Abstract
While high-frequency transcranial magnetic stimulation (HF-rTMS) is now included in the armamentarium to treat chronic neuropathic pain (NP), direct-current anodal stimulation (a-tDCS) to the same cortical targets may represent a valuable alternative in terms of feasibility and cost. Here we performed a head-to-head, randomized, single-blinded, cross-over comparison of HF-rTMS versus a-tDCS over the motor cortex in 56 patients with drug-resistant NP, who received 5 daily sessions of each procedure, with a washout of at least 4 weeks. Daily scores of pain, sleep, and fatigue were obtained during 5 consecutive weeks, and functional magnetic resonance imaging (fMRI) to a motor task was performed in a subgroup of 31 patients. The percentage of responders, defined by a reduction in pain scores of > 2 SDs from pre-stimulus levels, was similar to both techniques (42.0% vs. 42.3%), while the magnitude of “best pain relief” was significantly skewed towards rTMS. Mean pain ratings in responders decreased by 32.6% (rTMS) and 29.6% (tDCS), with half of them being sensitive to only one technique. Movement-related fMRI showed significant activations in motor and premotor areas, which did not change after 5 days of stimulation, and did not discriminate responders from non-responders. Both HF-rTMS and a-tDCS showed efficacy at 1 month in drug-resistant NP, with magnitude of relief slightly favoring rTMS. Since a significant proportion of patients responded to one procedure only, both modalities should be tested before declaring a patient as unresponsive.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01303-x.
Keywords: rTMS, tDCS, Neuropathic pain, fMRI, Non-invasive stimulation
Introduction
Shortly after the description of the neurosurgical procedure of epidural motor cortex stimulation for neuropathic pain (NP) control [1], repetitive transcranial magnetic stimulation (rTMS) was proposed as a non-invasive method to mimic epidural stimulation and predict its subsequent effectiveness. The potential value of rTMS as a pain therapy in its own right was soon recognized, and the use of rTMS as a full-fledged pain-relieving procedure has received considerable support in the last 10 years [2]. Although methodological drawbacks limited the quality of evidence of early studies due to low patients’ samples, absent blinding, lack of randomization and follow-up, etc. [3, 4], a number of well-conducted studies using single or double-blinded methodology, randomization, and inclusion of more than 20 patients in active groups have been recently reported in chronic NP of various origins, with positive results when using stimulus frequencies of at least 10 Hz [5–11]. Accordingly, recent reviews concluded to a significant superiority over placebo of high-frequency (HF) motor cortex rTMS in chronic neuropathic pain [12–15], and clinical recommendations have now included HF-rTMS of the motor cortex as a “third line” therapeutic option, at the same level as spinal cord stimulation [16]. In the same line, a recent report of the US Department of Veterans Affairs which analyzed rTMS data under a “best-evidence approach” (multisite studies, control of potential confounding factors) concluded that rTMS may reduce symptoms in NP and could be a treatment option for patients who have exhausted standard available options [12].
Transcranial direct current (galvanic) stimulation (tDCS), i.e., the non-invasive transcranial flow of electric charge that does not change direction, modulates the neuronal resting membrane state without eliciting action potentials, and has been empirically applied for medical purposes since the Roman Antiquity (Scribonious Largus ~70 AC, https://prabook.com/web/scribonius.largus/3727651). Modern research showed that surface anodal polarization of the cortex increases spontaneous unit discharges in rodents and felines [17, 18] and enhances human motor cortical excitability with magnitude and duration comparable to those observed with rTMS [19, 20]. Anodal tDCS appears therefore as a promising tool, able to emulate the analgesic effects of conventional motor cortex stimulation, with practical advantages over rTMS including its lower cost, the paucity of safety issues, and the availability of home-based long-lasting protocols. However, because of the limited quality of most published reports, the level of evidence regarding tDCS effects in chronic neuropathic pain remains very low and highly conflicting, despite a large number of studies published [2, 21, 22].
One single study comparing the short-term effect of 3 sessions of anodal tDCS versus HF-rTMS in lumbosacral radiculopathy concluded to the superiority of rTMS [23], while a very recent report in 12 patients with brachial plexus injuries found similar results from both techniques [24]. Head-to-head studies directly comparing the efficacy of HF-rTMS and a-tDCS for chronic, drug-resistant NP in large patients’ series are therefore warranted. In the present study, we report the results of a full head-to-head, randomized, prospective, single-blinded, cross-over study comparing HF-rTMS versus anodal tDCS over the motor cortex in a large series of patients with drug-resistant NP of different etiologies. To make the results directly comparable and maximize their clinical significance, each patient could benefit consecutively from the two techniques, separated by an adequate wash out period, and daily quotations of pain and pain-related items were obtained from written diaries during the full follow-up. In addition, functional magnetic resonance imaging (fMRI) during a motor task involving the painful area was obtained before and after the procedure in a subset of patients, to investigate the possible relations between clinical efficacy of the neurostimulation techniques and changes in the activity level of task-related motor networks.
Patients and Methods
Study Population
This bi-centric protocol was conducted in the Neurological Hospital of the Hospices Civils de Lyon, France, and in the Pain Center of the Grenoble Alpes University Hospital, France, from February 2013 to December 2020. The study was approved in both centers by the Institutional Review Boards Sud-Est IV Lyon (N° 10,619) and Sud-Est V Grenoble (N° 6705), France, and was registered with clinicaltrials.gov (NCT02120326, NCT02854332). All patients approved and signed an informed consent prior to entering the protocol. Equipment, stimulation protocol, and pain evaluation methods were identical in both sites, with the exception of the diameter of tDCS electrodes ( 6.2 cm soaked sponges in Grenoble, 1 cm Gel electrodes in Lyon, both by the same manufacturer Neuroelectrics®, both validated in terms of safety and without difference of effectiveness) ([25] and see results “Primary Outcome”).
Sixty-eight patients aged 18 to 80 years, suffering from lateralized pharmaco-resistant chronic neuropathic pain for more than 1 year, without any change in medical treatment since at least 1 month, were included in this study. Mean pain duration was 5 ± 3.8 years. Diagnosis of neuropathic pain followed IASP NeuPSIG guidelines [26], and a level of probable to definite neuropathic pain was required for inclusion [27]. The patients were classified into five groups according to the origin of pain: central post-stroke pain, central cancer and vascular pain, spinal cord injury, facial pain, and brachial plexus injury (Table 1). They were not included if they had a history of epilepsy, drug-addiction, migraine, intracranial ferromagnetic material, or implanted stimulator.
Table 1.
