Abstract
Background
It has been suggested that chronic hypertension is a risk factor for negative maternal and fetal outcomes during pregnancy and postpartum. We aimed to estimate the association of chronic hypertension on adverse maternal and infant outcomes and assess the impact of antihypertensive treatment and these outcomes.
Methods and Results
Using data from the French national health data system, we identified and included in the CONCEPTION cohort all women in France who delivered their first child between 2010 and 2018. Chronic hypertension before pregnancy was identified through antihypertensive medication purchases and by diagnosis during hospitalization. We assessed the incidence risk ratios (IRRs) of maternofetal outcomes using Poisson models. A total of 2 822 616 women were included, and 42 349 (1.5%) had chronic hypertension and 22 816 were treated during pregnancy. In Poisson models, the adjusted IRR (95% CI) of maternofetal outcomes for women with hypertension were as follows: 1.76 (1.54–2.01) for infant death, 1.73 (1.60–1.87) for small gestational age, 2.14 (1.89–2.43) for preterm birth, 4.58 (4.41–4.75) for preeclampsia, 1.33 (1.27–1.39) for cesarean delivery, 1.84 (1.47–2.31) for venous thromboembolism, 2.62 (1.71–4.01) for stroke or acute coronary syndrome, and 3.54 (2.11–5.93) for maternal death postpartum. In women with chronic hypertension, being treated with an antihypertensive drug during pregnancy was associated with a significantly lower risk of obstetric hemorrhage, stroke, and acute coronary syndrome during pregnancy and postpartum.
Conclusions
Chronic hypertension is a major risk factor of infant and maternal negative outcomes. In women with chronic hypertension, the risk of pregnancy and postpartum cardiovascular events may be decreased by antihypertensive treatment during pregnancy.
Keywords: antihypertensive agents, blood pressure, epidemiology, hypertension, pregnancy complication
Subject Categories: High Blood Pressure, Hypertension, Epidemiology, Pregnancy
Nonstandard Abbreviations and Acronyms
- CONCEPTION
Cohort of Cardiovascular Diseases in Pregnancy
- IRR
incidence risk ratio
Clinical Perspective.
What Is New?
Of primiparous women, 1.5% had chronic hypertension, and 54% of women with hypertension were treated with antihypertensive drugs during pregnancy.
In primiparous women, chronic hypertension was associated with infant death, small gestational age, preterm birth, preeclampsia, cesarean delivery, venous thromboembolism, stroke or acute coronary syndrome, and maternal death postpartum.
In women with chronic hypertension, being treated with an antihypertensive drug during pregnancy was associated with a significantly lower risk of obstetric hemorrhage, stroke, and acute coronary during pregnancy and postpartum.
What Are the Clinical Implications?
Chronic hypertension is a major risk factor of infant and maternal negative outcomes.
In women with chronic hypertension, the risk of pregnancy and postpartum cardiovascular events may be decreased by antihypertensive treatment during pregnancy.
Chronic hypertension in pregnancy, defined as hypertension predating pregnancy or diagnosed before 20 weeks of gestation, concerns 1% to 4% of pregnancies in the United States and 1.7% of pregnancies in France. 1 , 2 , 3 Previous studies reported an increasing temporal trend in the prevalence of chronic hypertension and other hypertensive disorders of pregnancy, presumably correlated with older maternal age at first birth and an increased prevalence of obesity. 1 , 4 , 5 , 6 It has been suggested that chronic hypertension is a risk factor for negative maternal and fetal outcomes during pregnancy and postpartum 7 , 8 , 9 , 10 , 11 , 12 and that these correlations are not entirely mediated by the excess risk of preeclampsia. 8 A recent systematic review and meta‐analysis of 94 studies found that chronic hypertension was significantly associated with many negative maternal and perinatal outcomes, including preeclampsia, cesarean delivery, maternal mortality, preterm birth, stillbirth, and small‐for‐gestational age (SGA). 7 However, few studies to date have assessed the association between chronic hypertension and maternal death or major cardiovascular events, such as acute coronary syndrome (ACS), 13 , 14 stroke, 15 and thromboembolism, 16 during pregnancy and postpartum.
Current guidelines for the management of hypertension and cardiovascular diseases during pregnancy recommend initiating pharmacological treatment in all women with severe hypertension and those with mild to moderate hypertension and a high cardiovascular risk. 17 , 18 , 19 However, the impact of antihypertensive treatment during pregnancy on maternal and fetal outcomes is debated, and evidence remains scarce. 20 The most recent Cochrane review on this topic concluded that antihypertensive drug therapy for mild to moderate hypertension during pregnancy probably halves the risk of developing severe hypertension but may have little or no effect on other clinically important outcomes, including child and mother death, preeclampsia, preterm birth, and fetal growth restriction. 21 In the CHAP (Chronic Hypertension and Pregnancy) randomized controlled trial, Tita et al 22 found that a strategy of targeting a blood pressure of <140/90 mm Hg was associated with better pregnancy outcomes than a strategy of reserving treatment only for severe hypertension, with no increase in the risk of SGA birth weight.
To the best of our knowledge, the impact of antihypertensive treatment for chronic hypertension on the occurrence of major cardiovascular events during pregnancy (ACS, stroke, venous thromboembolism) has never been assessed.
