Abstract
Emergency department patient boarding is associated with hospital mortality and increased hospital length of stay. The objective of the present study is to describe the impact of deploying an Intensive Care team in the ED and its association with sepsis mortality and ICU length of stay. Patients admitted to ICU through the ED with an ICD-10 CM diagnosis of sepsis were included. Preintervention and postintervention phases included 4 and 15 months, respectively. Sepsis time zero, SEP-1 compliance, and lag time from time zero to antibiotic administration were compared. Outcomes of interest were mortality and ICU LOS. 1021 septic patients were included. Sixty-six percent fulfilled compliance with 3 h SEP-1 bundle. Lag time from time zero to antibiotic administration was 75 min. Multivariate analysis showed no association between ICU team in the ED and hospital mortality (Log OR 0.94, CI 0.67–1.34; p = 0.73). The ICU team in the ED was associated with prolonged ICU LOS (Log OR 1.21, CI 1.13–1.30; p < 0.01). Septic shock and ED boarding time were associated with prolonged ICU LOS. Compliance with SEP-1 bundle was associated with its reduction. Implementation of an ICU team in the ED for the treatment of septic patients during high volume hospitalizations is not associated with a reduction of mortality or ICU LOS.
Keywords: Emergency department, Intensive care unit, Mortality, Length of stay, Outcomes
Introduction
Sepsis affects 750,000 patients each year in the United States and is considered the leading cause of death in critically ill patients. Among these patients, 15% complicate with septic shock presenting a mortality rate higher than 50% [1]. The definition of sepsis has evolved over time. In 2004, the Surviving Sepsis Campaign guidelines adopted a definition consisting on 3 different degrees of severity: Sepsis was defined as the presence of at least 2 systemic inflammatory response syndrome (SIRS) criteria plus a source of infection; severe sepsis was defined as sepsis with organ dysfunction and/or lactate level > 2 mmol/L; and septic shock was defined as fluid-resistant hypotension requiring vasopressors or a lactate level of at least 4 mmol/L [2]. Based on the aforementioned data, in October 2015 the National Quality Forum (NQF) and the US Centers for Medicare and Medicaid Services (CMS) implemented nationwide processes for the early detection and treatment of sepsis. The resulting CMS SEP-1 quality measure aimed at standardizing early management and treatment of sepsis and septic shock with the objective of improving survival [3, 4]. The proposed bundle included steps to be accomplished within 3 h and 6 h of a defined time zero. Since its publication, the SEP-1 overall hospital performance has been publicly available on the CMS website and important efforts to improve institutional compliance with each one of its elements have been attempted [5]. Despite those efforts, many experts and medical organizations have questioned the SEP-1 clinical relevance, as some of the core recommendations have not been supported by strong levels of evidence [6–8]. Conversely, other publications showed that a rapid completion of the 3 h bundle (particularly early antibiotic administration) was associated with a reduction of mortality, justifying the efforts to achieve high SEP-1 bundle compliance [9]. In 2016, the Sepsis-3 committee issued an updated sepsis definition utilizing the sequential organ failure assessment (SOFA) or its quick version (qSOFA) [10]. Nevertheless, CMS continues to recommend the utilization of the previously described criteria and the implementation of the SEP-1 bundle.
Several publications demonstrated an association between emergency department (ED) patient boarding and an increase in hospital mortality and delay in appropriate treatment [11, 12]. Whereas ED boarding exposes all patients to diminished quality of care, critically ill patients remain particularly vulnerable. In fact, some studies revealed an increase of 1.5% in the risk of ICU death for each 1 h-delay in admitting patients from ED to the intensive care unit (ICU) [13]. Among critically ill patients, those presenting with sepsis, severe sepsis or septic shock present unique challenges. First, there is a pressing need for an early diagnosis and consequent determination of time zero. Second, the implementation of the SEP-1 bundle is time-sensitive. Last, ED boarding of non-septic patients may affect timely delivery of care to septic ones, complicating their overall trajectory. As our hospital has experienced significant ED boarding of critically ill patients, our Pulmonary and Critical Care Medicine Division designed a quality improvement (QI) project consisting on the implementation of an ICU team devoted to the care of critically ill patient staying in the ED. The aforementioned Medical Intensive Care team was not restricted to a particular ED area, but delivered ICU care ‘without borders’ within the entire ED geographic space. The present article describes the impact of the deployment of the Medical Intensive Care team in the ED and its association with sepsis, severe sepsis, and septic shock mortality and ICU length of stay (LOS).
