Abstract
Objectives
The non-essential amino acids serine, glycine, and alanine, as well as diverse sphingolipid species, are implicated in inherited neuro-retinal disorders and are metabolically linked by serine palmitoyltransferase (SPT), a key enzyme in membrane lipid biogenesis. To gain insight into the pathophysiological mechanisms linking these pathways to neuro-retinal diseases we compared patients diagnosed with two metabolically intertwined diseases: macular telangiectasia type II (MacTel), hereditary sensory autonomic neuropathy type 1 (HSAN1), or both.
Methods
We performed targeted metabolomic analyses of amino acids and broad sphingolipids in sera from a cohort of MacTel (205), HSAN1 (25) and Control (151) participants.
Results
MacTel patients exhibited broad alterations of amino acids, including changes in serine, glycine, alanine, glutamate, and branched-chain amino acids reminiscent of diabetes. MacTel patients had elevated 1-deoxysphingolipids but reduced levels of complex sphingolipids in circulation. A mouse model of retinopathy indicates dietary serine and glycine restriction can drive this depletion in complex sphingolipids. HSAN1 patients exhibited elevated serine, lower alanine, and a reduction in canonical ceramides and sphingomyelins compared to controls. Those patients diagnosed with both HSAN1 and MacTel showed the most significant decrease in circulating sphingomyelins.
Conclusions
These results highlight metabolic distinctions between MacTel and HSAN1, emphasize the importance of membrane lipids in the progression of MacTel, and suggest distinct therapeutic approaches for these two neurodegenerative diseases.
Keywords: Amino acids, Sphingolipids, Macular telangiectasia, Retinopathy, Peripheral neuropathy, HSAN1
Highlights
-
•
Comprehensive quantification of sphingolipids & amino acids in human sera, including two rare neuropathies.
-
•
The retinopathy, MacTel, is a metabolic disease of impaired amino acid metabolism, mirroring aspects of diabetes.
-
•
Serine and glycine impact sphingolipid metabolism via multiple mechanisms.
-
•
Reduced membrane sphingomyelin and glycosphingolipids in circulation are associated with MacTel.
-
•
Opposing amino acid and sphingolipid changes in MacTel and HSAN1 suggest distinct therapeutic approaches.
1. Introduction
Altered amino acid metabolism is increasingly appreciated as a key driver in the pathology of multiple diseases, including metabolic syndrome, cancer, and neurological disease. Sphingolipids (SLs) are synthesized from serine and fatty acyl-CoAs by serine palmitoyltransferase (SPT) and are critical signaling molecules and membrane components that are enriched in the nervous system and retina. When serine levels are low, alanine (or glycine) is used as a substrate by SPT to yield non-canonical 1-deoxysphingolipids (doxSLs) that drive neuropathy and cellular dysfunction through diverse mechanisms. This highlights a potential mechanism for crosstalk between amino acid metabolism and SL biosynthesis in the context of neurological dysfunction [1]. Numerous heritable neurological and retinal disorders are causative or linked to mutations in genes encoding SL-metabolizing enzymes, including amyotrophic lateral sclerosis (ALS), Tay-Sachs, Niemann-Pick disease, Gaucher disease, Macular telangiectasia type II (MacTel), and hereditary sensory and autonomic neuropathy type 1 (HSAN1) [2].
MacTel is a rare (∼1:10,000) retinal degenerative disease leading to impairment of central vision and marked by reduced circulating serine and glycine levels and altered SL abundances [3,4]. In addition to common single nucleotide polymorphisms associated with serine and amino acid metabolism, a small fraction of MacTel cases (<3.5%) are driven by rare heterozygous coding variants in 3-phosphoglycerate dehydrogenase (PHGDH) or the SPT long chain subunits SPTLC1 and SPTLC2 [[5], [6], [7]]. HSAN1 is an ultra-rare (1:500,000) autosomal dominant disease caused by either SPTLC1 or SPTLC2 variants that induce preferential use of alanine over serine, which promotes doxSL accumulation and drives peripheral sensorimotor neuropathy [8]. Although clinical analyses of 25 HSAN1 patients showed high penetrance of MacTel in this population, more recent studies have identified HSAN1 patients lacking macular defects [9], suggesting the biochemical links between these diseases are more complex than first anticipated.
Altered amino acid and ceramide levels are also linked to more common diseases like type 2 diabetes (T2D), heart disease, cancer, multiple sclerosis, and Alzheimer's disease [2,[10], [11], [12], [13], [14], [15]]. Therefore, detailed analyses of amino acids and SL species in patients with such rare diseases may shed light on the molecular driver(s) of pathogenesis and biochemical regulation relevant to MacTel, HSAN1, and more common diseases. To gain insight into the role of amino acids and ceramides in cellular health and disease, we performed a comprehensive metabolic analysis of unaffected controls, MacTel and HSAN1 patient sera (151 Controls, 205 MacTel, and 25 HSAN1), quantifying SLs, doxSLs, and amino acids via targeted mass spectrometry. Given the centrality of serine in sphingolipid metabolism, we hypothesized that SLs beyond doxSLs would be altered in MacTel and that these lipids might distinguish HSAN1 patients with and without MacTel. Our results suggests that defects in both amino acid and membrane SL homeostasis may be critical for progression of both these diseases.
2. Methods
2.1. Study design and study population
To investigate the levels of amino acids and SLs, fasted serum was collected from MacTel and HSAN1 patients and control participants. Each sample was quantified for amino acids using gas chromatography–mass spectrometry (GC–MS) and a broad panel of SLs via liquid chromatography–mass spectrometry (LC–MS/MS) (Supplemental Table 1).
All study participants (including MacTel, HSAN1, and unaffected Controls) read and signed informed consent forms approved by local Institutional Review Boards, and all clinical research complied with the Declaration of Helsinki and HIPAA privacy regulations. Fasted serum samples were collected between 2016 and 2020 at six registered MacTel clinical sites (Scripps Health, La Jolla CA USA; Moran Eye Center, Salt Lake City UT USA; Moorfields Eye Hospital, London UK; Centre for Eye Research, Melbourne VIC AUS; Save Sight Institute, Sydney NSW AUS; University of Bonn, Bonn GER). Clinical diagnoses of MacTel and HSAN1 were made by experienced retina and neuropathy specialists and were subsequently confirmed by masked readers at the Moorfields Eye Hospital MacTel Reading Centre in London. Diagnoses of HSAN1 was confirmed by Sanger sequencing of the SPT gene. Unaffected probands without any signs of retinal disease served as Controls.
2.2. Serum metabolite quantification
2.2.1. Amino acid GC-MS analysis
In parallel to the SL extraction, 10 μL of patient or control serum was aliquoted and extracted as previously described [16]. Briefly, 10 μL of diluted Cambridge Isotopes internal standard mix (MSK-A2-1.2) was added, followed by 80 μL of methanol. Samples were vortexed for 30 s, then centrifuged at 21,000 g for 10 min at 4 °C. 80 μL of supernatant was collected and evaporated to dryness in a 4 °C centrivap. Dried polar metabolites were derivatized in 2% (w/v) methoxyamine hydrochloride in pyridine and incubated at 45 °C for 60 min. Samples were then silylated with N-tertbutyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) with 1% tert-butyldimethylchlorosilane (tBDMCS) (Regis Technologies) at 45 °C for 30 min. Polar derivatives were analyzed by GC-MS using a DB-35MS column (30 m × 0.25 mm i.d. × 0.25 μm, Agilent J&W Scientific) installed in an Agilent 7890 B gas chromatograph (GC) interfaced with an Agilent 5977 A MS with an XTR ion source. The MS source was held at 230 °C and the quadrupole at 150 °C with helium as the carrier gas. The GC oven was held at 100 °C for 2 min, increased to 300 °C at 10 °C/min, held at 300 °C for 4 min, then held at 325 °C for 3 min.
2.2.2. Sphingolipid LC–MS/MS analysis
Human sera were randomly assigned across eight batches for extraction. Each batch contained 50 patient samples and 11 quality control serum samples. Batches were not significantly different in terms of patient age or sex.
