SUMMARY
Multiple sclerosis (MS) is a chronic inflammatory and degenerative disease of the central nervous system afflicting nearly three million individuals worldwide. Neuroimmune interactions between glial, neural, and immune cells play important roles in MS pathology and offer potential targets for therapeutic intervention. Here, we review underlying risk factors, mechanisms of MS pathogenesis, available disease modifying therapies, and examine the value of emerging technologies, which may address unmet clinical needs and identify novel therapeutic targets.
INTRODUCTION
Multiple Sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS) characterized by neuroinflammation and neurodegeneration. In 2020, the MS atlas reported that one individual with an average age of 32 years is diagnosed with MS every 5 min around the world, adding to about 2.8 million individuals currently living with the disease. Strikingly, MS worldwide prevalence has risen by 30% since 2013.1 This increase may be partially attributed to improved reporting and diagnosis, but much of its underlying factors remain unknown. As for MS therapies, despite an improvement in efficacy and options, they remain mostly limited to relapsing-remitting MS. In this review, we summarize the current understanding of MS predisposing factors, mechanisms of disease pathology, and therapeutic interventions.
DISEASE PRESENTATION AND PATHOLOGY
Historical records describe a disease reminiscent of MS as early as the 14th century.2 In 1868, Jean-Martin Charcot named this disease sclérose en plaques, later adapted to multiple sclerosis.3 Charcot and collaborators established that the pathological hallmarks of MS include lesions in CNS white and gray matter areas with variable degrees of demyelination, perivascular immune cell infiltration, reactive gliosis, and/or neurodegeneration.3 Axonal transection and blood-brain barrier (BBB) disturbances were later described as additional features of these lesions.4
MS diagnosis and monitoring
MS is diagnosed based on clinical, biochemical, and radiological features that indicate a dissemination in time and space of CNS lesions.5 These features include symptoms of motor, sensory, and/or cognitive dysfunction; the presence of oligoclonal bands (OCBs) in the cerebrospinal fluid (CSF)-antibodies indicative of CNS inflammation-; and the detection of CNS lesions by magnetic resonance imaging (MRI) using T2-weigthed scans to provide information about lesion load; or the contrast agent gadolinium to detect active inflammatory lesions. MRI advances have improved the monitoring of CNS damage and, consequently, disease activity, progression, and response to therapy.6,7 Moreover, recent developments in positron emission tomography (PET) imaging have enabled the examination of microglial and astrocyte responses linked to MS progression.8,9
A persistent challenge in MS diagnosis and management is the need for blood biomarkers. However, recent large population studies and the identification of relevant confounding factors may enable the clinical use of serum neurofilament light chain (sNfL), a marker of neuronal damage sNfL, for disease monitoring.10,11 Other biomarkers such as serum glial fibrillary acid protein (GFAP),12 antibodies,13,14 and metabolites15 may provide additional tools to monitor disease progression and response to therapy.
MS disease forms
Relapsing remitting MS (RRMS) is the most common clinical form of MS diagnosed in 85%–90% of patients between the ages 20–40, characterized by symptomatic attacks (relapses) followed by periods of recovery (remissions). Clinically isolated syndrome (CIS) refers to a first episode of neurologic symptoms followed by complete or partial recovery. The incidental detection of MS-like CNS lesions in the absence of symptoms often leads to a diagnosis of radiologically isolated syndrome, considered a pre- or sub-clinical form of MS.16
RRMS often transitions into secondary progressive MS (SPMS), characterized by the progressive and irreversible accumulation of neurologic disability. Furthermore, 10%–15% of patients display a progressive disease course from diagnosis (primary-progressive MS, PPMS). However, early sub-clinical symptoms may interfere with the differentiation of SPMS and PPMS. In addition, relapses may hide disease progression, which may be present even during early MS stages.17 Thus, it has been suggested that specific disease outcomes, such as progression independent of relapse activity, might be more clinically useful.18 While RRMS is 2–4 times more frequent in females than in males, PPMS shows increased prevalence in males,1 suggesting that sex-specific factors (e.g., hormones) influence disease onset and progression.19
MS pathology
CNS lesions at their earliest stage are called active lesions, featuring myelin loss, myelin debris, immune cell infiltration (particularly in the perivascular space), BBB disruption, reactive gliosis, and axonal damage. Active lesions are mostly linked to early disease in RRMS patients.20 Inactive lesions depict areas of old demyelinating injury with minimal glia and immune cell presence and activity; they become more abundant in patients with extended disease duration.20
Mixed active/inactive lesions–also known as chronic active or smoldering lesions–are also demyelinated but show a border of activated microglia/macrophages with foamy or ramified phagocytic phenotypes, axonal damage, subtle BBB disruption, and some immune cell infiltration adjacent to a largely hypocellular center with minimal microglia/macrophage presence and moderate T cell infiltration.20 Interestingly, active/inactive lesions have been associated with MS progression and are more frequent in older patients.5 These and other findings suggest a role for age as a determinant of disease progression, probably because of deficits in CNS repair mechanisms and/or pathways that regulate peripheral and compartmentalized CNS inflammation. These findings also suggest that active/inactive lesions may be useful to monitor the response of therapies aimed to arrest MS progression.
Please note that CNS pathology in MS is not restricted to focal lesions. Diffuse injury, atrophy, BBB dysregulation, perivascular astrogliosis, and/or non-demyelinating inflammation are detected in both white and gray matter.20 Moreover, inflammatory immune cell infiltrates and ectopic lymphoid follicles are detected in the meninges of MS patients, in association with severe cortical pathology, neurodegeneration, and a progressive disease course.21,22 Thus, multiple CNS regions and tissue microenvironments are involved in MS pathology, even within the same patient, reflecting the contribution of multiple mechanisms to disease pathology.
MS PREDISPOSING FACTORS
MS develops as a result of a dynamic interplay between genetic, environmental, and lifestyle factors23 (Figure 1). These factors modulate pathogenic processes even before MS diagnosis, in a prodromal phase that may display depression, anxiety, gastrointestinal issues, and elevated sNfL levels indicative of neuroaxonal degeneration.24
Figure 1. Genetic and environmental factors that shape MS pathogenesis.
(A) Genetic studies linked MS susceptibility to autosomal and X chromosome genes. HLA variants were shown to promote pathogenic cross-talk between B and T lymphocytes. Autosomal variations are comparatively understudied, but increasing evidence suggest their involvement in dysregulated lymphocyte and glial responses. The impact of X-linked gene variants remains to be determined.
(B) The gut microbiome is altered in MS. Specific bacterial species have been shown to promote pathogenic T and B cell responses while limiting Tregs.
(C) EBV infection has been postulated to be a trigger of CNS autoimmunity in MS by boosting the production of pathogenic factors by B cells, by increasing the APC function of B cells, and by triggering cross-reactive responses with CNS antigens by molecular mimicry.
(D) MS-promoting responses of immune and glial cells are influenced by environmental factors (e.g., exposure to chemicals) and lifestyle choices (e.g., diet).
Genetics
The lifetime risk of developing MS in first-degree relatives and monozygotic twins of affected individuals was calculated to be more than 7-fold and 100-fold higher than in the general population, respectively, suggesting that genetic factors control MS susceptibility. Initial studies linked MS susceptibility to the MHC locus, paving the way for the identification of additional genetic factors25 (Table 1). The HLA gene cluster in the polymorphic MHC locus is considered a major genetic MS risk factor, with notable variants in class II HLA-DRB1 (e.g., HLA-DRB1*15:01) and HLA-DPB1 genes and class I HLA-A and HLA-B genes.25 In addition, a recent large study identified 233 genetic variants linked to MS susceptibility, 200 of which are located outside the MHC region, and one in the X chromosome26 (Figure 1A). Several of these non-MHC MS susceptibility genes play immune functions (e.g., IL2RA, IL7RA, and CD58) and are enriched in adaptive and innate immune cells (e.g., T cells and microglia).26 Interestingly, known gene variants only explain ~48% of MS genetic predisposition,26 suggesting that additional predisposing genetic factors are yet to be identified. Ongoing studies aim to elucidate the mechanisms through which genetic variants linked to MS contribute to disease pathogenesis and progression. For example, the HLA-DR15 haplotype was shown to promote the activation of autoreactive T cells by antigen-presenting B cells,27,28 while MS risk variants linked to NF-κB signaling29 and MERTK30 were linked to astrocyte and microglial responses linked to MS progression.
Table 1.
Representative genes linked to MS
| Chromosome | Key Susceptibility Loci |
Function(s) | Expression | Mechanism | p-value of Genome Wide Significancea |
|---|---|---|---|---|---|
| 6 | HLA Class II and I regions (E.g. HLA-DRB1, HLA-DPB1, HLA-A, HLA-B) | Adaptive immune regulation and function | Ubiquitous | Antigen presentation | 1.62E-1916 - 4.95E-08 |
| 16 | CLEC16A (C-type lectin domain containing 16) | Regulator of mitophagy | Ubiquitous | Antigen presentation; inflammatory response | 3.70E-71 - 4.09E-09 |
| 1 | CD58 (lymphocyte function-associated antigen 3, LFA3) | CD2 ligand; cell adhesion molecule; costimulatory molecule | Macrophages; dendritic cells | T cell activation | 4.55E-70 |
| 10 | IL2RA (IL-2 receptor alpha or CD25) | High affinity receptor for cytokine IL-2 | T and B cells | T cell activation | 2.96E-65 - 1.76E-15 |
| 12 | TNFRSF1A (TNF receptor superfamily member 1A, CD120a) | TNFα receptor | Ubiquitous | Inflammatory response | 2.24E-47 - 4.13E-10 |
| 3 | CD86 (B7-2) | Ligand for CD28 and CTLA-4; costimulatory molecule | Macrophages; dendritic cells; B cells | T cell activation | 6.98E-43 - 8.32E-10 |
| 1 | EVI5 (ecotropic viral integration Site 5) | Regulator of cell cycle and cell division | Ubiquitous | Unclear | 2.95E-30 |
| 11 | CD6 | ALCAM ligand; costimulatory receptor | T cells | T cell activation; cell trafficking | 2.00E-29 |
| 5 | IL7R (IL-7 receptor, CD127) | IL-7 receptor | Ubiquitous | T cell proliferation | 1.58E-28 |
| 17 | STAT3 (signal transducer and activator of transcription 3) | Transcription factor | Ubiquitous | T cell polarization | 2.32E-28 |
| 11 | CXCR5 (C-X-C motif chemokine receptor 5, CD185) | CXCL13 receptor | B cells | Cell trafficking | 4.53E-26 - 2.10E-19 |
| 1 | Vascular cell adhesion molecule 1 (VCAM1) | VLA-4 ligand; cell trafficking molecule | Ubiquitous | Cell trafficking | 1.11E-22 |
| 20 | CYP24A1 (Cytochrome P450 24A1) | Mitochondrial enzyme | Ubiquitous | Vitamin D metabolism | 1.92E-19 - 3.13E-10 |
| 20 | CD40 | Receptor for CD40L; costimulatory molecule | Macrophages; dendritic cells; B cells | T cell activation | 5.28E-19 |
| 16 | IRF8 (interferon regulatory factor 8) | Transcription factor | Macrophages; dendritic cells; B cells | Transcription control | 2.83E-17 |
| 3 | EOMES (Eomesodermin, TBR2) | Transcription factor | T lymphocytes and NK cells | T cell response | 1.55E-16 |
| 6 | ATXN1 (ataxin 1) | DNA binding protein | Ubiquitous | B-cell modulation | 1.62E-13 |
| 2 | MERTK (MER proto-oncogene tyrosine kinase) | Kinase | Microglia | Phagocytosis | 1.11E-11 |
| X | VGLL1 (vestigial like family member 1) | Transcription cofactor | Unclear | Unclear | 6.86E-09 |
p values obtained from the International Multiple Sclerosis Genetics Consortium.26
Microbiome
The microbiome has gained major attention in MS.31,32 In particular, the intestinal microbiome has been shown to promote the development of CNS-reactive pathogenic T cells33 and modulate the activity of CNS-resident cells.15,34,35 Interestingly, the transplantation of intestinal microbiota from MS patients worsened experimental autoimmune encephalomyelitis (EAE), a pre-clinical MS model.36,37 Indeed, analyses of the MS intestinal microbiome revealed alterations linked to pro- and anti-inflammatory responses (Figure 1B). Notably, the genera Akkermansia was found to be elevated in RRMS and progressive MS and promote T helper 1 responses.36-38 However, other studies linked Akkermansia to lower MS clinical disability39 and showed that the administration of specific Akkermansia strains ameliorate EAE.39,40 These findings suggest strain-specific effects of Akkermansia on mechanisms of MS pathogenesis.
