Abstract
Attention-deficit/hyperactivity disorder (ADHD) a common neurodevelopmental disorder of childhood and often comorbid with other externalizing disorders (EDs). There is evidence that externalizing behaviors share a common genetic etiology. Recently, a genome-wide, multigenerational sample linked variants in the Lphn3 gene to ADHD and other externalizing behaviors. Likewise, limited research in animal models has provided converging evidence that Lphn3 plays a role in EDs. This study examined the impact of Lphn3 deletion (i.e., Lphn3−/−) in rats on measures of behavioral control associated with externalizing behavior. Impulsivity was assessed for 30 days via a differential reinforcement of low rates (DRL) task and working memory evaluated for 25 days using a delayed spatial alternation (DSA) task. Data from both tasks were averaged into 5-day testing blocks. We analyzed overall performance, as well as response patterns in just the first and last blocks to assess acquisition and steady-state performance, respectively. “Positive control” measures on the same tasks were measured in an accepted animal model of ADHD–the spontaneously hypertensive rat (SHR). Compared with wildtype controls, Lphn3−/− rats exhibited deficits on both the DRL and DSA tasks, indicative of deficits in impulsive action and working memory, respectively. These deficits were less severe than those in the SHRs, who were profoundly impaired on both tasks compared with their control strain, Wistar-Kyoto rats. The results provide evidence supporting a role for Lphn3 in modulating inhibitory control and working memory, and suggest additional research evaluating the role of Lphn3 in the manifestation of EDs more broadly is warranted.
Keywords: Adgrl3, ADHD, CRISPR/Cas9, delayed spatial alternation, differential reinforcement of low rates, externalizing behavior, inhibitory control, Latrophilin-3, Lphn3, working memory
1 |. INTRODUCTION
Attention deficit/hyperactivity disorder (ADHD) is characterized by persistent problems with inattention, impulsivity, and/or hyperactivity lasting for at least 6 months and resulting in functional impairment in a variety of settings.1 Based on the 2016 National Survey of Children’s Health an estimated 9.4% of all US children (2–17 years of age) received an ADHD diagnosis2 making it the most common neurodevelopmental disorder diagnosed in childhood.3,4 ADHD can persist into adulthood and is often comorbid with other externalizing disorders (EDs) such as oppositional defiant disorder and conduct disorder, which typically present during childhood or early adolescence,1,5 as well as antisocial personality disorder and substance use disorder (SUD) which usually manifest during late adolescence or young adulthood.6,7
In addition, to documented shared liability for EDs,8–11 there is also evidence that externalizing behaviors may stem from a common genetic etiology.12,13 While the heritability of ADHD alone is high (h2 = 74%),14 much of it is common to other EDs, so much so that a single highly heritable (h2 ~ 80%) externalizing factor accounts for a larger percentage of the genetic variation than ADHD alone.12,15 Data from a genome-wide, multigenerational sample identified linkage of ADHD as well as co-segregation of ADHD and other externalizing behaviors with markers on chromosome 4q13.2.16–19 Mapping of this region revealed variants in the Lphn3 (Adgrl3) gene [OMIM 616417] that predisposed individuals to ADHD,20–22 predicted ADHD severity and response to treatment,22–24 and predicted SUD in ADHD patients.25 Similar studies in other populations replicated the finding that Lphn3 gene variants contribute to ADHD susceptibility.26–32 In addition, in SUD patients, Lphn3 was one of 86 “risk genes” identified.33
Research in animal models has provided converging evidence that the Lphn3 gene plays a role in EDs.34 For example, Lphn3−/− knockout (KO) mice exhibit hyperactivity in the open field test, increased premature responses (indicative of impulsivity) on a continuous performance test, and are more sensitive to the locomotor stimulant effects of cocaine.35,36 Similarly, Lphn3 KO rats were also hyperactive,37 while zebrafish that lacked lphn3.1 (one of two lphn3 zebrafish orthologs) exhibited increased locomotion, nighttime hyperactivity, and episodes of motor impulsivity.38
Research evaluating the impact of Lphn3 gene expression variants on behavioral assays associated with EDs is limited. The present experiments examined the impact of Lphn3 deletion on operant measures of behavioral control including impulsivity and working memory. Impulsivity is a core symptom of ADHD1 and is also a known risk factor for SUD.39–41 Increasing working memory load has been argued to increase impulsive behavior.42,43 Not surprisingly, working memory deficits are commonly reported in ADHD children44–47 and are associated with drug-seeking in rats48–51 and SUD in humans.52–54 In the current study, impulsivity was assessed for 30 days, while working memory was assessed for 25 days. To determine the suitability of Lphn3−/− (KO) rats as a prospective animal model of ADHD and assess their relative degree of impairment, we also report “positive control” measures of the same behaviors in Spontaneously Hypertensive Rats (SHRs) as numerous studies have shown their utility as an animal model of ADHD.55–59 In line with previous research, we expected to find an overall deficit in both impulsive action and working memory in the SHRs relative to Wistar-Kyoto (WKY) rats (i.e., the SHR control strain) as well as in the Lphn3 KO rats relative to their Lphn3+/+ (WT) controls. Additionally, we examined whether observed deficits in performance occurred during response acquisition and/or during steady-state performance.
2 |. MATERIAL AND METHODS
2.1 |. Subjects
The subjects were 24 SHRs (12 male, 12 female) and 24 WKY (12 male, 12 female) rats purchased from Charles River (Kingston, NY) as well as 17 Lphn3−/− (eight male, nine female) and 18 Lphn3+/+ (nine male, nine female) rats on a Sprague Dawley background (SD-IGS, strain 001, Charles River, Charleston, NC). The SHR and WKY rats were shipped to the University of Memphis when they were 45 ± 2 days old. The Lphn3−/− rats were generated at the Cincinnati Children’s Transgenic Animal and Genome Editing Core by using CRISPR/Cas9 to delete exon 3.37 The Lphn3−/− founders were then bred with Lphn3+/+ rats to establish the lines and heterozygote crossings (i.e., Lphn3+/− x Lphn3+/−) were used to generate the KO and WT rats used for these experiments. Ear punches were collected from offspring at postnatal day (P)7 for genotyping using three primers: (1) AAAGGGTCATAGCATCCGGC, (2) CTAACGTGGCTTTTTGTCTTCT, and (3) GCTCGACAGACAGTGTGGAT. HotStarTaq Master Mix kit (Qiagen, Hilden, Germany) was used per manufacturer’s recommendations. Thermocycler parameters were: (1) 95°C, 5 min; (2) 94°C, 1 min; (3) 61.5°C, 1 min; (4) 72°C, 2 min; (5) steps 2–4 were repeated 34 more times, followed by (6) 72°C, 10 min; and (7) held at 4°C until the product was run on a 2% agarose gel with ethidium bromide staining. The WT band appears at ~320 bp and the KO band at ~452 bp.37 Only one rat per genotype per sex per litter was randomly selected and shipped to the University of Memphis when 30 ± 2 days old.
Upon arrival at the University of Memphis Psychology vivarium, same-sex rats of the same strain or the same genotype were housed 2–3 per cage in standard plastic cages (45 cm × 24 cm × 30 cm) with corn cob bedding and ad libitum tap water in a room with a 12 h reverse light/dark cycle (lights off 0700 h) that was temperature- and humidity-controlled. Rats remained on free feed (Teklad, 2018) until all rats in a shipment reached P60 after which they were put on a food restriction schedule to maintain 85%–90% of their free-feeding weight. Adjustments were made bi-weekly to account for growth. All animal use was approved by the Institutional Animal Care and Use Committee at the University of Memphis and was in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals.60 Personnel were blinded to the genotype or strain of the rats during behavioral testing.
2.2 |. Apparatus
Behavioral tests were performed in automated, rat operant chambers (Med Associates Inc., St. Albans, VT) housed in sound attenuated wooden boxes. The test chambers were 17.5 cm tall with a 24 cm × 20 cm stainless steel grid floor that rests above a tray filled with corn cob bedding. Dustless precision pellets (BioServ product F0165) were dispensed into a food magazine centered 2.5 cm above the floor. A retractable lever with a cue light located above was located on each side of the food magazine and a house light was located on the wall opposite the food magazine. White noise was presented during testing to mitigate the intrusion of outside sounds. Med-PC V software was used to present the testing programs and record data.
2.3 |. Procedure
When all rats in a shipment reached P70, behavioral testing began and followed the testing sequence below. Rats received one session/day and were tested at the same time of day 0800–1100 h, 7 days/week.
2.3.1 |. Autoshaping
This program is designed for rats to learn the basics of the operant chamber, such as the location of food and the association between lever presses and food delivery. Upon activation of the autoshaping program, two levers were extended. When a lever was pressed, a pellet was dispensed into the magazine. If neither lever was pressed within a span of 3 min, a free pellet was dispensed. The program ended after 60 min or after 100 pellets were dispensed. Rats remained on autoshaping until no free pellets were delivered (~2–3 sessions).
2.3.2 |. Fixed ratio training
This program strengthened the lever-press response and ensured there was not a side preference for either lever. At the start of each fixed ratio (FR) session, the right lever was extended and the right cue light was illuminated. As with autoshaping, each lever press on an extended lever resulted in a food pellet. However, after five pellets were dispensed, the right lever was retracted and the right cue light turned off. At the same time, the left lever was extended and left cue light activated until five reinforcers were delivered. The response requirement then returned to the other side and this pattern of five lever presses followed by alternation was repeated until 100 total reinforcers were delivered. FR training lasted for three sessions.
2.3.3 |. Differential reinforcement of high rates
For differential reinforcement of high rates (DRH), only the left response lever was extended. To earn a reinforcer, the rat was required to make a minimum number of responses within a specific time interval. For example, during DRH 2:1 a reinforcer was delivered if two responses were made within 1 s. Only three sessions of DRH testing were conducted, including 1 day of DRH 2:1, 1 day of DRH 4:2, and one day of DRH 8:4. Each session terminated after 200 reinforcers were delivered or 90 min had elapsed, whichever occurred first.
2.3.4 |. Differential reinforcement of low rates
Three differential reinforcement of low rates (DRL) programs were conducted. At the start of each trial, the right lever was extended and the rat was required to press the lever to start the response timer. Once pressed, the rat had to wait for a set inter-response time (IRT) to elapse before pressing the lever again. If the lever was pressed after the set IRT elapsed, a food pellet was dispensed. However, if the lever was pressed before the allotted IRT passed, no reinforcer was delivered, and the rat was required to press the lever again to start a new trial. The IRTs included 2 days with a 5 s IRT (DRL 5), 2 days with a 10 s IRT (DRL 10), and 30 days with a 15 s IRT (DRL 15).
2.3.5 |. Cued alternation
Rats were trained to alternate their lever presses from one lever to the other from trial to trial. At the beginning of each cued alternation (CA) session, both levers were extended and both cue lights illuminated. Once a lever was pressed, the levers retracted and the alternate cue light was illuminated and both levers re-extended. To receive a reinforcer, the rat had to press the lever below the illuminated cue light, which alternated from one trial to the next. There was no delay imposed between trails except for the time needed for the levers to retract and re-extend at the start of the next trial. The rats completed 200 trials in each session. Rats remained on CA, until they met a performance criterion of 60% correct.
2.3.6 |. Noncued alternation
Rats had to alternate from one lever to the next from one trial to the next but did so without the assistance of cue lights. Each noncued alternation (NCA) session had 200 trials. Ten NCA sessions were conducted.
