ABSTRACT
In 1985 to 1986, the CARDIA (Coronary Artery Risk Development in Young Adults) study enrolled 5115 Black or White participants, including 2788 women, aged 18 to 30 years. Over the following 35 years, the CARDIA study amassed extensive longitudinal data on women's reproductive milestones, spanning menarche to menopause. Although not initially conceived as a study of women's health, >75 CARDIA study publications address relationships between reproductive factors and events with cardiovascular and metabolic risk factors, subclinical and clinical cardiovascular disease, and social determinants of health. The CARDIA study was one of the earliest population‐based reports to note Black‐White differences in age at menarche and associations with cardiovascular risk factors. Adverse pregnancy outcomes, particularly gestational diabetes and preterm birth, have been assessed along with postpartum behaviors, such as lactation. Existing studies have examined risk factors for adverse pregnancy outcomes and lactation, as well as their relationship to future cardiovascular and metabolic risk factors, diagnoses, and subclinical atherosclerosis. Ancillary studies examining components of polycystic ovary syndrome and ovarian biomarkers, such as anti‐Müllerian hormone, have facilitated examination of reproductive health in a population‐based cohort of young adult women. As the cohort transitioned through menopause, examination of the importance of premenopausal cardiovascular risk factors along with menopause has improved our understanding of shared mechanisms. The cohort is now aged in the 50s to mid‐60s, and women will begin to experience a greater number of cardiovascular events as well as other conditions, such as cognitive impairment. Thus, in the next decade, the CARDIA study will provide a unique resource for understanding how the women's reproductive life course epidemiology informs cardiovascular risk, as well as reproductive and chronological aging.
Keywords: fertility, lactation, menarche, menopause, polycystic ovary syndrome, pregnancy
Subject Categories: Pregnancy, Women, Race and Ethnicity, Epidemiology
Nonstandard Abbreviations and Acronyms
- APO
adverse pregnancy outcome
- ARIC
Atherosclerosis Risk in Communities Study
- CARDIA
Coronary Artery Risk Development in Young Adults
- CWS
CARDIA Women's Study
- GDM
gestational diabetes
- HDP
hypertensive disorder of pregnancy
- MESA
Multi‐Ethnic Study of Atherosclerosis
- NAFLD
nonalcoholic fatty liver disease
- OCP
oral contraceptive pill
- PCOS
polycystic ovary syndrome
- VMS
vasomotor symptoms
- WISE
Women's Ischemia Syndrome Evaluation
Examination of the impact of women's reproductive health on cardiovascular disease (CVD) risk began in the 1950s. One of the earliest reports was in Circulation in 1953. 1 In that issue, John H. Wuest Jr and colleagues reported that women who had undergone bilateral oophorectomy had significantly greater coronary atherosclerosis than control women, although less than control men. 1 In 1958, Warren Winkelstein Jr and colleagues noted that the male/female ratio in death rates changed at approximately the age of 45 years and could potentially be attributable to shifts in androgens and estrogens. 2 The authors stated that “We thought that a first approximation to such endocrine differences could be obtained by a study of menstrual and pregnancy patterns of patients with coronary artery disease. The preliminary results appeared sufficiently interesting to warrant reporting.” 2 The subsequent text comments on the significant associations between surgical menopause and miscarriages with myocardial infarction. It was not until the 1980s that the more extensive epidemiologic reports from the NHS (Nurses' Health Study) were published. These reports were notable for their longitudinal examination of associations between contraception, parity, and menopause with future CVD. 3 , 4 Epidemiologic studies, including the CARDIA (Coronary Artery Risk Development in Young Adults) study, were initiated to observe factors associated with CVD risk beginning in early adulthood.
The CARDIA study began enrolling participants aged 18 to 30 years in 1985 to 1986, 5 , 6 approximately the time that these epidemiologic studies were published. Original aims of the CARDIA study were to examine the distribution and determinants of CVD risk factors in Black and White young men and women and to identify associated risk factors, particularly lifestyle habits and behaviors, and eventually psychosocial factors and social determinants of health. Participants were recruited from 4 centers, located in Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA, to achieve a representative sample of Black and White individuals in the United States. At each field center, participants were recruited to achieve balance by sex, self‐reported race (Black and White), socioeconomic status (ie, more than high school education compared with high school or less), and age (<25 versus >25 years).
At baseline, Black women and White women had different mean levels of education (13.1 versus 14.5 years, respectively), marital status (21% versus 29%, respectively), full‐time employment (46% versus 62%, respectively), residence in impoverished neighborhoods (13% versus 10%, respectively), and difficulty paying for basics (39% versus 32%, respectively). 7 Follow‐up examinations occurred every 2 to 3 years during the first decade of the study and approximately every 5 years thereafter, with telephone contact with participants occurring every 6 months. As of September 2022, death rates per 1000 since 1985 were 109.0 in Black women versus 68.2 in White women. Retention of the survivors has been high, with >71% of surviving participants attending the year 30 examination. 5 , 6 Mortality in Black women exceeded that in White men and White women. At the year 30 examination, Black women (n=957) and White women (n=957) had different mean levels of education (14.5 versus 16.0 years, respectively); 29% of Black women and 14% of White women had less than a high school education.
Along with standard anthropometric measures, blood pressure assessments, and laboratory measures, the CARDIA study has collected medical and reproductive history, extensive nutritional information, physical fitness assessments, body composition imaging, ectopic fat imaging, medications, and CVD events. Participants have also undergone repeated imaging for assessment of vascular calcification, carotid intima‐media thickness, echocardiography, and brain structure and function. Ancillary studies have collected additional information on nontraditional risk factors, including oxidative stress and inflammation, and characterization of the genome, epigenome, transcriptome, proteome, and metabolome.
The recruitment of women in their reproductive years has provided a unique opportunity to assess longitudinally common exposures and characteristics in Black and White women with a range of social determinants of health using the core and ancillary measures of cardiovascular risk and a biorepository of stored samples. The repeated measures provided an opportunity to examine trajectories in risk factors from before conception and after pregnancy and lactation. Beginning in year 2, the CARDIA study has repeatedly assessed estrogen use through detailed inquiries about oral contraceptive pill (OCP) use and other hormonal and nonhormonal reproductive measures. The CARDIA study has also collected detailed survey information about fertility and pregnancy characteristics and outcomes, particularly adverse pregnancy outcomes (APOs), such as preterm delivery 8 and gestational diabetes (GDM), 9 as well as lactation duration for sequential pregnancies. One ancillary study focused on polycystic ovary syndrome (PCOS), including symptoms, biochemical measures of androgens, 10 and radiographic imaging of the ovaries and uterus. 11 Another ancillary study of parous women measured adipokines and inflammatory and endothelial function markers in stored blood from multiple examinations beginning with the year 2 examination. 12 As the cohort transitioned through menopause, the CARDIA study assessed menstrual history and vasomotor symptoms (VMS), 13 CVD risk factor changes around the final menstrual period, 14 and relationships with subclinical measures of disease. 14
Four decades after the CARDIA study began enrolling participants, interest in women's reproductive health and impact on CVD health has grown. In 2021, >500 studies examining reproductive exposures in women and subsequent CVD risk were cited in PubMed. Although initial studies focused on changes in traditional CVD risk factors, more recent reports examine the possible shared mechanisms between reproductive factors and CVD risk. These investigations have been enabled by the measurement of CVD risk factors at multiple time points before and after reproductive events. In this review article, we describe CARDIA studies for each reproductive milestone. We include all of the CARDIA studies that addressed reproductive milestones (ie, menarche, contraception, PCOS, pregnancy, GDM, preterm delivery, hypertensive disorders of pregnancy, preeclampsia, gestational hypertension, lactation, breastfeeding, menopause, reproductive aging, anti‐Müllerian hormone, infertility, hot flashes, and VMS). A total of 77 reports are listed in Tables 1, 2, 3, 4, 5. A brief summary of relevant literature accompanies each description. We also identify content that could be explored further on the relationships between these reproductive milestones and CVD risk, which is particularly relevant as the cohort is transitioning through middle age. Investigations could be conducted through collaborations with CARDIA study investigators as well as through public use data sets.
Table 1.
CARDIA Studies Examining Menarche, Contraception, and Cardiovascular Risk Factors
| Year | Menarche | References |
|---|---|---|
| 1992 | Black women have earlier menarche than White women | Burke 15 |
| 2015 | Each 1‐y earlier age at menarche associated with higher BMI | Dreyfus 18 |
| Each 1‐y earlier age at menarche associated with higher glucose, triglycerides, incident impaired fasting glucose, and metabolic syndrome, with associations stronger in White than Black women | ||
| 2015 | Each 1‐y earlier age at menarche associated with higher prevalence of NAFLD | Mueller 19 |
| Each 1‐y earlier at menarche associated with greater visceral adiposity |
| Contraception | ||
|---|---|---|
| 1992 | OCP use associated with higher white blood cell count | Friedman 46 |
| 1993 | OCP use associated with higher fibrinogen | Folsom 47 |
| 1996 | OCP use differed between Black and White women | Bild 45 |
| 2002 | OCP use associated with lower glucose levels and lower odds of diabetes | Kim 43 |
BMI indicates body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; NAFLD, nonalcoholic fatty liver disease; and OCP, oral contraceptive pill.
Table 2.
CARDIA Studies Examining PCOS and Its Components
| Year | PCOS components | References |
|---|---|---|
| 2008 | Changes in BMI and androgenicity are concurrent | Sternfeld 67 |
| 2010 | Androgens predict CAC | Calderon‐Margalit 10 |
| 2011 | PCOS predicts diabetes and dyslipidemia | Wang 69 |
| 2012 | PCOS predicts greater left ventricular mass | Wang 70 |
| 2014 | PCOS predicts CAC | Calderon‐Margalit 66 |
| 2019 | PCOS associated with depression | Greenwood 71 |
| 2016 | Cortisol and testosterone predict atherosclerosis better than testosterone alone | Lee 68 |
| 2017 | Testosterone predicts NAFLD | Sarkar 10 |
BMI indicates body mass index; CAC, coronary artery calcification; CARDIA, Coronary Artery Risk Development in Young Adults; NAFLD, nonalcoholic fatty liver disease; and PCOS, polycystic ovary syndrome.
Table 3.
CARDIA Studies Examining Pregnancy and Cardiovascular Risk Factors
| Year | Pregnancy and parity | References |
|---|---|---|
| 1992 | Black women have earlier age at first childbirth than White women, which may contribute to greater lifetime risk of obesity in Black women. | Burke 15 |
| 1994 | Pregnancy associated with 2–3 kg of persistent weight gain | Smith 85 |
| First pregnancy associated with changes in waist/hip ratio | ||
| Black women have greater gains than White mothers | ||
| 1994 | Parity associated with changes in skinfold distribution in Blacks | Lewis 86 |
| Parity not associated with BMI in younger mothers | ||
| 1996 | Pregnancy associated with decreases in HDL‐C | Lewis 91 |
| 2004 | HDL‐C decrements associated with a first birth pregnancy persist over time | Gunderson 92 |
| 2004 | Parity associated with cumulative increases in waist circumference | Gunderson 88 |
| 2004 | Smoking during pregnancy reduces weight gain during pregnancy | Gunderson 84 |
| 2005 | HDL‐C decrements associated with pregnancy vary by apoE phenotype | Gunderson 93 |
| 2008 | First birth associated with decreased long‐term BP | Gunderson 83 |
| 2008 | Pregnancy is associated with increased visceral fat independent of overall adiposity | Gunderson 87 |
| 2017 | Pregnancy not associated with maternal telomere length | Lane‐Cordova 137 |
| 2022 | Prepregnancy weight gain predicts gestational weight gain | Catov 116 |
| Preterm birth | ||
|---|---|---|
| 2004 | Black women reporting racial discrimination have higher risk of preterm birth and low birth weight deliveries | Mustillo 98 |
| 2009 | Prepregnancy depressive mood is a risk factor for preterm birth and contributes to increased risk of preterm birth in Black women. | Gavin 99 |
| 2010 | Adverse lipids predict preterm birth | Catov 8 |
| 2013 | Preterm delivery predicts higher maternal BP | Catov 107 |
| 2016 | Preterm delivery predicts incident metabolic syndrome | Catov 106 |
| 2018 | Endothelial dysfunction does not predict preterm birth | Lane‐Cordova 100 |
| 2018 | Cardiorespiratory fitness does not predict preterm birth | Lane‐Cordova 102 |
| 2018 | Women with preterm birth have adverse BP patterns and higher risk of CAC | Catov 108 |
| 2019 | Prepregnancy kidney function not associated with preterm birth | Harville 103 |
| 2020 | Oxidative stress does not predict preterm birth | Harville 101 |
| 2020 | Preterm and small for gestational age birth associated with higher maternal ASCVD risk in White women | Lane‐Cordova 189 |
| 2020 | Preterm birth associated with increases in diastolic BP and greater increases in weight after childbearing period | Sun 105 |
| GDM, metabolic syndrome, and diabetes | ||
|---|---|---|
| 1995 | Greater waist/hip ratio increases risk of incident GDM | Zhang 114 |
| 2007 | Higher parity does not increase risk of diabetes, except among women with a history of GDM | Gunderson 9 |
| 2009 | Childbearing associated with increased risk of metabolic syndrome, with greater impact on women with GDM | Gunderson 94 |
| 2010 | Adverse cardiometabolic risk factor profiles before pregnancy predict GDM | Gunderson 113 |
| 2013 | Women with and without GDM have unhealthy behaviors postpartum | Bennett 90 |
| 2018 | Fitness predicts incident GDM | Whitaker 117 |
| 2019 | White women with GDM have lower diabetes risk than Black women with GDM | Shen 120 |
| 2020 | Women with GDM gain weight faster before pregnancy than women without GDM | Catov 115 |
| GDM and CVD risk | ||
|---|---|---|
| 2014 | GDM increases risk of early atherosclerosis based on carotid intima‐media thickness | Gunderson 121 |
| 2016 | GDM associated with increasing left ventricular mass and risk of impaired systolic function | Appiah 122 |
| 2016 | GDM associated with increased risk of nonalcoholic fatty liver disease | Ajmera 124 |
| 2018 | GDM predicts incident chronic kidney disease in Black women | Dehmer 125 |
| 2021 | GDM history is associated with 2‐fold higher risk of CAC, even if glucose levels normalize, prediabetes, or type 2 diabetes | Gunderson 123 |
| 2021 | History of GDM increases risk of ectopic fat deposition | Appiah 139 |
ApoE indicates apolipoprotein E; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; BP, blood pressure; CAC, coronary artery calcification; CARDIA, Coronary Artery Risk Development in Young Adults; GDM, gestational diabetes; and HDL‐C, high‐density lipoprotein cholesterol.
