ABSTRACT
Streptococcus pneumoniae can produce a wide breadth of antigenically diverse capsule types, a fact that poses a looming threat to the success of vaccines that target pneumococcal polysaccharide (PS) capsule. Yet, many pneumococcal capsule types remain undiscovered and/or uncharacterized. Prior sequence analysis of pneumococcal capsule synthesis (cps) loci suggested the existence of capsule subtypes among isolates identified as “serotype 36” according to conventional capsule typing methods. We discovered these subtypes represent two antigenically similar but distinguishable pneumococcal capsule serotypes, 36A and 36B. Biochemical analysis of their capsule PS structure reveals that both have the shared repeat unit backbone [→5)-α-d-Galf-(1→1)-d-Rib-ol-(5→P→6)-β-d-ManpNAc-(1→4)-β-d-Glcp-(1→] with two branching structures. Both serotypes have a β-d-Galp branch to Ribitol. Serotypes 36A and 36B differ by the presence of a α-d-Glcp-(1→3)-β-d-ManpNAc or α-d-Galp-(1→3)-β-d-ManpNAc branch, respectively. Comparison of the phylogenetically distant serogroup 9 and 36 cps loci, which all encode this distinguishing glycosidic bond, revealed that the incorporation of Glcp (in types 9N and 36A) versus Galp (in types 9A, 9V, 9L, and 36B) is associated with the identity of four amino acids in the cps-encoded glycosyltransferase WcjA. Identifying functional determinants of cps-encoded enzymes and their impact on capsule PS structure is key to improving the resolution and reliability of sequencing-based capsule typing methods and discovering novel capsule variants indistinguishable by conventional serotyping methods.
KEYWORDS: Streptococcus pneumoniae, capsule polysaccharide, serotype, cps locus typing
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is a ubiquitous Gram-positive pathobiont that typically colonizes the human nasopharynx (NP), but is also responsible for ~14.5 million serious infections per year and ~826,000 pediatric deaths globally (1, 2). The most abundant component of the pneumococcal surface is a protective polysaccharide (PS) capsule, whose production facilitates survival in the NP and propagation among human hosts. Conversely, host antibodies that target capsule PS mediate bacterial opsonophagocytic killing, protect against pneumococcal disease, and prevent NP recolonization via multiple mechanisms (3, 4). This results in capsule PS-specific immunity that likely played a role in the evolution of over 100 antigenically diverse capsule types (5–10). Similarly, the introduction of pneumococcal conjugate vaccines (PCVs) containing a few of the most clinically relevant capsule types has reduced pneumococcal disease globally (11–14) but has also spurred a relative upsurge in the prevalence by nonvaccine capsule types (6, 15, 16). Since capsule PS diversity allows pneumococci to evade natural and vaccine-induced immunity, being able to accurately recognize the full range of capsule types is key to continuing the success of prevention efforts.
Almost all pneumococci produce capsule through a highly organized, Wzy-dependent process mediated by type-specific genes located in the capsule synthesis (cps) locus (17). Briefly, a type-specific oligosaccharide repeat unit (RU) is sequentially synthesized on a lipid carrier via the coordinated activity of cps glycosyltransferases (GTs). Some GTs utilize nonhousekeeping donor substrates that must be synthesized by other cps enzymes, e.g., the cps-encoded GT WchO uses UDP-N-acetylmannosamine synthesized by the cps-encoded MnaA synthetase. Completed RUs are exported to the bacterial surface by a Wzx flippase and then polymerized into glycan chains by a Wzy polymerase. The capsule PS of many pneumococcal capsule types can also be modified by cps O-acetyltransferases. Ultimately, mature glycan chains are covalently anchored to the cell wall, forming a glycocalyx that envelops the entire bacterium.
Each capsule type is defined according to the unique biochemical identity of its PS glycan chain. However, biochemical identification of capsule PS is too cumbersome for surveillance purposes, so pneumococcal capsule types have conventionally been assigned a “serotype” according to reactivity with reference antisera, and serotypes sharing antigenic properties are organized in “serogroups.” Serotyping relies on predefined antigenic markers semiarbitrarily chosen according to established serotype standards – many of which have not been epidemiologically revalidated in decades. As result, antigenically similar, but biochemically distinct capsule variants have been mistakenly grouped as a single capsule type, masking very relevant distinctions in their epidemiological behaviors (5, 8, 18). For example, serotypes 6A and 6C were mistakenly identified as a single capsule type “6A” prior to 2007, so an increase in 6C prevalence following the introduction of PCV7 in 2000 was mistakenly interpreted that PCV7 does not offer cross-protection against 6A (19, 20).
Leveraging the link between cps genes and capsule PS structure, some emergent capsule typing methods classify pneumococci according to cps locus nucleotide sequence (21), herein called “cps typing.” We recently showed how cps typing can identify isolates expressing biochemically distinct capsules that are otherwise indistinguishable via conventional serotyping and lead to the discovery of new capsule types (7). We presently report our investigation of two cps types initially identified among “serotype 36” isolates as part of the Global Pneumococcal Sequencing (GPS) project (22) (Table 1). We defined the antigenic profile and biochemical structures of their capsule PS, assigned putative functions to their cps genes, and identified amino acid positions that potentially mediate GT specificity and capsule identity. Altogether, we provide the biochemical, antigenic, and molecular determinants of newly named pneumococcal capsule types 36A and 36B.
TABLE 1.
Serogroup 36 isolates identified in the Global Pneumococcal Sequencing project
| Strain information |
Host information |
Epidemiology |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolate name | cps | Serotypeb | Capsule typec | MLSTd | Sourcee | Gender | Age (yrs) | Country | Isolation yr | Calculated |
| typea | Prevalencef | |||||||||
| GPS_NP_1196 | 36X | 36 | 36B | 8150 | NP swab | Male | Unknown | Nepal | 2009 | 0.25% |
| GPS_US_PATH4269 | 36X | 36 | 36B | 6499 | NP swab | Unknown | Unknown | Ethiopia | Unknown | 1.11% |
| GPS_RU_10 | 36 | 16/36 | 36A | 4841 | CSF | Female | 59 | Russia | 2012 | 1.13% |
| GPS_IN_PN17 | 36 | 9 | 36A | 11394 | Blood | Male | 1 | India | 2011 | 0.62% |
| GPS_IN_PO_H45 | 36 | 36 | 36A | Unknown | NP swab | Female | 55 | India | 2016 | |
| GPS_IN_PO_H151 | 36 | 28F | 36A | Unknown | NP swab | Female | 67 | India | 2016 | |
According to genome sequence alignment to reference capsule biosynthetic (cps) locus nucleotide sequences.
