Abstract
The relationship between gut microbiota, short chain fatty acid (SCFA) metabolism, and obesity is still not well understood. Here we investigated these associations in a large (n=1904) African origin cohort from Ghana, South Africa, Jamaica, Seychelles, and the US. Fecal microbiota diversity and SCFA concentration were greatest in Ghanaians, and lowest in the US population, representing the lowest and highest end of the epidemiologic transition spectrum, respectively. Obesity was significantly associated with a reduction in SCFA concentration, microbial diversity and SCFA synthesizing bacteria. Country of origin could be accurately predicted from the fecal microbiota (AUC=0.97), while the predictive accuracy for obesity was inversely correlated to the epidemiological transition, being greatest in Ghana (AUC = 0.57). The findings suggest that the microbiota differences between obesity and non-obesity may be larger in low-to-middle-income countries compared to high-income countries. Further investigation is needed to determine the factors driving this association.
Introduction
Obesity, which affects more than 600 million adults worldwide (“Obesity and Overweight” n.d.), over a third of Americans (Hales et al. 2020), and accounts for over 60% of deaths related to high body mass index (BMI) (Tseng and Wu 2019), remains an ongoing global health epidemic that continues to worsen at an alarming rate. A major driver of obesity is the adoption of a western lifestyle, which is characterized by excessive consumption of ultra-processed foods. Obesity is a major risk factor for type 2 diabetes, and according to the most recent National Diabetes Statistics Report almost 13% of the adult US population now have diabetes. Not only do 49.6% of adult African Americans present with obesity but over 17% of them now have diabetes, and are 1.5 times as likely to present with type 2 diabetes compared to whites (“National Diabetes Statistics Report” 2022). Populations of African origin outside of the US are experiencing similar fates, as the prevalence of obesity among adults living in Sub-Saharan Africa is greater than 13%, and higher than the global obesity prevalence for adults (Agyemang et al. 2016). This has been accompanied by dramatic increases in the prevalence of non-communicable diseases such as type two diabetes and hypertension among people of African origin (Roth et al. 2020; Gouda et al. 2019). Therefore, disrupting the rapidly expanding obesity epidemic, particularly among African origin populations is critical to controlling the cardiometabolic disorder epidemic (Geng et al. 2022). However, successfully managing and treating obesity and its comorbidities, and specifically maintaining weight loss long-term, is particularly challenging due to an incomplete understanding of the heterogeneous and complex etiopathology, as well as additional challenges facing populations experiencing rapid urbanization (Nordmo, Danielsen, and Nordmo 2020; Geng et al. 2022; Barone et al. 2022). The epidemiologic transition is a model able to capture these shifts in dietary and rural to urban movements and is characterized by diets that are high in ultra-processed foods with a significant loss in fiber, as evidenced in the US, where less than 50% of the population meet dietary fiber recommendations (Dahl and Stewart 2015).
Gut microbial ecology and metabolism play pivotal roles in the onset and progression of obesity and its related metabolic disorders (Ley 2010). Obese and lean individuals have reported differences in the composition and functional potential of the gut microbiome, with an overall reduction in species diversity in the obese gut (Dugas, Bernabé, et al. 2018; Greenblum, Turnbaugh, and Borenstein 2012; Jumpertz et al. 2011; Ley et al. 2006; Turnbaugh et al. 2009; Le Chatelier et al. 2013), additionally, fecal microbiota transfer from obese donors to mouse models can recapitulate the obese phenotype (Turnbaugh et al. 2006, 2008; Ridaura et al. 2013). Further, fecal microbiota transplant from healthy donors into patients with obese and metabolic syndrome has been shown to improve markers of metabolic health in the recipients (Vrieze et al. 2012). While these studies suggest that modification of microbial ecology may offer new options for the treatment and prevention of obesity, the mechanism that drives the microbiota-obesity relationship is not fully understood. The microbiota may facilitate greater energy exploitation from food, and storage capacity by the host (Turnbaugh et al. 2006; DiBaise et al. 2008), influencing adipose tissue composition and fat mass gain, as well as providing chronic low-grade inflammation and insulin resistance (Cani and Delzenne 2009; J. L.Sonnenburg and Bäckhed 2016).
Among the numerous microbial metabolites modulating obesity, there is an ever-growing interest in the role of short-chain fatty acids (SCFAs), which includes butyrate, acetate, and propionate as potential biomarkers for metabolic health as well as therapeutic targets. SCFAs derive primarily from microbial fermentation of non-digestible dietary fiber in the colon. They have many effects on host metabolism including serving as an energy source for host colonocytes, used as precursors for the biosynthesis of cholesterol, lipids, proteins and regulating gut barrier activities (Dalile et al. 2019; Koh et al. 2016; van der Hee and Wells 2021). Human and animal studies demonstrate a protective role of SCFAs in obesity and metabolic disease. In experimental animal models, SCFA supplementation reduces body weight, improves insulin sensitivity, and reduces obesity-associated inflammation (Vinolo et al. 2011; Gao et al. 2009; Henagan et al. 2015; Lu et al. 2016; Bonomo et al. 2020). In humans, increased gut production of butyrate correlates with improved insulin response after an oral glucose-tolerance test (Sanna et al. 2019). Although increased SCFA levels are generally observed as positive for health (Valdes et al. 2018), other studies have suggested that overproduction may promote obesity, possibly resulting from greater energy accumulation (Schwiertz et al. 2010; Rahat-Rozenbloom et al. 2014; Teixeira et al. 2013). Indeed, a previous study observed greater fecal SCFA concentrations to be linked with obesity, increased gut permeability, metabolic dysregulation, and hypertension in a human cohort (de la Cuesta-Zuluaga, Mueller, et al. 2018).
The conflicting obesity role of SCFAs identified by existing studies may result from the variation in the gut microbiota, which is shaped by lifestyle and diet. Adequately powered studies in well-characterized populations may permit more rigorous assessments of individual differences. Prior comparative epidemiological studies have broadly focused on either contrasting the gut microbiota of extremely different populations, such as the traditional hunter-gatherers and urban-westernized countries, or ethnically homogenous populations (Pasolli et al. 2019; He et al. 2018; Peters et al. 2018; Zhernakova et al. 2016). Demographic factors represent one of the largest contributors to the individualized nature of the gut microbiome (Falony et al. 2016; Zhernakova et al. 2016; Yatsunenko et al. 2012). The five diverse, well-defined cohorts from the Modeling the Epidemiologic Transition Study (METS) offers a unique opportunity to examine the issues since they are more representative of most of the world’s population. METS has longitudinally followed an international cohort of approximately 2,500 African origin adults spanning the epidemiologic transition from Ghana, South Africa, Jamaica, Seychelles, and the US since 2010 to investigate differences in health outcomes utilizing the framework of the epidemiologic transition. Pioneering microbiome studies from the METS cohorts reveal that cardiometabolic risk factors including obesity is significantly associated with reduced microbial diversity, and the enrichment of specific taxa and predicted functional traits in a geographic-specific manner (Dugas, Bernabé, et al. 2018; Fei et al. 2019). While yielding valuable descriptions of the connections between the gut microbiota ecology and disease, particularly obesity, as well as pioneering the efforts of microbiome studies of populations of African origin on different stages of the ongoing nutritional epidemiologic transitions, these studies, however, have applied small sample size (N=100 to N=655), and also did not utilize all the countries in the METS cohort. Thus, uncertainties remain as to the precise interpretation of the microbiome-obesity associations, which hampers further progress towards diagnostic and clinical applications.
