This cohort study examines associations between cumulative exposure to various ranges of glycated hemoglobin concentrations and dementia risk among adults aged 50 years or older with type 2 diabetes.
Key Points
Question
What are the optimal glycemic targets associated with reduced dementia risk in older people with type 2 diabetes?
Findings
In this cohort study of 253 211 people aged 50 years or older with type 2 diabetes, those with a majority (>50%) of glycated hemoglobin concentrations of 9% or greater had the greatest risk of dementia.
Meaning
The results of this study support the common clinical guideline recommendations for relaxed glycemic targets in older people.
Abstract
Importance
The levels of glycemic control associated with the lowest risk of dementia in people with type 2 diabetes are unknown. This knowledge is critical to inform patient-centered glycemic target setting.
Objective
To examine the associations between cumulative exposure to various ranges of glycated hemoglobin (HbA1c) concentrations with dementia risk across sex and racial and ethnic groups and the association of current therapeutic glycemic targets with dementia risk.
Design, Setting, and Participants
This cohort study included members of the Kaiser Permanente Northern California integrated health care system with type 2 diabetes who were aged 50 years or older during the study period from January 1, 1996, to September 30, 2015. Individuals with fewer than 2 HbA1c measurements during the study period, prevalent dementia at baseline, or less than 3 years of follow-up were excluded. Data were analyzed from February 2020 to January 2023.
Exposures
Time-updated cumulative exposure to HbA1c thresholds. At each HbA1c measurement, participants were categorized based on the percentage of their HbA1c measurements that fell into the following categories: less than 6%, 6% to less than 7%, 7% to less than 8%, 8% to less than 9%, 9% to less than 10%, and 10% or more of total hemoglobin (to convert percentage of total hemoglobin to proportion of total hemoglobin, multiply by 0.01).
Main Outcomes and Measures
Dementia diagnosis was identified using International Classification of Diseases, Ninth Revision codes from inpatient and outpatient encounters. Cox proportional hazards regression models estimated the association of time-varying cumulative glycemic exposure with dementia, adjusting for age, race and ethnicity, baseline health conditions, and number of HbA1c measurements.
Results
A total of 253 211 participants were included. The mean (SD) age of participants was 61.5 (9.4) years, and 53.1% were men. The mean (SD) duration of follow-up was 5.9 (4.5) years. Participants with more than 50% of HbA1c measurements at 9% to less than 10% or 10% or more had greater risk of dementia compared with those who had 50% or less of measurements in those categories (HbA1c 9% to <10%: adjusted hazard ratio [aHR], 1.31 [95% CI, 1.15-1.51]; HbA1c≥10%: aHR, 1.74 [95% CI, 1.62-1.86]). By contrast, participants with more than 50% of HbA1c concentrations less than 6%, 6% to less than 7%, or 7% to less than 8% had lower risk of dementia (HbA1c<6%: aHR, 0.92 [95% CI, 0.88-0.97]; HbA1c 6% to <7%: aHR, 0.79 [95% CI, 0.77-0.81]; HbA1c 7% to <8%: aHR, 0.93 [95% CI, 0.89-0.97]).
Conclusions and Relevance
In this study dementia risk was greatest among adults with cumulative HbA1c concentrations of 9% or more. These results support currently recommended relaxed glycemic targets for older people with type 2 diabetes.
Introduction
Although type 2 diabetes is associated with increased dementia risk,1,2,3 it is unclear whether glycemic control mediates this risk in people in middle to later life.4,5,6,7 Observational studies have reported that hyperglycemia and duration of diabetes are associated with increased dementia risk.5,8 However, studies of interventions with aggressive glycemic targets suggest that attempting to achieve strict glycemic control may increase the risk of harm, including death, particularly in older patients.6
The harm associated with intensive glucose control has led the American Diabetes Association, American Geriatrics Society, Endocrine Society, and US Department of Veterans Affairs to recommend that glycemic targets for people in middle to later life be individualized and to consider risk of hypoglycemia, number and severity of comorbidities, functional independence, cognitive impairment, and life expectancy.9,10,11,12 Each of these organizations differs with regard to the exact therapeutic target recommended and encourages this to be developed based on a person’s individual circumstances.
To help inform patient-centered glycemic target setting, it is essential to understand the contribution of glycemic control to dementia risk. Long-term glycemic control, measured using cumulative glycemic exposure via multiple glycated hemoglobin (HbA1c) measurements over time, provides a more nuanced understanding of glycemic control than mean HbA1c concentrations by incorporating the HbA1c concentration and frequency at that concentration. This method has been previously used to evaluate the association of glycemic trajectories with diabetes complications in people with type 2 diabetes13 and dementia risk in people with type 1 diabetes.14 As the influence of risk factors for dementia may vary by sex15,16,17 and by race and ethnicity,18 it is important to understand whether the associations between glycemic control and dementia risk vary across diverse groups. We therefore aimed to examine the associations between cumulative exposure to various ranges of HbA1c with dementia risk across sex and racial and ethnic groups and explore the association of current therapeutic glycemic targets with dementia risk.