2.3.7 |. Delayed spatial alternation
After NCA, rats were tested on delayed spatial alternation (DSA) for 25 days. This task was identical to NCA except that a delay of 0, 5, 10, or 20 s was imposed between trials. These delays were presented randomly with the exception that a given delay never occurred consecutively for more than three trials. All delays were equally distributed throughout a session such that each delay was presented 50 times for a total of 200 trials.
2.4 |. Design and analyses
Data were analyzed using SPSS (IBM) version 26.0.
2.4.1 |. Differential reinforcement of high rates
Three different dependent measures were analyzed for DRH including total number of lever presses, reinforcers earned, and efficiency (reinforcers earned × DRH response requirement ÷ total number of lever presses). Each dependent measure was analyzed using a 2 (genotype or strain) × 2 (sex) × 3 (schedule) mixed ANOVA where genotype and sex were between-subjects factors and schedule (i.e., 2:1, 4:2, and 8:4) was a repeated measures factor.
2.4.2 |. Differential reinforcement of low rates
For DRL 5 and DRL 10, the ratio of reinforced to nonreinforced lever presses as well as the total number of reinforcers dispensed and number of trials completed were analyzed separately using a 2 (genotype or strain) × 2 (sex) × 2 (day) mixed ANOVA with day a repeated-measures factor. For the 30 days of DRL 15, the analyses were similar except that each dependent measure was averaged into 6, 5-day testing blocks and included in the mixed ANOVA as the repeated measure factor. Weincluded all blocks in the analyses of the ratio of reinforced to nonreinforced lever presses, total number of reinforcers dispensed, and the number of trials completed during DRL 15. This was done to assess whether genotype or strain affected task acquisition (i.e., how quickly the task was learned) and/or steady-state responding (i.e., how well the task was learned).
2.4.3 |. DRL response pattern analysis
Response pattern analysis was conducted whereby the proportions of responses that fell within 2.5 s IRT bins were analyzed via a 2 (genotype or strain) × 2 (sex) × 2 (day or block) × (IRT bin) mixed ANOVA. IRT bins were 2.5 s in size. For DRL 5, there were four: <2.5 s, 2.5–5.0 s, 5.0–7.5 s, and > 7.5 s. In a similar fashion, DRL 10 had 6 IRT bins and DRL 15 had 8 IRT bins. DRL 5 and 10 included both days in the analysis. To simplify interpretation but allow for response pattern analysis during acquisition and steady-state responding, DRL 15 included the first testing block (i.e., days 1–5) and the last testing block (i.e., days 25–30). Note that only the last two IRT bins for each DRL schedule were reinforced. Lastly, peak deviation analyses61–63 including the parameters of burst ratio, peak location, and peak area were also conducted on the last block of DRL 15 data. Each parameter was analyzed separately using a 2 (genotype or strain) × 2 (sex) between-subjects ANOVA.
2.4.4 |. Cued alternation
For CA, the number of sessions to reach criterion (i.e., 60% correct) was analyzed with a 2 (genotype or strain) × 2 (sex) between-subjects ANOVA.
2.4.5 |. Noncued alternation
The percent correct responses during NCA was analyzed using a 2 (genotype or strain) × 2 (sex) × 10 (day) mixed ANOVA which included day as a repeated-measures factor.
2.4.6 |. Delayed spatial alternation
The percent correct responses during DSA was analyzed similarly to NCA except that data over the 25 days of testing were averaged into 6, 5-day testing blocks and block was included in the mixed ANOVA as the repeated measure factor instead of day. Again, we included all blocks in the analysis of the percent correct during DSA to assess task acquisition and steady-state performance.
2.4.7 |. DSA trial-by-trial analyses
The types of errors made during DSA task acquisition (block 1) and steady-state performance (block 5) were also analyzed. These included win-stay errors and lose-stay errors. A “win” was defined as a correct response and happened when a rat correctly alternated levers. If the rat then “stayed” on the same lever, as the previous trial, this generated an incorrect response. Thus, a win–stay error indicated that the rat responded correctly on the n-1 trial but incorrectly on the nth trial by failing to alternate between the two levers.64 In addition, a “lose” was defined as a trial in which the rat responded incorrectly because it failed to alternate. Thus, a lose–stay error indicated that the rat responded incorrectly on the n-1 trial, and also on the nth trial by staying on the same lever. Therefore, a lose–stay error represents at least three consecutive responses on the same lever and is considered a perseverative response.64 Win-stay and lose-stay errors were analyzed separately using a 2 (genotype or strain) × 2 (sex) × 2 (first block, fifth block)mixed ANOVA.
3 |. RESULTS
Two female SHRs were euthanized for health reasons prior to completion of NCA and DSA. If a sphericity violation was found for any within-subjects effect, a Greenhouse–Geisser correction was used to reduce the risk of a Type I error because ε < 0.75 in all cases.65 In the interest of brevity, detailed explanation of the significant genotype-, strain-, or sex-related main effects and interactions are only reported for DRL 15 and DSA as these were our primary assessments of impulsive action and working memory. However, supplemental figures are included detailing the statistical findings for DRH, DRL 5, DRL 10, CA, and NCA. Briefly, for DRH, significant genotype and strain differences were observed wherein female KO and SHRs of both sexes performed better than controls when a high response rate exclusive of response inhibition was required (Supplemental Figure 1). However, KO rats and SHRs exhibited impaired performance during DRL 5 (Supplemental Figures 2–3) and DRL 10 (Supplemental Figures 4–6), which both required response inhibition. Supplemental Figure 7 shows the “debursted” relative proportion of responses for the pause IRTs. No genotype or strain differences were observed during cued alternation (Supplemental Figure 8) when the correct response lever was illuminated. Surprisingly, while the KO and WT rats did not differ during NCA (i.e., correct lever not illuminated), SHRs exhibited impaired performance compared with WKY rats across all 10 days of testing (Supplemental Figure 9) even though there was no delay between lever retractions at the end of a trial to re-extension for the next trial.
3.1 |. DRL 15
3.1.1 |. Ratio of reinforced to nonreinforced responses
The ratio of reinforced to nonreinforced trials across all DRL 15 testing blocks is presented in Figure 1A for the KO and WT rats. Higher values indicate better performance. There was a significant main effect of genotype, [F(1, 31) = 4.84, p = 0.035] and of sex [F(1, 31) = 4.18, p = 0.049]. KO rats had a lower ratio of reinforced: nonreinforced trials than WT rats (Figure 1B) and males had a higher ratio than females. The reinforced: nonreinforced ratio across all blocks for the SHR/WKY rats is presented in Figure 1C. There was a significant main effect of strain [F(1, 44) = 190.4, p < 0.001] and of sex [F(1, 44) = 5.5, p = 0.024]. Males had a higher ratio than females and the SHRs had a lower overall ratio than the WKY rats (Figure 1D). The strain × block interaction was also significant [F(1.8, 134.8) = 34.58, p < 0.001]. The SHRs had a significantly lower ratio than the WKY rats in every block (Figure 1C) with the magnitude of the difference increasing across blocks (Figure 1C).
FIGURE 1.
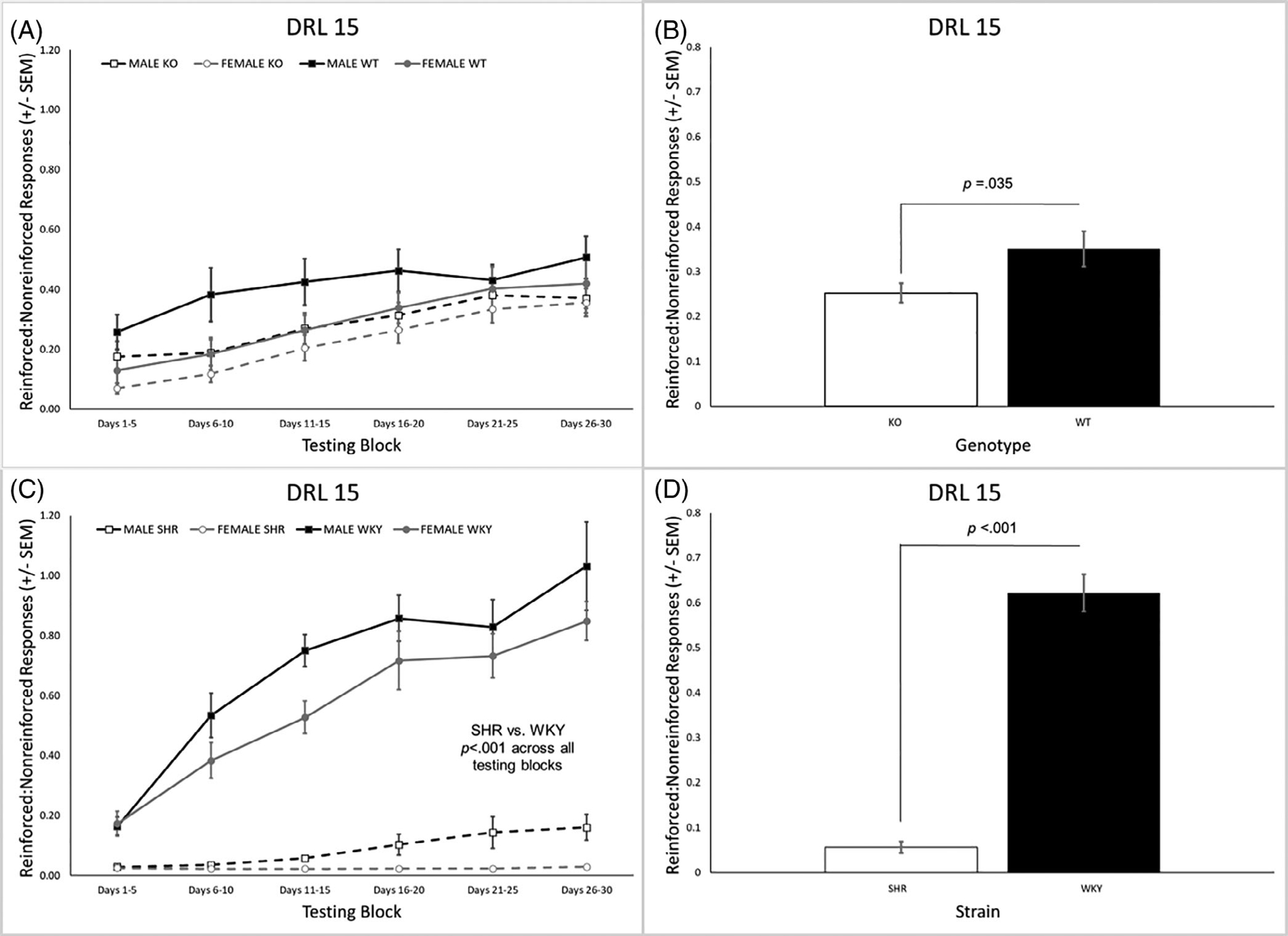
(A) The ratio of reinforced to nonreinforced trials across all testing blocks of DRL 15 for the Lphn3 KO and WT rats. (B) Lphn3 KO rats had a significantly lower ratio of reinforced to nonreinforced trials than the WT rats. (C) The ratio of reinforced to nonreinforced trials was significantly lower for the SHRs than the WKY within each of the six testing blocks. (D) SHRs had a significantly lower ratio of reinforced to nonreinforced trials than WKY rats overall. DRL, differential reinforcement of low rates; KO, knockout; SEM, standard error of the mean; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto; WT, wildtype
3.1.2 |. Reinforcers earned
The main effect of genotype on reinforcers earned was not significant, but the main effect of sex was [F(1, 31) = 6.65, p = 0.015], with males earning more than females (Figure 2A). For the SHR/WKY rats, there were significant main effects of strain [F(1, 44) = 303.94, p < 0.001] and sex [F(1, 44) = 12.28, p = 0.001] and significant strain × block [F(2.2, 96.2) = 20.16, p < 0.001] and strain × sex × block [F(2.2, 96.2) = 3.34, p = 0.035] interactions. As can be seen in Figure 2B, SHRs earned fewer reinforcers overall than the WKY rats. This was driven by the finding that SHR males earned significantly fewer reinforcers than WKY males (p < 0.001) in all testing blocks, with a similar effect in the females (p < 0.001; Figure 2C). In addition, SHR females earned fewer reinforcers than SHR males during blocks 3–6 (Figure 2C).
