Summary
Background
With the growing notion of patient-focused drug development, the quality of life and other patient-reported outcomes (PROs) of cancer patients are gaining considerable attention. Several drug regulatory agencies, including the U.S. Food and Drug Administration (FDA), are calling attention to PROs. This review aims to comprehensively characterise the application of PROs and regulatory considerations for PROs in the FDA-approved novel oncology drugs.
Methods
The FDA review documents and labels for novel oncology drugs approved from July 2017 to July 2022 were retrieved. We collected and analysed drug approval information, types of endpoints for PROs, PRO measures, designs of trials including PROs, and regulatory comments on PRO-related contents.
Findings
Results demonstrated that PROs were used more commonly for solid tumours than hematologic malignancies, which might be correlated with the disease characteristics. We further categorised and analysed existing PRO measures, providing insight for tool selection in future oncology trial design. Our findings also indicated that PROs currently do not play a significant role in oncology drug approvals. The major deficiencies related to PROs commented on by FDA reviewers were analysed, followed by recommendations for improvements.
Interpretation
This review demonstrates that PROs currently do not play a significant role in oncology drug marketing review, and how they can be used to support the approval of new oncology drugs is still in the exploratory stage. This current situation is not only related to the deficiencies in the design and implementation of PRO-related contents in oncology trials, but more importantly, it is a reminder that we should pay more attention to patient experience in the development of oncology drugs.
Funding
This study was not supported by any funding.
Keywords: Patient-reported outcome, Novel oncology drug
Research in context.
Evidence before this study
Patient-reported outcomes (PROs) in oncology have been continuously explored, but little previous research has focused on regulatory considerations regarding PROs. In fact, the role of PROs in supporting regulatory decisions in novel oncology drug is limited. There is a lack of studies that comprehensively evaluating the role of PROs in the marketing review of new oncology drugs and regulatory consideration of PRO-related content.
Added value of this study
This study presents a comprehensive analysis of incorporating PRO into the holistic clinical evidence during drug evaluation for newly approved oncology drugs in the US in the recent five years. On this basis, this study delineated future prospects for applications of PROs in oncology trials and raised feasible recommendations for the use of PROs in future oncology clinical trials from the perspective of regulatory considerations.
Implications of all the available evidence
All available evidence indicates that the role of PROs is currently limited in the FDA marketing reviewing of novel oncology drugs. However, the importance of the personal feelings and perspectives of oncology patients is well recognised. In the future development of novel oncology drugs, more attention could be paid to PRO-related studies.
Introduction
The value of patient-reported outcomes (PROs) has been recognised by both clinical experts and regulatory authorities. PRO measures (PROMs) can be recruited as a supportive means for the evaluation of disease-related symptoms, psychological and social functions, therapy-related adverse events and satisfaction with treatment.1 By reporting PROs, cancer patients can participate more actively in the treatment and provide important references for physicians' decision -making. In the clinical practice of oncological departments, it is important to prolong survival and to improve the quality of life (QoL) for the patients under cancer treatment. QoL and other patient experiences can be well reflected by PROs. Health-related quality of life (HRQoL) measures and other patient-reported outcomes in cancer trials could generate valuable data to assist in assessing the risks and benefits of treatment and to promote patient-focused cancer care.
While QoL is increasingly valued in the treatment of oncology patients, current oncology drug approvals still predominantly rely on survival-related endpoints. In recent years, a large number of new oncology drugs have been launched, often based on their use of overall survival (OS) as the gold standard for clinical benefit in oncology patients. However, from many recent reviews of the post-marketing clinical value of oncology drugs in the literature, many approved oncology drugs did not yield high clinically meaningful benefits in terms of QoL or OS in post-marketing clinical practice compared to placebo or pre-existing regimens.2, 3, 4, 5 Several studies have shown that many oncology drugs do not provide satisfactory improvements in QoL or that there are many deficiencies in the assessment of QoL in oncology clinical trials.6,7
From a regulatory perspective, the concept of patient-focused drug development has been widely accepted, with a main gateway of collecting patient experience data (PED). PED refers to all information on patients' experiences, perspectives and preferences regarding the disease and related treatments, with PRO as one of the key presentations. The demand for PROs to assist the development of novel oncology drugs has become increasingly evident as multinational drug regulatory agencies have become more enthusiastic about exploring patient-focused drug development.8,9 Sponsors and regulators showed increased interest in patients’ perception of disease conditions and treatments. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and many regulatory agencies are exploring how to incorporate patient experiences and perspectives into drug development. US Food and Drug Administration (FDA) acknowledged the use of PROs as clinical trial endpoints in cancer drugs and biologics development.10 FDA also published a series of guidance to support patient-focused drug development.11, 12, 13 However, challenges do exist in the collection, analysis and interpretation of PROs. Previous studies have shown that PROs were rarely included in the labelling of oncology products approved by the FDA,14,15 and how regulators view the results of PROs is yet equivocal. There is no sufficient clarity on how PRO is used to inform benefit-risk assessment and regulatory decision-making in novel oncology product applications, and current PROMs in the oncology field still stand against critical challenges to play a significant role in marketing reviews.
