Abstract
Background:
Exposure to air pollution in prenatal period is associated with prelabor rupture of membranes (PROM). However, the sensitive exposure time windows and the possible biological mechanisms underlying this association remain unclear.
Objective:
We aimed to identify the sensitive time windows of exposure to air pollution for PROM risk. Further, we examined whether maternal hemoglobin levels mediate the association between exposure to air pollution and PROM, as well as investigated the potential effect of iron supplementation on this association.
Method:
From 2015 to 2021, 6,824 mother–newborn pairs were enrolled in the study from three hospitals in Hefei, China. We obtained air pollutant data [particulate matter (PM) with aerodynamic diameter (), PM with aerodynamic diameter (), sulfur dioxide (), and carbon monoxide (CO)] from the Hefei City Ecology and Environment Bureau. Information on maternal hemoglobin levels, gestational anemia, iron supplementation, and PROM was obtained from medical records. Logistic regression models with distributed lags were used to identify the sensitive time window for the effect of prenatal exposure to air pollutant on PROM. Mediation analysis estimated the mediated effect of maternal hemoglobin in the third trimester, linking prenatal air pollution with PROM. Stratified analysis was used to investigate the potential effect of iron supplementation on PROM risk.
Results:
We found significant association between prenatal exposure to air pollution and increased PROM risk after adjusting for confounders, and the critical exposure windows of , , and CO were the 21th to 24th weeks of pregnancy. Every increase in and , increase in , and increase in CO was associated with low maternal hemoglobin levels [ (95% confidence interval (CI): , ), (95% CI: , ), (95% CI: , ), and (95% CI: , ), respectively] in the third trimester. The proportion of the association between air pollution and PROM risk mediated by hemoglobin levels was 20.61% [average mediation effect (95% CI): 0.02 (0.01, 0.05); average direct effect (95%): 0.08 (0.02, 0.14)]. The PROM risk associated with exposure to low-medium air pollution could be attenuated by maternal iron supplementation in women with gestational anemia.
Conclusions:
Prenatal exposure to air pollution, especially in the 21st to 24th weeks of pregnancy, is associated with PROM risk, which is partly mediated by maternal hemoglobin levels. Iron supplementation in anemia pregnancies may have protective effects against PROM risk associated with exposure to low–medium air pollution. https://doi.org/10.1289/EHP11134
Introduction
Air pollution is associated with health problems, including pregnancy-associated complications and increased adverse pregnancy outcomes.1–3 Prelabor rupture of membranes (PROM) affects 3% to 21% of all pregnant women globally4,5; it is a serious problem for maternal and infant health and may result in maternal mortality and premature birth.6–8 Thus, it is essential to identify factors that lead to PROM and their potential pathways.
Few studies have suggested a link between exposure to air pollution and PROM. A study conducted in Australia found that exposure to during the second trimester of pregnancy increased the risk of PROM by 3%.9 The prevalence of PROM has shown a small but significant decline in Australia; however, it continues to increase in China.9,10 A cohort study conducted in China between 2015 and 2017 indicated that daily levels (median concentrations: ) were more than 2-fold the limit recommended by the World Health Organization (WHO) ( annually)11,12; this could partly explain the rise in PROM in China.11 Because no consistent evidence is available, the association between chronic (during the entire period of pregnancy) exposure to air pollution and an increased risk of PROM needs to be investigated.
Recent studies have focused on the association of PROM with maternal factors, such as low maternal hemoglobin levels and anemia.13,14 Low maternal hemoglobin concentration may induce infections and predispose women to PROM.15 Several studies have indicated that maternal anemia may be responsible for the increased risk of PROM.13,15,16 In addition, few studies have suggested that fine particles might increase the risk of anemia and cause a decrease in hemoglobin levels in children and elder.17,18 However, evidence of the relationship between exposure to air pollution and hemoglobin levels and anemia in pregnant women is limited.
Therefore, this prospective cohort study aimed to investigate the associations of weekly exposure to air pollution during pregnancy with PROM risk, identify the windows of susceptibility, and calculate the cumulative effect of the window. Furthermore, we examined whether maternal hemoglobin levels have a mediator effect on these associations, as well as the potential effect of iron supplementation.
Research Design and Method
Study Participants
Our study was based on a maternal and infant health cohort study in Hefei (MIH-Hefei), China. Details of data collection and recruitment have been described previously.19,20 From March 2015 to September 2021, a total of 9,320 pregnant women were recruited for the study, provided they were between 13 and 23 gestational weeks, had a single pregnancy, lived in Hefei for at least 2 y, and gave birth at obstetrical examination hospitals.
Subjects who met the following criteria were excluded: participants pregnant using assisted reproductive techniques, participants experiencing serious pregnancy complications (including hyperemesis gravidarum and abnormal heart or liver function), pregnant mothers with uncertain address of residence, and pregnant women whose questionnaires showed missing data on birth outcome. Finally, 6,824 pregnant women were enrolled for the study at three hospitals in Hefei (Hefei First People’s Hospital, Anhui Maternal and Child Care Hospital, and the First Affiliated Hospital of Anhui Medical University). The annual outpatient volume of obstetrics in the three hospitals included in our study ranks in the top three in Hefei and accounts for of all hospitals in Hefei.21 Protocols used in our study were approved by the Ethics Committee of Anhui Medical University (number: 2015002).
Exposure to Air Pollution
The ambient air pollution data for , , , and CO levels in urban Hefei, estimated at 11 air monitoring points in five air monitoring stations (Shushanqu, Baohequ, Luyangqu, Yaohaiqu, and Xinzhanqu), are available on the Hefei Environment Projection Administration website (http://sthjj.hefei.gov.cn/index.html). We then converted the current address of the participants to map coordinates using Baidu Maps. The distance from the subjects’ home addresses to the matching monitor stations was calculated based on the coordinates, and 93.1% (mean: , range: ) of participants lived within of the nearest station. We estimated full gestational and trimester exposure to air pollution by calculating the daily averages and averaging the daily exposures throughout the total gestational and trimester-specific periods. Trimester periods were estimated based on the first day of the mother’s previous menstrual cycle and were verified by ultrasound examination of gestational age. The first, second, and third trimesters refer to gestational weeks (GW) 1–13, 14–26, and 27–40 or at birth, whichever was earlier.22
Assessment of PROM and Anemia during Pregnancy
Women with PROM (including preterm and term PROM) were identified from clinical and extract this information from the medical records from the three hospitals mentioned above; in our study, PROM was named code O42 and could be found in the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Preterm and term PROM is defined as rupture of fetal membranes prior to and at 37 wk or after 37 wk of gestation, respectively.
Maternal hemoglobin levels were measured during GW 24–28 and 32–36 in hospitals. Hemoglobin levels measured during 24–28 wk were used for the diagnosis of anemia according to the WHO guidelines.23 Women with hemoglobin levels lower than were diagnosed as having anemia during pregnancy according to WHO criteria. Daily iron supplements ( of elemental iron) were recommended to pregnant women with anemia.24 Hemoglobin levels measured between 32–36 wk were used for subsequent analysis.