FIGURE 2.
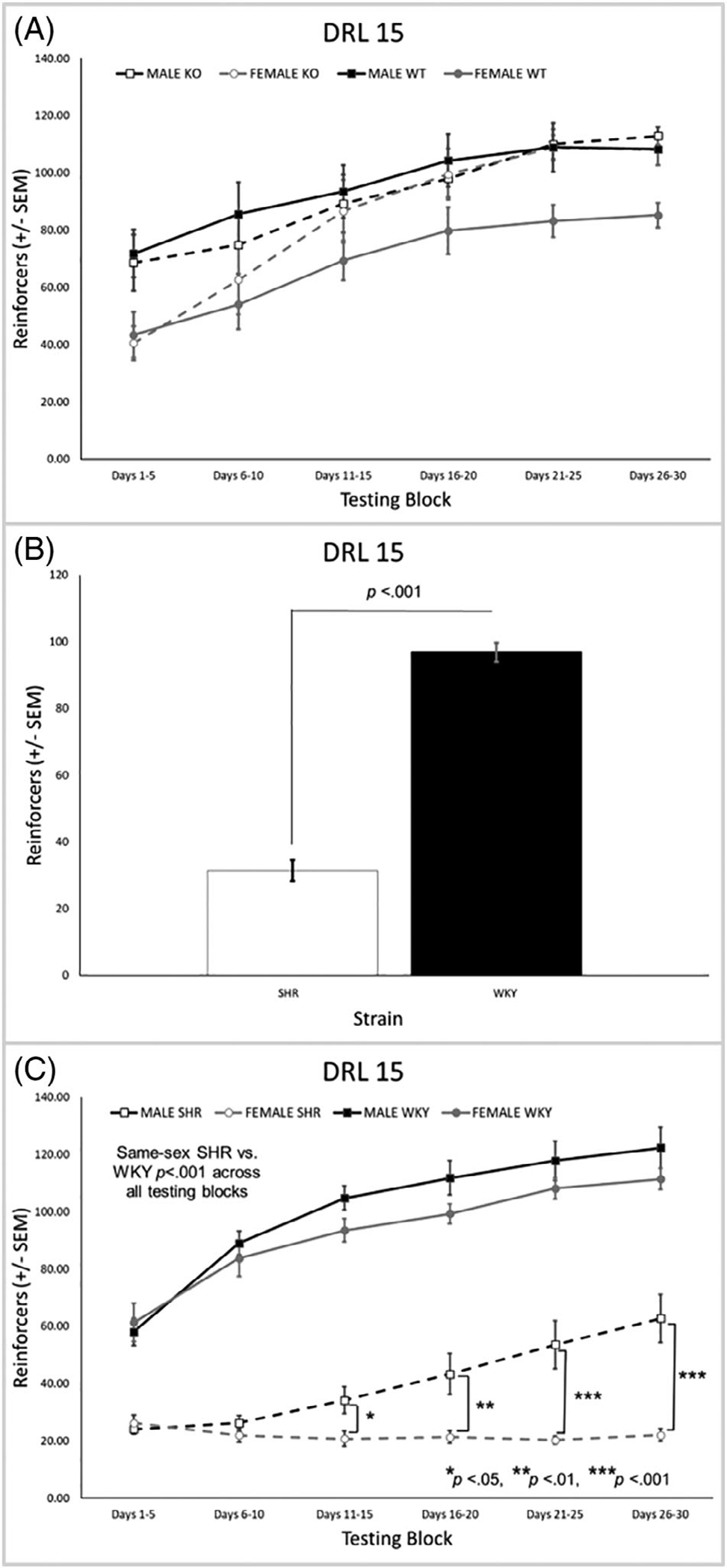
(A) There were no differences between the Lphn3 KO and WT rats on reinforcers earned, but there was a significant main effect of sex with males earning more than females (p = 0.015). (B) SHRs earned fewer reinforcers overall than WKY rats. (C) SHR males earned significantly fewer reinforcers than WKY males with a similar effect in the females. In addition, SHR females earned fewer reinforcers than SHR males in blocks 3–6. DRL, differential reinforcement of low rates; KO, knockout; SEM, standard error of the mean; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto; WT, wildtype
3.1.3 |. Total number of trials completed
There was a significant main effect of genotype [F(1, 31) = 19.22, p < 0.001] and a significant genotype × block interaction [F(2.4, 73.8) = 3.15, p = 0.040]. KO rats completed a higher number of trials than the WT rats overall (Figure 3A) and in all testing blocks (Figure 3B). There was also a significant main effect of sex [F(1, 31) = 4.83, p = 0.036] where females completed a significantly higher number of trials than males [data not shown]. For the SHR/WKY rats, there were significant main effects of strain [F(1, 44) = 162.8, p < 0.001] and sex [F(1, 44) = 12.61, p = 0.001] as well as significant strain × sex [F(1, 44) = 10.16, p = 0.003] and strain × block [F(2.3, 101.4) = 8.24, p < 0.001] interactions. SHRs completed significantly more trials than WKY rats overall (Figure 3C)–an effect that was present in each testing block (Figure 3D) and evident for each sex (Figure 3E). Females completed significantly more trials than males overall, particularly SHR females versus SHR males (Figure 3E).
FIGURE 3.
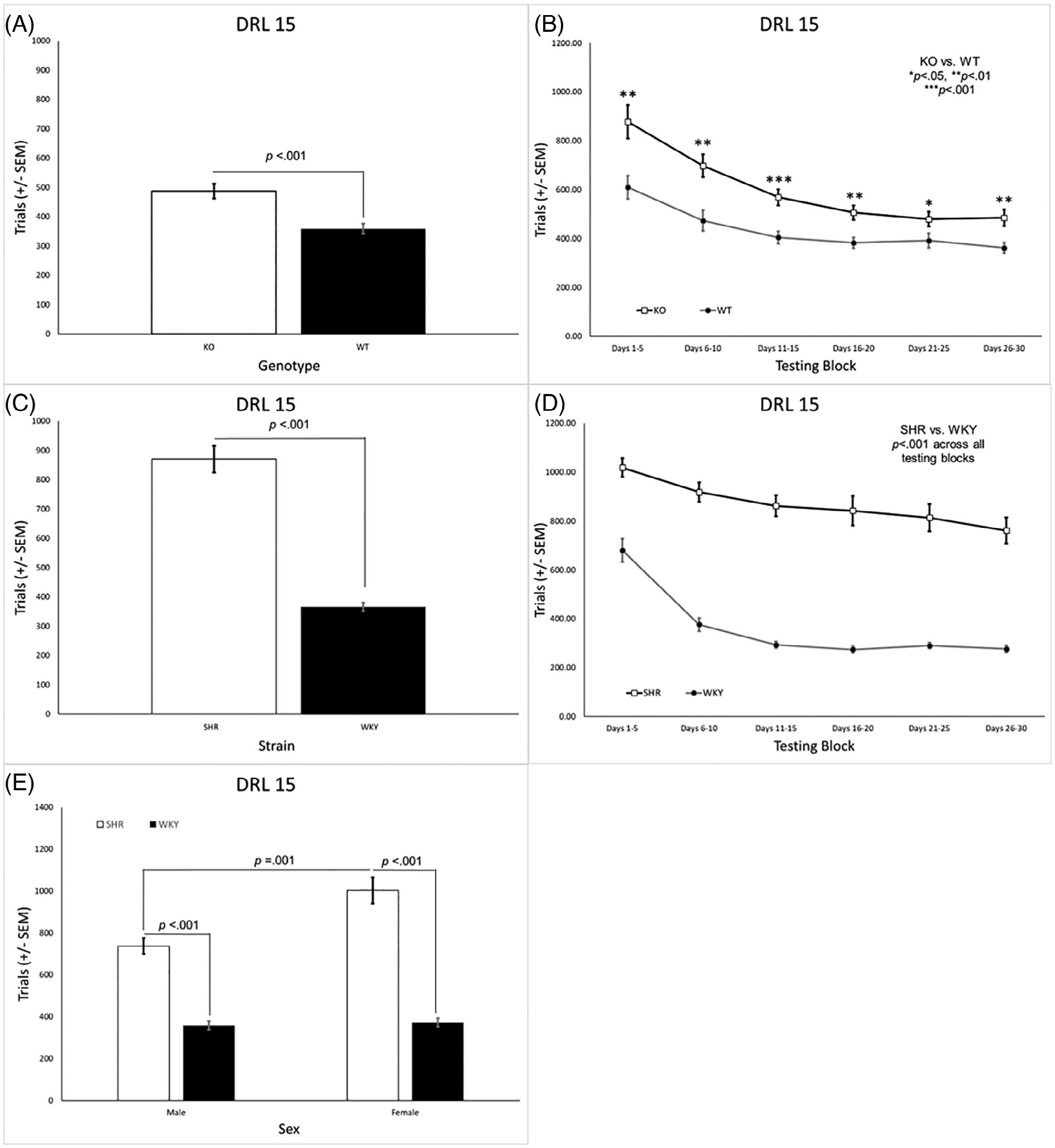
(A) Lphn3 KO rats completed a significantly higher number of trials than the WT rats overall. (B) The difference between Lphn3 KO and WT rats was present in all testing blocks. (C) SHRs completed significantly more trials then WKY rats. (D) The difference between SHR and WKY rats was present in all testing blocks. (E) SHR males and females completed more trials than their same-sex WKY counterparts. SHR females also completed more trials than SHR males. DRL, differential reinforcement of low rates; KO, knockout; SEM, standard error of the mean; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto; WT, wildtype
3.1.4 |. Response pattern analysis
The genotype × IRT [F(2.2, 67.6) = 9.59, p < 0.001] and genotype × sex × IRT [F(2.2, 67.6) = 3.68, p = 0.027] interactions were significant. Post hoc analyses done separately for each sex revealed a significant genotype × IRT interaction in the females (p < 0.001) but not males (Figure 4A,B, respectively). Compared with WT females, KO females exhibited a significantly higher proportion of burst responses in the shortest IRT bin (i.e., <2.5 s), but a lower proportion of responses in IRT bins ranging from 7.5–15.0 s.
FIGURE 4.