Table 4.
CARDIA Studies Examining Lactation and Cardiovascular Risk Factors
| Year | Lactation | References |
|---|---|---|
| 2007 | Longer lactation attenuated unfavorable changes in weight, lipids, and insulin associated with pregnancy | Gunderson 141 |
| 2010 | Longer duration of lactation is associated with reduced risk of incident metabolic syndrome | Gunderson 12 |
| 2015 | Women with less abdominal fat before pregnancy and less gestational weight gain are more likely to lactate | Kierkegard 82 |
| 2015 | Longer duration of lactation predicts less carotid intima‐media thickness | Gunderson 140 |
| 2018 | Longer duration of lactation is associated with reduced risk of incident diabetes in women with GDM and women without GDM | Gunderson 138 |
| 2019 | Longer lactation is associated with lower NAFLD prevalence | Ajmera 124 |
| 2021 | Longer lactation is associated with less ectopic fat (visceral and pericardial) | Appiah 139 |
CARDIA indicates Coronary Artery Risk Development in Young Adults; GDM, gestational diabetes; and NAFLD, nonalcoholic fatty liver disease.
Table 5.
CARDIA Studies Examining Reproductive Aging
| Year | Reproductive aging | References |
|---|---|---|
| 2008 | Black women more likely to experience infertility than White women | Wellons 160 |
| 2009 | Black women are more likely to undergo hysterectomy than White women | Bower 155 |
| 2013 | Prediction of future menopause based on menstrual cycles alone is limited | Whitham 13 |
| 2013 | AFC predicts menopause | Wellons 11 |
| 2015 | AMH predicts menopause | Nair 156 |
| 2015 | Hysterectomy does not predict future CVD risk factor levels, once prehysterectomy levels are considered | Appiah 14 |
| 2016 | Hypertension associated with CRP during premenopause | Ebong 161 |
| 2017 | AMH predicts menopause better than AFC or FSH | Kim 157 |
| 2017 | Surgical menopause (oophorectomy) does not predict left ventricular function, once presurgical risk factors are considered | Appiah 166 |
| 2019 | Hypertension associated with earlier age at natural menopause, BMI, and waist circumference with older age at menopause | Costanian 162 |
| 2019 | Fibroids not associated with CAC | Laughlin‐Tommaso 165 |
| 2020 | Lower AMH associated with greater oxidative stress | Kim 158 |
| 2021 | Menopause is associated with adverse left ventricular indexes, although adjustment for premenopausal risk factors is limited | Ying 163 |
| 2021 | Premature menopause is not associated with CAC | Freaney 167 |
| 2022 | Age at natural menopause is not associated with left ventricular structure and function once premenopausal factors are considered | Appiah 168 |
| 2022 | Postmenopausal women have higher oxidative stress than premenopausal women | Heravi 164 |
| 2022 | AMH is not associated with aging markers, including telomeres, mitochondrial DNA copy number, or epigenetic age | Kim 159 |
AFC indicates antral follicle count; AMH, anti‐Müllerian hormone; BMI, body mass index; CAC, coronary artery calcification; CARDIA, Coronary Artery Risk Development in Young Adults; CRP, C‐reactive protein; CVD, cardiovascular disease; and FSH, follicle‐stimulating hormone.
MENARCHE
At the time of baseline data collection in the CARDIA study, women were aged 18 to 30 years and had already experienced menarche about 5 to 13 years earlier. Thus, misclassification of age at menarche is possible. However, in the CARDIA study, recall of the age at menarche had adequate precision to note a racial discrepancy in menarche, 12.45 years in Black women and 12.73 years in White women 15 (Table 1). Subsequently, the racial discrepancy in age at menarche between Black and White women was reported in other cohorts. 16 , 17 In the CARDIA study, each earlier year of menarche was associated with greater body mass index (BMI) 18 as well as visceral adiposity. 19 After adjustment for BMI, earlier menarche predicted adverse glucose and lipid levels 18 as well as nonalcoholic fatty liver disease (NAFLD), 19 with associations stronger in White than Black women.
The CARDIA study findings for the association between earlier age at menarche and poorer CVD risk factor profiles are generally congruent with previous studies, but unique in examinations by Black compared with White women. Similar findings were reported in the Fels Longitudinal Study, 20 a cohort of White adolescents. Other reports noted that women who were older at baseline and who recalled early age menarche (<12 years) had increased risk of CVD events and mortality. 21 , 22 , 23 , 24 These cohorts consisted of primarily White or East Asian women. 21 , 22 , 23 , 24 Two reports noted that menarche after the age of 17 years was also associated with slightly increased risk. 22 , 23 However, examination of interactions by race or ethnicity have not been reported for those studies.
The associations between menarche and puberty with CVD risk factors have been attributed to several mechanisms (Figure 1). Childhood socioeconomic status and household structure have long been recognized to predict both early menarche 25 as well as later life CVD events, 26 , 27 although the extent to which these operate through or apart from subsequent lifestyle behaviors and traditional CVD risk factors is not completely understood. These pathways may include greater childhood BMI, 28 catch‐up growth during infancy, 29 and associated adipokine dysregulation, 30 which confer risk of early menarche and long‐term adiposity, as well as the other exposures indicated in Figure 1. The prolonged exposure to estrogen, which accompanies early age at menarche, was not associated with higher CVD risk in the WISE (Women's Ischemia Syndrome Evaluation) study. 22
Figure 1. Potential shared mechanisms between sexual maturation and cardiovascular risk.
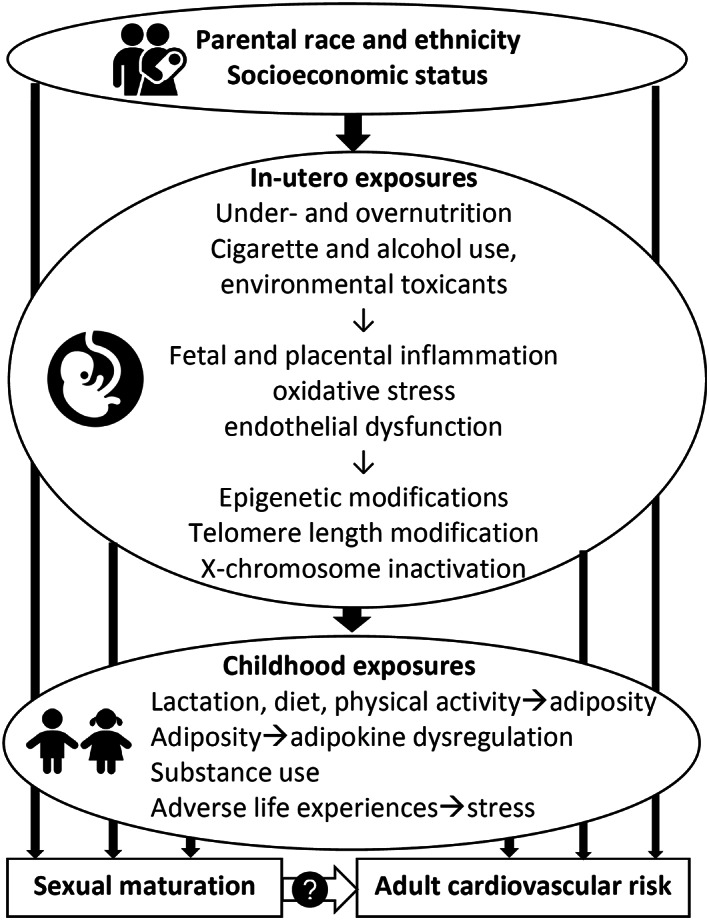
Two hypotheses comment on the impact of the in utero nutritional environment on offspring CVD risk profiles and have been supported by epidemiologic studies. The Barker or “thrifty‐phenotype” hypothesis proposes that fetal deprivation in combination with an enriched nutritional environment in later childhood is associated with CVD risk. 31 , 32 The Pederson hypothesis notes that fetal overnutrition (maternal overweight or hyperglycemia) can modify fetal metabolism, 33 and maternal overweight and obesity have since been noted to be associated with risk of offspring metabolic syndrome 34 and of CVD events as well. 35 , 36 , 37 , 38 Interestingly, both fetal undernutrition 39 as well as overnutrition 40 have also been associated with earlier onset of puberty, suggesting that in utero exposures may modify sexual development as well as offspring cardiovascular risk. Epigenetic modifications in utero or after birth may be a shared mechanism through which early sexual maturation 41 and CVD risk 42 occur, although the precise methylation sites and their role in fetal and infant metabolism and development are still not completely understood. As the CARDIA study cohort ages, such shared mechanisms examining menarche (as well as other reproductive milestones) and CVD risk factors, subclinical atherosclerosis, CVD events, and differences by race should be explored further.
CONTRACEPTION
The CARDIA study has collected data on OCPs and other hormonal contraception at each examination until the cohort transitioned through menopause. Questions have included length of use and no use since the last examination. At the year 10 examination, when women were on average aged 34 (range, 28–40) years, the CARDIA study surveyed women's lifetime history of specific hormonal contraceptive formulations. 43 Subsequent examinations also collected information on contraceptive formulations. Thus, it is possible to link women's CVD risk factor profile with OCP use by age at the time of use, duration, and formulation. 44
Contraceptive studies in the CARDIA study have focused on OCPs (Table 1). OCP use increased between baseline and the year 7 examination (between 1985 and 1992) in White women, but not in Black women. 45 OCP use at the time of examination was correlated with higher levels of inflammatory markers, including white blood cell count 46 and fibrinogen. 47 However, subsequent CARDIA study reports did not observe that OCP use was cross‐sectionally associated with metabolic dysfunction (ie, elevations in insulin, glucose, blood pressure, or lipids) as a pathway to CVD (Table 1). In fact, OCP use was associated with lower levels of glucose, possibly attributable to changes in OCP formulations over the past 30 years. 43 These associations persisted after adjustment for confounders, including age, education, weight, and race, and could represent residual confounding or a true protective effect.
Epidemiologic studies in other cohorts have focused on short‐term, rather than long‐term, risk of thromboembolic events 48 and ischemic heart disease. 48 , 49 Beginning in the 1960s, the earliest forms of estrogen‐progestin pills contained 150 μg of mestranol or ethinyl estradiol, but the dosage of ethinyl estradiol was decreased because of common adverse effects, including nausea and vomiting, and adverse effects, including thromboembolism. 50 The vast majority of OCPs prescribed today have <35 μg of ethinyl estradiol, including OCPs with <20 μg of ethinyl estradiol. In studies of French 51 and Danish 52 women, 20 μg OCPs were associated with significantly lower risk for pulmonary embolism, ischemic stroke, and myocardial infarction than formulations with 30 to 40 μg of ethinyl estradiol. Pills containing third‐generation progestins desogestrel and gestodene were associated with higher risk of pulmonary events than pills with levonorgestrel (the second‐generation progestin that also is included in hormonal intrauterine devices and implantable rods). 51 This research is congruent with older studies noting that lower doses of estrogen are linked with lower risk. 53 Therefore, OCPs are not recommended for women at increased risk for thromboembolic disease, including women who smoke and are aged >35 years or women with uncontrolled hypertension, thrombophilia, or history of thromboembolic disease. 54
Whether OCPs and other hormonal contraception affect atherosclerosis and longer‐term CVD risk is less clear. There was substantial variation in OCP use within CARDIA study women during follow‐up. The repeated measures, however, provide an opportunity to conduct time‐varying exposure analyses. The adverse associations with blood pressure 55 and the favorable associations with concurrent glucose levels and diabetes in the CARDIA study are similar in their reports of neutral 56 or beneficial associations 57 , 58 in other studies. Therefore, prospective studies examining cumulative impact on longer‐term risk factor profiles, atherosclerosis markers, and events need to be conducted. Examination of transdermal forms, including the vaginal ring and levonorgestrel formulations, should also be undertaken. Such studies will eventually be possible in the CARDIA study. This is particularly relevant for women who use long‐term hormonal contraception for its favorable effects on bleeding, hirsutism, and acne, such as women with PCOS. 59
POLYCYSTIC OVARY SYNDROME
PCOS is believed to be the most common endocrinopathy among reproductive‐aged women, with an estimated prevalence of 5% to 10%. 60 Because it is diagnosed on the basis of hyperandrogenism, oligomenorrhea, and polycystic ovaries, PCOS is typically first identified in the reproductive years. In 2021, a National Heart, Lung, and Blood Institute workshop concluded that women with PCOS commonly were affected by obesity, type 2 diabetes, dyslipidemia, hypertension, NAFLD, and sleep‐disordered breathing, but evidence for independent associations between PCOS and CVD was not conclusive. 60 In part, this uncertainty stems from the fact that diagnostic criteria for PCOS and recommendations for screening have evolved over the past several decades. 61 , 62 , 63 Figure 2 shows classification schemes based on symptoms and biochemical measures, 62 , 64 as well as more recent categories based on unsupervised machine‐learning algorithms and genome‐wide association studies. 65
Figure 2. Polycystic ovary syndrome (PCOS) classification schemes.
