Significance
Cyclin-dependent kinase 5 (Cdk5) hyperactivity is an important driver of pathology in neurodegeneration. Normally, Cdk5 is regulated by association with its co-activators p35 or p39. Cdk5 hyperactivity is caused by calpain-mediated cleavage of p35 into the truncated activator p25, which binds to Cdk5 and leads to prolonged activation and altered substrate specificity. Preventing p25 production by destroying the calpain cleavage site in p35 abolishes neurodegenerative phenotypes in mouse models, but this genetic approach does not present a viable therapeutic strategy. Here, we report a 12-amino acid long Cdk5-derived peptide that interferes with the Cdk5/p25 complex and ameliorates neurodegenerative phenotypes in cell and mouse models of Cdk5 hyperactivity. This small peptide is a promising candidate for a biotherapeutic against neurodegenerative diseases.
Keywords: Cdk5, Alzheimer’s disease, tauopathy, neurodegenerative disease
Abstract
Aberrant activity of cyclin-dependent kinase (Cdk5) has been implicated in various neurodegenerative diseases. This deleterious effect is mediated by pathological cleavage of the Cdk5 activator p35 into the truncated product p25, leading to prolonged Cdk5 activation and altered substrate specificity. Elevated p25 levels have been reported in humans and rodents with neurodegeneration, and the benefit of genetically blocking p25 production has been demonstrated previously in rodent and human neurodegenerative models. Here, we report a 12-amino-acid-long peptide fragment derived from Cdk5 (Cdk5i) that is considerably smaller than existing peptide inhibitors of Cdk5 (P5 and CIP) but shows high binding affinity toward the Cdk5/p25 complex, disrupts the interaction of Cdk5 with p25, and lowers Cdk5/p25 kinase activity. When tagged with a fluorophore (FITC) and the cell-penetrating transactivator of transcription (TAT) sequence, the Cdk5i-FT peptide exhibits cell- and brain-penetrant properties and confers protection against neurodegenerative phenotypes associated with Cdk5 hyperactivity in cell and mouse models of neurodegeneration, highlighting Cdk5i’s therapeutic potential.
Cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine/threonine kinase that becomes activated while complexing with its activators p35 and p39 (1, 2). Unlike other Cdks, Cdk5 is most active in the brain in adult mice (3). Cdk5 governs various cellular processes in the brain, regulating cortical development, axonal guidance, synaptic plasticity, and learning and memory (4–7). Notably, Cdk5 phosphorylates a wide array of synaptic proteins modulating synaptic transmission and scaling (5, 8). Cdk5 knockout mice are not viable, underscoring the critical role for Cdk5 in brain development (9–11).
Dysregulation of Cdk5 activity has been associated with neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and frontotemporal dementia (FTD) (4, 12). Neurodegenerative stimuli can trigger abnormal increases in intracellular calcium levels, leading to activation of the protease calpain (13, 14) which then cleaves the N-terminal regulatory motif of the Cdk5-activator p35. The resulting p25 fragment activates Cdk5 with a longer half-life than p35 does (14). Calpain also removes myristoylated residues that normally tether Cdk5/p35 to membrane sites (14, 15), liberating Cdk5/p25 from the membrane and allowing it to access additional kinase substrates in the cytosol and nuclear compartments (16, 17). The increased half-life and distinct subcellular localization of Cdk5/p25 suggest it as a target for reducing Cdk5 hyperactivity and thus Cdk5/p25-mediated pathology. Genetic manipulation to generate a mutant p35 that is noncleavable by calpain while still being functional (Δp35) has demonstrated the beneficial effects of blocking aberrant Cdk5 activity/p25 production in the context of neurodegeneration (18, 19). Replacement of p35 by Δp35 reduced AD pathology in 5XFAD mice (18) and also conferred protection against mutant Tau-mediated neurodegeneration in mouse and human organoid-based FTD models (19).
While the Δp35 transgenic model has been critical to understanding the pathological functions of Cdk5/p25, the translational potential of targeting Cdk5/p25 through a genetic approach is very limited. The use of inhibitory peptides has presented a promising alternative approach, offering advantages such as high specificity/affinity and low toxicity (20, 21). Notably, the vast majority of marketed peptide drugs contain less than 10 amino acids (22), indicating that the length of a peptide is an important factor in its translational potential. Of the two peptide inhibitors of Cdk5 identified since 2002 (P5 and CIP), the shorter one (P5—24 amino acids) proved indeed more effective (23, 24). TFP5, a derivative of the P5 peptide that is conjugated with a cell-penetrating transactivator of transcription (TAT) sequence and a FITC tag, reduced hyperactivation of Cdk5 and synaptic/cognitive impairments in mouse models of neurodegeneration (25, 26). While these studies serve as proof-of-principle that peptide inhibition of Cdk5 activity provides benefit in mouse neurodegeneration models, the identification of a Cdk5 inhibitory peptide that is closer in size to the 10 or less amino acids desirable for clinical applications remains an important goal.
Here, we report a 12-amino-acid-long Cdk5 inhibitory peptide (hereafter referred to as “Cdk5i peptide”) that has a high binding affinity toward Cdk5/p25, disrupts the interaction of Cdk5 with p25, and reduces Cdk5/p25 kinase activity. The Cdk5i-FT peptide, a Cdk5i peptide tagged with FITC and the TAT sequence, protected cultured neurons against pathologies associated with Cdk5 hyperactivity. The Cdk5i-FT peptide also reduced hyperphosphorylation of Tau both in cultured neurons overexpressing mutant Microtubule Associated Binding Protein Tau (MAPT) P301L and in cerebral organoids derived from induced pluripotent stem cells (iPSC) from an FTD patient carrying the MAPT P301L mutation. Importantly, when delivered by intraperitoneal injection to mouse models of neurodegenerative disease [CK-p25 mice and Tau P301S mice (27, 28)], the Cdk5i-FT peptide ameliorated brain pathologies and improved cognition. Overall, this work underscores the therapeutic potential of the Cdk5i peptide for the treatment of neurodegeneration.
Results
Cdk5i Peptide Disturbs Cdk5/p25 Complex Formation and Reduces Cdk5/p25 Kinase Activity In Vitro.
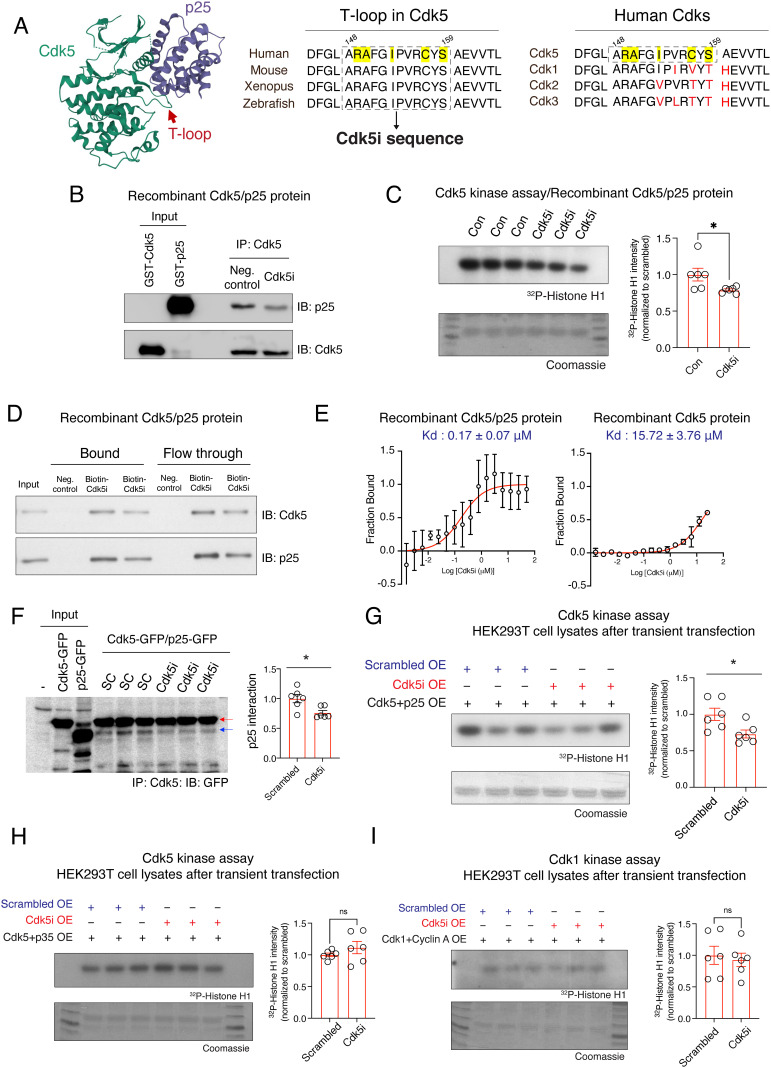
Utilizing crystal structure-based analysis, we previously revealed that p25 tethers the T-loop of Cdk5, stretching the conformation of Cdk5 into an active state (29). Given this evidence that the T-loop (144D-171D; 28 amino acids) is critical for the interaction of Cdk5 with p25, we selected a 12-amino-acid sequence (148ARAFGIPVRCYS159) from the T-loop to generate the Cdk5i peptide (Fig. 1A). This 12-amino-acid sequence is highly conserved in Cdk5 across species, yet not present in other Cdk family members. Importantly, the Cdk5i sequence includes key residues (R149, A150, I153, C157, and S159; highlighted in yellow in Fig. 1A) that are directed toward the p25 surface and are predicted to mediate intermolecular interactions between Cdk5 and p25 (29).
