Summary
Background
The cytokine interleukin-2 (IL-2) can stimulate both effector immune cells and regulatory T (Treg) cells. The ability of selectively engaging either of these effects has spurred interest in using IL-2 for immunotherapy of cancer and autoimmune diseases. Thus, numerous IL-2-based biologic agents with improved bias or delivery towards effector immune cells or Treg cells have been developed. This study systematically reviews clinical results of improved IL-2-based compounds.
Methods
We searched the ClinicalTrials.gov database for registered trials using improved IL-2-based agents and different databases for available results of these studies.
Findings
From 576 registered clinical trials we extracted 36 studies on different improved IL-2-based compounds. Adding another nine agents reported in recent literature reviews and based on our knowledge totalled in 45 compounds. A secondary search for registered clinical trials of each of these 45 compounds resulted in 141 clinical trials included in this review, with 41 trials reporting results.
Interpretation
So far, none of the improved IL-2-based compounds has gained regulatory approval for the treatment of cancer or autoimmune diseases. NKTR-214 is the only compound completing phase 3 studies. The PIVOT IO-001 trial testing the combination of NKTR-214 plus Pembrolizumab compared to Pembrolizumab monotherapy in metastatic melanoma missed its primary endpoints. Also the PIVOT-09 study, combining NKTR-214 with Nivolumab compared to Sunitinib or Cabozantinib in advanced renal cell carcinoma, missed its primary endpoint. Trials in autoimmune diseases are currently in early stages, thus not allowing definite conclusions on efficacy.
Funding
This work was supported by public funding agencies.
Keywords: Interleukin 2, IL-2, Cancer, Autoimmune disease, Immunotherapy, Systematic review
Research in context.
Evidence before this study
High-dose interleukin-2 (IL-2) was the first approved immunotherapy for treatment of cancer. Apart from activating effector immune cells, IL-2 also stimulates regulatory T (Treg) cells, thus potentially limiting its efficacy as cancer treatment but offering opportunities to treat autoimmune diseases. This spurred efforts to develop improved IL-2 formulations specifically targeting either tumour-reactive effector or immunomodulatory Treg cells, with numerous compounds in clinical development. By searching the ClinicalTrials.gov database for the terms “interleukin-2”, “IL-2”, and “Proleukin” we retrieved 576 clinical trials with study start between 01.01.2010 and 31.10.2022. Of these, 36 trials fulfilled our inclusion criteria testing improved IL-2 compounds, to which we added nine compounds from previous reviews and our knowledge, totalling in 45 compounds evaluated within this systematic review.
Added value of this study
Through a systematic review of improved IL-2 compounds in clinical development, combined with structural and mechanistic data on these compounds, we summarize available clinical results from early-stage to phase 3 clinical trials, thus allowing direct comparison of different approaches aimed at improved IL-2 immunotherapy.
Implications of all the available evidence
The herein reported findings will help to guide future development of IL-2-based compounds for treatment of cancer and autoimmune diseases.
Introduction
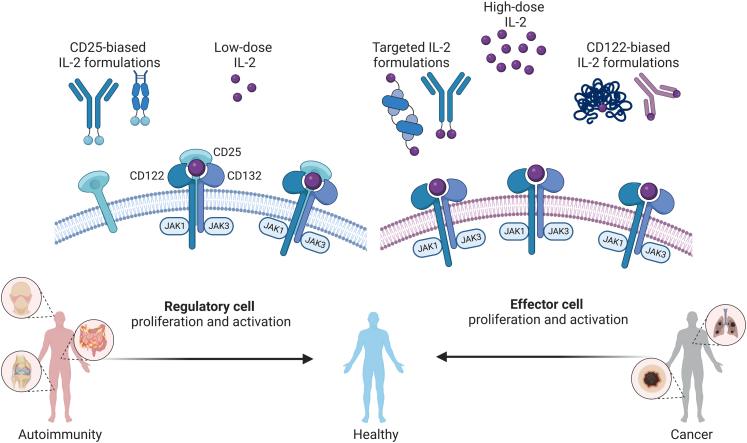
IL-2 is a small 15-kDa cytokine with pleiotropic effects on the immune system. Low doses of IL-2 preferentially bind to the trimeric IL-2 receptor (IL-2R), consisting of IL-2Rα (CD25), IL-2Rβ (CD122), and the common gamma chain (CD132), which is mainly expressed on immunosuppressive regulatory T (Treg) cells. Trimeric IL-2Rs are also referred to as high-affinity IL-2Rs because their affinity for IL-2 is about 10–100 times higher than that of dimeric IL-2Rs.1 Once the limited amounts of trimeric IL-2Rs on these cells are saturated, IL-2 also very efficiently associates with and stimulates dimeric IL-2Rs, made of CD122 and CD132, that are principally present on resting antigen-experienced (memory) effector T (Teff) and natural killer (NK) cells (Fig. 1).1,2 The stimulatory effect of IL-2 on Teff and NK cells motivated trials of high-dose IL-2 for the treatment of cancer, with recombinant human IL-2 (Aldesleukin) becoming the first US Food and Drug Administration approved immunotherapy for the treatment of metastatic renal cell carcinoma (RCC) and metastatic melanoma in 1992 and 1998, respectively.3,4 However, the stimulatory effects of high-dose IL-2 on Treg cells, which dampen immune responses against self-antigens including certain tumour antigens, as well as the considerable adverse side effects of IL-2 at high doses due to vascular leak syndrome limited its efficacy in cancer. Moreover, the short in vivo half-life of IL-2 in the range of minutes requires frequent applications.5 Thus, only 9.3% of patients with RCC and 4.0% with metastatic melanoma achieved complete responses (CR) on high-dose IL-2 monotherapy.6,7 These shortcomings of high-dose IL-2 treatment motivated the development of improved IL-2-based biologic agents with higher selectivity for effector immune cell subsets, reduced toxicity, and prolonged half-life.8 Furthermore, due to the success of immune checkpoint inhibitors, including monoclonal antibodies (mAbs) directed against programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), IL-2 immunotherapy regained the interest of the pharmaceutical industry. On this point, it is worth noting the complementary mode of action of checkpoint inhibitors and IL-2 immunotherapy. Whereas checkpoint inhibitors show limited efficacy in non-immunogenic and poorly immune cell-infiltrated tumours, IL-2 might render these tumours immunogenic and thus amenable to checkpoint inhibitor treatment due to indirect stimulation of and infiltration by dendritic cells.9 Thus, combination strategies targeting both axes could be particularly attractive.
Fig. 1.
Biology of interleukin-2 (IL-2). Low-dose IL-2 and improved IL-2 formulations with CD25 bias preferentially stimulate the trimeric IL-2 receptor consisting of CD25, CD122, and CD132, thus expanding regulatory T (Treg) cells. Treg cell expansion restores immune balance in patients with autoimmune diseases, including systemic lupus erythematosus and inflammatory bowel disease (left panel). On the other hand, high doses of IL-2 or CD122-biased IL-2 formulations preferentially stimulate the dimeric IL-2 receptor consisting of CD122 and CD132 and expressed on effector-type lymphocytes, such as resting antigen-experienced (memory) T and natural killer cells. Stimulation of these effector immune cells improves anti-tumour responses in cancer patients (right panel). Another approach focuses on delivery of IL-2 to either the tumour microenvironment or anti-tumour effector immune cells by using targeted IL-2 formulations (right panel).
On the other hand the discovery of highly IL-2-dependent immunosuppressive Treg cells in 1995 and subsequent research efforts reporting Treg cell deficiencies in various autoimmune diseases prompted first clinical trials testing low-dose IL-2 immunotherapy for the treatment of chronic graft-versus-host disease (GVHD) and cryoglobulinaemic vasculitis.10, 11, 12, 13, 14, 15 For steroid-refractory chronic GVHD, recently published cumulative results of five clinical trials reported response rates of 53.3% after 12 weeks of low-dose IL-2 treatment.16 Following these seminal trials, controlled clinical trials testing low-dose IL-2 in systemic lupus erythematosus (SLE) confirmed expansion of Treg cells by IL-2 immunotherapy, but these trials formally missed their predefined primary endpoints on clinical efficacy compared to control groups.17,18 However, the first trial could not show a significant clinical improvement at week 12 but at week 24,17 and the second trial did not meet the primary endpoint in the intention-to-treat population due to a 100% response rate in the placebo group at two sites in one country, but showed clinical response in the per-protocol population excluding these two sites.18 Another contributing factor could be the imperfect bias of low-dose IL-2, which can stimulate Teff and NK cells, apart from Treg cells. This rationale has supported current endeavours of developing improved IL-2-based compounds with increased CD25 bias for selective activation and proliferation of Treg cells.
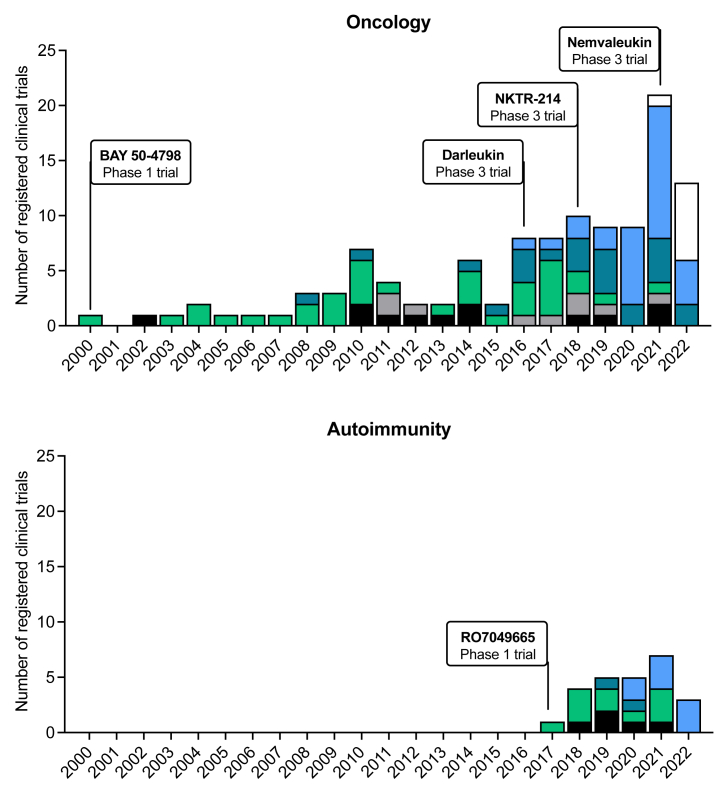
With registered clinical trials on improved IL-2-based agents increasing markedly during the past years (Fig. 2), the present study aims to provide a comprehensive review systematically summarizing our current knowledge on the available clinical efficacy data, compared to their molecular structure, of improved IL-2-based compounds that are currently in clinical trials.
Fig. 2.
Registered clinical trials testing improved IL-2-based compounds. Top panel displays trials in cancer and bottom panel trials in autoimmune diseases.
Methods
Study design and protocol registration
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table S1)19 and was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) prior to the initiation of the literature search (Registration number: INPLASY2022110086).
Search strategy
The ClinicalTrials.gov database was searched on 01.11.2022 for registered clinical trials by applying the “Intervention/treatment” search terms “interleukin-2” OR “interleukin 2” OR “IL-2” OR “IL2”. The results were filtered for clinical trials with study start between 01.01.2010 and 31.10.2022. The list of compounds was amended with missing IL-2-based formulations identified by recent literature reviews and based on the authors knowledge.4,20 During the revision process an additional search was conducted with the term “Proleukin” on 04.02.2023, which resulted in 29 additional clinical trials, with none of them meeting the inclusion criteria.
Eligibility criteria
Included were improved IL-2-based compounds in clinical development with registered clinical trials on ClinicalTrials.gov or reported results. Improved IL-2-based compounds were defined as IL-2-based biologic agents with a skewed bias towards or delivery to either effector immune cells or Treg cells compared to native IL-2. Clinical trials testing native IL-2, including Aldesleukin, or non-agonistic IL-2 formulations were excluded.
Study selection, data collection process and analysis
Two authors (MR and UK) independently conducted the primary search and screened clinical trials testing improved IL-2-based compounds for inclusion. The resulting separate lists of clinical trials were compared, and improved IL-2-based compounds selected. In case of disagreement, a third author (DS) was involved in the discussion to reach final consensus of whether to include or not a given trial. To find all registered clinical trials for identified improved IL-2 compounds, a second round of search was performed for each of the compounds by using the ClinicalTrials.gov database, including alternative names of compounds. All identified clinical trials were subsequently listed in Table 1, Table 2 and amended with available information on the clinical development status. For all studies, the trial phase, treatment arms and where available the results were retrieved from the ClinicalTrials.gov database. For completed trials with no results available on ClinicalTrials.gov, a targeted PubMed and internet search was conducted to retrieve study results. Non-retrievable trial results were confirmed by an independent search by a second author.
Table 1.