Associations between the MS intestinal microbiome and regulatory T cells (Tregs) have also been described. The genera Parabacteroides, which is reduced in MS patients, was shown to stimulate IL-10+ Tregs.36 Moreover, transplantation of microbiota from MS patients reduces IL-10 production in EAE.37 Conversely, it was recently shown that IgA-producing plasma cells induced in the gut migrate to the CNS and suppress inflammation via IL-10 production.41 IgA-producing plasma cells reactive with the intestinal microbiome were recently detected in active MS lesions.42 Collectively, these studies highlight the role of the microbiome in the control of MS pathology. Future studies should translate these findings into therapeutic interventions, while extending these investigations to the microbiome in other tissues. For example, it was recently shown that the lung microbiome regulates CNS inflammation in rat EAE,43 but overall, little is known about lung microbiome perturbations in MS.
Microbial infections
Charcot’s associate, Pierre Marie, noted that MS is often brought about during an infection.44 Epstein-Barr virus (EBV) infection is associated with MS, as highlighted by the recent analysis of a large cohort of blood samples obtained before MS diagnosis, which established that the risk of MS is increased 32-fold after infection with EBV but not with other viruses.45 The mechanisms through which EBV may contribute to MS development are many, including the triggering of CNS autoimmunity because of dysregulated T cell activation and/or molecular mimicry with EBV antigens (Figure 1C). Because of its tropism for B cells, EBV may boost their ability to activate pathogenic T cells. In addition, EBV-reactive T cells can cross-react with CNS antigens,46,47 and EBV was also found to induce a self-reactive B cell immune response targeting the oligodendrocyte and astrocyte antigen GlialCAM.48 However, these findings do not imply that EBV causes MS, but rather that immune mechanisms linked to EBV infection might cooperate with other environmental and/or genetic factors to trigger disease initiation in susceptible individuals. Novel vaccines may enable preventive interventions targeting EBV in MS and other diseases,49 with the major caveat that vaccination should be performed at an early age, posing an important challenge for clinical trial design and overall feasibility.
Other factors
Race, ethnicity, latitude, diet, obesity, smoking, and environmental exposures (e.g., sunlight, pollutants) are also thought to contribute to MS development.23,50 Studies of the mechanisms involved in the environmental regulation of MS showed, for example, that the nutrient methionine promotes CNS inflammation via the epigenetic reprogramming of CD4+ T lymphocytes,51 and that the herbicide linuron boosts astrocyte pathogenic activities driven by the transcription factor XBP1.52 Thus, combined epidemiologic and mechanistic studies may identify both novel MS predisposing factors and mechanisms of disease pathogenesis.
MECHANISMS OF DISEASE PATHOLOGY
Extensive analyses of patient samples, interventional clinical trials and pre-clinical model studies established a central role for peripheral leukocytes (T cells, B cells, myeloid cells) in MS pathology. Their aberrant responses damage oligodendrocytes and neurons, while activating CNS-resident cells such as microglia and astrocytes. Thus, MS pathology can be linked to four interdependent processes controlled by genetic and environmental factors: (1) activation of self-reactive lymphocytes, (2) CNS immune invasion, (3) immunoregulation, and (4) progressive neurodegeneration. Targeting these diverse processes with combinatorial approaches is needed for the effective therapeutic management of MS.
Activation of self-reactive lymphocytes
Decades of basic, translational, and clinical research established MS as an autoimmune disease. Seminal reports on the induction of EAE by immunization with CNS antigens supported a role for T cell autoimmunity in MS pathogenesis, while providing useful pre-clinical models. It should be kept in mind that different EAE models recapitulate specific aspects of MS, including an isolated acute CNS autoimmune attack induced in the C57BL/6 mouse strain by immunization with myelin oligodendrocyte glycoprotein (MOG)35-55, a relapsing-remitting course induced in SJL mice by immunization with proteolipid protein (PLP)139-151, or secondary progressive disease induced in non-obese diabetic (NOD) mice by immunization with MOG35-55. Other EAE models are based on the adoptive transfer of myelin-specific T cells, or the transgenic expression of myelin-specific T cell receptor, which results in spontaneous EAE development. Indeed, no EAE model captures all the inflammatory and neurodegenerative processes linked to MS. One additional limitation is that most EAE models are driven by CD4+ T cells targeting one or a few CNS antigens, and may not recapitulate the complex autoimmune responses driven by CD4+ cells, CD8+ T cells and B cells in MS.
Self-reactive lymphocytes
CD8+ and CD4+ T cells reactive with myelin and other antigens are detected in active MS lesions.53 Important insights on the role of T cells in MS were provided by reports of EAE induction in naive animals by the adoptive transfer of myelin-reactive T cells.54,55 Although CD8+ T cells are dominant in MS lesions, more is known about CD4+ T cell specificity and regulation in MS, reflecting technical limitations for the analysis of human CD8+ T cells and the paucity of pre-clinical models for their investigation. However, recent developments have re-invigorated the study of CD8+ T cells in EAE.56,57
CD4+ T helper (TH) cells are classified based on the expression of cytokines, transcription factors and effector functions. IFNγ+ TH1 cells controlled by the transcription factor Tbet, and IL-17+ TH17 cells controlled by the transcription factor RORγt play pivotal roles in MS and EAE pathology58 (Figure 2A). Importantly, these TH subtypes include functionally different subsets controlled by additional transcriptional regulators. For example, non-pathogenic TH17 cells show a limited ability to induce tissue pathology and express transcriptional modules linked to immune regulation, which include Il10 and Ahr.59,60 Conversely, pathogenic TH17 cells promote tissue destruction and express IL-23-driven transcriptional programs which include Csf2, Ifng and Tbx21 among other genes. Interestingly, increased TH1 and TH17 cell responses in MS have been linked to acute relapses and other disease outcomes.61,62 Further analyses of TH subsets reported increased pathogenic IFNγ+ IL-17+ TH17 cells in MS relapses, and increased IL-10+ IL-17+ TH17 cells in stable RRMS patients.63 When analyzing these findings in the context of disease monitoring and treatment, it should be kept in mind that these TH17 subsets may be interconvertible in vivo, as suggested by the AHR-induced conversion of pathogenic TH17 cells into IL-10+ anti-inflammatory T cells,64 and the IL-23-driven conversion of intestinal non-pathogenic TH17 cells into EAE-promoting pathogenic CXCR6+ TH17 cells.65 Thus, the analysis of a few cytokines or transcription factors may fail to assign TH cells to a specific subtype and more in-depth analyses may be needed, for example, single-cell epigenetic-based lineage tracing.66
Figure 2. Immune mechanisms and CNS invasion in MS.
(A) APCs (DCs, macrophages, B cells) and T cells first interact in the periphery, leading to activation and dysregulation of peripheral immunity and the secretion of inflammatory molecules.
(B) Peripheral immune cells invade the CNS through the BLMB, BCSFB, and BBB where they can interact with local APC subsets.
(C) Impaired Treg suppressive activity contributes to MS pathogenesis.
The adoptive transfer of CNS-specific TH1 or TH17 cells induces EAE, but different TH subtypes induce pathology in different CNS locations, suggesting differences in homing and tissue interactions.67 Indeed, the mechanisms used by TH1 and TH17 cells to promote CNS pathology are diverse, involving crosstalk with peripheral immune cells and CNS-resident cells. For example, TH1 cells stimulate macrophages and microglia and hinder oligodendrocyte precursor cell survival and differentiation.68-70 TH17 cells disrupt the BBB, promote inflammatory phagocyte infiltration, modulate glial functions, and interact with oligodendrocytes and neurons to promote axonal damage and neurodegeneration.70-72
Interactions between myeloid and CD4+ T cells are important contributors to MS pathogenesis (Figure 2A). Pathogenic T cells in MS and EAE produce granulocyte-macrophage colony-stimulating factor (GM-CSF),73-77 which triggers the release of Ly6Chi classical monocytes from the bone marrow and boosts their pathogenic activities,78 highlighting the role of CD4+ T cell-monocyte interactions in CNS pathology. Within the CNS, GM-CSF promotes microglial activation,79 and in combination with IFNγ, it differentiates Ly6Chi monocytes into inflammatory macrophages.68 In addition, GM-CSF boosts the differentiation of disease-promoting astrocyte subsets,72 while interfering with anti-inflammatory astrocyte functions.35 Interestingly, chronic inflammation induces GM-CSF production by astrocytes,15,52,74,80 suggesting a positive feedback loop that perpetuates pathogenic myeloid cell and astrocyte responses in progressive MS.
CD8+ T cells constitute the predominant T cell population in MS lesions57 (Figure 2A). CD8+ T cells bearing tissue-resident memory phenotypes were detected in mixed active/inactive lesions and shown to be clonally expanded in the CSF of MS patients.81,82 Interestingly, tissue resident CD8+ T cells were recently identified as putative drivers of compartmentalized autoimmune CNS damage.83,84 Thus, novel experimental approaches85-87 and pre-clinical models should enable a better characterization of CD8+ T cell responses and their dysregulation in MS.
B cells contribute to MS pathology through multiple mechanisms (Figure 2A). OCBs are a classic feature of MS, but the contribution of antibodies to disease pathology is still debated. Interestingly, the accumulation of B cells in meningeal ectopic follicles is linked to cortical pathology in MS,22 probably reflecting the contribution of B-cell produced GM-CSF, TNFα, IL-6, IL-12, IL-15 and other soluble factors in the promotion of inflammation88 and neurotoxicity.89 In addition, B cells play important roles as antigen-presenting cells (APCs).90 Indeed, the beneficial effects of B-cell targeting antibodies in MS have been linked to the suppression of T cell activation.90 However, it is still not clear whether these B cell targeted therapies interfere with T cell activation in CNS ectopic follicles, and whether additional therapeutic targets should be identified to target specific B cell populations.
Antigen presentation
The peripheral priming of naive T cells and their reactivation in the CNS are essential steps in EAE and MS pathology (Figures 2A and 2B). The analysis of clinical samples identified antigens recognized by the autoimmune T cell response in MS, including myelin basic protein (MBP), MOG, and PLP, among others (Table 2).27,48,53 The breadth of the autoimmune response distinguishes MS from other more focalized CNS demyelinating diseases, such as neuromyelitis optica (characterized by antibodies to aquaporin 4), MOG antibody disease, and acute disseminated encephalomyelitis (both characterized by antibodies to MOG)91 (Table 2).
Table 2.