Confounding Variables
Demographic characteristic covariates were obtained through a face-to-face questionnaire used in a standardized interview of pregnant women at enrollment, including maternal age (, 25–34, y), education (junior high school, high school, or bachelor’s degree and above), average family income [, 4,000–7,999, renminbi (RMB)/month], and parity (primipara, multipara). Self-reported information on lifestyle factors included the frequency of fruit and dessert intake (cake, ice cream, store-bought sweet rolls, etc.), vegetable intake during second trimester, maternal folic acid supplementation frequency (, d/wk) during first trimester, maternal passive smoking status (ever or never) during second trimester, and maternal iron supplementation frequency (, d/wk) during third trimester. The International Physical Activity Questionnaire25 was used to evaluate moderate physical activity (including table tennis, badminton, and vigorous walking) for at least 30 min per day. Pregnant women were divided into groups of or d/wk, based on iron supplementation frequency. Health-related characteristics were extracted from medical records during the second and third trimester, including prepregnancy body mass index (BMI) (, , or ), gestational diabetes mellitus (GDM) (yes or no); hypertension during pregnancy, including chronic hypertension (hypertension that preceded pregnancy), and preeclampsia (hypertension along with thrombocytopenia, systemics impairment such as liver function damage, progressing renal insufficiency, etc.), gestational hypertension (hypertension happening after 20 GW without the systemic findings aforementioned or proteinuria),26 and vaginitis (yes or no). After delivery, further details such as the season of delivery (spring/summer/autumn/winter), gestational weeks of delivery, and preterm status ( GW) were collected from the medical records. Information on the daily temperature (°C) in the month before delivery was obtained from the China Meteorological Administration (https://data.cma.cn/).
Statistical Analysis
The demographic characteristics of the participants with and without PROM were summarized using descriptive statistics. Chi-square () tests were used to compare the characteristics of women with and without PROM and characteristics of women included and excluded in the analysis. Spearman’s correlation analysis was used to examine the associations between , , , and CO levels. Confounders related to prenatal air pollution exposure, maternal hemoglobin levels, and PROM were determined using a directed acyclic graph (DAG) to visualize these relationships (Supplemental Figure 1).
Distributed lag nonlinear models were used to examine the sensitive windows of weekly , , , and CO exposure throughout GW 1–37 related to PROM in unadjusted and adjusted models.27,28 Maternal age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature were incorporated in the models. We built cross-basis matrices to model the exposure–lag–response association. The exposure–response function was assumed to be linear, and the lag structure was modeled using a natural cubic spline with degrees of freedom (df) based on the Akaike information criterion. We used the distributed lag model (DLM) to calculate the cumulative estimates for the first trimester (GW 1–13), second trimester (GW 14–26), third trimester (after GW 27) by incorporating weekly exposure during pregnancy. The models were adjusted for several covariates, including maternal age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature. We further constructed a logistic regression model to estimate the trimesters-specific exposure with PROM risk. In addition, a logistic regression model was used to estimate the associations of average exposure to air pollutant during the three trimesters with the risk of preterm PROM, term PROM, and PROM in unadjusted and adjusted models. The models were adjusted for several covariates, including maternal age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature. The logistic models that additionally included the trimester adjustment were done. Furthermore, we preliminarily evaluated the collinearity of four air pollution concentrations using correlation analysis among four air pollutants. Considering the impact of collinearity on the effect estimate, the copollutant models were used in the sensitivity analysis.
Multiple linear regression was used to estimate the association of each air pollutant (per in and , per in , and per in CO; per quartile increase) throughout the second and third trimesters with maternal hemoglobin levels in the third trimester. Adjustments for covariates included age, education, income, activity, passive smoking, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, and temperature. The odds ratio (OR) of PROM was calculated per unit increase in air pollution exposure (per in and , per in , and per in CO) stratified by anemia status () according to WHO standards23 based on logistic regression models. We examined the potential exposure–response relationships between maternal hemoglobin levels in the third trimester and PROM risk using logistic regression models after adjusting for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature. Considering the WHO cutoff points of maternal hemoglobin levels () for the diagnosis of anemia and the sample size, women were classified into nine groups according to change. Women with hemoglobin levels of (reference) and were defined as the highest and lowest level groups, respectively.
Principal component analysis (PCA) accounts for interactions between all pollutants and attributes these interactions to a principal component.29–31 PCA was used as a tool capable of providing an overview of the interdependencies and variability of prenatal air pollutants. After calculating the principal component scores of four pollutants, a mediation analysis was performed using the “mediation” package in R to estimate the role of hemoglobin levels during the third trimester in association with exposure to four pollutants in the second trimester, the third trimester, and throughout the second trimester and third trimester as well as its contribution to PROM risk. The total effect, including the average mediation effect (AME) and average direct effect (ADE), was calculated.32 The AME referred to the indirect effect of prenatal exposure to four air pollutants on PROM mediated by hemoglobin in the third trimester, and the ADE referred to the effect of prenatal exposure to four air pollutants on PROM, excluding the effect of hemoglobin during the third trimester. The counterfactual framework for mediation analysis was used to examine the causal mediation assumptions. Mediator models (), outcome models () were specified, and the “treat” (air pollution) as well as “mediator” variables were specified (Supplemental Figure 1).33–34 Adjustments for covariates included age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature.
Logistic regression models were used to estimate the association between maternal iron supplementation and PROM risk stratified by air pollution in women with anemia. Low-medium air pollution was defined as air pollution concentration percentile. Women with gestational anemia were classified into following four groups: a) low–medium air pollution ( percentile, P50) and iron supplementation, b) low–medium air pollution () and no iron supplementation, c) high air pollution () and iron supplementation, and d) high air pollution () and no iron supplementation. High air pollution () and no iron supplementation group was considered as the reference group, and OR for PROM for the other three groups were calculated. The average exposure levels of air pollutants selected at P50 were similar to those seen in previous studies in China.11 The -trend for maternal hemoglobin levels and the prevalence of PROM across four groups were calculated by general linear regression model and Mantel-Haenszel chi-square test, respectively. Furthermore, we conducted a sensitivity analysis based on the exposure levels of air pollution at P75 (75th percentile).
Statistical significance was considered as a two-sided . We performed all analyses in R (version 3.5.0; R Core Development Team) using the R statistical packages “ggplot2,” “dlnm,” and “mediation” and the SPSS statistical software (Statistical Package for the Social Sciences version 23.0; IBM Corp.).
Results
The characteristics of the study population are summarized in Table 1. Of the 6,824 women who agreed to participate in the study, 1,439 (21.1%) had PROM. Women with PROM were more likely to be younger and to have a lower frequency of folic acid supplementation during pregnancy than those without PROM (Table 1). In comparison with women without PROM, higher proportions of primipara, GDM, gestational anemia, and delivery in winter and spring were observed in women with PROM (Table 1). The excluded women had incomplete covariates on birth outcomes and had a higher proportion of giving birth in winter and spring (Supplement Table 1).
Table 1.
The general characteristics of the study population during 2015 to 2021 in Hefei [ (%)].