Samples were thawed in a 4 °C cold room and vortexed quickly to remove gradients. 100 μL of patient or control serum was aliquoted, and 20 μL of I.S. mix (Supplemental Table 1) was added, followed by 980 μL of 1:1 1-Butanol:Methanol (BUME). Samples were vortexed for 2 min in a cold room, then sonicated for 30 min in a cold room. Samples were then centrifuged at 4 °C for 5 min at 14,000 g. Supernatant was dried under nitrogen gas and immediately resuspended in 100 μL buffer containing 100% methanol, 1 mM ammonium formate and 0.2% formic acid (Buffer B) followed by a quick vortex, 5 min sonication, and 5 min 4 °C centrifugation at 14,000 g. Supernatant was transferred to a fused glass vial and loaded onto the LC–MS/MS.
SL species were determined via LC-MS/MS using an Agilent 6460 QQQ. SLs were separated on a C8 column (Spectra 3 μm C8SR 150 × 3 mm ID, Peeke Scientific, CA) utilizing transitions from Burla et al. [17] and quality control described in Broadhurst et al. [18]. Mobile phase A was composed of 100% HPLC grade water containing 2 mM ammonium formate and 0.2% formic acid and mobile phase B consisted of 100% methanol containing 0.2% formic acid and 1 mM ammonium formate. The gradient elution program held at 82% B for 3 min, raised to 90% B over 1 min and linearly increased to 99% B over 14 min, maintained for 7 min, and returned to 82% B over 2 min. The 82% mobile phase B was maintained for 3 min followed by re-equilibration for 10 min. The capillary voltage was set to 3.5 kV, the drying gas temperature was 350 °C, the drying gas flow rate was 10 L/min, and the nebulizer pressure was 60 psi.
A new C8 column was installed immediately prior to Batch 1 and was used for all eight batches. Prior to each batch, fresh buffer was made, the source was cleaned, and 100% acetonitrile was flushed through the column for 30–45 min. The column was equilibrated with 14 total injections of quality control samples, a procedure blank, and internal standard mixture [18]. Quality control samples were injected throughout the sequence to identify any machine drift. Blank injections were never performed to minimize variation of sample matrix in the column. SL species were analyzed by SRM of the transition from precursor to product ions at associated optimized collision energies and fragmentor voltages (Supplemental Table 1). SLs were then normalized to an internal standard of known concentration followed by batch correction and further statistical analyses.
2.3. Mouse husbandry, diet, and metabolite quantification
All animal procedures were approved by The Scripps Research Institute Animal Care Committee and we adhered to all federal animal experimental guidelines. At eight weeks of age, male C57/Bl6J mice were fed amino acid-adjusted diets for 3–5 weeks as indicated. Mice were fed ad libitum isocaloric and isonitrogenous control or serine- and glycine-free custom diets (Envigo Teklad Diets, Madison, WI) (Supplemental Table 2). Blood was collected at 6 AM, and serum was isolated after centrifugation at 2,000 g.
As described above for the human serum, 10 μL of mouse serum was extracted for amino acid quantification via GCMS and 50 μL of serum was extracted for SL quantification using LCMS. All samples were run in a single batch and quantified using internal standards described.
Mouse serum was extracted using 500 μL MeOH, 400 μL saline, 100 μL of water, and 19 μL of internal standard mix containing C13-dihydroceramide-d7, C15-ceramide-d7, d18:1-d7/15:0 glucosylceramide, d18:1-d7/15:0 lactosylceramide, and d18:1/18:1-d9 sphingomyelin. One mL of −20 °C chloroform was added. Samples were vortex mixed for 5 min and spun down for 5 min at 4 °C at 15,000 g. The organic phase was collected and 2 μL of formic acid was added to the remaining polar phase which was re-extracted with 1 mL of −20 °C chloroform. Combined organic phases were dried and the pellet was resuspended in 100 μL of buffer containing 100% methanol with 1 mM ammonium formate and 0.2% formic acid.
2.4. Imaging quantification
Imaging data sets included color fundus photographs (CFP), optical coherence tomography scans (OCT; volume scans of 15° × 10° [high-resolution mode, 97 scans] and 30° × 25° [high-speed mode, 61 scans]; Spectralis; Heidelberg Engineering, Heidelberg, Germany), fundus fluorescein angiograms (FFA), blue wavelength autofluorescence images (BAF), and blue-light reflectance images (BLR; all HRA Spectralis; Heidelberg Engineering, Heidelberg, Germany). Macular pigment optical density (MPOD) was measured using dual-wavelength autofluorescence (HRA Spectralis; Heidelberg Engineering, Heidelberg, Germany). Only complete, high quality image datasets were considered for the final analysis. CFP were graded for the presence of retinal crystals, pigment plaques, and neovascular membranes. The presence of retinal crystals was additionally verified on blue-light reflectance images as previously described [19]. OCT volume scans were analyzed for the presence of ellipsoid zone (EZ)-loss, outer retinal hyper-reflective changes, subretinal/sub-RPE neovascular membranes, and neovascular exudation. Eyes were classified into stages 0–4 using a simplified OCT-based disease staging system that was modified according to the classification proposed by Chew et al. [20]. Stage 0 was defined as no visible break of the EZ, no outer retinal hyper-reflectivity (ORHR), and no neovascularization; stage 1 as break of the EZ but absence of ORHR; stage 2 as break of the EZ, presence of ORHR, but absence of subretinal/sub-RPE neovascular membranes; stage 3 as break of the EZ, presence or absence of ORHR, presence of neovascular membranes without detectable neovascular activity. Stage 4 was defined as break of the EZ, presence or absence of ORHR, and presence of exudative neovascular membranes and/or fibrotic neovascular scars. Additionally, eyes were graded for hyper-reflectivity, and classified into three different categories (classes 0–2). Class 0 was defined as lack of ORHR and neovascular membranes; class 1 as presence of ORHR, but absence of neovascular membranes; and class 2 as presence of neovascular membranes. Definitions for ORHR, subretinal/sub-RPE neovascularization and neovascular exudation are in accordance with previously published descriptions [21].
For quantification of EZ-loss, OCT volume scans were analyzed using the 3D view panel as previously described [22]. MPOD maps were analyzed and eyes were assigned to MPOD-classes 1 to 3 according to the classification proposed by Zeimer et al. [23]. In short, this classification differentiates between three distinct distribution patterns (“MPOD-classes”) of macular pigment and outlines a depletion of macular pigment that increases with MPOD-classes.
2.5. Statistical analysis
To estimate machine measurement drift (analytical variation) over time in each machine-batch-metabolite group, mass spectral abundances were log-transformed, and a third-order polynomial equation was fitted using all experimental samples. As a small proportion of metabolite measurements were missing across the dataset (average 1.2%, SD 4.1%), we performed imputation using the GSimp R package [24] under a ‘missing not at random’ assumption—that is, the missing metabolite abundances were assumed to fall near the lower limit of detection. Comparison of the imputation results for the total data, compared with a subset excluding HSAN1 patients, indicated negligible differences (Supplemental Fig. 1A). Using the lme4 package [25], a mixed effects linear model was fitted to test the association between metabolite abundance and disease status correcting for age, sex, T2D status, batch and machine drift (fixed effects), with a random intercept for groups of related individuals. The three disease groups were first compared with unaffected controls. Subjects with MacTel only, or MacTel and HSAN1, were then compared to HSAN-only subjects. Each of the five contrasts were independently corrected for false discovery rate using the Benjamini Hochberg method. The partial residuals from subjects with any of the three diseases were calculated for plotting and for metabolic co-abundance analysis. The corrplot package was used to construct disease-specific metabolite co-abundance plots, and the R tidyverse suite of packages [26] were used for general data transformation, residualization and visualization.
Mouse metabolic levels where log2 transformed and scaled to have zero mean and one standard deviation. Association between mouse metabolic levels and diet was assessed via linear regression modeling. Association models were performed separately for amino acids and lipids. Correction for false discovery rate was performed using the Benjamini Hochberg method.
3. Results
3.1. MacTel patients exhibit altered levels of non-essential amino acids and sphingolipids
To investigate amino acid and SL changes in MacTel patients, we compared metabolite abundance in fasted serum from 205 MacTel patients and 151 Controls (Table 1; Supplemental Table 3). Differences in age, sex, and prevalence of diabetes were corrected for when testing for association. Testing for interaction between sex and MacTel disease did not reveal any differential MacTel dysregulations between males and females. To validate the ability of the model to correct for the discrepancy in the T2D prevalence between MacTel and Controls, we restricted the analysis to subjects without diabetes and returned extremely similar results (not shown). Consistent with prior results, MacTel patients had significantly lower levels of circulating serine and glycine compared to Controls (Figure 1A). More than 75% of the MacTel patients had lower glycine levels than the mean level for Control participants (Supplemental Fig. 1B). MacTel patients also had higher circulating levels of alanine, glutamate, and the branched-chain amino acids (BCAAs) leucine and valine, suggesting that amino acid dysregulation in these patients is similar to that observed in individuals with diabetes [27] (Figure 1A).
