Abstract
Endometrial cancer is the most common gynaecological malignancy in developed countries. Over 382,000 new cases were diagnosed worldwide in 2018, and its incidence and mortality are constantly rising due to longer life expectancy and life style factors including obesity. Two major improvements are needed in the management of patients with endometrial cancer, i.e., the development of non/minimally invasive tools for diagnostics and prognostics, which are currently missing. Diagnostic tools are needed to manage the increasing number of women at risk of developing the disease. Prognostic tools are necessary to stratify patients according to their risk of recurrence pre-preoperatively, to advise and plan the most appropriate treatment and avoid over/under-treatment. Biomarkers derived from proteomics and metabolomics, especially when derived from non/minimally-invasively collected body fluids, can serve to develop such prognostic and diagnostic tools, and the purpose of the present review is to explore the current research in this topic. We first provide a brief description of the technologies, the computational pipelines for data analyses and then we provide a systematic review of all published studies using proteomics and/or metabolomics for diagnostic and prognostic biomarker discovery in endometrial cancer. Finally, conclusions and recommendations for future studies are also given.
Keywords: endometrial cancer, proteomics, metabolomics, biomarker, machine learning
1. Introduction
1.1. Endometrial cancer – The need for minimally invasive diagnostic and prognostic biomarkers
Endometrial cancer (EC) is the most common gynaecological neoplasm in developed countries, and over 382,000 new cases were diagnosed worldwide in 2018 (1). In general, EC is diagnosed in postmenopausal women (85% of cases) and its incidence is rising due to longer life expectancy and life style associated risk factors. Women with BMI above 35 have an odds ratio of 5.7 for developing EC, with an increase of 1.1 odds ratio per BMI unit (2, 3). Exposure to unopposed estrogens or tamoxifen or genetic aberrations associated with Lynch syndrome confer a cumulative risk up to 70% (4, 5). Finally, endocrine disruptors and other environmental pollutants can also increase EC risk (6, 7). Therefore, an alarmingly high number of women in the general population is exposed to risk factors for developing EC.
In this context, screening programs would be extremely beneficial for these women, but, unfortunately, no minimally- or non-invasive diagnostic tool for EC exists today, and diagnosis relies on invasive endometrial biopsy and pathology investigation.
A second unmet clinical need in EC is the necessity to accurately stratify patients. EC is diagnosed at an early FIGO stage in 80% of the cases, and the five-year survival of FIGO stage 1a is around 95%. However, a proportion of women diagnosed with early-stage EC develop recurrent disease, which dramatically decreases survival rates (8). This represents a challenge as recent projections indicate that the worldwide EC mortality will increase by 70% by 2040 (Global Cancer Observatory, World Health Organisation - https://gco.iarc.fr).
Therefore, prognostic biomarkers to reliably predict patient prognosis are needed, both prior to any intervention - to decide on the most appropriate treatment and if needed optimally plan the surgical procedure - as well as post-operatively, to define the most appropriate adjuvant treatment, and avoid over-treatment and under-treatment. A number of prognostic markers like histological assessment of tumour type and grade, hormone receptor status, PTEN expression, mismatch repair proteins (MLH1, PMS2, MSH2, MSH6), POLE exon 3 mutation, CTTNB1 mutation, L1CAM overexpression, and TP53 aberrations allow stratification of patients according to their risk of recurrence (9–20). In particular, the recent introduction of The Cancer Genome Atlas (TCGA) molecular classification improved the risk stratification at the postsurgical (21, 22), but also improved the concordance between presurgical biopsy and pathology assessment at hysterectomy (23), which has been a problem in the past (24). This classification groups EC patients in four clusters with distinct prognosis and a number of studies demonstrated the reliability and the clinical applicability of this classification using surrogate analyses (i.e., IHC and POLE gene mutation analyses). Patients with POLE mutated tumours have the best prognosis, followed by mismatch repair deficient tumours and with the final groups having an intermediate and the worse prognosis (no specific molecular profile and p53 mutated, respectively) (14). Recently, also classification methods fully based on IHC, hence applicable also in centres with limited access to molecular infrastructures, showed robustness and reliability (19).
Nevertheless, these methods require invasive biopsies, and women consider the presurgical biopsy procedures discomforting and painful (25). Therefore, non- or minimally-invasive prognostic tools applicable presurgically are urgently needed.
Proteomic and metabolomic profiles are attractive approaches for identifying biomarkers that can be detected in tissues or body fluids obtained via non-, minimaly or semi--invasive procedures. The purpose of the present review is to explore the current research on the use of proteomics and metabolomics in the context of EC. This review provides a brief introduction to the wet-lab technologies, the computational pipelines for data analyses and a systematic review of all published studies aimed at using proteomics and/or metabolomics for diagnostic and prognostic biomarker discovery in EC. This is followed by conclusions and recommendations for future studies.
1.2. Proteomic and metabolomic approaches for biomarker discovery
Proteomics and metabolomics represent fields that have grown significantly in the last decades, thanks to the important technological advances that allow accurate and sensitive analyses. Both approaches have been extensively used for biomarker discovery in various disorders (26–31).
1.2.1. Targeted and non-targeted proteomics
Large-scale proteomics mainly relies on two different methodological approaches, namely immune-based, targeted protein microarrays and (non-targeted and targeted) mass spectrometry (MS). Making use of antibody-protein specific binding, protein microarrays can be seen as miniaturized conventional assays, thereby allowing multiplexing and high throughput. Protein microarrays relevant for biomarker discovery fall into three categories: analytical microarrays, reverse phase protein array (RPPA), and bead-based microarrays. Analytical protein microarrays are also called capture or antibody microarray because proteins from complex protein lysates are captured by antibodies or aptamers, which have been previously immobilized on the surface of an array. Conversely, RPPA is based on the immobilization of complex samples on a surface and subsequent probing by pre-selected antibodies. Bead-based microarrays use capture antibodies immobilised on microbeads combined with secondary, detection antibodies. Protein microarrays are highly sensitive and highly specific assays, which allow relative quantification among different clinical sample groups. While multiplexity is usually higher in analytical microarrays compared to RPPA and bead-based microarrays, all methods share simple sample processing allowing high throughput. A further immune-based method, Olink technology, uses antibodies that are labelled with ssDNA and detect proteins in a sample by proximity extension assay (PEA). Pairwise antibodies are linked with complementary ssDNA which upon binding the target protein are hybridized and extended using a DNA polymerase. Despite being targeted hypothesis-driven approaches, antibody-based technologies like protein arrays are solid and promising tools for biomarker discovery and verification (32–34).
Mass spectrometry measures mass-to-charge ratios of ionized peptides in order to analyse proteins. Ionization of proteins can be achieved by electrospray ionization (ESI) or by matrix-assisted laser desorption/ionization (MALDI). ESI allows the creation of ions in solution, while in MALDI, ions are created by laser light pulsing on matrix embedded proteins. A variation of MALDI is SELDI (surface-enhanced laser desorption/ionization), where the proteins are applied on a modified matrix surface allowing binding of specific proteins or proteins classes (35). Mass analysis of proteins is primarily conducted using TOF (time-of-flight) or quadrupoles. Sample preparation for mass spectrometry is a complex process. Upon cellular lysis, it includes subcellular fractionation, depletion of highly abundant proteins, enrichment of target proteins, denaturation and protein digestion. Resolving and denaturation of proteins can also be achieved by SDS polyacrylamide gel electrophoresis, 1D or 2D polyacrylamide gel electrophoresis (PAGE) or difference gel electrophoresis (DIGE). Mass spectrometry is not inherently quantitative but different types of labelling (isobaric tags for relative and absolute quantitation - iTRAQ; isotope-coded affinity tag - ICAT, stable isotope labelling by amino acids in cell culture - SILAC) allow relative and absolute quantification. Label-free quantification, based on signal intensity, is an alternative, cost-efficient option but with a relatively low throughput (36).
Non-targeted mass spectrometry is widely used for biomarker discovery because of its suitability for hypothesis-free approaches. Due to the complexity of the workflow, the number of samples analysed in a discovery setting is usually quite limited, especially when plasma samples are used. Furthermore, fractionation, depletion of high abundant proteins or digestion could bias the results and limit the sensitivity in the untargeted approach. In general, only a small number of candidates undergo clinical validation using orthogonal platforms and even fewer are tested in clinical studies (37, 38) as these studies need first the transition of MS data into immunobased assays to analyse a sufficiently large and statistically relevant number of samples. In this regard, protein microarrays for discovery present the advantage that such translation is not necessary (33).
Proteomics displays a large panel of different tools, which can be combined for discovery and validation phases and subsequently integrated in multi-omics approaches (39).
1.2.2. Targeted and non-targeted metabolomics
Metabolomics is the most recent ‘omics’-technology and strives to measure ideally all metabolites in a given biological sample (40). Since metabolites are final downstream products of all cellular processes, metabolomics is closest to the phenotype compared to the other ‘omics’-techniques.
Similar to proteomics, two approaches with different objectives are used (41), namely non-targeted and targeted metabolomics. Non-targeted metabolomics (profiling metabolomics) is a hypothesis-free approach, which aims to detect simultaneously as many metabolites as possible. Depending on the analytical platform, non-targeted metabolomics reveals metabolites from a wide range of metabolite classes (42), which are annotated after the measurement. Thus, the detection of unknown metabolites not yet annotated in metabolite databases is common in non-targeted metabolomics. Although being comprehensive, non-targeted metabolomics does not allow absolute quantification, but can provide at best only semiquantitative results (42).
Targeted metabolomics is hypothesis-driven and aims to quantify the absolute concentrations of a predefined set of metabolites (42). Since all measured metabolites are pre-selected, a standard calibration curve for accurate quantification can be prepared for each metabolite. Stable-isotope labelled internal standards are added at known concentrations to all samples, allowing compensation for any analytical interferences. With its advantages such as validated analytical performance and the results delivered in absolute concentrations, targeted metabolomics is often used for biomarker validation (42). However, the limited number of simultaneously quantified metabolites in targeted metabolomics increases the risk of missing relevant biological processes.
Metabolomic approaches usually use MS or nuclear magnetic resonance spectroscopy (NMR). While MS offers high mass accuracy, high resolution, high dynamic range and high sensitivity (43), NMR is less sensitive but is superior in terms of structural information content, robustness, and reproducibility (44, 45). However, current analytical methods are not able to cover the entire metabolome (46). To achieve a high metabolite coverage combined with quantitative data, the integration of different metabolomic techniques (multiplatform approaches) is necessary (46, 47).
1.3. Bioinformatics and statistical approaches for constructing diagnostic and prognostic algorithms
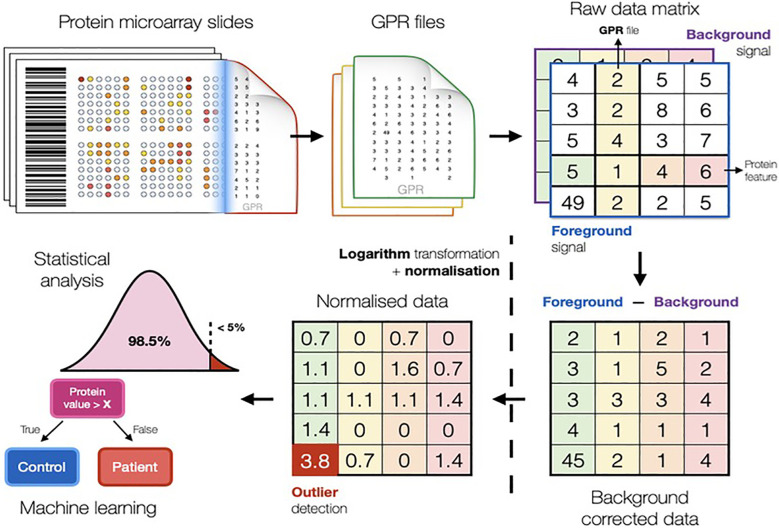
Data from proteomic and metabolomic experiments can reveal molecules that can possibly serve as diagnostic or prognostic markers. However, even if well-designed and executed, experiments often result in noisy, biased and incomplete data due to a multitude of uncontrollable factors. Therefore, thorough data analysis needs to be performed to eliminate technical noise, while preserving genuine biological variation between samples. A set of computational methods used to analyse data are typically bundled together into one unified data analysis pipeline ( Figure 1 ), which treats raw data files as an input while providing the list of potential biomarkers as the output.
Figure 1.
Scheme illustrating the typical computational methods used in biomarker discovery studies to analyse data. The pipeline in figure refers to the conventional protein microarray analysis (Fishman et al. https://arxiv.org/abs/2201.06074).
Proteomic and metabolomic raw data is first processed by background correction, signal transformation, outlier detection, and normalisation. Pre-processing is essential to minimise unwanted technical bias and enable comparisons of samples. Further, integration of clinical information enables comparisons of average metabolite and protein signals between phenotypic groups of interest (48–50).
Background correction addresses different effects in proteomics and metabolomics. In protein microarrays, it is challenging to correctly quantify the fluorescent signal produced by the biological reaction avoiding local residuary background (51–53). In metabolomics, background (or baseline) correction is used to eliminate low frequency artefacts and differences generated by the measuring instrument (54). Data log-transformation is common practice, as this renders fold changes symmetric around zero, reduces potential skew in the data and provides a good approximation for the normal distribution, which is a prerequisite for most computational methods (52, 55), especially for linear models. Following background correction, outlier detection is performed by the three standard deviations technique and subsequently removed or replaced.
In large-scale studies based on MS proteomics or metabolomics, samples will be distributed into several analytical batches, which may introduce instrumental variabilities into the data set. Such batch effects can be very destructive as they render comparison between phenotypic groups ineffective. Normalisation strategies for metabolomic and proteomic experiments make use of control samples and control molecules (52, 56, 57), which are usually assumed to exhibit constant signal levels. Any differences in signal values are considered to be technogenic and thus, corrected for. The most popular normalisation strategies are global scaling, quantile normalisation, cyclic loess and the ones involving linear models (56).
After appropriate pre-processing, the data is used for statistical analysis, where a large number of techniques are available. Characteristics of data and the research question determine the choice of the statistical method. In biomarker discovery studies, molecules that can reliably distinguish between two (or more) groups, like disease versus controls, are referred to as significantly differential and can be used as biomarker candidates. The process of identifying such molecules is termed differential analysis (52, 58). Classical univariate statistics such as Student’s t-test (requiring normal data distribution) or Mann-Whitney U test (non-parametric test that does not rely on parameterized data distribution) can be used for differential analyses. Differential analyses have a low probability (usually less than 5%) to deliver significant results by mistake; however, if repeated multiple times, as for omics studies, can result in the generation of false significant hits. Therefore, the number of tests performed needs to be taken into account. The simplest and one of the most popular methods for multiple testing correction is ‘Bonferroni correction’ (59), which adjusts the p-value threshold by dividing it by the number of tests. This is a conservative approach that may result in a high number of potential biomarkers being ignored, hence, less stringent methods can be considered (like Benjamini and Hochberg False Discovery Rate correction) that keeps the number of falsely significant results at a predefined level (e.g., 5%).