Improved IL-2-based compounds for the treatment of cancer.
| Targeting | Compound and company | Structure | Compound description and development status | ClinicalTrials.gov identifier | Study design | Status | Clinical results | References |
|---|---|---|---|---|---|---|---|---|
| Unbiased IL-2-based compounds | ||||||||
| Untargeted | BNT153BioNTech |  |
Liposome-encapsulated mRNA encoding native IL-2 attached to a pharmacokinetic modifying group. Development status: Clinical trials ongoing (Phase 1). |
1. NCT04710043 | Phase 1 study of BNT153 + BNT152 (mRNA encoding native IL-7) in cancer patients (EE: 112 patients). | Recruiting (first posted 14.01.2021) | NA | CT 21 |
| Untargeted | AVB-001Avenge Bio |  |
Native human IL-2 produced by polymer encapsulated cells for local cytokine delivery. Development status: Clinical trials in preparation (Phase 1/2). |
1. NCT05538624 | Phase 1/2 study of intraperitoneally administered AVB-001 in patients with serous adenocarcinoma of the ovary (EE: 44 patients). | Not yet recruiting (first posted 14.09.2022) | NA | CT 22 |
| Untargeted | TG4010, MVA-MUC1-IL2 Transgene |  |
TG4010 is a recombinant viral vaccine of modified vaccinia of Ankara virus expressing mucin-1 and IL-2 aimed at targeting mucin-1 overexpressing tumours. Development status: No active clinical trials registered with the last trial being completed in 2021. |
1. NCT00004881 | Phase 1 study of TG4010 in advanced cancers (AE: 13 patients). | Completed May 2004 | 4/12 evaluable patients with SD for 6–9 months, 8/12 with PD. | CT 23, 24, 25, 26 |
| 2. NCT00040170 | Phase 2 study of two-dosing schedules of TG4010 in prostate adenocarcinoma (AE: 40 patients). | Terminated, last-updated 02.11.2006 | 0% ORR defined as a decrease of 50% or more in the prostate-specific antigen level. | |||||
| 3. NCT00415818 | Phase 2b RCT of TG4010 + chemotherapy vs. chemotherapy in NSCLC (AE: 148 patients). | Completed March 2010 | Phase 2 results: 6-month PFS of TG4010 + chemotherapy 43.2% (95% CI 33.4–53.5) and of chemotherapy alone 35.1% (95% CI 25.9–45.3; one-sided p = 0.01). | |||||
| 4. NCT01383148 | Phase 2/3 RCT of chemotherapy + TG4010 vs. chemotherapy + placebo in NSCLC (AE: 222 patients). | Terminated after phase 2 in July 2016 | Phase 2: PFS 5.9 months (95% CI 5.4–6.7) in the TG4010 group and 5.1 months (4.2–5.9) in the placebo group (HR 0.74 [95% CI 0.55–0.98]; one-sided p = 0.019). | |||||
| 5. NCT03353675 | Phase 2 study of first-line chemotherapy + TG4010 + Nivolumab in NSCLC (AE: 44 patients). | Completed 17.02.2021 | 32.5% ORR at 15 months (90% CI 20.4–46.6), median OS 14.9 months (95% CI 8.0–NA). | |||||
| 6. NCT02823990 | Phase 2 study of TG4010 + Nivolumab in NSCLC (AE: 13 patients). | Completed 24.02.2021 | NA | |||||
| Untargeted | SJNB-JF–IL2Baylor College of Medicine |  |
Vaccine of gene modified neuroblastoma cell line secreting IL-2 and lymphotactin. Development status: Clinical trials not recruiting, clinical development likely not continued. |
1. NCT00703222 | Phase 1/2 study of immunizations with SJNB-JF–IL2 and SJNB-JF–LTN cells co-administered with two dose levels of unmodified SKNLP neuroblastoma cell lines in neuroblastoma patients (AE: 7 patients). | Active, not recruiting (first posted 23.06.2008) | NA | CT |
| 2. NCT01192555 | Phase 1/2 study of immunizations with SJNB-JF–IL2 and SJNB-JF–LTN cells co-administered with unmodified SKNLP neuroblastoma cell lines + oral Cytoxan in neuroblastoma patients (AE: 11 patients). | Active, not recruiting (first posted 01.09.2010) | NA | |||||
| Untargeted | AML Cell VaccineKing's College London |  |
Vaccine of acute myeloid leukaemia cell line expressing CD80 and IL-2. Development status: Clinical trial not recruiting, clinical development likely not continued. |
1. NCT02493829 | Phase 1 study of AML Cell Vaccine for high-risk myeloid dysplastic syndrome and acute myeloid leukaemia (EE: 10 patients). | Unknown (first posted 10.07.2015) | NA | CT 27 |
| Untargeted | Saltikva, Salmonella-IL2 Salspera |  |
Attenuated Salmonella Typhimurium secreting unmodified IL-2. Development status: Clinical trials ongoing (Phase 2). |
1. NCT04589234 | Phase 2 study of Saltikva + FOLFIRINOX or Saltikva + Gemcitabine and Abraxane in pancreatic cancer (EE: 60 patients). | Recruiting (first posted 19.10.2020) | NA | CT 28 |
| Untargeted | IL-2-expressing SalmonellaMasonic Cancer Center, University of Minnesota |  |
Attenuated Salmonella Typhimurium secreting unmodified IL-2. Development status: Clinical trials completed. |
1. NCT01099631 | Phase 1 study of IL-2-expressing salmonella applied orally for advanced liver cancer (AE: 22 patients). | Completed 20.07.2020 | 0/22 patients with CR at 8 weeks. | CT |
| Untargeted | ProscavaxOncBioMune Pharmaceuticals |  |
Prostate cancer vaccine of prostate-specific antigen, IL-2, and GM-CSF. Development status: Unknown status, clinical development likely discontinued. |
1. NCT02058680 | Phase 1 study of Proscavax in prostate-specific antigen recurrent prostate cancer (EE: 48 patients). | Completed December 2018 | 9/14 evaluable patients with increased prostate-specific antigen doubling time at median follow-up of 31 months. | CT 29 |
| 2. NCT03579654 | Phase 2 RCT of Proscavax vs. active surveillance in prostate adenocarcinoma (EE: 120 patients). | Unknown (first posted 06.07.2018) | NA | |||||
| Tumour-targeted | ALT-801Altor BioScience |  |
TCR domain specific for amino acids 264–272 of human p53 linked to IL-2. Development status: All trials completed, terminated, or withdrawn. Clinical development likely not continued. |
1. NCT01478074 | Phase 1 study of ALT-801-activated natural killer cells after FLAG induction for acute myeloid leukaemia (EE: 68 patients). | Withdrawn (no subject was found eligible after screening over 30 subjects) | NA | CT 30,31 |
| 2. NCT00496860 | Phase 1 study of ALT-801 in metastatic malignancies (AE: 26 patients). | Completed October 2009 | 10/26 SD, 16/26 PD or withdrawn at week 11. | |||||
| 3. NCT01029873 (QUILT-2.008) | Phase 1/2 study of ALT-801 + Cisplatin in metastatic melanoma (AE: 25 patients). | Completed September 2013 | NA | |||||
| 4. NCT01670994 (QUILT-3.020) | Phase 1/2 study of ALT-801 in relapsed or refractory multiple myeloma (AE: 6 patients). | Terminated September 2015 | No benefit from single agent treatment. | |||||
| 5. NCT01326871 | Phase 1/2 study of ALT-801 + Cisplatin + Gemcitabine or ALT-801 + Gemcitabine in muscle invasive or metastatic urothelial cancer (EE: 90 patients). | Unknown (last update posted 13.04.2016) | NA | |||||
| 6. NCT01625260 | Phase 1/2 study of ALT-801 + Gemcitabine in Bacillus Calmette-Guerin failure non-muscle invasive bladder cancer (EE: 52 patients). | Unknown (last update posted 24.01.2017) | NA | |||||
| Tumour-targeted | Hu14.18-IL2, EMD 273063, APN-301 Lexigen Research Center Corporation |  |
IL-2 conjugated to anti-ganglioside GD2 antibody. Tumours of neuroectodermal origin, including neuroblastoma and melanoma, overexpress GD2. Development status: All trials completed, suspended, or withdrawn. Clinical development likely not continued. |
1. NCT00003750 | Phase 1 study of Hu14.18-IL2 in neuroblastoma and other GD2 positive tumours (AE: 28 patients). | Completed September 2005 | No measurable complete or partial responses. Within median follow-up of 20 months 57% of patients deceased. | CT 32, 33, 34, 35 |
| 2. NCT03209869 | Phase 1 study of ex vivo expanded haploidentical NK cells + Hu14.18-IL2 in neuroblastoma and osteosarcoma (AE: 0 patients). | Withdrawn 07.09.2022 | NA | |||||
| 3. NCT03958383 | Phase 1/2 study of Hu14.18-IL2 + local irradiation + Nivolumab + Ipilimumab in melanoma (EE: 61 patients). | Suspended 26.10.2022 | NA | |||||
| 4. NCT00082758 | Phase 2 study of Hu14.18-IL2 in recurrent or refractory neuroblastoma (AE: 39 patients). | Completed May 2012 | No response in 13/13 evaluable patients with bulky disease. CR in 5/23 evaluable patients with disease detectable only by (123I)metaiodobenzylguanidine scintigraphy and/or bone marrow histology. | |||||
| 5. NCT01334515 | Phase 2 study of Hu14.18-IL2 + GM-CSF + Isotretinoin in relapsed or refractory neuroblastoma (AE: 52 patients). | Completed 31.12.2013 | Overall RECIST response in 1/15 patients with bulky disease and 6/30 patients with disease detectable only by (123I)metaiodobenzylguanidine scintigraphy and/or bone marrow histology. | |||||
| 6. NCT00109863 | Phase 2 study of Hu14.18-IL2 in advanced melanoma (AE: 14 patients). | Completed February 2014 | 1/14 PR, 4/14 SD, and 9/14 PD. | |||||
| 7. NCT00590824 | Phase 2 study of Hu14.18-IL2 in completely resectable stage III or IV melanoma (AE: 23 patients). | Completed 20.09.2018 | Median PFS in 18 evaluable patients 5.73 months (95% CI 1.80–NA). Median OS in 20 evaluable patients 61.6 months (95% CI 13.7–NA). | |||||
| Tumour-targeted | DI-Leu16–IL2Alopexx Oncology |  |
Fusion protein of DI-Leu anti-CD20 antibody and IL-2. Development status: All trials completed or terminated. Clinical development likely not continued. |
1. NCT00720135 | Phase 1 study of DI-Leu16–IL2 + Rituximab in patients with B cell non-Hodgkin lymphoma (AE: 6 patients). | Completed July 2014 | 1/6 CR, 1/6 PR, 1/6 possible PR, 2/6 SD, 1/6 PD. | CT 36, 37, 38 |
| 2 & 3: NCT01874288 & NCT02151903 | Phase 1/2 study of DI-Leu16–IL2 in B cell non-Hodgkin lymphoma (AE: 24 patients & 5 patients). | Terminated 16.11.2016 due to noted clinical benefit in the earlier portion of the trial | Mean change of longest tumour diameter in 15 evaluable patients with −30.7% (SDEV 60.3) at 1 mg/m2 dose, −27.4% (SDEV 49.3) at 2 mg/m2 dose, 1.40% (SDEV 43.5) at 4 mg/m2 dose, and 28.5% (SDEV 22.5) at 6 mg/m2 dose. | |||||
| Tumour-targeted | Darleukin, L19-IL2 Philogen |  |
Fusion protein of IL-2 and single-chain variable fragment of the L19 antibody. L19 recognizes the ED-B domain of fibronectin, which is expressed in tumour vessels. | 1. NCT01198522 | Phase 1 study of L19-IL2 + Gemcitabine in pancreatic cancer (AE: 28 patients). | Terminated November 2014 (lack of recruitment) | NA | CT 39, 40, 41, 42, 43, 44 |
| 2. NCT02086721 | Phase 1 study of L19-IL2 + radiotherapy in patients with oligometastatic tumours (AE: 18 patients). | Completed May 2017 | Mean PFS 20 months and mean OS 39.3 months at 37-month follow-up. | |||||
| 3. NCT02076646 | Phase 1/2 study of L19-IL2 + Dacarbazine in metastatic melanoma (EE: 96 patients). | Active, not recruiting (first posted 03.03.2014) | NA | |||||
| 4. NCT02957019 | Phase 1/2 study of L19-IL2 + Rituximab in B cell lymphoma (AE: 6 patients). | Active, not recruiting (first posted 06.11.2016) | NA | |||||
| 5. NCT01058538 | Phase 1/2 study of L19-IL2 in advanced solid tumours (AE: 33 patients). | Completed November 2019 | SD in 51% in solid tumour cohort and 83% in metastatic RCC cohort. Median PFS in metastatic RCC cohort of 8 months (range 1.5–30.5). | |||||
| 6. NCT01055522 | Phase 2 study of L19-IL2 + Dacarbazine in metastatic melanoma (AE: 32 patients). | Completed February 2013 | 29 patients evaluable for response assessment. RECIST OR in 28%, median OS in 26 recommended dose treated patients of 14.1 months. | |||||
| 7. NCT01253096 | Phase 2 study of intratumoural L19-IL2 in melanoma (AE: 25 patients). | Completed September 2013 | 24 evaluable patients, OR in 53%, CR in 44.4%, PR in 9.5%, SD in 36.5%, and PD in 9.5%. | |||||
| 8. NCT02076633 | Phase 2 study of L19-IL2/L19-TNF in patients with malignant melanoma of the skin amenable to intratumoural injection (AE: 22 patients). | Completed May 2015 | 20 evaluable for response. 1/20 CR, 10/20 PR, 5 SD, 4 PD at week 12. | |||||