Fig. 1.
Design and characterization of the Cdk5/p25 complex-specific inhibitory peptide. (A) The design of the Cdk5/p25 complex-specific inhibitory peptide (Cdk5i peptide). Cdk5i sequence contains key residues predicted to modulate the interaction between Cdk5 and p25 (yellow highlighting) that were conserved across species but not across family members (black vs. red letters). (B) Recombinant GST-p25 proteins were preincubated with the Cdk5i peptide before the addition of GST-Cdk5 and immunoprecipitation (IP) with Cdk5 antibody, and the reaction mixture was used for a Cdk5 pulldown assay. IB: target for immunoblotting. (C) Recombinant Cdk5/p25 proteins were incubated with Cdk5i or solvent control overnight and subjected to IP-linked Cdk5 kinase assay. Quantification plot shows relative amount of 32P incorporated into the Cdk5 substrate histone H1. Six replicates from two independent experiments. (D) Recombinant Cdk5/p25 complex was incubated with biotin-conjugated Cdk5i overnight and subjected to pull-down assay with streptavidin-coated beads. (E) Microscale thermophoresis-based biophysical assays assessing binding of Cdk5i peptide to recombinant Cdk5/p25 complex (two replicates) or Cdk5 protein alone (two replicates). (F) Cell lysates from HEK293T cells cooverexpressing Cdk5-GFP, p25-GFP, and Cdk5i or scrambled peptide were used for IP with Cdk5 antibody. Red and blue arrows indicate the position of Cdk5-GFP and p25-GFP, respectively. Bar graph shows the amount of p25 normalized to Cdk5 after IP. Six replicates from two independent experiments. (G–I) Cell lysates from HEK293T cells cooverexpressing Cdk5i or scrambled peptide and (G) Cdk5-GFP and p25-GFP, (H) Cdk5-GFP and p35-myc, or (I) Cdk1-HA and Cyclin A-Venus-Flag were used for IP-linked Cdk5 kinase assays. Bar graphs show the relative amount of 32P incorporated into the substrate histone H1. Six replicates from two independent experiments.
We first asked whether the Cdk5i peptide interferes with Cdk5/p25 complex formation. Recombinant p25 protein was incubated with Cdk5i peptide prior to the addition of recombinant Cdk5 protein, and the reaction mixture was subjected to a Cdk5i pull-down assay. As expected, preincubation with Cdk5i peptide decreased the interaction of Cdk5 with p25 (Fig. 1B). Next, we assessed whether the Cdk5i peptide impacts the kinase activity of a recombinant Cdk5/p25 complex. Compared to the Cdk5/p25 complex incubated with solvent control, Cdk5/p25 incubated with Cdk5i peptide showed significantly (~22%) reduced phosphorylation of histone H1, a known substrate of Cdk5 (Fig. 1C).
Cdk5i Peptide Shows Cdk5/p25 Complex-Specific Binding.
To characterize the strength and specificity of Cdk5i peptide binding, we next performed pull-down assays using recombinant Cdk5/p25 protein and biotin-tagged Cdk5i peptide for capture on streptavidin-coated beads. We found that the Cdk5i peptide pulled down not only p25 but also Cdk5 (Fig. 1D). To determine if Cdk5 pull-down was mediated by Cdk5i binding to p25 within the Cdk5/p25 complex, we performed a microscale thermophoresis assay to determine the affinity of Cdk5i peptide binding to the Cdk5/p25 complex and to Cdk5 alone. We found that the Cdk5i peptide bound to the Cdk5/p25 complex with more than 92-fold higher affinity than to Cdk5 alone (Fig. 1E, Kd = 0.17 μM vs. Kd = 15.72 μM), suggesting that binding of Cdk5i to the Cdk5/p25 complex is predominantly mediated by the presence of p25. We verified the strong binding interaction between the Cdk5i peptide and the Cdk5/p25 complex using biolayer interferometry, which yielded a similar affinity measure (Kd = 0.22 μM, SI Appendix, Fig. S1).
Expression of Cdk5i Peptide Interferes with Cdk5/p25 Complex Formation and Lowers Cdk5/p25 Kinase Activity in Mammalian Cells.
To investigate the effects of the Cdk5i peptide on the Cdk5/p25 complex in mammalian cells, we coexpressed Cdk5/p25 together with Cdk5i or with a scrambled peptide in HEK293T cells. The scrambled peptide (AFRSPCARIGYV), which contained the same amino acids as the Cdk5i peptide but in a different order, was included as a control for Cdk5i. Western blot analysis confirmed that coexpression of Cdk5i did not change the amounts of Cdk5 and p25 protein present in cells (SI Appendix, Fig. S2). However, Cdk5i coexpression did reduce both the interaction of Cdk5 with p25 (~25%) and Cdk5 kinase activity (~27%) (Fig. 1 F and G), while it did not significantly impact the kinase activity of Cdk5/p35 and Cdk1/Cyclin A complexes (Fig. 1 H and I). Together, our findings show that the Cdk5i peptide perturbs the interaction of Cdk5 with p25 and decreases Cdk5/p25 kinase activity in cells.
Cdk5i-FT Peptide Confers Protection against Pathology Associated with Cdk5 Hyperactivity in Cultured Neurons.
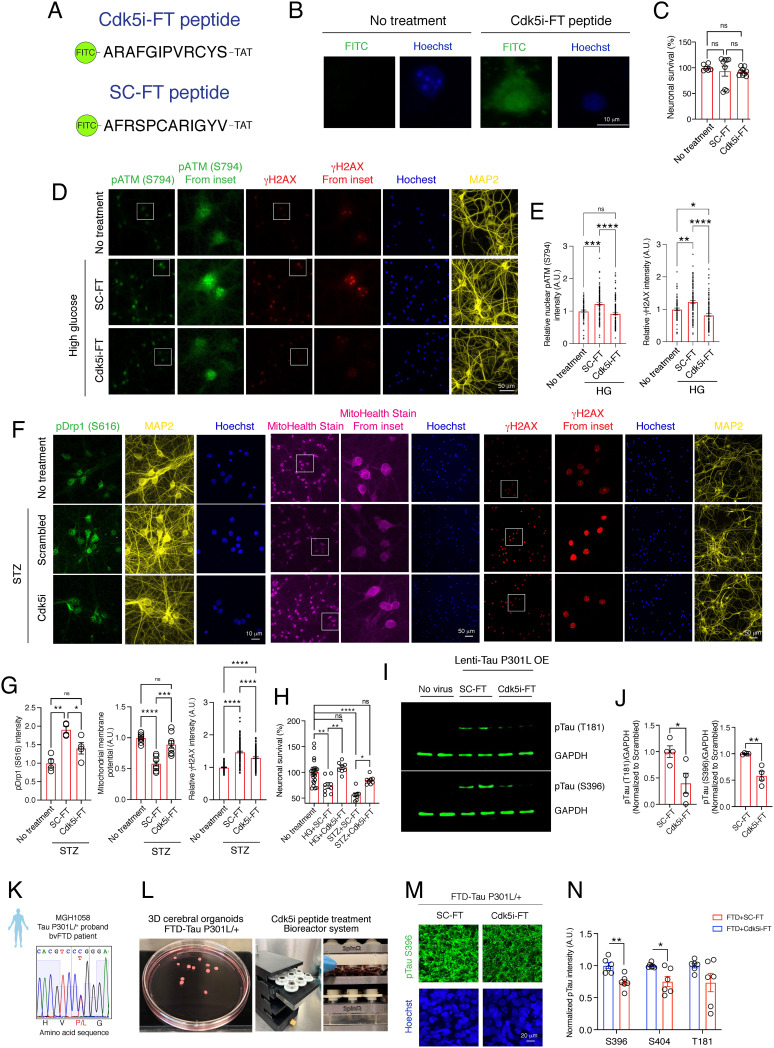
To investigate the effects of Cdk5i peptide on neurodegeneration-related phenotypes in cultured mouse neurons, we attached the Cdk5i and scrambled peptides to the cell-penetrating TAT sequence and conjugated a FITC tag, generating “Cdk5i-FT” and “SC-FT” peptides (Fig. 2A). Cultured neurons treated with Cdk5i-FT peptide exhibited FITC signals in the cytosolic and nuclear compartments, indicative of successful peptide delivery (Fig. 2B). Moreover, the Cdk5i-FT peptide had no effects on neuronal viability (Fig. 2C). We then tested the Cdk5i-FT peptide in two independent models of Cdk5 hyperactivity in cultured neurons, using both treatment with the SC-FT peptide and treatment with PBS as controls where experimentally feasible. Since both control treatments had comparable effects in all cases (SI Appendix, Fig. S3), SC-FT peptide treatment was used as the main control. In the first model, we challenged cultured neurons with high glucose, a condition known to induce p25 generation and lead to neuronal death through the pATM S794/γH2AX pathway (30, 31). High-glucose challenge in the presence of SC-FT peptide significantly increased the phosphorylation of ATM at S794 (~1.227-fold) and subsequent phosphorylation of H2AX at S139 (γH2AX, ~1.231-fold) (Fig. 2 D and E). However, treatment with Cdk5i-FT peptide attenuated this phosphorylation upon high-glucose challenge by ~25% (pATM S794) and ~33% (γH2AX), respectively (Fig. 2 D and E).