Methods
Study Population
This cohort study used data from Kaiser Permanente Northern California (KPNC), a large, integrated health care delivery system that provides comprehensive medical care to more than 4 million members representing approximately 30% of the surrounding geographic region. Members of KPNC are representative of the general population with respect to race and ethnicity and socioeconomic status except at the extreme tails of income distribution.19,20 Kaiser Permanente Northern California maintains a diabetes registry that identifies all members with diabetes using a combination of pharmacy and laboratory information, hospitalization records, and outpatient diagnoses.21 Within the diabetes registry, we restricted the sample to individuals aged 50 years or older during the study period (January 1, 1996, to September 30, 2015) and identified individuals with type 2 diabetes using the following criteria: at least 2 type 2 diabetes International Classification of Diseases, Ninth Revision (ICD-9) diagnoses or 50% or more of the individual’s diabetes-related diagnostic codes indicating type 2 diabetes. We excluded individuals with fewer than 2 HbA1c measurements during the study period, prevalent dementia at baseline, or less than 3 years of follow-up. Cohort entry was the first date between January 1, 1996, and September 30, 2015, that the patient was aged 50 years or older and had type 2 diabetes based on the aforementioned criteria. People in this dynamic cohort were followed up until 1 of the following occurred: diagnosis of dementia, KPNC membership lapse of 90 days or longer, death, or the end of the study period. This study was approved by the KPNC internal review board and was deemed exempt from requiring informed consent because it consisted of secondary data analyses and all data were deidentified. We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Outcome and Exposure
Dementia diagnoses were identified from electronic inpatient and outpatient records from January 1, 1996, to September 30, 2015, based on the following ICD-9 codes: Alzheimer disease (331.0), nonspecific dementia (290.0x, 290.1x, 290.2x, 290.3x, 294.1x, 294.2x, and 294.8), and vascular dementia (290.4x). For each patient, we extracted every HbA1c value measured during follow-up from the KPNC laboratory database. Beginning with their first available HbA1c measurement, each patient was categorized based on the percentage of their HbA1c measurements that fell into the following categories: less than 6% (42 mmol/mol), 6% to less than 7% (42 to <53 mmol/mol), 7% to less than 8% (53 to <64 mmol/mol), 8% to less than 9% (64 to <75 mmol/mol), 9% to less than 10% (75 to <86 mmol/mol), and 10% or more (86 mmol/mol) (to convert percentage of total hemoglobin to proportion of total hemoglobin, multiply by 0.01). Each time a new value was captured, we recalculated the individual’s cumulative glycemic exposure. We operationalized the amount of time exposed to the thresholds in 2 ways: (1) a binary indicator of whether more than 50% of an individual’s HbA1c measures fell within the aforementioned HbA1c categories and (2) a categorical variable representing the percentage of HbA1c measurements (<10% [reference group], 10 to <25%, 25 to <75%, or ≥75%) within the aforementioned HbA1c categories. Additionally, we examined the percentage of HbA1c measurements within current guidelines for glycemic targets in older patients with diabetes.9,10,11,12
Covariates
Demographic characteristics such as age, sex, race, and ethnicity were recorded at cohort entry. Race and ethnicity categories, ascertained by self-report via the electronic medical record, were Asian, Black, Hispanic, White, and other (not broken down further due to small proportions). We obtained the following baseline health condition diagnoses from electronic health records: peripheral artery disease, nephropathy, retinopathy, neuropathy, stroke, myocardial infarction, and severe hypoglycemic and hyperglycemic events resulting in an emergency department visit or hospitalization (ICD-9 and ICD-10 codes are given in eTable 1 in Supplement 1). At each HbA1c measurement, we also calculated the total number of HbA1c measurements and the rate of HbA1c measurements for each patient, defined as the total number of HbA1c measurements divided by the total follow-up time.
Statistical Analysis
Data were analyzed from February 2020 to January 2023. We examined the distribution of patient demographics and comorbidities at baseline in the overall sample and by dementia status, sex, and race and ethnicity. We first estimated age-adjusted dementia incidence rates by category of cumulative glycemic exposure. We then specified separate time-updated Cox proportional hazards regression models (with age as the time scale) to estimate the association between each category of cumulative glycemic exposure and risk of dementia. We repeated these models stratified by sex and by race and ethnicity. We started the follow-up for any given time-updated exposure 3 years after the end of that exposure period.
Our primary analysis included 2 sets of models that examined each of the following HbA1c thresholds separately, looking first at the majority of HbA1c measurements within a range and then at the percentage of measurements in those ranges: HbA1c less than 6%, 6% to less than 7%, 7% to less than 8%, 8% to less than 9%, 9% to less than 10%, or 10% or more. Each set included 3 models. The first was adjusted for age (as a time scale), sex, and race and ethnicity. The second was adjusted for baseline health conditions, including peripheral artery disease, nephropathy, neuropathy, retinopathy, stroke, myocardial infarction, and prior severe hypoglycemic and hyperglycemic hospitalization events. The third was additionally adjusted for rate of HbA1c measurements. Potential effect modification by sex or race and ethnicity was assessed using interaction terms between the exposure of interest and the possible modifier as well as through subgroup analyses.
We performed 3 additional sensitivity analyses. First, in individuals with at least 75% of HbA1c measurements within a given category, we compared dementia hazard in each category with dementia hazard in the lowest HbA1c category (<6%). Second, we repeated our sex-stratified analysis comparing dementia hazard in those with a majority of HbA1c measurements in each category with hazard in those with a minority of HbA1c measurements in each category, limited to those with at least 10 HbA1c measurements. Finally, across all HbA1c categories, we compared dementia hazard in those with 75% or more of measurements within the same glycemic category with hazard in those with less stable HbA1c measurements (<75% of measurements in the same category).