Much has been written about the design and implantation of PROs in clinical trials, and international standards for the inclusion and analysis of PROs are getting in place.16, 17, 18 Nevertheless, studies that systematically analyse regulatory considerations are still lacking. This study therefore set out to characterise the utilisation of PROs in clinical trials of a wide range of novel oncology drugs recently approved by the FDA and make an in-depth analysis of regulatory considerations for PRO data. This article will further provide feasible recommendations for applying PROs in clinical trials to support marketing review, thereby promoting a clinical value-oriented and patient-focused oncology drug development philosophy.
Methods
Data screening
This cross-sectional analysis screened all new molecular entities (NMEs) of new drug applications (NDAs) and biologic license applications (BLAs) approved by the FDA between July 01, 2017 and July 01, 2022. The reason for choosing July 01, 2017 as the inclusion start time is that FDA required a ‘Patient Experience Data Table’ in review documents for drug and biologic marketing applications received after June 12, 2017.19 Only novel therapeutic products in oncology were identified for analysis, excluding diagnostic products, vaccines, blood and blood products, and cellular and gene therapy products. FDA clinical review documents and labels were collected from Drugs@FDA, the FDA-approved drugs database. The screening process was conducted by two researchers independently and verified by a third author.
Data collection
For each product and document identified, we collected basic drug approval information (the first approved indication, expedited approval pathways, orphan drug status), PED included in review documents, PROMs, design of trials using PRO, analytical methods on PROMs, FDA reviewer comments regarding PRO and the inclusion of PRO information in the original labelling. Whether the review report contains PED information mainly referred to the “Patient Experience Data Table” in the FDA review documents. For the documents without the “Patient Experience Data Table”, two researchers independently verified the full text of these review documents for the inclusion of PED. All information was extracted independently by two different researchers according to a pre-determined proforma and verified by a third researcher.
Statistical analysis
The statistical analysis methods in this paper include descriptive statistics and the chi-square test. Data analyses and graphing were done with RStudio (version 2022.12.0) and Adobe Illustrator (version 27.0.1).
Role of funding
This study was not supported by any funding. All authors had access to the raw data and approved the manuscript for publication.
Results
Characteristics of included novel oncology drug approvals and pivotal trials
From July 2017 to July 2022, the FDA approved a total of 67 novel oncology therapeutic products. Among the 67 approvals, the most frequent indications were lung cancers (n = 10, 14.9%), breast cancers (n = 8, 11.9%), leukemia (n = 10, 14.9%) and lymphoma (n = 9, 13.4%). Information on all approved drugs included in this article is provided in Supplementary Table S1. For the 67 medical review documents of oncology NMEs and BLAs, we summarised the PED categories in 45 review documents that contained PED information (Supplementary Table S2). The most frequent type of PED was PRO, while a small number of clinician-reported outcomes (ClinROs), performance outcomes (PerfOs) and other patient-focused drug development or other stakeholder meeting summary reports also were identified. Therefore, we focused on the analysis and discussion of PROs in this article. As shown in Table 1, of these 67 approvals, 45 (67.2%) included PROs in FDA clinical review documents, including 33 NMEs and 12 BLAs. The inclusion of PROs in the review documents in different categories of applications was analysed. There was a slightly higher proportion of non-orphan drugs (75.0%) compared to orphan drugs (59.6%) and a slightly higher proportion of accelerated approvals (73.5%) compared to regular approvals (60.6%) that contain PRO information. The application of PROs in the medical reviews of products for different indications also varied. Approvals of solid tumour products were more likely to incorporate PROs than that of hematologic malignancies (82.5% vs 44.4%, p < 0.001).
Table 1.
Characteristics of oncology approvals reviewed in this article.
| Oncology NDA-NMEs and BLAs without PRO information in the medical review documents (N = 22) | Oncology NDA-NMEs and BLAs with PRO information in the medical review documents (N = 45) | p | |
|---|---|---|---|
| Application type | |||
| NDA-NME (n = 45) | 12 (26.7%) | 33 (73.3%) | 0.124 |
| BLA (n = 22) | 10 (45.5%) | 12 (54.5%) | |
| Orphan status | |||
| Orphan (n = 47) | 19 (40.4%) | 28 (59.6%) | 0.043 |
| Non-Orphan (n = 20) | 3 (15.0%) | 17 (75.0%) | |
| Recommendation on regulatory action | |||
| AA (n = 34) | 9 (26.5%) | 25 (73.5%) | 0.260 |
| RA (n = 33) | 13 (39.4%) | 20 (60.6%) | |
| Indication | |||
| Solid tumours (n = 40) | 7 (17.5%) | 33 (82.5%) | 0.001 |
| Haematological malignancies (n = 27) | 15 (55.6%) | 12 (44.4%) |
Abbreviations: BLA, biologic license application; NDA, new drug application; NME, new molecular entity; PRO, patient-reported outcome; RA, regular approval; AA, accelerated approval.
Of these 45 medical review documents, a total of 45 clinical trials containing PRO information were extracted and analysed. Nearly half of these trials were single-arm trials, and most were open-label trials (77.8%). Among the trials, PROs were typically used as secondary or exploratory endpoints (Table 2), with only one trial in which PROs were used as a coprimary endpoint but resulted in insufficient evidence (Supplementary Table S3). In several trials, PROs had also been utilised as a safety or tolerability assessment and recognised by FDA reviewers. The design details of these included trials were collected in Supplementary Table S3.
Table 2.