Fig. 2.
Cdk5i-FT peptide treatment counteracts pathological consequences linked to Cdk5 hyperactivity in cultured neurons and in models of tauopathy. (A) For cellular assays, Cdk5i and scrambled peptides were conjugating to FITC at the N-terminus and the TAT-sequence at the C-terminus. (B) Representative images of peptide penetration (FITC signal: green; Hoechst: blue) into 14 DIV cultured neurons after Cdk5i-FT peptide treatment. (C) Bar graphs represent the viability of cultured neurons (at least six replicates) after 6-h peptide treatment (10 nM). (D) Representative images of high-glucose challenged cultured neurons stained for pATM S794 (green), γH2AX (red), MAP2 (yellow), and Hoechst (blue). (E) Bar graph represents the intensity of pATM S794 and γH2AX (at least 76 nuclei were analyzed from two coverslips). (F) Representative images of STZ-challenged cultured neurons stained for pDrp1 S616 (green), MitoHealth dye (magenta), γH2AX (red), and Hoechst (blue). (G) Bar graphs represent the intensity of pDrp1 S616 (four replicates from two coverslips per group), MitoHealth stain (eight replicates from four coverslips, two experiments), and γH2AX (107 to 154 nuclei were analyzed from two coverslips). (H) Viability assay of cultured neurons was performed 24 h after high glucose or STZ insults; at least eight replicates in each group. (I) Representative blot images of pTau T181, pTau S396, and GAPDH. (J) Bar graphs represent the relative immunoreactivity for pTau normalized to the SC-FT-treated group. Four replicates from three independent experiments. (K) Sanger sequencing confirms that the FTD patient iPSC line carrying a heterozygous Tau P301L mutation. (L) Pictures of cerebral organoids and 3D-printed bioreactor system. (M) Representative images of 4-month-old cerebral organoids stained for pTau (S396) (green) and Hoechst (blue). (N) Bar graph represents the immunoreactivity for pTau normalized to SC-FT treatment. At least three organoids and two sections per organoid were analyzed in each group.
In the second model, we used the DNA-alkylating agent streptozotocin (STZ) to induce mitochondrial dysfunction in neurons through the Cdk5/pDrp1 S616 signaling pathway (32). STZ challenge in the presence of SC-FT peptide up-regulated (~1.9-fold) the phosphorylation of Drp1 at S616 (Fig. 2 F and G), but Cdk5i-FT peptide treatment reduced this upregulation significantly (~26%; Fig. 2 F and G). We next examined the mitochondrial membrane potential using MitoHealth stain. STZ insults in the presence of SC-FT peptide reduced the total intensity of the MitoHealth stain by ~50% (Fig. 2 F and G), whereas treatment with the Cdk5i-FT peptide rescued STZ-induced mitochondrial dysfunction (Fig. 2 F and G). Similarly, Cdk5i-FT peptide treatment partially reversed the increase in γH2AX, a marker for DNA double-strand breaks, observed in STZ-challenged cultured neurons (Fig. 2 F and G).
High glucose and STZ insults in the presence of SC-FT peptide also led to 25% and 44% neuronal death, respectively. Importantly, Cdk5i-FT peptide treatment conferred protection against both high-glucose and STZ-induced neurotoxicity (Fig. 2H). These results suggest that the Cdk5i-FT peptide protects cultured neurons against the pathological consequences associated with Cdk5 hyperactivity caused by two mechanistically distinct insults.
Cdk5i-FT Peptide Ameliorates Tau Hyperphosphorylation in Mouse and Human Cell Models of Tauopathy.
Cdk5 is a Tau protein kinase (33, 34) and hyperphosphorylation of Tau leads to pathological changes that compromise brain functions (35). We thus sought to determine if the Cdk5i-FT peptide can reduce pathological Tau phosphorylation. We transduced cultured neurons with lentivirus expressing human Tau P301L, an FTD-associated genetic variant, and later subjected them to either Cdk5i-FT or SC-FT peptide treatment before examining Tau phosphorylation. Compared to treatment with SC-FT peptide, treatment with the Cdk5i-FT peptide caused a significant reduction of Tau phosphorylation at both T181 (~55%) and S396 (~45%) (Fig. 2 I and J), two residues known to be phosphorylated by Cdk5 (34).
We next utilized iPSCs derived from an FTD patient carrying a heterozygous Tau P301L mutation (Fig. 2K). We generated cerebral organoids from the Tau P301L iPSC line and performed peptide treatment using 3D-printed bioreactors (36) (Fig. 2L). Compared to Tau P301L organoids that were treated with SC-FT peptide, we found that the levels of pTau S396 and pTau S404 were significantly reduced (Fig. 2 M and N) in Tau P301L organoids treated with Cdk5i-FT peptide. We also observed that the levels of pTau T181 exhibited a strong trend toward reduction after Cdk5i-FT peptide treatment (Fig. 2 M and N). These findings show that the Cdk5i-FT peptide counteracts pathological Tau hyperphosphorylation both in a mouse and in a human cell model of tauopathy.
Cdk5i-FT Peptide Prevents Pathological Phenotypes in CK-p25 Mice.
We next sought to examine the effects of the Cdk5i-FT peptide in vivo using mouse models of neurodegeneration. We first tested the peptide in CK-p25 mice, which inducibly overexpress p25 in forebrain neurons, resulting in Cdk5 hyperactivation (17, 27, 37). Two weeks of p25 induction induces DNA damage and microglial activation (37–39), and by 6 wk post p25 induction, the mice show neuronal/synaptic loss and cognitive impairment (17, 27, 40). To determine whether Cdk5i-FT peptide crosses the blood–brain barrier, we administrated the Cdk5i-FT and SC-FT peptides intraperitoneally (IP injection, 20 mg/kg body weight) into wild-type mice and probed for FITC signals in the brain. We detected FITC signals in the mouse hippocampus, indicating that both peptides crossed the blood–brain barrier (SI Appendix, Fig. S4).
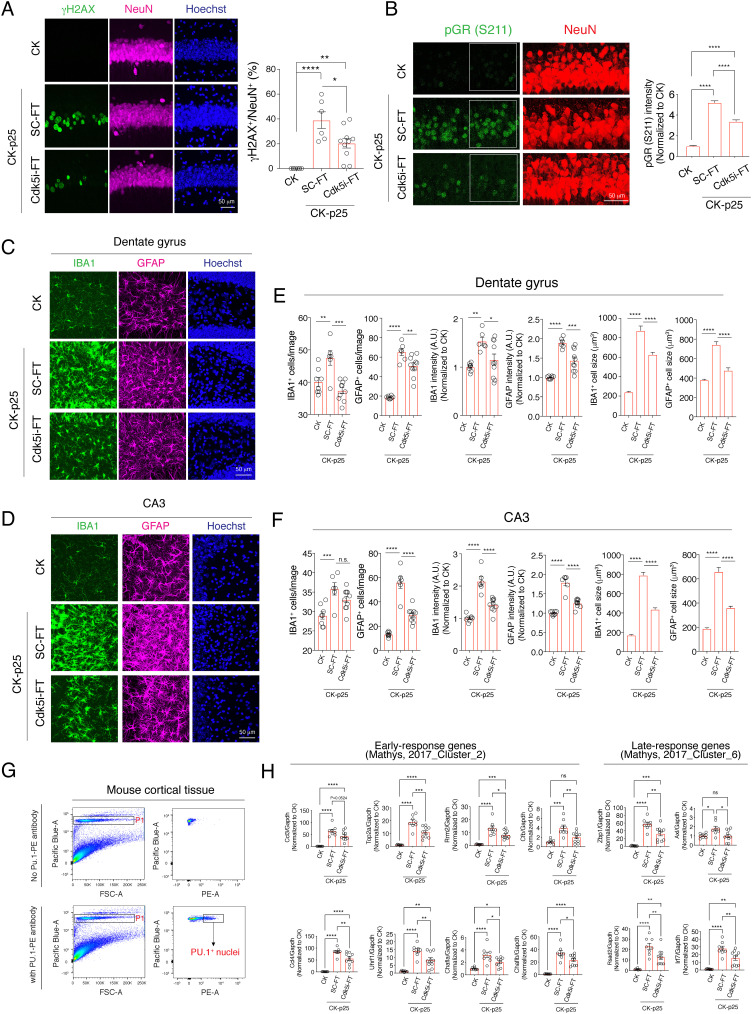
We next treated CK-p25 mice with the Cdk5i-FT peptide or, as controls, with the SC-FT peptide or saline every other day (IP injection, 20 mg/kg body weight) during the 2-wk p25 induction period. Saline and SC-FT treatments had comparable effects (SI Appendix, Fig. S5). Consistent with previous reports (37), we observed DNA damage in ~20 to 40% of the hippocampal CA1 at 2 wk of p25 induction in SC-FT-treated mice, as detected by immunoreactivity for γH2AX (Fig. 3A). Treatment with Cdk5i-FT peptide reduced the number of γH2AX-positive neurons by nearly 50% (Fig. 3A). Next, we examined the phosphorylation of a known in vivo Cdk5/p25 substrate, the glucocorticoid receptor (GR) (41). pGR S211 intensity was increased fivefold in SC-FT-treated CK-p25 mice after 2 wk of p25 induction (Fig. 3B). Administration of Cdk5i-FT peptide reduced this upregulation by 35% (Fig. 3B).