Table 1.
Study cohort characteristics.
| Controls | MacTel (without HSAN1) | HSAN1 | |
|---|---|---|---|
| N | 151 | 205 | 25 |
| Age, mean ± SD | 58.7 ± 14.3 | 63.5 ± 10.4 | 50.5 ± 12.3 |
| Sex, % female (N) | 57% (86) | 62% (127) | 48% (12) |
| Diabetes status, % T2D (N) | 12.6% (19) | 37.1% (76) | 0 |
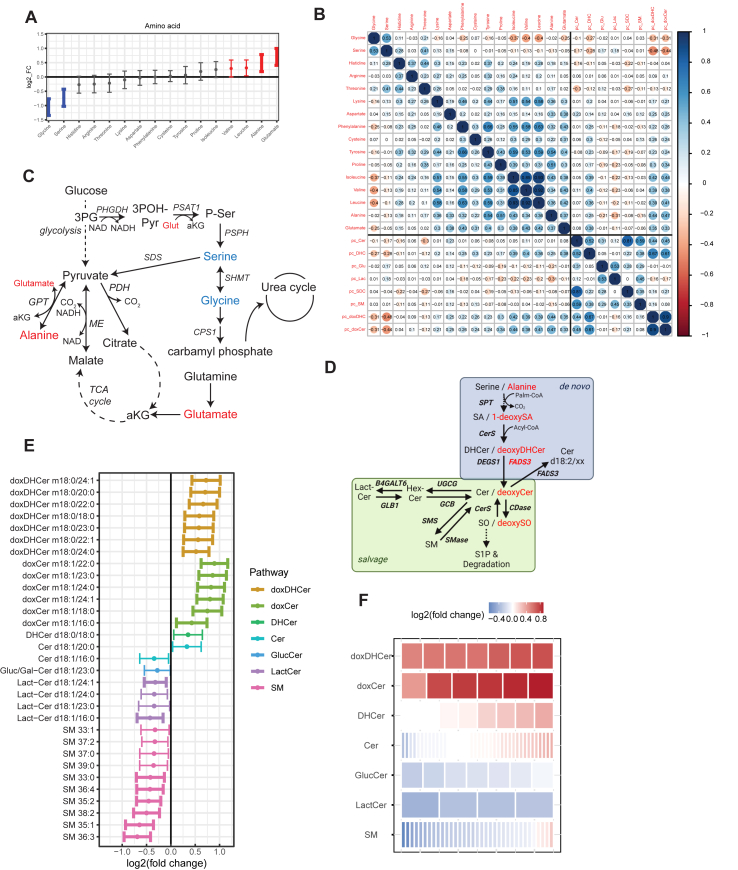
Figure 1.
MacTel patients exhibit altered levels of non-essential amino acids and sphingolipids. (A) Log-fold change of nominally significant metabolites in 16 amino acids in MacTel subjects compared to Controls. Points denote the log-fold change and bars extend to the 95% confidence interval. Bold bars indicate significance after correction for FDR. (B) Correlation table of amino acids and lipids principal components in Controls. Numbers indicate Pearson's correlation coefficient (R). Abbreviations in this panel: Cer, ceramide; DHC, dihydroceramide; Glu, glucosyl-ceramide; Lac, lactosyl-ceramide; SDC, ceramide (d18:2/xx); SM, sphingomyelin; doxDHC, deoxydihydroceramide; doxCer, deoxyceramide. (C) Simplified metabolic map demonstrating the connections between glycolysis, TCA cycle, amino acid metabolism, and the urea cycle. (D) Map of SL metabolism with the de novo and salvage pathways indicated. (E) Change in all measured sphingolipids (SL) between MacTel subjects compared to Controls, grouped by SL class. The tiles in each row represent lipids contained in that class, colored by log fold change in MacTel compared to Controls. The color blue represents depletion and red represents increased abundance. (F) Changes in abundance across SL classes. Each row is composed of tiles representing lipids contained in the group. The color of each tile represents the mean log fold change of that lipid in MacTel patients to Controls. The color blue represents depletion and red represents increased abundance. Abbreviations: doxDHCer, deoxydihydroceramide; doxCer, deoxyceramide; DHCer, dihydroceramide; Cer, ceramide; Gluc/Gal-Cer or GlucCer, glucosyl/galactosyl-ceramide; Lact-Cer, lactosyl-ceramide; SM, sphingomyelin.
We also analyzed pairwise correlations within MacTel and Control groups between all 16 measured amino acids (Figure 1B and Supplemental Fig. 1C). Interestingly, alanine and glutamate correlated positively to each other (as well as with other essential and non-essential amino acids) in Control participants, and this association was diminished in MacTel patients (Figure 1B and Supplemental Fig. 1C). A similar trend was also noted with glycine, which correlated negatively with BCAAs in Control participants but exhibited a weaker association in MacTel patients (Figure 1B and Supplemental Fig. 1C). Many of these associations are expected and arise due to transaminase activity in the liver or kidney, which are hubs for nitrogen metabolism that exchange nitrogen between glutamate and specific amino acids. The weaker correlations in MacTel patients could be indicative of diminished nitrogen homeostasis, as CPS1, an enzyme participating in the urea cycle, has also been identified as having a protective effect in MacTel patients [3] (Figure 1C).
SPT synthesizes sphingoid bases by condensing serine and fatty acyl-CoAs but can also use alanine as a substrate when mass action favors this reaction, producing 1-deoxysphingoid bases. These can be metabolized to deoxy (dihydro)ceramides but not further along the canonical SL pathway (Figure 1D). We previously demonstrated that 1-deoxysphinganine (m18:0) from hydrolyzed serum sphingolipids was elevated in MacTel patients and correlated strongly with serine and alanine levels [5]. To assess how intact canonical and non-canonical SLs were altered, we performed a targeted analysis of species using LC-MS/MS (Figure 1E–F). Normalized abundances of all lipid species based on class-specific internal standards were determined in all patient groups (Supplemental Figs. 2A–D, Supplemental Table 4). As expected, all detected deoxydihydroceramides and deoxyceramides were significantly elevated in MacTel patients, and these species were 35–85% higher compared to Control subjects, correlating strongly with serine and alanine (Figure 1E–F, Supplemental Figs. 2A and E). We quantified eight dihydroceramide species and 20 ceramides (13 with 18:1 bases and seven with 18:2 bases). Despite having reduced serine in circulation, dihydroceramides and ceramides (d18:1 and d18:2) were not broadly decreased in MacTel patients (Figure 1E–F, Supplemental Fig. 2B). In contrast to doxSLs, canonical ceramides are further metabolized in the Golgi to form glycosphingolipids and sphingomyelins. We quantified seven glucosyl/galactosyl-ceramides (Gluc/Gal-Cer), four lactosylceramides (Lact-Cer) and 35 sphingomyelin (SM) species, and many (15/46) were significantly decreased in MacTel patients compared to Control participants (Figure 1D–F). In particular, glycosphingolipids of relatively high abundance such as Lact-Cer d18:1/24:1, Lact-Cer d18:1/24:0, and Lact-Cer d18:1/16:0 were significantly decreased in MacTel patients (Figure 1E–F and Supplemental Fig. 2C). On the other hand, lower abundance but distinct (odd-chain or unsaturated) SM species were reduced nominally in MacTel patients (Figure 1E–F and Supplemental Fig. 2D). The decrease in complex SLs without concomitant decreases in the dihydroceramides may indicate an SPT-independent mechanism is driving these reductions.
To better understand how these circulating amino acids and SLs might be co-regulated, we performed a correlation analysis between each amino acid and the principal components derived from each SL class. Correlations for Control subjects, MacTel patients, and the differences between them are depicted in Figure 1B and Supplemental Fig. 1C. Serine, glycine, alanine, and to a lesser extent the BCAAs all strongly correlated with doxSL abundances. On the other hand, canonical SLs showed minimal correlation with amino acids aside from dihydroceramides, which showed a positive relationship with BCAAs and a negative correlation with serine. SL classes correlated strongly with one another (>0.5) when they were separated by a single reaction (i.e dihydroceramide and ceramide or Gluc/Gal-Cer and Lact-Cer). These results may reflect the flux topology of SL metabolic networks, where synthesis and recycling of complex SLs occurs at higher rates than sphingoid synthesis/turnover (and the lack thereof for doxSLs). However, they may also suggest that amino acid dysregulation in MacTel patients may be unrelated to alterations in canonical membrane SLs.
