Abstract
The population that has not received a SARS-CoV-2 vaccine is at high risk for infection whereas vaccination prevents COVID-19 severe disease, hospitalization, and death. In Argentina, to date, more than 50 million doses of vaccines against SARS-CoV-2 have been administered. The three main vaccines applied are Sputnik V, Oxford–AstraZeneca, and Sinopharm. In this study, we have compared the antibody response of voluntary individuals at day 0 (first dose vaccination day) and at 21–25 days post first and second dose. Our results indicate that at 21–25 days after the administration of the first doses of Sputnik V the large majority of the people vaccinated 80% (n = 15) presented high humoral responses as determined by the measurement of IgG against the Spike protein and the Receptor Binding Domain (RBD). In the case of those vaccinated with AstraZeneca, the percentage was 80% (n = 15) whereas this value was reduced to only 25% (n = 16) in persons that received Sinopharm. However, after the second doses, most of the recipients had significant levels of antibodies.
The virus neutralizing capacity of the antibodies generated was evaluated using a pseudotyped VSV-SARS-CoV2 Spike expressing eGFP and the data was analyzed by fluorescence microscopy and flow cytometry. The results indicate that a good correlation exists between the levels of IgG and the neutralizing capacity of the antibodies against the recombinant virus.
Our results stand out the importance of applying the second dose of Sinopharm. Thus, the present report provides data that will contribute to decisions making about the vaccine implementation plans of action for, not only our region but our country to support the fight against the COVID-19 global pandemic.
Keywords: COVID-19, Vaccine, Antibody, SARS-CoV-2, Virus
1. Introduction
Several criteria are used to evaluate the efficiency of vaccines including the efficacy to prevent death and critical disease. In the present study, we have evaluated the humoral response after receiving the first and second doses of three different vaccines. The anti-SARS-CoV-2 vaccines analyzed in this study were the main vaccines applied in Argentina since the beginning of 2020. The Gam-Covid-Vac known as Sputnik V is a non-replicative vector viral vaccine developed in The Gamaleya National Center of Epidemiology and Microbiology (Rusia) to be applied to people from 18-year-old or older [1]. The AstraZeneca vaccine, ChAdOx1 nCoV-19 vaccine (AZD1222) AstraZeneca-Oxford – United Kingdom, is a non-replicative vector viral vaccine for 18-year-old and older [2]. And the Sinopharm SARS-CoV-2 virus inactivated vaccine was made by the Beijing Institute of Biological Products (China) for 3 years old and older [3].
Vaccination in Argentina began on December 29th, 2020, with the Sputnik V vaccine, for healthcare personnel. The main aim was to vaccinate 100% of the population in a staggered and progressive manner, taking into account the resource availability (vaccine) and prioritizing the risk population. Because the vaccine doses availability was limited, it was required to establish a priority order of the population to be vaccinated, defined in stages and groups. The criteria established, taking into account a bioethical framework, to build the different stages in order to vaccinate the population, were based on the risk to develop severe disease and complications from COVID-19, the higher probability of exposure to the virus, the need to mitigate the impact of COVID-19 on carrying out socioeconomic activities and the possibility of influencing the chain of transmission.
As examples of the order of priority, we will mention: health personnel (grading based on the risk stratification of the activity), adults 70-year-old and over/Seniors residing in long-stay homes, adults 60 to 69-year-old age, Armed Forces, Security Forces, and Penitentiary Services Personnel, Teaching and non-teaching staff (initial, primary, and secondary levels schools), and so on.
The important advance of the promoted Strategic Plan for Vaccination against SARS-CoV-2 in our country allowed reaching 70% of the total population with a complete vaccination scheme after one year of implementation.
Currently, given the possibility of having different vaccines authorized in Argentina against COVID-19, progress is being made in the vaccination of different population groups from 3 years of age as well as the application of additional doses and boosters. www.argentina.gob.ar.
In the present report, we have determined the seroconversion of individuals at three time points: base lane (i.e. vaccination day, time 0) and at 21–25 days post first dose and at 21–25 days post second one for the each three different vaccines described above. These vaccines were the first to be applied in Argentina. In addition, we have evaluated the neutralizing capacity of the antibodies generated using an eGFP labeled pseudotyped virus VSV-SARS-CoV-2 expressing the protein Spike. Our results indicate that the Sputnik V and AstraZeneca vaccines were the most efficient in inducing high antibody titers after the first doses whereas a lower percentage of vaccinated individuals responded to the first dose of Sinopharm. However, after the application of the second dose the seroconversion titer increased. Our data emphasis that the two doses of Sinopharm must be applied to generate a humoral response.
2. Materials and methods
2.1. Participant volunteers and samples
The humoral immune response over time in individuals immunized with Sputnik V, AstraZeneca, or Sinopharm vaccines was determined by ELISA using the test COVIDAR (see below). The study was taken in Mendoza, Argentina with random volunteers donors. The participating volunteers signed an informed consent, prior to obtaining the samples, approved by the Board of Directors from the Hospital Universitario (HU) and the ethic committee of the Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina (FCM). The informed consent forms with the personal information were kept anonymous and assigned a serial number. The blood samples were acquired by venipuncture at HU and FCM vaccinating center, and then collected into serum separator tubes (SSTs). After the blood clot was formed, the samples were subjected to centrifugation and the serum was stored at −20 °C. Those individuals that had Covid-19 or were reactive at time 0 (vaccination day) were excluded from this study. All the blood samples were collected during the year 2021, since February to October.
The general characteristics of the volunteers are summarized in Supplementary Table 1. The number of volunteers correspond to all the volunteers who joined the study, in the two vaccination centers, during the period of time that the work lasted.
2.2. Cell lines used and culture conditions
Vero-CCL81 cells (ATCC) and 293T ACE2/TMPRSS2 cells (kindly provided by Benhur Lee) were used. Cells were cultured at 37 °C in 5% CO2 in Dulbecco's modified Eagle's high-glucose medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (NATOCOR).
2.3. SARS-CoV-2 antibody ELISA
The measurements of the IgG antibodies against SARS-CoV-2 were performed using the test COVIDAR developed in Argentina, a well-established immunoenzymatic assay previously described [4]. The test COVIDAR is an ELISA assay, based on the use of antigens generated by DNA recombinant technology, corresponding to the protein Spike and the Receptor Binding Domain (RBD), from now on anti-Spike IgG, present in the viral protein, which binds to the ACE2 receptor. For the assays, a quantitative COVIDAR test was used according to the manufacturer's instructions. To express IgG titers in Internationals Units/ml (IU/ml), the 450 nm OD value obtained were extrapolated on a calibration curve [5].