Fig. 3.
Cdk5i-FT peptide treatment prevents pathological phenotypes in CK-p25 mice brain. (A) Representative image of hippocampal CA1 region of CK and peptide-treated CK-p25 mice stained for γH2AX (green), NeuN (magenta), and Hoechst (blue). Bar graph represents the percentage of γH2AX-positive neurons in each image. Mice analyzed in each group: 4, 3, 5; two sections/mouse. (B) Representative image of hippocampal CA1 region of CK and peptide-treated CK-p25 mice stained for pGR S211 (green), NeuN (red), and Hoechst (blue). Mice analyzed in each group: 4, 3, 5; two sections/mouse. NeuN-positive nuclei were used to analyze pGR intensity. Nuclei analyzed in each group: 190, 241, 289. (C and D) Representative image of hippocampal dentate gyrus (C) and CA3 region (D) of CK and peptide-treated CK-p25 mice stained for IBA1 (green), GFAP (magenta), and Hoechst (blue). (E and F) Bar graphs represent the number of microglia/astrocytes, the intensity of IBA1/GFAP, and the cell volume of microglia/astrocytes. Mice analyzed in each group: 4, 3, 5; two sections/mouse. (G) Gating strategy for the isolation of PU.1+ microglia from mouse cortical tissue. (H) RT-qPCR analysis indicates the expression of early and late-response genes in microglia purified from CK or CK-p25 mice after peptide treatment. Mice analyzed in each group: 8, 8, 10.
Since profound gliosis is an additional early pathology resulting from p25 induction, we then examined microglia and astrocyte changes following peptide treatments. Compared to CK control mice, CK-p25 mice treated with SC-FT peptide showed increased numbers and cell sizes for IBA1-positive microglia (Fig. 3 C–F) and GFAP-positive astrocytes (Fig. 3 C–F). Cdk5i-FT peptide treatment markedly reduced the number of microglia and astrocytes and reversed the increase in size of these cells (Fig. 3 C–F). We next assessed the impact of the Cdk5i-FT peptide on p25-related upregulation of gene expression in microglia. In previous studies (38, 39), we found that p25 induction led to the upregulation of early-response genes, which include cytokines and genes involved in cell proliferation, and late-response genes, which include interferon response pathway and major histone compatibility genes (39). Here, we used fluorescence-activated cell sorting to isolate PU.1-positive microglia from the cortical tissue of 2-wk induced CK-p25 mice (Fig. 3G). We then evaluated the expression of early- and late-response genes by qRT-PCR analysis. As expected, the levels of Ccl3, Ccl4, Rrm2, Top2a, Chaf1a/b, Uhrf1, and Cfb were up-regulated in induced CK-p25 mice treated with SC-FT peptide when compared to CK control mice. Treatment with the Cdk5i-FT peptide notably attenuated the induction of these early-response genes (Fig. 3H). Similarly, the upregulation of late-response genes (Axl, Rsad2, Irf7, and Zbp1) was also reduced by Cdk5i-FT peptide treatment. Together, these data suggest that the Cdk5i-FT peptide counteracts Cdk5/p25-mediated pathology such as DNA damage, GR phosphorylation, and gliosis in vivo.
Cdk5i-FT Peptide Ameliorates Tauopathy in Tau P301S Mice.
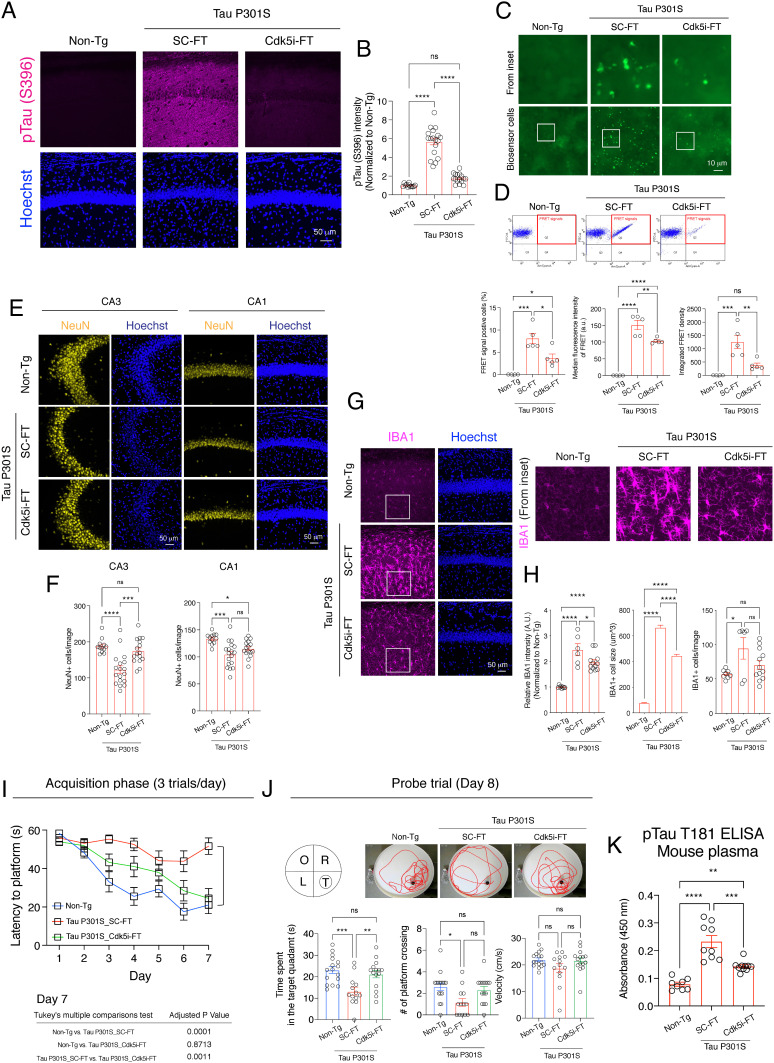
We have previously demonstrated that Tau hyperphosphorylation and seeding activity are attenuated when endogenous p25 generation is abolished in the compound Tau P301S;Δp35 transgenic mice (19). To determine whether Cdk5i-FT peptide similarly ameliorates tauopathy in vivo, we conducted a series of pathology assessments in 7- to 9-month-old Tau P301S mice after peptide treatment. We first performed immunostaining to examine the phosphorylation of Tau at S396, a known target of Cdk5. Compared to nontransgenic control mice (non-Tg controls), the intensity of pTau S396 was increased by ~5.6-fold in Tau P301S mice treated with SC-FT peptide, but only by 1.7-fold in Tau P301S mice treated with Cdk5i-FT peptide (Fig. 4 A and B). This finding indicates that Cdk5i-FT peptide treatment attenuates Tau hyperphosphorylation in Tau P301S mice.
Fig. 4.
Cdk5i-FT peptide ameliorates Tau-mediated neurodegenerative phenotypes in 7- to 9-month-old Tau P301S mice. (A) Representative image of hippocampal CA1 region of non-Tg and peptide-treated Tau P301S mice stained for pTau S396 (magenta) and Hoechst (blue). Bar graphs represent the intensity of pTau S396 in each image. Mice analyzed in each group: 6, 9, 8; two sections/mouse. (B) Representative image of Tau biosensor cells 48 h posttransduction with the brain lysates prepared from non-Tg controls and from Tau P301S mice treated with Cdk5i or scrambled. (C) Flow cytometry was used to measure FRET signals in each sample. Cells in quadrant (Q) 2 are FRET-positive cells. (D) Bar graphs represent the percentage of FRET-positive cells, median fluorescence intensity of FRET-positive cells, and integrated FRET density. Mice analyzed in each group: 4, 5, 5. (E) Representative image of hippocampal CA3 and CA1 region of non-Tg and peptide-treated Tau P301S mice stained for NeuN (yellow) and Hoechst (blue). (F) Bar graphs represent the number of NeuN-positive cells in each image. Mice analyzed in each group: 6, 9, 8; two sections/mouse. (G) Representative image of hippocampal CA1 region of non-Tg and peptide-treated Tau P301S mice stained for IBA (magenta) and Hoechst (blue). (H) Bar graphs represent the intensity of IBA1, the cell volume of microglia, and the number of microglia in each image. Mice analyzed in each group: 6, 9, 8; two sections/mouse. (I) Plot shows the latencies to hidden platform during the acquisition phase of the MWM test. (J) Bar graphs represent time spent in each Q, # of platform crossing, and swim velocity during the probe trial. Images show the location of each Q and swim trace of mouse during the probe trial. T = target, O = opposite, R = right, L = left. Animals analyzed were 16, 13, 15. (K) Bar graph represents plasma levels of pTau T181 in each group. Animals analyzed were 8, 9, 10.