In this context, we aimed to estimate the impact of chronic hypertension on adverse maternal and infant outcomes, including cardiovascular events, in women in France and assess the impact of antihypertensive treatment on these outcomes.
METHODS
Data Source
CONCEPTION (Cohort of Cardiovascular Risk in Pregnancy) is an ongoing prospective cohort including all women resident in France who gave birth in France between January 1, 2010, and December 31, 2018. A detailed description of the cohort protocol is available in previous articles. 2 , 23 Cohort data were extracted from the French national health insurance information system database (Système National des Données de Santé), 21 , 23 , 24 which contains comprehensive information on all health care expenditures reimbursed by France's national health insurance system for the entire population. Specifically, it contains information about all public and private hospital stays, including diagnosis and medical interventions, as well as information on outpatient care, including all reimbursements for drug purchases.
In line with the French national regulations and ethics committee, participant consent and institutional review board approval were not required for this study. Santé Publique France—the French public health agency—has full and permanent access to the Système National des Données de Santé (governmental deliberation No. 2016–316, October 13, 2016). We cannot share national health data system data as they are only available on a secure portal. Authorization to access this portal needs registration and clearance.
Study Population
The present study included all first deliveries of women enrolled in the CONCEPTION cohort (hereafter referred to as primiparous) who gave birth in a hospital after 22 weeks of gestation between January 1, 2010, and December 31, 2018. Before 22 weeks of gestation, fetal losses are defined as miscarriages and are therefore not always identifiable in the Système National des Données de Santé. Women who underwent a termination of pregnancy for a maternal or fetal reason were excluded as were those with a history of stroke, ACS, or heart failure after January 1, 2006. The study population was divided into 2 subpopulations according to the presence or not of chronic hypertension before pregnancy. The latter was defined as the dispensing of antihypertensive medication on at least 3 different dates between 1 year preceding the pregnancy and 20 weeks of gestation or on 2 different dates if at least 1 large package of antihypertensive drugs was dispensed or if they were hospitalized with a primary diagnosis of preexisting chronic hypertension (International Classification of Diseases, Tenth Revision [ICD‐10] codes: O10, O11) during pregnancy or postpartum. Chronic hypertension was considered treated during pregnancy if antihypertensive medication had been dispensed at least once between 20 weeks of gestation and delivery. Antihypertensive treatment initiated after ACS, stroke, or heart failure during pregnancy was presumed to have been prescribed as a secondary prevention treatment and was therefore not considered in the identification of treated hypertension during pregnancy. 2
Outcomes
We searched for adverse maternal outcomes from the date of pregnancy to the sixth week postpartum by identifying hospitalizations with the following ICD‐10 codes: delivery and postpartum hemorrhage, hereafter referred to as obstetric hemorrhage (O46, O67, O72), stroke (I60 to I64), venous thromboembolism (I80 to I82, O223, O871, O879, I676, I636, O873, O225, O229, O882, I26), ACS (I20 to I23), and preeclampsia (O14). Gestational diabetes was identified using an algorithm combining the delivery of insulin and glucose strips or a diagnosis of diabetes during pregnancy (E10 to E14, O24) with no preexisting diabetes. Because the study included only women who delivered, maternal death was recorded only during the postpartum period.
The following infant and maternal outcomes and covariates were identified from hospital maternity discharge summaries: multiple pregnancy, delivery mode (vaginal or cesarean delivery), infant death, premature birth defined as a live birth before 37 weeks of gestation and SGA (ie, <10th percentile for sex and gestational age).
Women's Characteristics and Covariates
Women who benefited from Universal Medical Coverage (Couverture médicale universelle complémentaire), a social benefit in France for those whose income is below a certain ceiling, were defined as living in social deprivation. Smoking was identified by specific coding during hospitalization (F17, Z716, Z720, T652), by the reimbursement of payments for nicotine replacement treatments before or during pregnancy, or by a “newborn affected by maternal use of tobacco” (P042) diagnosis. Previous diabetes was identified by the dispensing of at least 3 antidiabetic prescriptions on 3 different dates (or on 2 dates if at least 1 large package of antidiabetic drugs was dispensed) in the year preceding pregnancy. A personal history of venous thromboembolic disease was identified between January 1, 2006, and the date of pregnancy using the criteria cited previously (see Outcomes). Obesity was identified from maternity hospital discharge reports. Gestational age was expressed in completed weeks of amenorrhea.
For women with chronic hypertension before pregnancy, we identified the dispensing of antihypertensive medications, which we divided into the following 5 categories: (1) angiotensin‐converting enzyme inhibitors and angiotensin II receptor antagonists, (2) β‐blockers, (3) diuretics, (4) calcium channel blockers, and (5) other.
Antihypertensive Drugs During Pregnancy
For women with chronic hypertension treated during pregnancy, we identified the dispensing of antihypertensive drugs at each trimester of pregnancy and during the 6 weeks postpartum period. Angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, and aliskiren were considered contraindicated drugs during pregnancy (formally contraindicated in the second and third trimesters). Methyldopa, nicardipine, labetalol, and nifedipine were considered indicated drugs during pregnancy. All other molecules were considered nonindicated during pregnancy. 25
Statistical Analysis
We described mothers' and children's characteristics in the total population and according to a diagnosis of chronic hypertension before pregnancy by calculating numbers and percentages for categorical variables and mean and standard deviation for the age. Maternal and infant outcomes were described according to the existence of chronic hypertension and the treatment of hypertension during pregnancy. The frequency of these outcomes was compared using a χ2 test between the chronic hypertension and no hypertension groups and between the treated hypertension and untreated hypertension groups. The distribution of different gestational ages at birth was described by curves according to the existence of chronic hypertension and the treatment of hypertension during pregnancy.