The presence or absence of a specific IgG antibody against the SARS-CoV-2 virus is determined by taking into account the Decision Limit (Cut-off value). This is calculated by obtaining the average of the optical density (OD) of the Negative Control + 0.150. Samples with an OD value of 450 nm within the Cut-off value ± 10% are considered in the gray area. Samples with an OD 450 nm value below the lower limit of the gray zone would be considered non-reactive for antibodies against the SARS-CoV-2 virus. Samples with an OD 450 nm value higher than the upper limit of the gray zone would be considered reactive for antibodies against the SARS-CoV-2 virus. The calibration curve for quantitative determination was constructed following the manufacturer instructions [4].
2.4. Recombinant pseudotyped SARS-CoV-2 virus
Replication-competent SARS-CoV-2 pseudotyped particles generated in Dr. Sean Whelan's laboratory in St. Louis MO, USA [6] were used for the neutralization assays. Briefly, these recombinant pseudotyped viruses were constructed using the vesicular stomatitis virus (VSV) in which the glycoprotein gene G was replaced with the Spike protein of SARS-CoV-2. In addition, these recombinant viruses express eGFP allowing the visualization of the infected cells by fluorescence microscopy. Whelan and collaborators have shown that using these recombinant viruses, to test the neutralizing activities of both, monoclonal or polyclonal antibodies, show an elevated degree of concordance against a clinical isolate of SARS-CoV-2. These findings validate the use of the pseudotyped virus to assay the neutralizing activity of the serum samples. The assays were performed in a biosafety level 2 (BSL2) containment.
2.5. Generation and titration of virus stocks
A virus aliquot was amplified in our laboratory. For this purpose, HEK 293T cells overexpressing ACE2/TMPRSS2 were seeded in a T75 were grown to a 70% of confluency in DMEM containing 10% FBS at 37 °C and 5% CO2. The cells were infected with the pseudotyped virus in DMEM 2% FBS at a MOI of 0.01 for 1h at 37 °C. Afterwards the inoculum was replaced by DMEM 2% FBS. The infection was evaluated by fluorescence microscopy and cell culture supernatants were collected when cytopathic effects are observed in the cell monolayers. The samples were centrifuged to eliminate cellular debris and subsequently aliquoted and preserved at −80 °C.
Viral stock titration was performed using Vero CCL81 cells seeded in 24 wells and incubated with serial dilutions of the viral stock. After 1h, low melting agarose was added to each well and the formation of the bald-spots was observed after 72–96 h of incubation. Cells were fixed with 10% paraformaldehyde and stained with crystal violet.
2.6. Amplification of virus
A virus aliquot was amplified in our laboratory. For this purpose, HEK 293T cells overexpressing ACE2/TMPRSS2 were seeded in a T75 were grown to a 70% of confluency in DMEM containing 10% FBS at 37 °C and 5% CO2. The cells were infected with the pseudotyped virus in DMEM 2% FBS at a MOI of 0.01 for 1h at 37 °C. Afterwards the inoculum was replaced by DMEM 2% FBS. The infection was evaluated by fluorescence microscopy and cell culture supernatants were collected when cytopathic effects are observed in the cell monolayers. The samples were centrifuged to eliminate cellular debris and subsequently aliquoted and preserved at −80 °C.
Viral stock titration was performed using Vero CCL81 cells seeded in 24 wells and incubated with serial dilutions of the viral stock. After 1h, low melting agarose was added to each well and the formation of the bald spots was observed after 72–96 h of incubation. Cells were fixed with 10% paraformaldehyde and stained with crystal violet.
Fluorescence forming units per milliliter (FFU) were determined as previously described [6].
2.7. Pseudovirus neutralization assay
To determine the neutralizing capacity of the antibodies present in the serum of the tested individuals, Vero CCL81 cells were seeded in a 24 well culture plate in a final volume of 250 μl of alpha-MEM containing 10% FBS. After 24 h, cells were infected with 102 PFU of pseudovirus VSVΔg-Spike-GFP pre-incubated with serial tenth dilutions of patient serum for 1h at 37 °C. The patient serums were previously heat-inactivated at 56 °C for 30 min. After 24h incubation samples were fixed with 2% paraformaldehyde and evaluated by fluorescence microscopy to visualize the infected fluorescent cells (qualitative assay). For quantified assay, the infected fluorescent cells were washed, fixed in 1% paraformaldehyde, and analyzed with a Becton Dickinson FACSAria III. Data were collected using Facs Diva software and analyzed with Cyflogic. The populations were quantified based on fluorescent labeling and the FSC/SSC profile. The following controls are used, pseudovirus pre-incubated with negative serum for anti SARS CoV2 tests as a negative control of neutralization and as a positive control of infection, the pseudovirus was developed alone (Supplementary Fig. 1 and Supplementary Fig. 2).
2.8. Statistical analysis
Data from samples that do not follow normal distribution were analyzed with the non-parametric Friedman test and Dunn's Multiple Comparison post-test, Kruskal Wallis test and Dunn's Multiple Comparison post-test. The nonparametric correlation Spearman test was used to analyze the correlation between anti-Spike IgG production and infection percentage. The Graph Pad Prism Software version 9 was used. A p value less than 0.05 was considered statistically significant.
2.9. IC50 determination
The infection rate was plotted against the log anti-Spike IgG concentration after normalizing the infection from 0 to 100%. The curves were fitted to a nonlinear sigmoidal function to determine the IC50 values. Curve fitting was performed using GraphPad Prism (version 9.02, GraphPad Software, San Diego, CA).
3. Results
3.1. Humoral response variations for the complete vaccination schemes of Sputnik V, AstraZeneca and Sinopharm vaccines
We have determined the seroconversion in volunteer individuals that received vaccination among the months of February and October in Mendoza, Argentina. As shown in Table 1, 109 serum samples were collected and analyzed by the ELISA test COVIDAR as described in Materials and Methods. Approximately 30% of the people tested presented detectable antibodies against the Spike protein and the Receptor Binding Domain (RBD) at time 0, vaccination day, indicating that have been infected with the virus previously (Covid-19 convalescents), and this group of people COVID-19 convalescents were excluded for the following studies. As depicted in Table 1 from 96 samples obtained at 21–25 days after the first dose application 69 were reactive. After the second dose, 95.9% of individuals that attended to the serum extraction were reactive (Table 1), indicating seroconversion.
Table 1.
Summary results of seroconversion following SARS-CoV-2 vaccination.
| Sample collection | Tested | Seroconversion (SARS CoV2 Spike IgG)a |
|---|---|---|
| 21–25 days after dose 1 | 96 | 69 (71.9%)b |
| 21–25 days after dose 2 | 47 | 45 (95.7%)b |
ELISA detection of anti-SPIKE IgG§-SARS-CoV-2 in blood serum.
Displayed are the number of individuals and the percentage of seroconverted among the tested individuals.