| Characteristics | All () | PROM () | Non-PROM () | -Valuea |
|---|---|---|---|---|
| Sociodemographic characteristics [ (%)] | ||||
| Age (y) | — | — | — | |
| 1,666 (24.4) | 406 (28.2) | 1,260 (23.4) | — | |
| 25–34 | 4,476 (65.6) | 912 (63.4) | 3,564 (66.2) | — |
| 682 (10.0) | 121 (8.4) | 561 (10.4) | — | |
| Education | — | — | — | 0.433 |
| Junior high school | 941 (13.8) | 184 (12.8) | 757 (14.1) | — |
| High school | 1,655 (24.3) | 348 (24.2) | 1,307 (24.3) | — |
| Bachelor’s degree and above | 4,228 (62.0) | 907 (63.0) | 3,321 (61.7) | — |
| Family income (RMB/month) | — | — | — | 0.231 |
| 2,149 (31.5) | 460 (32.0) | 1,689 (31.4) | — | |
| 4000–7999 | 4,132 (60.6) | 878 (61.7) | 3,254 (60.4) | — |
| 543 (8.0) | 101 (7.0) | 442 (8.2) | — | |
| Parity | — | — | — | |
| Primipara | 2,611 (38.3) | 642 (44.6) | 1,969 (36.6) | — |
| Multipara | 4,213 (61.7) | 797 (55.4) | 3,416 (63.4) | — |
| Season of delivery | — | — | — | 0.003 |
| Spring | 1,756 (25.7) | 386 (26.8) | 1,370 (25.4) | — |
| Summer | 1,800 (26.4) | 331 (23.0) | 1,469 (27.3) | — |
| Autumn | 1,730 (25.4) | 403 (28.0) | 1,327 (24.6) | — |
| Winter | 1,538 (22.5) | 319 (22.2) | 1,219 (22.6) | — |
| Enrollment years | — | — | — | 0.080 |
| 2015–2016 | 2,385 (35.0) | 538 (37.4) | 1,846 (34.3) | — |
| 2017–2018 | 2,569 (37.6) | 517 (35.9) | 2,052 (38.1) | — |
| 2019–2021 | 1,870 (27.4) | 384 (26.7) | 1,486 (27.6) | — |
| Perinatal health lifestyle factors [ (%)]b | ||||
| Vegetable intake (times/week) | — | — | 0.982 | |
| 231 (3.4) | 49 (3.4) | 182 (3.4) | — | |
| 6,593 (96.6) | 1,390 (96.6) | 5,203 (96.6) | — | |
| Fruit intake (times/week) | — | — | 0.917 | |
| 470 (6.9) | 100 (6.9) | 370 (6.9) | — | |
| 6,354 (93.1) | 1,339 (93.1) | 5,015 (93.1) | — | |
| Dessert intake (times/week) | — | — | 0.991 | |
| 5,633 (82.5) | 1,188 (82.6) | 4,445 (82.5) | — | |
| 1,191 (17.5) | 251 (17.4) | 940 (17.5) | — | |
| Physical activity (days/week) | — | — | — | 0.281 |
| 5,394 (79.0) | 1,154 (80.2) | 4,240 (78.7) | — | |
| 1,430 (21.0) | 285 (19.8) | 1,145 (21.3) | — | |
| Folic acid supplementation (days/week) | — | — | 0.047 | |
| 4,387 (64.2) | 893 (62.1) | 3,494 (64.9) | — | |
| 2,437 (35.7) | 546 (37.9) | 1,891 (35.1) | — | |
| Iron supplementation (days/week) | — | — | — | 0.075 |
| 5,847 (85.7) | 1,254 (87.1) | 4,593 (85.3) | — | |
| 977 (14.3) | 185 (12.9) | 792 (14.7) | — | |
| Passive smoking | — | — | — | 0.113 |
| Never | 5,598 (82.0) | 1,201 (83.5) | 4,397 (81.7) | — |
| Ever | 1,226 (18.0) | 238 (16.5) | 988 (18.3) | — |
| Perinatal health status [ (%)] | ||||
| Prepregnancy BMI () | — | — | — | 0.709 |
| 985 (14.4) | 216 (15.0) | 769 (14.3) | — | |
| 18.5–23.9 | 4,816 (70.6) | 1,014 (70.5) | 3,802 (70.6) | — |
| 1,023 (15.0) | 209 (14.5) | 814 (15.1) | — | |
| Hypertension during pregnancy | 139 (2.0) | 37 (2.6) | 102 (1.9) | 1.106 |
| Vaginitis | 783 (11.5) | 151 (10.5) | 632 (11.7) | 0.189 |
| Gestational diabetes mellitus | 1,420 (20.8) | 255 (17.7) | 1,165 (22.1) | 0.001 |
| Premature birth | 229 (3.4) | 111 (7.7) | 118 (2.2) | |
| Maternal anemia | 2,290 (33.6) | 591 (41.1) | 1,699 (31.6) | |
Note: There were no missing values for covariates. —, no data; PROM, prelabor rupture of membranes; RMB, renminbi.
Based on the chi-square test.
The frequency of vegetable intake, fruit intake, dessert intake, physical activity was during second trimester. The frequency of folic acid supplementation intake was during the first trimester. The frequency of iron supplementation was during the third trimester.
A strong correlation between four air pollutants in three trimesters was observed (Spearman correlation ranged from 0.01 to 0.94; Supplement Table 2). The mean (SD) gestational exposure to , , , and CO in the second and third trimester was (15.1), (13.5), (4.0), and (0.2), whereas that in the first trimester was (18.6), (17.0), (4.9), and (0.2) (Table 2).
Table 2.
Cumulative and average effects between air pollutants exposure and PROM risk in distributed lag models and average exposure model.
| Pollution | Mean | SD | OR (95% CI) of PROMd | |
|---|---|---|---|---|
| Distributed lag modele | Average exposure modele | |||
| First trimester | ||||
| ()a | 57.7 | 18.6 | 1.01 (0.97, 1.04) | 1.01 (0.98, 1.04) |
| ()a | 87.0 | 17.0 | 1.02 (0.98, 1.06) | 1.02 (0.98, 1.06) |
| ()b | 17.8 | 4.9 | 1.06 (0.98, 1.16) | 1.08 (1.01, 1.14) |
| CO ()c | 0.9 | 0.2 | 1.03 (0.99, 1.07) | 1.04 (1.01, 1.08) |
| Second trimester | ||||
| ()a | 54.6 | 18.1 | 1.11 (1.04, 1.19) | 1.14 (1.09, 1.19) |
| ()a | 84.1 | 16.3 | 1.14 (1.07, 1.22) | 1.17 (1.12, 1.22) |
| ()b | 12.1 | 4.4 | 1.13 (1.03, 1.24) | 1.22 (1.14, 1.31) |
| CO ()c | 0.9 | 0.2 | 1.10 (1.04, 1.16) | 1.13 (1.09, 1.18) |
| Third trimester | ||||
| ()a | 54.0 | 19.0 | 1.04 (1.01, 1.08) | 1.06 (1.03, 1.09) |
| ()a | 82.2 | 16.9 | 1.05 (1.01, 1.09) | 1.07 (1.04, 1.11) |
| ()b | 11.5 | 4.2 | 1.14 (1.05, 1.25) | 1.16 (1.08, 1.24) |
| CO ()c | 0.9 | 0.2 | 1.06 (1.02, 1.10) | 1.08 (1.04, 1.12) |
| Second and third trimesters | ||||
| ()a | 54.2 | 15.1 | 1.13 (1.06, 1.22) | 1.11 (1.06, 1.15) |
| ()a | 83.2 | 13.5 | 1.18 (1.10, 1.27) | 1.14 (1.09, 1.20) |
| ()b | 11.8 | 4.0 | 1.16 (1.06, 1.28) | 1.22 (1.13, 1.31) |
| CO ()c | 0.9 | 0.2 | 1.10 (1.04, 1.15) | 1.11 (1.07, 1.16) |
Note: BMI, body mass index; CI, confidence interval; CO, carbon monoxide; OR, odds ratio; PROM, prelabor rupture of membranes; SD, standard deviation.
Per increase in .
Per increase in .
Per increase in .
Estimated by distributed lag models using weekly mean exposures and by mean air pollution during specific exposure windows (average exposure model).
The models were adjusted for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature.