Our new study METS-Microbiome investigated associations between the gut microbiota composition and functional patterns, concentrations of fecal SCFAs and obesity in a large (N = 1,904) adult population cohort of African origin, comprised of Ghana, South Africa, Jamaica, Seychelles, and the US spanning the epidemiologic transition (Dugas, Lie, et al. 2018; Luke et al. 2011). The central hypothesis is that shifts towards the highest end of the epidemiologic transition spectrum is associated with alterations in microbiota diversity and community composition, reductions in levels of fecal SCFAs and obesity.
Results
Obesity differs significantly across the epidemiological transition.
From 2018–2019, the METS-Microbiome study recruited 2,085 participants (~60% women) ages 35–55 years old from five different sites (Ghana, South Africa, Jamaica, Seychelles, and US). Of these participants, 1,249 have been followed on a yearly basis since 2010 under the parent METS study. Data from 1,867 participants with complete data sets were used in this analysis. Overall mean age was 42.5 ± 8.0 years (Table 1). Mean fasted blood glucose was 105.2 ± 39.4 mg/dL, mean systolic blood pressure was 123.4±18.1 mm Hg and mean diastolic blood pressure was 77.2 ± 13.1 (Table 1). When compared to the high-income countries (Jamaica, Seychelles, and US), both women and men from the lower- and middle-income countries (Ghana and South Africa) had significantly lower BMI, fasted blood glucose and blood pressure (systolic and diastolic). Mean BMI was lowest in the South African men (22.3 kg/m2 ± 4.1) and highest in US women (36.3 kg/m2 ± 8.8). When compared to the US, all sites had significantly lower prevalence of obesity (p<0.001 for all sites except for Seychelles: p=0.02). Prevalence of hypertension was lowest in Ghanaian men (33.1%) and highest in US men (72.7%). Prevalence of diabetes was lowest in South African women and men (3.5% for women and men) and highest for Seychellois men (22.8%). When compared to the US, prevalence of hypertension and diabetes was significantly lower in countries at the lower end of the spectrum of HDI (i.e., Ghana and South Africa) when compared to the US (p<0.001).
Table 1.
METS-Microbiome participant characteristics from Ghana, South Africa, Jamaica, Seychelles and US. Data are presented as mean ± standard deviation for continuous variables and percentages (%) for categorical variables.
| Women | |||||
|---|---|---|---|---|---|
| Ghana | South Africa | Jamaica | Seychelles | US | |
| n=254 | n=228 | n=263 | n=196 | n=213 | |
| Age (years) | 40.74 ± 8.1 | 35.56 ± 7.8 | 45.16 ± 7.5 | 43.84 ± 6.1 | 45.44 ± 6.4 |
| BMI (kg/m2) | 28.30 ± 5.9 | 33.42 ± 8.6 | 32.12 ± 7.3 | 30.32 ± 7.2 | 36.34 ± 8.8 |
| Obese (%) | 45,0% | 61,0% | 60,4% | 49,5% | 74,7% |
| SBP (mm Hg) | 117.1 ± 18.5 | 115.20 ± 17.1 | 126.08 ± 19.0 | 123.28 ± 17.8 | 124.19 ± 18.4 |
| DBP (mm Hg) | 70.53 ± 12.2 | 75.20 ± 12.1 | 79.41 ± 12.6 | 79.37 ± 14.4 | 81.52 ± 12.1 |
| Hypertensive (%) | 37,5% | 37,3% | 57,4% | 55,5% | 65,4% |
| Glucose (mg/dL) | 110.45 ± 62.7 | 89.17 ± 20.0 | 107.46 ± 39.1 | 111.35 ± 27.2 | 107.07 ± 44.0 |
| Diabetic (%) | 10,0% | 3,5% | 12,9% | 13,9% | 19,9% |
| Men | |||||
| Ghana | South Africa | Jamaica | Seychelles | US | |
| n=117 | n=171 | n=133 | n=164 | n=107 | |
| Age (years) | 43.92 ± 8.7 | 36.53 ± 7.2 | 44.42 ± 7.5 | 44.57 ± 5.1 | 47.12 ± 5.5 |
| BMI (kg/m2) | 23.7 ± 4.4 | 22.26 ± 4.1 | 24.8 ± 5.3 | 28.46 ± 5.5 | 30.37 ± 8.2 |
| Obese (%) | 13,4% | 5,3% | 15,7% | 39,2% | 44,4% |
| SBP (mm Hg) | 121.28 ± 15.4 | 122.71 ± 15.5 | 129.23 ± 17.1 | 130.43 ± 16.2 | 130.67 ± 16.0 |
| DBP (mm Hg) | 68.02 ± 13.0 | 75.32 ± 11.1 | 78.07 ± 11.5 | 81.64 ± 12.1 | 82.37 ± 12.2 |
| Hypertensive (%) | 33,1 % | 45,0% | 50,3% | 65,9% | 72,7% |
| Glucose (mg/dL) | 100.52 ± 19.4 | 94 ± 23.4 | 99.04 ± 33.1 | 124.26 ± 44.2 | 107 ± 36.2 |
| Diabetic (%) | 4,6% | 3,5% | 4,8% | 22,8% | 17,5% |
Microbial community composition and predicted metabolic potential differs significantly between countries and correlates with obesity.
