Abstract
Background
Pelvic floor dysfunction and urinary incontinence are two of the most frequent gynecological problems, and pelvic floor muscle training is recommended as a first‐line treatment, with new approaches such as hypopressive exercises. This study aimed to analyze the efficacy of an 8‐week supervised training program of hypopressive exercises on pelvic floor muscle strength and urinary incontinence symptomatology.
Design
Blinded randomized controlled trial.
Settings
Women with pelvic floor dysfunction and urinary incontinence symptoms, aged 18–60 years.
Participants
A total of 117 participants were randomly allocated to the hypopressive exercises group (n = 62) or a control group that received no intervention (n = 55) and completed the study.
Main Outcome Measures
Clinical and sociodemographic data were collected, as well as pelvic floor muscle strength (using the Modified Oxford Scale); the genital prolapse symptoms, colorectal symptoms, and urinary symptoms (with the Pelvic Floor Distress Inventory [PFDI‐20]); the impact of pelvic floor disorders (PFD) on women's lives (with the Pelvic Floor Impact Questionnaire [PFIQ‐7]); and the severity of urinary incontinence symptoms (using the International Consultation on Incontinence Questionnaire [ICIQ]).
Results
The results showed an improvement in the hypopressive group in the pelvic floor muscle strength F (1117) = 89.514, p < 0.001, a significantly lower score for the PFIQ7 total score, t (112) = 28.895, p < 0.001 and FPDI20 t (112) = 7.037, p < 0.001 as well as an improvement in ICIQ‐SF values after 8 weeks of intervention in comparison with the control group.
Conclusions
After performing an 8‐week of hipopressive exercises intervention, a decrease in pelvic floor disorders associated symptoms can be observed. In addition, pelvic floor muscle contractility is improved and a decrease in severity and symptoms associated with urinary incontinence has been reported.
Keywords: hypopressive exercise, pelvic floor muscle, pelvic floor muscle training, pelvic organ prolapse, urinary incontinence
1. INTRODUCTION
Pelvic floor dysfunction (PFD) is one of the most common gynecological problems among women worldwide. 1 It is caused by weakness of the pelvic floor (PF) supporting tissues, grouping a set of conditions such as urinary incontinence (UI), pelvic organ prolapse (POP), fecal incontinence, sexual dysfunction, and other urogenital symptoms. 1 This condition is related to a decrease in the quality of life (QoL), with social, health, and economic repercussions. 2 The symptom's severity can be enhanced by childbirth, obesity, constipation, pelvic surgery, heavy lifting activities, and the influence of a genetic history. 3 Pelvic floor muscle training (PFMT) is recommended as a first‐line treatment. 4 The UI has a direct impact on women's QoL, being a major determinant of their physical, mental, and social functioning. 2 However, in many instances, it can be improved with an appropriate treatment. 4 PFMT is often combined with behavioral treatments, bladder training, and suppression techniques to treat urge UI. 5 This intervention, not only helps develop greater strength and tone of the pelvic floor musculature (PFM) but also facilitates suppression of urgency, noting that detrusor muscle contraction can be inhibited by using rapid PFM movements to actively contract the sphincter, which in turn causes reflex relaxation of the detrusor muscle. 6 Therefore, the objective of physiotherapy is based on re‐educating the perineal reflex before exertion, promoting awareness and correct contraction of PFM, as well as activation and coordination of the abdominal muscles. 7 PFMT has been recommended because it promotes PFM strength, endurance, and proprioception. 7 However, the popularity of new approaches, such as hypopressive exercises (HE), has increased due to the reported benefits such as PFM strength and endurance, postural control, deep trunk muscle activation, and ventilatory capacity. 8 This technique is related to a decrease in intra‐abdominal pressure in the thoracic, abdominal, and perineal compartments, and may play an important role in the activation of striated muscle fibers of the PFM and deep trunk muscles. 9 Despite being widely used as a therapeutic exercise modality for PFM, there is still controversy about its clinical efficacy in the treatment of PFD, because QoL and PFM function can be increased with PFMT, being even superior to HE intervention. 10
The main hypothesis of the present study was that the intervention based on HE will be effective in the management of PFM and UI due to the described muscle activation of the pelvic floor and abdominal muscles. Therefore, this study aims to analyze the efficacy of an 8‐week supervised HE training program on PFM strength and UI symptomatology.