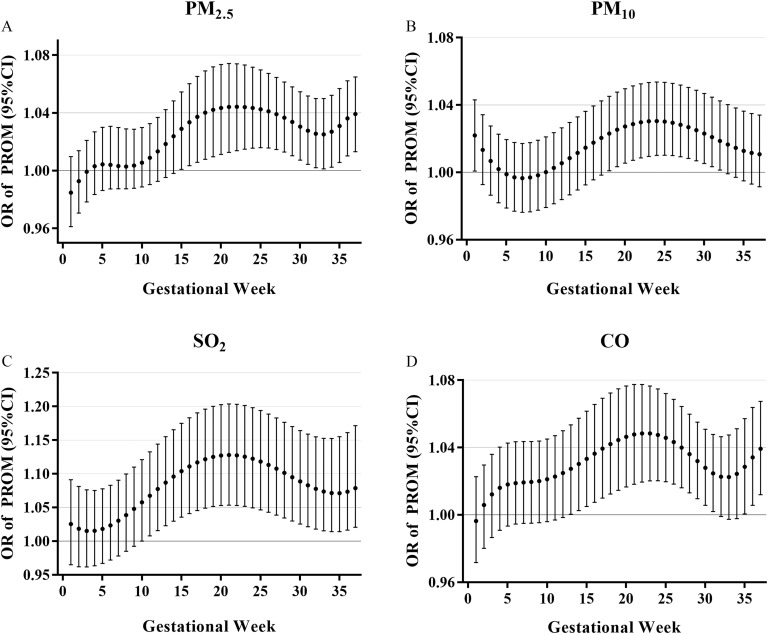
Distributed lag nonlinear models were used to examine the sensitive windows of weekly , , and CO exposure related to PROM in the unadjusted and adjusted models (Figure 1; Supplement Table 3). The weekly air pollutant exposures were significantly associated with increased PROM risk: The window of susceptibility for (per increase) was between GW 15 and 37, and the maximum effect was in the 21th–23th GW; for (per increase) the susceptibility window was between GW 18 and 32, and the maximum effect was in the 24th GW; for (per increase) the susceptibility window was between GW 10 and 37, and the maximum effect was in the 21th GW; and for CO (per increase) the window of susceptibility was between GW 13 and 31, and the maximum effect was in the 21th–24th GW. Table 2 depicts the estimated trimester cumulative PROM risk in the DLMs and average models in logistic regression with covariates adjusted, as with the unadjusted model (Supplement Table 4). The estimated cumulative risk of PROM was significantly associated with prenatal exposure to air pollutants throughout the second and third trimesters (Table 2). For example, the cumulative OR for the second and third trimester was 1.13 per increase in (95% CI: 1.06, 1.22). Similarly, significant associations were observed with per increase in [ (95% CI: 1.10, 1.27)], per increase in [ (95% CI: 1.06, 1.28)], and per increase in CO [ (95% CI: 1.04, 1.15)]. DLM estimates were consistent with the results from the average exposure models. We find similar effect estimates in the unadjusted model presented in Supplement Table 4. In addition, adjustment for trimesters air exposure in Supplement Table 5 did not change the pattern of estimates from those in Table 2. The associations between prenatal exposure to air pollution and term PROM and preterm PROM are presented in Supplement Table 6. However, prenatal exposure to air pollution was not associated with preterm PROM. For sensitivity analysis, we further evaluated the correlation in the copollution models during same exposure period, and the results are presented in Supplement Table 7. Results of copollution models for estimating the effect for , , CO, and were generally consistent with those of the single-pollutant model exposure to same period.
Figure 1.
The PROM risk in association with week-specific prenatal air pollution exposure during pregnancy. Week-specific estimates are provided as the OR of PROM (with 95% CI) for a increment of exposure (A). Week-specific estimates are provided as the OR of PROM (with 95% CI) for a increment of exposure (B). Week-specific estimates are provided as the OR of PROM (with 95% CI) for a increment of exposure (C). Week-specific estimates are provided as the OR of PROM (with 95% CI) for a increment of CO exposure (D). Models were based on a distributed lag (nonlinear) model and adjusted for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature. The numerical results are presented in Supplement Table 3. Note: BMI, body mass index; CI, confidence interval; CO, carbon monoxide; OR, odds ratio; PROM, prelabor rupture of membranes.
As presented in Figure 2A and Supplement Table 8, effect of each air pollutant (per in and , per in , and per in CO) throughout the second trimester and third trimester was negatively associated with low maternal hemoglobin levels in third trimester [ with 95% CI for : (95% CI: , ); for : (95% CI: , ); for : (95% CI: , ); and for CO: (95% CI: , )] upon adjustment for age, education, income, activity, passive smoking, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, and temperature. Significant associations with per quartile increase for each air pollutant were observed in Figure 2A. The results from the copollution models showed similar negative associations with the effect estimation for , , , and CO in the single-pollutant model in Supplement Table 9. We further examined the potential exposure–response relationships between maternal hemoglobin levels during the third trimester and PROM risk (Figure 2B; Supplement Table 10). After adjusting for age, education, income, parity, activity, passive smoking folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature, higher risk of PROM was observed in women with hemoglobin below (; 95% CI: 1.73, 3.46), women with a hemoglobin range of (; 95% CI: 1.72, 3.41), women with a hemoglobin range of (; 95% CI: 1.73, 3.34), women with a hemoglobin range of (; 95% CI: 1.42, 2.73), women with a hemoglobin range of (; 95% CI: 1.27, 2.45), women with a hemoglobin range of (; 95% CI: 1.23, 2.42), and women with a hemoglobin range of (; 95% CI: 1.05, 2.21) when compared with that in women in the conference group (hemoglobin higher than ).
Figure 2.
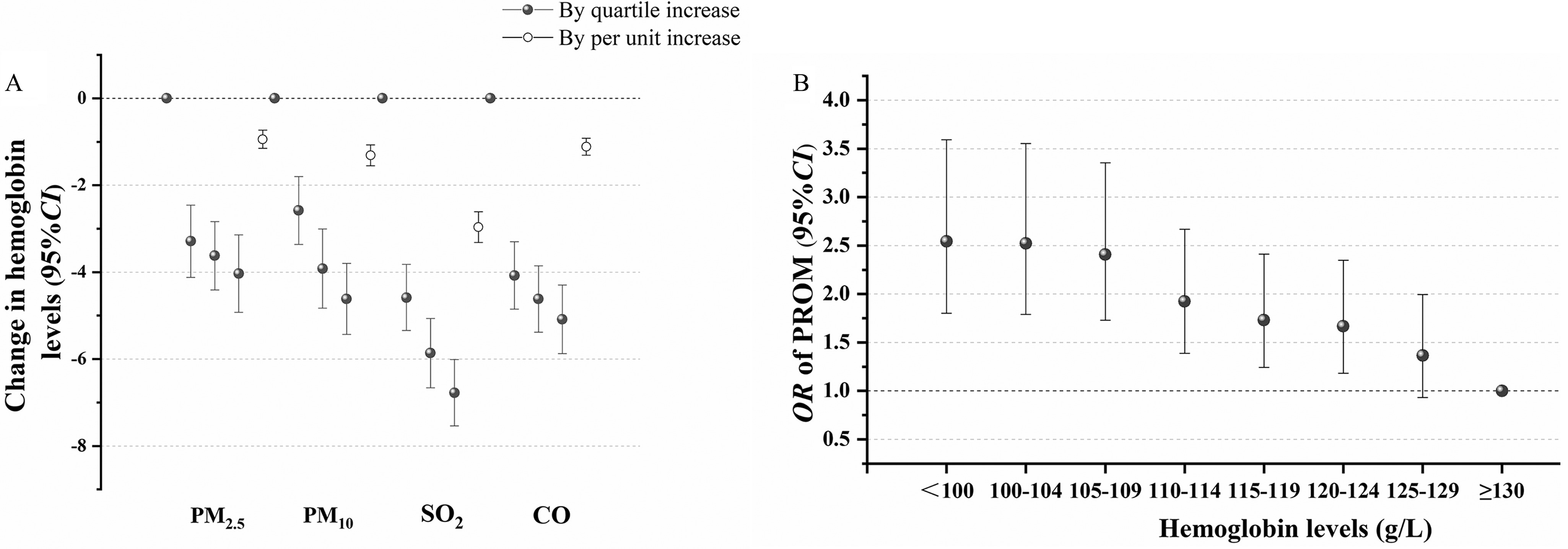
The association among air pollution exposure, hemoglobin levels, and PROM risk. The estimated change in hemoglobin levels was calculated for each quartile and each unit increment in , , , and CO during the second and third trimesters in linear regression model (A). The model was based on the line regression model and adjusted for age, education, income, activity, passive smoking, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, and temperature. The hemoglobin level per increase in and was , the hemoglobin level per increase in was , and the hemoglobin level per increase in CO was . The numerical results are presented in Supplement Table 8. The relationship between hemoglobin levels and PROM (B). The model was based on the logistic regression model and adjusted for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature. Air pollution was in the second and third trimesters. The numerical results are presented in Supplement Table 10. Note: BMI, body mass index; CO, carbon monoxide; PROM, prelabor rupture of membranes.