According to quellung reaction or latex agglutination using reference antisera.
Capsule type name given in the current study.
MLST, multilocus sequence typing.
NP, nasopharynx; CSF, cerebrospinal fluid.
Percentage of total GPS project isolates from the respective country putatively expressing the capsule type.
MATERIALS AND METHODS
Bacterial strains and cultivation.
Pneumococcal carriage isolates GPS_NP_1196 (“NP1196,” isolated in Nepal, 2009) and GPS_US_PATH4269 (“PATH4269,” isolated in Ethiopia, year unknown) were characterized as a part of the GPS project (https://www.pneumogen.net/gps/), as previously described (22). Reference strains SSISP36/2 and SSISP24F/2 (an arbitrarily chosen strain to act as a negative control) representing serotypes 36A and 24F, respectively, were obtained from Statens Serum Institut (SSI, Copenhagen, Denmark). Unless noted otherwise, strains were cultured on blood agar plates (BAP) supplemented with 5% sheep blood (Remel Laboratories, Lenexa. KS) or Todd-Hewitt broth with 5% yeast extract (THYb) and incubated at 37°C in 5% CO2. Bacteria stocks were stored in THYb with 15% glycerol at −80°C. The pneumococcal identity of all strains was confirmed using colony morphology, BAP hemolytic activity, and optochin susceptibility (23, 24).
Isolate serotyping.
Quellung, slide agglutination, and flow cytometry serotyping assays (FCSA) were performed as previously described (6, 7, 25, 26) using the following panel of polyclonal rabbit antisera obtained from the SSI (Copenhagen, Denmark): Omnisera (Lot: YOM1A1), Pool D (Lot: CD12B1), Type 36 (Lot: p3613R1), and Factor Serum 9e (Lot: X9e11A1). Macroscopic slide agglutination results were confirmed using microscopy. For FCSA, frozen bacterial stocks were thawed, washed, and incubated in FCSA buffer (phosphate-buffered saline [PBS], 3% fetal bovine serum [FBS], 0.1% NaN3) containing 1:900 dilution of Factor Serum 9e or 1:5,000 dilutions of each other polyclonal rabbit antisera, for 30 min at 4°C. After washing, bound immunoglobulin (Ig) was stained with 1:1,000 dilution of phycoerythrin-labeled antirabbit Ig antibody (Southern Biotech, Birmingham, AL) in FCSA buffer, and detected by flow cytometry using BD Accuri C6 Plus (BD Biosciences, Franklin Lakes, USA) and FCS Express software (Pasadena, USA).
Capsule polysaccharide purification and detection.
Capsule PS was purified from NP1196, PATH4269, and SSISP36/2, as described previously (6, 7). Briefly,10 mL of the culture of each strain was inoculated into 1 L of a chemically defined medium (27) supplemented with choline chloride (1 g/L), sodium bicarbonate (2.5 g/L), and cysteine HCl (0.73 g/L) and incubated at 37°C for 16 h without shaking. Following centrifugation (15,344 × g, for 30 min at 4°C) and removal of the supernatant, bacterial pellets were lysed by incubation in 0.9% aqueous NaCl containing sodium deoxycholate (0.05%) and mutanolysin (100 U/mL), for 72 h at 37°C. Lysates were centrifuged, dialyzed against 4 L of 5 mM Tris (pH 7.3) with 3,500-molecular-weight cutoff dialysis tubing, and applied to a DEAE Sepharose (GE Healthcare, Uppsala, Sweden) anion exchange column. Elution was performed with a linear gradient of NaCl ranging from 0 M to 0.4 M.
The fractions containing capsule PS from SSISP36/2 were detected and quantified with an inhibition-type enzyme-linked immunosorbent assay (ELISA) as described previously (7) using 1 μg/mL of type 36 (36A) purified capsule PS (SSI, Copenhagen, Denmark) as a coating antigen and type 36 antiserum at 1:2,000 dilutions as a serological marker. Capsule PS from NP1196 or PATH4269 could not be detected in the above ELISA. Thus, NP1196 or PATH4269 capsule PS-containing fractions were identified by a classical colorimetric “anthrone reactivity test” which detects and semiquantifies carbohydrates in an acidic solution (28). We pooled fractions containing high levels of capsule PS but low levels of teichoic acid, as determined by inhibition-ELISA testing binding of a phosphocholine-specific monoclonal antibody, HPCG2b (29, 30), to plates coated with pneumococcal teichoic acid (SSI, Copenhagen, Denmark) and other nonglycan contaminants were detected via absorbance at 260 and 280 nm. Pooled fractions were desalted by dialysis, lyophilized, and stored at −20°C until analyzed.
Glycosyl composition analysis.
Glycosyl composition analysis of NP1196 and SSISP36/2 capsule PS was performed at the Complex Carbohydrate Research Center, University of Georgia, Athens, GA. Approximately, 1 mg of capsule PS samples were dephosphorylated by incubating in 48% hydrofluoric acid (HF) for 48 h at 4°C. Samples were dried and depolymerized by acidic methanolysis (1 M HCl in methanol for 16 h at 80°C), followed by re-N-acetylation with pyridine and acetic anhydride to enable the detection of amino sugars. Samples were then per-O-trimethylsilylated (TMS) by treatment with Tri-Sil HTP reagent (ThermoFisher Scientific) for 30 min at 80°C. The TMS derivatives were analyzed by gas chromatography-mass spectrometry (GC-MS) using Agilent 8890 GC interfaced to a 5977B MSD (mass selective detector, electron impact ionization mode). Separation was performed with Supelco Equity-1 fused silica capillary column (30 m × 0.25 mm internal diameter [ID]) (31).
NMR spectroscopy.
Approximately, 5 mg of capsule PS samples were dissolved in 0.6 mL of 99.99% D2O (Cambridge Isotope Laboratories). NMR data were collected at 35°C on Bruker Avance III-HD (1H, 600, or 850 MHz) spectrometers equipped with cryogenic triple-resonance probes. Complete assignment of 1H and 13C signals was achieved by two-dimensional nuclear Overhauser spectroscopy (1H-1H NOESY), correlation spectroscopy (1H-1H COSY), total correlation spectroscopy (1H-1H TOCSY), heteronuclear multiple quantum coherence (1H-13C HMQC), and heteronuclear multiple bond correlation (1H-13C HMBC) spectra. NMR data were processed with NMRPIPE (32) and analyzed with NMRVIEW (33). HDO signal was used as a reference. The 1H-31P HMBC NMR data were collected at the Complex Carbohydrate Research Center at the University of Georgia (Athens, GA) at 35°C on a Varian Inova DD2 NMR spectrometer (1H, 600 MHz) equipped with a room-temperature 3 mm inverse broad-band probe. 1H-31P HMBC spectra were collected with 2000 points, 64 increments, four scans per increment, and spectral widths of 7,184 (1H) and 4,856 Hz (31P). The HMBC long-range transfer delay corresponded to a coupling of 8 Hz. 31P chemical shifts were referenced to 85% H3PO4 at 0 ppm.