Representative MS autoantigens
| Autoantigen | Expression | Function | Reacting Cell Types |
|---|---|---|---|
| Myelin basic protein (MBP)a | Oligodendrocytes | Myelin-associated | T and B cells |
| Myelin oligodendrocyte glycoprotein (MOG)b | Oligodendrocytes | Myelin-associated | T and B cells |
| Proteolipid protein (PLP) | Oligodendrocytes | Myelin-associated | T and B cells |
| Myelin-associated glycoprotein (MAG) | Oligodendrocytes | Myelin-associated | T and B cells |
| Myelin-associated oligodendrocyte basic protein (MOBP) | Oligodendrocytes | Myelin-associated | T cells |
| 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) | Oligodendrocytes | Myelin-associated | T and B cells |
| Transaldolase | Oligodendrocytes | Enzyme | T and B cells |
| Transketolase | Oligodendrocytes | Enzyme | B cells |
| KIR4.1 | Oligodendrocyte; astrocytes | Potassium channel | B cells |
| GlialCAM (HEPACAM)a | Oligodendrocytes; astrocytes | Cell adhesion | B cells |
| Reticulon 4 (NOGO-A) | Oligodendrocytes; neurons | Inhibitor of neurite outgrowth | B cells |
| Contactin-2 | Neurons | Cell adhesion | T cells |
| Neurofilament light chain | Neurons | Cytoskeleton | B cells |
| Tubulin | Neurons | Cytoskeleton | B cells |
| Neurofascin | Neurons | Cell adhesion | B cells |
| RAS guanyl-releasing protein 2 (RASGRP2) | Neurons | Nucleotide exchange factor | T cells |
| β-synuclein (SNCB) | Neurons | Synaptic protein | T cells |
| Prokineticin 2 (PROK2) | Neurons | Circadian clock | T cells |
| Reticulon 3 (RTN3) | Neurons | Protein transporter | T cells |
| Synaptosome associated protein 91 (CALM) | Neurons | Vesicle transport | T cells |
| Anoctamin 2 (TMEM16B)a | Neurons; astrocytes | Chlorine channel | B cells |
| S100β | Astrocytes | Ca2+ binding protein | T cells |
| Aquaporin 4c | Astrocytes | Water channel | B cells |
| Fatty acid. binding protein 7 (FABP7) | Astrocytes | Hydrophobic ligand transporter | T cells |
| GDP-l-fucose synthase (GFUS) | Ubiquitous | Enzyme | T cells |
| αB-crystallin (CRYAB) | Ubiquitous | Small heat shock protein | T and B cells |
autoantigens cross-reactive with EBV antigens.
also in MOG antibody disease and acute disseminated encephalomyelitis.
also in neuromyelitis optica spectrum disorders.
The specific autoantigens targeted by the immune response changes during the course of MS as a result of mechanisms of intra- and inter-molecular epitope spreading.58 Indeed, the analysis of pediatric MS samples with antigen microarrays established epitope spreading as an early pathogenic event, probably driven by self-immunization against antigens released from tissue damaged by the autoimmune response.92 In addition, infections and the commensal microbiome also influence the specificity of the MS autoimmune response via molecular mimicry,48,53 further promoting patient-to-patient heterogeneity. However, autoimmune targets linked to specific MS pathogenic processes have been identified,13,93,94 suggesting that novel antigen identification methods85-87 may enable patient staging and monitoring, and guide the development of antigen-specific immunomodulatory therapies.
Pre-clinical studies and the analysis of patient samples detected the drainage of antigens and dendritic cells drain from the CNS into cervical lymph nodes (CLNs), where they initiate T cell responses.95 Reports on meningeal lymphatic vessels draining antigens and immune cells from the CSF and the meninges into the CLNs further support these findings.96,97 However, a recent publication reported a central role for APCs in the leptomeninges, but not in the dura or CLNs, in T cell activation in EAE and MS.98 Thus, future studies should evaluate the relative contribution of these anatomic locations in the acute and chronic activation of CNS-reactive T cells in MS, and in other neurologic diseases.
Strikingly, anatomically distant sites participate in the control of CNS-reactive T cells. For example, in EAE, T cells home to different CNS locations as a function of their priming in inguinal or mesenteric lymph nodes.99 Indeed, commensal bacteria in mucosal compartments such as the gut, skin and lungs modulate effector or regulatory activities in myelin-reactive T cells.33,65,100-102 These findings highlight potential roles of the microbiome in the control of autoimmune responses through the production of factors that modulate T cell activation and polarization, and via the expression of cross-reactive microbial antigens which may activate self-reactive T cells.
APCs
Dendritic cells (DCs), macrophages, and B cells are considered major APCs involved in T cell activation in MS and EAE. Surprisingly, recent data suggest that microglia do not play a central APC role, at least in acute EAE models.103,104
Peripheral monocytes that cross the BBB differentiate into perivascular DCs that promote TH1 and TH17 responses.105,106 DCs containing myelin debris are detected in the perivascular space of active MS lesions and in the meninges near infiltrating T lymphocytes.106,107 In EAE, conventional type 2 DCs (cDC2s) dwell in the meninges and reactivate pathogenic T cells.103,105,108 DCs are also detected in the choroid plexus in EAE and MS but their capacity to reactivate encephalitogenic T cells remains to be determined.103,109 Indeed, some of these T cell-APC interactions may dampen T cell autoimmunity, rather than boost it.110
A macrophage subset capable of antigen presentation to T cells was identified on the abluminal side of BBB blood vessels and also in active and smoldering MS lesions.111,112 Macrophages were identified as the largest meningeal APC subset at peak EAE, surpassing DCs by ~4-fold and promoting CNS T cell infiltration.102 In MS, leptomeningeal macrophages were associated with cortical lesions and with meningeal lymphoid-like structures linked to worse disease outcome.22 More recently, MHC-II+ macrophages in dural sinuses were shown to present CNS antigens to T cells during homeostatic CNS surveillance, in addition to cDC2s.108 DCs and macrophages are also present in the choroid plexus. Thus, the relative contribution of dural and choroidal APCs to MS pathogenesis remains to be defined.
Mechanistic studies on B-cell-targeting disease modifying therapies (DMTs) underscore the role of B cells as APCs in MS.88,90 Indeed, B cells induce the proliferation of CNS-recruited TH1 and TH17 cells in MS.27 Moreover, B cells are detected in the perivascular space and in meningeal lymphoid-like structures adjacent to subpial cortical lesions.22,113 Collectively, these findings highlight the potential of DCs, macrophages, and B cells as targets for the therapeutic induction of antigen-specific tolerance. However, reports on T cell activation by type 3 innate lymphoid cells and neutrophils in autoimmune neuroinflammation suggest that additional APCs may also play important roles in the control of autoimmune T cells in MS114,115; but little is known about their contribution to different disease stages and therapeutic potential.
CNS immune invasion
CNS T cell infiltration involves three major CNS barriers (Figure 2B); a fourth meningeal layer named the subarachnoid lymphatic-like membrane has been recently identified in the mouse and human subarachnoid space,116 but its role in EAE and MS remains to be determined: (1) The BBB is the main gateway to the CNS parenchyma.117 (2) The blood-leptomenin-geal barrier (BLMB) provides access to subpial CNS regions and, to some extent, the CSF.118 More recently, immune cell trafficking from the skull bone marrow into the meninges was described, potentially through channels whose roles in disease pathogenesis are not clearly defined.119,120 (3) The choroid plexus represents the main portal linking the peripheral blood with the CSF.121
Migration through these CNS barriers involves cell-cell interactions mediated by cell adhesion molecules, chemokines, and their receptors. These interactions are mediated by classical molecules such as ICAM-1/LFA-1 and VCAM-1/VLA-4, and more recently identified ones such as DICAM, MCAM, ALCAM, and JAML.122 Importantly, these molecules are potential therapeutic targets to block the access of peripheral lymphocytes to the CNS, as exemplified by the VLA-4 blocking antibody natalizumab. However, unspecific therapeutic approaches, such as natalizumab, also interfere with physiologic mechanisms of CNS immune surveillance that limit the reactivation of common viruses, and thus could enable the development of progressive multifocal leukoencephalopathy.123
Immunoregulation
Multiple mechanisms prevent tissue damage caused by dysregulated immune responses. CD4+ FoxP3+ Tregs and IL-10-producing type 1 regulatory (Tr1) cells have been extensively studied in MS and EAE.50,124-127 In EAE, Tregs have been shown to suppress the development of autoimmune neuroinflammation and promote remyelination 128,129 while Tr1 cells have been assigned a more dominant role in the resolution of CNS inflammation, for example through their ability to suppress microglial and astrocyte responses via IL-10 production.130,131 Indeed, the induction of IL-10+ Tr1 cells by nasal anti-CD3 is undergoing clinical testing in MS. Similarly, IL-4 is reportedly neuroprotective in EAE,132 suggesting its potential for MS treatment.
Several observations suggest Treg dysfunction in MS, including reports of Treg decreased abundance, suppressive function, stability, migratory capacity and/or the relative resistance of effector T cells.124 In addition, it has been suggested that epitope spreading gives rise to pathogenic T cell clones that evade regulation by antigen-specific Tregs and may be associated with MS relapses.58 Interestingly, genetic variants linked to MS map to genes associated with immunoregulation, such as IL2RA and CTLA4.133
CD8+ T cells endowed with regulatory function have also been described.134-136 A decrease in their frequency and function has been reported in MS patients during relapses and to some extent in progressive MS patients.137,138 However, less is known about the regulatory mechanisms used by CD8+ regulatory T cells and their clinical relevance, in part due to the lack of specific markers to unequivocally define, characterize and study them. The recent characterization of CD8+ regulatory T cells characterized by the expression of inhibitory killer immunoglobulin-like receptors (KIRs) is likely to open new avenues for their investigation in MS.139
B cells also limit CNS inflammation, while promoting oligodendrogenesis and remyelination,140-142 but it is still unclear whether defects in B cell-driven immune regulation are linked to specific MS stages. Nevertheless, IL-10+ B cells controlled by the intestinal microbiome were recently reported to migrate to the CNS and suppress MS pathogenesis.41 The characterization of these and other immunoregulatory mechanisms may guide the development of antigen-specific therapies for MS and other autoimmune diseases.
CNS INFLAMMATION AND MS PROGRESSION
A pressing challenge in MS research is the identification of mechanisms of disease progression and therapeutic targets to arrest it. Indeed, most therapies targeting peripheral immune cells show limited efficacy in progressive MS, suggesting that compartmentalized CNS inflammation drives disease progression.143,144 Astrocytes, microglia, and oligodendrocytes play largely non-overlapping roles in the control of neurotransmission, phagocytosis, inflammation, neurodegeneration, and myelination.145-147 Importantly, each of these glial cell types is comprised of specialized cell subsets.72,148-151 Hence, the development of therapies for progressive MS requires the identification of relevant cell subsets and targets for their specific modulation.
Astrocytes are the most abundant glial cell in the CNS, providing trophic and metabolic support for neurons, while recycling neurotransmitters from the extracellular space.145 Microglia are resident macrophages that seed the CNS early during neurodevelopment and play key roles in the removal of cell debris and, together with astrocytes, the modulation of inflammation.146 Finally, oligodendrocytes myelinate CNS neuronal axons to ensure proper conduction of action potentials, while providing additional support for neuron metabolism. Oligodendrocytes are also the main target of the autoimmune response in MS.152 Recent studies have highlighted not only the heterogeneity of these cell types across different CNS regions and activation states, but also their functional interactions.
