GRB2 has numerous documented binding partners and is implicated in the regulation of several signaling responses in diverse cell types. Here, we show that the main function of GRB2 in thymocytes is to facilitate THEMIS-mediated inactivation of the tyrosine phosphatase SHP1.
Abstract
The T-lineage restricted protein THEMIS has been shown to play a critical role in T cell development. THEMIS, via its distinctive CABIT domains, inhibits the catalytic activity of the tyrosine phosphatase SHP1 (PTPN6). SHP1 and THEMIS bind to the ubiquitous cytosolic adapter GRB2, and the purported formation of a tri-molecular THEMIS–GRB2–SHP1 complex facilitates inactivation of SHP1 by THEMIS. The importance of this function of GRB2 among its numerous documented activities is unclear as GRB2 binds to multiple proteins and participates in several signaling responses in thymocytes. Here, we show that similar to Themis−/− thymocytes, the primary molecular defect in GRB2-deficient thymocytes is increased catalytically active SHP1 and the developmental block in GRB2-deficient thymocytes is alleviated by deletion or inhibition of SHP1 and is exacerbated by SHP1 overexpression. Thus, the principal role of GRB2 during T cell development is to promote THEMIS-mediated inactivation of SHP1 thereby enhancing the sensitivity of TCR signaling in CD4+CD8+ thymocytes to low affinity positively selecting self-ligands.
Introduction
THEMIS is a recently identified T lineage restricted protein that has been shown to perform an important function in T cell development (Choi et al., 2017b; Fu et al., 2009; Johnson et al., 2009; Kakugawa et al., 2009; Lesourne et al., 2009; Patrick et al., 2009). In the absence of THEMIS, thymocyte maturation is partially blocked at the stage associated with positive selection when immature CD4+CD8+ (double positive; DP) thymocytes receive T cell antigen receptor (TCR) signals from self-peptide/self MHC (self-pMHC) interactions that promote maturation to the CD4+CD8− (CD4 single positive; SP) or CD4−CD8+ (CD8 SP) stage (Fu et al., 2009; Johnson et al., 2009; Kakugawa et al., 2009; Lesourne et al., 2009; Patrick et al., 2009).
THEMIS contains tandem copies of a novel sequence designated the CABIT (Cysteine-containing-All-Beta-in-THEMIS) domain (Johnson et al., 2009). CABIT domains were initially identified in THEMIS, which is the prototype of a large family of single or dual CABIT proteins in metazoans (Johnson et al., 2009). Although the biological function of the THEMIS CABIT domain was initially unknown, we demonstrated that it performs an entirely new function in the TCR signaling pathway (Choi et al., 2017b). Specifically, the THEMIS CABIT domains bind to the hematopoietic protein tyrosine phosphatase SHP1 (PTPN6) and facilitate or stabilize oxidation of the SHP1 catalytic cysteine by cellular reactive oxygen species (ROS), thereby inactivating SHP1 phosphatase activity (Choi et al., 2017b). Consistent with this function, deletion of Ptpn6 (the gene encoding SHP1) alleviates the late developmental partial block in Themis−/− thymocytes, restoring near-normal positive selection (Choi et al., 2017b).
THEMIS also contains a C-terminal proline-rich sequence (PRS) that is required for its constitutive interaction with the ubiquitous cytosolic adapter protein GRB2 (Lesourne et al., 2012; Paster et al., 2013). GRB2 is a small (25 kD) protein that consists of a central SH2 domain flanked by SH3 domains. Deletion of the THEMIS PRS, which binds to the GRB2 C-terminal SH3 domain, disrupts THEMIS:GRB2 binding and renders THEMIS-ΔPRS incapable of rescuing the developmental block in Themis−/− thymocytes (Paster et al., 2013; Zvezdova et al., 2014). Thus, these findings identify an important role for the THEMIS:GRB2 interaction for THEMIS function. SHP1 binds to the N-terminal SH3 domain of GRB2, and it has been proposed that formation of a tri-molecular THEMIS:GRB2:SHP1 complex facilitates inactivation of SHP1 by THEMIS as well as controlling cellular localization of THEMIS and SHP1 following TCR stimulation (e.g., via binding of the GRB2 SH2 domain to phosphotyrosine residues in LAT and other transmembrane proteins following T cell activation; Choi et al., 2017a; Choi et al., 2017b; Paster et al., 2015). In addition to its interaction with THEMIS and SHP1, GRB2 binds to several other signaling proteins in T lineage cells including VAV1, CD28, CBL, SOS1, and PLC-γ1 and regulates Ras signaling downstream of growth factor receptors (Jang et al., 2009; Voisinne et al., 2019). Although germline deletion of Grb2 results in embryonic lethality, conditional T lineage–specific Grb2 deletion (Grb2 T-cKO) results in a phenotype similar to that of Themis−/− mice, manifesting as a partial block in late thymocyte development and a strong reduction in CD4 SP thymocytes, a less severe reduction in CD8 SP thymocytes, and peripheral lymphopenia (Jang et al., 2010). Also, similar to Themis−/− mice, positive selection of TCR transgenic DP thymocytes is markedly impaired in Grb2 T-cKO mice (Jang et al., 2010; Zvezdova et al., 2016).
In this study, we compared the developmental and signaling defects in Themis−/− and Grb2 T-cKO mice. In addition to documenting their phenotypic similarities, we found that a shared prominent defect in both Themis−/− and Grb2 T-cKO thymocytes is an increase in catalytically active SHP1 resulting in reduced activation of early signaling intermediates including ZAP-70 and LAT in response to TCR engagement. Notably, the developmental defects in Grb2 T-cKO thymocytes were substantially corrected by deletion of Shp1 (Ptpn6) and were exacerbated by overexpression of SHP1. Together, these results indicate that although GRB2 interacts with multiple proteins and is implicated in numerous distinct cellular signaling responses, its principal function in DP thymocytes is to facilitate THEMIS-mediated inactivation of SHP1 thereby enhancing TCR signaling during positive selection.