Whole-genome sequencing.
Genomic DNA was extracted from SSISP36/2 using a Monarch Genomic DNA purification kit (New England Biolabs, Ipswitch, USA). DNA library construction and sequencing were performed by SeqCenter (Pittsburgh, USA). Raw reads were assembled into draft genomes using the de novo assembler Unicycler v0.4.7. Raw reads and assembled contigs are available on NCBI under BioProject number PRJNA906594. Scaffolds .fasta files were used for downstream analysis.
Comparative genetic analysis.
Genetic sequences used in our analysis and their descriptions and accession numbers are listed in Table S2. The Streptococcus mitis sequence was arbitrarily chosen among sequences of nonpneumococcal origin containing wcjA homologs. Nucleotide and amino acid sequences were compared, translated, and analyzed by Geneious prime v2020. Multiple Alignment using Fast Fourier Transform was run with a scoring matrix of 200 PAM/K of 2 and a gap open penalty of 1.5.
RESULTS
Type 36 cps subtypes demonstrate distinguishable antigenic profiles.
Prior cps typing of the six “serotype 36” isolates in the GPS project revealed that the cps loci of nasopharyngeal carriage isolates GPS_NP_1196 (herein “NP1196”) from Nepal and GPS_US_PATH4269 (herein “PATH4269”) from Ethiopia, both contained alleles of the cps genes wchO and wcjA that more closely resemble Streptococcus mitis alleles than the alleles in other pneumococcal serotype 36 isolates (22). These divergent cps types, which comprised 0.01% (2/18768) of all GPS isolates, were provisionally called “36X” (Table 1). We evaluated whether 36X isolates expressed capsule PS antigenically distinct from the serotype 36 reference isolate, SSISP36/2. Whole-genome sequencing of SSISP36/2 revealed it contains a cps locus that shares 100% identity with the reference serotype 36 cps locus (34) and differs from NP1196 and PATH4269 cps loci principally in the wchO and wcjA gene sequences (Fig. 1A). Accordingly, SSISP36/2 reacted with typing omnisera (recognizes all pneumococcal capsule types), Pool D antisera (recognizes serogroups 9, 11, 16, 36, and 37), and Type 36 antisera (recognizes “serotype 36”) in quellung assays, slide agglutination assays (Fig. 1B), and in our flow cytometric serotyping assay (FCSA) (Fig. 1C). In contrast, NP1196 and PATH4269 reacted only with omnisera in slide agglutination assays (Fig. 1B), but were also recognized by Pool D and Type 36 antisera in quellung and FCSA (Fig. 1C). In search of binary criteria to distinguish these putative subtypes, we tested other antisera used for the identification of Pool D serotypes. Notably, SSISP36/2, but not the other strains (NP1196, PATH4269), was recognized by Factor serum 9e (FS9e) according to quellung and FCSA. According to these distinctive antigenic properties, we provisionally assigned serotype 36A to SSISP36/2 and serotype 36B to NP1196 and PATH4269.
FIG 1.
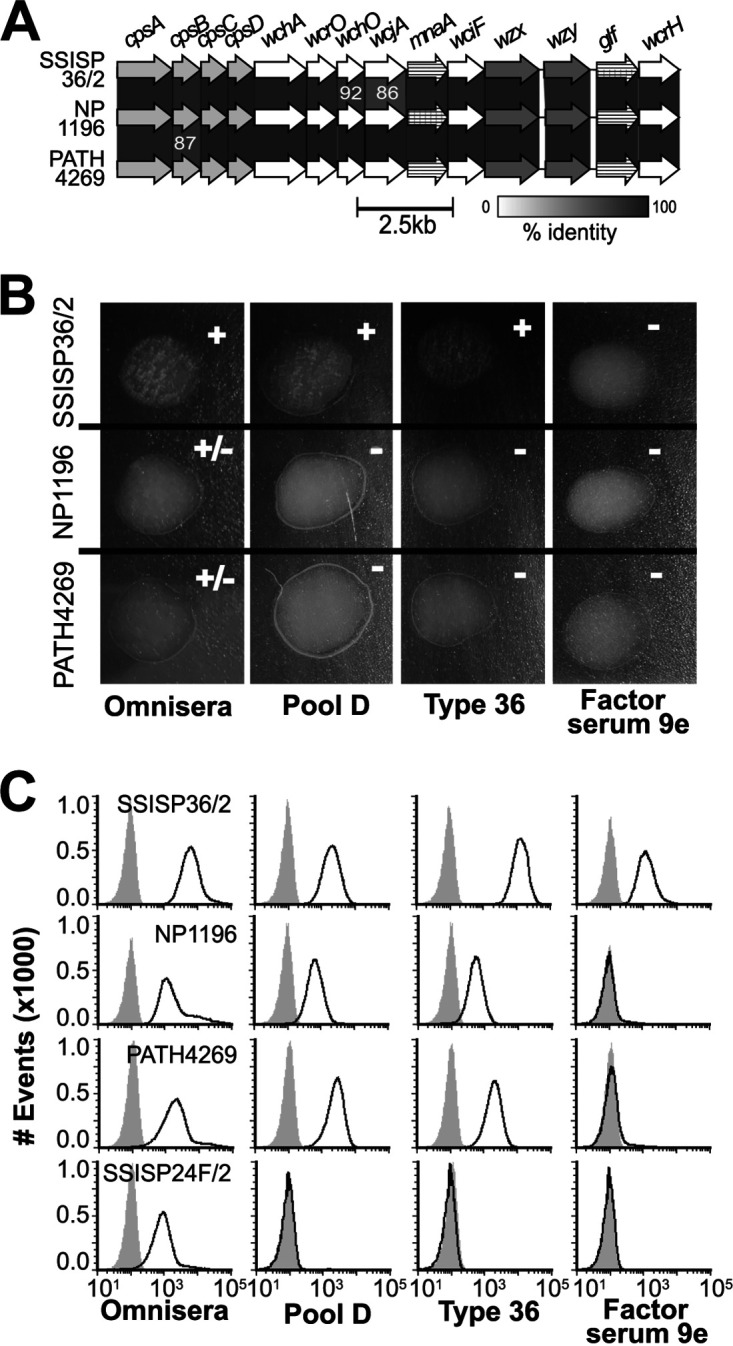
Genetic and antigenic comparison of serogroup 36 subtypes. (A) Alignment of the 36 (SSISP36/2) and 36X (NP1196 and PATH4269) cps loci of strains in this study. Genes encoding glycosyltransferases (white arrows), Wzx/Wzy enzymes (dark gray arrows), carbohydrate synthetases (striped arrows), and highly conserved biosynthetic elements (light gray arrows) are labeled at the top. Gene alignments with percent sequence identity below 93% are labeled in white. (B) Photographic results and interpretation (upper right) of slide agglutination assays using three serogroup 36 strains (left) and reference antisera (bottom). All macroscopic results were confirmed with microscopy. (C) FCSA histograms depicting antibody deposition on strains SSISP36/2 (36A), NP1196 (36B), PATH4269 (36B), and SSISP24F/2 (serotype 24F control). Black curves represent the fluorescence of bacteria incubated in different antisera (bottom labels), while gray-filled curves represent negative-control preparations incubated with secondary antibody only.