Hyperphosphorylation of Tau is associated with the propagation of tauopathy across cells by “seeding” the formation of intracellular aggregates (42–44). Therefore, we next utilized FRET biosensor HEK293T cells expressing human Tau carrying the P301S mutation and fused with either CFP or YFP to test Tau seeding activity (45). Compared to lysates from non-Tg controls, brain lysates from SC-FT-treated Tau P301S mice exhibited a robust seeding effect, demonstrated by an increase in the percentage of FRET-positive cells and in FRET median fluorescence intensity (Fig. 4 C and D). Importantly, both parameters were notably attenuated when Tau P301S mice had been treated with Cdk5i-FT peptide. Reduction of Tau seeding activity by Cdk5i-FT treatment became especially clear when considering the combined measure of integrated FRET density (percentage multiplied by the median fluorescence intensity) (Fig. 4D).
We next investigated whether the Cdk5i-FT peptide reverses neuronal loss and microglial activation in Tau P301S mice. Relative to non-Tg controls, SC-FT-treated Tau P301S mice showed reduced numbers of neurons in the hippocampal CA3 (~40%) and CA1 (~30%) regions (Fig. 4 E and F). In the CA3 region, this neuronal loss was rescued by Cdk5i-FT peptide treatment (Fig. 4 E and F). Furthermore, Cdk5i-FT peptide treatment significantly reduced microglial cell size (~33%) and caused a strong trend toward lower microglial cell numbers (~25%) in the hippocampus of Tau P301S mice, suggesting attenuation of microglial activation (Fig. 4 G and H). These findings indicate that Cdk5i-FT peptide treatment counteracts numerous pathological changes associated with the Tau P301S mutation in vivo, including Tau hyperphosphorylation, Tau seeding activity, neuronal loss, and microglial activation.
Cdk5i-FT Peptide Ameliorates Cognitive Deficits in Tau P301S Mice.
Given its neuroprotective effects, we next examined if Cdk5i-FT peptide treatment rescues the cognitive deficits in 7- to 9-month-old Tau P301S mice. In the Morris water maze task, Tau P301S mice treated with SC-FT peptide took longer (2.45-fold on day 7) to locate the hidden platform during training and spent less time (~57%) in the target Q during the probe trial, relative to non-Tg controls (Fig. 4 I and J). Importantly, Cdk5i-FT peptide treatment significantly improved the cognitive performance of Tau P301S mice, as evidenced by reduced escape time latency (~53%) during training and increased (1.59-fold) time spent in the target quadrant (Q) during the probe trial (Fig. 4J). Analysis of the total distance traveled during the probe trial showed that the cognitive enhancement observed in Tau P301S mice after Cdk5i-FT peptide treatment was not due to a change in swimming velocity (Fig. 4J).
Higher levels of plasma pTau T181 correlate with worse cognition in patients with AD and have been suggested as a reliable biomarker for AD (46). Accordingly, we collected plasma samples from an independent cohort of 7- to 9-month-old Tau P301S mice after peptide treatment for the measurement of plasma pTau T181. We observed an increase in plasma pTau T181 (~2.95-fold) in Tau P301S mice treated with SC-FT peptide relative to non-Tg controls, whereas Cdk5i-FT peptide treatment markedly reduced this increase (~40%) (Fig. 4K). Together, these findings demonstrate that Cdk5i-FT peptide treatment improves cognition and reduces plasma pTau T181 levels in Tau P301S mice.
Cdk5i-FT Peptide Reverses Gene Expression Changes in Tau P301S Mice.
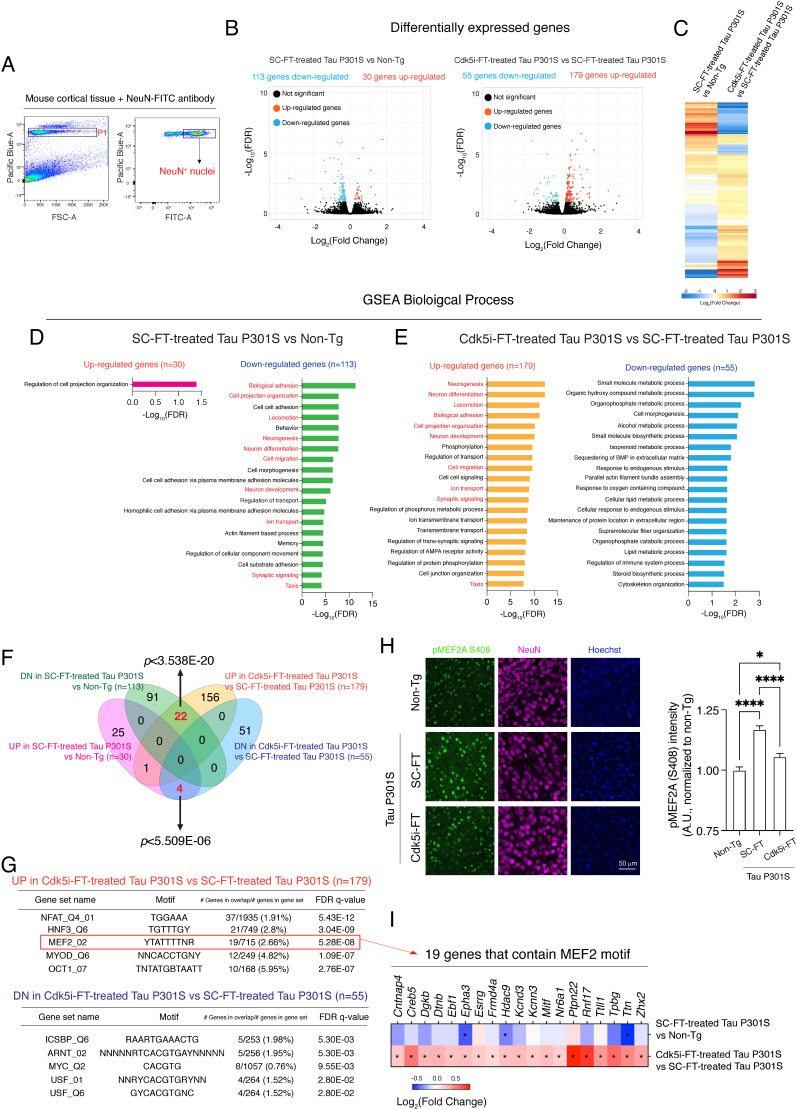
Gene expression alterations have been associated with disease manifestation and functional decline in the Tau P301S mouse model (47, 48). To assess whether Cdk5i-FT peptide rescues gene expression changes in Tau P301S mice, we performed RNA-seq analysis on NeuN+ nuclei isolated from 7- to 9-month-old Tau P301S mice after peptide treatment and from non-Tg controls (Fig. 5A). Compared to non-Tg controls, genes up-regulated in SC-FT-treated Tau P301S mice (n = 30) were involved in cell projection organization. By contrast, down-regulated genes (n = 113) were more specific to pathways that potentially impact cognition in Tau P301S mice, as indicated by the enrichment of gene ontology terms related to neurogenesis, neuron development/differentiation, ion transport, memory, and synaptic signaling (Fig. 5 B–D).
Fig. 5.
Cdk5i-FT peptide treatment reverses gene expression alterations in 7- to 9-month-old Tau P301S mice. (A) Gating strategy for the isolation of NeuN+ nuclei from mouse cortical tissue. (B and C) Volcano plot of DEGs that were identified in each comparison. Three animals were analyzed in each group. (D and E) Enriched GO terms of DEGs were annotated using MSigDB. (F) Venn diagram represents the overlap of DEGs between groups. (G) Known motifs identified in the promoter regions (2 kb upstream and downstream from the transcription start site) of the DEGs by MSigDB (TFT_Legacy). Top five motifs are shown. (H) Representative images of pMEF2A S408 (green), NeuN (magenta), and Hoechst (blue) in the cortical region of non-Tg controls, scrambled-treated, and Cdk5i-FT-treated Tau P301S mice. Bar graph represents the mean intensity of pMEF2 S408 in NeuN+ cells. Four mice and two sections per mouse were analyzed in each group, total nuclei analyzed: 1,111, 1,016, 1,011. (I) A heatmap showing the Log2(Fold change) of genes that predicted to have MEF2 motif (see G). Asterisk indicates FDR < 0.1.