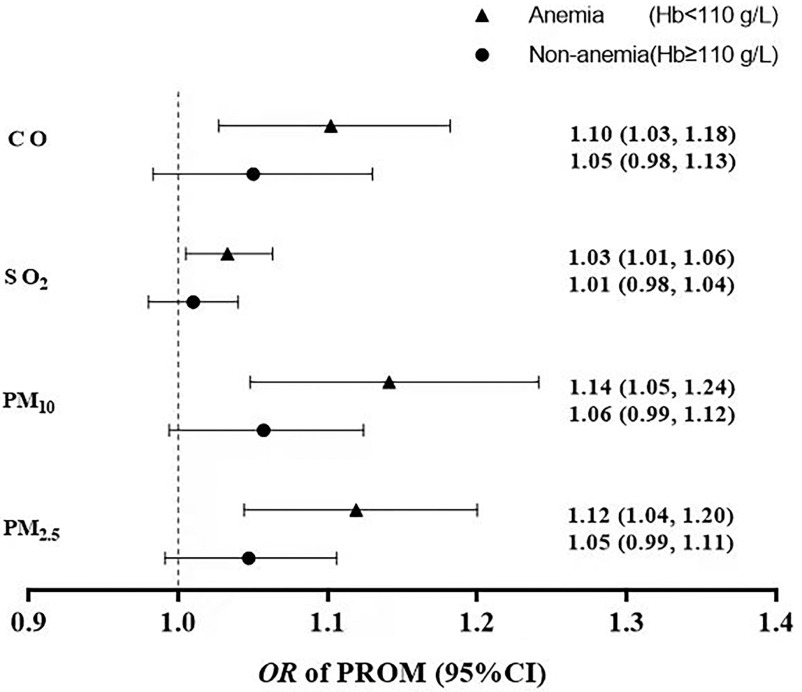
The OR of PROM was calculated with per unit increase in exposure to air pollution (per in and , per in , and per in CO) stratified by anemia status (Figure 3). Our results showed a decreased risk of PROM associated with per unit increase in exposure to , , , and CO in women without anemia, in comparison with that in women with anemia. For example, for , PROM risk in women without anemia [ (95% CI: 0.99, 1.11)] was lower than that in women with anemia [ (95% CI: 1.04, 1.20)].
Figure 3.
The relationship between air pollution and PROM risk in different hemoglobin levels. Air pollution was in the second and third trimesters. The hemoglobin level per increase in and was , the hemoglobin level per increase in was , and the hemoglobin level per increase in CO was . Models adjusted for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature. Note: BMI, body mass index; CO, carbon monoxide; Hb, hemoglobin; PROM, prelabor rupture of membranes.
In PCA, the calculated eigenvalue of the first principal component (PC1) was 3.615 (), providing 90.38% composite information. PC1 is mainly driven by CO, , , and (Supplement Table 11). Maternal hemoglobin levels in the third trimester mediated 20.61% (; 95% CI: 0.01, 0.05) of the contribution to the association of principal component of exposure to air pollutants throughout the second and third trimesters with PROM after adjusting for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature (Table 3).
Table 3.
Mediation effect by hemoglobin levels on third trimester air pollution associated with PROM risk.
| Exposure window | AME (95% CI) | -Value | ADE (95% CI) | -Value | Proportion (%)a |
|---|---|---|---|---|---|
| Second trimester | 0.02 (0.01, 0.03) | 0.08 (0.02, 0.10) | 17.31 | ||
| Third trimester | 0.02 (0.01, 0.04) | 0.06 (0.01, 0.08) | 0.044 | 24.26 | |
| Second and third trimester | 0.02 (0.01, 0.05) | 0.08 (0.02, 0.14) | 0.008 | 20.61 |
Note: Meditation effect by hemoglobin levels was calculated per . Air pollution was based on the total score of principal components of four air pollutant exposures (, , , and CO). ADE, average direct effect; AME, average mediation effect; BMI, body mass index; CI, confidence interval; CO, carbon monoxide; PROM, prelabor rupture of membranes.
Proportion (%): The extent to which the association between four air pollution exposures and PROM was mediated through hemoglobin in the third trimester. The models were adjusted for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature.
The 50th and 75th percentiles for the exposures during second and third trimester was and for , and for , and for , and for CO, respectively. (Table 4; Supplement Table 12). Data presented in Table 4 show that the risk of PROM was significantly attenuated by iron supplementation during pregnancy in women with gestational anemia under low–medium exposure to air pollution in adjusted and unadjusted models. Iron supplementation in women with low–medium exposure to air pollution resulted in a significantly lower incidence of PROM than that seen in women without iron supplementation. For , our analysis indicated a pattern toward lower incidence of PROM in women who were taking iron supplements in comparison with that in women not taking iron supplements under low–medium air pollution (16.0% vs. 18.5%) and high air pollution (21.4% vs. 23.4%). The decreasing trend toward PROM incidence in the above-mentioned groups persisted with exposure to concentration of , concentration of , and CO concentration of . Results from the sensitivity analysis using the cutoff point of exposure to air pollution at P75 remained robust, as shown in Supplement Table 12. [For example, incidence of PROM under low–medium pollution was 17.9% and 19.5% for women with iron supplementation, without iron supplementation; high pollution (21.7% vs. 23.6%)] (Supplement Table 12). We did not observe discrepancy in the results of unadjusted model using cutoff point of P50 in Table 4. [For example, (95% CI: 0.51, 0.83) for women with iron supplementation under low–medium pollution; (95% CI: 0.56, 1.03) for women without iron supplementation under low–medium pollution; (95% CI: 0.56, 1.43) for women with iron supplementation under high pollution] or P75 in Supplement Table 12 [OR with 95% CI: 0.70 (0.51, 0.98) for women with iron supplementation under low- to medium pollution; (95% CI: 0.58, 1.06) for women without iron supplementation under low–medium pollution; (95% CI: 0.55, 1.47) for women with iron supplementation under high pollution].
Table 4.
The association between iron supplementation and PROM risk stratified by air pollution levels in women diagnosed with anemia.
| Pollutionsa | Exposure level | Iron supplementation (days/week) | Hemoglobind | PROMd | |||
|---|---|---|---|---|---|---|---|
| , g/L | (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)e | ||||
| f | 531 | b | 85 (16.0)c | 0.65 (0.51, 0.83) | 0.62 (0.49, 0.79) | ||
| 817 | 151 (18.5) | 0.76 (0.56, 1.03) | 0.74 (0.53, 1.01) | ||||
| 309 | 66 (21.4) | 0.90 (0.56, 1.43) | 0.87 (0.54, 1.40) | ||||
| 633 | 148 (23.4) | 1.00f | 1.00f | ||||
| 555 | b | 90 (16.2)c | 0.69 (0.52, 0.92) | 0.68 (0.50, 0.93) | |||
| 821 | 156 (19.0) | 0.79 (0.59, 1.06) | 0.75 (0.56, 1.01) | ||||
| 285 | 60 (21.1) | 0.92 (0.65, 1.30) | 0.90 (0.62, 1.27) | ||||
| 629 | 144 (22.9) | 1.00f | 1.00f | ||||
| 587 | b | 95 (16.2)c | 0.68 (0.51, 0.91) | 0.66 (0.50, 0.87) | |||
| 790 | 149 (18.9) | 0.75 (0.53, 1.05) | 0.72 (0.51, 1.02) | ||||
| 253 | 50 (19.8) | 0.80 (0.56, 1.14) | 0.77 (0.54, 1.12) | ||||
| 660 | 156 (23.6) | 1.00f | 1.00f | ||||
| CO | 596 | b | 103 (17.3)c | 0.72 (0.54, 0.95) | 0.74 (0.55, 0.99) | ||
| 843 | 158 (18.7) | 0.79 (0.61, 1.02) | 0.81 (0.62, 1.05) | ||||
| 244 | 52 (21.4) | 0.93 (0.65, 1.34) | 0.93 (0.65, 1.34) | ||||
| 607 | 137 (22.6) | 1.00f | 1.00f | ||||
Note: CI, confidence interval; OR, odds ratio; PROM, prelabor rupture of membranes; SD, standard deviation.