| 9. NCT02735850 | Phase 2 RCT of L19-IL2 + radiotherapy in NSCLC (AE: 0 patients). | Withdrawn (first posted 13.04.2016) | NA | |||||
| 10. NCT03705403 | Phase 2 RCT of L19-IL2 + radiotherapy vs. standard of care in NSCLC (EE: 126 patients). | Recruiting (first posted 15.10.2018) | NA | |||||
| 11. NCT04362722 | Phase 2 study of intratumoural administration of L19-IL2/L19-TNF in non-melanoma skin cancer patients with presence of injectable lesions (EE: 40 patients). | Recruiting (first posted 27.04.2020) | NA | |||||
| 12. NCT05329792 | Phase 2 study of L19-IL2/L19-TNF in patients with malignant tumours of the skin amenable to intratumoural injection (EE: 70 patients). | Not yet recruiting (first posted 15.04.2022) | NA | |||||
| 13. NCT02938299 | Phase 3 RCT of L19-IL2/L19-TNF neoadjuvant intratumoural treatment followed by surgery vs. surgery alone in melanoma (EE: 214 patients). | Recruiting (first posted 19.10.2016) | NA | |||||
| 14. NCT03567889 | Phase 3 RCT of L19-IL2/L19-TNF neoadjuvant intratumoural treatment followed by surgery vs. surgery alone in melanoma (AE: 186 patients). | Recruiting (first posted 26.06.2018) | NA | |||||
| Tumour-targeted | F16-IL2Philogen |  |
Fusion protein of IL-2 and single-chain variable fragment of the F16 antibody. F16 recognizes the A1 domain of tenascin-C, which is overexpressed in the neovasculature of breast cancer. Development status: Clinical trials active but not recruiting. |
1. NCT02957032 | Phase 1 study of F16-IL2 + Cytarabin in acute myeloid leukaemia relapse (EE: 30 patients). | Active, not recruiting (first posted 06.11.2016) | NA | CT 45, 46, 47 |
| 2. NCT03207191 | Phase 1 study of F16-IL2 + anti-CD33 (BI 836858) in acute myeloid leukaemia relapse (AE: 15 patients). | Completed 26.03.2020 | 3/15 OR, 4/15 SD. | |||||
| 3. NCT01131364 | Phase 1/2 study of F16-IL2 + Doxorubicin in advanced solid tumours (AE: 29 patients). | Terminated December 2012 | Disease control rate 57% in 14 evaluable phase 1 patients and 67% in 9 evaluable phase 2 patients at 8 weeks. | |||||
| 4. NCT01134250 | Phase 1/2 study of F16-IL2 + Paclitaxel in advanced solid tumours (AE: 48 patients). | Completed 07.04.2014 | NA | |||||
| 5. NCT05468294 | Phase 1/2 study of F16-IL2 + Nivolumab in NSCLC (AE: 3 patients). | Active, not recruiting (first posted 21.07.2022) | NA | |||||
| 6. NCT02054884 | Phase 2 RCT of F16-IL2 + Paclitaxel vs. Paclitaxel in metastatic Merkel cell carcinoma (AE: 13 patients). | Terminated 15.12.2017 due to lack of enrolment. | NA | |||||
| Tumour-targeted |
TILT-123 TILT Biotherapeutics |
 |
Oncolytic adenovirus engineered to encode tumour necrosis factor alpha and IL-2. Development status: Clinical trials ongoing (Phase 1). |
1. NCT04217473 | Phase 1 study of TILT-123 + adoptive cell therapy in melanoma (EE: 15 patients). | Recruiting (first posted 03.01.2020) | NA | CT |
| 2. NCT04695327 | Phase 1 study of TILT-123 in patients with injectable solid tumours (EE: 15 patients). | Recruiting (first posted 05.01.2021) | NA | |||||
| 3. NCT05222932 | Phase 1 study of TILT-123 + Avelumab in advanced melanoma and HNSCC (EE: 15 patients). | Recruiting (first posted 03.02.2022) | NA | |||||
| 4. NCT05271318 | Phase 1 study of TILT-123 + Pembrolizumab in platinum-resistant or refractory ovarian cancer (EE: 15 patients). | Recruiting (first posted 09.03.2022) | NA | |||||
| Tumour-targeted | WTX-124, IL-2 Indukine Werewolf Therapeutics |  |
Half-life prolonged IL-2 linked to an inactivation domain with a linker, that is cleaved by tumour proteases. Development status: Clinical trials ongoing (Phase 1). |
1. NCT05479812 | Phase 1 study of WTX-124 and WTX-124 + Pembrolizumab in solid tumours (EE: 150 patients). | Recruiting (first posted 29.07.2022) | NA | CT 48 |
| CD25-biased IL-2 compounds | ||||||||
| Untargeted | BAY 50-4798Bayer |  |
IL-2 mutein with N88R mutation reducing affinity for CD122. Development status: All clinical trials completed. Clinical development not continued. |
Not registered. | Phase 1 trial in melanoma and RCC (AE: 45 patients). | Completed June 2002 | Patients with RCC 1/20 PR for 4 months, 13/20 SD for at least 2 months, 6/20 PD. | 49,50 |
| Tumour-targeted | NHS-IL2, NHS-IL2LT, EMD 521873, Selectikine Merck |  |
IL-2 mutein coupled to anti-DNA-histone complex antibody NHS76 targeting necrotic tumour core. Mutation: D20T. Development status: All clinical trials completed. Clinical development likely not continued. |
1. NCT01032681 | Phase 1 trial of NHS-IL2 or NHS-IL2 + Cyclophosphamide in solid tumours (AE: 66 patients). | Completed January 2012 | CR and PR in 0/48 evaluable patients, SD 12/48 for more than 6 weeks. | CT 51, 52, 53 |
| 2. NCT00879866 | Phase 1 single-arm trial of NHS-IL2 + local radiotherapy in NSCLC (AE: 15 patients). | Completed September 2012 | No objective RECIST response. Median PFS 2.9 months (95% CI 1.5–3.1) and median OS 8.6 months (95% CI 4.9–NA). | |||||
| Immune cell-targeted | IBI363, PD-1–IL2m Innovent Biologics (Suzhou) |  |
Modified IL-2 with attenuated binding to CD122 and partially attenuated CD132 binding fused to anti-PD-1 antibody. Development status: Clinical trials ongoing (Phase 1). |
1. NCT05290597 | Phase 1 study of IBI363 in advanced solid tumours or lymphoma (EE: 84 patients). | Not yet recruiting (first posted 22.03.2022) | NA | CT |
| 2. NCT05460767 | Phase 1 study of IBI363 in advanced solid tumours or lymphoma (EE: 260 patients). | Not yet recruiting (first posted 15.07.2022) | NA | |||||
| CD25/CD122-biased IL-2 formulations | ||||||||
| Untargeted | STK-012Synthekine |  |
PEGylated CD25/CD122-selective IL-2 mutein with CD25/CD122 selectivity. PEGylation for half-life extension. Mutations: L18R, Q22E and Q126K for reduced CD132 binding.54 Development status: Clinical trials ongoing (Phase 1). |
1. NCT05098132 | Phase 1 trial of STK-012 or STK-012 + Pembrolizumab in advanced solid tumours (EE: 202 patients). | Recruiting (first posted 28.10.2021) | NA | CT 55,56 |
| CD122-biased IL-2 formulations | ||||||||
| Untargeted | ANV419, NARA1leukin Anaveon |  |
IL-2 fused to anti-IL-2 antibody NARA1, obstructing the CD25-binding site of IL-2. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT04855929 | Phase 1 study in solid tumours (EE: 60 patients). | Recruiting (first posted 22.04.2021) | Interim results: 2/13 patients with SD beyond 10 weeks. | CT 57,58 |
| 2. NCT05578872 | Phase 1/2 study of ANV419 or ANV419 + Pembrolizumab or ANV419 + Ipilimumab in advanced melanoma (EE: 130 patients). | Not yet recruiting (first posted 13.10.2022) | NA | |||||
| Untargeted | AU-007Aulos |  |
IL-2 complexed with an anti-IL-2 antibody obstructing the CD25-binding site of IL-2. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT05267626 | Phase 1/2 study of AU-007 in advanced cancer (EE: 69 patients). | Recruiting, first posted 04.03.2022 | NA | CT |
| Untargeted | SLC-3010Selecxine |  |
SLC-3010 consists of IL-2 complexed to the anti-IL-2 antibody TCB2, which obstructs the CD25 binding site on IL-2. Development status: Clinical trials ongoing (Phase 1). |
1. NCT05525247 | Phase 1/2 study of SLC-3010 or SLC-3010 + Gemcitabine in advanced solid tumours (EE: 420 patients). | Not yet recruiting (first posted 01.09.2022) | NA | CT 59 |
| Untargeted | Nemvaleukin alfa, ALKS 4230 Alkermes |  |
Fusion protein of circularly permuted IL-2 and extracellular domain of CD25. Firstly, C-terminal Thr133 and N-terminal Ser 6 of Aldesleukin were covalently linked, followed by cut between Ser 75 and Gln 74 creating new N- and C-termini. Finally, new C-terminal Gln74 of circularly permuted IL-2 was fused to N-terminal Glu 1 of CD25 with a six amino-acid-linker. Development status: Clinical trials ongoing (Phase 3). |
1. NCT02799095 (ARTISTRY-1) | Phase 1/2 study of ALKS 4230 or ALKS 4230 + Pembrolizumab in advanced solid tumours (AE: 243 patients). | Active, not recruiting (first posted 14.06.2016) | Monotherapy melanoma cohort (6/46 PR, 31/46 SD), monotherapy RCC cohort (4/22 PR, 10/22 SD), combination with Pembrolizumab in PD-1/PD-L1 unapproved cancers cohort (2/36 CR, 4/36 PR, 14/36 SD), and combination with Pembrolizumab in PD-1/PD-L1 approved cancers cohort (1/43 CR, 7/43 PR, 17/43 SD). | CT 60, 61, 62 |
| 2. NCT03861793 (ARTISTRY-2) | Phase 1/2 study of ALKS 4230 or ALKS 4230 + Pembrolizumab in advanced solid tumours (AE: 185 patients). | Active, not recruiting (first posted 04.03.2019) | Interim results: 30/46 patients with 1 on-treatment scan with SD. | |||||
| 3. NCT04592653 (ARTISTRY-3) | Phase 1/2 study of ALKS 4230 or ALKS 4230 + Pembrolizumab in advanced solid tumours (EE: 78 patients). | Recruiting (first posted 19.10.2020) | NA | |||||
| 4. NCT04144517 | Phase 2 study of ALKS 4230 + Pembrolizumab in HNSCC (AE: 14 patients). | Active, not recruiting (first posted 30.10.2019) | NA | |||||
| 5. NCT04830124 | Phase 2 study of ALKS 4230 in advanced melanoma (EE: 176 patients). | Recruiting (first posted 02.04.2021) | NA | |||||
| 6. NCT05092360 (ARTISTRY-7) | Phase 3 RCT of ALKS 4230 + Pembrolizumab vs. standard chemotherapy in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer (EE: 376 patients). | Recruiting (first posted 25.10.2021) | NA | |||||
| Untargeted |
NKTR-214, Bempegaldesleukin Nektar Therapeutics, Bristol Myers Squibb |
 |
PEGylated IL-2 with approximately six PEG moieties added at lysine residues slightly more numerous in CD25-binding sites. Upon in vivo release of PEG chains, NKTR-214 becomes active. Development status: Clinical development of NKTR-214 + Nivolumab has been terminated. All other trials are either completed, terminated, withdrawn or active, but not recruiting. |
1. NCT03835533 | Phase 1 platform study of immunotherapy combinations including NKTR-214 in metastatic castration-resistant prostate cancer (AE: 43 patients). | Active, not recruiting (first posted 08.02.2019) | NA | CT 63, 64, 65, 66, 67, 68, 69 |
| 2. NCT04955262 | Phase 1 study of CD8 positron emission tomography tracer in advanced melanoma patients receiving NKTR-214 + Nivolumab (AE: 0 patients). | Withdrawn 08.07.2021 (business decision) | NA | |||||
| 3. NCT03745807 | Phase 1 study of NKTR-214 + Nivolumab in advanced solid tumours (AE: 3 patients). | Completed 18.12.2019 | NA | |||||
| 4. NCT04540705 (PIVOT IO 011) | Phase 1 study of NKTR-214 + Nivolumab + tyrosine kinase inhibitor vs. Nivolumab + tyrosine kinase inhibitor alone in untreated metastatic RCC (AE: 30 patients). | Active, not recruiting (first posted 07.09.2020) | NA | |||||
| 5. NCT03772288 | Phase 1 study of tyrosine kinase inhibitor TAK-659 + NKTR-214 in non-Hodgkin lymphoma (AE: 0 patients). | Withdrawn 17.11.2021 (business decision) | NA | |||||
| 6. NCT03548467 | Phase 1/2 study of individualized VB10.NEO vaccine + NKTR-214 in advanced solid tumours with incomplete response to standard immune checkpoint treatments (EE: 65 patients). | Active, not recruiting (first posted 07.06.2018) | NA | |||||
| 7. NCT02869295 | Phase 1/2 trial of NKTR-214 in advanced solid tumour malignancies (AE: 28 patients). | Completed 31.10.2018 | Clinical efficacy not assessed. | |||||
| 8. NCT04052204 | Phase 1/2 study of NKTR-214 + Avelumab in advanced HNSCC and NKTR-214 + Avelumab + Talazoparib or Enzalutamide in metastatic castration resistant prostate cancer (AE: 3 patients). | Terminated 29.09.2020 (business decision) | NA | |||||
| 9. NCT02983045 | Phase 1/2 of NKTR-214 + Nivolumab with or without other anti-cancer therapies in advanced solid tumours (AE: 557 patients). | Completed, 28.04.2022 | At 29 months median follow-up ORR 52.6% and CR 34.2%. | |||||