Following the removal of samples that had fewer than 6,000 reads and features less than ten reads in the entire dataset, a total of 433,364,873 16S rRNA gene sequences were generated from the 1,873 fecal samples which were clustered into 13,254 ASVs. Country of origin describes most of the variation in microbial diversity and composition, with significant differences in both alpha and beta diversity. Although there were major variations in alpha diversity between countries and large degree of inter-individual variation within countries, Ghana showed significantly greater diversity for all the alpha diversity metrics (Observed ASVs, Shannon Diversity and Faith’s phylogenetic diversity) when compared to all other countries. The Seychelles and US had the lowest alpha diversity (Fig. 1). The stool microbiota alpha diversity of non-obese individuals was significantly greater when compared with that of obese individuals (Fig. 1). Beta diversity was also significantly different between countries (Fig. 1, Supplementary Tables 2 & 3; principal coordinate analysis, weighted UniFrac distance; F-statistic =58.67; p < 0.001; unweighted UniFrac distance; F= 39.87; p < 0.001) and obese group (weighted UniFrac distance; F-statistic =2.39; p = 0.031; unweighted UniFrac distance; F=6.06; p < 0.001).
Figure 1. Variation in gut microbiome diversity.
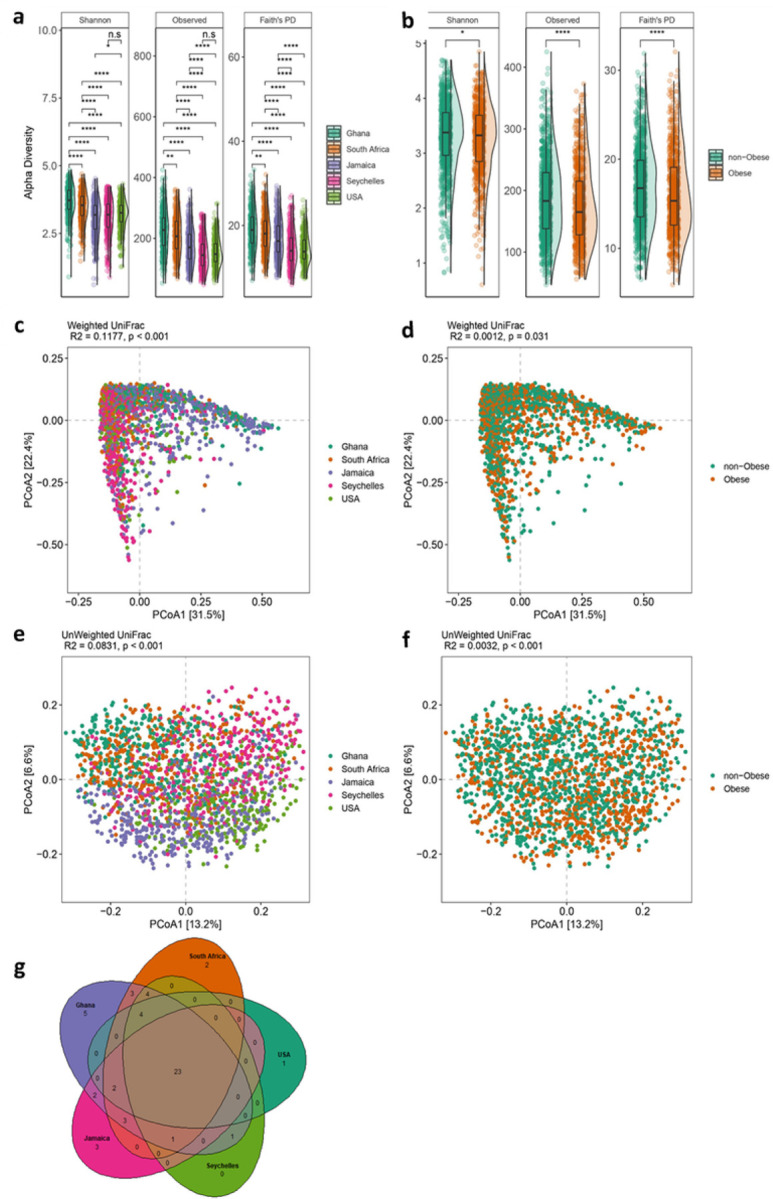
(a)Alpha diversity estimated by Shannon, Observed ASVs and Faith’s PD (Phylogenetic Diversity) between countries. (b) Alpha diversity estimated by Shannon, Observed ASVs and Faith’s PD (Phylogenetic Diversity) between obese and non-obese. Alpha diversity metrics (Faith’s PD, Observed ASVs and Shannon) are shown on the y-axis in different panels, while country or obese group are shown on the x-axis. (c) Beta diversity principal coordinate analysis based on weighted UniFrac distance between countries. (d) Beta diversity principal coordinate analysis based on weighted UniFrac distance between obese and non-obese. (e) Beta diversity principal coordinate analysis based on unweighted UniFrac distance between countries. (f) Beta diversity principal coordinate analysis based on unweighted UniFrac distance between obese and non-obese. Proportion of variance explained by each principal coordinate axis is denoted in the corresponding axis label. (g) Venn diagram of shared and unique ASVs between the five countries. Box plots show the interquartile range (IQR), the horizontal lines show the median values, and the whiskers extend from the hinge no further than 1.5*IQR. Each colored dot denotes a sample. Statistical significance adjusted for multiple comparisons using false discovery rate (FDR) correction are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ∗∗∗, P < 0.001 across countries and obese groups (Kruskal-Wallis test) for alpha diversity or by permutational multivariate analysis of variance (PERMANOVA) for beta diversity. Alpha diversity analysis for country, n=1873 samples (Ghana, n= 373; South Africa, n=390; Jamaica, n= 401; Seychelles, n= 396; USA, n= 313) and obesity status, n=1764 samples. For Beta diversity analysis, n=1764 samples.
Next, we compared fecal microbiota diversity between obese individuals with their non-obese counterparts within each country independently. Greater alpha diversity was detected in non-obese subjects in the Ghanaian (Observed ASVs, Faith PD; p<0.05) and South African cohorts (Observed ASVs; p<0.05) only (Supplementary Table 1). Similarly, significant differences in beta diversity between obese and non-obese microbiota were observed in Ghana (Unweighted UniFrac; p<0.05), South Africa (Unweighted UniFrac; p<0.05) and US (Weighted UniFrac; p<0.05) data sets (Supplementary Tables 2 & 3). These results suggest that the beta diversity differences observed in the Ghanaian and South African participants may partly be due to the presence of more abundant fecal microbiota taxa in the fecal samples whereas among the US participants, the differences may be related to the abundance of rare taxa. Collectively, these observations suggest that country is a major driver of the variance in gut microbiota diversity and composition among participants with or without obesity with marked contributions from Ghana and South Africa and modest contribution from the US in the overall cohort.
