Abstract
Type 2 immunity is orchestrated by a canonical group of cytokines primarily produced by innate lymphoid cells, group 2, and their adaptive counterparts, CD4+ helper type 2 cells, and elaborated by myeloid cells and antibodies that accumulate in response. Here, we review the cytokine and cellular circuits that mediate type 2 immunity. Building from insights in cytokine evolution, we propose that innate type 2 immunity evolved to monitor the status of microbe-rich epithelial barriers (outside) and sterile parenchymal borders (inside) to meet the functional demands of local tissue, and when necessary, to relay information to the adaptive immune system to reinforce demarcating borders to sustain these efforts. Allergic pathology likely results from deviations in local sustaining units caused by alterations imposed by environmental effects during postnatal developmental windows and exacerbated by mutations that increase vulnerabilities. This framework positions T2 immunity as central to sustaining tissue repair and regeneration and provides a context towards understanding allergic disease.
eTOC
Type 2 immunity is orchestrated by a canonical group of cytokines and both innate and adaptive immune cells. Locksley and Molofsky review the cytokine and cellular circuits that mediate type 2 immunity and propose a conceptual framework that places T2 immunity within the mechanisms that sustain tissue repair and regeneration. Allergic disease is discussed in this context.
Introduction
Allergic pathology consists of a constellation of syndromes - predominantly at barrier tissues – linked by immune responses to otherwise innocuous environmental antigens that result in exaggerated attempts to restrict offending agents to the external space while reinforcing avoidance behaviors to limit future exposure1. Whereas many allergic diseases, such as atopic dermatitis (eczema), conjunctivitis, hay fever (rhinitis), asthma and food allergy are managed by combinations of avoidance and therapeutics, control is often incomplete, and can be refractory and progressive, leading to life-threatening conditions such as anaphylaxis, airway mucus impaction and tissue fibrosis. Although avoidance mechanisms can have evolutionary advantages (e.g., pain, itch), the rising prevalence of diverse allergic disorders, particularly in developed countries, raises questions of how this arm of immunity has become increasingly dysregulated in modern humans.
Substantial research over the past decades has identified the key role for type 2 immunity and type 2 cytokines in allergic pathology. Although clinical endotypes exist across the spectrum of allergic diseases, with typical type 2 immunity more prominent in younger patients and less apparent in some adult populations2, the association of type 2 immunity with allergic pathology has been established by extensive animal research3, GWAS associations4, illuminative mutations of key transcription factors5,6, and the therapeutic successes of biologics that target components of type 2 immunity among patients across the allergic disease spectrum7,8. We focus this review on type 2 cytokines as effectors of allergic pathology with an attempt to understand how these cytokines evolved in vertebrate immunity; developmental windows that accompany the ordered waves of hematopoietic ontogeny and tissue residency by immune cells that express these cytokines, which could define periods of later vulnerability; and how insights regarding the positioning and elaboration of type 2 cytokines by innate lymphocytes might illuminate deployment of these programs by adaptive lymphocytes that could account for the prevalent conscription of type 2 immunity by allergic pathology occurring later in life.
Evolution and the type 2 cytokines
The crux of type 2 immunity resides within a 600 kb region of human chromosome 5q31 and the syntenic region on mouse chromosome 11 that encompasses the type 2 cytokine locus (Figure 1A). Here, the core type 2 cytokines IL-4, IL-13 and IL-5 reside bordered (~ 2.55 Mb telomeric of IL-4) by IL-9 (in human, although located at a syntenic region of chromosome 13 in mouse) and (centromeric) by GM-CSF and IL-3, which share signaling using the common beta chain with IL-5. Each are members of the short-chain 4α-helix bundle class I cytokines, all of which are involved in immunity, and distinct from long-chain class I cytokines, such as growth hormone, erythropoietin, prolactin, leptin and the gp130 family cytokines that have diverse roles in development, hormone regulation, hematopoiesis and neuropoietic differentiation, as well as immunity, and comprise the evolutionarily older members of this cytokine family9. Receptors for the short- and long-chain class I cytokines, as well as the class II cytokines, comprising type I, II and III Interferons and the IL-10 family members, signal primarily via associated JAK kinases to recruit and phosphorylate STAT proteins, which form homo- and heterodimers via SH2-phosphotyrosine interactions, translocate to the nucleus and bind to specific DNA elements to induce chromatin structural changes and target transcription of hundreds to thousands of genes10,11. Transcriptional targets include the SOCS (suppressors of cytokine signaling) family proteins that feedback to negatively regulate the pathway, which is also controlled during homeostasis by a variety of receptor, cytoplasmic and nuclear phosphatases.
Figure 1. The cytokines and core components of type 2 immunity.
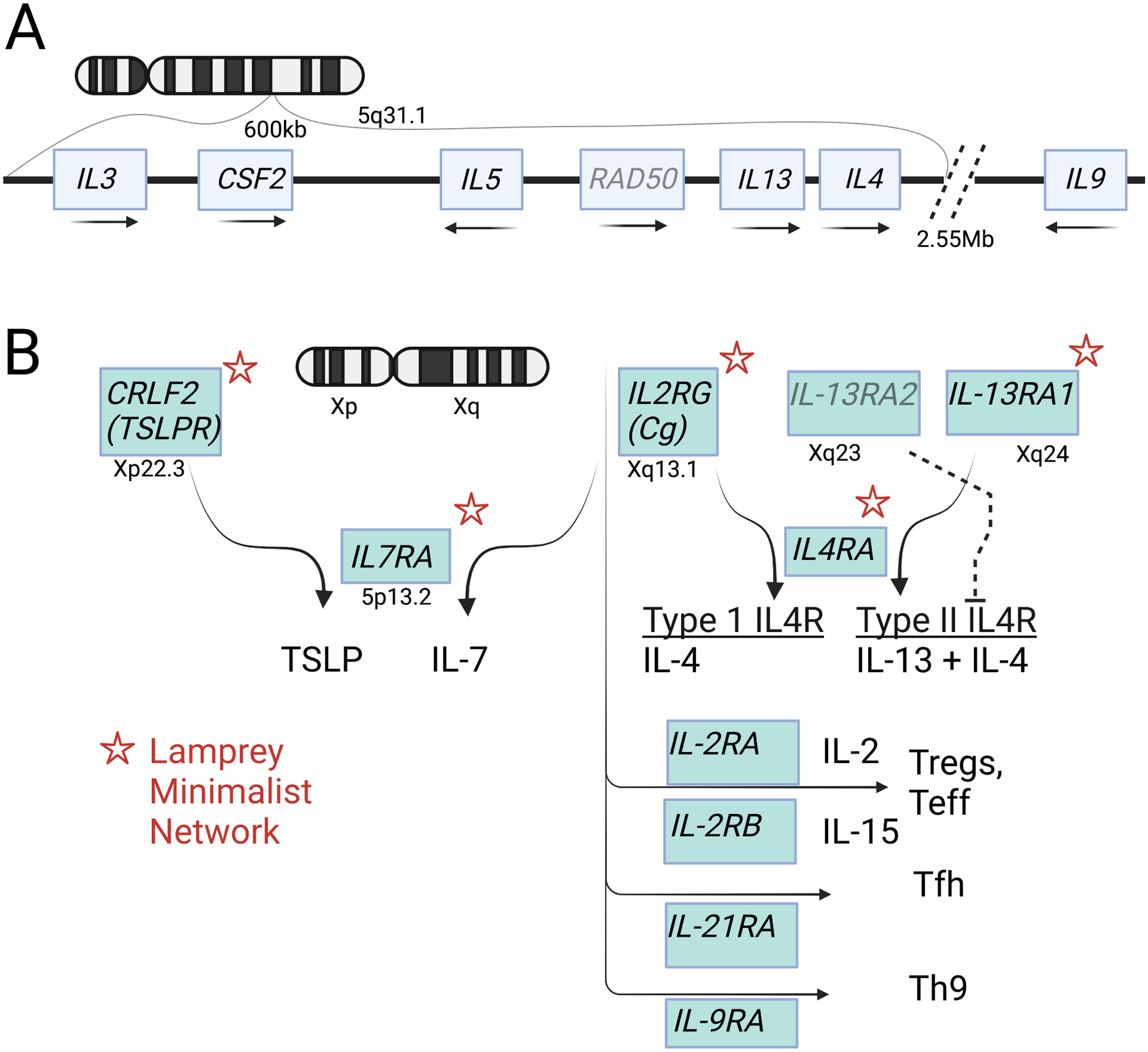
A. Genomic organization of the core type 2 cytokines on human chromosome 5q31.1. Transcription orientation indicated by arrows. B. Human cytokine signaling receptors on the X chromosome. Asterisks indicate homologs found in the lamprey cytokine receptor network. Further diversification of subsets of CD4 lymphocytes in jawed vertebrates is accompanied by appearance of additional partners for the common γ chain.
Although individual components appear earlier12, the composite cytokine/receptor/JAK-STAT/SOCs pathway appears evolutionarily first in Bilateria (animals with bilateral symmetry as embryos), as best studied in the fruit fly. Drosophila has three chromosomally-clustered class I cytokines - Upd (Unpaired), Upd2 and Upd3 – and a single receptor (Dome), JAK (Hopscotch), STAT (Stat92E), and SOCS (Socs36E). Although cytokine components of type 2 immunity appear coincident with adaptive immunity, the fly Upd cytokines, which are more closely related to IL-6 and leptin, illustrate early roles for the pathway in tissue maintenance and homeostasis in response to perturbation, including by pathogens, at least in part by interactions with tissue stem cell compartments through effects on cell fate determination, migration, proliferation and planar polarity13. In the midgut (small intestine equivalent), Upd2 from intestinal stem cells (SCs) and Upd2 and Upd3 from mature enterocytes cooperate with Notch signals to upregulate Sox21a, promoting transition to enterocytes from the intermediate enteroblasts, which accumulate in the absence of STAT92E14. Following injury or infection, Upd3 is upregulated in damaged enterocytes, prompting proliferation of intestinal SCs, in part through induction of epidermal growth factor receptor (EGFR) ligands from adjacent muscle cells, which accelerates transition of enteroblasts to enterocytes required for healing. Activation of body cavity phagocytic plasmatocytes (macrophages) induces Upd, which further upregulates visceral muscle EGFR ligands as well as antimicrobial peptides for dissemination from the fat body. Upd induction in healthy cells adjacent to damage is also required for proper regeneration after injury to the wing disc; additional examples exist in both somatic and germ cells15. Upd2 and Upd3 also link neural and hematopoietic perturbations with metabolic pathways targeting insulin sensitivity and mobilization of tissue glutamate and lipid stores necessary for redirecting resources to meet local demands16. Although caution is warranted in generalizing from evolutionarily early innate IL-6-like cytokines, class I cytokines likely evolved as local signals that sustain tissue homeostasis and are amplified when needed by recruited immune cells to regulate target cell proliferation, metabolism, survival and fate determination to restore structural and functional integrity. As such, intimate relationships with peripheral stem cell compartments might be expected17.
Type 2 cytokines are present in teleost fish, descendants of the earliest jawed vertebrates containing an adaptive immune system that depends on lymphocyte antigen receptors somatically diversified by RAG genes. Although IL-4 and IL-13 are separate in mammals, teleost fish have a single IL-4/IL-13 homolog that is present in multiple copies on different chromosomes, perhaps reflecting additional whole genome duplication in teleost, and that may represent the ancestral cytokine that duplicated to generate the distinct IL-4 and IL-13 loci18. The discovery of somatically diversified variable lymphocyte receptors (VLRs) mediated through activation-induced cytidine deaminase (AID)-driven gene conversion in jawless vertebrates (lampreys, hagfish) has furthered recognition of adaptive immunity as a hallmark of all vertebrates and facilitated identification of homologous cytokine networks that evolved to orchestrate the increasing complexity of the immune system. Despite their structural similarities, the short-chain class I cytokines lack interspecies sequence conservation, which has hindered identification of cytokine homologs in distant species. To circumvent this, an orthogonal approach was used to identify cytokine receptors instead of cognate cytokines to infer the ‘minimalist’ cytokine network needed to support adaptive immunity (Figure 1B). Genomic and transcriptomic mining of the lamprey genome identified five receptor orthologs of short-chain class I cytokines, including IL7RA, IL4RA and the three structurally similar X-linked (in human) genes, IL13RA, IL2RG (γC), and CRLF2 (TSLPR), among 23 total class 1 receptors (as compared to 34 in human)19. The authors predict existence of both type I (IL4RA/IL2RG) and type II (IL4RA/IL13RA) IL-4 receptors, but whether these are targets of a single IL-4/IL-13 homolog as found in teleosts or whether lamprey has diversified IL-4 and IL-13 by duplication remains unknown. Similarly, lamprey IL7RA/IL2RG and IL7RA/TSLPR predict the presence of IL-7 and TSLP, whereas absence of IL2RA and IL2RB predicts the absence of IL-2 and IL-15. The ancestral nature of TSLPR to CSF2RA (GM-CSF receptor) and IL4RA to CSF2RB (IL-3RB) suggests a GM-CSF/IL-3/IL-5-related cytokine and consistent with the historical identification of eosinophils in lamprey20,21. The deep evolutionary use of receptor swapping by IL2RG, IL13RA and TSLPR in lamprey suggests the possibility of novel cytokine-receptor pairings under conditions that remain undiscovered.
The presence of lamprey class II cytokine receptors for interferons and IL-10 as well as orthologs of Stat4 and Stat6 (along with Stat1 and Stat5) suggests that polarization to Th1 and Th2 cells was supported by this minimalist network. The later diversification of IL4RA to generate IL9RA (Th9), IL21RA (T follicular helper cells; Tfh) and IL2RB (regulatory T cells, Treg) that each pair with IL2RG to tune division of labor among adaptive helper T cells in jawed vertebrates was not apparent. Presumably, diversification was driven by evolution of private ligand-binding cytokine receptors capable of engaging IL2RG through its generally ‘bland’ interface devoid of highly charged bonds, thus promoting degenerate receptor sharing22. In summary, the core IL-4/IL-13/IL-5 type 2 cytokines and core signaling modules represented by IL4RA, IL13RA1, IL2RG and TSLPR have a deep evolutionary relationship with the emergence of adaptive immunity in vertebrates as supported by studies in jawless vertebrates that use alternative strategies to achieve antigen receptor diversification. Although none of the type 2 core cytokines are required for hematopoiesis, key roles for IL-4/IL-13 in alternative macrophage activation, IL-13/IL-5 in eosinophil generation and accumulation, IL-3/IL-4/IL-9 in mast cell/basophil biology, GM-CSF in myeloid cell activation, and IL-3 in plasmacytoid DC activation support mechanisms by which type 2 cytokines ‘tune’ resident and recruited hematopoietic cells to reshape local tissue environments, and may have contributed to diversification of the locus23.
Layered ontogeny and differentiation of type 2 lymphocytes
The type 2 cytokines are dominantly expressed by type 2 lymphocytes and allergic diseases are manifest predominantly at cutaneous and mucosal borders. We next consider how type 2 lymphocytes become positioned during development and the milieu in which they operate.
The concept of layered ontogeny of the immune system, proposed by the Herzenbergs in experiments revealing the derivation of B1 and B2 cells from hematopoietic stem cells purified from temporally separate periods of development24, is now known to apply to all immune cells and reflects the different needs of tissues during embryonic differentiation, independence after birth, and additional differentiation and maintenance through adult life. Organs are thus ‘layered’ by immune cells that originate during fetal life, that expand post-birth and that regenerate from diverse precursors across life. Different organs have different numbers of constituents from each developmental period as related to the capacity for self-renewal and turnover and dictated by functional needs of the local microenvironments that regulate niche size and access. Best characterized for macrophages, such ‘layering’ underpins the transition of immune cells from early developmental needs centered around growth and plasticity (e.g., synaptic pruning by brain primitive macrophages or microglia) to the increased ability to respond to injury or pathogens in post-natal life (e.g., blood-recruited monocyte-derived macrophages), in part reflecting alterations in tissue niches corresponding with developmental needs25. Thus, fetal-derived alveolar macrophages adeptly recycle surfactants and outcompete adult bone-marrow derived macrophages in fetal alveoli whereas adult monocyte-derived alveolar macrophages outcompete fetal macrophages in the adult niche and respond more aggressively to inflammatory airway perturbations, although both subserve core tissue homeostatic needs26,27. Improved fate-mapping tools in mouse have extended contributions by primitive hematopoiesis (yolk sac and embryonic hemogenic endothelial-derived precursors prior to production of definitive pluripotent hematopoietic stem cells (HSCs)) to adult tissue resident macrophage populations that are self-renewing and minimally replaced by blood monocytes not only in brain and skin, but in most tissues, particularly among macrophage populations along delimiting border tissues as opposed to interstitial cells, which derive largely from monocyte-derived precursors; similar populations are present in human fetal and adult tissues28. Comparable trajectories occur among mast cells, important contributors in type 2 immune pathology by virtue of IgE-mediated survival and activation pathways, with developmental heterogeneity among connective tissue and mucosal mast cells29,30.