While classical statistical methods analyse the significance of each molecule of interest independently (60), machine learning algorithms are able to efficiently assess the predictive performance of multiple proteins, metabolites, features and even their combinations. Machine learning is a field of computer science that studies algorithms capable of learning valuable relationships from data without being explicitly programmed. Myriads of machine learning algorithms have been developed over the past years (61) and are frequently used in biology to discover biomarkers for various diseases (49, 62). The most popular machine learning methods are decision tree (63), support vector machine, random forest (64) and gradient boosting machines (65).
It can be challenging to build reliable machine learning models, because most model algorithms can learn random patterns that can only explain data these models were exposed to. This phenomenon is known as overfitting and might cause models to report completely irrelevant biomarkers and thus, render the entire study obsolete. In order to account for potential overfitting and keep its influence at minimum, various strategies have been proposed (66). One of the most important techniques is k-fold cross validation. By using only one part of the data to build a model (training set) and the remaining part to assess its performance (test set), researchers can be confident that the biomarkers identified by the model are not random fluctuations in the training data.
2. Methods
2.1. Study design
With this systematic review we aimed to respond to the following question: Can proteomics and metabolomics contribute to identification of biomarkers for diagnostics and prognostics in EC? The review was conducted according to the PRISMA guidelines (67) and is registered at the ‘International prospective register of systematic reviews’ (PROSPERO, Registration number CRD42022245880).
2.2. Search strategy, data extraction and quality assessment
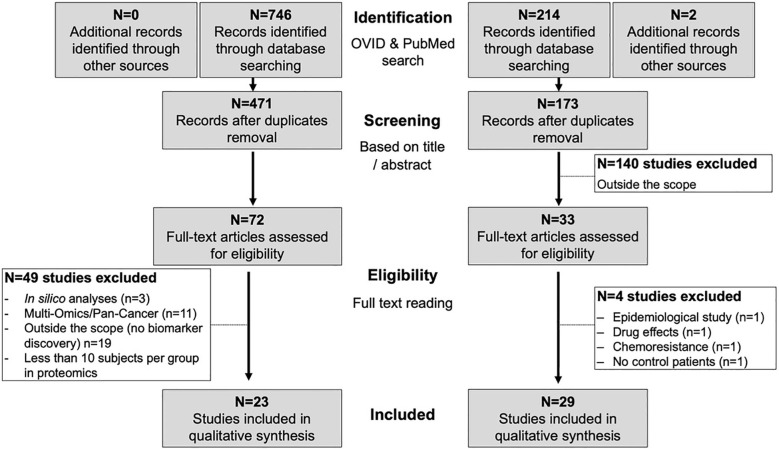
We performed a systematic search of the literature in the PubMed® and OVID® Embase databases on July 20, 2022 using the search terms listed in Supplementary Table S1 . We focused on proteomic and metabolomic studies performed in physiological fluids and tissue samples. There was no restriction on publication date. Reports were retrieved, and titles/abstracts were screened according to the inclusion and exclusion criteria ( Table 1 ) independently by authors AR and TLR. Disagreements were discussed and consensus was reached. The search strategy is provided in Table 2 .
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | research papers, papers in English, studies in humans, blood (plasma or serum), urine, other physiological fluids, tissue samples, at least 10 subjects per study group. |
| Exclusion criteria | abstracts, review papers, papers in other languages, studies in animals, studies in cell lines, studies including only unidentified metabolites, epidemiological studies, studies evaluating drug effects. |
Table 2.
Search strategy for identification of manuscripts in Pubmed and OVID Embase.
| Search Query | Search results | Selected manuscripts | Additional manuscripts | Included |
|---|---|---|---|---|
| Endometrial cancer and proteomics* |
746 total
570 PubMed 176 OVID 275 duplicates 471 total 224 removed/titles 247 total 175 removed/abstract Total 72 |
23
49 removed after full-text reading |
0 | 23 |
| Endometrial cancer and metabolomics* |
214 total
89 PubMed 125 OVID 43 duplicates 171 total 70 removed/titles 101 total 70 removed/abstract Total 31 |
31
4 removed after full-text reading |
2 | 29 |
*Search strategy is provided as Supplementary Table S1 .
The selected reports were read in detail and the following relevant data was extracted (when applicable): author and year of publication, country, fundings; sample: tissue (kind), plasma, serum or other body-fluid; study design; methods: omics approach, targeted/nontargeted proteomics/metabolomics; analytical methods; patient selection: case/control, stratification of patients according to reference test; patient characteristics: number of patients, characteristics of the enrolled patients with EC (e.g., mean age, body mass index [BMI], type of EC, histological differentiation, FIGO stage, menopausal status) and control patients or healthy women (e.g., mean age, BMI, diagnosis, menopausal status); study phase and statistical methods: discovery, validation phase, machine learning approaches used; differentially abundant proteins and metabolites in the study groups; diagnostic characteristics (e.g., sensitivity, specificity, area under the curve [AUC], positive predictive value [PPV], negative predictive value [NPV]) or prognostic characteristics (overall survival [OS], disease-free survival [DFS]), and hazard ratios [HR]; diagnostic or prognostic models; disclosures: affiliations with industry, industrial funds, patents.
Reporting was performed under the guidance of the PRISMA diagnostic test accuracy checklist (67). The risk of bias and quality of individual diagnostic accuracy studies were assessed following the QUADOMICS tool, an adaptation of QUADAS (68) that was designed specifically for omics studies (69) ( Supplementary Table S2 ). This tool focuses on study design, patient selection, index test, reference standards, flow of timing, pre-analytical and analytical procedures, and statistical analysis and nine questions per study were specifically answered ( Supplementary Tables S2–S4 ). Additional potential financial, commercial and conflict of interest biases were further examined ( Supplementary Tables S5 ).
3. Results
Systematic literature search led to the identification of 52 studies in EC, 23 on proteomics and 29 on metabolomics ( Figure 2 ).
Figure 2.
Workflow of the systematic search and paper selection.
3.1. Evaluation of the quality of published studies
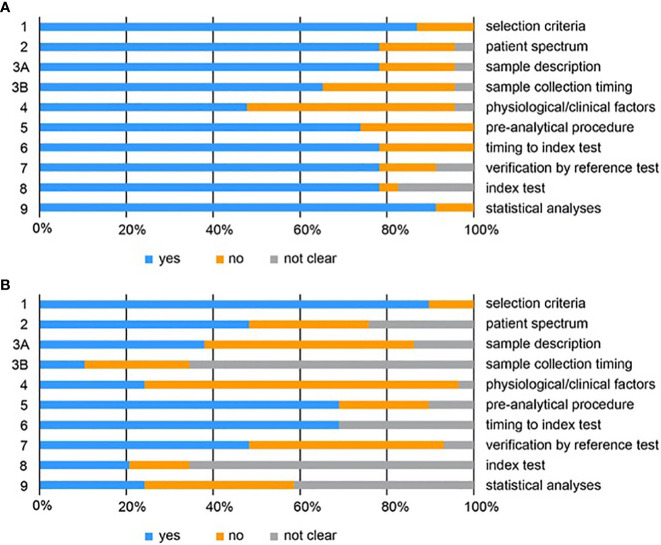
The quality of the studies included was assessed systematically according to the QUADOMICS tool ( Supplementary Tables S2–S4 ; Figure 3 ). We evaluated study design and pre-analytical, analytical, and post-analytical bias of all included studies. The majority of the studies described the criteria for patient selection (question 1) in appropriate detail. Approximately 50% of metabolomic and 20% of proteomic studies did not reflect the real clinical setting, because they compared patients with healthy women, who are not likely to need a diagnostic test (see discussion - question 2). The assessment of pre-analytical bias (questions 3A and 3B) revealed that only a fraction of all studies reported appropriate descriptions of the samples, including the procedures for sample collection and processing (e.g., centrifugation time, type of blood tube). Furthermore, the majority of the studies did not report any information about the time of sample collection, the time between blood draw and centrifugation, the time between sample acquisition and storage, and the number of freeze/thaw cycles. In 75% of the metabolomic and 51% of the proteomic studies sufficient information on the clinical and physiological factors that can affect -omics data was not provided (question 4; e.g., BMI, menopausal status, menstrual phase cycle, fasting status). Approximately 70% of the included studies reported detailed descriptions on sample storage and metabolite extraction (question 5). Almost all samples were stored at -80°C or in liquid nitrogen, but several studies failed to report this information. The time between the reference standard and the index test (metabolomics or proteomics) was not clear for 31% of the included metabolomic and 21% of the proteomic studies (question 6), while in 52% of the metabolomic and 22% of the proteomic studies, the verification by reference test was not performed in all patients (question 7). With respect to analytical biases, we observed that only 21% of all studies provided a detailed description of the metabolomic analysis (question 8). Most studies did not provide information on sample randomisation for MS-based metabolomics, for the use and type of quality control samples, and occasionally, important MS parameters were not given. In proteomics, 78% of all included studies described the index test in sufficient detail. Regarding post-analytical biases, we observed that 24% of the metabolomic studies described the statistical analysis in sufficient detail, while 42% of studies provided incomplete description. These studies failed to report information on missing value treatment, sample-to-sample normalization, data transformation and scaling and in one case also on model calculation and cross-validation. In proteomics, 91% of all included studies reported the statistical analysis in sufficient detail.
Figure 3.
QUADOMICS scoring of all studies included for proteomics (A) and metabolomics (B). Proportion of studies with answers “yes”, “no”, or “not clear” to each of the signalling questions. Each signalling question is numbered on the left and a short description of each question is given on the right. The detailed scoring is given in Supplementary Tables S3 and S4 .
3.2. Disclosure of financial and other potential conflicts of interest
We also evaluated whether studies clearly stated the financial support, disclosed any potential conflict of interest, whether authors were affiliated to industry and whether the studies complied with the open science policy and deposited their data on public repositories ( Supplementary Table S5 ). Although older studies tend not to report any information on the financial support or the presence of any conflict of interest, more recent studies provide this information. The source of funding was declared in 88% of the studies (19 out of 23 proteomic and 27 out of 29 metabolomic studies) and the presence of any potential conflict was declared in 77% of the studies (14 out of 23 proteomic and 26 out of 29 metabolomic studies). Three studies (6% of those declaring the source of funding) received industrial supports and four studies included authors affiliated to companies. Only 25% of the studies deposited the data in public repository (1 out of 23 proteomic and 12 out of 29 metabolomic studies) and four proteomic studies declare that data are available upon request.
3.3. Proteomics in endometrial cancer
From the systematic literature search, 72 research papers were selected based on title and abstract. From these, 19 papers were excluded because focusing on basic cell mechanisms of carcinogenesis with no further investigation on the diagnostic or prognostic potential (70–80), response to metformin (81), side effect to radiotherapy (82), racial disparities (83, 84), drug resistance (85–87), premenopausal endometrial physiology (88). Three studies were in silico analyses (89–91). Additionally, 11 papers (22, 92–101) were based on multi-omics approaches or focused on pan-cancer biomarkers and will be discussed in paragraph 3.4, thus resulting in 39 papers for review.
Papers that included less than 10 subjects per study group are not further discussed here (n=16; see Supplementary Table S6 for details). This resulted in a total of 23 papers focusing on proteomic biomarkers for diagnosis, prognosis, risk stratification or classification ( Table 3 and Supplementary Table S7 ). Ten studies used blood (serum or plasma), two studies used uterine aspirate whereas eight studies used fresh frozen tissues and three used formalin-fixed-paraffin-embedded (FFPE) tissues. Various technologies were used, with the most common being 2D-DIGE/MS based methods. Although studies on tissue proteomics preceded chronologically those in body fluid, since this review focus of diagnostic/prognostic biomarkers where body fluids represent the most suitable biomaterial, we will start in the next paragraphs describing studies using body fluids for biomarker discovery.
Table 3.
List of the 23 proteomic studies in endometrial cancer.
| Study | Study aim | Samples * | Study design |
|---|---|---|---|
| Zhu, 2006 (102) | Diagnostic Biomarkers | Serum | Case - Control |
| Kikuchi, 2007 (103) | Diagnostic Biomarkers | Serum | Case - Control |
| Zhu, 2008 (104) | Diagnostic Biomarkers | Serum | Case - Control |
| Qiu, 2010 (105) | Diagnostic Biomarkers | Serum | Case - Control |
| Wang, 2011 (106) | Diagnostic Biomarkers | Serum | Cases only |
| Enroth, 2018 (107) | Diagnostic Biomarkers | Plasma | Case - Control |
| Tarney, 2019 (108) | Diagnostic Biomarkers | Serum | Nested case-control |
| Ura, 2021 (109) | Diagnostic Biomarkers | Serum | Case - Control |
| Celsi, 2022 (110) | Diagnostic Biomarkers | Serum | Case - Control |
| Ura, 2022 (111) | Diagnostic Biomarkers | Serum | Case - Control |
| Martinez-Garcia, 2016 (112) | Diagnostic Biomarkers | uterine aspirate | Case - Control |
| Martinez-Garcia, 2017 (113) | Diagnostic Biomarkers Prognostic Biomarkers | uterine aspirate | Case - Control |
| Yoshizaki, 2005 (114) | Diagnostic Biomarkers | Frozen tissue | Case - Control |
| DeSouza, 2007 (115) | Diagnostic Biomarkers | Frozen tissue | Case - Control |
| Voisin, 2011 (116) | Diagnostic, Prognostic, Therapeutic Biomarkers | Frozen tissue | Case - Control |
| Shan, 2016 (117) | Diagnostic Biomarkers | Frozen tissue | Cases only |
| Ceylan, 2020 (118) | Diagnostic Biomarkers | Frozen tissue | Case - Control |
| Mauland, 2017 (119) | Prognostic Biomarker associated with obesity | Frozen tissue | Cases only |
| Akkour, 2022 (120) | Diagnostic Biomarkers | Frozen tissue | Case - Control |
| Kurimchak, 2020 (121) | Prognostic Biomarkers | Frozen tissue | Cases only |
| DeSouza, 2010 (122) | Diagnostic Biomarkers | FFPE tissue | Case - Control |
| Aboulouard, 2021 (123) | Prognostic Biomarkers | FFPE tissue | Case - Control |
| Janacova, 2020 (124) | Prognostic Biomarkers in the tamoxifen users | FFPE tissue | Cases only |
See Supplementary Table S7 for further details.
* FFPE: Formalin fixed paraffin embedded.