We also conducted a multivariable density analysis to examine the risk of dementia associated with changes in the proportion of HbA1c measurements within commonly used diabetes guidelines for older people (ie, 6% to <8%), holding constant the number of HbA1c measurements. The coefficients of this model enabled us to calculate the relative risk associated with the percentage of measurements outside the recommended guidelines to within the guidelines (eg, move 10% of HbA1c measurements from ≥9% to 6% to <8%), holding the number of measures within other ranges (<6%, 8% to <9%, and ≥9%) and the total number of HbA1c measurements constant. Estimates were obtained from Cox proportional hazards regression models with less than 6%, 8% to less than 9%, and 9% or greater concurrently in the model adjusting for age (as the time scale), demographics, and baseline health conditions. We used SAS, version 9.4 (SAS Institute Inc), for all analyses. Two-sided P < .05 was considered significant.
Results
A total of 409 108 individuals with type 2 diabetes were identified, of whom 155 897 (38.1%) met exclusion criteria. Overall, there were 253 211 people included, with a total of more than 4.6 million HbA1c measurements. The mean (SD) age at study entry was 61.5 (9.4) years; 53.1% of participants were men, and 46.9% were women. A total of 17.5% were Asian, 9.9% were Black, 14.6% were Hispanic, 50.6% were White, and 5.9% were other race and ethnicity (Table 1). The mean (SD) number of HbA1c values available for each participant was 11.6 (10.0). The mean (SD) duration of follow up was 5.9 (4.5) years. The sample characteristics stratified by race and ethnicity are presented in eTable 2 in Supplement 1.
Table 1. Baseline Characteristics of Analytic Cohort by Sex.
| Variables | Individualsa | ||
|---|---|---|---|
| Overall | Men | Women | |
| Patients | 253 211 (100.0) | 134 385 (53.1) | 118 826 (46.9) |
| Age at study entry, mean (SD) [range], y | 61.5 (9.4) [50.0-100.9] | 61.2 (9.1) [50.0-97.9] | 61.9 (9.6) [50.0-100.9] |
| Race and ethnicity | |||
| Asian | 44 390 (17.5) | 23 229 (17.3) | 21 161 (17.8) |
| Black | 25 163 (9.9) | 11 784 (8.8) | 13 379 (11.3) |
| Hispanic | 37 002 (14.6) | 19 306 (14.4) | 17 696 (14.9) |
| White | 128 099 (50.6) | 70 597 (52.5) | 57 502 (48.8) |
| Otherb | 14 857 (5.9) | 7251 (5.4) | 7606 (6.4) |
| Missing | 3700 (1.5) | 2218 (1.7) | 1482 (1.2) |
| Diabetes complications | |||
| Severe hypoglycemic event | 930 (0.4) | 444 (0.3) | 486 (0.4) |
| Severe hyperglycemic event | 3886 (1.5) | 2154 (1.6) | 1732 (1.5) |
| Myocardial infarction | 4430 (1.7) | 2999 (2.2) | 1431 (1.2) |
| Peripheral artery disease | 7009 (2.8) | 4318 (3.2) | 2691 (2.3) |
| Stroke | 7296 (2.9) | 3789 (2.8) | 3507 (3.0) |
| Neuropathy | 13 957 (5.5) | 5926 (4.4) | 8031 (6.8) |
| Nephropathy | 21 580 (8.5) | 11 780 (8.8) | 9800 (8.2) |
| Retinopathy | 31 212 (12.3) | 16 465 (12.3) | 14 747 (12.4) |
| Reason for end of follow-up | |||
| Death | 39 266 (15.5) | 22 548 (16.8) | 16 718 (14.1) |
| Dementia | 21 139 (8.3) | 9910 (7.4) | 11 229 (9.4) |
| Membership dropout | 53 881 (21.3) | 29 329 (21.8) | 24 552 (20.7) |
| Administrative censoring | 138 925 (54.9) | 72 598 (54.0) | 66 327 (55.8) |
| Age at dementia diagnosis, mean (SD) [range], y | 79.8 (8.0) [53.2-109.0] | 79.2 (7.9) [53.2-109.0] | 80.3 (8.0) [53.3-106.8] |
| HbA1c measurements over follow-up period, mean (SD) [range], No. | 11.6 (10.0) [1-126] | 11.4 (10.0) [1-126] | 11.7 (10.1) [1-122] |
Abbreviation: HbA1c, glycated hemoglobin.
Data are presented as number (percentage) of individuals unless otherwise indicated.
Other race and ethnicity was not broken down further due to small proportions.
The age-adjusted incidence of dementia by HbA1c category is presented in eTable 3 in Supplement 1. The lowest incidence of dementia was seen in individuals with 75% or more of HbA1c measurements in the category of 6% to less than 7% (42 to <53 mmol/mol) (10.24 per 1000 person-years). The greatest incidence of dementia was in those with 75% or more of HbA1c measurements in the range of 10% or greater (86 mmol/mol) (19.32 per 1000 person-years).