Serotypes 36A and 36B capsule PS differ in whether they contain branching glucose or galactose.
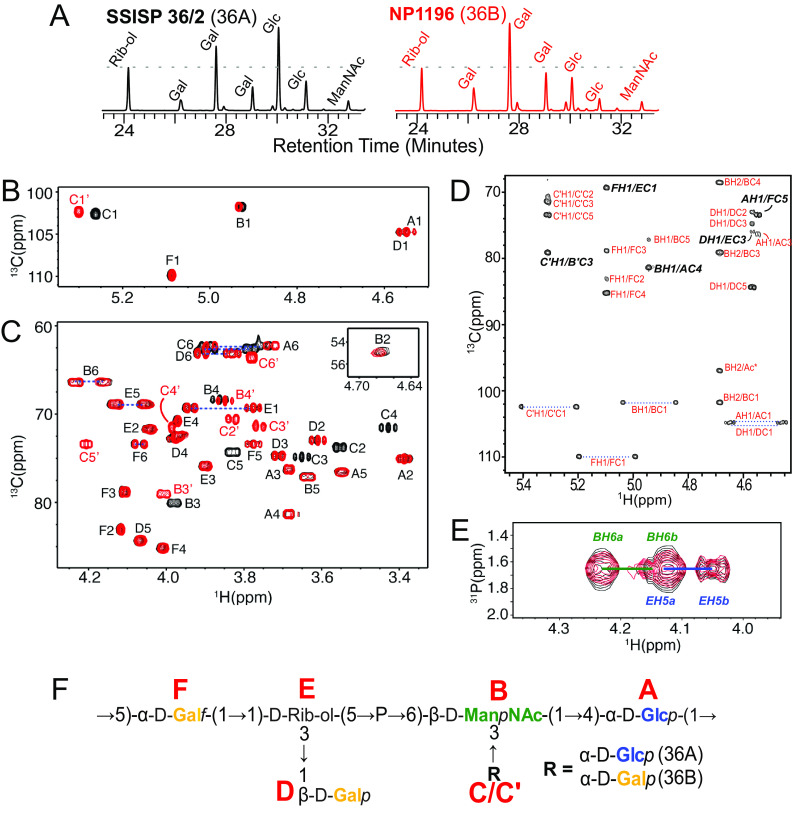
To identify the structural basis for their distinctive antigenic properties, we purified and biochemically analyzed capsule PS from SSISP36/2 (serotype 36A) and NP1196 (serotype 36B). Gas chromatography analysis of hydrofluoric acid-treated samples detected the presence of glucose (Glc), galactose (Gal), N-acetylmannosamine (ManNAc), and ribitol (Rib-ol) in approximately 2:2:1:1 and 1:3:1:1 ratios, in 36A and 36B capsule PS, respectively (Fig. 2A, Table S1), supporting that a Glc residue in the 36A capsule PS RU is substituted by Gal in 36B.
FIG 2.
Biochemical analysis of 36A and 36B capsule polysaccharide. (A) GC/MS analysis chromatograph revealing carbohydrate composition of HF-treated capsule PS purified from SSISP36/2 (36A) and NP1196 (36B). Dotted lines are drawn at the intensity value of ribitol (Rib-ol) for comparison. Relative quantities of each carbohydrate calculated according to the integration of curves corresponding are provided in Table S1. (B and C) Overlay of 1H-13C HMQC NMR spectra of regions containing chemical shifts corresponding to anomeric (B) and ring (C) positions in 36A (black) and 36B (red) capsule PS. Peak assignments are provided in Table 2. (D) 1H-13C HMBC NMR spectrum for 36B capsule PS. (E) Overlay of 1H-31P HMBC NMR spectra for 36A (black) and 36B (red) capsule PS. Cross peaks corresponding to interresidue glycosidic bonds (italicized and bolded) are shown in panels D and E. (F) Structure of 36A and 36B capsule PS. Red letters denote the residue name assigned to each carbohydrate in the NMR spectra.
On further evaluating the capsule PS structures by NMR, we were able to assign all signals in the 36A and 36B capsule PS heteronuclear multiple quantum coherence (HMQC) spectra to six spin systems (labeled residues A to F) corresponding to five anomeric signals and a ribitol (Fig. 2B and C). Complete assignment of 1H, 13C, and 31P chemical shifts by homo- and heteronuclear 2D NMR spectra (Fig. 2D and E) revealed a backbone containing Glcp (residue A), galactofuranose (Galf, residue F), and a rib-ol (residue E) phosopholinked to the 6 position of a ManpNAc (residue B) (Table 2). Furthermore, each RU contained a branching galactopyranose (Galp, residue D) and, consistent with GC/MS findings described above, either a Glcp (residue C in 36A) or a second Galp (residue C’ in 36B) branching off the 3 position of ManpNAc (Fig. 2F). 1H NMR spectrum of capsule PS purified from PATH4269 was indistinguishable from NP1196 capsule PS and shared key chemical shifts distinctive to 36B but not 36A capsule PS (Fig. S1). In summary, except for the presence of either a Glcp (36A) or Galp (36B) branching from the backbone ManpNAc, serotypes 36A and 36B pneumococci express otherwise identical capsule PS (Fig. 2F).