Baseline characteristics of patients according to the allocated group
| Total (n = 56) | rTMS then tDCS (n = 26) | tDCS then rTMS (n = 30) | P-value | |
|---|---|---|---|---|
| Age (year), mean (SD) | 58.6 (13.2) | 60.2 (13.7) | 57.3 (12.9) | 0.407 |
| Female, n (%) | 27 (48%) | 12 (46%) | 15 (50%) | 0.774 |
| Washout period (week), median (IQR) | 10.0 (9.0, 12.1) | 10.0 (9.0, 12.0) | 10.7 (9.0, 12.1) | 0.904 |
| Disease history | ||||
| Pain syndrome duration (year), median (IQR) | 5 (3, 8) | 5 (3, 8) | 5 (3, 7) | 0.391 |
| Pain origin, n (%): | 0.569 | |||
| Brachial plexus injury | 4 (7%) | 2 (8%) | 2 (7%) | |
| Spinal cord injury | 6 (11%) | 4 (15%) | 2 (7%) | |
| Central post-stroke pain | 21 (37.5%) | 7 (27%) | 14 (46%) | |
| Brain tumor, vascular and other pain | 4 (7%) | 2 (8%) | 2 (7%) | |
| Orofacial pain | 21 (37.5%) | 11 (42%) | 10 (33%) | |
| Summary of pharmacological treatment | ||||
| Number of drugs/patient, median (IQR) | 2 (2, 3) | 2 (2, 3) | 2 (2, 2) | 0.340 |
| Drug class, n (%): | ||||
| Antiepileptics | 39 (70%) | 18 (69%) | 21 (70%) | 0.950 |
| Antidepressants | 36 (64%) | 17 (65%) | 19 (63%) | 0.873 |
| Strong opioids | 6 (11%) | 2 (8%) | 4 (13%) | 0.496 |
| Weak opioids | 22 (39%) | 11 (42%) | 11 (37%) | 0.666 |
| Non-opioid analgesics | 15 (27%) | 9 (35%) | 6 (20%) | 0.218 |
| Clinical scorea during the week pre-stimulation | ||||
| Pain score, mean (SD) | 6.4 (2.0) | 6.0 (2.1) | 6.7 (2.0) | 0.180 |
| Sleep score, mean (SD) | 4.7 (2.5) | 4.8 (2.6) | 4.7 (2.6) | 0.876 |
| Fatigue score, mean (SD) | 5.5 (2.3) | 5.0 (2.4) | 5.9 (2.2) | 0.170 |
SD standard deviation, IQR interquartile range
aNumerical rating scale (NRS). The NRS score ranges from 0 to 10, with 0 indicating no pain/sleep disorder/fatigue and 10 the worst imaginable pain/sleep disorder/fatigue
All patients (except one who had discontinued all drugs due to inefficacy before entering the study) were taking one or more analgesic treatments (anti-epileptic drugs, antidepressants, and/or painkillers levels 1, 2, or 3) (Table 1). Patients were asked to maintain their ongoing analgesic treatment unchanged for the duration of the protocol, but were allowed to take medication for breakthrough pain if needed.
Study Design
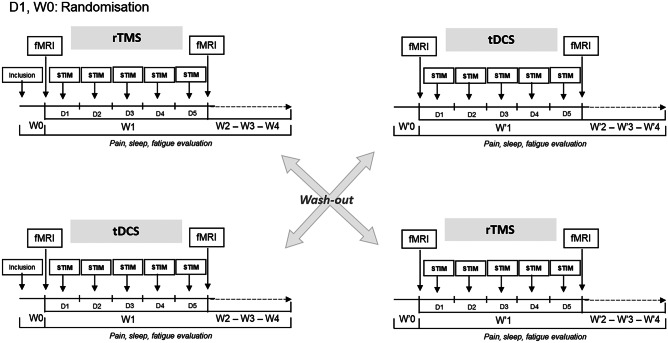
Eligible patients were randomized 1:1 to one of the two treatment sequences: rTMS followed by tDCS (group I) or tDCS followed by rTMS (group II) through simple randomization (random numbers generated by computer). Each patient benefited from two stimulation cycles of 1 week each (rTMS and tDCS) at least 4 weeks apart, each cycle comprising 5 daily sessions of stimulation (Fig. 1). Patients filled a diary evaluating pain intensity using a numerical rating scale (NRS) ranging from 0 (no pain) to 10 (the worst pain possible) every day during 5 weeks: from 1 week (W0) before stimulation week (W1) to 3 weeks after the end of stimulation (W2, W3, and W4). In addition, patients were also asked to provide daily ratings of sleep quality, fatigue, and “rescue” medication [28]. A minimal wash-out period of 4 weeks preceded the second phase, with identical design but different stimulus modality. At the end of the second phase, patients sent their notebook to a nurse different from the investigators and continued medical follow-up with their pain physician. Investigators did not have access to the patients’ ratings before the end of each trial.
Fig. 1.
Study design. Abbreviations: rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; fMRI, functional magnetic resonance imaging; D, day; W0, baseline; W1, stimulation sessions; W2-W3-W4, follow-up period
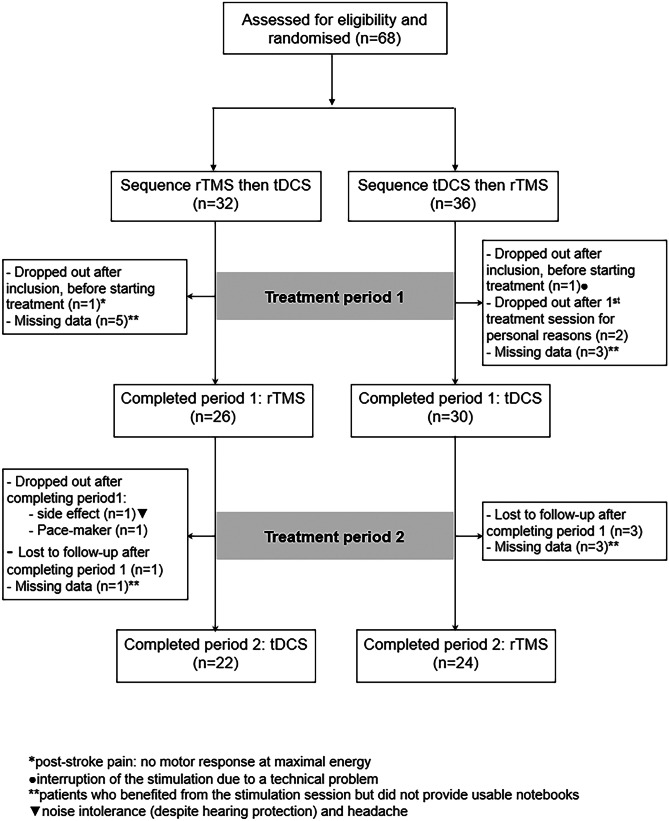
Chronic drug intake was maintained unchanged during the whole study period, with the exception of punctual “rescue” drugs for breakthrough pain, if needed, which must be reported in the patients’ logbook. From 68 patients initially entering the study, 56 completed all 5 weeks of data for at least one mode of stimulation, and 46 completed all follow-up from both techniques (Fig. 2).