Characteristics of the clinical trials that included PRO.
| Number of trials | |
|---|---|
| Randomisation | |
| Randomised | 26 (57.8%) |
| Single-arm | 19 (42.2%) |
| Blinding status | |
| Blinded | 10 (22.2%) |
| Open-label | 35 (77.8%) |
| PRO related endpoint type | |
| Primary | 1 (2.2%) |
| Key secondary | 2 (4.4%) |
| Secondary or exploratory | 42 (93.3%) |
Summary and classification of PROMs used in oncology trials
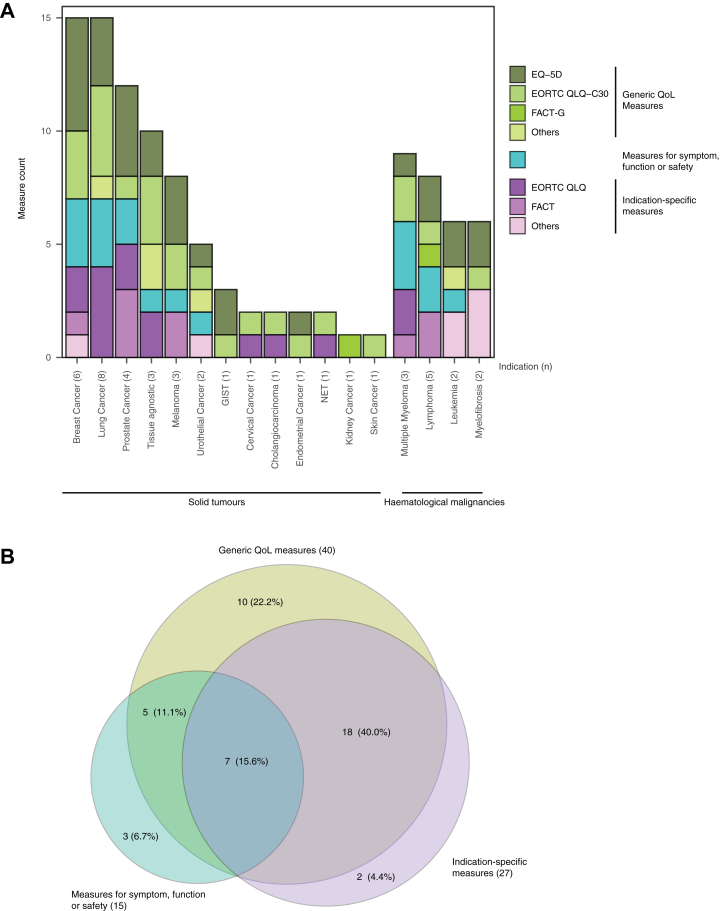
To further investigate the application of PROs in the oncology trials of NDA, all PROMs applied in the 45 pivotal trials included in this study were sorted out and classified. As shown in Fig. 1A, 36 different PROMs were identified and distributed into three categories, including (1) generic QoL measures, (2) other generic measures for symptoms, function or safety, (3) indication-specific measures. The frequency of application of different types of PROs in each category was plotted according to the indication. Of the 36 PROMs, 5 were generic QoL measures. The EuroQol-5 Dimensions (EQ-5D) and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-core questionnaire (EORTC QLQ-C30) were the most broadly used measures, which were applied in 28 (62.2%) and 24 (53.3%) trials respectively. In addition to the generic QoL measures described above, there were 11 measures designed to assess specific symptoms, function or safety. And these measures were also generic and applicable to a wide range of indications. Besides the generic PROMs, 20 indication-specific PROMs were used across the included trials (Table 3). We further analysed the strategy of selecting PROs in the oncology trial design. Across the 45 trials that conducted PRO assessments, 40 (88.9%) included generic QoL measures, 15 (33.3%) included generic measures for symptom, function or safety, and 27 (60.0%) included indication-specific measures (Fig. 1B). The results showed that the majority of oncology trials made the PRO assessment using the combination of different PROMs. The most common strategies are the combination of generic QoL measures and indication-specific PROMs (55.6%), or the combination of generic QoL measures and measures for key symptoms, functions or safety (26.7%) PROMs.
Fig. 1.
Use of different types of PROM in oncology trials with different indications. PROMs are classified into three major categories, generic quality of life (QoL) measures (olive), measures for symptom, function or safety (cyan) and indication-specific measures (purple). (A) The measure counts (y axis) are plotted versus the number of pivotal trials corresponding to indications mentioned on the x axis. Generic QoL measures are further divided into EQ-5D, EORTC QLQ-C30, FACT-G and other generic QoL measures; Indication-specific measures are divided into EORTC QLQ modular, FACT modular and other indication-specific measures. (B) The numbers in the Venn diagram indicate the number of included pivotal trials, using the Venn diagram to represent the use of multiple different types of PROMs in a single trial. Abbreviations: EQ-5D, EuroQol Five Dimensions Questionnaire; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; FACT, the Functional Assessment of Cancer Therapy; EORTC QLQ-C30, EORTC QLQ-core questionnaire; FACT-G, FACT- General.
Table 3.