Results and discussion
Deletion of Themis or Grb2 results in a similar phenotype
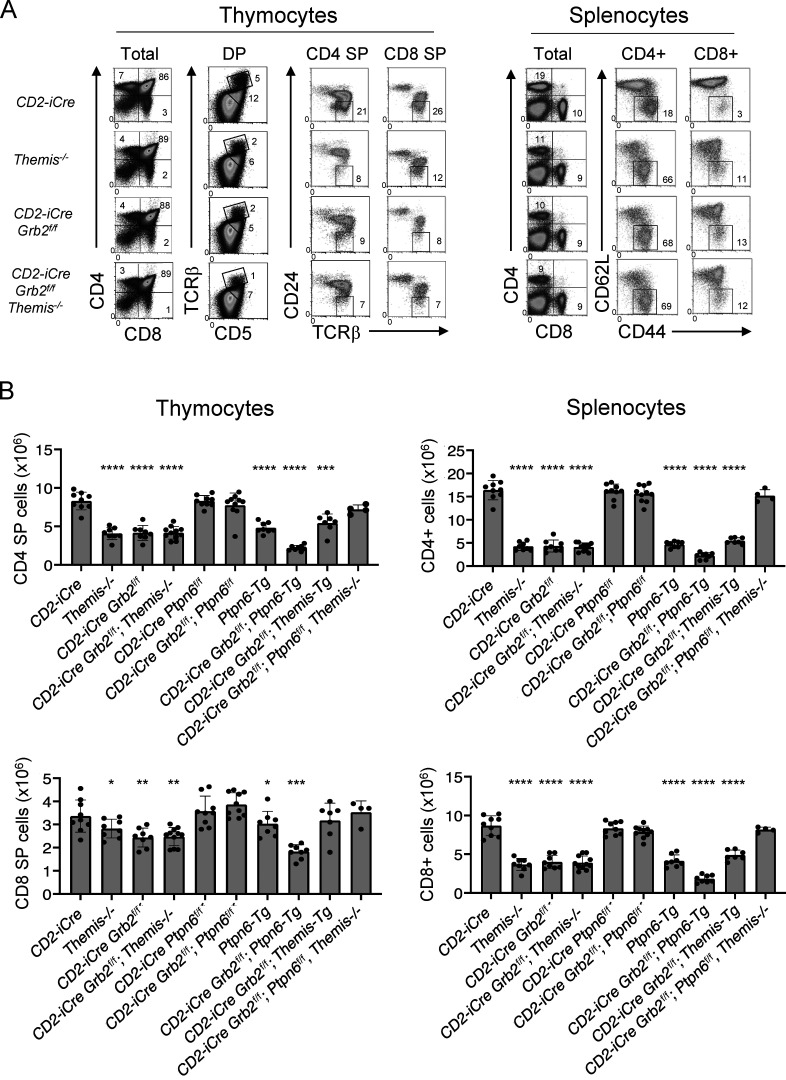
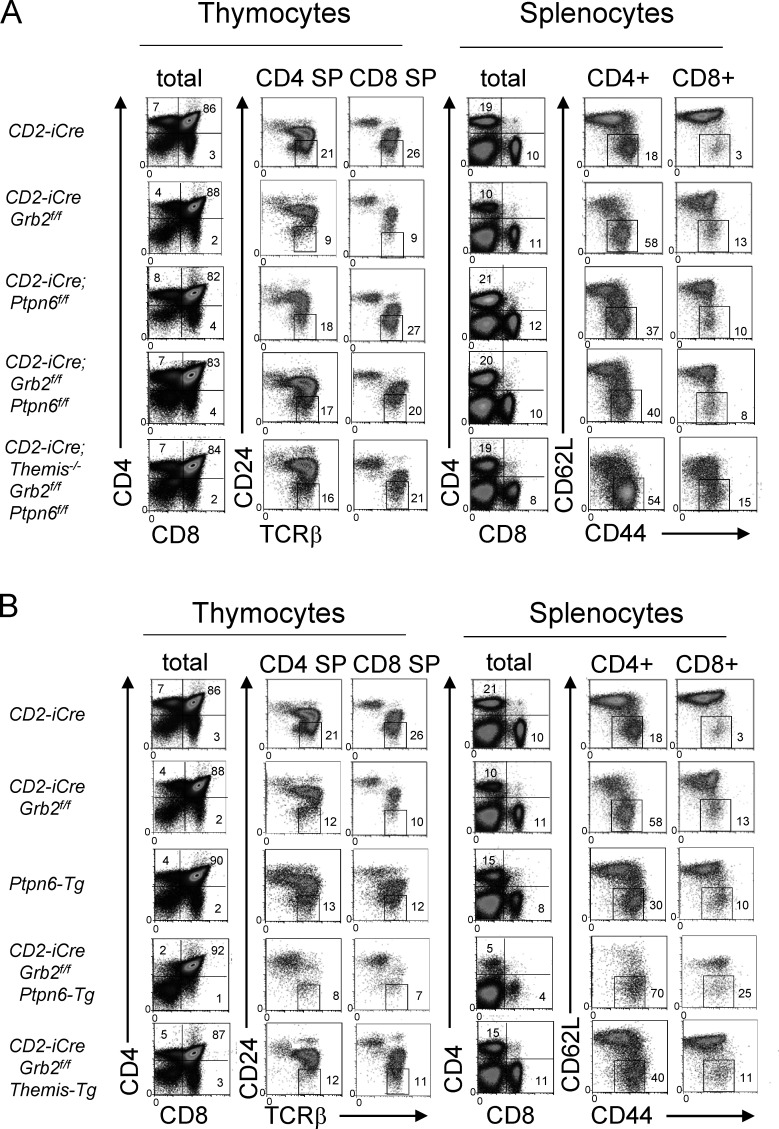
Comparison of the phenotype of Themis germline knockout and conditional T lineage Grb2-deficient mice (using the CD2-iCre transgene which begins to be expressed in immature CD4−CD8−; double negative [DN] thymocytes; de Boer et al., 2003) revealed striking similarities (Fig. 1 A). In both cases, total thymocyte numbers were slightly reduced (data not shown) and the percentage and number of CD4 SP thymocytes and CD4+ splenocytes were markedly reduced while CD8 SP thymocytes and CD8+ splenocyte percentages and numbers were significantly but less strongly reduced (Fig. 1 B). A late defect in CD4 SP and CD8 SP development in both Themis−/− and CD2-iCre;Grb2f/f thymocytes, reflected by a decrease in TCRhi CD5hi DP thymocytes and mature TCRhi CD24lo SP thymocytes, was observed in mice of each genotype (Fig. 1 A). A high percentage of mature CD4+ and CD8+ T cells from both Themis−/− and CD2-iCre;Grb2f/f mice exhibited a “memory-like” CD62Llo CD44hi phenotype likely due to lymphopenia-induced expansion (Goldrath et al., 2004; Jameson, 2002; Fig. 1, A and B). Interestingly, the thymocyte developmental defect in mice lacking both Themis (germline) and Grb2 (T cell specific) was not more severe than the single Themis knockout or Grb2 knockout, suggesting that THEMIS and GRB2 may act at the same point in the signal transduction pathway downstream of the TCR (Fig. 1, A and B).
Figure 1.
Themis-deficient and Grb2-deficient mice exhibit a similar phenotype. (A) Phenotype of thymocytes (left panels) and splenocytes (right panels) from the indicated mice. Numbers are percentage of cells in the indicated gate. Data shown are representative of at least eight mice of each genotype. Efficient Cre-mediated deletion was confirmed in all cases where appropriate by Western blot. (B) Numbers of CD4 SP and CD8 SP thymocytes (left panels) or CD4+ and CD8+ splenocytes (right panels) from all experiments. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. T test, two tailed, Type 2. Error bars show SD.