Neuroimmune cross-talk within the CNS in MS
Astrocytes, oligodendrocytes, microglia, and neurons control development, inflammation, metabolism, and neuronal activity through complex cell-cell interactions (Figure 3).145-147 Studies in MS patients and animal models identified vulnerabilities in defined neuron subsets (Figure 3A). An analysis of cortical lesions identified a population of layer 2-layer 3 cortical neurons that degenerate in MS,149 which displayed the activation of pathways linked to cell stress and disrupted metabolism, including CLU, NEFL, PPIA, and long non-coding RNAs. These findings suggest that stress-associated programs in neurons are linked to disease pathology, although it remains to be determined whether these programs promote or limit pathology. Moreover, studies in a cortical lesion animal model suggest that the activation of neuronal activity precedes neurodegeneration.153 For instance, elevated calcium signaling in dendritic spines precedes their elimination by macrophages or microglia. Consistent with these data, neural network dysfunction is detected in EAE (even in its remission phase) and in MS patients.154,155 These findings suggest that perturbations in neuronal networks drive neurodegeneration-promoting programs. However, it is still not clear to what extent spinal cord neurodegeneration in pre-clinical models recapitulates findings in the cortex of MS patients.
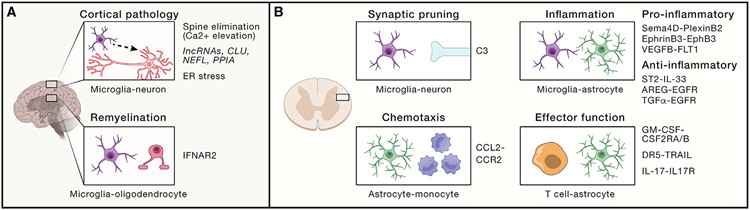
Figure 3. Cell-cell interactions in the CNS.
(A) Mechanisms driving cortical pathology identified in pre-clinical models and clinical samples. Remyelination deficits have been linked to microglia-oligodendrocyte cross-talk mediated via a type I interferon response. Additional mechanisms linked to neuron dysfunction include long non-coding RNAs, mitochondrial and endoplasmic reticulum-associated signaling pathways, and synaptic pruning controlled by calcium signaling.
(B) Mechanisms that mediate cross-talk among CNS-resident and CNS-recruited immune cells. C3 has been implicated in the microglial pruning of neuronal synapses (top-left). Astrocyte-produced chemokines promote the recruitment of pro-inflammatory CCR2+ monocytes via CCL2 (bottom-left). Ligand-receptor pairs implicated in the cross-talk among CNS-resident astrocytes and microglia promote regulatory or pro-inflammatory signals (top-right). Bidirectional modulation of astrocyte-T cell responses can boost or limit MS pathogenesis via epigenetic modifications, apoptosis, and pro-inflammatory cytokine signaling (bottom-right).
CNS-infiltrating immune cells and resident glial cells promote neurodegeneration and contribute to MS progression156 (Figure 3B). Indeed, pro-inflammatory monocyte recruitment to the CNS is correlated with EAE severity157; astrocytes promote monocyte recruitment to the CNS.158 In addition, inflammation-induced perturbations in astrocyte metabolism interfere with neuronal metabolic support.159,160 Interestingly, dysfunctional redox metabolism activates the unfolded protein response (UPR),161 which can boost pathology-promoting astrocyte responses in multiple contexts.52,162,163 The activation of the UPR-associated XBP1 transcription factor increases pro-inflammatory cytokine and chemokine production by astrocytes.52 Conversely, the specific ablation of XBP1 or its upstream activators IRE1a or SigmaR1 in astrocytes, ameliorates EAE, decreasing microglial activation and CNS infiltration by pro-inflammatory monocytes.52,163 Collectively, these data suggest that the therapeutic modulation of CNS-resident cells and their interactions may identify novel therapeutic targets for progressive MS.
Astrocytes and cell-cell interactions are attractive candidates for therapeutic intervention in MS and other neurologic diseases,145 but specific astrocyte responses and subsets have to be identified first. For example, the ablation of reactive astrocytes during the early phase of NOD EAE worsens the disease,80 but astrocyte ablation in the chronic progressive phase leads to disease amelioration, suggesting the need to target specific astrocyte pathogenic activities and/or functional subsets.80 Indeed, these pathogenic roles of astrocytes were driven by the dysregulation of sphingolipid metabolism through a signaling pathway involving cPLA2 and the MAVS protein, classically involved in anti-viral immune responses.159 cPLA2-MAVS signaling boosted pro-inflammatory NF-κB signaling and decreased production of lactate, which supports neuronal metabolism. Interestingly, MAVS+ astrocytes also express complement C3, a marker of an astrocyte subset that secretes neurotoxic lipids.164,165 C3 may also contribute to MS pathology by participating in microglial synaptic pruning.166
Additional microglial and astrocyte functions have also been linked to disease pathology in single-cell analyses of MS CNS samples.111 These studies agree with scRNA-seq analyses of microglia in MS patients148 and reports of microbiome-regulated communication between specific astrocyte and microglial subsets.34 Importantly, MS-associated microglial subpopulations bear transcriptional signatures similar to those found in other neurologic diseases.167
Finally, it should be kept in mind that some glial subsets actively limit CNS pathology. For example, microglial subsets have been found to support myelin integrity,168 limit neurodegeneration driven by oxidized phosphatidylcholine169 and actively engulf pathogenic TH17 cells.170 Similarly, a subset of TRAIL+ astrocytes has been shown to induce apoptosis in pathogenic T cells.35 Collectively, these data highlight the diversity of glial responses relevant to MS pathology, and the need to develop approaches for the monitoring and therapeutic modulation of specific glial subsets.
Interrogation of CNS-resident cell subsets in MS
The extent to which disease-associated microglial and astrocyte subsets interact to coordinate and regulate their responses is still not fully understood. However, new tools have enabled the study of glial subset interactions in health and disease. For instance, advanced sequencing methods have provided new insights. One example is PIC-seq, a scRNA-seq-based tool for the analysisof interacting cells isolated based on defined surface markers.171 PIC-seq was initially deployed to study DC-T cell biology, but it has been recently used to study CNS neuroimmune interactions.172
Likewise, novel barcoding approaches have enabled the comprehensive investigation of cell-cell interactions in the CNS. For example, RABID-seq labels interacting cells in vivo using a virus-delivered RNA-encoded barcode that can be detected by scRNA-seq.160 RABID-seq recently uncovered multiple interactions and communication mechanisms between astrocytes and microglia in the context of CNS inflammation, identifying Sema4D-PlexinB1/2 and EphrinB3-EphB3 signaling as candidate therapeutic targets for MS.
These and other approaches for the study of cell-cell interactions in vivo are complemented by novel platforms for the comprehensive investigation of mechanisms of cell communication in forward genetic screens, classic approaches to first screen phenotypes following systematic or random genetic perturbations and then identify the perturbed gene responsible. In particular, the need to enable forward genetic screens of cell-cell interaction mechanisms led to the development of SPEAC-seq (Systematic Perturbation of Encapsulated Associated Cells followed by sequencing). SPEAC-seq is based on the co-encapsulation in droplets of two cells: cell 1 harbors a random single guide RNA derived from a genome-wide CRISPR library while cell 2 expresses a reporter that indicates the effect that gene inactivation in cell 1 has on cell 2.173 Combining SPEAC-seq with an in vivo Perturb-seq screen, we recently identified microglia-produced amphiregulin as a suppressor of EAE-promoting astrocyte responses. The microglial production of amphiregulin was induced by ST2 signaling triggered by IL-33 released from astrocytes during EAE. This regulatory loop was also functional in human astrocytes and microglia. These and other tools for the study of cell-cell interactions, their perturbations, and in-depth characterization of the subsets involved are likely to identify novel mechanisms of disease pathogenesis and therapeutic targets for progressive MS.
Similarly, microglial subsets inhibit remyelination through their interactions with oligodendrocytes,174 such as via interferon alpha and beta receptor 2 (Figure 3A). It is possible that these microglial subsets cross-talk with oligodendrocyte subsets previously identified in EAE and MS, which express CDH20, MHC-II and other molecules that facilitate immune cell interactions.150,151 Although the specific role of MHC-II expressed by this oligodendrocyte subset remains to be further investigated, the modulation of the interaction between oligodendrocytes and immune cells may enable effective remyelination therapies for MS.152 Indeed, secreted factors including IGF-1, antibodies, and axon guidance cues, regardless of their cellular source, control remyelination in pre-clinical MS models and may guide the development of new therapies.5
Finally, an important concept is the distinction between bona fide cell populations and transcriptional activation states. For example, multiple astrocyte subsets have been identified in EAE and MS,72,111,149,150 but it is still unknown if they constitute populations with defined functions.145 Analogously, microglial subsets have been identified in MS and other disorders based on scRNA-seq transcriptional signatures,148,167 but recent studies suggest that some of these subsets include myeloid cells of different origins, with opposing roles in CNS pathology.175 Follow up studies on the origin, regulation and function of glial subsets identified in scRNA-seq analyses is needed. In this context, FIND-seq (Focused Interrogation of cells by Nucleic acid Detection and Sequencing) integrates nucleic acid cytometry and microfluidics to enable in-depth analyses of cell subsets of interest based on nucleic acid markers (RNA or DNA). In combination with genetic and perturbation approaches, FIND-seq may reveal the role of these subsets in the pathology of MS and other neurologic diseases.
THERAPEUTIC INTERVENTIONS
The development of disease modifying therapies (DMTs) for MS has been spectacular over the last three decades. Following IFN-β-1b approval in 1993, over 15 DMTs have been approved176 (Table 3). Most DMTs target the peripheral immune system, with mechanisms of action broadly defined as immunomodulatory, anti-trafficking or immunodepleting.
Table 3.
Current MS therapies
| Drug | Indication | Main Impact | Mechanism of Action |
|---|---|---|---|
| IFN-β-1a | Relapsing MS | Immunomodulation | Inhibits T cell division and CNS infiltration; Induces Tregs and suppressor B cells |
| Glatiramer acetate | Relapsing MS | Immunomodulation | Competes with peptide binding to MHC |
| Teriflunomide | Relapsing MS | Immunomodulation | Pyrimidine synthesis inhibitor |
| Dimethyl fumarate | Relapsing MS | Immunomodulation | NRF2 activation/antioxidant signaling |
| Diroximel fumarate | Relapsing MS | Immunomodulation | NRF2 activation/antioxidant signaling |
| Fingolimod | Relapsing MS | Inhibition of immune cell trafficking | Sphingosine-1-phosphate (S1P) receptor modulator |
| Siponimod | Relapsing MS SPMS | Inhibition of immune cell trafficking | Sphingosine-1-phosphate (S1P) receptor modulator |
| Ozanimod | Relapsing MS | Inhibition of immune cell trafficking | Sphingosine-1-phosphate (S1P) receptor agonist |
| Ponesimod | Relapsing MS | Inhibition of immune cell trafficking | Sphingosine-1-phosphate receptor 1 (S1PR1) modulator |
| Natalizumab | RRMS | Inhibition of immune cell trafficking | Anti-VLA-4 monoclonal antibody |
| Cladribine | Relapsing MS | Immunodepletion | Purine analog |
| Alemtuzumab | Relapsing MS | Immunodepletion | Anti-CD52 monoclonal antibody |
| Ocrelizumab | Relapsing MS PPMS | Immunodepletion | Anti-CD20 monoclonal antibody |
| Ofatumumab | Relapsing MS | Immunodepletion | Anti-CD20 monoclonal antibody |
Immunomodulatory therapies represent the earliest DMTs approved for MS. Their clinical efficacy results from targeting multiple immune cell subsets (mainly T cells) to re-balance the ratio of pro-to anti-inflammatory responses.177,178 These therapeutic agents include IFN-β, glatiramer acetate, teriflunomide and dimethyl fumarate.
