To the Editor:
Significant sex differences exist in the prevalence and severity of asthma across the life span. Asthma, which affects 8% of the U.S. population, is more prevalent and severe in pubescent boys than girls but worse in girls and women after puberty (1). Population-based studies from the United States and the United Kingdom revealed significant associations between sex hormones and asthma (2, 3). This suggests that sex and sex hormones play an important role in asthma pathogenesis. The cumulative genetic burden of asthma susceptibility loci also appears to differ by sex (4). In this study, we identify sex-specific associations between germline genetic variation and asthma susceptibility to gain insight into mechanisms that drive disease-related sexual dimorphism.
We analyzed data from the UK Biobank, a large-scale biomedical database, which includes genomic data from 500,000 participants from the United Kingdom (5). We only included non-Hispanic white (∼90%) participants because of small sample size in other ancestral populations. The diagnosis of asthma was made based on self-reported questionnaire information. Patients with other chronic lung diseases were excluded. We first conducted genotype quality control at both the individual level and the SNP level by omitting samples with mismatch between genetic and putative sex, excess heterozygosity, >5% missing genotypes, close relatedness (coefficient of relationship > 0.2), and ancestry outliers as determined using principal components and omitting variants with minor allele frequency < 0.01, >3% missing genotypes, or P < 10−5 from an exact test of Hardy-Weinberg proportions. Y chromosome variants were excluded from comparison.
Sex-stratified genome-wide association analyses were then performed using logistic regression adjusted for age, body mass index, smoking status, pack-years smoking history, batch effect, and the first 10 principal components of ancestry as covariates. Genetic variants that were significantly associated with asthma (P < 5 × 10−8) in either sex-stratified genome-wide association analysis were identified. Of those 1,342 independent significant SNPs, 154 lead SNPs were mapped to Ensembl gene IDs using the biomaRt Bioconductor package (6). For each of these 154 lead SNPs, the odds ratio of asthma genetic risk susceptibility was then compared between women and men using the Wald test, where the z-score equals the difference in odds ratio between men and women divided by the square root of the sum of the squared SE in men and squared SE in women or . P values were computed using R version 4.0.5 (R Project for Statistical Computing), and multiple testing was adjusted for using false discovery rate. Genes with significant sex differences in the effect size of the association with asthma were included in subsequent pathway enrichment analysis using Ingenuity Pathway Analysis (Ingenuity Systems) software. This research has been conducted using the UK Biobank Resource under Application Number 44578.
Inclusion criteria were met in 220,673 women and 187,584 men enrolled in the UK Biobank. Participants with asthma (26,198 women and 19,055 men) had a median age of 57.0 years (interquartile range, 49.0–63.1 yr), and 57.9% were females. Although age did not differ between both sexes (P = 0.257), women had a lower body mass index (median [interquartile range], 27.0 [24.0–31.1] vs. 27.4 [25.1–30.4]; P < 0.001) than men.