Like macrophages, tissue-resident lymphocytes are also comprised of sequential contributions by primitive and definitive hematopoiesis. Lineage-tracing with inducible VE-cadherin, which temporally and indelibly marks hematopoietic descendants of hemogenic endothelia, reveals that mouse dendritic epidermal T cells, or DETCs, are yolk sac-derived, self-renewing and poorly replaced by adult bone marrow precursors31. In human thymus, fetal but not adult HSCs are intrinsically poised to develop relatively invariant germline-encoded sequences and mature effector function, in part due to suppression of terminal deoxynucleotidyl transferase (TdT) by RNA binding protein Lin28b32,33. Lympho-myeloid precursors (LMPs) are present among yolk sac-derived precursors34,35 and clonal multipotent progenitors with lymphoid potential can be derived from human induced pluripotent stem cells that resembled progenitors in mouse yolk sac and had γδ T cell potential36. Studies in human fetus show widespread accumulation of both myeloid and lymphoid progenitors and mature cells in tissues, including macrophages, mast cells and NK cells, that had acquired tissue-specific transcript signatures at 10–12 weeks post-conception, as well as thymic PLZF+ ILCs that were absent from postnatal thymus37. Importantly, developmental layering also occurs among CD4 and CD8 αβ T cells, reflecting the bifurcated origins from pro-definitive, lineage-restricted HSCs and definitive pluripotent HSCs before development in the thymus. In general, naive fetal T cells express a less-diversified TCR repertoire, distribute into tissues and acquire the capacity for rapid effector responses and activation by cytokines and pattern recognition receptors that resemble nonconventional T cells and ILCs, in part through developmental expression of microRNA switches that regulate TCR sensitivity and metabolic activity38. The use of promiscuous germline-like receptors, poised effector function and lower activation thresholds positions these cells for rapid responses but also lower thresholds for peripheral Treg cell conversion or anergy in response to self-antigens or non-threatening antigens encountered after birth, and consistent with original observations of perinatal tolerance by Medawar39 and later studies in humans40. Fate-mapping and transfer experiments of both CD4 and CD8 neonatal cells in mice revealed retention into adulthood as major constituents of induced tissue CD4 Treg cells41 and rapidly responding tissue CD8 T cells that generated short-term effectors but not long-lived memory cells42. Developmental layering of adaptive T cells populates the neonatal environment with poised effector cells highly responsive to tissue perturbations but restrained by lower thresholds for regulatory conversion, ensuring the capacity to respond quickly to danger after birth while preserving tolerance to self and innocuous newly encountered environmental antigens. Long-term memory is not necessary in the developing fetus but becomes critical in adults, where CD8 T cells can persist as functional, long-lived memory cells well beyond a single lifespan43.
ILC2s, like neonatal CD4 T cells, are poised for rapid effector responses. Temporally controlled fate-mapping reveals three waves of ILC2 accumulation in tissues during fetal, early post-natal and adult life in the mouse44. Fetal-derived ILC2s labelled between E16.5–18.5 are variably replaced over time in different tissues, although retention of discrete populations of self-renewing cells is apparent. Most ILC2s appear during the post-natal wave of expansion (d3-24 in mice) that occurs concomitant with activation of the effector program marked by expression of canonical type 2 cytokines and acquisition of tissue-specific transcriptomes. ILC2s from fetal liver or adult bone marrow derive from common lymphoid precursors (CLPs) through a series of intermediate precursors that sequentially extinguish alternative ILC fates, including α4β7 lymphoid precursors (α-LPs) and common helper-like ILC progenitors (CHILPs) before restriction into ILC2 precursors (ILC2p) and mature ILC2s45. Genes necessary for pan-ILC development, such as Gata3, Id2, Nfil3, Tox and Tcf7, are positioned in actively transcribed areas of euchromatin in CLPs, as opposed to genes involved in T and B cell commitment like Bcl11b and Ebf1, suggesting that default genomic organization in CLPs favors ILC development46, with ILC2p differentiation and cytokine production mediated in part by utilization of unique GATA3 enhancer elements47. Commitment in ILC2p cells requires interactions of the Id2 promoter via DNA loops with cis-regulatory elements, designated locus control region-1 and -2 (LCR1, LCR2), that bound GATA3 and RORα, which are required for ILC2 development48,49; deletion of LCR1 or GATA3 and RORα binding sites in LCR1 ablates ILC2s with development block at the ILC2p stage that is rescued by Id2 lentivirus. LCR1-deficient mice are ILC2-deficient and show attenuated responses in lung models of allergic inflammation, including house dust mite, papain and IL-33; Th2 cells are normal but diminished in numbers at the conclusion of the experiments although in vitro Th2 differentiation is normal, revealing sustained expression of Id2 through LCR1 as critical for ILC2 differentiation but not for activation of the type 2 cytokine effector program46. Thus, the absence of developmental positioning and activation of ILC2s had effects on the temporal accumulation of effector Th2 cells but not their function.
The perinatal period in mouse is also notable by the appearance of a population of IL7R+ lymphoid-primed multipotent progenitors (LMPPs) biased to produce ILC2s and T cells as compared to IL7R− LMPPs that generated common lymphoid progenitors (CLPs)50. Some IL7R+ LMPPs express Rag and CCR9 consistent with early T cell precursors primed for thymic entry before the postnatal period of massive T cell expansion. Although generated in Rag-deficient mice, some ILC2s, like other ILCs and NK cells51, can be fate-mapped for Rag expression and tissue resident ILC2s can express transcripts for rearranged TCR gamma chain with frameshift and premature stop mutations suggesting potential origin as non-selected T cells52, and consistent with the appearance of embryonic thymic ILC2s from shared T cell precursors53. Although further work is needed with improved time- and lineage-stamping methods to exclude contamination by small numbers of ILC progenitors, these data suggest the possibility that ILC2s that expand in mice after birth derive both from direct seeding of hemogenic endothelial-derived tissue precursors and from thymic T cell-committed precursors, some of which fail γδ TCR selection, thus creating heterogeneity that is little explored54. Of interest, even such thymic-derived ILC2s express type 2 cytokines whereas successfully selected γδ T cells are primed to produce type 3 (IL-17A) or type 1 (IFNγ) cytokines55.
While acknowledging the need for more definitive study, ILCs likely first emerge from hemogenic endothelial precursors during the transition of pro-definitive to definitive hematopoiesis and seed developing tissues essentially concurrent with colonization of fetal liver during a narrow developmental window, potentially creating bifurcating pathways for establishing distributed tissue ILC precursors while seeding fetal liver with fetal and pluripotent HSCs that support the needs of fetal and postnatal life, respectively (Figure 2). Although circulating ILC2p cells are more prevalent in human than mouse56,57,58, the longer gestation in humans is associated with colonization of peripheral tissues by adaptive T cells that outnumber resident ILC2s at birth. Bone marrow-derived CLPs generated from definitive HSCs can adoptively replenish tissue ILC2 stores in mice, like systemic human precursors56, by processes hastened by perturbation and resembling trajectories for monocyte-derived macrophages59. Despite the capacity for substantial functional tissue adaptation by adult ILC2 precursors44,59, however, it is likely that specific properties of fetal-derived cells and/or fetal niches are temporally restricted to developmental windows and not fully recapitulated by adult precursors, as noted for macrophages25, mast cells60, LTic61, nonconventional T cells62,63, B1a cells24 and adaptive T cells38. Hematopoietic reconstitution of humans genetically deficient in ILCs resulted in the slow reappearance of blood ILCs, including ILC2s64,65, and loss of normal nasal mucosal homeostasis, thus hinting at non-redundancies of function66. As discussed below, the transition during birth and early postnatal life may be susceptible to misalignment between developmental and environmental demands on the immune system that can create pathways for subsequent organ pathology as predicted by consequences of ontogenic layering25,67,68.
Figure 2. Layered ontogeny underlies the distributed network of type 2 immune lymphocytes.
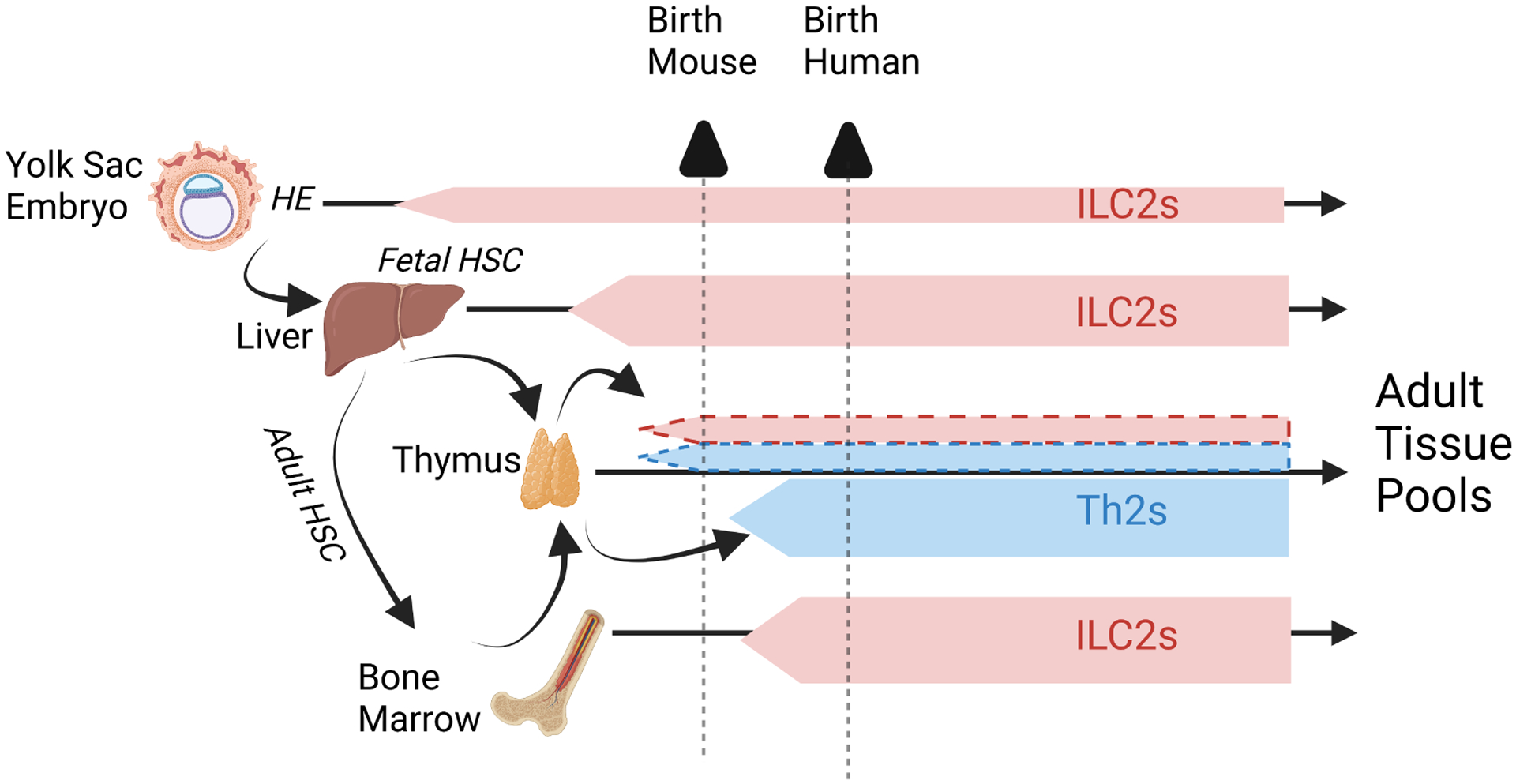
Although tissue resident ILC2s and Th2s derive from upstream precursors, we figuratively represent their origins from populations designated ILC2s or Th2s for illustrative purposes. Tissue ILC2s are comprised of populations originating from primitive hematopoiesis originating in yolk sac and liver, and bone marrow definitive HSCs. Additional heterogeneity may reflect derivation of some ILC2s from thymic pre-T cell precursors (dotted red borders). Adaptive Th2s arise from definitive adult bone marrow HSCs after thymic education and export as naive T cell precursors. Neonatal adaptive T cells can also arise from fetal HSCs, comprising a small population of tissue cells with rapid effector function (dotted blue borders). The triangles at top denote the different layering of peripheral tissues at the time of birth in mouse and human.
Cellular and humoral aspects of type 2 immunity
Although not absolute, an intriguing difference in type 2 cytokine expression by ILC2s and in vitro-generated Th2 cells is the predominant expression of IL-13 and IL-5 by ILC2s (consistent with a role in maintaining eosinophils69,70) and IL-4 and IL-13 by Th2 cells71 (where IL-5 expression increases with additional rounds of TCR stimulation72). Early studies used crossspecies comparisons of conserved noncoding sequences and DNase hypersensitivity sites to map key regulatory elements across the type 2 cytokine locus, leading to the epigenetic model of stepwise Th subset differentiation, including Th2 cells73. Incorporation of a locus control region in the 3’-end of the RAD50 gene between IL-13 and IL-5 in proximity to cytokine transcriptional domains was necessary to confer lymphocyte-specific expression74. Recent use of sophisticated methods for assessing gene regulation have added insights. Short-term activation of mouse lung ILC2s with IL-33 and Neuromedin U led to production of IL-13 and IL-5, whereas activation of splenic-derived, in vitro-generated Th2 cells with CD28/CD3 generated IL-4 and IL-13. Unexpectedly, stimulation resulted in ILC2 or Th2 lineage-specific three-dimensional reorganization of the locus imposing cohesion-associated insulator domains that apposed IL-13 and IL-5 in ILC2s but IL-4 and IL-13 in Th2 cells, respectively75. Thus, stimulation-dependent transcription factors acutely enforce geometrically divergent activation domains in the two cell types, which the authors tentatively attribute to lower SatB1 (special AT-rich sequence binding protein 1) levels in ILC2s as compared to Th2 cells. As the authors acknowledge, in vitro-generated Th2 cells are unlikely to mirror Th2 cells that mature in tissues with ILC2s and take on innate-like functions76.
Lineage-tracing in transgenic mouse models reinforce the distinct functional and regional specialization of type 2 cytokine-producing lymphocytes. Cytokine-driven cell deletion by diphtheria toxin using IL-13-Cre or IL-5-Cre essentially phenocopy in the N. brasiliensis mouse model, with loss of tissue (goblet cell hyperplasia, eosinophilia) and functional (worm clearance) components of type 2 immunity, and implicating IL-13/IL-5-expressing ILC2s and Th2 cells. Of note, IL-4-mediated IgE production was unchanged or even enhanced, indicating little contribution by IL-13-producing cells to the humoral arm of type 2 immunity in this acute model71,77. Single-cell mRNA sequencing of cytokine-reporter+ Th2 cells from lung or mesenteric lymph node of N. brasiliensis-infected mice confirmed the segregation of IL-4+ Tfh lymph node cells from IL-4/IL-13+ and IL-4/IL-13/IL-5+ Th2 cells in lung tissue; tissue Th2s expressed markers typical of resident lung ILC2s, including CD25, ST2 (Il1rl1 component of the IL-33 receptor), Cysltr 1, and effectors like amphiregulin and IL-1078.