We also examined whether country of origin or obesity relates to the presence of specific microbial genera frequently used to stratify humans into enterotypes (Arumugam et al. 2011). As expected, large differences in enterotype between the countries were observed. The Prevotella enterotype (P-type) was enriched on the African continent, with 81% and 62% in Ghanaians and South Africans respectively while Bacteroides enterotype (B-type) was dominant in the US (75%), Jamaican cohorts (68%), and comparable proportions of both enterotypes among individuals from Seychelles. Further, obese individuals displayed a greater abundance of B-type whereas a higher proportion of the P-type associated with the non-obese group (Supplementary Table 4). Consistent with this observation, the abundance of B-type correlated with higher BMI (p=0.004) than P-type. Significantly greater diversity and increased levels of total SCFA were observed in participants in the P-type (Supplementary Table 4). The relative abundance of shared and unique features between the different countries illustrated by the Venn diagram showed that Ghana carries the largest proportion of unique taxa than the other countries, and US the lowest (Fig. 1).
Microbial taxa differ significantly between countries and between lean and obese individuals.
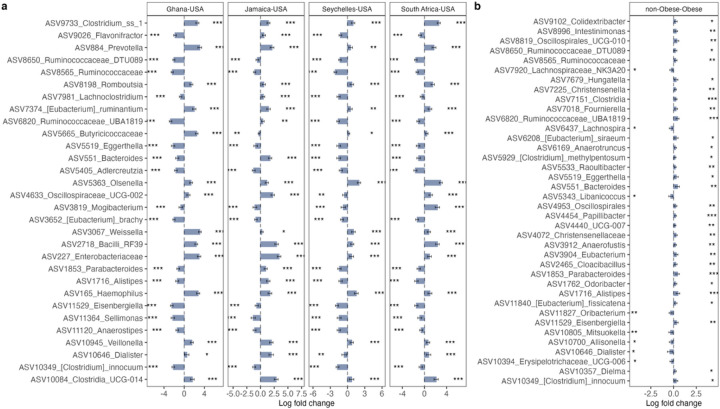
In comparison with the US, South African fecal microbiota had a significantly greater proportion of Clostridium, Olsenella, Bacilli and Mogibacterium; Jamaican samples had a significantly greater proportion of Bacilli, Bacteroides, Clostridia, Dialister, Enterobacteriaceae, and Oscillospiraceae; Seychelles samples had a significantly greater proportion of Clostridium, Olsenella and Haemophilus; and Ghanaian samples had a significantly greater proportion of Clostridium, Prevotella, Weisella, Enterobacteriaceae and Butyricicoccaceae. The US samples had a significantly greater proportion of Aldercreutzia, Anaerostipes, Clostridium, Eggerthella, Eisenbergiella, Ruminococcaceae and Sellimonas compared to the 4 countries (Fig. 2 and Supplementary Fig. 1).
Figure 2. Variation in gut microbiome composition.
Differentially abundant taxa among (a) countries and (b) obese group adjusted for BMI, age, sex and country using ANCOM-BC. Bars represent ANCOM-BC estimated log fold change between compared groups and error bars, with the 95% confidence interval. Representative ASVs with log fold change >1.4 in at least one group are shown for country. FDR-adjusted (p < 0.05) effect sizes are indicated by *, ** and ***, corresponding to p < 0.05, <0.01 and <0.001 respectively. n= 1694 samples. FDR= False Discovery Rate.
When adjusted for country, age, and sex (p < 0.05; false discovery rate (fdr)-corrected), 38 Amplicon Sequence Variants (ASVs) were significantly different between obese and non-obese groups. The obese group was characterized by an increased proportion of Allisonella, Dialister, Oribacterium, Mitsuokella, and Lachnospira, whereas non-obese microbiota had a significantly greater proportion of Alistipes, Bacteroides, Clostridium, Parabacteroides, Christensenella, Oscillospira, Ruminococcaceae (UBA1819), and Oscillospiraceae (UCG010) (Fig. 2).
Microbial taxonomic features predict obesity overall and within each country.
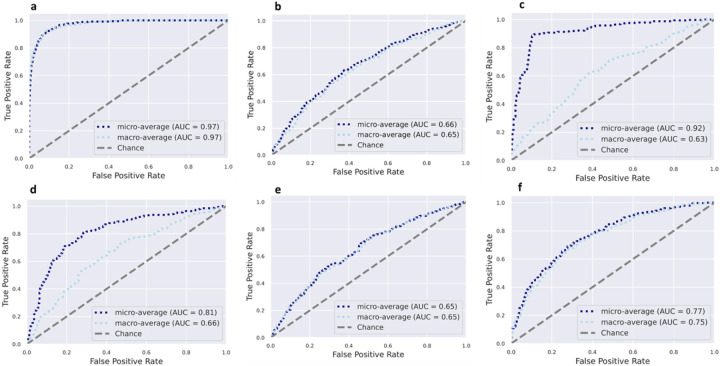
Using supervised Random Forest machine learning, the predictive capacity of the gut microbiota features in stratifying individuals to country of origin, sex, or with metabolic phenotypes were assessed. The predictive performance of the model was calculated by area under the receiver operating characteristic curve (AUC) analysis, which showed a high accuracy for country of origin (AUC = 0.97), and a comparatively lower level of predictive accuracy for obese state (AUC = 0.65) (Fig. 3). Sex was predicted with AUC = 0.75, the diabetes status with AUC = 0.63, hypertensive status with AUC = 0.65 and glucose status with AUC = 0.66. Random Forest analysis was also used to identify the top 30 microbial taxonomic features that differentiate between countries and obese states. Similar to the ANCOMBC results, Prevotella and Streptococcus were at a greater proportion in the microbiota of Ghanaian and non-obese individuals, whereas Mogibacterium was at a greater proportion in the South African cohort. A greater proportion of Megasphaera was associated with the Jamaican cohort, while a greater proportion of Ruminococcaceae was observed in the American microbiota. Weisella, which was identified as having a significantly greater proportion in the Ghanaian cohort using ANCOMBC, was observed to be a discriminatory feature for Seychelles microbiota using Random Forest (Supplementary Fig. 2).
Figure 3. Receiver operating characteristic curves showing the classification accuracy of gut microbiota in a Random Forest model.
Classification accuracy for estimating (a). All countries (n=1694); (b) Obesity status (n=1694), (c). Diabetes status (n=1657); (d). Glucose status (n=1657); (e). Hypertensive status (1694); (f). Sex (n=1694) are presented. AUC= area under the curve
Similarly, the predictive capacity of the gut microbiota features in stratifying individuals by obese state was assessed at each of the five study sites. The predictive performance of the model was calculated by AUC analysis, which showed a moderate accuracy for obese state for all sites, namely, Ghana (AUC = 0.57), South Africa (AUC = 0.52), Jamaica (AUC = 0.48), Seychelles (AUC = 0.43) and US (AUC = 0.52) (Supplementary Fig. 3).
Predicted genetic metabolic potential differs by country and obesity status.