2. MATERIALS AND METHODS
2.1. Study design
A blinded randomized controlled trial (RCT) registered on Clinicaltrial.gov (NCT04343599), was conducted between February–June 2019 at the University of Jaén (Spain). The guidelines of the CONSORT 11 statement and the Consensus on Exercise Reporting Template (CERT) 12 were followed.
2.2. Participants
Participants were recruited in the province of Jaén (Spain). Inclusion criteria were: women aged 18 to 60 years and the presence of PFD symptomatology lasting >6 months. Exclusion criteria were: previous experience with HE, conservative treatment for PFD during the previous year, pelvic or abdominal surgery, and medical contraindication for HE such as pregnancy, uncontrolled hypertension, hiatal hernia, or cardiorespiratory disease. The selected participants signed the informed consent form and were randomly assigned to the Experimental Group (EG) or Control Group (CG) using the OxMaR system in a 1:1 ratio. 13
2.3. Ethical considerations
The recommendations of the Declaration of Helsinki were followed by ethical principles for research on human subjects. Obtained data were administered following the Organic Law 3/2018, of December 5, on the Protection of Personal Data and Guarantee of Digital Rights. All participants signed informed consent to be part of the study that was approved by the Human Ethics Committee of the University of Jaén (02/2019).
2.4. Measurements
Clinical and sociodemographic data were collected, such as age, weight, height, body mass index (BMI), the number of pregnancies, the number and type of deliveries (vaginal and/or cesarean), and smoking habits. On the other hand, participants were considered physically active if they performed a minimum of 150 min of moderate activity per week. 14
PFM strength and function: assessed by digital palpation with the Modified Oxford Scale (MOS) proposed by Laycock, 15 scored from 0 to 5: 0 no contraction; one mild contraction with no movement; two mild contraction and movement (weak); three moderate contraction, intravaginal pressure and finger compression with slight vaginal wall elevation (moderate); four strong contractions against resistance (good); and five contractions against maximum resistance and maintained (strong).
Pelvic Floor Distress Inventory (PFDI‐20): consists of 20 questions divided into three symptom scales, questions 1–6 are related to genital prolapse symptoms (POPDI), questions 7–14 to anal colorectal symptoms (CRADI), and questions 15–20 to urinary symptoms (UDI), indicating the degree to which they are bothersome on a Likert‐type scale from 1 (not at all) to 4 (very much). 16
Pelvic Floor Impact Questionnaire (PFIQ‐7): contains 7 items that assess the effect on activities, relationships, or feelings of urinary (UIQ), colorectal‐anal (CRAIQ), and genital prolapse (POPIQ) symptoms, scored on a Likert‐type scale from 0 (not at all) to 3 (very much). 16
International Consultation on Incontinence Questionnaire (ICIQ): it evaluates the severity of specific UI symptoms and the impact on QoL. It consists of 3 items: quantity, frequency, and affectation, scored on a Likert‐type scale: frequency from 0 (never) to 5 (continuously), quantity from 0 (nothing escapes me) to 6 (a lot), and affectation from 0 (nothing) to 10 (a lot). Its final score ranges from 0 to 21 points, where any score above 0 is considered incontinence. 17
2.5. Intervention
The intervention consisted of an 8‐week HE program, performed twice a week for 20 min. The HE program followed the methodology described by Rial and Pinsach, 18 including initial training in breathing patterns, rib cage proprioception, apnea familiarization, and abdominal vacuum. The sessions were conducted in small groups (10–12 participants), allowing adequate supervision of the participants. All hypopressive postures followed the same postural indications (axial elongation, neutral pelvis, center of gravity projection, ankle dorsiflexion, and activation of the shoulder girdle). 18 The progression of postures included a dynamic progression of HE performed in standing, seated, quadruped, and supine (see Figure 1). The CG did not receive treatment and was advised to maintain their usual daily activity and refrain from any physical intervention.
Figure 1.

Fypopressive exercise poses
2.6. Data collection
Data were collected between February‐June 2019. Two blinded investigators external to the study performed the data collection as well as its analysis. Assessment of PFM was performed by manual vaginal palpation assessment, in the lithotomy position, and voluntary PFM contraction was assessed by MOS. 15 Participants were asked to maintain a maximal voluntary PFM contraction for 5 s, repeated three times with 10‐s rest intervals. The score was recorded from 0 to 5 and the best score of the three attempts was considered for analysis. In addition, all participants completed PFDI‐20, PFIQ‐7, and ICIQ.