We performed univariate and multivariate Poisson regression models to estimate the incidence risk ratios (IRRs) of maternal and infant outcomes according to chronic hypertension versus no hypertension, treated hypertension versus no hypertension, and untreated hypertension versus no hypertension, with a 95% CI. Multivariate Poisson models were performed corrected for overdispersion and adjusted for maternal age (years), social deprivation, obesity, tobacco use, history of diabetes, multiple pregnancy, gestational diabetes, and preeclampsia. Models estimating the IRRs of gestational diabetes and preeclampsia were adjusted on these covariates except for gestational diabetes.
As a sensibility analysis, we computed a propensity score of being treated during pregnancy for women with chronic hypertension before pregnancy based on a logistic regression model. All previously cited covariates were included in the propensity score plus the year of childbirth. We then estimated the odds ratios of maternal and infant outcomes using logistic regression models (crude and weighted on the inverse probability of treatment). The purpose of this sensibility analysis was to assess whether the associations between mother and child outcomes and antihypertensive treatment during pregnancy persist when a propensity score instead of a multiple adjustment controls the indication bias.
For women with chronic hypertension treated during pregnancy, we calculated the numbers and percentages of women who took only indicated antihypertensive drugs, nonindicated drugs, or contraindicated drugs.
RESULTS
We identified 2 833 376 primiparous women who gave birth in France during the period from January 1, 2010, to December 31, 2018 (Figure 1). After the exclusion of 7847 terminations of pregnancy for maternal or fetal medical indication and 2913 women with histories of stroke, ACS, or heart failure, the analysis population included 2 822 616 women. Of these, 42 349 (1.5%) had chronic hypertension before pregnancy, and 22 816 were treated for this condition during pregnancy (54% of women with chronic hypertension). Table 1 shows the population's characteristics according to the existence of chronic hypertension before pregnancy. Women with chronic hypertension were, on average, >3 years older and were more likely to smoke, live in social deprivation, be obese and diabetic, and have previous venous thromboembolism medical history.
Figure 1. Study flowchart.
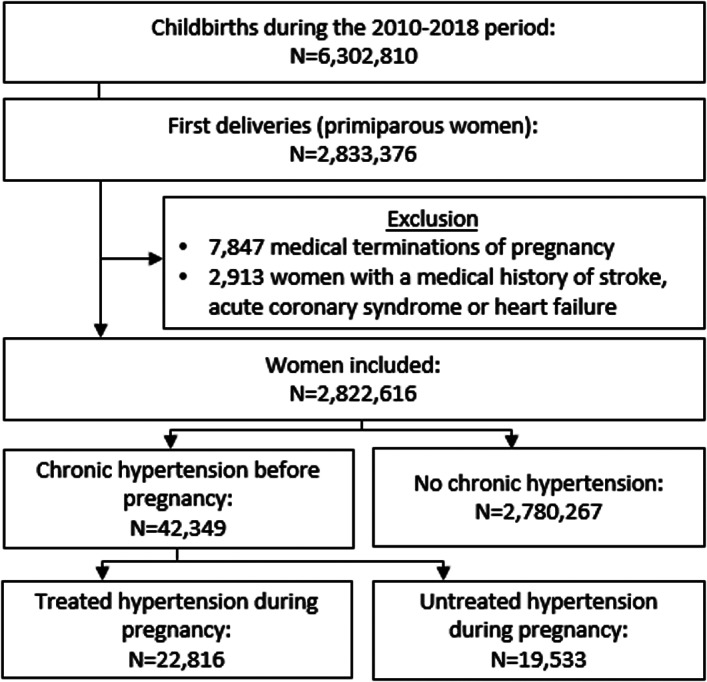
Table 1.
Population Characteristics and Dispensing of Antihypertensive Drugs
| Total, N=2 822 616 | Chronic hypertension before pregnancy | |||||
|---|---|---|---|---|---|---|
| No, n=2 780 267 | Yes, n=42 349 | |||||
| No. or mean | Percentage or SD | No. or mean | Percentage or SD | No. or mean | Percentage or SD | |
| Maternal characteristic | ||||||
| Maternal age, y | 28.29 | (5.38) | 28.29 | (5.36) | 28.29 | (6.01) |
| Multiple pregnancy | 56 544 | 2.00 | 55 210 | 1.99 | 1334 | 3.15 |
| Smoking | 251 303 | 8.90 | 246 635 | 8.87 | 4668 | 11.02 |
| Social deprivation | 389 005 | 13.78 | 382 864 | 13.77 | 6141 | 14.50 |
| Medical history | ||||||
| Obesity | 116 556 | 4.13 | 110 410 | 3.97 | 6146 | 14.51 |
| Diabetes | 14 510 | 0.51 | 12 783 | 0.46 | 1727 | 4.08 |
| Previous veinous thromboembolism | 3491 | 0.12 | 3321 | 0.12 | 170 | 0.40 |
| Antihypertensive drugs dispensing before 20 wks of gestation* | ||||||
| ACE inhibitors/AIIRA | … | … | … | … | 8078 | 19.07 |
| β‐blockers | … | … | … | … | 22 171 | 52.35 |
| Diuretics | … | … | … | … | 3696 | 8.73 |
| Calcium channel blockers | … | … | … | … | 8194 | 19.35 |
| Other | … | … | … | … | 5908 | 13.95 |
| None | … | … | … | … | 33 463 | 79.02 |
SD data are shown in parentheses. ACE indicates angiotensin‐converting enzyme; and AIIRA, angiotensin II receptor antagonist.