The predicted potential microbial functional traits resulting from the compositional differences in microbial taxa between countries and obese state were assessed. PICRUSt2 predicted a total of 372 MetaCyc functional pathways. ANCOM-BC analysis adjusted for sex, age and BMI identified 67 pathways (p< 0.05; false discovery rate (fdr)-corrected), LFC>1.4) that accounted for discriminative features between the 4 different countries with the US (Supplementary Fig. 4). In comparison with US, MetaCyc pathways differentially increased in Ghana and Jamaica include methylgallate degradation, norspermidine biosynthesis (PWY-6562), gallate degradation I pathway, gallate degradation II pathway, histamine degradation (PWY-6185), and toluene degradation III (via p-cresol) (PWY-5181). South African samples had a greater proportion of L-glutamate degradation VIII (to propanoate) (PWY-5088), isopropanol biosynthesis (PWY-6876), creatinine degradation (PWY-4722), adenosyl cobalamin biosynthesis (anaerobic) (PWY-5507), respiration I (cytochrome c) (PWY-3781). MetaCyc pathways linked to norspermidine biosynthesis (PWy-6562), mycothiol biosynthesis (PWY1G-0), were at a greater proportion in the Seychelles samples, whereas reductive acetyl coenzyme A (CODH-PWY), and chorismate biosynthesis II (PWy-6165) were depleted in the US samples. ANCOM-BC analysis adjusted for site, sex and age identified 24 predicted pathways that differentiated between obese and non-obese individuals (Supplementary Fig. 4). Notably, the microbiota of non-obese individuals had a greater proportion of predicted pathways including the TCA cycle, amino acid metabolism (P162-PWY, PWY-5154, PWY-5345), ubiquinol biosynthesis-related pathways (PWY-5855, PWY-5856, PWY-5857, PWY-6708, UBISYN-PWY), cell structure biosynthesis and nucleic acid processing (PWY0 845, PYRIDOXSYN-PWY).
Next, KEGG orthology (KO) involved in pathways related to butanoate (butyrate) metabolism and LPS biosynthesis were investigated. Predicted genes involved in butyrate biosynthesis pathways showed that enoyl-CoA hydratase enzymes (K01825, K01782, K01692), lysine, glutarate /succinate enzymes (K07250, K00135, K00247), glutarate/Acetyl CoA enzymes (K00175, K00174, K00242, K00241 K01040, K01039) were differentially abundant in participants from Ghana, South Africa, Jamaica, and Seychelles in comparison to the US cohort. The relative abundance of succinic semialdehyde reductase (K18122) was significantly increased only in South Africa, Jamaica, and Seychelles population. Further, predicted genes proportionally abundant only in specific countries were observed. For instance, succinate semialdehyde dehydrogenase (K18119) was enriched only in the Ghanaian cohort, 4-hydroxybutyrate CoA-transferase (K18122) enriched among South African participants and lysine/glutarate/succinate enzyme (K14268) differentially abundant within the Seychelles population. The relative abundance of predicted genes encoded for enzymes such as maleate isomerase (K10799), 3-oxoacid CoA-transferase(K01027) and pyruvate/acetyl CoA (K00171, K00172, K00169) were greater in the US participants compared with participants from the 4 countries (Supplementary Fig. 5). The non-obese exhibited a significantly greater abundance of genes that catalyze the production of butyrate via the fermentation of pyruvate or branched amino-acids such as enoyl-CoA hydratase enzyme (K0182), Leucine/Acetyl CoA enzyme (K01640) and pyruvate/acetyl CoA enzyme (K00171, K00172, K00169, K1907) by contrast obese individuals were differentially enriched for succinyl-CoA:acetate CoA-transferase (K18118) (Supplementary Fig. 5). All analyses were adjusted for country, sex, BMI and age (fdr-corrected p < 0.05).
Several gut microbial predicted genes involved in LPS biosynthesis differentially enriched among the countries (p< 0.05; false discovery rate (fdr)-corrected) were identified. In particular, the relative abundance of specific LPS genes (K02560, K12973, K02849, K12979, K12975, K12974) were significantly enriched in Ghana, South Africa, Jamaica, and Seychelles when compared with US. Higher proportions of LPS genes including K12981, K12976 K09953, K03280 were significantly increased in Seychelles samples in comparison with US samples and also significantly increased in the US cohorts in comparison with participants from Ghana, South Africa, and Seychelles. US samples had a greater proportion of the following genes (K15669, K09778, K07264, K03273, K03271) in comparison with the other 4 countries (Supplementary Fig. 6). Non-obese individuals had a greater abundance of predicted genes encoding LPS biosynthesis (K02841, K02843, K03271, K03273, K19353, K02850) whereas only 1 LPS gene (K02841) differentially elevated in the non-obese group (Supplementary Fig. 6). All analyses were adjusted for country, sex, BMI and age (fdr-corrected p < 0.05).
Microbial community composition and taxonomy correlate with observed fecal SCFA concentrations.
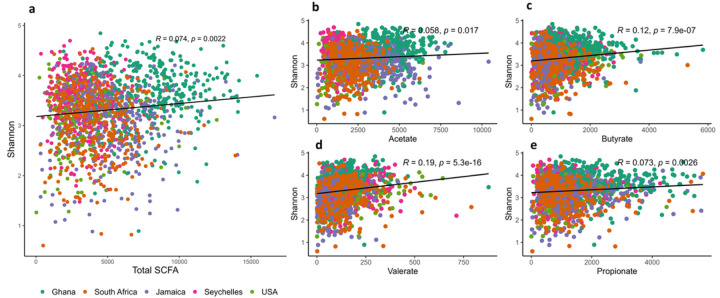
All countries had significantly higher weight-adjusted fecal total SCFA levels when compared to the US participants (p<0.001), with Ghanaians having the highest weight-adjusted fecal total SCFA levels (Supplementary Table 5). When compared to their obese counterparts, non-obese participants had significantly higher weight-adjusted fecal total and individual SCFA levels (Supplementary Table 6). Total SCFA levels displayed weak, but significantly positive correlation with Shannon diversity (r = 0.0.074). A similar trend was observed in the different individual SCFAs, namely valerate (r = 0.19), butyrate (r = 0.12), propionate (r = 0.073) and acetate (r = 0.058) (Fig. 4). Observed ASVs were not significantly correlated with total SCFAs (p>0.05). Levels of acetate, butyrate and propionate exhibited strong significant correlations with total SCFA, whereas valerate levels significantly correlated negatively (r = −0.09) with total SCFAs. Next, we assessed if levels of total SCFAs could be predicted by a mixed model. Country explained 45.7% of the variation in SCFAs. No significant effect was explained either by obesity or Shannon diversity.