2.7. Data analysis
Statistical analysis was carried out with the statistical program SPSS, version 21.0. Categorical variables are described with frequencies and percentages, and the continuous variables are as means and standard deviations (SD). The Kolmogorov–Smirnov test was employed to analyze normality for continuous variables. For the descriptive analysis of the quantitative (continuous) and qualitative (categorical) variables, Student's t‐test and χ 2 test were applied respectively. Analysis of variance (ANOVA) with a 2 × 2 design was used to assess the differences between groups (EG vs. CG) and between assessments (baseline vs. 8 weeks). η 2 was applied to calculate the effect size of the specific group × time interactions.
3. RESULTS
3.1. Selection of the sample of participants
A total of 254 participants were selected, of whom 76 did not meet the inclusion criteria and 53 did not agree to participate in the study. A total of 125 women were randomly assigned to each group (64 to the EG and 60 to the CG); five CG participants did not complete the last assessment and two EG women dropped out due to time incompatibility. Finally, a total of 117 participants completed the study (see Figure 2).
Figure 2.
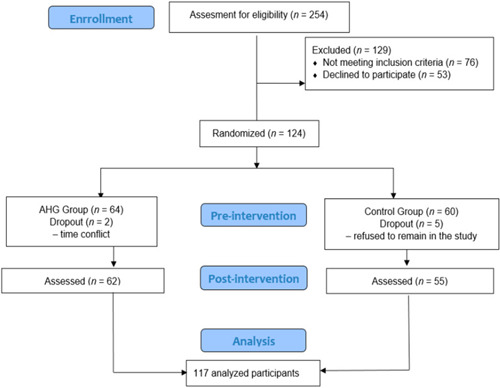
Flow chart of participant's allocation
3.2. Characteristics of the participants
Of the participants who completed the study, the mean age was 45.65(8.86), having a BMI of 24.03(3.63). There were no differences between the groups at the beginning of the study in terms of sociodemographic variables (see Table 1).
Table 1.
Demographic characteristics of participants at baseline (n = 117)
| Total (n = 117)mean (SD) | EG (n = 62)mean (SD) | CG (n = 55) mean(SD) | p Value | ||
|---|---|---|---|---|---|
| Age (years) | 45.65(8.86) | 44.54(10.40) | 46.89(6.59) | 0.149 | |
| Weight (Kg) | 63.59(10.59) | 62.67(11.05) | 64.62(10.04) | 0.318 | |
| Height (cm) | 162.56(5.95) | 161.78(5.99) | 163.45(5.83) | 0.128 | |
| BMI (kg/m2) | 24.03(3,63) | 23.93(3.92) | 24.15(3.31) | 0.742 | |
| N° pregnancies | 1.54(1.07) | 1.38(1.07) | 1.71(1.06) | 0.090 | |
| N° deliveries | 1.47(1.05) | 1.33(1.05) | 1.63(1.04) | 0.130 | |
| Delivery type | |||||
| Nulliparous | N (%) 28 (23.5) | N (%) 18 (64.3) | N (%) 10 (35.7) | 0.511 | |
| Vaginal | 68 (57.1) | 34 (50) | 34 (50) | ||
| Cesarean | 12 (10.1) | 5 (41.7) | 7 (58.3) | ||
| Vaginal‐cesarean | 10 (8.4) | 6(54.5) | 5(45.5) | ||
| Smoker | No | 100 (84) | 51(51) | 49(49) | 0.330 |
| Yes | 19 (16) | 12(63.2) | 7(36.8) | ||
| Physically active | No | 56 (47.1) | 29(51.8) | 27(48.2) | 0.812 |
| Yes | 63 (52.9) | 34(54) | 29(46) | ||
Note: Quantitative variables are presented as mean and standard deviation. Qualitative variables are presented as frequency and percentage.
Abbreviations: BMI, body mass index; cm, centimeters; Kg, kilograms; N°, number; SD, standard deviation.
3.3. Outcome measures
In the study of perineal function, through PFM contractility assessed by MOS, the values of the postintervention test were higher in the EG 3.14(1.20) than the CG 1.57(0.85) (see Figure 3). The results revealed significant changes in the variable Group: F (1.117) = 22.967, p < 0.001, η 2 = 0.171; in the variable Measurement time: F (1.117) = 14.424, p < 0.001, η 2 = 0.115 and in the interaction of both (Group × Time): F (1,117) = 89.514, p < 0.001, η 2 = 0.446. After analysis of this interaction, statistically, significant differences were observed between both groups in PFM contractility after HE, MOS: t (112) = 6.763, p < 0.001 with an effect size difference (Cohen's d = 1.53). In addition, EG participants showed significant differences from pre‐intervention values, t (62) = −9.059, p < 0.001, with a moderate effect size (Cohen's d = 0.79) (see Table 2).