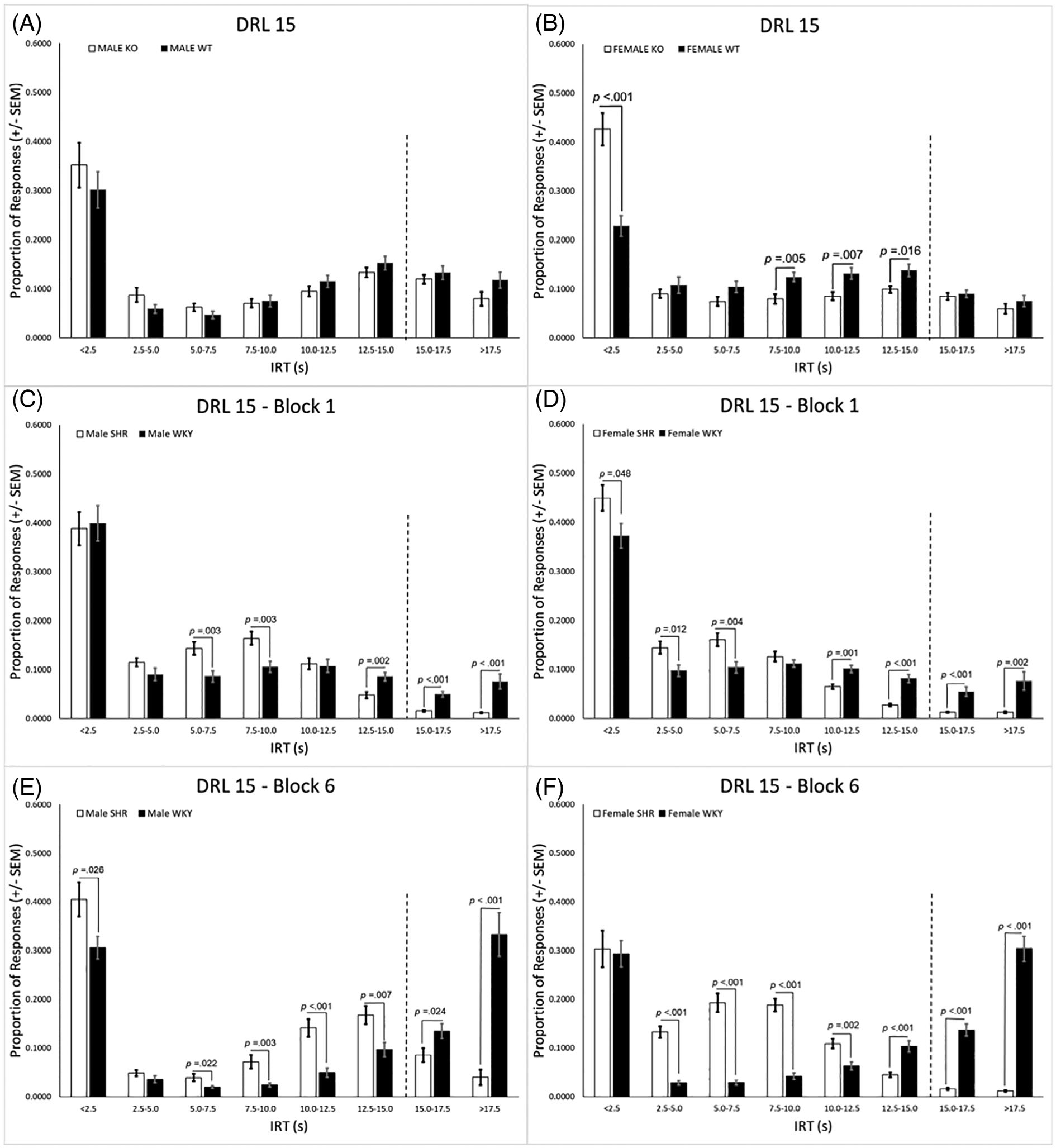
(A) The genotype x IRT interaction was not significant in the males. (B) Compared with WT females, KO females exhibited a significantly higher proportion of burst responses in the shortest IRT bin, but a lower proportion of responses in bins ranging from 7.5–15.0 s. (C) In block 1, SHR males had a higher proportion of responses in intermediate IRT bins ranging from 5.0–10.0 s, but a lower proportion of responses in IRT bins greater than 12.0 s when compared with WKY males. (D) SHR females in block 1 had a significantly higher proportion of responses than WKY females in the IRT bins that were less than 7.5, but a lower proportion of responses in IRT bins greater than 10.0 s. (E) In block 6 the SHR males exhibited a significantly higher proportion of responses than WKY males in all but one of the IRT bins less than 15.0 s, as well as a lower proportion of responses in both bins greater than 15.0 s. (F) SHR females in block 6 exhibited a significantly higher proportion of responses than WKY females in all bins ranging from 2.5–12.5 s, but a significantly lower proportion in the three longest IRT bins. DRL, differential reinforcement of low rates; KO, knockout; SEM, standard error of the mean; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto; WT, wildtype
Response pattern analysis in the SHR/WKY rats revealed significant interactions of strain × block [F(1, 44) = 12.56, p = 0.001], strain × IRT [F(3.6, 160.4) = 40.17, p < 0.001], strain × sex × IRT [F(6.6, 160.4) = 6.59, p < 0.001], strain × block × IRT [F(3.8, 165.0) = 26.37, p < 0.001], and strain × sex × block × IRT [F(3.8165.0) = 3.28, p = 0.015]. Separate post hoc analyses were conducted for each sex and testing block. A significant genotype × IRT interaction was found in both the males (p = 0.046) and females (p < 0.001) in block 1. Compared with WKY males, SHR males had a higher proportion of responses in intermediate IRT bins ranging from 5.0–10.0 s, but a lower proportion of responses in longer IRT bins ≤12.0 s (Figure 4C). SHR females in block 1 had a significantly higher proportion of responses than WKY females in all IRT bins <7.5 s, but a lower proportion of responses in IRT bins ≤10.0 s (Figure 4D). A significant genotype × IRT interaction was also found for the males (p < 0.001) and females (p < 0.001) in block 6. The SHR males had a significantly higher proportion of responses than WKY males in all but one of the IRT bins <15.0 s, as well as a lower proportion of responses in bins >15.0 s (Figure 4E). A similar trend in block 6 was observed in the SHR females who had a significantly higher proportion of responses than WKY females in all bins ranging from 2.5–12.5 s, but a significantly lower proportion in the three longest IRT bins ≥12.5 s; Figure 4F).
3.1.5 |. Peak deviation analysis
The negative exponential curves and the “debursted” relative proportion of responses for the pause IRTs (i.e., IRTs >2.5 s) for each group, as well as graphs showing the difference curves for the different genotypes and different strains used for peak deviation analysis [see 62, 63], are included in Supplemental Figure 7. Only responses that were < 2.5 s were considered burst responses, while all others were considered pause responses. The analyses for burst ratio revealed a significant main effect of genotype [F(1, 31) = 4.55, p = 0.041]. The Lphn3 KO rats exhibited a significantly higher burst ratio than the WT rats (Figure 5A). Although the genotype × sex interaction was not significant [F(1, 31) = 1.60, p = 0.215], visual inspection of the data suggests the genotype main effect was driven primarily by the very low burst ratio in the WT females. The SHRs also had a higher burst ratio than the WKY rats (Figure 5B), although the main effect for strain did not quite exceed the criterion for significance [F(1, 44) = 3.89, p = 0.055]. Again, the effect of strain appears to be impacted by sex, (i.e., the SHR males had a higher burst ratio than the other groups), but the strain × sex interaction was not significant [F(1, 44) = 1.44, p = 0.237]. There were no significant genotype-related effects for peak location or peak area (Figure 5C, E). There were, however, strain-related effects on both of these parameters. For peak location, there was a main effect of strain [F(1, 44) = 305.35, p < 0.001], a main effect of sex [F(1, 44) = 76.337, p < 0.001], and a strain × sex interaction [F(1, 44) = 64.145, p < 0.001]. As seen in Figure 5D, the peak location of the SHR males and females occurred sooner than that of their same-sex WKY counterparts (p < 0.001 in both cases). In addition, the peak location for the SHR females occurred sooner than it did for the SHR males (p < 0.001). There was a similar trend in the SHR/WKY rats for peak area, but the strain × sex interaction was not significant [F(1, 44) = 2.48, p = 0.122]. Rather, there were only main effects for strain [F(1, 44) = 87.587, p < 0.001] and sex [F(1, 44) = 8.34, p = 0.006], which was lower in the SHRs and the females (Figure 5F).
FIGURE 5.
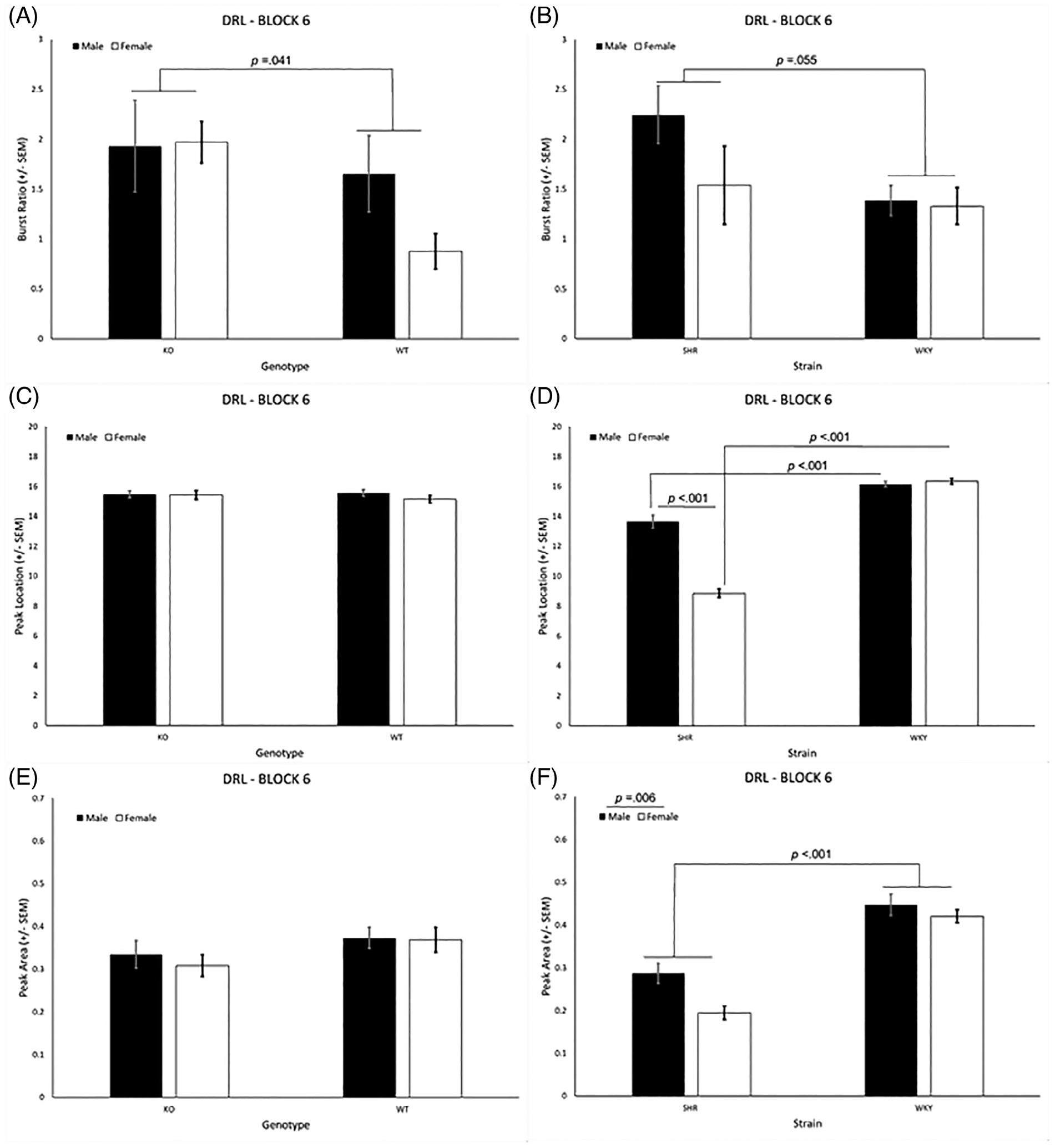
(A) The Lphn3 KO rats exhibited a significantly higher burst ratio than the WT rats. (B) The SHRs exhibited a significantly higher burst ratio than the WKY rats. (C) Genotype did not affect peak location. (D) Peak location occurred sooner in the SHR males and females compared with their same-sex WKY counterparts. In addition, the peak location of SHR females occurred earlier than for SHR males. (E) Genotype did not affect peak area. (E) The peak area was larger in the WKY rats than the SHRs. DRL, differential reinforcement of low rates; KO, knockout; SEM, standard error of the mean; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto; WT, wildtype
3.2 |. Delayed spatial alternation
3.2.1 |. Percent correct
Analysis of the percent correct during DSA revealed a significant main effect of genotype [F(1, 31) = 11.48, p = 0.002], as well as a significant genotype × delay [F(1.7, 52.6) = 7.14, p = 0.003] and genotype × delay × block [F(6.9, 213.6) = 2.93, p = 0.006] interaction. The WT rats had a higher overall percent correct than the KO rats (Figure 6A). Post hoc analysis revealed no genotype differences at the 0 s delay (see Figure 5B). With a 5 s delay, however, WT rats had a higher percentage correct than KO rats during the second and third testing blocks. Increasing the delay to 10 s, resulted in a significant genotype difference during all testing blocks. Increasing the delay even more to 20 s, caused both groups to perform at chance levels during the first testing block where the KO rats remained for the remaining blocks. The WT rats, however, improved such that they were performing significantly better than the KO rats as early as the second testing block.
