Abstract
Breast cancer (BC) is a heterogeneous disease, encompassing a diverse spectrum of tumours with varying morphological, biological, and clinical phenotypes. Although tumours may show phenotypic overlap, they often display different biological behaviour and response to therapy. Advances in high‐throughput molecular techniques and bioinformatics have contributed to improved understanding of BC biology and refinement of molecular taxonomy with the identification of specific molecular subclasses. Although the traditional pathological morphological classification of BC is of paramount importance and provides diagnostic and prognostic information, current interest focusses on the use of a single gene and multigene assays to stratify BC into distinct groups to guide decisions on systemic therapy. This review considers approaches to the classification of BC, including their limitations, and with particular emphasis on the fundamental role of morphology in establishing an accurate diagnosis of primary invasive carcinoma of breast origin. This forms the basis for further morphological characterization and for all other approaches to BC classification that are used to provide prognostic and therapeutic predictive information.
Keywords: breast cancer, classification, clinical, differentiation, grade, molecular, outcome, stage
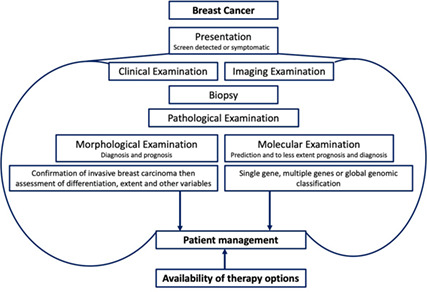
Background
Breast cancer (BC) comprises a heterogeneous group of tumours that displays marked variation in clinical presentation, morphology, molecular features, biological behaviour, and response to therapy. Despite major advances in our understanding and management of BC, BC remains a major public health problem and continues to pose significant challenges worldwide.
The diagnosis of BC dates back 3500 years, 1 when it was classified according to visible signs and symptoms of the disease. The realization, in the mid‐18th century, that cancer is a local disease that progresses in stages rather than a de novo systemic disease led to proposals of early surgical removal of a breast tumour before it had spread to the axillary lymph nodes. Mastectomy continued to be the mainstay of treatment of BC until the second half of the 20th century. Until then, the pathological examination of excised breast tissue was primarily an exercise in confirming the diagnosis of BC without pathological prognostic stratification of the disease. 2 , 3
The 1960s heralded the discovery of the biological significance of hormone receptor status in BC leading to the approval of the anti‐oestrogen drug Tamoxifen. The 1980s witnessed the introduction of population based mammographic screening. 4 The refined criteria for histological assessment of tumour type 5 , 6 and grade 7 , 8 and their prognostic significance, in addition to the prognostic importance of other pathological variables, 9 , 10 were also published. In the 1990s, taxanes and capecitabine, important chemotherapeutic drugs, were approved for the adjuvant management of BC. During that decade, sentinel lymph node biopsy was introduced as an alternative to full axillary lymph node clearance for the staging of BC, and the specific inherited mutations in the tumour suppressor genes, BRCA1 and BRCA2, 1 were identified. Towards the end of 1990s, the first targeted anti‐HER2 drug, trastuzumab (Herceptin) was approved for the management of metastatic BC. These changes were accompanied by a significant improvement in the pathological diagnostic and prognostic classification of BC, which included detailed histomorphological assessment in addition to evaluation of the hormone receptor and human epidermal growth factor receptor 2 (HER2).
In the early 2000s, the concept of the molecular classification of BC was introduced with the recognition of intrinsic molecular subtypes and the development of multigene signatures, representing a significant advance in our understanding of BC. Despite the importance of the well‐established morphological prognostic variables in BC, this focus has dominated BC research in the last two decades, facilitated by the development of high‐throughput molecular techniques, such as microarrays and next‐generation sequencing, and is rapidly expanding in response to the increasing availability of targeted therapy and the move towards precision and personalized medicine.
These important and continuously evolving advances in our understanding of the biology and clinical management of BC, together with improved BC detection and an appreciation of its significant heterogeneity, have emphasized the importance of a patient‐focussed pathological classification with biological and clinical relevance (Table 1). This includes complex and expanding systems of classification that are based on the diagnosis, prognostic evaluation, and predictive stratification in addition to the stratification of BC into other clinically relevant groups to assist disease monitoring, genetic counselling, and risk factor assessment.
Table 1.
Main classification systems of breast cancer
| Classifier | Variables |
|---|---|
| Presentation |
|
| Imaging | Mass shape, margin, depth, and site, breast composition, calcification, axillary findings, laterality |
| Pathological | |
| Morphological classification (mainly diagnostic and prognostic) | |
| Tumour differentiation | |
| Tumour type: | Several tumour types (currently at least 18 tumour types) are described and some types include multiple variants 20 based on the combination of cytological, architecture features, and secretory activity and stromal features |
| Tumour grade | Three grades 19 based on the degree of differentiation and similarity to TDLUs |
| Disease extent | |
| Tumour stage | Invasive tumour size, infiltration of other tissues, lymph node status, and assessment of lesions at distant sites |
| Other factors | |
| Lymphovascular invasion (present or absent), presence and extent of the in situ lesions (DCIS), stromal features such as TILs, Paget's disease, focality, bilaterality, and excision status | |
| Molecular classification (mainly predictive but can provide diagnostic and prognostic value) | |
| Single gene classifier |
Oestrogen receptor and HER2 are the most important classifiers to guide treatment decision with the addition of PDL1 Other markers include progesterone receptor (PR), KI67 as prognostic markers Familial predisposition genes such as BRCA1, BRCA2, and PALB2. |
| Multiple gene classifier | Multigene prognostic signatures are composed of multiple genes assessed together to assess risk in certain BC groups mainly the luminal class. |
| Global gene expression and genomic classification |
|
| Therapy classification |
|
Following initial diagnosis and confirmation of a primary breast tumour, further histological classification is typically based on the type and degree of differentiation (tumour type and histological grade), by examination of haematoxylin and eosin (H&E)‐stained slides, taking account the gross findings and supported by special stains, immunohistochemistry (IHC), and other molecular assays, as required. The assessment of prognostic markers and other parameters such as tumour stage, lymphovascular invasion (LVI), margin status, and the identification of coexisting and precursor lesions, guide further management 11 and complement the diagnostic classification of BC. For instance, the demonstration of LVI or lymph node metastasis confirm the invasive nature of the tumour in addition to being of prognostic value. Similarly, documentation of the molecular features of BC, which are primarily used for predictive purposes, may also have diagnostic and prognostic value. In this review we address the pathological classification systems in BC with emphasis on morphological and molecular classification. Clinical and epidemiological classification are beyond the scope of this review.
