Abstract
Objectives
To conduct an evidence map on self-management interventions and patient-relevant outcomes for adults living with overweight/obesity.
Methods
Following Arksey and O’Malley methodology, we searched in five electronical databases including randomized controlled trials (RCTs) on SMIs for overweight/obesity. We used the terms “self-management”, “adult” and “obesity” for content. Two independent reviewers assessed eligible references; one reviewer extracted data, a second checked accuracy.
Results
We identified 497 RCTs (58% US, 20% Europe) including 99,741 (median 112, range 11–5145) adults living with overweight/obesity. Most research evaluated clinical outcomes (617, 55%) and behaviors adherence (255, 23%). Empowerment skills, quality of life and satisfaction were less targeted (8%, 7%, 0.2%, respectively). The most frequent techniques included sharing information (858, 99%), goal setting (619, 72%) and self-monitoring training (614, 71%), provided face-to-face (386, 45%) or in combination with remote techniques (256, 30%). Emotional management, social support and shared-decision were less frequent (18%, 26%, 4%). Socio-economic status, minorities or health literacy were seldom reported.
Conclusion
There is a need of widening the scope of research by focusing on outcomes important to patients, assessing emotional/social/share-decision support, exploring remote techniques and including vulnerable populations.
Highlights
-
•
First evidence map on self-management interventions on overweight or obesity.
-
•
Research focused mostly in clinical outcomes and less in quality of life.
-
•
Self-management techniques such stress/emotional/social support were less frequent.
-
•
Research gaps found on vulnerable populations and emotional/social support.
-
•
“COMPAR-EU” comprehensive taxonomy helps to identify further research areas.
Practice Implications
Using “COMPAR-EU” taxonomy can help stakeholders identifying research areas to be addressed.
1. Introduction
More than 1.9 billion adults (≥ 18 years and older) worldwide are overweight and of these, 650 million adults are obese [1]. Living with overweight or obesity leads to a multitude of adverse health events such as morbidity, disability, premature death, poor mental health, stigma and discrimination [2], [3]. Many of these conditions increase the risk of several non-communicable diseases, including cardiovascular diseases, diabetes, musculoskeletal disorders and some cancers [4].
People living with overweight or obesity may have to perform a number of daily tasks such as dietary changes, exercise and activity, behaviour changes, weight loss medication or other optional treatments to cope with their condition. All of these activities and tasks and their interactions are part of what is called self-management [5].
Self-management has been defined as: ‘actions that individuals, families, and communities engage in to promote, maintain, or restore health with or without the support of health professionals, and including but not limited to self-prevention, self-diagnosis, self-medication, and coping with illness and disability’ [6]. In practice, managing a long-term condition such as obesity requires confidence in coping with the condition, which includes having the skills to monitor symptoms and clinical markers, understand their implications and adjust behaviours appropriately. Drawing on this definition, self-management interventions (SMIs) can be characterised as supportive interventions that healthcare professionals, peers, or laypersons systematically provide to increase patients’ skills and confidence in their ability to manage their chronic conditions. Therefore, SMIs aim to equip patients (and, where appropriate, informal caregivers) in such a way that they can actively participate in the management of their own conditions [7]. Evidence has shown that SMIs can help people living with overweight or obesity in managing their disease, and improve clinical outcomes such as weight loss [8], [9]. Overweight and obesity are conditions often treated jointly by the same lifestyle interventions such as healthy diet and regular physical activity [10], [13]. Despite the growing evidence of SMIs implementation, to our knowledge, there is not systematic evidence on the knowledge gaps in self-management for people with overweight or obesity.
“COMPAR-EU” project (https://self-management.eu) is an EU Horizon 2020 international research project that aims to identify, compare, and rank the most effective and cost-effective SMIs for adults, living with one of four high-priority chronic conditions: type 2 diabetes, obesity, chronic obstructive pulmonary disease, and heart failure [10]. We developed and validated a taxonomy of SMIs addressing the needs of people living with chronic conditions, to help identifying key characteristics and facilitate design, reporting and comparisons of SMIs. The taxonomy includes four domains: target population characteristics, intervention characteristics, expected patient (or informal caregiver) self-management behaviors and patient-relevant outcomes [11]. For selecting the outcomes, we developed a Core Outcome Set (COS) specifically for SMIs for adult people living with overweight or obesity in Europe [12]. This COS was developed with patients, patient representatives and healthcare professionals, including a systematic review on patients and informal caregiver priorities [12]. Within “COMPAR-EU” project, a comprehensive systematic review and network meta-analysis was performed on any SMI for type 2 diabetes, obesity, chronic obstructive pulmonary disease, and heart failure. The present study aims to synthesize and describe current evidence on SMIs for patient-important outcomes for adults living with overweight or obesity [9], [13].
2. Methods
We followed evidence mapping methodology [14]. An evidence map is a systematic synthesis that provides an overview of the available evidence on a specific topic, by highlighting its characteristics and gaps [14]. Evidence mapping is becoming a common research tool. It does not only provide a synthesis of the available literature, but it also graphically illustrates what is available [14]. Having a clear overview of the available evidence is important to understand the coverage of the literature, and to develop suggestions and strategies for future research. Evidence maps, through a user-friendly format, facilitate communication targeted to a range of stakeholders, such as policy makers, researchers, and health professionals [15], both at national and international level [16].
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews checklist [17] for developing this evidence map (Table S1). The review methodology was established before data extraction and was registered in PROSPERO (CRD42020155441) and published [10].
[About here link to Table S1. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist].
We used the Arksey and O’Malley methodological framework [18], modified by Levac et al. [19] for conducting the evidence map which includes six stages:
2.1. Stage 1. Identifying the research question
To synthetize and describe the reported experimental research on self-management literature for people living with overweight or obesity, the research question to be addressed using an evidence map was: What are the existing SMIs for people living with overweight or obesity, how were they delivered to patients in randomized controlled trials (RCTs), and which patient-relevant outcomes were addressed?
2.2. Stage 2. Identifying relevant studies
To identify relevant RCTs on SMIs for overweight or obesity, we searched the databases of a completed European project (i.e., PRO-STEP [6]) that had previously identified systematic reviews on SMIs for overweight or obesity. From these databases, we included studies from 2000 onwards (date of the last systematic review included). We updated this search with articles from 2010 up to December 5th, 2018, searching in PubMed CINAHL, Embase, Cochrane, and PsycINFO. The search string consisted of at least the key terms “self-management”, “adults” “obesity” for content and “randomized controlled trials” for study type. MESH terms were used to find relevant synonyms. The search algorithms were adapted to the requirements of each database. The full search strategy for each database is attached as an additional file (Table S2). We included RCTs assessing SMI for overweight or obesity in adults. [About here link to Table S2. Search strategy].