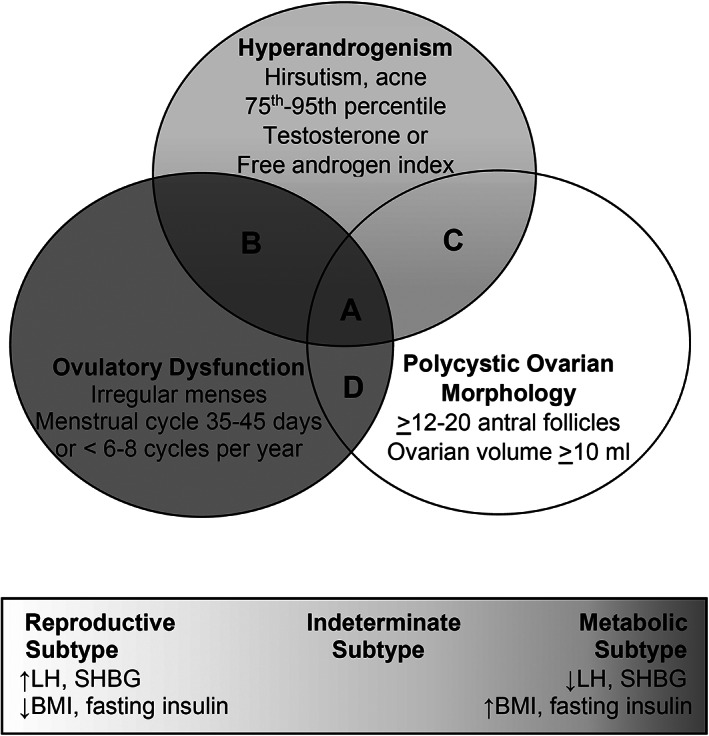
Top panel: Phenotype “A” consists of hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. Phenotype “B” consists of hyperandrogenism and ovulatory dysfunction. Phenotype “C” consists of hyperandrogenism and polycystic ovarian morphology. Phenotype “D” consists of ovulatory dysfunction and polycystic ovarian morphology. Bottom panel: PCOS subtypes include a reproductive subtype, a metabolic subtype, and an indeterminate subtype distinguished by levels of luteinizing hormone (LH), sex hormone–binding globulin (SHBG), body mass index (BMI), and fasting insulin. (Levels of testosterone were elevated compared with controls but did not distinguish between PCOS subtypes.)
Another factor contributing to our incomplete understanding of CVD in PCOS is a lack of prospective and long‐term longitudinal studies that include participants with well‐characterized PCOS. 60 Accordingly, investigators have sought to use the CARDIA study to gain an understanding of the trajectory of cardiometabolic features in participants with PCOS as they transition to their postreproductive years. In the CWS (CARDIA Women's Study), an ancillary study to the CARDIA study, women participating in the year 15 examination were invited to a year 16 ancillary study visit. The CWS participants consented to measurements of their androgens (testosterone and sex hormone–binding globulin) from the year 2 examination, when they were about 27 years of age, as well as from the year 10 and year 16 examinations. 66 Women were also asked to characterize their menstrual cycles, excess hirsutism, and OCP use between the ages of 20 and 30 years. Initial CWS reports examined relationships between androgens and cardiometabolic risk factors and outcomes, including one report showing that changes in androgens were tightly correlated with changes in BMI 67 (Table 2). In another study, free testosterone levels predicted carotid intima‐media thickness. 68 Subsequent studies have further defined PCOS by identifying subjects with both hyperandrogenism (either defined by excess hair growth or biochemical hyperandrogenemia) and oligomenorrhea. Using this approach, investigators found that participants with PCOS had stronger associations with diabetes, 69 dyslipidemia, 69 subclinical atherosclerosis, 10 , 66 and left ventricular mass compared with those without PCOS. These associations were independent of lifestyle behaviors, such as diet and physical activity, and similar associations were not present when subjects with either isolated hyperandrogenism or oligomenorrhea were considered, suggesting the independence and importance of the full syndrome in predicting cardiometabolic outcomes. 70 However, the relationship between isolated hyperandrogenism and oligomenorrhea with other outcomes, such as APOs and lactation duration, may warrant future investigation.
Other CARDIA studies have reported links between PCOS and factors that may contribute to CVD outcomes. Notably, depression risk, 71 which has predicted adverse cardiovascular health in other CARDIA studies, is also increased in those with PCOS in the CARDIA study, with the highest symptom burden noted in Black women. In non‐CARDIA studies, reports using hospitalization data, 72 electronic medical record data, 73 and other cohorts 73 note that PCOS may also be linked with peripartum cardiovascular complications as well as GDM and hypertensive disorders of pregnancy (HDPs), which in and of themselves confer CVD risk (Figure 3). Thus, CARDIA study data could also be used to examine how various life course events, including reproductive events and mood disorders, contribute to the relationships between PCOS and CVD.
Figure 3. Reproductive life course events may be associated with each other and share precursors with cardiovascular disease (CVD) and jointly contribute to CVD.
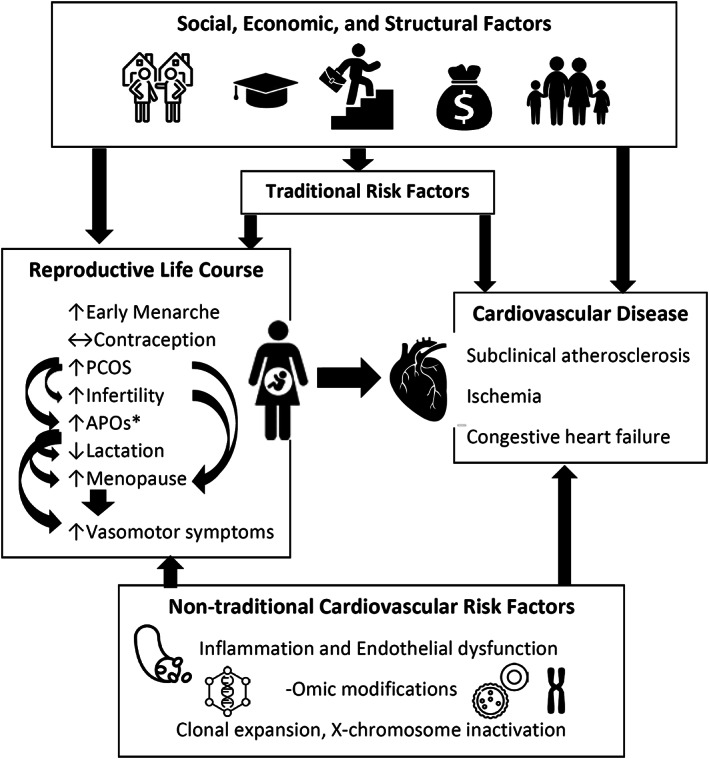
Upward arrows indicate higher associated CVD risk, and downward arrows indicate lower associated CVD risk. *Adverse pregnancy outcomes (APOs) include gestational diabetes, hypertensive disorders of pregnancy, and preterm birth. PCOS indicates polycystic ovary syndrome.
Although CARDIA study reports contribute to the growing body of work supporting a link between PCOS and intermediate cardiometabolic outcomes, 74 , 75 , 76 , 77 whether PCOS ultimately leads to increased rates of CVD events has not been definitively shown. The ages of participants in most epidemiologic studies that enroll reproductive‐aged women, including the CARDIA study, have not been sufficiently advanced to achieve this while the cohort was premenopausal. Furthermore, the magnitude of the associations between PCOS and cardiometabolic outcomes and whether all PCOS subsets are at risk remain unclear. One factor contributing to these uncertainties is the heterogeneity inherent to PCOS and differences in ascertainment of PCOS across various studies. 62 , 65 , 78 , 79 Indeed, studies that rely on physician diagnosis may not identify all patients: In one 2020 study of electronic medical records in the United Kingdom, 80 approximately half of women who met PCOS criteria based on diagnoses of irregular menses, polycystic ovaries, or hyperandrogenism were not diagnosed. Similarly, another study from Australia reported that ≈70% of women meeting PCOS criteria were not diagnosed. 81 Therefore, studies relying on a clinical diagnosis may be subject to various forms of selection bias, including overrepresentation of subjects with more adverse features, and/or improved health care access. Improved strategies to identify PCOS in epidemiologic studies are needed to improve our understanding of PCOS and CVD risk across a more diverse sample. Toward this end, recent genome‐wide association studies suggest that ‐omics data could potentially serve as an adjunct to clinical criteria in identifying subtypes of women with PCOS at high risk for chronic diseases, 65 including CVD and malignancy.
ADVERSE PREGNANCY OUTCOMES
The CARDIA study has examined the relationships between several adverse pregnancy outcomes with CVD risk factors (Table 3). Pregnancy features were reported at every examination, and the CARDIA validation studies have demonstrated excellent maternal recall of infant birth weight, preterm birth, gestational weight gain, and GDM. 8 , 9 , 82 Although women tended to overreport instances of HDPs, 83 the sensitivity of maternal recall of HDP was high, thus providing reliable data for normotensive pregnancies. Because of its periodic assessment of BMI and anthropometrics as well as laboratory measurements, the CARDIA study is one of the few longitudinal cohorts able to examine prepregnancy, postpregnancy, and postlactation cardiometabolic health. This has been particularly useful for examination of racial disparities in relation to APOs and childbearing as they influence CVD risk factors.
The CARDIA study was one of the earliest studies to note that first pregnancy resulted in permanent weight gain of several kilograms, except in smokers, 84 and not in women aged <25 years, and that pregnancy might adversely affect distribution of adiposity as well as total number of kilograms. 15 , 85 , 86 , 87 , 88 Waist circumference and visceral fat increases were cumulative with each child, independent of overall adiposity, 87 which may be partially attributable to less physical activity and increased caloric intake. 89 , 90 After adjustment for weight gain and lifestyle behavior changes after pregnancy, the CARDIA study has also reported that pregnancy is linked with lower high‐density lipoprotein cholesterol following a first birth 91 , 92 and varies by apolipoprotein E phenotype, 93 and parity is associated with increased risk of incident metabolic syndrome. 94 In contrast, increasing parity was not associated with future risk of developing overt diabetes, unless women had a history of GDM. 9 Having at least one birth was also associated with lower long‐term blood pressure in the CARDIA study, but only among women who did not develop HDPs. 83 Biologic plausibility of a beneficial relationship of pregnancy with blood pressure has been supported by smaller studies that demonstrated that the characteristic decreases in peripheral resistance during pregnancy persist postpartum. 95 , 96
Preterm births, pregnancies characterized by delivery before 37 weeks of gestation, are a key contributor to Black‐White disparities in infant mortality in the United States. 97 The CARDIA study was one of the earliest studies to report that racial discrimination contributes to preterm birth risk and low birth weight risks and to note the Black‐White disparity in preterm birth rates, even after accounting for known risk factors, such as tobacco and substance use, as well as gestational weight gain. 98 Prepregnancy depressed mood also likely contributed to racial differences in preterm birth. 99 In the CARDIA study, adverse risk profiles predicted poorer pregnancy outcomes, including preterm birth. 8 Interestingly, hypothesized predictors, such as endothelial dysfunction, 100 oxidative stress, 101 and cardiorespiratory fitness 102 or chronic kidney disease, 103 did not predict preterm birth. Apolipoprotein E2 allele was also associated with greater risk of miscarriage. 104
The CARDIA studies have also noted that preterm birth was independently associated with future adverse CVD risk profiles 105 and metabolic syndrome 106 as well as inflammatory markers and subclinical atherosclerotic markers, including carotid intima‐media thickness. 107 , 108 These studies are congruent with studies from Norwegian cohorts on the associations between preterm delivery and risk of future adverse CVD risk factor levels 109 and CVD events. 110 In these Norwegian women, 10‐year CVD risk scores were higher in women with histories of APOs than in women without APOs. 111 Taken together, these studies suggest that preterm birth itself may modify CVD risk. Alternatively, preterm birth and CVD could potentially share precursors, such as CVD risk factors, but risk is incremental, 112 and novel modifiable targets are not currently known.
The CARDIA study has also examined the relationship of prepregnancy CVD risk factors with the risk of future GDM. In the CARDIA study, cardiometabolic risk factors independently associated with several fold higher risk of future GDM included elevated fasting glucose (ie, prediabetes), fasting insulin, and lower high‐density lipoprotein cholesterol levels independent of prepregnancy BMI and waist circumference. 113 These findings were the first to show that dysmetabolism strongly predicted GDM better than overall adiposity. Others showed that women with greater waist/hip ratio 114 and greater speed of weight gain before pregnancy 115 had greater risk of gestational weight gain 116 and incident GDM, even after adjustment for BMI. Along similar lines, the CARDIA study women with poorer CVD risk factor profiles 113 and lower study‐assessed fitness levels 117 were more likely to develop GDM. Others later reported findings that were similar in large cohorts of women, particularly for blood pressure and weight. 118 , 119
In the CARDIA study, GDM was associated with a higher risk of developing metabolic syndrome, and a 4‐fold higher risk of incident diabetes after pregnancy, 94 particularly in Black women. 120 In the CARDIA study, GDM history has also been associated with higher carotid artery intima‐media thickness in midlife among women who did not develop the metabolic syndrome, prediabetes, or overt diabetes. 121 Even after consideration of BMI and traditional CVD risk factors, a history of GDM may still be associated with higher risk of impaired systolic ejection fraction. 122 A history of GDM was associated with a 2‐fold higher relative risk of coronary artery calcification for all levels of glucose tolerance (euglycemia, prediabetes, and overt diabetes) in midlife, suggesting that other established CVD risk factors may contribute substantially to atherosclerotic CVD risk. 121 Risk was elevated even when normoglycemia persisted many years after delivery. 123 In the CARDIA study, GDM history was also associated with adverse outcomes, such as the metabolic syndrome, 94 NAFLD, 124 and chronic kidney disease. 125 These studies are congruent with studies from other cohorts demonstrating associations between GDM and adverse outcomes, particularly diseases related to greater adiposity, including the metabolic syndrome, type 2 diabetes, CVD, NAFLD, and breast cancer. 126 , 127 At least part of this risk is attributable to preconception BMI as well as higher postpartum weights among women with GDM, which tend to be higher compared with women without GDM. 128
Maternal recall of HDP in the CARDIA study, as in other epidemiologic studies enrolling women in perimenopause or postmenopause, is not adequate to distinguish between subtypes of HDPs (prepregnancy chronic hypertension, gestational hypertension, preeclampsia/eclampsia, and preeclampsia superimposed on chronic hypertension) or accurately ascertain a history of HDPs. These subtypes of HDPs likely have different pathophysiology and different CVD sequelae. 129 Thus, examination of such studies has been limited in the CARDIA study. Studies from other cohorts have demonstrated that women with gestational hypertension have greater risk of CVD events compared with women who are normotensive during pregnancy, 130 , 131 , 132 and the link with future CVD is particularly marked for women who have preeclampsia.