Morphological classification of BC
Over the last few decades, the classification of BC has moved from a simple pathological diagnosis, based on confirmation of cancer and a description of type such as “adenocarcinoma of the breast” or “scirrhous breast carcinoma”, to comprehensive synoptic reports that now include more than 20 core items in addition to a series of noncore items. Several national organizations, including the United Kingdom Royal College of Pathologists (RCPath) and the College of American Pathologists (CAP), have published BC datasets and guideline recommendations to help pathologists involved in reporting BC to improve concordance and so to enhance patient care. 12 , 13 , 14 In further recognition of the importance of pathology reports in providing the fundamental information required for the management of BC patients, the International Collaboration on Cancer Reporting (ICCR) was founded by major pathology organizations from around the world to produce internationally standardized and evidence‐based datasets to improve cancer patient outcomes worldwide and to advance international benchmarking in cancer management (http://www.iccr‐cancer.org/).
Pathological staging classification
BC stage is the most important prognostic variable. Early‐stage BC has 10‐year survival rates of over 90%. In contrast, metastatic BC, which accounts for approximately 6–7% of de novo presentations and develops in ~30% of women with early‐stage BC at diagnosis, is associated with 5‐year relative survival rates of ~25%, and a median overall survival (OS) of ~2 years. 15 This difference in the outcome is observed regardless of the histological type, grade, or molecular features of the disease. Differences in outcome, to a lesser degree, are also observed with local versus regional disease extent. Despite the importance of the clinical and radiological staging of BC, pathological staging remains the gold standard and provides detailed staging information including confirmation of primary tumour size, infiltration of local structures, the presence of lymph node metastases, and estimation of nodal disease burden.
Several staging classifications have been published, acknowledging the importance and the clinical relevance of BC disease extent. The Tumour Node Metastasis (TNM) system, currently the most widely used staging system, classifies extent according to the primary tumour (T) size, nodal (N) involvement, and metastasis (M) based on clinical and pathological evaluations. In addition, the TNM recognizes some specific clinical presentations including inflammatory carcinoma (T4d) and skin ulceration (T4b), as these have distinct BC behavioural patterns, and the presence and pattern of local spread including chest wall infiltration (T4a) and the presence of ipsilateral macroscopic satellite nodules (T4b). The TNM system was developed and is maintained by the Union for International Cancer Control (UICC) and is also used by the American Joint Committee on Cancer (AJCC). 16 This staging classification system is updated on a regular basis and is used by pathologists worldwide.
The latest 8th edition of the AJCC Staging Manual, in addition to retaining traditional anatomic staging, recognized the importance of the biological and molecular variables. It introduced a prognostic staging system, which incorporates tumour grade, hormone receptors (oestrogen receptor [ER], and progesterone receptor [PR]) and oncogene status (HER2), further modified to include multigene panel results in a subset of patients to amend the anatomical stage. 16 This new approach, aimed to better reflect BC prognosis, will result in the restaging of some patients, but has limited applications in some scenarios such as triple‐negative and advanced‐stage BC. 17 In many parts of the world, biological markers and multigene panels are not routinely available, limiting the worldwide applicability of such a staging system. Many countries, including the UK, continue to rely on the anatomical staging system.
The Nottingham Prognostic Index (NPI 18 ), the first BC prognostic staging system to be developed, based on the lymph node stage (1–3), histological grade (1–3), 19 and the primary tumour size, is still used in many centres and provides one of the most cost‐efficient and easy‐to‐use prognostic tools in BC. BC staging is primarily used to risk stratify patients for consideration for therapy, rather than to determine the specific type of therapy. It is also important to note that therapy may modify the behaviour of tumours and the original estimated risk may change with treatment. Therefore, the risk classification of BC includes two predicted estimates: the original therapy‐naïve risk and the posttreatment risk.
Classification based on tumour differentiation
Despite the clinical importance of BC staging, the histomorphological classification plays the key role in BC diagnosis and provides the basis for all other classification systems. This classification system relies heavily on the performance and expertise of pathologists, with limited input from molecular tests. Daily challenges include the distinction between in situ and invasive disease, tumour typing and grading, and the distinction of primary breast cancers from their mimics. No staging or molecular classification system is of value without the histological confirmation of BC and the accuracy of diagnosis.
The histological diagnosis of BC is based on the evaluation of certain features, individually and in combination, including the cytological and architectural features of the proliferating cells, tumour‐associated stroma, demonstration of the presence or absence of myoepithelial cells at the epithelial stroma interface, using H&E‐stained slides, supported by the use of IHC and other molecular assays in certain situations. The confirmation of invasive BC of primary breast origin is followed by the assessment of tumour differentiation. Differentiation in BC can be measured morphologically using tumour histological grade and type. Histological grade, which measures the similarity of a tumour to the normal breast terminal duct lobular units (TDLUs), 20 reflects the degree of differentiation, whereas tumour type reflects the type of differentiation. 19 , 21 BCs are graded using the Nottingham grading system, which involves the semiquantitative evaluation of three important biology‐dependent morphological features: (i) degree of tubule or gland formation, (ii) nuclear pleomorphism, and (iii) mitotic count. 22 Although the Nottingham tumour grade is prognostically relevant in all BC histological subtypes, some BCs have a certain histological grade determined by their type, e.g. tubular, invasive cribriform carcinoma, low‐grade adenosquamous carcinoma, and fibromatosis like metaplastic carcinoma are grade 1 by definition, whereas IBC with medullary features and basal‐like BC are grade 3 tumours. Most BCs, including invasive ductal carcinoma – no special type (IBC‐NST), lobular, mucinous, and metaplastic carcinoma show a spectrum of histological grade while maintaining specific tumour type characteristics.
Tumour type is determined according to specific tumour characteristics, including cytological features, tumour growth pattern and architecture, secretory activity, and stromal features. Identification of tumour type is important for diagnosis and confirmation of primary breast origin. Although some tumour types are associated with distinct clinical behavioural patterns, the individual tumour type is of variable prognostic and predictive value. This is mainly due to the presence of several histological types of BC and the existence of several variants of the more common types, e.g. IBC‐NST, lobular and metaplastic carcinomas. IBC‐NST accounts for most primary BCs (60–75%), while some special type tumours comprise <2% of all BCs. Despite this, the prognostic significance of tumour type can be improved if the tumour types are grouped into prognostic groups (Table 2), combined with the tumour grade, 5 , 22 , 23 , 24 and ideally with receptor status and tumour size. HER2‐positive and triple‐negative tumours are likely to have a poorer prognosis compared to ER‐positive tumours of the same type and stage. Very small tumours (<5 mm) typically have a very good prognosis, regardless of the tumour type. 25 Some tumour types such as lobular and metaplastic carcinomas are likely to show less response to chemotherapy compared with IBC‐NST.