FIGURE 6.
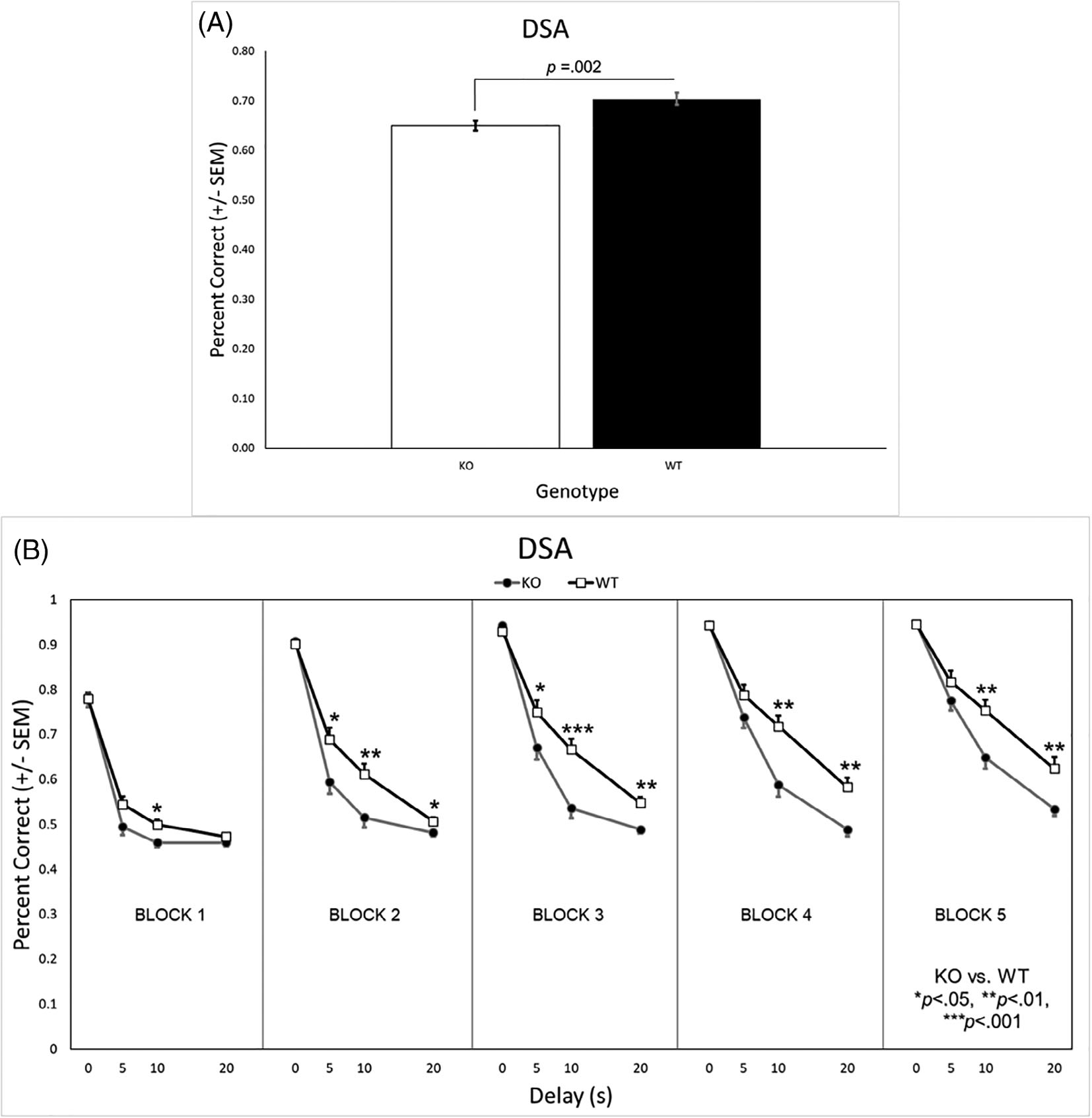
(A) WT rats had a significantly higher overall percentage correct on the DSA task that KO rats. (B) There were no differences on the DSA task when there was no delay. However, at the 5 s delay, WT rats had a higher percentage correct than KO rats during the second and third testing blocks (days 6–15) and when the delay was 10 s, a genotype difference was present during all testing blocks. While both genotypes were performing at chance during the first testing block (i.e., acquisition), when the delay was 20 s, the WT rats improved and were performing significantly better than the KO rats by the second testing block (days 6–10). DSA, delayed spatial alternation; KO, knockout; SEM, standard error of the mean; WT, wildtype
Similar analysis in the SHR/WKY rats revealed a significant main effect of strain [F(1, 42) = 57.00, p < 0.001], as well as significant strain × block [F(2.4, 98.9) = 8.65, p < 0.001], strain × delay [F(2.2, 90.7) = 3.49, p = 0.031], and strain × delay × block [F(6.9, 292.2) = 9.74, p < 0.001] interactions. The WKY rats had a higher overall percent correct than the SHRs (Figure 6A). Post hoc analysis revealed the WKY rats outperformed the SHRs during all blocks when the delay was 0 s (see Figure 7B). When there was a 5, 10, or 20 s delay, the WKY rats had a higher percentage correct than SHRs in all but the first testing block.
FIGURE 7.
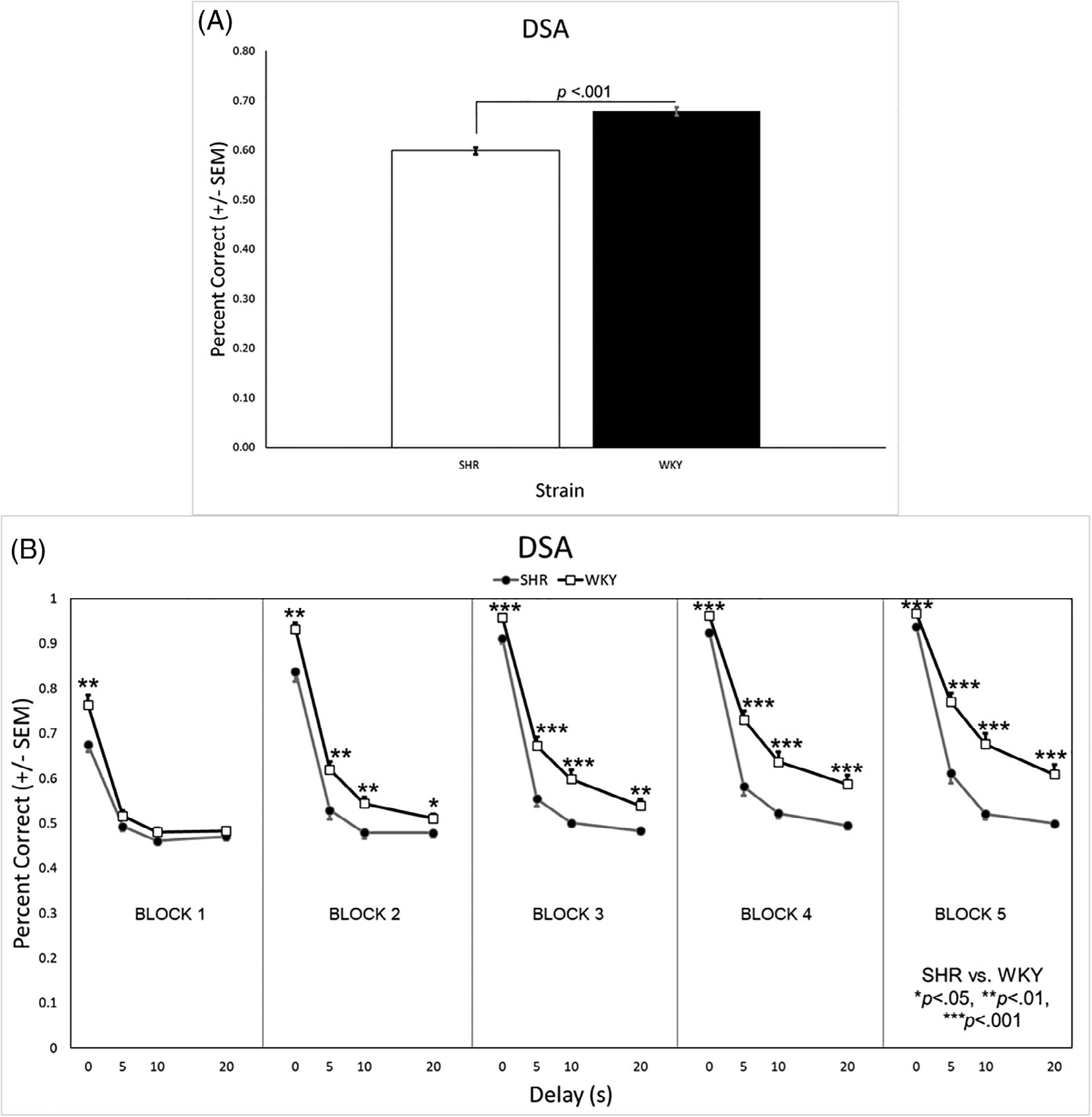
(A) WKY rats had a significantly higher overall percentage correct on the DSA task than SHRs. (B) The WKY rats had a higher percent correct across all blocks when the delay was 0 s. At the 5 s, 10 s, and 20 s delays, the WKY rats had a higher percent correct in all but the first testing block. DSA, delayed spatial alternation; SHR, spontaneously hypertensive rat; SEM, standard error of the mean; WKY, Wistar-Kyoto
3.2.2 |. Trial-by-trial analyses
Analysis of win-stay errors in the first and last testing blocks revealed significant main effects of genotype [F(1, 31) = 11.125, p = 0.002] and sex [F(1, 31) = 5.271, p = 0.029] as well as significant genotype × block [F(1, 31) = 11.223, p = 0.002], sex × block [F(1, 31) = 5.091, p = 0.031], and genotype × sex × block [F(1, 31) = 4.682, p = 0.038] interactions. KO rats had significantly more win-stay errors overall, but this was primarily due to a difference between the male KO and WT rats during the last testing block (days 21–25; Figure 8A). Analysis of lose-stay errors revealed a significant main effect of genotype [F(1, 31) = 5.497, p = 0.026] and a significant sex × block interaction [F(1, 31) = 7.591, p = 0.010]. KO rats committed more lose-stay errors overall (Figure 8B). In addition, while the number of lose-stay errors was similar for males and females during block 1 (40.9 ± 1.9 and 40.1 ± 1.3, respectively), males made significantly fewer lose-stay errors than females during block 5 (9.9 ± 1.4 and 15.8 ± 1.5, respectively).