Air pollution was in the second and third trimesters.
The for trend of hemoglobin levels across four groups was , , , , respectively.
The for trend of PROM prevalence across four groups was , 0.006, 0.012, 0.004. respectively.
The test for -trend was performed using general linear regression model and Mantel-Haenszel chi-square test in hemoglobin levels and PROM prevalence across the above four groups.
Models adjusted for age, education, income, parity, activity, passive smoking, folic acid supplementation, iron supplementation, prepregnancy BMI, hypertension during pregnancy, gestational diabetes mellitus, vaginitis, and temperature. The 50th percentile for the exposure during second and third trimester was for , for , for , and for CO, respectively.
Reference group.
Discussion
A positive association between exposure to air pollution and a statistically significance showed in this multicenter, prospective cohort study. Our mediation analysis suggested that maternal hemoglobin levels in the third trimester could partly mediate the association between prenatal exposure to air pollution and PROM. In addition, the potential association of iron supplementation on PROM risk was observed in women with gestational anemia under low–medium levels of air pollution. Therefore, iron supplementation during pregnancy could possibly affect PROM associated with prenatal exposure to air pollution in women with anemia.
The relationship between elevated air pollution and PROM has been examined but with inconsistent results.35–37 Contrasting results presented in these studies may be attributed to apparent differences in levels of air pollution, inconsistent domestic conditions, and differences in population characteristics. A prospective cohort study conducted in Wuhan, China, indicated that levels during pregnancy increased the risk of PROM, and the estimated exposure concentration was during the entire pregnancy, which is similar to the finding in our current study.11 In recent years, some researchers have proposed that considering longer exposure periods does not take into account the potential windows of exposure that may span different periods of pregnancy, resulting in deviations in inferred susceptible windows of exposure. Therefore, it is necessary to further refine the exposure period to identify the window of the effect of exposure to air pollution more accurately.38 Results of the present study showed that the time windows for the maximum effect of , , CO, and were in the 21th to 24th week of pregnancy. Our study confirmed the association between a higher prenatal exposure to air pollution and PROM during pregnancy and more accurate time windows of maximum effects of exposure to pollutants. Accelerated fetal membrane aging and higher levels of immune responses from early- to mid-pregnancy could account for the sensitivity time windows of exposure to prenatal air pollution associated with risk to PROM.22,39,40
Anemia during pregnancy (hemoglobin ) is associated with poor maternal outcomes (such as postpartum infections) and infant outcomes (such as neonatal perinatal and mortality).41,42 Lower levels of hemoglobin have been considered as a marker in the condition of subclinical infections and inflammation.15 In our study, we found that exposure to air pollution during mid- to late pregnancy was negatively associated with maternal hemoglobin levels. This evidence indicates that changes in hemoglobin levels with increased prenatal exposure to air pollution could be an indicator of increased risk of adverse pregnancy outcomes. These findings are consistent with prior results reported for women with an average age of 69.6 y.17 Honda et al. reported that mean concentrations of exposure and hemoglobin levels were and , respectively. They found that a increase in annual-average was associated with a decrease in hemoglobin levels. In accordance with our findings, these results indicate a significant negative association between air pollution and hemoglobin levels. Studies in Ethiopia have suggested that indoor air pollution is associated with anemia during pregnancy.43 Consistently, our study emphasized that exposure to air pollution during pregnancy is related to lower levels of maternal hemoglobin, thus contributing to prenatal anemia. Furthermore, our study found a significant correlation between anemia and an increased risk of PROM in pregnancy. Mediation analysis suggested that maternal hemoglobin levels could represent a potential factor contributing to increased risk of PROM posed by prenatal air pollution exposure. The assumption of unmeasured exposure–mediator confounding (e.g., wearing N95 masks for mitigating inhaled particulate air pollution, etc.),44 unmeasured mediator–outcome confounding (maternal diet, etc.), and unmeasured exposure–outcome confounding (genetic risk, etc.) could bias our results, although major covariates were adjusted for in our analyses.33,34,41
Iron deficiency may result from dramatically increased demands for iron in pregnancy to meet rapid fetal growth, as well as a decrease in serum iron triggered by inflammation, increased numbers of red blood cells, and increased plasma volume.45 Such factors may predispose pregnant women to anemia. Iron deficiency also accounts for adverse pregnancy and birth outcomes. In our study, we found a possible association of iron supplementation on the correction of anemia and PROM risk. Furthermore, in a stratified analysis using air pollution levels, the possible association of iron supplementation on PROM risk was detected only at low- to medium air pollution levels, showing that the potential positive effect of iron supplementation on PROM risk because of prenatal exposure to air pollution in women with anemia was limited. Our study suggests that PROM risk posed by exposure to low- to medium air pollution could be attenuated by maternal iron supplementation to some extent, especially in women with gestational anemia.
The mechanism through which hemoglobin may mediate the association of prenatal air pollution on PROM is unknown. We hypothesize the following potential mechanisms: Air pollutants cause a systemic inflammatory response and directly affect bone marrow function, and inflammation decreases renal erythropoietin secretion and increases bone marrow endogenous erythropoietin resistance, leading to the reduction in erythrocyte and hemoglobin production.46,47 In addition, air particles may cause hemolysis and destruction of red blood cells. Low hemoglobin levels is a known marker of inflammation,48 and therefore is associated with impaired immune function as well as dysregulation of functions of natural killer cells, T-cells, and neutrophils. Furthermore, impaired immune function has been identified in anemia, contributing to a higher susceptibility to bacterial infection, and it has been hypothesized to be associated with air pollution–related PROM.14
In the present study, the rates of preterm birth among included vs. excluded participants are 3.4% vs. 4.1%, respectively. The overall preterm rate in this study (3.4%) is lower than the rates in the overall China population49 (6.1%) but is similar with the rates in Anhui province50 (4.1%). Although several studies in China showed similar incidences of PROM from Nanjing5 (20.8%) and Shanghai51 (22.0%), the incidence of PROM in our study (21.0%) appears to be higher in comparison with that of the United States52 (12.0%). It could be explained by the discrepancies in air pollution levels, race, and socioeconomic factors.10,15,53 Moreover, considering the lower rate of preterm birth in this study, the proportion of preterm PROM (7.7%) of all PROM appears to be relatively low.
Our study is significant for several reasons. Our study could add to the growing body of literature pertaining to demonstrate the relationship between chronic ambient exposure to air pollution and PROM mediated by maternal anemia as well as decreasing hemoglobin levels based on results from our large-scale prospective cohort of pregnant women. Moreover, our finding that iron supplementation possibly alleviates the association of air pollution on PROM may drive further studies in this field. Second, we used data from our multicenter, prospective cohort study with adjustment for several confounding variables. Third, we simultaneously observed the influence of maternal anemia and possible association of iron supplementation. Finally, a more accurate time window for the effect of exposure of air pollution on PROM was identified in our study.