These and other data support the compartmentalization of cellular and humoral aspects of type 2 immunity. The latter is dominantly mediated by IL-4 production (with some production of IL-13) by Tfh cells, together with B cell IgE production and FcεR1 on mast cells, basophils and populations of activated dendritic cells. In contrast, cellular type 2 immunity is dominantly promoted by IL-13 and IL-5 produced by ILC2s and Th2s (with some production of IL-4) in tissues, which mirrors widespread expression of the type II IL4RA/IL13RA1 receptor on diverse tissue cell types, including epithelia, endothelia, fibroblasts, neurons and muscle, but more limited expression on hematopoietic cells, like macrophages. Despite roles for IL-13 in human and mouse B cell IgE class-switching79,80, the therapeutic efficacy of anti-IL4RA therapy (which blocks both cytokines) in targeting human allergic diseases may reflect the capacity to attenuate both arms of type 2 immunity, including IgE production and tissue eosinophilia that depends on IL-13-mediated stimulation of eotaxins and localizing endothelial adhesins that promote tissue eosinophil entry81; indeed, anti-IL4Ra therapy is often accompanied by increases in circulating eosinophils. Selective anti-IL-13 therapeutics were less effective in asthma, perhaps due to lesser effects on IL-4-dependent IgE production (despite expression of IL-13Ra1 on human B cells) and/or interactions that affect binding to IL-13RA2, the decoy IL-13 receptor82; greater efficacy is seen in atopic dermatitis, where JAK inhibitors are also effective83. Diseases with high levels of tissue eosinophils, such as hypereosinophilic syndromes, eosinophilic vasculitis or locally infiltrative diseases like eosinophilic asthma or esophagitis, benefit from treatment directed at IL-5 or IL-5R that diminish tissue eosinophil burden directly7,8, potentially limiting inflammation provoked by Charcot-Leyden crystals of galectin-10 oligomers84. Importantly, each of the biologics approved in the US for asthma (anti-IgE, anti-IL5, anti-IL5R, anti-IL-4RA, anti-TSLP), or atopic dermatitis (anti-IL4RA, anti-IL-13, JAK inhibitors), shows efficacy, that, while incomplete, corroborates the central role of the type 2 cytokines in allergic pathogenesis.
The ‘alarmins’ – thresholding type 2 immunity
The recognition of ILC2s was hastened by the earlier discoveries of IL-25 and IL-33, so-called ‘alarmins’ and members of the evolutionary ancient IL-17 and IL-1 cytokine families, respectively; each cause robust type 2 immune responses when injected into mice85,86. First described as a cell population during studies of IL-2585,87, ILC2s were initially recognized as a distinct population of mesenteric adipose tissue-associated ILCs in mice and humans that released IL-5 and IL-6 spontaneously when maintained in IL-7 and markedly increased amounts of these cytokines with IL-13 when stimulated with IL-2 and either IL-33 or IL-2588. The cells were clustered along vessels in peritoneal mesentery surrounded by adipocytes and were activated by helminth infection to drive goblet cell hyperplasia and B1 cell expansion in association with increases in serum IL-5 and IL-13. Two publications that rapidly followed used engineered type 2 cytokine reporters to extend observations from this seminal paper. The first89 used IL-13-eGFP reporter mice to make several sentient observations: (1) administration of IL-25 or IL-33 to SPF mice each resulted in redundant proliferation of IL-13-expressing innate lymphocytes, implicating ILC2s as the major responding cells; (2) ILC2s were activated in response to intestinal helminth infection (N. brasiliensis) by IL-25R- (driving early control) and IL-33R-dependent (additively affecting late clearance) mechanisms, revealing temporal nuances; (3) wild-type (WT) ILC2s but not IL-13-deficient ILC2s restored reactivity of IL-25 in IL-25R-deficient mice and restored the tissue type 2 response to N. brasiliensis in IL-25R-deficient and IL-25R/IL-33R-deficient mice, including goblet cell hyperplasia, eosinophilia and worm expulsion, implicating ILC2s as the necessary cell target driving early IL-13 release by alarmins; (4) the temporal delay of IL-13-producing Th2 cells in mesenteric lymph nodes in the absence of IL-25R was restored by either WT or IL-13-deficient ILC2s, indicating IL-13-independent effects of ILC2s on early CD4 T cell type 2 responses that ultimately developed even in the absence of ILC2 IL-13 production, and implicating ILC2s in optimizing the subsequent type 2 response. The third report90 used IL-13-Cre mice to mark and delete IL-13-producing cells, identifying ILC2s as the major responding cells to systemic IL-25, IL-33 and after N. brasiliensis infection, while emphasizing the systemic presence of these cells in most tissues and scarcity in blood, presaging recognition of their dispersed, tissue resident phenotype91.
Taken together, these observations (and many since) establish ILC2s as non-redundant sources of early tissue IL-13 and IL-5 that can be triggered by alarmins IL-25 and IL-33 to elicit type 2 immunity. As noted above, tissue ILC2s in mice accumulate predominantly during postnatal expansion driven by de novo generation of new cells and massive proliferation over the first 3–4 weeks of life in association with type 2 cytokine expression44. Although initial expansion of ILC2s is essentially normal in the absence of IL-33 or IL-25, activation is variably attenuated in different tissues. This period is characterized by widespread IL-33 expression implicated in post-birth developmental processes including mechanical deformation during breathing and alveolarization of the lung92,93, neuronal development sculpted by brain microglia94,95, and establishment of thermogenic properties in beige and brown fat96. IL-33 is a marker for human arterial hemogenic endothelia97 and can drive maturation of endothelial-derived stromal cells to support bone marrow hematopoiesis98 and mobilization of ILC2p cells from bone marrow99. Tissue Treg cells are deposited during this same period to adjudicate indifference to self-antigens by tissue adapted, clonally restricted ST2+ Treg cells that participate in homeostasis and repair in adipose, skeletal muscle, and other organs100 and mediate tolerance by attenuating IL-33 sensitivity among conventional naïve T cells during the neonatal period101. Peripheral Treg cells are induced at mucosal and cutaneous borders to facilitate tolerance to colonizing microbes and food antigens102,103, enabled by a neonatal wave of specialized antigen-presenting cells to control type 3 immune responses104. As a nuclear-localized cytokine, the mechanisms facilitating release of IL-33 after cell damage or creation of transient pore-forming pathways remain actively investigated, while enhancement of IL-33 activity following cleavage by endogenous and environmental (allergen-associated) proteases are consistent with an active role in tissue remodeling in development and repair105,106. Although ILC2s have not been directly implicated in these processes, their accumulation across tissues during this developmental wave suggests coordination with systemically driven signal(s). Variants in IL-33 constitute a highly replicated risk for human allergic disease, particularly asthma, across diverse populations, and were localized to a 5 kb enhancer-blocking element with surrounding cohesion and insulator CCCTC-binding factor (CTCF) sites that supported long-range looping to the IL-33 promoter, potentially through enhanced binding of the transcription factor OCT-1107. IL-33 signaling promotes rapid increases of cytokine mRNAs by down-regulation of the mRNA degrading protein tristetraprolin108. Conversely, a rare loss-of-function IL-33 allele is associated with reduced numbers of blood eosinophils and diminished asthma risk109.
In contrast to the widespread expression of IL-33, IL-25 is produced mainly by epithelial tuft cells, rare chemosensory cells primarily in mucosa, which in small intestine develop with weaning and maturation of the hepatic bile acid cycle to become important regulators of ILC2 activation while contributing to ILC2 responses at other sites during inflammatory states110,111. The basal ‘inflammatory’ state of ILC2s in the small intestine lamina propria reflects residence at a highly microbial-colonized border requiring high epithelial turnover; lamina propria eosinophils also display an unusual activated, regulatory phenotype, in part driven by IL-33, that suggests an integrated type 2 response to maintain homeostasis at a site critical for nutrient acquisition and commensal control112. TSLP, a third alarmin that can activate ILC2s, particularly in synergy with other alarmins, is expressed among subsets of stromal, epithelial and dendritic cells, and is induced in keratinocytes in response to inflammation caused by vitamin D-like analogs113. Epidermal skin residency by ILC2s is regulated by both IL-7 and TSLP and the CCR6-CCL20 axis that mediates accumulation of epidermal Treg cells in neonatal mice where ILC2 activation impacts sebaceous gland homeostasis and itch perception by sensory neurons114,115. Differentiated tissue ILC2s express many positive- and negative-regulating receptors for eicosanoids, neuropeptides, neurotransmitters, cytokines and hormones that titrate activation thresholds by integrating inputs from multiple cell types that act synergistically with episodic signals from alarmins116–122. Although ILC2 numbers and cytokine transcripts are little affected in germfree or mice deficient in IL-25, ST2 or TSLPR123, ILC2 effector function becomes attenuated in the latter, suggesting roles for alarmins in maintaining responsiveness among tissue ILC2s; similar effects are seen in tissue Th2 cells. Studies using cohorts of ST2-, IL-25- and TSLPR-deficient mice in various combinations revealed combinatorial effects on ILC2 activation and Th2 cytokine secretion; triple-deficient (TKO; ST2/IL-25/TSLPR-deficient) mice displayed defects in elaboration of type 2 cytokines in tissue resident ILC2s and Th2 cells77,123,124, with loss of all 3 alarmins additively blocking type 2-mediated immune pathology125, and consistent with initial studies using alarmin blockade in humans with allergic disease7,8. Alarmins thus constitute checkpoints regulating activation of ILC2s and Th2 cells in tissues. Of note, humoral type 2 immunity as assessed by IgE responses, Tfh differentiation and the appearance of IL-4-expressing cells in lymph nodes was normal in alarmin TKO mice71,77.
Type 2 immune cell niches – barriers, borders and adventitial boundaries
Accumulation of ILC2s occurs at locations providing survival factors like IL-7 in combination with alarmins that threshold activation; interfering with the former precludes postnatal expansion44,126. Although mechanistic details are incomplete, stromal cells likely guide initial developmental positioning of ILC2p cells, in part by providing chemokines, tethering, and terminal differentiation signals like Notch. Following post-birth expansion in mice, ILC2s accumulate in at least three areas (Figure 3). (1) Barrier ILC2s localize beneath non-sterile epithelia in the cutaneous epidermis, upper respiratory tract, lamina propria of the intestines and the reproductive and lower genitourinary tracts. (2) Border ILC2s are distributed more uniformly across natural demarcations between tissues, including the dermal and submucosal fascial planes in skin and bowel that separate these tissues from underlying structures and along the epithelial-like mesothelial linings that surround the body cavities and internal organs of the chest and abdomen, such as the pleura, pericardium and mesentery. (3) Boundary ILC2s, present in most organs, are delimited by perivascular adventitial cuffs supported by specialized fibroblasts that constitutively express IL-33 and TSLP, often accompanied by lymphatic endothelial cells that express IL-7127.
Figure 3. The ins- and-outs of tissue surveillance by type 2 immune lymphocytes – an organizational model.
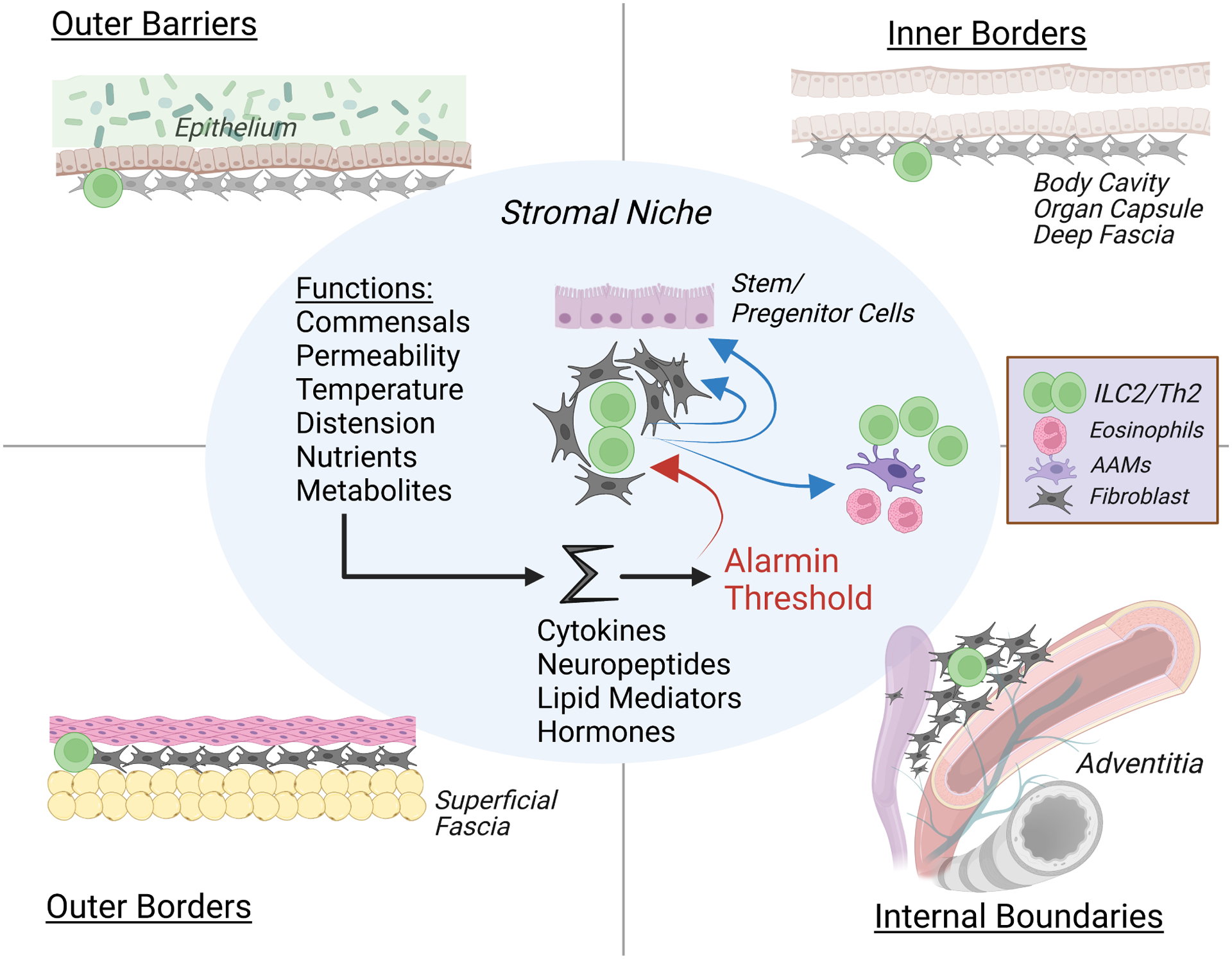
The Center grouping depicts core shared components of ILC2s in a stromal niche that likely applies to resident Th2s in the adapted state. Tissue function is translated by many cell types into signaling pathways that are integrated and thresholded by resident alarmin signals, transmitted in part by stromal niche fibroblasts to ILC2s which release type 2 cytokines that feedback on niche stromal cells and regulate myeloid cell activation and recruitment. With sufficient perturbation, ILC2s proliferate, increase cytokine outputs and migrate from the niche to interact with resident cells and tissues. Outer Barriers (upper left) are externally-oriented epidermis in skin and mucosa, where ILC2s can be activated by barrier-specific alarmins, like IL-25 in small intestine or TSLP/IL-18 in skin. Outer Barrier tissues are supported by Outer Borders (lower left) consisting of subepithelial fascial planes that support resident ILC2s that when activated can facilitate niche enlargement to house recruited Th2 cells and myeloid cells that increase local collagen deposition and reinforce the physical border internal to the external barrier. Inner Borders (upper right) represent serosal mesothelial linings surrounding internal organs, which also support ILC2/stromal niches and use type 2 cytokines to enlarge the niche and enhance structural support. Internal Boundaries (lower right) depict organization of ILC2s in proximity to vascular and ductal structures that allow monitoring of regional homeostasis in tissues.
