Abstract
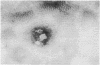
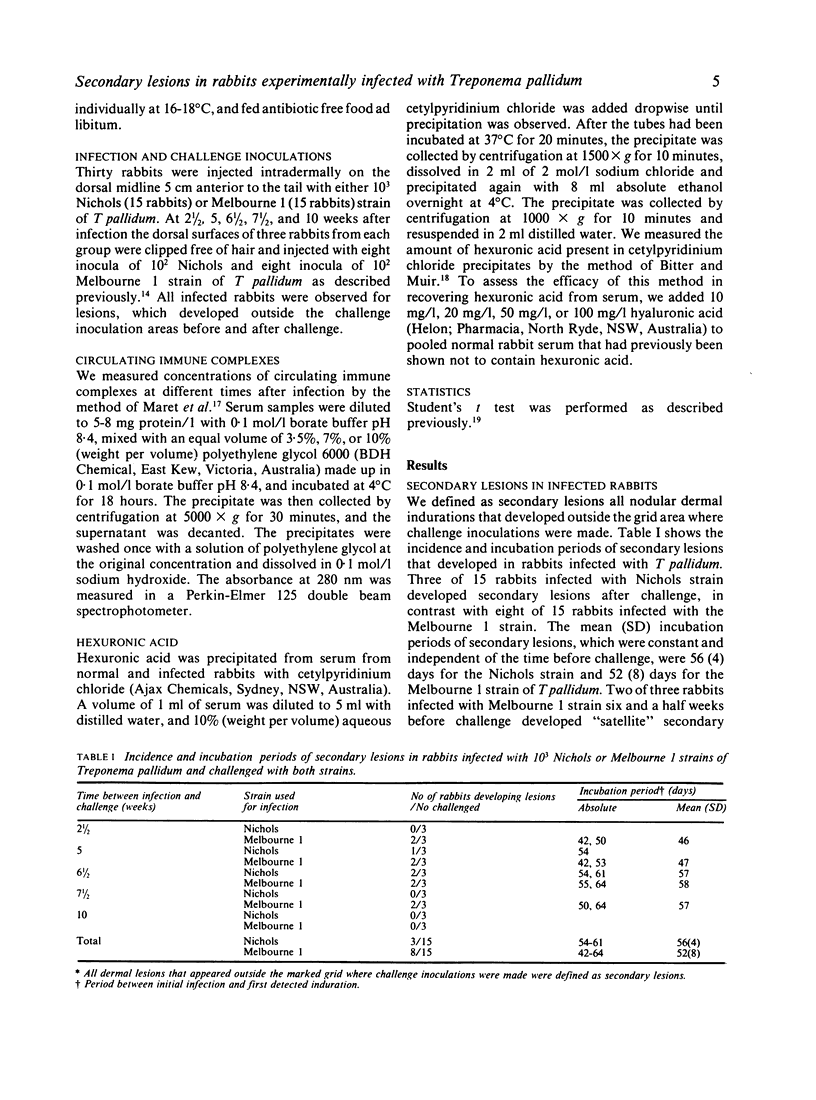
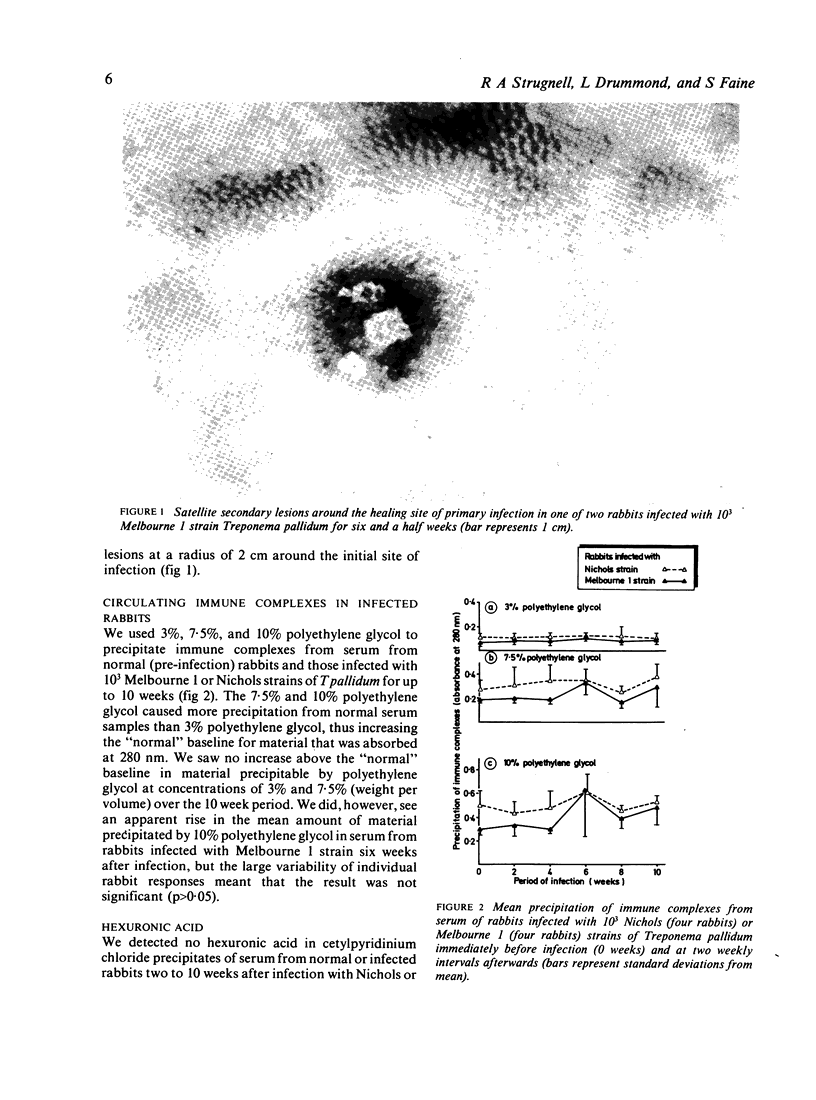
Thirty rabbits infected with 10(3) of either Nichols or Melbourne 1 strains of Treponema pallidum were observed for the development of secondary lesions, which appeared outside areas inoculated with viable treponemes. More rabbits infected with Melbourne 1 strain (eight of 15 rabbits) than were infected with the Nichols reference strain (three of 15 rabbits) developed secondary lesions. The mean (SD) incubation periods of secondary lesions were 52 (8) days for rabbits infected with Melbourne 1 and 56 (4) days for rabbits infected with Nichols strain. These mean incubation periods did not correlate with appreciably increased concentrations of immune complexes or glycosaminoglycans in the serum of infected rabbits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Reappraisal of lymphocyte responsiveness to concanavalin A during experimental syphilis: evidence that glycosaminoglycans in the sera and tissues interfere ith active binding sites on the lectin and not with the lymphocytes. Infect Immun. 1982 Feb;35(2):552–559. doi: 10.1128/iai.35.2.552-559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Tung K. S., Musher D. M. Detection of circulating immune complexes in the sera of rabbits with experimental syphilis: possible role in immunoregulation. Infect Immun. 1980 Aug;29(2):575–582. doi: 10.1128/iai.29.2.575-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey R. F., Johnson R. C., Fitzgerald T. J. Suppression of lymphocyte response to concanavalin A by mucopolysaccharide material from Treponema pallidum-infected rabbits. Infect Immun. 1979 Oct;26(1):64–69. doi: 10.1128/iai.26.1.64-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhorade M. S., Carag H. B., Lee H. J., Potter E. V., Dunea G. Nephropathy of secondary syphilis. A clinical and pathological spectrum. JAMA. 1971 May 17;216(7):1159–1166. [PubMed] [Google Scholar]