Number of women diagnosed with chronic hypertension before pregnancy with at least 1 dispensing of antihypertensive drugs between 1 year before the date of pregnancy and 20 weeks of amenorrhea.
All pregnancy and maternal outcomes were statistically more frequent in women with chronic hypertension than in women without chronic hypertension (Table 2). Singletons born in 2013 or after had mean birth weights of 3214.5 g and 2963.8 g for mothers without and with chronic hypertension, respectively (mean difference = 251 g).
Table 2.
Numbers and Proportions of Infant and Maternal Adverse Events, According to Hypertension Status
| No hypertension, n=2 780 267 | Chronic hypertension, n=42 349 | Chronic hypertension treated during pregnancy, n=22 816 | Chronic hypertension untreated during pregnancy, n=19 533 | Chronic hypertension vs no hypertension, P value | Treated hypertension vs untreated hypertension, P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage | No. | Percentage | No. | Percentage | No. | Percentage | |||
| Infant events | ||||||||||
| Infant death | 12 136 | 0.44 | 355 | 0.84 | 201 | 0.88 | 154 | 0.79 | <0.0001 | 0.2977 |
| Small‐for‐gestational age* | 220 449 | 13.96 | 5203 | 24.38 | 3223 | 28.77 | 1980 | 19.54 | <0.0001 | <0.0001 |
| Premature delivery threat | 277 722 | 9.99 | 5514 | 13.02 | 3288 | 14.41 | 2226 | 11.40 | <0.0001 | <0.0001 |
| Preterm birth† | 201 767 | 7.30 | 8010 | 19.11 | 4857 | 21.52 | 3153 | 16.30 | <0.0001 | <0.0001 |
| Preterm birth stages† | <0.0001 | <0.0001 | ||||||||
| Moderate preterm (≥32 WG) | 170 722 | 6.17 | 6093 | 14.54 | 3682 | 16.32 | 2411 | 12.47 | ||
| Very preterm (27–31 WG) | 24 271 | 0.88 | 1569 | 3.74 | 983 | 4.36 | 586 | 3.03 | ||
| Extremely preterm (<27 WG) | 6774 | 0.24 | 348 | 0.83 | 192 | 0.85 | 156 | 0.81 | ||
| Maternal events | ||||||||||
| Gestational diabetes | 229 593 | 8.26 | 7110 | 16.79 | 4547 | 19.93 | 2563 | 13.12 | <0.0001 | <0.0001 |
| Preeclampsia | 75 222 | 2.71 | 7237 | 17.09 | 4124 | 18.08 | 3113 | 15.94 | <0.0001 | <0.0001 |
| Cesarean delivery | 736 920 | 26.51 | 20 864 | 49.27 | 12610 | 55.27 | 8254 | 42.26 | <0.0001 | <0.0001 |
| Obstetric hemorrhage | 158 957 | 5.72 | 2982 | 7.04 | 1582 | 6.93 | 1400 | 7.17 | <0.0001 | 0.35 |
| Venous thromboembolism | 3281 | 0.12 | 121 | 0.29 | 62 | 0.27 | 59 | 0.30 | <0.0001 | 0.56 |
| ACS or stroke | 770 | 0.03 | 45 | 0.11 | 16 | 0.07 | 29 | 0.15 | <0.0001 | 0.01 |
| Death postpartum | 152 | 0.01 | 13 | 0.03 | 9 | 0.04 | 4 | 0.02 | <0.0001 | 0.27 |
ACS indicates acute coronary syndrome; and WG, weeks of gestation.
Birth weight was available from 2013; percentages for small for gestational age are calculated for available data for the period from 2013 to 2018.
Preterm percentages are calculated for live births.
In fully adjusted Poisson models (Figure 2), the IRRs (95% CI) of infant outcomes for women with chronic hypertension were 1.76 (1.54–2.01) for infant death, 1.73 (1.60–1.87) for SGA, and 2.14 (1.89–2.43) for preterm birth compared with women without chronic hypertension. The adjusted IRRs for maternal outcomes were 1.29 (1.25–1.33) for gestational diabetes, 4.58 (4.41–4.75) for preeclampsia, 1.33 (1.27–1.39) for cesarean delivery, 1.09 (1.05–1.14) for obstetric hemorrhage, 1.84 (1.47–2.31) for venous thromboembolism, 2.67 (1.71–4.01) for stroke or ACS, and 3.54 (2.11–5.93) for maternal death postpartum. IRRs were higher in women with treated hypertension during pregnancy for gestational diabetes and preeclampsia. Figure 3 shows the frequency curves of gestational age at birth according to the existence of chronic hypertension and hypertension treatment during pregnancy. Women with chronic hypertension gave birth on average 1 week earlier than women without chronic hypertension (37.9 weeks of amenorrhea versus 39.0 weeks).