Anti-trafficking therapies interfere with immune cell migration into the CNS at two different stages. The sphingosine-1-phosphate (S1P) receptor modulators fingolimod and siponimod sequester lymphocytes within lymph nodes, although S1P signaling also affects CNS-resident cells such as astrocytes.179,180 In contrast, natalizumab directly inhibits the recruitment of circulating leukocytes into the CNS by blocking VLA-4. Of note, this mechanism of action interferes with physiologic CNS immune patrolling and has been linked to John Cunningham virus reactivation and progressive multifocal leukoencephalopathy in natalizumab-treated patients.181
Immunodepleting therapies target adaptive immune cells. Approved to treat RRMS and active progressive MS, cladribine (purine analogue) and alemtuzumab (anti-CD52) target T cells and B cells, whereas ocrelizumab, ofatumumab and ublituximab (anti-CD20) specifically deplete B cells.140,182,183 In 2017, ocrelizumab became the first DMT approved for PPMS.184 B-cell directed therapies targeting CD20 show strong suppressive effects on pathogenic T cell responses. However, it should be noted that patients on anti-CD20 therapy and fingolimod show reduced responses to vaccination against COVID-19.185 In addition, the recombinant protein atacicept, which blocks B-cell function and proliferation, failed to control MS relapses and triggered disease exacerbations,186 probably because of its activity against IL-10+ anti-inflammatory IgA+ B cells,41 which are not targeted by anti-CD20 antibodies. These findings highlight the role of B cells in the control of T cell autoimmunity in MS, as well as the challenges linked to their therapeutic targeting.
NOVEL THERAPEUTIC APPROACHES AND TARGETS
Currently available DMTs show limited success in halting MS progression.144 However, numerous promising treatments are currently in advanced clinical trials. One example is that of Bruton tyrosine kinase (BTK) inhibitors, which target B cells and peripheral myeloid cells, limiting their pathogenic cross-talk with T cells.187 In addition, due to their capacity to cross the BBB, some BTK inhibitors may suppress microglial pathogenic functions and promote remyelination,188,189 offering hope for the treatment of progressive MS.
One desired feature of novel therapies for MS and other autoimmune diseases is the re-establishment of antigen-specific tolerance, without impacting protective immunity against infectious agents and tumors. However, the development of these tolerogenic approaches is not risk free, as indicated by disease exacerbation in MS patients treated with a modified myelin peptide thought to be endowed with tolerogenic activity.190 Promising approaches for the induction of antigen-specific tolerance involve cell- or nanoparticle-based methods. The former includes the administration of antigen-loaded tolerogenic DCs, autoantigen-specific Treg cells or chimeric antigen receptor T cell-based approaches.191,192 However, logistic challenges limit the clinical implementation of cell-based therapies.
Alternatively, antigen-specific immune tolerance has been induced with nanoparticles loaded with myelin antigens and tolerogenic adjuvants such as aryl hydrocarbon receptor agonists and other factors.130,193,194 More recently, mRNA vaccines modified to prevent immune activation were shown to induce immune tolerance in EAE,195 recapitulating previous observations made with DNA vaccines.196 Interestingly, these nanoparticle- and mRNA-based approaches induce bystander suppression, an important feature considering the challenge imposed by epitope spreading and the heterogeneity of the autoantigens targeted in different MS patients. It remains to be seen whether the lack of an adjuvant enforcing tolerance in mRNA vaccines induces tolerogenic responses strong enough to arrest the ongoing inflammatory response in MS.
Finally, the identification of environmental factors associated with MS development opens new therapeutic avenues ranging from EBV vaccines49 to supplementation with specific probiotics, their metabolites, or even synthetic probiotics engineered to produce therapeutic molecules.197 Additional therapeutic avenues for MS could stem from studies focused on circadian biology and pollution. For example, the reported effects of melatonin and pollutants on regulatory and pro-inflammatory responses,52,198 may provide further insight into environmentally controlled pathways that play important but less well understood roles in MS pathogenesis.23
Conclusions
Halting MS progression remains an unmet clinical need. Recent single-cell studies have provided unique insights into MS pathology, uncovering CNS genomic signatures associated with disease.35,72,111,148-150,163 The integration of these findings with in situ profiling methods will enable the identification of perturbed neuroimmune interactions relevant for MS progression amenable to therapeutic targeting. It should be kept in mind, however, that age is strongly associated with MS progression, reflecting the impact of senescence on pro-inflammatory, neurodegenerative, and tissue repair responses.199 For instance, senescence in progressive MS was recently linked to impaired oligodendrocyte progenitor cell maturation and remyelination.200 Thus, the study of aging may provide novel insights on the biology of MS progression.
In summary, we have witnessed remarkable progress in our understanding of MS pathogenesis and the development of DMTs. In addition, groundbreaking studies have uncovered environmental and genetic factors relevant for MS pathogenesis. The identification of mechanisms of disease progression, and biomarkers to monitor and target specific cell-subsets involved in MS, may provide new therapeutic strategies for MS.
ACKNOWLEDGMENTS
Work in the Quintana lab is supported by grants NS102807, ES02530, ES029136, AI126880 from the NIH; RG4111A1 and JF2161-A-5 from the NMSS; RSG-14-198-01-LIB from the American Cancer Society; and PA-1604-08459 from the International Progressive MS Alliance. M.C. is supported by a postdoctoral fellowship from the MS Society of Canada. M.A.W. was supported by the NIH (R01MH130458, R00NS114111, F32NS101790), a training grant from the NIH and Dana-Farber Cancer Institute (T32CA207201), a traveling neuroscience fellowship from the Program in Interdisciplinary Neuroscience at Brigham and Women’s Hospital, and the Women’s Brain Initiative at Brigham and Women’s Hospital. We apologize to investigators whose research we could not cite because of space limitations.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, et al. (2020). 26, . Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler 26, 1816–1821. 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray TJ (2009). The history of multiple sclerosis: the changing frame of the disease over the centuries. J. Neurol. Sci 277 (Suppl 1), S3–S8. 10.1016/S0022-510X(09)70003-6. [DOI] [PubMed] [Google Scholar]
- 3.Charcot J-M (1868). Histologie de la sclèrose en plaques. Gaz. Hosp 41, 554–556. [Google Scholar]
- 4.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, and Bo L (1998). Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med 338, 278–285. 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 5.Reich DS, Lucchinetti CF, and Calabresi PA (2018). Multiple sclerosis. N. Engl. J. Med 378, 169–180. 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshaghi A, Young AL, Wijeratne PA, Prados F, Arnold DL, Narayanan S, Guttmann CRG, Barkhof F, Alexander DC, Thompson AJ, et al. (2021). Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat. Commun 12, 2078. 10.1038/s41467-021-22265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolb H, Absinta M, Beck ES, Ha SK, Song Y, Norato G, Cortese I, Sati P, Nair G, and Reich DS (2021). 7T MRI differentiates remyelinated from demyelinated multiple sclerosis lesions. Ann. Neurol 90, 612–626. 10.1002/ana.26194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodini B, Tonietto M, Airas L, and Stankoff B (2021). Positron emission tomography in multiple sclerosis - straight to the target. Nat. Rev. Neurol 17, 663–675. 10.1038/s41582-021-00537-1. [DOI] [PubMed] [Google Scholar]
- 9.Poutiainen P, Jaronen M, Quintana FJ, and Brownell AL (2016). Precision medicine in multiple sclerosis: future of PET imaging of inflammation and reactive astrocytes. Front. Mol. Neurosci 9, 85. 10.3389/fnmol.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benkert P, Meier S, Schaedelin S, Manouchehrinia A, Yaldizli Ö, Maceski A, Oechtering J, Achtnichts L, Conen D, Derfuss T, et al. (2022). Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 21, 246–257. 10.1016/S1474-4422(22)00009-6. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KC, Sotirchos ES, Smith MD, Lord HN, DuVal A, Mowry EM, and Calabresi PA (2022). Contributors to serum NfL levels in people without neurologic disease. Ann. Neurol 92, 688–698. 10.1002/ana.26446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelhak A, Huss A, Kassubek J, Tumani H, and Otto M (2018). Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci. Rep 8, 14798. 10.1038/s41598-018-33158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintana FJ, Farez MF, Viglietta V, Iglesias AH, Merbl Y, Izquierdo G, Lucas M, Basso AS, Khoury SJ, Lucchinetti CF, et al. (2008). Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 105, 18889–18894. 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar-Or A, Vollmer T, Antel J, Arnold DL, Bodner CA, Campagnolo D, Gianettoni J, Jalili F, Kachuck N, Lapierre Y, et al. (2007). Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch. Neurol 64, 1407–1415. 10.1001/archneur.64.10.nct70002. [DOI] [PubMed] [Google Scholar]
- 15.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, et al. (2016). Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 22, 586–597. 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Stefano N, Giorgio A, Tintoré M, Pia Amato M, Kappos L, Palace J, Yousry T, Rocca MA, Ciccarelli O, Enzinger C, et al. (2018). Radiologically isolated syndrome or subclini calmultiple sclerosis: MAGNIMS consensus recommendations. Mult. Scler 24, 214–221. 10.1177/1352458517717808. [DOI] [PubMed] [Google Scholar]
- 17.Hauser SL, and Cree BAC (2020).Treatment of multiple sclerosis: a review. Am. J. Med 133, 1380–1390.e2. 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, Model F, Koendgen H, Manfrini M, Belachew S, and Hauser SL (2020). Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 77, 1132–1140. 10.1001/jamaneurol.2020.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voskuhl R, and Itoh Y (2022). The X factor in neurodegeneration. J. Exp. Med 219, e20211488. 10.1084/jem.20211488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, Mandrekar J, Bramow S, Metz I, Brück W, et al. (2015). Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol 78, 710–721. 10.1002/ana.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, and Lassmann H (2005). Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712. 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 22.Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, and Reynolds R (2010). A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol 68, 477–493. 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 23.Olsson T, Barcellos LF, and Alfredsson L (2017). Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol 13, 25–36. 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 24.Bjornevik K, Munger KL, Cortese M, Barro C, Healy BC, Niebuhr DW, Scher AI, Kuhle J, and Ascherio A (2020). Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 77, 58–64. 10.1001/jamaneurol.2019.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranzini SE, and Oksenberg JR (2017). The genetics of multiple sclerosis: from 0 to 200 in 50 years. Trends Genet. 33, 960–970. 10.1016/j.tig.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Multiple Sclerosis Genetics Consortium (2019). Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, eaav7188. 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, Madjovski A, Ruhrmann S, Faigle W, Frauenknecht K, et al. (2018). Memory B cells activate brain-homing, autoreactive CD4(+) T cells in multiple sclerosis. Cell 175, 85–100.e23. 10.1016/j.cell.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Jelcic I, Mühlenbruch L, Haunerdinger V, Toussaint NC, Zhao Y, Cruciani C, Faigle W, Naghavian R, Foege M, et al. (2020). HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 183, 1264–1281.e20. 10.1016/j.cell.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponath G, Lincoln MR, Levine-Ritterman M, Park C, Dahlawi S, Mubarak M, Sumida T, Airas L, Zhang S, Isitan C, et al. (2018). Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat. Commun 9, 5337. 10.1038/s41467-018-07785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen K, Reichelt M, Kyauk RV, Ngu H, Shen YAA, Foreman O, Modrusan Z, Friedman BA, Sheng M, and Yuen TJ (2021). Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 34, 108835. 10.1016/j.celrep.2021.108835. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki A, and Quintana FJ (2020). The Gut-CNS axis in multiple sclerosis. Trends Neurosci. 43, 622–634. 10.1016/j.tins.