PROMs of different categories reviewed in this article.
| Approvals (n) | Generic QoL measures (n) | Other generic measures for symptom, function or safety (n) | Indication specific measures (n) |
|---|---|---|---|
| Solid tumours (33) | EORTC QLQ-C30 (20)/EQ-5D (20)/FACT-G (2)/PedsQL (2)/PGIC (1) | Bowel diaries (1)/BPI-SF (4)/FACIT-F (1)/HRU (1)/PGIC (Symptoms) (1)/WPAI-GH (1) | EORTC QLQ-BIL21 (1)/EORTC QLQ-BR23 (2)/EORTC QLQ-CX24 (1)/EORTC QLQ-G.I.NET21 (1)/EORTC QLQ-LC13 (5)/EORTC QLQ-PR25 (2)/FACT-B (1)/FACT-M (2)/FACT-P (3)/NFBSI-16 (1)/SMQ (1) |
| Haematological malignancies (12) | EORTC QLQ-C30 (4)/EQ-5D (7)/FACT-G (1)/PGIC (1) | NEI-VFQ-25 (1)/FACIT-F (1)/OSDI (1)/TINAS (1)/PRO-CTCAE (2)/Skindex-29 (1) | EORTC QLQ-MY20 (2)/FACT-Lym (2)/FACT-MM (1)/MFSAF (1)/MDASI-CML (1)/MPN-SAF (1)/WPAI-CML (1) |
Abbreviations: BPI-SF, (modified) Brief Pain Inventory-Short Form; EQ-5D, EuroQol Five Dimensions Questionnaire; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-BIL21, EORTC QLQ- Cholangiocarcinoma and Gallbladder Cancer; EORTC QLQ-BR23, EORTC QLQ-Breast Cancer; EORTC QLQ-CX24, EORTC QLQ-Cervical Cancer; EORTC QLQ-C30, EORTC QLQ-core questionnaire; EORTC QLQ-G.I.NET21, EORTC QLQ-Gastrointestinal neuroendocrine tumours; EORTC QLQ-LC13, EORTC QLQ-Lung Cancer; EORTC QLQ-MY20, EORTC QLQ-Multiple Myeloma; EORTC QLQ-PR25, EORTC QLQ-Prostate Cancer; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; FACT, the Functional Assessment of Cancer Therapy; FACT-B, FACT-Breast; FACT-G, FACT-General; FACT-Lym, FACT-Lymphoma; FACT-M, FACT-Melanoma; FACT-MM, FACT-Multiple Myeloma; FACT-P, FACT-Prostate; HRU, Health Resource Utilization; MDASI-CML, MD Anderson Symptom Inventory-Chronic Myeloid Leukemia; MFSAF, Myelofibrosis Symptom Assessment Form; MPN-SAF, Myeloproliferative Neoplasm Symptom Assessment Form; NEI-VFQ-25, National Eye Institute Visual Function Questionnaire; NFBSI-16, National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy-Breast Cancer Symptom Index; OSDI, Ocular Surface Disease Index; PedsQL, Paediatric Quality of Life Inventory; PGIC, Patients' Global Impression of Change; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; SMQ, Symptom Measurement Questionnaire; TINAS, Therapy-Induced Neuropathy Assessment Scale; WPAI, Work Productivity and Activity Impairment questionnaire; WPAI-CML, WPAI-Chronic Myeloid Leukemia; WPAI-GH, WPAI-General Health.
Critical comments on PROs clearly identified in the review documents
Although PRO information was included in the clinical review documents of the 45 products, the vast majority of these PRO results (43/45) were not included in the original label. PRO information was included in the original label for only 2 out of the total 45 products. To explore why PROs were rarely endorsed by FDA reviewers and failed to meet the criteria of labelling claims, we comprehensively reviewed all the review documents containing PRO information and collected the PRO-related deficiencies explicitly pointed out by reviewers in their comments. The most common deficiencies were presented in Fig. 2 and categorised into issues regarding trial design, PROM selection, statistical analysis and PRO data collection. The trial design issue was likely to be addressed when PROs were generated in a single-arm or open-label study. The PROM selection issue was addressed when the elements in the PROMs did not align with the potential treatment-related outcomes, and therefore, referred to as questionable PROMs content validity. Specific deficiencies related to statistical analysis and PRO data collection were presented in a descriptive manner in the figure, including type I error control, not adequately power, lack of minimal clinically important difference (MCID) or baseline assessment, low PROM completion rate and missing data due to patients discontinued study. The detailed excerpts of the critical language of PRO-related deficiencies commented by FDA reviewers were summarised in Supplementary Table S4.
Fig. 2.
Key comments and regulatory decision on PRO results. The key comments extracted from the clinical review documents were categorised and shown on the top of the figure. Study designs of the pivotal trials supporting each drug approval were colour-coded and indicated on the left of the figure. FDA's decisions on PRO results were indicated by a symbol after the established name of listed products. A circle indicated that the PRO result was not considered part of the efficacy benefit evidence but were considered as important data for the review of safety and tolerability, and a star indicated that it supported efficacy benefit and was included in the original labelling. The absence of a circle or a star indicated that the PRO results were not endorsed by the FDA. Issues that were not addressed by the FDA reviewers could also exist in the related studies. Abbreviations: FDA, US Food and Drug Administration; MCID, minimal clinically important difference; PROM, patient-reported outcome measures.
