. Soc. 2013;135:11299–11308. doi: 10.1021/ja4047872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDaniel JR, Radford DC, Chilkoti A. A unified model for de novo design of elastin-like polypeptides with tunable inverse transition temperatures. Biomacromolecules. 2013;14:2866–2872. doi: 10.1021/bm4007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer DE, Chilkoti A. Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides. Biomacromolecules. 2004;5:846–851. doi: 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- 64.MacKay JA, Callahan DJ, FitzGerald KN, Chilkoti A. Quantitative model of the phase behavior of recombinant pH-responsive elastin-like polypeptides. Biomacromolecules. 2010;11:2873–2879. doi: 10.1021/bm100571j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christensen T, Hassouneh W, Trabbic-Carlson K, Chilkoti A. Predicting transition temperatures of elastin-like polypeptide fusion proteins. Biomacromolecules. 2013;14:1514–1519. doi: 10.1021/bm400167h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hassouneh, W., MacEwan, S.R. & Chilkoti, A. in Methods in Enzymology Vol. 502 (eds Wittrup, K. D. & Verdine, G. L.) 215–237 (Elsevier, 2012). [DOI] [PMC free article] [PubMed]
- 67.Christensen T, et al. Fusion order controls expression level and activity of elastin‐like polypeptide fusion proteins. Protein Sci. 2009;18:1377–1387. doi: 10.1002/pro.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trabbic‐Carlson K, et al. Effect of protein fusion on the transition temperature of an environmentally responsive elastin‐like polypeptide: a role for surface hydrophobicity? Protein Eng. Des. Sel. 2004;17:57–66. doi: 10.1093/protein/gzh006. [DOI] [PubMed] [Google Scholar]
- 69.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally responsive polypeptides. Nat. Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 70.Roberts S, Dzuricky M, Chilkoti A. Elastin-like polypeptides as models of intrinsically disordered proteins. FEBS Lett. 2015;589:2477–2486. doi: 10.1016/j.febslet.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simon JR, Carroll NJ, Rubinstein M, Chilkoti A, López GP. Programming molecular self-assembly of intrinsically disordered proteins containing sequences of low complexity. Nat. Chem. 2017;9:509–515. doi: 10.1038/nchem.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muiznieks LD, Sharpe S, Pomès R, Keeley FW. Role of liquid–liquid phase separation in assembly of elastin and other extracellular matrix proteins. J. Mol. Biol. 2018;430:4741–4753. doi: 10.1016/j.jmb.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Knowles TPJ, et al. Observation of spatial propagation of amyloid assembly from single nuclei. Proc. Natl Acad. Sci. USA. 2011;108:14746–14751. doi: 10.1073/pnas.1105555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michaels TC, et al. Dynamics of oligomer populations formed during the aggregation of Alzheimer’s Aβ42 peptide. Nat. Chem. 2020;12:445–451. doi: 10.1038/s41557-020-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miao M, et al. Sequence and structure determinants for the self-aggregation of recombinant polypeptides modeled after human elastin. J. Biol. Chem. 2003;278:48553–48562. doi: 10.1074/jbc.M308465200. [DOI] [PubMed] [Google Scholar]
- 76.Berendsen, H. J., Postma, J. P., van Gunsteren, W. F. & Hermans, J. in Intermolecular Forces (ed. Pullman, B.) 331–342 (Springer, 1981).