Majority Time at Each Threshold and Dementia Hazard
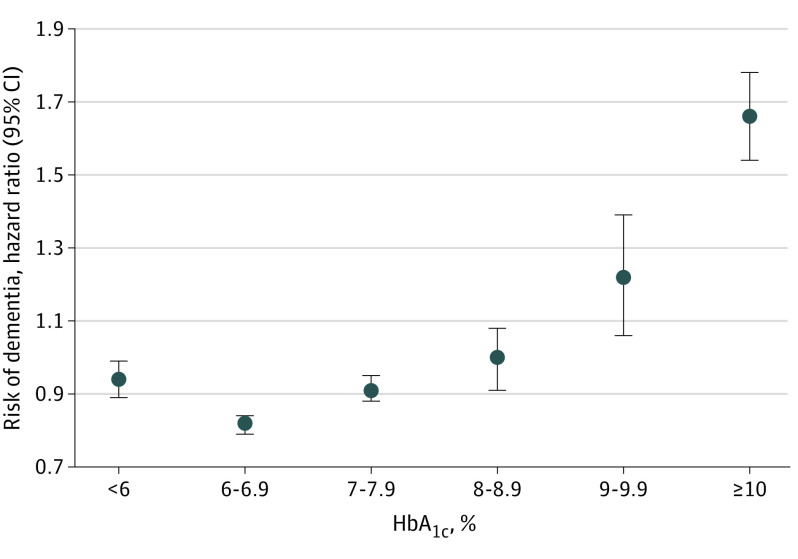
Adjusting for age, sex, and race and ethnicity, majority exposure (ie, >50% of HbA1c measurements) to HbA1c concentrations less than 6%, 6% to less than 7%, or 7% to less than 8% was associated with decreased hazard of dementia compared with less than 50% of measurements in each respective range (HbA1c<6%: adjusted hazard ratio [aHR], 0.92 [95% CI, 0.88-0.97]; HbA1c 6% to <7%: aHR, 0.79 [95% CI, 0.77-0.81]; and HbA1c 7% to <8%: aHR, 0.93 [95% CI, 0.89-0.97]) (eTable 4 in Supplement 1). Majority exposure to HbA1c concentrations of 8% to less than 9% was not significantly associated with hazard of dementia (aHR, 1.07; 95% CI, 0.98-1.16). Majority exposure to HbA1c concentrations of 9% to less than 10% was associated with an increased hazard of dementia (aHR, 1.31; 95% CI, 1.15-1.51). The greatest hazard of dementia was observed in those with majority HbA1c measurements of 10% or more (aHR, 1.74; 95% CI, 1.62-1.86) compared with those with less than 50% of measurements in that range. Point estimates were similar in models further adjusting for baseline dysglycemic events, microvascular and macrovascular disease, and number of HbA1c measurements (Figure 1). Models including an interaction term between the exposure category of interest and sex were statistically significant for HbA1c thresholds less than 8% (eTable 4 in Supplement 1). In subgroup analyses, having a majority of HbA1c values less than 6% was associated with a lower hazard of dementia than having less than 50% of HbA1c values in that range in men (aHR, 0.88; 95% CI, 0.81-0.95) (Table 2), but there was no association in women (aHR, 1.01; 95% CI, 0.94-1.09) (P = .002 for sex × HbA1c<6%). In men and women, compared with those with less than 50% of HbA1c measurements between 6% and less than 7%, those with majority of HbA1c concentrations in this range had the lowest hazard of dementia (men: aHR, 0.80 [95% CI, 0.77-0.84]; women: aHR, 0.83 [95% CI, 0.80-0.87]; P = .007 for sex × HbA1c 6% to <7%). Having a majority of HbA1c measurements in the range of 7% to less than 8% was associated with a lower hazard of dementia than was having less than 50% of HbA1c measures in this range in women (aHR, 0.86; 95% CI, 0.81-0.91), but there was no association in men (aHR, 0.98; 95% CI, 0.93-1.04) (P = .002 for sex × HbA1c 7% to <8%). We did not find a statistically significant association between majority HbA1c measurements in the range of 8% to less than 9% and dementia hazard in either men or women compared with less than 50% of HbA1c measurements in that range. Men and women with majority HbA1c concentrations of 10% or more had an elevated hazard of dementia compared with those with less than 50% of measurements within that range (men: aHR, 1.70 [95% CI, 1.54-1.88]; women: aHR, 1.61 [95% CI, 1.46-1.78]; P = .57 for sex × HbA1c ≥10%). When we stratified by racial and ethnic groups, we found broadly similar patterns to those described in the overall sample (Figure 2 and eTable 5 in Supplement 1).
Figure 1. Dementia Hazard by Majority Glycated Hemoglobin (HbA1c) Exposure.
Cox proportional hazards regression adjusted for age (as time scale), sex, race and ethnicity, and baseline hyperglycemic and hypoglycemic events, myocardial infarction, peripheral artery disease, neuropathy, nephropathy, stroke, and retinopathy. Whiskers indicate 95% CIs. To convert percentage of total hemoglobin to proportion of total hemoglobin, multiply by 0.01.