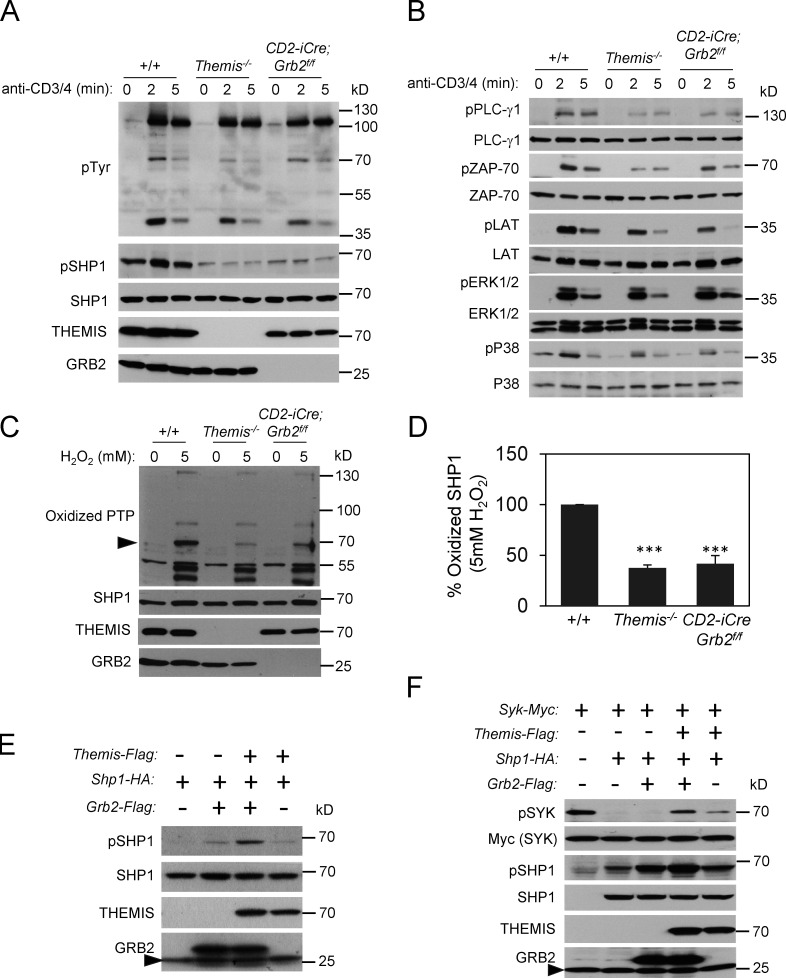
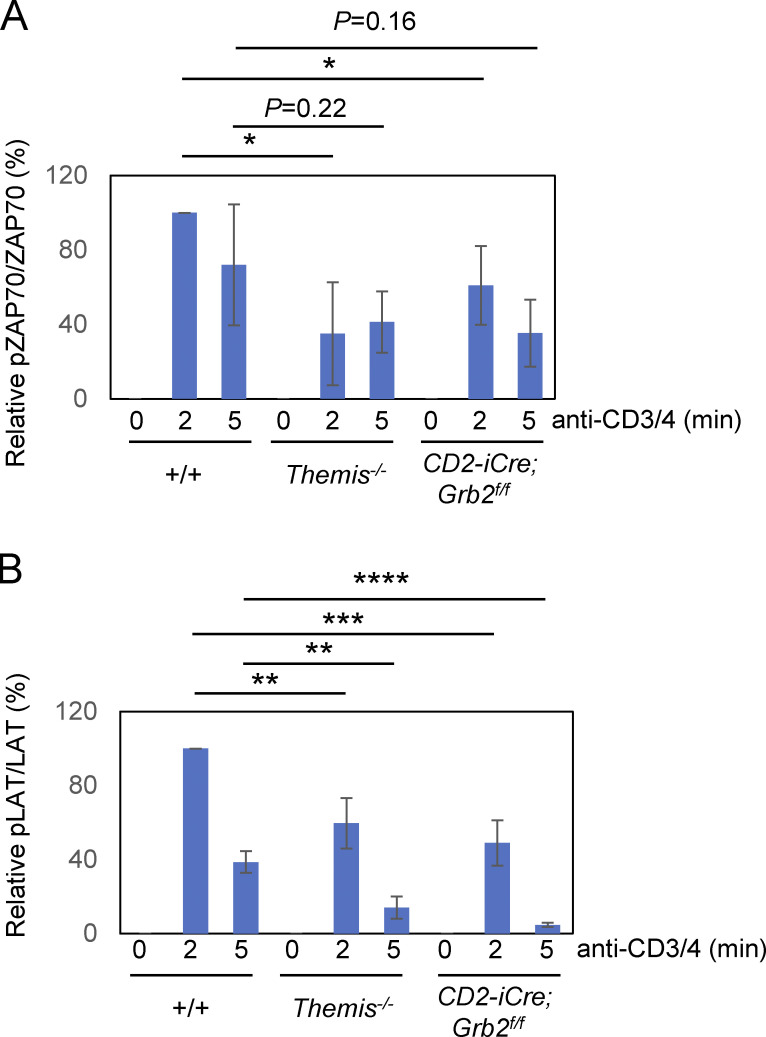
Stimulation of DP-enriched thymocytes from Themis−/− and CD2-iCre;Grb2f/f mice with anti-CD3 and anti-CD4 elicited similar signaling responses with slightly to moderately reduced activation (phosphorylation) of PLC-γ1, ZAP-70, LAT, and p38 but not ERK relative to +/+ controls (Fig. 2, A and B and Fig. S1) similar to what was reported for Lck-Cre–mediated Grb2 T-cKO thymocytes (Jang et al., 2010). Interestingly, THEMIS protein levels were reduced in CD2-iCre;Grb2f/f thymocytes (Fig. 2 A). In a previous study, we demonstrated a role for THEMIS in stabilizing and preventing the degradation of GRB2 (Zvezdova et al., 2016). The present findings are consistent with other results suggesting a reciprocal role for GRB2 in THEMIS stabilization (Garreau et al., 2017).
Figure 2.
TCR signaling responses are impaired and SHP1 tyrosine phosphatase activity is increased in Grb2-deficient thymocytes. (A and B) CD3+CD4 antibody activation of DP enriched +/+, Themis−/−, and CD2-iCre;Grb2f/f thymocytes. Thymocytes were rested for 6 h at 37°C, pre-incubated with biotinylated anti-CD3 and anti-CD4 antibodies, then streptavidin was added for the indicated times at 37°C. Cells were lysed at 4°C and proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes then blotted with the indicated antibodies. Results shown are representative of three experiments. (C) Oxidized SHP1 in Themis−/− and CD2-iCre;Grb2f/f thymocytes following treatment with H2O2. SHP1 (arrowhead) was identified by its mobility on SDS-PAGE. (D) Summary of experiments as shown in C. +/+ was arbitrarily set to 100% for comparison. Error bars show SD. ***P < 0.005. T test, two tailed, Type 2. Data shown are from four experiments. (E and F) GRB2 and THEMIS cooperate to inhibit SHP1 tyrosine phosphatase activity. HEK-293 cells were transfected with the indicated plasmids and Western blots of cell lysates were performed with the indicated antibodies. Arrowhead designates endogenous GRB2. Source data are available for this figure: SourceData F2.
Figure S1.
Quantitation of pZAP-70 bands and pLAT bands from three experiments as shown in Fig. 2 B. Bands were quantitated by densitometry relative to total ZAP-70 (A) or LAT (B) bands, respectively, for each time point. The value for +/+ at 2 min was arbitrarily set at 100% and all results are shown relative to that. Error bars show SD. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. T test, two tailed, Type 2.
A consistent finding in CD2-iCre;Grb2f/f thymocytes compared to control, +/+ thymocytes, observed in both ex vivo un-activated and activated (TCR stimulated) thymocytes, was a marked reduction in tyrosine phosphorylated SHP1 relative to Grb2+/+ controls (Fig. 2 A). Again, this phenotype closely resembled that of Themis−/− thymocytes (Fig. 2 A). Indeed, reduced tyrosine phosphorylated SHP1 (pSHP1) was the most prominent and consistent biochemical defect observed in Themis−/− thymocytes (Choi et al., 2017b; Fu et al., 2013).