Figure 3.
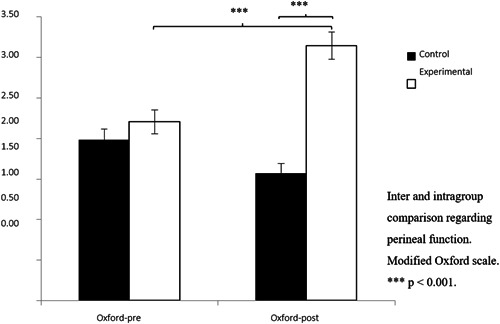
Modified Qxford Scale change scores
Table 2.
Between and within‐group changes scores
| Experimental group | Control group | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre (n = 63) | Post (n = 63) | Pre (n = 51) | Post (n = 51) | Group | Time | Group × time | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | Valor‐ p | η 2 | F | p Value | η2 | F | p Value | η 2 | |
| Oxford Score | 2.21 | 1.17 | 3.1 14 | 1.20 | 1.98 | 0.97 | 1.57 | 0.85 | 22.967 | <0.001 | 0.171 | 14.424 | <0.001 | 0.115 | 89.514 | <0.001* | 0.446 |
| UIQ7 | 12.98 | 12.55 | 3.02 | 6.96 | 11.30 | 19.59 | 13.61 | 20.17 | 2.686 | 0.104 | 0.024 | 20.987 | <0.001 | 0.159 | 53.077 | <0.001* | 0.323 |
| CRAIQ7 | 8.10 | 14.75 | 0.98 | 2.72 | 6.81 | 13.03 | 8.06 | 11.85 | 2.372 | 0.126 | 0.021 | 8.750 | 0.004 | 0.073 | 16.375 | <0.001* | 0.129 |
| POPIQ7 | 5.62 | 9.65 | 0.60 | 2.18 | 6.44 | 16.21 | 8.26 | 17.19 | 3.895 | 0.051 | 0.034 | 4.678 | 0.033 | 0.040 | 22.146 | <0.001* | 0.166 |
| PFIQ7 | 27.61 | 32.38 | 4.76 | 10.04 | 24.44 | 38.61 | 29.22 | 40.09 | 3.804 | 0.054 | 0.033 | 18.587 | <0.001 | 0.143 | 43.400 | <0.001* | 0.281 |
| POPDI6 | 16.00 | 14.16 | 6.80 | 9.18 | 13.15 | 13.73 | 17.53 | 14.53 | 2.911 | 0.091 | 0.026 | 7.833 | 0.006 | 0.066 | 54.182 | <0.001* | 0.328 |
| CRADI8 | 15.28 | 14.94 | 7.65 | 9.28 | 13.90 | 15.49 | 18.02 | 15.56 | 3.876 | 0.051 | 0.034 | 3.167 | 0.078 | 0.028 | 37.815 | <0.001* | 0.254 |
| UDI6 | 21.95 | 17.13 | 8.41 | 10.24 | 17.31 | 17.73 | 23.36 | 19.83 | 3.111 | 0.081 | 0.027 | 7.639 | 0.007 | 0.064 | 58.271 | <0.001* | 0.344 |
| FPDI20 | 53.23 | 39.18 | 22.85 | 21.64 | 45.18 | 35.85 | 59.41 | 37.18 | 5.612 | 0.020 | 0.048 | 13.515 | <0.001 | 0.109 | 105.099 | <0.001* | 0.486 |
Note: Quantitative variables are presented as mean and standard deviation (SD).
Abbreviations: CRADI8, Colorectal‐Anal Discomfort Inventory; CRAIQ7, Colorectal‐Anal Impact Questionnaire; FPDI20, Pelvic Floor Discomfort Inventory Classification Form; ICIQSF, International Consultation on Incontinence Questionnaire‐Urinary Incontinence Short Form; PFIQ7, pelvic floor impact questionnaire classification form; POPDI6, Pelvic Organ Prolapse Distress Inventory; POPIQ7, Pelvic Organ Prolapse Impact Questionnaire; UDI6, Urogenital Discomfort Inventory; UIQ7, Urinary Incontinence Impact Questionnaire.
Statistically significant.