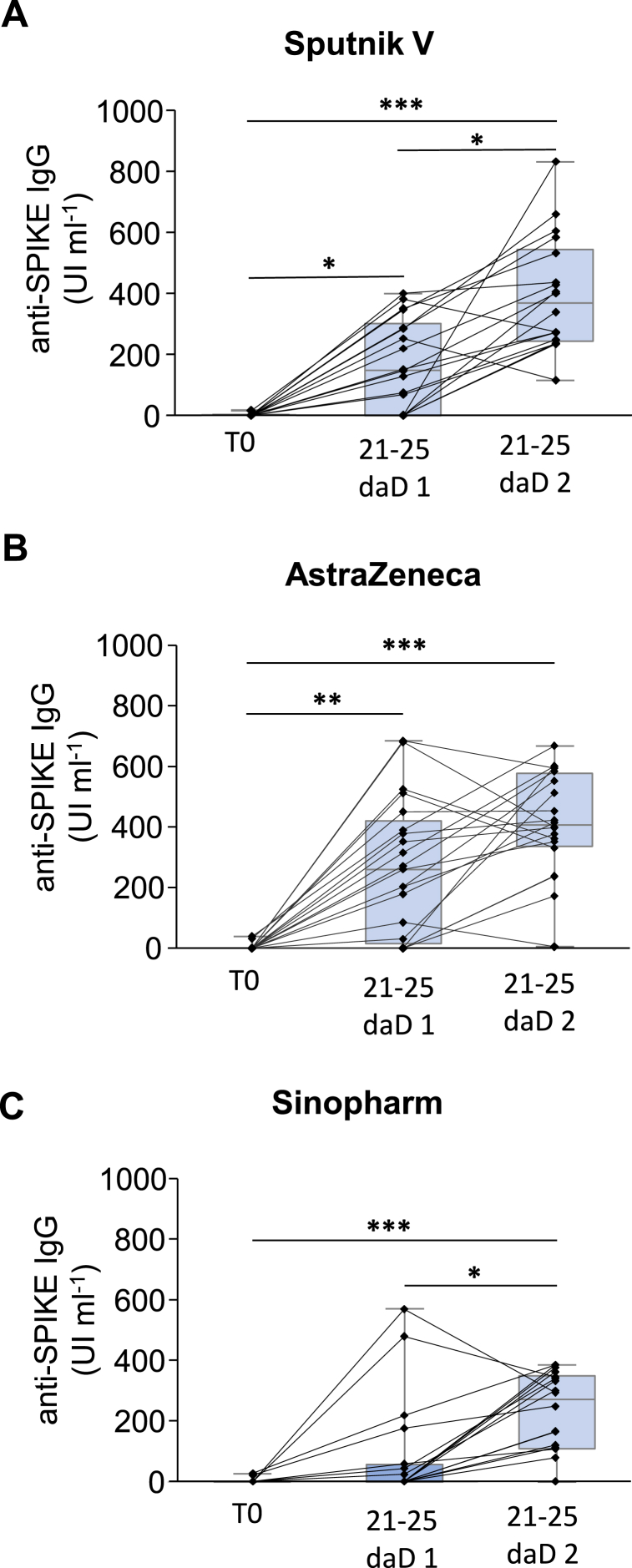
The responsivity of each individual receiving Sputnik V, AstraZeneca or Sinopharm is shown in Fig. 1 (A, B, and C respectively) depicting that the majority of the persons tested presented an increase in the antibody's titer expressed in IU ml−1 after the application of the second dose, which is also evident in the case of Sinopharm (Fig. 1C).
Fig. 1.
Responsivity of each individual receiving SARS-CoV-2 Vaccination. Each single black line connecting bars represent the evolution of the anti-SPIKE IgG titer for a particular individual before vaccination (T0), 21–25 days after receive the dose 1 (21-25 daD 1), and 21–25 days after receive the dose 2 (21-25 daD 2) for the vaccines A Sputnik V, B AstraZeneca, or C Sinopharm. *: p < 0,05; **: p < 0,01; ***: p < 0,001 Friedman statistic test with Dunn post test for multiple comparisons.
When these determinations were discriminated in naïve individuals according to the vaccine received (see Table 2), after the administration of the first doses of Sputnik V, 75% (n = 16) of the people vaccinated presented IgG antibodies. In the case of those vaccinated with Oxford/AstraZeneca the percentage was 80% (n = 15) whereas this value was significantly lower, 25% (n = 16), in people that received Sinopharm. After the second dose application, the percentage of reactive samples was 100% for Sputnik V and AstraZeneca. In the case of Sinopharm, the responsivity increased to 88% of the cases.
Table 2.
Summary results of seroconversion discriminating according to the SARS-CoV-2 vaccine received.
| Dose 1 | Dose 2 | ||||
|---|---|---|---|---|---|
| COVID19 Vaccine | anti-SPIKE IgGa | IgGb (UI ml−1) | anti-SPIKE IgGa | IgGb (UI ml−1) | n |
| Sputnik | 75% | 164 ± 39 | 100% | 414 ± 49 | 16 |
| AstraZeneca | 80 | 250 ± 56 | 100% | 416 ± 44 | 15 |
| Sinopharm | 25% | 70 ± 45 | 88% | 213 ± 36 | 16 |
n Individuals tested.
Percentages of seroconversion post-COVID 19 vaccination.
Anti-SPIKE IgG titer in blood serum.
During the course of this study some individuals who received Sputnik V vaccine as the first dose were vaccinated with Moderna vaccine for the second dose. The seroconversion titer reached was comparable to the homologous vaccination (data not shown).
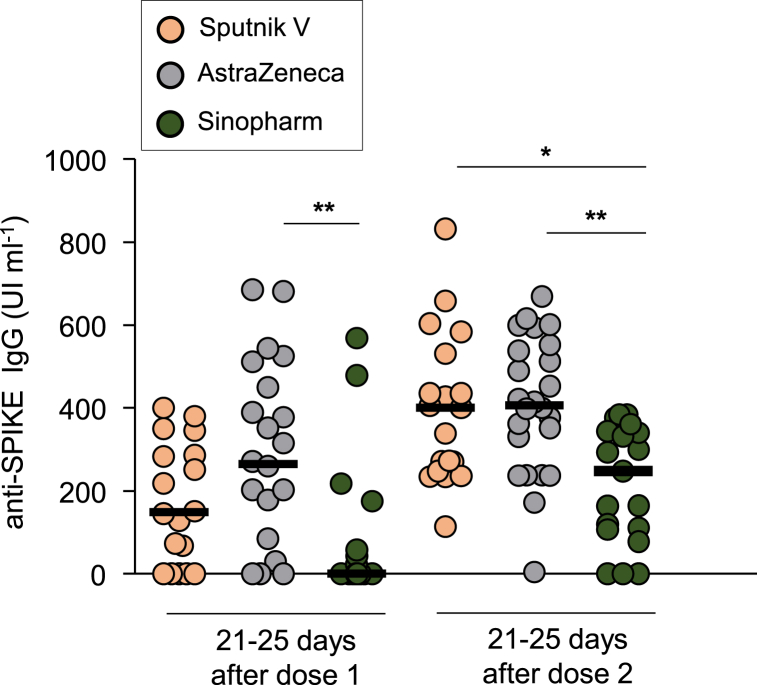
These data are also presented in Fig. 2, which indicates that after the first dose the medians for Sputnik V (orange circles) and AstraZeneca (gray circles) were higher with respect to Sinopharm (green circles). However, after the second dose, although the levels of antibodies to Sputnik V and AstraZeneca remained higher, an increase in the levels of antibodies to Sinopharm was observed (Fig. 2 and Table 2). For comparative purpose, the antibodies levels detected in Covid-19 convalescent sera were included in Supplementary Fig. 3; showing that the seroconversion in individuals vaccinated with Sputnik V and AstraZeneca vaccines was higher than the Covid-19 convalescent patients.