The CARDIA study has previously validated women's self‐report of pregnancy metrics compared with medical records and found high correlation for most outcomes, including diabetes and weight. 9 , 83 In general, although self‐report has limited accuracy to distinguish subtypes of HDPs in the CARDIA study and other studies, maternal report of key pregnancy metrics has high correlation with birth certificates 133 and electronic medical records. For example, one report 133 noted that 98% of mothers noted gestational age within 1 week of the date noted on the birth certificate, and sensitivity and specificity of self‐report of preterm birth were 99% and for low birth weight were also high. Correlation between women's self‐report of GDM, 134 gestational age, and birth weight also had high correlation with medical records, years after diagnosis. 135 Obviously, examination of particular exposures during pregnancy, such as alcohol or tobacco use, may be subject to social desirability biases and might limit the accuracy of such analyses.
In general, the implications of APOs for prevention and treatment of future maternal chronic disease are not entirely elucidated. In the studies of Norwegian women, differences in the proportion of women who would be classified in different risk categories based on APOs was low. 111 In other words, the findings did not support reclassifying women with histories of APOs for different CVD screening procedures, beyond existing recommendations for CVD risk factor screening. However, interventions target preconception, prenatal, and postpartum periods as potentially sensitive windows in which interventions could be useful. 136 Future potential studies in the CARDIA study could serve as exploratory studies leveraging the multiple ancillary studies in the CARDIA study to determine whether novel risk factors, such as epigenetic factors and telomere length, 137 might differ between women with normotensive pregnancies and women with HDPs. As the cohort ages, examining the relationships of specific types and severity of APOs with measures of later‐life functioning, particularly cognitive functioning, will be possible.
LACTATION
The CARDIA study was one of the first epidemiologic studies to examine the beneficial associations of breastfeeding on metabolic outcomes, such as diabetes. Gunderson and colleagues noted that lactation was related to ~50% lower risk of incident metabolic syndrome 12 as well as overt diabetes, 138 independent of preconception cardiometabolic risk profiles, BMI, perinatal outcomes, social factors, and follow‐up lifestyle behaviors, as well as strong protective associations among Black women, and among women with histories of GDM. The CARDIA study also noted that breastfeeding behavior may be related to other factors, including fat distribution, apart from BMI. 82 Independent of these factors, longer duration of lactation was associated with reduced odds of pericardial and visceral fat deposition, 139 NAFLD, 124 and carotid intima‐media thickness 140 (Table 4). CARDIA's prospective studies across the childbearing years show much stronger protective association for lactation than studies that obtained information on lactation close to or after the end of the reproductive lifespan. These cohorts have also reported longer duration of lactation to be favorable 141 and associated with improved glucose uptake, 142 , 143 insulin sensitivity, 142 , 143 and lipid metabolism, 142 , 144 , 145 leading to the reversal of some of the adverse metabolic changes brought on by pregnancy, 146 particularly lower risk of future diabetes among women with previous GDM. 147 These studies reported weaker associations of 10% relative risk reduction per year of lactation than observed in CARDIA, perhaps attributable to lack of adjustment for antecedent cardiometabolic risk factors before pregnancy or history of APOs. The positive effects of lactation on maternal metabolic health have been reported to last for >7 to 15 years after the last birth, 148 and are associated with lower risk of CVD, 149 although the extent to which these findings are independent of preconception risk factors and history of APOs is unclear.
The mechanisms through which lactation may improve maternal health are not completely understood. To some extent, the favorable association may also reflect the fact that mothers who are obese before pregnancy face greater challenges to breastfeed their offspring, arising from medical conditions and adverse maternal and infant outcomes. These mothers are less likely to receive education on the benefits of lactation or be offered lactation support compared with normal weight mothers. 150 , 151 Interestingly, in Sprague‐Dawley rats, lactation has favorable effects on oxidative stress markers. 152 Oxidative markers are available at several time points in the CARDIA study, including before and after pregnancy, and could assist in investigations of whether lactation modifies these markers significantly.
Finally, the benefits of lactation in combination with healthy infant feeding practices for childhood obesity have been well documented. 153 Studies from other cohorts have noted that women with GDM may have reduced breastmilk production as well as different quality breastmilk than women without GDM. 154 Whether the quality of breastmilk among infants with GDM has independent adverse effects on infant growth is not yet established.
REPRODUCTIVE AGING AND MENOPAUSE
When the CARDIA study women were on average aged 40 years at the year 16 CWS ancillary study examination, 1163 women underwent transvaginal ultrasonography assessing ovarian volume, cysts, and uterine structure. The presence of uterine fibroids on the imaging also was noted. In the CARDIA study, Black women were more likely to undergo hysterectomy than White women. 155 The CARDIA study has used the ultrasound information along with surveys on menstrual frequency and ovarian markers (anti‐Müllerian hormone and follicle‐stimulating hormone) to expand prediction of menopause and understanding of nontraditional risk factors (Table 5). In addition to reporting associations between these markers and menopause, 156 , 157 the CARDIA study reports also noted that anti‐Müllerian hormone does correlate with oxidative stress, 158 but does not correlate with other aging markers 159 that have been associated with CVD risk. These data were also used to examine infertility and race‐specific explanations. The CARDIA study women were asked whether they had not conceived during the past year despite unprotected intercourse. 160 Black women were more likely to have experienced infertility compared with White women, even after considering fibroids, ovarian volume, and smoking. 160
Because the CARDIA study was able to collect data on CVD risk factors before premenopause, the CARDIA study reports have been able to examine the relationships between premenopausal risk factors, menopausal status, and CVD risk. 161 , 162 , 163 , 164 One of the most interesting findings from the CARDIA study is that surgical menopause and age at natural menopause after 45 years were not strong predictors of CVD risk factors, subclinical atherosclerosis, or left ventricular indexes once presurgical or premenopausal risk factors were considered. 165 , 166 , 167 , 168 The CARDIA study's reproductive aging reports complement those from studies that enrolled perimenopausal women, such as the SWAN (Study of Women Across the Nation), or perimenopausal and postmenopausal women enrolled in cardiovascular cohorts, such as the ARIC (Atherosclerosis Risk in Communities Study) and MESA (Multi‐Ethnic Study of Atherosclerosis). 169 , 170 The CARDIA study and SWAN have both reported that risk factors are generally less favorable in the postmenopausal period than in the premenopausal period. Such changes are largely attributable to linear changes in aging, with the exception of lipids, which may change more rapidly during the perimenopausal transition. 171 , 172
An exception to this relationship is menopause occurring before the age of 40 years, which may represent a particularly high‐risk population. 169 , 170 For instance, in other investigations of premature menopause, menopause before the age of 35 years doubles the risk of an incident CVD event. 173 The CARDIA study adds to these studies in noting that premature menopause does not seem to be associated with greater risk of coronary artery calcification, suggesting that the relationship between premature menopause and CVD events occurs through pathways other than atherosclerosis. 167 In addition, other examinations suggest that inclusion of premature menopause as a CVD risk factor does not significantly improve assessment of risk based on traditional CVD risk factors. 174 However, CARDIA study data could be used to reveal causal pathways that enhance accelerated aging, because the CARDIA study measured repeated nontraditional risk factors before and after the last menstrual period. These pathways likely do not involve estrogen per se. Although the menopausal transition ultimately results in declines in endogenous estrogen levels, estrogen supplementation has had only slight beneficial to neutral impact on markers of subclinical atherosclerosis when given in the 5 years after the final menstrual period in KEEPS (Kronos Early Estrogen Prevention Study) 175 and the ELITE (Early Versus Late Intervention Trial With Estradiol). 176 Aging mechanisms contributing both to early menopause as well as CVD risk in postmenopausal populations have included clonal hematopoiesis of indeterminate potential, 177 epigenetic age acceleration, 178 and telomere length, 179 which could potentially be assessed using premenopausal and postmenopausal measures in the CARDIA study.
Early menopause has also predicted greater risk of heart failure in other cohorts. 180 , 181 , 182 , 183 , 184 , 185 As more heart failure cases are observed in the CARDIA study, there will be an opportunity to replicate these findings while taking into account premenopausal measurements of obesity in CARDIA study women. The relationship between menopause and heart failure has been further validated by studies involving NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), which is a known biomarker for heart failure diagnosis and prognosis. 186 , 187 , 188 In cohorts of primarily perimenopausal and postmenopausal women, associations between age at the final menstrual period and sex hormones with NT‐proBNP have been demonstrated. 186 , 187 , 188 The CARDIA study has measurements of sex hormones and will be measuring heart failure biomarkers, and future studies can determine whether such associations exist during the reproductive years.
Finally, the CARDIA study has collected information on VMS, the severity of which were independent predictors of CVD risk. 189 Interestingly, such risk was synergistic with history of preterm birth, suggesting shared pathways spanning the reproductive years. Other studies, including SWAN, as well as meta‐analyses note that VMS are associated with increased risk of CVD by as much as 28%, even after adjustment for standard CVD risk factors. 190 , 191 However, the specificity of VMS, the importance of length of symptoms and onset in relation to the final menstrual period, and the added benefit for reclassification of CVD risk have not been examined.
CONCLUSIONS
The CARDIA study has contributed to our understanding of how premenopausal reproductive events are associated with CVD risk factors and subclinical markers of atherosclerosis. As the CARDIA study cohort finishes its current round of data collection, the current age range of the surviving women is roughly 55 to 65 years. Thus, life course analyses can examine contributions to existing marked racial disparities in women's health. Such analyses can examine associations between reproductive span and pregnancy‐lactation outcomes on CVD outcomes in the near future (Table 6).
Table 6.
Limitations of CARDIA for Reproductive Milestone Analyses and Future Directions
| Limitations |
|---|
| Represents Black and White populations in 4 metropolitan areas and may not extend to other areas of the United States |
| Information on contraception and pregnancy obtained through survey and less accurate for distinguishing between hypertensive disorders of pregnancy |
| Frequently repeated measurements during reproductive transitions (such as during each pubertal stage, pregnancy trimester, or each menopausal stage) not performed compared with studies designed to assess these transitions |
| Strengths and future directions |
|---|
| Examinations of interactions between trajectories of social determinants of health and reproductive milestones |
| Examination of clusters of reproductive milestones and joint relationships with CVD risk |
| Examination of relationships between reproductive milestones and cognitive and physical outcomes, including brain structure and cognitive performance |
| Combining data with other observational cohorts to increase representativeness and statistical power for examination of reproductive milestones. |
CARDIA indicates Coronary Artery Risk Development in Young Adults; and CVD, cardiovascular disease.
As an observational cohort, CARDIA study analyses cannot prove causal relationships between reproductive milestones and CVD risk. However, such examinations may serve as the basis through which to question potentially harmful practices, such as the routine performance of bilateral oophorectomy before epidemiologic analyses that such practices might be harmful for CVD health. In addition, the CARDIA study and other cohort studies may help to identify which reproductive factors might benefit from modification by examining dose‐response relationships, as with lactation or length of OCP use. Observational studies can also identify specific disorders and populations who might be useful to target in future randomized trials or through policy, such as provision of mental health services for women with preterm deliveries.
In addition to the suggestions for additional explorations noted under each reproductive milestone, there are general areas for which rich data are available but not fully leveraged. In consortia using data from multiple cohorts, CARDIA study genome‐wide association study data have been used to identify single‐nucleotide polymorphisms associated with several reproductive characteristics, including fibroids, 192 age at menarche, 193 age at natural menopause, 194 and sex hormones, such as sex hormone–binding globulin. 195 In combination with closer examination of social determinants of health, such data could be used to reduce residual confounding by unmeasured sociocultural, economic, or psychosocial factors when examining racial differences in such outcomes. The CARDIA study has serial assessments of social determinants of health in a cohort stratified by both education and Black versus White race. Trajectory analyses examining structural factors, including wealth, employment status, single parenthood, neighborhood‐level determinants, and access to health care, during a time of economic change (1985‐present), policies, and social support networks for postdelivery care and relationships with a broad range of CVD outcomes are possible; such analyses have been performed to some extent for Black and White participants overall but not by both race and sex. These issues affect women and men differently, and comparisons by sex could be conducted. The CARDIA study has recently reported that the higher risk of premature CVD in Black versus White adults was statistically explained by clinical and neighborhood factors in women, compared with clinical and socioeconomic factors in men. 7 Such factors include mental health, discrimination, and substance use, which have been collected repeatedly in the CARDIA study and whose relationship with reproductive milestones could provide rich areas for exploration.