Increased catalytically active SHP1 in Grb2-deficient thymocytes
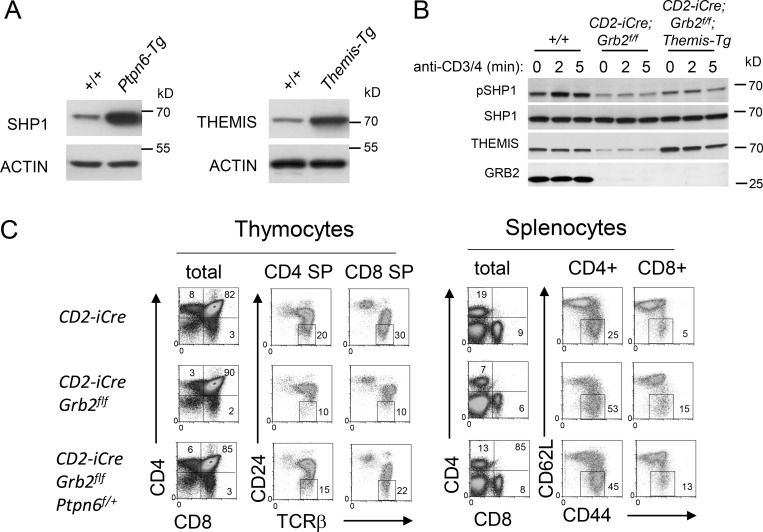
Our previous analysis of Themis−/− thymocytes demonstrated that the reduction in pSHP1 was associated with increased SHP1 tyrosine phosphatase activity; the decrease in pSHP1 being attributed to enhanced auto-/trans-dephosphorylation of SHP1 by increased catalytically active SHP1 (Choi et al., 2017b). Evaluation of the redox state of SHP1 in CD2-iCre;Grb2f/f thymocytes by Western blot revealed a lower level of oxidized (catalytically inactive) enzyme following treatment with the ROS H2O2 compared to control Grb2+/+ thymocytes, again similar to what was observed in Themis−/− thymocytes (Fig. 2, C and D; Choi et al., 2017b). Several findings suggested a cooperative effect of THEMIS and GRB2 on the inhibition of SHP1 phosphatase activity. In transfection experiments performed in HEK-293 cells, expression of THEMIS with SHP1 resulted in a slight increase in pSHP1, whereas co-expression of both THEMIS and GRB2 with SHP1 further increased the amount of pSHP1 (Fig. 2 E). Similar results were observed when we evaluated the effect of THEMIS and GRB2 co-expression on inhibition of SHP1 activity by assaying the phosphorylation status of the SHP1 target SYK (Fig. 2 F). Together, these results demonstrated that both THEMIS and GRB2 regulate the catalytic activity (and consequently the tyrosine phosphorylation state) of SHP1. Consistent with prior data demonstrating a direct interaction between THEMIS and SHP1 (Choi et al., 2017b), overexpression of THEMIS in Themis transgenic (Themis-tg) CD2-iCre;Grb2f/f thymocytes (Fig. S2 A) resulted in a slight but reproducible increase in basal pSHP1 in unstimulated thymocytes compared to CD2-iCre;Grb2f/f thymocytes (Fig. S2 B).
Figure S2.
GRB2 and THEMIS cooperate to inactivate SHP1. (A) Protein expression in Ptpn6-tg (left) and Themis-tg (right) thymocytes. (B) Overexpression of THEMIS in CD2-iCre; Grb2f/f; Themis-tg mice results in an increase in phosphorylated SHP1. (C) Ptpn6 (Shp1) haploinsufficiency (±) partially rescues the developmental defect in Grb2-deficient thymocytes. Shown are phenotypes of thymocytes (left panels) and splenocytes (right panels) from the indicated mice. Numbers are percentage of cells within each gate. Data shown in B and C are representative of four mice for each genotype. For all experiments, efficient Cre-mediated deletion was confirmed by Western blot. Source data are available for this figure: SourceData FS2.
Deletion of Ptpn6 restores T cell development in CD2-iCre;Grb2f/f mice
Deletion of the gene encoding SHP1 (Ptpn6) substantially rescues positive selection and T cell development in Themis−/− mice confirming the hypothesis that the developmental defects caused by loss of THEMIS are due to enhanced SHP1 activity in thymocytes undergoing positive selection (Choi et al., 2017b). To determine whether deletion of Shp1 could also reverse the developmental block in CD2-iCre;Grb2f/f mice, we generated CD2-iCre;Grb2f/f;Ptpn6f/f mice where both Grb2 and Ptpn6 were deleted in early DN thymocytes. As shown in Fig. 1 B and Fig. 3 A, CD2-iCre;Grb2f/f;Ptpn6f/f mice generated normal (similar to +/+ mice) numbers of CD4 SP and CD8 SP thymocytes and CD4+ and CD8+ splenocytes. We noted that although lympho-replete, both CD2-iCre;Ptpn6f/f and CD2-iCre;Grb2f/f;Ptpn6f/f mice contained higher percentages of memory-like (CD62L− CD44+) CD4+ and CD8+ T cells, a phenotype previously noted to be associated with deletion of Ptpn6 (Johnson et al., 2013). SHP1 haploinsufficiency (CD2-iCre;Grb2f/f;Ptpn6f/+ mice) resulted in a partial rescue of the Grb2-deficient phenotype suggesting that this was due to a reduction in enzymatic SHP1 activity rather than loss of a structural (protein interaction) requirement for SHP1 (Fig. S2 C). Finally, deletion of Ptpn6 rescued the developmental defect in T lineage cells lacking both Themis and Grb2, again consistent with the notion that the maturational defect caused by THEMIS deficiency or GRB2 deficiency is due to enhanced SHP1 activity (Fig. 1 B and Fig. 3 A).
Figure 3.
The developmental defect in Grb2-deficient thymocytes is rescued by deletion of Shp1 (Ptpn6) and exacerbated by overexpression of SHP1. (A and B) Phenotype of thymocytes (left panels) and splenocytes (right panels) from the indicated mice. Numbers are percentage of cells in the indicated gate. Data shown are representative of at least four mice of each genotype. Efficient Cre-mediated deletion was confirmed in all cases where appropriate by Western blot.
The developmental defect in GRB2-deficient thymocytes is exacerbated by overexpression of SHP1 and alleviated by inhibition of SHP1 activity
Transgenic overexpression of SHP1 (Ptpn6-tg) by approximately fourfold in thymocytes (Fig. S2 A; Markovics et al., 2020) resulted in a phenotype that closely resembled that of CD2-iCre;Grb2f/f and Themis−/− mice (Fig. 1, A and B and Fig. 3 B). Moreover, the thymocyte developmental defect in CD2-iCre;Grb2f/f thymocytes was markedly worsened by transgenic overexpression of SHP1, and was slightly but significantly alleviated by transgenic overexpression of THEMIS (Fig. 1 B and Fig. 3 B). These results further supported the idea that the defect in positive selection observed in Grb2-deficient thymocytes is due to enhanced SHP1 phosphatase activity.
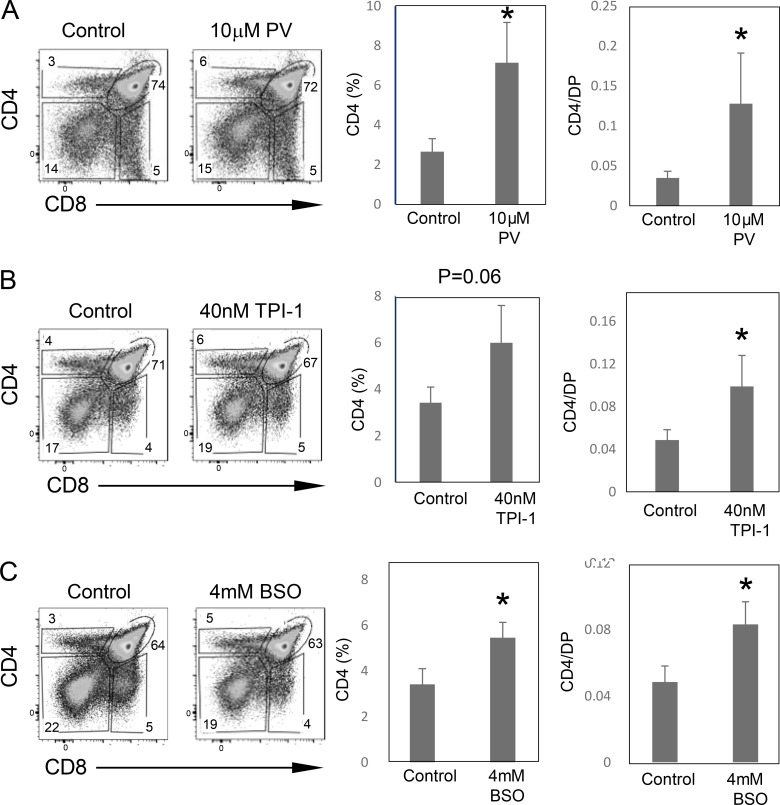
To determine whether the defect in thymocyte maturation in CD2-iCre;Grb2f/f mice could be alleviated by inhibition of SHP1 phosphatase activity, we next performed fetal thymic organ culture (FTOC) experiments with CD2-iCre;Grb2f/f thymi in the presence of known phosphatase inhibitors. Addition of the pan-phosphatase inhibitor pervanadate to FTOCs resulted in a significant rescue of CD4 SP thymocyte development in CD2-iCre;Grb2f/f thymi (Fig. S3 A). A similar partial rescue of CD4 SP thymocyte development was observed in the presence of the SHP1-specific phosphatase inhibitor, Tyrosine Phosphatase Inhibitor-1 (TPI-1; Fig. S3 B). Finally, culturing of fetal thymi in the presence of L-buthionine-sulfoximine (BSO), a cell permeable inhibitor of glutathione synthesis that delays/reduces the reduction and reactivation of reversibly ROS-oxidized protein tyrosine phosphatases, also partially restored CD4 SP thymocyte maturation in CD2-iCre;Grb2f/f thymi (Fig. S3 C).