Fig. 2.
Comparison of Responsivity of different SARS-CoV-2 vaccine brands. Each dot represents the anti-SPIKE IgG titer for a particular individual 21–25 days after receive the dose 1 and dose 2 for the vaccine Sputnik V (orange dots), AstraZeneca (gray dots) or Sinopharm (green dots). The horizontal lines show the median for each group. *:p < 0,05, **: p < 0,01 Kruskal–Wallis test with Dunn post test for multiple comparisons.
3.2. There is a positive correlation between neutralization serum effectiveness and levels of antibodies
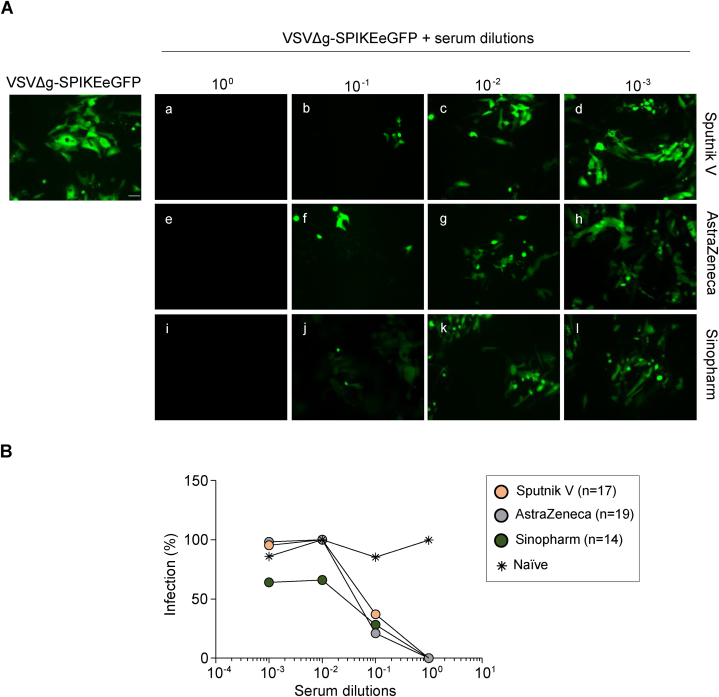
Subsequently, the neutralizing capacity of the detected antibodies was determined using a pseudotyped VSV-SARS-CoV2 expressing the protein Spike and also the fluorescent eGFP protein (VSVΔg-Spike eGFP) which allows the visualization of the virus inside the infected cells by fluorescent microscopy. Thus, we were able to show that pre-incubation of the pseudovirus with serial dilutions of the immunized individuals serum hampered the entry of the virus to the cell in comparison to the serum of non-vaccinated as depicted in Fig. 3A. These results were quantified by flow cytometry taken advantage of the fluorescence emitted by the infected cells. As shown in Fig. 3B, all the samples analyzed presented neutralizing capacity against the infection with the pseudotyped virus. Interestingly, the serum of the Sinopharm vaccinated individual seems to present a slightly more neutralizing capacity than the others.
Fig. 3.
Comparison of the neutralizing capacity of serum from individuals vaccinated with different SARS-CoV-2 vaccine. A Pre-incubation of the pseudovirus VSVΔg expressing the protein SPIKE and the fluorescent eGFP protein, with serial dilutions of serum from individuals vaccinated with Sputnik V (a–d), AstraZeneca (e–h) and Sinopharm (i–l) resulted in a clear inhibition of the infection of the Vero CCL81 cells. Bar: 20 μm B. The neutralization capacity of serum samples from individuals that received different vaccines, or non-vaccinated individuals were measured by flow cytometry and the eGFP fluorescence was interpreted as a percentage of infection. Each dot represents the median value in each group of vaccinated * Naïve: non-vaccinated, non-infected with SARS-CoV-2 individuals.
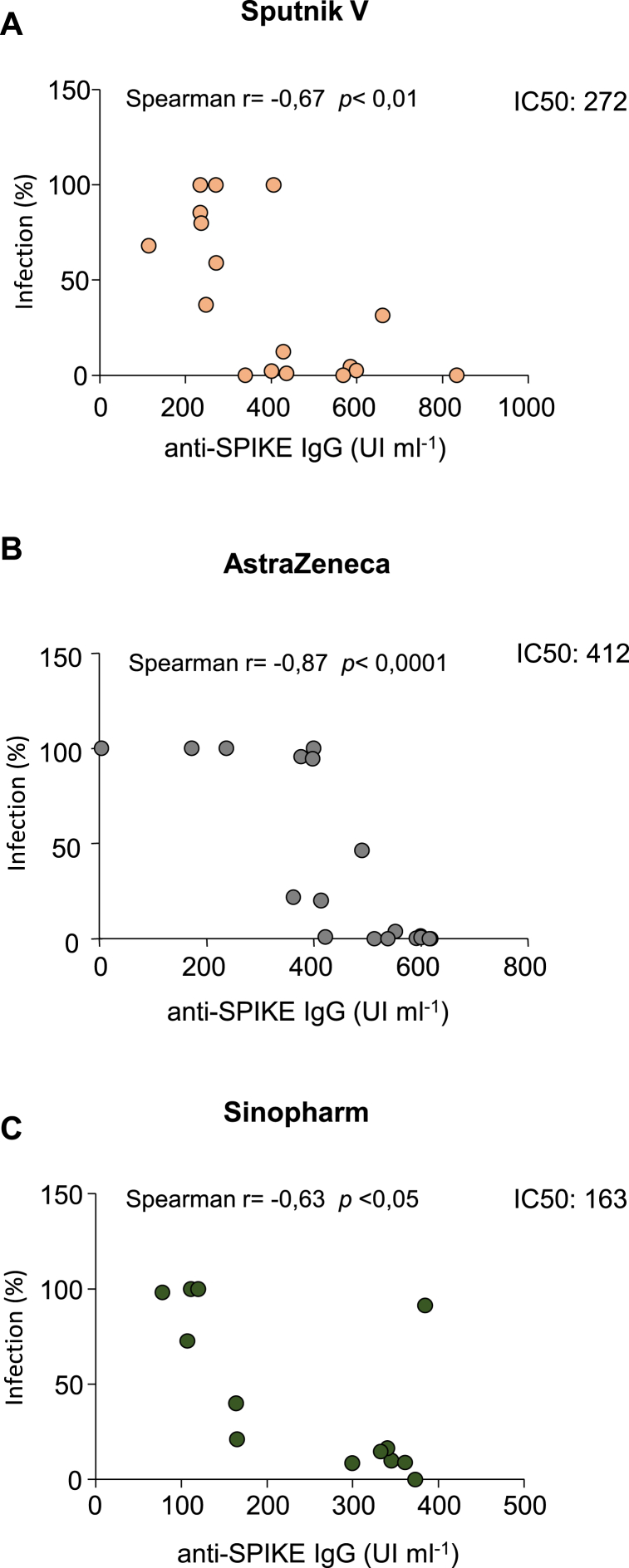
In addition, we have observed that there is a significant correlation between the anti-Spike generation and the neutralizing capacity of the serum of the vaccinated cohort as published by Ojeda et al. (Fig. 4A–C), [4]. We have found that Sinopharm vaccine inhibits the entry of the virus with lower titer of antibodies respect to Sputnik V and AstraZeneca.