The study of the impact of PFD on health was analyzed using the PFIQ‐7 and FPDI‐20 questionnaires. In the results of the study of the main effects, shown in Table 2, there were significant effects concerning the main variable Group only for the scores obtained in the FPDI‐20 questionnaire. Significant results were found for the Time of measurement effect for all variables, except for the subscale on anal colorectal symptoms CRADI‐8, showing a significant Group × Time effect for all variables analyzed.
In the analysis of the interactions observed in the Group × Time analysis concerning the PFIQ‐7 questionnaire and its subscales (see Figure 4), the EG obtained a significant decrease in values after the intervention: t (62) = 7. 987, p < 0.001 (UIQ7), t (62) = 4.157, p < 0.001 (CRAIQ7), t (62) = 4.356, p < 0.001 (POPIQ7), and t (62) = 6.349, p < 0.001 (PFIQ7). The effect sizes of these differences were large, with Cohen's d values of 1.08 for the PFIQ7 total score, and 1.02, 0.82, and 0.85 for the UIQ7, CRAIQ7, and POPIQ7 domains respectively. In addition, EG was significantly lower on the postintervention measure for the PFIQ7 total score, t (112) = 28.895, p < 0.001, with a large effect size (Cohen's d = 0.98). There were significant differences between the three domains: t (112) = 22.846, p = 0.001 Cohen's d = 0.78 (UIQ7), t (112) = 27.567, p < 0.001, Cohen's d = 0.97 (CRAIQ7), and t (112) = 27.084, p = 0.003, Cohen's d = 0.79 (POPIQ7) (see Table 2).
Figure 4.
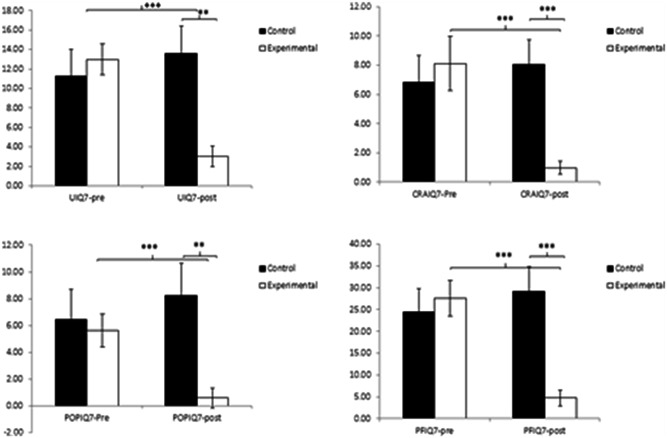
Inter‐ and intragroup comparison regarding the impact of pelvic floor dysfunctions on quality of life PFIQ‐7 and subscales (UIQ7, CRAIQ7 y POPIQ7). CRAIQ7, Colorectal‐Anal Impact Quessionnery; PFIQ7, Plevic Floor Impact Quessionnery Sort Form; POPIQ7, Pelvice Qrgan Prolapse Impact Quessionnaire; UIQ7, Urinary Incountinence Impact Questionnairy. **p < 0.01, ***p < 0.001.
Regarding the analysis of the Group × Time interaction of the FPDI‐20 questionnaire (see Figure 4), the results showed a significant decrease in the total score in the EG after performing HE: t (62) = 9.00, p < 0.001 (Cohen's d = 1.00). Similarly, it was observed how the scores of its subscales also decreased significantly in this group: t (62) = 7.375, p < 0.001 (POPDI6, Cohen's d = 0.79), t (62) = 5.316, p < 0.001 (CRADI8, Cohen's d = 0.63), and t (62) = 7.249, p < 0.001 (UDI6, Cohen's d = 0.99). In the postintervention values, the EG obtained significantly lower values than the CG in both the FPDI20 total score, t(112) = 7.037, p < 0.001 (Cohen's d = 1. 24) as well as in the subscales or domains POPDI6, t(112) = 8.982, p < 0.001 (Cohen's d = 0.90), CRADI8, t(112) = 14.236, p < 0.001, Cohen's d = 0.83), and UDI6, t(112) = 18.039, p < 0.001 (Cohen's d = 0.99).
In relation to the severity and symptoms associated with UI in QoL using the ICIQ‐SF, the analysis of the postintervention data showed statistically significant differences for the variable Group: F(1,117) = 5.017, p = 0.027, η2 = 0.043, Time of measurement: F(1,117) = 16.301, p < 0.001, η2 = 0.128 and for the interaction Group × Time: F (1,117) = 64.451, p < 0.001, η2 = 0.128. 367. After analysis of the Group × Time interactions, the EG showed significantly lower scores on the ICIQSF both after the intervention period, t (62) = 10.975, p < 0.001, and in the comparison between groups post‐intervention, t(112) = 7.04, p < 0.001, in both cases with a high effect size difference (Cohen's d = 0.84 and 0.96, respectively) (see Figure 5).