Nevertheless, this study has several limitations. First, we did not consider indoor air pollution, such as indoor dust, kitchen cooking oil fumes, indoor cigarette smoke, and sleep air quality. We failed to assess traffic-related air and noise pollution during pregnancy, which was demonstrated to be related to PROM.54 Second, we performed an observational study rather than a randomized controlled trial to elucidate the effect of iron supplementation. Moreover, the residual confounding and uncontrolled risk factors, such as prior history of PROM, could bias our results. In addition, given the cumulative effect of secular trends in air pollution before conception on hemoglobin levels in women, our study possibly overestimated the effect of air pollution exposure during pregnancy. Information on the change of addresses during pregnancy was not available, which may cause some bias in the effect estimates. Finally, the exposure data were assigned to the nearest air quality monitor rather than the estimated personal exposure to air pollution, which could provide incorrect results.
Conclusion
Prenatal exposure to ambient air pollution (, , , and CO), especially in 21th to 24th week of pregnancy, is positively associated with PROM risk, partly mediated by maternal hemoglobin levels. Iron supplementation in anemia pregnancy potentially has a positive association on low- to medium air pollution-related PROM. Screening and treatment of gestational anemia could provide novel insights into the prevention of low-to medium air pollution–related PROM.
Supplementary Material
Acknowledgments
This research received financial support from the National Key R&D Program of China (2022YFC2702901), the National Natural Science Foundation of China (81872631, 82173531) and Foundation for Scientific Research Improvement of Anhui Medical University (2021xkjT009).
L.W.: Analyzing-data, Writing—original draft. W.-J.Y.: Writing—original draft. L-J.Y., Y.-H.W., X.-M.J., Y.Z.: Investigation. F.-B.T.: Writing—review and editing. R.-X.T.: Supervision, project administration. P.Z.: Writing—review and editing; designing study.
References
- 1.Wang YY, Li Q, Guo Y, Zhou H, Wang X, Wang Q, et al. . 2018. Association of long-term exposure to airborne particulate matter of 1 μm or less with preterm birth in China. JAMA Pediatr 172(3):e174872, PMID: , 10.1001/jamapediatrics.2017.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwag Y, Kim MH, Oh J, Shah S, Ye S, Ha EH. 2021. Effect of heat waves and fine particulate matter on preterm births in Korea from 2010 to 2016. Environ Int 147:106239, PMID: , 10.1016/j.envint.2020.106239. [DOI] [PubMed] [Google Scholar]
- 3.Smith RB, Beevers SD, Gulliver J, Dajnak D, Fecht D, Blangiardo M, et al. . 2020. Impacts of air pollution and noise on risk of preterm birth and stillbirth in London. Environ Int 134:105290, PMID: , 10.1016/j.envint.2019.105290. [DOI] [PubMed] [Google Scholar]
- 4.Committee on Practice Bulletins-Obstetrics. 2018. ACOG Practice Bulletin No. 188: Prelabor Rupture of Membranes. Obstet Gynecol 131(1):e1–e14, PMID: , 10.1097/AOG.0000000000002455. [DOI] [PubMed] [Google Scholar]
- 5.Han Y, Wang W, Wang X, Dong T, van Donkelaar A, Martin RV, et al. . 2020. Prenatal exposure to fine particles, premature rupture of membranes and gestational age: a prospective cohort study. Environ Int 145:106146, PMID: , 10.1016/j.envint.2020.106146. [DOI] [PubMed] [Google Scholar]
- 6.Conde-Agudelo A, Belizán JM. 2000. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ 321(7271):1255–1259, PMID: , 10.1136/bmj.321.7271.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcellin L, Goffinet F. 2012. Are biological markers relevant for the diagnosis and the prognosis of preterm premature rupture of membranes (PPROM)? Clin Chem Lab Med 50(6):1015–1019, PMID: , 10.1515/cclm-2011-1850. [DOI] [PubMed] [Google Scholar]
- 8.Morris JM, Roberts CL, Bowen JR, Patterson JA, Bond DM, Algert CS, et al. . 2016. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet 387(10017):444–452, PMID: , 10.1016/S0140-6736(15)00724-2. [DOI] [PubMed] [Google Scholar]
- 9.Pereira G, Bell ML, Belanger K, de Klerk N. 2014. Fine particulate matter and risk of preterm birth and pre-labor rupture of membranes in Perth, Western Australia 1997–2007: a longitudinal study. Environ Int 73:143–149, PMID: , 10.1016/j.envint.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace ME, Grantz KL, Liu D, Zhu Y, Kim SS, Mendola P. 2016. Exposure to ambient air pollution and premature rupture of membranes. Am J Epidemiol 183(12):1114–1121, PMID: , 10.1093/aje/kwv284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Tian Y, Zheng H, Shan S, Zhao X, Liu C. 2019. Maternal exposure to ambient fine particulate matter and risk of premature rupture of membranes in Wuhan, Central China: a cohort study. Environ Health 18(1):96, PMID: , 10.1186/s12940-019-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Guidelines Approved by the Guidelines Review Committee. 2021. WHO Global Air Quality Guidelines: Particulate Matter (PM25 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 13.Chu FC, Shaw SW, Lo LM, Hsieh TT, Hung TH. 2020. Association between maternal anemia at admission for delivery and adverse perinatal outcomes. J Chin Med Assoc 83(4):402–407, PMID: , 10.1097/JCMA.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 14.Archabald K, Lopes V, Anderson B. 2015. Impact of antepartum anemia on the development of chorioamnionitis at term. Am J Perinatol 32(3):283–288, PMID: , 10.1055/s-0034-1384638. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson SE, Smith GN, Salenieks ME, Windrim R, Walker MC. 2002. Preterm premature rupture of membranes. Nutritional and socioeconomic factors. Obstet Gynecol 100(6):1250–1256, PMID: , 10.1016/s0029-7844(02)02380-3. [DOI] [PubMed] [Google Scholar]
- 16.Gonzales GF, Tapia V, Gasco M, Carrillo CE, Fort AL. 2012. Association of hemoglobin values at booking with adverse maternal outcomes among Peruvian populations living at different altitudes. Int J Gynaecol Obstet 117(2):134–139, PMID: , 10.1016/j.ijgo.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Honda T, Pun VC, Manjourides J, Suh H. 2017. Anemia prevalence and hemoglobin levels are associated with long-term exposure to air pollution in an older population. Environ Int 101:125–132, PMID: , 10.1016/j.envint.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris AM, Sempértegui F, Estrella B, Narváez X, Egas J, Woodin M, et al. . 2011. Air pollution and anemia as risk factors for pneumonia in Ecuadorian children: a retrospective cohort analysis. Environ Health 10:93, PMID: , 10.1186/1476-069X-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao M, Liu Y, Jin D, Yin W, Ma S, Tao R, et al. . 2020. Relationship between temporal distribution of air pollution exposure and glucose homeostasis during pregnancy. Environ Res 185:109456, PMID: , 10.1016/j.envres.2020.109456. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Li L, Xie J, Jiao X, Hu H, Zhang Y, et al. . 2021. Foetal 25-hydroxyvitamin D moderates the association of prenatal air pollution exposure with foetal glucolipid metabolism disorder and systemic inflammatory responses. Environ Int 151:106460, PMID: , 10.1016/j.envint.2021.106460. [DOI] [PubMed] [Google Scholar]
- 21.Hefei Municipal Health Commission. 2019. The table of obstetric bed usage in the secondary midwifery medical institutions in Hefei. http://wjw.hefei.gov.cn/xwzx/tzgg/17313697.html [accessed 8 November 2022].