Table 2.
Prognostic tumour type groups
| Prognostic groups | Types |
|---|---|
| Very indolent (excellent prognosis similar to locally infiltrative lesions with limited metastatic potential) |
Pure low‐grade adenosquamous carcinoma, pure fibromatosis like metaplastic carcinoma, pure low‐grade mucoepidermoid, adenoid cystic and secretory carcinomas 81 , * Other special type tumours which include encapsulated and solid papillary carcinomas that lack myoepithelial cells but staged as in situ disease (pTis), and other lesions such as atypical adenomyoepithelioma and malignant adenomyoepithelioma in situ. 82 |
| Excellent prognosis group (low metastatic potential. Mainly lymph node metastasis) | Pure tubular and invasive cribriform carcinoma of limited size (<3 cm)* |
| Good prognosis group | Grade 1 invasive lobular, mucinous, invasive papillary and IBC‐NST, and tubulolobular carcinoma. |
| Moderate prognosis group | Grade 2 IBC‐NST, and invasive lobular carcinoma classical type. |
| Poor prognosis group | High grade IBC‐NST, solid and other high‐grade invasive lobular carcinoma, high‐grade matrix producing and squamous cell metaplastic carcinomas. |
| Very poor prognosis group | High grade spindle cell metaplastic carcinoma, small cell carcinoma and high‐grade triple‐negative IBC‐NST of large size. |
These tumours should be small (<3 cm). During tumourigenesis, the cancer cells undergo replication and mutation, thereby increasing the tumour size is often associated with increasing invasiveness of the tumour. 83 Larger size tumours are likely to have different tumour components and the behaviour is likely to relate to the other (more aggressive) carcinoma component. The basaloid and solid variant of adenoid cystic carcinoma is more aggressive. Also, some secretory carcinomas in older patients may behave less indolently.
Tumour typing is a dynamic process and new entities are described and old entities are renamed or combined with other tumour types. Some pathologists consider tumours with certain features as new or distinct entities (‘splitting’), while others will link the features to a more common and well‐established tumour type and consider these tumours as variants of the main tumour type (‘lumping’). The recognition of new entities usually starts with the publication of case reports and/or case series reporting on the histological features of certain breast lesions and the behaviour of such lesions. In contrast, the recognition that a rare tumour type overlaps histologically with another more common tumour type and the lack of distinct clinical value in specific recognition may result in grouping these tumours together, best exemplified by the recent inclusion of medullary carcinoma into the IBC‐NST category. Similarly, basal‐like carcinoma was proposed as a special tumour type following the description of the basal‐like/triple‐negative molecular subtype. It was subsequently shown that these tumours showed morphological overlap with other high‐grade IBC‐NST tumours and that some basal‐like tumours defined using molecular assays did not display the same histological features.
The World Health Organization (WHO) series on the Classification of Tumours (also known as the WHO Blue Books) is regarded as the gold standard for the diagnosis of tumours and provide indispensable international standards for classification of breast tumours worldwide. 20 The 5th edition (2019) WHO Classification of Breast Tumours continues to recognize several special types of BC, which together account for up to 25% of all invasive BCs. 20 Knowledge of these special types helps pathologists to recognize that a tumour is of primary breast origin and may provide clinically relevant information. For example, a diagnosis of invasive lobular carcinoma (ILC) on core needle biopsy (CNB) usually leads to further preoperative imaging due to the increased incidence of multifocality and bilaterality. Invasive lobular carcinoma is also less likely to respond to chemotherapy, which is important in patient selection for neoadjuvant chemotherapy. Metaplastic carcinoma is generally associated with a poor prognosis and a limited response to neoadjuvant chemotherapy.
The 5th edition WHO working group 20 introduced some changes concerning tumour typing, reflecting not only improved understanding of tumour biology but also challenges in achieving diagnostic concordance. Some rare tumours, e.g. tall cell carcinoma of breast with reversed polarity and mucinous cystadenocarcinoma are now recognized as special type BCs (Figures 1 and 2). Other rare tumour types including salivary gland‐like tumours, apocrine carcinoma, and invasive papillary carcinoma continue to be recognized as special types with distinct molecular and clinical features. Knowledge of these entities will avoid misclassification as metastatic tumour and provide information on likely biological behaviour. However, other tumour types, considered to represent end of differentiation of IBC‐NST, have been reassigned to the IBC‐NST category with a designation of special morphological pattern including medullary/medullary‐like carcinomas, glycogen‐rich, lipid‐rich, sebaceous, and oncocytic carcinomas. In addition to morphological overlap with IBC‐NST, the prognosis of these tumours does not differ from grade matched classical IBC‐NST.
Figure 1.
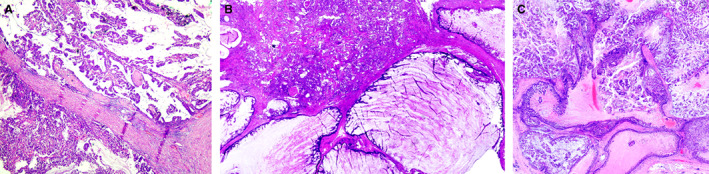
A case of mucinous cystadenocarcinoma featuring complex papillary growth pattern (A) and prominent cystic spaces (B) with mucinous differentiation (C) mimicking mucinous cystadenocarcinoma of the ovary and some other organs.
Figure 2.
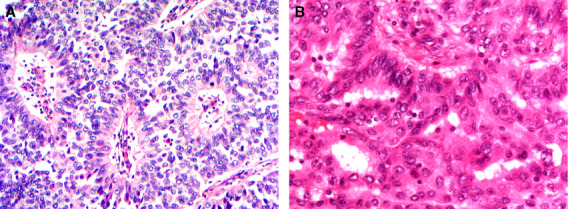
A case of tall cell carcinoma with reversed polarity featuring nuclei placed away from the basal border (A), nuclear groove and nuclear overlapping (B) similar to the tall cell variant of papillary thyroid carcinoma.