Figure S3.
Inhibition of SHP1 tyrosine phosphatase activity enhances positive selection of CD2-iCre; Grb2f/f thymocytes. Gestation day 16 CD2-iCre; Grb2f/f fetal thymus lobes were cultured in medium alone for 2 d followed by medium alone or medium containing Pervanadate (10 μM), TPI-1 (40 nM), or BSO (4 mM) for 3 d. Thymocytes were harvested and analyzed by FACS (left panels). Shown on right are %CD4 SP thymocytes or %CD4 SP/%DP thymocytes. Error bars indicate SD (T test). All comparisons are to untreated controls. *P < 0.05. Summary results (right panels) are from four to six thymus lobes for each genotype. The genotype of fetal thymi was confirmed by PCR.
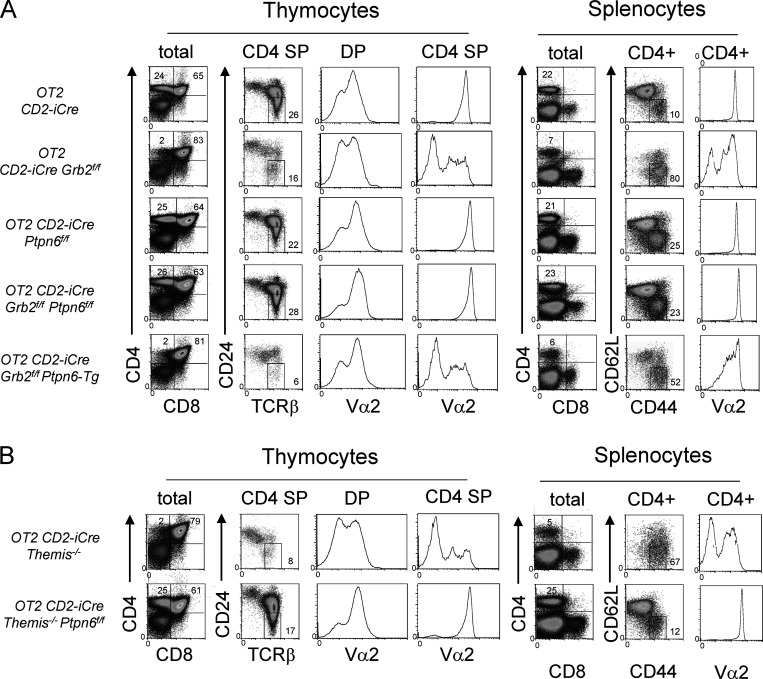
Since it was possible that deletion of Shp1 rescues thymocyte development in mice lacking GRB2 by altering the TCR repertoire of positively selected cells, we fixed the specificity of the TCR on thymocytes by introducing the MHC Class II restricted OT2 Tcrα/β transgene into CD2-iCre;Grb2f/f and CD2-iCre;Grb2f/f;Ptpn6f/f mice. Notably, the strong block in positive selection in OT2; CD2-iCre;Grb2f/f mice, evident from the marked reduction in Vα2+ CD4 SP thymocytes and CD4+ T cells compared with OT2+/+ mice, was reversed in OT2; CD2-iCre;Grb2f/f;Ptpn6f/f mice (Fig. 4 A). On the other hand, the developmental defect in OT2;CD2-iCre;Grb2f/f mice was only slightly aggravated by overexpression of SHP1 (Ptpn6-tg), possibly indicating that the effect of unrestrained SHP1 activity was already maximal (Fig. 4 A). Finally, we noted a striking similarity in the phenotype of OT2; CD2-Cre;Themis−/− and OT2; CD2-iCre;Grb2f/f mice (Fig. 4, A and B) and in the phenotype of OT2; CD2-Cre;Themis−/−;Ptpn6f/f and OT2; CD2-iCre;Grb2f/f;Ptpn6f/f mice (Fig. 4, A and B), consistent with a similar function of THEMIS and GRB2 in regulating SHP1 activity.
Figure 4.
The developmental defect in OT2 TCR transgenic Grb2-deficient thymocytes is rescued by deletion of Shp1 (Ptpn6) and exacerbated by overexpression of SHP1. (A) Phenotype of thymocytes (left panels) and splenocytes (right panels) from the indicated mice. (B) Phenotype of OT2 TCR transgenic Themis−/− mice and rescue of the developmental defect in OT2 TCR transgenic Themis−/− mice by deletion of Shp1 (Ptpn6) shown for comparison to A. Numbers indicate percentage of cells in the indicated gate. Data shown are representative of at least four mice for each genotype. Efficient Cre-mediated deletion was confirmed in all cases where appropriate by Western blot.
Deletion of Ptpn6 alleviates the signaling defect in CD2-iCre;Grb2f/f thymocytes
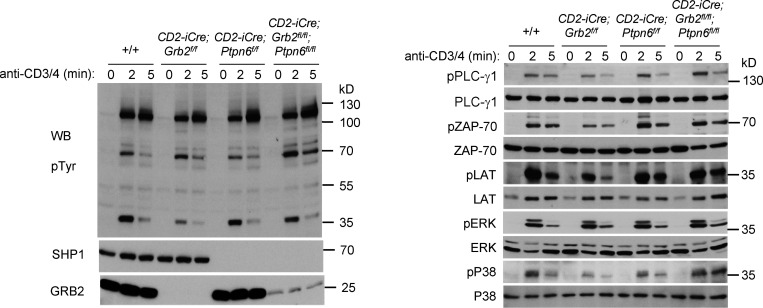
Although GRB2 interacts with several proteins that participate in the TCR signaling response in addition to SHP1, the restoration of positive selection and late thymocyte development in CD2-iCre;Grb2f/f;Ptpn6f/f mice suggested that deletion of Ptpn6 may result in a significant normalization of the TCR signaling response. To investigate this, we evaluated signaling in response to CD3+CD4 engagement in CD2-iCre;Grb2f/f;Ptpn6f/f thymocytes (Fig. 5). Activation of the proximal TCR signaling effectors ZAP-70 and LAT was substantially restored in CD2-iCre;Grb2f/f;Ptpn6f/f thymocytes (Fig. 5). In addition, activation of proteins involved in more distal signaling responses including PLC-γ1 and P38 MAP kinase was also enhanced in CD2-iCre;Grb2f/f;Ptpn6f/f thymocytes (Fig. 5). A near-normal TCR signaling response was also restored in THEMIS-deficient thymocytes by deletion of Ptpn6 (data not shown).
Figure 5.