Figure 4. Shannon index correlates positively with fecal short chain fatty acids.
Correlations between Shannon diversity (n = 1764) and concentrations (n=1704) of the different types of fecal short chain fatty acids (SCFAs) namely (a) total SCFA; (b) Acetate; (c) Butyrate; (d) Propionate; (e) Valerate among countries. Each colored dot represents a sample of specific country and the horizontal line on scatterplot denotes line of best t.
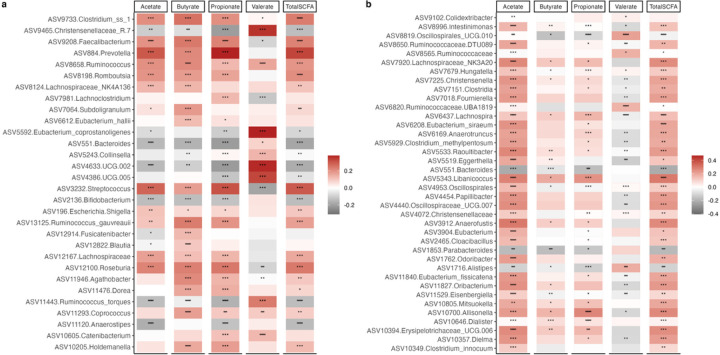
To explore the connection between SCFAs with gut microbiota, Spearman correlations between taxa that were proportionally significantly different between countries and concentrations of SCFAs were determined. Valerate negatively correlated with the proportion of Clostridium, Prevotella, Faecalibacterium, Roseburia and Streptococcus, which were all positively correlated with acetate, propionate, and butyrate. Similarly, the proportions of Christensenellaceae, Eubacterium, and UCG 002 (Ruminococcaceae) were significantly positively associated with valerate, and negatively correlated with acetate, propionate, and butyrate. In addition, only a single ASV annotated to Ruminococcus was observed to be positively associated with all 4 SCFAs (Fig. 5). Similarly, Spearman’s rank correlation coefficients were calculated between the differentially abundant ASVs identified between obese and non-obese group with concentrations of SCFAs. Broadly, the proportions of most ASVs were significantly positively associated with acetate in comparison with the other 3 SCFAs. Consistent with the correlations mentioned above, valerate negatively correlated with most ASVs that were found to be positively correlated with the three major SCFAs, acetate, propionate, and butyrate and vice versa. The relative proportions of ASVs belonging to Allisonella, Erysipelotrichaceae and Libanicoccus positively correlated with acetate, propionate, and butyrate, whereas significantly negative relationships were observed between Parabacteroides and Bacteroides abundances with the aforementioned SCFAs. Valerate showed significantly positive associations with Oscillospiralles and Ruminococcaceae abundances and significantly negative correlations with Lachnospira and Eggerthella abundances (Fig. 5).
Figure 5. Associations of gut microbiota ASVs with concentrations of short chain fatty acids (SCFAs).
(a) Heatmap of Spearman’s correlation between concentrations of SCFAs (n=) and top 30 differentially abundant ASVs (identified by ANCOM-BC) among countries. (b) Heatmap of Spearman’s correlation between concentrations of SCFAs and differentially abundant ASVs (identified by ANCOM-BC) for obese. Correlations are identified by Spearman’s rank correlation coefficient. Brick red squares indicate positive correlation, gray squares represent negative correlation and white squares are insignificant correlation. Mapping from FDR adjusted p values are denoted as: *, ** and ***, corresponding to p < 0.05, <0.01 and <0.001 respectively.
Discussion
By leveraging a well characterized large population-based cohort of African origin residing in geographically distinct regions of Ghana, South Africa, Jamaica, Seychelles, and the US, we examined the relationships between gut microbiota, SCFAs and adiposity. Our data revealed profound variations in gut microbiota, which are reflected in the significant changes in community composition, structure, and predicted functional pathways as a function of population obesity and geography, despite their shared ancestral background. Our data further revealed an inverse relation between fecal SCFA concentrations, microbial diversity, and obesity; importantly, the utility of the microbiota in predicting whether an individual was lean or obese was inversely correlated with the income-level of the country of origin. Overall, our findings are important for understanding the complex relationships between the gut microbiota, population lifestyle and the development of obesity, which may set the stage for defining the mechanisms through which the microbiome may shape health outcomes in populations of African origin.
As reported previously our data showed that geographic origin can modulate the composition of the gut microbiota. Our findings were also consistent with our previous METS studies (Fei et al. 2019; Dugas, Bernabé, et al. 2018) and other large scale continental cohort studies (De Filippo et al. 2010, 2017; Yatsunenko et al. 2012; Schnorr et al. 2014; Clemente et al. 2015; Rampelli et al. 2015; Gomez et al. 2016; Mancabelli et al. 2017), that report a higher bacterial diversity and composition/microbial richness in traditionally non-western groups that distinguish them from urban-industrialized individuals whose diets are low in fiber and high in saturated fats (E. D.Sonnenburg and Sonnenburg 2019; Kolodziejczyk, Zheng, and Elinav 2019). Although we observe enrichment in the relative abundance of several taxa associated with country of origin in our cohorts, we also detect a pattern where the gut microbiota of Ghanaian and South African cohort tends to share many features, while the gut microbiota of the Jamaican cohort shared many features with all 4 countries, possibly reflecting the ongoing epidemiological transitional nature of their communities represented by the overlap with western and traditionally non-western populations. Notably, traditionally non-western associated taxa including Prevotella, Butyrivibrio, Weisella and Romboutsia were enriched in participants from Ghana and South Africa, as suggested previously (Mancabelli et al. 2017). Western-associated taxa such as Bacteroides and Parabacteroides were enriched in individuals from Jamaica and the US (Mancabelli et al. 2017; Kao et al. 2015), while an ASV annotated as Olsenella was proportionally abundant in Seychelles microbiota. Bifidobacterium and Aldercreutzia were enriched in the US cohort. Clostridium sensu stricto 1 was over-represented in all 4 countries in comparison with the US. We also found greater enrichment of VANISH taxa including Butyricicoccus and Succinivibrio in the Ghanaian cohort, in line with individuals practicing traditional lifestyles (Pasolli et al. 2019). Prevotella is usually associated with plant-based diets rich in dietary fibers, while Bacteroides abundance broadly correlates with diets high in fat, animal protein, and sugars (Gupta, Paul, and Dutta 2017; Wu et al. 2011), which is in agreement with our enterotype analysis where a Prevotella-rich microbiota dominates the Ghanaian and South African gut, while a Bacteroides-rich microbiota dominated in the high-income countries. As Prevotella synthesizes SCFAs (T.Chen et al. 2017), its depletion may lead to the observed reduction in SCFA concentrations. Our results support a potential role for geography in reinforcing variations in the gut microbiota in our study cohort despite shared origin. Geography may reflect subtle shifts in lifestyle and/or environmental exposures including heterogeneity of dietary sources, exposure to medications, socioeconomic factors, medical history, and biogeographical patterns in microbial dispersion (Asnicar et al. 2021; Pasolli et al. 2019; Costello et al. 2012; He et al. 2018).