Materials and methods
Our hospital is an 844-bed tertiary care medical center, with 118 ICU beds, and approximately 39,000 admissions per year. On November 1st 2020, a Medical Intensive Care Team composed by a Pulmonary and Critical Care physician, an Advance Practitioner Provider, and a Pulmonary and Critical Care fellow was deployed in the ED. The ED has a total of 74 beds, distributed among 5 sections. Every critically ill patient who arrived in critical condition to any section of the ED triggered a Medical Intensive Care Team consultation. Based on a prior analysis of ED utilization at our institution, the highest number of ED arrivals happened between 10 AM and 10 PM. Therefore, the Medical Intensive Care Team working shift was allocated to match the previously mentioned hours. There was no ICU team coverage in ED from 10 PM to 10 AM. A Hospitalist physician would cross-cover the ICU patients and admit patients during the remaining hours, and the Medical Intensive Care team would resume coverage at 10 AM. On a rotational daily basis, the ED nursing staff was supported by an ICU nurse, who provided just-in-time training for ICU procedures and practices. This position provided resources and education to the ED nurses who were not familiar with assisting and providing care for ICU patients. The ED was already staffed by an on-site pharmacist and trained respiratory therapists prior the initiation of this QI project. In addition to provide consultations for new cases, the Medical Intensive Care team performed multidisciplinary rounds every morning at 10 AM and defined daily plans of care for those ICU patients still boarding in the ED. ED boarding patients were transferred to ICU as soon as an inpatient bed became available. Handoff differed between the two groups as patients were admitted by a Hospitalist in the pre-intervention group, and care was resumed by the Medical Intensive Care team on arrival to the ICU. The post-intervention team received handoff directly from the ED staff. To assess the impact of the deployment of the Medical Intensive Care team in the ED, a retrospective analysis of prospectively collected data of critically ill patients arriving to the ED before-and-after the aforementioned team implementation was performed. In this report, we focused on critically ill patients with diagnoses of sepsis, severe sepsis or septic shock. Of note, septic patients deemed non-critically ill were not included in this analysis, as the ICU consultation team was not activated in those cases. A software tool for data and analytic technology (Health Catalyst®, South Jordan, Utah) was implemented in our institution in July 2020. Specifically, on a daily basis, the analytic technology incorporated input from our electronic health record (Epic®. Verona, WI). All patients admitted to our hospital with an ICD-10 CM code corresponding to sepsis were included. The severity of each case (sepsis, severe sepsis, or septic shock) was categorized based on the ICD-10 CM, as well. Inpatient LOS was calculated based on admission and discharge dates. Patients admitted and discharged within same day (before midnight) were assigned LOS of 1 day. Emergency department LOS was calculated as the time the patient physically arrived in the ED through the ED departure time (in hours). The ICU LOS was calculated as the time between when the patient was physically roomed in the ICU through the ICU departure or expiration. If they were downgraded from ICU status or expired while in the ED, the ICU or expired while in the ED, the ICU time was included as zero. Sepsis time zero was defined as the date and time when the sepsis encounter begun, and it was based on a hierarchy of qualifying events (Table 1). The 3 h SEP-1 bundle compliance was deemed present whenever all 3 required elements were present within 3 h from time zero, including lactate level and blood culture collection, and appropriate antibiotic administration. Partial presence of