Fig. 2.
Participant flow diagram. Abbreviations: rTMS, high-frequency repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation. *One patient requested to stop the study after rTMS because of tension headache. **One patient was withdrawn from the study due to the implantation of a pace-maker
The protocol included two identical magnetic resonance imaging (MRI) sessions, respectively, preceding and immediately following the first week of stimulation, whatever its type. The sessions were performed on 3 T Philips Achieva-TX scanners with a 32-channel head coil at both Lyon and Grenoble sites. Each session included a first morphological 3D T1-weighted sequence eventually used for neuronavigation-based treatment, and then a set of four BOLD weighted fMRI runs. The fMRI runs included each a different movement task: a right hand movement of the Vth finger, a left hand movement of the Vth finger, a right zygomatic movement of the face, and a left zygomatic movement (half a smile). Each fMRI run consisted in a block design with 3 epochs of 30 s alternating with rest epochs of 30 s, for a total duration of 3 min and 30 s per run.
The complete procedure is schematized in Fig. 1.
Stimulation Parameters
Stimulation was carried out in the University Hospital Pain Center (CETD) of the Neurological Hospital of Lyon and in the Pain Centre of the Grenoble Alpes University Hospital. rTMS and tDCS were performed using the same stimulators in both experimental centers.
rTMS (Mag-Pro X100, MagVenture©) induced biphasic magnetic pulses via an eight-shaped coil (cool-B65 butterfly shape coil MagVenture©). The motor strip was localized in each patient using T1-3D MRI, and the stimulating coil was positioned perpendicular to the central sulcus, with postero-anterior orientation. The optimal position of the coil was determined using the MRI-Neuronavigation system with Visor© software (ANT©) and collecting EMG responses of the abductor digiti minimi. Motor threshold at rest was defined before each stimulation session as the lowest intensity that produced five responses with peak-to-peak amplitude of at least 50 µV in ten consecutive trials [29]. Each 10 Hz-rTMS session comprised 32 consecutive trains of 50 pulses, delivered at 90% of motor threshold, separated by inter-trains intervals of 25 s (i.e., a total of 1600 pulses during a 17-min session).
tDCS (DC stimulator NIC-Starstim®, Neuroelectrics®) was delivered in Grenoble via sponge electrodes soaked in salty solution and in Lyon via NG electrodes placed on prefixed positions on a neoprene cap with a conductive gel between the electrodes. Skin–electrode impedance levels below 5 kOhms were required to initiate stimulation. For each tDCS session, a 2 mA anodal stimulation was applied during 20 min over the motor cortex contralateral to pain, over C3/C4 positions of the international 10–20 system. The cathode was placed over the frontal-polar region, ipsilateral to pain, over Fp1/Fp2.
Outcome Variables and Statistical Analyses
From the week preceding the stimulation (Day 7) to the end of the four week following the stimulation period (Day 28), patients used a diary at home to record the following information: pain intensity, quality of sleep, and fatigue, using a 0–10 numerical rating scale (total of 5 weeks). Daily NRS ratings were averaged week per week.
The primary outcome was the analgesic effect of each stimulation modality compared to its own baseline (week before stimulation: W0 on Fig. 1). Secondary outcomes were the quality of sleep and fatigue for each stimulation modality compared to its own baseline (same analyses as for pain intensity).
To allow inter-subject comparisons, daily NRS was normalized using Z-scores [30, 31]. Thus, each daily pain rating was Z-transformed using the formula (Xi–Xbaseline) / SDbaseline, where Xi is the actual raw daily rating in day ‘‘i”, Xbaseline is the average rating from the pre-stimulation week in the same individual, and SDbaseline is the associated standard deviation of NRS values during this pre-stimulation week. Patients were considered “responders” if their pain ratings decreased by at least 2 standard deviations (SD) from baseline, during at least 1 week.
Continuous data are expressed as mean ± standard deviation (SD) or median (25th–75th centiles), and categorical data are expressed as numbers and percentages. Comparisons of baseline characteristics between allocated groups (rTMS/tDCS versus tDCS/rTMS) and responders versus non-responders to each modality were conducted by using the Chi-square or Fisher’s exact tests, Student’s t-test, or Wilcoxon-Mann–Whitney test. Homogeneity of the two groups regarding pain ratings was tested by comparing with a two-tailed paired t-test their respective NRS during the baseline week, before initiating the stimulation periods. NRS during the week before the first and second stimulation cycles (regardless of the stimulation type) was also compared with two-tailed paired t-test to check for possible carry-over effects. In the patients who completed the entire study (n = 46), normalized pain scores were compared using a 2-way repeated-measures ANOVA (time × stimulation mode). A p-value < 0.05 was considered significant after Greenhouse–Geisser correction when needed. Correlation between the magnitude of the analgesic effect from rTMS and tDCS was studied using Pearson-product-moment coefficients.
Data were analyzed using GraphPad Prism. A two-sided p value < 0.05, after correction when needed, was considered statistically significant.
fMRI Analysis
Using SPM12 software (The Welcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm/), image pre-processing was performed in each subject’s referential and included motion correction, slice timing, and registration to the anatomical image of the pre-treatment session. Doing so, both pre-treatment and post-treatment functional images are in the same referential. Individual statistical analysis was estimated using a linear generalized model to produce the following contrasts: (i) the individual contrasts for each task at each time point and (ii) the differential contrast for each task after vs. before the treatment. The anatomical image of the pre-treatment session of each subject was segmented into 6 classes of tissue (gray matter, white matter, cerebrospinal fluid, large vessels, meninges, and scalp) using the tissue probability maps provided by the software. In order to transform the images in a common referential for group analysis, we computed the deformation field to be applied to each individual to match a symmetrical template, provided by the CAT12 software (neuro.uni-jena.de/cat12-html/cat_versions.html). The DARTEL method was used to achieve a clear difference between primary motor and primary sensory cortices [32]. The deformation field computed for each subject was applied to individual contrast images. Since the pain lateralization is patient-dependent, so was the stimulated hemisphere. In order to be able to pool the stimulated hemispheres and to compare them to the non-stimulated hemispheres, the individual contrast images were left–right flipped when necessary so as to obtain the stimulated hemisphere on the left side of the image and the unstimulated hemisphere on the right side of the image.