A particular challenge encountered by pathologists in breast tumour typing is the recognition of tumours that are associated with biological behaviour like that observed in in situ breast carcinoma. The distinction between in situ and invasive carcinoma may be blurred and lacks a strong evidence base. The approach to categorisation varies depending on the specific tumour, resulting in a lack of diagnostic concordance among pathologists. Examples include solid and encapsulated papillary carcinomas that lack peripheral myoepithelial cells. These tumours are currently designated as in situ tumours, although they are likely to represent indolent invasive tumours with low metastatic potential. 26 , 27 In contrast, pure low‐grade adenosquamous carcinoma and pure low‐grade mucoepidermoid carcinoma of the breast do not demonstrate metastatic potential, but are managed as invasive tumours. Although low‐grade adenosquamous carcinoma and syringomatous tumour of the nipple share histological and molecular features, the first is designated as carcinoma, while the latter is considered benign. Neuroendocrine (NE) tumours of the breast have long been a source of confusion regarding cell of origin, terminology, diagnostic criteria, and the lack of distinction between invasive and in situ lesions with consequent management implications. Breast NE neoplasms (NENs) are currently described either as NE tumours or NE carcinoma, although all represent carcinomas. 20 Further refinement of the classification of these breast tumours is needed to improve their diagnostic reproducibility and consolidate the clinical significance of each diagnosis. 28
ILC is often considered to be a single tumour type characterized by loss of function of the cell adhesion protein, E‐cadherin, with subsequent cell dyscohesion but comprises a spectrum of tumours with different histological features and clinical behaviour. The classical variant is the most common subtype, typically Nottingham grade 2, and shows a distinct clinical behaviour. 29 Most of the data on the clinical behaviour of ILC are derived from this variant, with less available information on the clinical behaviour of other variants. The solid ILC variant is characterized by a solid growth pattern, shows high mitotic activity, and may be associated with aggressive clinical behaviour. The pleomorphic ILC variant is characterized by high‐grade cytological features and a poor prognosis, whereas the alveolar and tubulo‐lobular variants are characterized by a good prognosis. 30 Metaplastic breast carcinoma (MBC) comprises a heterogeneous group of tumours with a range of histological features and clinical behaviour, but is also sometimes considered by pathologists and clinicians to represent a single tumour type. MBC subtypes reflect variable differentiation pathways that are distinct from the adenocarcinoma differentiation pathways. Two main differentiation pathways are recognized in MBC: squamous and mesenchymal, the latter including spindle cell and matrix producing differentiation. MBC includes indolent low‐grade tumours such as fibromatosis‐like spindle cell MBC and low‐grade adenosquamous carcinoma and the aggressive high‐grade spindle cell MBC and high‐grade adenosquamous carcinoma. 31 , 32 Intermediate nuclear grade (grade 2) spindle cell metaplastic carcinoma can rarely be encountered, and these tumours have intermediate risk between the fibromatosis like and the high‐grade spindle cell MBC. In practice, we observe two main types of MBC: (1) tumours with metaplastic and adenocarcinomatous components with morphological and/or biomarker overlap, considered as MBC regardless of the percentage of the tumour occupied by metaplastic elements. In these tumours, the adenocarcinomatous component are typically high grade and shows a triple‐negative phenotype and the transition between the two components is gradual, and (2) tumours with a distinct metaplastic component (e.g. spindle cell, matrix producing or squamous) that may coexist with IBC‐NST or another special type component. The second component (IBC‐NST or special type) in these tumours may show receptor positivity and there is a clear distinction between such a component and the metaplastic tumour component. Although not specified in the WHO book, these tumours can be regarded as pure MBC if the metaplastic component exceeds 90%, and as mixed metaplastic and NST tumours if the metaplastic component accounts for >10% and < 90% of the tumour. In general, the presence of a high‐grade metaplastic element is associated with aggressive clinical behaviour and is likely to drive the behaviour of the mixed tumours regardless of percentage. Therefore, it is advised that the presence of high‐grade metaplastic component in mixed tumours to be stated even if it is a minor component.
Conventional IBC‐NST carcinoma occasionally contains minor components of other special type BCs. 33 When the special type component forms a recognizable proportion of the tumour (10–90%), the term mixed carcinoma is used. 20 The concordance of classifying mixed BC in clinical practice is low, 34 which may reflect the difficulty in distinguishing the special type from the nonspecial component.
Molecular classification
There are several lines of evidence to suggest that the clinical and morphological parameters currently available are insufficient to fully reflect the biological heterogeneity of BC, and that tumours with similar morphology and stage vary in clinical behaviour and response to therapy. Moreover, the widespread use of mammographic screening, improved understanding of the nature and biology of BC, and the increasing array of systemic therapy options (hormone, chemotherapy, anti‐HER2, and other targeted therapy and immunotherapy) 35 further emphasizes the need to utilize molecular prognostic and predictive markers. These are primary tumour molecular characteristics that can be used to determine tumour behaviour (prognostic) and response to specific therapy (predictive). 36 Molecular classifiers include genes and their products (RNA and protein) that can be assessed individually or in consort (e.g. molecular profiling).