We also inferred the metabolic capacity of the gut microbiota associated with the different countries. Several metabolic pathways linked to carrier, cofactor and vitamin biosynthesis, biosynthesis/degradation of amines, amino acids, aromatic xenobiotics, and tricarboxylic acid (TCA) cycle were differentially enriched between the different countries compared with the US. These pathways are involved in biochemical reactions that regulate several processes including energy metabolism, inflammation, epigenetic processes, and oxidative stress. Participants from Ghana and Jamaica were enriched for gallate degradation, which can result in phenolic catechin metabolites which are thought to alleviate obesity-related pathologies (Marchesi et al. 2016; Liu et al. 2021). Additionally, glutamate metabolism, which can be fermented to butyrate and propionate, was enriched in South Africans and Ghanaians compared to the US. In the Seychelles, actinobacterial mycothiol biosynthesis was upregulated, which is involved in antioxidant activity and the removal of toxic compounds from cells (Newton, Buchmeier, and Fahey 2008). We further identified an increase in SCFA synthesis pathways, e.g. acetyl coenzyme A pathway, threonine biosynthesis, and leucine degradation in the microbiomes of all four countries compared to the US. Further studies are required to evaluate the potential causal relations of these gut microbial functions with health outcomes using shotgun metagenomic sequencing.
Preclinical mouse models provided early causal links between gut microbial ecology and obesity (Ley et al. 2005; Bäckhed et al. 2007), suggesting the potential to predict obesity risk from the microbiome. However, prediction has proven difficult because results are conflicting (Finucane et al. 2014). As we observed, the bulk of evidence from prior studies show that obesity is associated with a less diverse bacterial community (Turnbaugh et al. 2009; Dugas, Bernabé, et al. 2018; Peters et al. 2018). In addition, we identified several SCFA producing bacteria that were significantly depleted in obese individuals, which may influence host energy metabolism. For example, Oscillospira and Christensenella which were statistically associated with increases SCFA concentrations and reduced obesity have previously been associated with a lean phenotype (Beaumont et al. 2016; Konikoff and Gophna 2016; Gophna, Konikoff, and Nielsen 2017; Morotomi, Nagai, and Watanabe 2012), and produce SCFAs (Konikoff and Gophna 2016; Gophna, Konikoff, and Nielsen 2017) including butyrate, which improves insulin sensitivity and reduces inflammation (M.-H.Kim et al. 2020). We also detected several butyrate producing ASVs including Eubacterium, Alistipes, Clostridium and Odoribacter to be proportionally enriched in individuals who were non-obese. We observed that obese individuals presented a greater abundance of Lachnospira, which does produce SCFAs, a finding also consistent with our prior study in the same population (Dugas, Bernabé, et al. 2018), and others (Lippert et al. 2017; Meehan and Beiko 2014; de la Cuesta-Zuluaga, Corrales-Agudelo, et al. 2018). However, other studies have observed the opposite (Companys et al. 2021; Stanislawski et al. 2017).
SCFA supplementation has been documented to protect against a high-fat diet-induced obesity in mice (H. V. Lin et al. 2012; Lu et al. 2016) as well as weight gain in humans (Chambers et al. 2015). Conversely, other studies, mostly from western populations, have reported that elevated SCFA concentrations in stool can associate with obesity (Schwiertz et al. 2010; Fernandes et al. 2014; Riva et al. 2017; de la Cuesta-Zuluaga, Mueller, et al. 2018). For instance, a Colombian cohort showed associations between elevated fecal SCFA levels, central obesity, gut permeability, and hypertension (de la Cuesta-Zuluaga, Mueller, et al. 2018). One potential explanation is that obese gut microbiota may lead to less efficient SCFA absorption, hence the increased SCFA excretion (de la Cuesta-Zuluaga, Mueller, et al. 2018). However, as we found diets high in fiber correlate positively with weight loss (Hu et al. 2013; Esposito et al. 2011) and increased levels of fecal SCFAs (De Filippis et al. 2016). One explanation may be in differences lifestyle factors, including medication, activity, and pollutant exposure, which could also impact intestinal absorption in western countries. We note that fecal SCFA concentrations are not a direct measure of intestinal SCFA production, but rather reflect a net result of the difference between production and absorption (Canfora, Jocken, and Blaak 2015). Studies using stable isotopes to measure SCFA dynamics would improve interpretation of dichotomy.
While SCFAs associate with obese phenotype, another mechanism underpinning obesity is metabolic endotoxemia. An increase in Proteobacteria, which often accompanies a high fat/high sugar diet, is often associated with an increase in circulating lipopolysaccharide (LPS) and H2S, which provoke low-grade inflammation, increased intestinal permeability, and clock gene disruption in the liver, which associate with adiposity (Zhao 2013; Cani et al. 2008; Leone et al. 2015). We identified an increase in Dialister in obese individuals which has been associated with increased LPS production (Yang et al. 2022; Boulangé et al. 2016), obesity (Zhang et al. 2021), sleep disruption and chronic inflammation (Yan et al. 2021; Karlsson et al. 2012; Fei and Zhao 2013; Cani et al. 2007; Fei et al. 2021). Collectively, our results demonstrate that obese individuals harbor a marked inflammatory state favoring the development of obesity, and this is in concordance with the associated metabolic endotoxemia pathway linking gut bacteria to obesity.