| 10. NCT03435640 | Phase 1/2 study of NKTR-214 + NKTR-262 (toll-like receptor 7 and 8 agonist) + Nivolumab in advanced solid tumours (AE: 64 patients). | Terminated after phase 1 09.05.2022 | PR and SD in 41.2%. | |||||
| 11. NCT04730349 (PIVOT IO 020) | Phase 1/2 study of NKTR-214 + Nivolumab in paediatric patients with recurrent or refractory malignancies (AE: 15 patients). | Completed 22.06.2022 | NA | |||||
| 12. NCT03138889 | Phase 1/2 study of NKTR-214 + Pembrolizumab with or without chemotherapy in advanced solid tumours (AE: 127 patients). | Completed 24.08.2022 | Dose expansion cohort: ORR 12/70, CR 2/70, PR 10/70, SD 24/70, PD 31/70, not evaluable 3/70. | |||||
| 13. NCT03282344 | Phase 2 study of NKTR-214 + Nivolumab in advanced sarcoma (AE: 88 patients). | Active, not recruiting (first posted 13.09.2017) | NA | |||||
| 14. NCT04936841 | Phase 2 study of NKTR-214 + Pembrolizumab and radiotherapy in advanced HNSCC (AE: 5 patients). | Active, not recruiting (first posted 23.06.2021) | NA | |||||
| 15. NCT03785925 (PIVOT IO-10) | Phase 2 study of NKTR-214 + Nivolumab in locally advanced or metastatic urothelial cancer (AE: 192 patients). | Completed 30.06.2022 | Did not reach efficacy threshold to support continuation of program. | |||||
| 16. NCT04969861 | Phase 2/3 RCT of NKTR-214 + Pembrolizumab vs. Pembrolizumab in PD-L1-positive HNSCC (AE: 1 patient). | Terminated 22.04.2022 (business decision) | NA | |||||
| 17. NCT03635983 (PIVOT IO-001) | Phase 3 RCT of NKTR-214 + Nivolumab vs. Nivolumab in untreated metastatic melanoma (AE: 783 patients). | Active, not recruiting (first posted 17.08.2018) | Missed primary endpoint with no significant difference in ORR, PFS, and OS. | |||||
| 18. NCT03729245 (PIVOT-09) | Phase 3 study of NKTR-214 + Nivolumab vs. Sunitinib or Cabozantinib in advanced RCC (AE: 623 patients). | Terminated 19.10.2022 (business decision) | Missed primary endpoint of ORR. | |||||
| 19. NCT04209114 | Phase 3 RCT of neoadjuvant and adjuvant NKTR-214 + Nivolumab vs. Nivolumab in muscle-invasive bladder cancer (AE: 114 patients). | Active, not recruiting (first posted 23.12.2019) | NA | |||||
| 20. NCT04410445 (PIVOT-12) | Phase 3 RCT of adjuvant NKTR-214 + Nivolumab vs. Nivolumab in resected melanoma (AE: 775 patients). | Terminated 19.09.2022 (business decision) | NA | |||||
| Untargeted | TransCon IL-2 β/γAscendis Pharma |  |
IL-2 permanently linked to PEG obstructing the CD25 binding site and thus inducing a CD122 bias. For half-life prolongation an additional PEG carrier protein is linked, which is slowly released in vivo. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT05081609 | Phase 1/2 trial of TransCon IL-2 β/γ monotherapy or in combination with chemotherapy, Pembrolizumab, or TransCon toll-like receptor 7/8 agonist (EE: 317 patients). | Recruiting (first posted 18.10.2021) | NA | CT 70 |
| Untargeted | THOR-707, SAR444245 Sanofi |  |
Site-specific (P65) PEGylated CD122-biased IL-2 mutein. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT04009681 | Phase 1/2 study of THOR-707 alone or combined with either a checkpoint inhibitor or an anti-epithelial growth factor receptor antibody (EE: 300 patients). | Recruiting (first posted 05.07.2019) | Interim results: 4/68 PR and 3/68 minor response. | CT 71,72 |
| 2. NCT04913220 | Phase 1/2 study of THOR-707 + Cemiplimab in advanced skin cancers (EE: 80 patients). | Active, not recruiting (first posted 04.06.2021) | NA | |||||
| 3. NCT04914897 | Phase 2 study of THOR-707 + Pembrolizumab in pleural mesothelioma and NSCLC (EE: 160 patients). | Active, not recruiting (first posted 07.06.2021) | NA | |||||
| 4. NCT05061420 | Phase 2 study of THOR-707 + Pembrolizumab or THOR-707 + Cetuximab with HNSCC (EE: 120 patients). | Active, not recruiting (first posted 29.09.2021) | NA | |||||
| 5. NCT05104567 | Phase 2 study of THOR-707 + Pembrolizumab or THOR-707 + Cetuximab in advanced gastrointestinal cancers (EE: 280 patients). | Active, not yet recruiting (first posted 03.11.2021) | NA | |||||
| 6. NCT05179603 | Phase 2 study of THOR-707 and THOR-707 + Pembrolizumab in relapsed B cell lymphoma (EE: 50 patients). | Active, not recruiting (first posted 05.01.2022) | NA | |||||
| 7. NCT05535023 | Phase 2 study of neoadjuvant THOR-707 + Cemiplimab in HPV related oropharyngeal cancer (EE: 26 patients). | Not yet recruiting (first posted 10.09.2022) | NA | |||||
| Untargeted | BNT151BioNTech |  |
Liposome-encapsulated mRNA encoding modified IL-2 fused with albumin for half-life prolongation. Mutations not disclosed but according to patent likely a CD122-biased IL-2. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT04455620 | Phase 1/2 study of BNT151 in advanced solid tumours (EE: 84 patients). | Recruiting (first posted 02.07.2020) | NA | CT 73 |
| Untargeted | MDNA11Medicenna |  |
IL-2 mutein linked at C-terminus to human serum albumin with a 15-amino acid flexible linker for extended half-life. Mutations: L80F, R81D, L85V, I86V and I92F for increased CD122 affinity (based on H9 superkine74), and F42A and E62A to abrogate CD25 binding. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT05086692 | Phase 1/2 study of MDNA11 alone or with checkpoint inhibitors in advanced solid tumours (EE: 100 patients). | Recruiting (first posted 21.10.2021) | NA | CT 75 |
| Untargeted | SHR-1916Jiangsu Hengrui Pharmaceuticals |  |
PEG-conjugated IL-2 mutein that promotes proliferation of CD8+ T cells and NK cells. Development status: Clinical trials ongoing (Phase 1). |
1. NCT04842630 | Phase 1 study of SHR-1916 in solid tumours (EE: 50 patients). | Recruiting (first posted 13.04.2021) | NA | CT |
| Untargeted | NL-201Neoleukin |  |
NL201 is the PEGylated half-life-extended form of Neo-2/15, which is a computationally-designed de novo IL-2 with fully abolished CD25 binding. Development status: Clinical trials ongoing (Phase 1). |
1. NCT04659629 | Phase 1 study of NL-201 alone or with Pembrolizumab in advanced solid tumours (EE: 310 patients). | Recruiting (first posted 09.12.2020) | NA | CT 76 |
| Immune cell-targeted | PD-1–IL2v, RO7284755, RG6279 Roche |  |
Dual approach targeting PD-1+ T cells with an anti-PD-1 antibody fused to IL-2 variant. Mutations: F42A, Y45A and L72G for CD122 selectivity. Development status: Clinical trials ongoing (Phase 1). |
1. NCT04303858 | Phase 1 study of PD-1–IL2v or PD-1–IL2v + Atezolizumab in advanced tumours (EE: 348 patients). | Recruiting (first posted 11.03.2020) | NA | CT 77 |
| Immune cell-targeted | CUE-101CUE Biopharma |  |
Fusion protein of HLA-A∗0201, Fc, and CD122-biased IL-2 variant (H16A and F42A mutations). Amino acids 11–20 of HPV-16 E7 protein are loaded on HLA for delivery of IL-2 to antigen-specific CD8+ T cells. Development status: Clinical trials ongoing (Phase 2). |
1. NCT03978689 | Phase 1 study of CUE-101 alone or in combination with Pembrolizumab in HPV16-positive HNSCC (EE: 85 patients). | Recruiting (first posted 07.06.2019) | Interim results: 49 patients received monotherapy (1/49 PR, 15/49 SD) and 9 patients received CUE-101 + Pembrolizumab (8 evaluable, 2/8 PR, 2/8 SD). | CT 78,79 |
| 2. NCT04852328 | Phase 2 study of CUE-101 before surgery or chemoradiation in HPV16-positive oropharyngeal squamous-cell carcinoma (EE: 30 patients). | Recruiting (first posted 21.04.2021) | NA | |||||
| Immune cell-targeted | GI-101GI Innovation |  |
Fusion protein of CD80, IgG4 Fc and IL-2 variant with CD122 bias (mutations not disclosed). CD80 is a decoy for the immune checkpoint CTLA-4. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT04977453 | Phase 1/2 study of GI-101 alone or in combination with Pembrolizumab, Lenvatinib, or local radiotherapy in advanced solid tumours (EE: 374 patients). | Recruiting (first posted 27.07.2021) | NA | CT 80 |
| Tumour-targeted | Cergutuzumabamunaleukin, RO6895882, RG7813, CEA-IL2v Roche |  |
CD122-biased IL-2 variant (mutations: F42A, Y45A and L72G) fused to the carcinoembryonic antigen (CEA)-specific antibody Cergutuzumab. CEA is a protein overexpressed by various tumours. Development status: Clinical development likely not continued. | 1. NCT02004106 | Phase 1 study of Cergutuzumab amunaleukin in advanced tumours (AE: 110 patients). | Completed 31.08.2016 | NA | CT 81,82 |
| 2. NCT02350673 | Phase 1 study of Cergutuzumab amunaleukin + Atezolizumab in advanced solid tumours (AE: 70 patients). | Completed 06.12.2019 | NA | |||||
| Tumour-targeted | Simlukafuspalfa, RO6874281, RG7461, FAP-IL2v Roche |  |
CD122-biased IL-2 variant (mutations: F42A, Y45A and L72G) fused to the 4B9 antibody targeting fibroblast activation protein-α (FAP). Cancer-associated fibroblasts highly express FAP. Development status: Clinical trials ongoing (Phase 1/2). | 1. NCT02627274 | Phase 1 study of Simlukafusp alfa + Trastuzumab or Cetuximab in solid tumours (AE: 134 patients). | Active, not recruiting (first posted 10.12.2015) | RECIST ORR 7% in HNSCC. | CT 83, 84, 85, 86 |
| 2. NCT03193190 | Phase 1/2 study of Simlukafusp alfa as part of multiple immunotherapy-based treatment combinations in pancreatic ductal adenocarcinoma (EE: 340 patients). | Recruiting (first posted 20.06.2017) | NA | |||||
| 3. NCT03063762 | Phase 1 study of Simlukafusp alfa + Pembrolizumab with or without Bevacizumab in RCC (AE: 69 patients). Arm A: Simlukafusp alfa weekly for 4 weeks, followed by Q2W thereafter combined with atezolizumab. Arm B: same as arm A but combined with Bevacizumab. Arm C: Simlukafusp alfa + Atezolizumab Q3W. Arm D: Simlukafusp alfa + Atezolizumab + Bevacizumab Q3W. |
Completed 15.06.2021 | 25 evaluable for therapeutic activity in arm A (ORR: 24% [6 PR; 90% CI 13.0–40.1]); 15 in arm B (46.7% [1 CR, 6 PR; 90% CI 27.7–66.7]); 3 in arm C (33.3% [1 PR; 90% CI 7.83–74.7]); and 23 in Arm D (47.8% [2 CR, 9 PR; 90% CI 35.7–68.2]). | |||||
| 4. NCT03386721 | Phase 2 study of Simlukafusp alfa + Atezolizumab in advanced solid tumours (AE: 256 patients). | Completed 30.12.2021 | 44 evaluable patients with cervical squamous cell carcinoma with 2/44 CR, 10/44 PR, and 19/44 SD. | |||||
| 5. NCT03875079 | Phase 1 study of Simlukafusp alfa + Pembrolizumab in advanced melanoma (AE: 83 patients). | Completed 14.07.2022 | NA | |||||
| Tumour-targeted | XTX202Xilio Therapeutics |  |
CD122-biased IL-2 mutein (mutations not disclosed) linked to an inactivation domain with a linker, that is cleaved by tumour proteases. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT05052268 | Phase 1/2 open-label trial of XTX202 in advanced tumours (EE: 189 patients). | Recruiting (first posted 22.09.2021) | NA | CT 87 |
Abbreviations: AE, Actual enrolment; CI, Confidence interval; CR, Complete response; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CT, ClinicalTrials.gov; EE, Estimated enrolment; FAP, fibroblast activation protein-α; FLAG, Chemotherapy of fludarabine, cytarabine and filgrastim; FOLFIRINOX, Chemotherapy of 5-Fluorouracil, Irinotecan und Oxaliplatin; GM-CSF, Granulocyte-macrophage colony-stimulating factor; HNSCC, Head and neck squamous-cell carcinoma; HPV, Human papilloma virus; HR, Hazard ratio; IL-2, Interleukin-2; NA, Not available; NSCLC, Non-small cell lung cancer; ORR, Objective response rate; OR, Objective response; OS, Overall survival; PD-1, Programmed cell death protein 1; PD-L1, Programmed cell death ligand 1; PD, Progressive disease; PEG, Polyethylene glycol; PFS, Progression free survival; Q2W, Every two weeks; Q3W, Every three weeks; RCC, Renal cell carcinoma; RCT, Randomized controlled trial; RECIST, Response evaluation criteria in solid tumours; SDEV, Standard deviation; SD, Stable disease; TCR, T cell receptor.
Table 2.