Boundary ILC2s reside in close apposition to fibroblasts, here called adventitial stromal cells (ASCs) but with many designations that share over-lapping characteristics (e.g.; mesenchymal stromal cells, fibro-adipogenic progenitor cells, multipotent stromal cells), in the outermost layers of intermediate-to-large blood vessels within an interstitial space accommodating inter-cellular interactions in an extracellular matrix (ECM)- and neuron-rich environment where interstitial fluid can accumulate before drainage by contiguous afferent lymphatics128. Perivascular adventitial cuffs are in proximity to conduits linked with the unique functionality of the tissues where they reside, such as airways in lung, ducts in liver and pancreas, and dural sinuses in the meninges127,129. ASCs are usually PDGFRa+, GP38+ (podoplanin), Sca-1+, although this phenotype is not entirely specific, and express IL-33, TSLP and CCL11 (eotaxin) in resting tissues. Their presence in multiple organs suggests an organizing principle for type 2 immunity; resident IL-5 reporter+ Th2 cells localize similarly in organs where this has been studied. ILC2s and Th2 cells in adventitial cuffs are generally ST2+TSLPR+ while expressing low levels of IL-25 and IL-18 receptors. Adventitial niches are dynamically regulated by stromal – immune cell communication, with IL-7 and TSLP sustaining and IL-33 driving activation and proliferation of type 2 immune cells; stable, prolonged expansion of IL-33+ ASCs after type 2 immune activation was curtailed by deletion of IL-5/IL-13-expressing immune cells127, emphasizing the role of reinforcing crosstalk in fibroblast-immune cell homeostasis130. In white adipose tissue, PDGFRa+ ASCs, also designated multipotent stromal cells or adipocyte progenitor cells, were the major source of adipose tissue IL-33 and CCL11131,132, where ILC2 activation was also mediated by cell-cell contacts between LFA1 on ILC2s and ICAM-1 on ASCs133 as well as by sympathetic neuron-mediated release of glial-derived neurotrophic factor (GDNF)134. Eosinophils recruited by activated ILC2s released nerve growth factor (NGF) that promoted sympathetic axonal outgrowth, potentially reinforcing the circuit135. At all sites, ILC2s accumulate in proximity to sensory and autonomic neurons and are tuned positively and negatively by neuropeptides116, which with eicosanoids amplify thresholds for alarmin signaling and control differentiation trajectories among effector subpopulations117,118. Thus, boundary ILC2s, and likely resident Th2s, are organized within innervated regional niches structured by ECM-promoting interactive domains and bathed by interstitial fluid and metabolites reporting tissue-specific homeostasis, and in proximity to endothelial vascular access and lymphatic egress, thereby establishing a microdomain poised for integrating perturbations from multiple cell types within small functional units. Upon localization in niches promoting their maintenance, ILC2s (and resident Th2s) proliferate, likely through intrinsic autocrine and paracrine interactions that regulate clonal expansion75,136,137,138. In mice, upregulation of integrin αvβ3 on activated naive T cells promoted IL-2 and CD25 expression, leading to autocrine and paracrine Stat5 signaling, and differentiation to IL-13- and IL-5-secreting Th2 cells139; ablation of the integrin attenuated accumulation of lung Th2 cells while IL-4 expression remained unaffected. Integrin interactions promoted T cell-T cell interactions, which could contribute to clonality seen in tissue resident cells in mice and humans78,140. Regulation of stromal niche size and occupancy are not well understood, although feed-forward circuits that expand alarmin-producing cells, as noted above, and the marked pliability of tissues for lymphocyte residency suggest the potential for dynamic changes141. Tertiary lymphoid organs (TLO) in lung (induced bronchus-associated lymphoid tissue, iBALT), adipose (fat-associated lymphoid clusters, FALC) and other tissues can form in continuity with adventitial niches and expand the pool of alarmin+ stromal cells resulting in protective or pathologic contributions to allergic disease in different contexts142,143. Roles for IL-13 and IL-22 in sequentially driving activation and proliferation of stromal precursors that nucleate TLO formation have been shown in both mice and humans144.
Aside from adventitial cuffs, ILC2s are distributed at tissue-demarcating borders surrounding organs and along fascial-adipocyte planes beneath the dermis and lamina propria of externally exposed barrier tissues. These border ILC2s are typically ST2+ and also in proximity to ASCs that can express IL-33127. In mesenteric adipose tissue, serosal IL-33+ mesothelial cells respond to injury by activating resident border ILC2s, revealing a responsive interface to announce disruption131. Finally, barrier ILC2s, prevalent in upper respiratory tract, intestinal lamina propria and epidermis, are characterized by high-turnover and basal responses to non-IL-33-mediated activation, as discussed in the next section. In small intestinal lamina propria, barrier ILC2s constitutively express the IL-25 receptor and TSLPR whereas skin epidermal ILC2s express TSLPR and the IL-18 receptor, suggesting barrier-specific regulatory activity123. On an organismal scale (Figure 3), ILC2s are arranged with an ‘outside-in’ and ‘inside-out’ perspective with a shared cored program; outer barrier ILC2s integrate external perturbations with tissue signals to regulate activation, whereas inner boundary ILC2s are positioned to integrate organ-specific perturbations with tissue signals to regulate activation, and each is reinforced by internal border ILC2s responsive to loss of homeostasis by the overlying domains.
Following disruptive perturbations that exceed the homeostatic capacity of the local cellular network, ILC2s integrate alarmins with signals from multiple cell types, proliferate and mature along trajectories that promote interactions with circulating hematopoietic cells recruited to the tissue59,145; proliferating, activated ILC2s can upregulate S-1-P receptors and undergo retrograde trafficking via lymphatics to enter blood, although whether emigrants represent a subset of the total population is unknown146. Border ILC2s, where studied, are typically resident and self-renewing, with no clear evidence for egress, and may even have precursor relationships with barrier or adventitial boundary ILC2s, although further study is needed. Proliferation leading to tissue eviction can be driven by IL-33 or IL-25, and perhaps other activating signals, dependent on geographic differences in expression of the relevant alarmin and alarmin receptors by resident ILC2s147. Egress of mature ILC2s from tissue is accompanied by differentiation of locally embedded ILC2 precursors and entry of blood-borne precursors from bone marrow or other tissues, perhaps by creating space or freeing survival ligands, although the precise contributions of ILC2 precursors versus proliferating, mature effectors remains unclear. Activated migratory ILC2s can enter distal tissues and affect target tissues for many months; whether epigenetically modified ‘memory’ tissue ILC2s represent local, recruited, or combinations of cells from both bone marrow and perturbed tissues requires more granularity59. Although cohesive in principle, much regarding the mechanistic underpinnings of these processes requires further study.
Type 2 immunity and tissue homeostasis
Contributions of ILCs, including ILC2s, to tissue homeostasis and repair after injury have been described in numerous organs, including the intestines, lung, adipose tissues, skin, muscle, heart, brain and bone marrow116,148. Most studies involve genetically homogeneous mice under SPF conditions, which rely on resident ILC2s rather than tissue resident Th2 cells likely present in ‘wildling’ mice and humans149. However, the overlapping phenotypes of these cells support comparable functions, with caveats regarding unsuspected developmental roles as noted above. Involvement of ILC2s and type 2 cytokines in regulation of epithelial barriers, repair and regeneration suggest proximity to peripheral tissue stem cell niches, specialized regenerative microdomains protected from external injury where intrinsic stem cell and extrinsic signals from cells and ECM interact to regulate stem cell fate in close approximation to lymphatic endothelia actively involved in these processes150,151; maintenance of tissue stem cell niches is critical for sustaining homeostasis and dysfunction is associated with senescence and aging152. Activated ILC2s can support aged or regenerating tissues153,154,155 and effects of ILC2s in altering trajectories of stem cell compartments to affect barrier physiology reveal shared aspects that hint at principles that might guide this process.
The capacity for barrier ILC2s to alter physiology in response to external stimuli is well illustrated in the small intestine. Epithelial tuft cells in mucosal epithelia express multiple GPCRs, including GPR91, which binds succinate, a metabolite secreted by the protist Tritrichomonas muris, an intestinal commensal of wild mice156. Following detection of luminal succinate, tuft cells release IL-25, which activates lamina propria ILC2s, which constitutively express the IL-25 heterodimeric receptor, IL-17RA/IL-17RB, and release type 2 cytokines and amphiregulin157,158. In turn, IL-13 acts directly on crypt transit-amplifying cells to slow transit time, thus altering the tempo at which cells move into the gradient of BMPs that increases as cells ascend the villi159. Secretory cell fates arise after fewer divisions than absorptive enterocytes such that prolonging ‘dwell-time’ at critical thresholds for differentiation favors entrance into the secretory cell lineage and increased numbers of goblet and tuft cells. The secretory cell bias, together with increased gut motility, underpins the ‘weep-and-sweep’ response to luminal parasite infestation160,161. Helminths like N. brasiliensis produce succinate but engage additional tuft cell receptors responsible for IL-25 release158, while Heligmosomoides, which enters the intestinal lumen after maturation from subepithelial granulomas, targets the BMP gradient to attenuate the host epithelial response162. Epithelial tuft cells express many tissue-specific GPCRs; type 2 taste receptor cells (sweet, bitter, umami) share features with tuft cells, including developmental dependence on Pou2f3, expression of IL-25, and overlapping signal transduction cascades, underscoring the adaptable capacity of this sentinel chemosensory system163. The small intestinal ILC2 circuit is restrained intrinsically by negative feedback on IL-25 receptor signaling, primarily by A20 (TNFAIP3) but also CISH and SOCS1157,164,165, and is affected in BALB/c mice, which lack a key co-factor required with Pou2f3 for optimal tuft cell differentiation and are thus less responsive to Tritrichomonas166.
Two additional aspects of the small intestinal ILC2 response are noteworthy. First, despite intrinsic down-regulation, epithelial alterations induced by commensals like Tritrichomonas and H. polygyrus in C57BL/6 mice are themselves long-lived, persisting after resolution of infection and manifest as ‘memory’ responses to secondary homologous or even heterologous luminal infestation; more dramatic effects can be elicited by deletion of A20 in ILC2s, revealing the dedicated role for intrinsic IL-25 expression in small intestinal physiology157 (of note, lampreys express IL-25 but not IL-33167). Whether memory is mediated at the level of crypt long-lived epithelial stem cells, niche stromal cells and/or ILC2s through epigenetic alterations remains incompletely studied. In human chronic allergic rhinosinusitis with nasal polyps, basal epithelial progenitor cells expressed an IL-13-mediated transcriptional signature and showed altered differentiation trajectories168. Success in treating polyposis non-surgically by antibodies blocking type 2 cytokines, however, suggests a need for extrinsic immune signals to sustain the pathology. ILC2 activation in response to helminth infection drives proliferation and migration of IL25R+ ILC2s into circulation, where these cells access distal tissues, including lung and conjunctivae, and establish protective responses in uninvolved mucosa146,169. The ability of migratory ILC2s to alter mucosal responses in distal tissues suggests that extrinsic signals can drive epithelial changes in heterologous tissues. Further study is required to assess whether lasting epigenetic changes are induced in target epithelial stem cell or stromal populations, as noted in inflammatory states170, or rather are sustained by ‘memory’ ILC2s171,172 or resident Th2 cells at these sites.
ILC2 involvement in the hair follicle response to cutaneous injury induced by the commensal mite, Demodex, also suggests a relationship with regenerative compartments. Common inhabitants of mammalian hair follicles, Demodex are contained at small numbers and spread by host-to-host transmission. In the absence of IL-4/IL-13 or IL-13Ra1, control of Demodex infestation is lost, resulting in mite overgrowth and inflammatory dermopathy with hair follicle damage; transfer of cytokine-competent ILC2s was sufficient to re-establish control of mites in IL-4/IL-13-deficient mice173. Of note, blepharitis, inflammation of eyelid hair follicles, can be associated with outgrowth of Demodex mites in patients treated for atopic dermatitis with antibodies that block cytokine binding to IL-4Ra174. Like systemically arrayed ILC2s, epidermal ILC2s were IL-5+IL-13+ at birth, but subsequently produced IL-13 synchronously with onset of anagen, the hair follicle growth phase. In the absence of IL-13 or its receptor, hair follicle stem cell proliferation increased, consistent with a role for IL-13 in attenuating processive differentiation of transiting stem cells. Perturbations of the skin by Demodex colonization or tape stripping in the absence of IL-4/IL-13 signaling were associated with loss of dermal integrity and cytokine- or receptor-deficient mice with Demodex developed premature skin senescence with hair loss, dermal collagen deposition and skin stiffening. Although ILC2s expanded in response to inflammation, Th2 cells and Tregs also accumulated173. Studies investigating transient depletion of Tregs in mice during the period of postnatal expansion in skin noted IL-13-mediated accumulation of IL33+ subdermal fibroblasts, leading to development of an enlarged subdermal collagenous, fascia associated with recruited Th2 cells and eosinophils. These perinatal alterations, otherwise uneventful and morphologically resolving over time, created an altered border milieu with impact on skin responses in adult animals: full-thickness cutaneous wounds healed quicker and antigen-driven cutaneous challenges generated biased type 2 immune responses175. Taken together, activated epidermal skin ILC2s impact stem cell compartments to mediate hair follicle homeostasis. When perturbations overwhelm local control, IL-33+ ‘border’ fascial fibroblasts, potentially in response to IL-13, activate a structural transition to expand the niche for type 2 innate and adaptive immune cells and reinforce the deeper barrier to protect internal tissues. As revealed in this study, perturbation of immune regulation during the neonatal window drove establishment of a memory state that manifested as a heightened type 2 immune response to subsequently encountered antigens in adult mice.
Adipose represents a tissue rich in ILC2s, as noted in the initial description of ILC2s88, and ILC2 production of IL-13, IL-5 and Met-enkephalin has been implicated in sustaining thermogenic responses of adipocyte beiging and resident myeloid populations like eosinophils and alternatively activated macrophages176,177,178; the loss of type 2 immunity was associated with diminished insulin sensitivity and infiltration by inflammatory cells, promoting obesity. After intestinal H. polygyrus infection, Th2 cells infiltrated mesenteric adipose adjacent to granulomas where larval forms matured and produced amphiregulin and TGFβ in response to stromal multipotent progenitor cells expressing TSLP and IL-33, resulting in expansion of the stromal cell population and consistent with the crosstalk noted above179. Th2-stromal cell alterations persisted many months after drug cure of infection, suggesting a self-sustaining state associated with declining numbers of mature adipocytes and improved systemic metabolic homeostasis, as previously noted after helminth infection180 and consistent with effects of IL-13 on muscle fatty acid oxidation and mitochondrial biogenesis181. Single-cell mRNA analysis of resident Th2 cells revealed expression of genes typical of resident ILC2s, including PPARγ, Neuromedin 1 receptor, CALCA, KLRG1 and Arg-1, revealing reiterative adaption imposed by the tissue on type 2 immune lymphocytes of both innate and adaptive lineages77,179. Mechanistically, amphiregulin from activated Th2 cells inhibited maturation of multipotent progenitors to mature adipocytes, suggesting consistent effects of type 2 immunity in altering proliferation and differentiation of tissue stem cells. Functionally, increased collagen deposition at sites of granuloma-induced injury resulted in barrier reinforcement, revealing a highly localized response to helminth-mediated perturbation accompanied by re-direction of body metabolic resources to support the structural alterations.
These examples suggest some organizational principles underlying the deployment of type 2 immunity (Figure 3). Barrier ILC2s relay signals from epithelial sensory cells, such as keratinocytes in skin or tuft cells and enteroendocrine cells in gut, and respond to tissue-specific activation cues, such as TSLP/IL-18 or IL-25, respectively, to produce canonical outputs, including IL-13, IL-5 and amphiregulin, that act on the stem cell compartment to alter the composition and physical properties of the barrier while sustaining macrophage and eosinophil populations and stromal alarmin+ support cells, thus constraining type 2 lymphocytes to relevant niches and avoiding pathology. We envision that upstream physical signals that trigger activation cues are linked to tissue function, such as vitamin D metabolites or inflammasome assembly and IL-18 production in skin in response to sun exposure and injury, GPCR engagement and acetylcholine release in upper respiratory tract, or IL-25 and eicosanoid release in small intestine in response to luminal constituents. When perturbations exceed the ability to maintain homeostasis, ILC2s proliferate, differentiate and spread systemically while cDC2s are also activated to migrate and initiate an adaptive type 2 immune response, leading to production of Th2 cells and IgE. Type 2 cytokines produced during ILC2 expansion and Th2 recruitment feed-forward to expand the bordering stromal network of IL-33+ cells, thereby priming the perturbed tissue by enlarging barrier and border niches and the carrying capacity for type 2 lymphocytes while reinforcing subepithelial and serosal borders by inducing collagen deposition in deeper tissues. Migratory ILC2s in blood can enter distal sites and alter stroma, stem cells and mucosa, reinforcing barriers in the presence of environmental perturbants.