Deletion of Ptpn6 (Shp1) alleviates the signaling defect in GRB2-deficient thymocytes. CD3+CD4 antibody activation of +/+, CD2-iCre;Grb2f/f, CD2-iCre;Ptpn6f/f, and CD2-iCre;Grb2f/f;Ptpn6f/f thymocytes. Thymocytes were rested for 6 h at 37°C, pre-incubated with biotinylated anti-CD3 and anti-CD4 antibodies, then streptavidin was added for the indicated times at 37°C. Cells were lysed at 4°C and proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, then blotted with the indicated antibodies. Results shown are representative of three independent experiments. Source data are available for this figure: SourceData F5.
The ubiquitous cytosolic adapter GRB2, first identified 30 yr ago in Caenorhabditis elegans (Clark et al., 1992), has been extensively studied in multiple cell types from diverse organisms (Tari and Lopez-Berestein, 2001). More than 70 proteins have been shown to interact with GRB2 and its expression is known to be essential for early embryogenesis, cell-cycle progression, cell motility, growth factor receptor signaling, and coordination of tyrosine kinase-mediated signal transduction (Belov and Mohammadi, 2012; Caron et al., 2017; Jang et al., 2009; Voisinne et al., 2019). In thymocytes, GRB2 has been ascribed important functions in regulating TCR signaling and positive and negative selection by facilitating the activation of MAPKs, LCK, ZAP-70, PLC-γ1, and VAV1 (Gong et al., 2001; Jang et al., 2009). In addition to these functions, more recent data have identified a role for GRB2 in activation-induced LAT oligomerization in response to TCR engagement (Houtman et al., 2006).
In the present study, we confirm a requirement for GRB2 for normal activation of ZAP-70 and LAT, each of which are less activated (tyrosine phosphorylated) in GRB2-deficient thymocytes following TCR/CD4 engagement compared with +/+ thymocytes. The relatively mild signaling defects in CD2-iCre;Grb2f/f thymocytes were reminiscent of those observed in Themis−/− thymocytes, and indeed, the phenotypes of CD2-iCre;Grb2f/f and Themis−/− mice were remarkably similar and of equal severity (Choi et al., 2017b). Of note, the most evident and reproducible biochemical finding in both CD2-iCre;Grb2f/f and Themis−/− thymocytes was a reduction in the amount of pSHP1 in both “resting” cells and following TCR stimulation. We found that similar to Themis−/− thymocytes, the reduction in pSHP1 in CD2-iCre;Grb2f/f thymocytes was due to an increase in catalytically active SHP1, which provides an explanation for the observed impairment in activation of the TCR signaling effector proteins ZAP-70 and LAT, which are targets of SHP1 (Lorenz, 2009; Pao et al., 2007a).
Taken together, our findings support a model in which formation of a tri-molecular GRB2:THEMIS:SHP1 complex is required for efficient inactivation of SHP1 in thymocytes undergoing positive selection. We previously demonstrated that the binding of THEMIS, via its novel CABIT domains, to the SHP1 catalytic domain promotes or stabilizes oxidation of the SHP1 catalytic cysteine (Choi et al., 2017b). Although THEMIS was capable of binding to and inactivating SHP1 in the absence of GRB2, SHP1 inactivation is markedly enhanced in the presence of GRB2 which may correctly position THEMIS and SHP1, stabilize the THEMIS:SHP1 interaction, or possibly render the SHP1 catalytic domain more assessable to the CABIT domains. In thymocytes, GRB2 recruits THEMIS and SHP1 to the transmembrane scaffolding adapter LAT (Paster et al., 2013). Although this interaction has been speculated to position SHP1 near its putative targets (Gascoigne and Acuto, 2015), another effect of LAT recruitment is that it positions SHP1 near the cell membrane where entry of ROS occurs through aquaporin and ion channels thereby localizing SHP1 near its catalytic inhibitors (Belikov et al., 2015; Fisher, 2009).
Considering the extremely large number of GRB2 interacting proteins that have been identified, it was unexpected and surprising that deletion of only one of its known partners, SHP1, effectively reversed the developmental block in CD2-iCre;GRB2f/f thymocytes. This may reflect thymocyte-specific properties. For example, GRB2 has been shown to play an important role in Ras activation in most cells through its interaction with the nucleotide exchange factor SOS; however, in thymocytes, Ras-GRP functions as a GRB2-independent mechanism for MAPK activation (Jang et al., 2009). Although our results do not imply that formation of GRB2:THEMIS:SHP1 complexes to enable SHP1 inactivation is the only function of GRB2 during positive selection (and GRB2 likely also performs important THEMIS and SHP1 independent signaling functions in mature T cells), it identifies this particular activity as especially critical at this stage of T cell development.
An accumulating body of work suggests that the population of DP thymocytes which undergoes positive selection is rendered especially sensitive to TCR signaling enabling activation by low affinity self-pMHC, whereas mature T cells are unresponsive to the same stimuli (Davey et al., 1998; Gaud et al., 2018). Interestingly, expression of THEMIS and SHP1 as well as several other signaling molecules including TESPA, PKD2/3, and TRAF3IP3 is developmentally regulated to specifically enhance the responsiveness of DP thymocytes and reduce the responsiveness of mature T cells (Gaud et al., 2018). The identification of a critical role for GRB2 in THEMIS-mediated suppression of SHP1 activity at the stage of positive selection underscores the importance of this stage-specific “tuning” of the TCR signaling response to enable activation and positive selection in response to very low affinity pMHC interactions.
Materials and methods
Mice
Themis−/− mice were described previously (Choi et al., 2017b). Grb2f/f mice (Jang et al., 2010) were provided by Hua Gu, McGill University, Montreal, Canada. Shp1f/f (Ptpn6f/f) mice (Pao et al., 2007b) were provided by Markus Muschen, University of California, San Francisco, San Francisco, CA, USA. CD2-iCre mice (Strain# 008520) and OT2 TCR transgenic mice (Strain# 004194) were obtained from Jackson Labs. Shp1 transgenic (Ptpn6-tg) mice were generated as described (Markovics et al., 2020). All experiments were conducted in compliance with the National Institutes of Health Animal Care and Use Committee; National Institute of Child Health and Human Development Animal Study Protocol 21-020.
FTOC
Thymic lobes were excised from fetuses at a gestational age of day 16 (the detection of the vaginal plug is considered as day 1). The lobes were put on polycarbonate membranes in 12-well transwell plate (cat. 3401; Costar) in DMEM supplemented with 10% FBS, penicillin, streptomycin, 2 mM glutamine, and 10 mM β-mercaptoethanol. After 2 d, media was exchanged and reagents were added to the culture at the indicated concentrations. After 3 more days in culture, thymocytes were released from the lobes by pressing the tissue through nylon mesh. Cells were then analyzed by flow cytometry.