For inference at the group level, several statistical tests were performed. First, a comparison between the post- and pre-stimulation contrasts using paired t-test for the two types of treatment, each type of treatment and between type of treatment, to investigate the general effect of stimulation, the effect of each type of stimulation, and the differential effect between both types of stimulation. Second, to check whether the effect of stimulation differed in responders vs. non-responders, a comparison between the post-stimulation and the pre-stimulation was tested between contrasts according to the responding status, using two sample t-test. Third, in order to check whether movements performed in the painful and the non-painful sides generated differential brain activation patterns, a comparison between movements in both sides previous to any treatment was tested using paired t-test. Finally, in order to check whether the pattern corresponding to movement in the painful side could predict the response to treatment, the contrast corresponding to movement in the painful side before treatment was compared between subgroups of responders and non-responders. Differences were considered significant when p < 0.05 after correction for multiple comparisons using family-wise error at voxel level and the probability for the extent of activation cluster to be find by chance was below 0.05.
Results
Flowchart of the Study
Figure 2 depicts the flowchart of the study participants. Sixty-eight patients were initially recruited and randomly assigned to study groups: “rTMS then tDCS” (group I) or “tDCS then rTMS” (group II). Fifty-six patients completed the first phase of the protocol (26 group I and 30 group II) and 46 patients completed both phases of study (22 group I and 24 group II). Functional imaging (fMRI) study comparing motor-evoked activations pre- and post-neurostimulation was performed in 31 of the 56 patients who completed the protocol. The MRI study could not be completed in the others for patient-dependent reasons (unavailability for the 2nd session) or organizational difficulties in connection with time-slot availability in the radiology department. Despite such difficulties, the sample presented here is to our knowledge the largest group of patients described so far with motor-related fMRI performed before and immediately after a series of motor cortical stimulation for neuropathic pain.
Baseline Demographics and Clinical Characteristics
The demographics and baseline characteristics of the 56 patients who completed the 5-week follow-up assessment of the first phase are shown in Table 1. The mean age of the patients was 58.6 ± 13.2 years. No significant differences at baseline were found between groups I and II (starting with rTMS or tDCS) according to age, sex, and origin of pain or clinical scores during the week pre-stimulation.
Primary Outcome
Patients were defined as “responders” if pain scores decreased by at least 2 SDs relative to baseline values (W0) during 1 week or more (see Methods), and these criteria were met by 21/50 patients (42.0%) for rTMS and 22/52 (42.3%) for tDCS, the difference being non-significant. The number of responders was also similar in the 46 patients receiving both techniques (two-sided Fisher’s exact test: p = 0.76). Of notice, almost half of these patients (21/46) responded to one modality exclusively (12 responded only to rTMS and 9 only to tDCS). We did not find any significant difference between responders and non-responders regarding their characteristics at baseline, including age, sex, origin or intensity of pain, quality of sleep, and fatigue scores (Table 2).
Table 2.
Baseline characteristics of responders versus non-responders
| rTMS | tDCS | |||||
|---|---|---|---|---|---|---|
| Responders (n = 21) | Non-responders (n = 29) | P-value | Responders (n = 22) | Non-responders (n = 30) | P-value | |
| Age (year), mean (SD) | 61.1 (12.1) | 57.0 (14.9) | 0.299 | 59.5 (12.6) | 58.6 (12.6) | 0.817 |
| Female, n (%) | 9 (43%) | 14 (48%) | 0.704 | 12 (55%) | 14 (47%) | 0.575 |
| Disease history | ||||||
| Pain syndrome duration (year), median (IQR) | 5 (3, 8) | 5 (3, 7) | 0.945 | 5 (3, 8) | 5 (3, 7) | 0.886 |
| Pain origin, n (%): | 0.513 | 0.839 | ||||
| Brachial plexus injury | 2 (10%) | 2 (7%) | 1 (4.5%) | 3 (10%) | ||
| Spinal cord injury | 3 (14%) | 2 (7%) | 2 (9%) | 3 (10%) | ||
| Central post-stroke pain | 6 (29%) | 11 (38%) | 9 (41%) | 12 (40%) | ||
| Central cancer, vascular and other pain | 3 (14%) | 1 (3%) | 1 (4.5%) | 3 (10%) | ||
| Facial pain | 7 (33%) | 13 (45%) | 9 (41%) | 9 (30%) | ||
| Summary of pharmacological treatment | ||||||
| Number of drugs/patient, median (IQR) | 2 (1, 3) | 2 (2, 3) | 0.651 | 2 (1, 2) | 2 (2, 3) | 0.136 |
| Drug class, n (%): | ||||||
| Antiepileptics | 14 (67%) | 21 (72%) | 0.662 | 13 (59%) | 26 (87%) | 0.023 |
| Antidepressants | 14 (67%) | 17 (59%) | 0.563 | 15 (68%) | 19 (63%) | 0.717 |
| Strong opioids | 3 (14%) | 2 (7%) | 0.390 | 2 (9%) | 3 (10%) | 0.913 |
| Weak opioids | 5 (24%) | 14 (48%) | 0.079 | 7 (32%) | 12 (40%) | 0.545 |
| Non-opioid analgesics | 6 (29%) | 8 (28%) | 0.939 | 4 (18%) | 8 (27%) | 0.473 |
| Clinical scorea during the week pre-stimulation | ||||||
| Pain score, mean (SD) | 6.7 (1.8) | 6.1 (1.9) | 0.297 | 6.7 (2.2) | 5.8 (2.0) | 0.144 |
| Sleep score, mean (SD) | 4.7 (2.6) | 4.8 (2.3) | 0.916 | 4.2 (2.5) | 4.6 (2.7) | 0.602 |
| Fatigue score, mean (SD) | 5.5 (2.2) | 5.3 (2.5) | 0.825 | 5.7 (2.1) | 5.3 (2.6) | 0.509 |
SD standard deviation, IQR interquartile range
aNumerical rating scale (NRS). The NRS score ranges from 0 to 10, with 0 indicating no pain/sleep disorder/fatigue and 10 the worst imaginable pain/sleep disorder/fatigue
In the 46 patients who received both stimulation modalities, a 2-way, rm-ANOVA (time × stimulation mode) on Z-normalized pain changes between W0 and W4 showed a significant effect of time (F(4,360) = 10.98; p < 10−3) but no effect of stimulation mode (F(1,360) = 0.46; p = 0.50) and no interaction (F(4,360) = 51.10; p = 0.35). Figure 3 illustrates the evolution of Z-normalized pain scores during the 4-week post-stimulation according to response status.