Fig. 4.
Relationship between the antibody generation and their neutralizing capacity for each vaccine used in this study. The A, B and C graphics show the correlation between the blocking ability for the virus entry (infection %) related to the antibody titer production (UI/ml) from the serum of individuals immunized with the different vaccines. IC50 values were estimated; A Sputnik V 272 IU ml−1; B AstraZeneca 412 IU ml−1; C Sinopharm 163 IU ml−1 respectively.
IC50 values as the concentration of anti-SPIKE IgG to block a half maximal infection of the virus were estimated; Sputnik V 272 IU ml−1 342; AstraZeneca 412 IU ml−1; Sinopharm 163 IU ml−1 343 respectively. Moreover, the convalescent serums were also effective in neutralizing the virus (Supplementary Fig. 4 A-C) but a higher titer of produced antibodies respect to the ones generated after vaccines application is required.
4. Discussion
In this work, we have reported relevant data about the humoral response in individuals who were inoculated with any of the three vaccines studied. Our results indicate that after the first dose of the Sputnik V vaccine the participants of this study presented a high seroconversion rate in agreement with the previous publication by Rossi et al. [7]. Similar results were obtained with AstraZeneca. However, after the first dose of Sinopharm, the percentage of individuals that produced circulating antibodies was much lower respect to the other ones (Fig. 1). Nevertheless, these differences were partially alleviated in the cohort receiving the second dose of Sinopharm (Fig. 1). To the best of our knowledge, there are very limited studies comparing the immunogenicity of the vaccine Sputnik V in contrast with other vaccines. In a recent paper, the authors show similar results to us in antibody activity [8].
As we show in Supplementary Fig. 3, the seroconversion in individuals vaccinated with Sputnik V and AstraZeneca vaccines was higher than in the Covid-19 convalescent patients. Do to it is well known that antibodies levels decline with time. Then, it is likely that these differences could be due to the time elapsed after the infection with SARS-CoV-2 in the convalescent patients. In contrast, in the case of the vaccinated people, the antibody levels were determined at a specific time, 21–25 days after receiving the first or the second dose.
This report highlight that, in the case of Sinopharm, is critical to apply the second dose, to acquire an adequate level of protective antibodies against SARS-CoV-2. Furthermore, since the seroconversion levels reached with the second dose of Sinopharm is lower than with the Sputnik V and AstraZeneca vaccines (Fig. 2), it would be recommended a booster dose. Nevertheless, as mentioned above, the neutralizing capacity of the antibodies produced by the Sinopharm vaccine is moderately higher than the other two vaccines (Figs. 3 and 4C), probably due to the nature of the Sinopharm vaccine, which is made-up with whole inactivated virus.
One possibility is that besides the RBD domain other domains of the S protein may be involved in virus entry into the cells. The amino-terminal (N-terminal) domain (NTD), constituted by four stacked β-sheets, connected by flexible loops containing glycan molecules may possibly have a role in virus entry (for a review see Jackson et al. Nature Reviews 2022 [9]). Although today the role of NTD in SARS-CoV-2 entry is unknown, the glycan molecules present in other NTD domains (i.e. bovine coronavirus or MERS-CoV) seem to facilitate virus attachment. Thus, since in the case of the Sinopharm vaccine the whole inactivated virus is used, we can speculate that the antibodies generated may have a slightly higher neutralizing capacity because antibodies are also generated against other domains of the S protein. Indeed, some potent neutralizing antibodies target the NTD of SARS-CoV-2 [6,10]. Therefore, it is likely that proper insertion of the Spike protein in the native virus may result in exposure to certain sites which may not be exposed when the recombinant S protein is generated in the other vaccines.
In summary, the three vaccines generated specific antibodies but differences among them were observed. The most notorious case was shown for the first dose of Sinopharm, where the antibody production was very low respect to the Sputnik V and AstraZeneca vaccines (Fig. 1A–C); highlighting the importance of completing the vaccine scheme.
Despite the limited number of volunteers who satisfied the inclusion criteria, the implementation of heterologous vaccination due to the delay in the arrival of vaccines in the country, in addition to the third wave of Covid-19 that affected a large number of the initial participants, our observations demonstrated that there is a significant correlation between the level of antibodies generated by each vaccine and its neutralizing capacity, showing all of them are capable of neutralizing the entry of the pseudotyped virus into the cell.
Finally, these findings not only will contribute to the implementation of more accurate vaccination schemes goes on the hand that Sinopharm, as an example, produces a lower humoral response, then, a booster dose should be recommended. In addition, we have observed that some individuals did not develop antibodies at all after the first dose with any of the other vaccines tested, then the application of the second dose is highly advised. Also, it is relevant to remark that our conclusions are exclusively related to our country, specifically to Mendoza province.
Production notes
Author contribution statement
Constanza Giai, PhD; Betiana Nebai Salassa, PhD; Valeria Zarelli, PhD: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Oscar Bello, PhD: Analyzed and interpreted the data.
María Cristina Vanrell, PhD: Performed the experiments; Analyzed and interpreted the data.
Diego Ojeda, PhD: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Andrea Gamarnik, PhD: Contributed reagents, materials, analysis tools or data.
María Isabel Colombo, PhD: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was partly supported by a grant MINCYT-MZA 06 COVID FEDERAL EX 2020-39069598 APN-DDYGD#MECCYT.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no competing interests.