Single gene classifiers
Of the individual molecular markers, ER and HER2 have proven of predictive and prognostic value. ER and HER2 status, which are an essential part of the diagnostic workup of all BC patients, are determined using standardized techniques according to well‐defined published guidelines. 12 , 37 , 38 , 39 Clinically, all invasive BCs are grouped into following biomarker‐defined subtypes/groups for treatment purposes: (1) ER‐positive, HER2‐negative, (2) ER‐positive, HER2‐positive, (3) ER‐negative, HER2‐positive, and (4) ER‐negative, HER2‐negative cancers. 20 It is currently recognized that the main consideration for adjuvant treatment of BC is potential tumour endocrine responsiveness. Adjuvant hormone therapy accounts for almost two‐thirds of the overall benefit of adjuvant therapy in patients with ER‐positive BC. Tumour ER expression predicts response to hormone therapy in 30–60%, while ER‐negativity, which accounts for 20–30% of breast cancer, identifies a population of patients who will not benefit from endocrine therapy. 40 , 41 , 42 , 43 Approximately 30% of ER‐positive tumours are PR‐negative and ER+/PR− tumours are generally less responsive than ER+/PR+ tumours. 44 , 45 , 46 Lack of PR expression in ER‐positive tumours may be a surrogate marker of aberrant growth factor signalling that could contribute to tamoxifen resistance. Multiple studies have provided evidence for the prognostic and predictive importance of PR in BC. 46 , 47 , 48 , 49 TheER−/PR+ phenotype is rare but exists and is not purely a staining artefact (ER false‐negative or PR false‐positive on CNB). Earlier studies have reported 10% or more of BC show ER−/PR+ 50 ; however, recent data indicate that this phenotype comprises ~1−2%. 51 It is our experience that few cases (<1%) remain as biologically relevant ER−/PR+ phenotype (convincingly PR‐positive (moderate to strong nuclear staining in >10% of the tumour cells) and ER‐negative (<1%) when the staining is repeated on CNB or the excision specimens. It is our opinion that weak PR staining in 1–10% of the tumour cells is unlikely to have clinical or biological significance on ER− BC in terms of response to therapy, or clinical behaviour. The outcome of ER−/PR+ BC is not clear, but it is likely worse than ER+/PR+ tumours. 52 , 53
Although for management purpose, a cutoff of 1% is used to define ER positivity and define eligibility for endocrine therapy, the level of ER expression in BC is variable (the intensity of expression varies from weak to strong and the frequency of positive cell varies from 1% to 100%). This has prognostic significance in terms of better outcome and response to endocrine therapy in BC, showing strong diffuse nuclear expression. To categorize patients into prognostic groups, multiple scoring systems are developed including the Quick score, Allred Score, and H. score, which consider a combination of intensity of staining and the percentage of positive cells to produce a score that can be categorized into subgroups of prognostic significance. 54
Use of anti‐HER2 therapy is based on HER2 status, determined using IHC and/or in situ hybridisation (ISH) studies, combined with risk stratification. 55 Amplification of HER2 gene occurs in 12–20% of BCs and more than half (~55%) of these tumours are ER‐negative. 56 , 57 Numerous studies have shown that HER2 gene amplification/protein overexpression is a predictor of poor prognosis and response to certain types of chemotherapy. 58 , 59 , 60 ER and HER2 are assessed in daily practice to provide information on response to endocrine therapy and anti‐HER2 targeted therapy, respectively. However, expression of these biomarkers overlaps and their prognostic and predictive value can be improved by using them in combination 61 and also in combination with PR status and the proliferation marker KI67. 62 Most IHC studies have used a combination of ER, PR, and HER2 with or without KI67 as IHC surrogates to define the molecular classes initially identified by gene expression profiling (intrinsic subtypes). ER positivity is a surrogate for luminal class, HER2 expression for HER2‐positive tumours, and the triple‐negative (ER‐, PR‐, HER2‐) phenotype is used to define the basal‐like molecular class. 63 Some other genes, which are assessed individually in BC, such as PR and KI67, 64 have been shown to be of specific clinical utility in classifying luminal tumours as luminal A or B. 65
More recently, PDL1 is being used as a predictive marker for potential response to immunotherapy. Other genes that are used to classify BC include BRCA1, BRCA2, and PIK3CA, the latter is also used to guide systemic treatment. It is likely that additional genes will be used to guide therapy decision‐making in the future.
Multigene classifiers
The introduction of the concept that BC can be classified using global gene expression profiling in 2000 66 (molecular taxonomy) has revolutionized BC research. The total gene expression pattern of a given sample is known as a gene expression profile, often referred to as a ‘signature’ or ‘portrait’. Most tumours display expression signatures/profiles that are unique and related to specific biological features. 67 , 68 Although the molecular classification system provides prognostic value and possible predictive information and has contributed to our current understanding of BC molecular complexity, its application in the clinical setting and influence on BC therapeutic decision‐making remains less than was anticipated. The added value of these studies over the routinely assessed products of individual genes, ER, HER2, PR, and KI67, is currently limited.
ER‐positive luminal and HER2‐positive tumours had been characterized before the advent of gene expression molecular taxonomy. The basal‐like group attracted particular attention as a novel class characterized by triple‐negative phenotype, poor outcome, and the generally similar molecular profile of these tumours that clustered together at the molecular level. Subsequent studies have, however, demonstrated several molecular subclasses in this basal/triple‐negative BC group, including luminal androgen receptor type tumours. 69 , 70 However, the clinical and therapeutic relevance of these subclasses and the additional value of performing androgen receptor and basal marker expression is not yet clear. Similarly, patients with HER2‐positive BC determined using IHC and/or ISH studies are likely to be offered in anti‐HER2 therapy, regardless of molecular portrait, while it is not current practice to offer this treatment to patients with HER‐2‐positive BC based on molecular portrait alone.
Clinical relevance needs to be considered and factored into any emerging classification system to ensure that patients are treated appropriately. Moreover, it remains unknown how many molecular subclasses exist and, more important, how many can be reliably identified with currently available technology. The four main molecular classes frequently reported may represent an oversimplification of a novel molecular classification system that does not greatly advance our knowledge of the likely biological behaviour of BC. BC has also been classified using integrated analysis of gene copy number (DNA) and gene expression using gene transcripts (RNA). 71 Classification based on the expression of several genes using IHC and tissue microarrays may also help to identify key molecular classes. 72
Multigene signatures
In addition to the molecular classes, a few prognostic multigene signatures have been identified based on the differential expression of a selected set of genes in a specific subgroup of tumours. Multigene signatures include “prognostic gene signatures” that can predict outcome. Other gene signatures have been developed based on prediction of response to specific therapy and are used as predictive signatures. 73 , 74 A common character shared by all these signatures is the use of combinations of genes, rather than using single genes, to predict a certain outcome that appears to reflect the overall genetic derangements underlying the complex tumour biology. To date, these signatures have not replaced the currently used prognostic and predictive factors in the management of BC, but provide useful complementary information to traditional clinicopathological parameters in the clinically intermediate risk group of patients, in particular those with ER‐positive, HER2‐negative early‐stage BC. The prognostic value of these tests in ER‐negative and HER2‐positive BC remains limited.