FIGURE 8.
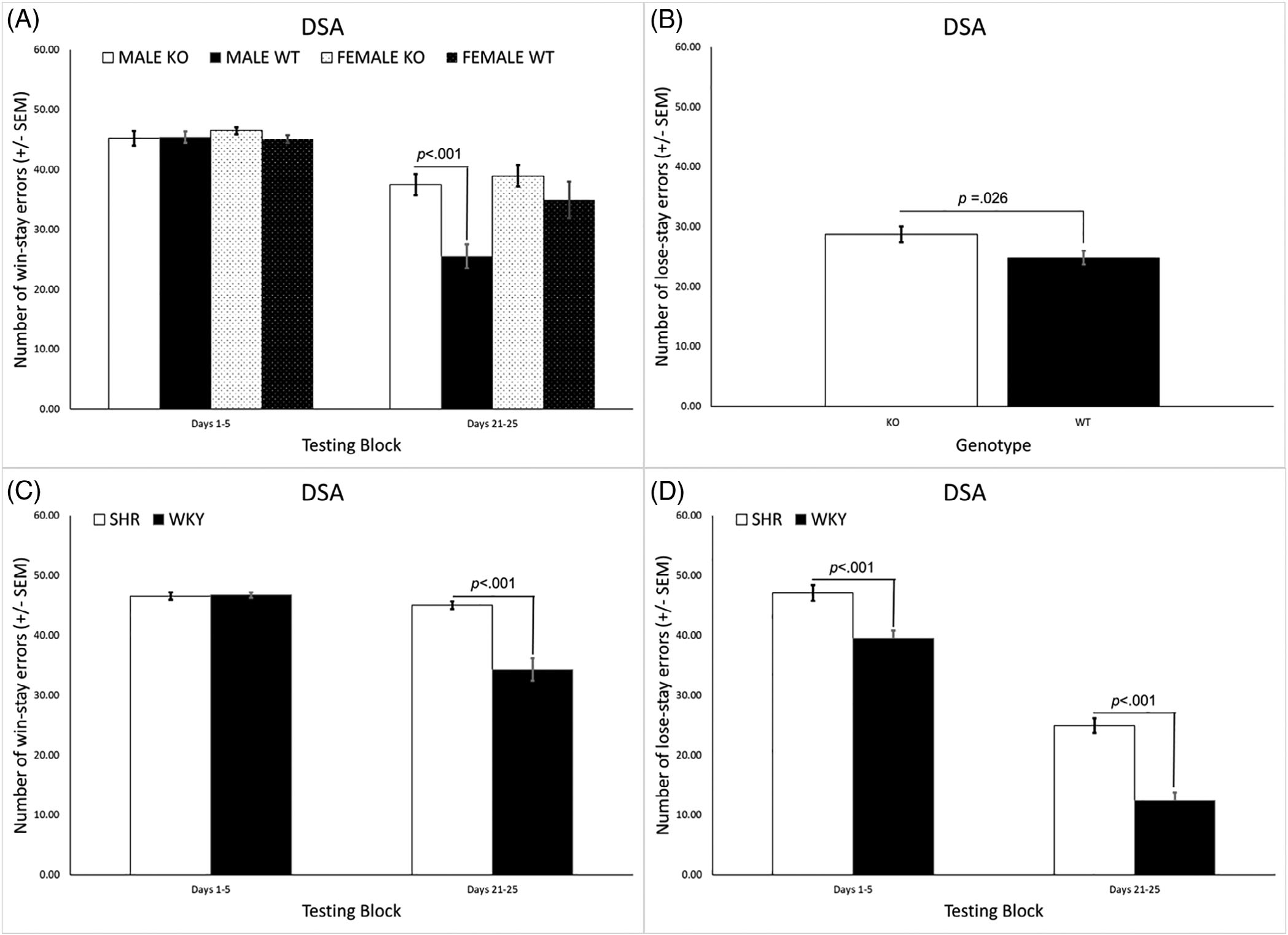
(A) KO rats committed a significantly larger overall number of win-stay errors compared with WT rats but this was primarily due to a difference between the male KO and WT rats during the last testing block (days 21–25). (B) KO rats also committed a significantly larger overall number of lose-stay errors compared with WT rats. (C) The SHRs had significantly more win-stay errors overall, primarily due to a difference from WKY rats during the last testing block (days 21–25). (D) The SHRs committed more lose-stay errors overall and during both the first and last testing block, with the difference from the WKY rats being larger during the latter (days 21–25). DSA, delayed spatial alternation; SHR, spontaneously hypertensive rat; SEM, standard error of the mean; WKY, Wistar-Kyoto
In the SHR/WKY rats the analysis of win-stay errors revealed significant main effects of strain [F(1, 42) = 27.064, p < 0.001] and sex [F(1, 42) = 4.750, p = 0.035] and a significant strain × block interaction [F(1, 42) = 26.484, p < 0.001]. SHRs had significantly more win-stay errors overall, primarily due to a difference from WKY rats during the last testing block (days 21–25; Figure 8C). Analysis of lose-stay errors revealed a main effect of strain [F(1, 42) = 61.398, p < 0.001] and a significant strain × block interaction [F(1, 42) = 4.4085, p = 0.042]. SHRs committed more lose-stay errors overall and during both the first and last testing block, with the difference from the WKY rats being larger during the last testing block (Figure 8D).
4 |. DISCUSSION
Evidence of behavioral disinhibition was present in both the Lphn3 KO rats as well as the SHRs. Relative to their controls, Lphn3 KO rats and SHRs exhibited impaired inhibitory control performance during the DRL tasks due to a higher proportion of responses with short IRTs that was accompanied by a lower proportion of responses with longer IRTs, the latter of which were necessary to be earn a reinforcer. Likewise, the burst ratio was higher in the Lphn3 KO rats and SHRs, although these effects appeared to be differentially modulated by sex. In the case of genotype, the effect was driven by the lower burst ratio in the female WT rats, while for strain, the SHR males had a higher burst ratio than the other groups. Looking across DRL 15 testing blocks, the SHRs appear to be more profoundly affected than the Lphn3 KO rats (Figure 1A, C). This interpretation is based not only on the smaller KO vs. WT difference (Figure 1B) in comparison with the SHR vs. WKY difference (Figure 1D), but also by directly comparing the ratio of reinforced: nonreinforced responses of the KO rats to the SHRs (white bars in Figures 1B, D). Furthermore, the Lphn3 KO rats were able to earn an equal number of reinforcers as WT rats during DRL 15 by compensating for their decreased efficiency by completing a larger number of trials. This was not the case for the SHRs, who also had decreased efficiency, but were not able to earn an equivalent number of reinforcers even when completing more trials. In other words, while the Lphn3 KO rats were able to work harder to overcome their efficiency deficit, the SHR rats – especially the females - were not. Lastly, no genotype effects were seen for peak location or peak area, while the peak location was earlier and peak area was lower in the SHRs, especially the SHR females.
Working memory deficits were also present in both the Lphn3 KO rats and SHRs, with the relative degree of impairment again being greater in the SHRs. During NCA (Supplemental Figure 9) and the 0 s delay of DSA, the Lphn3 KO rats did not differ from the WT rats. Rather, persistent differences between the genotypes were evident only during the longer 10 and 20 s DSA delays (Figure 6). This was not the case for the SHRs, who had significantly poorer performance than the WKY rats across all delays (0, 5, 10, and 20 s) of DSA (Figure 7) and even during NCA (Supplemental Figure 9). This discrepancy is also apparent by directly comparing overall DSA performance of the Lphn3 KO rats with the SHRs (white bars in Figures 6A, 7A).
5 |. RULING OUT A MOTOR IMPAIRMENT
Importantly, the DRL and DSA impairments exhibited by the Lphn3 KO rats and SHRs do not represent a motor impairment. During DRH, when the response requirement was high, the Lphn3 KO rats and SHRs were not impaired. In fact, the Lphn3 female KO rats earned more reinforcers than the WT females. Similarly, the SHRs (no sex difference) earned more reinforcers and exhibited greater DRH efficiency than the WKY rats (Supplemental Figure 1). Likewise, during CA (Supplemental Figure 8) the Lphn3 KO rats and SHRs were able to alternate levers by following the cue light just as effectively as their WT and WKY counterparts, respectively.
5.1 |. Response acquisition versus steady-state responding
During DRL, the difference in the ratio of reinforced: nonreinforced trials between the Lphn3 KO and WT rats was relatively consistent across testing blocks (Figure 1A) such that the difference between the strains was manifested only as a main effect of genotype (Figure 1B). In other words, the genotypes learned at a similar rate across blocks, but the WT rats performed consistently better. The ratio of reinforced: nonreinforced trials for the WKY rats rose sharply across testing blocks compared with the SHRs who showed only a modest increase in performance across testing blocks. Steady-state performance of the WKY rats was much higher than the SHRs as well. Said differently, the WKY rats learned to complete the DRL 15 task faster and better than the SHRs (Figure 1C) yielding higher performance overall (Figure 1D).
The above pattern was also prevalent during DSA. During the first block, both the Lphn3 KO and WT rats were at (or slightly above) chance performance when any delay (5, 10, or 20 s) occurred. Both genotypes improved across testing blocks when presented with the 5, 10, and 20 s delays, with the relative difference observed between the WT and KO rats being fairly consistent across blocks (Figure 6B). Again, there was no obvious difference in the rate of learning between the genotypes, but there was a large discrepancy in how well the two genotypes learned the task. On the other hand, the WKY rats increased their performance across testing blocks to a greater degree than the SHRs, whose performance did not change much across testing blocks, particularly at the longer delays (Figure 7B). As was the case during DRL, SHRs exhibited both acquisition and steady-state performance deficits on DSA compared with their WKY control strain.
5.2 |. Utility of the Lphn3 KO rat as an animal model of ADHD
Like the SHRs, the Lphn3 KO rats exhibited deficits in inhibitory control and working memory, albeit the relative degree of impairment was greater in the SHRs. Inhibitory control and working memory are part of a collection of top-down, higher-order cognitive processes known as executive functions (EF).66 Attention is also an EF,66 that is often impaired in individuals with ADHD.67 Notably, ADHD is characterized by two primary presentations - hyperactivity/impulsivity and inattention – which can occur individually or together, thereby resulting in three unique ADHD sub-types: hyperactive/impulsive, inattentive, or combined.1 The SHRs appear to represent the combined ADHD sub-type as they are hyperactive, impulsive, and inattentive. 57,68 On the other hand, data collected to date, including the current study and others that have used Lphn3 KO models,35–38,69,70 only provide evidence for the hyperactive/impulsive subtype. Direct measures of attention are limited, but suggest it is not affected following Lphn3 deletion. For example, Mortimer et al.35 used a touchscreen operant response to assess Lphn3 KO and WT mice on a continuous performance task. If mice pressed the screen when the target was presented, they received a food reinforcer and the response was classified as a “hit”. If they failed to press when the target was presented (i.e., they omitted a trial), this response was classified as a “miss.” If the Lphn3 KO mice had an attentional deficit, this would have increased the number of misses. This was not the case, however, as Mortimer and colleagues reported that the hit rate (i.e., hits / [hits + misses]) did not differ between the genotypes.