In parenchymal tissues like lung and liver, adventitial boundary ILC2s are positioned in proximity to cells that can monitor interstitial fluids in proximity to conduits related to tissue-specific functions, such as bile or pancreatic duct content or oxygen exchange. Although the nature of such sensors remains unknown, we speculate that such biologic outputs are translated through dynamic signals, like eicosanoids and neuropeptides, that contribute to the basal activity of tissue ILC2s69,70. With disturbances that trigger alarmin release, ILC2s relay cytokines to vessels and lymph nodes to alter the cellular milieu and flag areas (via cytokine-mediated induction of adhesins and chemokines) for deployment of myeloid and adaptive Th2 cells to reinforce functional integrity by cytokine and growth factor regulation through cellular and tissue containment mediated by myeloid cells, including AAMs and eosinophils (Figure 3). Homeostasis is restored in microdomains using all aspects of recently proposed mechanistic pathways underlying immunological ‘scars’ - cellular reprogramming, reconfiguration of cellular content and structural remodeling68 – while systemically altering metabolic homeostasis to provide energetic support to sustain tissue functionality. Clearly, further understanding is needed, particularly at reproductive and genitourinary tract borders where inflammatory effects and oncogenic transformation have profound negative effects on functional integrity of the tissues that are likely countered by homeostatic regulation by type 2 immunity.
Developmental windows and temporal vulnerabilities drive allergic pathology
Type 2 immune responses are associated with intestinal and migratory helminth infections and provide host protection against mucosal injury through epithelial reorganization and barrier reinforcement, as noted above, and their downstream effects, including metabolic adaptation and colonization resistance183. Given the reliance of helminths on host survival for reproduction and transmission, evolution has sculpted interactions by which parasites induce type 2 responses to enhance their own survival and minimize damage, particularly at nutrient interfaces in small intestine. Although results are mixed, therapeutic infections with non-replicating or self-limited helminths have been used in attempts to attenuate inflammatory bowel disease183. Morbidity of intestinal soil-transmitted and vector-transmitted organisms remains high, but much of the debilitation occurs after accumulation of repetitive injury over many years and often at post-reproductive ages. Efforts to understand the protective effects of tissue type 2 responses may lead to mechanistic understanding that minimizes cumulative damage to organs and tissues. Despite association of IL-13 with pathologic fibrosis, attenuating type 2 immunity can increase tissue damage in some models of fibrosis184 and anti-IL-13 therapeutics have not shown efficacy in human pulmonary interstitial fibrosis185. Further work is warranted.
We envision at least two mechanisms predisposing to development of allergies in early life (Figure 4). First, perturbations that affect developmental windows during which deposition and expansion of ILC2s (and Th2s during adaptive responses) occur in tissues could alter niche size, composition or quality and affect type 2 cytokine outputs involved in homeostasis. Second, temporal vulnerability windows when environmental perturbations create stress on resident immune cells that drive unbalanced immune and regenerative responses resulting in allergic pathology. Allergic mechanisms through both pathways reflect gene-environment interactions orchestrated by type 2 immunity and consistent with genetic studies implicating enhancers of immune genes in allergic pathogenesis with roles that include epithelial responses to infectious agents186, and likely contributing to the risk of allergy early in life when stabilization of the barrier microbiota remains fluid187.
Figure 4. Vulnerabilities to type 2 pathology.
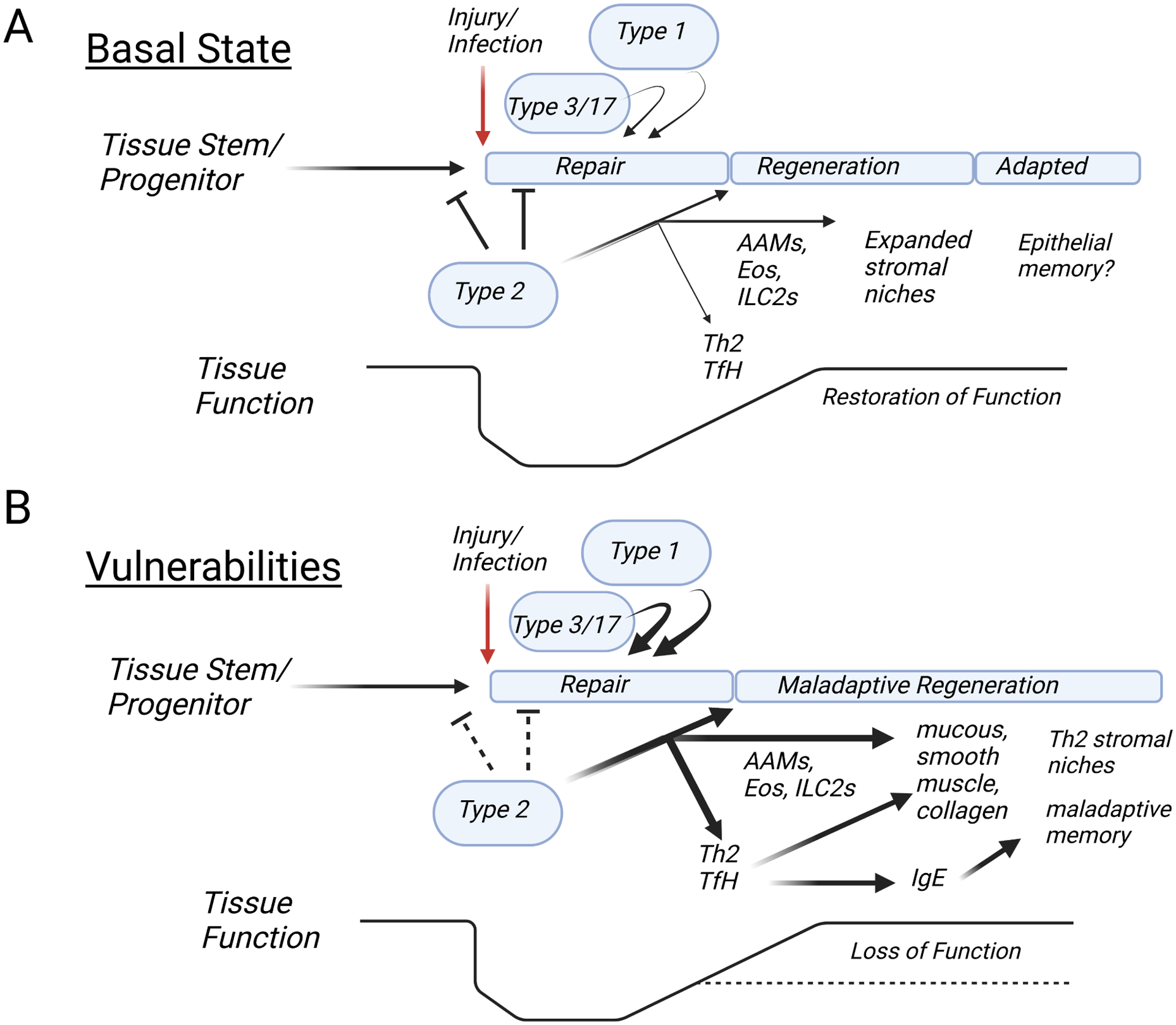
A. In the basal state, injury induces alarmin-mediated ILC2/Th2 outputs that inhibit normal stem cell transitions while the epithelium regenerates by engaging immediate type 3 responses and later type 1 immunity to enforce training. With repair, type 2 cytokines activate myeloid cells (AAMs, eosinophils) to mediate regulatory control and expand the type 2 immune cell niche using structural reinforcement as necessary. Systemic spread of type 2 lymphocytes contributes to metabolic homeostasis needed to support the altered tissue state and local function is sustained or even improved, depending on the context of the inciting event. B. Vulnerabilities occur when mutations interact with environmental exposures during critical developmental windows that affect local tissue functions or output of type 2 cytokines. The tempo of repair becomes altered, resulting in an excess of tissue AAMs, eosinophils and alarmin+ stroma that support an enlarged niche with potential for development of tertiary lymphoid structures promoting local Tfh and Th2 differentiation. The misalignment of repair and regeneration with immune signals, particularly as affected by underlying mutations, can lead to aberrant epithelial and tissue responses manifest as allergic pathology and loss of function.
Developmental windows reflect periods when the relevant cell types, including ILC2s, stromal fibroblasts, macrophages and mast cells, are deposited and mature in tissues, thus creating microdomains where type 2 cytokines bridge vascular access and macrophage-mediated interactions with fibroblasts and parenchymal tissues. Genetic and maternal effects on cell types that affect ILC2 niche size or composition could cause changes in outputs during the perinatal expansion with the potential to enlarge or misposition the type 2 niche due to positive feedback on alarmin+ stromal cells188. Such alterations would be consistent with effects of gain-of-function or loss-of-function genes5,6,109 involved with expression of alarmins or type 2 cytokines and potentially those that impact mast cell function189, but also effects of maternal nutrition, infections and transfer and quality of antibodies like IgE.
Temporal vulnerability windows reflect periods when environmental exposures to infectious agents or inflammatory moieties occur during post-birth transitional states, particularly during periods of increased energetic needs that drive decisions between tolerance and resistance190. Human energetic needs follow stereotyped inflection points over lifespan191. After rapid increase to a peak at the first year of life, energy expenditures decline to adult levels after puberty, follow a stable plateau from ages 20–60, and then progressively decline, revealing a large footprint for resource needs in the initial perinatal period. Resource allocations may be less tolerant of deviations during this period and explain the epidemiologic associations of asthma and early life exposures to respiratory viruses3, inflammatory stimuli envisioned by the ‘hygiene hypothesis’ or exposure to antibiotics, potentially damaging food additives or novel chemicals192, and the proclivity towards tissue Th2 development in response to airway perturbation193. Epigenetic alterations in mucosal niches may lead to fixed areas of exuberant type 2 responses, as in chronic allergic rhinosinusitis with nasal polyps or recurrent, fixed mucus airway plugging in asthma168,194. Mutations in genes affecting epithelial integrity (e.g., filaggrin) or leading to lymphopenia, dysregulated T cell signaling or Treg function195 would similarly increase pressure on epithelial stem cell compartments during periods when tissue-intrinsic developmental windows become stressed by fluctuations in microbial control during colonization post-birth or loss of tolerance to innocuous environmental antigens196. Mutations in B cell receptor signaling affecting IgE receptor-mediated apoptosis in IgE plasma cells could impede normal tolerance mechanisms197. Inhibition and niche confinement of ILC2s by interferons is important in facilitating optimal parenchymal responses to inflammatory organisms198,199, but whether this mechanism is operative during the period of post-natal ILC2 (mice) or Th2 expansion (humans) is unknown. Interferons induced in SPF mice co-housed with pet store mice waned after ~2 months when the microbiota stabilized and inhibition of innate type 2 immune responses in lung was largely, but not completely, recovered, although long-term commensals like helminths and protists were not explored200. Type 2 niches typically exclude inflammatory type 1 cells by unknown mechanisms, but in lung, severe viral-induced inflammation drove niche invasion by inflammatory lymphocytes, in part through increased IL-7 induction in ASCs, resulting in damage to alveolar stem cells and structural compromise201. As with susceptibility to infectious diseases, where the impact of underlying genetic mutations in shaping vulnerability increasingly supports early epidemiologic observations202,203, allergic diseases are likely to be defined increasingly by the impact of mutations on environmental windows of vulnerability at the nexus of immune-mediated regeneration and repair195.
While many illustrative mutations exist, here we briefly review loss-of-function mutations in type 17 immunity due to dominant-negative Stat3 mutations, biallelic mutations in ZNF341, which regulates Stat3 transcription, and mutations in IL6ST (gp130) implicated in IL-6 and IL-11 signaling that lead to canonical hyper-IgE syndrome204. Complicated by cutaneous and mucosal Staphylococcal and fungal infections, patients develop eosinophilia but little food allergy or anaphylaxis in part due to diminished mast cell degranulation due to attenuated Stat3 activation. While not entirely clear, loss of negative regulation of IgE class switching by IL-21 on B cells may contribute to the hyper-IgE response205. Loss of control during colonization by cutaneous commensals normally constrained by IL-17 and type 3 responses may lead to activation of ILC2s and Th2 cells in response to injury-induced stem cell proliferation and reinforcement of alarmin+ stromal cells, accounting for the high IgE, eosinophilia and atopic dermatitis that occur. Over-lapping phenotypes with mutations in the TGFβR signaling pathway revealed cross-regulation between Stat3 and induction of a negative regulator of SMAD2/3 nuclear localization resulting in increased TGFβ signaling with enhanced IL-4Ra and GATA3 expression in T cells associated with eosinophilia, hyper-IgE and atopic syndromes that respond to therapies targeting type 2 cytokines206. Periods of immune dissonance when two pathogens require antagonistic Th subset responses also expose temporal windows of vulnerability. In SPF mice (immunologically akin to newborn humans), viral challenges at the peak of early type 2 immune responses to parasitic helminths can lead to loss of virologic control and poor outcomes whereas prior helminth infections given time to establish mucosal homeostasis protect against subsequent challenges207,208,209.
Closing remarks
We focused here on innate type 2 immunity as elaborated by core type 2 cytokines by lymphoid cells in attempting to construct an evolutionary-based model for this pathway in vertebrate health that might be subject to deviation early in life and the increased risk for atopy. Clearly, many other cells and pathways are involved in both homeostasis and allergic pathology, including Treg, non-conventional T cells, B cells and other antigen-presenting cells, and but briefly discussed here. We focused on the core type 2 cytokines due to the successful therapeutic targeting of this pathway in human allergic diseases, while acknowledging key roles for mast cells and IgE (and IgG) antibodies in food allergy, anaphylaxis, urticaria and itch syndromes that can be driven by alternative pathways189,210, and addressed elsewhere in this Issue. The tissue adaptability of ILC2s and Th2 cells and the evolutionarily honed diversification by domain swapping among the core type 2 cytokines and their receptors create strategies for synthetic receptor engineering211 and cell-based therapies212 to exploit regenerative pathways while reducing inflammatory costs to tissue213. Further study may yield unexpected opportunities to improve health by targeted deployment of components of type 2 immunity during critical windows of need while enlightening the ins and outs of allergic diseases.