Classification based on mutational signatures
The BC genome is a record of the mutagenic activity that has occurred throughout the development of a tumour. The clinical significance of BC mutations includes not only driver gene mutations but also passenger mutational signatures, gene rearrangement, the imprints of DNA damage, and DNA repair processes. 75 , 76 The existence of mutational signatures in BC was first described utilizing more than 183 thousand substitutions in 21 whole BC genomes. 77 This was followed by a large study exploring 560 BCs that identified a total of 12 substitution signatures from over 3,479 thousand mutations. 78 Some of these signatures are variously associated with age at diagnosis, BRCA1/BRCA2 deficiency, the activity of the APOBEC cytidine deaminases, or with mismatch repair deficiency. Importantly, these mutational signatures do not appear to demonstrate specificity to BC subtype, whether classified by ER status or intrinsic molecular subtype. The mutational signatures not only include base substitutions, small insertions, and deletions (indels), but they have also extended to structural variation (genome rearrangement signatures). 78 Rearrangements were classified into clustered (at specific loci reporting driver amplicons or simply at sites of chromothripsis 79 ) or dispersed (equivalent number of rearrangements that are widely distributed throughout the genome), then divided according to rearrangement class (tandem duplication, deletion, inversion, or translocation) and size. 78 Following this classification system six rearrangement signatures and seven major subgroups that exhibited distinct associations with other genomic, histological, gene expression, and clinical features were described. 76
BC can also be classified based on the genetics of familial predisposition including the key predisposition genes, BRCA1, BRCA2, and PALB2, in addition to other genes based on penetrance and frequency in the population. 20 There is increasing interest in classifying BC using multigene BC susceptibility and polygenic risk scores. Patients with germline and/or somatic mutations in BRCA1 and/or BRCA2, show sensitivity to poly (ADP‐ribose) polymerase (PARP) inhibitors. 80
Conclusion
BC is a heterogeneous disease that can be classified using several classification systems. These include clinical, imaging, and pathological morphological and molecular classifications, each with further subclassification systems. Knowledge of systems is likely to increase concordance of BC diagnosis and standardization of BC management. Although the clinical and molecular classification systems are important for determination of prognosis and prediction of response to therapy decisions, it is the pathological morphological diagnosis that is the foundation of other classification systems, used to confirm the diagnosis of malignancy, characterise as an invasive tumour of breast origin, and provide information on tumour type, grade, and other key prognostic variables. There is an increasing focus on the use of single genes, multiple genes, and global gene expression BC classifiers, which provide varying degrees of predictive information and act as companion diagnostics in the BC management workup. Applications of next‐generation sequencing to BC research are expanding and may change the way we understand and treat BC in the future. Despite the enormous amount of work that has been carried out to develop and refine BC molecular prognostic and predictive assays, this is still evolving. With the increasing use of more sophisticated molecular techniques, large amounts of data will continue to emerge, which could potentially lead to identification of novel therapeutic targets and allow more precise classification systems that can more accurately predict patient outcome and response to therapy.
Acknowledegement
N/A
Author Contributions
Emad Rakha, drafted the article, reviewed, and approved the final version. Cecily Quin reviewed and amended the article and approved the final version. Gary Tse reviewed and approved the final version of the article.
Ethical Approval and Consent to Participate
N/A
Funding
N/A
Conflict of Interest
The author's have no conflicts of interest to declare.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCE
- 1. Lukong KE. Understanding breast cancer ‐ the long and winding road. BBA Clin. 2017; 7; 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloom HJG. Further studies on prognosis of breast carcinoma. Br. J. Cancer 1950b; 4; 347–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cutler SJ, Black MM, Friedell GH, Vidone RA, Goldenberg IS. Prognostic factors in cancer of the female breast. II. Reproducibility of histopathologic classification. Cancer 1966; 19; 75–82. [DOI] [PubMed] [Google Scholar]
- 4. Biesheuvel C, Weigel S, Heindel W. Mammography screening: Evidence, history and current practice in Germany and other European countries. Breast Care (Basel) 2011; 6; 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer. II. Histological type. relationship with survival in a large study with long‐term follow‐up. Histopathology 1992; 20; 479–489. [DOI] [PubMed] [Google Scholar]
- 6. Black MM, Barclay THC, Hankey BF. Prognosis in breast cancer utilising histologic characteristics of the primary tumour. Cancer 1975; 36; 2048–2055. [DOI] [PubMed] [Google Scholar]
- 7. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. the value of histological grade in breast cancer: experience from a large study with long‐term follow‐up. Histopathology 1991; 19; 403–410. [DOI] [PubMed] [Google Scholar]
- 8. Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br. J. Cancer 1957; 11; 359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallgren A, Silfversward C, Eklund G. Prognostic factors in mammary carcinoma. Acta Radiol. Ther. Phys. Biol. 1976; 15; 1–16. [DOI] [PubMed] [Google Scholar]
- 10. Blamey RW, Davies CJ, Elston CW, Johnson J, Haybittle JL, Maynard PV. Prognostic factors in breast cancer: the formation of a prognostic index. Clin. Oncol. 1979; 5; 227–236. [PubMed] [Google Scholar]
- 11. Elston CW, Ellis IO, Pinder SE. Pathological prognostic factors in breast cancer. Crit. Rev. Oncol. Hematol. 1999; 31; 209–223. [DOI] [PubMed] [Google Scholar]
- 12. Ellis IO, Rakha EA, Lee AHS et al. Pathology reporting of breast disease in surgical excision specimens incorporating the dataset for histological reporting of breast cancer. London: The Royal College of Pathologists, 2016. [Google Scholar]
- 13. Fitzgibbons PL, Connolly J, Rose S et al. Protocol for the examination of resection specimens from patients with invasive carcinoma of the breast. Northfield (IL): College of American Pathologists; Version: InvasiveBreast 4.1.0.0 Protocol Posting Date: January 2018. [Accessed July 14th, 2022]. https://documents.cap.org/protocols/cp‐breast‐invasive‐18protocol‐4100.pdf. 2018.