Figure 2. Adjusted IRRs of infant (A) and maternal (B) outcomes according to prepregnancy hypertension and the treatment of hypertension during pregnancy.
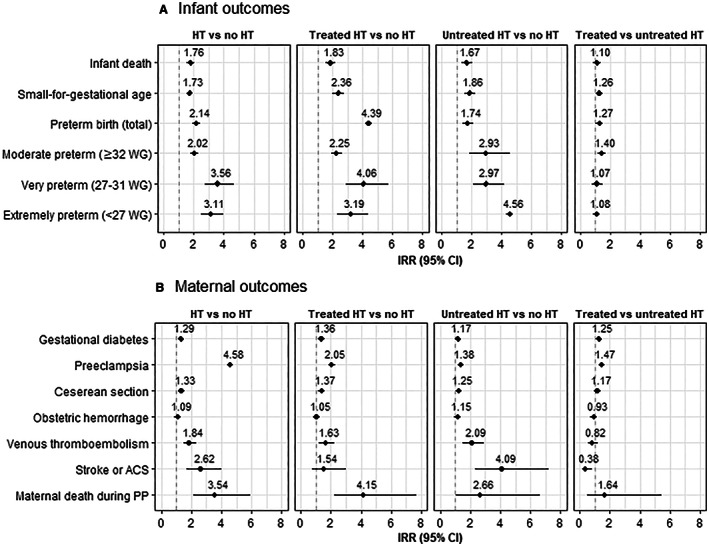
IRRs of preeclampsia and gestational diabetes were adjusted for maternal age (years), deprivation (Couverture médicale universelle complémentaire), obesity, tobacco use, history of diabetes, and multiple pregnancy. Other IRRs were adjusted for factors cited previously plus gestational diabetes. Birth weight was available from 2013, low birth weight IRRs were calculated for available data at the period from 2013 to 2018. Preterm IRRs were calculated for live births. ACS indicates acute coronary syndrome; HT, hypertension; IRR, incidence risk ratio; PP, postpartum; and WG, weeks of gestation.
Figure 3. Distribution of gestational age at birth according to chronic hypertension and antihypertensive treatment.
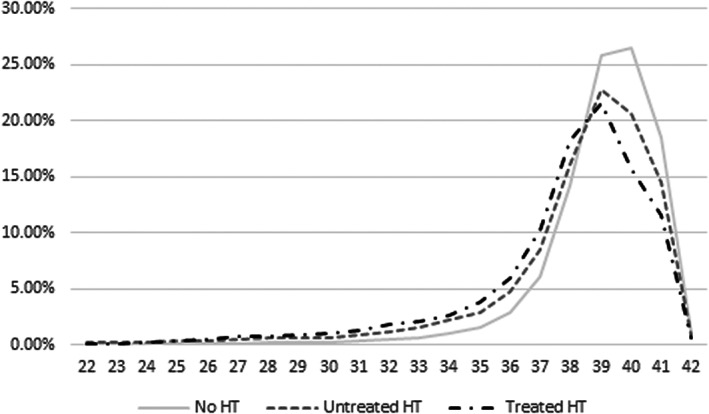
HT indicates hypertension.
In women with chronic hypertension, being treated with an antihypertensive drug during pregnancy was associated with a significant excess risk (adjusted IRR [95% CI]) of SGA (1.47 [1.39–1.57]), preterm birth (1.26 [1.17–1.36]), cesarean delivery (1.17 [1.14–1.20]), gestational diabetes (1.25 [1.21–1.30]), and preeclampsia (1.08 [1.04–1.13]) but not of infant death (1.10 [0.87–1.38]). On the contrary, being treated with an antihypertensive drug during pregnancy was associated with a decreased risk of hemorrhage, venous thromboembolism, and stroke or ACS during pregnancy and postpartum. However, this difference only reached statistical significance for hemorrhage (0.93 [0.86–0.99]) and the composite end point “stroke or ACS” (0.38 [0.18–0.83]). In a sensitivity analysis in which the 2913 women with a medical history of stroke, ACS, or heart failure were not excluded, these lower adjusted IRRs were not significant (data not shown).
In women with chronic hypertension, the probability of being treated during pregnancy was estimated using a logistic regression, which comprised all previously cited covariates plus the year of childbirth. The density of the propensity score showed an important overlap between the treated and untreated groups (Figure S1). The associations between antihypertensive treatment and mother and child outcomes estimated by logistic regression weighted on inverse probability of treatment were similar to those estimated by adjusted Poisson regression models (Table S1).
With respect to the 22 816 women treated for hypertension, an antihypertensive drug was dispensed for 13 795 (60.5%) of them during the first trimester, 16 473 (72.2%) during the second trimester, and 14 779 (64.8%) during the third trimester. A contraindicated drug (angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, or aliskiren) was dispensed for 328 (2.0%) of all 22 816 women during the second trimester and for 105 (0.7%) women during the third trimester (Table 3).
Table 3.