some of the SEP-1 bundle elements was considered non-compliant. Due to its particular relevance, lag time from time zero to antibiotic administration was individually collected, as well [14, 15]. For the purpose of this study, only patients admitted to the ICU through the ED were included. Furthermore, only patients with a primary diagnosis of sepsis, severe sepsis, or septic shock were included. Patients admitted to the ICU from other locations, such as rapid responses or out-of-hospital transfers were not included in our study. Also, patients admitted with other diagnosis (non-sepsis) complicated with sepsis during their hospitalization were excluded. Patients with a diagnosis of COVID-19 were excluded from the study. This was decided to be able to evaluate the impact of our intervention in sepsis mortality without confounders. To calculate adjusted mortality, our organization used the Midas plus (Simplr®, Houston, TX) artificial intelligence and machine learning tool. For modeling technique, upon patient’s discharge, Midas plus analyzes a variety of patient data, such as diagnosis-related group (MS DRG), principal diagnosis and procedures, clinical cluster (among 309 options), clinical cluster category, groups, and service lines. Once patients are categorized within the aforementioned frame, data are subsequently analyzed using Lasso, a regression analysis method to select those variables that will enhance the prediction accuracy of a specific outcome, such as expected mortality and hospital or ICU LOS for each discharged patient. Hence, based on these data, standardized mortality and LOS ratios (SMR and SLOR, respectively) were calculated by dividing observed/expected mortality or ICU LOS for all included patients on a monthly basis. The primary outcome of interest were SMR and mortality. Secondary outcomes were ICU SLOR and ICU length of stay. We compared these outcomes between preintervention vs. postintervention phases. The preintervention phase included 4 months (July 2020 to October 2020) based on the timeframe when the new software tool was implemented, and the postintervention phase included 15 months (November 2020 to January 2022). A study protocol for this project was submitted and approved by the Internal Review Board (Protocol # 021–212).
Table 1.
Qualifying events to determine sepsis time zero
| 1. Organ dysfunction with chronic conditions removed. Earliest resulted date/time is used if multiple criteria are met |
|---|
| Abnormal lactate: > 2 or intermediate or critical |
| SBP < 90 |
| MAP < 65 |
| Creatinine > 2 exclude renal failure |
| Platelet < 100,000 |
| INR > 1.5 excluding patients on warfarin |
| Vent in place (mechanical vent) |
| Bilirubin > 2 excluding liver failure |
| Diagnostic sepsis order sets ordered date/time. Earliest order date/time is used if multiple criteria are met |
| ED triage completion date/time |
Statistical analysis
Patient demographics, criteria to determine time zero, sepsis severity, SEP-1 compliance and ED boarding time and outcome variables were summarized using means and SD or median and IQR for continuous variables, and counts and percentages for categorical ones. Wilcoxon rank sum test and Chisq-test were utilized to compare differences between pre- and post- team implementation on the corresponding continuous and categorical variables. Multivariate analyses were also performed. Multiple logistic regression model was used to model for mortality, and Multiple Poisson regression models were used to model for ICU LOS towards the pre- and post- implementation points and other variables. Odds ratios, 95% Confidence Intervals of the odds ratios, and P-values were estimated from the logistic and Poisson models.
Results
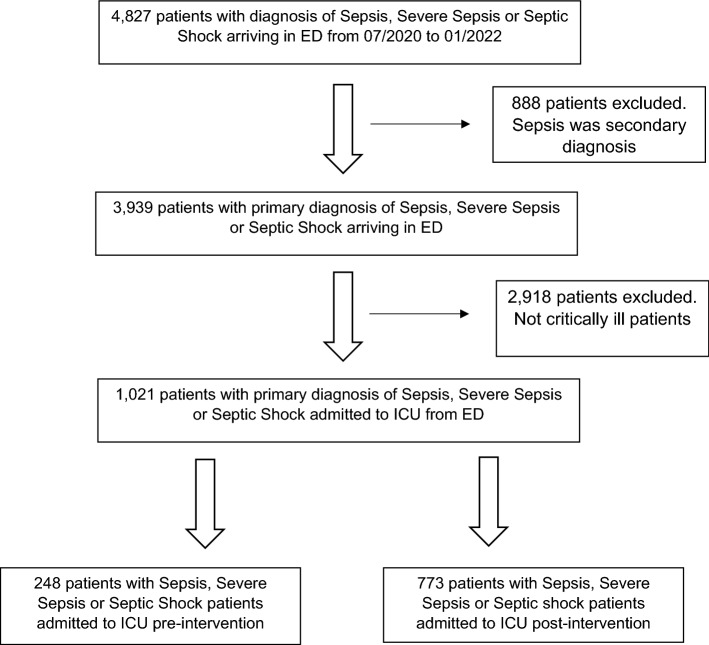