Fig. 3.
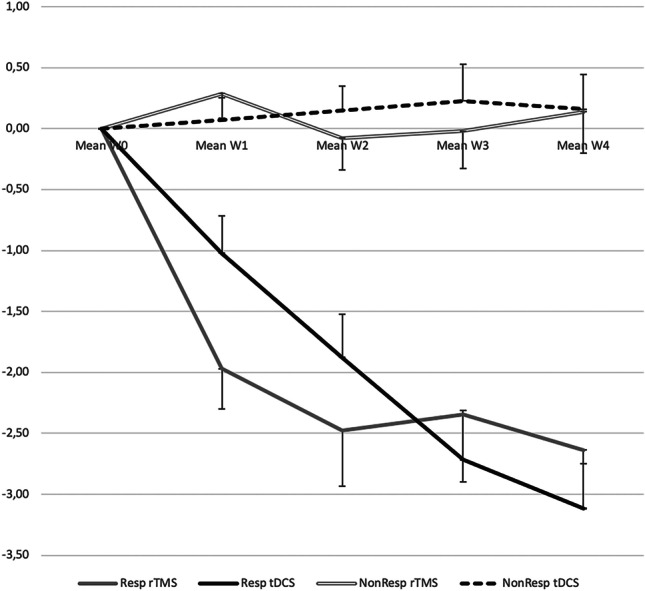
Evolution of Z-normalized pain scores during the 4 weeks post-stimulation. Evolution of pain scores in responders and non-responders during the 4 weeks (W1 to W4) post-stimulation. Pain reports were normalized as z-scores relative to values during the baseline pre-stimulation week (W0; see Methods), and patients were considered as “responders” if their scores decreased by more than 2 SD relative to baseline. The groups of responders and not-responders were clearly differentiated since the first week post-stimulation, with no overlap. Abbreviations: rTMS, high-frequency repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation. Resp rTMS, responders to rTMS; Resp tDCS, responders to tDCS; NonResp rTMS, non-responders to rTMS; NonResp tDCS, non-responders to tDCS
The percentage of pain decrease at the “best week” (the week with most prominent changes) for each modality was significantly correlated (r = 0.34; p = 0.005). However, as illustrated in Fig. 4, the slope of the regression line (β = 0.34, 95% CI = [0.10–0.54]) was significantly biased in favor of rTMS relative to the theoretical equivalence slope (β = 1). No significant difference was found in the level of pain decrease according to the type of electrode used for tDCS in the two experimental sites.
Fig. 4.
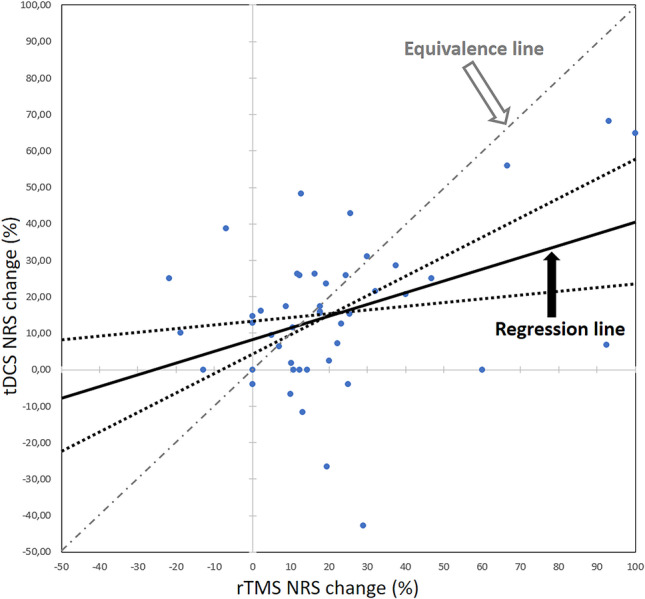
Correlation between NRS percentage changes after rTMS and tDCS. Correlation between maximal percentage changes of numerical pain reports (NRS) after rTMS and tDCS. Although NRS changes to both techniques were correlated, the slope of the regression line was significantly skewed towards rTMS, with confidence limits (dotted lines) which did not reach the theoretical equivalence line of β = 1. Abbreviations: rTMS, high-frequency repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; NRS, numerical rating scale
Pain scores during the baseline preceding the second stimulation session were decreased relative to those preceding the first one (6.4 ± 1.9 vs. 5.9 ± 2.3; two-tailed paired t-test: p = 0.03) reflecting a possible carry-over effect independent of the stimulus mode. However, NRS baseline values were not significantly different when preceding rTMS or tDCS (two-tailed paired t-test: p = 0.44).
Secondary Outcomes
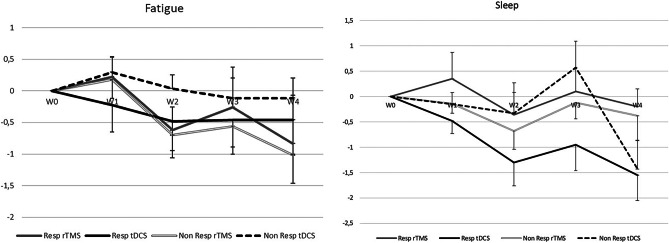
Quality of Sleep and Fatigue
Self-assessment of sleep and fatigue changed very little during the 4-week post-stimulation, and no significant differences between techniques were observed (Fig. 5). A 2-way, rmANOVA (time × stimulation mode) on Z-normalized fatigue changes between W0 and W4 showed a significant and favorable effect of time (F(4,336) = 3.13; p = 0.01) but no effect of stimulation mode (F(1,336) = 0.00; p = 0.97) and no interaction (F(4,336) = 0.71; p = 0.58. Comparable results were obtained for sleep changes: 2-way, rmANOVA (time × stimulation mode) on Z-normalized changes between W0 and W4 showed a significant effect of time (F(4,320) = 6.75; p < 10−3) but no effect of stimulation mode (F(1,320) = 0.11; p = 0.74) and no interaction (F(4,320) = 0.66; p = 0.62).
Fig. 5.