Comparisons between Black and White women of trajectories of social determinants of health and how these interact with reproductive milestones should also be examined. Specifically, how disparities in CVD risk factors in Black versus White women are influenced by reproductive disparities could be examined more closely. Although it is a plausible hypothesis that higher‐risk reproductive milestones are associated with poorer social determinants of health and CVD risk factors, which, in turn, influence future reproductive outcomes and CVD outcomes, such lifecourse analysis has not been conducted. Such analyses have the potential to influence not only epidemiologic analyses but also health policies. For example, the examination of the impact of single parenthood on cardiovascular health has been limited, because of inadequate data on cardiovascular risk before and after parenthood, whether such parenthood was modified by divorce and social support systems, and potential mediators, such as lifestyle behaviors and access to health care. Exploring these questions in the CARDIA study along with other observational studies would increase representativeness as well as statistical power.
Comprehensive reproductive life course models that take into account the correlations between fertility, adverse pregnancy outcomes, and VMS may have greater impact than models considering only one of these dimensions (Figure 3). In particular, the linkages between adverse pregnancy outcomes, such as preterm birth, early age at menopause, and presence of VMS, may confer a particularly high‐risk CVD profile. Another high‐risk grouping may be represented by PCOS, GDM, and absence of lactation, which would be of value to examine because of lactation as a potentially modifiable behavior that might ameliorate CVD risk. Combining CARDIA study data with other observational studies would increase statistical power.
The CARDIA study has collected information on COVID‐19 infection and vaccination. As the cohort ages, the next wave of data collection involves collection of data relevant to aging. Future studies might examine the relationship between reproductive events on cognition, fitness, and aging metrics. Ancillary studies have already characterized lung function, sleep apnea, cardiometabolic disease, as well as cognitive functioning, bone markers, and physical functioning. Linkages between reproductive exposures with epigenetic, genetic, and metabolomic markers that have already been measured in CARDIA study subsets have not been fully explored. Thus, the contribution of reproductive milestones to these other aspects of health will be possible as the cohort ages.
Sources of Funding
The CARDIA (Coronary Artery Risk Development in Young Adults) study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). Additional support was provided by R01HL065622, K23HL087114, R03HL135453, R21HL145419, R21HL145419, R01DK106201, and R01DK090047.
Disclosures
None.
Acknowledgments
This article has been reviewed by the CARDIA (Coronary Artery Risk Development in Young Adults) study for scientific content.
For Sources of Funding and Disclosures, see page 14.
References
- 1. Wuest J Jr, Dry T, Edwards J. The degree of coronary atherosclerosis in bilaterally oophorectomized women. Circulation. 1953;7:801–809. doi: 10.1161/01.cir.7.6.801 [DOI] [PubMed] [Google Scholar]
- 2. Winkelstein W Jr, Stenchever M, Lilienfeld A. Occurrence of pregnancy, abortion, and artificial menopause among women with coronary artery disease: a preliminary study. J Chronic Dis. 1958;7:273–286. doi: 10.1016/0021-9681(58)90085-7 [DOI] [PubMed] [Google Scholar]
- 3. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801 [DOI] [PubMed] [Google Scholar]
- 4. Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. Am J Epidemiol. 1987;126:861–870. doi: 10.1093/oxfordjournals.aje.a114723 [DOI] [PubMed] [Google Scholar]
- 5. Cutter G, Burke G, Dyer A, Friedman G, Hilner J, Hughes G, Hulley S, Jacobs D Jr, Liu K, Manolio T. Cardiovascular risk factors in young adults: the CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4 [DOI] [PubMed] [Google Scholar]
- 6. Friedman G, Cutter G, Donahue R, Hughes G, Hulley S, Jacobs D Jr, Liu K, Savage P. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 7. Shah N, Ning H, Petito L, Kershaw K, Bancks M, Reis J, Rana J, Sidney S, Jacobs D Jr, Kiefe C, et al. Associations of clinical and social risk factors with racial differences in premature cardiovascular disease. Circulation. 2022;146:201–210. doi: 10.1161/CIRCULATIONAHA.121.058311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catov J, Ness R, Wellons M, Jacobs D Jr, Roberts J, Gunderson E. Prepregnancy lipids related to preterm birth risk: the coronary artery risk development in young adults study. J Clin Endocrinol Metab. 2010;95:3711–3718. doi: 10.1210/jc.2009-2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gunderson E, Lewis C, Tsai A, Chiang V, Carnethon M, Quesenberry C Jr, Sidney S. A 20‐year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007;56:2990–2996. doi: 10.2337/db07-1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calderon‐Margalit R, Schwartz S, Wellons M, Lewis C, Daviglus M, Schreiner P, Williams O, Sternfeld B, Carr J, O'Leary D, et al. Prospective association of serum androgens and sex hormone‐binding globulin with subclinical cardiovascular disease in young adult women: the "Coronary Artery Risk Development in Young Adults" Women's Study. J Clin Endocrinol Metab. 2010;95:4424–4431. doi: 10.1210/jc.2009-2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wellons M, Bates G, Schreiner P, Siscovick D, Sternfeld B, Lewis C. Antral follicle count predicts natural menopause in a population‐based sample: the CARDIA Women's Study. Menopause. 2013;20:825–830. doi: 10.1097/GME.0b013e31827f06c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gunderson E, Jacobs D Jr, Chaing V, Lewis C, Feng J, Quesenberry C, Sidney S Jr. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20‐year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes. 2010;59:495–504. doi: 10.2337/db09-1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whitham H, Maclehose R, Harlow B, Wellons M, Schreiner P. Assessing the utility of methods for menopausal transition classification in a population‐based cohort: the CARDIA Study. Maturitas. 2013;75:289–293. doi: 10.1016/j.maturitas.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Appiah D, Schreiner P, Bower J, Sternfeld B, Lewis C, Wellons M. Is surgical menopause associated with future levels of cardiovascular risk factors independent of antecedent levels? The CARDIA Study. Am J Epidemiol. 2015;182:991–999. doi: 10.1093/aje/kwv162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burke G, Savage P, Manolio T, Sprafka J, Wagenknecht L, Sidney S, Perkins L, Liu K, Jacobs D Jr. Correlates of obesity in young black and white women: the CARDIA Study. Am J Public Health. 1992;82:1621–1625. doi: 10.2105/ajph.82.12.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freedman D, Khan L, Serdula M, Dietz W, Srinivasan S, Berenson G. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43 [DOI] [PubMed] [Google Scholar]
- 17. Biro F, McMahon R, Striegel‐Moore R, Crawford P, Obarzanek E, Morrison J, Barton B, Falkner F. Impact of timing of pubertal maturation on growth in black and white female adolescents: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–643. doi: 10.1067/mpd.2001.114476 [DOI] [PubMed] [Google Scholar]
- 18. Dreyfus J, Jacobs D Jr, Mueller N, Schreiner P, Moran A, Carnethon M, Demerath E. Age at menarche and cardiometabolic risk in adulthood: the Coronary Artery Risk Development in Young Adults Study. J Pediatr. 2015;167:344–352. doi: 10.1016/j.jpeds.2015.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mueller N, Pereira M, Demerath E, Dreyfus J, MacLehose R, Carr J, Terry J, Jacobs D Jr. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: the CARDIA study. Obesity (Silver Spring). 2015;23:468–474. doi: 10.1002/oby.20950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Remsberg K, Demerath E, Schubert C, Chumlea W, Sun S, Siervogel R. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90:2718–2724. doi: 10.1210/jc.2004-1991 [DOI] [PubMed] [Google Scholar]
- 21. Lakshman R, Forouhi N, Sharp S, Luben R, Bingham S, Khaw K, Wareham N, Ong K. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–4960. doi: 10.1210/jc.2009-1789 [DOI] [PubMed] [Google Scholar]
- 22. Lee J, Cook‐Wiens G, Johnson B, Braunstein G, Berga S, Stanczyk F, Pepine C, Bairey Merz C, Shufelt C. Age at menarche and risk of cardiovascular disease outcomes: findings from the National Heart Lung and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation. J Am Heart Assoc. 2019;8:e012406. doi: 10.1161/JAHA.119.012406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Canoy D, Beral V, Balkwill A, Wright F, Kroll M, Reeves G, Green J, Cairns B. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131:234–244. doi: 10.1161/CIRCULATIONAHA.114.010070 [DOI] [PubMed] [Google Scholar]
- 24. Chen X, Liu Y, Sun X, Yin Z, Li H, Liu X, Zhang D, Cheng C, Liu L, Liu F, et al. Age at menarche and risk of all‐cause and cardiovascular mortality: systematic review and dose‐response meta‐analysis. Menopause. 2018;26:670–676. doi: 10.1097/GME.0000000000001289 [DOI] [PubMed] [Google Scholar]
- 25. Aghaee S, Deardorff J, Greenspan L, Quesenberry C Jr, Kushi L, Kubo A. Early life household intactness and timing of pubertal onset in girls: a prospective cohort study. BMC Pediatr. 2020;20:464. doi: 10.1186/s12887-020-02345-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Devareddy A, Sarraju A, Rodriguez F. Health disparities across the continuum of ASCVD risk. Curr Cardiol Rep. 2022;24:1129–1137. doi: 10.1007/s11886-022-01736-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawlor D, Davey Smith G, Ebrahim S. Association between childhood socioeconomic status and coronary heart disease risk among postmenopausal women: findings from the British Women's Heart and Health Study. Am J Public Health. 2004;94:1386–1392. doi: 10.2105/ajph.94.8.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang J, Yuan J, Zhang D, Liu W, Su P, Wan Y, Zhang Z, Tao F, Sun Y. Casual associations and shape between prepuberty body mass index and early onset of puberty: a Mendelian randomization and dose‐response relationship analysis. Front Endocrinol (Lausanne). 2022;13:853494. doi: 10.3389/fendo.2022.853494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aghaee S, Quesenberry C Jr, Deardorff J, Kushi L, Greenspan L, Ferrara A, Kubo A. Associations between infant growth and pubertal onset timing in a multiethnic prospective cohort of girls. BMC Pediatr. 2022;22:171. doi: 10.1186/s12887-022-03242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinkney J, Streeter A, Hosking J, Mostazir M, Jeffery A, Wilkin T. Adiposity, chronic inflammation, and the prepubertal decline of sex hormone binding globulin in children: evidence for associations with the timing of puberty (Earlybird 58). J Clin Endocrinol Metab. 2014;99:3224–3232. doi: 10.1210/jc.2013-3902 [DOI] [PubMed] [Google Scholar]
- 31. Barker D, Winter P, Osmond C, Margetts B, Simmonds S. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1 [DOI] [PubMed] [Google Scholar]
- 32. Zanetti D, Tikkanen E, Gustafsson S, Priest J, Burgess S, Ingelsson E. Birthweight, type 2 diabetes mellitus, and cardiovascular disease: addressing the barker hypothesis with Mendelian randomization. Circ Genom Precis Med. 2018;11:e002054. doi: 10.1161/CIRCGEN.117.002054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pedersen J. Weight and length at birth in infants of diabetic mothers. Acta Endocrinol. 1954;16:330–342. doi: 10.1530/acta.0.0160330 [DOI] [PubMed] [Google Scholar]
- 34. Boney C, Verma A, Tucker R, Vohr B. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808 [DOI] [PubMed] [Google Scholar]
- 35. Razaz N, Villamor E, Muraca G, Edstedt Bonamy A, Cnattingius S. Maternal obesity and risk of cardiovascular diseases in offspring: a population‐based cohort and sibling‐controlled study. Lancet Diabetes Endocrinol. 2020;8:572–581. doi: 10.1016/S2213-8587(20)30151-0 [DOI] [PubMed] [Google Scholar]
- 36. Lee K, Raja E, Lee A, Bhattacharya S, Bhattacharya S, Norman J, Reynolds R. Maternal obesity during pregnancy associates with premature mortality and major cardiovascular events in later life. Hypertension. 2015;66:93–844. doi: 10.1161/HYPERTENSIONAHA.115.05920 [DOI] [PubMed] [Google Scholar]
- 37. Reynolds R, Allan K, Raja E, Bhattacharya S, McNeill G, Hannaford P, Sarwar N, Lee A, Bhattacharya S, Norman J. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow‐up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bianco M, Josefson J. Hyperglycemia during pregnancy and long‐term offspring outcomes. Curr Diab Rep. 2019;19:143. doi: 10.1007/s11892-019-1267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Persson I, Ahlsson F, Ewald U, Tuvemo T, Qingyuan M, von Rosen D, Proos L. Influence of perinatal factors on the onset of puberty in boys and girls. Implications for interpretation of link with risk of long term diseases. Am J Epidemiol. 1999;150:747–755. doi: 10.1093/oxfordjournals.aje.a010077 [DOI] [PubMed] [Google Scholar]
- 40. Zhou J, Zhang F, Zhang S, Li P, Qin X, Yang M, Teng Y, Huang K. Maternal pre‐pregnancy body mass index, gestational weight gain, and pubertal timing in daughters: a systematic review and meta‐analysis of cohort studies. Obes Rev. 2022;23:e13418. doi: 10.1111/obr.13418 [DOI] [PubMed] [Google Scholar]