We next compared gene expression changes in Tau P301S mice between SC-FT and Cdk5i-FT peptide treatment. Cdk5i-FT peptide treatment down-regulated genes (n = 55) associated with metabolic pathways, including isoprenoid and lipid metabolic processes (Fig. 5 B, C, and E). Interestingly, Cdk5i-FT peptide treatment rescued several pathways (highlighted in red) that were down-regulated in SC-FT-treated Tau P301S mice, such as neuron development/differentiation, neurogenesis, ion transport, and synaptic signaling (Fig. 5E). A similar effect of Cdk5i-FT peptide treatment could also be observed on the gene level. Compared to non-Tg controls, 113 genes were down-regulated in SC-FT-treated Tau P301S mice, and 22 out of these 113 genes (~19.4%) became up-regulated after Cdk5i-FT peptide treatment (hypergeometric tests: P < 3.538E-20) (Fig. 5F). Thirty genes were up-regulated in SC-FT-treated Tau P301S mice, and four out of these 30 genes (~13.3%) became down-regulated after Cdk5i-FT peptide treatment (hypergeometric tests: P < 5.509E-06) (Fig. 5F). Together, these findings indicate that Cdk5i-FT peptide treatment partially rescues the gene expression changes in cortical neurons of Tau P301S mice.
To better understand how Cdk5i-FT peptide reverses gene expression changes in Tau P301S mice, we next performed motif enrichment analysis in the promoter regions of differentially expressed genes (DEGs) observed between SC-FT and Cdk5i-FT peptide treatment. Motif analysis indicated the enrichment of binding sites for the transcription factors NFAT, HNF3, MEF2, MYOD, and OCT1 for genes that were up-regulated and ICSBP, ARNT, MYC, and USF for genes that were down-regulated after Cdk5i-FT peptide treatment (Fig. 5G). Interestingly, previous studies have reported family members of MEF2, MYC, and USF as Cdk5 substrates, with Cdk5-directed phosphorylation negatively regulating the activity of MEF2 members and positively regulating that of MYC and USF members (49–51). MEF2 motifs were indeed enriched among the down-regulated genes when performing motif analysis on DEGs between SC-FT-treated Tau P301S mice and non-Tg controls, (SI Appendix, Fig. S6), although not ranked among the top five motifs. These findings suggest that the activity of MEF2 may be attenuated in Tau P301S mice, leading to the downregulation of MEF2 target genes, but rescued by Cdk5i-FT peptide treatment, reversing the downregulation of MEF2 target genes.
We next sought to investigate whether the Cdk5i-FT peptide preserves MEF2 activity in Tau P301S mice by regulating its phosphorylation. We chose to focus on MEF2A and MEF2C, two MEF members with high expression in NeuN+ nuclei based on our dataset (SI Appendix, Fig. S7). We examined the levels of phosphorylation at the Cdk5 target sites MEF2A S408 and MEF2C S387 in 7- to 9-month-old Tau P301S mice after peptide treatment. While immunolabeling of pMEF2C S387 showed mainly cytoplasmic signals (SI Appendix, Fig. S8), immunolabeling of pMEF2A S408 displayed nuclear signals. Compared to non-Tg controls, SC-FT-treated Tau P301S mice exhibited augmented pMEF2A S408 intensity (~1.16-fold) in cortical neurons, but this induction was attenuated after Cdk5i-FT peptide treatment (Fig. 5H). This observation indicates that MEF2A transactivation activity is repressed (due to phosphorylation) in Tau P301S mice relative to non-Tg controls, but becomes de-repressed after Cdk5i-FT peptide treatment. Indeed, when we focused on the 19 genes predicted to have an MEF2 motif among genes up-regulated in Tau P301S mice after Cdk5i-FT peptide treatment (Fig. 5G), three of these 19 genes (15.78%, Epha3, Hdac9, and Ttn) showed a significantly reduced level in SC-FT-treated Tau P301S mice relative to non-Tg controls (Fig. 5I). Collectively, our findings suggest that Cdk5i-FT peptide treatment rescues gene expression alterations in Tau P301S mice in part through its modulation of MEF2 activity.
Discussion
In this study, we report a 12-amino-acid Cdk5-derived peptide that binds to the Cdk5/p25 complex, interferes with p25 binding to Cdk5, and reduces Cdk5/p25 kinase activity. We show that this Cdk5i peptide ameliorates pathologies associated with Cdk5 hyperactivity in cell and animal models of neurodegeneration. The Cdk5i peptide recapitulates many of the effects of the noncleavable mutant p35 but holds much more promise as a treatment for neurodegeneration. While therapeutic application of genetic modifications in humans is limited due to technical challenges and ethical concerns (52), peptides are highly selective and relatively safe agents for therapeutic intervention (20). Importantly, the Cdk5i peptide is much smaller than previously reported peptide inhibitors of Cdk5, the 126-amino-acid CIP and 24-amino-acid P5 peptides (23, 24). The majority of marketed peptide drugs are 8 to 10 amino acids long (22), indicating that shorter length is important for peptide drugs. Notably, the high cost of production is a key technical hurdle to the development of peptide-based therapeutics, and shorter peptides are cheaper to synthesize (20, 21). Moreover, smaller peptides are also more amenable for future development and modifications, such as grafting onto scaffolds for increased peptide stability (53, 54).
While we see clear effects of the Cdk5i peptide on Cdk5/p25 kinase activity and phenotypes, the precise mode of action remains uncertain. Since the Cdk5i peptide consists of a stretch of Cdk5 residues predicted to mediate the interaction with p25, we hypothesized that it would bind primarily to p25. We indeed observed a greater binding affinity of the Cdk5i peptide to Cdk5/p25 than to Cdk5 alone, suggesting that p25 is crucial for Cdk5i peptide binding. However, we were unable to determine the binding affinity of Cdk5i peptide to p25 alone since the Cdk5i peptide and p25 protein mixture proved too viscous for the microscale thermophoresis assay. Additional optimization or alternative approaches will be required to overcome this technical limitation. In our streptavidin pull-down assay, we detected an interaction of Cdk5i peptide with recombinant Cdk5 protein. Because pull-down occurred in the presence of p25 and the Cdk5i peptide shows high binding affinity to the Cdk5/p25 complex, the interaction between the Cdk5i peptide and Cdk5 could be mediated by binding of the Cdk5i peptide to p25 in the context of the Cdk5/p25 complex. Given that the Cdk5i peptide is derived from part of the T-loop in Cdk5, the Cdk5i peptide could dock at the interface of the Cdk5/p25 complex, presumably distorting the interaction between Cdk5 and p25 and preventing Cdk5 from assuming an active conformation. Moreover, the T-loop of Cdk5 is essential for the binding of both p25 and of substrate to Cdk5. It is possible that, by interfering with the interaction between p25 and the T-loop, the Cdk5i peptide could alter substrate accessibility to Cdk5. A recent study has successfully predicted the binding modes of Cdk5 with the P5 peptide, such as near the ATP-binding site of Cdk5 without occluding ATP access (55). Conducting similar investigations on how Cdk5i peptide docks at the Cdk5/p25 complex will surely advance our understanding of this peptide and will provide further mechanistic insights into targeting the Cdk5/p25 complex.
Overexpression of Cdk5i peptide reduces Cdk5/p25 kinase activity but has no impact on the activity of Cdk5/p35 and Cdk1/Cyclin A complexes. Several factors could potentially contribute to this specificity of inhibition. Selectivity for Cdk5/p25 over Cdk1/Cyclin A is likely due to the fact that the sequence of the Cdk5i peptide is derived from Cdk5 and not present in Cdk1. In contrast, selectivity for Cdk5/p25 over Cdk5/p35 could reflect preferential access to Cdk5/p25 within the cell and/or preferential inhibition of aberrant Cdk5 kinase activity over its normal physiological activity. While Cdk5/p35 complexes reside at the cell membrane, Cdk5/p25 complexes are localized in cytosol and nucleus (14). We found a strong FITC signal in the nucleus after adding Cdk5i-FT peptide to cultured neurons, suggesting that the Cdk5i-FT peptide is most abundant in the nucleus, where only the Cdk5/p25 complex, but not the Cdk5/p35 complex is present. Nuclear localization would allow the Cdk5i-FT peptide to inhibit Cdk5/p25-dependent phosphorylation of transcription factors and thus prevent subsequent gene expression changes. Nuclear localization of the Cdk5i-FT peptide may depend on the TAT sequence, which is likely to enhance cell- and brain-penetrant properties of the peptide. In addition, the Cdk5i peptide could act as an S-nitrosylation sink since it contains the cysteine 157 residue found to be S-nitrosylated under pathological conditions. Increased S-nitrosylation of Cdk5 has been observed in brain samples of patients with AD, and S-nitrosylation of Cdk5 (C157) positively stimulates Cdk5/p25 kinase activity (56). By providing excess substrate, the Cdk5i peptide could prevent pathological S-nitrosylation and subsequent aberrant activity of Cdk5 in the disease context.