3.2. Low serine and glycine levels reduce circulating ceramides and sphingolipids in mice
The lack of correlation between serine and the canonical SLs might suggest that the decrease in more complex SLs such as Gluc/Gal-Cer and Lact-Cer observed in MacTel is a serine-independent change. To test if reduced levels of serine and glycine (as observed in MacTel) can cause changes in canonical and complex SL levels, we fed mice control or amino acid-altered diets, which lacked serine and glycine but included higher levels of other non-essential amino acids to make them isonitrogenous and isocaloric (Figure 2A, Supplemental Table 2). Mice fed the serine/glycine deficient diet had a 58% and 75% reduction in circulating serine and glycine levels, respectively, compared to mice fed the control diet and no other amino acids were significantly altered (Figure 2B). As expected, the mice fed the serine/glycine deficient diet had elevated serum levels of deoxydihydroceramides and deoxyceramides compared to control mice. The reduction in serine and glycine also led to a broad decrease in the canonical and complex SLs (Figure 2B, Supplemental Fig. 3A–D). Distinct from the MacTel patients, dihydroceramides showed a trend to be reduced and almost all ceramides were nominally or significantly decreased (Figure 2B). Complex SLs also were broadly reduced, with all four Gluc/Gal-Cer species significantly reduced and a trend for Lact-Cer and SM to be decreased.
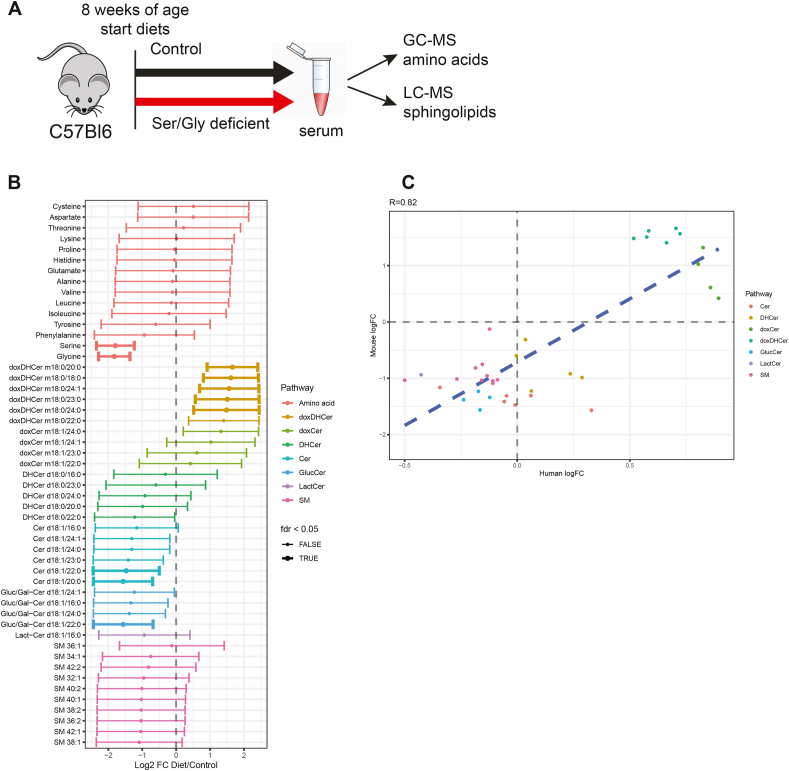
Figure 2.
Low serine and glycine levels reduce circulating ceramides and complex SL in mice. (A) Log-fold change of all tested metabolites in diet mice subjects compared to control diet mice. Points denote the log-fold change and bars extend to the 95% confidence interval. Bold bars indicate significance after correction for FDR. (B) Correlation plot between human and mice Log-fold change. Pearson correlation coefficient is reported at the top of the figure and the correlation line is displayed in blue. Amino-acids are not contained in this figure as they were artificially altered by design in the mice experiment. Abbreviations: doxDHCer, deoxydihydroceramide; doxCer, deoxyceramide; DHCer, dihydroceramide; Cer, ceramide; Gluc/Gal-Cer or GlucCer, glucosyl/galactosyl-ceramide; Lact-Cer, lactosyl-ceramide; SM, sphingomyelin.
We compared SL changes in the mice driven by low serine and glycine to those of the MacTel patients and found strong agreement (Pearson's R = 0.82) (Figure 2C). Although a reduction in simple ceramides in glycine/serine-deprived mice is not recapitulated in MacTel subjects, these results indicate the reduction in complex SLs (Gluc/Gal-Cer, Lact-Cer and SM) observed in MacTel can be induced by limiting serine and glycine availability.
3.3. MacTel patients presenting neo-vascularization have reduced levels of sphingomyelins
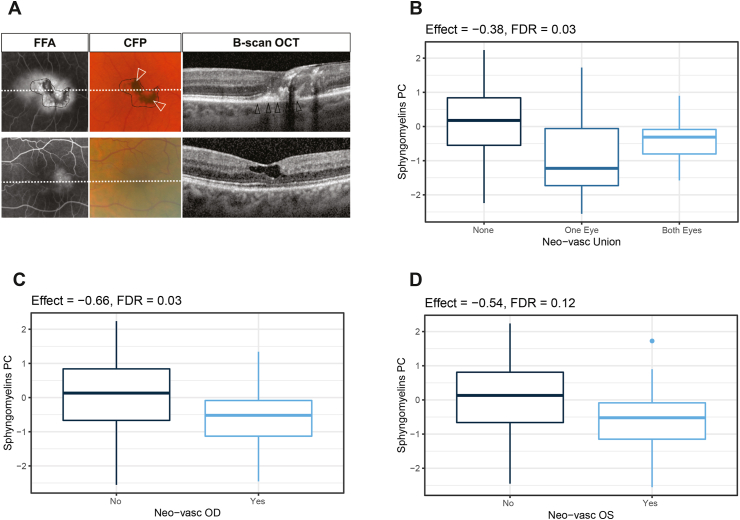
To explore potential associations between metabolite levels and MacTel patient disease status, we evaluated retinal imaging markers known to be associated with disease severity [[21], [22], [23],[28], [29], [30]] in multimodal retinal images of 200 eyes from 100 MacTel patients within this cohort (Figure 3A). These patients were selected at random and exhibited no significant differences in measured metabolite levels relative to the non-selected patients. Age of disease onset and rate of disease progression were not available for this cohort. We subsequently compared the graded phenotypic values to patient metabolite levels. Individual metabolites and principal components derived from lipid classes were tested for retinal phenotype associations. Both individual eye values (as well as their sum) were tested (Supplemental Tables 5 and 6). We found that SM negatively correlated with neovascularization, a phenotype frequently observed in MacTel and indicative of late-stage disease (Figure 3B–D) [21,29,31,32]. Additionally, hyper-reflectivity and disease stage were also found to be negatively correlated with SM levels, although this association was predominantly driven by their relationship with neovascularization (Supplemental Fig. 4).
Figure 3.
MacTel patients presenting neovascularization have reduced levels of sphingomyelins. (A) Representative left eyes of two MacTel patients with low (top) and high (bottom) sphingomyelin serum levels. Eye with a large subretinal/sub-RPE neovascular membrane (black arrowheads) and pigment accumulation (white arrowheads) (top) and eye with mild parafoveal leakage on fundus fluorescein angiography (FFA) and lack of hyper-reflective changes on OCT (bottom). (B) Association between sphingomyelins principal component score and neovascularization in both eyes (left + right). Center line indicates the median and box boundaries denote the 25th and 75th percentiles. (C) Association between sphingomyelins principal component score and neovascularization in right eyes (OD) only. Center line indicates the median and box boundaries denote the 25th and 75th percentiles. (D) Association between sphingomyelins principal component score and neovascularization in left eyes (OS) only. Center line indicates the median and box boundaries denote the 25th and 75th percentiles.