The current study provides indirect evidence on attention. While not a direct measure of attention, the NCA task has a minimal working memory requirement and can provide cursory information about the ability to “stay on task.” While there were no effects of genotype on NCA performance, this was not the case in the SHRs who exhibited a performance decrement across all 10 days of NCA (see Supplemental Figure 8). Ongoing research is assessing attention in SHRs and Lphn3 KO rats more directly and comparing their data with WKY and Lphn3 WT rats to better assess whether the two former exemplify different ADHD subtypes.
5.3 |. Lphn3, executive function, and catecholamines
Proper modulation of catecholamine neurotransmission is essential for guiding optimal prefrontal cortex (PFC) function – the neural structure that gives rise to EF.71 Lphn3 gene expression affects catecholamine neurotransmission. Research on zebrafish larvae has demonstrated down-regulation of lphn3.1 (one of the two zebrafish lphn3 orthologs) resulted in a decrease and misplacement of dopamine (DA) (but not norepinephrine or serotonin) neurons,38 as well as decreased locomotor sensitivity to DA agonists and antagonists.70 The hyperactivity seen in lphn3.1 mutant larvae was rescued by the commonly prescribed ADHD catecholamine-regulating medications: methylphenidate and atomoxetine.38 Compared with WT mice, Lphn3 mutant mice had increased differential gene expression of DA receptor D4 (DRD4), serotonin receptor 2a (5-HT2A), the serotonin transporter (5-HTT), and tyrosine hydroxylase (TH; required for the synthesis of DA) on P0, as well as increased striatal DA and serotonin when 4–6 weeks of age.36 In Lphn3 KO rats,37 no changes in the levels of DA, norepinephrine, serotonin, or their major metabolites were seen in striatum, hippocampus, or PFC when measured by HPLC. Likewise, the expression of DRD2, DRD4, and the vesicular monoamine transporter (VMAT-2) did not differ between the genotypes. However, TH and aromatic L-amino acid decarboxylase (AADC) were increased, while DRD1 was decreased,37 suggesting greater DA and/or NE availability.
In Lphn3 mutant mice and rats, there are differential expression levels of the DA transporter (DAT) gene (Slc6a3) and the protein itself. Adult Lphn3 KO rats have increased DAT expression37 and increased reuptake of DA (i.e., functional implication for increase in DAT)72 in the dorsal striatum. Likewise, adult Lphn3−/− mice demonstrate overexpression of Slc6a3 in whole brain36 and in the dorsal striatum,34 but downregulation of Slc6a3 in the PFC.35,69 DAT expression is associated with ADHD, cocaine addiction, and alcoholism (see ref. [73] for review] making these results noteworthy.
The exact mechanism whereby alterations in Lphn3 gene expression alters catecholamine neurotransmission is still being investigated. LPHN3 is an adhesion G-protein coupled receptor (GPCR) that is expressed in the brain.74,75 It is more robustly expressed early in development, while expression decreases as the brain matures.23 The LPHN3 receptor binds endogenous ligand fibronectin and leucine-rich repeat transmembrane protein 3 (FLRT3).76 FLRT3 is involved in cell adhesion, neuronal signaling, and synaptic organization, and disruptions in FLRT3 function lead to reductions in excitatory synapse density and a weakened afferent input.69 The interaction between LPHN3 and FLRT3 plays an important role in transmembrane signaling, Ca2+ mobilization from presynaptic stores, neuronal structure, and synaptic development.69,77,78 The LPHN3 receptor also has the ability to interact with SHANK proteins,79 which is significant as interference in SHANK protein functioning is linked to abnormal structural changes in dendritic spines and excitatory synapses, as well as defects in corticostriatal circuitry.80–82
5.4 |. A Role for Lphn3 in EDs
As previously mentioned, impulsivity is a behavior that is common to EDs including ADHD and SUD1,7,39–41 and deficits in working memory are believed to contribute to impulsivity.42,43 The current results demonstrate Lphn3 expression is involved in regulating response inhibition and working memory. Numerous studies have implicated Lphn3 in the etiology of ADHD.17,20–24,26–32 While less is known about whether Lphn3 contributes to the cause of other EDs, there is some evidence suggesting this is the case.13,25,33,69 Future research in our lab will assess whether differences in drug self-administration (as a proxy measure for SUD) exist between Lphn3 KO and WT rats. Given the high degree of comorbidity1,5–7 and shared heritability12,15 of EDs, as well the role that Lphn3 plays in neurodevelopment, it seems plausible that Lphn3 may be part of a shared mechanism.
Supplementary Material
ACKNOWLEDGMENTS
Appreciation is extended to Donny Ray for assistance with lab animal care and Dr. Karyl Buddington for her excellent veterinary support. This work was supported by bridge funding from The University of Memphis as well as a grant from The University of Memphis Faculty Research Grant Fund, neither of which imply endorsement by the University of the research conclusions. This research was also supported by a Dissertation Completion Award from the Dean of the Graduate School, University of Cincinnati and NIH grant R01 ES032270 (Charles V. Vorhees and Michael T. Williams). All authors declare no conflicts of interest.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.American Psychiatric Association. Disruptive, Impulse-Control, and Conduct Disorders. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199–212. doi: 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou R, Bitsko RH, Blumberg SJ, et al. Mental health surveillance among children–United States, 2005–2011. MMWR Supp. 2013;62:1–35. [PubMed] [Google Scholar]
- 4.Hansen BH, Oerbeck B, Skirbekk B, Petrovski B, Kristensen H. Neurodevelopmental disorders: prevalence and comorbidity in children referred to mental health services. Nord J Psychiatry. 2018;72:285–291. doi: 10.1080/08039488.2018.1444087 [DOI] [PubMed] [Google Scholar]
- 5.Frick PJ, Nigg JT. Current issues in the diagnosis of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Ann Rev Clinic Psychol. 2012;8:77–107. doi: 10.1146/annurev-clinpsy-032511-143150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Personality Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 7.American Psychiatric Association. Substance-Related and Addictive Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 8.Palacio JD, Castellanos FX, Pineda DA, et al. Attention-deficit/hyperactivity disorder and comorbidities in 18 paisa Colombian multigenerational families. J Am Acad Child Adolesc Psychiatry. 2004;43:1506–1515. doi: 10.1097/1501.chi.0000142279.0000179805.dc [DOI] [PubMed] [Google Scholar]
- 9.Babinski LM, Hartsough CS, Lambert NM. Childhood conduct problems, hyperactivity-impulsivity, and inattention as predictors of adult criminal activity. J Child Psychol Psychiatry. 1999;40:347–355. doi: 10.1111/1469-7610.00452 [DOI] [PubMed] [Google Scholar]
- 10.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.1164.1110.1145 [DOI] [PubMed] [Google Scholar]
- 11.Fergusson DM, Boden JM, Horwood LJ. The developmental antecedents of illicit drug use: evidence from a 25-year longitudinal study. Drug Alcohol Dependence. 2008;96:165–177. doi: 10.1016/j.drugalcdep.2008.1003.1003 [DOI] [PubMed] [Google Scholar]
- 12.Barr PB, Dick DM. The genetics of externalizing problems. Curr Top Behav Neurosci. 2020;47:93–112. doi: 10.1007/7854_2019_120 [DOI] [PubMed] [Google Scholar]
- 13.Arcos-Burgos M, Vélez JI, Solomon BD, Muenke M. A common genetic network underlies substance use disorders and disruptive or externalizing disorders. Hum Genet. 2012;131:917–929. doi: 10.1007/s00439-012-1164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24:562–575. doi: 10.1038/s41380-018-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. doi: [DOI] [PubMed] [Google Scholar]
- 16.Jain M, Palacio LG, Castellanos FX, et al. Attention-deficit/hyperactivity disorder and comorbid disruptive behavior disorders: evidence of pleiotropy and new susceptibility loci. Biol Psychiatry. 2007;61:1329–1339. doi: 10.1016/j.biopsych.2006.1306.1026 [DOI] [PubMed] [Google Scholar]
- 17.Jain M, Velez JI, Acosta MT, et al. A cooperative interaction between LPHN3 and 11q doubles the risk for ADHD. Mol Psychiatry. 2012;17:741–747. doi: 10.1038/mp.2011.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcos-Burgos M, Castellanos FX, Pineda D, et al. Attention-deficit/hyperactivity disorder in a population isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am J Human Genetics. 2004;75:998–1014. doi: 10.1086/426154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcos-Burgos M, Muenke M. Toward a better understanding of ADHD: LPHN3 gene variants and the susceptibility to develop ADHD. Atten Defic Hyperact Disord. 2010;2:139–147. doi: 10.1007/s12402-010-0030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta MT, Castellanos FX, Bolton KL, et al. Latent class subtyping of attention-deficit/hyperactivity disorder and comorbid conditions. J Am Acad Child Adolesc Psychiatry. 2008;47:797–807. doi: 10.1097/CHI.1090b1013e318173f318170b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta MT, Swanson J, Stehli A, et al. ADGRL3 (LPHN3) variants are associated with a refined phenotype of ADHD in the MTA study. Mol Genet Genomic Med. 2016;4:540–547. doi: 10.1002/mgg1003.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta MT, Velez JI, Bustamante ML, Balog JZ, Arcos-Burgos M, Muenke M. A two-locus genetic interaction between LPHN3 and 11q predicts ADHD severity and long-term outcome. Transl Psychiatry. 2011;1:e17. doi: 10.1038/tp.2011.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arcos-Burgos M, Jain M, Acosta MT, et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry. 2010;15:1053–1066. doi: 10.1038/mp.2010.1056 [DOI] [PubMed] [Google Scholar]
- 24.Bruxel EM, Salatino-Oliveira A, Akutagava-Martins GC, et al. LPHN3 and attention-deficit/hyperactivity disorder: a susceptibility and pharmacogenetic study. Genes Brain Behav. 2015;14:419–427. doi: 10.1111/gbb.12224 [DOI] [PubMed] [Google Scholar]
- 25.Arcos-Burgos M, Velez JI, Martinez AF, et al. ADGRL3 (LPHN3) variants predict substance use disorder. Trans Psychiatry. 2019;9:42. doi: 10.1038/s41398-41019-40396-41397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang IW, Lim MH, Kwon HJ, Jin HJ. Association of LPHN3 rs6551665 a/G polymorphism with attention deficit and hyperactivity disorder in Korean children. Gene. 2015;566:68–73. doi: 10.1016/j.gene.2015.04.033 [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Zhang Q, Gu X, et al. LPHN3 gene variations and susceptibility to ADHD in Chinese Han population: a two-stage case-control association study and gene-environment interactions. Eur Child Adolesc Psychiatry. 2019;28:861–873. doi: 10.1007/s00787-018-1251-8 [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Sanchez CI, Riveiro-Alvarez R, Soto-Insuga V, et al. Attention deficit hyperactivity disorder: genetic association study in a cohort of Spanish children. Behav Brain Funct. 2016;12:2. doi: 10.1186/s12993-015-0084-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribases M, Ramos-Quiroga JA, Sanchez-Mora C, et al. Contribution of LPHN3 to the genetic susceptibility to ADHD in adulthood: a replication study. Genes Brain Behav. 2011;10:149–157. doi: 10.1111/j.1601-183X.2010.00649.x [DOI] [PubMed] [Google Scholar]