Improved IL-2-based compounds for the treatment of autoimmune diseases.
| Targeting | Compound and company | Structure | Compound description and development status | ClinicalTrials.gov identifier | Study design | Status | Clinical results | References |
|---|---|---|---|---|---|---|---|---|
| CD25-biased IL-2 compounds | ||||||||
| Untargeted | NKTR-358, LY3471851, Rezpegaldesleukin Nektar, Eli Lilly |  |
PEGylated IL-2 (Aldesleukin) with attenuated affinity for CD25, CD122 and heterodimeric CD25/CD122. Development status: Clinical trials ongoing (Phase 1/2). |
1. NCT04380324 | Phase 1 RCT of NKTR-358 vs. placebo in healthy (AE: 100 participants). | Completed 20.01.2019 | NA | CT 88,89 |
| 2. NCT03556007 | Phase 1 RCT of NKTR-358 vs. placebo in systemic lupus erythematosus (AE: 48 patients). | Completed 29.08.2019 | No treatment related changes in SLEDAI score or joint counts. Dose-dependent reduction in CLASI-A score. | |||||
| 3. NCT04081350 | Phase 1 RCT of NKTR-358 vs. placebo in atopic dermatitis (AE: 48 patients). | Completed 24.06.2022 | NA | |||||
| 4. NCT04133116 | Phase 1 RCT of NKTR-358 vs. placebo in Caucasian and Japanese healthy (AE: 36 participants). | Completed 06.03.2020 | Biomarker results published with NCT03556007. Clinical efficacy not assessed. | |||||
| 5. NCT04433585 | Phase 2 RCT of NKTR-358 vs. placebo in systemic lupus erythematosus (EE: 280 patients). | Active, not recruiting (first posted 16.06.2020) | NA | |||||
| 6. NCT04119557 | Phase 1 RCT of NKTR-358 vs. placebo in psoriasis (AE: 30 patients). | Completed 21.07.2021 | NA | |||||
| 7. NCT05565729 | Phase 1 RCT of subcutaneous NKTR-358 ± levocetirizine vs. placebo in healthy (EE: 42 participants). | Recruiting (first posted 04.10.2022) | NA | |||||
| 8. NCT04998487 | Phase 1 RCT of subcutaneous NKTR-358 in healthy (AE: 71 participants). | Completed 06.07.2022 | NA | |||||
| 9. NCT04677179 | Phase 2 RCT of NKTR-358 vs. placebo in ulcerative colitis (AE: 81 patients). | Terminated due to enrolment futility on 09.08.2022 | NA | |||||
| Untargeted |
RO7049665, RG-7835 Roche |
 |
CD25-biased IL-2 harbouring the N88D mutation and fused to IgG1. Development status: All clinical trials completed or terminated. |
1. NCT03221179 | Phase 1 RCT of RO7049665 vs. placebo in healthy (AE: 49 participants). | Completed 05.07.2019 | NA | CT 90 |
| 2. NCT03943550 | Phase 1 RCT of RO7049665 vs. placebo in ulcerative colitis (AE: 45 patients). | Terminated 22.07.2021 due to lack of efficacy | NA | |||||
| 3. NCT04790916 | Phase 2 RCT of RO7049665 vs. placebo in autoimmune hepatitis (AE: 2 patients). | Terminated 18.11.2021 due lack of efficacy in NCT03943550 | NA | |||||
| Untargeted | CC-92252, DEL-106 BMS, Celgene |  |
CD25-biased IL-2 mutein-Fc fusion protein. Development status: All clinical trials terminated. Clinical development likely discontinued. |
1. NCT03971825 | Phase 1 RCT of CC-92252 vs. placebo in healthy and in psoriasis (AE: 131 patients). | Terminated 05.08.2021 | Did not meet progression criteria. Detailed results not available. | CT |
| Untargeted |
XmAb27564 Xencor |
 |
CD25-biased IL-2 mutein-Fc fusion protein. Attenuated interaction with CD122 and strengthened interaction with CD25. Development status: Clinical trial ongoing (Phase 1). |
1. NCT04857866 | Phase 1 RCT of XmAb27564 vs. placebo in healthy (EE: 48 participants). | Recruiting (first posted 23.04.2021) | NA | CT 91 |
| Untargeted | MK-6194, PT101 Merck |  |
CD25-biased IL-2-Fc fusion protein. Mutein with decreased interaction with CD122 and increased CD25 affinity (mutations not disclosed). Development status: Clinical trials ongoing (Phase 1). |
1. NCT04924114 | Phase 1 RCT of MK-6194 vs. placebo in ulcerative colitis (EE: 30 patients). | Recruiting (first posted 11.06.2021) | NA | CT 92 |
| 2. NCT05450198 | Phase 1 RCT of MK-6194 vs. placebo in atopic dermatitis (EE: 72 patients). | Recruiting (first posted 08.07.2022) | NA | |||||
| Untargeted |
AMG592, Efavaleukin alfa Amgen |
 |
Mutated IL-2-Fc fusion protein with increased Treg selectivity. Development status: Clinical trials ongoing (Phase 2). |
1. NCT04987333 | Phase 1 study of AMG592 in healthy (AE: 32 participants). | Completed 03.10.2022 | NA | CT 93, 94, 95 |
| 2. NCT03451422 | Phase 1 RCT of AMG592 vs. placebo in systemic lupus erythematosus (AE: 35 patients). | Completed 12.10.2021 | Clinical response not evaluated. | |||||
| 3. NCT03410056 | Phase 1/2 RCT of AMG592 vs. placebo in rheumatoid arthritis (AE: 36 patients). | Terminated 13.05.2020 (business decision) | Clinical response not evaluated. | |||||
| 4. NCT03422627 | Phase 1/2 study of AMG592 in chronic graft-versus-host disease (AE: 32 patients). | Completed 13.10.2022 | NA | |||||
| 5. NCT04680637 | Phase 2 RCT of AMG592 vs. placebo in systemic lupus erythematosus (EE: 320 patients). | Recruiting (first posted 23.12.2020) | NA | |||||
| 6. NCT04987307 | Phase 2 RCT of AMG592 vs. placebo in ulcerative colitis (EE: 320 patients). | Recruiting (first posted 03.08.2021) | NA | |||||
| Untargeted |
CUG252 Cugene, AbbVie |
 |
Mutated IL-2-Fc fusion protein with increased Treg selectivity. Development status: Clinical trial ongoing (Phase 1). |
1. NCT05328557 | Phase 1 RCT of CUG252 vs. placebo in healthy (AE: 32 participants). | Active, not recruiting (first posted 14.04.2022) | NA | CT 96 |
| Untargeted |
mRNA-6231 Moderna |
 |
mRNA coding for human serum albumin fused to IL-2 mutein with increased Treg selectivity. Development status: No active clinical trials. |
1. NCT04916431 | Phase 1 study of mRNA-6231 in healthy (AE: 18 participants). | Completed 02.08.2022 | NA | CT |
| Untargeted | NNC0361-0041National Institute of Diabetes and Digestive and Kidney Diseases |  |
Recombinant supercoiled plasmid encoding four human proteins: pre-proinsulin, transforming growth factor β1, interleukin-10, and IL-2. Development status: Clinical trial ongoing (Phase 1). |
1. NCT04279613 | Phase 1 RCT of NNC0361-0041 vs. placebo in type I diabetes (EE: 48 patients). | Recruiting (first posted 21.02.2020) | NA | CT |
Abbreviations: AE, Actual enrolment; CLASI-A, Cutaneous lupus erythematosus disease area and severity index; CT, ClinicalTrials.gov; EE, Estimated enrolment; NA, Not available; RCT, Randomized controlled trial; SLEDAI, Systemic lupus erythematosus disease activity index; Treg, Regulatory T cell.
Risk of bias assessment
A Modified Downs and Black tool was applied to assess bias of retrieved randomized controlled trials reporting clinical results (Table S2).20 Scoring the studies according to the categories (a) reporting, (b) external validity, (c) internal validity and (d) power resulted in ranks of high (23–27 points), medium (15–22 points), and low (0–14 points) quality. We searched the ClinicalTrials.gov database for all studies registered and conducted a secondary search for results available for these trials on PubMed, company websites, news sites, and other websites, reducing publication bias.
Principal summary measures and synthesis of results
We aimed to provide a systematic review of clinical results of improved IL-2-based compounds for the treatment of cancer and autoimmune diseases. To avoid exclusion of IL-2-based compounds, we refrained from further specification of these endpoints.
Role of funders
This work was solely supported by public funding agencies, as reported in the acknowledgements, and was conducted independent of pharmaceutical companies. The funding agencies did not have any role in study design, data collection, data analysis, interpretation, writing of report, and the decision to publish the results.
Results
Study selection and characteristics
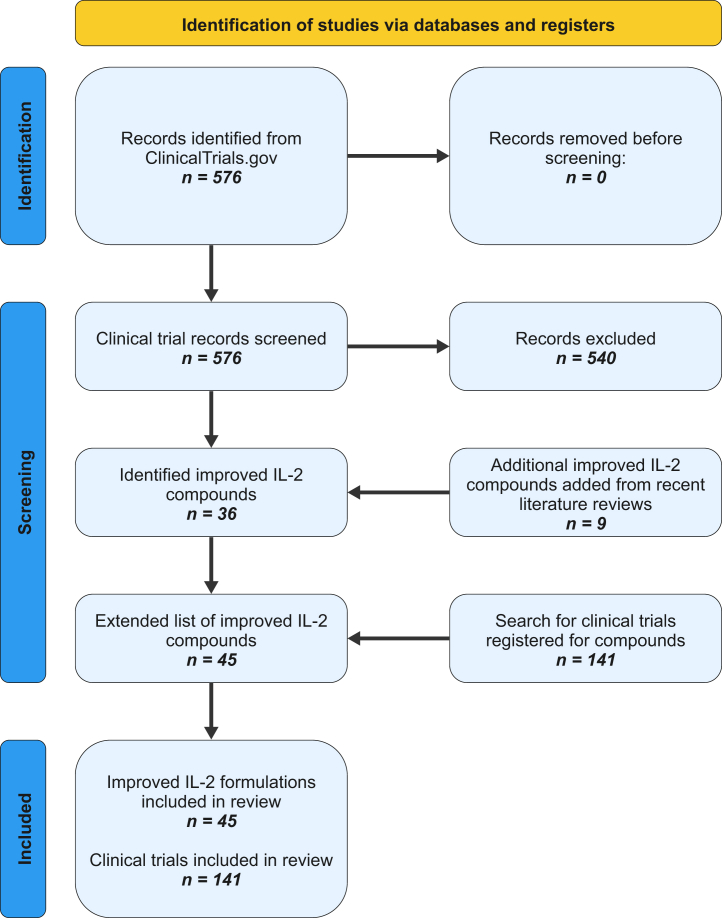
Applying the predefined search terms, we identified and screened 576 clinical trials registered on ClincialTrials.gov. Of these, 540 records were excluded resulting in 36 individual improved IL-2-based compounds. We added another nine compounds identified by recent literature reviews and based on our knowledge, resulting in a total of 45 compounds. A secondary search for clinical trials of each individual compound resulted in a total of 141 clinical trials that were included in this systematic review, of which 41 had available clinical results (Fig. 3).
Fig. 3.
PRISMA flow chartof literature research.
Synthesized findings
The findings for improved IL-2-based compounds for the treatment of cancer are synthesized in Table 1, and those for the treatment of autoimmune diseases in Table 2.