3.4. HSAN1 patients have altered circulating levels of canonical ceramides and amino acids
Given the links between MacTel, HSAN1, and doxSLs, we analyzed serum from 25 HSAN1 patients carrying six different variants in either SPTLC1 or SPTLC2 (Table 2 and Supplemental Table 7). Patients with the SPTLC1 C133Y or SPTLC2 S384F variants have both peripheral neuropathy and MacTel. Patients with the remaining four variants studied here, while having severe peripheral neuropathy, do not show signs of retinopathy (Table 2 and Supplemental Table 7) [9]. While several patients carrying the SPTLC1 C133Y variant and one with the C133W variant were taking serine to treat their disease at the time of serum collection (Supplemental Table 7), we compared effect sizes from analyses with and without these patients and found that exclusion of these patients had little effect on the overall group statistics other than elevating serine itself (R = 0.94, p < 1E−10) (Supplemental Fig. 5). Comparing HSAN1 subjects (independent of their MacTel status) to Control participants, neuropathy patients exhibited high abundances of deoxydihydroceramides and deoxyceramides with a variety of acyl chains lengths (Figure 4A–B, Supplemental Fig. 6A). Interestingly, the canonical ceramides were broadly reduced in HSAN1 patient sera compared to controls, including dihydroceramides, d18:1 and, most strikingly, d18:2 ceramides (Figure 4A–B, Supplemental Fig. 6B). While SMs were also broadly decreased, the other complex SLs, Gluc/Gal-Cer and Lact-Cers, were not uniformly altered (Figure 4A–B, Supplemental Fig. 6C–D). While the numbers of patients with individual variants are low, these changes were uniform across variants (Supplemental Fig. 7A–G). These findings suggest HSAN1 patients have decreased canonical activity owing to SPTLC1 and SPTLC2 variants and highlight potential decreases in circulating canonical SLs that also may be relevant for HSAN1 pathophysiology.
Table 2.
Incidence of SPTLC1 and SPTLC2 variants across HSAN1 patient cohort.
| Variant | HSAN1 patients included | MacTel affected/predicted | MacTel unaffected |
|---|---|---|---|
| SPTLC1 C133Y | 8 | 8 | – |
| SPTLC1 C133W | 11 | – | 11 |
| SPTLC1 V144D | 1 | – | 1 |
| SPTLC2 A182P | 2 | – | 2 |
| SPTLC2 N177H | 1 | – | 1 |
| SPTLC2 S384F | 2 | 1 | 1 |
| TOTAL | 25 | 9 | 16 |
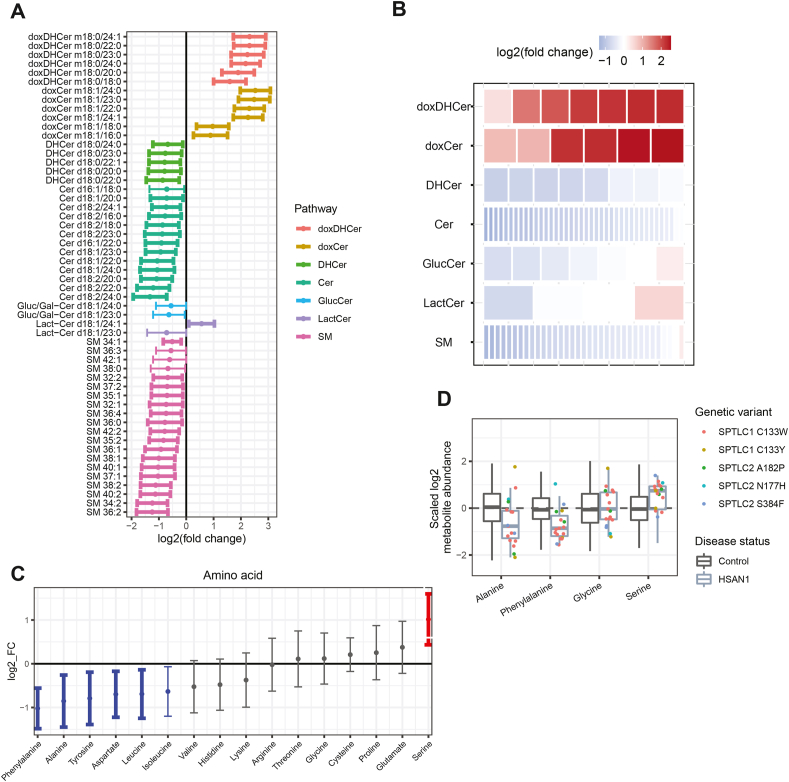
Figure 4.
HSAN1 patients have altered circulating levels of canonical ceramides and amino acids. (A) Representation of all significantly different SL species (p < 0.05) comparing HSAN1 to Controls. Color indicates the lipid class and bold bars indicate significance after correction for FDR. (B) Changes in abundance across SL classes. Each row is composed of tiles representing lipids contained in the group. The color of each tile represents the mean log fold change of that lipid in HSAN1 patients to Controls. The color blue represents depletion and red represents increased abundance. (C) Change in 16 amino acids comparing HSAN1 to Controls. Points indicate mean log fold change and bars extend to the 95% confidence interval of the mean. Red or blue colors indicate nominal significance (p < 0.05), and bold bars indicate significance after correction for FDR. (D) Distribution of alanine, phenylalanine, glycine, and serine levels in HSAN1 subjects (excluding those on serine supplementation) compared to Controls. HSAN1 subject metabolite abundance (points) are colored according to genetic variant. Individual data points for Controls are not shown. Boxplot central line indicates the median and box boundaries denote the 25th and 75th percentiles. Whiskers extend to the most extreme values within 1.5× the interquartile range. Abbreviations: doxDHCer, deoxydihydroceramide; doxCer, deoxyceramide; DHCer, dihydroceramide; Cer, ceramide; Gluc/Gal-Cer or GlucCer, glucosyl/galactosyl-ceramide; Lact-Cer, lactosyl-ceramide; SM, sphingomyelin; SPTLC1 and SPTLC2, serine-palmitoyl transferase long chain subunit 1 or 2.
Intriguingly, HSAN1 patients exhibited higher serine levels and lower alanine levels compared to controls, showing the opposite effect as observed for MacTel patients (Figure 4C). These findings are not driven by a specific SPTLC variant and are observed with or without the serine-supplemented patients (Figure 4D, Supplemental Fig. 7H). Consequently, the correlations between serine or alanine and doxSLs were not present in these patients (Supplemental Fig. 2C). HSAN1 patients also showed lower circulating phenylalanine, tyrosine, aspartate, and leucine concentrations, with a trend for reduced levels of the other BCAAs (Figure 4C). These amino acid changes suggest potential compensatory mechanisms to deal with the increased doxSL levels (higher serine/lower alanine) and highlight distinctions in how these analytes might contribute to MacTel versus HSAN1.
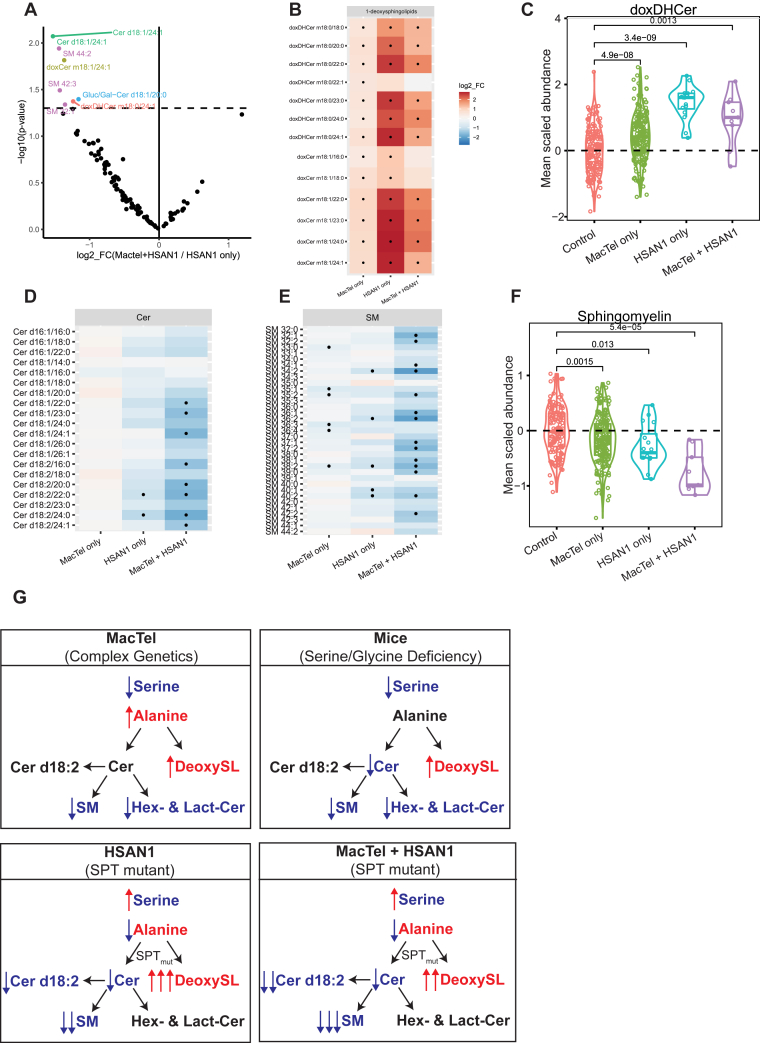
3.5. MacTel patients have distinct sphingolipid changes compared to HSAN1 patients
To determine if circulating levels of amino acid and SL can distinguish HSAN1 patients with or without MacTel (Table 2) we compared metabolite levels between these groups. This analysis identified several metabolites that were significantly different (uncorrected p < 0.05) in HSAN1 patients ± MacTel. These species were all lipids and included deoxydihydroceramide (doxDHCer) m18:0/24:1, deoxyceramide (doxCer) m18:1/24:1, and ceramide (Cer) d18:1/24:1 that were less abundant in HSAN1 patients with MacTel (Figure 5A; Supplemental Table 6). Interestingly, these relatively abundant molecules all have a common N-acyl chain in nervonic acid (24:1). Other species that were lower in HSAN1 patients with MacTel patients included canonical SMs and lower abundant hexosylceramide (d18:1/20:0). This comparison suggests that HSAN1 patients with MacTel have more severe decreases in circulating canonical sphingolipids.