- Braunstein G. D., Lewis E. J., Galvanek E. G., Hamilton A., Bell W. R. The nephrotic syndrome associated with secondary syphilis. An immune deposit disease. Am J Med. 1970 May;48(5):643–648. doi: 10.1016/0002-9343(70)90016-1. [DOI] [PubMed] [Google Scholar]
- Graves S. R., Sandok P. L., Jenkin H. M., Johnson R. C. Retention of motility and virulence of Treponema pallidum (Nichols strain) in vitro. Infect Immun. 1975 Nov;12(5):1116–1120. doi: 10.1128/iai.12.5.1116-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S., Alden J. Limited protection of rabbits against infection with Treponema pallidum by immune rabbit sera. Br J Vener Dis. 1979 Dec;55(6):399–403. doi: 10.1136/sti.55.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Maret S. M., Rauchbach A. S., Folds J. D. Immune complexes in experimental T. pallidum infection in rabbits. J Clin Lab Immunol. 1982 May;8(1):47–50. [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S., Powell H. C. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982 Apr;46(4):355–364. [PubMed] [Google Scholar]
- Sell S., Norris S. J. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- Strugnell R. A., Faine S., Graves S. Response of syphilitic rabbits to reinfection with homologous and heterologous Treponema pallidum strains. Infect Immun. 1984 Sep;45(3):561–565. doi: 10.1128/iai.45.3.561-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugnell R. A., Handley C. J., Drummond L., Faine S., Lowther D. A., Graves S. R. Polyanions in syphilis: evidence that glycoproteins and macromolecules resembling glycosaminoglycans are synthesised by host tissues in response to infection with Treponema pallidum. Br J Vener Dis. 1984 Apr;60(2):75–82. doi: 10.1136/sti.60.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]