Acknowledgements
The authors thanks KM Ansel and RR Ricardo-Gonzalez for comments, and H-E Liang for pointing out papers on lamprey eosinophils. We acknowledge support from HHMI (RML), the National Institutes of Health (ABM, RML), and the SABRE Center at UCSF (ABM,RML). RML is a member of the Scientific Advisory Board for Genentech and a participant on the Editorial Advisory Board at Immunity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palm NW, Rosenstein RK, Medzhitov R. 2012. Allergic host defenses. Nature 484:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahy JV. 2015. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol 15:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammad H, Lambrecht BN. 2021. The basic immunology of asthma. Cell 184:1469–1485. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Lee PH, Chaffin MD, Chung W, Loh P-R, Lu Q, Christiani, Liang L. 2018. A genome-wide cross trait analysis from UK Biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat Genet 50:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma M, Leung D, Momenilandi M, Jones LCW, Pacillo L, James AE, Murrell JR, Delafontaine S, Maimaris J, Vaseghi-Shanjani M, Del Bel KL, Lu HY, Chua GT, Di Cesare S, Fornes O, Liu Z, De Matteo G, Fu MP, Amodio D, et al. 2023. Human germline heterozygous gain-of-function STAT6 variants cause severe allergic disease. J Exp Med 220:20221437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang R, Weisshaar M, Mele F, Benhsaien I, Dorgham K, Han J, Croft CA, Notarbartolo S, Rosain J, Bastard P, Puel A, Fleckenstein B, Glimcher LH, Di Santo JP, Ma CS, Gorochov G, Bousfiha A, Abel L, Tangye SG, Casanova J-L, Bustamante J, Sallusto F. 2021. High Th2 cytokine levels and upper airway inflammation in human inherited T-bet deficiency. J Exp Med 218:e20202726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brusselle GG, Koppelman GH. 2022. Biologic therapies for severe asthma. New Engl J Med 386:157–171. [DOI] [PubMed] [Google Scholar]
- 8.Morita H, Matsumoto K, Saito H. 2022. Biologics for allergic and immunologic diseases. J Allergy Clin Immunol 150:766–777. [DOI] [PubMed] [Google Scholar]
- 9.Boulay J-L, O’Shea JJ, Paul WE. 2003. Molecular phylogeny within type 2 cytokines and their cognate receptors. Immunity 19:159–163. [DOI] [PubMed] [Google Scholar]
- 10.Philips RL, Wang Y, Cheon HJ, Kanno Y, Gadina M, Sartoreilli, Horvath CM, Darnell JE Jr., Stark GR, O’Shea JJ. 2022. The JAK-STAT pathway at 30: much learned, much more to do. Cell 185:3857–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris R, Kershaw NJ, Babon JJ. 2018. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci 27:1984–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liongue C, Sertori R, Ward AC. 2016. Evolution of cytokine receptor signaling. J Immunol 197:11–18. [DOI] [PubMed] [Google Scholar]
- 13.Hou SX, Zheng Z, Chen X, Perrimon N. 2002. The JAK/STAT pathway in model organisms: emerging roles in cell migration. Dev Cell 3:765–778. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. 2009. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137:1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera SC, Bach EA. 2019. JAK/STAT signaling in stem cells and regeneration: from Drosophila to vertebrates. Development 146: dev167643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Luo F, Xu Y, Zhang Y, Jin LH. 2022. Drosophila innate immunity involves multiple signaling pathways and coordinated communication between different tissues. Front Immunol 13:905370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik S, Larsen SB, Cowley CJ, Fuchs E. 2018. Two to tango: dialog between immunity and stem cells in health and disease. Cell 175:908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sequeida A, Maisey K, Imarai M. 2017. Interleukin 4/13 receptors: an overview of genes, expression and functional role in teleost fish. Cytokine and Growth Factors Rev 38:66–72. [DOI] [PubMed] [Google Scholar]
- 19.Boulay J-L, Du Pasquier L, Cooper MD. 2022. Cytokine receptor diversity in the lamprey predicts minimal essential cytokine networks of vertebrates. J Immunol 209:1013-1-20. [DOI] [PubMed] [Google Scholar]
- 20.Piavis GW, Hiatt JL. 1971. Blood cell lineage in the sea lamprey, Petromyzon marinus (Pisces: Petromyzontidae). Copeia 1971:722–728. [Google Scholar]
- 21.Kay AG. 2019. Was Thomas Wharton Jones FRS, assistant to the infamous Dr Knox, the first to recognize the blood eosinophil? J Roy Coll Physicians Edinb 49:78–83. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Pupardus P, Laporte SL, Garcia KC. 2009. Structural biology of shared cytokine receptors. Annu Rev Immunol 27:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougan M, Dranoff G, Dougan SK. 2019. GM-CSF, IL-3 and IL-5 family of cytokines: regulators of inflammation. Immunity 50:796–811. [DOI] [PubMed] [Google Scholar]
- 24.Herzenberg LA, Herzenberg LA. 1989. Toward a layered immune system. Cell 59:953–954. [DOI] [PubMed] [Google Scholar]
- 25.Park MD, Silvin V, Ginhoux F, Merad M. 2022. Macrophages in health and disease. Cell 185:4259–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evern E, Ringqvist E, Doisne J-M, Thaller A, Sleiers N, Flavell RA, Di Santo JP, Willinger T. 2021. CD116+ fetal precursors migrate to the perinatal lung and give rise to human alveolar macrophages. J Exp Med 219:e20210987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, Piattini F, Pohlmeier L, Feng Q, Rehrauer H, Kopf M. 2022. Monocyte-derived alveolar macrophages autonomously determine severe outcome of respiratory infection. Sci Immunol 7:eabj5761. [DOI] [PubMed] [Google Scholar]
- 28.Dick SA, Wong A, Hamidzada H, Nejat S, Nechanitzky R, Vohra, Mueller B, Zaman R, Kantores C, Aronoff L, Momen A, Nechanitzky D, Li WY, Ramachandran P, Crome SQ, Becher B, Cybulsky MI, Billia F, Keshavjee S, Mital S, Robbins CS, Mak TW, Epelman S. 2022. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci Immunol 7:eabf7777. [DOI] [PubMed] [Google Scholar]
- 29.Gentek R, Ghigo C, Hoeffel G, Bulle MJ, Msallam R, Gautier G, Launay P, Chen J, Ginhoux F, Bajenoff M. 2018. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity 48:1160–1171. [DOI] [PubMed] [Google Scholar]
- 30.St John AL, Rathore APS, Ginhoux F. 2023. New perspectives on the origin and heterogeneity of mast cells. Nature Rev Immunol 23:55–68. [DOI] [PubMed] [Google Scholar]
- 31.Gentek R, Ghigo C, Hoeffel G, Jorquera A, Msallam R, Wienert S, Klauschen F, Ginhoux F, Bajenoff M. 2018. Epidermal γδ T cell originate from yolk sac hematopoiesis and clonally self-renew in the adult. J Exp Med 215:2994–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo S. 2012. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tieppo P, Papadopoulou M, Gatti D, McGovern N, Chan JKY, Gosselin F, Goetgeluk G, Weening K, Ma L, Dauby N, Cogan A, Donner C, Ginhoux F, Vandekerckhove B, Vermijlen D. 2020. The human fetal thymus generates invariant effector γδ T cells. J Exp Med 217:e20190580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palacios R, Imhof BA. 1993. At day 8–8.5 of mouse development the yolk sac, not the embryo proper, has lymphoid precursor potential in vivo and in vitro. Proc Natl Acad Sci USA 90:6581–6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boiers C, Carrelha J, Lutteropp M, Luc S, Green JCA, Azzoni E, Woll PS, Mead AJ, Hultquist A, Swiers G, Perdiguero EG, Macaulay IC, Melchiori L, Luis TC, Kharazi S, Bouriez-Jones T, Deng Q, Ponten A, Atkinson D, Jensen CT, Sitnicka E, Geissmann F, Godin I, Sandberg R, de Bruijn MFTR, Jacobsen SEW. 2013. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 13:535–548. [DOI] [PubMed] [Google Scholar]
- 36.Atkins MH, Scarfo R, McGrath KE, Yang D, Palis J, Ditadi A, Keller GM Modeling human yolk sac hematopoiesis with pluripotent stem cells. 2021. J Exp Med 2019:e20211924.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suo C, Dann E, Goh I, Jardine L, Kleshchevnikov V, Park J-E, Botting RA, Stephenson E, Engelbert J, Tuong ZK, Polanski K, Yayon N, Xu C, Suchanek O, Elmentaite R, Conde CD, He P, Pritchard S, Miah M, Moldovan C, Steemers AS, Mazin P, Prete M, Horsfall D, Marioni JC, Clatworthy MR, Haniffa M, Teichmann SA. 2022. Mapping the developing human immune system across organs. Science 376:eabo0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudd BD. 2020. Neonatal T cells: a reinterpretation. Annu Rev Immunol 38:229–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billingham RE, Brent L, Medawar PB. 1953. Actively acquired tolerance of foreign cells. Nature 172:603–606. [DOI] [PubMed] [Google Scholar]
- 40.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. 2010. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 330:1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. 2015. Immune tolerance: regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith NL, Patel RK, Reynaldi A, Grenier JK, Wang J, Watson NB, Nzingha K, Yee Mon KJ, Peng SA, Grimson A, Davenport MP, Rudd BD. 2018. Developmental origin governs CD8 T cell fate decisions during infection. Cell 174:117–130. [DOI] [PubMed] [Google Scholar]
- 43.Soerens AG, Kunzli M, Quarnstrom CF, Scott MC, Swanson L, Locquiao JJ, Ghoneim HE, Zehn D, Youngblood B, Vezys V, Masopust D. 2023. Functional T cell are capable of supernumerary cell division and longevity. Nature 614:762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider C, Lee J, Koga S, Ricardo-Gonzalez RR, Nussbaum JC, Smith LK, Villeda SA, Liang H-E, Locksley RM. 2019. Tissue-resident groups 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity 50:1425–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seillet C, Mielke LA, Amann-Zalcenstein D, Su S, Gao J, Almeida FF, Shi W, Ritchie ME, Naik SH, Huntington ND, Carotta S, Belz GT. 2016. Deciphering the innate lymphoid cell transcriptional program. Cell Rep 17:436–447. [DOI] [PubMed] [Google Scholar]
- 46.Michieletto MF, Tello-Cajiao JJ, Mowel MK, Chandra A, Yoon S, Joannas L, Clark ML, Jimenez MT, Wright JM, Lundgren P, Williams A, Thaiss CA, Vahedi G, Henao-Mejia J. 2023. Multiscale 3D genome organization underlies ILC2 ontogenesis and allergic airway inflammation. Nat Immunol 24:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasal DN, Liang Z, Hollinger MK, O’Leary CY, Lisicka W, Sperling AI, Bendelac A. 2021. A Gata3 enhancer necessary for ILC2 development and function. Proc Natl Acad Sci USA 118:e2106311118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoyler T, Klose CSN, Souabni A,Turqueti-Neves A, Pfeifer K, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. 2012. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37:634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie ANJ. 2012. Transcription factor RORα is critical for nuocyte development. Nat Immunol 13:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghaedi M, Steer CA, Martinez-Gonzalez I, Halim TYF, Abraham N, Takei F. 2016. Common-lymphoid-progenitor-independent pathways of innate and T lymphocyte development. Cell Reports 15:471–480. [DOI] [PubMed] [Google Scholar]
- 51.Karo JM, Schatz DG, Sun JC. 2014. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell 159:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin SB, Lo BC, Ghaedi M, Scott RW, Li Y, Messing M, Hernaez DC, Cait J, Murakami T, Hughes MR, Leslie KB, Underhill RM, Takei F, McNagny KM. 2020. Abortive γδ TCR rearrangements suggest blood ILC2s are derived from T cell precursors. Blood Adv 4:5362–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferreira ACF, Szeto ACH, Heycock MWD, Clark PA, Walker JA, Crisp A, Barlow JL, Kitching S, Lim A, Gogoi M, Berks R, Daly M, Jolin HE, McKenzie AN. 2021. RORα is a critical checkpoint for T cells and ILC2 commitment in the embryonic thymus. Nat Immunol 22:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kogame T, Egawa G, Nomura T, Kabashima K. 2022. Waves of layered immunity over innate lymphoid cells. Front Immunol 13:957711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribot JC, Lopes N, Silva-Santos B. 2021. γδ T cells in tissue physiology and surveillance. Nature Rev Immunol 21:221–232. [DOI] [PubMed] [Google Scholar]
- 56.Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, Serafini N, Puel A, Bustamante J, Surace L, Masse-Ranson G, David E, Strick-Marchand H, Le Bourhis L, Coocchi R, Topazio D, Graziano P, Muscarella LA, Rogge L, Norel X, Sallenave JM, Allez M, Graf T, Hendriks RW, Casanova JL, Amit I, Yssel H, Di Santo JP. 2017. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 168:1086–1100. [DOI] [PubMed] [Google Scholar]
- 57.Kokkinou E, Pandey RV, Mazzurana L, Gutierrez-Perez I, Tibbitt CA, Weigel W, Soini T, Carrasco A, Rao A, Nagasawa M, Bal SM, Jangard M, Griberg D, Lindforss U, Nordenvall C, Ljunggren M, Haapaniemi S, Keita AV, Soderholm J, Hedin C, Spits H, Bryceson YT, Mjosberg J. 2022. CD45RA+CD62L- ILCs in human tissues represent a quiescent local reservoir for the generation of differentiated ILCs. Sci Immunol 7:eabj8301. [DOI] [PubMed] [Google Scholar]
- 58.Spits H, Mjosberg J. 2022. Heterogeneity of type 2 innate lymphoid cells. Nature Rev Immunol 22:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeis P, Lian M, Fan X, Herman JS, Hernandez DC, Gentek R, Elias S, Symowski C, Knopper K, Peltokangas N, Friedrich C, Doucst-Ladeveze R, Kabat AM, Locksley RM, Voehringer D, Bajenoff M, Rudensky AY, Romagnani C, Grun D, Gasteiger G. 2020. In situ maturation and tissue adaptation of type 2 innate lymphoid cell progenitors. Immunity 53:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshimoto M, Kosters A, Cornelius S, Valiente N, cheng H, Latorre A, Nishida C, Ghosn EEB, Kobayashi M. 2022. Mast cell repopulating ability is lost during the transition from pre-HSC to FL HSC. Front Immunol 13:896396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simic M, Manasolva I, Spinelli L, Gentek R, Shayan RR, Siret C, Girard-Madoux M, Wang S, de Fabritus L, Verschoor J, Kerdiles YM, Bajenoff M, Stumm R, Golub R, van de Pavert SA. 2020. Distinct waves from hemogenic endothelium give rise to layered lymphoid tissue inducer cell ontogeny. Cell Rep 32:1008004. [DOI] [PubMed] [Google Scholar]
- 62.Constantinides MG, Belkaid Y. 2021. Early-life imprinting of unconventional T cells and tissue homeostasis. Science 374:eabf0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayassi T, Barreiro LB, Rossjohn J, Jabri B. 2021. A multilayered immune system through the lens of unconventional T cells. Nature 595:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vely F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Perchet T, Petit M, Yessaad N, Touzot F, Gruneau J, Mahlaoui N, Zucchini N, Farnarier C, Michel G, Moshous D, Blanche S, Dujardin A, Spits H, Distler JHW, Ramming A, Picard C, Golub R, Fischer A, Vivier E. 2016. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol 17:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Lier YF, Krabbendam L, Haverkate NJE, Zeerleder SS, Rutten CE, Blom B, Spits H, Hazengerg MD. 2022. GATA2 haplosufficiency patients lack innate lymphoid cells that arise after hematopoietic cell transplantation. Front Immunol 13:1020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goncalves P, Doisne J-M, Eri T, Charbit B, Bondet V, Posseme C, Llibre A, Casrouge A, Lenoir C, Neven B, Duffy D, Fischer A, Di Santo JP, Milieu Interieur Consortium. 2022. Defects in mucosal immunity and nasopharyngeal dysbiosis in HSC-transplanted SCID patients with IL2RG/JAK3 deficiency. Blood 139:2585–2600. [DOI] [PubMed] [Google Scholar]
- 67.Mass E, Gentek R. 2021. Fetal-derived immune cells at the roots of lifelong pathophysiology. Front Cell Dev Biol 9:648313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halper-Stromberg A, Jabri B. 2022. Maladaptive consequences of inflammatory events shape individual immune identity. Nat Immunol 23:1675–1686. [DOI] [PubMed] [Google Scholar]
- 69.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, Locksley RM. 2013. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jarick KJ, Topczewska PM, Jakob MO, Yano H, Arifuzzaman M, Gao X, Boulekou S, Stokic-Trtica V, Leclere PS, Preuber A, Rompe ZA, Stamm A, Tsou AM, Chu C, Heinrich FR, Guerra GM, Durek P, Ivanov A, Beule D, Helfirch S, Duerr CU, Kuhl AA, Stehle C, Romagnani C, Mashreghi M-F, Diefenbach A, Artis D, Klose CSN. 2022. Non-redundant functions of group 2 innate lymphoid cells. Nature 611:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang H-E, Reinhardt RL, Bando JK, Sullivan BS, Ho I-C, Locksley RM. 2012. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol 13:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Upadhyaya B, Yin Y, Hill BJ, Douek DC, Prussin C. 2011. Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J Immunol 187:3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ansel KM, Djuretic I, Tanasa B, Rao A. 2006. Regulation of Th2 differentiation and IL-4 locus accessibility. Annu Rev Immunol 24:607–656. [DOI] [PubMed] [Google Scholar]
- 74.Lee GR, Kim ST Spilianakis CG, Fields PE, Flavell RA. 2006. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity 24:369–79. [DOI] [PubMed] [Google Scholar]