Improved IL-2-based compounds in clinical trials for the treatment of cancer
Unbiased IL-2-based compounds
The category of unbiased and untargeted IL-2-based compounds comprises several compounds. BNT153 is a liposome-encapsulated messenger RNA (mRNA) formulation encoding native IL-2.21 Limited published data are available from one registered phase 1 clinical trial in cancer patients testing BNT153 in combination with BNT152, the latter containing mRNA encoding native IL-7, with no results available as of this date. AVB-001 is a polymer encapsulated IL-2-producing cellular cytokine factory designed for local delivery.22 A phase 1/2 trial of intraperitoneally administered AVB-001 in ovarian serous adenocarcinoma was recently initiated, with no results available yet. Another approach consists in the delivery of IL-2 by viral vectors. TG4010 is a recombinant vaccine based on modified vaccinia virus Ankara expressing mucin-1 and IL-2, targeting mucin-1-overexpressing tumours, assessed within six registered clinical trials.23, 24, 25, 26 The phase 2/3 randomized-controlled trial (RCT) NCT01383148 tested standard chemotherapy with TG4010 or placebo in 222 non-small cell lung cancer (NSCLC) patients, reporting progression-free survival (PFS) of 5.9 months (95% confidence interval [CI] 5.4–6.7) in the TG4010 group and 5.1 months (95% CI 4.2–5.9) in the placebo group (hazard ratio [HR] 0.74 [95% CI 0.55–0.98]; one-sided p = 0.019). The trial was terminated during phase 3 in July 2016. The latest phase 2 single-arm trial NCT03353675 testing chemotherapy with TG4010 and the anti-PD-1 mAb Nivolumab in NSCLC reported 32.5% objective response rate (ORR) at 15 months (90% CI 20.4–46.6) and median overall survival (OS) of 14.9 months (95% CI 8.0 to not available [NA]). Currently, no active clinical trials are registered using TG4010, with the last trial completed in 2021. SJNB-JF–IL2 and AML Cell Vaccine are vaccines based on genetically modified IL-2-secreting neuroblastoma and acute myeloid leukaemia cell lines, respectively (Table 1).27 Three clinical trials were registered in 2008, 2010, and 2015, respectively, and are listed active but are not recruiting or of unknown status. Modified Salmonella typhimurium bacteria secreting IL-2 are found in Saltikva, which is tried in combination with chemotherapy in pancreatic cancer patients within a phase 2 trial registered in October 2020 and currently recruiting.28 A very similar compound of IL-2-expressing Salmonella was developed at the University of Minnesota and tested in advanced liver cancer, reporting CR in none of the 22 patients enrolled in the trial (Table 1). Proscavax, a vaccine based on prostate cancer cells secreting prostate-specific antigen, IL-2, and granulocyte-macrophage colony-stimulating factor (GM-CSF) is registered with one clinical trial reporting in 9 of 14 evaluable patients an extension of the time needed for doubling the serum concentration of prostate-specific antigen (Table 1).29
Another category of improved IL-2-based compounds comprises tumour-targeting molecules. ALT-801 consists of native IL-2 linked to a single-chain T cell receptor (TCR) domain recognizing amino acids 264–272 of human p53 antigen, thus shuttling IL-2 to the tumour.30,31 Six clinical trials are registered with all of them either completed, terminated, or withdrawn, indicating that clinical development has been suspended. Results are available for the NCT00496860 single-arm phase 1 trial testing ALT-801 in 26 patients with metastatic malignancies reporting stable disease (SD) in 10 of 26, and progressive disease (PD) or withdrawn in 16 of 26 patients. The IL-2 fusion protein Hu14.18-IL2 consists of two IL-2 molecules fused to an anti-ganglioside GD2 antibody.32, 33, 34, 35 GD2 is expressed in tumours of neuroectodermal origin, including neuroblastoma and melanoma. Of the seven registered clinical trials all are either completed, suspended, or withdrawn. The most advanced single-arm phase 2 trial (NCT00590824) testing Hu14.18-IL2 as adjuvant treatment in completely resectable stage III or IV melanoma patients reported PFS of 5.73 months (95% CI 1.80–NA) in 18 evaluable patients and median OS of 61.6 months (95% CI 13.7–NA) in 20 evaluable patients. Another fusion protein of IL-2 and a CD20-targeting antibody, DI-Leu16–IL2, was tested in a phase 1/2 trial for the treatment of B cell non-Hodgkin lymphoma.36, 37, 38 The trial NCT02151903 reported in 15 evaluable patients a reduction in tumour diameter of up to 30.7% (standard deviation [SDEV] 60.3) at the 1 mg/m2 dose. This trial was completed in November 2016 and no additional trials are registered. An alternative strategy used fusion proteins of IL-2 molecules linked to antibody light chains either targeting the ED-B domain of an angiogenesis-associated isoform of fibronectin overexpressed in tumour vessels (Darleukin)39, 40, 41, 42, 43, 44 or the alternatively spliced A1 domain of tenascin-C strongly expressed in neovascular stroma of breast cancer (F16-IL2).45, 46, 47 Darleukin or L19-IL2 lists 14 registered phase 1 to 3 clinical trials with four reporting clinical results. The phase 1 trial NCT02086721 observed mean PFS of 20 months and mean OS of 39.3 months at 37-month follow-up in patients with oligometastatic tumours. Results of the phase 1/2 study NCT01058538 in advanced solid tumours showed SD in 51% in the solid tumour cohort and 83% in the metastatic RCC cohort, with median PFS of 8 months (1.5–30.5 months) in the metastatic RCC cohort. The more advanced phase 2 trial NCT01253096 testing intratumoural injection of Darleukin in melanoma patients reported in 24 evaluable patients OR in 53%, CR in 44.4%, PR in 9.5%, SD in 36.5%, and PD in 9.5%, and the phase 2 study NCT01055522 of Darleukin plus Dacarbazine in metastatic melanoma reported OR in 28% of 29 patients evaluable for response evaluation criteria in solid tumours (RECIST) response assessment and median OS of 14.1 months in 26 patients treated with the recommended dose.39, 40, 41, 42, 43 F16-IL2 lists six registered clinical trials with the phase 1/2 study NCT01131364 testing F16-IL2 with Doxorubicin in advanced solid tumours reporting a disease control rate of 57% in 14 evaluable phase 1 patients and 67% in 9 evaluable phase 2 patients. Several clinical trials are listed active but are currently not recruiting.45, 46, 47 TILT-123 is an oncolytic adenovirus encoding tumour necrosis factor (TNF) and IL-2 and thus delivering these cytokines to the tumour microenvironment. Four phase 1 clinical trials are registered and currently recruiting patients (Table 1). WTX-124, which contains half-life prolonged IL-2 with a linker to an inactivation domain that is cleaved by proteases in the tumour microenvironment should allow preferential delivery of IL-2 to the tumour.48 One clinical trial of WTX-124 alone or in combination with Pembrolizumab is ongoing and recruiting.
CD25-biased IL-2 compounds
Due to the adverse effects of high-dose native IL-2, which was initially believed to be due to excessive activation of CD122-expressing NK cells, the first improved IL-2 compound developed was a CD25-biased IL-2 mutein harbouring the N88R mutation, termed BAY 50-4798.49 BAY 50-4798 was tested in a phase 1 trial in patients with RCC reporting PR in 1 of 20 patients (5%), SD in 65% for at least 2 months, and PD in 30%.50 Due to limited efficacy and emergence of neutralizing anti-drug antibodies directed against the mutated sequence of IL-2 further clinical development of BAY 50-4798 was stopped.97
Another CD25-biased IL-2 fusion protein, termed NHS-IL2, consists of an anti-DNA-histone complex antibody coupled to an IL-2 mutein for targeting the necrotic tumour core. NHS-IL2 was tested in two phase 1 trials. NCT00879866 testing NHS-IL2 with radiotherapy in NSCLC reported no objective RECIST response, and NCT01032681 testing NHS-IL2 either alone or combined with cyclophosphamide observed only SD in 12 of 48 patients with no CR or PR.51, 52, 53 All registered clinical trials have been completed.
Building on the success of checkpoint inhibitors, fusing IL-2 to an anti-PD-1 mAb thus shuttling IL-2 to effector T cells could be promising. IBI363 is such a fusion protein with CD25 bias and is currently tested in 2 ongoing phase 1 studies (Table 1).
CD25/CD122-biased IL-2 compounds
STK-012 is a pegylated IL-2 mutein with suggested CD25/CD122 selectivity due to mutations reducing binding to CD132.55,56 One phase 1 trial of STK-012 alone compared to STK-012 combined with Pembrolizumab in advanced solid tumours is ongoing with no results available yet.
CD122-biased IL-2 compounds
The largest category of improved IL-2-based compounds comprises CD122-biased IL-2. The first such compounds developed were so-called IL-2–anti-IL-2 mAb complexes, or briefly referred to as IL-2 complexes.98 ANV419 is a second-generation IL-2 complex fusion protein of IL-2 fused to the anti-IL-2 mAb NARA1, the latter of which covers with high affinity the CD25-binding site of IL-2, thus obstructing association with CD25.57,99 Interim results of the NCT04855929 phase 1 trial showed SD beyond 10 weeks in 2 of 13 patients.58 Two first-generation IL-2 complexes, AU-007 and SLC-3010, where IL-2 is non-covalently complexed to specific anti-IL-2 mAbs are also in clinical development. AU-007 and SLC-3010 have each registered one phase 1/2 study (Table 1).59
Nemvaleukin alfa is a fusion protein of IL-2 fused to the extracellular domain of CD25, thus obstructing the CD25-binding site and inducing a CD122 bias.60, 61, 62 Six clinical trials are registered, ranging from phase 1–3. Available results of the ARTISTRY-1 trial (NCT02799095) show four PR within 22 melanoma patients receiving monotherapy, two CR and four PR in 36 patients with PD-1/PD-L1-unapproved cancers treated with a combination of Nemvaleukin alfa and Pembrolizumab, and one CR and seven PR in 43 patients with PD-1/PD-L1-approved cancers receiving a combination of Nemvaleukin alfa and Pembrolizumab. A phase 3 trial testing Nemvaleukin alfa combined with Pembrolizumab against standard chemotherapy in platinum-resistant ovarian, fallopian tube, and primary peritoneal cancer was initiated in October 2021.
Another approach to induce CD122 bias is achieved by adding polyethylene glycol (PEG) moieties to CD25-binding sites on IL-2 by a process called PEGylation. Apart from inducing IL-2R bias, PEGylation also prolongs the in vivo half-life of IL-2. The first pegylated IL-2-based compound was NKTR-214, also known as Bempegaldesleukin.63, 64, 65, 66, 67, 68, 69 With 20 registered clinical trials NKTR-214 is the most studied IL-2-based compound, however, due to a lack of efficacy reported in two phase 3 trials clinical development of NKTR-214 was terminated in 2022.65,66 Detailed clinical results have not been released yet. A more targeted PEGylation approach was used to generate TransCon IL-2 βγ.70 One clinical trial is registered and recruiting patients. Another molecule, THOR-707, contains a 30-kDa PEG moiety attached to the P65 site of IL-2, thus obstructing CD25 binding.71,72 Six clinical trials are registered, all of them not yet recruiting patients. According to the recently released 2022 third quarter results by Sanofi, the phase 2 platform of THOR-707 was closed for dose optimization within a phase 1 trial.100
Very commonly, specific mutations are introduced into IL-2 to either increase binding affinity to CD122 and/or decrease affinity to CD25, resulting in IL-2 muteins. Similar to BNT153, BioNTech developed BNT151 which is a liposome-encapsulated mRNA formulation encoding a mutated IL-2 with CD122 bias fused to albumin for half-life prolongation.73 One phase 1/2 clinical trial is recruiting cancer patients.
MDNA-11 and SHR-1916, two other IL-2 muteins with increased CD122 bias fused to a half-life prolonging domain are both tested in ongoing phase 1/2 trials in advanced solid tumours, with patients currently being recruited (Table 1).75NL-201 is a computationally-designed de novo IL-2 with fully abolished CD25 binding linked to a PEG moiety for half-life prolongation.76 One phase 1 trial of NL-201 alone or combined with pembrolizumab in advanced solid tumours is ongoing.
The IL-2–anti-PD-1 mAb fusion protein PD-1–IL2v is currently in clinical development and tested alone or combined with Atezolizumab in advanced tumours.77 CUE-101 is a fusion protein consisting of an IL-2 variant with increased CD122 bias, an Fc fragment, and a human leukocyte antigen molecule loaded with amino acids 11–20 of human papilloma virus (HPV)-16 E7.78,79 Of the two registered trials, interim results of the phase 1 trial in HPV-16-positive head and neck squamous cell carcinoma reports PR in 1 of 49 and SD in 15 of 49 patients receiving CUE-101 monotherapy, and PR in 2 of 8 and SD in 2 of 8 patients receiving CUE-101 plus Pembrolizumab. A phase 2 trial is currently recruiting patients. The CD80–IgG4-Fc–IL-2 variant fusion protein GI-101 uses CD80 as a decoy for CTLA-4 and an IL-2 with CD122 bias for increased stimulation of Teff cells.80 One phase 1/2 study of GI-101 alone or in combination with Pembrolizumab, Lenvatinib or local radiotherapy is currently recruiting patients.
Two tumour-targeting compounds consisting of an IL-2 variant with increased CD122 bias coupled to either the carcinoembryonic antigen-specific antibody Cergutuzumab (Cergutuzumab amunaleukin) or the fibroblast activation protein-α-targeting antibody 4B9 (Simlukafusp alfa) have been developed by Roche.81, 82, 83, 84, 85, 86 For Cergutuzumab amunaleukin two phase 1 trials have been completed in 2016 and 2019, respectively, with results not yet available. For Simlukafusp alfa five trials are registered with results of the phase 2 study NCT03386721 testing Simlukafusp alfa combined with Atezolizumab in advanced solid tumours reporting CR in 2 of 44, PR in 10 of 44, and SD in 19 of 44 patients. Finally, XTX202 is a CD122-biased IL-2 mutein linked to an inactivation domain, which becomes cleaved in the tumour microenvironment, thus activating IL-2.87 One phase 1/2 trial in advanced tumours is currently recruiting patients.
Improved IL-2-based compounds in clinical trials for the treatment of autoimmune diseases
CD25-biased IL-2 compounds
For the treatment of autoimmune diseases only nine compounds are being tested in registered clinical trials, with all of these compounds harbouring an IL-2 molecule with improved CD25 bias. NKTR-358 is a PEGylated IL-2 with attenuated binding to CD25, CD122, and the heterodimer of CD25 and CD122, thus inducing a Treg cell bias. This is claimed due to decreased affinity of NKTR-358 for CD25 monomer, CD122 monomer, and the heterodimer of CD25 and CD122, which disfavours binding to cells expressing the dimeric IL-2R.88 Of the nine registered trials testing NKTR-358 only one phase 1 study (NCT03556007) reports clinical results, which did not reveal any significant treatment-related changes in the validated SLE Disease Activity Index (SLEDAI) or the counts of affected joints in SLE patients. However, a dose-dependent improvement of skin manifestations measured by using the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI-A) was observed.89 RO7049665 is a CD25-biased IL-2 mutein harbouring the N88D mutation fused to IgG for prolonged half-life. Three registered clinical trials in healthy individuals, patients with ulcerative colitis, and patients with autoimmune hepatitis are all terminated or completed with clinical results not available.90 The IL-2 mutein-Fc fusion protein CC-92252 was tested in one phase 1 RCT in healthy individuals and psoriasis patients, which was terminated early, as the progression criteria for continuing the trial was not met. Detailed results are not available (Table 2). Another IL-2 mutein-Fc fusion protein named XmAb27564 lists one registered clinical trial currently recruiting healthy participants.91 MK-6194, an IL-2 mutein with preserved CD25 but decreased CD122 binding is tested in two clinical trials in ulcerative colitis and atopic dermatitis, both currently recruiting patients.92 The half-life-extended IL-2 mutein (mutations not disclosed) AMG592 has six registered phase 1–2 trials with four of them completed (in healthy individuals, SLE patients, and chronic GVHD patients) or terminated (in rheumatoid arthritis patients) and two currently recruiting (in SLE and ulcerative colitis patients). Results are not available yet or clinical response was not assessed.93, 94, 95 CUG252 is a half-life-extended and mutated molecule tested in one phase 1 RCT currently recruiting SLE patients.96 mRNA-6231 is an mRNA formulation coding for an IL-2 mutein fused to human albumin for prolonged half-life and was tested in a phase 1 study in healthy individuals, which was completed, but no results are available (Table 2). The supercoiled plasmid NNC0361-0041 encoding for pre-proinsulin, transforming growth factor β1, interleukin-10 (IL-10), and IL-2 is tested within a phase 1 trial in patients with type-1 diabetes mellitus and is currently recruiting patients (Table 2).