- 41. Binder A, Corvalan C, Mericq V, Pereira A, Santos J, Horvath S, Shepherd J, Michels K. Faster ticking rate of the epigenetic clock is associated with faster pubertal development in girls. Epigenetics. 2018;13:85–94. doi: 10.1080/15592294.2017.1414127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang R, Lillycrop K, Beilin L, Godfrey K, Anderson D, Mori T, Rauschert S, Craig J, Oddy W, Ayonrinde O, et al. Epigenetic age acceleration in adolescence associates with BMI, inflammation, and risk score for middle age cardiovascular disease. J Clin Endocrinol Metab. 2019;104:3012–3024. doi: 10.1210/jc.2018-02076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim C, Siscovick D, Sidney S, Lewis C, Kiefe C, Koepsell T. Oral contraceptive use and association with glucose, insulin, and diabetes in young adult women: the CARDIA study. Coronary Artery Risk Development in Young Adults. Diabetes Care. 2002;25:1027–1032. doi: 10.2337/diacare.25.6.1027 [DOI] [PubMed] [Google Scholar]
- 44. Cobb K, Kelsey J, Sidney S, Ettinger B, Lewis C. Oral contraceptives and bone mineral density in white and black women in CARDIA. Osteoporos Int. 2002;13:893–900. doi: 10.1007/s001980200123 [DOI] [PubMed] [Google Scholar]
- 45. Bild D, Jacobs D Jr, Liu K, Williams O, Hilner J, Perkins L, Marcovina S, Hulley S. Seven‐year trends in plasma low‐density‐lipoprotein‐cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6:235–245. doi: 10.1016/1047-2797(96)00005-1 [DOI] [PubMed] [Google Scholar]
- 46. Friedman G, Tekawa I, Grimm R, Manolio T, Shannon S, Sidney S. The leucocyte count: correlates and relationship to coronary risk factors: the CARDIA study. Int J Epidemiol. 1990;19:889–893. doi: 10.1093/ije/19.4.889 [DOI] [PubMed] [Google Scholar]
- 47. Folsom A, Qamhieh H, Flack J, Hilner J, Liu K, Howard B, Tracy R. Plasma fibrinogen: levels and correlates in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1993;138:1023–1036. doi: 10.1093/oxfordjournals.aje.a116821 [DOI] [PubMed] [Google Scholar]
- 48. Brabaharan S, Veettil S, Kaiser J, Rao V, Wattanayingcharoenchai R, Maharajan M, Insin P, Talungchit P, Anothaisintawee T, Thakkinstian A, et al. Association of hormonal contraceptive use with adverse health outcomes: an umbrella review of meta‐analyses of randomized clinical trials and cohort studies. JAMA Netw Open. 2022;5:e2143730. doi: 10.1001/jamanetworkopen.2021.43730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sidney S, Siscovick D, Petitti D, Schwartz S, Quesenberry C Jr, Psaty B, Raghunathan T, Kelaghan J, Koepsell T. Myocardial infarction and use of low‐dose oral contraceptives: a pooled analysis of 2 U.S. studies. Circulation. 1998;98:1053–1063. doi: 10.1161/01.cir.98.11.1058 [DOI] [PubMed] [Google Scholar]
- 50. Liao P, Dollin J. Half a century of the oral contraceptive pill. Can Fam Phys. 2012;58:e757–e760. [PMC free article] [PubMed] [Google Scholar]
- 51. Weill A, Dalichampt M, Raguideau F, Ricordeau P, Blotiere P, Rudant J, Alla F, Zureik M. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002. doi: 10.1136/bmj.i2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lidegaard O, Lokkegaard E, Jensen A, Skovlund C, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257–2266. doi: 10.1056/NEJMoa1111840 [DOI] [PubMed] [Google Scholar]
- 53. Roach R, Helmerhorst F, Lijfering W, Stijnen T, Algra A, Dekkers O. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;8:CD011054. doi: 10.1002/14651858.CD011054.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindley K, Bairey Merz C, Davis M, Madden T, Park K, Bello N; Group ACoCCDiWCatC‐OW . Contraception and reproductive planning for women with cardiovascular disease: JACC focus seminar 5/5. J Am Coll Cardiol. 2021;77:1823–1834. doi: 10.1016/j.jacc.2021.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chasan‐Taber L, Willett W, Manson J, Spiegelman D, Hunter D, Curhan G, Colditz G, Stampfer M. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94:483–489. doi: 10.1161/01.cir.94.3.483 [DOI] [PubMed] [Google Scholar]
- 56. Lopez L, Grimes D, Schulz K. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev. 2014;30:CD006133. doi: 10.1002/14651858.CD006133.pub4 [DOI] [PubMed] [Google Scholar]
- 57. Chasan‐Taber L, Willett W, Stampfer M, Hunter D, Colditz G, Spiegelman D, Manson J. A prospective study of oral contraceptives and NIDDM among U.S. women. Diabetes Care. 1997;20:330–335. doi: 10.2337/diacare.20.3.330 [DOI] [PubMed] [Google Scholar]
- 58. Yao W, Dong X, Yu X, Luo J, Zhang D. The use of oral contraceptive is inversely associated with the risk of type 2 diabetes mellitus among middle‐aged women. Gynecol Endocrinol. 2021;37:758–763. doi: 10.1080/09513590.2021.1932802 [DOI] [PubMed] [Google Scholar]
- 59. Fraison E, Kostova E, Moran L, Bilal S, Ee C, Venetis C, Costello M. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;8:CD005552. doi: 10.1002/14651858.CD005552.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. National Heart L, and Blood Institute . Cardiovascular risk across the lifespan for polycystic ovary syndrome workshop. 2021. https://www.nhlbi.nih.gov/events/2021/cardiovascular‐risk‐across‐lifespan‐polycystic‐ovary‐syndrome‐workshop
- 61. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz B. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod. 2016;31:2841–2855. doi: 10.1093/humrep/dew218 [DOI] [PubMed] [Google Scholar]
- 62. Azziz R, Carmina E, Dewailly D, Diamanti‐Kandarakis E, Escobar‐Morreale H, Futterweit W, Janssen O, Legro R, Norman R, Taylor A, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456. doi: 10.1016/j.fertnstert.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 63. Teede H, Misso M, Costello M, Dokras A, Laven J, Moran L, Piltonen T, Norman R; International PCOS Network . Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33:1602–1618. doi: 10.1093/humrep/dey256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Azziz R, Carmina E, Dewailly D, Diamanti‐Kandarakis E, Escobar‐Morreale H, Futterweit W, Janssen O, Legro R, Norman R, Taylor A, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178 [DOI] [PubMed] [Google Scholar]
- 65. Dapas M, Dunaif A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev. 2022;43:927–965. doi: 10.1210/endrev/bnac001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Calderon‐Margalit R, Siscovick D, Merkin S, Wang E, Daviglus M, Schreiner P, Sternfeld B, Williams O, Lewis C, Azziz R, et al. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid‐intima‐media thickness. Arterioscler Thromb Vasc Biol. 2014;34:2688–2694. doi: 10.1161/ATVBAHA.114.304136 [DOI] [PubMed] [Google Scholar]
- 67. Sternfeld B, Liu K, Quesenberry C Jr, Wang H, Jiang S, Daviglus M, Fornage M, Lewis C, mahan J, Schreiner P, et al. Changes over 14 years in androgenicity and body mass index in a biracial cohort of reproductive‐age women. J Clin Endocrinol Metab. 2008;93:2158–2165. doi: 10.1210/jc.2007-2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee J, Colangelo L, Schwartz J, Yano Y, Siscovick D, Seeman T, Schreiner P, Liu K, Lloyd‐Jones D, Greenland P. Associations of cortisol/testosterone and cortisol/sex hormone‐binding globulin ratios with atherosclerosis in middle‐age women. Atherosclerosis. 2016;248:203–209. doi: 10.1016/j.atherosclerosis.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang E, Calderson‐Margalit R, Cedars M, Daviglus M, Merkin S, Schreiner P, Sternfeld B, Wellons M, Schwartz S, Lewis C, et al. Polycystic ovary syndrome and risk for long‐term diabetes and dyslipidemia. Obstet Gynecol. 2011;117:6–13. doi: 10.1097/AOG.0b013e31820209bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang E, Ku I, Shah S, Daviglus M, Schreiner P, Konety S, Williams O, Siscovick D, Bibbins‐Domingo K. Polycystic ovary syndrome is associated with higher left ventricular mass index: the CARDIA Women's Study. J Clin Endocrinol Metab. 2012;97:4656–4662. doi: 10.1210/jc.2012-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greenwood E, Yaffe K, Wellons M, Cedars M, Huddleston H. Depression over the lifespan in a population‐based cohort of women with polycystic ovary syndrome: longitudinal analysis. J Clin Endocrinol Metab. 2019;104:2809–2819. doi: 10.1210/jc.2019-00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zahid S, Khan M, Gowda S, Faza N, Honigberg M, Vaught A, Guan C, Minhas A, Michos E. Trends, predictors and outcomes of cardiovascular complications associated with polycystic ovary syndrome during delivery hospitalizations: a National Inpatient Sample Analysis (2002–2019). JAHA. 2022;11:e025839. doi: 10.1161/JAHA.121.025839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Riestenberg C, Jagasia A, Markovic D, Buyalos R, Azziz R. Health care‐related economic burden of polycystic ovary syndrome in the United States: pregnancy‐related and long‐term health consequences. J Clin Endocrinol Metab. 2022;107:575–585. doi: 10.1210/clinem/dgab613 [DOI] [PubMed] [Google Scholar]
- 74. Kakoly N, Kohmami M, Joham A, Cooray S, Misso M, Norman R, Harrison C, Ranasinha S, Teede H, Moran L. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta‐regression. Hum Reprod Update. 2018;24:455–467. doi: 10.1093/humupd/dmy007 [DOI] [PubMed] [Google Scholar]
- 75. Wekker V, van Dammen L, Koning A, Heida K, Painter R, Limpens J, Laven J, van Lennep J, Roseboom T, Hoek A. Long‐term cardiometabolic disease risk in women with PCOS: a systematic review and meta‐analysis. Hum Reprod Update. 2020;26:942–960. doi: 10.1093/humupd/dmaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Groot P, Dekkers O, Romijn J, Dieben S, Helmerhorst F. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta‐analysis. Hum Reprod Update. 2011;17:495–500. doi: 10.1093/humupd/dmr001 [DOI] [PubMed] [Google Scholar]
- 77. Huddleston HG, Quinn MM, Kao CN, Lenhart N, Rosen MP, Cedars MI. Women with polycystic ovary syndrome demonstrate worsening markers of cardiovascular risk over the short‐term despite declining hyperandrogenaemia: results of a longitudinal study with community controls. Clin Endocrinol (Oxf). 2017;87:775–782. doi: 10.1111/cen.13497 [DOI] [PubMed] [Google Scholar]
- 78. Azziz R, Sanchez L, Knochenhauer E, Moran C, Lazenby J, Stephens K, Taylor K, Boots L. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462. doi: 10.1210/jc.2003-031122 [DOI] [PubMed] [Google Scholar]
- 79. Day F, Karaderi T, Jones M, Meun C, He C, Drong A, Kraft P, Lin N, Huang H, Broer L, et al. Large‐scale genome‐wide meta‐analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14:e1007813. doi: 10.1371/journal.pgen.1007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ding T, Baio G, Hardiman P, Petersen I, Sammon C. Diagnosis and management of polycystic ovary syndrome in the UK (2004–2014): a retrospective cohort study. BMJ Open. 2016;6:e012461. doi: 10.1136/bmjopen-2016-012461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. March W, Moore V, Willson K, Phillips D, Norman R, Davies M. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399 [DOI] [PubMed] [Google Scholar]
- 82. Kirkegaard H, Nohr E, Rasmussen K, Stovring H, Sorensen T, Lewis C, Gunderson E. Maternal prepregnancy waist circumference and BMI in relation to gestational weight gain and breastfeeding behavior: the CARDIA study. Am J Clin Nutr. 2015;102:393–401. doi: 10.3945/ajcn.114.099184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gunderson E, Chiang V, Lewis C, Catov J, Quesenberry C Jr, Sidney S, Wei G, Ness R. Long‐term blood pressure changes measured from before to after pregnancy relative to nonparous women. Obstet Gynecol. 2008;112:1294–1302. doi: 10.1097/AOG.0b013e31818da09b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gunderson E, Quesenberry C Jr, Lewis C, Tsai A, Sternfeld B, Smith West D, Sidney S. Development of overweight associated with childbearing depends upon smoking habit: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Obes Res. 2004;12:2041–2053. doi: 10.1038/oby.2004.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Smith D, Lewis C, Caveny J, Perkins L, Burke G, Bild D. Longitudinal changes in adiposity associated with pregnancy: the CARDIA Study. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- 86. Lewis C, Smith D, Caveny J, Perkins L, Burke G, Bild D. Associations of body mass and body fat distribution with parity among African‐American and Caucasian women: the CARDIA Study. Obes Res. 1994;2:517–525. doi: 10.1002/j.1550-8528.1994.tb00100.x [DOI] [PubMed] [Google Scholar]
- 87. Gunderson E, Sternfeld B, Wellons M, Whitmer R, Chiang V, Quesenberry C Jr, Lewis C, Sidney S. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity. 2008;16:1078–1084. doi: 10.1038/oby.2008.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gunderson E, Murtagh M, Lewis C, Quesenberry C, West D, Sidney S. Excess gains in weight and waist circumference associated with childbearing: the CARDIA study. Int J Obstet. 2004;28:525–535. doi: 10.1038/sj.ijo.0802551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Murtaugh M, Jacobs D, Moran A, Steinerger J, Sinaiko A. Relation of birth weight to fasting insulin, insulin resistance, and body size in adolescence. Diabetes Care. 2003;26:187–192. doi: 10.2337/diacare.26.1.187 [DOI] [PubMed] [Google Scholar]
- 90. Bennett W, Liu S, Yeh H, Nicholson W, Gunderson E, Lewis C, Clark J. Change in weight and health behaviors after pregnancies complicated by gestational diabetes mellitus: the CARDIA Study. Obesity (Silver Spring). 2013;21:1269–1275. doi: 10.1002/oby.20133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lewis C, Funkhouser E, Raczynski J, Sidney S, Bild D, Howard B. Adverse effect of pregnancy on high‐density lipoprotein (HDL) cholesterol in young adult women. Am J Epidemiol. 1996;144:247–254. doi: 10.1093/oxfordjournals.aje.a008919 [DOI] [PubMed] [Google Scholar]
- 92. Gunderson E, Lewis C, Murtaugh M, Quesenberry C, Smith‐West D, Sidney S. Long‐term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2004;159:1028–1039. doi: 10.1093/aje/kwh146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gunderson E, Whitmer R, Lewis C, Quesenberry C Jr, West D, Sidney S. Do long‐term HDL‐C declines associated with a first birth vary by apoE phenotype? The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Womens Health (Larchmt). 2005;14:917–928. doi: 10.1089/jwh.2005.14.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gunderson E, Jacobs D Jr, Chiang V, Lewis C, Tsai A, Quesenberry C Jr, Sidney S. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA Study. Am J Obstet Gynecol. 2009;201:e171–e179. doi: 10.1016/j.ajog.2009.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Clapp J III, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80:1469–1473. doi: 10.1016/s0002-9149(97)00738-8 [DOI] [PubMed] [Google Scholar]