TABLE 2.
1H and 13C chemical shifts (ppm) of 36A and 36B capsule PS obtained at 35°Ca
| Serotype | Residue | Label | NMR chemical shift (proton/carbon shift) (ppm) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a,H6b/C6 | N-Ac | 31P | |||
| 36A | →4-α-d-Glcp-(1→ | A | 4.54/104.8 | 3.38/75.1 | 3.68/76.3 | 3.68/81.4 | 3.55/76.6 | 3.74,3.89/62.3 | ||
| →6-β-d-ManNAc-(1→ | B | 4.92/101.9 | 4.67/55.1 | 3.98/80.1 | 3.87/68.5 | 3.63/77.1 | 4.16,4.23/66.4 | 2.11/24.3 | 1.66 | |
| α-d-Glcp-(1→ | C | 5.26/102.5 | 3.56/73.9 | 3.65/75.0 | 3.43/71.6 | 3.83/74.3 | 3.78,3.88/62.6 | |||
| β-d-Galp-(1→ | D | 4.56/104.8 | 3.61/73.1 | 3.71/74.8 | 3.97/72.7 | 4.06/84.4 | 3.83,3.91/63.1 | |||
| →1-d-Rib-ol-(5→ | E | 3.77,3.94/69.3 | 4.04/71.8 | 3.90/75.9 | 3.97/70.8 | 4.05,4.13/69.0 | ||||
| →5-α-d-Galf-(1→ | F | 5.09/109.9 | 4.11/83.0 | 4.11/78.8 | 4.00/85.2 | 3.77/73.4 | 4.06,4.08/73.5 | |||
| 36B | →4-α-d-Glcp-(1→ | A | 4.54/104.8 | 3.38/75.1 | 3.68/76.3 | 3.68/81.4 | 3.55/76.6 | 3.74,3.89/62.3 | ||
| →6-β-d-ManNAc-(1→ | B′ | 4.93/101.9 | 4.68/55.1 | 4.00/79.1 | 3.85/68.5 | 3.63/77.2 | 4.16,4.23/66.4 | 2.11/24.3 | 1.66 | |
| α-d-Galp-(1→ | C′ | 5.30/102.5 | 3.83/70.6 | 3.76/71.5 | 3.99/71.5 | 4.20/73.4 | 3.77,3.78/63.7 | |||
| β-d-Galp-(1→ | D | 4.56/104.8 | 3.61/73.1 | 3.71/74.8 | 3.97/72.7 | 4.06/84.4 | 3.83,3.91/63.1 | |||
| →1-d-Rib-ol-(5→ | E | 3.77,3.94/69.3 | 4.04/71.8 | 3.90/75.9 | 3.97/70.8 | 4.05,4.13/69.0 | ||||
| →5-α-d-Galf-(1→ | F | 5.09/109.9 | 4.11/83.0 | 4.11/78.8 | 4.00/85.2 | 3.77/73.4 | 4.06,4.08/73.5 | |||
Each carbohydrate residue is labeled with a unique letter for 36A (A to F) and 36B (A to F). Proton and carbon are indicated by letters H and C, respectively, and the numbers associated with them indicate their respective chemical shift values. A slash separates the proton and carbon chemical shifts (ppm). For each residue, the table shows the chemical shifts of every proton and carbon molecule attached to it at different positions. N-Ac, N-acetylation. 31P, phosphate.
36A/36B capsule PS structure is compatible with assigned putative biosynthetic roles of their cps genes.
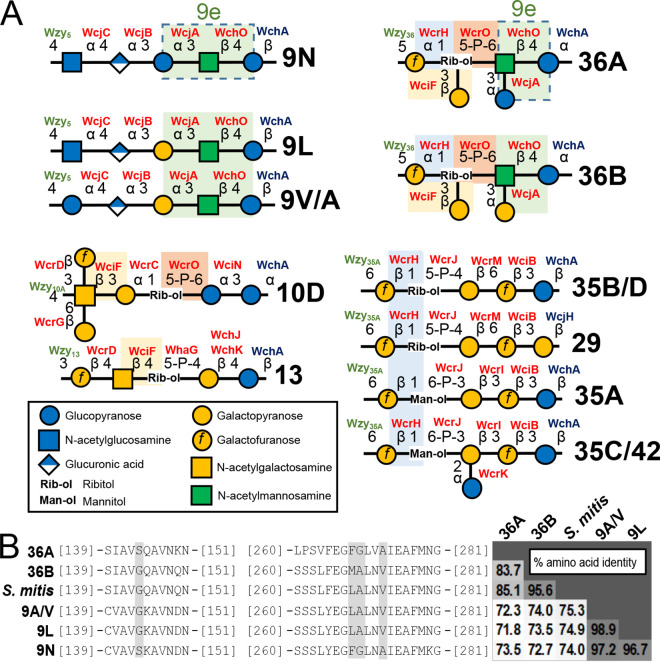
Our de novo derived 36A/36B capsule PS structure shares little similarity with any other described pneumococcal cps (35). To validate our conclusions, we assigned functional roles to all 36A/36B cps-encoding genes via correlation with similar capsule PS features and cps gene functions previously reported in other capsule types (Fig. 3A). First, the presence of ManpNAc and Galf in capsule PS requires biosynthesis of nonhousekeeping donor molecules UDP-ManpNAc and UDP-Galf by MnaA and Glf, respectively (36), and incorporation of the reducing end Glcp is determined by the initial transferase WchA in most pneumococcal capsule types (34, 36). N-Acetylmannosamine is likely added to the backbone by WchO, which putatively mediates the β-d-ManpNAc-(1→4)-β-d-Glcp linkage in serogroup 9, 19, and 36 RUs (34, 36). WcrO is the only predicted phosphotransferase encoded in 36A/36B cps loci (36) and putatively mediates the d-Rib-ol-(5→P→6)-β-d-ManpNAc linkage, similar to its assigned function in serotype 10D (Fig. 3A, orange boxes) (7). WcrH is predicted to be a Galf-polyol transferase for various capsule types and can be assigned to the α-d-Galf-(1→1)-d-Rib-ol linkage (Fig. 3A, blue boxes). The α-d-Glcp side chain is likely formed by WcjA, which is associated with the characteristic α-d-Glcp/Galp-(1→3)-β-d-ManpNAc bond in serogroup 9 and 36 (Fig. 3A, green boxes) (34, 36). By elimination, the β-d-Galp-(1→3)-d-Rib-ol side chain is assigned to WciF. While WciF homologs are associated with β-Galp/GalpNAc-(1→3)-Galp linkage in other capsule types, including serogroups 10 and 33 (7, 36), it is also linked to the β-GalpNAc-(1→4)-d-Rib-ol bond reported in serotype 13 capsule PS (36, 37) (Fig. 3A, yellow boxes). Last, the 36A/36B Wzy polymerase can be assigned to the β-d-Glcp-(1→5)-α-d-Galf glycosidic bond that mediates glycan extension and defines the capsule PS glycosidic backbone. Altogether, our derived capsule PS structures are consistent with 36A/36B cps loci content.