- 77.Rauscher S, et al. Proline and glycine control protein self-organization into elastomeric or amyloid fibrils. Structure. 2006;14:1667–1676. doi: 10.1016/j.str.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Cinar H, et al. Temperature, hydrostatic pressure, and osmolyte effects on liquid–liquid phase separation in protein condensates: physical chemistry and biological implications. Chemistry. 2019;25:13049–13069. doi: 10.1002/chem.201902210. [DOI] [PubMed] [Google Scholar]
- 79.Käfer F, et al. LCST and UCST in one: double thermoresponsive behavior of block copolymers of poly(ethylene glycol) and poly(acrylamide-co-acrylonitrile) Langmuir. 2015;31:8940–8946. doi: 10.1021/acs.langmuir.5b02006. [DOI] [PubMed] [Google Scholar]
- 80.Dutta NK, et al. A genetically engineered protein responsive to multiple stimuli. Angew. Chem. Int. Ed. 2011;50:4428–4431. doi: 10.1002/anie.201007920. [DOI] [PubMed] [Google Scholar]
- 81.Bracha D, et al. Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell. 2018;175:1467–1480.e13. doi: 10.1016/j.cell.2018.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitrea DM, et al. Self-interaction of NPM1 modulates multiple mechanisms of liquid–liquid phase separation. Nat. Commun. 2018;9:842. doi: 10.1038/s41467-018-03255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feric M, et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang P, et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell. 2020;181:325–345.e28. doi: 10.1016/j.cell.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guillén-Boixet J, et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell. 2020;181:346–361.e17. doi: 10.1016/j.cell.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shin Y, et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell. 2017;168:159–171.e14. doi: 10.1016/j.cell.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seim I, et al. Dilute phase oligomerization can oppose phase separation and modulate material properties of a ribonucleoprotein condensate. Proc. Natl Acad. Sci. USA. 2022;119:e2120799119. doi: 10.1073/pnas.2120799119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H, et al. RNA controls PolyQ protein phase transitions. Mol. Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Babinchak WM, Surewicz WK. Liquid–liquid phase separation and its mechanistic role in pathological protein aggregation. J. Mol. Biol. 2020;432:1910–1925. doi: 10.1016/j.jmb.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai Y, et al. Programmable synthetic biomolecular condensates for cellular control. Nat. Chem. Biol. 2023;19:518–528. doi: 10.1038/s41589-022-01252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alberti S, Gladfelter A, Mittag T. Considerations and challenges in studying liquid–liquid phase separation and biomolecular condensates. Cell. 2019;176:419–434. doi: 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kedersha N, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jain S, et al. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–498. doi: 10.1016/j.cell.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu S, et al. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat. Commun. 2021;12:502. doi: 10.1038/s41467-020-20768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao EM, et al. Light-based control of metabolic flux through assembly of synthetic organelles. Nat. Chem. Biol. 2019;15:589–597. doi: 10.1038/s41589-019-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho W-K, et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361:412–415. doi: 10.1126/science.aar4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boija A, et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 2018;175:1842–1855.e16. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Henninger JE, et al. RNA-mediated feedback control of transcriptional condensates. Cell. 2021;184:207–225.e24. doi: 10.1016/j.cell.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brangwynne CP, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 100.Updike D, Strome S. P Granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 2010;31:53–60. doi: 10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei M-T, et al. Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 2017;9:1118–1125. doi: 10.1038/nchem.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brangwynne CP. Soft active aggregates: mechanics, dynamics and self-assembly of liquid-like intracellular protein bodies. Soft Matter. 2011;7:3052–3059. doi: 10.1039/c0sm00981d. [DOI] [Google Scholar]
- 103.Serrano L. Synthetic biology: promises and challenges. Mol. Syst. Biol. 2007;3:158. doi: 10.1038/msb4100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu CC, Jewett MC, Chin JW, Voigt CA. Toward an orthogonal central dogma. Nat. Chem. Biol. 2018;14:103–106. doi: 10.1038/nchembio.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu DR, Magliery TJ, Pastrnak M, Schultz PG. Engineering a tRNA and aminoacyl-tRNA synthetase for the site-specific incorporation of unnatural amino acids into proteins in vivo. Proc. Natl Acad. Sci. USA. 1997;94:10092–10097. doi: 10.1073/pnas.94.19.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chin JW. Expanding and reprogramming the genetic code. Nature. 2017;550:53–60. doi: 10.1038/nature24031. [DOI] [PubMed] [Google Scholar]