Figure 5.
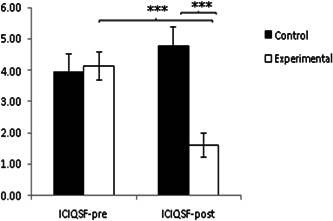
Inter‐ and intragroup comparison of severity and symptoms associated with urinary incontinence on women's quality of life ICIQSF: international Consultation on Incontinence Questionnaire_Urinary Incontinence Short Form.***p < 0.001.
It should be noted that no adverse effects were observed and treatment adherence was high (97%), with only two dropouts in the EG.
4. DISCUSSION
This study aimed to analyze the efficacy of an 8‐week supervised HE training program on PFM strength and UI symptomatology. Significant differences were found in maximal PFM contraction with a moderate effect size compared to CG. These findings agree with previous research where it has been observed an improvement in MOS after a 12‐week of HE program 19 and improvements in PFM contraction, UI tone, body image, and sense of well‐being, with high satisfaction with the intervention after 2‐month of HE exercise program. 20
Despite the existing skepticism about HE and its effect on PFM, it is evidenced that after a HE protocol the PFM strength increases, observing an improvement in PF function. 20 In the HE group of this study, was observed an improvement of the perineal contraction in participants allocated to the HE group after the intervention, with reported values ≥3 in MOS in the absence of parasitic contractions. These data are supported by the results of Costa et al. 21 where women with SUI increased perineal strength and progressed in MOS after performing three individual HE sessions. These findings could be attributed to the direct action of the hypopressive breathing maneuver on the thoracic diaphragm and its close relationship with PFM. 22
Assessment of perineal contractility provides information on muscle weakness and is the basis for planning specific programs for patients with PFD, but there is no definite consensus on the most suitable method of assessment. 23 MOS is one of the most commonly used manual techniques and has been employed in this trial because it is widely employed in clinical practice. 23 Whereas other tools such as manometry or dynamometry could be useful no superiority when assessing PFM has been reported. 23 Several authors, have compared PFM function and strength when performing HE and PFMT alone or in combination. 19 , 24 Jose‐Vaz et al. 19 reported an improvement in UI symptoms, quality of life impact, and PFM function after 12 weeks of PFMT or HE with better scores in the PFMT group. On the other hand, there is evidence that both treatment models produce similar improvements in both baseline PFM strength and tone in women with PFD. 24 Although there is a discrepancy between treatment methods and results could be related to the fact that PFMT offers a specific force increase in PFM and during HE no direct contraction of PFM is requested, but its activation is due to a synergic action between the thoracic diaphragm and the deep abdominal musculature. 19
Regarding the impact of PFDs on QoL, although their effectiveness has been identified in women with UI, 21 its effect in terms of urogynecological symptoms affecting the QoL had not been reported. EG group improved 30.38 (±21.64) points on the PFDI‐20 and 22.85 (±10.04) points on the PFIQ‐7, with a decrease of at least 45 points on the PFDI‐20 questionnaire and 36 points on the PFIQ‐7. These scores are considered to be the minimum clinically important difference in women who have undergone surgery for PFD. These differences can be explained by the sample characteristics. The participants showed mild PFD, with low scores in the questionnaires at the beginning of the study, and were higher than the observed in previous research. 24 Regarding the monitoring of the severity and symptoms associated with UI in QoL, assessed with ICIQ‐SF, the EG showed improvement after the intervention. These positive effects were also reported by another study 20 where after 24 groups of HE sessions of 30 min, the mean ICIQ‐SF score decreased. Therefore, the established HE protocol in this RCT improved symptoms associated with PFD and UI in EG women. These findings could be justified by the improvement of the perineal function and normalization of the intra‐abdominal pressure management, as a consequence of diaphragmatic suctioning coupled with hypopressive postural fundamentals.
In summary, an 8‐week HE program can provide benefits in perineal function and decrease symptoms related to PFD and UI. In addition, HE has no adverse effects and treatment adherence was high (97%). Therefore, it could be considered that a correctly performed HE program could provide a PFM stimulus despite not performing a direct contraction of this musculature.