Neuronal death occurs during disease progression and is associated with memory impairment in neurodegeneration. Under pathological conditions, overactivated Cdk5 phosphorylates MEF2 members, which leads to neuronal apoptosis (49). We observed that Cdk5i-FT peptide treatment attenuates neuronal loss, memory impairment, and pMEF2A S408 induction in Tau P301S mice. Given the established role of the Cdk5/MEF2 pathway in neuronal apoptosis, we reason that the Cdk5i-FT peptide treatment rescues pathological alterations, including neuronal loss and memory deficits in Tau P301S mice, in part by its modulation of MEF2 activity. A recent study revealed that MEF2 members, in particular MEF2A and MEF2C, are key regulators of cognitive potential and confer resilience to neurodegeneration (48). Here, we focused on MEF2A, a known target of Cdk5. However, we cannot rule out the possible contributions from other MEF2 members, such as MEF2C. It remains unclear whether the cytosolic signals observed for pMEF2C S387 labeling in Tau P301S mice reflected its low abundance in the neuronal nuclei or technical limitations.
Based on our experiments, the rescue effect of Cdk5i-FT peptide treatment appears to increase with the complexity of the model system. This may reflect differences between Cdk5 substrates as well as the role a substrate plays within the cell. For example, while we find only modest inhibition of the ability of Cdk5 to phosphorylate Histone H1 in our in-vitro assay (Fig. 1 C and G), our data show more pronounced inhibition of Tau phosphorylation in cellular models (Fig. 2J) and an even greater effect on Tau seeding—downstream of tau phosphorylation—in the Tau P301S mouse model (Fig. 4 C and D). Such amplification effects may be especially strong for Cdk5/p25 substrates that themselves act as regulatory proteins, such as the transcription factor MEF2A, helping to explain the unexpectedly broad effect of Cdk5i-FT on disease pathology. This complex cross-talk occurring between Cdk5 and other signaling pathways could turn Cdk5/p25 activity into a signaling hub for accelerating neurodegeneration, amplifying the rescue effects of Cdk5/p25 inhibition.
The effect of Cdk5i-FT peptide treatment on specific pathological features may also depend on treatment timing. In 7- to 9-mo-old Tau P301S mice, 1 mo of treatment effectively rescues neuronal loss and memory deficits (Fig. 4 E, F, I, and J) but only partially reverses microglial activation (Fig. 4 G and H), a pathological feature that already develops at the age of three months (28). These findings suggest that the treatment may be more effective when the Cdk5i-FT peptide is administered near the onset of the respective pathology. We also find that treatment with the Cdk5i-FT peptide is more protective in Tau P301S mice than in CK-p25 mice. We believe that this difference could be due to higher Cdk5 kinase activity in the brains of CK-p25 mice than in those of Tau P301S mice, relative to their respective control mice (19, 57), suggesting that more inhibition results in more protection. However, this may only hold true up to a threshold value since the complete abrogation of Cdk5 activity is associated with neurodegenerative phenotypes such as neuronal loss, microglial activation, and memory impairment (58–60). In this context, nuclear localization of the Cdk5i-FT peptide may prove especially beneficial for selective suppression of aberrant Cdk5/p25 activity.
Additional modes of action for Cdk5i are also conceivable, and further experiments will be required to conclusively demonstrate the precise actions of this peptide. Although we saw beneficial effects of the Cdk5i-FT peptide in vivo, it is likely that these functions can be improved further by identifying the key residues important for its action and making the peptide even smaller. Furthermore, adding auxiliary sequences may allow for even more effective delivery to the brain, increase stability, and prevent unwanted targeting by enzyme modifiers (61, 62).
In this study, we report a 12-amino-acid-long peptide derived from Cdk5 that exhibits high binding affinity to the Cdk5/p25 complex, interferes with the interaction of Cdk5 with p25, and reduces Cdk5/p25 kinase activity. By using cell and mouse models of Cdk5 hyperactivity, we show that treatment with Cdk5i-FT peptide ameliorates multiple pathological phenotypes related to neurodegeneration, highlighting its therapeutic potential. Importantly, the Cdk5i peptide has potential to be applied not only to neurodegenerative diseases such as AD, PD, and FTD, but also to other diseases associated with aberrant Cdk5 activity including cancer, stroke, diabetes, and neuropathic pain.
Methods
Cdk5i and Scrambled Peptides.
The Cdk5i peptide consists of 12 amino acids (ARAFGIPVRCYS) derived from the Cdk5 T loop. The scrambled peptide contains the same amino acids but in scrambled order (AFRSPCARIGYV). For the pull-down assay, biotin was linked at N-terminus of both peptides. For intraperitoneal injection in mice and treatment of cultured neurons, FITC along with a 6-aminohexanoic acid linker (Ahx) was added at the N-terminus and the TAT-sequence (YGRKKRRQRRR) at the C-terminus of both peptides. All peptides were synthesized by Peptide 2.0 Inc.
IP-Linked Kinase Assay.
Recombinant Cdk5/p25 complex or Human embryonic kidney (HEK) cell lysates were incubated with anti-Cdk5 or anti-Cdk1 antibodies for overnight at 4 °C, and the immunocomplex was prepared as described previously (18) and loaded on a 12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis gel and resolved immediately. After running, the gel was carefully dried with plastic wrap and filter paper to avoid wrinkles, and the dry gel was exposed onto a film for several hours to detect 32P-Histone H1. Band intensity was analyzed using ImageJ software. Coomassie staining was performed to confirm that equal amounts of protein were loaded for each group.
Expression Constructs.
Oligos that contain Cdk5i or scrambled peptide sequences were synthesized by Integrated DNA Technologies. The FUGW plasmid was a gift from David Baltimore (Addgene plasmid # 14883), and the oligos that contain Cdk5i or scrambled peptide sequences were cloned into the FUGW plasmid. Oligo sequences used can be found in SI Appendix, Table S1.
iPSCs Cultures.
A skin biopsy was obtained during life by B.C.D. and D.E.L. from a man with an autosomal dominant family history of FTD; he developed behavioral variant FTD at age 53, was determined to have a MAPT P301L mutation, and died at age 56. Autopsy confirmed FTLD tau neurodegenerative changes as the sole pathologic change in the patient’s brain. Fibroblasts from this sample were used to derive the iPSC line. The work carried out in this study was approved by the Mass General Brigham Institutional Review Board. Subjects are consented for extensive use of the samples such as but not limited to creation of pluripotent cells, differentiation of pluripotent cells into cells representing many tissue types, use of differentiated cells to identify underlying genetic causes and pathological correlates of neurodegenerative conditions, and in the discovery of novel therapeutic targets, use of high-throughput chemical screening for the identification of novel therapeutic agents. For subjects unable to give consent due to mental status, consent will be obtained from their legal guardian, healthcare proxy, power of attorney, or adult next of kin, in this order. Capacity to consent will be determined by the potential subject’s neurologist or physician, who has knowledge of the subject’s condition. In determining an individual’s ability to consent/assent, the physician will take into account the age, maturity, level of functioning, and psychological state of the subject. In combination with the professional opinion of an appropriate clinician, competency will also be determined (as needed) by MMSE and/or CDR threshold scores. As an additional way of gauging understanding and capacity to consent, the consenting study staff member will ask the subject to explain in his/her own words what the study is about and what participating means. If the physician determines that the subject’s decision-making capacity is impaired, we will require the assent of the subject when possible and the consent of the subject’s legal guardian, healthcare proxy, power of attorney, or adult next of kin, in this order.
Animals and In Vivo Drug Administration.
All animal experiments were performed with approval from the MIT Committee on Animal Care. For intraperitoneal injection, mice received a dose of 20 mg peptide/kg body weight every other day. Cdk5i-FT or SC-FT peptides were dissolved in normal saline to make 5 mg/mL solution and stored at −20 °C. CK-p25 mice were raised on doxycycline-containing diet until 2 mo of age and then switched to normal diet for 2 wk along with IP injection. Tau P301S mice were treated for a month. Tau P301S mice were obtained from the Jackson Laboratory.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
We thank E. McNamara for mouse colony maintenance. We thank BioMicroCenter and Kock Institute Flow Cytometry core at Massachusetts Institute of Technology for their help and guidance on RNA-seq and sorting experiments. We thank Harvard’s Center for Macromolecular Interactions for access to instrumentation for Biolayer Interferometry and Microscale Thermophoresis. We thank Lorenzo Bozzelli, Mary Mazzanti, Mitchell Murdock, and members of Tsai lab for discussion and valuable comments on manuscript. S.J.H. received funding from the Tau Consortium, Stuart & Suzanne Steele MGH Research Scholars Program, and Souvien Therapeutics. This work was supported by NIH grants R37 NS051874 (L.-H.T.), R21 NS085487 (B.C.D. and S.J.H.), and R21 NS084156 (B.C.D.).
Author contributions
P.-C.P., J.S., and L.-H.T. designed research; P.-C.P., J.S., A. Lee, O.K., D.P., A. Loon, M.X.Y., M.Y., and S.J.H. performed research; P.-C.P., J.S., A. Lee, O.K., D.P., R.M.R., M.X.Y., M.B., and M.Y. analyzed data; M.C.S., D.E.L., J.F.G., B.C.D., and S.J.H. generated an FTD patient-derived iPSC line; and P.-C.P., J.S., J.P., U.G., and L.-H.T. wrote the paper.