Discussion
In this study, we analysed the use of PROs in the NDAs and BLAs in a wide range of oncology drugs and summarised the common PRO-related deficiencies proposed by the FDA reviewers. Across the approvals of oncology products reviewed here, PROs were found to have a minor role in supporting the benefit-risk assessment. Historically, cases in which PROs have played a significant supportive role in oncology drug approval have been sporadic, such cases include the approval of Porfimer, Gemcitabine, Mitoxantrone, Imatinib, Palifermin, etc.20 From the results of this study, the application of PROs was higher in solid tumors (n = 33, 82.5%) than in haematological malignancies (n = 12, 44.4%). Among them, breast cancer products applied the highest number of PROs, followed by lung cancer and prostate cancer (Supplement Table S1). This could be related to the treatment development and disease characteristics of different tumor types. For instance, with the establishment of molecular subtypes of breast cancer and the development of new drugs, the 5-year survival rate has reached 90%.21 Patients with breast cancer often experience chronic pain and fatigue, given the palliative nature of many advanced breast cancer treatments, health-related QoL (HRQoL) is an important factor in assessing the risk–benefit profile of treatments. Similarly, the number of men surviving after being diagnosed with prostate cancer has increased rapidly in high-income countries. The quality of survival experienced, with the definition of the specific effects of the disease and its treatment, must be robustly measured to facilitate appropriate care provision.22 However, in haematological malignancies, clinical trials included in this study, the use of PROs is less than that in solid tumors. This may be because response rates have been widely and reliably used for approval of haematological malignancy drugs, where the complete response was associated with decreased transfusion requirements, decrease in infections, and increased survival.23 This article further provided an in-depth analysis of the FDA reviewers' evaluation of PROs and summarises the drawbacks of the current use of PROs in oncology trials. The analysis was performed based on a large number of review documents to derive trends. Further in-depth studies on PROs in oncology drug approval are hinged due on several factors. The deficiencies of PRO studies are not raised in a systemic manner and issues that are not highlighted by FDA reviewers may also exist in the relevant studies. Meanwhile, despite that PRO-related content is currently presented in a specific section of the review documents, in most cases, the details about the PROs in the oncology trials are not disclosed owing to their exploratory nature. In addition, there is a risk that suboptimal PRO results will not be published publicly, leading to the details in the design and implementation of PRO studies in the related oncology trials could be submerged. In order to advance patient-focused drug development, it is necessary to disclose more detailed PRO-related content. On the other hand, the comments from the FDA reviewers have provided some insights on the refinement of PRO evaluation for oncology drugs. Although there are some guidelines issued by different regulatory agencies regarding patient-focused drug development, the inclusion of PROs in clinical trials is not a mandatory requirement. Therefore, PROs in oncology trials cannot be overly demanding and currently exist in many oncology drug trials as exploratory studies. However, if it is hoped that PRO-related content will play a greater role in supporting regulatory review, a sound trial design is needed. In the following section, we proposed potential improvements for each specific deficiency to mitigate the risk of inappropriate use of PROs in future oncology clinical trials.
Regarding the study design issues, FDA reviewers frequently mentioned that PRO results in single-arm and open-label trials were difficult to interpret and could not be claimed to support efficacy assessment on this basis. Previous FDA guidance did suggest that PRO results in unblinding trials are rarely adequate to be included in labelling, and unintentional unblinding should be avoided.1 PROs are subjectively assessed metrics that are susceptible to placebo effects or reporter bias from non-blinded settings. However, open-label and single-arm trials are very common in the development of novel oncology drugs, and a large proportion of the accelerated approvals are based on single-arm trials.24 Therefore, it would be meaningful to address the limitations of unblinded designs and establish the acceptance criteria for incorporating PRO data into the benefit-risk assessment of single-arm or open-label trials. Previously, FDA members have brought up their vision regarding the bias that might be introduced into PROs of open-label trials and identified several debatable points. They suggested that it is possible to investigate some of the key concerns and a more targeted assessment needs to be devoted.25 However, a recent study comparing PRO results in blinded or open-label trials for same cancer has not found the bias of PRO results arising from open-label settings.26 Another study has also provided evidence supporting the validity of PRO results from open-label randomised controlled trials by assessing 538 randomised cancer trials with PRO endpoints.27 In respect of single-arm trials, the PRO evaluation could potentially be refined by utilizing external controls, which could be generated by standardised collection of PRO data from oncology patients. Nevertheless, the actual implementation of this strategy can be extremely challenging, since the concepts of interests of PRO evaluation are likely to vary among different cancer stages, target populations and available treatment, even for the same indication. By contrast, the conduction of PRO evaluation in the post-marketing studies seemed more feasible. However, the driving force of such studies is yet to be defined.