Abs and reagents
The following Abs were used for flow cytometry at a 1/500 dilution: anti-CD4-Qdot 605 (Q10092) and anti-CD8-APC eFluor 780 (47-0081-82) Invitrogen; anti-CD5-PerCP (100616) Biolegend; anti-CD24-FITC (553261), anti-CD44-APC (559250), anti-CD62L-PerCP Cy5.5 (560513), and anti-TCRα(Vα2)-FITC (553288), BD Biosciences; anti-TCRβ-eFluor 450 (48-5961-82) eBioscience. For stimulation, biotin-anti-CD3 (553060), biotin- anti-CD4 (553728), and biotin-anti-CD28 (553296), used at 1 μg each per 1 × 107 thymocytes, were from BD Biosciences. For immunoblot analysis, anti-pTyr (05321) and anti-LAT (06807) EMD Millipore; anti-SHP1 (MA5-111669) and anti-pTyr783-PLC-γ1 (44-696 G) Invitrogen; anti-pTyr564-SHP1 (8849), anti-pTyr171-LAT (3581), anti-pERK (9101), anti-ERK (4695), anti-pp38 MAPK (9211), and anti-p38 MAPK (9212) were from Cell Signaling Technology; anti-pTyr319-ZAP-70 (612574), anti-ZAP-70 (610240), and anti-GRB2 (610111) were from BD Biosciences; anti-actin (SC58673) and anti-PLC-γ1 (SC81) were from Santa Cruz Biotechnology; and anti-oxidized PTP active site mAb (MAB2844) was from R&D Systems. The antibodies for immunoblot analysis were diluted 1:1,000. The rabbit polyclonal antiserum to THEMIS has been described (Lesourne et al., 2009). Streptavidin was purchased from Southern Biotechnology. Mouse IgGkBP-HRP (SC516102) and anti-rabbit IgG-HRP (SC2357) were purchased from Santa Cruz Biotechnology. BSO (B2515) was purchased from Sigma-Aldrich. TPI-1 (22480) was purchased from Cayman Chemical. 1 mM Pervanadate was made with 10 μl of 100 mM Na3VO4 and 5 μl of 3% H2O2 (cat. 88597; Sigma-Aldrich) in 1 ml H2O.
Flow cytometry
Cells were treated with Fc blocking antibody 2.4G2 (BD Biosciences) at 1/1,000 dilution of stock. Antibody cocktails were prepared in HBSS containing 1% BSA Fraction V (MP Biomedicals), 0.1% NaN3 (Sigma-Aldrich), and cells were labeled for 30 min at 4°C while shielded from light. Samples were washed and resuspended in the described buffer and acquired on an LSRFortessa X-20 cell analyzer (BD Biosciences). Analysis was conducted on FlowJo software (v10.6.1; TreeStar Inc.).
Plasmids and constructs
The construct for Flag-tagged THEMIS was subcloned into pFLAG-CMV2 vector by PCR with THEMIS-eGFP plasmid. Myc-SYK plasmid was purchased from OriGene. HA-tagged SHP1 were subcloned into pCDNA3-HA vector by PCR with human cDNA for SHP1 from Addgene. Flag-tagged Grb2 was subcloned into pFLAG-CMV2 vector by PCR with human cDNA for Grb2 from Addgene. Subcloned plasmids were confirmed by DNA sequencing.
Immunoblot analysis
DP enriched thymocytes were obtained by positive selection with anti-CD8 magnetic beads (Miltenyi). Cells used for biochemistry experiments were 97–99% DP, the remaining cells were CD8 SP. Thymocytes were stimulated with anti-CD3 biotin plus anti-CD4 biotin followed by cross-linking with streptavidin. Cells were then washed in ice-cold PBS and lysed in standard lysis buffer (1% Nonidet P-40, 10 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, and protease inhibitors). Immunoblot analysis was performed as described (Choi et al., 2017b).
Transient transfections
HEK-293 cells were cultured in DMEM supplemented with 10% (vol/vol) FBS and 2 mM glutamine, plus penicillin and streptomycin (100 U/ml each). 1 × 106 cells were co-transfected with the appropriate plasmid using Lipofectamine 2000 (Thermo Fisher Scientific). HEK-293 cells were incubated for 24 h and then washed in ice-cold PBS and lysed in standard lysis buffer.
Analysis of SHP1 oxidation after stimulation with H2O2
Analysis of SHP1 oxidation was performed as described (Choi and Love, 2018). Briefly, thymocytes were stimulated with 5 mM H2O2 for 5 min at room temperature. Cells were then washed with degassed 1 × PBS and lysed in degassed standard lysis buffer including 10 mM iodoacetamide and 10 mM N-Ethylmaleimide. Immunoblot analysis of SHP1 oxidation was performed with antibody to oxidized protein tyrosine phosphatase active site.
Statistics
For the detection of oxidized SHP1, FTOC, and cell (thymocyte and lymphocyte) counts, significance was calculated by t test, two-tailed, type 2 (unpaired equal variance).
Supplementary Material
is the source file for Fig. 2.
is the source file for Fig. 5.
is the source file for Fig. S2.
Acknowledgments
This paper is dedicated to the memory of Tibor Glant, MD, PhD, Department of Orthopedic Surgery, Rush University Medical Center.
This work was supported by intramural National Institutes of Health funding (P. Love): Project number: 1 ZIAHD001803-28.
Author Contributions: S. Choi, T. Hatzihristidis, G. Gaud, A. Dutta, J. Lee, A. Arya, L.M. Clubb, and D.B. Stamos performed experiments, A. Markovics and K. Mikecz generated Ptpng-Tg mice, S.Choi and P. Love designed experiments, and P. Love wrote the paper.
Data availability
The data underlying Figs. 1, 2, 3, 4, and 5 are available in the published article and its online supplemental material.
Online supplemental material
Fig. S1 shows quantitation of pZAP-70 and pLAT in three replicate experiments by densitometry. Fig. S2 shows expression of Ptpn6 and Themis transgenes; effect of Themis transgene on pSHP1 levels in Grb2-deficient thymocytes; and phenotype of CD2-iCre;Grb2f/f;Ptpn6f/+ mice. Fig. S3 shows the effect of tyrosine phosphatase inhibitors (pervanadate, TPI-1) or BSO on thymocyte development in FTOCs.
References
- Belikov, A.V., Schraven B., and Simeoni L.. 2015. T cells and reactive oxygen species. J. Biomed. Sci. 22:85. 10.1186/s12929-015-0194-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov, A.A., and Mohammadi M.. 2012. Grb2, a double-edged sword of receptor tyrosine kinase signaling. Sci. Signal. 5:pe49. 10.1126/scisignal.2003576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron, E., Roncagalli R., Hase T., Wolski W.E., Choi M., Menoita M.G., Durand S., García-Blesa A., Fierro-Monti I., Sajic T., et al. 2017. Precise Temporal Profiling of Signaling Complexes in Primary Cells Using SWATH Mass Spectrometry. Cell Rep. 18:3219–3226. 10.1016/j.celrep.2017.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S., Cornall R., Lesourne R., and Love P.E.. 2017a. THEMIS: Two Models, Different Thresholds. Trends Immunol. 38:622–632. 10.1016/j.it.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S., and Love P.E.. 2018. Detection of intracellular reduced (catalytically active) SHP-1 and analyses of catalytically inactive SHP-1 after oxidation by pervanadate or H2O2. Bio Protoc. 8. e2684. 10.21769/BioProtoc.2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S., Warzecha C., Zvezdova E., Lee J., Argenty J., Lesourne R., Aravind L., and Love P.E.. 2017b. THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat. Immunol. 18:433–441. 10.1038/ni.3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.G., Stern M.J., and Horvitz H.R.. 1992. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 356:340–344. 10.1038/356340a0 [DOI] [PubMed] [Google Scholar]
- Davey, G.M., Schober S.L., Endrizzi B.T., Dutcher A.K., Jameson S.C., and Hogquist K.A.. 1998. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J. Exp. Med. 188:1867–1874. 10.1084/jem.188.10.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer, J., Williams A., Skavdis G., Harker N., Coles M., Tolaini M., Norton T., Williams K., Roderick K., Potocnik A.J., and Kioussis D.. 2003. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33:314–325. 10.1002/immu.200310005 [DOI] [PubMed] [Google Scholar]