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.iMSMS Consortium. Electronic address: sergio.baranzini@ucsf.edu, iMSMS Consortium (2022). Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell 185, 3467–3486.e16. 10.1016/j.cell.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, and Krishnamoorthy G (2011). Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 34.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, Li Z, Giovannoni F, Batterman KV, Scalisi G, et al. (2021). Gut-licensed IFNgamma(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature 590, 473–479. 10.1038/s41586-020-03116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, et al. (2017). Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 114, 10713–10718. 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, et al. (2017). Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 114, 10719–10724. 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, et al. (2016). Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun 7, 12015. 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox LM, Maghzi AH, Liu S, Tankou SK, Dhang FH, Willocq V, Song A, Wasén C, Tauhid S, Chu R, et al. (2021). Gut microbiome in progressive multiple sclerosis. Ann. Neurol 89, 1195–1211. 10.1002/ana.26084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, Song A, Dhang FH, Wei Z, Costamagna G, and Weiner HL (2019). Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding akkermansia muciniphila. Cell Host Microbe 26, 779–794.e8. 10.1016/j.chom.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rojas OL, Pröbstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, et al. (2019). Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 176, 610–624.e18. 10.1016/j.cell.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pröbstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, Sellrie K, Bischof A, Kim K, Ramesh A, et al. (2020). Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol 5, eabc7191. 10.1126/sciimmunol.abc7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosang L, Canals RC, van der Flier FJ, Hollensteiner J, Daniel R, Flügel A, and Odoardi F (2022). The lung microbiome regulates brain autoimmunity. Nature 603, 138–144. 10.1038/s41586-022-04427-4. [DOI] [PubMed] [Google Scholar]
- 44.Marie P (1884). Sclérose en plaques et Maladies infectieuses. Prog. Med. : journal de médecine, de chirurgie et de pharmacie 12. [Google Scholar]
- 45.Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, and Ascherio A (2022). Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301. 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 46.Lang HLE, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, et al. (2002). A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol 3, 940–943. 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 47.Wucherpfennig KW, and Strominger JL (1995). Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80, 695–705. 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, Fernandes RA, Gomez AM, Nadj GS, Bartley CM, et al. (2022). Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603, 321–327. 10.1038/s41586-022-04432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei CJ, Bu W, Nguyen LA, Batchelor JD, Kim J, Pittaluga S, Fuller JR, Nguyen H, Chou TH, Cohen JI, and Nabel GJ (2022).A bivalent Epstein-Barr virus vaccine induces neutralizing antibodies that block infection and confer immunity in humanized mice. Sci. Transl. Med 14, eabf3685. 10.1126/scitranslmed.abf3685. [DOI] [PubMed] [Google Scholar]
- 50.Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B, Ysrraelit MC, Zhu C, et al. (2015). Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162, 1338–1352. 10.1016/j.cell.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy DG, Chen J, Mamane V, Ma EH, Muhire BM, Sheldon RD, Shorstova T, Koning R, Johnson RM, Esaulova E, et al. (2020). Methionine metabolism shapes T helper cell responses through regulation of epigenetic reprogramming. Cell Metab. 31, 250–266.e9. 10.1016/j.cmet.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Wheeler MA, Jaronen M, Covacu R, Zandee SEJ, Scalisi G, Rothhammer V, Tjon EC, Chao CC, Kenison JE, Blain M, et al. (2019). Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596.e18. 10.1016/j.cell.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hohlfeld R, Dornmair K, Meinl E, and Wekerle H (2016). The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 15, 198–209. 10.1016/S1474-4422(15)00334-8. [DOI] [PubMed] [Google Scholar]
- 54.Ben-Nun A, Wekerle H, and Cohen IR (1981). The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur. J. Immunol 11, 195–199. 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- 55.Zamvil S, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, and Steinman L (1985). T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature 317, 355–358. 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 56.Saligrama N, Zhao F, Sikora MJ, Serratelli WS, Fernandes RA, Louis DM, Yao W, Ji X, Idoyaga J, Mahajan VB, et al. (2019). Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572, 481–487. 10.1038/s41586-019-1467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner CA, Roqué PJ, Mileur TR, Liggitt D, and Goverman JM (2020). Myelin-specific CD8+ T cells exacerbate brain inflammation in CNS autoimmunity. J. Clin. Invest 130, 203–213. 10.1172/JCI132531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinman L (2014). Immunology of relapse and remission in multiple sclerosis. Annu. Rev. Immunol 32, 257–281. 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- 59.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. (2012). Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol 13, 991–999. 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, and Cua DJ (2007). TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol 8, 1390–1397. 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 61.Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, Ferrero B, Eid P, and Novelli F (2009). T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann. Neurol 65, 499–509. 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 62.Huber AK, Wang L, Han P, Zhang X, Ekholm S, Srinivasan A, Irani DN, and Segal BM (2014). Dysregulation of the IL-23/IL-17 axis and myeloid factors in secondary progressive MS. Neurology 83, 1500–1507. 10.1212/WNL.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu D, Notarbartolo S, Croonenborghs T, Patel B, Cialic R, Yang TH, Aschenbrenner D, Andersson KM, Gattorno M, Pham M, et al. (2017). Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10gene expression in multiple sclerosis. Nat. Commun 8, 1600. 10.1038/s41467-017-01571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, et al. (2015). Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225. 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schnell A, Huang L, Singer M, Singaraju A, Barilla RM, Regan BML, Bollhagen A, Thakore PI, Dionne D, Delorey TM, et al. (2021). Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 184, 6281–6298.e23. 10.1016/j.cell.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bielecki P, Riesenfeld SJ, Hütter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, Lian M, Amezcua Vesely MC, Kroehling L, Xu H, et al. (2021). Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 592, 128–132. 10.1038/s41586-021-03188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jäger A, Dardalhon V, Sobel RA, Bettelli E, and Kuchroo VK (2009). Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol 183, 7169–7177. 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amorim A, De Feo D, Friebel E, Ingelfinger F, Anderfuhren CD, Krishnarajah S, Andreadou M, Welsh CA, Liu Z, Ginhoux F, et al. (2022). IFNgamma and GM-CSF control complementary differentiation programs in the monocyte-to-phagocyte transition during neuroinflammation. Nat. Immunol 23, 217–228. 10.1038/s41590-021-01117-7. [DOI] [PubMed] [Google Scholar]
- 69.Murphy AC, Lalor SJ, Lynch MA, and Mills KHG (2010). Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun 24, 641–651. 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 70.Moore CS, Cui QL, Warsi NM, Durafourt BA, Zorko N, Owen DR, Antel JP, and Bar-Or A (2015). Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. J. Immunol 194, 761–772. 10.4049/jimmunol.1401156. [DOI] [PubMed] [Google Scholar]
- 71.Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, et al. (2010). In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33, 424–436. 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 72.Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V, et al. (2020). MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599. 10.1038/s41586-020-1999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, and Becher B (2011). RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol 12, 560–567. 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 74.Komuczki J, Tuzlak S, Friebel E, Hartwig T, Spath S, Rosenstiel P, Waisman A, Opitz L, Oukka M, Schreiner B, et al. (2019). Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1beta. Immunity 50, 1289–1304.e6. 10.1016/j.immuni.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Galli E, Hartmann FJ, Schreiner B, Ingelfinger F, Arvaniti E, Diebold M, Mrdjen D, van der Meer F, Krieg C, Nimer FA, et al. (2019). GM-CSF and CXCR4 define a T helper cell signature in multiple sclerosis. Nat. Med 25, 1290–1300. 10.1038/s41591-019-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasouli J, Casella G, Yoshimura S, Zhang W, Xiao D, Garifallou J, Gonzalez MV, Wiedeman A, Kus A, Mari ER, et al. (2020).A distinct GM-CSF(+) T helper cell subset requires T-bet to adopt a T(H)1 phenotype and promote neuroinflammation. Sci. Immunol 5, eaba9953. 10.1126/sciimmunol.aba9953. [DOI] [PubMed] [Google Scholar]
- 77.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, and Rostami A (2011). The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol 12, 568–575. 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, and Becher B (2015). The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43, 502–514. 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, and Dittel BN (2007). GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol 178, 39–48. 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 80.Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisäkk P, Kallas K, et al. (2014). Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med 20, 1147–1156. 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Machado-Santos J, Saji E, Tröscher AR, Paunovic M, Liblau R, Gabriely G, Bien CG, Bauer J, and Lassmann H (2018). The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141, 2066–2082. 10.1093/brain/awy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fransen NL, Hsiao CC, van der Poel M, Engelenburg HJ, Verdaasdonk K, Vincenten MCJ, Remmerswaal EBM, Kuhlmann T, Mason MRJ, Hamann J, et al. (2020). Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 143, 1714–1730. 10.1093/brain/awaa117. [DOI] [PubMed] [Google Scholar]
- 83.Frieser D, Pignata A, Khajavi L, Shlesinger D, Gonzalez-Fierro C, Nguyen XH, Yermanos A, Merkler D, Höftberger R, Desestret V, et al. (2022). Tissue-resident CD8(+) T cells drive compartmentalized and chronic autoimmune damage against CNS neurons. Sci. Transl. Med 14, eabl6157. 10.1126/scitranslmed.abl6157. [DOI] [PubMed] [Google Scholar]
- 84.Vincenti I, Page N, Steinbach K, Yermanos A, Lemeille S, Nunez N, Kreutzfeldt M, Klimek B, Di Liberto G, Egervari K, et al. (2022). Tissue-resident memory CD8(+) T cells cooperate with CD4(+) T cells to drive compartmentalized immunopathology in the CNS. Sci. Transl. Med 14, eabl6058. 10.1126/scitranslmed.abl6058. [DOI] [PubMed] [Google Scholar]
- 85.Gee MH, Han A, Lofgren SM, Beausang JF, Mendoza JL, Birnbaum ME, Bethune MT, Fischer S, Yang X, Gomez-Eerland R, et al. (2018). Antigen identification for orphan T cell receptors expressed on tumor-infiltrating lymphocytes. Cell 172, 549–563.e16. 10.1016/j.cell.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kula T, Dezfulian MH, Wang CI, Abdelfattah NS, Hartman ZC, Wucherpfennig KW, Lyerly HK, and Elledge SJ (2019). T-Scan: a genome-wide method for the systematic discovery of T cell epitopes. Cell 178, 1016–1028.e13. 10.1016/j.cell.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li G, Bethune MT, Wong S, Joglekar AV, Leonard MT, Wang JK, Kim JT, Cheng D, Peng S, Zaretsky JM, et al. (2019). T cell antigen discovery via trogocytosis. Nat. Methods 16, 183–190. 10.1038/s41592-018-0305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li R, Patterson KR, and Bar-Or A (2018). Reassessing B cell contributions in multiple sclerosis. Nat. Immunol 19, 696–707. 10.1038/s41590-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 89.Lisak RP, Nedelkoska L, Benjamins JA, Schalk D, Bealmear B, Touil H, Li R, Muirhead G,and Bar-Or A (2017). B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J. Neuroimmunol 309, 88–99. 10.1016/j.jneuroim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Zamvil SS, and Hauser SL (2021). Antigen presentation by B cells in multiple sclerosis. N. Engl. J. Med 384, 378–381. 10.1056/NEJMcibr2032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, Amato MP, Asgari N, Banwell B, Bennett J, et al. (2021). Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 20, 762–772. 10.1016/S1474-4422(21)00218-0. [DOI] [PubMed] [Google Scholar]