4.1. Strengths and weaknesses
The results of this study should be considered in the context of a series of limitations, highlighting that the participants could not be blinded because it was an intervention in which they were aware of whether or not they were receiving the intervention, nor was there any follow‐up of the medium‐ or long‐term effects. On the other hand, the strength of the present study was the sample size of 62 participants in the EG, as opposed to other studies with 31, 24 and 21 participants. 25 Another strength was the standardization and description of the methodology as well as the continued instruction through small face‐to‐face groups compared to previous studies 10 , 25 which may be associated with high adherence to treatment, with only two dropouts in the EG.
4.2. Clinical implications
The management of pelvic floor dysfunctions requires a comprehensive approach involving abdominal muscles, diaphragm, and pelvic floor muscles. The observed improvement in PFM and UI, support that HE should be recommended to this population group. Another important clinical application consists of the fact that it's possible to make group sessions. This may lead to a democratization of access to specific pelvic floor treatment for patients that otherwise might not have been possible due to the lack of information regarding HE, pelvic floor dysfunctions, and taboo aspects that are still present in our days. Furthermore, this modality of treatment could be combined with physiotherapy and manual therapy intervention.
5. CONCLUSIONS
After performing an 8‐week HE‐based training program in women with PFD it can be observed that EG women decreased PFD‐associated symptoms and there were differences between groups postintervention. In addition, women who performed HE showed improved PFM contractility and decreased severity and symptoms associated with UI.
AUTHOR CONTRIBUTIONS
David Cruz‐Diaz conceived the study, and all authors participated in the study design. Yolanda Castellote‐Caballero collected the data. Fidel Hita‐Contreras analyzed the data. Mar Moreno‐Muñoz, Tamara R. RebullidoB, Marco Bergamin, and Stefano Gobbo drafted the manuscript. Guadalupe Molina‐Torres and David Cruz‐Diaz writing, review and editing. All authors gave comments on the earlier versions of the manuscript. All authors edited the manuscript and approved the final version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was registered on Clinicaltrial.gov (NCT04343599) and all the participants signed informed consent to be part of the study that was approved by the Human Ethics Committee of the University of Jaén (02/2019).
ACKNOWLEDGMENTS
Authors would like to express their gratitude to Low‐Pressure Fitness and Camilo Villanueva for their cooperation, Fisioelite and Forus Jaén for the facilities, and especially to all the participants of the study.
Molina‐Torres G, Moreno‐Muñoz M, Rebullido TR, et al. The effects of an 8‐week hypopressive exercise training program on urinary incontinence and pelvic floor muscle activation: a randomized controlled trial. Neurourol Urodyn. 2023;42:500‐509. 10.1002/nau.25110
Guadalupe Molina‐Torres and Mar Moreno‐Muñoz contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Xu P, Wang X, Guo P, Zhang W, Mao M, Feng S. The effectiveness of eHealth interventions on female pelvic floor dysfunction: a systematic review and meta‐analysis. Int Urogynecol J. 2022;33:3325‐3354. 10.1007/s00192-022-05222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hage‐Fransen MAH, Wiezer M, Otto A, et al. Pregnancy‐ and obstetric‐related risk factors for urinary incontinence, fecal incontinence, or pelvic organ prolapse later in life: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2021;100(3):373‐382. 10.1111/aogs.14027 [DOI] [PubMed] [Google Scholar]
- 3. Nygaard I. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311‐1316. 10.1001/jama.300.11.1311.Prevalence [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murray AS. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Res Nurs Health. 2019;42:234‐235. 10.1002/nur.21946 [DOI] [PubMed] [Google Scholar]
- 5. Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract. 2009;63(8):1177‐1191. 10.1111/j.1742-1241.2009.02078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrison JF. The excitability of the micturition reflex. Scand J Urol Nephrol Suppl. 1995;175:21‐25. [PubMed] [Google Scholar]