Acknowledgments
We are grateful to Dr. Sean Whelan (St. Louis, MO, USA) for providing the VSV-eGFP-SARS-CoV-2 pseudovirus. The aliquots were originally received by Andrea Garmarnik's laboratory and afterwards they were sent to our laboratory in Mendoza. We thank the Dean of the FCM Dr. Roberto Miatello and the Vice Dean Dr. Viviana Parra as well as Dr. Patricia Ramírez for allowing the use of a facility to obtain the blood samples at the vaccination center of the FCM. We also thank Dr. Gonzalo Nalda, Dr. Sebastián Suarez and Joel Gabrielli from the Hospital Universitario.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15211.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Pseudovirus VSVg-SPIKEeGFP infection in VERO CCL81 cells. The infection of pseudovirus VSVΔg expressing the protein SPIKE and the fluorescent eGFP protein in Vero CCL81 cells were determined by fluorescent microscopy (a, b) and flow cytometry (c-e). The percentage of positive cells (c, d) and mean fluorescence intensity (MFI) (e) were determined by flow cytometry. Representative histograms are shown. Open histograms: cells infected with the pseudovirus, solid histograms: mock. Bar: 20μm.
VSVg-SPIKEeGFP pseudovirus infection pre-incubated with negative serum for anti-SARS-CoV2 antibodies in VERO CCL81 cells. The infection of pseudovirus VSVΔg and the fluorescent eGFP protein in Vero CCL81 cells were determined by fluorescent microscopy (a-d) and flow cytometry (e-i). The percentage of positive cells (e-h) and mean fluorescence intensity (MFI) (i) were determined by flow cytometry. Representative histograms are shown. Open histograms: cells infected with the pseudovirus and negative serum dilution, solid histograms: mock. Bar: 20μm.
Comparison of the production of IgG anti-SPIKE among vaccinated and convalescent of COVID-19 individuals. Each dot represents the anti-SPIKE IgG titer for a particular individual 21-25 days after receiving the dose 2 of the Sputnik V, AstraZeneca or Sinopharm vaccines and the seroconverted titer of post-COVID-19 infected individuals (convalescent). The horizontal lines show the median for each group. **: p<0,01 Kruskal–Wallis test with Dunn post test for multiple comparisons.
Comparison of the neutralizing capacity of serum from convalescent of Covid-19 individuals. A. a-d. Fluorescence images from figure 3A depicting the neutralizing entry of the virus are shown to compare the neutralizing capacity of the Covid-19 convalescent serums (m-p). Bar: 20μm B. The neutralization capacity of serum samples from Covid-19 convalescent and naïve individuals were measured by flow cytometry and the eGFP fluorescence was interpreted as a percentage of infection. Each dot represents the median value in each group of vaccinated. C. The graph represents the correlation among anti-SPIKE IgG (UI/ml) production and the percentage of virus infection. * Naïve: non-vaccinated, non-infected with SARS-CoV-2 individuals.
References
- 1.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., Simakova Y.V., Tokarskaya E.A., Lubenets N.L., Egorova D.A., Shmarov M.M., Nikitenko N.A., Morozova L.F., Smolyarchuk E.A., Kryukov E.V., Babira V.F., Borisevich S.V., Naroditsky B.S., Gintsburg A.L. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3/ATTACHMENT/90399439-F921-499F-82CF-3819253FE494/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O'Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N.A., Smith R., Song M.D., Snape E., Sprinz R.K., Sutherland R., Tarrant E.C., Thomson M.E., Török M., Toshner D.P.J., Turner J., Vekemans T.L., Villafana M.E.E., Watson C.J., Williams A.D., Douglas A.V.S., Hill T., Lambe S., Gilbert C., Pollard A.J., Aban M., Abayomi F., Abeyskera K., Aboagye J., Adam M., Adams K., Adamson J., Adelaja Y.A., Adewetan G., Adlou S., Ahmed K., Akhalwaya Y., Akhalwaya S., Alcock A., Ali E.R., Allen L., Allen T.C.D., C S., Almeida M.P.S., Alves F., Amorim F., Andritsou R., Anslow M., Appleby E.H., Arbe-Barnes M.P., Ariaans B., Arns L. Arruda, Azi P., Azi L., Babbage G., Bailey C., Baker K.F., Baker M., Baker N., Baker P., Baldwin L., Baleanu I., Bandeira D., Bara A., Barbosa M.A.S., Barker D., Barlow G.D., Barnes E., Barr A.S., Barrett J.R., Barrett J., Bates L., Batten A., Beadon K., Beales E., Beckley R., Belij-Rammerstorfer S., Bell J., Bellamy D., Bellei N., Belton S., Berg A., Bermejo L., Berrie E., Berry L., Berzenyi D., Beveridge A., Bewley K.R., Bexhell H., Bhikha S., Bhorat A.E., Bhorat Z.E., Bijker E., Birch G., Birch S., Bird A., Bird O., Bisnauthsing K., Bittaye M., Blackstone K., Blackwell L., Bletchly H., Blundell C.L., Blundell S.R., Bodalia P., Boettger B.C., Bolam E., Boland E., Bormans D., Borthwick N., Bowring F., Boyd A., Bradley P., Brenner T., Brown P., Brown C., Brown-O’Sullivan C., Bruce S., Brunt E., Buchan R., Budd W., Bulbulia Y.A., Bull M., Burbage J., Burhan H., Burn A., Buttigieg K.R., Byard N., Cabera Puig I., Calderon G., Calvert A., Camara S., Cao M., Cappuccini F., Cardoso J.R., Carr M., Carroll M.W., Carson-Stevens A., Carvalho Y. de M., Carvalho J.A.M., Casey H.R., Cashen P., Castro T., Castro L.C., Cathie K., Cavey A., Cerbino-Neto J., Chadwick J., Chapman D., Charlton S., Chelysheva I., Chester O., Chita S., Cho J.S., Cifuentes L., Clark E., Clark M., Clarke A., Clutterbuck E.A., Collins S.L.K., Conlon C.P., Connarty S., Coombes N., Cooper C., Cooper R., Cornelissen L., Corrah T., Cosgrove C., Cox T., Crocker W.E.M., Crosbie S., Cullen L., Cullen D., Cunha D.R.M.F., Cunningham C., Cuthbertson F.C., Da Guarda S.N.F., da Silva L.P., Damratoski B.E., Danos Z., Dantas M.T.D.C., Darroch P., Datoo M.S., Datta C., Davids M., Davies S.L., Davies H., Davis E., Davis J., Davis J., De Nobrega M.M.D., De Oliveira Kalid L.M., Dearlove D., Demissie T., Desai A., Di Marco S., Di Maso C., Dinelli M.I.S., Dinesh T., Docksey C., Dold C., Dong T., Donnellan