In terms of the selection and utilisation of PROMs, we innovatively classified PROMs into three categories, including generic QoL measures, other generic measures for symptom, function or safety and indication-specific measures. From the results of this study, generic QoL measures are the most widely used in oncological PRO investigations. PROs generated from one generic measure for symptoms and one indication-specific measure for symptoms became the only two PROs included in the prescription label in our dataset. PROMs assessing disease-specific symptoms are more favoured to support labelling claims, in accordance with previous studies.15,20 For PRO studies, the concepts of specific symptoms or domains are likely to be lucid and easily justified. However, despite the complexity, the QoL remains the primary focus of PRO evaluation. Many QoL scales have been widely used in clinical settings with good reliability and validity, but their appropriateness for new drug reviews is still debatable. The most common generic QoL measures included in oncology trials, such as EORTC-QLQ-C30, EQ-5D and FACT-G, have multiple domains covering the evaluation of physical, functional, social, sexual and emotional well-being.28, 29, 30 Nevertheless, such multidomain assessments are not tailored for the evaluation of the therapeutic effect of new drugs.31 The content validity of these measures, if noted, is almost always questioned. The multi-domain nature of generic QoL measures means that items affected by variable factors (e.g. emotional well-being) could be introduced into the PRO analysis. Even for indication-specific PROMs, the inclusion of treatment-irrelevant items (e.g. satisfaction with sexual life) is considered unfavourable. To make the PRO analysis interpretable, the potential effects of the drugs as well as the clinical features of the target population should be input into the item generation of the PROMs. Tailoring PROMs for novel oncology drugs is indeed challenging, the selection of items from pre-existing PROMs or item banks may help with this process.32
The lack of prespecified statistical analysis plans (SAP) is another major deficiency in the reviewed documents. The lack of explicit hypotheses and statistical analysis plans of PRO endpoints in oncology clinical trials has also been raised in previous studies.33,34 In many cases, the establishment of statistical analysis plans is hindered by the study design which can only be refined in post-marketing studies. On the other hand, for blinded RCTs, it is important to choose proper statistical analyses (e.g. time-to-event and longitudinal analysis) to represent the prespecified concepts of interest. The SAPs of PRO endpoints should be designed based on the characteristics of different indications and therapeutical approaches. Statistical analyses of PRO endpoints in clinical trials for different cancer types have already been systematically reviewed,35, 36, 37 which could potentially be recruited as references for future PRO studies. Across the approvals included in this review, the vast majority of PROs were presented in an exploratory manner. However, the FDA guidance stated that exploratory PRO results that are not included in the statistical hierarchy results are considered descriptive and will be evaluated for inclusion in the label.10 In addition, the lack of minimal clinically important difference (MCID) was frequently brought up by the FDA reviewers. Not only are SAPs and statistical differences needed to support the recognition of PRO results in oncology trials as evidence to support benefit, statistically different results that also reach MCID are more desirable. To enable PROs to better support benefit-risk evaluation, more efforts need to be made in the design and justification of statistical methods, sensitivity tests and MCID settings.
Issues regarding PRO data collection are attributed to baseline assessment, questionnaire completion rate, and sample size. It has been proposed in the literature to encourage PROs to be used in early-stage clinical trials. The early use of PROs could facilitate the early identification of challenges in completing PROs and avoid low completion rates in later pivotal trials.38 Previous literature also suggested that considering and adequately addressing the respondent burden associated with PRO collection could minimise missing PRO data and ensure the quality of PRO data.39 The rollout of electronic PRO (ePRO) will also bring great promise for the application of PROs in clinical trials, streamlining the information collection process and enabling easier recording and processing of large sample size information.40 The incorporation of ePRO data collection further improved the feasibility and reliability of PRO assessment.
Not only can PROs be used for an evaluation endpoint in the therapeutic effect, but the role of PROs in safety evaluation should not be neglected. Analysis of this study showed that FDA reviewers mentioned in multiple cases that while they do not recognize the role of PRO in supporting efficacy benefits, they do recognize the role of PRO in supporting safety evaluations. The usefulness of PRO for the evaluation of drug-related adverse events (AE) has been demonstrated in previous publications, and patient self-reports might more adequately reflect AE than physician observation of symptoms.41,42 Currently, the collection of adverse event information relies on Common Terminology Criteria for Adverse Events (CTCAE). The PRO-CTCAE, which collects AE in the form of patient reports, is also gradually gaining recognition and being used.43 From the analysis of previous FDA review documents in this study, the regulators not only recognised the PRO-CTCAE for AE assessment,44 but also suggested that some other QoL questionnaires could also support the safety evaluation of treatment to some extent. Moreover, there is variation in regulatory consideration of PRO results to support the efficacy or to support safety. From the analysis in this paper, PRO results are subject to more rigorous scrutiny if they are used to support drug effectiveness in label claims than support for safety assessments.
The comprehensive and detailed summarisation and classification of the PROs in oncology trials in this study could inspire the design of subsequent oncology trials. However, there are still some limitations in this study. Firstly, because only FDA review documents were analysed, this article cannot provide a comprehensive review of globally overreaching regulatory agencies. Secondly, pivotal trials of NDA are usually limited in time duration and enrolled population with excluding some certain populations. Nevertheless, it is beyond the scope of this study to examine the application of PROs in investigator-initiated oncology drug trials or real-world studies. Thirdly, only pivotal trials in the NME or BLA were included in this study because the FDA tends to have more detailed review documents of these applications and it is also more informative for the use of PRO in the first marketing review of novel drugs. The use of PROs in other supporting clinical trials or abbreviated new drug application (ANDA) filings were not included in this study. Finally, though this study encompassed bunches of oncology products, the generalisability of these findings to other non-oncology drugs is not clear. From the current status, there is a lack of clear and unified consensus for PROs in oncology drug trials in the regulatory review, and industry and academia need clearer guidelines to incorporate patient perspectives into novel oncology drug development. To develop a full picture of patient-focused drug development, all parties need to work together to enhance the focus on the oncology patients’ own perspectives and to improve the quality of life of oncology patients.