Risk of bias assessment
Risk of bias of RCTs reporting clinical results was assessed using a modified Downs and Black checklist, reproduced in Table S2.
Discussion
Summary of main findings
With 36 improved IL-2-based compounds in clinical trials, cancer comprises the main indication of current endeavours bringing a more specific IL-2 compound to the clinic. Although many early-phase trials reported promising results, a recent press release revealed negative results from two phase 3 studies using NKTR-214. These included the PIVOT IO-001 trial, testing NKTR-214 versus NKTR-214 combined with Pembrolizumab in metastatic melanoma, which failed its primary endpoints of ORR, PFS, and OS.65 Likewise, the PIVOT-09 study, assessing NKTR-214 combined with Nivolumab compared to Sunitinib or Cabozantinib in advanced RCC, missed its primary endpoint of improved ORR (Table 1). Unfortunately, detailed results have not been made publicly available so far. We speculate on two reasons for the failure of NKTR-214. Firstly, the targeted indication might be wrong, as melanoma is considered an immunogenic tumour already showing high response rates to checkpoint inhibitor treatment, where NKTR-214 might not provide additional benefit. Accordingly, poorly immunogenic tumours with low response rates to checkpoint inhibitors might be a more suitable indication for IL-2-based treatments, as IL-2 might render tumours more immunogenic and thus amenable to checkpoint inhibitor treatment.9 Secondly, PEGylation of IL-2 by approximately six PEG moieties, which are slowly released in vivo and positioned at lysine residues of IL-2, with such residues found slightly more abundant on the CD25-binding site than on the CD122 epitope of IL-2, might overall result in suboptimal CD122 bias or even a CD25 bias, depending on which PEG moieties are released first. The future will show if other improved IL-2-based compounds can hold up to their promise and show improved anti-tumour responses, either by relying on a better design, by their selectivity for immune or tumour cells, or by being tried in less immunogenic tumours. The only other compound with available RCT results is TG4010, showing significant efficacy when combined with chemotherapy versus chemotherapy alone in two separate phase 2 studies in NSCLC (NCT01383148 and NCT00415818). As currently no active clinical trials are registered, we suspect that development of TG4010 was suspended; this could likely be due to the recent success of targeted therapeutics in NSCLC, which have replaced chemotherapy as the standard-of-care.
For the treatment of autoimmune diseases results reporting clinical response are only available for NKTR-358 where a phase 1 trial in SLE did not show improvement in the clinical SLEDAI score, although a dose-dependent improvement in the CLASI-A score focused on cutaneous manifestations has been reported.
Limitations
This study comprises a comprehensive systematic review summarizing the rapidly expanding field of improved IL-2-based compounds. To minimize bias, we applied standard systematic review techniques and included all registered trials into the synthesis of this review. However, this review has several limitations. By limiting the search to ClinicalTrials.gov, trials registered in other registries might have been missed. Moreover, the majority of the retrieved trials were in early phases with only few reporting phase 3 results. Furthermore, due to the commercial interest many trials did not provide clinical results, potentially inducing a reporting bias. Finally, comparison of results from different trials is complex, as trials were conducted in different disease entities with different outcome measures assessed at different time points and different dosing schemes and combination treatments. A conclusive statement on the most promising compounds is thus not possible.
Conclusions
In summary, most improved IL-2-based compounds are currently developed for the treatment of cancer with some compounds in early phase clinical trials for the treatment of autoimmune diseases. Only limited results of RCTs have been published so far, not allowing a clear conclusion on efficacy of improved IL-2-based compounds. However, the very large number of compounds and registered clinical trials reflects the considerable interest in developing improved IL-2 compounds and commitment to bring these to clinical application.
Contributors
MR and OB: conception of the study. MR, UK, and DS: data collection. MR, UK, DS, and OB: analysis and interpretation of data. MR and OB: writing of the first draft of the article with critical revision and approval of the final version by all authors. All reported data have been verified by at least two authors, MR and DS for the cancer data, and MR and UK for the data on autoimmune diseases.
Data sharing statement
The full dataset generated for this study is available upon request from the corresponding authors.
Declaration of interests
MR, UK, and OB hold patents on improved IL-2-based compounds and OB is a shareholder of Anaveon AG. MR discloses paid consulting activities for Urogen. DS states no competing interests related to this work.
Acknowledgements
This work was supported by the Swiss National Science Foundation (310030-172978 and 310030-200669; to OB), Hochspezialisierte Medizin Schwerpunkt Immunologie (HSM-2-Immunologie; to OB), the Clinical Research Priority Program CYTIMM-Z of University of Zurich (to OB), a Young Talents in Clinical Research Project Grant by the Swiss Academy of Medical Sciences and G. & J. Bangerter-Rhyner Foundation (YTCR 08/20; to MR), and a Filling the Gap Fellowship by University of Zurich (to MR). Cartoons were created with BioRender.com.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104539.
Contributor Information
Miro E. Raeber, Email: miro.raeber@uzh.ch.
Onur Boyman, Email: onur.boyman@uzh.ch.
Appendix A. Supplementary data
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Risk of bias assessment.
References
- 1.Boyman O., Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 2.Yu A., Snowhite I., Vendrame F., et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes. 2015;64(6):2172–2183. doi: 10.2337/db14-1322. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez R., Poder J., LaPorte K.M., Malek T.R. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. 2022;22(10):614–628. doi: 10.1038/s41577-022-00680-w. [DOI] [PubMed] [Google Scholar]
- 5.Donohue J.H., Rosenberg S.A. The fate of interleukin-2 after in vivo administration. J Immunol. 1983;130(5):2203–2208. [PubMed] [Google Scholar]
- 6.Bright R., Coventry B.J., Eardley-Harris N., Briggs N. Clinical response rates from interleukin-2 therapy for metastatic melanoma over 30 Years' experience: a meta-analysis of 3312 patients. J Immunother. 2017;40(1):21–30. doi: 10.1097/CJI.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg S.A., Yang J.C., White D.E., Steinberg S.M. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228(3):307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg C., Letourneau S., Pantaleo G., Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A. 2010;107(26):11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raeber M.E., Rosalia R.A., Schmid D., Karakus U., Boyman O. Interleukin-2 signals converge in a lymphoid-dendritic cell pathway that promotes anticancer immunity. Sci Transl Med. 2020;12(561) doi: 10.1126/scitranslmed.aba5464. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 11.Koreth J., Matsuoka K., Kim H.T., et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadoun D., Rosenzwajg M., Joly F., et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365(22):2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 13.Humrich J.Y., von Spee-Mayer C., Siegert E., et al. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann Rheum Dis. 2015;74(4):791–792. doi: 10.1136/annrheumdis-2014-206506. [DOI] [PubMed] [Google Scholar]
- 14.Klatzmann D., Abbas A.K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15(5):283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 15.Grasshoff H., Comduhr S., Monne L.R., et al. Low-dose IL-2 therapy in autoimmune and rheumatic diseases. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.648408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H.T., Koreth J., Whangbo J., et al. Organ-specific response after low-dose interleukin-2 therapy for steroid-refractory chronic graft-versus-host disease. Blood Adv. 2022;6(15):4392–4402. doi: 10.1182/bloodadvances.2022007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J., Zhang R., Shao M., et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79(1):141–149. doi: 10.1136/annrheumdis-2019-215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humrich J.Y., Cacoub P., Rosenzwajg M., et al. Low-dose interleukin-2 therapy in active systemic lupus erythematosus (LUPIL-2): a multicentre, double-blind, randomised and placebo-controlled phase II trial. Ann Rheum Dis. 2022;81(12):1685–1694. doi: 10.1136/ard-2022-222501. [DOI] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raeber M.E., Sahin D., Boyman O. Interleukin-2-based therapies in cancer. Sci Transl Med. 2022;14(670) doi: 10.1126/scitranslmed.abo5409. [DOI] [PubMed] [Google Scholar]
- 21.Sahin U., Kranz L., Vormehr M., Diken M., Kreiter S., Tillmann B. 2019. Treatment using cytokine encoding RNA. Patent number EP2019053134W. [Google Scholar]
- 22.Nash A.M., Jarvis M.I., Aghlara-Fotovat S., et al. Clinically translatable cytokine delivery platform for eradication of intraperitoneal tumors. Sci Adv. 2022;8(9) doi: 10.1126/sciadv.abm1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quoix E., Lena H., Losonczy G., et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212–223. doi: 10.1016/S1470-2045(15)00483-0. [DOI] [PubMed] [Google Scholar]
- 24.Quoix E., Ramlau R., Westeel V., et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12(12):1125–1133. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 25.Dreicer R., Stadler W.M., Ahmann F.R., et al. MVA-MUC1-IL2 vaccine immunotherapy (TG4010) improves PSA doubling time in patients with prostate cancer with biochemical failure. Invest New Drugs. 2009;27(4):379–386. doi: 10.1007/s10637-008-9187-3. [DOI] [PubMed] [Google Scholar]
- 26.Rochlitz C., Figlin R., Squiban P., et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5(8):690–699. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 27.Chan L., Hardwick N., Darling D., et al. IL-2/B7.1 (CD80) fusagene transduction of AML blasts by a self-inactivating lentiviral vector stimulates T cell responses in vitro: a strategy to generate whole cell vaccines for AML. Mol Ther. 2005;11(1):120–131. doi: 10.1016/j.ymthe.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Saltzman D., Moradian E. Poster presented at the 27th congress of the European Association for Cancer Research virtual meeting. 9 June 2021. A phase 2 study of Saltikva (Salmonella-IL2) in metastatic pancreatic cancer. [Google Scholar]
- 29.OncBioMune . 2017. OncBioMune meets primary objective in trial of ProscaVax immunotherapy vaccine for prostate cancer. Press release published on OncBioMune. [Google Scholar]
- 30.Card K.F., Price-Schiavi S.A., Liu B., et al. A soluble single-chain T-cell receptor IL-2 fusion protein retains MHC-restricted peptide specificity and IL-2 bioactivity. Cancer Immunol Immunother. 2004;53(4):345–357. doi: 10.1007/s00262-003-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishman M.N., Thompson J.A., Pennock G.K., et al. Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A∗0201 complex, in patients with advanced malignancies. Clin Cancer Res. 2011;17(24):7765–7775. doi: 10.1158/1078-0432.CCR-11-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hank J.A., Surfus J.E., Gan J., et al. Activation of human effector cells by a tumor reactive recombinant anti-ganglioside GD2 interleukin-2 fusion protein (ch14.18-IL2) Clin Cancer Res. 1996;2(12):1951–1959. [PubMed] [Google Scholar]
- 33.Shusterman S., London W.B., Gillies S.D., et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osenga K.L., Hank J.A., Albertini M.R., et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res. 2006;12(6):1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albertini M.R., Hank J.A., Gadbaw B., et al. Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol Immunother. 2012;61(12):2261–2271. doi: 10.1007/s00262-012-1286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillies S.D., Lan Y., Williams S., et al. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood. 2005;105(10):3972–3978. doi: 10.1182/blood-2004-09-3533. [DOI] [PubMed] [Google Scholar]
- 37.Lansigan F., Nakamura R., Quick D.P., et al. DI-Leu16-IL2, an anti-CD20-interleukin-2 immunocytokine, is safe and active in patients with relapsed and refractory B-cell lymphoma: a report of maximum tolerated dose, optimal biologic dose, and recommended phase 2 dose. Blood. 2016;128(22):620. [Google Scholar]
- 38.Bionews Services Immuno-Oncology News. https://immuno-oncologynews.com/di-leu16-il2/?cn-reloaded=1
- 39.Carnemolla B., Borsi L., Balza E., et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–1665. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 40.Weide B., Eigentler T., Catania C., et al. A phase II study of the L19IL2 immunocytokine in combination with dacarbazine in advanced metastatic melanoma patients. Cancer Immunol Immunother. 2019;68(9):1547–1559. doi: 10.1007/s00262-019-02383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johannsen M., Spitaleri G., Curigliano G., et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 42.Eigentler T.K., Weide B., de Braud F., et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 43.Van Limbergen E.J., Hoeben A., Lieverse R.I.Y., et al. Toxicity of L19-interleukin 2 combined with stereotactic body radiation therapy: a phase 1 study. Int J Radiat Oncol Biol Phys. 2021;109(5):1421–1430. doi: 10.1016/j.ijrobp.2020.11.053. [DOI] [PubMed] [Google Scholar]