F.R., Dos Santos T., dos Santos T.G., Dos Santos E.P., Douglas N., Downing C., Drake J., Drake-Brockman R., Driver K., Drury R., Dunachie S.J., Durham B.S., Dutra L., Easom N.J.W., van Eck S., Edwards M., Edwards N.J., El Muhanna O.M., Elias S.C., Elmore M., English M., Esmail A., Essack Y.M., Farmer E., Farooq M., Farrar M., Farrugia L., Faulkner B., Fedosyuk S., Felle S., Ferreira Da Silva C., Field S., Fisher R., Flaxman A., Fletcher J., Fofie H., Fok H., Ford K.J., Fowler J., H A P., Fraiman E., Francis M.M., Franco J., Frater M.S.M., Freire S.H., Fry S., Fudge J., Furze M., Fuskova P., Galian-Rubio E., Galiza H., Garlant M., Gavrila A., Geddes K.A., Gibbons C., Gilbride H., Gill S., Glynn, Godwin K., Gokani K., Goldoni U.C., Goncalves M., Gonzalez I.G.S., Goodwin J., Goondiwala A., Gordon-Quayle K., Gorini G., Grab J., Gracie L., Greenland M., Greenwood N., Greffrath J., Groenewald M.M., Grossi L., Gupta G., Hackett M., Hallis B., Hamaluba M., Hamilton E., Hamlyn J., Hammersley D., Hanrath A.T., Hanumunthadu B., Harris S.A., Harris C., Harris T., Harrison T.D., Harrison D., Hart T.C., Hartnell B., Hassan S., Haughney J., Hawkins S., Hay J., Head I., Henry J., Hermosin Herrera M., Hettle D.B., Hill J., Hodges G., Horne E., Hou M.M., Houlihan C., Howe E., Howell N., Humphreys J., Humphries H.E., Hurley K., Huson C., Hyder-Wright A., Hyams C., Ikram S., Ishwarbhai A., Ivan M., Iveson P., Iyer V., Jackson F., De Jager J., Jaumdally S., Jeffers H., Jesudason N., Jones B., Jones K., Jones E., Jones C., Jorge M.R., Jose A A., Joshi E.A.M.S., Júnior J., Kadziola R., Kailath, Kana F., Karampatsas K., Kasanyinga M., Keen J., Kelly E.J., Kelly D.M., Kelly D., Kelly S., Kerr R D., de Á Kfouri L Khan B., Khozoee S., Kidd A., Killen J., Kinch P., Kinch L.D., King W., King T.B., Kingham L., Klenerman P., Knapper F., Knight J.C., Knott D., Koleva S., Lang M., Lang G., Larkworthy C.W., Larwood J.P.J., Law R., Lazarus E.M., Leach A., Lees E.A., Lemm N.M., Lessa A., Leung S., Li Y., Lias A.M., Liatsikos K., Linder A., Lipworth S., Liu S., Liu X., Lloyd A., Lloyd S., Loew L., Ramon R. Lopez, Lora L., Lowthorpe V., Luz J.K., MacDonald C., MacGregor G., Madhavan M.D.O., Mainwaring E., Makambwa R., Makinson M., Malahleha R., Malamatsho G., Mallett K., Mansatta T., Maoko K., Mapetla N.G., Marchevsky S., Marinou E., Marlow G., Marques N., Marriott R.P., Marshall J., Marshall L., Martins F.J., Masenya M., Masilela M., Masters S.K., Mathew M., Matlebjane H., Matshidiso K., Mazur O., Mazzella A., McCaughan H., McEwan J., McGlashan J., McInroy L., McIntyre Z., McLenaghan D., McRobert N., McSwiggan S., Megson C., Mehdipour S., Meijs W., Mendonça R.N.Á., Mentzer A.J., Mirtorabi N., Mitton C., Mnyakeni S., Moghaddas F., Molapo K., Moloi M., Moore M., Moraes-Pinto M.I., Moran M., Morey E., Morgans R., Morris S., Morris S., Morris H.C., Morselli F., Morshead G., Morter R., Mottal L., Moultrie A., Moya N., Mpelembue M., Msomi S., Mugodi Y., Mukhopadhyay E., Muller J., Munro A., Munro C., Murphy S., Mweu P., Myasaki C.H., Naik G., Naker K., Nastouli E., Nazir A., Ndlovu B., Neffa F., Njenga C., Noal H., Noé A., Novaes G., Nugent F.L., Nunes G., O'Brien K., O'Connor D., Odam M., Oelofse S., Oguti B., Olchawski V., Oldfield N.J., Oliveira M.G., Oliveira C., Oosthuizen A., O'Reilly P., Osborne P., Owen D.R.J., Owen L., Owens D., Owino N., Pacurar M., Paiva B.V.B., Palhares E.M.F., Palmer S., Parkinson S., Parracho H.M.R.T., Parsons K., Patel D., Patel B., Patel F., Patel K., Patrick-Smith M., Payne R.O., Peng Y., Penn E.J., Pennington A., Peralta Alvarez M.P., Perring J., Perry N., Perumal R., Petkar S., Philip T., Phillips D.J., Phillips J., Phohu M.K., Pickup L., Pieterse S., Piper J., Pipini D., Plank M., Du Plessis J., Pollard S., Pooley J., Pooran A., Poulton I., Powers C., Presa F.B., Price D.A., Price V., Primeira M., Proud P.C., Provstgaard-Morys S., Pueschel S., Pulido D., Quaid S., Rabara R., Radford A., Radia K., Rajapaska D., Rajeswaran T., Ramos A.S.F., Ramos Lopez F., Rampling T., Rand J., Ratcliffe H., Rawlinson T., Rea D., Rees B., Reiné J., Resuello-Dauti M., Reyes Pabon E., Ribiero C.M., Ricamara M., Richter A., Ritchie N., Ritchie A.J., Robbins A.J., Roberts H., Robinson R.E., Robinson H., Rocchetti T.T., Rocha B.P., Roche S., Rollier C., Rose L., Ross Russell A.L., Rossouw L., Royal S., Rudiansyah I., Ruiz S., Saich S., Sala C., Sale J., Salman A.M., Salvador N., Salvador S., Sampaio M., Samson A.D., Sanchez-Gonzalez A., Sanders H., Sanders K., Santos E., Santos Guerra M.F.S., Satti I., Saunders J.E., Saunders C., Sayed A., Schim van der Loeff I., Schmid A.B., Schofield E., Screaton G., Seddiqi S., Segireddy R.R., Senger R., Serrano S., Shah R., Shaik I., Sharpe H.E., Sharrocks K., Shaw R., Shea A., Shepherd A., Shepherd J.G., Shiham F., Sidhom E., Silk S.E., da Silva Moraes A.C., Silva-Junior G., Silva-Reyes L., Silveira A.D., Silveira M.B.V., Sinha J., Skelly D.T., Smith D.C., Smith N., Smith H.E., Smith D.J., Smith C.C., Soares A., Soares T., Solórzano C., Sorio G.L., Sorley K., Sosa-Rodriguez T., Souza C.M.C.D.L., Souza B.S.D.F., Souza A.R., Spencer A.J., Spina F., Spoors L., Stafford L., Stamford I., Starinskij I., Stein R., Steven J., Stockdale L., Stockwell L.V., Strickland L.H., Stuart A.C., Sturdy A., Sutton N., Szigeti A., Tahiri-Alaoui A., Tanner R., Taoushanis C., Tarr A.W., Taylor K., Taylor U., Taylor I.J., Taylor J., te Water Naude R., Themistocleous Y., Themistocleous A., Thomas M., Thomas K., Thomas T.M., Thombrayil A., Thompson F., Thompson A., Thompson K., Thompson A., Thomson J., Thornton-Jones V., Tighe P.J., Tinoco L.A., Tiongson G., Tladinyane B., Tomasicchio M., Tomic A., Tonks S., Towner J., Tran N., Tree J., Trillana G., Trinham C., Trivett R., Truby A., Tsheko B.L., Turabi A., Turner R.C., Turner M., Ulaszewska B.R., Underwood, Varughese R., Verbart D., Verheul M., Vichos I., Vieira T., Waddington C.S., Walker L., Wallis E., Wand M., Warbick D., Wardell T., Warimwe G., Warren S.C., Watkins B., Watson E., Webb S., Webb-Bridges A., Webster A., Welch J., Wells J., West A., White C., White R., Williams P., Williams R.L., Winslow R., Woodyer M., Worth A.T., Wright D., Wroblewska M., Yao A., Zimmer R., Zizi D., Zuidewind P. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. ATTACHMENT/BD910DFE-2C8A-4512-A277-6B1DBA6322EE/MMC2.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Y., Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4/ATTACHMENT/73FC01E3-9C65-42C8-A9D7-8B45A431E97F/MMC2.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojeda D., Gonzalez Lopez Ledesma M., Pallarés H., Costa Navarro G., Sanchez L., Perazzi B., Villordo S., Alvarez D., Echavarria M., Oguntuyo K., Stevens C., Lee B., Carradori J., Caramelo J., Yanovsky M., Gamarnik A. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17 doi: 10.1371/JOURNAL.PPAT.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristiansen P., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., Plotkin S., Knezevic I. 2021. WHO International Standard For Anti-SARS-Cov-2 Immunoglobulin, Lancet (London, England) pp. 1347–1348. 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Case J., Rothlauf P., Chen R., Liu Z., Zhao H., Kim A., Bloyet L., Zeng Q., Tahan S., Droit L., Ilagan M., Tartell M., Amarasinghe G., Henderson J., Miersch S., Ustav M., Sidhu S., Virgin H., Wang D., Ding S., Corti D., Theel E., Fremont D., Diamond M., Whelan S. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.05.18.102038. the Preprint Server for Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi A., Ojeda D., Varese A., Sanchez L., Gonzalez lopez ledesma M., Mazzitelli I., Alvarez juliá A., Oviedo Rouco S., Pallarés H., Costa Navarro G., Rasetto N., Garcia C., Wenker S., Ramis L., Bialer M., de Leone M., Hernando C., Sosa S., Bianchimano L., Rios A., Treffinger Cienfuegos M., Caramelo J., Longueira Y., Laufer N., DE D., Alvarez, Carradori, Pedrozza D., Rima A., Echegoyen C., Ercole R., Gelpi P., Marchetti S., Zubieta M., Docena G., Kreplak N., Yanovsky M., Geffner J., Pifano M., Gamarnik A. Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose, cell reports. Medicine. 2021;2 doi: 10.1016/J.XCRM.2021.100359[7].[7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dashdorj N.J., Wirz O.F., Röltgen K., Haraguchi E., Buzzanco A.S., Sibai M., Wang H., Miller J.A., Solis D., Sahoo M.K., Arunachalam P.S., Lee A.S., Shah M.M., Liu J., Byambabaatar S., Bat-Ulzii P., Enkhbat A., Batbold E., Zulkhuu D., Ochirsum B., Khurelsukh T., Dalantai G., Burged N., Baatarsuren U., Ariungerel N., Oidovsambuu O., Bungert A.S., Genden Z., Yagaanbuyant D., Mordorj A., Pulendran B., Chinthrajah S., Nadeau K.C., Jardetzky T., Wilbur J.L., Wohlstadter J.N., Sigal G.B., Pinsky B.A., Boyd S.D., Dashdorj N.D. Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia. Cell Host Microbe. 2021;29:1738–1743. doi: 10.1016/J.CHOM.2021.11.004. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells, nature reviews. MCB (Mol. Cell. Biol.) 2022;23:3–20. doi: 10.1038/S41580-021-00418-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., Chen Z., Guo Y., Zhang J., Li Y., Song X., Chen Y., Xia L., Fu L., Hou L., Xu J., Yu C., Li J., Zhou Q., Chen W. vol. 369. 2020. pp. 650–655. (A Neutralizing Human Antibody Binds to the N-Terminal Domain of the Spike Protein of SARS-CoV-2, Science (New York, N.Y.). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pseudovirus VSVg-SPIKEeGFP infection in VERO CCL81 cells. The infection of pseudovirus VSVΔg expressing the protein SPIKE and the fluorescent eGFP protein in Vero CCL81 cells were determined by fluorescent microscopy (a, b) and flow cytometry (c-e). The percentage of positive cells (c, d) and mean fluorescence intensity (MFI) (e) were determined by flow cytometry. Representative histograms are shown. Open histograms: cells infected with the pseudovirus, solid histograms: mock. Bar: 20μm.
VSVg-SPIKEeGFP pseudovirus infection pre-incubated with negative serum for anti-SARS-CoV2 antibodies in VERO CCL81 cells. The infection of pseudovirus VSVΔg and the fluorescent eGFP protein in Vero CCL81 cells were determined by fluorescent microscopy (a-d) and flow cytometry (e-i). The percentage of positive cells (e-h) and mean fluorescence intensity (MFI) (i) were determined by flow cytometry. Representative histograms are shown. Open histograms: cells infected with the pseudovirus and negative serum dilution, solid histograms: mock. Bar: 20μm.
Comparison of the production of IgG anti-SPIKE among vaccinated and convalescent of COVID-19 individuals. Each dot represents the anti-SPIKE IgG titer for a particular individual 21-25 days after receiving the dose 2 of the Sputnik V, AstraZeneca or Sinopharm vaccines and the seroconverted titer of post-COVID-19 infected individuals (convalescent). The horizontal lines show the median for each group. **: p<0,01 Kruskal–Wallis test with Dunn post test for multiple comparisons.
Comparison of the neutralizing capacity of serum from convalescent of Covid-19 individuals. A. a-d. Fluorescence images from figure 3A depicting the neutralizing entry of the virus are shown to compare the neutralizing capacity of the Covid-19 convalescent serums (m-p). Bar: 20μm B. The neutralization capacity of serum samples from Covid-19 convalescent and naïve individuals were measured by flow cytometry and the eGFP fluorescence was interpreted as a percentage of infection. Each dot represents the median value in each group of vaccinated. C. The graph represents the correlation among anti-SPIKE IgG (UI/ml) production and the percentage of virus infection. * Naïve: non-vaccinated, non-infected with SARS-CoV-2 individuals.
Data Availability Statement
Data will be made available on request.