Figure 5.
MacTel patients have distinct SL alterations compared to patients with HSAN1. (A) Volcano plot comparing HSAN1 patients with MacTel, to HSAN1 patients without MacTel. Dashed line represents p = 0.05. Only metabolites below this threshold are labeled and coloured by metabolite class. (B) Depiction of the log2 fold change of 1-deoxysphingolipids in MacTel only, HSAN1 only, and HSAN1 patients with MacTel, compared to Controls. Tiles indicate the mean log fold change in abundance between each cohort and controls. Black points indicate FDR-significant changes. Red colors indicate increased relative abundance, and blue colors indicate decreased abundance. (C) Scaled abundance of averaged dihydroxyceramide levels in Controls, MacTel only, HSAN1 only, and HSAN1 patients with MacTel. Center line indicates the median and box limits indicate the 25th and 75th percentiles. (D) Depiction of the log2 fold change of d18:2 ceramides in MacTel only, HSAN1 only, and HSAN1 patients with MacTel, compared to Controls. Tiles rendered as for panel B. (E) Depiction of the log2 fold change of sphingomyelins in MacTel only, HSAN1 only, and HSAN1 patients with MacTel compared to Controls. Tiles rendered as for panel B. (F) Scaled abundance of averaged sphingomyelin levels in Controls, MacTel only, HSAN1 only, and HSAN1 patients with MacTel. Center line indicates the median and box limits indicate the 25th and 75th percentiles. (G) Summary of the amino acid and SL alterations in MacTel, HSAN1, and HSAN1 patients with MacTel compared to Controls. Red indicates increased, and blue indicates decreased abundance. Abbreviations: doxDHCer, deoxydihydroceramide; doxCer, deoxyceramide; DHCer, dihydroceramide; Cer, ceramide; Gluc/Gal-Cer or GlucCer, glucosyl/galactosyl-ceramide; Lact-Cer, lactosyl-ceramide; SM, sphingomyelin.
We next compared each patient subgroup (MacTel without SPT variants: MacTel only, n = 205; HSAN1 without MacTel: HSAN1 only, n = 16; HSAN1 with MacTel: MacTel + HSAN1, n = 9) to Control subjects, n = 151. As noted above, HSAN1 patients (Figure 4C) had distinct amino acid changes compared to the MacTel group (Figure 1A) with serine increasing and alanine decreasing in HSAN1 patients, and these trends were maintained regardless of the MacTel status in the HSAN1 cohort (Supplemental Fig. 8A, Supplemental Table 8). Although doxSLs were high in all cohorts compared to Controls, they were generally more elevated in HSAN1 only patients than MacTel + HSAN1, or MacTel only (Figure 5B–C). This finding is consistent with in vitro results indicating higher activity of SPTLC1C133W versus the SPTLC1C133Y variants [[33], [34], [35]]. MacTel + HSAN1 patients showed a broader trend for having lower circulating canonical ceramides and SMs compared to those patients with HSAN1 only (Figure 5D, Supplemental Figs. 8B–C), and these patients had the lowest SM of all patient subgroups (Figure 5E–F), while no general trends were evident for the Lact-Cer and Gluc/Gal-Cer in these comparisons (Supplemental Figs. 8D–E). Collectively, these results suggest that patients with HSAN1 and MacTel have more significant depletions in circulating canonical SLs and highlight roles for both altered amino acids and membrane lipid homeostasis in driving MacTel.
4. Discussion
Here we comprehensively profiled serum amino acids and SL species in subgroups of patients with a rare polygenic macular disease (MacTel) and an even more rare autosomal dominant disease (HSAN1), two diseases that show clinical, metabolic, and genetic overlap. These metabolite groups intersect at an interesting biochemical node linking amino acid metabolism to membrane lipids important for nervous system function. In expanding the cohort and analytical complexity, we have confirmed metabolic changes in these patient populations (reduced serine/glycine and increased doxSLs) and identified novel metabolite classes likely involved in disease pathology. The significant increases observed in alanine and glutamate levels in MacTel patients is further indication that amino acid dysregulation is a key contributor to MacTel. These amino acids become elevated in the context of mitochondrial dysfunction [36,37] and highlight potential links between MacTel, doxSLs, and mitochondrial function [36,[38], [39], [40]]. Furthermore, we also find reduced serine and glycine and elevated BCAAs in MacTel patients versus Control subjects, a pattern shared with obese and insulin-resistant patients [27]. MacTel patients have an increased prevalence of T2D [41], which was reflected in our cohort and controlled for statistically. As such, these results provide additional evidence that MacTel and T2D share genetic drivers that impact amino acid metabolism through related mechanisms [6,42]. In this cohort, participants were classified as having diabetes or not, which does not capture the complexity of the disease. Future studies will benefit from quantitative metrics, like HbA1c levels, to further explore the intersection of diabetes and MacTel. In recent work we found that insulin resistance or deficiency drives reduced serine and glycine in mice that is associated with elevated peripheral doxSLs and sensory neuropathy, highlighting a biochemical (rather than genetic) link to this phenotype [1].
In participants without SPT variants, we find that serine, glycine, and alanine strongly correlate with deoxydihydroceramide, but not the far more abundant canonical dihydroceramides. Consequently, monitoring doxSL levels could function as a more stable and reliable biomarker of serine levels than the amino acid itself, which varies with diet and time of day [5]. This could be beneficial in both characterizing chronic serine levels and monitoring a patient's response to treatment.
We observed distinct changes in canonical ceramides and complex SLs in MacTel and HSAN1 patients. As summarized in Figure 5G, MacTel patients are typified by altered serine, glycine, and alanine levels and have elevated doxSLs and broad decreases in complex membrane SLs but not ceramides. The HSAN1 patients, with altered SPT function, have elevated doxSLs, reduced ceramides and SMs, but the other complex SLs were not generally affected. The mice with very low serine and glycine levels have elevated doxSLs and a reduction of all canonical SLs, with ceramides and Gluc/Gal-Cers being the most reduced. The common features between the three models are elevation of doxSLs and reduction in SM, suggesting that doxSLs might negatively influence SM levels. While no correlation was found between doxSLs and SM in the patient cohort, this mechanism fits with recent work by Clark et al., showing that HSAN1 patient cells had impaired membrane SL function [43]. Although the HSAN1 cohort is relatively small, those HSAN1 patients with MacTel had even lower levels of SM in circulation. On the other hand, we observed reduced Gluc/Gal-Cers and Lact-Cers specifically in MacTel patients and in the dietary mouse model, but not in HSAN1 patients. Since the amino acid levels are a distinguishing feature between the MacTel and HSAN1 patients, we speculate that low serine and glycine levels may be responsible for regulating these glycosylated complex SLs. Furthermore, since ceramides are not reduced in MacTel, it is likely via an SPT-independent mechanism.