2.3. Stage 3. Selection of articles
After a calibration exercise, two reviewers independently screened the search results to select potentially eligible records based on title and abstract. Subsequently, two reviewers independently confirmed eligibility based on the full text articles of the relevant selected records. In case of disagreement, they reached consensus by discussion or involving a third reviewer. We used Covidence© (www.covidence.org) for the article’s selection. Through the screening process, a team of experienced researchers monitored consistency among reviewers and could be asked for help in case of doubts. Information of multiple publications from the same study were merged to be analysed as a single case.
Our inclusion criteria included: i) Population, adults (≥18 years of age) with a diagnosis of overweight (defined as having a BMI ≥ 25 kg/m2 or ≥ 23 kg/m2 in case of Asian population) or obesity (defined as having a BMI ≥ 30 kg/m2 or ≥ 25 kg/m2 in case of Asian population) and their informal caregivers. When a study had a mixed population of patients (i.e., not only people living with overweight or obesity) and did not report the outcomes per condition separately, it was included if at least 80% of the population targeted the chronic condition of interest; ii) Intervention, SMIs; iii) Comparison, usual care (usual care or usual care plus if included self-management support techniques), other SMIs (head-to-head) and not SMIs (excluding surgery); iv) Outcomes, studies must have reported at least one of the outcomes from the “COMPAR-EU” predefined COS [12]; v) Study design, RCTs; quasi-randomized studies were excluded. We included only studies published in English or Spanish [10].
2.4. Stage 4. Charting the data
After calibration, one reviewer extracted the relevant data from eligible studies, and a second reviewer checked accuracy. A handbook was developed following Cochrane guidance and training sessions were held for those responsible for collecting data. We extracted information regarding the characteristics of study population and the study setting, characteristics of the SMIs, outcome results and variables to assess the risk of bias, using a pre-designed extraction platform. In case of disagreement, consensus was reached by discussion or by involving a third reviewer. Senior researchers team monitored the consistency of the data extraction between reviewers in all cases and could always be asked for help in case of doubts.
SMIs characteristics were coded in a structured way, following the pre-developed taxonomy [11], according to the type of support technique (techniques or methods that are used to provide care and encouragement to people with chronic conditions and their carers to help them understand their central role in managing their condition, make informed decisions about care and engage in appropriate behaviours.), the type of recipient, the type of provider, the type of encounter, the delivery method, the intensity of the intervention, the time of communication and the location (Table 1). The level of adaptation or modification of the SMI when delivered to the study population (tailoring) was as well assessed and extracted. The list of outcomes considered for extraction were classified into basic empowerment, adherence to self-management behaviours, clinical outcomes, patient/informal caregiver quality of life, care perception/satisfaction and costs (see Table 1 for more detail) [12].
Table 1.
COMPAR-EU self-management interventions taxonomy and core outcome set for obesity.
| Self-management interventions characteristics [11] | |
|---|---|
| Type of support technique | |
| Education | Sharing information (with/without written materials) |
| Skills training | |
| Monitoring techniques | Self-monitoring training and feedback |
| Use of prompts and reminders | |
| Action-based behavioral change techniques | Enhancing problem solving skills |
| Goal setting and action planning | |
| Emotional-based behavioral change techniques | Coaching and motivational interviewing |
| Stress and/or emotional management | |
| Social support | Social support |
| Shared decision-making | Shared decision-making |
| Use of external resources | Encourage use of service |
| Provision of equipment | |
| Type of recipient | Groups |
| Individual | |
| Combined | |
| Type of provider | Peers and laypersons |
| Nurses and educators | |
| Physicians | |
| Type of encounter | Clinical visits |
| Support sessions | |
| Self-guided | |
| Combination | |
| Mode of delivery | Face-to-face |
| Remote | |
| Combined | |
| Intensity | High (≥ 10 h) |
| Low (< 10 h) | |
| Time of communication | Synchronous |
| Asynchronous | |
| Location | Outpatient care |
| Homecare | |
| Hospital care | |
| Virtual | |
| Community-based care | |
| Obesity core outcome set [12] | |
| Basic empowerment | Self-efficacy |
| Self-monitoring (including self-recording) | |
| Patient activation | |
| Participation and decision-making | |
| Knowledge | |
| Health literacy | |
| Adherence to self-management behaviors | Physical activity |
| Healthy nutrition habits / personalized nutrition (e.g., dietary planning of healthy eating pattern, eating management, consumption of unhealthy beverages, mindful eating) | |
| Adherence to program | |
| Clinical outcomes | Weight management |
| Comorbidity management (e.g., blood pressure control) | |
| Mortality | |
| Patient/informal caregiver quality of life | Quality of life (physical and psychological functioning, including depression) |
| Coping with the disease (e.g., how well a person feels capable to cope with stress and other disease-related difficulties, or tasks) | |
| Social interactions (e.g., relationship with friends, social activities) | |
| Sex functioning | |
| Integration at work (e.g., being able to do work task or to take up paid employment) | |
| Sleep quality | |
| Care perceptions / satisfaction | Patient- Provider relationship |
| Cost | Cost-effectiveness for the health system – value-based outcomes |
[About here Table 1. COMPAR-EU self-management interventions taxonomy for overweight/obesity].
Risk of bias was assessed in included studies using Cochrane’s tool [20]. We rated the risk of bias as low, high or unclear risk in each of the five items from the Cochrane’s risk of bias tool [20].
2.5. Stage 5. Collating, summarizing, and reporting the results
We summarized the results using tables and figures (i.e., bubble plot) to show the evidence landscape on SMIs for overweight and obesity, and to elucidate knowledge clusters and gaps. Descriptive data are presented focusing on the number of studies or in the total number of intervention arms depending on the variable reported.
We tabulated the included studies, summarizing reported study characteristics, patient characteristics, patient-relevant outcomes and SMIs characteristics. Furthermore, to cluster the evidence and identify research gaps, we used the program Tableau © (https://www.tableau.com) to create bubble plots. The basis for the bubble plot was a crosstabulation, showing the number of arms that included one or two or more self-management support techniques or self-management support techniques in relation to patient-relevant outcomes and the mode of delivery of such techniques. Using bubble plots to visualize the results allows us to see at first glance the research that was done regarding the topic of interest (herein SMIs and patient-relevant outcomes) and presenting a clear image of evidence clusters and gaps in research.