Table 2. Dementia Hazard by Majority HbA1c Concentration Exposure Stratified by Sexa.
| HbA1c, % of total hemoglobin | Men | Women | ||||
|---|---|---|---|---|---|---|
| Events | Person-years | Adjusted HR (95% CI) | Events | Person-years | Adjusted HR (95% CI) | |
| <6 | 750 | 59 070 | 0.88 (0.81-0.95) | 748 | 40 511 | 1.01 (0.94-1.09) |
| 6 to <7 | 2958 | 219 183 | 0.80 (0.77-0.84) | 3716 | 215 574 | 0.83 (0.80-0.87) |
| 7 to <8 | 1241 | 96 017 | 0.98 (0.93-1.04) | 1428 | 99 768 | 0.86 (0.81-0.91) |
| 8 to <9 | 244 | 28 310 | 0.93 (0.82-1.06) | 304 | 24 900 | 1.06 (0.94-1.19) |
| 9 to <10 | 110 | 13 004 | 1.35 (1.12-1.63) | 100 | 10 514 | 1.10 (0.90-1.34) |
| ≥10 | 416 | 45 561 | 1.70 (1.54-1.88) | 429 | 41 036 | 1.61 (1.46-1.78) |
Abbreviations: HbA1c, glycated hemoglobin; HR, hazard ratio.
SI conversion factor: To convert percentage of total hemoglobin to proportion of total hemoglobin, multiply by 0.01.
Cox proportional hazards regression adjusted for age (as time scale), sex, race and ethnicity, baseline hyperglycemic and hypoglycemic events, myocardial infarction, peripheral artery disease, neuropathy, nephropathy, stroke, and retinopathy.
Figure 2. Dementia Hazard by Majority Glycated Hemoglobin (HbA1c) Exposure by Race and Ethnicity and Sex.
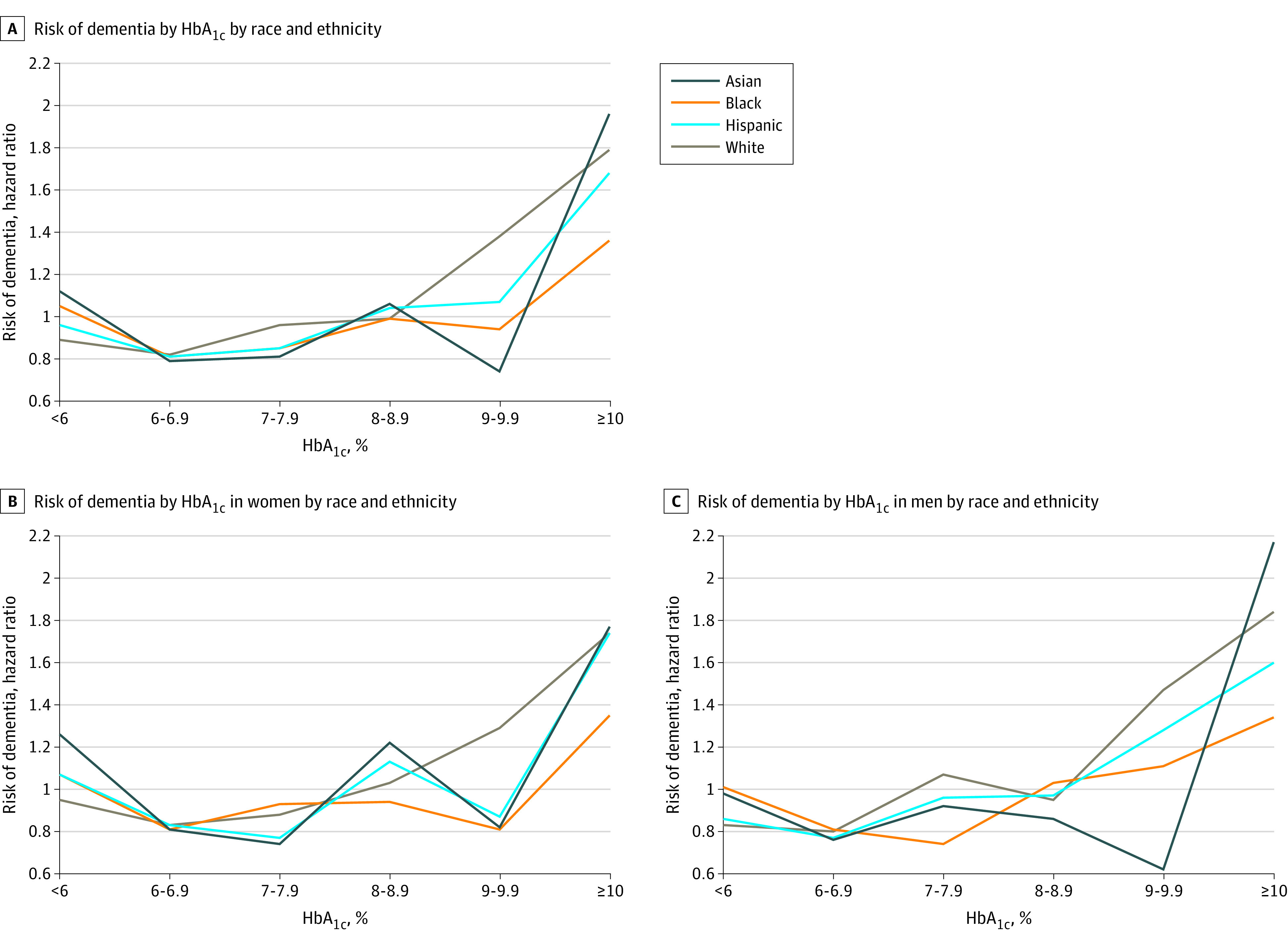
Cox proportional hazards regression adjusted for age (as time scale), baseline hyperglycemic and hypoglycemic events, myocardial infarction, peripheral artery disease, neuropathy, nephropathy, stroke, and retinopathy. Dementia hazard for race and ethnicity × HbA1c interactions: less than 6%, P = .07; 6% to less than 7%, P = .74; 7% to less than 8%, P = .33; 8% to less than 9%, P = .06; 9% to less than 10%, P = .27; 10% or greater, P = .03. To convert percentage of total hemoglobin to proportion of total hemoglobin, multiply by 0.01.