3.3.1. Blood proteomics
Under the rationale that protein fragments/peptides are produced in the tissue microenvironment by proteolytic processes and released into the blood, the first proteomic studies based on blood (serum or plasma) were published during the first decade of 2000. Zhu and co-workers (102) performed a biomarker discovery study using SELDI-TOF-MS on 40 patients and 30 age-matched healthy controls and identified 13 m/z protein-peaks that were found in different levels between patients and healthy women. The sensitivity of each single peak ranged from 40-95%. The authors further built a decision tree-based algorithm that correctly identified 95.7% (70) of the samples (30/33 healthy women and 37/40 ECs; Supplementary Table S7 ). The same authors further improved the model using only four m/z protein peaks resulting in sensitivity and specificity of 100% and 92.3%, respectively, in the training set and 60% and 75%, respectively, in an independent validation cohort (104). The authors did not identify the proteins corresponding to the m/z spectra peaks ( Supplementary Table S7 ).
A relatively large study including 199 serum samples from untreated EC patients (n=92), patients with prolapsed uterus (n=16), healthy women (n=17) (n=33), and uterine fibroids (n=74) identified 507 peaks with m/z values ranging from 2,000 to 30,000 by MALDI QTOF-MS (103). Based on predefined stringent criteria (P < 0.00001, AUC value > 0.80) three peaks were differentially abundant between the case and the control groups and showed sensitivity and specificity of 65.2% and 93.9%, respectively ( Supplementary Table S7 ). Surgical stage of patients could not be discriminated by the selected m/z peaks but patients with EC and patients with uterine fibroids could be distinguished ( Supplementary Table S7 ).
Qiu and co-workers (105) performed proteomics on 30 EC patients and 30 control patients on serum collected pre-operatively and identified 147 differential peaks. They further used different algorithms based on various peaks (from two to 10) and reported specificities and sensitivities up to 97% and 100%, respectively ( Supplementary Table S7 ).
In another study, Wang and colleagues (106) performed a pilot study to compare the serum proteomics in patients with distinct stages of endometrial disease, from simple endometrial hyperplasia (n=6), complex hyperplasia (n=4), hyperplasia with atypia (n=4) and with early-stage EC (n=6). The authors identified 74 proteins including potential biomarkers ( Supplementary Table S7 ), but the number of samples included was very limited.
A large nested case-control study aiming at identifying early detection biomarkers for EC was based on the UK Prostate, Lung, Colorectal, and Ovarian cancer screening trial (n =78,216 subjects), including 112 incident EC cases and 112 matched postmenopausal controls (108). Among cases who received an EC diagnosis less than two years after inclusion (n=31), 1,100 total proteins were identified, 565 of which were co-quantified across all patient samples and 47 proteins resulted altered compared with controls. Six candidate protein biomarkers were used to build a diagnostic algorithm with over 45% sensitivity and 96% specificity ( Supplementary Table S7 ). A recent study employed PEA proteomics (PCR-based) and Olink Multiplex assays to search for candidate diagnostic biomarkers in gynaecologic malignancies, including EC (107). The authors compared malignant cases with both a group of healthy controls and with a group of women with benign tumours. The abundance of 441 unique proteins in plasma was first evaluated in a discovery phase that resulted in 16 potential protein biomarkers. The diagnostic value of nine out of these 16 proteins was validated in a replication cohort and resulted in sensitivities and specificities above 64% and 67%, respectively, to distinguish EC from healthy women or from patients with benign tumours ( Supplementary Table S7 ).
Three proteomics studies using serum (109–111) were performed by an Italian group. In 2021, the authors performed a pilot study using the serum of 15 EC patients and 15 [non-cancer patients (109)] and identified 16 proteins with diagnostic potential ( Supplementary Table S7 ), four of which (ITIH4, CLU, SERPIN1, and C1R) were validated by western blotting. One year later, the study was extended to a larger cohort including 60 non-EC controls and 44 EC patients (110). Proteomic analyses was performed on 10 controls and 10 EC. It is not stated in the study whether the study population and the samples used for proteomics overlaps with the previous investigation from the same team (109). The authors further validated the observed downregulation of SBSN in serum of patients by western blotting and in silico analysis of the TCGA database. In a subsequent study, the authors included 44 EC cases and 44 non-oncologic patients (111) - the study does not specify whether this study population overlaps with the previously studied groups (109, 110). By using PEA on two distinct protein panels (Immuno-oncology panel and Target 96 Oncology III panel), the authors identified several differentially expressed proteins and proposed different models resulting in AUCs up to 0.96 ( Supplementary Table S7 ).
3.3.2. Other body fluids
For diagnostics, another potentially interesting minimally invasively obtained body fluid is the uterine aspirate, which has the advantage to capture the tumour heterogeneity better than a presurgical biopsy (current standard diagnostic method). Uterine aspirate for proteomic biomarker discovery was used by Martinez-Garcia and collaborators (112, 113). Since the LC-PRM targeted proteomics technique allows the quantification of a limited number of predefined proteins, the authors adopted a sequential workflow: 506 candidate biomarkers were first extracted from a literature search. Subsequently, the authors determined the presence of these biomarkers in uterine aspirates by LC-MS/MS and confirmed the presence of 158 proteins. After method optimisation, a list of 52 candidate biomarkers was selected for PRM design/development and 26 proteins were differentially expressed between cases and controls (112). The same set of 52 proteins was subsequently tested on an independent prospective cohort of 116 women entering the EC diagnostic workup due to EC suspicion (113). A diagnosis of EC was confirmed in 69 women and 28 proteins elevated in EC versus controls had an AUC >0.75. Various tests and combinations of the five best individual biomarkers were assessed, resulting in diagnostic and prognostic models with sensitivities and specificities above 89% and 83%, respectively ( Table 3 ).
3.3.3. Tissue proteomics
3.3.3.1. Frozen tissue
Pioneering studies were conducted as early as 2005 using iTRAQ. After determining the feasibility and comparing the performance of iTRAQ and cICAT for proteomics (authors used less than 10 samples per group; Supplementary Table S6 , (125), the authors used iTRAQ to analyse 40 frozen tissue samples including proliferative, secretory endometrium and EC (115). Over 1,000 proteins were identified among which six candidate markers (PK, PIGR, CPN10, MIF, AAT, CKB, and TAGLN) were confirmed as differentially expressed from their previous pilot investigation (125). Fourteen proteins were selected for further analyses and after assessing the associations of each individual protein with malignant or benign status using the two-sample t-test (p<0.005), four proteins (PK, CPN10, AAT and CKB) were selected to build a prediction model. Although these proteins used as single markers reached maximum AUC of 0.95 (sensitivity: 85%; specificity: 90%; PV: 87%; PPV: 89%), the use of three markers (AAT, PK and CPN10) resulted in improved performance and an AUC of 0.96 ( Table 3 ). The validity of these biomarkers was further confirmed by two-thirds/one-third cross-validation, and also by using dot-blot and IHC on a panel of independent samples (115). In a subsequent study, the authors verified five of the identified markers (CPN10, S100A8, PIGR, PK-M2 and AAT) and one additional marker (TIMP-1) by IHC on a tissue microarray (TMA), including 148 samples (two simple hyperplasia; eight complex hyperplasia; 39 endometrioid EC; 13 serous papillary/clear cell or Type II EC; one carcinosarcoma: 85 benign endometrium samples of which 25 proliferative, 25 secretory, 25 atrophic, and 10 menstrual). They further showed that CPN10 and PK-M2 could distinguish hyperplasia and EC cases versus controls (sensitivity and specificity of 77% and 87%, respectively), whereas the combination of AAT, CPN10, and PK-M2 resulted in sensitivity and specificity of 85% and 93%, respectively, in distinguishing EC versus control patients (126). The same authors subsequently performed a pilot study on 10 EC patients and 10 control patients using a novel strategy (drill-down coupled to iTRAQ) to improve their ability to detect novel proteins and identified 1,529 proteins, among which 40 candidate biomarkers. The PPV and AUC of these proteins used as single diagnostic biomarkers ranged between 62%-100% and 0.60-1.00, respectively ( Supplementary Table S7 ) (116).
In parallel to these studies based on iTRAQ, the first studies analysing fresh/frozen EC tissues using 2D gel separation followed by MS were published in 2005. By applying SELDI-TOF-MS to 19 cases of EC and 20 control patients, the authors identified one peak (m/z 9,600) consistently upregulated and a second peak (m/z 11,300) consistently downregulated in case group versus control group (114).
Additional biomarker discovery studies were published in subsequent years, with most of them being pilot or feasibility in nature and including less than 40 samples. Shan and co-workers (117) compared EC versus adjacent normal tissue in 10 cases with iTRAQ-based proteomics and identified 1,266 proteins, 103 of which were upregulated and 30 downregulated in cancer versus control tissue ( Supplementary Table S7 ). Results were confirmed by western blotting, qRT-PCR and functional studies using cell lines. Ceylan and co-workers (118) also performed a diagnostic biomarker discovery study based on 2D-DIGE-MALDI-TOF and compared controls (pre- and post-menopausal women), hyperplasia and EC. Several proteins were differentially expressed between controls and EC, controls and hyperplasia ( Supplementary Table S7 ), or were associated with advanced-stage disease (CAH1, PPIB, K2C8, and UAP56).
Mauland and colleagues (119) explored the levels of 163 proteins using RPPA in relation to prognosis and obesity. The authors used patient cohorts from different geographical regions: a group of samples collected in Norway in two different periods served as training (n=272 collected between 2001-2013) and validation (n=68 collected between 2011-2015) cohorts and a third cohort collected in Texas (USA) was used as extra validation (n=178 collected between 2000-2009). Beside correlation with BMI, several proteins were associated with patient prognosis, including proteins indicative of a low PI3K activation in non-obese early-stage ER-positive tumours. Data was further validated by RNA (correlation) and IHC ( Table 3 ). Akkour and colleagues (120) used 2D-DIGE to analyse tissues from patients with hyperplasia (n=12), EC (n=12) and age-matched control patients (n=12) and identified 87 differentially expressed proteins (26 between controls and hyperplasia and 32 between EC and hyperplasia; Table 3 ). Further modelling was not performed. In a recent study, Kurimchak et al. (121) used an innovative approach based on Multiplexed Inhibitor Beads (MIB) and MS to chart the kinase network in EC (n=20) and adjacent normal tissues (n=16). The MIB binding value was measured for 347 kinases, 300 of which were quantitated by both LFQ and s-SILAC, whereas 37 and 10 by LFQ and s-SILAC, respectively. These analyses showed that SRPK1 was overexpressed in cancer tissue ( Table 3 ). IHC on TMAs (39 serous and 18 endometrioid and 12 normal endometrial tissues), functional/loss of function studies in vitro and the TGCA and CPTAC datasets confirmed that SRPK1 is associated with EC and with poor patient survival.
3.3.3.2. Formalin-Fixed-Paraffin-Embedded (FFPE) tissue
A number of studies investigated the potential use of FFPE material for proteomics ( Supplementary Table 6 ), but only three of them met our selection criteria ( Table 3 ; Supplementary Table S ). DeSouza and colleagues confirmed the feasibility of mTRAQ targeted proteomics using FFPE tissues (122). The authors laser-capture-microdissected tissue and examined the tissue of interest from 10 ECs and 15 proliferative endometrium samples and detected 13 out of the 17 targeted proteins across 12 samples ( Table 3 ; Supplementary Table 7 ).
Janacova and colleagues (124) explored archival material from 36 EC patients, 15 of whom had received tamoxifen adjuvant treatment for breast cancer, whereas 21 were never exposed to tamoxifen previously. The authors explored with LC−MS/MS in SWATH-MS mode 34 tumour samples (each from one subject) and 11 myometrial tissues adjacent to the tumours. The proteomic approach targeted over 1,100 different proteins, of which over 900 were consistently identified. The authors compared clinical features in the tamoxifen versus tamoxifen naïve patients and identified six upregulated and 22 downregulated proteins. The expression of CAPS and STMN1 was confirmed with IHC and STMN1 was also associated with poor patient prognosis ( Table 3 ). Using a very innovative approach (123), Aboulouard and coworkers compared the proteome profile in EC and sentinel-lymph-node SLN tissues ( Table 3 ; Supplementary Table 7 ). Regions of interest were first microdissected, then analysed with NanoLC-ESI-MS and a number of potential biomarkers indicative of lymph node disease were identified.
3.4. Metabolomics in endometrial cancer
Our literature search identified 29 studies using metabolomics in EC ( Table 4 and Supplementary Table S8 ), with the majority evaluating the metabolic profiles in blood samples (10 serum, seven plasma, one serum and plasma, one dried blood). Seven studies focused on endometrial tissue samples, one on cervical lavage, one on endometrial brushing and one study on urine samples. Most studies aimed to identify diagnostic and/or prognostic biomarkers, better understanding the mechanisms of carcinogenesis (studies in tissue samples), and also to determine associations between metabolic profiles and EC (131, 132). Plasma, serum and urine represent appropriate sources for discovery of diagnostic/prognostic biomarkers (155). However, also metabolic profiles of cervical lavage, brushing endometrial samples, and tissue samples (if obtained as pre-surgical biopsy) may be of clinical relevance. Non-targeted metabolomics was more commonly applied (20 studies) as compared to targeted metabolomics (10 studies). Only six studies used NMR analysis, and there was one study that combined NMR with the most commonly used LC-MS/MS (138). The majority of the targeted metabolomic studies focused on lipids and amino acids.
Table 4.
List of the 29 metabolomic studies in endometrial cancer.
| Study | Study aim | Samples | Study design |
|---|---|---|---|
| Ihata, 2014 (127) | Diagnostic Biomarkers | Plasma | Case-control |
| Knific, 2018 (128) | Diagnostic Biomarkers Prognostic Biomarkers |
Plasma | Case-control |
| Strand, 2019 (129) | Prognostic Biomarkers | Plasma | Cases only |
| Njoku, 2021 (130) | Diagnostic Biomarkers Prognostic Biomarkers |
Plasma | Case-control |
| Kliemann, 2021 (131) | Association | Plasma & serum | Nested case-control |
| Dossus, 2021 (132) | Association | Plasma | Nested case-control |
| Breeur, 2022 (133) | Association | Plasma & serum | Case-control study |
| Audet-Delage, 2018 (134) | Diagnostic Biomarkers Prognostic Biomarkers |
Serum | Case-control |
| Audet-Delage, 2018 (135) | Diagnostic Biomarkers Prognostic Biomarkers |
Serum | Case-control |
| Troisi, 2018 (136) | Diagnostic Biomarkers Prognostic Biomarkers |
Serum | Case-control |
| Shi, 2018 (137) | Exploratory | Serum | Case-control |
| Bahado-Singh, 2017 (138) | Diagnostic Biomarkers | Serum | Case –control |
| Lunde, 2020 (139) | Exploratory | Serum | Cases only |
| Kozar, 2021 (140) | Exploratory | Serum | Prospective observational study |
| Gu, 2021 (141) | Diagnostic Biomarkers Prognostic Biomarkers |
Serum | Case-control |
| Yan, 2022 (142) | Diagnostic Biomarkers Prognostic Biomarkers |
Serum | Case-control |
| Schuhn, 2022 (143) | Diagnostic Biomarkers | Serum | Case-control |
| Troisi, 2020 (144) | Diagnostic Biomarkers | Dried blood samples | Multicenter prospective cohort study |
| Shao, 2016 (145) | Diagnostic Biomarkers | Urine | Case-control |
| Cheng, 2019 (146) | Diagnostic Biomarkers | Cervicovaginal fluid | Case-control |
| Jove, 2016 (147) | Diagnostic Biomarkers | Tissue | Case-control |
| Altadill, 2017 (148) | Diagnostic Biomarkers | Tissue | Case-control |
| Trousil, 2014 (149) | Diagnostic Biomarkers | Tissue | Case-control |
| Cummings, 2019 (150) | Diagnostic Biomarkers | Tissue | Case-control |
| Skorupa, 2021 (151) | Diagnostic Biomarkers | Tissue | Case-control |
| Arda Düz, 2022 (152) | Diagnostic Biomarkers | Tissue | Case-control |
| Gatius, 2022 (153) | Diagnostic Biomarkers | Tissue from Biobank | Cases only |
| Shafiee, 2020 (154) | Diagnostic Biomarkers | Plasma & tissue | Cros-sectional study |
| Yi, 2022 (101) | Diagnostic Biomarkers | Tissue & Urine | Case-control |
See Supplementary Table S8 for further details.