Number and Type of Antihypertensive Drugs Dispensed to Women With Chronic Hypertension Treated During Pregnancy at All 3 Trimesters of the Pregnancy and Postpartum
| Indication* | 1 year before pregnancy | First trimester | Second trimester | Third trimester | Postpartum | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage | No. | Percentage | No. | Percentage | No. | Percentage | No. | Percentage | |
| Contraindicated drug | 4563 | 31.7 | 1498 | 10.9 | 328 | 2.0 | 105 | 0.7 | 1584 | 12.8 |
| Nonindicated drug | 8684 | 60.3 | 7144 | 51.8 | 7022 | 42.6 | 5013 | 33.9 | 4382 | 35.3 |
| Indicated drug | 1143 | 7.9 | 5153 | 37.4 | 9123 | 55.4 | 9661 | 65.4 | 6436 | 51.9 |
| All drugs | 14 390 | 100 | 13 795 | 100 | 16 473 | 100 | 14 779 | 100 | 12 402 | 100 |
Angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, and aliskiren were considered contraindicated drugs during pregnancy (formally contraindicated in the second and third trimesters). Methyldopa, nicardipine, labetalol, and nifedipine were considered indicated drugs during pregnancy. All other molecules were considered nonindicated during pregnancy.
DISCUSSION
In the large‐scale nationwide prospective French cohort CONCEPTION, which included, among others, 2 833 376 primiparous women who gave birth between 2010 and 2018, we found that chronic hypertension before pregnancy was an important risk factor for adverse pregnancy outcomes, cardiovascular diseases, and death both during pregnancy and postpartum. This excess risk was significant irrespective of whether hypertension was treated during pregnancy. When treated during pregnancy, women with chronic hypertension had a significantly lower risk of obstetric hemorrhage and stroke or ACS than their untreated counterparts.
Although several studies have found an association between chronic hypertension and adverse infant outcomes, 7 , 8 , 9 , 11 , 26 , 27 evidence concerning the association between chronic hypertension and cardiovascular diseases during pregnancy is scarce. 7 , 10 We found that chronic hypertension was associated with preeclampsia, obstetric hemorrhage, thromboembolic venous disease, and stroke/ACS. It was also associated with a 3½ times higher risk of maternal death during postpartum, which is consistent with previous reports. 6 , 7 , 10 Although the absolute risks of major cardiovascular events during pregnancy and postpartum remain low, these events have dramatic consequences on both mothers and children, including physical or mental disabilities and death. They have become the main cause of maternal mortality in developed countries. 28 , 29 Given the increasing trend of chronic hypertension before pregnancy, this global public health issue is likely to worsen in the next few years, which highlights the need to improve the prevention, screening, and multidisciplinary management of chronic hypertension in women of childbearing age.
Previous studies reported that antihypertensive treatment is beneficial in preventing several negative maternal outcomes. 7 , 21 , 30 Particularly, the CHIPS trial (Control of Hypertension in Pregnancy Study) found that tight versus less‐tight control of hypertension in pregnancy did not improve maternal and infant outcomes, except the risk of severe maternal hypertension, thrombocytopenia, and elevated liver enzymes. 30 However, a post hoc analysis of CHIPS data found that severe hypertension was associated with all outcomes except for maternal readmission. Recently, the CHAP trial found that, in women with chronic hypertension, a pharmacological treatment targeting a blood pressure of <140/90 mm Hg was associated with a decreased risk of a composite outcome combining severe preeclampsia, medically indicated preterm birth, placental abruption, or fetal or neonatal death. 22
In our study, antihypertensive treatment during pregnancy was significantly associated with a higher risk of cesarean delivery, SGA, and preterm birth but not of infant death. Nevertheless, it remains unclear whether antihypertensive drugs have a negative causal effect on pregnancy outcomes or if they only reflect the severity of hypertension. Indeed, we were not able to adjust our models for the severity of hypertension because blood pressure measurements are not available for the cohort. Among all studied outcomes, the association between antihypertensive treatment and SGA was the strongest and could be partly explained by the prescription of β‐blockers, which has already been associated with fetal growth restriction. 31 , 32
Moreover, we found that being treated with an antihypertensive drug during pregnancy was associated with a significantly lower risk of delivery or postpartum hemorrhage and of stroke/ACS during pregnancy and postpartum. To the best of our knowledge, this is the first study to report such results. Nevertheless, they must be considered with caution. Given the observational design of this study, a causal relationship between the treatment of chronic hypertension during pregnancy and a lower risk of hemorrhage or stroke cannot be asserted. In a sensibility analysis using a propensity score, these associations were unchanged, but the discriminating power of this propensity score was flawed by the lack of blood pressure measurements.
Nonetheless, this finding remains valuable because a clinical trial would require a considerable number of participants to assess the effect of antihypertensive treatment on such rare events and therefore would be unethical and difficult to conduct.
Our results highlight the importance of preconceptional care for women with chronic hypertension to optimize pregnancy planning. This would allow providing women information about the excess risk of negative maternal and infant outcomes and the importance of blood pressure control during the pregnancy, especially considering the results of the CHIPS and CHAP clinical trials. Our findings also support previous recommendations that women with chronic hypertension be monitored closely for the potential development of adverse complications during pregnancy.