- 92.Quintana FJ, Patel B, Yeste A, Nyirenda M, Kenison J, Rahbari R, Fetco D, Hussain M, O’Mahony J, Magalhaes S, et al. (2014). Epitope spreading as an early pathogenic event in pediatric multiple sclerosis. Neurology 83, 2219–2226. 10.1212/WNL.0000000000001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quintana FJ, Farez MF, Izquierdo G, Lucas M, Cohen IR, and Weiner HL (2012). Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology 78, 532–539. 10.1212/WNL.0b013e318247f9f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, and Steinman L (2007). Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 448, 474–479. 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 95.van Zwam M, Huizinga R, Melief MJ, Wierenga-Wolf AF, van Meurs M, Voerman JS, Biber KPH, Boddeke HWGM, Höpken UE, Meisel C, et al. (2009). Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J. Mol. Med 87, 273–286. 10.1007/s00109-008-0421-4. [DOI] [PubMed] [Google Scholar]
- 96.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. (2018). CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci 21, 1380–1391. 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, et al. (2019). Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66. 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- 98.Merlini A, Haberl M, Strauß J, Hildebrand L, Genc N, Franz J, Chilov D, Alitalo K, Flügel-Koch C, Stadelmann C, et al. (2022). Distinct roles of the meningeal layers in CNS autoimmunity. Nat. Neurosci 25, 887–899. 10.1038/s41593-022-01108-3. [DOI] [PubMed] [Google Scholar]
- 99.Hiltensperger M, Beltrán E, Kant R, Tyystjärvi S, Lepennetier G, Domínguez Moreno H, Bauer IJ, Grassmann S, Jarosch S, Schober K, et al. (2021). Skin and gut imprinted helper T cell subsets exhibit distinct functional phenotypes in central nervous system autoimmunity. Nat. Immunol 22, 880–892. 10.1038/s41590-021-00948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WEF, Flügel-Koch C, Issekutz TB, Wekerle H, and Flügel A (2009). Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98. 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 101.Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WEF, et al. (2012). T cells become licensed in the lung to enter the central nervous system. Nature 488, 675–679. 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 102.Schläger C, Körner H, Krueger M, Vidoli S, Haberl M, Mielke D, Brylla E, Issekutz T, Cabañas C, Nelson PJ, et al. (2016). Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353. 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 103.Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, and Becher B (2019). Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol 4, eaau8380. 10.1126/sciimmunol.aau8380. [DOI] [PubMed] [Google Scholar]
- 104.Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N, et al. (2019). Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554. 10.1126/science.aat7554. [DOI] [PubMed] [Google Scholar]
- 105.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, and Becher B (2005). Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med 11, 328–334. 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 106.Ifergan I, Kébir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, Arbour N, and Prat A (2008). The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain 131, 785–799. 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- 107.Kooi EJ, van Horssen J, Witte ME, Amor S, Bo L, Dijkstra CD, van der Valk P, and Geurts JJG (2009). Abundant extracellular myelin in the meninges of patients with multiple sclerosis. Neuropathol. Appl. Neurobiol 35, 283–295. 10.1111/j.1365-2990.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 108.Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, Papadopoulos Z, Kanamori M, Salvador AF, Baker W, et al. (2021). Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000–1016.e27. 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodríguez-Lorenzo S, Konings J, van der Pol S, Kamermans A, Amor S, van Horssen J, Witte ME, Kooij G, and de Vries HE (2020). Inflammation of the choroid plexus in progressive multiple sclerosis: accumulation of granulocytes and T cells. Acta Neuropathol. Commun 8, 9. 10.1186/s40478-020-0885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu M, Laaker C, Madrid A, Herbath M, Choi YH, Sandor M, and Fabry Z (2022). Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate. Nat. Immunol 23, 581–593. 10.1038/s41590-022-01158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, Fitzgerald KC, Song A, Liu P, Lin JP, et al. (2021). A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714. 10.1038/s41586-021-03892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fabriek BO, Van Haastert ES, Galea I, Polfliet MMJ, Döpp ED, Van Den Heuvel MM, Van Den Berg TK, De Groot CJA, Van Der Valk P, and Dijkstra CD (2005). CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia 51, 297–305. 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- 113.Ramaglia V, Sheikh-Mohamed S, Legg K, Park C, Rojas OL, Zandee S, Fu F, Ornatsky O, Swanson EC, Pitt D, et al. (2019). Multiplexed imaging of immune cells in staged multiple sclerosis lesions by mass cytometry. Elife 8, e48051. 10.7554/eLife.48051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grigg JB, Shanmugavadivu A, Regen T, Parkhurst CN, Ahmed A, Joseph AM, Mazzucco M, Gronke K, Diefenbach A, Eberl G, et al. (2021). Antigen-presenting innate lymphoid cells orchestrate neuroinflammation. Nature 600, 707–712. 10.1038/s41586-021-04136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whittaker Hawkins RF, Patenaude A, Dumas A, Jain R, Tesfagiorgis Y, Kerfoot S, Matsui T, Gunzer M, Poubelle PE, Larochelle C, et al. (2017). ICAM1+ neutrophils promote chronic inflammation via ASPRV1 in B cell-dependent autoimmune encephalomyelitis. JCI Insight 2, e96882. 10.1172/jci.insight.96882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Møllgård K, Beinlich FRM, Kusk P, Miyakoshi LM, Delle C, Plá V, Hauglund NL, Esmail T, Rasmussen MK, Gomolka RS, et al. (2023). A mesothelium divides the subarachnoid space into functional compartments. Science 379, 84–88. 10.1126/science.adc8810. [DOI] [PubMed] [Google Scholar]
- 117.Daneman R, and Prat A (2015). The blood-brain barrier. Cold Spring Harb. Perspect. Biol 7, a020412. 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alves de Lima K, Rustenhoven J, and Kipnis J (2020). Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol 38, 597–620. 10.1146/annurev-immunol-102319-103410. [DOI] [PubMed] [Google Scholar]
- 119.Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J, et al. (2018). Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci 21, 1209–1217. 10.1038/s41593-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mazzitelli JA, Smyth LCD, Cross KA, Dykstra T, Sun J, Du S, Mamuladze T, Smirnov I, Rustenhoven J, and Kipnis J (2022). Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat. Neurosci 25, 555–560. 10.1038/s41593-022-01029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lun MP, Monuki ES, and Lehtinen MK (2015). Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci 16, 445–457. 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Charabati M, Grasmuck C, Ghannam S, Bourbonnière L, Fournier AP, Lécuyer MA, Tastet O, Kebir H, Rébillard RM, Hoornaert C, et al. (2022). DICAM promotes TH17 lymphocyte trafficking across the blood-brain barrier during autoimmune neuroinflammation. Sci. Transl. Med 14, eabj0473. 10.1126/scitranslmed.abj0473. [DOI] [PubMed] [Google Scholar]
- 123.Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, et al. (2006). Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med 354, 924–933. 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dominguez-Villar M, and Hafler DA (2018). Regulatory T cells in autoimmune disease. Nat. Immunol 19, 665–673. 10.1038/s41590-018-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, and Kuchroo VK (2010). The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol 11, 854–861. 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, and Quintana FJ (2010). Activation of thearyl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol 11, 846–853. 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, et al. (2015). Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med 21, 638–646. 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, and Lafaille JJ (2008). Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29, 114–126. 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 129.Dombrowski Y, O’Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, Fleville S, Eleftheriadis G, Zhao C, Naughton M, et al. (2017). Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci 20, 674–680. 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kenison JE, Jhaveri A, Li Z, Khadse N, Tjon E, Tezza S, Nowakowska D, Plasencia A, Stanton VP Jr., Sherr DH, and Quintana FJ (2020). Tolerogenic nanoparticles suppress central nervous system inflammation. Proc. Natl. Acad. Sci. USA 117, 32017–32028. 10.1073/pnas.2016451117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mayo L, Cunha APD, Madi A, Beynon V, Yang Z, Alvarez JI, Prat A, Sobel RA, Kobzik L, Lassmann H, et al. (2016). IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain 139, 1939–1957. 10.1093/brain/aww113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vogelaar CF, Mandal S, Lerch S, Birkner K, Birkenstock J, Bühler U, Schnatz A, Raine CS, Bittner S, Vogt J, et al. (2018). Fast direct neuronal signaling via the IL-4 receptor as therapeutic target in neuroinflammation. Sci. Transl. Med 10, eaao2304. 10.1126/scitranslmed.aao2304. [DOI] [PubMed] [Google Scholar]
- 133.International Multiple Sclerosis Genetics Consortium, Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PIW, Gabriel SB, Mirel DB, et al. (2007). Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med 357, 851–862. 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 134.Jiang H, and Chess L (2006). Regulation of immune responses by T cells. N. Engl. J. Med 354, 1166–1176. 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 135.Kim HJ, Verbinnen B, Tang X, Lu L, and Cantor H (2010). Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467, 328–332. 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lider O, Reshef T, Beraud E, Ben-Nun A, and Cohen IR (1988). Anti-idiotypic network induced by T cell vaccination against experimental autoimmune encephalomyelitis. Science 239, 181–183. 10.1126/science.2447648. [DOI] [PubMed] [Google Scholar]
- 137.Baughman EJ, Mendoza JP, Ortega SB, Ayers CL, Greenberg BM, Frohman EM, and Karandikar NJ (2011). Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J. Autoimmun 36, 115–124. 10.1016/j.jaut.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balashov KE, Khoury SJ, Hafler DA, and Weiner HL (1995). Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J. Clin. Invest 95, 2711–2719. 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li J, Zaslavsky M, Su Y, Guo J, Sikora MJ, van Unen V, Christophersen A, Chiou SH, Chen L, Li J, et al. (2022). KIR(+)CD8(+) T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 376, eabi9591. 10.1126/science.abi9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cencioni MT, Mattoscio M, Magliozzi R, Bar-Or A, and Muraro PA (2021). B cells in multiple sclerosis - from targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol 17, 399–414. 10.1038/s41582-021-00498-5. [DOI] [PubMed] [Google Scholar]
- 141.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, and Anderton SM (2002). B cells regulate autoimmunity by provision of IL-10. Nat. Immunol 3, 944–950. 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 142.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, and Tedder TF (2012). Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268. 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thompson A, and Ciccarelli O (2020). Towards treating progressive multiple sclerosis. Nat. Rev. Neurol 16, 589–590. 10.1038/s41582-020-00421-4. [DOI] [PubMed] [Google Scholar]
- 144.Dangond F, Donnelly A, Hohlfeld R, Lubetzki C, Kohlhaas S, Leocani L, Ciccarelli O, Stankoff B, Sormani MP, Chataway J, et al. (2021). Facing the urgency of therapies for progressive MS - a progressive MS alliance proposal. Nat. Rev. Neurol 17, 185–192. 10.1038/s41582-020-00446-9. [DOI] [PubMed] [Google Scholar]