2.6. Stage 6. Consultation
Through the project, several stakeholders have been involved providing insights beyond those found in the literature. Patients were consulted during all stages of the process. The taxonomy of SMIs [11] was refined and validated through expert consultation, including patients’ representatives and allowed us to define the components that helped us extract the information about the interventions from the included studies. The obesity “COMPAR-EU” COS [12] was developed together with patients, patients’ representatives and professionals and also included a systematic review on patients and informal caregiver priorities regarding treatment [21]. Descriptive findings displayed in this study have been presented and discussed in a “COMPAR-EU” patient panel lead by the European Patient Forum.
3. Results
A total of 8707 citations were identified by the searches. Following title and abstract screening, 1322 remained for full-text screening. Seven hundred and eighty-one articles were selected for extraction, of which 497 studies were finally included and extracted. The main reasons for exclusion during the extraction phase (n = 284) were being part of a multiple publication (n = 122), invalid article type (n = 36), not including adults living with overweight or obesity (n = 32), not being an RCT (n = 26) or not being a SMI (n = 11) (Table S3). A PRISMA flowchart (Fig. S1) describes the process in more detail [22].
[About here link to Table S3. Excluded studies and reasons for exclusion].
[About Fig. S1. Evidence map PRISMA flowchart].
3.1. Key characteristics of the included studies and participants
The 497 included studies comprising 99,741 (median 112, range 11–5145) adults living with overweight or obesity were conducted in 43 different countries. By far, most of them were performed in the United States (n = 288, 58%), followed by the United Kingdom (n = 34, 7%) and Australia (n = 27, 5%). One hundred (20%) studies were performed in a European country. There were only two studies that were conducted in more than one country [23], [24]. Fifty-four percent of the studies concerned single centre studies, whereas 31% were conducted in multiple centres.
Almost all studies were implemented on an individual patient level (95%) compared to population interventions. And almost all focused-on patients (n = 494, 99%), with only three studies focusing on both patients and informal caregivers [25], [26]. Participants were mostly female (median 84%, IQR 68–100%). Median age across studies was 48 years old (IQR 44 – 54 years). Data on socio-economic status, belonging to a minority group or health literacy was seldom reported for most patients in the studies. Regarding the severity of the condition, when more than one measure was given, BMI was the preferred measure to extract though we included studies with other measures reported (e.g., waist circumference). Almost all studies (99%) provided information on severity of obesity with 97% providing information on BMI at baseline (median 34, IQR 31.5 – 36.3). Only five studies reported the time since diagnosis of people living with overweight or obesity [27], [28], [29], [30], [31]. In 18% (n = 91) of the studies information on comorbidity was given. The number of other conditions next to overweight/obesity ranged from 1 to 6; in 27% of these 91 studies, the type of comorbidity was not specified. Diabetes (7%), hypertension (2%), depression (1%), osteoarthritis (1%) and cancer (1%) were the most common comorbidities encountered.
Table 2 and Table S4 show key characteristics of the included studies.
Table 2.
Summary of the characteristics from included studies (n = 497).
| Variable |
n (%) | |
|---|---|---|
| Type of population | Patient Patient and informal caregiver |
494 [99] 3 (0.6) |
| Type of comparison | Head-to-head interventions Intervention vs usual care |
244 (49) 253 (51) |
| Type of usual care (n = 253) | Usual care Usual care plus |
140 (55) 113(45) |
| Study site. Type of centre. | Single centre Multi-centre Unclear Not reported |
269 (54) 156 (31) 57(12) 15 (3) |
| Study site. Continent of implementation | Europe Outside Europe |
100 (20) 392 (80) |
| Sex (n = 480) | Sex as inclusion criteria Median (IQR) percentage of females |
137(28) 84 (68–100) |
| Age (n = 493) | Age as inclusion criteria Median (IQR) age |
415 (84) 48 (44–54) |
| Socio-economic status data included | 16 (3) | |
| Minority cultural groups data included | 48 (10) | |
| Health literacy data included | 1 (0.2) | |
| Time since diagnosis (years) | Time since diagnosis - (data available) Time since diagnosis as inclusion criteria Median (IQR) years |
5 (1) 2 (0.4) 6.5 (3.9–8.2) |
| Measure of disease control (n = 490) | BMI as disease control measure | 477 (97) |
| Presence of other condition | Presence of other condition (data available) | 91(18) |
| Presence of other condition as inclusion criteria | 80 (16) | |
| Type of condition described | Diabetes – 33 (7) Hypertension – 12 (2) Cancer – 7 (1) Depression – 6 (1) Osteoarthritis – 5 (1) |
|
[About here Table 2. Summary of the characteristics from included studies].
[About here link to Table S4. Key characteristics of the included studies].
3.2. Risk of bias of the included studies
Most studies had a low risk of bias in the sequence generation of the random number for allocation of participants, but there was a lack of clarity reporting the methods for concealment of the allocation. The main methodological limitation of the included studies was the lack of blinding of the intervention as very few studies (n = 48, 8%) incorporated a procedure to occult the active group from participants or care personnel or used a “sham” intervention to reduce the influence of being aware of which arm participants were allocated. This limitation also affected the assessment of the subjective outcomes (i.e., quality of life), and objective outcomes that might be influenced by the assessor (i.e., blood pressure). We considered that objective outcome from laboratory tests or based in clearly observed events (i.e., mortality, hospitalization) were not affected by the lack of blinding. Around 48% of the studies also have a relevant number of drop-out during follow-up that raised concern of high risk of bias due to attrition in those studies. The risk of selective reporting was more difficult to evaluate as few studies made available their protocols before the publication of the results, however we considered that most studies had low risk of bias (Table S5).
[About here link to Table S5. Risk of bias of the included studies].
3.3. Outcomes reported in the included studies
Table 3 shows the frequency in which outcomes were used in the 497 included studies using the “COMPAR-EU” obesity core outcome set [12]. More than a half of studies (55%) focused on clinical outcomes, being weight management the most frequently studied in this group, or on adherence to self-management behaviours (23%) as physical activity or healthy nutrition. Basic empowerment skills were less targeted (8%), being self-efficacy the most studied in this group; RCTs on patient activation and participation in decision-making were limited, and no RCT had a direct focus on health literacy. Patient or informal caregiver quality of life were reported in less than 14% of the studies. Most frequently they were focused on overall quality of life, while aspects related to coping with their condition or with daily life as sex functioning or sleep quality were hardly reported as well as those related with social functioning, such as integration at work or social interaction, including stigma, taking into account these outcomes were considered as important by patients and professionals in the COS development process. Important policy issues as cost-effectiveness of the interventions were only gathered in less than 1% of the studies.