- 107.Reinkemeier CD, Girona GE, Lemke EA. Designer membraneless organelles enable codon reassignment of selected mRNAs in eukaryotes. Science. 2019;363:eaaw2644. doi: 10.1126/science.aaw2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reinkemeier CD, Lemke EA. Dual film-like organelles enable spatial separation of orthogonal eukaryotic translation. Cell. 2021;184:4886–4903.e21. doi: 10.1016/j.cell.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nielsen J, Keasling JD. Engineering cellular metabolism. Cell. 2016;164:1185–1197. doi: 10.1016/j.cell.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 110.Garabedian MV, et al. Designer membraneless organelles sequester native factors for control of cell behavior. Nat. Chem. Biol. 2021;17:998–1007. doi: 10.1038/s41589-021-00840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo H, et al. Spatial engineering of E. coli with addressable phase-separated RNAs. Cell. 2022;185:3823–3837.e23. doi: 10.1016/j.cell.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 112.Kar M, et al. Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions. Proc. Natl Acad. Sci. USA. 2022;119:e2202222119. doi: 10.1073/pnas.2202222119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burke KA, Janke AM, Rhine CL, Fawzi NL. Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol. Cell. 2015;60:231–241. doi: 10.1016/j.molcel.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shin Y, et al. Liquid nuclear condensates mechanically sense and restructure the genome. Cell. 2018;175:1481–1491.e13. doi: 10.1016/j.cell.2018.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanenbaum ME, et al. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159:635–646. doi: 10.1016/j.cell.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gouveia B, et al. Capillary forces generated by biomolecular condensates. Nature. 2022;609:255–264. doi: 10.1038/s41586-022-05138-6. [DOI] [PubMed] [Google Scholar]
- 117.Roberts S, et al. Injectable tissue integrating networks from recombinant polypeptides with tunable order. Nat. Mater. 2018;17:1154–1163. doi: 10.1038/s41563-018-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roberts S, et al. Complex microparticle architectures from stimuli-responsive intrinsically disordered proteins. Nat. Commun. 2020;11:1342. doi: 10.1038/s41467-020-15128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alshareedah I, Moosa MM, Raju M, Potoyan DA, Banerjee PR. Phase transition of RNA–protein complexes into ordered hollow condensates. Proc. Natl Acad. Sci. USA. 2020;117:15650–15658. doi: 10.1073/pnas.1922365117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanford C. Theory of micelle formation in aqueous solutions. J. Phys. Chem. 1974;78:2469–2479. doi: 10.1021/j100617a012. [DOI] [Google Scholar]
- 121.Hassouneh W, Zhulina EB, Chilkoti A, Rubinstein M. Elastin-like polypeptide diblock copolymers self-assemble into weak micelles. Macromolecules. 2015;48:4183–4195. doi: 10.1021/acs.macromol.5b00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dreher MR, et al. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J. Am. Chem. Soc. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruff KM, Dar F, Pappu RV. Polyphasic linkage and the impact of ligand binding on the regulation of biomolecular condensates. Biophys. Rev. 2021;2:021302. doi: 10.1063/5.0050059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Linsenmeier M, et al. Dynamic arrest and aging of biomolecular condensates are modulated by low-complexity domains, RNA and biochemical activity. Nat. Commun. 2022;13:3030. doi: 10.1038/s41467-022-30521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McNally JG. Quantitative FRAP in analysis of molecular binding dynamics in vivo. Methods Cell Biol. 2008;85:329–351. doi: 10.1016/S0091-679X(08)85014-5. [DOI] [PubMed] [Google Scholar]
- 126.Schaaf MJ, Cidlowski JA. Molecular determinants of glucocorticoid receptor mobility in living cells: the importance of ligand affinity. Mol. Cell. Biol. 2003;23:1922–1934. doi: 10.1128/MCB.23.6.1922-1934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo YE, et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature. 2019;572:543–548. doi: 10.1038/s41586-019-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klein IA, et al. Partitioning of cancer therapeutics in nuclear condensates. Science. 2020;368:1386–1392. doi: 10.1126/science.aaz4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schuster BS, et al. Biomolecular condensates: sequence determinants of phase separation, microstructural organization, enzymatic activity, and material properties. J. Phys. Chem. B. 2021;125:3441–3451. doi: 10.1021/acs.jpcb.0c11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kilgore HR, Young RA. Learning the chemical grammar of biomolecular condensates. Nat. Chem. Biol. 2022;18:1298–1306. doi: 10.1038/s41589-022-01046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Folkmann AW, Putnam A, Lee CF, Seydoux G. Regulation of biomolecular condensates by interfacial protein clusters. Science. 2021;373:1218–1224. doi: 10.1126/science.abg7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaur T, et al. Sequence-encoded and composition-dependent protein–RNA interactions control multiphasic condensate morphologies. Nat. Commun. 2021;12:872. doi: 10.1038/s41467-021-21089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alshareedah I, Moosa MM, Pham M, Potoyan DA, Banerjee PR. Programmable viscoelasticity in protein–RNA condensates with disordered sticker-spacer polypeptides. Nat. Commun. 2021;12:6620. doi: 10.1038/s41467-021-26733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Costa SA, et al. Photo-crosslinkable unnatural amino acids enable facile synthesis of thermoresponsive nano- to microgels of intrinsically disordered polypeptides. Adv. Mater. 2018;30:1704878. doi: 10.1002/adma.201704878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 136.Kim TH, et al. Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science. 2019;365:825–829. doi: 10.1126/science.aax4240. [DOI] [PubMed] [Google Scholar]
- 137.Simon JR, Eghtesadi SA, Dzuricky M, You L, Chilkoti A. Engineered ribonucleoprotein granules inhibit translation in protocells. Mol. Cell. 2019;75:66–75.e65. doi: 10.1016/j.molcel.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry (Macmillan, 2008).