Percentage of Time Within Each Glycemic Range and Dementia Hazard
A greater proportion of measurements within each HbA1c bracket below 8% was associated with lower dementia hazard (Table 3). Those with 75% or more of time spent in the HbA1c bracket of 6% to less than 7% had the lowest hazard of dementia (aHR, 0.69; 95% CI, 0.66-0.72). Conversely, a greater proportion of time spent within each bracket above 8% was associated with a greater dementia hazard. Those with 75% or more of time spent in the HbA1c bracket of 10% or more had the highest hazard of dementia (aHR, 1.76; 95% CI, 1.60-1.93). In sensitivity analyses, across all HbA1c ranges, having stable HbA1c (≥75% of all measurements within a particular range) was associated with a lower hazard of dementia than was having HbA1c measures that were less consistent (<75% in a particular range) (aHR, 0.86; 95% CI, 0.83-0.89).
Table 3. Cumulative Glycemic Exposure and Risk of Dementia.
| Adjusted HR (95% CI) | |||
|---|---|---|---|
| Age, race and ethnicity, and sex | Age, race and ethnicity, sex, and baseline health conditionsa | Age, race and ethnicity, sex, baseline health conditions, and number of HbA1c measurementsa | |
| HbA1c measurements <6%, % | |||
| <10 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 10 to <25 | 1.04 (1.00-1.09) | 1.06 (1.02-1.11) | 1.07 (1.02-1.11) |
| 25 to <75 | 0.95 (0.91-0.99) | 0.97 (0.93-1.01) | 0.97 (0.93-1.01) |
| ≥75 | 0.91 (0.85-0.97) | 0.93 (0.87-0.99) | 0.93 (0.87-0.99) |
| HbA1c measurements 6% to <7%, % | |||
| <10 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 10 to <25 | 0.94 (0.90-0.99) | 0.97 (0.92-1.01) | 0.97 (0.92-1.01) |
| 25 to <75 | 0.82 (0.80-0.85) | 0.86 (0.83-0.89) | 0.86 (0.83-0.89) |
| ≥75 | 0.69 (0.66-0.72) | 0.73 (0.70-0.76) | 0.73 (0.70-0.76) |
| HbA1c measurements 7% to <8%, % | |||
| <10 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 10 to <25 | 1.10 (1.06-1.15) | 1.10 (1.05-1.14) | 1.10 (1.05-1.14) |
| 25 to <75 | 1.05 (1.02-1.08) | 1.04 (1.01-1.07) | 1.04 (1.01-1.07) |
| ≥75 | 0.93 (0.87-1.00) | 0.91 (0.85-0.98) | 0.91 (0.85-0.98) |
| HbA1c measurements 8% to <9%, % | |||
| <10 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 10 to <25 | 1.18 (1.14-1.22) | 1.16 (1.12-1.20) | 1.16 (1.12-1.20) |
| 25 to <75 | 1.26 (1.21-1.31) | 1.20 (1.16-1.25) | 1.20 (1.16-1.25) |
| ≥75 | 1.12 (0.98-1.27) | 1.04 (0.91-1.19) | 1.04 (0.91-1.19) |
| HbA1c measurements 9% to <10%, % | |||
| <10 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 10 to <25 | 1.32 (1.27-1.37) | 1.28 (1.23-1.33) | 1.28 (1.23-1.33) |
| 25 to <75 | 1.37 (1.30-1.44) | 1.30 (1.24-1.37) | 1.30 (1.23-1.37) |
| ≥75 | 1.43 (1.20-1.70) | 1.33 (1.11-1.58) | 1.32 (1.11-1.58) |
| HbA1c measurements ≥10%, % | |||
| <10 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 10 to <25 | 1.26 (1.20-1.31) | 1.23 (1.18-1.29) | 1.23 (1.18-1.29) |
| 25 to <75 | 1.56 (1.49-1.63) | 1.51 (1.44-1.58) | 1.51 (1.44-1.58) |
| ≥75 | 1.76 (1.60-1.93) | 1.68 (1.53-1.84) | 1.68 (1.53-1.84) |
Abbreviations: HbA1c, glycated hemoglobin; HR, hazard ratio.
SI conversion factor: To convert percentage of total hemoglobin to proportion of total hemoglobin, multiply by 0.01.
Each of the following baseline health conditions was adjusted for in the model: history of hyperglycemic and hypoglycemic events, myocardial infarction, peripheral artery disease, neuropathy, nephropathy, stroke, and retinopathy.
Sensitivity Analyses
Restricting the sample to only individuals with more than 75% of measurements in 1 particular HbA1c category resulted in findings similar to those of the main analysis. The dementia hazard among those with at least 75% of HbA1c measurements 6% to less than 7% (aHR, 0.93; 95% CI, 0.85-1.01), 7% to less than 8% (aHR, 0.97; 95% CI, 0.87-1.10), or 8% to less than 9% (aHR, 1.08; 95% CI, 0.92-1.28) were not statistically significantly different from those with at least 75% of HbA1c measurements in the category of less than 6%. Compared with the category of less than 6%, the hazard of dementia in the 9% to less than 10% and 10% or greater groups was 1.44 (95% CI, 1.18-1.75) and 1.68 (95% CI, 1.47-1.92), respectively. These associations remained following adjustment for comorbidities and number of HbA1c measurements. Repeating these analyses in men and women with at least 10 HbA1c measurements resulted in similar point estimates of dementia hazard as reported but with widening of the 95% CIs (eTable 6 in Supplement 1).