Intriguingly, serine levels were generally elevated in HSAN1 patients, whereas canonical ceramides were broadly reduced compared to Controls. In addition to dihydroceramide (d18:0) and ceramide (d18:1), the d18:2 ceramides were particularly impacted in the HSAN1 patients. This is interesting given that the enzyme responsible for their synthesis, FADS3, is also used in deoxyceramide production [44]. The elevation of serine and low canonical ceramide levels suggest that sphingolipid supplementation (rather than serine) may be most therapeutically beneficial for these rare patients [11,34].
These analyses indicate that while elevated circulating doxSLs are associated with MacTel, they alone (as in HSAN1) are not sufficient to drive this macular phenotype. Here we find that reduction in membrane SLs might be an additional metabolic stressor. Indeed, our analysis of retinal imaging in MacTel patients indicated that low SM correlated with retinal neovascularization. Though no direct correlations have previously been described between neovascularization and SM, others have observed associations between ceramides and sphingosine 1-phosphate with ocular neovascularization and angiogenesis [45]. These results, combined with the reduction in glycosylated SLs, further link MacTel pathogenesis to dysregulation of membrane lipids [46]. These data further support the concept that MacTel patients may benefit more directly from serine supplementation to improve amino acid and SL homeostasis compared to HSAN1 patients. However, further studies and more longitudinal data are needed to better understand the impact of systemic metabolic changes on the retinal phenotype and disease progression in MacTel.
5. Conclusions
To our knowledge, this targeted metabolomic analysis is the most comprehensive quantitation of sphingolipids in human sera, including the largest cohorts of two rare diseases, MacTel and HSAN1. Despite the overlapping phenotypes and the convergence on sphingolipid metabolism, we found MacTel and HSAN1 patients to have highly distinct metabolite profiles. While MacTel patients have low serine and glycine, and elevated alanine (mirroring changes observed in T2D), the opposite is found in HSAN1 patients. The elevation of serine in HSAN1 suggests existing physiological compensation and treatment with downstream sphingolipids might be beneficial. Despite the shared elevation of toxic 1-deoxysphingolipids in both diseases, our results suggest that they are not sufficient to drive MacTel pathology. Complex membrane sphingolipids such as glycosphingolipids and sphingomyelin are particularly reduced in subjects with MacTel, suggesting membrane lipid metabolism contributes to macular disease and warrants further study.
Author contributions
C.R.G.: conceptualization, formal analysis, data curation, investigation, methodology, writing - original draft, writing - review and editing, and visualization; R.B.: conceptualization, software, formal analysis, data curation, investigation, writing - original draft, writing - review and editing, and visualization; B.R.E.A.: conceptualization, software, formal analysis, data curation, investigation, writing - original draft, writing - review & editing, supervision project administration; S.T.: formal analysis, investigation, writing-original draft; M.K.H.: investigation, writing - review and editing; G.H.M.: investigation, writing - review and editing; B.H.: investigation; J.T.: investigation; M.M.R.: investigation; P.S.B.: investigation; writing - review and editing, C.E.: investigation; M. Fru.: investigation; M.W.: conceptualization, methodology, writing-review & editing; M.B.: review & editing, supervision project administration and supervision; M.Fri.: resources, writing-review & editing, supervision project administration and supervision; C.M.M.: conceptualization, resources, supervision project administration and supervision, writing - review & editing; M.L.G.: conceptualization, data curation, formal analysis, investigation, project administration, resources, supervision, visualization, writing - original draft, review & editing.
Funding
This work was funded by the Lowy Medical Research Institute with additional support to individual investigators.
6. Acknowledgements
We thank the Lowy family for their support of the MacTel Project and the Lowy Medical Research Institute. M.B. was supported by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant [grant number GNT 1195236]. B.R.E.A. was supported by a NHMRC Early Career Fellowship [grant number GNT 1157776]. This work was also supported by the Victorian Government's Operational Infrastructure Support Program and the NHMRC Independent Research Institute Infrastructure Support Scheme (IRIISS) and by an unrestricted departmental grant from Research to Prevent Blindness to the Moran Eye Center of the University of Utah. This work was also supported by grants to C.M.M. from the National Institutes of Health [grant number R01CA234245] and National Science Foundation [grant number 1454425]. Finally, the authors wish to thank the MacTel patients, HSAN1 patients and their families for their involvement in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101716.
Contributor Information
Christian M. Metallo, Email: metallo@salk.edu.
Marin L. Gantner, Email: mgantner@lmri.net.
CONFLICT OF INTEREST
The authors have no financial disclosures or conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be available through public database: Metabolomics Workbench
References
- 1.Handzlik M.K., Gengatharan J.M., Frizzi K.E., McGregor G.H., Martino C., Rahman G., et al. Insulin-regulated serine and lipid metabolism drive peripheral neuropathy. Nature. 2023;614(7946):118–124. doi: 10.1038/s41586-022-05637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn T.M., Tifft C.J., Proia R.L. A perilous path: the inborn errors of sphingolipid metabolism. J Lipid Res. 2019;60(3):475–483. doi: 10.1194/jlr.S091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scerri T.S., Quaglieri A., Cai C., Zernant J., Matsunami N., Baird L., et al. Genome-wide analyses identify common variants associated with macular telangiectasia type 2. Nat Genet. 2017;49(4):559–567. doi: 10.1038/ng.3799. [DOI] [PubMed] [Google Scholar]
- 4.Bonelli R., Woods S.M., Ansell B.R.E., Heeren T.F.C., Egan C.A., Khan K.N., et al. Systemic lipid dysregulation is a risk factor for macular neurodegenerative disease. Sci Rep. 2020;10(1):12165. doi: 10.1038/s41598-020-69164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gantner M.L., Eade K., Wallace M., Handzlik M.K., Fallon R., Trombley J., et al. Serine and lipid metabolism in macular disease and peripheral neuropathy. N Engl J Med. 2019;381(15):1422–1433. doi: 10.1056/NEJMoa1815111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonelli R., Ansell B.R.E., Lotta L., Scerri T., Clemons T.E., Leung I., et al. Genetic disruption of serine biosynthesis is a key driver of macular telangiectasia type 2 aetiology and progression. Genome Med. 2021;13(1):39. doi: 10.1186/s13073-021-00848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eade K., Gantner M.L., Hostyk J.A., Nagasaki T., Giles S., Fallon R., et al. Serine biosynthesis defect due to haploinsufficiency of PHGDH causes retinal disease. Nat Metab. 2021;3(3):366–377. doi: 10.1038/s42255-021-00361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penno A., Reilly M.M., Houlden H., Laura M., Rentsch K., Niederkofler V., et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem. 2010;285(15):11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues F.G., Pipis M., Heeren T.F.C., Fruttiger M., Gantner M., Vermeirsch S., et al. Description of a patient cohort with Hereditary Sensory Neuropathy type 1 without retinal disease Macular Telangiectasia type 2 – implications for retinal screening in HSN1. J Peripher Nerv Syst. 2022;27(3):215–224. doi: 10.1111/jns.12508. [DOI] [PubMed] [Google Scholar]