Table 3.
Frequency of outcomes from the core outcome set cited in the included studies (n = 497).
| Obesity core outcome set | n (%) | |
|---|---|---|
| Basic empowerment 87 (8) |
Self-efficacy | 67 (14) |
| Self-monitoring | 11 (2) | |
| Patient activation | 6 (1) | |
| Participation and decision-making | 3 (1) | |
| Knowledge | 0 (0) | |
| Health literacy | 0 (0) | |
| Adherence to self-management behaviours 255 (23) |
Physical activity | 223 (45) |
| Healthy nutrition | 177 (36) | |
| Adherence to program | 32 (6) | |
| Clinical outcomes 617 (55) |
Weight management | 456 (92) |
| Comorbidities management | 154 (31) | |
| Mortality | 7 (1) | |
| Patient / informal caregiver quality of life 161 (14) |
Quality of life | 107 (22) |
| Coping with the disease | 30 (6) | |
| Social interactions | 15 (3) | |
| Sex functioning | 7 (1) | |
| Integration at work | 1 (0.2) | |
| Sleep quality | 1 (0.2) | |
| Care perceptions / satisfaction 1 (0.2) |
Patient – Provider relationship | 1 (0.2) |
| Cost 5 (1) |
Cost-effectiveness | 5[1] |
[About here Table 3. Frequency of outcomes from the core outcome set in the included studies].
3.4. Characteristics of the SMIs reported in included studies
In half of the studies a SMI was compared to usual care (n = 253, 51%); two hundred and forty-four studies (49%) compared two or more intervention arms. In total, we found 866 intervention arms (77%) and 253 (23%) usual care arms.
In 21% of the active intervention arms (n = 866), the intervention was tailored, i.e., the content of the intervention or the way it was delivered was personalized to the study population (e.g., educational material of an existing intervention that was simplified for participants with low health literacy or translated to Spanish to adapt it to Spanish speaking population or adapted based on cultural preferences of diet). The median duration of the SMIs across studies was 180 min (IQR 90–360).
Table 4 shows the SMIs characteristics reported in the included intervention study arms (n = 866). The three most frequent self-management support techniques were sharing information (n = 858, 99%), goal setting and action planning (n = 619, 72%) and self-monitoring training and feedback (n = 614, 71%). Stress and/or emotional management (n = 157, 18%)) or social support (n = 228, 26%) were less frequently described; shared decision-making (n = 36, 4%), encourage use of services (n = 94, 11%) and the use of prompts and reminders (n = 146, 17%) were the least reported self-management support techniques.
Table 4.
Summary of the characteristics of the self-management interventions in the included study arms (n = 866).
| Type of support technique | Type of support technique | n (%) | |
|---|---|---|---|
| Education | Sharing information | 858 (99) | |
| Skills training | 364 (42) | ||
| Monitoring techniques | Self-monitoring training and feedback | 614 (71) | |
| Use of prompts and reminders | 146 (17) | ||
| Action-based behavioural change | Enhancing problem solving skills | 305 (35) | |
| Goal setting and action planning | 619 (72) | ||
| Emotional-based behavioural change techniques | Coaching and motivational interviewing | 304 (35) | |
| Stress and/or emotional management | 157 (18) | ||
| Social support | 228 (26) | ||
| Shared decision-making | 36 (4) | ||
| Use of external resources | Encourage use of service | 94 (11) | |
| Provision of equipment | 287 (33) | ||
| Type of encounter | n (%) | Type of communication | n (%) |
| Clinical visits | 15 (2) | Synchronous | 498 (56) |
| Support sessions | 373 (43) | Asynchronous | 131 (15) |
| Self-guided | 133 (15) | Combination | 236 (27) |
| Combination | 342 (40) | Not reported | 1 (0.1) |
| Not reported | 3 (0.4) | ||
| Mode of delivery | n (%) | Type of recipient | n (%) |
| Face-to-face | 386 (45) | Groups | 452 (52) |
| Remote | 185 (21) | Individual | 413 (48) |
| Combination | 256 (30) | Not reported | 1 (0) |
| Non-reported | 39 (5) | ||
| Location (Top 5) | n (%) | Provider (Top 5) | n (%) |
| Virtual | 271 (31) | Nutritionist | 264 (30) |
| Outpatient care | 220 (26) | Educator | 214 (25) |
| Community care | 183 (21) | Service | 152 (18) |
| Home care | 174 (20) | Psychologist | 117 (14) |
| Unclear | 126 (15) | Unclear | 115 (13) |
Most of the interventions were made in specifically designed support sessions encounters (n = 373, 43%) or using a combination of different formats including self-guiding (n = 342, 40%); very few were delivered in the regular clinical visits. This was also confirmed by the type of professional involved, whereas only in 71 (8%) and 46 (5%) arms physicians and nurses were reported to be the main professional supporting the intervention, respectively. Half of the interventions were produced synchronously (n = 498, 56%) and face-to- face (n = 386, 45%), being this the most frequent delivery setting, followed by a combination of face-to-face and remote activities (n = 256, 30%).
[About here Table 4. Summary of the characteristics of the self-management interventions in the included study arms].
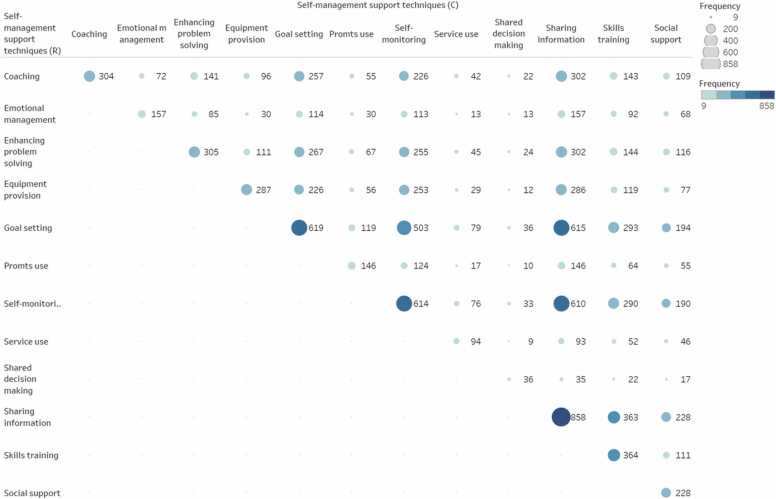
Studies used different types of support techniques to provide care and encouragement to people living with overweight or obesity and their informal caregivers to help them understand their central role in managing their condition, make informed decisions about care and engagement in appropriate behaviors. Intervention arms focused on a single or combination of different types of support techniques. In the intervention arms the number of self-management support techniques used varied between one and 12 (Median 5, IQR 4–7). Fig. 1 shows a bubble plot with the frequency in which specific self-management support techniques were combined across studies. Most frequent combinations included self-monitoring training and feedback, goal setting and action planning, sharing information, enhancing problem solving skills and coaching and motivational interviewing.