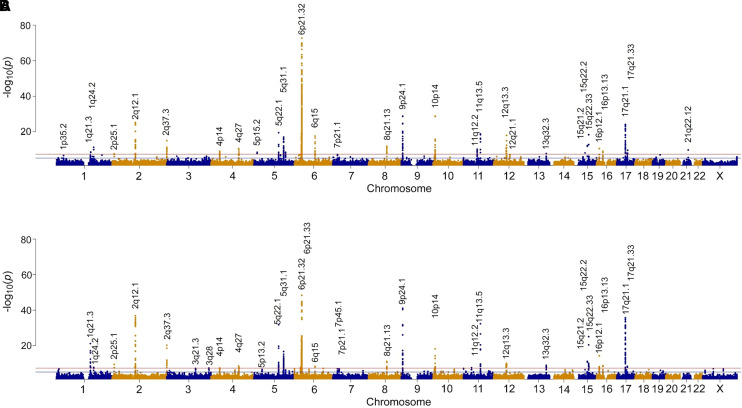
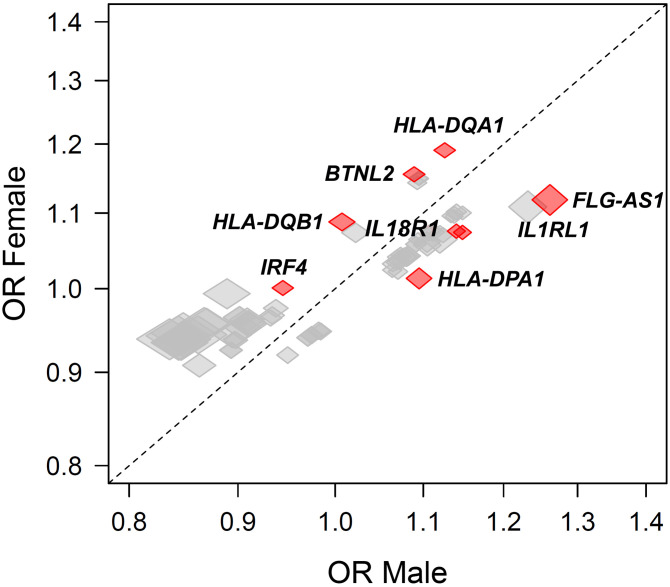
Sex-stratified genome-wide association analyses are demonstrated in Figure 1. Of the 803,113 genetic variants that passed the genotype quality control, 1,342 variants reached genome-wide significance at P < 5 × 10−8 in either male- (n = 961; n = 301 unique to male sex) or female- (n = 1,041; n = 381 unique to female sex) stratified genome-wide association analyses mapping to 161 unique genes. However, sex differences in the effect size of such associations were present in 76 genes (P < 0.05). Of those, sex differences were present in only eight genes (HLA-DQA1, HLA-DQB1, IL1RL1, FLG-AS1, BTNL2, IL18R1, HLA-DPA1, and IRF4 [Interferon Regulatory Factor 4]) at a false discovery rate < 0.05 (colored in red in Figure 2). Pathway enrichment analysis highlighted several biological pathways, particularly T-helper cell type 1 (Th1) and Th2 activation pathway, antigen presentation pathway, glucocorticoid receptor signaling, and IL-4 signaling. Although these reached statistical significance, the difference in the effect size between genetic susceptibility and asthma association in men and women was small.
Figure 1.
Manhattan plot of results of genome-wide association testing are shown for (A) female, and (B) male UK Biobank participants. The horizontal line indicates the stringent genome-wide significance threshold (P < 5 × 10−8).
Figure 2.
Estimated effect sizes stratified by sex. The size of each marker reflects confidence intervals (with height reflecting the confidence interval along the y-axis and width reflecting the confidence interval along the x-axis). Comparisons reaching P < 0.05 are labeled and colored in red. BTNL2 = Butyrophilin Like 2; FLG-AS1 = Filaggrin Antisense RNA 1; HLA-DPA1 = Major Histocompatibility Complex, Class II, DP Alpha 1; HLA-DQA1 = Major Histocompatibility Complex, Class II, DQ Alpha 1; HLA-DQB1 = Major Histocompatibility Complex, Class II, DQ Beta 1; IL18R1 = Interleukin 18 Receptor 1; IL1RL1 = Interleukin 1 Receptor Like 1; IRF4 = interferon regulatory factor 4; OR = odds ratio.
These data highlight that sex differences in asthma genetic susceptibility are strongly related to immunologic pathways, including Th1 and Th2 pathway activation, and differences in the immune response to viral infections. HLA-DQA1 [Major Histocompatibility Complex, Class II, DQ Alpha 1], involved in Th cell signaling and Th17 differentiation, was highly associated with asthma in previous studies (7). IRF4 (Interferon Regulatory Factor 4)—a transcription factor that drives dendritic cells to promote Th2 differentiation—has previously been linked to sex differences in the immune response to viral infections (triggering asthma exacerbations) (8).