A contraindicated treatment (angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, or aliskiren) had been dispensed during the second or third trimesters of pregnancy for a small proportion of the women in our study. These drugs can lead to fetal complications or death. The importance of stopping or substituting these drugs for others during pregnancy should therefore be emphasized for both mothers and clinicians. 19
This study has many strengths. The use of a national database enabled us to create a near‐exhaustive nationwide cohort of women who gave birth in France at some point during a 9‐year time period and to avoid inclusion bias. The large sample size of this cohort ensured optimal statistical power to study rare events such as maternal cardiovascular events and death during pregnancy or postpartum, compare treated and untreated pregnant women with chronic hypertension, and adjust our models for several covariates to limit confounding bias. The combination of data on hospital‐based diagnoses and outpatient drug dispensing enabled us to study not only infant outcomes but also maternal cardiovascular events and analyze the consumption of each antihypertensive drug class during pregnancy and postpartum. Moreover, the use of hospital records and drug‐dispensing data to identify outcomes (diagnoses and treatments) lowered the risk of classification bias, such as recall bias.
This study also had limitations. Because chronic hypertension was identified by the dispensing of antihypertensive drugs and hospital diagnoses, untreated hypertension not reported during hospitalization may have been missed and therefore underestimated. Moreover, we cannot exclude the possibility that a small proportion of women may have taken antihypertensive drugs for indications other than hypertension (eg, kidney failure, migraine). Having said that, apart from migraine, such indications are rare in young women. These possible misclassifications would likely lead to an underestimation of the IRRs of perinatal and maternal outcomes in women with chronic hypertension. Although we comprehensively identified every medication dispensing event, we can only assume that these treatments were actually taken. This assumption could therefore lead to an underestimation of the potential effect of antihypertensive drugs on perinatal and maternal outcomes. Likewise, the compliance to antihypertensive drugs could not be assessed because we could not know how many drugs women were taking. Moreover, the Système National des Données de Santé is a medico‐administrative database and therefore lacks clinical data such as weight or blood pressure, resulting in residual confounding. Similarly, no data are available for rates of postpartum hypertension and rates of readmissions in the postpartum period. Finally, as our analysis was conducted on women who delivered for the first time, these results cannot be generalized to subsequent pregnancies.
CONCLUSIONS
Chronic hypertension is a major risk factor of perinatal negative outcomes, including infant death and maternal venous thromboembolic or cardiovascular events during pregnancy and postpartum. Women treated with antihypertensive drugs during pregnancy were at higher risk of negative perinatal outcomes and at lower risk of postpartum cardiovascular events, yet no causal inference can be established between the antihypertensive treatment and these outcomes given the observational design of this study. Further studies should be done on how antihypertensive therapy affects not just pregnancy outcomes but also long‐term outcomes such as ACS, cardiovascular diseases, and death among these women.
Sources of Funding
This work was supported by the French Hypertension Society, the Hypertension Research Foundation, and the French Cardiology Federation through the call for scientific projects titled “Thematic Grant 2019: Cardiovascular Diseases in Women.” The funders had no role in the study design, data collection, data analysis, decision to publish, or drafting of the manuscript.
Disclosures
Dr Kretz reports, outside the submitted work, nonfinancial support from Lilly France, Novonordisk, Novartis Pharma, Roche diabetes care, Lifescan, Abbott France, Sanofi, ViiV Healthcare, Servier, and Becton Dickinson and personal fees from Icomed, Pascaleo, BT3SI, and M3global research. Dr Mounier‐Vehier reports, outside the submitted work, personal fees and nonfinancial support from Servier, Lundbeck, Astrazeneca, Merck & Co, and Boehringer. Dr Blacher reports, outside the submitted work, personal fees from Abbott, Bayer, Bottu, Ferring, Steripharma, Kantar, and Teriak; personal fees and nonfinancial support from Pfizer and Quantum Genomics; and personal fees from Sanofi and Servier. All other authors declare no conflict of interest.
Supporting information
Table S1
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027266
For Sources of Funding and Disclosures, see page 9.