Fig. 1.
Frequency in which self-management support techniques are combined across intervention arms. This bubble plot shows the frequency in which specific self-management support techniques were combined across studies (n = 497). The size and colour of the bubble indicates the number of studies including each combination presented.
[About here Fig. 1. Frequency in which self-management support techniques are combined across intervention arms].
In most cases, usual care included regular visits (n = 33, 13%) and a form of education (n = 153, 60%). When-face-to-face or when the mode of delivery was a combination of face-to-face and remote, 98% (n = 249) of usual care arms were considered low intensity (less than of 10 h of duration of the interventions), while it was 56% (n = 483) in intervention arms. In remote interventions, the median intensity was 30.0 (15.0–60.0) minutes for usual care while it was 160.0 (60−380) minutes for intervention arms (p = 0.038).
3.5. Self-management support techniques, mode of delivery and outcomes measured
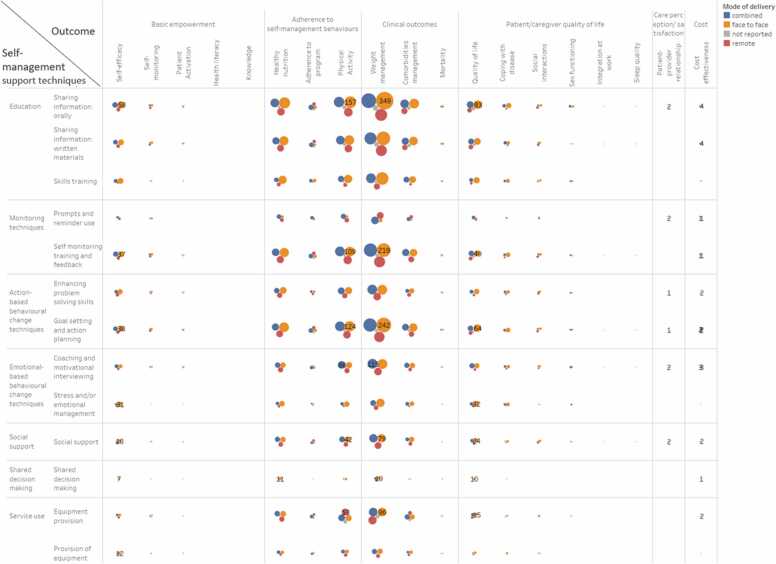
Fig. 2 shows the bubble plot including all the intervention arms (n = 866) included in the 497 studies and classified according to the self-management support techniques and mode of delivery used as well as the type of outcome measured. Our clustering approach shows that most of the research in self-management literature is concentrated in clinical outcomes (e.g., weight management) and adherence to self-management behaviors (e.g., adherence to physical activity followed by adherence to healthy nutrition) through education (e.g., sharing information orally), using monitoring (e.g., self-monitoring training and feedback) and action-based behavioral change techniques (e.g., goal setting and action planning) provided face-to-face or in combination with remote techniques. Remote mode of delivery was mainly applied to enhance weight management and physical activity, mainly for education and providing information purposes as well as for monitoring or following the action plans.
Fig. 2.
Type of self-management support technique by type of outcome and mode of delivery. This bubble plot shows the frequency of intervention arms (n = 866) categorized by self-management support technique, mode of delivery (indicated by color) and type of outcome measured. The size of the bubble indicates the number of intervention arms including each combination presented.
[About here Fig. 2. Type of self-management support technique by type of outcome and mode of delivery].
4. Discussion and conclusion
This evidence map provides a systematic overview on SMIs for adults living with overweight or obesity reported in published RCTs including patient-relevant outcomes. This work describes the existing evidence landscape regarding reported SMIs in terms of their main characteristics, such as the included self-management support techniques, mode of delivery as well the outcomes measured.
4.1. Evidence clusters and knowledge gaps
We identified 497 RCTs, half of them conducted in United States, and less than one third in countries across Europe. Participants were mostly middle-aged females with a median BMI of 34 at the study inclusion (grade I obesity according to WHO [32]).
A very limited number of RCTs had a focus on social vulnerability aspects (i.e., socio-economic status, minority groups or health literacy). However, social inequalities in people living with overweight or obesity are important, especially among women. In a study conducted by the Organization for Economic Co-operation and Development (OECD) study in 2017 [33], four of the eight countries for which data were available, less educated women were two to three times more likely to be living with overweight than those with a higher level of education. Numerous efforts have been made to systematically understand and address population and individual inequalities, including poor and low literacy populations [34], [35] and to identify their specific research needs [36]. For that reason, it is critical to provide further knowledge on how to address self-management in the most vulnerable groups of persons affected by this condition.
Overall, clinical outcomes (e.g., weight management) were the most frequently reported outcomes, followed by adherence to self-management behaviors. Our findings also indicate less studies focusing on overall quality of life and a paucity of studies focusing on specific aspects reported as important for patients, as we gathered after developing the “COMPAR-EU” obesity COS [12], such as those related to daily life (e.g., sleep quality, sex functioning), social functioning including stigma and those related to facilitate managing the condition as participation in decision-making and coping with their condition. A few years ago, the Cochrane Collaboration, WHO and governments [37] stated that research priority setting is a collective social activity stressing the need of involving all stakeholders. To ensure the incorporation of patients and citizens views in research and in the priority setting process, several guidelines are available as the Guidance for Reporting Involvement of Patients and the Public (GRIPP). Despite these recommendations, patients are not regularly included in the selection of topics and priorities for research. In a recent systematic review on how priorities are being developed in obesity research, the authors found that in half of them, public had not been included in any phase of the process [38], whether other stakeholders such as policy makers, researchers and healthcare professionals were always included [39], [40]. The evidence clusters and gaps shown in this evidence map may reflect this lack of representation of patients/civil society in research development.
Cost-effectiveness of the SMIs were hardly gathered as well. However, today one in five adults is obese in OECD countries and the OECD has estimated an average impact of 8.4% on health expenditure on overweight and related conditions for 2020–2050 (France, 5%; USA, 14%) [33]. So, although cost seems important, costs as an outcome of SMIs was only reported by 1% of studies making it difficult to support important policy decisions in the coming years.