Competing interests
S.J.H. is a member of the scientific advisory board of Psy Therapeutics, Frequency Therapeutics, Vesigen Therapeutics, 4M Therapeutics, and Sensorium Therapeutics, none of which is involved in the present study. S.J.H. and L.-H.T. are also co-founders and members of the Scientific Advisory Board of Souvien Therapeutics. J.S., S.J.H., and L.-H.T. have licensed intellectual property related to Cdk5 inhibitory peptide. B.C.D. has served as a consultant for Acadia, Alector, Arkuda, Biogen, Denali, Eisai, Genentech, Lilly, Merck, Takeda, and Wave Lifesciences; he receives royalties from Cambridge University Press, Elsevier, and Oxford University Press, none of which is relevant to the present study. The remaining authors declare no competing interests.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
The source data are provided as a Source Data file. Neuronal RNA-seq data are publicly available and can be downloaded at the NCBI Gene Expression Omnibus (GSE222327) (63). All other data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Tsai L. H., Delalle I., Caviness V. S., Chae T., Harlow E., p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371, 419–423 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Tang D., et al. , An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J. Biol. Chem. 270, 26897–26903 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Tsai L. H., Takahashi T., Caviness V. S., Harlow E., Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development 119, 1029–1040 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Pao P. C., Tsai L. H., Three decades of Cdk5. J. Biomed. Sci. 28, 79 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su S. C., Tsai L. H., Cyclin-dependent kinases in brain development and disease. Annu. Rev. Cell Dev. Biol. 27, 465–491 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Lai K. O., Ip N. Y., Recent advances in understanding the roles of Cdk5 in synaptic plasticity. Biochim. Biophys. Acta 1792, 741–745 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Cheng K., Ip N. Y., Cdk5: A new player at synapses. NeuroSignals 12, 180–90 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Cheung Z. H., Fu A. K. Y., Ip N. Y., Synaptic roles of Cdk 5: Implications in higher cognitive functions and neurodegenerative diseases. Neuron 50, 13–18 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Gilmore E. C., Ohshima T., Goffinet A. M., Kulkarni A. B., Herrup K., Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci. 18, 6370–6377 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohshima T., et al. , Migration defects of cdk5(-/-) neurons in the developing cerebellum is cell autonomous. J. Neurosci. 19, 6017–6026 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohshima T., et al. , Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. U.S.A. 93, 11173–11178 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung Z. H., Ip N. Y., Cdk5: A multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 22, 169–175 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Lee M. S., et al. , Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360–364 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Patrick G. N., et al. , Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 (1999), 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 15.Kusakawa G. I., et al. , Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J. Biol. Chem. 275, 17166–17172 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Cheung Z. H., Ip N. Y., Cdk5: Mediator of neuronal death and survival. Neurosci. Lett. 361, 47–51 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Cruz J. C., et al. , p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid β in vivo. J. Neurosci. 26, 10536–10541 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo J., et al. , Activity-dependent p25 generation regulates synaptic plasticity and aβ-induced cognitive impairment. Cell 157, 486–498 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo J., et al. , Inhibition of p25/Cdk5 attenuates tauopathy in mouse and iPSC models of frontotemporal dementia. J. Neurosci. 37, 9917–9924 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L., et al. , Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 7, 48 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apostolopoulos V., et al. , A global review on short peptides: Frontiers and perspectives. Molecules 26, 430 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craik D. J., Fairlie D. P., Liras S., Price D., The future of peptide-based drugs. Chem. Biol. Drug Des. 81, 136–147 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y. L., et al. , A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J. Biol. Chem. 285, 34202–34212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y. L., Li B. S., Amin N. D., Albers W., Pant H. C., A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur. J. Biochem. 269, 4427–4434 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Shukla V., et al. , A truncated peptide from p35, a Cdk5 activator, prevents alzheimer’s disease phenotypes in model mice. FASEB J. 27, 174–186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla V., et al. , TFP5, a peptide inhibitor of aberrant and hyperactive Cdk5/p25, attenuates pathological phenotypes and restores synaptic function in CK-p25Tg mice. J. Alzheimer’s Dis. 56, 335–349 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz J. C., Tseng H. C., Goldman J. A., Shih H., Tsai L. H., Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40, 471–483 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Yoshiyama Y., et al. , Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Tarricone C., et al. , Structure and regulation of the CDK5-p25nck5a complex. Mol. Cell 8, 657–669 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Bk B., et al. , TFP5, a peptide derived from p35, a Cdk5 neuronal activator, rescues cortical neurons from glucose toxicity. J. Alzheimer’s Dis. 39, 899–909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian B., Yang Q., Mao Z., Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat. Cell Biol. 11, 211–218 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J., et al. , Streptozotocin induces alzheimer’s disease-like pathology in hippocampal neuronal cells via CDK5/Drp1-mediated mitochondrial fragmentation. Front. Cell. Neurosci. 14, 235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishiguro K., et al. , Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J. Biol. Chem. 267, 10897–10901 (1992). [PubMed] [Google Scholar]
- 34.Kimura T., Ishiguro K., Hisanaga S. I., Physiological and pathological phosphorylation of tau by Cdk5. Front. Mol. Neurosci. 7, 65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noble W., et al. , Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 38, 555–565 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Qian X., et al. , Generation of human brain region–specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 13, 565–580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D., et al. , Deregulation of HDAC1 by p25/Cdk5 in Neurotoxicity. Neuron 60, 803–817 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gjoneska E., et al. , Conserved epigenomic signals in mice and humans reveal immune basis of alzheimer’s disease. Nature 518, 365–369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathys H., et al. , Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 21, 366–380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer A., Sananbenesi F., Wang X., Dobbin M., Tsai L. H., Recovery of learning and memory is associated with chromatin remodelling. Nature 447, 78–82 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Gräff J., et al. , An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483, 222–226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frost B., Jacks R. L., Diamond M. I., Propagation of Tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284, 12845–12852 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clavaguera F., et al. , Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo J. L., Lee V. M. Y., Seeding of normal tau by pathological tau conformers drives pathogenesis of alzheimer-like tangles. J. Biol. Chem. 286, 15317–15331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes B. B., et al. , Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. U.S.A. 111, E4376–E4385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barthélemy N. R., Horie K., Sato C., Bateman R. J., Blood plasma phosphorylated-tau isoforms track CNS change in alzheimer’s disease. J. Exp. Med. 217, e20200861 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adaikkan C., et al. , Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron 102, 929–943 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker S. J., et al. , MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration. Sci. Transl. Med. 13, eabd7695 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong X., et al. , Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 38, 33–46 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Haeng R. S., Kim J., Bae S., Soh J. W., Lee Y. S., Cdk5-mediated phosphorylation of c-Myc on Ser-62 is essential in transcriptional activation of cyclin B1 by cyclin G1. J. Biol. Chem. 283, 15601–15610 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chi T. F., Horbach T., Götz C., Kietzmann T., Dimova E. Y., Cyclin-dependent kinase 5 (CDK5)-mediated phosphorylation of upstream stimulatory factor 2 (USF2) contributes to carcinogenesis. Cancers (Basel) 11, 523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H., et al. , Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 5, 1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gould A., Ji Y., Aboye T. L., Camarero J. A., Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery. Curr. Pharm. Des. 17, 4294–4307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gracy J., et al. , KNOTTIN: The knottin or inhibitor cystine knot scaffold in 2007. Nucleic Acids Res. 36, D314–D319 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tammareddy T., et al. , Computational study of the allosteric effects of p5 on CDK5−p25 hyperactivity as alternative inhibitory mechanisms in neurodegeneration. J. Phys. Chem. B 126, 5033–5044 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Qu J., et al. , S-nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by β-amyloid peptide. Proc. Natl. Acad. Sci. U.S.A. 108, 14330–14335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sananbenesi F., et al. , A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat. Neurosci. 10, 1012–1019 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan J. S., et al. , Cdk5 is required for memory function and hippocampal plasticity via the camp signaling pathway. PLoS One 6, e25735 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Utreras E., et al. , Suppression of neuroinflammation in forebrain-specific Cdk5 conditional knockout mice by PPARγ agonist improves neuronal loss and early lethality. J. Neuroinflammation 11, 28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudenko A., et al. , Loss of cyclin-dependent kinase 5 from parvalbumin interneurons leads to hyperinhibition, decreased anxiety, and memory impairment. J. Neurosci. 35, 2372–2383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fosgerau K., Hoffmann T., Peptide therapeutics: Current status and future directions. Drug Discov. Today 20, 122–128 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Kaspar A. A., Reichert J. M., Future directions for peptide therapeutics development. Drug Discov. Today 18, 807–817 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Pao P. C., Raju R. M., Tsai L. H., Data from “Cdk5i peptide treatment in aged Tau P301S mice”. GSE222327. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE222327. Deposited 6 January 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
The source data are provided as a Source Data file. Neuronal RNA-seq data are publicly available and can be downloaded at the NCBI Gene Expression Omnibus (GSE222327) (63). All other data are included in the article and/or SI Appendix.