Weekly evolution of fatigue and sleep scores in responders and non-responders during the 4 weeks (W1 to W4) post-stimulation. A global trend to a decrease of severity with time was observed, with no significant difference either according to the stimulation modality or according to the response to treatment. Abbreviations: rTMS, high-frequency repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation
Secondary Outcomes: fMRI
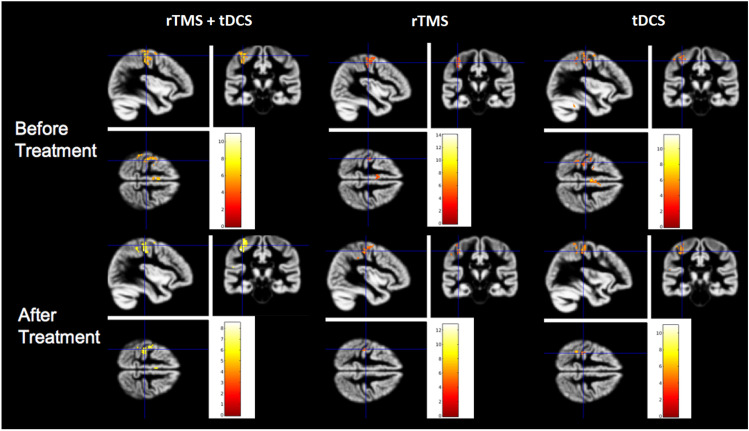
At the group-level, the activation pattern for the finger movement contralateral to the pain involved as expected the primary motor and supplementary motor areas within the motor network (Fig. 6). The pattern of motor-related activation did not show statistically significant differences for the painful and the non-painful sides. This pattern was similar before and after the treatment, and not statistically different following rTMS or tDCS, nor when both treatments were pooled together. The distribution and magnitude of motor-related activation after the treatment were equivalent when compared between responders and non-responders. In order to disclose any pre-stimulation feature in motor activation that could be predictive of cortical stimulation efficacy, we checked for differences in activation patterns in responders and non-responders before treatment, but no significant differences were detected between sub-groups. Taken together, this set of analyses showed the expected patterns of brain activation during voluntary movement, but failed to demonstrate significant differences in fMRI motor activity patterns at group level, neither between techniques nor between responders and non-responder patients.
Fig. 6.
BOLD activation patterns during the motor task. The left column depicts the results combined for both rTMS and tDCS while the middle column illustrates the results for rTMS only and the right column for tDCS. The motor task induced significant activations in the contralateral sensori-motor cortex and the supplementary motor area (SMA), with no significant differences before (upper panel) or after one week of daily motor cortex stimulation (lower panel)
Adverse Effects
No serious adverse effects were reported during or following any of the two interventions, but minor side effects were noted very occasionally. One patient complained of noise intolerance (despite wearing hearing protection) and headaches during the rTMS session and discontinued the study. One patient reported tension headache immediately after rTMS, and 3 patients complained about this symptom several days after the end of the stimulation week (1 week and 2 weeks after the end of rTMS, and 2 days after the end of tDCS). These headaches were relieved by a level-1 analgesic (paracetamol) and did not recur. Skin irritation was observed under a tDCS electrode on 3 occasions, without recurrence the following days.
Discussion
Both rTMS and tDCS decreased pain levels in this series of patients with pharmaco-resistant neuropathic pain. Although pain decrease was overall significant at the group level for the two interventions, individual analysis showed that only 42% of the patients achieved a significant level of pain decrease, defined as a change equal or superior to 2 SDs relative to the average ratings in the week previous to stimulation. Analysis of the consecutive weekly pain ratings showed that there was no overlap between the timelines of responders and non-responders: pain ratings in responders progressively decreased during the week of stimulation, and then during the 3 following weeks, whereas in non-responders the sequence of changes in pain ratings was virtually horizontal and around zero to either procedure (Fig. 3).
Best pain decrease in responders was moderate when considering raw NRS values, about 31% of initial values in the average. This is consistent with a number of previous reviews and meta-analyses [3, 4, 12] and represents a significant but moderate improvement according to IMMPACT consensus statement criteria of “clinically meaningful” treatment [33]. It has been suggested that more than 5 consecutive stimulation sessions may be necessary to induce a maximal analgesic effect, both for rTMS [34] and tDCS [35], and iteration of maintenance sessions at longer intervals has proved useful to maintain or enhance the analgesic effects [5, 34]. Of notice, although the changes observed here may not appear impressive, they concern patients who had previously proven to be drug-resistant to both 1st and 2nd line drugs for neuropathic pain, in many cases for more than 1 year. Furthermore, normalized pain ratios warranted that in any patient qualified as “responder,” pain had abated by at least 2 SDs relative to pre-stimulus values, and often reaching up to −3 SD, which ensured a sizeable effect size at the individual level. Indeed, the Z-score of normalization is individually tailored and takes into account the intrinsic variability of pain reports at baseline in each patient, hence minimizing any changes in scores that do not exceed significantly such pre-stimulus variability. Z-score normalization is also the preferred method in other domains in pain research, notably when describing changes in quantitative sensory testing [28, 36, 37].
When tested as a group, the overall magnitude of changes in pain scores due to rTMS and tDCS did not differ significantly, and their respective levels were highly correlated. However, as illustrated in Fig. 5, the regression line between the best level of pain relief in both techniques appeared skewed toward rTMS, and significantly different from the theoretical equivalence slope (45°, or β = 1), suggesting a slightly superior level of pain relief for rTMS at individual level, and under the conditions tested here. We cannot rule out the possibility that the number of stimulation sessions to achieve a given level of relief may be different for tDCS and rTMS. Although there is not, to our knowledge, a direct comparison of both techniques in this respect, the number of sessions considered sufficient to obtain maximal analgesic effects was estimated as 7 for rTMS by Hodaj et al. [34], and as high as 15 for tDCS by Castillo-Saavedra et al. [35]; such disparities may have influenced the present results.
The percentage of responders to either technique was almost identical (42%), but the individual patients responding to each procedure were not the same. This is an indirect indication that the mechanisms underlying the pain-relieving effect of both techniques may differ, at least in their initial “induction” phase. In support of this view, a lack of effect of tDCS has been described in patients previously responding to rTMS [38], and conversely a patient with chronic NP not responding to rTMS could be improved in the long term by anodal tDCS [39]. A very recent report comparing these two techniques in 12 patients with brachial plexus avulsion also found that different patients may be differentially sensitive to one or the other [24]. Together with these previous reports, the present study in a larger sample of NP patients appears clinically relevant in that it highlights the possibility of using one technique if the other fails, thereby increasing the probability of a positive response, which currently tops out at about 50% for rTMS [2, 3, 14].