It is widely accepted that obesity is a complex and multifaceted condition. Self-management usually requires people to adopt new behaviors. Several behavioral theories have been used to guide the development of SMIs (e.g., social cognitive theory, social influence, stress coping framework etc.). In our study, self-management techniques described were mainly focused on education, monitoring and action-based behavioral change techniques. Coaching was the most reported emotional-based behavioral change technique while emotional management, motivational interviews or social support were less frequently included though they seem highly promising [41], [42], [43].
Face-to-face alone followed by a combination with remote mode of delivery were the most prevalent approach. Despite the growth of digital health in recent years, only 185 studies (21%) were reported to be performed completely online and those were mainly focused in weight management and training self-monitoring. Complete remote interventions were less reported though there is a wide range of technologies available. Generally, these interventions appear to be acceptable and feasible though its long-term engagement seems to be a challenge [44]. Probably specific tailored remote interventions need to be further studied to facilitate its access to some groups of patients or to support emotional based behavioral change techniques or social support. We recommend further research to include number of sessions, duration, professionals participating and follow-up time in the description of all self-management RCTs.
4.2. Comparison with other studies
To our knowledge, this is the first evidence map providing details on the characteristics of existing SMI for people living with overweight or obesity. We recently performed the description of SMIs components in RCTs for chronic obstructive pulmonary disease within “COMPAR-EU” project following the rapid reviews method proposed by Cochrane [45]. Again, self-management support techniques like education, self-monitoring and goal setting were the most frequently used. However, outcomes considered important by patients were hardly taken into account [45]. We identified one previous evidence map that focused on describing obesity related determinants, specifically how psychological factors were associated with the development of obesity, but did not provide details of existing SMIs [46]. On the other hand, we identified a recent systematic review describing behavior lifestyle interventions for moderate and severe obesity [47]. This systematic review described how interventions were delivered, and what components were included, and made conclusions on the effectiveness of such interventions by reporting that comprehensive and intensive behavioral interventions can result in clinically significant, albeit modest, weight loss in this obese subpopulation but may not result in significant improvements of other cardiometabolic risk factors [47].
4.3. Strengths and limitations of the current review
This evidence map summarizes an extensive body of research, providing a searchable database of RCTs on SMIs for people living with overweight and obesity, along with detailed descriptive information. Using the “COMPAR-EU” comprehensive taxonomy contributed to better reporting of the interventions and using the COS for obesity and overweight allowed us to describe how align is research with outcomes that matter most to patients.
Our evidence map has some limitations. Incomplete reporting of the evidence in already published RCTs is an important limitation for analyzing the available evidence and to make recommendations for future research. Our study includes literature published until 2018; therefore, it is possible that some of the areas with limited information reported have improved in recent years, though in some cases there were so few studies that it is likely that the detected gaps will still be valid. Recently published systematic reviews seem to confirm the gaps found, specially related to the lack of research in this field with a focus on vulnerable population [48], that included patient-relevant outcomes [49] or cost-effectiveness analysis [49], [50] or tested SMIs other than education, self-monitoring and goal setting [49]. We found only one systematic review that found low-quality evidence that weight loss SMIs, mostly multimodal and including psychosocial support, appeared to result in greater reductions of clinical outcomes such as body weight, but as well quality of life in overweight and obese breast cancer survivors [50]. Furthermore, we have not been able to separate studies focusing only on overweight or obesity due that most of RCTs did not do so.
To make our review more feasible, extracting the data from eligible studies was performed by one person after a previous calibration process which was checked by a second reviewer and supervised by a senior team that monitored consistency among reviewers through a random sample of cases. The main methodological limitation of the included studies was the lack of blinding of the intervention. This limitation also affected the assessment of the subjective and objective outcomes that might have been influenced by the assessor.
4.4. Conclusions, practical implications, and future directions
Great amount of RCTs on SMIs have been reported from the seminal work from Coulter and Ellis in 2006 [51]. The results from this evidence map suggest the need of widening the scope of self-management research to enhance the focus on vulnerable persons and to include areas that are important to patients such as emotional and social support, and further explore feasibility of using only remote support techniques. Resource use information was not usually reported and constitutes an important information for taking needed policy decisions in this area. This evidence map could guide further research by exploring the gaps identified and therefore to enhance our understanding of SMIs. We believe that using the “COMPAR-EU” comprehensive taxonomy can help all stakeholders to identify further research areas needing to be addressed in the future. More detailed results can also be found at the COMPAR-EU platform (https://platform.self-management.eu/).
Funding
This work was supported by the European Union’s Horizon 2020 research and innovation program [grant agreement no. 754936, 2017].
Author contributions
Conceptualization, systematic search, and double screening: RS, MB, MH, MvdG, CCA, DM, OG, PA and CO. Data extraction: AIGG, RP, CR, MLG, KS, EN, CK, MS, CS, PG, SM, FW, IC, JB, AK, JW, TL, IK, JB and JZ. Data analysis: AAV, SZ, GS and DM. RS, AIGG and CV wrote the manuscript; MB, LS, MH, RP, MvdG, JZ, OG and CO edited the manuscript. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare no competing interests.
Acknowledgements
The COMPAR-EU group for their valuable assistance in conducting this research:
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pec.2023.107647.
Contributor Information
Rosa Sunol, Email: rsunol@fadq.org.
Ana Isabel González-González, Email: aigonzalez@fadq.org.
Claudia Valli, Email: cvalli@fadq.org.
Marta Ballester, Email: mballester@fadq.org.
Laura Seils, Email: mir@fadq.org.
Monique Heijmans, Email: m.heymans@nivel.nl.
Rune Poortvliet, Email: r.poortvliet@nivel.nl.
Marieke van der Gaag, Email: m.vandergaag@nivel.nl.
Claudio Rocha, Email: claudiorochacalderon@gmail.com.
Montserrat León-García, Email: mleong@santpau.cat.
Karla Salas-Gama, Email: karla.salas@vallhebron.cat.
Ena Niño de Guzman, Email: e.nino@iconcologia.net.
Chrysoula Kaloteraki, Email: xkaloter@gmail.com.
Marilina Santero, Email: msantero@santpau.cat.
Cristina Spoiala, Email: cristina.spoiala@ons.gov.uk.
Pema Gurung, Email: p.gurung@library.leidenuniv.nl.
Saida Moaddine, Email: s.moaddine@lumc.nl.
Fabienne Wilemen, Email: fabiennewillemen@live.nl.