From July 2020 to January 2022, 4827 patients with primary diagnosis of sepsis, severe sepsis or septic shock were admitted through our ED. Figure 1 shows the flow chart of patients included and excluded from this study. A total of 1,021 critically ill septic patients were included. Out of these patients, the average age was 62 years, 54% were men, and the mean body-mass index was 28. The three most common qualifying events to determine time zero for sepsis diagnosis were abnormal lactate level, systolic blood pressure (SBP) < 90 mm Hg, or need of mechanical ventilation in 386 (38%), 355 (35%), and 120 (12%) patients, respectively. Of note, 676 (66%) of patients fulfilled compliance with the 3 h SEP-1 bundle. Furthermore, the average lag time from time zero to antibiotic administration was 75 min. In terms of sepsis severity, 179 (17%) presented with sepsis, 179 (18%) severe sepsis, and 663 (65%) septic shock. Mortality rate for all septic patients was 24%. Table 2 shows a univariate analysis comparing demographic information, criteria for time zero determination, severity of sepsis, and compliance with SEP-1 bundle between pre- and post-implementation groups. Of note, there were more septic patients with time zero defined by a hemodynamic reason in the post-intervention group. Specifically, there was a 13% higher rate of SBP lower than 90 mm Hg as a trigger for sepsis diagnosis in the post-intervention group (p < 0.01). Furthermore, there was a 6% higher rate of septic shock diagnosis post-intervention. Nevertheless, this difference did not reach statistical significance. Interestingly, there was a median delay of antibiotic administration of 8 min in the post-intervention group, compared with pre-intervention (p = 0.04). However, in both groups, antibiotic administration was achieved in a median time within 1 h of determined time zero. Table 3 reveals a univariate analysis comparing outcome variables between pre- and post-intervention groups. Despite the fact that the ICU SLOS ratio resulted lower than 1 in both groups, there was a statistically significant difference between them. Particularly, the ICU SLOS ratio was 0.11 points higher post-intervention. Furthermore, the median ICU LOS was 12 h higher post-intervention. No differences in mortality or SMR were found between groups. A multivariate analysis to assess the impact of individual variables in patient mortality showed no association between the deployment of an MICU team in the ED and the aforementioned outcome (Table 4). Interestingly, the presence of the MICU team in the ED was significantly associated with prolonged ICU LOS. Other variables associated with prolonged ICU LOS were diagnosis of septic shock and ED boarding time. There was no difference between timing of antibiotic administration between groups. Conversely, the rate of compliance with the SEP-1 bundle was associated with a reduction of the ICU LOS (Table 5).
Fig. 1.
Flowchart of included and excluded patients
Table 2.
Summary of explanatory variables pre- and post-intervention
| Pre- intervention (N = 248) | Post-intervention (N = 773) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) mean (SD) | 62 (15.97) | 62 (15.76) | 0.60 |
| Gender, male (n/%) | 136 (55%) | 419 (54%) | 0.88 |
| BMI mean (SD) | 29 (1056) | 28 (9.73%) | 0.22 |
| Criterion to determine time zero (n/%) | |||
| Bilirubin increase | 10 (4%) | 23 (3%) | 0.41 |
| Creatinine increase | 8 (3%) | 12 (2%) | 0.11 |
| INR prolonged | 13 (5%) | 36 (5%) | 0.73 |
| Lactate level | 107 (43%) | 279 (36%) | 0.05 |
| ED documentation | 9 (4%) | 23 (3.0%) | 0.67 |
| Low platelets | 3 (1%) | 15 (2%) | 0.58 |
| Systolic blood pressure | 63 (25%) | 291 (38%) | < 0.01 |
| Ventilator use | 34 (14%) | 86 (11%) | 0.30 |
| Severity of sepsis (n/%) | |||
| Sepsis | 41 (17%) | 138 (18%) | 0.70 |
| Severe sepsis | 57 (23%) | 122 (16%) | 0.01 |
| Septic shock | 150 (60%) | 513 (66%) | 0.09 |
| SEP-1 compliance and ED boarding time | |||
| Compliance with SEP-1 (n/%) | 165 (67%) | 511 (66%) | 0.94 |
| Abx time (mins) median (IQR) from time zero | 44 (17,90) | 52 (22,108) | 0.04 |
| ED LOS (hours) ED median (IQR) | 10 (6,15) | 9 (6, 15) | 0.19 |
Table 3.