The brain activation pattern during motor tasks mainly involved as expected the primary motor and supplementary motor areas, and remained unchanged from the beginning to the end of the stimulation week, for both rTMS and tDCS. Such lack of evidence for motor-related plasticity after 1 week of stimulation might indicate that the second fMRI was conducted too early to detect possible motor-related effects triggered by the procedures, which may have occurred later. Indeed, post-intervention fMRI data were acquired at the end of the stimulation week, whereas the maximal effects on pain in responders were not obtained until 1 week later or more (Fig. 3). If plastic changes in the cortex develop in parallel with the decrease in pain, they may have gone unnoticed in our patients because of the different temporality of recordings. Although technically challenging, future studies should consider the importance that fMRI data be acquired in close connection with changes in pain reports.
The relation of local changes in motor networks and pain relief from cortical neurostimulation remains a subject of debate, as it remains unclear whether rTMS entails sizeable changes in intracortical motor circuits under the conditions used to treat pain. Indeed, while stimulation at levels above motor threshold induced clear metabolic activation in M1, such activation was found to subside or disappear at the sub-threshold levels commonly applied for pain relief [40, 41]. Also, although a correlation was initially described between intracortical motor inhibition and rTMS-induced pain relief [42], later studies in NP patients failed to reproduce such effects [43, 44], and rTMS analgesia could be blocked pharmacologically in the absence of motor excitability changes [45]. Therefore, while widespread long-distance changes in cortical and subcortical structures after rTMS/tDCS have received consistent support, the relevance of motor cortex excitability for rTMS analgesic effects remains unconfirmed.
Limitations
An obvious limitation of this study is the lack of long-term follow-up beyond 5 weeks [4]. The long-term maintenance of pain relief in responding patients is a major challenge for all non-invasive stimulation methods, and different procedures are being currently tested, mostly based on the progressive spacing out of consecutive sessions [2, 46]. Future studies should consider providing assessment of pain relief during months or years if these techniques are to be accepted as routine treatments for chronic pain [12]. The head-to-head design of the study, comparing tDCS to a reference active stimulation (rTMS) instead of a placebo, also entails interpretative limitations. According to current literature, rTMS can now be considered a validated procedure for drug-resistant neuropathic pain [2, 12, 14] that has been incorporated to standard guidelines for NP therapy [16] and could in our view act as a valid reference. This also made it possible to avoid subjecting patients suffering from drug-resistant pain for many years to a placebo. Although the placement of the anode tDCS was centered on the motor cortex, the size of the electrodes and the standard motor-prefrontal montages entail a current distribution covering a region much more extended than the focalized figure-of-eight rTMS coil, and could render the comparison hazardous [47]. New high-definition tDCS montages could permit more focalized current distribution around the motor cortex, but their use in neuropathic pain remains anecdotal [48]. Finally, our fMRI analysis was restricted to the activations induced by a motor task. Analysis of resting state, pain-related activity, and connectivity changes before and after stimulation may prove in the future much better approaches to investigate the brain activities accompanying and/or supporting neurostimulation–related pain relief [49].
Conclusion
In patients with drug-resistant neuropathic pain, five daily sessions of tDCS or rTMS over the motor cortex showed a similar pattern of efficacy at one month. Each technique entailed significant effects in about half of the patients, and half of them responded to one procedure only; therefore, both techniques deserve being tested before declaring a patient as unresponsive. Pain relief was not paralleled by motor-related plasticity on fMRI. Since duration of pain relief after 5 daily sessions often does not exceed some weeks (2,44), prolonging the beneficial effects of neurostimulation in responders remains a crucial issue. Potential solutions include delivering maintenance sessions at progressively longer intervals, autonomous stimulation at home, and/or neurosurgical implanted stimulation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr Jean-Pierre Alibeu and Koichi Hagiwara for their contribution to the work leading to this article, the medical staff of the University Pain Centers of Lyon and Grenoble, who referred to us many of the patients treated herein, and the technical staff that provided logistic assistance.
Abbreviations
- NP
Neuropathic pain
- HF-rTMS
High-frequency repetitive transcranial magnetic stimulation
- tDCS
Transcranial direct current stimulation
- fMRI
Functional magnetic resonance imaging
- IASP
International association for the study of pain
- NRS
Numerical rating scale
- rmANOVA
Repeated measures analyses of variance
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This work was supported in part by the “Fondation APICIL,” “Agir à Dom,” and LABEX CORTEX (ANR-11-LABX-0042) of Université de Lyon, within the program “Investissements d’Avenir” (ANR-11-IDEX-0007) conducted by the French National Research Agency (ANR).
Declarations
Competing Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nathalie André-Obadia and Hasan Hodaj contributed equally to this work.
References
- 1.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–139. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Larrea L, Quesada C. Cortical stimulation for chronic pain: from anecdote to evidence. Eur J Phys Rehabil Med. 2022;58(2):290–305. 10.23736/S1973-9087.22.07411-1. [DOI] [PMC free article] [PubMed]
- 3.Cruccu G, Garcia-Larrea L, Hansson P, et al. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol. 2016;23:1489–1499. doi: 10.1111/ene.13103. [DOI] [PubMed] [Google Scholar]
- 4.O'Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018;4:CD008208. doi: 10.1002/14651858.CD008208.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quesada C, Pommier B, Fauchon C, et al. New procedure of high-frequency repetitive transcranial magnetic stimulation for central neuropathic pain: a placebo-controlled randomized crossover study. Pain. 2020;161:718–728. doi: 10.1097/j.pain.0000000000001760. [DOI] [PubMed] [Google Scholar]
- 6.Hosomi K, Sugiyama K, Nakamura Y, et al. A randomized controlled trial of 5 daily sessions and continuous trial of 4 weekly sessions of repetitive transcranial magnetic stimulation for neuropathic pain. Pain. 2020;161:351–360. doi: 10.1097/j.pain.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao CG, Sun W, Ju F, et al. Analgesic effects of directed repetitive transcranial magnetic stimulation in acute neuropathic pain after spinal cord injury. Pain Med. 2020;21:1216–1223. doi: 10.1093/pm/pnz290. [DOI] [PubMed] [Google Scholar]