Iza Cools, Email: izacools@hotmail.com.
Julia Bleeker, Email: julia.bleeker@live.nl.
Angelina Kancheva, Email: angelina.k.kancheva@gmail.com.
Julia Ertl, Email: juliaertl@hotmail.com.
Tajda Laure, Email: laure@essb.eur.nl.
Ivana Kancheva, Email: ivanakirilova.kancheva@gmail.com.
Areti Angeliki Veroniki, Email: areti-angeliki.veroniki@unityhealth.to.
Stella Zevgiti, Email: stella.zevgiti@gmail.com.
Jessica Beltrán, Email: jbeltranp@santpau.cat.
Carlos Canelo-Aybar, Email: ccanelo@santpau.cat.
Jessica Hanae Zafra-Tanaka, Email: j.zafra.t@gmail.com.
Georgios Seitidis, Email: g.seitidis@uoi.gr.
Dimitris Mavridis, Email: dimi.mavridis@gmail.com.
Oliver Groene, Email: o.groene@optimedis.de.
Pablo Alonso-Coello, Email: palonso@santpau.cat.
Carola Orrego, Email: corrego@fadq.org.
Appendix A. Supplementary material
Supplementary materialFigure S1. Evidence Map PRISMA Flowchart
.
Supplementary material.
.
Supplementary material.
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.World Health Organisation. Obesity and overweight. [Internet]. 2021 [cited 2022 Aug 6]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/%0Dobesity-and-overweight
- 2.Atlantis E., Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int J Obes. 2008;32(6):881–891. doi: 10.1038/ijo.2008.54. [DOI] [PubMed] [Google Scholar]
- 3.Puhl R.M., Moss-Racusin C.A., Schwartz M.B., Brownell K.D. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res. 2007;23(2):347–358. doi: 10.1093/her/cym052. [DOI] [PubMed] [Google Scholar]
- 4.Kyrou I., Randeva H., Tsigos C. Clinical problems caused by obesity [Internet]. ENDOTEX. 2018 [cited 2022 Aug 6]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278973/.
- 5.Lorig K.R., Holman H.R. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 6.PRO-STEP Consortium. Promoting self-management for chronic diseases in the EU-PROSTEP project. 2018.
- 7.Tattersall R. The expert patient: a new approach to chronic disease management for the twenty-first century. Clin Med (North Il) 2002;2(3):227–229. doi: 10.7861/clinmedicine.2-3-227. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann‐Boyce J., Johns D.J., Jebb S.A., Summerbell C., Aveyard P. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta‐analysis. Obes Rev. 2014;15(11):920–932. doi: 10.1111/obr.12220. Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franz M.J., Boucher J.L., Rutten-Ramos S., VanWormer J.J. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115(9):1447–1463. doi: 10.1016/j.jand.2015.02.031. (Sep) [DOI] [PubMed] [Google Scholar]
- 10.Ballester M., Orrego C., Heijmans M., Alonso-Coello P., Versteegh M.M., Mavridis D., et al. Comparing the effectiveness and cost-effectiveness of self-management interventions in four high-priority chronic conditions in Europe (COMPAR-EU): a research protocol. BMJ Open. 2020;10(1) doi: 10.1136/bmjopen-2019-034680. Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orrego C., Ballester M., Heymans M., Camus E., Groene O., Niño de Guzman E., et al. Talking the same language on patient empowerment: Development and content validation of a taxonomy of self‐management interventions for chronic conditions. Heal Expect. 2021;24(5):1626–1638. doi: 10.1111/hex.13303. Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valli C., Suñol R., Orrego C., Niño de Guzmán E., Strammiello V., Adrion N., et al. The development of a core outcomes set for self‐management interventions for patients living with obesity. Clin Obes. 2022;12(1) doi: 10.1111/cob.12489. Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez Puente J.M., Martínez-Marcos M. Sobrepeso Y obesidad: eficacia de las intervenciones en adultos. Enferm Clínica. 2018;28(1):65–74. doi: 10.1016/j.enfcli.2017.06.005. (Jan) [DOI] [PubMed] [Google Scholar]
- 14.Miake-Lye I.M., Hempel S., Shanman R., Shekelle P.G. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5(1):28. doi: 10.1186/s13643-016-0204-x. Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bragge P., Clavisi O., Turner T., Tavender E., Collie A., Gruen R.L. The global evidence mapping initiative: scoping research in broad topic areas. BMC Med Res Method. 2011;11(1):92. doi: 10.1186/1471-2288-11-92. Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saran A., White H. Evidence and gap maps: a comparison of different approaches. Campbell Syst Rev. 2018;14(1):1–38. doi: 10.4073/cmdp.2018.2. Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467. doi: 10.7326/M18-0850. Oct 2. [DOI] [PubMed] [Google Scholar]
- 18.Arksey H., O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Method. 2005;8(1):19–32. (Feb) [Google Scholar]
- 19.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. doi: 10.1186/1748-5908-5-69. Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2) doi: 10.1136/bmj.d5928. Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niño de Guzmán E., Martínez García L., González A.I., Heijmans M., Huaringa J., Immonen K., et al. The perspectives of patients and their caregivers on self-management interventions for chronic conditions: a protocol for a mixed-methods overview. F1000Research. 2020;9:120. doi: 10.12688/f1000research.22125.1. Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. Mar 29;n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jebb S.A., Ahern A.L., Olson A.D., Aston L.M., Holzapfel C., Stoll J., et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378(9801):1485–1492. doi: 10.1016/S0140-6736(11)61344-5. (Oct) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhurandhar E.J., Dawson J., Alcorn A., Larsen L.H., Thomas E.A., Cardel M., et al. The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am J Clin Nutr. 2014;100(2):507–513. doi: 10.3945/ajcn.114.089573. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris L., Hankey C., Jones N., Pert C., Murray H., Tobin J., et al. A cluster randomised control trial of a multi-component weight management programme for adults with intellectual disabilities and obesity. Br J Nutr. 2017;118(3):229–240. doi: 10.1017/S0007114517001933. Aug 14. [DOI] [PubMed] [Google Scholar]
- 26.Sorkin D.H., Mavandadi S., Rook K.S., Biegler K.A., Kilgore D., Dow E., et al. Dyadic collaboration in shared health behavior change: the effects of a randomized trial to test a lifestyle intervention for high-risk Latinas. Heal Psychol. 2014;33(6):566–575. doi: 10.1037/hea0000063. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.-Y., Oh S., Steinhubl S., Kim S., Bae W.K., Han J.S., et al. Effectiveness of 6 months of tailored text message reminders for obese male participants in a worksite weight loss program: randomized controlled trial. JMIR mHealth uHealth. 2015;3(1) doi: 10.2196/mhealth.3949. Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo B.K., Han K.A., Ahn H.J., Jung J.Y., Kim H.C., Min K.W. The effects of total energy expenditure from all levels of physical activity vs. physical activity energy expenditure from moderate-to-vigorous activity on visceral fat and insulin sensitivity in obese Type 2 diabetic women. Diabet Med. 2010;27(9):1088–1092. doi: 10.1111/j.1464-5491.2010.03045.x. (Sep) [DOI] [PubMed] [Google Scholar]
- 29.Pedersen S.D. Portion control plate for weight loss in obese patients with type 2 diabetes mellitus. Arch Intern Med. 2007;167(12):1277. doi: 10.1001/archinte.167.12.1277. Jun 25. [DOI] [PubMed] [Google Scholar]
- 30.Seif-Barghi T., Akbari-Fakhrabadi M., Teimori M.P., Tashk A., Alizadeh Z., Memari A.H. Cognitive behavior therapy’s effect in a weight loss program among obese Iranian women. Nutr Today. 2018;53(4):174–178. (Jul) [Google Scholar]
- 31.Harrigan M., Cartmel B., Loftfield E., Sanft T., Chagpar A.B., Zhou Y., et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. J Clin Oncol. 2016;34(7):669–676. doi: 10.1200/JCO.2015.61.6375. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO). Obesity and overweight [Internet]. 2022 [cited 2022 Aug 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 33.World Health Organization (WHO). Obesity Update 2017. Vol. 13, OECD Better Policies for Better Lives. 2017.