Summary of outcome variables between pre- vs. post- intervention
| Pre-intervention (N = 248) | Post-intervention (N = 773) | P-value | |
|---|---|---|---|
| Mortality (n/%) | 60 (24%) | 187 (24%) | > 0.99 |
| ICU LOS (days) median (IQR) | 2.2 (1.15,5.07) | 2.7 (1.45,5.52) | 0.02 |
| Standardized mortality ratio | 1.04 | 0.78 | 0.21 |
| Standardized LOS ratio | 0.72 | 0.83 | 0.02 |
ICU Intensive care unit, LOS Length of stay
Table 4.
Mortality vs. multivariate factors via logistics regression
| Log odds ratio | 95% C.I | P-value | |
|---|---|---|---|
| Time (Post- vs. pre-intervention) | 0.94 | (0.67,1.34) | 0.73 |
| Age | 1.00 | (0.99,1.01) | 0.53 |
| Gender | 1.41 | (1.04,1.92) | 0.03 |
| BMI | 1.01 | (0.995,1.02) | 0.17 |
| Severe sepsis | 0.99 | (0.503,1.97) | 0.99 |
| Septic shock | 3.90 | (2.40,6.66) | < 0.01 |
| Compliance with SEP-1 bundle | 0.82 | (0.59,1.14) | 0.24 |
| Abx time from time zero | 1.00 | (1.00,1.00) | 0.51 |
| ED LOS | 0.99 | (0.98,1.01) | 0.36 |
Abx Antibiotic, ED Emergency department, LOS Length of stay
Table 5.
ICU LOS vs. multivariate factors via poisson regression
| Log odds ratio | 95% C.I | P-value | |
|---|---|---|---|
| Time (Post- vs. pre-intervention) | 1.21 | (1.13,1.30) | < 0.01 |
| Age | 0.99 | (0.99,1.00) | 0.52 |
| Gender | 0.99 | (0.94,1.05) | 0.86 |
| BMI | 1.00 | (0.99,1.00) | 0.52 |
| Severe sepsis | 0.97 | (0.87,1.07) | 0.56 |
| Septic shock | 1.17 | (1.08,1.27) | < 0.01 |
| Compliance with SEP-1 | 0.84 | (0.79,0.89) | < 0.01 |
| Abx time from time zero | 1.000 | (0.99,1.00) | 0.52 |
| ED LOS | 1.02 | (1.01,1.02) | < 0.01 |
Abx Antibiotic, ED Emergency department, LOS Length of stay
Discussion
The present study shows that the implementation of an ICU team in the ED for the assessment and treatment of septic patients is not associated with reduction in mortality and/or ICU LOS. Conversely, the ICU LOS was prolonged an average of 12 h post-implementation of the ICU team. Our results are discordant with those published by other groups. Specifically, an ED-based ICU implemented at the University of Michigan was associated with a reduction of short-stay ICU admission (adjusted OR, 0.70; 95% CI, 0.62–0.80) [16]. In that report, short-stay ICU was defined as an ICU LOS shorter than 24 h. The authors attributed those gains to an absolute increase of 19.3% in the proportion of patients receiving ICU-level care within 6 h of arrival. While part of the discordance may be attributed to the fact that we measured the entire ICU LOS (rather than short-stay), other factors might have contributed to our outcome. Particularly, baseline ED care of critically ill patients at each institution may have accounted for differences found on the impact of the implementation an ICU teams. Furthermore, as our study focused exclusively on septic patients, it is likely that protocols already employed in our ED aimed at achieving high SEP-1 bundle compliance may have reduced the impact of our ICU team presence in that location. Surprisingly, after the implementation of the ICU team, the ICU LOS was prolonged. This result might have been associated with a higher percentage of patients with hemodynamic compromise in the post-implementation group. In fact, time zero determined by SBP lower than 90 mm Hg was 13% higher post-implementation. Furthermore, although non-statistically significant, the percentage of patients with septic shock was 6% higher post-ICU team implementation. It is possible that these imbalances might have caused a higher level of invasive interventions, such as central venous access and arterial catheter placement, as well as monitoring time, prolonging the consequent ICU stay for several hours. Another possibility for prolonged ICU LOS in the post-implementation