- 145.Lee HG, Wheeler MA, and Quintana FJ (2022). Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov 21, 339–358. 10.1038/s41573-022-00390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prinz M, Masuda T, Wheeler MA, and Quintana FJ (2021). Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu. Rev. Immunol 39, 251–277. 10.1146/annurev-immunol-093019-110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Linnerbauer M, Wheeler MA, and Quintana FJ (2020). Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622. 10.1016/j.neuron.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Scheiwe C, Nessler S, Kunz P, van Loo G, Coenen VA, and Reinacher PC (2019). Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392. 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 149.Schirmer L, Velmeshev D, Holmqvist S, Kaufmann M, Werneburg S, Jung D, Vistnes S, Stockley JH, Young A, Steindel M, et al. (2019). Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82. 10.1038/s41586-019-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jäkel S, Agirre E, Mendanha Falcão A, van Bruggen D, Lee KW, Knuesel I, Malhotra D, Ffrench-Constant C, Williams A, and Castelo-Branco G (2019). Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547. 10.1038/s41586-019-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, Floriddia EM, Vanichkina DP, Ffrench-Constant C, Williams A, and Guerreiro-Cacais AO (2018). Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med 24, 1837–1844. 10.1038/s41591-018-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Franklin RJM, and Ffrench-Constant C (2017). Regenerating CNS myelin - from mechanisms to experimental medicines. Nat. Rev. Neurosci 18, 753–769. 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 153.Jafari M, Schumacher AM, Snaidero N, Ullrich Gavilanes EM, Neziraj T, Kocsis-Jutka V, Engels D, Jürgens T, Wagner I, Weidinger JDF, et al. (2021). Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat. Neurosci 24, 355–367. 10.1038/s41593-020-00780-7. [DOI] [PubMed] [Google Scholar]
- 154.Chard DT, Alahmadi AAS, Audoin B, Charalambous T, Enzinger C, Hulst HE, Rocca MA, Rovira À, Sastre-Garriga J, Schoonheim MM, et al. (2021). Mind the gap: from neurons to networks to outcomes in multiple sclerosis. Nat. Rev. Neurol 17, 173–184. 10.1038/s41582-020-00439-8. [DOI] [PubMed] [Google Scholar]
- 155.Ellwardt E, Pramanik G, Luchtman D, Novkovic T, Jubal ER, Vogt J, Arnoux I, Vogelaar CF, Mandal S, Schmalz M, et al. (2018). Maladaptive cortical hyperactivity upon recovery from experimental autoimmune encephalomyelitis. Nat. Neurosci 21, 1392–1403. 10.1038/s41593-018-0193-2. [DOI] [PubMed] [Google Scholar]
- 156.Healy LM, Stratton JA, Kuhlmann T, and Antel J (2022). The role of glial cells in multiple sclerosis disease progression. Nat. Rev. Neurol 18, 237–248. 10.1038/s41582-022-00624-x. [DOI] [PubMed] [Google Scholar]
- 157.Ajami B, Samusik N, Wieghofer P, Ho PP, Crotti A, Bjornson Z, Prinz M, Fantl WJ, Nolan GP, and Steinman L (2018). Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci 21, 541–551. 10.1038/s41593-018-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Locatelli G, Theodorou D, Kendirli A, Jordão MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, et al. (2018). Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat. Neurosci 21, 1196–1208. 10.1038/s41593-018-0212-3. [DOI] [PubMed] [Google Scholar]
- 159.Chao CC, Gutiérrez-Vázquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, Zandee SEJ, Blain M, de Lima KA, Takenaka MC, et al. (2019). Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell 179, 1483–1498.e22. 10.1016/j.cell.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clark IC, Gutiérrez-Vázquez C, Wheeler MA, Li Z, Rothhammer V, Linnerbauer M, Sanmarco LM, Guo L, Blain M, Zandee SEJ, et al. (2021). Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230. 10.1126/science.abf1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bettigole SE, and Glimcher LH (2015). Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol 33, 107–138. 10.1146/annurev-immunol-032414-112116. [DOI] [PubMed] [Google Scholar]
- 162.Smith HL, Freeman OJ, Butcher AJ, Holmqvist S, Humoud I, Schätzl T, Hughes DT, Verity NC, Swinden DP, Hayes J, et al. (2020). Astrocyte unfolded protein response induces a specific reactivity state that causes non-cell-autonomous neuronal degeneration. Neuron 105, 855–866.e5. 10.1016/j.neuron.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Clark IC, Wheeler MA, Lee HG, Li Z, Sanmarco LM, Thaploo S, Polonio CM, Shin SW, Scalisi G, Henry AR, et al. (2023). Identification of astrocyte regulators by nucleic acid cytometry. Nature 614, 326–333. 10.1038/s41586-022-05613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guttenplan KA, Weigel MK, Prakash P, Wijewardhane PR, Hasel P, Rufen-Blanchette U, Münch AE, Blum JA, Fine J, Neal MC, et al. (2021). Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 599, 102–107. 10.1038/s41586-021-03960-y. [DOI] [PubMed] [Google Scholar]
- 166.Werneburg S, Jung J, Kunjamma RB, Ha SK, Luciano NJ, Willis CM, Gao G, Biscola NP, Havton LA, Crocker SJ, et al. (2020). Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52, 167–182.e7. 10.1016/j.immuni.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, et al. (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566– 581.e9. 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McNamara NB, Munro DAD, Bestard-Cuche N, Uyeda A, Bogie JFJ, Hoffmann A, Holloway RK, Molina-Gonzalez I, Askew KE, Mitchell S, et al. (2023). Microglia regulate central nervous system myelin growth and integrity. Nature 613, 120–129. 10.1038/s41586-022-05534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong Y, D’Mello C, Pinsky W, Lozinski BM, Kaushik DK, Ghorbani S, Moezzi D, Brown D, Melo FC, Zandee S, et al. (2021). Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci 24, 489–503. 10.1038/s41593-021-00801-z. [DOI] [PubMed] [Google Scholar]
- 170.Wasser B, Luchtman D, Löffel J, Robohm K, Birkner K, Stroh A, Vogelaar CF, Zipp F, and Bittner S (2020). CNS-localized myeloid cells capture living invading T cells during neuroinflammation. J. Exp. Med 217, e20190812. 10.1084/jem.20190812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Giladi A, Cohen M, Medaglia C, Baran Y, Li B, Zada M, Bost P, Blecher-Gonen R, Salame TM, Mayer JU, et al. (2020). Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol 38, 629–637. 10.1038/s41587-020-0442-2. [DOI] [PubMed] [Google Scholar]
- 172.Kumar P, Lim A, Hazirah SN, Chua CJH, Ngoh A, Poh SL, Yeo TH, Lim J, Ling S, Sutamam NB, et al. (2022). Single-cell transcriptomics and surface epitope detection in human brain epileptic lesions identifies pro-inflammatory signaling. Nat. Neurosci 25, 956–966. 10.1038/s41593-022-01095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wheeler MA, Clark IC, Lee HG, Li Z, Linnerbauer M, Rone JM, Blain M, Faust Akl C, Piester G, Giovannoni F, et al. Droplet-based forward genetic screening identifies regulatory mechanisms of astrocytemicroglia cross-talk. Science (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lloyd AF, Davies CL, Holloway RK, Labrak Y, Ireland G, Carradori D, Dillenburg A, Borger E, Soong D, Richardson JC, et al. (2019). Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci 22, 1046–1052. 10.1038/s41593-019-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Silvin A, Uderhardt S, Piot C, Da Mesquita S, Yang K, Geirsdottir L, Mulder K, Eyal D, Liu Z, Bridlance C, et al. (2022). Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity 55, 1448–1465.e6. 10.1016/j.immuni.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 176.Baecher-Allan C, Kaskow BJ, and Weiner HL (2018). Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 97, 742–768. 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 177.Li R, Rezk A, Ghadiri M, Luessi F, Zipp F, Li H, Giacomini PS, Antel J, and Bar-Or A (2017). Dimethyl fumarate treatment mediates an anti-inflammatory shift in B cell subsets of patients with multiple sclerosis. J. Immunol 198, 691–698. 10.4049/jimmunol.1601649. [DOI] [PubMed] [Google Scholar]
- 178.Klotz L, Eschborn M, Lindner M, Liebmann M, Herold M, Janoschka C, Torres Garrido B, Schulte-Mecklenbeck A, Gross CC, Breuer J, et al. (2019). Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci. Transl. Med 11, eaao5563. 10.1126/scitranslmed.aao5563. [DOI] [PubMed] [Google Scholar]
- 179.Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, Chao CC, Wilz A, Blain M, Healy L, et al. (2017). Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc. Natl. Acad. Sci. USA 114, 2012–2017. 10.1073/pnas.1615413114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Colombo E, Di Dario M, Capitolo E, Chaabane L, Newcombe J, Martino G, and Farina C (2014). Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann. Neurol 76, 325–337. 10.1002/ana.24217. [DOI] [PubMed] [Google Scholar]
- 181.Cortese I, Reich DS, and Nath A (2021). Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol 17, 37–51. 10.1038/s41582-020-00427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee DSW, Rojas OL, and Gommerman JL (2021). B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Discov 20, 179–199. 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Steinman L, Fox E, Hartung HP, Alvarez E, Qian P, Wray S, Robertson D, Huang D, Selmaj K, Wynn D, et al. (2022). Ublituximab versus teriflunomide in relapsing multiple sclerosis. N. Engl. J. Med 387, 704–714. 10.1056/NEJMoa2201904. [DOI] [PubMed] [Google Scholar]
- 184.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, et al. (2017). Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med 376, 209–220. 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 185.Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, Rezk A, Patterson KR, Espinoza DA, Kadri JC, et al. (2021). Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med 27, 1990–2001. 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kappos L, Hartung HP, Freedman MS, Boyko A, Radü EW, Mikol DD, Lamarine M, Hyvert Y, Freudensprung U, Plitz T, et al. (2014). Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 13, 353–363. 10.1016/S1474-4422(14)70028-6. [DOI] [PubMed] [Google Scholar]
- 187.Li R, Tang H, Burns JC, Hopkins BT, Le Coz C, Zhang B, de Barcelos IP, Romberg N, Goldstein AC, Banwell BL, et al. (2022). BTK inhibition limits B-cell-T-cell interaction through modulation of B-cell metabolism: implications for multiple sclerosis therapy. Acta Neuropathol. 143, 505–521. 10.1007/s00401-022-02411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Martin E, Aigrot MS, Grenningloh R, Stankoff B, Lubetzki C, Boschert U, and Zalc B (2020). Bruton’s tyrosine kinase inhibition promotes myelin repair. Brain Plast. 5, 123–133. 10.3233/BPL-200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yong VW (2022). Microglia in multiple sclerosis: Protectors turn destroyers. Neuron 110, 3534–3548. 10.1016/j.neuron.2022.06.023. [DOI] [PubMed] [Google Scholar]
- 190.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, et al. (2000). Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med 6, 1167–1175. 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 191.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, et al. (2013). IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol 14, 1054–1063. 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yi J, Miller AT, Archambault AS, Jones AJ, Bradstreet TR, Bandla S, Hsu YS, Edelson BT, Zhou YW, Fremont DH, et al. (2022). Antigen-specific depletion of CD4(+) T cells by CAR T cells reveals distinct roles of higher- and lower-affinity TCRs during autoimmunity. Sci. Immunol 7, eabo0777. 10.1126/sciimmunol.abo0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, et al. (2015). Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. USA 112, E156–E165. 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yeste A, Nadeau M, Burns EJ, Weiner HL, and Quintana FJ (2012). Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 109, 11270–11275. 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, Akilli-Öztürk Ö, Kranz LM, Berger H, Petschenka J, et al. (2021). A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153. 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 196.Robinson WH, Fontoura P, Lee BJ, de Vegvar HEN, Tom J, Pedotti R, DiGennaro CD, Mitchell DJ, Fong D, Ho PPK, et al. (2003). Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol 21, 1033–1039. 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 197.Scott BM, Gutiérrez-Vázquez C, Sanmarco LM, da Silva Pereira JA, Li Z, Plasencia A, Hewson P, Cox LM, O’Brien M, Chen SK, et al. (2021). Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med 27, 1212–1222. 10.1038/s41591-021-01390-x. [DOI] [PubMed] [Google Scholar]
- 198.Sanmarco LM, Chao CC, Wang YC, Kenison JE, Li Z, Rone JM, Rejano-Gordillo CM, Polonio CM, Gutierrez-Vazquez C, Piester G, et al. (2022). Identification of environmental factors that promote intestinal inflammation. Nature 611, 801–809. 10.1038/s41586-022-05308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kuhlmann T, Moccia M, Coetzee T, Cohen JA, Correale J, Graves J, Marrie RA, Montalban X, Yong VW, Thompson AJ, and Reich DS; International Advisory Committee on Clinical Trials in Multiple Sclerosis (2023). Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 22, 78–88. 10.1016/s1474-4422(22)00289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nicaise AM, Wagstaff LJ, Willis CM, Paisie C, Chandok H, Robson P, Fossati V, Williams A, and Crocker SJ (2019). Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc. Natl. Acad. Sci. USA 116, 9030–9039. 10.1073/pnas.1818348116. [DOI] [PMC free article] [PubMed] [Google Scholar]