- 34.Michou M., Panagiotakos D.B., Costarelli V. Low health literacy and excess body weight: a systematic review. Cent Eur J Public Health. 2018;26(3):234–241. doi: 10.21101/cejph.a5172. Sep 30. [DOI] [PubMed] [Google Scholar]
- 35.El-Sayed A.M., Scarborough P., Galea S. Unevenly distributed: a systematic review of the health literature about socioeconomic inequalities in adult obesity in the United Kingdom. BMC Public Health. 2012;12(1):18. doi: 10.1186/1471-2458-12-18. Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens J., Pratt C., Boyington J., Nelson C., Truesdale K.P., Ward D.S., et al. Multilevel interventions targeting obesity: research recommendations for vulnerable populations. Am J Prev Med. 2017;52(1):115–124. doi: 10.1016/j.amepre.2016.09.011. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry R.F., Charles E., Purdy B., Sanford A. An analysis of research priority-setting at the World Health Organization – how mapping to a standard template allows for comparison between research priority-setting approaches. Heal Res Policy Syst. 2018;16(1):116. doi: 10.1186/s12961-018-0391-0. Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler A.R., Astbury N.M., Goddard L., Hajizadeh A., Seeber P., Crawley B., et al. Setting the top 10 priorities for obesity and weight-related research (POWeR): a stakeholder priority setting process. BMJ Open. 2022;12(7) doi: 10.1136/bmjopen-2021-058177. Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iqbal H., McEachan R.R.C., West J., Haith-Cooper M. Research priority setting in obesity: a systematic review. J Public Health (Bangk) 2021 doi: 10.1007/s10389-021-01679-8. Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassi L., Lahatte A., Rafols I., Sautier P., de Turckheim É. Improving fitness: mapping research priorities against societal needs on obesity. J Inf. 2017;11(4):1095–1113. (Nov) [Google Scholar]
- 41.Martín-Vicario L., G-P L. The role of social support in obesity online health communities: a literature review. Rev Commun Res. 2022 [Google Scholar]
- 42.Carraça E., Encantado J., Battista F., Beaulieu K., Blundell J., Busetto L., et al. Effective behavior change techniques to promote physical activity in adults with overweight or obesity: a systematic review and meta‐analysis. Obes Rev. 2021;22(S4) doi: 10.1111/obr.13258. Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glenny A.-M., O’Meara S., Melville A., Sheldon T., Wilson C. Review: The treatment and prevention of obesity: a systematic review of the literature. Int J Obes. 1997;21(9):715–737. doi: 10.1038/sj.ijo.0800495. Sep 18. [DOI] [PubMed] [Google Scholar]
- 44.Harvey-Berino J., Pintauro S., Buzzell P., DiGiulio M., Casey Gold B., Moldovan C., et al. Does using the Internet facilitate the maintenance of weight loss? Int J Obes. 2002;26(9):1254–1260. doi: 10.1038/sj.ijo.0802051. Sep 21. [DOI] [PubMed] [Google Scholar]
- 45.Heijmans M., Poortvliet R., Van der Gaag M., González-González A.I., Beltran Puerta J., Canelo-Aybar C., et al. Using a taxonomy to systematically identify and describe self-management interventions components in randomized trials for COPD. Int J Environ Res Public Health. 2022;19(19):12685. doi: 10.3390/ijerph191912685. Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson E., Roberts C., Vainik U., Jones A. The psychology of obesity: an umbrella review and evidence-based map of the psychological correlates of heavier body weight. Neurosci Biobehav Rev. 2020;119:468–480. doi: 10.1016/j.neubiorev.2020.10.009. (Dec) [DOI] [PubMed] [Google Scholar]
- 47.Lv N., Azar K.M.J., Rosas L.G., Wulfovich S., Xiao L., Ma J. Behavioral lifestyle interventions for moderate and severe obesity: a systematic review. Prev Med (Balt) 2017;100:180–193. doi: 10.1016/j.ypmed.2017.04.022. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tully A., Smyth S., Conway Y., Geddes J., Devane D., Kelly J.P., et al. Interventions for the management of obesity in people with bipolar disorder. Cochrane Database Syst Rev. 2020;2020(7) doi: 10.1002/14651858.CD013006.pub2. Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conley M.M., McFarlane C.M., Johnson D.W., Kelly J.T., Campbell K.L., MacLaughlin H.L. Interventions for weight loss in people with chronic kidney disease who are overweight or obese. Cochrane Database Syst Rev. 2021;2021(3) doi: 10.1002/14651858.CD013119.pub2. Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaikh H., Bradhurst P., Ma L.X., Tan S.Y.C., Egger S.J., Vardy J.L. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. 2020;2020(12) doi: 10.1002/14651858.CD012110.pub2. Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coulter A., Ellins J. Patient-focused interventions: a review of the evidence. London. 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materialFigure S1. Evidence Map PRISMA Flowchart
Supplementary material.
Supplementary material.
Supplementary material
Supplementary material
Supplementary material