group is the limited skilled nursing facility and long-term acute care beds, and therefore patients may have stayed longer when previously there would not have been a delay. It is also possible that the ICU LOS was more prolonged in the post-intervention group because of the higher proportion of critically ill patients with covid-19 and acute respiratory distress syndrome who were excluded from this study, and therefore may have consumed more time than septic patients. Interestingly, compliance with SEP-1 bundle was inversely associated with ICU LOS. Prior studies showed benefits in SEP-1 compliance in relationship with clinical outcomes. Particularly, Townsend et al. demonstrated that when SEP-1 bundles were successfully delivered, there was a reduction in mortality from 30.3 to 21.7% (p < 0.001) [17]. Other reports showed improvement in hospital LOS associated with higher SEP-1 compliance [18]. Of note, our report did not show a correlation between delay of antibiotic administration and mortality and/or ICU LOS. This result might have been related to the mix of patient severity, as our study just included 63% of patients within the septic shock category. Studies showed that the impact of antibiotic administration delay becomes relevant depending on sepsis severity. Specifically, a randomized trial that compared differential time of antibiotics for sepsis found no difference in mortality despite showing higher than 90 min difference between study arms in time-to-antibiotics. Notably, fewer than 4% of included patients had septic shock [19]. Conversely, within a group of septic shock patients, data suggest a clear association between antibiotic administration delay and mortality [20]. Our study also shows an association between ED boarding time and prolonged ICU LOS. Prior studies revealed similar findings. Specifically, a retrospective analysis from Saudi Arabia that included 940 boarding ICU patients admitted to ICU within 6 h, between 6 and 24 h, and later than 24 h revealed a direct association between boarding times and mortality and hospital LOS [21]. Another study, which included 50,000 patients admitted to 120 ICUs located in the United States revealed similar results, increasing hospital LOS for those patients boarding in ED for more than 6 h [22]. Finally, a study that included 4 cohort studies from North America and Europe involving patients admitted with community-acquired pneumonia to ICU, demonstrated an increased odds ratio for hospital LOS for those patients admitted to ICU with delay, compared with those rapidly transferred from ED to ICU [23]. In our study, SEP-1 compliance was 66 and 67% in the pre and post-intervention group, which was not significant. These data are higher than both the national average (48%) and state average for Texas (50.8%), according to 2018 CDC data [24]. Hospitals with better SEP-1 performance are smaller, for-profit and with an intermediate ICU size, however, our hospital is a larger tertiary center that is non-profit with a large ICU capacity [25]. Therefore, we may already have more protocols and systems in place than other hospitals of our size. Our study presents many strengths. First, it includes a specific population of patients admitted through the ED with primary diagnosis of sepsis, excluding other confounding diagnosis. Second, due to the use of analytic technology, we were able to accurately gather data related to compliance with SEP-1, ED boarding time, and adjusted predicted outcomes (SMR, SLOS). Third, the study was performed within the implementation of QI project, involving the deployment of an ICU team in the ED. Therefore, the results of our experience may be relevant for its applicability in other organizations.