FIG 3.
Structural and genetic comparative analysis identifies putative determinants of 36A versus 36B capsule types. (A) Graphical representation of structurally resolved pneumococcal capsule polysaccharide (PS) repeat units that contain features putatively mediated by 36A/36B capsule biosynthetic (cps) locus gene homologs. For clarity, capsule PS O-acetylation is not depicted, and capsule types with identical de-O-acetylated capsule PS structures (e.g., 9V and 9A, etc.) are listed together. Structural features shared by different capsule types are highlighted by alike colored boxes. Glycosidic bonds predicted to be catalyzed by each cps-encoded glycosyltransferase (GT) (see the text) are labeled in red. Features dictated by initial GTs (blue) and polymerases (green) are also labeled. Dotted boxes in the 36A and 9N repeat units denote the predicted factor 9e antigenic determinant (“9e”). (B) Alignment of amino acid sequences encoded by wcjA alleles from cps loci representing listed serotypes and from Streptococcus mitis (S. mitis). For clarity, only portions containing residues associated with factor 9e (highlighted in gray) are shown. The grid on the right shows pairwise percent amino acid identity between the complete wcjA-encoded GTs.
The type-defining 9N/36A antigenic determinant is likely mediated by distinct WcjA polymorphisms.
Though FS9e is reported to exclusively recognize pneumococcal serotype 9N, its cross-reactivity with SSISP36/2 strongly suggests that 36A and 9N capsule PS share an antigenic determinant that is absent from the capsule PS of 36B and other FS9e nonreactive serogroup 9 capsule types (i.e., 9A, 9V, and 9L). Indeed, while all other capsule PS structures contain a α-d-Galp-(1→3)-β-d-ManpNAc-(1→4)-Glcp, 9N and 36A capsule PS harbor a α-d-Glcp-(1→3)-β-d-ManpNAc-(1→4)-Glcp (Fig. 3A, dotted boxes), herein called “factor 9e.” As the synthesis of factor 9e in serogroups 9 and 36 is putatively mediated by WcjA (see above), we evaluated whether any WcjA allelic features correlated with its putative specificity for adding Glcp versus Galp. Alignment of translated amino acids of wcjA alleles from representative cps loci and S. mitis found that despite a relatively low overall WcjA identity between serogroups (71.8 to 74.0%, compared to 83.7 to 98.9% within serogroups), the 9N and 36A WcjA shared four polymorphisms, S144, F268, G269, and A273, compared to G144, M/L268, A269 and V273 in all other alleles (Fig. 3B). Thus, across phylogenetically diverse alleles, factor 9e presence correlates to the identity of four WcjA amino acids.
DISCUSSION
We report the biochemical, antigenic, and genetic determinants of newly named pneumococcal capsule types 36A and 36B. We opted to follow recent trends in capsule type nomenclature (10) and assigned the initial member of this newly defined serogroup “36A,” in contrast to prior conventions that used “F” to denote the “first” member. 36A and 36B share very similar capsule PS structures, though they can be distinguished serologically by the presence of factor 9e on 36A. This antigen is mediated by the presence of a 36A and 9N type-defining α-d-Glcp-(1→3)-β-d-ManpNAc, instead of an α-d-Galp-(1→3)-β-d-ManpNAc present in 36B and other serogroup 9 capsule PS. This shared capsule PS structural determinant is in turn associated with the identity of four amino acid positions (i.e., 144, 268, 269, and 273) in the cps locus-encoded GT WcjA. Though the predicted role of these WcjA residues requires confirmatory tests, capsule PS variation commonly arises from minor GT alterations. For example, three residues in the serogroup 6 cps GT WciP dictate whether 6A or 6B capsule PS is expressed (38), while a single amino acid substitution in the cps GTs WciN or WcrL converts serotypes 6A to 6F or 11A to 11D, respectively (39, 40).
Regarding the evolutionary origins of 36A and 36B, strains putatively expressing capsule type 36A have been isolated decades apart and across multiple continents (22, 34), reflecting an ability for this capsule type to persist in the global pneumococcal pool. In contrast, the two 36B isolates (i.e., NP1196 and PATH4269) were obtained in separate countries and represent two phylogenetic independent lineages (22), so additional isolates are required to confirm whether 36B is capable of clonal propagation. Alignment analysis revealed that approximately 3.7 kb region containing wcrO, wchO, wcjA, and mnaA in the 36B cps locus shares >95% nucleotide identity with other streptococcal species, such as S. mitis (22), but some of these genes share only 86 to 92% identity with the 36A cps locus (Fig. 1A). This suggests 36B arose as result of a homologous recombination event between a 36A recipient and a nonpneumococcal donor, though it could also mean that a 36B donor was the source for these capsule synthesis genes in other streptococcal species. Regardless, this demonstrates that 36A and 36B likely diverged by means of horizontal gene transfer, as opposed to incremental accumulation of polymorphisms in wcjA.
It is difficult to estimate the true prevalence of 36A and 36B among global populations. Prior studies employing conventional serotyping schemes would have been unable to distinguish 36A and 36B and may have even misclassified 36B due to the apparent lack of enough cross-linking antibodies to mediate agglutination/quellung in at least one lot of Pool D antisera (Fig. 1B). Given the limited access to assays sensitive enough to detect antibody binding to capsule regardless of cross-linking capability (e.g., FCSA, etc.), efforts should develop Pool D antiserum and FS9e with enough reactivity to 36B and 36A bacteria, respectively, to identify these capsule types in conventional agglutination and quellung assays. Alternatively, we propose future surveys distinguish 36A and 36B by wcjA sequencing and focus on encoded polymorphisms of residues 144, 268, 269, and 273.