- 139.Lafontaine DL, Riback JA, Bascetin R, Brangwynne CP. The nucleolus as a multiphase liquid condensate. Nat. Rev. Mol. Cell Biol. 2021;22:165–182. doi: 10.1038/s41580-020-0272-6. [DOI] [PubMed] [Google Scholar]
- 140.Abbas M, Lipiński WP, Nakashima KK, Huck WTS, Spruijt E. A short peptide synthon for liquid–liquid phase separation. Nat. Chem. 2021;13:1046–1054. doi: 10.1038/s41557-021-00788-x. [DOI] [PubMed] [Google Scholar]
- 141.Kelley FM, Favetta B, Regy RM, Mittal J, Schuster BS. Amphiphilic proteins coassemble into multiphasic condensates and act as biomolecular surfactants. Proc. Natl Acad. Sci. USA. 2021;118:e2109967118. doi: 10.1073/pnas.2109967118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dine E, Gil AA, Uribe G, Brangwynne CP, Toettcher JE. Protein phase separation provides long-term memory of transient spatial stimuli. Cell Syst. 2018;6:655–663.e5. doi: 10.1016/j.cels.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rhys GG, et al. De novo designed peptides for cellular delivery and subcellular localisation. Nat. Chem. Biol. 2022;18:999–1004. doi: 10.1038/s41589-022-01076-6. [DOI] [PubMed] [Google Scholar]
- 144.Molinari S, et al. A synthetic system for asymmetric cell division in Escherichia coli. Nat. Chem. Biol. 2019;15:917–924. doi: 10.1038/s41589-019-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin D-W, et al. Construction of intracellular asymmetry and asymmetric division in Escherichia coli. Nat. Commun. 2021;12:888. doi: 10.1038/s41467-021-21135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee MJ, et al. Engineered synthetic scaffolds for organizing proteins within the bacterial cytoplasm. Nat. Chem. Biol. 2018;14:142–147. doi: 10.1038/nchembio.2535. [DOI] [PubMed] [Google Scholar]
- 147.Lasker K, et al. Selective sequestration of signalling proteins in a membraneless organelle reinforces the spatial regulation of asymmetry in Caulobacter crescentus. Nat. Microbiol. 2020;5:418–429. doi: 10.1038/s41564-019-0647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Klosin A, et al. Phase separation provides a mechanism to reduce noise in cells. Science. 2020;367:464–468. doi: 10.1126/science.aav6691. [DOI] [PubMed] [Google Scholar]
- 149.Lin J, Amir A. Homeostasis of protein and mRNA concentrations in growing cells. Nat. Commun. 2018;9:4496. doi: 10.1038/s41467-018-06714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang W, et al. Molecular determinants for regulation of G3BP1/2 phase separation by the SARS-CoV-2 nucleocapsid protein. Cell Discov. 2021;7:69. doi: 10.1038/s41421-021-00306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang J, et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 2018;174:688–699.e16. doi: 10.1016/j.cell.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li B, Alonso DOV, Bennion BJ, Daggett V. Hydrophobic hydration is an important source of elasticity in elastin-based biopolymers. J. Am. Chem. Soc. 2001;123:11991–11998. doi: 10.1021/ja010363e. [DOI] [PubMed] [Google Scholar]
- 153.Jin X, et al. Membraneless organelles formed by liquid–liquid phase separation increase bacterial fitness. Sci. Adv. 2021;7:eabh2929. doi: 10.1126/sciadv.abh2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lasker K, et al. The material properties of a bacterial-derived biomolecular condensate tune biological function in natural and synthetic systems. Nat. Commun. 2022;13:5643. doi: 10.1038/s41467-022-33221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]