- Fisher, A.B. 2009. Redox signaling across cell membranes. Antioxid. Redox Signal. 11:1349–1356. 10.1089/ars.2008.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, G., Casas J., Rigaud S., Rybakin V., Lambolez F., Brzostek J., Hoerter J.A., Paster W., Acuto O., Cheroutre H., et al. 2013. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature. 504:441–445. 10.1038/nature12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, G., Vallée S., Rybakin V., McGuire M.V., Ampudia J., Brockmeyer C., Salek M., Fallen P.R., Hoerter J.A., Munshi A., et al. 2009. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat. Immunol. 10:848–856. 10.1038/ni.1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreau, A., Blaize G., Argenty J., Rouquié N., Tourdès A., Wood S.A., Saoudi A., and Lesourne R.. 2017. Grb2-Mediated Recruitment of USP9X to LAT Enhances Themis Stability following Thymic Selection. J. Immunol. 199:2758–2766. 10.4049/jimmunol.1700566 [DOI] [PubMed] [Google Scholar]
- Gascoigne, N.R., and Acuto O.. 2015. THEMIS: a critical TCR signal regulator for ligand discrimination. Curr. Opin. Immunol. 33:86–92. 10.1016/j.coi.2015.01.020 [DOI] [PubMed] [Google Scholar]
- Gaud, G., Lesourne R., and Love P.E.. 2018. Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 18:485–497. 10.1038/s41577-018-0020-8 [DOI] [PubMed] [Google Scholar]
- Goldrath, A.W., Luckey C.J., Park R., Benoist C., and Mathis D.. 2004. The molecular program induced in T cells undergoing homeostatic proliferation. Proc. Natl. Acad. Sci. USA. 101:16885–16890. 10.1073/pnas.0407417101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Q., Cheng A.M., Akk A.M., Alberola-Ila J., Gong G., Pawson T., and Chan A.C.. 2001. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat. Immunol. 2:29–36. 10.1038/83134 [DOI] [PubMed] [Google Scholar]
- Houtman, J.C., Yamaguchi H., Barda-Saad M., Braiman A., Bowden B., Appella E., Schuck P., and Samelson L.E.. 2006. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat. Struct. Mol. Biol. 13:798–805. 10.1038/nsmb1133 [DOI] [PubMed] [Google Scholar]
- Jameson, S.C. 2002. Maintaining the norm: T-cell homeostasis. Nat. rev. Immunol. 2:547–556. 10.1038/nri853 [DOI] [PubMed] [Google Scholar]
- Jang, I.K., Zhang J., Chiang Y.J., Kole H.K., Cronshaw D.G., Zou Y., and Gu H.. 2010. Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc. Natl. Acad. Sci. USA. 107:10620–10625. 10.1073/pnas.0905039107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, I.K., Zhang J., and Gu H.. 2009. Grb2, a simple adapter with complex roles in lymphocyte development, function, and signaling. Immunol. Rev. 232:150–159. 10.1111/j.1600-065X.2009.00842.x [DOI] [PubMed] [Google Scholar]
- Johnson, A.L., Aravind L., Shulzhenko N., Morgun A., Choi S.Y., Crockford T.L., Lambe T., Domaschenz H., Kucharska E.M., Zheng L., et al. 2009. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat. Immunol. 10:831–839. 10.1038/ni.1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, D.J., Pao L.I., Dhanji S., Murakami K., Ohashi P.S., and Neel B.G.. 2013. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J. Exp. Med. 210:1419–1431. 10.1084/jem.20122239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakugawa, K., Yasuda T., Miura I., Kobayashi A., Fukiage H., Satoh R., Matsuda M., Koseki H., Wakana S., Kawamoto H., and Yoshida H.. 2009. A novel gene essential for the development of single positive thymocytes. Mol. Cell. Biol. 29:5128–5135. 10.1128/MCB.00793-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesourne, R., Uehara S., Lee J., Song K.D., Li L., Pinkhasov J., Zhang Y., Weng N.P., Wildt K.F., Wang L., et al. 2009. Themis, a T cell-specific protein important for late thymocyte development. Nat. Immunol. 10:840–847. 10.1038/ni.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesourne, R., Zvezdova E., Song K.D., El-Khoury D., Uehara S., Barr V.A., Samelson L.E., and Love P.E.. 2012. Interchangeability of Themis1 and Themis2 in thymocyte development reveals two related proteins with conserved molecular function. J. Immunol. 189:1154–1161. 10.4049/jimmunol.1200123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, U. 2009. SHP-1 and SHP-2 in T cells: Two phosphatases functioning at many levels. Immunol. Rev. 228:342–359. 10.1111/j.1600-065X.2008.00760.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovics, A., Toth D.M., Glant T.T., and Mikecz K.. 2020. Regulation of autoimmune arthritis by the SHP-1 tyrosine phosphatase. Arthritis Res. Ther. 22:160. 10.1186/s13075-020-02250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao, L.I., Badour K., Siminovitch K.A., and Neel B.G.. 2007a. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu. Rev. Immunol. 25:473–523. 10.1146/annurev.immunol.23.021704.115647 [DOI] [PubMed] [Google Scholar]
- Pao, L.I., Lam K.P., Henderson J.M., Kutok J.L., Alimzhanov M., Nitschke L., Thomas M.L., Neel B.G., and Rajewsky K.. 2007b. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 27:35–48. 10.1016/j.immuni.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Paster, W., Brockmeyer C., Fu G., Simister P.C., de Wet B., Martinez-Riaño A., Hoerter J.A., Feller S.M., Wülfing C., Gascoigne N.R., and Acuto O.. 2013. GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J. Immunol. 190:3749–3756. 10.4049/jimmunol.1203389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster, W., Bruger A.M., Katsch K., Grégoire C., Roncagalli R., Fu G., Gascoigne N.R., Nika K., Cohnen A., Feller S.M., et al. 2015. A THEMIS:SHP1 complex promotes T-cell survival. EMBO J. 34:393–409. 10.15252/embj.201387725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick, M.S., Oda H., Hayakawa K., Sato Y., Eshima K., Kirikae T., Iemura S., Shirai M., Abe T., Natsume T., et al. 2009. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc. Natl. Acad. Sci. USA. 106:16345–16350. 10.1073/pnas.0908593106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tari, A.M., and Lopez-Berestein G.. 2001. GRB2: A pivotal protein in signal transduction. Semin. Oncol. 28:142–147. 10.1016/S0093-7754(01)90291-X [DOI] [PubMed] [Google Scholar]
- Voisinne, G., Kersse K., Chaoui K., Lu L., Chaix J., Zhang L., Goncalves Menoita M., Girard L., Ounoughene Y., Wang H., et al. 2019. Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics. Nat. Immunol. 20:1530–1541. 10.1038/s41590-019-0489-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvezdova, E., Lee J., El-Khoury D., Barr V., Akpan I., Samelson L., and Love P.E.. 2014. In vivo functional mapping of the conserved protein domains within murine Themis1. Immunol. Cell Biol. 92:721–728. 10.1038/icb.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvezdova, E., Mikolajczak J., Garreau A., Marcellin M., Rigal L., Lee J., Choi S., Blaize G., Argenty J., Familiades J., et al. 2016. Themis1 enhances T cell receptor signaling during thymocyte development by promoting Vav1 activity and Grb2 stability. Sci. Signal. 9:ra51. 10.1126/scisignal.aad1576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
is the source file for Fig. 2.
is the source file for Fig. 5.
is the source file for Fig. S2.
Data Availability Statement
The data underlying Figs. 1, 2, 3, 4, and 5 are available in the published article and its online supplemental material.
Online supplemental material
Fig. S1 shows quantitation of pZAP-70 and pLAT in three replicate experiments by densitometry. Fig. S2 shows expression of Ptpn6 and Themis transgenes; effect of Themis transgene on pSHP1 levels in Grb2-deficient thymocytes; and phenotype of CD2-iCre;Grb2f/f;Ptpn6f/+ mice. Fig. S3 shows the effect of tyrosine phosphatase inhibitors (pervanadate, TPI-1) or BSO on thymocyte development in FTOCs.