- 10.Sun H., Olson K.C., Gao C., Prosdocimo D.A., Zhou M., Wang Z., et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation. 2016;133(21):2038–2049. doi: 10.1161/CIRCULATIONAHA.115.020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fridman V., Suriyanarayanan S., Novak P., David W., Macklin E.A., McKenna-Yasek D., et al. Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology. 2019;92(4):e359–e370. doi: 10.1212/WNL.0000000000006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi R.H., Tatum S.M., Symons J.D., Summers S.A., Holland W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol. 2021;18(10):701–711. doi: 10.1038/s41569-021-00536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filippatou A.G., Moniruzzaman M., Sotirchos E.S., Fitzgerald K.C., Kalaitzidis G., Lambe J., et al. Serum ceramide levels are altered in multiple sclerosis. Mult Scler. 2021;27(10):1506–1519. doi: 10.1177/1352458520971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury M.R., Jin H.K., Bae J.S. Diverse roles of ceramide in the progression and pathogenesis of Alzheimer's disease. Biomedicines. 2022;10(8) doi: 10.3390/biomedicines10081956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Douce J., Maugard M., Veran J., Matos M., Jego P., Vigneron P.A., et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in Alzheimer's disease. Cell Metabol. 2020;31(3):503–517 e508. doi: 10.1016/j.cmet.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Cordes T., Metallo C.M. Quantifying intermediary metabolism and lipogenesis in cultured mammalian cells using stable isotope tracing and mass spectrometry. Methods Mol Biol. 2019:219–241. doi: 10.1007/978-1-4939-9236-2_14. 1978. [DOI] [PubMed] [Google Scholar]
- 17.Burla B., Muralidharan S., Wenk M.R., Torta F. Sphingolipid analysis in clinical research. Methods Mol Biol. 2018;1730:135–162. doi: 10.1007/978-1-4939-7592-1_11. [DOI] [PubMed] [Google Scholar]
- 18.Broadhurst D., Goodacre R., Reinke S.N., Kuligowski J., Wilson I.D., Lewis M.R., et al. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics. 2018;14(6):72. doi: 10.1007/s11306-018-1367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallo F.B., Leung I., Chung M., Wolf-Schnurrbusch U.E., Dubra A., Williams D.R., et al. Retinal crystals in type 2 idiopathic macular telangiectasia. Ophthalmology. 2011;118(12):2461–2467. doi: 10.1016/j.ophtha.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chew E.Y., Peto T., Clemons T.E., Pauleikhoff D., Sallo F.B., Heeren T., et al. A New Classification for Macular Telangiectasia type 2 based on multi-modal imaging. Investig Ophthalmol Vis Sci. 2019;60(9) 1335–1335. [Google Scholar]
- 21.Tzaridis S., Hess K., Heeren T.F.C., Bonelli R., Holz F.G., Friedlander M. Hyperreflectivity on optical coherence tomography in macular telangiectasia type 2. Retina. 2021;41(7):1428–1437. doi: 10.1097/IAE.0000000000003111. [DOI] [PubMed] [Google Scholar]
- 22.Heeren T.F.C., Kitka D., Florea D., Clemons T.E., Chew E.Y., Bird A.C., et al. Longitudinal correlation of ellipsoid zone loss and functional loss in macular telangiectasia type 2. Retina. 2018;38(Suppl 1):S20–S26. doi: 10.1097/IAE.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeimer M.B., Kromer I., Spital G., Lommatzsch A., Pauleikhoff D. Macular telangiectasia: patterns of distribution of macular pigment and response to supplementation. Retina. 2010;30(8):1282–1293. doi: 10.1097/IAE.0b013e3181e096dd. [DOI] [PubMed] [Google Scholar]
- 24.Wei R., Wang J., Jia E., Chen T., Ni Y., Jia W. GSimp: a Gibbs sampler based left-censored missing value imputation approach for metabolomics studies. PLoS Comput Biol. 2018;14(1) doi: 10.1371/journal.pcbi.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bates D. 2010. Lme4: mixed-effects modeling with R. [Google Scholar]
- 26.Wickham H., Averick M., Bryan J., Chang W., McGowan L.D.A., François R., et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. [Google Scholar]
- 27.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabol. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gass J.D., Blodi B.A. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology. 1993;100(10):1536–1546. [PubMed] [Google Scholar]
- 29.Charbel Issa P., Gillies M.C., Chew E.Y., Bird A.C., Heeren T.F., Peto T., et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013;34:49–77. doi: 10.1016/j.preteyeres.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller S., Charbel Issa P., Heeren T.F.C., Thiele S., Holz F.G., Herrmann P. Macular pigment distribution as prognostic marker for disease progression in macular telangiectasia type 2. Am J Ophthalmol. 2018;194:163–169. doi: 10.1016/j.ajo.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Mueller S., Gunnemann F., Rothaus K., Book M., Faatz H., Bird A., et al. Incidence and phenotypical variation of outer retina-associated hyperreflectivity in macular telangiectasia type 2. Br J Ophthalmol. 2021;105(4):573–576. doi: 10.1136/bjophthalmol-2020-317997. [DOI] [PubMed] [Google Scholar]
- 32.Krivosic V., Lavia C., Aubineau A., Tadayoni R., Gaudric A. OCT of outer retinal hyperreflectivity, neovascularization, and pigment in macular telangiectasia type 2. Ophthalmol Retina. 2021;5(6):562–570. doi: 10.1016/j.oret.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Eichler F.S., Hornemann T., McCampbell A., Kuljis D., Penno A., Vardeh D., et al. Overexpression of the wild-type SPT1 subunit lowers desoxysphingolipid levels and rescues the phenotype of HSAN1. J Neurosci. 2009;29(46):14646–14651. doi: 10.1523/JNEUROSCI.2536-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garofalo K., Penno A., Schmidt B.P., Lee H.J., Frosch M.P., von Eckardstein A., et al. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J Clin Invest. 2011;121(12):4735–4745. doi: 10.1172/JCI57549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bode H., Bourquin F., Suriyanarayanan S., Wei Y., Alecu I., Othman A., et al. HSAN1 mutations in serine palmitoyltransferase reveal a close structure-function-phenotype relationship. Hum Mol Genet. 2016;25(5):853–865. doi: 10.1093/hmg/ddv611. [DOI] [PubMed] [Google Scholar]
- 36.Lim E.W., Handzlik M.K., Trefts E., Gengatharan J.M., Pondevida C.M., Shaw R.J., et al. Progressive alterations in amino acid and lipid metabolism correlate with peripheral neuropathy in Polg(D257A) mice. Sci Adv. 2021;7(42) doi: 10.1126/sciadv.abj4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke C., Xiao R., Place E., Zhang Z., Sondheimer N., Bennett M., et al. Mitochondrial respiratory chain disease discrimination by retrospective cohort analysis of blood metabolites. Mol Genet Metabol. 2013;110(1–2):145–152. doi: 10.1016/j.ymgme.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alecu I., Tedeschi A., Behler N., Wunderling K., Lamberz C., Lauterbach M.A., et al. Localization of 1-deoxysphingolipids to mitochondria induces mitochondrial dysfunction. J Lipid Res. 2017;58(1):42–59. doi: 10.1194/jlr.M068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zucker C.L., Bernstein P.S., Schalek R.L., Lichtman J.W., Dowling J.E. A connectomics approach to understanding a retinal disease. Proc Natl Acad Sci U S A. 2020;117(31):18780–18787. doi: 10.1073/pnas.2011532117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birtel J., von Landenberg C., Gliem M., Gliem C., Reimann J., Kunz W.S., et al. Mitochondrial retinopathy. Ophthalmol Retina. 2022;6(1):65–79. doi: 10.1016/j.oret.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Clemons T.E., Gillies M.C., Chew E.Y., Bird A.C., Peto T., Wang J.J., et al. Medical characteristics of patients with macular telangiectasia type 2 (MacTel Type 2) MacTel project report no. 3. Ophthalmic Epidemiol. 2013;20(2):109–113. doi: 10.3109/09286586.2013.766757. [DOI] [PubMed] [Google Scholar]
- 42.Bonelli R., Jackson V.E., Prasad A., Munro J.E., Farashi S., Heeren T.F.C., et al. Identification of genetic factors influencing metabolic dysregulation and retinal support for MacTel, a retinal disorder. Commun Biol. 2021;4(1):274. doi: 10.1038/s42003-021-01788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark A.J., Kugathasan U., Baskozos G., Priestman D.A., Fugger N., Lone M.A., et al. An iPSC model of hereditary sensory neuropathy-1 reveals L-serine-responsive deficits in neuronal ganglioside composition and axoglial interactions. Cell Rep Med. 2021;2(7):100345. doi: 10.1016/j.xcrm.2021.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karsai G., Lone M., Kutalik Z., Brenna J.T., Li H., Pan D., et al. FADS3 is a Delta14Z sphingoid base desaturase that contributes to gender differences in the human plasma sphingolipidome. J Biol Chem. 2020;295(7):1889–1897. doi: 10.1074/jbc.AC119.011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terao R., Kaneko H. Lipid signaling in ocular neovascularization. Int J Mol Sci. 2020;21(13) doi: 10.3390/ijms21134758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cordes T., Kuna R.S., McGregor G.H., Khare S.V., Gengatharan J., Muthusamy T., et al. 1-deoxysphingolipid synthesis compromises anchorage-independent growth and plasma membrane endocytosis in cancer cells. J Lipid Res. 2022:100281. doi: 10.1016/j.jlr.2022.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available through public database: Metabolomics Workbench