3.4.1. Blood metabolomics
Metabolomic studies on serum samples from EC patients have been performed from 2017 and identified a series of metabolites in differential concentrations between study groups ( Supplementary Table S8 ). Audet-Delage and co-workers (134) reported that the levels of 115 acylcholines, monoacylglycerols, and acylcarnitines were increased while the levels of 22 free fatty acids were decreased in 26 postmenopausal EC patients (type I and II, recurrent and non-recurrent) versus 18 patients with benign conditions. The authors identified a series of metabolites specific for recurrent EC, where bile acids were increased in type I and sphingolipids in type II recurrent EC. The authors constructed a diagnostic model (including the levels of spermine, isovalerate, glycylvaline and gamma-glutamyl-2-aminobutyrate) with an AUC of 0.92 and a prognostic model (including 2-oleoylglycerol and TAG 42.2-GA12:0) that separated between recurrent and non-recurrent EC with an AUC of 0.90. Troisi and colleagues (136) used a GC-MS approach and determined the metabolic profiles in 118 EC patients and 130 healthy women and control patients. Using several machine learning approaches and distinct patient cohorts, they constructed and validated a diagnostic model (EC versus healthy women) with accuracy of 0.99 and a prognostic model (type I/type II) with accuracy of 0.93. The first was based on increased levels of lactic acid, homocysteine, 3-hydroxybutyrate, and decreased levels of linoleic acid, stearic acid, myristic acid, threonine, valine and progesterone, whereas the latter on increased levels of progesterone and decreased levels of lactic acid, cystine, serine, malate, glutamate and homo-cysteine. Bahado-Singh and co-workers (138) performed NMR analysis in 56 stage I-IV EC patients and 60 healthy women, divided in discovery (33 ECs and 36 healthy women) and validation phase (23 EC and 24 healthy women) and constructed several diagnostic logistic regression models based on lipid levels with an AUC above 0.8. The highest AUC (0.83) was reported for the combination of C14:2, PCae C38:1 and 3-hydroxybutyric acid. This model separated also between stage I-II EC and healthy women (AUC = 0.82). An exploratory MS analysis by Kozar et al. in 15 EC and 21 control patients (140) reported a Random Forest model including Cer 34:1;2, Cer 40:1;2, AC 16:1-OH and 1-methyladenosine with AUC of 0.92, but reported no validation. Yan et al. performed a MS-based study (142) which included 23 EC patients, 30 healthy women, 30 patients with endometrial polyps and 12 patients with endometrial hyperplasia in the discovery phase and 50 EC patients (stage I-IV) and 195 healthy women, 171 polyps and 40 hyperplasia patients in validation phase. Their logistic regression models for separation between EC and endometrial polyps included 6-keto PGF1α, PA(37:4), LysoPC (20:1) and PS (36:0) and showed good characteristics with AUC > 0.90. A recent MS/MS targeted metabolomic study by Schuhn et al. performed in 20 EC patients, 157 healthy women and 14 control patients (143) reported that individual metabolites (carnitines and amino acids) allow stratification between EC and healthy women, and EC and control patients, with AUCs of 0.82 and 0.85 for malonylcarnitine and threonine, respectively ( Supplementary Table S8 ). The study by Lunde et al. (139) performed NMR analysis in serum samples from 78 EC patients stored in Danish Cancer Biobank to determine metabolic profiles that allow identification of patients with chronic pelvic pain after hysterectomy. Using different machine learning approaches on metabolites with different levels in the two groups ( Supplementary Table S8 ), several models were built, with the best diagnostic characteristics (AUC of 0.87) seen for linear support vector model.
Due to potential variability in the composition of serum, plasma represents the preferred source for biomarker discovery. However, so far a minority of the studies on blood metabolomics were performed using plasma. The first study by Ihata et al. (127) used MS to analyse plasma from 80 EC patients (stages I-IV), 122 patients with benign gynaecological diseases and 240 healthy women using training (40 EC and 120 healthy women) and validation sets (40 EC and 120 healthy women and 122 control patients). The authors built logistic regression diagnostic models based on panels of amino acids (histidine, isoleucine, valine and proline) that separated EC from healthy women (AUC > 0.91) and EC from control patients (AUC = 0.83; Supplementary Table S8 ). In a study by Knific et al. (128), 61 EC patients and 65 patients with benign uterine conditions were included. By employing LC-MS/MS analysis in training and test sets, the authors constructed diagnostic logistic regression models to separate EC from controls (AUC = 0.84) and prognostic models that allowed stratification of patients with lymphovascular invasion (LVI; AUC = 0.94) and myometrial invasion (AUC = 0.86). These were the first diagnostic and prognostic models of EC that included metabolite ratios. Strand et al. (129) used the same methodological approach but focused on prognostic biomarkers to identify metabolic differences between 20 EC patients with long versus 20 EC patients with short survival, where patients were matched for stage, grade, age, and BMI. Using Partial Least-Squares Discriminant Analysis (PLS-DA), three models with AUC up to 0.96 were constructed but none has been validated yet ( Supplementary Table S8 ). Another MS-based study in plasma samples (130) focused on diagnosis of EC in obese patients (BMI > 30) and included 67 EC patients and 69 control patients (test and training sets). RF algorithms including 20 metabolites separated all EC patients from controls (AUC = 0.95) and showed even better characteristics for separation of stage I EC from control patients (AUC = 0.98). Individual metabolites showed potential as prognostic biomarkers and separated EC patients with/without LVI (AUC = 0.83; Supplementary Table S8 ). Other studies on serum/plasma metabolome in EC patients reported only different levels of metabolites in EC patients (133, 135, 137, 141) and associations of individual metabolites with EC (131, 132).
A well-designed GC-MS discovery study analysed dried blood samples analysed 50 postmenopausal EC patients and 70 patients without EC and validated prospectively the results among 1,430 postmenopausal women including 16 incident EC patients ( Supplementary Table S8 ). Their ensemble machine-learning algorithm included 10 different classification models with accuracy of 99.9% (144). Among studies reporting serum and/or plasma metabolic profiles in EC patients, only few diagnostic/prognostic models have been validated in large multicenter studies, and the majority still awaits appropriate validation.
3.4.2. Metabolomics in other physiological fluids
Only two studies searched for biomarkers of endometrial cancer in urine samples ( Supplementary Table S8 ). Shao et al. (145) used nontargeted metabolomics to determine differences in urine metabolic profiles from 25 EC patients, 25 healthy women and 10 endometrial hyperplasia patients and constructed PLS-DA and Support Vector Machine models, but provided no diagnostic characteristics. Yi et al. (101) analysed urine, tissue samples, and brushing endometrial samples and identified 285 metabolites in differential levels in urine samples from 10 EC patients compared with 10 control patients. PLS-DA based on the top 100 metabolites showed an AUC of 0.81. The cervicovaginal fluid from 21 EC patients and 33 non-EC controls was analysed by NMR using training and test sets (146). The levels of 29 metabolites differed between groups and RF and SVM models with accuracy up to 0.78 were constructed ( Supplementary Table S8 ). These studies in urine samples and cervicovaginal fluid included a small number of samples thus future attempts for biomarker discovery should include respective metabolomics profiles from larger group of EC and control patients.
3.4.3. Tissue metabolomics
Seven studies explored the metabolic profiles in EC tissue. The first study was published in 2014 (149) and included 10 ECs and 10 control patients. NMR analysis revealed deviated concentration of of several amino acids, phosphocholine, glutathione, scyllo-inositol, myo-inositol, and inosine/adenosine in EC tissue ( Supplementary Table S8 ) and the authors built a PLS-DA model with an AUC of 0.99. Arda Düz et al. (152) employed NMR to analyse tissues from 17 ECs and 18 control patients, and reported a number of candidate metabolite biomarkers e.g., lactate, alanina, phenylalanine and ratios glutamate/glutamine/methionine and leucine/isoleucine with AUCs up to 0.88 ( Supplementary Table S8 ). A recent non-targeted NMR analysis on 64 EC tissue (patients with different grades of disease) and 10 tissues from patients with benign uterine diseases, identified using OPLS-DA the levels of a number of metabolites differentiating the patient groups (151). The concentrations of dimethylsulfone and phosphocholine were higher whereas the concentrations of glycerophosphocholine and glutamine were lower in low grade EC. In grade 1/2 EC, the levels of myoinositol were decreased and in grade 3 there were higher levels of 3-hydroxybutyrate, alanine, and betaine. The models constructed based on individual metabolites allowed separation between different grades of tumours with AUCs above 0.90 ( Supplementary Table S8 ).
Other studies that investigated the metabolic profiles in cancerous tissues contributed mainly to a better understanding of the pathophysiology of EC, as these studies reported differential metabolites and dysregulated pathways in EC. Jove et al. (147) analysed 27 EC tissue samples and 15 normal endometrium samples by MS/MS and identified 44 differential metabolites including increased levels of stearamide, monoolein, hypoxanthine, 1,2-dihexadecanoyl-sn-glycerol ( Supplementary Table S8 ). Comparison of cancer tissue of different grades identified 26 metabolites with increased levels of taurine and erythriol and decreased levels of oleamide. Importantly, this nontargeted metabolomic study used a novel approach as the authors examined the differences between surface EC and the myometrial invasion front and reported 104 differential metabolites (147). Altadil et al. (148) used non-targeted MS/MS to analyse 39 EC tissues and 17 control samples from postmenopausal women with stage I-III EC and benign diseases, respectively. Eighty metabolites, with 42 exhibiting differential levels, were identified with increased levels of taurine and erythriol and decreased levels of oleamide ( Supplementary Table S8 ). Specific metabolites had different levels between cancers and controls (glutamate-phenylalanine-arginine-tryptophan, palmic amide, stearamide, oleamide, 2-phosphatidylserine, phosphatidylglycerol, inosine, and picolinic acid) or between stage I/II and stage III disease (phosphatidylcholines, phosphatidylethanolamines, and arachidonic acid; ( Supplementary Table S8 ). Cummings et al. (150) performed a targeted metabolomic study on 108 cancer tissues, 53 samples of normal endometrium, 33 atrophic endometrium and 31 samples of atypical hyperplasia. The authors showed decreased concentrations of a number of metabolites including 13,14-dihydro-15-keto PGE2 in type 1 and 2 EC versus normal endometrium and 12-HETE in EC type 2 versus type 1; Supplementary Table S8 ). Shafiee et al. (154) focused on the pathophysiology of EC and compared 34 cancerous tissues with 34 control endometrial tissues from patients with polycystic ovarian syndrome (PCOS). Their nontargeted MS-based analysis revealed changes mainly among lipids. Yi et al. (101) performed nontargeted metabolomics in urine, intrauterine brushing, and tissues from 24 EC patients and 18 control patients where PLS-DA identified 74 metabolites of which 47 were found in higher levels and 27 in lower levels. Comparison of metabolic profiles in tissue samples to urine and brushing samples showed that 49 of 74 metabolites were also detected in urine samples and 21 of 74 metabolites in intrauterine brushing samples, which supports the potential of urine metabolomics profiles for non-invasive diagnostic/prognosis ( Supplementary Table S8 ). A recent study explored the metabolic profiles in biobanked tissue samples from endometrioid (n=20) and serous EC (n=11) (153). Using non-targeted MS analysis, 232 metabolic differences could be characterised ( Supplementary Table S8 ).
Three metabolomic studies using tissue samples (149, 151, 152) identified individual metabolites and constructed diagnostic models with promising AUC values; however, these studies included very limited number of patients.
3.5. Combined metabolomics/proteomics
There was only one study that employed a combined omics approach (101) and performed nontargeted metabolomics on 24 cancer and 20 control tissue samples ( Supplementary Table S7 ) and also nontargeted proteomics on a subset of 12 cancer and 9 control tissue samples by LC-MS/MS. The authors identified 1,445 proteins significantly up- or down-regulated in the EC group compared with the control group (adj. p<0.05, FC<1.5). To further characterise any relation between the metabolic and proteomic profiles, the authors performed network analysis that showed 28 metabolites and 135 proteins with 212 connections. Glutamine, dopamine, noradrenaline, adenosine-5-monophospate, and guanosine-5’-monophosphate were the major centres of sub-networks showing differences in amino acid and nucleotide metabolism.
3.6. Additional multi-omics or pan-cancer studies
A number of studies explored the proteome in patients with EC, but such analyses were part of a larger multi-omics approach, or part of systems-biology/pan-cancer approaches to study human disease or to establish databases and repositories. Most of these studies did not use proteomics for biomarker discovery, but as tools to understand the pathophysiological processes.
Five studies aiming at improved patient classification were performed within the TCGA consortium and used RPPA as proteomic method (22, 92, 94, 95, 99). Two studies demonstrated the utility of functional proteomics based on RPPA next to genomics and transcriptomics (92) and further created the bioinformatic resource ‘The Cancer Proteome Atlas’ (TCPA; (94)). The study of Kandoth and co-workers (13) explored a cohort of 373 EC patients, including 307 endometrioid and 53 serous or mixed histology cases to assess somatic mutations, copy number alterations, RNA expression, protein expression, DNA methylation and micro-RNA expression. With regard to proteomics, 293 samples were analysed by RPPA and several differentially expressed proteins were associated with other specific molecular tumour features (22). One of the latter two TCGA studies did not use proteomics (99), whereas the other study (95) explored 57 carcinosarcomas and -also in this case-, protein analyses were used to confirm other features identified with the molecular analyses (like EMT transitions, PI3K/AKT pathway activation, low steroid hormone receptor signalling). Similar to the TCPA (initiated within TCGA), the Clinical Proteomic Tumor Analysis Consortium (CTPAC) generated large proteomic datasets across various tumour types, and further characterised the proteogenomic landscape in EC (100). A pan-cancer study (98) aimed to characterise the actionable mutations across different solid tumours (including EC) and used RPPA to demonstrate PI3K and MAPK signalling pathways, whereas one study was focussed on human diseases other than cancer (97).