Limitations
Our study also has several limitations. First, due to the retrospective nature of the analysis, it is possible that selection and/or information bias occurred. Second, we were unable to collect information regarding practices before vs. after implementation, such as rate of venous or arterial access placement. Therefore, the resulting prolonged ICU LOS post-implementation due to higher level of invasiveness delivered by the ICU team, it is hypothesis generating. Nevertheless, it cannot be confirmed at this point. As previously described, the ICU team admitted critically ill patients between the hours of 10 AM and 10 PM since that interval had the highest rates of ICU admissions. We were unable to obtain exact information on the percentage of patients admitted by the hospitalist compared to intensivist in the post-implementation group. The ICU nurse who was deployed in the ED did not directly take care of patients, but was used mostly as a resource for the ED staff for unfamiliar procedures and processes that are considered commonplace in the ICU environment. Another limitation is that we do not have the ED volume data for either group, therefore, we cannot analyze if there was a heavier volume in the post-intervention group to affect outcomes. Our study was designed at a time when many hospital systems were overwhelmed with the covid pandemic, which may have impacted the study outcomes as there were significant disruptions that our data may not have been able to include. For example, travel nurses not familiar with the hospital system or material shortages. Lastly, we were only able to collect partial information pertaining to the first 3 h of the SEP-1 bundle (antibiotic administration, blood culture collection, lactate level). We were unable to collect other relevant information, which could have affected the results, such as intravenous crystalloid use, vasopressor needs, and/or subsequent lactate level monitoring.
Conclusion
In conclusion, our study shows that the implementation of an ICU team in the ED for the evaluation and treatment of septic patients during the high volume hospitalizations from the covid pandemic is not associated with a reduction of mortality or ICU LOS. Sepsis severity, compliance with SEP-1 bundle, and ED boarding remained the only factors associated with reduction in ICU LOS.
Take home message
Implementation of an ICU team in the ED has been shown in prior studies to improve mortality and reduce ICU length of stay. Due to a higher burden of patients than normal boarding in the ED, our hospital executed this change during the covid pandemic and looked specifically at the treatment of septic patients, finding no association with mortality reduction or ICU length of stay.
Acknowledgements
Not applicable.
Author contributions
ET and AM: were responsible for the conception and design of the study, drafting the manuscript, reviewing and analyzing literature and were responsible for the manuscripts’ revisions. XW: was responsible for data analysis. All authors read and approved the final manuscript.
Funding
This work was not supported by any foundation.
Data availability
The datasets used and analyze during the current study are available on reasonable request.
Declarations
Conflict of interest
Authors do not have conflicts of interest. No authors have no competing of interest nor any financial interests in the paper.
Ethics approval and consent to participate
The study was approved by Internal Review Board (Protocol # 021–212) at Baylor University Medical Center in Dallas, Texas.
Consent for publication
All authors approved the final version submitted for publication and are accountable for the accuracy and integrity of the work.
Human and animal rights statement and informed consent
Informed consent was waived by the institution for reasons of the retrospective design and anonymization of patient identifiers before analysis. There are no animal subjects in this article and informed consent is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5 year study. Intens Care Med. 2014;40(11):1623–1633. doi: 10.1007/s00134-014-3496-0. [DOI] [PubMed] [Google Scholar]
- 3.Klompas M, Rhee C. The CMS sepsis mandate: right disease. Wrong Measure Ann Intern Med. 2016;165(7):517–518. doi: 10.7326/M16-0588. [DOI] [PubMed] [Google Scholar]