- 14. Cho SY, Park SY, Bae YK et al. Standardized pathology report for breast cancer. J. Breast Cancer 2021; 24; 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonotto M, Gerratana L, Poletto E et al. Measures of outcome in metastatic breast cancer: insights from a real‐world scenario. Oncologist 2014; 19; 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amin MB, Greene FL, Edge SB et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017; 67; 93–99. [DOI] [PubMed] [Google Scholar]
- 17. He J, Tsang JY, Xu X et al. AJCC 8th edition prognostic staging provides no better discriminatory ability in prognosis than anatomical staging in triple‐negative breast cancer. BMC Cancer 2020; 20; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham prognostic index in primary breast cancer. Breast Cancer Res. Treat. 1992; 22; 207–219. [DOI] [PubMed] [Google Scholar]
- 19. Rakha EA, Reis‐Filho JS, Baehner F et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010; 12; 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The WHO Classification of Tumours Editorial Board . Breast Tumours. Fifth ed. Lyon: IARC press, 2019. [Google Scholar]
- 21. Rakha EA, Alsaleem M, ElSharawy KA et al. Visual histological assessment of morphological features reflects the underlying molecular profile in invasive breast cancer: a morphomolecular study. Histopathology 2020; 77; 631–645. [DOI] [PubMed] [Google Scholar]
- 22. Rakha EA, El‐Sayed ME, Lee AH et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J. Clin. Oncol. 2008; 26; 3153–3158. [DOI] [PubMed] [Google Scholar]
- 23. Pereira H, Pinder SE, Sibbering DM et al. Pathological prognostic factors in breast cancer. IV: should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology 1995; 27; 219–226. [DOI] [PubMed] [Google Scholar]
- 24. Rakha E, Toss M, Quinn C. Specific cell differentiation in breast cancer: a basis for histological classification. J. Clin. Pathol. 2022; 75; 76–84. [DOI] [PubMed] [Google Scholar]
- 25. Johnson KC, Quiroga D, Sudheendra P, Wesolowski R. Treatment of small (T1mic, T1a, and T1b) node‐negative HER2+ breast cancer ‐ a review of current evidence for and against the use of anti‐HER2 treatment regimens. Expert Rev. Anticancer Ther. 2022; 22; 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rakha EA, Gandhi N, Climent F et al. Encapsulated papillary carcinoma of the breast: an invasive tumor with excellent prognosis. Am. J. Surg. Pathol. 2011; 35; 1093–1103. [DOI] [PubMed] [Google Scholar]
- 27. Rakha EA, Varga Z, Elsheik S, Ellis IO. High‐grade encapsulated papillary carcinoma of the breast: an under‐recognized entity. Histopathology 2015; 66; 740–746. [DOI] [PubMed] [Google Scholar]
- 28. Rakha E, Tan PH. Head to head: do neuroendocrine tumours in the breast truly exist? Histopathology 2022; 81; 2–14. [DOI] [PubMed] [Google Scholar]
- 29. Rakha EA, El‐Sayed ME, Menon S, Green AR, Lee AH, Ellis IO. Histologic grading is an independent prognostic factor in invasive lobular carcinoma of the breast. Breast Cancer Res. Treat. 2008; 111; 121–127. [DOI] [PubMed] [Google Scholar]
- 30. Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin. Diagn. Pathol. 2010; 27; 49–61. [DOI] [PubMed] [Google Scholar]
- 31. van Deurzen CH, Lee AH, Gill MS et al. Metaplastic breast carcinoma: tumour histogenesis or dedifferentiation? J. Pathol. 2011; 224; 434–437. [DOI] [PubMed] [Google Scholar]
- 32. Rakha EA, Tan PH, Varga Z et al. Prognostic factors in metaplastic carcinoma of the breast: a multi‐institutional study. Br. J. Cancer 2015; 112; 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaufman MW, Marti JR, Gallager HS, Hoehn JL. Carcinoma of the breast with pseudosarcomatous metaplasia. Cancer 1984; 53; 1908–1917. [DOI] [PubMed] [Google Scholar]
- 34. Rakha EA, Bennett RL, Coleman D, Pinder SE, Ellis IO, Pathology UKNCCfB . Review of the national external quality assessment (EQA) scheme for breast pathology in the UK. J. Clin. Pathol. 2017; 70; 51–57. [DOI] [PubMed] [Google Scholar]
- 35. Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20‐69 years. Lancet 2000; 355; 1822. [DOI] [PubMed] [Google Scholar]
- 36. Hayes DF, Bast RC, Desch CE et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J. Natl. Cancer Inst. 1996; 88; 1456–1466. [DOI] [PubMed] [Google Scholar]
- 37. Rakha EA, Pinder SE, Bartlett JM et al. Updated UK recommendations for HER2 assessment in breast cancer. J. Clin. Pathol. 2015; 68; 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010; 28; 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rakha EA, Chmielik E, Schmitt FC, Tan PH, Quinn CM, Gallagy G. Assessment of predictive biomarkers in breast cancer: challenges and updates. Pathobiology 2022; 1–15. [DOI] [PubMed] [Google Scholar]
- 40. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. Early breast cancer Trialists' collaborative group. N. Engl. J. Med. 1988; 319; 1681–1692. [DOI] [PubMed] [Google Scholar]
- 41. Adjuvant tamoxifen in the management of operable breast cancer: the Scottish trial. Report from the breast cancer trials committee, Scottish cancer trials office (MRC), Edinburgh. Lancet 1987; 2; 171–175. [PubMed] [Google Scholar]
- 42. Tamoxifen for early breast cancer: an overview of the randomised trials. Early breast cancer Trialists' collaborative group. Lancet 1998; 351; 1451–1467. [PubMed] [Google Scholar]
- 43. Fisher B, Dignam J, Bryant J et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor‐positive tumors. J. Natl. Cancer Inst. 1996; 88; 1529–1542. [DOI] [PubMed] [Google Scholar]
- 44. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J. Clin. Oncol. 2003; 21; 1973–1979. [DOI] [PubMed] [Google Scholar]
- 45. Arpino G, Weiss H, Lee AV et al. Estrogen receptor‐positive, progesterone receptor‐negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J. Natl. Cancer Inst. 2005; 97; 1254–1261. [DOI] [PubMed] [Google Scholar]
- 46. Rakha EA, El‐Sayed ME, Green AR et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J. Clin. Oncol. 2007; 25; 4772–4778. [DOI] [PubMed] [Google Scholar]
- 47. Colomer R, Beltran M, Dorcas J et al. It is not time to stop progesterone receptor testing in breast cancer. J. Clin. Oncol. 2005; 23; 3868–3869. author reply 9‐70. [DOI] [PubMed] [Google Scholar]
- 48. Ryden L, Jonsson PE, Chebil G et al. Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: a randomised, controlled trial with long‐term follow‐up. Eur. J. Cancer 2005; 41; 256–264. [DOI] [PubMed] [Google Scholar]
- 49. Regan MM, Viale G, Mastropasqua MG et al. Re‐evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J. Natl. Cancer Inst. 2006; 98; 1571–1581. [DOI] [PubMed] [Google Scholar]