Despite limitations in current typing techniques, it is apparent that serogroup 36 strains are overall uncommon. However, the characterization of low-prevalence capsule types can offer important insight into capsule biology. First, 36A and 36B represent a novel capsule PS structural motif containing a backbone d-Rib-ol with a branching monosaccharide (Fig. 3A), a feature that to our knowledge has not been described in any microbial glycan. Characterizing rare capsule types and describing the full breadth of capsule structures is important, as these uncommon structural features could become future targets in ongoing vaccine efforts or could be exploited for bioengineering applications.
Second, investigating a variety of capsule types facilitates identifying the determinants of capsule PS structure encoded in synthesis genes. Genetic typing methods, e.g., cps typing, etc, are powerful tools regularly being employed to type glycans in multiple microbial species, especially those for which serological methods are unavailable (21, 41, 42). However, most of these tools determine capsule PS structure according to nucleotide identity with curated reference sequences. This approach has limitations, as divergent cps sequences do not necessarily result in different capsule types, as highlighted by the fact that many strains predicted to express different capsules according to cps locus sequence alone in our GPS study (i.e., 9X, 11X, 16X, 18X2, 18X3, and 29X) (22), actually produced capsule PS that is serologically and biochemically identical to the recognized capsule PS (unpublished findings). Conversely, as discussed above, only 1 to 3 amino acid substitutions are sufficient to result in differences in capsule PS structure with very significant biological implications (5, 8, 18, 43). Genetic glycan typing methods must incorporate amino acid functional determinants encoded in biosynthesis genes, and identifying these determinants is facilitated by aligning sequence across phylogenetically distinct gene sets regardless of epidemiological prevalence, as demonstrated in our comparison of wcjA alleles in serogroups 9 and 36.
Last, despite their scarcity in pneumococcal studies, capsules serologically and genetically similar to pneumococcal 36A/36B capsules types have been identified among oral streptococcal species, Streptococcus mitis and Streptococcus infantis (44). This strongly suggests multiple species produce capsule PS identical or similar to pneumococcal serogroup 36. The capsule repertoires of nonpneumococcal commensal species arguably play major roles in shaping natural anticapsule PS herd immunity overall, consequently fueling a selective pressure that prevents overlapping capsule types from becoming prevalent among pneumococcal populations. Thus, the low frequency of a capsule PS among pneumococci does not mean humans are not routinely exposed to these microbial glycans or that they play a marginal role in shaping human health.
ACKNOWLEDGMENTS
We thank Rama Kandasamy and Andrew Pollard for providing the Nepalese isolate.
This work was supported by the Global Pneumococcal Sequencing project funded by the Bill and Melinda Gates Foundation (grant code OPP1034556). The High-Field NMR facility at the University of Alabama at Birmingham was established through the NIH (1S10RR026478) and is supported by the UAB Comprehensive Cancer Center (NCI grant P30 CA013148). J.J.C. is supported by an NIH grant (K08 AI148582). The NMR facility at the Complex Carbohydrate Research Center, University of Georgia is supported by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, U.S. Department of Energy (grant no. DE-SC0015662 to Parastoo Azadi).
UAB has Intellectual Property rights on some reagents used in the study. F.A.G., J.S.S., J.J.C., and M.H.N. are UAB employees. We declare that they have no other relevant conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
[This article was published on 27 March 2023 with errors in Fig. 1 and 3. Figure 1 was updated in the version posted on 28 March 2023. Figure 3 was updated in the current version, posted on 30 March 2023.]
Supplemental material is available online only.
Contributor Information
Moon H. Nahm, Email: mnahm@uabmc.edu.
Sandra S. Richter, Mayo Clinic
REFERENCES
- 1.Jimbo-Sotomayor R, Armijos-Acurio L, Proano-Espinosa J, Segarra-Galarza K, Sanchez-Choez X. 2020. Morbidity and mortality due to pneumococcal disease in children in ecuador from 2005 to 2015. J Glob Infect Dis 12:124–128. 10.4103/jgid.jgid_125_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal global burden of disease study team . 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Doyle CR, Pirofski LA. 2016. Reduction of Streptococcus pneumoniae colonization and dissemination by a nonopsonic capsular polysaccharide antibody. mBio 7:e02260-15–e02215. 10.1128/mBio.02260-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musher DM, Groover JE, Rowland JM, Watson DA, Struewing JB, Baughn RE, Mufson MA. 1993. Antibody to capsular polysaccharides of Streptococcus pneumoniae: prevalence, persistence, and response to revaccination. Clin Infect Dis 17:66–73. 10.1093/clinids/17.1.66. [DOI] [PubMed] [Google Scholar]
- 5.Calix JJ, Nahm MH. 2010. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J Infect Dis 202:29–38. 10.1086/653123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganaie F, Maruhn K, Li C, Porambo RJ, Elverdal PL, Abeygunwardana C, van der Linden M, Duus JO, Sheppard CL, Nahm MH. 2021. Structural, genetic, and serological elucidation of Streptococcus pneumoniae serogroup 24 serotypes: discovery of a new serotype, 24C, with a variable capsule structure. J Clin Microbiol 59:e0054021. 10.1128/JCM.00540-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganaie F, Saad JS, McGee L, van Tonder AJ, Bentley SD, Lo SW, Gladstone RA, Turner P, Keenan JD, Breiman RF, Nahm MH. 2020. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio 11:e00937-20. 10.1128/mBio.00937-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol 45:1225–1233. 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calix JJ, Nahm MH, Zartler ER. 2011. Elucidation of structural and antigenic properties of pneumococcal serotype 11A, 11B, 11C, and 11F polysaccharide capsules. J Bacteriol 193:5271–5278. 10.1128/JB.05034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, Nahm MH. 2012. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem 287:27885–27894. 10.1074/jbc.M112.380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen R, Levy C, Ouldali N, Goldrey M, Bechet S, Bonacorsi S, Varon E. 2021. Invasive disease potential of pneumococcal serotypes in children after PCV13 implementation. Clin Infect Dis 72:1453–1456. 10.1093/cid/ciaa917. [DOI] [PubMed] [Google Scholar]