- 4.Kalantari A, Mallemat H, Weingart SD. Sepsis definitions: the search for gold and what CMS got wrong. West J Emerg Med. 2017;18(5):951–956. doi: 10.5811/westjem.2017.4.32795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepper DJ, Jaswal D, Sun J, Welsh J, Natanson C, Eichacker PQ. Evidence underpinning the centers for medicare & medicaid services’ severe sepsis and septic shock management bundle (SEP-1): a systematic review. Ann Intern Med. 2018;168(8):558–568. doi: 10.7326/M17-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goal-directed resuscitation for patients with early septic shock. Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 7.Pro CI, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 9.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr BG, Kaye AJ, Wiebe DJ, Gracias VH, Schwab CW, Reilly PM. Emergency department length of stay: a major risk factor for pneumonia in intubated blunt trauma patients. J Trauma. 2007;63(1):9–12. doi: 10.1097/TA.0b013e31805d8f6b. [DOI] [PubMed] [Google Scholar]
- 12.Goldhill DR, McNarry AF, Hadjianastassiou VG, Tekkis PP. The longer patients are in hospital before intensive care admission the higher their mortality. Intens Care Med. 2004;30(10):1908–1913. doi: 10.1007/s00134-004-2386-2. [DOI] [PubMed] [Google Scholar]
- 13.Cardoso LT, Grion CM, Matsuo T, Anami EH, Kauss IA, Seko L, Bonametti AM. Impact of delayed admission to intensive care units on mortality of critically ill patients: a cohort study. Crit Care. 2011;15(1):R28. doi: 10.1186/cc9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 16.Gunnerson KJ, Bassin BS, Havey RA, Haas NL, Sozener CB, Medlin RP, Jr, Gegenheimer-Holmes JA, Laurinec SL, Boyd C, Cranford JA, et al. Association of an emergency department-based intensive care unit with survival and inpatient intensive care unit admissions. JAMA Netw Open. 2019;2(7):e197584. doi: 10.1001/jamanetworkopen.2019.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend SR, Phillips GS, Duseja R, Tefera L, Cruikshank D, Dickerson R, Nguyen HB, Schorr CA, Levy MM, Dellinger RP, et al. Effects of compliance with the early management bundle (SEP-1) on mortality changes among medicare beneficiaries with sepsis: a propensity score matched cohort study. Chest. 2022;161(2):392–406. doi: 10.1016/j.chest.2021.07.2167. [DOI] [PubMed] [Google Scholar]
- 18.Lasater KB, Sloane DM, McHugh MD, Cimiotti JP, Riman KA, Martin B, Alexander M, Aiken LH. Evaluation of hospital nurse-to-patient staffing ratios and sepsis bundles on patient outcomes. Am J Infect Control. 2021;49(7):868–873. doi: 10.1016/j.ajic.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam N, Oskam E, Stassen PM, Exter PV, van de Ven PM, Haak HR, Holleman F, Zanten AV, Leeuwen-Nguyen HV, Bon V, et al. Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med. 2018;6(1):40–50. doi: 10.1016/S2213-2600(17)30469-1. [DOI] [PubMed] [Google Scholar]
- 20.Kashiouris MG, Zemore Z, Kimball Z, Stefanou C, Fowler AA, 3rd, Fisher B, de Wit M, Pedram S, Sessler CN. Supply chain delays in antimicrobial administration after the initial clinician order and mortality in patients with sepsis. Crit Care Med. 2019;47(10):1388–1395. doi: 10.1097/CCM.0000000000003921. [DOI] [PubMed] [Google Scholar]
- 21.Al-Qahtani S, Alsultan A, Haddad S, Alsaawi A, Alshehri M, Alsolamy S, Felebaman A, Tamim HM, Aljerian N, Al-Dawood A, et al. The association of duration of boarding in the emergency room and the outcome of patients admitted to the intensive care unit. BMC Emerg Med. 2017;17(1):34. doi: 10.1186/s12873-017-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP. group D-Es: Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35(6):1477–1483. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 23.Renaud B, Santin A, Coma E, Camus N, Van Pelt D, Hayon J, Gurgui M, Roupie E, Herve J, Fine MJ, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community-acquired pneumonia. Crit Care Med. 2009;37(11):2867–2874. doi: 10.1097/CCM.0b013e3181b02dbb. [DOI] [PubMed] [Google Scholar]
- 24.Kempker JA, Kramer MR, Waller LA, Wang HE, Martin GS. State-level hospital compliance with and performance in the centers for medicaid & medicare services’ early management severe sepsis and septic shock bundle. Crit Care. 2019;23(1):92. doi: 10.1186/s13054-019-2382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbash IJ, Davis B, Kahn JM. National performance on the medicare SEP-1 sepsis quality measure. Crit Care Med. 2019;47(8):1026–1032. doi: 10.1097/CCM.0000000000003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyze during the current study are available on reasonable request.