- 44.Danielli R., Patuzzo R., Di Giacomo A.M., et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marlind J., Kaspar M., Trachsel E., et al. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res. 2008;14(20):6515–6524. doi: 10.1158/1078-0432.CCR-07-5041. [DOI] [PubMed] [Google Scholar]
- 46.Catania C., Maur M., Berardi R., et al. The tumor-targeting immunocytokine F16-IL2 in combination with doxorubicin: dose escalation in patients with advanced solid tumors and expansion into patients with metastatic breast cancer. Cell Adh Migr. 2015;9(1–2):14–21. doi: 10.4161/19336918.2014.983785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berdel A.F., Rollig C., Wermke M., et al. A phase I trial of the antibody-cytokine fusion protein F16IL2 in combination with anti-CD33 immunotherapy for posttransplant AML relapse. Blood. 2021;138:2345. [Google Scholar]
- 48.Nirschl C.J., Brodkin H.R., Hicklin D.J., et al. Discovery of a conditionally activated IL-2 that promotes antitumor immunity and induces tumor regression. Cancer Immunol Res. 2022;10(5):581–596. doi: 10.1158/2326-6066.CIR-21-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shanafelt A.B., Lin Y., Shanafelt M.C., et al. A T-cell-selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nat Biotechnol. 2000;18(11):1197–1202. doi: 10.1038/81199. [DOI] [PubMed] [Google Scholar]
- 50.Margolin K., Atkins M.B., Dutcher J.P., et al. Phase I trial of BAY 50-4798, an interleukin-2-specific agonist in advanced melanoma and renal cancer. Clin Cancer Res. 2007;13(11):3312–3319. doi: 10.1158/1078-0432.CCR-06-1341. [DOI] [PubMed] [Google Scholar]
- 51.Gillies S.D., Lan Y., Hettmann T., et al. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin Cancer Res. 2011;17(11):3673–3685. doi: 10.1158/1078-0432.CCR-10-2921. [DOI] [PubMed] [Google Scholar]
- 52.Gillessen S., Gnad-Vogt U.S., Gallerani E., et al. A phase I dose-escalation study of the immunocytokine EMD 521873 (Selectikine) in patients with advanced solid tumours. Eur J Cancer. 2013;49(1):35–44. doi: 10.1016/j.ejca.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 53.van den Heuvel M.M., Verheij M., Boshuizen R., et al. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med. 2015;13:32. doi: 10.1186/s12967-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitra S., Ring A.M., Amarnath S., et al. Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity. 2015;42(5):826–838. doi: 10.1016/j.immuni.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emmerich J., Kauder S., McCauley S.A., Oft M. 2021. Biased IL2 muteins methods and compositions. Patent number WO2021146436A2. [Google Scholar]
- 56.Emmerich J., Ratti N., Bauer M., et al. Poster presented at the 2021 annual meeting of the American Association for Cancer Research, virtual meeting. 10 April 2021. STK-012, an alpha/beta selective IL-2 mutein for the activation of antigen-activated T cells in solid tumors (Poster 1744) [Google Scholar]
- 57.Sahin D., Arenas-Ramirez N., Rath M., et al. An IL-2-grafted antibody immunotherapy with potent efficacy against metastatic cancer. Nat Commun. 2020;11(1):6440. doi: 10.1038/s41467-020-20220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garralda E., Alonso G., Lopez J., et al. Poster presented at the 2022 annual meeting of the American Association for Cancer Research, New Orleans, LA. 11 April 2022. ANV419, an IL-2R-beta-gamma targeted antibody-IL-2 fusion protein, induces selective effector cell proliferation in patients with progressed cancer (Poster CT140) [Google Scholar]
- 59.Kim G.-A., Kim D.-E., Kim Y.-J., et al. Abstract 689: strong anti-tumor activity and stability of IL-2/anti IL-2 conjugate SLC-3010 in preclinical experiments. Cancer Res. 2021;81(13_Supplement):689. [Google Scholar]
- 60.Lopes J.E., Fisher J.L., Flick H.L., et al. ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaishampayan U.N., Tomczak P., Muzaffar J., et al. Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors: ARTISTRY-1. J Clin Oncol. 2022;40(16_suppl):2500. [Google Scholar]
- 62.Hamid O., Liu S.V., Boccia R.V., et al. Selection of the recommended phase 2 dose (RP2D) for subcutaneous nemvaleukin alfa: ARTISTRY-2. J Clin Oncol. 2021;39(15_suppl):2552. [Google Scholar]
- 63.Charych D.H., Hoch U., Langowski J.L., et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res. 2016;22(3):680–690. doi: 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
- 64.Bentebibel S.E., Hurwitz M.E., Bernatchez C., et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rbetagamma-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019;9(6):711–721. doi: 10.1158/2159-8290.CD-18-1495. [DOI] [PubMed] [Google Scholar]
- 65.Nektar Therapeutics and Bristol Myers Squibb . 2022. Bristol Myers Squibb and Nektar announce update on phase 3 PIVOT IO-001 trial evaluating Bempegaldesleukin (BEMPEG) in combination with Opdivo (Nivolumab) in previously untreated unresectable or metastatic melanoma. Press release published on Business Wire. [Google Scholar]
- 66.Nektar Therapeutics, Bristol Myers Squibb . 2022. Nektar and Bristol Myers Squibb announce update on clinical development program for Bempegaldesleukin (BEMPEG) in combination with Opdivo (Nivolumab) Press release published on Cision PR Newswire. [Google Scholar]
- 67.Diab A., Tykodi S.S., Daniels G.A., et al. Bempegaldesleukin plus Nivolumab in first-line metastatic melanoma. J Clin Oncol. 2021;39(26):2914–2925. doi: 10.1200/JCO.21.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diab A., Curti B., Bilen M., et al. 368 REVEAL: phase 1 dose-escalation study of NKTR-262, a novel TLR7/8 agonist, plus bempegaldesleukin: local innate immune activation and systemic adaptive immune expansion for treating solid tumors. J Immunother Cancer. 2020;8:A224–A225. [Google Scholar]
- 69.Felip E, Spigel D, Juan-Vidal OJ, et al. Preliminary results from propel: a phase 1/2 study of bempegaldesleukin (BEMPEG: NKTR-214) plus pembrolizumab with or without chemotherapy in patients with metastatic NSCLC. Poster presented at the ESMO Immuno-Oncology Congress 2021, Virtual Meeting, 8-11 December 2021.
- 70.Rosen D.B., Kvarnhammar A.M., Laufer B., et al. TransCon IL-2 beta/gamma: a novel long-acting prodrug with sustained release of an IL-2Rbeta/gamma-selective IL-2 variant with improved pharmacokinetics and potent activation of cytotoxic immune cells for the treatment of cancer. J Immunother Cancer. 2022;10(7) doi: 10.1136/jitc-2022-004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ptacin J.L., Caffaro C.E., Ma L., et al. An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat Commun. 2021;12(1):4785. doi: 10.1038/s41467-021-24987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janku F., Abdul-Karim R., Azad A., et al. Abstract LB041: THOR-707 (SAR444245), a novel not-alpha IL-2 as monotherapy and in combination with pembrolizumab in advanced/metastatic solid tumors: interim results from HAMMER, an open-label, multicenter phase 1/2 Study. Cancer Res. 2021;81(13_Supplement):LB041. [Google Scholar]
- 73.Sahin U., Vormehr M., Kranz L., et al. 2020. IL-2 agonists. Patent number WO2020020783A1. [Google Scholar]
- 74.Levin A.M., Bates D.L., Ring A.M., et al. Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'. Nature. 2012;484(7395):529–533. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merchant R., Galligan C., Munegowda M.A., et al. Fine-tuned long-acting interleukin-2 superkine potentiates durable immune responses in mice and non-human primate. J Immunother Cancer. 2022;10(1) doi: 10.1136/jitc-2021-003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silva D.A., Yu S., Ulge U.Y., et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature. 2019;565(7738):186–191. doi: 10.1038/s41586-018-0830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Codarri Deak L., Nicolini V., Hashimoto M., et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8(+) T cells. Nature. 2022;610(7930):161–172. doi: 10.1038/s41586-022-05192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quayle S.N., Girgis N., Thapa D.R., et al. CUE-101, a novel E7-pHLA-IL2-Fc fusion protein, enhances tumor antigen-specific T-cell activation for the treatment of HPV16-driven malignancies. Clin Cancer Res. 2020;26(8):1953–1964. doi: 10.1158/1078-0432.CCR-19-3354. [DOI] [PubMed] [Google Scholar]
- 79.Chung C.H., Colevas A.D., Adkins D., et al. A phase 1 dose-escalation and expansion study of CUE-101, a novel HPV16 E7-pHLA-IL2-Fc fusion protein, given alone and in combination with pembrolizumab in patients with recurrent/metastatic HPV16+ head and neck cancer. J Clin Oncol. 2022;40(16_suppl):6045. [Google Scholar]
- 80.Pyo K-H, Koh YJ, Synn C-B, et al. Abstract 1826: comprehensive preclinical study on GI-101, a novel CD80-IgG4-IL2 variant protein, as a therapeutic antibody candidate with bispecific immuno-oncology target. Poster presented at the 2021 Annual Meeting of the American Association of Cancer Research, virtual meeting, 10 April 2021.
- 81.Klein C., Waldhauer I., Nicolini V.G., et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology. 2017;6(3) doi: 10.1080/2162402X.2016.1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Brummelen E., Lassen U., Melero I., et al. 4747 - pharmacokinetics (PK) and Pharmacodynamics (PD) of cergutuzumab amunaleukin (CA), a carcinoembryonic antigen (CEA)-targeted interleukin 2 variant (IL2v) with abolished binding to CD25. Ann Oncol. 2017;28:v403–v427. [Google Scholar]
- 83.Waldhauer I., Gonzalez-Nicolini V., Freimoser-Grundschober A., et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs. 2021;13(1) doi: 10.1080/19420862.2021.1913791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Italiano A., Verlingue L., Prenen H., et al. Clinical activity and safety of simlukafusp alfa, an engineered interleukin-2 variant targeted to fibroblast activation protein-α, combined with atezolizumab in patients with recurrent or metastatic cervical cancer. J Clin Oncol. 2021;39(15_suppl):5510. [Google Scholar]
- 85.Hansen A.R., Gomez-Roca C.A., Lolkema M.P., et al. 906P Simlukafusp α and cetuximab combination in patients with recurrent, unresectable or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2021;32:S805. [Google Scholar]
- 86.Perez-Gracia J.L., Hansen A.R., Eefsen R.H.L., et al. Randomized phase Ib study to evaluate safety, pharmacokinetics and therapeutic activity of simlukafusp α in combination with atezolizumab ± bevacizumab in patients with unresectable advanced/metastatic renal cell carcinoma (RCC) ( NCT03063762) J Clin Oncol. 2021;39(15_suppl):4556. [Google Scholar]
- 87.O’Neil J., Guzman W., Yerov O., et al. Poster presented at the 2021 Annual meeting of the American Society of Clinical Oncology, virtual meeting. 4 June 2021. Tumor-selective activity of XTX202, a protein-engineered IL-2, in mice without peripheral toxicities in nonhuman primates. [Google Scholar]
- 88.Dixit N., Fanton C., Langowski J.L., et al. NKTR-358: a novel regulatory T-cell stimulator that selectively stimulates expansion and suppressive function of regulatory T cells for the treatment of autoimmune and inflammatory diseases. J Transl Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fanton C., Furie R., Chindalore V., et al. Selective expansion of regulatory T cells by NKTR-358 in healthy volunteers and patients with systemic lupus erythematosus. J Transl Autoimmun. 2022;5 doi: 10.1016/j.jtauto.2022.100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peterson L.B., Bell C.J.M., Howlett S.K., et al. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J Autoimmun. 2018;95:1–14. doi: 10.1016/j.jaut.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varma R., Liu K., Avery K., et al. Regulatory T cell selective IL-2-Fc fusion proteins for the treatment of autoimmune diseases. Blood. 2018;132(Supplement 1):3709. [Google Scholar]
- 92.Visweswaraiah J., Sampson E.R., Kiprono T., et al. Generation of PT101, a highly selective IL-2 mutein for treatment of autoimmune diseases. J Immunol. 2021;206:66.14. [Google Scholar]
- 93.Ghelani A., Bates D., Conner K., et al. Defining the threshold IL-2 signal required for induction of selective Treg cell responses using engineered IL-2 muteins. Front Immunol. 2020;11:1106. doi: 10.3389/fimmu.2020.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tchao N., Amouzadeh H., Sarkar N., et al. Efavaleukin alfa, a novel IL-2 mutein, selectively expands regulatory T cells in patients with SLE: interim results of a phase 1b multiple ascending dose study. Arthritis Rheumatol. 2021;73:3613–3615. [Google Scholar]
- 95.Tchao N., Gorski K.S., Yuraszeck T., et al. Amg 592 is an investigational IL-2 mutein that induces highly selective expansion of regulatory T cells. Blood. 2017;130:696. [Google Scholar]
- 96.Hsieh Y.T., Hubeau C., Massa V., et al. Emerging best-in-class IL-2 variant highlights Treg-directed therapy for autoimmune disease. Ann Rheum Dis. 2020;79(Suppl 1):195. [Google Scholar]
- 97.Arenas-Ramirez N., Woytschak J., Boyman O. Interleukin-2: Biology, design and application. Trends Immunol. 2015;36(12):763–777. doi: 10.1016/j.it.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Boyman O., Kovar M., Rubinstein M.P., Surh C.D., Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 99.Arenas-Ramirez N., Zou C., Popp S., et al. Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci Transl Med. 2016;8(367) doi: 10.1126/scitranslmed.aag3187. [DOI] [PubMed] [Google Scholar]
- 100.Sanofi Third quarter 2022 results. https://www.sanofi.com/en/investors/financial-results-and-events/financial-results/Q3-results-2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Risk of bias assessment.