- 50. Yi M, Huo L, Koenig KB et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann. Oncol. 2014; 25; 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y, Yang D, Yin X et al. Clinicopathological characteristics and breast cancer‐specific survival of patients with single hormone receptor‐positive breast cancer. JAMA Netw. Open 2020; 3; e1918160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rakha EA, Ellis IO. Does estrogen receptor‐negative/progesterone receptor‐positive breast carcinoma exist? In reply. J. Clin. Oncol. 2008; 26; 336–338. [DOI] [PubMed] [Google Scholar]
- 53. Zheng H, Ge C, Lin H et al. Estrogen receptor‐negative/progesterone receptor‐positive and her‐2‐negative breast cancer might no longer be classified as hormone receptor‐positive breast cancer. Int. J. Clin. Oncol. 2022; 27; 1145–1153. [DOI] [PubMed] [Google Scholar]
- 54. Brouckaert O, Paridaens R, Floris G, Rakha E, Osborne K, Neven P. A critical review why assessment of steroid hormone receptors in breast cancer should be quantitative. Ann. Oncol. 2013; 24; 47–53. [DOI] [PubMed] [Google Scholar]
- 55. Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann. Oncol. 2005; 16; 1569–1583. [DOI] [PubMed] [Google Scholar]
- 56. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science 1987; 235; 177–182. [DOI] [PubMed] [Google Scholar]
- 57. Dandachi N, Dietze O, Hauser‐Kronberger C. Chromogenic in situ hybridization: a novel approach to a practical and sensitive method for the detection of HER2 oncogene in archival human breast carcinoma. Lab. Invest. 2002; 82; 1007–1014. [DOI] [PubMed] [Google Scholar]
- 58. Wolff AC, Hammond ME, Schwartz JN et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007; 25; 118–145. [DOI] [PubMed] [Google Scholar]
- 59. Bartlett J, Mallon E, Cooke T. The clinical evaluation of HER‐2 status: which test to use? J. Pathol. 2003; 199; 411–417. [DOI] [PubMed] [Google Scholar]
- 60. Kaufmann M, von Minckwitz G, Bear HD et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann. Oncol. 2007; 18; 1927–1934. [DOI] [PubMed] [Google Scholar]
- 61. Rakha EA, Reis‐Filho JS, Ellis IO. Combinatorial biomarker expression in breast cancer. Breast Cancer Res. Treat. 2010; 120; 293–308. [DOI] [PubMed] [Google Scholar]
- 62. Cuzick J, Dowsett M, Wale C et al. Prognostic value of a combined ER, PgR, Ki67, HER2 immunohistochemical (IHC4) score and comparison with the GHI recurrence score – Results from TransATAC. Cancer Res. 2009; Suppl 24; Abstract 74. [Google Scholar]
- 63. Carey LA, Perou CM, Livasy CA et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. AMA ‐ J. Am. Med. Assoc. 2006; 295; 2492–2502. [DOI] [PubMed] [Google Scholar]
- 64. Aleskandarany MA, Rakha EA, Macmillan RD, Powe DG, Ellis IO, Green AR. MIB1/Ki‐67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups. Breast Cancer Res. Treat. 2010; 127; 591–599. [DOI] [PubMed] [Google Scholar]
- 65. Viale G, Regan MM, Mastropasqua MG et al. Predictive value of tumor Ki‐67 expression in two randomized trials of adjuvant chemoendocrine therapy for node‐negative breast cancer. J. Natl. Cancer Inst. 2008; 100; 207–212. [DOI] [PubMed] [Google Scholar]
- 66. Perou CM, Sorlie T, Eisen MB et al. Molecular portraits of human breast tumours. Nature 2000; 406; 747–752. [DOI] [PubMed] [Google Scholar]
- 67. Bertucci F, Finetti P, Cervera N, Maraninchi D, Viens P, Birnbaum D. Gene expression profiling and clinical outcome in breast cancer. OMICS 2006; 10; 429–443. [DOI] [PubMed] [Google Scholar]
- 68. Cowin PA, Anglesio M, Etemadmoghadam D, Bowtell DD. Profiling the cancer genome. Annu. Rev. Genomics Hum. Genet. 2010; 11; 133–159. [DOI] [PubMed] [Google Scholar]
- 69. Lehmann BD, Bauer JA, Schafer JM et al. PIK3CA mutations in androgen receptor‐positive triple‐negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014; 16; 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lehmann BD, Bauer JA, Chen X et al. Identification of human triple‐negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 2011; 121; 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Curtis C, Shah SP, Chin SF et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486; 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Green AR, Soria D, Powe DG et al. Nottingham prognostic index plus (NPI+) predicts risk of distant metastases in primary breast cancer. Breast Cancer Res. Treat. 2016; 157; 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang Y, Klijn JG, Zhang Y et al. Gene‐expression profiles to predict distant metastasis of lymph‐node‐negative primary breast cancer. Lancet 2005; 365; 671–679. [DOI] [PubMed] [Google Scholar]
- 74. Paik S, Shak S, Tang G et al. A multigene assay to predict recurrence of tamoxifen‐treated, node‐negative breast cancer. N. Engl. J. Med. 2004; 351; 2817–2826. [DOI] [PubMed] [Google Scholar]
- 75. Denkert C, Untch M, Benz S et al. Reconstructing tumor history in breast cancer: signatures of mutational processes and response to neoadjuvant chemotherapy (small star, filled). Ann. Oncol. 2021; 32; 500–511. [DOI] [PubMed] [Google Scholar]
- 76. Nik‐Zainal S, Morganella S. Mutational signatures in breast cancer: the problem at the DNA level. Clin. Cancer Res. 2017; 23; 2617–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nik‐Zainal S, Alexandrov LB, Wedge DC et al. Mutational processes molding the genomes of 21 breast cancers. Cell 2012; 149; 979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nik‐Zainal S, Davies H, Staaf J et al. Landscape of somatic mutations in 560 breast cancer whole‐genome sequences. Nature 2016; 534; 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stephens PJ, Greenman CD, Fu B et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011; 144; 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lyons TG, Robson ME. Resurrection of PARP inhibitors in breast cancer. J. Natl. Compr. Canc. Netw. 2018; 16; 1150–1156. [DOI] [PubMed] [Google Scholar]
- 81. Cserni G, Quinn CM, Foschini MP et al. Triple‐negative breast cancer histological subtypes with a Favourable prognosis. Cancers (Basel) 2021; 13; 5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rakha E, Tan PH, Ellis I, Quinn C. Adenomyoepithelioma of the breast: a proposal for classification. Histopathology 2021; 79; 465–479. [DOI] [PubMed] [Google Scholar]
- 83. Beerenwinkel N, Antal T, Dingli D et al. Genetic progression and the waiting time to cancer. PLoS Comput. Biol. 2007; 3; e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