- 12.Galanis I, Lindstrand A, Darenberg J, Browall S, Nannapaneni P, Sjostrom K, Morfeldt E, Naucler P, Blennow M, Ortqvist A, Henriques-Normark B. 2016. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. Eur Respir J 47:1208–1218. 10.1183/13993003.01451-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedson DS. 2014. Preventing non bacteremic pneumococcal pneumonia in older adults: historical background and considerations for choosing between PCV13 and PPV23. Hum Vaccin Immunother 10:1322–1330. 10.4161/hv.28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen R, Varon E, Doit C, Schlemmer C, Romain O, Thollot F, Bechet S, Bonacorsi S, Levy C. 2015. A 13-year survey of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 and PCV13 implementation. Vaccine 33:5118–5126. 10.1016/j.vaccine.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Hausdorff WP, Hanage WP. 2016. Interim results of an ecological experiment - Conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother 12:358–374. 10.1080/21645515.2015.1118593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. 2018. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis 18:441–451. 10.1016/S1473-3099(18)30052-5. [DOI] [PubMed] [Google Scholar]
- 17.Yother J. 2011. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annu Rev Microbiol 65:563–581. 10.1146/annurev.micro.62.081307.162944. [DOI] [PubMed] [Google Scholar]
- 18.Nahm MH, Lin J, Finkelstein JA, Pelton SI. 2009. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis 199:320–325. 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park IH, Moore MR, Treanor JJ, Pelton SI, Pilishvili T, Beall B, Shelly MA, Mahon BE, Nahm MH, Active Bacterial Core Surveillance Team . 2008. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis 198:1818–1822. 10.1086/593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs MR, Bajaksouzian S, Bonomo RA, Good CE, Windau AR, Hujer AM, Massire C, Melton R, Blyn LB, Ecker DJ, Sampath R. 2009. Occurrence, distribution, and origins of Streptococcus pneumoniae Serotype 6C, a recently recognized serotype. J Clin Microbiol 47:64–72. 10.1128/JCM.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epping L, van Tonder AJ, Gladstone RA, The Global Pneumococcal Sequencing C, Bentley SD, Page AJ, Keane JA. 2018. SeroBA: rapid high-throughput serotyping of Streptococcus pneumoniae from whole genome sequence data. Microb Genom 4:e000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Tonder AJ, Gladstone RA, Lo SW, Nahm MH, Du Plessis M, Cornick J, Kwambana-Adams B, Madhi SA, Hawkins PA, Benisty R, Dagan R, Everett D, Antonio M, Klugman KP, von Gottberg A, Breiman RF, McGee L, Bentley SD, The Global Pneumococcal Sequencing C . 2019. Putative novel cps loci in a large global collection of pneumococci. Microb Genom 5:e000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song JY, Eun BW, Nahm MH. 2013. Diagnosis of pneumococcal pneumonia: current pitfalls and the way forward. Infect Chemother 45:351–366. 10.3947/ic.2013.45.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govindan V, Ganaie F, Nagaraj G, Hussain A, Rk KL. 2016. Laboratory based identification of pneumococcal infections: current and future. Pediatr Infect Dis 8:76–78. [Google Scholar]
- 25.Henrichsen J. 1995. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol 33:2759–2762. 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geno KA, Saad JS, Nahm MH. 2017. Discovery of novel pneumococcal serotype 35D, a natural WciG-deficient variant of serotype 35B. J Clin Microbiol 55:1416–1425. 10.1128/JCM.00054-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Rijn I, Kessler RE. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun 27:444–448. 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashwell G. 1957. Colorimetric analysis of sugars. Methods Enzymol 3:73–105. 10.1016/S0076-6879(57)03350-9. [DOI] [Google Scholar]
- 29.Nahm MH, Brissac T, Kilian M, Vlach J, Orihuela CJ, Saad JS, Ganaie F. 2020. Pneumococci can become virulent by acquiring a new capsule from oral streptococci. J Infect Dis 222:372–380. 10.1093/infdis/jiz456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer BL, Saad JS, Shenoy AT, Orihuela CJ, Nahm MH. 2017. Position of O-acetylation within the capsular repeat unit impacts the biological properties of pneumococcal serotypes 33A and 33F. Infect Immun 85:e00132-17. 10.1128/IAI.00132-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santander J, Martin T, Loh A, Pohlenz C, Gatlin DM, Curtiss R. 2013. Mechanisms of intrinsic resistance to antimicrobial peptides of Edwardsiella ictaluri and its influence on fish gut inflammation and virulence. Microbiology (Reading) 159:1471–1486. 10.1099/mic.0.066639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 33.Johnson BA, Blevins RA. 1994. Nmr View - a computer-program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614. 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 34.Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet 2:e31. 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. 2015. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 28:871–899. 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aanensen DM, Mavroidi A, Bentley SD, Reeves PR, Spratt BG. 2007. Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J Bacteriol 189:7856–7876. 10.1128/JB.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamerling JP. 2000. Pneumococcal polysaccharides: a chemical view, p 81–114. In Tomasz A (ed), Streptococcus pneumoniae molecular biology & mechanisms of disease. Mary Ann Liebert, Inc., Larchmont, NY. [Google Scholar]
- 38.Mavroidi A, Godoy D, Aanensen DM, Robinson DA, Hollingshead SK, Spratt BG. 2004. Evolutionary genetics of the capsular locus of serogroup 6 pneumococci. J Bacteriol 186:8181–8192. 10.1128/JB.186.24.8181-8192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliver MB, van der Linden MP, Kuntzel SA, Saad JS, Nahm MH. 2013. Discovery of Streptococcus pneumoniae serotype 6 variants with glycosyltransferases synthesizing two differing repeating units. J Biol Chem 288:25976–25985. 10.1074/jbc.M113.480152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver MB, Jones C, Larson TR, Calix JJ, Zartler ER, Yother J, Nahm MH. 2013. Streptococcus pneumoniae serotype 11D has a bispecific glycosyltransferase and expresses two different capsular polysaccharide repeating units. J Biol Chem 288:21945–21954. 10.1074/jbc.M113.488528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyres KL, Cahill SM, Holt KE, Hall RM, Kenyon JJ. 2020. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb Genom 6:e000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyres KL, Wick RR, Gorrie C, Jenney A, Follador R, Thomson NR, Holt KE. 2016. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb Genom 2:e000102. 10.1099/mgen.0.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brady AM, Calix JJ, Yu J, Geno KA, Cutter GR, Nahm MH. 2014. Low invasiveness of pneumococcal serotype 11A is linked to ficolin-2 recognition of O-acetylated capsule epitopes and lectin complement pathway activation. J Infect Dis 210:1155–1165. 10.1093/infdis/jiu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorensen UB, Yao K, Yang Y, Tettelin H, Kilian M. 2016. Capsular polysaccharide expression in commensal Streptococcus species: genetic and antigenic similarities to Streptococcus pneumoniae. mBio 7:e01844-16. 10.1128/mBio.01844-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download jcm.00024-23-s0001.pdf, PDF file, 0.2 MB (218KB, pdf)