Although the sexual dimorphism of asthma is well described, little is known about the effect of sex or sex hormones on the biological mechanisms underlying asthma and severe asthma (SA) (1). Testosterone attenuates both Th2-induced and IL17A-associated allergic inflammation (9), whereas estradiol and progesterone increase IL-17A production from TH17 cells, providing potential mechanisms for sex differences in asthma (10). In the National Heart, Lung, and Blood Institute SARP (Severe Asthma Research Program), an ongoing multicenter program to investigate the pathogenesis of SA (1), we demonstrated that increasing serum androgens, but not estradiol concentrations, were associated with better lung function (11). Similarly, our data suggested that the androgen receptor signaling pathway plays an important role in asthma pathogenesis. In fact, airway androgen receptor expression was lower in patients with asthma than in control subjects in both sexes, and androgen receptors negatively correlated with nitrosative airway inflammation (11, 12).
Our results add to the existing body of evidence highlighting the role of sex differences in asthma susceptibility. Yet, the effect of sex on the genomic drivers of asthma is marginal, suggesting that other mechanisms, such as gene regulation, gene-by-gene interaction, and gene-by-environment interactions (such as the hormonal milieu or outdoor exposures) may also play an important role. Although understanding the pathobiology of asthma and SA has been a public health priority (1), defining key sex-specific hormonal and genomic drivers of asthma risk and severity is critical to implementing personalized, hormone-based risk stratification to refine treatment approaches in women and men with asthma and SA.
Acknowledgments
Acknowledgment
The method of this manuscript was recommended and guided by Robert P. Igo, Ph.D. Dr. Igo was a professor of statistical genetics at Case Western Reserve University, who died during the COVID-19 pandemic.
Footnotes
Supported by National Institutes of Health National Heart, Lung, and Blood Institute grants K08 HL133381 (J.G.Z.), R01 HL161674 (J.G.Z), and 1P01HL158507 (B.G.).
Author contributions: J.G.Z., P.B., and V.O. made substantial contributions to the conception or design of the work. J.G.Z. and P.B. acquired, analyzed, and interpreted the data for the work. J.G.Z., P.B., D.M., E.B., B.G., B.H., A.A., and V.O. contributed to drafting the work or revising it critically for important intellectual content. J.G.Z. agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. J.G.Z., P.B., D.M., E.B., B.G., B.H., A.A., and V.O. gave the final approval of the version to be published.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract . 2018;6:545–554.e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han YY, Forno E, Celedón JC. Sex steroid hormones and asthma in a nationwide study of U.S. adults. Am J Respir Crit Care Med . 2020;201:158–166. doi: 10.1164/rccm.201905-0996OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han YY, Yan Q, Yang G, Chen W, Forno E, Celedon JC. Serum free testosterone and asthma, asthma hospitalisations and lung function in British adults. Thorax . 2020;75:849–854. doi: 10.1136/thoraxjnl-2020-214875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y, Jia Q, Jahani PS, Hurrell BP, Pan C, Huang P, et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat Commun. 2020;11:1776. doi: 10.1038/s41467-020-15649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature . 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smedley D, Haider S, Ballester B, Holland R, London D, Thorisson G, et al. BioMart: biological queries made easy. BMC Genomics . 2009;10:22. doi: 10.1186/1471-2164-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. GABRIEL Consortium A large-scale, consortium-based genomewide association study of asthma. N Engl J Med . 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carreras E, Turner S, Frank MB, Knowlton N, Osban J, Centola M, et al. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood . 2010;115:238–246. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuseini H, Yung JA, Cephus JY, Zhang J, Goleniewska K, Polosukhin VV, et al. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol . 2018;201:1843–1854. doi: 10.4049/jimmunol.1800293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol . 2015;136:1025–1034.e11. doi: 10.1016/j.jaci.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zein JG, McManus JM, Sharifi N, Erzurum SC, Marozkina N, Lahm T, et al. Benefits of airway androgen receptor expression in human asthma. Am J Respir Crit Care Med. 2021;204:285–293. doi: 10.1164/rccm.202009-3720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McManus JM, Gaston B, Zein J, Sharifi N. Association between asthma and reduced androgen receptor expression in airways. J Endocr Soc . 2022;6:bvac047. doi: 10.1210/jendso/bvac047. [DOI] [PMC free article] [PubMed] [Google Scholar]