- 8.Zhao CG, Sun W, Ju F, et al. Analgesic effects of navigated repetitive transcranial magnetic stimulation in patients with acute central poststroke pain. Pain Ther. 2021;10:1085–1100. doi: 10.1007/s40122-021-00261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.André-Obadia N, Magnin M, Garcia-Larrea L. Theta-burst versus 20 Hz repetitive transcranial magnetic stimulation in neuropathic pain: a head-to-head comparison. Clin Neurophysiol. 2021;132:2702–2710. doi: 10.1016/j.clinph.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Mori N, Hosomi K, Nishi A, et al. Exploratory study of optimal parameters of repetitive transcranial magnetic stimulation for neuropathic pain in the lower extremities. Pain Rep. 2021;13(6):e964. doi: 10.1097/PR9.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attal N, Poindessous-Jazat F, De Chauvigny E, et al. Repetitive transcranial magnetic stimulation for neuropathic pain: a randomized multicentre sham-controlled trial. Brain. 2021;144:3328–3339. doi: 10.1093/brain/awab208. [DOI] [PubMed] [Google Scholar]
- 12.Anderson J, Parr NJ, Vela K. Evidence brief: transcranial magnetic stimulation (TMS) for chronic pain, PTSD, TBI, opioid addiction, and sexual trauma. Washington (DC): Department of Veterans Affairs (US); 2020. PMID: 33502837. Bookshelf ID: NBK566938. [PubMed]
- 13.Gatzinsky K, Bergh C, Liljegren A, et al. Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review. Scand J Pain. 2020;21:8–21. doi: 10.1515/sjpain-2020-0054. [DOI] [PubMed] [Google Scholar]
- 14.Lefaucheur JP, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018) Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang KL, Yuan H, Wu FF, et al. Analgesic effect of noninvasive brain stimulation for neuropathic pain patients: a systematic review. Pain Ther. 2021;10:315–332. doi: 10.1007/s40122-021-00252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moisset X, Bouhassira D, Attal N. French guidelines for neuropathic pain: an update and commentary. Rev Neurol (Paris) 2021;177:834–837. doi: 10.1016/j.neurol.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- 18.Bindman LJ, Lippoldand OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5(3):175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knotkova H, Hamani C, Sivanesan E, et al. Neuromodulation for chronic pain. Lancet. 2021;397(10289):2111–2124. doi: 10.1016/S0140-6736(21)00794-7. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Chang MC. Transcranial direct current stimulation for the management of neuropathic pain: a narrative review. Pain Physician. 2021;24:E771–E781. doi: 10.36076/ppj.2021.24.E771. [DOI] [PubMed] [Google Scholar]
- 23.Attal N. Ciampi De Andrade D, Mhalla A, et al, Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: a randomized sham-controlled comparative study. Pain. 2016;157:1224–1231. doi: 10.1097/j.pain.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 24.Bonifacio de Assis ED, Martins WKN, de Carvalho CD, et al. Effects of rTMS and tDCS on neuropathic pain after brachial plexus injury: a randomized placebo-controlled pilot study. Sci Rep. 2022;12:1440. doi: 10.1038/s41598-022-05254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruffini G, Dubreuil Vall L. tDCS clinical research - highlights: safety of transcranial Current Stimulation. Neuroelectrics White Paper WP201501;1–8. https://www.neuroelectrics.com/wiki/index.php/Neuroelectrics_White_Papers. Released: April 24th 2015. Accessed 15 Aug 2022.
- 26.Jensen TS, Baron R, Haanpää M, et al. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.André-Obadia N, Mertens P, Gueguen A, Peyron R, Garcia-Larrea L. Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology. 2008;71:833–840. doi: 10.1212/01.wnl.0000325481.61471.f0. [DOI] [PubMed] [Google Scholar]
- 29.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 30.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Larrea L, Perchet C, Hagiwara K, André-Obadia N. At-home cortical stimulation for neuropathic pain: a feasibility study with initial clinical results. Neurotherapeutics. 2019;16:1198–1209. doi: 10.1007/s13311-019-00734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzagalli F, Auzias G, Delon-Martin C, Dojat M. Local landmark alignment for high-resolution fMRI group studies: toward a fine cortical investigation of hand movements in human. J Neurosci Methods. 2013;15(218):83–95. doi: 10.1016/j.jneumeth.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Hodaj H, Payen JF, Hodaj E, et al. Long-term treatment of chronic orofacial, pudendal, and central neuropathic limb pain with repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 2020;131:1423–1432. doi: 10.1016/j.clinph.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain. 2016;17:14–26. doi: 10.1016/j.jpain.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151:598–605. doi: 10.1016/j.pain.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Lötsch J, Dimova V, Hermens H, et al. Pattern of neuropathic pain induced by topical capsaicin application in healthy subjects. Pain. 2015;156:405–414. doi: 10.1097/01.j.pain.0000460328.10515.c9. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill F, Sacco P, Bowden E, et al. Patient-delivered tDCS on chronic neuropathic pain in prior responders to TMS (a randomized controlled pilot study) J Pain Res. 2018;11:3117–3128. doi: 10.2147/JPR.S186079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodaj H, Payen JF, Lefaucheur JP. A case of long-term treatment of chronic pain syndrome by anodal tDCS of the motor cortex, previously resistant to high-frequency rTMS and implanted spinal cord stimulation. Brain Stimul. 2016;9:618–620. doi: 10.1016/j.brs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol. 1998;79:1102–1107. doi: 10.1152/jn.1998.79.2.1102. [DOI] [PubMed] [Google Scholar]
- 41.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- 42.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006;67:1568–1574. doi: 10.1212/01.wnl.0000242731.10074.3c. [DOI] [PubMed] [Google Scholar]
- 43.Hosomi K, Kishima H, Oshino S, et al. Cortical excitability changes after high-frequency repetitive transcranial magnetic stimulation for central poststroke pain. Pain. 2013;154:1352–1357. doi: 10.1016/j.pain.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Jetté F, Côté I, Meziane HB, Mercier C. Effect of single-session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil Neural Repair. 2013;27:636–643. doi: 10.1177/1545968313484810. [DOI] [PubMed] [Google Scholar]
- 45.Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Repetitive transcranial magnetic stimulation induced analgesia depends on N-methyl-D-aspartate glutamate receptors. Pain. 2014;155:598–605. doi: 10.1016/j.pain.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Lefaucheur JP, Nguyen JP. A practical algorithm for using rTMS to treat patients with chronic pain. Neurophysiol Clin. 2019;49:301–307. doi: 10.1016/j.neucli.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Hodaj H, Payen JF, Mick G, et al. Long-term prophylactic efficacy of transcranial direct current stimulation in chronic migraine. A randomised, patient-assessor blinded, sham-controlled trial. Brain Stimul. 2022;15:441–453. doi: 10.1016/j.brs.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Deblieck C, Smeijers S, Morlion B, Datta A, Thomas C, Theys T. Case report: initial evidence of safety and efficacy of high definition-transcranial direct current stimulation in a patient with neuropathic pain and implanted spinal cord stimulator. Front Pain Res (Lausanne) 2021;2:753464. doi: 10.3389/fpain.2021.753464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Miesen MM, Lindquist MA, Wager TD. Neuroimaging-based biomarkers for pain: state of the field and current directions. Pain Rep. 2019;4:e751. doi: 10.1097/PR9.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.