- 96. Morris E, Hale S, Badger G, Magness R, Bernstein I. Pregnancy induces persistent changes in vascular compliance in primiparous women. Am J Obstet Gynecol. 2015;212:633.e1–633.e6. doi: 10.1016/j.ajog.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Riddell C, Harper S, Kaufman J. Trends in differences in U.S. mortality rates between black and white infants. JAMA Pediatr. 2017;171:911–913. doi: 10.1001/jamapediatrics.2017.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mustillo S, Krieger N, Gunderson E, Sidney S, McCreath H, Kiefe C. Self‐reported experiences of racial discrimination and Black‐White differences in preterm and low‐birthweight deliveries: the CARDIA study. Am J Public Health. 2004;94:2125–2131. doi: 10.2105/ajph.94.12.2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gavin A, Chae D, Mustillo S, Kiefe C. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA Study. J Womens Health (Larchmt). 2009;18:803–811. doi: 10.1089/jwh.2008.0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lane‐Cordova A, Gunderson E, Carnethon M, Catov J, Reiner A, Lewis C, Dude A, Greenland P, Jacobs D Jr. Pre‐pregnancy endothelial dysfunciton and birth outcomes: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertens Res. 2018;41:282–289. doi: 10.1038/s41440-018-0017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Harville E, Lewis C, Catov J, Jacobs D Jr, Gross M, Gunderson E. A longitudinal study of pre‐pregnancy antioxidant levels and subsequent perinatal outcomes in black and white women: the CARDIA Study. PLoS One. 2020;15:e0229002. doi: 10.1371/journal.pone.0229002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lane‐Cordova A, Carnethon M, Catov J, Montag S, Lewis C, Schreiner P, Dude A, Sternfeld B, Badon S, Greenland P, et al. Cardiorespiratory fitness, exercise haemodynamics and birth outcomes: the Coronary Artery Risk Development in Young Adults Study. BJOG. 2018;125:1127–1134. doi: 10.1111/1471-0528.15146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Harville E, Catov J, Lewis C, Bibbins‐Domingo K, Gunderson E. Pre‐pregnancy kidney function and subsequent adverse pregnancy outcomes. Pregnancy Hypertens. 2019;15:195–200. doi: 10.1016/j.preghy.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gamundi‐Segura S, Torres‐Perez E, Sanz‐Paris A, Arbones‐Mainar J. Interaciton of apoliprotein E gene polymorphisms on miscarriage risk in black and white American women. Fertil Steril. 2016;105:1554–1560. doi: 10.1016/j.fertnstert.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 105. Sun B, Bertolet M, Brooks M, Hubel C, Lewis C, Gunderson E, Catov J. Life course changes in cardiometabolic risk factors associated with preterm delivery: the 30‐year CARDIA study. J Am Heart Assoc. 2020;9:e015900. doi: 10.1161/JAHA.119.015900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Catov J, Althouse A, Lewis C, Harville E, Gunderson E. Preterm delivery and metabolic syndromeinwomen followed pregnancy through 25 years later. Obstet Gynecol. 2016;127:1127–1134. doi: 10.1097/AOG.0000000000001434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Catov J, Lewis C, Lee M, Wellons M, Gunderson E. Preterm birth and future maternal blood pressure, inflammation, and intimal‐medial thickness: the CARDIA Study. Hypertension. 2013;61:641–646. doi: 10.1161/HYPERTENSIONAHA.111.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Catov J, Snyder G, Fraser A, Lewis C, Liu K, Althouse A, Bertolet M, Gunderson E. Blood pressure patterns and subsequent coronary artery calcification in women who delivered preterm births. Hypertension. 2018;72:159–166. doi: 10.1161/HYPERTENSIONAHA.117.10693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Crump C, Sundquist J, Sundquist K. Preterm delivery and long‐term risk of hypertension in women. JAMA Cardiol. 2022;7:65–74. doi: 10.1001/jamacardio.2021.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Crump C, Sundquist J, Howell E, McLaughlin M, Stroustrup A, Sundquist K. Pre‐term delivery and risk of ischemic heart disease in women. J Am Coll Cardiol. 2020;76:57–67. doi: 10.1016/j.jacc.2020.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fraser A, Markovitz A, Haug E, Horn J, Romundstad P, Dalen H, Rich‐Edwards J, Asvold B. Ten‐year cardiovascular disease risk trajectories by obstetric history: a longitudinal study in the Norwegian Hunt Study. J Am Heart Assoc. 2022;11:e021733. doi: 10.1161/JAHA.121.021733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Retnakaran R, Shah B. Patterns of cardiovascular risk factors in the years before pregnancy in nulliparous women with and without preterm birth and small‐for‐gestational‐age delivery. J Am Heart Assoc. 2021;10:e021321. doi: 10.1161/JAHA.121.021321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gunderson E, Quesenberry C Jr, Jacobs D Jr, Feng J, Lewis C, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: the CARDIA study. Am J Epidemiol. 2010;172:1131–1143. doi: 10.1093/aje/kwq267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhang S, Folsom A, Flack J, Liu K. Body fat distribution before pregnancy and gestational diabetes: findings from coronary artery risk development in young adults (CARDIA) study. BMJ. 1995;311:1139–1140. doi: 10.1136/bmj.311.7013.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Catov J, Sun B, Bertolet M, Snyder G, Lewis C, Allen N, Shikany J, Ingram K, Appiah D, Gunderson E. Changes in cardiometabolic risk factors before and after gestational diabetes: a prospective life‐course analysis in CARDIA women. Obesity (Silver Spring). 2020;28:1397–1404. doi: 10.1002/oby.22848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Catov J, Sun B, Lewis C, Bertolet M, Gunderson E. Prepregnancy weight change associated with high gestational weight gain. Obesity (Silver Spring). 2022;30:524–534. doi: 10.1002/oby.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Whitaker K, Ingram K, Appiah D, Nicholson W, Bennett W, Lewis C, Reis J, Schreiner P, Gunderson E. Prepregnancy fitness and risk of gestational diabetes: a longitudinal analysis. Med Sci Sports Exerc. 2018;50:1613–1619. doi: 10.1249/MSS.0000000000001600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hedderson M, Williams M, Holt V, Weiss N, Ferrara A. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2008;198 409:e401–e407. doi: 10.1016/j.ajog.2007.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hedderson M. High blood pressure before and during early pregnancy is associated with an increased risk of gestational diabetes mellitus. Diabetes Care. 2008;31:2362–2367. doi: 10.2337/dc08-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shen Y, Hou L, Liu H, Wang L, Leng J, Li W, Hu G. Racial differences of incident diabetes postpartum in women with a history of gestational diabetes. J Diabetes Complications. 2019;33:107472. doi: 10.1016/j.jdiacomp.2019.107472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gunderson E, Chiang V, Pletcher M, Jacobs D Jr, Quesenberry C, Sidney S, Lewis C. History of gestational diabetes mellitus and future risk of atherosclerosis in mid‐life: the Coronary Artery Risk Development in Young Adults Study. J Am Heart Assoc. 2014;3:e000490. doi: 10.1161/JAHA.113.000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Appiah D, Schreiner P, Gunderson E, Konety S, Jacobs D Jr, Nwabuo C, Ebong I, Whitham H, Goff D Jr, Lima J, et al. Association of gestational diabetes mellitus with left ventricular structure and function: the CARDIA Study. Diabetes Care. 2016;39:400–407. doi: 10.2337/dc15-1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gunderson E, Sun B, Catov J, Carnethon M, Lewis C, Allen N, Sidney S, Wellons M, Rana J, Hou L, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA Study. Circulation. 2021;143:974–987. doi: 10.1161/CIRCULATIONAHA.120.047320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ajmera V, Gunderson E, VanWagner L, Lewis C, Carr J, Terrault N. Gestational diabetes mellitus is strongly associated with non‐alcoholic fatty liver disease. Am J Gastroenterol. 2016;111:658–664. doi: 10.1038/ajg.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dehmer E, Phadnis M, Gunderson E, Lewis C, Bibbins‐Domingo K, Engel S, Jonsson Funk M, Kramer H, Kshirsagar A, Heiss G. Association between gestational diabetes and incident maternal CKD: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Kidney Dis. 2018;71:112–122. doi: 10.1053/j.ajkd.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kramer C, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta‐analysis. Diabetologia. 2019;62:905–914. doi: 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- 127. Madsen L, Gerdøe‐Kristensen S, Lauenborg J, Damm P, Kesmodel U, Lynge E. Long‐term follow‐up on morbidity among women with a history of gestational diabetes mellitus: a systematic review. J Clin Endocrinol Metab. 2022. doi: 10.1210/clinem/dgac373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Moon J, Kwak S, Jung H, Choi S, Lim S, Cho Y, Park K, Jang H, Cho M. Weight gain and progression to type 2 diabetes in women with a history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015;100:3548–3555. doi: 10.1210/JC.2015-1113 [DOI] [PubMed] [Google Scholar]
- 129. Roberts J, Rich‐Edwards J, McElrath T, Garmire L, Myatt L; Global Pregnancy Collaboration . Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. 2021;77:1430–1441. doi: 10.1161/HYPERTENSIONAHA.120.14781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Leon L, McCarthy F, Direk K, Gonzalez‐Izuierdo A, Priet‐Merino D, Casas J, Chappell L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER Study. Circulation. 2019;140:1050–1060. doi: 10.1161/CIRCULATIONAHA.118.038080 [DOI] [PubMed] [Google Scholar]
- 131. Haug E, Horn J, Markovitz A, Fraser A, Klykken B, Dalen H, Vatten L, PR R, Rich‐Edwards J, Asvold B. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the Nord‐Trøndelag Health Study. JAMA CArdiol. 2019;4:628–635. doi: 10.1001/jamacardio.2019.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Grandi S, Filion K, Yoon S, Ayele H, Doyle C, Hutcheon J, Smith G, Gore G, Ray J, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 133. Wise L, Wang T, Wesselink A, Willis S, Chaiyasarikul A, Levinson J, Rothman K, Hatch E, Savitz D. Accuracy of self‐reported birth outcomes relative to birth certificate data in an Internet‐based prospective cohort study. Paediatr Pernat Epidemiol. 2021;35:590–595. doi: 10.1111/ppe.12769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hinkle S, Rawal S, Zhu Y, Grewal J, Albert P, Zhang C. Validation of self‐reported diagnosis of gestational diabetes at 6‐weeks postpartum. Epidemiology. 2017;28:747–752. doi: 10.1097/EDE.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chin H, Baird D, McConnaughey R, Weinberg C, Wilcox A, Jukic A. Long‐term recall of pregnancy‐related events. Epidemiology. 2017;28:575–579. doi: 10.1097/EDE.0000000000000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jowell A, Sarma A, Gulati M, Michos E, Vaught A, Natarajan P, Powe C, Honigberg M. Interventions to mitigate risk of cardiovascular disease after adverse pregnancy outcomes: a review. JAMA Cardiol. 2022;7:346–355. doi: 10.1001/jamacardio.2021.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lane‐Cordovia A, Puterman E, Gunderson E, Chan C, Hou L, Carnethon M. Gravidity is not associated with telomere length in a biracial cohort of middle‐aged women: the Coronary Artery Risk Development in Young Adults (CARDIA) study. PLoS One. 2017;12:e0186495. doi: 10.1371/journal.pone.0186495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gunderson E, Lewis C, Lin Y, Sorel M, Gross M, Sidney S, Jacobs D Jr, Shikany J, Quesenberry C Jr. Lactation duration and progression to diabetes in women across the childbearing years: the 30‐year CARDIA Study. JAMA Intern Med. 2018;178:328–337. doi: 10.1001/jamainternmed.2017.7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Appiah D, Lewis C, Jacobs D Jr, Shikany J, Quesenberry C Jr, Gross M, Carr J, Sidney S, Gunderson E. The association of lactation duration with visceral and pericardial fat volumes in parous women. J Clin Endocrinol Metab. 2021;106:1821–1831. doi: 10.1210/clinem/dgaa980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gunderson E, Quesenberry C Jr, Ning X, Jacobs D Jr, Gross M, Goff D Jr, Pletcher M, Lewis C. Lactation duration and midlife atherosclerosis. Obstet Gynecol. 2015;126:381–390. doi: 10.1097/AOG.0000000000000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gunderson E, Lewis C, Weis G, Whitmer R, Quesenberry C, Sidney S. Lactation and changes in maternal metabolic risk factors. Obstet Gynecol. 2007;109:729–738. doi: 10.1097/01.AOG.0000252831.06695.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gunderson E, Hedderson M, Chiang V, Crites Y, Walton D, Azevedo R, Fox G, Elmasian C, Young S, Salvador N, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012;35:50–56. doi: 10.2337/dc11-1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gunderson E, Crites Y, Chiang V, Walton D, Azevedo R, Fox G, Elmasian C, Young S, Salvador N, Lum M, et al. Influence of breastfeeding during the postpartum oral glucose tolerance test on plasma glucose and insulin. Obstet Gynecol. 2012;120:136–143. doi: 10.1097/AOG.0b013e31825b993d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang Z, Lai M, Piro A, Alexeeff S, Rost H, Dai F, Wheeler M, Gunderson E. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: the SWIFT Study. BMC Med. 2021;19:241. doi: 10.1186/s12916-021-02095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Knopp R, Bergelin R, Wahl P, Walden C. Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes. 1985;34:71–77. doi: 10.2337/diab.34.2.s71 [DOI] [PubMed] [Google Scholar]