- 7. Caufriez M, Fernández JC, Fanzel R, Snoeck T. Efectos de un programa de entrenamiento estructurado de Gimnasia Abdominal Hipopresiva sobre la estática vertebral cervical y dorsolumbar. Fisioterapia. 2006;28(4):205‐216. 10.1016/S0211-5638(06)74048-2 [DOI] [Google Scholar]
- 8. Juez L, Núñez‐Córdoba JM, Couso N, Aubá M, Alcázar JL, Mínguez JÁ. Hypopressive technique versus pelvic floor muscle training for postpartum pelvic floor rehabilitation: a prospective cohort study. Neurourol Urodyn. 2019;38(7):1924‐1931. 10.1002/nau.24094 [DOI] [PubMed] [Google Scholar]
- 9. Moreno‐Muñoz MM, Hita‐Contreras F, Estudillo‐Martínez MD, et al. The effects of abdominal hypopressive training on postural control and deep trunk muscle activation: a randomized controlled trial. Int J Environ Res Public Health. 2021;18(5):2741. 10.3390/ijerph18052741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Resende APM, Bernardes BT, Stüpp L, et al. Pelvic floor muscle training is better than hypopressive exercises in pelvic organ prolapse treatment: an assessor‐blinded randomized controlled trial. Neurourol Urodyn. 2019;38(1):171‐179. 10.1002/nau.23819 [DOI] [PubMed] [Google Scholar]
- 11. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on Exercise Reporting Template (CERT): explanation and elaboration statement. Br J Sports Med. 2016;50(23):1428‐1437. 10.1136/bjsports-2016-096651 [DOI] [PubMed] [Google Scholar]
- 13. Guillaumes S, O'Callaghan CA. Versión en español del software gratuito OxMaR para minimización y aleatorización de estudios clínicos. Gac Sanit. 2019;33(4):395‐397. 10.1016/j.gaceta.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 14. Ferry B. Sports medicine: athletes and physically active people with diabetes. FP essentials. 2022;518:29‐34. [PubMed] [Google Scholar]
- 15. Laycock J. Clinical evaluation of the pelvic floor. Pelvic Floor Re‐Education, 1994. [Google Scholar]
- 16. Sánchez‐Sánchez B, Torres‐Lacomba M, Yuste‐Sánchez MJ, et al. Cultural adaptation and validation of the Pelvic Floor Distress Inventory Short Form (PFDI‐20) and Pelvic Floor Impact Questionnaire Short Form (PFIQ‐7) Spanish versions. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):281‐285. 10.1016/j.ejogrb.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 17. Espuña Pons M, Rebollo Álvarez P, Puig Clota M. Validación de la versión española del International Consultation on Incontinence Questionnaire‐Short Form. Un cuestionario para evaluar la incontinencia urinaria. Med Clin (Barc). 2004;122(8):288‐292. 10.1016/s0025-7753(04)74212-8 [DOI] [PubMed] [Google Scholar]
- 18. Rial T. Pinsach P. Practical manual low pressure fitness level 1. International Hypopressive & Physical Therapy Institute; Vigo, Spain. 2017.
- 19. Jose‐Vaz LA, Andrade CL, Cardoso LC, Bernardes BT, Pereira‐Baldon VS, Resende APM. Can abdominal hypropressive technique improve stress urinary incontinence? An assessor‐blinded randomized controlled trial. Neurourol Urodyn. 2020;39(8):2314‐2321. 10.1002/nau.24489 [DOI] [PubMed] [Google Scholar]
- 20. Soriano L, González‐Millán C, Álvarez Sáez MM, Curbelo R, Carmona L. Effect of an abdominal hypopressive technique programme on pelvic floor muscle tone and urinary incontinence in women: a randomised crossover trial. Physiotherapy. 2020;108:37‐44. 10.1016/j.physio.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 21. Costa TF, Paula Resende AM, Seleme MR, et al. Hypopressive gymnastics as a resource for perineal proprioception in women with urinary incontinence. Fisioter Bras. 2011;12(5):365‐369. [Google Scholar]
- 22. Ithamar L, de Moura Filho AG, Benedetti Rodrigues MA, et al. Abdominal and pelvic floor electromyographic analysis during abdominal hypopressive gymnastics. J Bodyw Mov Ther. 2018;22(1):159‐165. 10.1016/j.jbmt.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 23. Mateus‐Vasconcelos ECL, Ribeiro AM, Antônio FI, Brito LGO, Ferreira CHJ. Physiotherapy methods to facilitate pelvic floor muscle contraction: a systematic review. Physiother Theory Pract. 2018;34(6):420‐432. 10.1080/09593985.2017.1419520 [DOI] [PubMed] [Google Scholar]
- 24. Niederauer S, de Gennaro J, Nygaard I, Petelenz T, Hitchcock R. Development of a novel intra‐abdominal pressure transducer for large scale clinical studies. Biomed Microdevices. 2017;19:80. 10.1007/s10544-017-0211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stüpp L, Resende APM, Petricelli CD, Nakamura MU, Alexandre SM, Zanetti MRD. Pelvic floor muscle and transversus abdominis activation in abdominal hypopressive technique through surface electromyography. Neurourol Urodyn. 2011;30:1518‐1521. 10.1002/nau [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