- 75.Nagashima H, Petermann F, Pekowska A, Chaitankar V, Kanno Y, O’Shea JJ. 2022. Remodeling of IL-4-IL13-IL5 locus underlies selective gene expression. BioRxiv 10.1101/2022.07.22.501024. [DOI] [Google Scholar]
- 76.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF Jr, Paul WE. 2015. Innate immunological function of Th2 cells in vivo. Nat Immunol 16:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang H-E, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, Erle DJ, Locksley RM. 2016. A tissue checkpoint regulates type 2 immunity, Nat Immunol 17:1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radtke D, Thuma N, Schulein C, Kirchner P, Ekici AB, Schober, Voehringer D. 2022. Th2 single-cell heterogeneity and clonal distribution at distant sites in helminth-infected mice. eLife 11:e74183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Defrance T, Carayon P, Billian G, Guillemot JC, Minty A, Caput D, Ferrara P. 1994. Interleukin 13 is a B cell stimulating factor. J Exp Med 179:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gowthaman U, Chen JS, Zhang B, Blynn WF, Lu Y, Song W, Joseph J, Gertie JA, Xu L, Collet MA, Grassmann JDS, Simoneau T, Chiang D, Berin MC, Craft JE, Weinstein JS, Williams A, Eisenbarth SC. 2019. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365:eaaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foster PS, Mould AW, Mackenzie J, Mattes J, Hogan SP, Mahalingam S, McKenzie AN, Rothenberg ME, Young IG, Matthaei KI, Webb DC. 2001. Elemental signals regulating eosinophil accumulation in the lung. Immunol Rev 179:173–181. [DOI] [PubMed] [Google Scholar]
- 82.De Boever EH, Ashman C, Cahn AP, Locantore NW, Overend P, Pouliquen IJ, Serone AP, Wright TJ, Jenkins MM, Panesar IS, Thiagarajah SS, Wenzel SE. 2014. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol 133:989–996. [DOI] [PubMed] [Google Scholar]
- 83.Shawky AM, Almalki FA, Abdall AN, Abdelazeem AH, Gouda AM. 2022. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics 14:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Persson EK, Verstraete K, Heyndrickx I, Gevaert E, Aegerter H, Percier JM, Deswarte K, Verschueren KHG, Dansercoer A, Gras D, Chanez P, Bachert C, Goncalves A, Van Grop H, De Haard H, Blanchetot C, Saunders M, Hammad H, Savvides SN, Lambrecht BN. 2019. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 364:eaaw4295. [DOI] [PubMed] [Google Scholar]
- 85.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. 2001. IL-25 induces IL-4, IL-5 and IL-13 and Th2-associated pathologies in vivo. Immunity 15:985–995. [DOI] [PubMed] [Google Scholar]
- 86.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals the IL-1 receptor-related protein ST2 and induces helper type 2-associated cytokine. Immunity 23:479–490. [DOI] [PubMed] [Google Scholar]
- 87.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. 2002. New IL-17 family members promote th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 169:443–453. [DOI] [PubMed] [Google Scholar]
- 88.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furuwawa J, Ohtani M, Fujii H, Koyasu S. 2010. Innate production of Th2 cytokine by adipose tissue-associate c-kit+Sca-1+ lymphoid cells. Nature 463:540–544. [DOI] [PubMed] [Google Scholar]
- 89.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie ANJ. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464:1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA 107:11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. 2015. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350:981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.deKleer IM, Kool M, de Bruijn MJ, Willart M, van Moorleghem J, Schuijs MJ, Plantinga M, Beyaert R, Fallon PG, Hammad H, Hendriks RW, Lambrecht B. 2016. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 45:1285–1298. [DOI] [PubMed] [Google Scholar]
- 93.Saluzzo S, Gorki A-D, Rana BMJ, Martins R, Scanlon S, Starkl P, Lakovits K, Hladik A, Korosec A, Sharif O, Warszawska JM, Jolin H, Mesteri I, McKenzie ANJ, Knapp S. 2017. First-breath-induced type 2 pathway shape the lung immune environment. Cell Rep 18:1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, Akil O, Joshita S, Barres B, Paz JT, Molofsky AB, Molofsky AV. 2020. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Science 359:1269–1273. [Google Scholar]
- 95.He D, Wu H, Zhang H, Tang R, Lan Y, Xing R, Li S, Christian E, Hou Y, Lorello P, Caldarone B, Ding J, Nguyen L, Dionne D, Thakore P, Schnell A, Huh JR, Rozenblatt-Rosen O, Regev A, Kuchroo VJ. 2022. Disruption of the IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting metabolic adaptation and phagocytic function. Immunity 55:159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Odegaard JI, Lee MW, Sogawa Y, Bertholet AM, Locksley RM, Weinberg DE, Kirichok Y, Deo RC, Chawla A. 2017. Perinatal licensing of thermogenesis by IL-33 and ST2. Cell 166:841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Calvanese V, Capellera-Garcia S, Ma F, Fares I, Liebscher S, Ng ES, Ekstrand S, Aguade-Gorgorio J, Vavilina A, Lefaudeux D, Nadel B, Li JY, Wang Y, Lee YK, Ardehali R, Iruela-Arispe ML, Pellegrini M, Stanley EG, Elefanty AG, Schenke-Layland K, Mikkola HKA. 2022. Mapping human hematopoietic stem cells from hemogenic endothelium to birth. Nature 604:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kenswil KJG, Pisterzi P, Sanchez-Duffhues G, van Dijk C, Lolli A, Knuth C, Vanchin B, Jaramillo AC, Hoogenboezem RM, Sanders MA, Feyen J, Cupedo T, Costa IG, Li R, Bindels EMJ, Lodder K, Blom B, Bos PK, Goumans M-J, Ten Pijke P, Farrell E, Krenning G, Raaijmakers MHGP. 2021. Endothelium-derived stromal cells contribute to hematopoietic bone marrow niche formation. Cell Stem Cell 28:653–670. [DOI] [PubMed] [Google Scholar]
- 99.Stier MT, Zhang J, Goleniewska, Cephus JY, Rusznak M, Wu L, Van Kaer L, Zhou B, Newcomb DC, Peebles RS Jr.. 2018. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J Exp Med 215:263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panduro M, Benoist C, Mathis D. 2016. Tissue Tregs. Annu Rev Immunol 34:609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tuncel J, Benoist C, Mathis D. 2019. T cell anergy in perinatal mice is promoted by Treg cells and prevented by IL-33. J Exp Med 216:1328–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Lohning M, Belkaid Y, Fallon PG, Powrie F. 2014. The alarmin IL-33 promotes regulatory T cell function in the intestine. Nature 513:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, Abbas AK, Fischbach MA, Rosenblum MD. 2015. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43:1011–0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akagbosu B, Tayyebi Z, Shibu G, Paucar Iza YA, Parisotto YF, Fisher L, Pasolli HA, thevin V, Elmentaite R, Knott M, Hemmers S, Jahn L, Friedrich C, Verter J, Wang ZM, van den Brink M, Gasteiger G, Grumewald TGP, Marie JC, Leslie C, Rudensky AY, Brown CC. 2022. Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota. Nature 610:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen W, Chen S, Yan C, Zhang Y, Zhang R, Chen M, Zhong S, Fan W, Zhu S, Zhang D, Lu X, Zhang J, Huang Y, Zhu L, Li X, Lv D, Fu Y, Iv H, Ling Z, Ma L, Jiang H, Long G, Zhu J, Wu D, Wu B, Sun B. 2022. Allergen-protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion. Nat Immunol 23:1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cayrol C, Girard J-P. 2022. Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 156:155891. [DOI] [PubMed] [Google Scholar]
- 107.Aneas I, Decker DC, Howard CL, Sobreira DR, Sakabe NJ, Blaine KM, Stein MM, Hrusch CL, Montefiori LE, Tena J, Magnaye KM, Clay SM, Gern JE, Jackson DJ, Altman MC, Naureckas ET, Hogarth DK, White SR, Gomez-Skarmeta JL, Schoetler N, Ober C, Sperling AI, Nobrega MA. 2021. Asthma-associated genetic variants induce IL-33 differential expression through an enhancer-blocking regulatory region. Nat Comm 12:6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hikichi Y, Motomura Y, Takeuchi O, Moro K. 2021. Posttranscriptional regulation of ILC2 homeostatic function via tristetraprolin. J Exp Med 218:e20210181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith D, Helgason H, Sulem P, Bjornsdottir US, Lim AC, Sveinbjornsson G, Fasegawa H, Brown M, Ketchem RR, Gavala M, Garrett L, Jonasdottir A, Jonasdottir A, Sigurdsson A, Magnusson OT, Eyjolfsson GI, Olafsson I, Onundarson PT, Sigurdardottir O, Gislason D, Gislason T, Ludviksson BR, Lukviksdottir D, Boezen HM, Heinzmann A, Krueger M, Porsbjerg C, Ahluwalia TS, Waage J, Backer V, Deichmann KA, Koppelman GH, Bonnelykke K, Bisgaard H, Masson G, Thorsteinsdottir U, Gudbjartsson DF, Johnston JA, Jonsdottir I, Stefansson K. 2017. A rare IL33 loss-of-function allele mutation reduces blood eosinophil counts and protects from asthma. PLoS Genet 13:e1006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schneider C, O’Leary CE, Locksley RM. 2019. Regulation of immune responses by tuft cells. Nat Rev Immunol 19:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O’Leary CE, Sbierski-Kind J, Kotas ME, Wagner JC, Liang H-E, Schroeder AW, de Tenorio JC, von Moltke J, Ricardo-Gonzalez RR, Eckalbar WL, Molofsky AB, Schneider C, Locksley RM. 2022. Bile acid-sensitive tuft cells regulate biliary neutrophil influx. Sci Immunol 7:eabj1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gurtner A, Borrelli C, Gonzalez-Perez I, Bach K, Acar IE, Nunez NG, Crepaz D, Handler K, Vu VP, Lafzi A, Stirm K, Raju D, Gschwend J, Basler K, Schneider C, Slack E, Valenta T, Becher B, Krebs P, Moor AE, Arnold IC. 2022. Active eosinophils regulate host defense and immune responses in colitis. Nature 612: 10.1038/s41586-022-05628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li M, Hener P, Zhang Z, Kato S, Metzger D, Cambon P. 2006. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA 103:11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.107. Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo J-H, Shih H-Y, Truong A, Doebel T, Sakamoto K, Chi C-Y, Schlessinger D, Moro K, Nakae S, Horiuchi K, Zhu J, Leonard WJ, Kong HH, Nagao K. 2019. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 176:982–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, Luo J, Gao X, Yang L, Hamilton SL, Wang PL, Brestoff JR, Council ML, Brasington R, Schaffer A, Brombacher F, Hsieh C-S, Gereau RW 4th, Miller MJ, Chen Z-F, Hu H, Davidson S, Liu Q, Kim BS. 2017. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 171:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klose CSN, Artis D. 2020. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res 30:475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGinty JW, Ting HA, Billipp TE, Nadjsombati MS, Khan DM, Barrett NA, Liang HE, Matsumoto I, von Moltke J. 2020. Tuft cell-derived leukotrienes drive rapid anti-helminth immunity in the small intestine but are dispensable for anti-protist immunity. Immunity 52:528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM. 2017. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activated ILC2s. J Exp Med 214:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, Viega-Fernandes H. 2017. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar A-L, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, Shen X, Kostenis E, Konig GM, Senda T, Carpenter D, Farber DL, Artis D. 2017. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549:282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour REE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, Haas BJ, Tickle TL, Trombetta JJ, Baral P, Klose CSN, Mahlakoiv T, Artis D, Rozenblatt-Rosen O, Chiu IM, Levy BD, Kowalczyk MS, Regev A, Kuchroo VK. 2017. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsou AM, Yano H, Parkhurst CN, Mahlakoiv T, Chu C, Zhang W, He Z, Jarick KJ, Zhong C, Putzel GG, Hatazaki M, JRI IBD Live Cell Bank Consortium; Lorenz IC, Andrew D, Balderes P, Klose CSN, Lira SA, Artis D. 2022. Neuropeptide regulation of non-redundant ILC2 responses at barrier surfaces. Nature 611:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang H-E, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, Locksley RM. 2018. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 19:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van Dyken SJ, Mohapatra A, Nussbaum JC, Nussbaum JC, Thornton EE, Ziegler SF, McKenzie ANJ, Krummel MF, Liang H-E, Locksley RM. 2018. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity 40:414–424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vannella KM, Ramalingam TR, Borthwick LA, Barron L, Hart KM, Thompson RW, Kindrachuk KN, Cheever AW, White S, Budelsky AL, Corneau MR, Smith DE, Wynn TA. 2016. Combinatorial targerting of TSLP, IL-25 and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci Transl Med 8:337ra65. [DOI] [PubMed] [Google Scholar]
- 126.Koga S, Hozumi K, Hirano K, Yazawa M, Terooatea T, Minoda A, Nagasawa T, Koyasu S, Moro K. 2018. Peripheral PDGFRalpha(+)gp38(+) mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med 215:1609–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dahlgren MW, Jones SW, Cautivo KM, Dubinin A, Ortiz-Carpena JF, Farhat S, Yu KS, Lee K, Wang C, Molofsky AV, Tward AD, Krummel MF, Peng T, Molofsky AB. 2019. Adventitial stromal cell define group 2 innate lymphoid tissue niches. Immunity 50:707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. 2013. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 75:23–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gadani SP, Smirnov I, Wiltbank AT, Overall CC, Kipnis J. 2017. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med 214:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Fang J, Liu B, Shao C, Shi Y. 2022. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 29:1515–1530. [DOI] [PubMed] [Google Scholar]
- 131.Mahlakoiv T, Flamar AL, Johnston LK, Moriyama S, Putzel GG, Bryce PJ, Artis D. 2019. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol 4:eaax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, Benoist C, Mathis D. 2019. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol 4:eeaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rana BMJ, Jou E, Barlow JL, Rodriguez-Rodriguez N, Walker JA, Knox C, Jolin HE, Hardman C, Sivasubramaniam M, Szeto A, Cohen ES, Scott IC, Sleeman MA, Chidomere CI, Cruz Mignon S, Caamano J, Jorgensen HF, Carobbio S, Vidal-Puig A, McKenzie ANJ. 2019. A stromal cell niche sustains ILC2-mediate type-2 conditioning in adipose tissue. J Exp Med 216:1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cardoso F, Klein Wolterink RGJ, Godinho-Silva C, Domingues RG, Ribeiro H, Alves da Silva J, Mahu I, Domingos AI, Veiga-Fernandes H. 2021. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature 597:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meng X, Qian X, Ding X, Wang W, Yin X, Zhuang G, Zeng W. 2022. Eosinophils regulate intra-adipose axonal plasticity. Proc Natl Acad Sci USA 119:e2112281119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maazi H, Patel N, Sankaranayayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, Akbari O. 2015. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42:538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Halim TYF, Rana BMJ, Walker JA, Kershcer B, Knolle MD, Jolin HE, Serrao EM, Haim-Vilmovsky L, Teichmann SA, Rodewald H-R, Botto M, Vyse TJ, Fallon PG, Li Z, Withers DR, McKenzie ANJ. 2018. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity 48:1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knipfer L, Schulz-Kuhnt A, Kindermann M, Greif V, Symowski C, Voehringer D, Neurath MF, Atreya I, Wirtz S. 2019. A CCL1/CCR8-dependent feed-forward mechanism drives ILC2 functions in type 2-mediated inflammation. J Exp Med 216:2763–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Szeto ACH, Ferreira ACF, Mannion J, Clark PA, Sivasubramaniam M, Heycock MWD, Crisp A, Jolin HE, Kozik P, Knolle MD, McKenzie ANJ. 2023. An αvβ3 integrin checkpoint is critical for efficient Th2 cell cytokine polarization and potentiation of antigen-specific immunity. Nat Immunol 24:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poon MML, Caron DP, Wang Z, Wells SB, Chen D, Meng W, Szabo PA, Lam N, Kubota M, Matsumoto R, Rahman A, Luning Prak ET, Shen Y, Sims PA, Farber DL. 2023. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat Immunol 24:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wijeyesinghe S, Beura KL, Pierson MJ, Stolley JM, Adam OA, Ruscher R, Steinert EM, Rosato PC, Vezys V, Masopust D. 2021. Expansible residence decentralizes immune homeostasis. Nature 592:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Silva-Sanchez A, Randall TD. 2020. Role of iBALT in respiratory immunity. Curr Top Microbial Immunol 426:21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Okano M, Hirahara K, Kiuchi M, Onoue M, Iwamura C, Kokubo K, Hishiya T, Morimoto Y, Ikehara Y, Murakami A, Ebihara N, Nakayama T. 2022. Interleukin-33-activated neuropeptide CGRP-producing memory Th2 cells cooperate with somatosensory neurons to induce conjunctival itch. Immunity 55:2352–2368. [DOI] [PubMed] [Google Scholar]