4. Discussion
In this study, we systematically reviewed all papers that explored the proteome or the metabolome in search for candidate biomarkers for prognostic or diagnostic purposes in EC. After screening the retrieved publications, we included 23 studies on proteomics (serum, plasma, uterine aspirate or tissue) and 29 studies on metabolomics (serum, plasma, urine, intra-uterine brushing, dried blood, cervicovaginal fluid or tissue).
Proteomic studies on body fluids and tissues (first fresh frozen then FFPE) have been published from second half of 2000. Initial studies were pilot in nature, and enrolled only a few patients ( Supplementary Table S6 ). The first metabolomic studies were published one decade later, and study populations were in general larger than those used for proteomics. Seven out of 23 proteomic and 14 out of 19 metabolomic studies reported the performance of the models as AUCs, and several candidate biomarkers show great potential with AUC values above 0.8. However, the majority of the reported proteins or metabolites and corresponding models represent biomarker candidates that still require validation. The models developed need evaluation of their statistical performance by splitting the data into training and test sets (so called “statistical validation”) and further experimental validation on independent cohort is essential (156).
With regard to statistical validation, this was performed by two proteomic studies (two-thirds/one-third cross-validation (115); leave-one-out-cross-validation (113) and four metabolomic studies (127, 128, 136, 142). Data on differentially expressed proteins was also confirmed experimentally using alternative methodologies like dot-blot (115), IHC (119, 121), RNA/qRT-PCR (117, 119), western blotting (110, 117), in vitro functional studies (117, 121) or using existing databases and repositories (119, 121).
In the context of proteomics, four studies only validated their data using independent sample cohorts (104, 107, 113, 119), and one study (115), validated their proteomic-based model in a subsequent publication using TMAs (126). Three metabolomic studies performed similar validations in independent cohorts (138, 142, 144), and one study in particular successfully validated the metabolic profiles identified in serum samples (136) also using dried blood and reported excellent diagnostic characteristics (144). For validation, the authors adopted a prospective design on a very large study cohort (over 1,000 subjects), therefore, candidate biomarkers identified in this study bear great potential for translation into clinical practice. Of interest, these biomarkers include steroids, which were also selected as candidate molecules in other studies (135, 157)
A caveat in a number of the included studies on proteomics (102, 104, 108) or metabolomics (136, 138, 142, 143) is the use of healthy (not age/comorbidity matched) women, likely resulting in an overestimated diagnostic accuracy. Also, if pre-menopausal controls are included, this may induce biases as EC is predominantly a postmenopausal disease (114–116, 122, 147, 149, 154). Additionally, biomarker discovery preferably includes a relevant population needing diagnostic tests such as women with postmenopausal uterine bleeding, or high-risk women, e.g., patients treated with tamoxifen or with Lynch syndrome, but also women with PCOS and obesity. Some proteomic studies focussed on these target groups, like obese subjects (119), or women with previous exposure to tamoxifen (124). One metabolomic study was performed in an obese population, and reported a Random Forest-based diagnostic model combining the top 10 performing metabolites that stratified stage I EC from other obese patients with an AUC of 0.98 (130), whereas the second metabolomic study included patients with PCOS (154).
A final relevant confounder is the ethnic background, known to affect the proteome profiles (83, 84). However, only one study used a cohort from a different geographical background, although still Caucasian to validate their data (119).
In a diagnostic/preoperative workup, ideal biomarkers should be present in easily and minimally invasively obtained body specimens. The proteomic studies included in this review predominantly used blood (serum of plasma) or uterine aspirate. The pilot studies using urine, although possibly the ideal body fluid for biomarker detection, were too small to be included ( Supplementary Table S6 ). Also in the context of metabolomics, only a few studies were performed on physiological fluids other than blood. One study explored cervicovaginal fluid (146) and two studies used urine samples, both of which have important limitations as they either used healthy women as controls (145) or included a few samples only (10 EC patients and 10 control patients) (101). Despite these limitations, these studies are also promising and future attempts for biomarker discovery in urine or cervicovaginal fluids using larger group of EC and control patients are warranted.
Importantly, although research groups were often able to validate their own candidate biomarkers in subsequent studies, rarely data was confirmed by independent authors/researchers, most probably due to methodological issues and also the abovementioned biases associated with geographical locations/ethnicity and lifestyles. Of note, there were candidate biomarkers that were validated in independent studies, and these represent highly promising molecules. Besides lipids, phospholipids and steroids, candidate proteins were reported as well. ANXA1 is described upregulated by three independent groups in EC tissues, uterine aspirates, and lymph nodes contaminated with EC cells (112, 113, 118, 123). ANXA1, annexin A1, plays an important role in immunity and inflammation and is associated with various diseases and cancers (158). HSPB1 was found upregulated in EC tissues and uterine aspirates by two teams (112, 113, 118). The HSPB1 gene encodes for Heat Shock Protein Family B (Small) Member 1, a protein that is associated with gynaecological cancers (159). In addition, SERPINC1, APOA4, APOE and ITIH4 are described deviated in the serum of hyperplasia or cancer patients by two teams (106, 109). In metabolomic studies, both molecule levels but also the ratio between levels of molecules proved to be good biomarkers, and a number of studies included metabolite ratios in modelling/analyses (128, 130, 132, 148, 152).
Overall, the major limitations of the studies published up to data are: i) the use of small study cohorts; ii) the diagnostic or prognostic accuracy was seldom compared with other known biomarkers or reference molecules (e.g., CA-125). iii) the 95% confidence intervals for AUC values, sensitivity and specificity were rarely reported; iv) as outlined above, validation in independent cohorts was done by a few studies only; specifically in metabolomic studies, validation using other technologies was never performed.
4.1. Strengths and limitations of the present study
The limitations reported above related to the papers retrieved and reviewed are reflected also in the present study, i.e., small study cohorts, potenial pre-analytical and analytical bias, potential bias due to ethnic background, lifestyle; lack of validations; no comparison with a reference or gold standard. This, in combination with the heterogeneity in study designs and in the technologies adopted precluded us making any meta-analyses of identified candidate biomarkers, whose potential can be assessed at a qualitative level only. This implies the impossibility to make any clinically relevant recommendation or conclusion at this moment. Nevertheless, the strength of the present study is that, due to the rigorous systematic approach we adopted, it offers a balanced and realistic view of the potential of these technologies for the future. It sets the milestones in proteomic and metabolomic biomarker discovery research, and indicates the path to follow in the future (see paragraph 4.2. Recommendations for further biomarker discovery).
It should be also noted that recently systematic reviews focused on metabolomic and/or proteomic biomarkers for diagnosis of EC (160), and liquid biomarkers for diagnosis of EC (161). However, our systematic review has additional unique strengths: we did not limit our analyses on one biospecimen only, but focused on metabolomics and proteomics in different biospecimens; we rigorously assessed the study quality using QUADOMICS and analysed additional potential conflicts of interest. The study quality was also assessed by Karkia and co-workers (161), however, these authors included only studies published in the two years prior to the publication, whereas we did not set a time limit for publication. Additionally, we used strict inclusion and exclusion criteria, which were defined and deposited in the PROSPERO repository prior to the start of our work. We finally provide comprehensive tables with all available diagnostic accuracy data (AUC, sensitivity, specificity).
4.2. Recommendations for further biomarker discovery
As thoroughly discussed, body fluids and liquid biopsies represent the most suitable material for diagnostic and prognostic biomarkers (162, 163), as they capture the disease heterogeneity better than a biopsy and they are non- or minimally invasive, thus create less anxiety in patients. In the context of the most appropriate body material, plasma preparation is less prone to technical (pre-analytical) biases than serum. Therefore, plasma represents the preferred source at least for the discovery phase of non-invasive diagnostic/prognostic biomarkers. Urine also represents an important clinical sample for non-invasive diagnostics (155), calling for further biomarker discovery studies. However, urine poses a challenge for biobanking, as large sample volumes are needed for analyses (this applies not only if 24-hour urine needs to be collected, but also for morning urine, which is common in biomarker discovery studies).
In terms of methodology, statistical and experimental validation in independent cohorts should be an intrinsic part of biomarker discovery studies, and inclusion of study populations with distinct lifestyles, geographical regions of origin and ethnic backgrounds is essential to identify candidate biomarkers truly associated with diseases. Strict standard operation procedures for sample collection and processing should be prepared by experts and rigorously followed (164). The importance of adhering to quality standards is also emphasized in a recent narrative review on biomarker discovery in EC (165).
5. Conclusions and future prospects
Clinically, there is a great need for non/minimally invasive biomarkers of EC that could serve as replacement test for endometrial biopsy or a triage test to select patients for further invasive diagnosis. Tissue biomarkers are also needed to allow preoperative stratification of patients and further individualised treatment. Recent advances in analytical technologies and computational approaches that can handle increasingly larger numbers of features offer unprecedented potentials to develop diagnostic and prognostic tools. The studies performed so far were in most cases pilot or explorative in nature, and heterogeneous in terms of study design, technology, and methodologies. These aspects need harmonisation for the future, and the study quality should be scrupulously monitored by journals, reviewers, and stakeholders in order to ensure translationability of the discoveries.
BIOENDOCAR
Members of the consortium besides the authors: Špela Smrkolj, Luka Roškar, Boštjan Pirš, Department of Gynecology, University Medical Centre Ljubljana, Slovenia; Aneta Adamiak-Godlewska, Sara Wawrysiuk and Ola Kaminska, Department of Gynaecology, Lublin Medical University, Poland. External collaborators: Jure Knez, Monika Sobočan and Iztok Takač, University Medical Centre Maribor, Slovenia; Fabio Barra, Department of Obstetrics and Gynaecology, IRCCS Ospedale Sacro Cuore - Don Calabria, Genoa, Italy; Simone Ferrero, Academic Unit of Obstetrics and Gynaecology, IRCCS Ospedale Policlinico San Martino; Vit Weinberger and Petra Vinklerova, Department of Gynecology and Obstetrics, University Hospital Brno, Czechia.
Author contributions
AR, TR, HW, AS, CL, CS, AG, JA, DF, and JT contributed to design the study, AR and TR to the literature search, AR, TR, HW, AS, CL, AG, DF and JT to writing the manuscript and issuing the figures. All authors contributed to the article and approved the submitted version.
Acknowledgments
The BioEndoCar consortium was a EU-H2020 funded Transcan2 ERA-Net project (2018–2021), with involvement of the national funding agencies: MIZS, Ministry of Education, Science and Sports Slovenia, grant no. C3330-18-252005; KWF, Dutch Cancer Society grant number 8303 German Federal Ministry for Education and Research (BMBF; 01KT1806A); Estonian Research Council grants PRG1095, PSG59; National Centre for Research and Development Poland NCBiR grant ERA-NET TRANSCAN/03/2018. The authors would also like to acknowledge ELIXIR and the European Regional Development Fund through EXCITE Center of Excellence and would like to thank the librarian Nana Turk from Institute of Bioinformatics, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia for creating literature search strategy.