References
- 1. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre‐eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 2. Olie V, Moutengou E, Grave C, Deneux‐Tharaux C, Regnault N, Kretz S, Gabet A, Mounier‐Vehier C, Tsatsaris V, Plu‐Bureau G, et al. Prevalence of hypertensive disorders during pregnancy in France (2010–2018): the Nationwide CONCEPTION Study. J Clin Hypertens (Greenwich). 2021;23:1344–1353. doi: 10.1111/jch.14254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803 [DOI] [PubMed] [Google Scholar]
- 4. Blondel B, Lelong N, Kermarrec M, Goffinet F; National Coordination Group of the National Perinatal S . Trends in perinatal health in France from 1995 to 2010. Results from the French National Perinatal Surveys. J Gynecol Obstet Biol Reprod. 2012;41:e1–e15. doi: 10.1016/j.jgyn.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 5. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–1306. doi: 10.1097/AOG.0b013e3181a45b25 [DOI] [PubMed] [Google Scholar]
- 6. Bateman BT, Bansil P, Hernandez‐Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206(134):e131–e138. doi: 10.1016/j.ajog.2011.10.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al Khalaf SY, O'Reilly EJ, Barrett PM, BL DF, Pawley LC, FP MC, Khashan AS. Impact of chronic hypertension and antihypertensive treatment on adverse perinatal outcomes: systematic review and meta‐analysis. J Am Heart Assoc. 2021;10:e018494. doi: 10.1161/JAHA.120.018494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynaecol. 1996;103:123–129. doi: 10.1111/j.1471-0528.1996.tb09662.x [DOI] [PubMed] [Google Scholar]
- 9. Bramham K, Parnell B, Nelson‐Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta‐analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbert WM, Young AL, Danielsen B. Pregnancy outcomes in women with chronic hypertension: a population‐based study. J Reprod Med. 2007;52:1046–1051. [PubMed] [Google Scholar]
- 11. Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410–416. doi: 10.1016/0002-9378(94)90276-3 [DOI] [PubMed] [Google Scholar]
- 12. Li F, Wang T, Chen L, Zhang S, Chen L, Qin J. Adverse pregnancy outcomes among mothers with hypertensive disorders in pregnancy: a meta‐analysis of cohort studies. Pregnancy Hypertens. 2021;24:107–117. doi: 10.1016/j.preghy.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 13. Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population‐based study. Obstet Gynecol. 2005;105:480–484. doi: 10.1097/01.AOG.0000151998.50852.31 [DOI] [PubMed] [Google Scholar]
- 14. James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population‐based study. Circulation. 2006;113:1564–1571. doi: 10.1161/CIRCULATIONAHA.105.576751 [DOI] [PubMed] [Google Scholar]
- 15. Karjalainen L, Tikkanen M, Rantanen K, Aarnio K, Korhonen A, Saaros A, Laivuori H, Gissler M, Ijas P. Stroke in pregnancy and puerperium: validated incidence trends with risk factor analysis in Finland 1987–2016. Neurology. 2021;96:e2564–e2575. doi: 10.1212/WNL.0000000000011990 [DOI] [PubMed] [Google Scholar]
- 16. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311–1315. doi: 10.1016/j.ajog.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 17. Regitz‐Zagrosek V, Roos‐Hesselink JW, Bauersachs J, Blomstrom‐Lundqvist C, Cifkova R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340 [DOI] [PubMed] [Google Scholar]
- 18. Mounier‐Vehier C, Amar J, Boivin J‐M, Denolle T, Fauvel J‐P, Plu‐Bureau G, Tsatsaris V, Blacher J. Hypertension and pregnancy. Expert consensus statement from the French Society of Hypertension, an affiliate of the French Society of Cardiology. Presse Med. 2016;45:682–699. doi: 10.1016/j.lpm.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 19. American College of O, Gynecologists' Committee on Practice B‐O . ACOG practice bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–e50. doi: 10.1097/AOG.0000000000003020 [DOI] [PubMed] [Google Scholar]
- 20. Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, Rana S, Vermunt JV, August P; American Heart Association Council on H , et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association. Hypertension. 2022;79:e21–e41. doi: 10.1161/HYP.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2018;10:CD002252. doi: 10.1002/14651858.CD002252.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, Lawrence K, Hughes BL, Bell J, Aagaard K, Edwards RK, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781–1792. doi: 10.1056/NEJMoa2201295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boucheron P, Lailler G, Moutengou E, Regnault N, Gabet A, Deneux‐Tharaux C, Kretz S, Grave C, Mounier‐Vehier C, Tsatsaris V, et al. Hypertensive disorders of pregnancy and onset of chronic hypertension in France: the nationwide CONCEPTION study. Eur Heart J. 2021;43:3352–3361. doi: 10.1093/eurheartj/ehab686 [DOI] [PubMed] [Google Scholar]
- 24. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286–290. doi: 10.1016/j.respe.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 26. Fisher SC, Van Zutphen AR, Romitti PA, Browne ML; National Birth Defects Prevention S . Maternal hypertension, antihypertensive medication use, and small for gestational age births in the national birth defects prevention study, 1997–2011. Matern Child Health J. 2018;22:237–246. doi: 10.1007/s10995-017-2395-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al Khalaf SY, O'Reilly EJ, McCarthy FP, Kublickas M, Kublickiene K, Khashan AS. Pregnancy outcomes in women with chronic kidney disease and chronic hypertension: a National cohort study. Am J Obstet Gynecol. 2021;225:298.e1–298.e20. doi: 10.1016/j.ajog.2021.03.045 [DOI] [PubMed] [Google Scholar]
- 28. Kotit S, Yacoub M. Cardiovascular adverse events in pregnancy: a global perspective. Glob Cardiol Sci Pract. 2021;2021:e202105. doi: 10.21542/gcsp.2021.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9 [DOI] [PubMed] [Google Scholar]
- 30. Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, et al. Less‐tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595 [DOI] [PubMed] [Google Scholar]
- 31. Duan L, Ng A, Chen W, Spencer HT, Lee MS. Beta‐blocker subtypes and risk of low birth weight in newborns. J Clin Hypertens (Greenwich). 2018;20:1603–1609. doi: 10.1111/jch.13397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kayser A, Beck E, Hoeltzenbein M, Zinke S, Meister R, Weber‐Schoendorfer C, Schaefer C. Neonatal effects of intrauterine metoprolol/bisoprolol exposure during the second and third trimester: a cohort study with two comparison groups. J Hypertens. 2020;38:354–361. doi: 10.1097/HJH.0000000000002256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