- 144.Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, Roulois D, Turner J, Sylvestre M, Asam S, Glaysher B, Bowman SJ, Fearon DT, Filer A, Tarte K, Luther SA, Fisher BA, Buckley CD, Coles MC, Barone F. 2019. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci USA 116:13490–13497. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bielecki P, Riesenfeld SJ, Hutter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, Lian M, Amezcua Vesely MC, Kroehling L, Xu H, Slyper M, Muus C, Ludwig LS, Christian E, Tao L, Kedaigle AJ, Steach HR, York AG, Skadow MH, Yaghoubi P, Dionne D, Jarret A, McGee HM, Porter CBM, Licona-Limon P, Bailis W, Jackson R, Gagliani N, Gasteiger G, Locksley RM, Regev A, Flavell RA. 2021. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 592:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang Y, Mao K, Chen X, Sun MA, Sawabe T, Li W, Usher N, Zhu J, Urban JF Jr, Paul WE, Germain RN. 2018. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ricardo-Gonzalez RR, Schneider C, Liao C, Lee J, Liang H-E, Locksley RM. 2020. Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J Exp Med 217:e20191172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H. 2018. Innate lymphoid cells: 10 years on. Cell 174:1054–1066. [DOI] [PubMed] [Google Scholar]
- 149.Rosshart SP, Herz J, Vassallo BG, Hunter A, Wall MK, Badger JH, McCulloch JA, Anastasakis DG, Sarashad AA, Leonardi I, collins N, Blatter JA, Han S-J, Tamoutounour S, Potapova S, Foster St Claire MB, Yuan W, Sen SK, Dreier MS, Hild B, Hafner M, Wang D, Iliev ID, Belkaid Y, Trinchieri G, Rehermann B. 2019. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365:eaaw4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, Fuchs E. 2019. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Niec RE, Chu T, Schernthanner M, Gur-Cohen S, Hidalgo L, Pasolli HA, Luckett KA, Wang Z, Bhalla SR, Cambuli F, Kataru RP, Ganesh K, Mehrara BJ, Pe’er D, Fuchs E. 2022. Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell Stem Cell 29:1067–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brunet A, Goodell MA, Rando TA. 2023. Ageing and rejuvenation of tissue stem cells and niches. Nat Rev Mol Cell Biol 24:45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fung ITH, Sankar P, Zhang Y, Robison LS, Zhao X, D’Souza SS, Salinero AE, Wang Y, Zian J, Kuentzel ML, Chittur SV, Temple S Zuloaga Yang Q. 2020. Activation of group 2 innate lymphoid cells alleviate aging-associated cognitive decline. J Exp Med 217:e20190915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sudo T, Motomura Y, Okuzaki D, Hasegawa T, Yokota T, Kikuta J, Ao T, Mizuno H, Matsui T, Motooka D, Yoshizawa R, Nagasawa T, Kanakura Y, Moro K, Ishii M. 2021. Group 2 innate lymphoid cells support hematopoietic recovery under stress conditions. J Exp Med 218:e20200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goldberg EL, Shchukina I, Youm Y-H, Ryu S, Tsusaka T, Young KC, Camell CD, Dlugos T, Artyomov MN, Dixit VD. 2021. IL-33 causes thermogenic failure in aging by expanding dysfunctional adipose ILC2. Cell Metab 33:2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Howitt MR, Lavoie S, M<ichaud M, Blum AM, Tran SV, Weinstonck JV, Galliini CA, Redding K, Margolskee RF, Osborne LC, Artis D, Garrett WS. 2016. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351:1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schneider C, O’Leary CE, von Moltke J, Liang H-E, Ang qy, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, Locksley RM. 2018. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 174:271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, Miller CN, Pollack JL, Nagana Gowda GA, Fontana MF, Erle DJ, Anderson MS, Locksley RM, Raftery D, von Moltke J. 2018. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sanman LE, Chen IW, Bieber JM, Steri V, Trentexaux C, Hann B, Klein OD, Wu LF, Altschuler SJ. 2021. Transit-amplifying cells coordinate changes in epithelial cell-type composition. Dev Cell 56:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.von Moltke J, Ji M, Liang HE, Locksley RM. 2016. Tuft cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. 2016. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lindholm HT, Parmar N, Drurey C, Campillo Poveda M, Vornewald PM, Ostrop J, Diez-Sanchez A, Maizels RM, Oudhoff MJ. 2022. BMP signaling in the intestinal epithelium drives a critical feedback loop to restrain IL-13-driven tuft cell hyperplasia. Sci Immunol 7:eabl6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.O’Leary CE, Schneider C, Locksley RM. 2019. Tuft cells – systemically dispersed sensory epithelia integrating immune and neural circuitry. Annu Rev Immunol 37:47–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kotas ME, Mroz NM, Koga S, Liang HE, Schroeder AW, Ricardo-Gonzalez RR, Schneider C, Locksley RM. 2021. CISH constrains the tuft-ILC2 circuit to set epithelial and immune tone. Mucosal Immunol 14:1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh PB, Pua HH, Happ HC, Schneider C, von Moltke J, Locksley RM, Baumjohann D, Ansel KM. 2017. MicroRNA regulation of type 2 innate lymphoid cell homeostasis and function in allergic inflammation. J Exp Med 214:3627–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nadjsombati MS, Niepoth N, Webeck LM Kennedy EA, Jones DL, Baldridge MT, Bendesdky A, von Moltke J. 2022. Genetic mapping reveals Pou2af2-dependent tuning of tuft cell differentiation and intestinal type 2 immunity. bioRxiv 2022.10.19.512785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kubick N, Klimovich P, Flournoy PH, Bienkowska I, Lazarczyk M, Sacharczuk M, Bhaumik S, Mickael M-E, Basu R. 2021. Interleukins and interleukin receptors: evolutionary history and origin in relation to CD4+ T cell evolution. Genes 12:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, Wadsworth II MH, Hughes TK, Kazer SW, Yoshimoto E, Cahill KN, Bhattacharyya N, Katz HR, Berger B, Laidlaw TM, Boyce JA, Barrett NA, Shalek AK. 2018. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 560:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Campbell L, Hepworth MR, Whittingham-Dowd J, Thompson S, Bancroft AJ, Hayes KS, Shaw TN, Dickey BF, Flamar AL, Artis D, Schwartz DA, Evans CM, Roberts IS, Thornton DJ, Grencis RK. 2019. ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J Exp Med 216:2714–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Niec RE, Rudensky AY, Fuchs E. 2021. Inflammatory adaptation in barrier tissues. Cell 184:3361–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trabanelli S, Ercolano G, Wyss T, Gomez-Cadena A, Falquet M, Cropp D, Imbratta C, Leblond MM, Salvestrini V, Curti A, Adotevi O, Jandus C, Verdeil G. 2022. C-Maf enforces cytokine production and promotes memory-like responses in mouse and human type 2 innate lymphoid cells. EMBO J 41:e109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verma M, Michalec L, Sripada A, McKay J, Sirohi K, Verma D, Sheth D, Martin R, Dyjack N, Seibold MA, Knapp JR, Tu TH, O’Connor BP, Gorska MM, Alam R. 2021. The molecular and epigenetic mechanisms of innate lymphoid cell (ILC) memory and its relevance for asthma. J Exp Med 218:e20201354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ricardo-Gonzalez RR, Kotas ME, O’Leary CE, Singh K, Damsky W, Liao C, Arouge E, Tenvooren I, Marquez DM, Schroeder AW, Cohen JN, Fassett MS, Lee J, Daniel SG, Bittinger K, Diaz RE, Fraser JS, Ali N, Ansel KM, Spitzer MH, Liang H-E, Locksley RM. 2022. Innate type 2 immunity controls hair follicle commensalism by Demodex mites. Immunity 55:1891–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Krakowski AC, Senft SC, Heymann WR. 2021. Demodex folliculitis and recent dupilumab administration. Pediatrics 147:e2020029520. [DOI] [PubMed] [Google Scholar]
- 175.Boothby IC, Kinet MJ, Boda DP, Kwan EY, Clancy S, Cohen JN, Habrylo I, Lowe MM, Pauli M, Yates AE, Chan JD, Harris HW, Neuhaus IM, McCalmont TH, Molofsky AB, Rosenblum MD. 2021. Early-life inflammation primes a T helper 2 cell-fibroblast niche in skin. Nature 599:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. 2015. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. 2013. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. 2015. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kabat AM, Hackl A, Sanin DE, Zeis P, Grzes KM, Baixauli F, Kyle R, Caputa G, Edwards-Hicks J, Villa M, Rana N, Curtis JD, Casatoldi A, Cupovic J, Dreesen L, Sibilia M, Pospisilik JA, Urban JF, Grun D, Pearce EL, Pearce EJ. 2022. Resident Th2 cells orchestrate adipose tissue remodeling at a site adjacent to infection. Sci Immunol 7:eadd3263. [DOI] [PubMed] [Google Scholar]
- 180.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Houihan HA, Bando JK, Chawla A, Locksley RM. 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Knudsen NH, Stanya KJ, Hyde AL, Chalom MM, Alexander RK, Liou Y-H, Starost KA, Gangl MR, Jacobi D, Liu S, Sopariwala DH, Fonseca-Pereira D, Li J, Hu FB, Garrett WS, Narkar VA, Orlund EA, Kim JH, Paton CM, Cooper JA, Lee C-H. 2020. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 368:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, Honda K, Gause WC, Blaser MJ, Bonneau RA, Lim YAL, Loke P, Cadwell K. 2016. Helminth infection promotes colonization resistance via type 2 immunity. Science 352:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wolff MY, Broadhurst MJ, Loke P. 2012. Helminthic therapy: improving mucosal barrier function. Trends Parasitol 28:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Van Dyken SJ, Liang H-E, Naiawadi RP, Woodruff PG, Wolters PJ, Erle DJ, Locksley RM. 2017. Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell 169:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Parker JM, Glaspole IN, Lancaster LH, Haddad TJ, She D, Roseti SL, Fiening JP, Grant EP, Kell CM, Flaherty KR. 2018. A phase 2 randomized controlled study of tralokinumab in subjects with idiopathic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 197:94–103. [DOI] [PubMed] [Google Scholar]
- 186.Demenais F, Margaritte-Jeannin P, Barnes KC, Cookson WOC, Altmuller J, Ang W, Barr RG, Beaty TH, Becker AB, Beilby J, et al. 2018. Multiancestry association study identifies new asthma risk loci that colocalize with immune cell enhancer marks. Nat Genet 50:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bogaert D, van Beveren GJ, de Koff EM, Parga PL, Lopez CEB, Koppensteiner L, Clerc M, Hasrat R, Arp K, Chu ML, de Groot PCM, Sanders EAM, van Houten MA, de Steenhuijsen Piters WAA. 2023. Mother-to-infant microbiota transmission and infant microbiota development across multiple body sites. Cell Host & Microbe 31:447–460. [DOI] [PubMed] [Google Scholar]
- 188.Krausgruber T, Fortelney N, Fife-Gernedl V, Senekowitsch, Schuster LC, Lercher A, Nemc A, Schmidl C, Rendeiro AF, Bergthaler A, Bock C. 2020. Structural cells are key regulators of organ-specific immune responses. Nature 583:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lyons JJ, Milner JD. 2028. Primary atopic disorders. J Exp Med 215:1009–1022. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang A, Luan HH, Medzhitov R. 2019. An evolutionary perspective on immunometabolism. Science 363:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pontzer H, Yamada Y, Sagayama H, Ainslie PN, Andersen LF, Anderson LJ, Arab L, Baddou I, Bedu-Addo K, Blaak EE, et al. 2021. Daily energy expenditure through the human life course. Science 373:808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Akdis CA. 2021. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions. Nat Rev Immunol 21:739–751. [DOI] [PubMed] [Google Scholar]
- 193.Saglani S, Gregory LG, Manghera AK, Branchett WJ, Uwadiae F, Entwistle LJ, Oliver RA, Vasiliou JE, Sherburn R, Lui S, Putter F, Voehringer D, Walker SA, Buckley J, Brychtol R, Fainardi V, Denney L, Byrne A, von Mutius E, Bush A, Lloyd CM. 2018. Inception of early-life allergen-induced airway hyperresponsiveness is reliant on IL-13 CD4 T cells. Sci Immunol 3:eaan4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, Raymond WW, Lachowicz-Scroggins ME, Di Maio S, Hoffman EA, Castro M, Fain SB, Jarjour NN, Israel E, Levy BD, Erzurum SC, Wenzel SE, Meyers DA, Bleecker ER, Phillips BR, Mauger DT, Gordon ED, Woodruff PG, Peters MC, Fahy JV; National Heart Lung and Blood Institute (NHLBI) Severe Asthma Research Program (SARP). 2018. J Clin Invest 128:997–1009.29400693 [Google Scholar]
- 195.Milner JD. 2020. Primary atopic disorders. Annu Rev Immunol 38:785–808. [DOI] [PubMed] [Google Scholar]
- 196.Torow N, Hand TW, Hornef MW. 2023. Programmed and environmental determinants driving neonatal mucosal immune development. Immunity 56:485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wade-Vallance AK, Yang Z, Libang JB, Robinson MJ, Tarlinton DM, Allen CDC. 2023. B cell receptor ligation induces IgE plasma cell elimination. J Exp Med 220:e20220964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M, Fukunaga K, Asano K, Betsuyaku T, Koyasu S. 2015. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 17:76–86. [DOI] [PubMed] [Google Scholar]
- 199.Cautivo KM, Matatia PR, Lizama CO, Mroz NM, Dahlgren MW, Yu X, Sbierski-Kind J, Taruselli MT, Brooks JF, Wade-Vallance A, Caryotakis SE, Chang AA, Liang HE, Zikherman J, Locksley RM, Molofsky AB. 2022. Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation. Immunity 55:254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Block KE, Iijima K, Pierson MJ, Walsh DA, Tei R, Kucaba TA, Xu J, Khan MH, Staley C, Griffith TS, McSorley HJ, Kita H, Jameson SC. 2022. Physiological microbial exposure transiently inhibits mouse lung ILC2 responses to allergens. Nat Immunol 23:1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang C, Hyams B, Allen NC, Cautivo K, Monahan K, Zhou M, Dahlgren MW, Lizama CO, Matthay M, Wolters P, Molofsky AB, Peng T. 2023. Dysregulated lung stroma drive emphysema exacerbation by potentiating resident lymphocytes to suppress an epithelial stem cell reservoir. Immunity 56, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sorensen TI, Nielsen GG, Anderson PK, Teasdale TW. 1988. Genetic and environmental influences on premature death in adult adoptees. New Engl J Med 318:727–732. [DOI] [PubMed] [Google Scholar]
- 203.Casanova J-L, Abel L 2022. From rare disorders of immunity to common determinants of infection: following the mechanistic thread. Cell 185:3086–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang Q, Boisson B, Beziat V, Puel A, Casanova JL. 2018. Human hyper-IgE syndrome: singular or plural? Mamm Genome 29:603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang Z, Wu CA, Targ S, Allen CDC. 2020. IL-21 is a broad negative regulator of IgE class switch recombination in mouse and human B cells. J Exp Med 217:e20190472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Droghini HR, Abonia JP, Collins MH, Milner JD, Lyons JJ, Freeman AF, Mukkada VA, Risma KA, Rothenberg ME, Schwartz JT. Targeted IL-4Ra blockade ameliorates refractory allergic eosinophilic inflammation in a patient with dysregulated TGF-β signaling due to ERBIN deficiency. J Allergy Clin Immunol Pract 10:1903–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Desai P, Janova H, white JP, Reynoso GV, Hickman HD, Baldridge MT, Urban JF Jr, Stappenbeck TS, Thackray LB, Diamond MS. 2021. Enteric helminth infection enhances host susceptibility to neurotropic flaviviruses via a tuft cell-IL-4 receptor signaling axis. Cell 184:1214–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ahrends T, Aydin B, Matheis F, Classon CH, Marchildon F, Furtado GC, Lira SA, Mucida D. 2021. Enteric pathogens induce tissue tolerance and prevent neuronal cell loss from subsequent infections. Cell 184:5715–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hilligan KL, Oyesola OO, Namasivayam S, Howard N, chlancy CS, Oland SD, Garza NL, Lafont BAP, Johnson RF, Mayer-Barber KD, Sher A, Loke PN. 2022. Helminth exposure protects against murine SARS-CoV-2 infection through macrophage dependent T cell activation. bioRxiv 2022.11.09.515832 [DOI] [PubMed] [Google Scholar]
- 210.Chia SL, Kapoor S, Carvalho C, Bajenoff M, Gentek R. 2023. Mast cell ontogeny: from fetal development to life-long health and disease. Immunol Rev 315: 10.1111/imr.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moraga I, Spangler JB, Mendoza JL, Gakovic M, Wehrman TS, Krutzik P, Garcia KC. 2017. Synthekines are surrogate cytokine and growth factor agonists that compel signaling through non-natural receptor dimers. eLife 6:e22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cobb LM, Verneris MR. 2021. Therapeutic manipulation of innate lymphoid cells. JCI Insight 6:e146006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mascharak S, Talbott HE, Januszyk M, Griffin M, Chen K, Davitt MF, Demeter J, Henn D, Bonham CA, Foster DS, Mooney N, cheng R, Jackson PK, Wan DC, Gurtner GC, Longaker MT. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 29:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]