Glossary
| AUC | area under the curve |
| BMI | body mass index |
| CPTAC | Clinical Proteomic Tumor Analysis Consortium |
| Da | Dalton |
| DFS | disease-free survival |
| DIGE | difference gel electrophoresis |
| EC | Endometrial cancer |
| ELISA | enzyme-linked immunosorbent assay |
| ER | estrogen receptor |
| ESI | electrospray ionization |
| FFPE | formalin-fixed-paraffin-embedded |
| FIGO | International federation of gynaecologic oncology |
| GC | Gas chromatography |
| HER2/Neu | Human Epidermal growth factor Receptor 2 |
| HR | hazard ratios |
| iCAT | Isotope-coded affinity tag |
| IHC | immunohistochemistry |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| LC | Liquid chromatography |
| LFQ | Label free quantification (LFQ) |
| LVI | lymphovascular invasion |
| MALDI | matrix-assisted laser desorption/ionization |
| MIB | Multiplexed Inhibitor Beads |
| MRM | multiple-reaction monitoring |
| MS | mass spectrometry |
| MSI | mass spectrometry imaging |
| NMR | nuclear magnetic resonance spectroscopy |
| NPV | negative predictive value |
| OS | overall survival |
| PAGE | polyacrylamide gel electrophoresis |
| PCOS | Poly Cystic Ovarian Syndrome |
| PEA | proximity extension assay |
| PLS-DA | Partial Least-Squares Discriminant Analysis |
| PPV | positive predictive value |
| PR | progesterone receptor |
| PRM | parallel reaction monitoring |
| PTEN | Phosphatase and tensin homolog |
| RPPA | reverse phase protein microarray |
| SD | Standard Deviation |
| SDS | sodium dodecyl sulphate |
| SELDI | surface-enhanced laser desorption/ionization |
| SILAC | Stable isotope labelling by amino acids in cell culture |
| SLN | sentinel-lymph-node |
| SRM | Selected-reaction monitoring |
| TCGA | The Cancer Genome Atlas |
| TCGA | The Cancer Proteome Atlas |
| TMA | tissue microarray |
| TOF | time-of-flight |
Conflict of interest
Authors CL, CS, and AG are or were employed by Sciomics GmbH. Author DF is employed by Quretec Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1120178/full#supplementary-material
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Renehan AG, Soerjomataram I, Leitzmann MF. Interpreting the epidemiological evidence linking obesity and cancer: a framework for population-attributable risk estimations in Europe. Eur J Cancer (2010) 46(14):2581–92. doi: 10.1016/j.ejca.2010.07.052 [DOI] [PubMed] [Google Scholar]
- 3. Rota M, Rumi F, Bagnardi V, Dal Maso L, Zucchetto A, Levi F, et al. Modelling body mass index and endometrial cancer risk in a pooled-analysis of three case-control studies. BJOG (2016) 123(2):285–92. doi: 10.1111/1471-0528.13717 [DOI] [PubMed] [Google Scholar]
- 4. Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, et al. Revised guidelines for the clinical management of lynch syndrome (Hnpcc): recommendations by a group of European experts. Gut (2013) 62(6):812–23. doi: 10.1136/gutjnl-2012-304356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the lynch syndrome. N Engl J Med (2006) 354(3):261–9. doi: 10.1056/NEJMoa052627 [DOI] [PubMed] [Google Scholar]
- 6. Mallozzi M, Leone C, Manurita F, Bellati F, Caserta D. Endocrine disrupting chemicals and endometrial cancer: An overview of recent laboratory evidence and epidemiological studies. Int J Environ Res Public Health (2017) 14(3):334. doi: 10.3390/ijerph14030334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adani G, Filippini T, Wise LA, Halldorsson TI, Blaha L, Vinceti M. Dietary intake of acrylamide and risk of breast, endometrial, and ovarian cancers: a systematic review and dose-response meta-analysis. Cancer Epidemiol Biomarkers Prev (2020) 29(6):1095–106. doi: 10.1158/1055-9965.EPI-19-1628 [DOI] [PubMed] [Google Scholar]
- 8. Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the corpus uteri. Int J Gynaecol Obstet (2018) 143 Suppl 2:37–50. doi: 10.1002/ijgo.12612 [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Patel L, Mills GB, Lu KH, Sood AK, Ding L, et al. Clinical significance of Ctnnb1 mutation and wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst (2014) 106(9). doi: 10.1093/jnci/dju245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stelloo E, Nout RA, Osse EM, Jurgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the portec cohorts. Clin Cancer Res (2016) 22(16):4215–24. doi: 10.1158/1078-0432.CCR-15-2878 [DOI] [PubMed] [Google Scholar]
- 11. Karnezis AN, Leung S, Magrill J, McConechy MK, Yang W, Chow C, et al. Evaluation of endometrial carcinoma prognostic immunohistochemistry markers in the context of molecular classification. J Pathol Clin Res (2017) 3(4):279–93. doi: 10.1002/cjp2.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurnit KC, Kim GN, Fellman BM, Urbauer DL, Mills GB, Zhang W, et al. Ctnnb1 (Beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol (2017) 30(7):1032–41. doi: 10.1038/modpathol.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kommoss FK, Karnezis AN, Kommoss F, Talhouk A, Taran FA, Staebler A, et al. L1cam further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br J Cancer (2018) 119(4):480–6. doi: 10.1038/s41416-018-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, et al. Final validation of the promise molecular classifier for endometrial carcinoma in a Large population-based case series. Ann Oncol (2018) 29(5):1180–8. doi: 10.1093/annonc/mdy058 [DOI] [PubMed] [Google Scholar]
- 15. Moroney MR, Davies KD, Wilberger AC, Sheeder J, Post MD, Berning AA, et al. Molecular markers in recurrent stage I, grade 1 endometrioid endometrial cancers. Gynecol Oncol (2019) 153(3):517–20. doi: 10.1016/j.ygyno.2019.03.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Leo A, de Biase D, Lenzi J, Barbero G, Turchetti D, Grillini M, et al. Arid1a and Ctnnb1/Beta-catenin molecular status affects the clinicopathologic features and prognosis of endometrial carcinoma: implications for an improved surrogate molecular classification. Cancers (Basel) (2021) 13(5). doi: 10.3390/cancers13050950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo M, Gong H, Nie D, Li Z. High L1cam expression predicts poor prognosis of patients with endometrial cancer: a systematic review and meta-analysis. Med (Baltimore) (2021) 100(13):e25330. doi: 10.1097/MD.0000000000025330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vrede SW, van Weelden WJ, Visser NCM, Bulten J, van der Putten LJM, van de Vijver K, et al. Immunohistochemical biomarkers are prognostic relevant in addition to the esmo-Esgo-Estro risk classification in endometrial cancer. Gynecol Oncol (2021) 161(3):787–94. doi: 10.1016/j.ygyno.2021.03.031 [DOI] [PubMed] [Google Scholar]
- 19. Perrone E, De Felice F, Capasso I, Distefano E, Lorusso D, Nero C, et al. The immunohistochemical molecular risk classification in endometrial cancer: a pragmatic and high-reproducibility method. Gynecol Oncol (2022) 165(3):585–93. doi: 10.1016/j.ygyno.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 20. Travaglino A, Raffone A, Raimondo D, Reppuccia S, Ruggiero A, Arena A, et al. Prognostic significance of Ctnnb1 mutation in early stage endometrial carcinoma: a systematic review and meta-analysis. Arch Gynecol Obstet (2022) 306(2):423–31. doi: 10.1007/s00404-021-06385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. Esgo/Estro/Esp guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer (2021) 31(1):12–39. doi: 10.1136/ijgc-2020-002230 [DOI] [PubMed] [Google Scholar]
- 22. Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497(7447):67–73. doi: 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talhouk A, Hoang LN, McConechy MK, Nakonechny Q, Leo J, Cheng A, et al. Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: earlier prognostic information to guide treatment. Gynecol Oncol (2016) 143(1):46–53. doi: 10.1016/j.ygyno.2016.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Visser NCM, Reijnen C, Massuger L, Nagtegaal ID, Bulten J, Pijnenborg JMA. Accuracy of endometrial sampling in endometrial carcinoma: a systematic review and meta-analysis. Obstet Gynecol (2017) 130(4):803–13. doi: 10.1097/AOG.0000000000002261 [DOI] [PubMed] [Google Scholar]
- 25. Narice BF, Delaney B, Dickson JM. Endometrial sampling in low-risk patients with abnormal uterine bleeding: a systematic review and meta-synthesis. BMC Fam Pract (2018) 19(1):135. doi: 10.1186/s12875-018-0817-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Messner CB, Demichev V, Wang Z, Hartl J, Kustatscher G, Mulleder M, et al. Mass spectrometry-based high-throughput proteomics and its role in biomedical studies and systems biology. Proteomics (2022) e2200013. doi: 10.1002/pmic.202200013 [DOI] [PubMed] [Google Scholar]
- 27. Aerqin Q, Wang ZT, Wu KM, He XY, Dong Q, Yu JT. Omics-based biomarkers discovery for alzheimer's disease. Cell Mol Life Sci (2022) 79(12):585. doi: 10.1007/s00018-022-04614-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neagu AN, Jayathirtha M, Whitham D, Mutsengi P, Sullivan I, Petre BA, et al. Proteomics-based identification of dysregulated proteins in breast cancer. Proteomes (2022) 10(4). doi: 10.3390/proteomes10040035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agregan R, Pateiro M, Kumar M, Franco D, Capanoglu E, Dhama K, et al. The potential of proteomics in the study of processed meat products. J Proteomics (2023) 270:104744. doi: 10.1016/j.jprot.2022.104744 [DOI] [PubMed] [Google Scholar]
- 30. Li W, Shao C, Zhou H, Du H, Chen H, Wan H, et al. Multi-omics research strategies in ischemic stroke: a multidimensional perspective. Ageing Res Rev (2022) 81:101730. doi: 10.1016/j.arr.2022.101730 [DOI] [PubMed] [Google Scholar]
- 31. Zou Z, Sun W, Xu Y, Liu W, Zhong J, Lin X, et al. Application of multi-omics approach in sarcomas: a tool for studying mechanism, biomarkers, and therapeutic targets. Front Oncol (2022) 12:946022. doi: 10.3389/fonc.2022.946022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ren AH, Diamandis EP, Kulasingam V. Uncovering the depths of the human proteome: Antibody-based technologies for ultrasensitive multiplexed protein detection and quantification. Mol Cell Proteomics (2021) 20:100155. doi: 10.1016/j.mcpro.2021.100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoheisel JD, Alhamdani MS, Schroder C. Affinity-based microarrays for proteomic analysis of cancer tissues. Proteomics Clin Appl (2013) 7(1-2):8–15. doi: 10.1002/prca.201200114 [DOI] [PubMed] [Google Scholar]
- 34. Solier C, Langen H. Antibody-based proteomics and biomarker research - current status and limitations. Proteomics (2014) 14(6):774–83. doi: 10.1002/pmic.201300334 [DOI] [PubMed] [Google Scholar]
- 35. Tang N, Tornatore P, Weinberger SR. Current developments in seldi affinity technology. Mass Spectrom Rev (2004) 23(1):34–44. doi: 10.1002/mas.10066 [DOI] [PubMed] [Google Scholar]
- 36. Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem (2007) 389(4):1017–31. doi: 10.1007/s00216-007-1486-6 [DOI] [PubMed] [Google Scholar]
- 37. Anderson NL. The clinical plasma proteome: A survey of clinical assays for proteins in plasma and serum. Clin Chem (2010) 56(2):177–85. doi: 10.1373/clinchem.2009.126706 [DOI] [PubMed] [Google Scholar]
- 38. Makawita S, Diamandis EP. The bottleneck in the cancer biomarker pipeline and protein quantification through mass spectrometry-based approaches: current strategies for candidate verification. Clin Chem (2010) 56(2):212–22. doi: 10.1373/clinchem.2009.127019 [DOI] [PubMed] [Google Scholar]
- 39. Torres S, Bartolome RA, Mendes M, Barderas R, Fernandez-Acenero MJ, Pelaez-Garcia A, et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res (2013) 19(21):6006–19. doi: 10.1158/1078-0432.CCR-13-1130 [DOI] [PubMed] [Google Scholar]
- 40. Tweeddale H, Notley-McRobb L, Ferenci T. Effect of slow growth on metabolism of escherichia coli, as revealed by global metabolite pool ("Metabolome") analysis. J Bacteriol (1998) 180(19):5109–16. doi: 10.1128/JB.180.19.5109-5116.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patti GJ, Yanes O, Siuzdak G. Innovation: Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol (2012) 13(4):263–9. doi: 10.1038/nrm3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tokarz J, Haid M, Cecil A, Prehn C, Artati A, Moller G, et al. Endocrinology meets metabolomics: achievements, pitfalls, and challenges. Trends Endocrinol Metab (2017) 28(10):705–21. doi: 10.1016/j.tem.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 43. Hocher B, Adamski J. Metabolomics for clinical use and research in chronic kidney disease. Nat Rev Nephrol (2017) 13(5):269–84. doi: 10.1038/nrneph.2017.30 [DOI] [PubMed] [Google Scholar]
- 44. Kostidis S, Addie RD, Morreau H, Mayboroda OA, Giera M. Quantitative nmr analysis of intra- and extracellular metabolism of mammalian cells: a tutorial. Anal Chim Acta (2017) 980:1–24. doi: 10.1016/j.aca.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 45. Schripsema J. Application of nmr in plant metabolomics: techniques, problems and prospects. Phytochem Anal (2010) 21(1):14–21. doi: 10.1002/pca.1185 [DOI] [PubMed] [Google Scholar]
- 46. Naz S, Vallejo M, Garcia A, Barbas C. Method validation strategies involved in non-targeted metabolomics. J Chromatogr A (2014) 1353:99–105. doi: 10.1016/j.chroma.2014.04.071 [DOI] [PubMed] [Google Scholar]
- 47. Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. The human serum metabolome. PloS One (2011) 6(2):e16957. doi: 10.1371/journal.pone.0016957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zheng Y. Study design considerations for cancer biomarker discoveries. J Appl Lab Med (2018) 3(2):282–9. doi: 10.1373/jalm.2017.025809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ottas A, Fishman D, Okas TL, Pussa T, Toomik P, Martson A, et al. Blood serum metabolome of atopic dermatitis: Altered energy cycle and the markers of systemic inflammation. PloS One (2017) 12(11):e0188580. doi: 10.1371/journal.pone.0188580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fishman D, Kisand K, Hertel C, Rothe M, Remm A, Pihlap M, et al. Autoantibody repertoire in apeced patients targets two distinct subgroups of proteins. Front Immunol (2017) 8:976. doi: 10.3389/fimmu.2017.00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duarte JG, Blackburn JM. Advances in the development of human protein microarrays. Expert Rev Proteomics (2017) 14(7):627–41. doi: 10.1080/14789450.2017.1347042 [DOI] [PubMed] [Google Scholar]
- 52. Abel L, Kutschki S, Turewicz M, Eisenacher M, Stoutjesdijk J, Meyer HE, et al. Autoimmune profiling with protein microarrays in clinical applications. Biochim Biophys Acta (2014) 1844(5):977–87. doi: 10.1016/j.bbapap.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 53. Diez P, Dasilva N, Gonzalez-Gonzalez M, Matarraz S, Casado-Vela J, Orfao A, et al. Data analysis strategies for protein microarrays. Microarrays (Basel) (2012) 1(2):64–83. doi: 10.3390/microarrays1020064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xi Y, Rocke DM. Baseline correction for nmr spectroscopic metabolomics data analysis. BMC Bioinf (2008) 9:324. doi: 10.1186/1471-2105-9-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Feng C, Wang H, Lu N, Chen T, He H, Lu Y, et al. Log-transformation and its implications for data analysis. Shanghai Arch Psychiatry (2014) 26(2):105–9. doi: 10.3969/j.issn.1002-0829.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sboner A, Karpikov A, Chen G, Smith M, Mattoon D, Freeman-Cook L, et al. Robust-Linear-Model normalization to reduce technical variability in functional protein microarrays. J Proteome Res (2009) 8(12):5451–64. doi: 10.1021/pr900412k [DOI] [PubMed] [Google Scholar]