Guideline Targets and Dementia Hazard
Greater time spent within the glycemic targets proposed by most geriatrics guidelines for healthier older people (6%-7.9%)9 was associated with reduced dementia hazard (eTable 7 in Supplement 1). For every additional 10 percentage points of HbA1c measurements from the less than 6% range replaced by an equivalent number of measurements in the recommended 6% to less than 8% range, the hazard of dementia decreased by 1% (aHR, 0.99; 95% CI, 0.98-1.00). For every additional 10 percentage points spent in the 6% to less than 8% range instead of the 8% to less than 9% range, the hazard of dementia decreased by 3% (aHR, 0.97; 95% CI, 0.96-0.98). For every additional 10 percentage points spent in the 6% to less than 8% range instead of the more than 9% range, the hazard of dementia decreased by 5% (aHR, 0.95; 95% CI, 0.93-0.97).
Discussion
In this large sample of older people with type 2 diabetes, we found that greater cumulative exposure to HbA1c concentrations in the range of 6% to less than 7% and 7% to less than 8% was associated with lower hazard of dementia. Importantly, we observed no significant change in hazard for individuals with HbA1c concentrations in the range of relaxed glycemic control recommended by the American Geriatrics Society and US Department of Veterans Affairs for older patients with multiple comorbidities, poor health, or limited life expectancy.10,12 Although there were subtle sex and race and ethnicity differences in the dementia hazard associated with cumulative exposure to increasing HbA1c concentrations, greater time spent in commonly recommended glycemic targets was associated with lower dementia hazard across all sexes and racial and ethnic groups.
These results are supported by and add to the results of a recent study5 of 457 902 predominantly White middle-aged people with type 2 diabetes that reported that greater mean HbA1c concentration over an average of 6 years of follow-up was associated with greater dementia risk. Our study builds on this work by considering cumulative exposure to various HbA1c thresholds. This methodologic approach, previously used in people with type 1 diabetes,14 does not assume that HbA1c concentrations are constant the way that an overall mean HbA1c measurement does and recognizes that wide variations above and below a mean differs from time spent near the mean. Greater HbA1c variability has been associated with various other health outcomes (eg, all-cause mortality, cardiovascular disease, kidney disease, and peripheral neuropathy), with many of these associations independent of glycemic control.22 Although not a focus of this study, our finding that dementia hazard was lower in those who appeared to have greater consistency of HbA1c concentrations in normoglycemic ranges further supports examination of the contribution of glycemic variability to dementia risk.
Strengths and Limitations
Our study has a number of strengths. We used a high-quality electronic health record with a low turnover rate, enabling us to capture multiple longitudinal HbA1c measurements, diabetes comorbidities, and incident dementia. We reduced the risk of underlying changes in cognition driving changes in glycemic control by starting follow-up for any given time-updated exposure 3 years after the end of that exposure period. However, this approach prevented us from examining equally important but different clinical questions, namely that of the potential association of dementia with glycemic control immediately prior to and following the diagnosis of dementia. The coexistence of both diabetes and dementia presents its own challenges4 and needs further research but was beyond the scope of this study.
This study also has limitations. We did not have information related to age at type 2 diabetes onset or duration of diabetes that occurred before individuals became KPNC members and/or before age 50 years. Similarly, we did not have information about HbA1c measurements earlier in life, which would result in an underestimate of the association of cumulative exposure to HbA1c thresholds with dementia hazard if we assume earlier glycemic control was associated with dementia risk. It is also possible that variations in the frequency of HbA1c measurements could introduce bias. For example, HbA1c monitoring standards improved over the period we examined, and it is likely that physicians measured HbA1c more frequently in people for whom there were clinical concerns. We attempted to minimize the risk of bias introduced by the number of HbA1c measurements available by including this as a covariable in our modeling as well as in sensitivity analyses restricted to individuals with at least 10 measurements. It is also unclear whether our results can be generalized to individuals younger than 50 years or those outside northern California. Data regarding other important factors, such as smoking and obesity, were also not available, which limited our ability to take these other factors into account. Related to this, we did not have neuroimaging or neuropathological data and were therefore unable to examine associations with specific dementia subtypes. Underdiagnosis of dementia was possible in the sample using health record data. However, the implications of this for our results are unclear. Finally, our operationalization of glycemic control also has limitations. Although we attempted to examine the association of HbA1c and consistency over time with dementia risk, we suggest caution if intending to directly apply our results to clinical practice. Mean glycemic control and variation in glycemic control are different constructs, and it is unclear whether they are complementary or if one should be given priority over the other at the individual patient level. For example, weighing the risks of attempting to lower the mean HbA1c concentration if it contributes to greater glycemic variability remains in the realm of an informed conversation between clinician and patient.