- 30.Martinez AF, Abe Y, Hong S, et al. An Ultraconserved brain-specific enhancer within ADGRL3 (LPHN3) underpins attention-deficit/hyperactivity disorder susceptibility. Biol Psychiatry. 2016;80:943–954. doi: 10.1016/j.biopsych.2016.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puentes-Rozo PJ, Acosta-López JE, Cervantes-Henríquez ML, et al. Genetic variation underpinning ADHD risk in a Caribbean community. Cells. 2019;8(8):907. doi: 10.3390/cells8080907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappel DB, Schuch JB, Rovaris DL, et al. ADGRL3 rs6551665 as a common vulnerability factor underlying attention-deficit/hyperactivity disorder and autism Spectrum disorder. Neuromol Med. 2019;21:60–67. doi: 10.1007/s12017-019-08525-x [DOI] [PubMed] [Google Scholar]
- 33.Liu QR, Drgon T, Johnson C, Walther D, Hess J, Uhl GR. Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes. Am J Med Genet B Neuropsychiatr Genet. 2006;141b:918–925. doi: 10.1002/ajmg.b.30436 [DOI] [PubMed] [Google Scholar]
- 34.Regan SL, Williams MT, Vorhees CV. Latrophilin-3 disruption: effects on brain and behavior. Neurosci Biobehav Rev. 2021;127:619–629. doi: 10.1016/j.neubiorev.2021.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortimer N, Ganster T, O’Leary A, et al. Dissociation of impulsivity and aggression in mice deficient for the ADHD risk gene Adgrl3: evidence for dopamine transporter dysregulation. Neuropharmacology. 2019;156:107557. doi: 10.1016/j.neuropharm.2019.02.039 [DOI] [PubMed] [Google Scholar]
- 36.Wallis D, Hill DS, Mendez IA, et al. Initial characterization of mice null for Lphn3, a gene implicated in ADHD and addiction. Brain Res. 2012;1463:85–92. doi: 10.1016/j.brainres.2012.04.053 [DOI] [PubMed] [Google Scholar]
- 37.Regan SL, Hufgard JR, Pitzer EM, et al. Knockout of latrophilin-3 in Sprague-Dawley rats causes hyperactivity, hyper-reactivity, under-response to amphetamine, and disrupted dopamine markers. Neurobiol Disease. 2019;130:104494. doi: 10.1016/j.nbd.2019.104494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lange M, Norton W, Coolen M, et al. The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol Psychiatry. 2012;17:946–954. doi: 10.1038/mp.2012.29 [DOI] [PubMed] [Google Scholar]
- 39.Kozak K, Lucatch AM, Lowe DJE, Balodis IM, MacKillop J, George TP. The neurobiology of impulsivity and substance use disorders: implications for treatment. Ann NY Acad Sci. 2018;1451:71–91. doi: 10.1111/nyas.13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee RSC, Hoppenbrouwers S, Franken I. A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychol Rev. 2019;29:14–26. doi: 10.1007/s11065-11019-09402-x [DOI] [PubMed] [Google Scholar]
- 41.Vassileva J, Conrod PJ. Impulsivities and addictions: a multidimensional integrative framework informing assessment and interventions for substance use disorders. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180137. doi: 10.1098/rstb.2018.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298 [DOI] [PubMed] [Google Scholar]
- 43.Baskin-Sommers AR, Wallace JF, MacCoon DG, Curtin JJ, Newman JP. Clarifying the factors that undermine behavioral inhibition system functioning in psychopathy. Personal Disord. 2010;1:203–217. doi: 10.1037/a0018950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS. ADHD and working memory: the impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. J Abnorm Child Psychol. 2010;38:149–161. doi: 10.1007/s10802-009-9357-6 [DOI] [PubMed] [Google Scholar]
- 45.Kofler MJ, Soto EF, Fosco WD, Irwin LN, Wells EL, Sarver DE. Working memory and information processing in ADHD: evidence for directionality of effects. Neuropsychology. 2020;34:127–143. doi: 10.1037/neu0000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson LA, Ryan M, Martin RB, et al. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychol. 2011;17:209–224. doi: 10.1080/09297049.2010.532204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasper LJ, Alderson RM, Hudec KL. Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev. 2012;32:605–617. doi: 10.1016/j.cpr.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 48.Jordan CJ, Andersen SL. Working memory and salivary brain-derived neurotrophic factor as developmental predictors of cocaine seeking in male and female rats. Addict Biol. 2018;23:868–879. doi: 10.1111/adb.12535 [DOI] [PubMed] [Google Scholar]
- 49.Gobin C, Shallcross J, Schwendt M. Neurobiological substrates of persistent working memory deficits and cocaine-seeking in the prelimbic cortex of rats with a history of extended access to cocaine self-administration. Neurobiol Learn Mem. 2019;161:92–105. doi: 10.1016/j.nlm.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 50.Hámor PU, Gobin CM, Schwendt M. The role of glutamate mGlu5 and adenosine A2a receptor interactions in regulating working memory performance and persistent cocaine seeking in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2020;103:109979. doi: 10.1016/j.pnpbp.2020.109979 [DOI] [PubMed] [Google Scholar]
- 51.Rao KN, Sentir AM, Engleman EA, et al. Toward early estimation and treatment of addiction vulnerability: radial arm maze and N-acetyl cysteine before cocaine sensitization or nicotine self-administration in neonatal ventral hippocampal lesion rats. Psychopharmacology (Berl). 2016;233:3933–3945. doi: 10.1007/s00213-016-4421-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khurana A, Romer D, Betancourt LM, Hurt H. Working memory ability and early drug use progression as predictors of adolescent substance use disorders. Addiction. 2017;112:1220–1228. doi: 10.1111/add.13792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramey T, Regier PS. Cognitive impairment in substance use disorders. CNS Spectr. 2019;24:102–113. doi: 10.1017/s1092852918001426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vo HT, Schacht R, Mintzer M, Fishman M. Working memory impairment in cannabis- and opioid-dependent adolescents. Subst Abus. 2014;35:387–390. doi: 10.1080/08897077.2014.954027 [DOI] [PubMed] [Google Scholar]
- 55.Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R, Gallegos-Cari A, Castillo C. Spontaneously hypertensive rat (SHR) as an animal model for ADHD: a short overview. Rev Neurosci. 2011;22:365–371. doi: 10.1515/rns.2011.024 [DOI] [PubMed] [Google Scholar]
- 56.Kantak KM, Singh T, Kerstetter KA, et al. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340 [DOI] [PubMed] [Google Scholar]
- 57.Russell VA. Overview of animal models of attention deficit hyperactivity disorder (ADHD). Curr Protoc Neurosci. 2011. Unit 9.35. doi: 10.1002/0471142301.ns0935s54 [DOI] [PubMed] [Google Scholar]
- 58.Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Brain Res Rev. 2003; 42:1–21. doi: 10.1016/S0165-0173(02)00274-6 [DOI] [PubMed] [Google Scholar]
- 59.Gallo EF, Posner J. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. Lancet Psychiatry. 2016;3:555–567. doi: 10.1016/S2215-0366(16)00096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NIH, Public Health Service Policy on Humane Care and Use of Laboratory Animals, NIH, Bethesda, MD, 2015. [Google Scholar]
- 61.Barthelemy OJ, Richardson MA, Cabral HJ, Frank DA. Prenatal, perinatal, and adolescent exposure to marijuana: relationships with aggressive behavior. Neurotoxicol Teratol. 2016;58:60–77. doi: 10.1016/j.ntt.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 62.Richards JB, Sabol KE, Seiden LS. DRL interresponse-time distributions: quantification by peak deviation analysis. J Exp Anal Behav. 1993;60:361–385. doi: 10.1901/jeab.1993.60-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richards JB, Seiden LS. A quantitative interresponse-time analysis of DRL performance differentiates similar effects of the antidepressant desipramine and the novel anxiolytic gepirone. J Exp Anal Behav. 1991;56:173–192. doi: 10.1901/jeab.1991.56-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roegge CS, Widholm JJ, Engeseth NJ, et al. Delayed spatial alternation impairments in adult rats following dietary n-6 deficiency during development. Neurotoxicol Teratol. 2005;27:485–495. doi: 10.1016/j.ntt.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 65.Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. 2nd ed. Lawrence Erlbaum Associates Publishers; 1999. [Google Scholar]
- 66.Diamond A Executive functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- 68.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 69.Orsini CA, Setlow B, DeJesus M, et al. Behavioral and transcriptomic profiling of mice null for Lphn3, a gene implicated in ADHD and addiction. Mol Genet Genomic Med. 2016;4:322–343. doi: 10.1002/mgg3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lange M, Froc C, Grunwald H, Norton WHJ, Bally-Cuif L. Pharmacological analysis of zebrafish lphn3.1 morphant larvae suggests that saturated dopaminergic signaling could underlie the ADHD-like locomotor hyperactivity. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:181–189. doi: 10.1016/j.pnpbp.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Regan SL, Cryan MT, Williams MT, Vorhees CV, Ross AE. Enhanced transient striatal dopamine release and reuptake in Lphn3 knockout rats. ACS Chem Nerosci. 2020;11:1171–1177. doi: 10.1021/acschemneuro.0c00033 [DOI] [PubMed] [Google Scholar]
- 73.Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez AF, Muenke M, Arcos-Burgos M. From the black widow spider to human behavior: Latrophilins, a relatively unknown class of G protein-coupled receptors, are implicated in psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2011;156b:1–10. doi: 10.1002/ajmg.b.31137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreno-Salinas AL, Avila-Zozaya M, Ugalde-Silva P, Hernández-Guzmán DA, Missirlis F, Boucard AA. Latrophilins: a neuro-centric view of an evolutionary conserved adhesion G protein-coupled receptor subfamily. Front Neurosci. 2019;13:700. doi: 10.3389/fnins.2019.00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Sullivan ML, de Wit J, Savas JN, et al. FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron. 2012;73:903–910. doi: 10.1016/j.neuron.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sando R, Jiang X, Südhof TC. Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science. 2019;363:eaav7969. doi: 10.1126/science.aav7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sando R, Südhof TC. Latrophilin GPCR signaling mediates synapse formation. eLife. 2021;10:e65717. doi: 10.7554/eLife.65717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kreienkamp HJ, Zitzer H, Gundelfinger ED, Richter D, Bockers TM. The calcium-independent receptor for alpha-latrotoxin from human and rodent brains interacts with members of the ProS-AP/SSTRIP/Shank family of multidomain proteins. J Biol Chem. 2000;275:32387–32390. doi: 10.1074/jbc.C000490200 [DOI] [PubMed] [Google Scholar]
- 80.Durand CM, Perroy J, Loll F, et al. SHANK3 Mutations identified in autism lead to modification of dendritic spine morphology via an Actin-dependent mechanism. Mol Psychiatry. 2012;17:71–84. doi: 10.1038/mp.2011.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monteiro P, Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18:147–157. doi: 10.1038/nrn.2016.183 [DOI] [PubMed] [Google Scholar]
- 82.Peça J, Feliciano C, Ting JT, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