- 57. Da Gama Duarte J, Goosen RW, Lawry PJ, Blackburn JM. Pma: Protein microarray analyser, a user-friendly tool for data processing and normalization. BMC Res Notes (2018) 11(1):156. doi: 10.1186/s13104-018-3266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. DeLuca DS, Marina O, Ray S, Zhang GL, Wu CJ, Brusic V. Data processing and analysis for protein microarrays. Methods Mol Biol (2011) 723:337–47. doi: 10.1007/978-1-61779-043-0_21 [DOI] [PubMed] [Google Scholar]
- 59. Bonferroni CE. Teoria statistica delle classi e calcolo delle probabilita. Pubblicazioni del R Istituto Superiore di Sci Economiche e Commericiali di Firenze (1936) 8:3–62. [Google Scholar]
- 60. Zumbo BD. Univariate tests. Netherlands: Springer; (2014) p. 6819–20. [Google Scholar]
- 61. Tarca AL, Carey VJ, Chen XW, Romero R, Draghici S. Machine learning and its applications to biology. PloS Comput Biol (2007) 3(6):e116. doi: 10.1371/journal.pcbi.0030116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nagele E, Han M, Demarshall C, Belinka B, Nagele R. Diagnosis of alzheimer's disease based on disease-specific autoantibody profiles in human sera. PloS One (2011) 6(8):e23112. doi: 10.1371/journal.pone.0023112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Quinlan JR. Induction of decision trees. Mach Learn (1986) 1(1):81–106. doi: 10.1007/BF00116251 [DOI] [Google Scholar]
- 64. Breiman RF. Vaccines as tools for advancing more than public health: perspectives of a former director of the national vaccine program office. Clin Infect Dis (2001) 32(2):283–8. doi: 10.1086/318461 [DOI] [PubMed] [Google Scholar]
- 65. Wu X, Kumar V, Ross Quinlan J, Ghosh J, Yang Q, Motoda H, et al. Top 10 algorithms in data mining. Knowledge Inf Syst (2008) 14(1):1–37. doi: 10.1007/s10115-007-0114-2 [DOI] [Google Scholar]
- 66. Walsh I, Fishman D, Garcia-Gasulla D, Titma T, Pollastri G, Group EMLF et al. Dome: Recommendations for supervised machine learning validation in biology. Nat Methods (2021) 18(10):1122–7. doi: 10.1038/s41592-021-01205-4 [DOI] [PubMed] [Google Scholar]
- 67. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the P-DTAG et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the prisma-dta statement. JAMA (2018) 319(4):388–96. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 68. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 69. Lumbreras B, Porta M, Marquez S, Pollan M, Parker LA, Hernandez-Aguado I. Quadomics: an adaptation of the quality assessment of diagnostic accuracy assessment (Quadas) for the evaluation of the methodological quality of studies on the diagnostic accuracy of '-Omics'-Based technologies. Clin Biochem (2008) 41(16-17):1316–25. doi: 10.1016/j.clinbiochem.2008.06.018 [DOI] [PubMed] [Google Scholar]
- 70. Fata CR, Seeley EH, Desouki MM, Du L, Gwin K, Hanley KZ, et al. Are clear cell carcinomas of the ovary and endometrium phenotypically identical? A proteomic analysis. Hum Pathol (2015) 46(10):1427–36. doi: 10.1016/j.humpath.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 71. Liu Z, Hong Z, Qu P. Proteomic analysis of human endometrial tissues reveals the roles of Pi3k/Akt/Mtor pathway and tumor angiogenesis molecules in the pathogenesis of endometrial cancer. BioMed Res Int (2020). doi: 10.1155/2020/5273969. (pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lopez-Janeiro A, Ruz-Caracuel I, Ramon-Patino JL, Rios VDL, Esparza MV, Berjon A, et al. Proteomic analysis of low-grade, early-stage endometrial carcinoma reveals new dysregulated pathways associated with cell death and cell signaling. Cancers (2021) 13(4):1–16. doi: 10.3390/cancers13040794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mariscal J, Fernandez-Puente P, Calamia V, Abalo A, Santacana M, Matias-Guiu X, et al. Proteomic characterization of epithelial-like extracellular vesicles in advanced endometrial cancer. J Proteome Res (2019) 18(3):1043–53. doi: 10.1021/acs.jproteome.8b00750 [DOI] [PubMed] [Google Scholar]
- 74. Raglan O, Assi N, Nautiyal J, Lu H, Gabra H, Gunter MJ, et al. Proteomic analysis of malignant and benign endometrium according to obesity and insulin-resistance status using reverse phase protein array. Transl Res (2020) 218:57–72. doi: 10.1016/j.trsl.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 75. Acland M, Arentz G, Mussared M, Whitehead F, Hoffmann P, Klingler-Hoffmann M, et al. Proteomic analysis of pre-invasive serous lesions of the endometrium and fallopian tube reveals their metastatic potential. Front Oncol (2020) 10:523989(no pagination). doi: 10.3389/fonc.2020.523989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hiramatsu K, Yoshino K, Serada S, Yoshihara K, Hori Y, Fujimoto M, et al. Similar protein expression profiles of ovarian and endometrial high-grade serous carcinomas. Br J Cancer (2016) 114(5):554–61. doi: 10.1038/bjc.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maxwell GL, Hood BL, Day R, Chandran U, Kirchner D, Kolli VS, et al. Proteomic analysis of stage I endometrial cancer tissue: identification of proteins associated with oxidative processes and inflammation. Gynecol Oncol (2011) 121(3):586–94. doi: 10.1016/j.ygyno.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Monge M, Doll A, Colas E, Gil-Moreno A, Castellvi J, Garcia A, et al. Subtractive proteomic approach to the endometrial carcinoma invasion front. J Proteome Res (2009) 8(10):4676–84. doi: 10.1021/pr900390t [DOI] [PubMed] [Google Scholar]
- 79. Seo G, Han H, Vargas RE, Yang B, Li X, Wang W. Map4k interactome reveals Strn4 as a key stripak complex component in hippo pathway regulation. Cell Rep (2020) 32(1):107860. doi: 10.1016/j.celrep.2020.107860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yoneyama K, Shibata R, Igarashi A, Kojima S, Kodani Y, Nagata K, et al. Proteomic identification of dihydrolipoamide dehydrogenase as a target of autoantibodies in patients with endometrial cancer. Anticancer Res (2014) 34(9):5021–7. [PubMed] [Google Scholar]
- 81. Bateman NW, Teng PN, Hope E, Hood BL, Oliver J, Ao W, et al. Jupiter Microtubule-associated homolog 1 (Jpt1): a predictive and pharmacodynamic biomarker of metformin response in endometrial cancers. Cancer Med (2020) 9(3):1092–103. doi: 10.1002/cam4.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kodell RL, Haun RS, Siegel ER, Zhang C, Trammel AB, Hauer-Jensen M, et al. Novel use of proteomic profiles in a convex-Hull ensemble classifier to predict gynecological cancer patients' susceptibility to gastrointestinal mucositis as side effect of radiation therapy. J Proteomics Bioinform (2015) 8(7):149–54. doi: 10.4172/jpb.1000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Javadian P, Xu C, Sjoelund V, Borden LE, Garland J, Benbrook DM. Identification of candidate biomarker and drug targets for improving endometrial cancer racial disparities. Int J Mol Sci (2022) 23(14). doi: 10.3390/ijms23147779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bateman NW, Dubil EA, Wang G, Hood BL, Oliver JM, Litzi TA, et al. Race-specific molecular alterations correlate with differential outcomes for black and white endometrioid endometrial cancer patients. Cancer (2017) 123(20):4004–12. doi: 10.1002/cncr.30813 [DOI] [PubMed] [Google Scholar]
- 85. Lin ST, Chou HC, Chang SJ, Chen YW, Lyu PC, Wang WC, et al. Proteomic analysis of proteins responsible for the development of doxorubicin resistance in human uterine cancer cells. J Proteomics (2012) 75(18):5822–47. doi: 10.1016/j.jprot.2012.07.047 [DOI] [PubMed] [Google Scholar]
- 86. Lin ST, Lo YW, Chang SJ, Wang WC, Chang MD, Lyu PC, et al. Redox-proteomic analysis of doxorubicin resistance-induced altered thiol activity in uterine carcinoma. J Pharm BioMed Anal (2013) 78-79:1–8. doi: 10.1016/j.jpba.2013.01.028 [DOI] [PubMed] [Google Scholar]
- 87. Lo YW, Lin ST, Chang SJ, Chan CH, Lyu KW, Chang JF, et al. Mitochondrial proteomics with sirna knockdown to reveal Acat1 and Mdh2 in the development of doxorubicin-resistant uterine cancer. J Cell Mol Med (2015) 19(4):744–59. doi: 10.1111/jcmm.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, Garcia-Velasco JA, et al. Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res (2009) 8(10):4622–32. doi: 10.1021/pr9004426 [DOI] [PubMed] [Google Scholar]
- 89. Monsivais D, Vasquez YM, Chen F, Zhang Y, Chandrashekar DS, Faver JC, et al. Mass-Spectrometry-Based proteomic correlates of grade and stage reveal pathways and kinases associated with aggressive human cancers. Oncogene (2021) 40(11):2081–95. doi: 10.1038/s41388-021-01681-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lai J, Xu T, Yang H. Protein-based prognostic signature for predicting the survival and immunotherapeutic efficiency of endometrial carcinoma. BMC Cancer (2022) 22(1):325. doi: 10.1186/s12885-022-09402-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang Q, Xia T, Qi C, Du J, Ye C. High expression of S100a2 predicts poor prognosis in patients with endometrial carcinoma. BMC Cancer (2022) 22(1). doi: 10.1186/s12885-022-09180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Akbani R, Ng PKS, Werner HMJ, Shahmoradgoli M, Zhang F, Ju Z, et al. A pan-cancer proteomic perspective on the cancer genome atlas. Nat Commun (2014) 5(3887). doi: 10.1038/ncomms4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Berger AC, Korkut A, Kanchi RS, Hegde AM, Lenoir W, Liu W, et al. A comprehensive pan-cancer molecular study of gynecologic and breast cancers. Cancer Cell (2018) 33(4):690–705. doi: 10.1016/j.ccell.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen MJM, Li J, Wang Y, Akbani R, Lu Y, Mills GB, et al. Tcpa V3.0: An integrative platform to explore the pan-cancer analysis of functional proteomic data. Mol Cell Proteomics (2019) 18(8):S15–25. doi: 10.1074/mcp.RA118.001260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cherniack AD, Shen H, Walter V, Stewart C, Murray BA, Bowlby R, et al. Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell (2017) 31(3):411–23. doi: 10.1016/j.ccell.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang W, Chen J, Weng W, Xiang Y, Shi H, Shan Y. Development of cancer prognostic signature based on pan-cancer proteomics. Bioengineered (2020) 11(1):1368–81. doi: 10.1080/21655979.2020.1847398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pietzner M, Wheeler E, Carrasco-Zanini J, Cortes A, Koprulu M, Worheide MA, et al. Mapping the proteo-genomic convergence of human diseases. Science (2021) 374(6569). doi: 10.1126/science.abj1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Toomey S, Carr A, Mezynski MJ, Elamin Y, Rafee S, Cremona M, et al. Identification and clinical impact of potentially actionable somatic oncogenic mutations in solid tumor samples. J Transl Med (2020) 18(1):99. doi: 10.1186/s12967-020-02273-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Iijima M, Banno K, Okawa R, Yanokura M, Iida M, Takeda T, et al. Genome-wide analysis of gynecologic cancer: The cancer genome atlas in ovarian and endometrial cancer. Oncol Lett (2017) 13(3):1063–70. doi: 10.3892/ol.2017.5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dou Y, Kawaler EA, Cui Zhou D, Gritsenko MA, Huang C, Blumenberg L, et al. Proteogenomic characterization of endometrial carcinoma. Cell (2020) 180(4):729–48.e26. doi: 10.1016/j.cell.2020.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yi R, Xie L, Wang X, Shen C, Chen X, Qiao L. Multi-omic profiling of multi-biosamples reveals the role of amino acid and nucleotide metabolism in endometrial cancer. Front Oncol (2022) 12:861142. doi: 10.3389/fonc.2022.861142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhu LR, Zhang WY, Yu L, Zheng YH, Zhang JZ, Liao QP. Serum proteomic features for detection of endometrial cancer. Int J Gynecol Cancer (2006) 16(3):1374–8. doi: 10.1111/j.1525-1438.2006.00561.x [DOI] [PubMed] [Google Scholar]
- 103. Kikuchi S, Honda K, Handa Y, Kato H, Yamashita K, Umaki T, et al. Serum albumin-associated peptides of patients with uterine endometrial cancer. Cancer Sci (2007) 98(6):822–9. doi: 10.1111/j.1349-7006.2007.00458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhu LR, Zhang WY, Yu L, Zheng YH, Hu J, Liao QP. Proteiomic patterns for endometrial cancer using seldi-Tof-Ms. J Zhejiang Univ Sci B (2008) 9(4):286–90. doi: 10.1631/jzus.B0710589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qiu F, Gao YH, Jiang CG, Tian YP, Zhang XJ. Serum proteomic profile analysis for endometrial carcinoma detection with maldi-tof Ms. Arch Med Sci (2010) 6(2):245–52. doi: 10.5114/aoms.2010.13903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang YS, Cao R, Jin H, Huang YP, Zhang XY, Cong Q, et al. Altered protein expression in serum from endometrial hyperplasia and carcinoma patients. J Hematol Oncol (2011) 4:15. doi: 10.1186/1756-8722-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Enroth S, Berggrund M, Lycke M, Lundberg M, Assarsson E, Olovsson M, et al. A two-step strategy for identification of plasma protein biomarkers for endometrial and ovarian cancer. Clin Proteomics (2018) 15:38. doi: 10.1186/s12014-018-9216-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tarney CM, Wang G, Bateman NW, Conrads KA, Zhou M, Hood BL, et al. Biomarker panel for early detection of endometrial cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Obstet Gynecol (2019) 221(5):472.e1–.e10. doi: 10.1016/j.ajog.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 109. Ura B, Biffi S, Monasta L, Arrigoni G, Battisti I, Di Lorenzo G, et al. Two dimensional-difference in gel electrophoresis (2d-dige) proteomic approach for the identification of biomarkers in endometrial cancer serum. Cancers (2021) 13(14). doi: 10.3390/cancers13143639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Celsi F, Monasta L, Arrigoni G, Battisti I, Licastro D, Aloisio M, et al. Gel-based proteomic identification of suprabasin as a potential new candidate biomarker in endometrial cancer. Int J Mol Sci (2022) 23(4). doi: 10.3390/ijms23042076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ura B, Capaci V, Aloisio M, Di Lorenzo G, Romano F, Ricci G, et al. A targeted proteomics approach for screening serum biomarkers observed in the early stage of type I endometrial cancer. Biomedicines (2022) 10(8). doi: 10.3390/biomedicines10081857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Martinez-Garcia E, Lesur A, Devis L, Campos A, Cabrera S, van Oostrum J, et al. Development of a sequential workflow based on lc-prm for the verification of endometrial cancer protein biomarkers in uterine aspirate samples. Oncotarget (2016) 7(33):53102–15. doi: 10.18632/oncotarget.10632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Martinez-Garcia E, Lesur A, Devis L, Cabrera S, Matias-Guiu X, Hirschfeld M, et al. Targeted proteomics identifies proteomic signatures in liquid biopsies of the endometrium to diagnose endometrial cancer and assist in the prediction of the optimal surgical treatment. Clin Cancer Res (2017) 23(21):6458–67. doi: 10.1158/1078-0432.Ccr-17-0474 [DOI] [PubMed] [Google Scholar]