In conclusion, patient-reported outcomes currently do not play a significant role in influencing the regulatory review of novel oncology drugs for marketing. It is related to the deficiencies in the design and implementation of oncology drug clinical trials incorporating patient experience data, and more so to the overall oncology drug development thinking and regulatory review considerations. This study summarised the current application of PROs in oncology trials and raises feasible recommendations for the use of PROs in future oncology clinical trials from the perspective of regulatory considerations. These findings will contribute to a deeper understanding of PROs in the development of novel oncology drugs.
Contributors
CG, KG, GL, and XC conceptualised the study, CG, KG, and YL collected the data, CG, KG, YL, GL, and XC contributed to write the manuscript, HZ, JY, YL, CY, SL, SX, and XC contributed to the interpretation of the results. XC and SX supervised the entire work. All authors accessed and verified the data. All authors reviewed the manuscript and provided feedback.
Data sharing statement
Data inventories are provided in supplementary material. All other relevant data for this study is available upon request to the corresponding author.
Declaration of interests
All authors declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101953.
Contributor Information
Songmei Xie, Email: xiesm@cde.org.cn.
Xiaoyuan Chen, Email: cxya02648@mail.tsinghua.edu.cn.
Appendix A. Supplementary data
References
- 1.U.S. Food and Drug Administration . 2009. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims.https://www.fda.gov/media/77832/download [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rupp T., Zuckerman D. Quality of life, overall survival, and costs of cancer drugs approved based on surrogate endpoints. JAMA Intern Med. 2017;177(2):276–277. doi: 10.1001/jamainternmed.2016.7761. [DOI] [PubMed] [Google Scholar]
- 3.Davis C., Naci H., Gurpinar E., Poplavska E., Pinto A., Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ. 2017;359:j4530. doi: 10.1136/bmj.j4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C., Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992–1994. doi: 10.1001/jamainternmed.2015.5868. [DOI] [PubMed] [Google Scholar]
- 5.Vivot A., Jacot J., Zeitoun J.D., Ravaud P., Crequit P., Porcher R. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000-2015. Ann Oncol. 2017;28(5):1111–1116. doi: 10.1093/annonc/mdx053. [DOI] [PubMed] [Google Scholar]
- 6.Marandino L., La Salvia A., Sonetto C., et al. Deficiencies in health-related quality-of-life assessment and reporting: a systematic review of oncology randomized phase III trials published between 2012 and 2016. Ann Oncol. 2018;29(12):2288–2295. doi: 10.1093/annonc/mdy449. [DOI] [PubMed] [Google Scholar]
- 7.Samuel J.N., Booth C.M., Eisenhauer E., Brundage M., Berry S.R., Gyawali B. Association of quality-of-life outcomes in cancer drug trials with survival outcomes and drug class. JAMA Oncol. 2022;8(6):879–886. doi: 10.1001/jamaoncol.2022.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluetz P.G., O'Connor D.J., Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19(5):e267–e274. doi: 10.1016/S1470-2045(18)30097-4. [DOI] [PubMed] [Google Scholar]
- 9.Lipscomb J., Gotay C.C., Snyder C.F. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clin. 2007;57(5):278–300. doi: 10.3322/CA.57.5.278. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration . 2021. Guidance for industry: core patient-reported outcomes in cancer clinical trials.https://www.fda.gov/media/149994/download [Google Scholar]
- 11.U.S. Food and Drug Administration . 2020. Patient-focused drug development: collecting comprehensive and representative input.https://www.fda.gov/media/139088/download [Google Scholar]
- 12.U.S. Food and Drug Administration . 2022. Patient-focused drug development: methods to identify what is important to patients.https://www.fda.gov/media/131230/download [Google Scholar]
- 13.U.S. Food and Drug Administration . 2022. Patient-focused drug development: selecting, developing, or modifying fit-for purpose clinical outcome assessments.https://www.fda.gov/media/159500/download [Google Scholar]
- 14.Gnanasakthy A., Barrett A., Evans E., D'Alessio D., Romano C.D. A review of patient-reported outcomes labeling for oncology drugs approved by the FDA and the EMA (2012-2016) Value Health. 2019;22(2):203–209. doi: 10.1016/j.jval.2018.09.2842. [DOI] [PubMed] [Google Scholar]
- 15.Gnanasakthy A., DeMuro C., Clark M., Haydysch E., Ma E., Bonthapally V. Patient-reported outcomes labeling for products approved by the office of hematology and oncology products of the US Food and Drug Administration (2010-2014) J Clin Oncol. 2016;34(16):1928–1934. doi: 10.1200/JCO.2015.63.6480. [DOI] [PubMed] [Google Scholar]
- 16.Bottomley A., Pe M., Sloan J., et al. Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol. 2016;17(11):e510–e514. doi: 10.1016/S1470-2045(16)30510-1. [DOI] [PubMed] [Google Scholar]
- 17.Coens C., Pe M., Dueck A.C., et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 2020;21(2):e83–e96. doi: 10.1016/S1470-2045(19)30790-9. [DOI] [PubMed] [Google Scholar]
- 18.Calvert M., Kyte D., Mercieca-Bebber R., et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319(5):483–494. doi: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 19.Eastern Research Group, Inc . 2021. Assessment of the use of patient experience data in regulatory decision-making.https://www.fda.gov/media/150405/download [Google Scholar]