- 146. McClure C, Catov J, Ness R, Schwarz E. Maternal visceral adiposity by consistency of lactation. Matern Child Health J. 2012;16:316–321. doi: 10.1007/s10995-011-0758-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hurston S, Ning X, Lo J, Crites Y, Walton D, Dewey K, Azevedo R, Young S, Fox G, Elmasian C, et al. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus: a prospective cohort study. Ann Intern Med. 2015;163:889–898. doi: 10.7326/M15-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stuebe A, Rich‐Edwards J, Willett W, Manson J, Michels K. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. doi: 10.1001/jama.294.20.2601 [DOI] [PubMed] [Google Scholar]
- 149. Tschiderer L, Seekircher L, Kunutsor S, Peters S, O'Keeffe L, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: systematic review and meta‐analysis involving data from 8 studies and 1,192,700 parous women. J Am Heart Assoc. 2022;11:e022746. doi: 10.1161/JAHA.121.022746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Stuebe A. Does breastfeeding prevent the metabolic syndrome, or does the metabolic syndrome prevent breastfeeding? Semin Perinatol. 2015;39:290–295. doi: 10.1053/j.semperi.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kair LR, Colaizy TT. Obese mothers have lower odds of experiencing pro‐breastfeeding hospital practices than mothers of normal weight: CDC pregnancy risk assessment monitoring system (PRAMS), 2004–2008. Matern Child Health J. 2016;20:593–601. doi: 10.1007/s10995-015-1858-z [DOI] [PubMed] [Google Scholar]
- 152. Hyatt H, Zhang Y, Hood W, Kavazis A. Physiological, mitochondrial, and oxidative stress differences in the presence or absence of lactation in rats. Reprod Biol Endocrinol. 2018;16. doi: 10.1186/s12958-12017-10317-12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vandyousefi S, Davis J, Gunderson E. Association of infant diet with subsequent obesity at 2–5 years among children exposed to gestational diabetes: the SWIFT study. Diabetologia. 2021;64:1121–1132. doi: 10.1007/s00125-020-05379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Choi Y, Nagel E, Kharoud H, Johnson K, Gallagher T, Duncan K, Kharbanda E, Fields D, Gale C, Jacobs K, et al. Getational diabetes mellitus is associated with differences in human milk and cytokine concentrations in a fully breastfeeding United States cohort. Nutrients. 2022;14:667. doi: 10.3390/nu14030667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bower J, Schreiner P, Sternfeld B, Lewis C. Black‐White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300–307. doi: 10.2105/AJPH.2008.133702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nair S, Slaughter J, Terry J, Appiah D, Ebong I, Wang E, Siscovick D, Sternfeld B, Schreiner P, Lewis C, et al. Anti‐mullerian hormone (AMH) is associated with natural menopause in a population‐based sample: the CARDIA Women's Study. Maturitas. 2015;81:493–498. doi: 10.1016/j.maturitas.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kim C, Slaughter J, Wang E, Appiah D, Schreiner P, Leader B, Calderon‐Margalit R, Sternfeld B, Siscovick D, Wellons M. Anti‐Mullerian hormone, follicle stimulating hormone, antral follicle count, and risk of menopause within 5 years. Maturitas. 2017;102:18–25. doi: 10.1016/j.maturitas.2017.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kim C, Slaughter J, Terry J, Jacobs D Jr, Parikh N, Appiah D, Leader B, Moravek M, Wellons M. Antimüllerian hormone and F2‐isoprostanes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Fertil Steril. 2020;114:646–652. doi: 10.1016/j.fertnstert.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kim C, Puterman E, Hou L, Slaughter J, Terry J, Wellons M. Antimüllerian hormone and leukocyte aging markers in the Coronary Artery Risk Development in Young Adults study. Fertil Steril. 2022;118:125–133. doi: 10.1016/j.fertnstert.2022.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wellons M, Lewis C, Schwartz S, Gunderson E, Schreiner P, Sternfeld B, Richman J, Sites C, Siscovick D. Racial differences in self‐reported infertility and risk factors for infertility in a cohort of black and white women: the CARDIA Women's Study. Fertil Steril. 2008;90:1640–1648. doi: 10.1016/j.fertnstert.2007.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ebong I, Schreiner P, Lewis C, Appiah D, Ghelani Z, Wellons M. The association between high‐sensitivity C‐reactive protein and hypertension in women of the CARDIA study. Menopause. 2016;23:662–668. doi: 10.1097/GME.0000000000000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Costanian C, McCague H, Edgell H, Ardern C, Tamim H. Changes in adiposity and other factors in relation to age at natural menopause: analyses from the CARDIA study. Menopause. 2019;26:162–171. doi: 10.1097/GME.0000000000001196 [DOI] [PubMed] [Google Scholar]
- 163. Ying W, Post W, Michos E, Subramanya V, Ndumele C, Ouyang P, Ambala‐Venkatesh B, De Vasconcellos H, Nwabuo C, Schreiner P, et al. Associations between menopause, cardiac remodeling, and diastolic function: the CARDIA study. Menopause. 2021;28:1166–1175. doi: 10.1097/GME.0000000000001815 [DOI] [PubMed] [Google Scholar]
- 164. Heravi A, Michos E, Zhao Y, Ambala‐Venkatesh B, Vasconcellos H, Lloyd‐Jones D, Schreiner P, Reis J, Wu C, Lewis C, et al. Oxidative stress and menopausal status: the Coronary Artery Risk Development in Young Adults Cohort study. J Womens Health (Larchmt). 2022;31:1057–1065. doi: 10.1089/jwh.2021.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Laughlin‐Tommaso S, Fuchs E, Wellons M, Lewis C, Calderon‐Margalit R, Stewart E, Schreiner P. Uterine fibroids and the risk of cardiovascular disease in the Coronary Artery Risk Development in Young Adult Women's Study. J Womens Health (Larchmt). 2019;28:46–52. doi: 10.1089/jwh.2018.7122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Appiah D, Schreiner P, Nwabuo C, Wellons M, Lewis C, Lima J. The association of surgical versus natural menopause with future left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Menopause. 2017;24:1269–1276. doi: 10.1097/GME.0000000000000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Freaney P, Petito L, Colangelo L, Lewis C, Schreiner P, Terry J, Wellons M, Kim C, Rana J, Lloyd‐Jones D, et al. Association of premature menopause with coronary artery calcium: the CARDIA study. Circ Cardiovasc Imaging. 2021;14:e012959. doi: 10.1161/CIRCIMAGING.121.012959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Appiah D, Nwabuo C, Ebong E, Vasconcellos H, Wellons M, Lewis C, Lima J, Schreiner P. The association of age at natural menopause with pre‐ to postmenopausal changes in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Menopause. 2022;29:564–572. doi: 10.1097/GME.0000000000001950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wellons M, Ouyang P, Schreiner P, Herrington D, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi‐Ethnic Study of Atherosclerosis (MESA). Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Muka T, Oliver‐Williams C, Kunutsor S, Laven J, Fauser B, Chowdhury R, Kavousi M, Franco O. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all‐cause mortality: a systematic review and meta‐analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415 [DOI] [PubMed] [Google Scholar]
- 171. Matthews K, Crawford S, Chae C, Everson‐Rose S, Sowers M, Sternfeld B, Sutton‐Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Matthews K, Gibson C, El Khoudary S, Thurston R. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: study of Women's Health Across the Nation. J Am Coll Cardiol. 2013;62:191–200. doi: 10.1016/j.jacc.2013.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhu D, Chung HF, Pandeya N, Dobson AJ, Hardy R, Kuh D, Brunner EJ, Bruinsma F, Giles GG, Demakakos P, et al. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. Eur J Epidemiol. 2019;34:235–246. doi: 10.1007/s10654-019-00490-w [DOI] [PubMed] [Google Scholar]
- 174. Freaney P, Ning H, Carnethon M, Allen N, Wilkins J, Lloyd‐Jones D, Khan S. Premature menopause and 10‐year risk prediction of atherosclerotic cardiovascular disease. JAMA Cardiol. 2021;6:1463–1465. doi: 10.1001/jamacardio.2021.3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. El Khoudary S, Zhao Q, Venugopal V, Manson J, Brooks M, Santoro N, Black D, Harman S, Cedars M, Hopkins P, et al. Effects of hormone therapy on heart fat and coronary artery calcification progression: secondary analysis from the KEEPS trial. J Am Heart Assoc. 2019;8:e012763. doi: 10.1161/JAHA.119.012763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hodis H, Mack W, Henderson V, Shoupe D, Budoff M, Hwang‐Levine J, Li Y, Feng M, Dustin L, Kono N, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374:1221–1231. doi: 10.1056/NEJMoa1505241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Honigberg M, Zekavat S, Niroula A, Griffin G, Bick A, Pirruccello J, Nakao T, Whitesel E, Farland L, Laurie C, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. 2021;143:410–423. doi: 10.1161/CIRCULATIONAHA.120.051775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lu A, Quach A, Wilson J, Reiner A, Aviv A, Raj K, Hou L, Baccarelli A, Li Y, Stewart J, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11:303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Shenassa E, Rossen L. Telomere length and age‐at‐menopause in the U.S. Maturitas. 2015;82:215–221. doi: 10.1016/j.maturitas.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ebong IA, Watson KE, Goff DC, Bluemke DA, Srikanthan P, Horwich T, Bertoni AG. Age at menopause and incident heart failure: the Multi‐Ethnic Study of Atherosclerosis. Menopause. 2014;21:585–591. doi: 10.1097/GME.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rahman I, Akesson A, Wolk A. Relationship between age at natural menopause and risk of heart failure. Menopause. 2015;22:12–16. doi: 10.1097/gme.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 182. Ebong IA, Wilson MD, Bertoni AG, Appiah D, Polonsky T, Michos ED, Ballantyne C, Chang P. High‐sensitivity cardiac troponin T and the risk of heart failure in postmenopausal women of the ARIC Study. Menopause. 2021;28:284–291. doi: 10.1097/gme.0000000000001705 [DOI] [PubMed] [Google Scholar]
- 183. Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of age at menopause with incident heart failure: a prospective cohort study and meta‐analysis. J Am Heart Assoc. 2016;5:e003769. doi: 10.1161/JAHA.116.003769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Li Y, Zhao D, Wang M, Sun JY, Liu J, Qi Y, Hao YC, Deng QJ, Liu J, Liu J, et al. Combined effect of menopause and cardiovascular risk factors on death and cardiovascular disease: a cohort study. BMC Cardiovasc Disord. 2021;21:109. doi: 10.1186/s12872-021-01919-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ebong IA, Wilson MD, Appiah D, Michos ED, Racette SB, Villablanca A, Breathett K, Lutsey PL, Wellons M, Watson KE, et al. Relationship between age at menopause, obesity, and incident heart failure: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2022;11:e024461. doi: 10.1161/jaha.121.024461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ebong IA, Watson KE, Goff DC, Bluemke DA, Srikanthan P, Horwich T, Bertoni AG. Association of menopause age and N‐terminal pro brain natriuretic peptide: the Multi‐Ethnic Study of Atherosclerosis. Menopause. 2015;22:527–533. doi: 10.1097/GME.0000000000000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ebong IA, Wilson MD, Chang P, Appiah D, Polonsky T, Ballantyne C, Bertoni AG. NT‐pro B‐type natriuretic peptide, early menopause, and incident heart failure in postmenopausal women of the ARIC study. Menopause. 2022;29:309–316. doi: 10.1097/gme.0000000000001916 [DOI] [PubMed] [Google Scholar]
- 188. Ying W, Zhao D, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Sharma K, Shah SJ, Heckbert SR, Lima JA, et al. Sex hormones and change in N‐terminal pro‐B‐type natriuretic peptide levels: the multi‐ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2018;103:4304–4314. doi: 10.1210/jc.2018-01437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lane‐Cordova A, Gunderson E, Greenland P, Catov J, Lewis C, Gabriel K, Wellons M, Carnethon M. Life‐course reproductive history and cardiovascular risk profile in late mid‐life: the CARDIA study. J Am Heart Assoc. 2020;9:e014859. doi: 10.1161/JAHA.119.014859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Muka T, Oliver‐Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, Kavousi M, Franco O. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: a systematic review and meta‐analysis. PLoS One. 2016;11:e0157417. doi: 10.1371/journal.pone.0157417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Thurston R, Aslanidou Vlachos H, Derby C, Jackson E, Brooks M, Matthews K, Harlow S, Joffe H, El Khoudary S. Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. J Am Heart Assoc. 2021;10:e017416. doi: 10.1161/JAHA.120.017416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Bray MJ, Wellons MF, Jones SH, Torstenson ES, Edwards TL, Velez Edwards DR. Transethnic and race‐stratified genome‐wide association study of fibroid characteristics in African American and European American women. Fertil Steril. 2018;110:737–745.e734. doi: 10.1016/j.fertnstert.2018.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Lunetta KL, Day FR, Sulem P, Ruth KS, Tung JY, Hinds DA, Esko T, Elks CE, Altmaier E, He C, et al. Rare coding variants and X‐linked loci associated with age at menarche. Nat Commun. 2015;6:7756. doi: 10.1038/ncomms8756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik‐Sullivan B, et al. Large‐scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1‐mediated DNA repair. Nat Genet. 2015;47:1294–1303. doi: 10.1038/ng.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Coviello AD, Haring R, Wellons M, Vaidya D, Lehtimäki T, Keildson S, Lunetta KL, He C, Fornage M, Lagou V, et al. A genome‐wide association meta‐analysis of circulating sex hormone‐binding globulin reveals multiple Loci implicated in sex steroid hormone regulation. PLoS Genet. 2012;8:e1002805. doi: 10.1371/journal.pgen.1002805 [DOI] [PMC free article] [PubMed] [Google Scholar]