- 114. Yoshizaki T, Enomoto T, Nakashima R, Ueda Y, Kanao H, Yoshino K, et al. Altered protein expression in endometrial carcinogenesis. Cancer Lett (2005) 226(2):101–6. doi: 10.1016/j.canlet.2004.11.053 [DOI] [PubMed] [Google Scholar]
- 115. DeSouza LV, Grigull J, Ghanny S, Dube V, Romaschin AD, Colgan TJ, et al. Endometrial carcinoma biomarker discovery and verification using differentially tagged clinical samples with multidimensional liquid chromatography and tandem mass spectrometry. Mol Cell Proteomics (2007) 6(7):1170–82. doi: 10.1074/mcp.M600378-MCP200 [DOI] [PubMed] [Google Scholar]
- 116. Voisin SN, Krakovska O, Matta A, DeSouza LV, Romaschin AD, Colgan TJ, et al. Identification of novel molecular targets for endometrial cancer using a drill-down lc-Ms/Ms approach with itraq. PloS One (2011) 6(1):e16352. doi: 10.1371/journal.pone.0016352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shan N, Zhou W, Zhang S, Zhang Y. Identification of Hspa8 as a candidate biomarker for endometrial carcinoma by using itraq-based proteomic analysis. Onco Targets Ther (2016) 9:2169–79. doi: 10.2147/ott.S97983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ceylan Y, Akpinar G, Doger E, Kasap M, Guzel N, Karaosmanoglu K, et al. Proteomic analysis in endometrial cancer and endometrial hyperplasia tissues by 2d-dige technique. J Gynecol Obstet Hum Reprod (2020) 49(2):101652. doi: 10.1016/j.jogoh.2019.101652 [DOI] [PubMed] [Google Scholar]
- 119. Mauland KK, Ju Z, Tangen IL, Berg A, Kalland KH, Oyan AM, et al. Proteomic profiling of endometrioid endometrial cancer reveals differential expression of hormone receptors and mapk signaling proteins in obese versus non-obese patients. Oncotarget (2017) 8(63):106989–7001. doi: 10.18632/oncotarget.22203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Akkour K, Alanazi IO, Alfadda AA, Alhalal H, Masood A, Musambil M, et al. Tissue-based proteomic profiling in patients with hyperplasia and endometrial cancer. Cells (2022) 11(13). doi: 10.3390/cells11132119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kurimchak AM, Kumar V, Herrera-Montavez C, Johnson KJ, Srivastava N, Davarajan K, et al. Kinome profiling of primary endometrial tumors using multiplexed inhibitor beads and mass spectrometry identifies Srpk1 as candidate therapeutic target. Mol Cell Proteomics (2020) 19(12):2068–89. doi: 10.1074/mcp.RA120.002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. DeSouza LV, Krakovska O, Darfler MM, Krizman DB, Romaschin AD, Colgan TJ, et al. Mtraq-based quantification of potential endometrial carcinoma biomarkers from archived formalin-fixed paraffin-embedded tissues. Proteomics (2010) 10(17):3108–16. doi: 10.1002/pmic.201000082 [DOI] [PubMed] [Google Scholar]
- 123. Aboulouard S, Wisztorski M, Duhamel M, Saudemont P, Cardon T, Narducci F, et al. In-depth proteomics analysis of sentinel lymph nodes from individuals with endometrial cancer. Cell Rep Med (2021) 2(6). doi: 10.1016/j.xcrm.2021.100318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Janacova L, Faktor J, Capkova L, Paralova V, Pospisilova A, Podhorec J, et al. Swath-Ms analysis of ffpe tissues identifies stathmin as a potential marker of endometrial cancer in patients exposed to tamoxifen. J Proteome Res (2020) 19(7):2617–30. doi: 10.1021/acs.jproteome.0c00064 [DOI] [PubMed] [Google Scholar]
- 125. DeSouza L, Diehl G, Rodrigues MJ, Guo J, Romaschin AD, Colgan TJ, et al. Search for cancer markers from endometrial tissues using differentially labeled tags itraq and cicat with multidimensional liquid chromatography and tandem mass spectrometry. J Proteome Res (2005) 4(2):377–86. doi: 10.1021/pr049821j [DOI] [PubMed] [Google Scholar]
- 126. Dube V, Grigull J, DeSouza LV, Ghanny S, Colgan TJ, Romaschin AD, et al. Verification of endometrial tissue biomarkers previously discovered using mass spectrometry-based proteomics by means of immunohistochemistry in a tissue microarray format. J Proteome Res (2007) 6(7):2648–55. doi: 10.1021/pr070087o [DOI] [PubMed] [Google Scholar]
- 127. Ihata Y, Miyagi E, Numazaki R, Muramatsu T, Imaizumi A, Yamamoto H, et al. Amino acid profile index for early detection of endometrial cancer: Verification as a novel diagnostic marker. Int J Clin Oncol (2014) 19(2):364–72. doi: 10.1007/s10147-013-0565-2 [DOI] [PubMed] [Google Scholar]
- 128. Knific T, Vouk K, Smrkolj Š, Prehn C, Adamski J, Rižner TL. Models including plasma levels of sphingomyelins and phosphatidylcholines as diagnostic and prognostic biomarkers of endometrial cancer. J Steroid Biochem Mol Biol (2018) 178:312–21. doi: 10.1016/j.jsbmb.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 129. Strand E, Tangen IL, Fasmer KE, Jacob H, Halle MK, Hoivik EA, et al. Blood metabolites associate with prognosis in endometrial cancer. Metabolites (2019) 9(12):302. doi: 10.3390/metabo9120302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Njoku K, Campbell AE, Geary B, MacKintosh ML, Derbyshire AE, Kitson SJ, et al. Metabolomic biomarkers for the detection of obesity-driven endometrial cancer. Cancers (Basel) (2021) 13(4). doi: 10.3390/cancers13040718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kliemann N, Viallon V, Murphy N, Beeken RJ, Rothwell JA, Rinaldi S, et al. Metabolic signatures of greater body size and their associations with risk of colorectal and endometrial cancers in the European prospective investigation into cancer and nutrition. BMC Med (2021) 19(1):101. doi: 10.1186/s12916-021-01970-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dossus L, Kouloura E, Biessy C, Viallon V, Siskos AP, Dimou N, et al. Prospective analysis of circulating metabolites and endometrial cancer risk. Gynecol Oncol (2021) 162(2):475–81. doi: 10.1016/j.ygyno.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Breeur M, Ferrari P, Dossus L, Jenab M, Johansson M, Rinaldi S, et al. Pan-cancer analysis of pre-diagnostic blood metabolite concentrations in the European prospective investigation into cancer and nutrition. BMC Med (2022) 20(1):351. doi: 10.1186/s12916-022-02553-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Audet-Delage Y, Villeneuve L, Grégoire J, Plante M, Guillemette C. Identification of metabolomic biomarkers for endometrial cancer and its recurrence after surgery in postmenopausal women. Front Endocrinol (2018) 9:87. doi: 10.3389/fendo.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Audet-Delage Y, Grégoire J, Caron P, Turcotte V, Plante M, Ayotte P, et al. Estradiol metabolites as biomarkers of endometrial cancer prognosis after surgery. J Steroid Biochem Mol Biol (2018) 178:45–54. doi: 10.1016/j.jsbmb.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 136. Troisi J, Sarno L, Landolfi A, Scala G, Martinelli P, Venturella R, et al. Metabolomic signature of endometrial cancer. J Proteome Res (2018) 17(2):804–12. doi: 10.1021/acs.jproteome.7b00503 [DOI] [PubMed] [Google Scholar]
- 137. Shi K, Wang Q, Su Y, Xuan X, Liu Y, Chen W, et al. Identification and functional analyses of differentially expressed metabolites in early stage endometrial carcinoma. Cancer Sci (2018) 109(4):1032–43. doi: 10.1111/cas.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bahado-Singh RO, Lugade A, Field J, Al-Wahab Z, Han B, Mandal R, et al. Metabolomic prediction of endometrial cancer. Metabolomics Off J Metabolomic Soc (2017) 14(1):6. doi: 10.1007/s11306-017-1290-z [DOI] [PubMed] [Google Scholar]
- 139. Lunde S, Nguyen HT, Petersen KK, Arendt-Nielsen L, Krarup HB, Søgaard-Andersen E. Chronic postoperative pain after hysterectomy for endometrial cancer: A metabolic profiling study. Mol Pain (2020) 16:1744806920923885. doi: 10.1177/1744806920923885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kozar N, Kruusmaa K, Dovnik A, Bitenc M, Argamasilla R, Adsuar A, et al. Identification of novel diagnostic biomarkers in endometrial cancer using targeted metabolomic profiling. Adv Med Sci (2021) 66(1):46–51. doi: 10.1016/j.advms.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 141. Gu M, Chen X, Sun Y, Wang L, Shu H, Qian C. A metabolomic signature of figo stage I and ii endometrial cancer. Neoplasma (2021) 68(6):1283–91. doi: 10.4149/neo_2021_210306N288 [DOI] [PubMed] [Google Scholar]
- 142. Yan X, Zhao W, Wei J, Yao Y, Sun G, Wang L, et al. A serum lipidomics study for the identification of specific biomarkers for endometrial polyps to distinguish them from endometrial cancer or hyperplasia. Int J Cancer (2022) 150(9):1549–59. doi: 10.1002/ijc.33943 [DOI] [PubMed] [Google Scholar]
- 143. Schuhn A, Tobar TW, Gahlawat AW, Hauke J, Baumann L, Okun JG, et al. Potential of blood-based biomarker approaches in endometrium and breast cancer: A case-control comparison study. Arch Gynecol Obstet (2022) 306(5):1623–32. doi: 10.1007/s00404-022-06482-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Troisi J, Raffone A, Travaglino A, Belli G, Belli C, Anand S, et al. Development and validation of a serum metabolomic signature for endometrial cancer screening in postmenopausal women. JAMA Network Open (2020) 3(9):e2018327. doi: 10.1001/jamanetworkopen.2020.18327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Shao X, Wang K, Liu X, Gu C, Zhang P, Xie J, et al. Screening and verifying endometrial carcinoma diagnostic biomarkers based on a urine metabolomic profiling study using uplc-Q-Tof/Ms. Clinica chimica acta; Int J Clin Chem (2016) 463:200–6. doi: 10.1016/j.cca.2016.10.027 [DOI] [PubMed] [Google Scholar]
- 146. Cheng SC, Chen K, Chiu CY, Lu KY, Lu HY, Chiang MH, et al. Metabolomic biomarkers in cervicovaginal fluid for detecting endometrial cancer through nuclear magnetic resonance spectroscopy. Metabolomics Off J Metabolomic Soc (2019) 15(11):146. doi: 10.1007/s11306-019-1609-z [DOI] [PubMed] [Google Scholar]
- 147. Jove M, Gatius S, Yeramian A, Portero-Otin M, Eritja N, Santacana M, et al. Metabotyping human endometrioid endometrial adenocarcinoma reveals an implication of endocannabinoid metabolism. Oncotarget (2016) 7(32):52364–74. doi: 10.18632/oncotarget.10564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Altadill T, Dowdy TM, Gill K, Reques A, Menon SS, Moiola CP, et al. Metabolomic and lipidomic profiling identifies the role of the rna editing pathway in endometrial carcinogenesis. Sci Rep (2017) 7(1):8803. doi: 10.1038/s41598-017-09169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Trousil S, Lee P, Pinato DJ, Ellis JK, Dina R, Aboagye EO, et al. Alterations of choline phospholipid metabolism in endometrial cancer are caused by choline kinase alpha overexpression and a hyperactivated deacylation pathway. Cancer Res (2014) 74(23):6867–77. doi: 10.1158/0008-5472.CAN-13-2409 [DOI] [PubMed] [Google Scholar]
- 150. Cummings M, Massey KA, Mappa G, Wilkinson N, Hutson R, Munot S, et al. Integrated eicosanoid lipidomics and gene expression reveal decreased prostaglandin catabolism and increased 5-lipoxygenase expression in aggressive subtypes of endometrial cancer. J Pathol (2019) 247(1):21–34. doi: 10.1002/path.5160 [DOI] [PubMed] [Google Scholar]
- 151. Skorupa A, Ponski M, Ciszek M, Cichon B, Klimek M, Witek A, et al. Grading of endometrial cancer using 1H hr-mas nmr-based metabolomics. Sci Rep (2021) 11(1):18160. doi: 10.1038/s41598-021-97505-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Arda Düz S, Mumcu A, Doğan B, Yılmaz E, İnci Çoşkun E, Sarıdogan E, et al. Metabolomic analysis of endometrial cancer by high-resolution magic angle spinning nmr spectroscopy. Arch Gynecol Obstet (2022) 306(6):2155–66. doi: 10.1007/s00404-022-06587-0 [DOI] [PubMed] [Google Scholar]
- 153. Gatius S, Jove M, Megino-Luque C, Alberti-Valls M, Yeramian A, Bonifaci N, et al. Metabolomic analysis points to bioactive lipid species and acireductone dioxygenase 1 (Adi1) as potential therapeutic targets in poor prognosis endometrial cancer. Cancers (Basel) (2022) 14(12). doi: 10.3390/cancers14122842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Shafiee MN, Ortori CA, Barrett DA, Mongan NP, Abu J, Atiomo W. Lipidomic biomarkers in polycystic ovary syndrome and endometrial cancer. Int J Mol Sci (2020) 21(13). doi: 10.3390/ijms21134753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rizner TL. Noninvasive biomarkers of endometriosis: Myth or reality? Expert Rev Mol Diagn (2014) 14(3):365–85. doi: 10.1586/14737159.2014.899905 [DOI] [PubMed] [Google Scholar]
- 156. Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics Off J Metabolomic Soc (2013) 9(2):280–99. doi: 10.1007/s11306-012-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tangen IL, Fasmer KE, Konings G, Jochem A, Delvoux B, Xanthoulea S, et al. Blood steroids are associated with prognosis and fat distribution in endometrial cancer. Gynecol Oncol (2019) 152:46–52. doi: 10.1016/j.ygyno.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 158. Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J (2007) 21(4):968–75. doi: 10.1096/fj.06-7464rev [DOI] [PubMed] [Google Scholar]
- 159. Wyciszkiewicz A, Kalinowska-Lyszczarz A, Nowakowski B, Kazmierczak K, Osztynowicz K, Michalak S. Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Sci Rep (2019) 9(1):9817. doi: 10.1038/s41598-019-46221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Raffone A, Troisi J, Boccia D, Travaglino A, Capuano G, Insabato L, et al. Metabolomics in endometrial cancer diagnosis: a systematic review. Acta Obstet Gynecol Scand (2020) 99(9):1135–46. doi: 10.1111/aogs.13847 [DOI] [PubMed] [Google Scholar]
- 161. Karkia R, Wali S, Payne A, Karteris E, Chatterjee J. Diagnostic accuracy of liquid biomarkers for the non-invasive diagnosis of endometrial cancer: a systematic review and meta-analysis. Cancers (Basel) (2022) 14(19). doi: 10.3390/cancers14194666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Muinelo-Romay L, Casas-Arozamena C, Abal M. Liquid biopsy in endometrial cancer: New opportunities for personalized oncology. Int J Mol Sci (2018) 19(8). doi: 10.3390/ijms19082311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pineiro-Perez R, Abal M, Muinelo-Romay L. Liquid biopsy for monitoring ec patients: Towards personalized treatment. Cancers (Basel) (2022) 14(6). doi: 10.3390/cancers14061405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Rizner TL, Adamski J. Paramount importance of sample quality in pre-clinical and clinical research-need for standard operating procedures (Sops). J Steroid Biochem Mol Biol (2019) 186:1–3. doi: 10.1016/j.jsbmb.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 165. Njoku K, Sutton CJ, Whetton AD, Crosbie EJ. Metabolomic biomarkers for detection, prognosis and identifying recurrence in endometrial cancer. Metabolites (2020) 10(8). doi: 10.3390/metabo10080314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.