- 20.Rock E.P., Kennedy D.L., Furness M.H., Pierce W.F., Pazdur R., Burke L.B. Patient-reported outcomes supporting anticancer product approvals. J Clin Oncol. 2007;25(32):5094–5099. doi: 10.1200/JCO.2007.11.3803. [DOI] [PubMed] [Google Scholar]
- 21.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Downing A., Wright P., Hounsome L., et al. Quality of life in men living with advanced and localised prostate cancer in the UK: a population-based study. Lancet Oncol. 2019;20(3):436–447. doi: 10.1016/S1470-2045(18)30780-0. [DOI] [PubMed] [Google Scholar]
- 23.Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics. 2018. https://www.fda.gov/media/71195/download [Google Scholar]
- 24.Beaver J.A., Howie L.J., Pelosof L., et al. A 25-year experience of US Food and Drug Administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–856. doi: 10.1001/jamaoncol.2017.5618. [DOI] [PubMed] [Google Scholar]
- 25.Roydhouse J.K., Fiero M.H., Kluetz P.G. Investigating potential bias in patient-reported outcomes in open-label cancer trials. JAMA Oncol. 2019;5(4):457–458. doi: 10.1001/jamaoncol.2018.6205. [DOI] [PubMed] [Google Scholar]
- 26.Chakravarti P.B., Basch E.M., Hirshfield K.M., et al. Exploring open-label bias in patient-reported outcome (PRO) emotional domain scores in cancer trials. J Clin Oncol. 2018;36(15_suppl):e18702. [Google Scholar]
- 27.Efficace F., Cella D., Aaronson N.K., et al. Impact of blinding on patient-reported outcome differences between treatment arms in cancer randomized controlled trials. J Natl Cancer Inst. 2022;114(3):471–474. doi: 10.1093/jnci/djab177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella D.F., Tulsky D.S., Gray G., et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 29.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 30.EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 31.Kluetz P.G., Slagle A., Papadopoulos E.J., et al. Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res. 2016;22(7):1553–1558. doi: 10.1158/1078-0432.CCR-15-2035. [DOI] [PubMed] [Google Scholar]
- 32.Garcia S.F., Cella D., Clauser S.B., et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25(32):5106–5112. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 33.Safa H., Tamil M., Spiess P.E., et al. Patient-reported outcomes in clinical trials leading to cancer immunotherapy drug approvals from 2011 to 2018: a systematic review. J Natl Cancer Inst. 2021;113(5):532–542. doi: 10.1093/jnci/djaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyte D., Retzer A., Ahmed K., et al. Systematic evaluation of patient-reported outcome protocol content and reporting in cancer trials. J Natl Cancer Inst. 2019;111(11):1170–1178. doi: 10.1093/jnci/djz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pe M., Dorme L., Coens C., et al. Statistical analysis of patient-reported outcome data in randomised controlled trials of locally advanced and metastatic breast cancer: a systematic review. Lancet Oncol. 2018;19(9):e459–e469. doi: 10.1016/S1470-2045(18)30418-2. [DOI] [PubMed] [Google Scholar]
- 36.Chakraborty R., Cannella L., Cottone F., Efficace F. Quality of patient-reported outcome reporting in randomised controlled trials of haematological malignancies according to international quality standards: a systematic review. Lancet Haematol. 2020;7(12):e892–e901. doi: 10.1016/S2352-3026(20)30292-1. [DOI] [PubMed] [Google Scholar]
- 37.Fiero M.H., Roydhouse J.K., Vallejo J., King-Kallimanis B.L., Kluetz P.G., Sridhara R. US Food and Drug Administration review of statistical analysis of patient-reported outcomes in lung cancer clinical trials approved between January, 2008, and December, 2017. Lancet Oncol. 2019;20(10):e582–e589. doi: 10.1016/S1470-2045(19)30335-3. [DOI] [PubMed] [Google Scholar]
- 38.Retzer A., Aiyegbusi O.L., Rowe A., et al. The value of patient-reported outcomes in early-phase clinical trials. Nat Med. 2022;28(1):18–20. doi: 10.1038/s41591-021-01648-4. [DOI] [PubMed] [Google Scholar]
- 39.Aiyegbusi O.L., Roydhouse J., Rivera S.C., et al. Key considerations to reduce or address respondent burden in patient-reported outcome (PRO) data collection. Nat Commun. 2022;13(1):6026. doi: 10.1038/s41467-022-33826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeBlanc T.W., Abernethy A.P. Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14(12):763–772. doi: 10.1038/nrclinonc.2017.153. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson T.M., Dueck A.C., Satele D.V., et al. Clinician vs patient reporting of baseline and postbaseline symptoms for adverse event assessment in cancer clinical trials. JAMA Oncol. 2020;6(3):437–439. doi: 10.1001/jamaoncol.2019.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kluetz P.G., Kanapuru B., Lemery S., et al. Informing the tolerability of cancer treatments using patient-reported outcome measures: summary of an FDA and critical path institute workshop. Value Health. 2018;21(6):742–747. doi: 10.1016/j.jval.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Basch E., Reeve B.B., Mitchell S.A., et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J., Singh H., Ayalew K., et al. Use of PRO measures to inform tolerability in oncology trials: implications for clinical review, IND safety reporting, and clinical site inspections. Clin Cancer Res. 2018;24(8):1780–1784. doi: 10.1158/1078-0432.CCR-17-2555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.