- 22.Martens DS, Cox B, Janssen BG, Clemente DBP, Gasparrini A, Vanpoucke C, et al. . 2017. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr 171(12):1160–1167, PMID: , 10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel JP, Souza JP, Mori R, Morisaki N, Lumbiganon P, Laopaiboon M, et al. . 2014. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 121 Suppl 1:76–88, PMID: , 10.1111/1471-0528.12633. [DOI] [PubMed] [Google Scholar]
- 24.WHO (World Health Organization). 2012. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 25.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. . 2003. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395, PMID: , 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 26.Hypertension in pregnancy. 2013. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122(5):1122–1131, PMID: , 10.1097/01.aog.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 27.Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29(21):2224–2234, PMID: , 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neven KY, Wang C, Janssen BG, Roels HA, Vanpoucke C, Ruttens A, et al. . 2021. Ambient air pollution exposure during the late gestational period is linked with lower placental iodine load in a Belgian birth cohort. Environ Int 147:106334, PMID: , 10.1016/j.envint.2020.106334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng Q, Fan L, Ni Y, Li G, Gu Q. 2020. Construction of AQHI based on the exposure relationship between air pollution and YLL in Northern China. Sci Total Environ 710:136264, PMID: , 10.1016/j.scitotenv.2019.136264. [DOI] [PubMed] [Google Scholar]
- 30.Voukantsis D, Karatzas K, Kukkonen J, Räsänen T, Karppinen A, Kolehmainen M. 2011. Intercomparison of air quality data using principal component analysis, and forecasting of PM10 and PM2.5 concentrations using artificial neural networks, in Thessaloniki and Helsinki. Sci Total Environ 409(7):1266–1276, PMID: , 10.1016/j.scitotenv.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 31.Tran H, Kim J, Kim D, Choi M, Choi M. 2018. Impact of air pollution on cause-specific mortality in Korea: results from Bayesian model averaging and principle component regression approaches. Sci Total Environ 636:1020–1031, PMID: , 10.1016/j.scitotenv.2018.04.273. [DOI] [PubMed] [Google Scholar]
- 32.Imai K, Keele L, Tingley D. 2010. A general approach to causal mediation analysis. Psychol Methods 15(4):309–334, PMID: , 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 33.Richiardi L, Bellocco R, Zugna D. 2013. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol 42(5):1511–1519, PMID: , 10.1093/ije/dyt127. [DOI] [PubMed] [Google Scholar]
- 34.VanderWeele TJ. 2016. Mediation analysis: a practitioner’s guide. Annu Rev Public Health 37:17–32, PMID: , 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 35.Dadvand P, Basagaña X, Figueras F, Martinez D, Beelen R, Cirach M, et al. . 2014. Air pollution and preterm premature rupture of membranes: a spatiotemporal analysis. Am J Epidemiol 179(2):200–207, PMID: , 10.1093/aje/kwt240. [DOI] [PubMed] [Google Scholar]
- 36.Song J, Lu M, An Z, Liu Y, Zheng L, Li Y, et al. . 2019. Estimating the acute effects of ambient ozone pollution on the premature rupture of membranes in Xinxiang, China. Chemosphere 227:191–197, PMID: , 10.1016/j.chemosphere.2019.04.062. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C, Li S, Guo GL, Hao JW, Cheng P, Xiong LL, et al. . 2021. Acute associations between air pollution on premature rupture of membranes in Hefei, China. Environ Geochem Health 43(9):3393–3406, PMID: , 10.1007/s10653-021-00833-1. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Li PH, Fan H, Li C, Zhang Y, Ju D, et al. . 2022. Weekly-specific ambient fine particular matter exposures before and during pregnancy were associated with risks of small for gestational age and large for gestational age: results from project ELEFANT. Int J Epidemiol 51(1):202–212, PMID: , 10.1093/ije/dyab166. [DOI] [PubMed] [Google Scholar]
- 39.Lannon SM, Vanderhoeven JP, Eschenbach DA, Gravett MG, Adams Waldorf KM. 2014. Synergy and interactions among biological pathways leading to preterm premature rupture of membranes. Reprod Sci 21(10):1215–1227, PMID: , 10.1177/1933719114534535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon R, Richardson LS, Lappas M. 2019. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 79:40–45, PMID: , 10.1016/j.placenta.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young MF, Oaks BM, Tandon S, Martorell R, Dewey KG, Wendt AS. 2019. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta-analysis. Ann NY Acad Sci 1450(1):47–68, PMID: , 10.1111/nyas.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemppinen L, Mattila M, Ekholm E, Pallasmaa N, Törmä A, Varakas L, et al. . 2021. Gestational iron deficiency anemia is associated with preterm birth, fetal growth restriction, and postpartum infections. J Perinat Med 49(4):431–438, PMID: , 10.1515/jpm-2020-0379. [DOI] [PubMed] [Google Scholar]
- 43.Andarge SD, Areba AS, Kabthymer RH, Legesse MT, Kanno GG. 2021. Is indoor air pollution from different fuel types associated with the anemia status of pregnant women in Ethiopia? J Prim Care Community Health 12:21501327211034374, PMID: , 10.1177/21501327211034374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kodros JK, O’Dell K, Samet JM, L’Orange C, Pierce JR, Volckens J. 2021. Quantifying the health benefits of face masks and respirators to mitigate exposure to severe air pollution. Geohealth 5(9):e2021GH000482, PMID: , 10.1029/2021GH000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georgieff MK. 2020. Iron deficiency in pregnancy. Am J Obstet Gynecol 223(4):516–524, PMID: , 10.1016/j.ajog.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bárány P. 2001. Inflammation, serum C-reactive protein, and erythropoietin resistance. Nephrol Dial Transplant 16(2):224–227, PMID: , 10.1093/ndt/16.2.224. [DOI] [PubMed] [Google Scholar]
- 47.Hsu CY, Bates DW, Kuperman GJ, Curhan GC. 2001. Relationship between hematocrit and renal function in men and women. Kidney Int 59(2):725–731, PMID: , 10.1046/j.1523-1755.2001.059002725.x. [DOI] [PubMed] [Google Scholar]
- 48.Sears DA. 1992. Anemia of chronic disease. Med Clin North Am 76(3):567–579, PMID: , 10.1016/s0025-7125(16)30340-6. [DOI] [PubMed] [Google Scholar]
- 49.Jing S, Chen C, Gan Y, Vogel J, Zhang J. 2020. Incidence and trend of preterm birth in China, 1990–2016: a systematic review and meta-analysis. BMJ Open 10(12):e039303, PMID: , 10.1136/bmjopen-2020-039303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li ZJ, Liang CM, Xia X, Huang K, Yan SQ, Tao RW, et al. . 2019. Association between maternal and umbilical cord serum cobalt concentration during pregnancy and the risk of preterm birth: the Ma’anshan birth cohort (MABC) study. Chemosphere 218:487–492, PMID: , 10.1016/j.chemosphere.2018.11.122. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Yu G, Menon R, Zhong N, Qiao C, Cai J, et al. . 2022. Acute and chronic maternal exposure to fine particulate matter and prelabor rupture of the fetal membranes: a nation-wide survey in China. Environ Int 170:107561, PMID: , 10.1016/j.envint.2022.107561. [DOI] [PubMed] [Google Scholar]
- 52.Pereira G, Evans KA, Rich DQ, Bracken MB, Bell ML. 2016. Fine particulates, preterm birth, and membrane rupture in Rochester, NY. Epidemiology 27(1):66–73, PMID: , 10.1097/EDE.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 53.Shree R, Caughey AB, Chandrasekaran S. 2018. Short interpregnancy interval increases the risk of preterm premature rupture of membranes and early delivery. J Matern Fetal Neonatal Med 31(22):3014–3020, PMID: , 10.1080/14767058.2017.1362384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yorifuji T, Naruse H, Kashima S, Murakoshi T, Doi H. 2015. Residential proximity to major roads and obstetrical complications. Sci Total Environ 508:188–192, PMID: , 10.1016/j.scitotenv.2014.11.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.