Conclusions
In this cohort study of a large sample of older people with type 2 diabetes, we found that increased exposure to HbA1c concentrations greater than or equal to 9% was associated with the greatest hazard of dementia. Additional work is needed to examine whether the observational associations we report are causal and are seen in other groups.
eTable 1. Diagnostic Codes Used to Identify Microvascular and Macrovascular Complications
eTable 2. Baseline Characteristics of Analytic Cohort by Race and Ethnicity
eTable 3. Person, Events, and Person-years of Available Data by HbA1c
eTable 4. Dementia Risk by Majority HbA1c Exposure and Possible Interaction by Sex
eTable 5. Dementia Risk by Majority HbA1c Exposure by Ethnicity and Sex
eTable 6. Dementia Hazard by Majority HbA1c Exposure Stratified by Sex in Those With at Least 10 HbA1c Measures
eTable 7. Hazard of Dementia for Each HbA1c Category Using Multivariable Density Analyses
Data Sharing Statement
References
- 1.Peila R, Rodriguez BL, Launer LJ; Honolulu-Asia Aging Study . Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51(4):1256-1262. doi: 10.2337/diabetes.51.4.1256 [DOI] [PubMed] [Google Scholar]
- 2.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64-74. doi: 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 4.Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction—towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8(6):535-545. doi: 10.1016/S2213-8587(20)30118-2 [DOI] [PubMed] [Google Scholar]
- 5.Zheng B, Su B, Price G, Tzoulaki I, Ahmadi-Abhari S, Middleton L. Glycemic control, diabetic complications, and risk of dementia in patients with diabetes: results from a large UK cohort study. Diabetes Care. 2021;44(7):1556-1563. doi: 10.2337/dc20-2850 [DOI] [PubMed] [Google Scholar]
- 6.Launer LJ, Miller ME, Williamson JD, et al. ; ACCORD MIND investigators . Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10(11):969-977. doi: 10.1016/S1474-4422(11)70188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray AM, Hsu FC, Williamson JD, et al. ; Action to Control Cardiovascular Risk in Diabetes Follow-On Memory in Diabetes (ACCORDION MIND) Investigators . ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia. 2017;60(1):69-80. doi: 10.1007/s00125-016-4118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WJ, Lee SJ, Lee E, Lee EY, Han K. Risk of incident dementia according to glycemic status and comorbidities of hyperglycemia: a nationwide population-based cohort study. Diabetes Care. 2022;45(1):134-141. doi: 10.2337/dc21-0957 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association . 12: older adults: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S168-S179. doi: 10.2337/dc21-S012 [DOI] [PubMed] [Google Scholar]
- 10.Moreno G, Mangione CM, Kimbro L, Vaisberg E; American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus . Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013;61(11):2020-2026. doi: 10.1111/jgs.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1520-1574. doi: 10.1210/jc.2019-00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Defense . VA/DoD Clinical Practice Guidelines: Management of Diabetes Mellitus in Primary Care. US Department of Veterans Affairs; 2017. [Google Scholar]
- 13.Laiteerapong N, Karter AJ, Moffet HH, et al. Ten-year hemoglobin A1c trajectories and outcomes in type 2 diabetes mellitus: the Diabetes & Aging Study. J Diabetes Complications. 2017;31(1):94-100. doi: 10.1016/j.jdiacomp.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacy ME, Gilsanz P, Karter AJ, Quesenberry CP, Pletcher MJ, Whitmer RA. Long-term glycemic control and dementia risk in type 1 diabetes. Diabetes Care. 2018;41(11):2339-2345. doi: 10.2337/dc18-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran C, Gilsanz P, Beeri MS, Whitmer RA, Lacy ME. Sex, diabetes status and cognition: findings from the study of longevity in diabetes. BMJ Open Diabetes Res Care. 2021;9(1):9. doi: 10.1136/bmjdrc-2020-001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 2018;14(9):1171-1183. doi: 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300-307. doi: 10.2337/dc15-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin W, Li W, Wang Q, et al. Race-related association between APOE genotype and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2021;83(2):897-906. doi: 10.3233/JAD-210549 [DOI] [PubMed] [Google Scholar]
- 19.Gordon NP, Kaplan GA. Some evidence refuting the HMO “favorable selection” hypothesis: the case of Kaiser Permanente. Adv Health Econ Health Serv Res. 1991;12:19-39. [PubMed] [Google Scholar]
- 20.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703-710. doi: 10.2105/AJPH.82.5.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: the Diabetes Study of Northern California (DISTANCE). Diabetes Care. 2013;36(3):574-579. doi: 10.2337/dc12-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Yi Q, Wang Y, et al. Long-term glycemic variability and risk of adverse health outcomes in patients with diabetes: a systematic review and meta-analysis of cohort studies. Diabetes Res Clin Pract. 2022;192:110085. doi: 10.1016/j.diabres.2022.110085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnostic Codes Used to Identify Microvascular and Macrovascular Complications
eTable 2. Baseline Characteristics of Analytic Cohort by Race and Ethnicity
eTable 3. Person, Events, and Person-years of Available Data by HbA1c
eTable 4. Dementia Risk by Majority HbA1c Exposure and Possible Interaction by Sex
eTable 5. Dementia Risk by Majority HbA1c Exposure by Ethnicity and Sex
eTable 6. Dementia Hazard by Majority HbA1c Exposure Stratified by Sex in Those With at Least 10 HbA1c Measures
eTable 7. Hazard of Dementia for Each HbA1c Category Using Multivariable Density Analyses
Data Sharing Statement