In obese individuals, as well as SCFA metabolism, we also detected marked depletion in pathways involved in cell structure, vitamin B6, NAD, and amino acid biosynthesis. This suggests that pathways important for growth and energy homeostasis are disrupted in individuals with obesity. We also noted an enrichment of the formaldehyde assimilation I (serine pathway) pathway. Endogenous formaldehyde produced at sufficient levels has carcinogenic properties and detrimental effects on genome stability. To counteract this reactive molecule, organisms have evolved a detoxification system that converts formaldehyde to formate, a less reactive molecule that can be used for nucleotide biosynthesis (Reingruber and Pontel 2018;N. H. Chen et al. 2016). Thus, we may infer that the pattern of increased formaldehyde assimilation pathway in our data might result from a defect or diminished capacity of formaldehyde detoxification system pathway, an assumption which requires further verification. A study reported increases in the abundance of formaldehyde assimilation pathway in a depressed group when compared with non-depressed controls (S.-Y. Kim et al. 2022). We are the first to show that the gut of obese participants is enriched in the formaldehyde assimilation pathway. Although we do not understand the mechanistic details, it is known that toxic formaldehyde is generated along with reactive oxygen species during inflammatory processes (N. H. Chen et al. 2016). Thus, an increased capacity for formaldehyde pathway may indicate a microbiome-induced increase in reactive oxygen species in the gut of obese individuals. Indeed, prior work has identified induction of oxygen stress by microbial perturbations as one of the mechanisms by which the microbiome can promote weight gain and insulin resistance (J. Qin et al. 2012). The specific alterations of the gut microbiota and the associated predicted functionality may constitute a potential avenue for the development of microbiome-based therapeutics to treat obesity and/or to promote and sustain weight loss.
Study strengths and limitations.
While our study has several strengths including a large sample size, diverse population along an epidemiological transition gradient with a comprehensive dataset that allowed the exclusion of the potential effects of origin as well as control of potential interpersonal covariates, and use of validated and standard tools for data collection, we acknowledge some limitations as well. First, the cross-sectional nature of our study design is unable to establish temporality or identify mechanisms by which the gut microbiome may causally influence the observed associations. In that regard, we expect that prospective data from the METS cohort study will provide the basis to assess the longitudinal association between gut microbiota composition, metabolites, and obesity, and we have an ongoing study exploring the potential correlations longitudinally. The use of 16S rRNA sequencing in our analysis for inferences on microbial functional ecology inherently has its limitations for drawing conclusions on species and strain level functionality due to its low resolution. Nevertheless, our results provide insight into the relationship between obesity, gut microbiota, and metabolic pathways in individuals of African origin across different geographies, stimulating further examination of large-scale studies using multi-omic approaches with deeper taxonomic and functional resolution and animal transplantation studies to investigate potentially novel microbial strains and to explore the clinical relevance of the observed metabolic differences.
Conclusion
Our study analyzed the relationship between gut microbiota composition, SCFA concentrations, and obesity in a cohort of African origin from different countries. Ghanaian participants had the most diverse microbiota, and the American cohort had the least. Obese individuals had different gut microbiota composition and function compared to non-obese individuals. Non-obese participants had more SCFA-producing microbes and higher total SCFA concentrations in feces. The predictive accuracy of the microbiota for obesity was greatest in low-income countries, suggesting that lifestyle traits in high-income countries may increase obesity risk even for lean individuals. Alterations in the gut microbiota and associated metabolic functions could guide the development of microbiome-based solutions to treat obesity. Further studies using multi-omic approaches are needed to confirm the identified taxonomic and metabolic signatures.
Methods
Study Cohort.
Since 2010, METS, and the currently funded METS-Microbiome study has longitudinally followed an international cohort of African origin adults spanning the epidemiologic transition from Ghana, South Africa, Jamaica, Seychelles, and US (Dugas, Lie, et al. 2018; Luke et al. 2011). METS utilizes the framework of the epidemiologic transition to investigate differences in health outcomes based on country of origin. The epidemiologic transition is defined using the United Nations Human Development Index (HDI) as an approximation of the epidemiologic transition. Ghana represents a lower-middle income country, South Africa represents a middle-income country, Jamaica and Seychelles represent high income countries and the US represents a very high-income country. This framework has allowed us to investigate aspects of increased Westernization throughout the world (ex. increased consumption of ultra-processed foods) are related to increased prevalence of obesity, diabetes and cardiometabolic diseases. Our data from the original METS cohort demonstrate that the epidemiologic transition has altered habitual diets in the international METS sites, and that reduced fiber intake is associated with higher metabolic risk, inflammation, and obesity across the epidemiologic transition (Mehta et al. 2021). Originally, 2,506 African origin adults (25–45 yrs), were enrolled in METS between January 2010 and December 2011 and followed on a yearly basis. In 2018, METS participants were recontacted and invited to participate in METS-Microbiome. Participants were excluded from participating in the original METS study if they self-reported an infectious disease, including HIV-positive individuals, pregnancy, breast-feeding or any condition which prevented the individual from participating in normal physical activities. METS-Microbiome was approved by the Institutional Review Board of Loyola University Chicago, IL, US; the Committee on Human Research Publication and Ethics of Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; the Research Ethics Committee of the University of Cape Town, South Africa; the Board for Ethics and Clinical Research of the University of Lausanne, Switzerland; and the Ethics Committee of the University of the West Indies, Kingston, Jamaica. All study procedures were explained to participants in their native languages, and participants provided written informed consent after being given the opportunity to ask any questions.
Participant anthropometry, sociodemographic and biochemical measurements.
Participants completed the research visits at the established METS research clinics located in the respective communities (Luke et al. 2011). Briefly, they presented themselves at the site-specific research clinic early in the morning, following an overnight fast. The weight of the participant was measured without shoes and dressed in light clothing to the nearest 0.1 kg using a standard digital scale (Seca, SC, USA). Height was measured using a stadiometer without shoes and head held in the Frankfort plane to the nearest 0.1 cm. Waist circumference was measured to the nearest 0.1 cm at the umbilicus, while hip circumference was measured to the nearest 0.1 cm at the point of maximum extension of the buttocks. Adiposity (% body fat) was assessed using BIA (Quantum, RJL Systems, Clinton Township, MI), and study specific equations (Luke et al. 2011). Blood pressure was measured using the standard METS protocol using the Omron Automatic Digital Blood Pressure Monitor (model HEM-747Ic, Omron Healthcare, Bannockburn, IL, USA), with the antecubital fossa at heart level. Participants were asked to provide a fecal sample using a standard collection kit (EasySampler stool collection kit, Alpco, NH). Fecal samples were placed within a −80° freezer immediately upon receipt at all the sites. Participants were requested to fast from 8 pm in the evening prior to the clinic examination, during which fasting capillary glucose concentrations were determined using finger stick (Accu-check Aviva, Roche).
Fecal Short Chain Fatty Acid quantification.
As in our previous studies (Nooromid et al. 2020; Lewandowski et al. 2021; Reiman, Layden, and Dai 2021; Barengolts et al. 2019; Navarro et al. 2018; Dugas, Bernabé, et al. 2018), fecal SCFAs were measured using LC-MC/MS at the University of Illinois-Chicago Mass Spectrometry Core using previously published methods (Moreau et al. 2003; Richardson et al. 1989). The LC-MC/MS analysis was completed on an AB Sciex Qtrap 5500 coupled to Agilent UPLC/HPLC system. All samples were analyzed by Agilent poroshell 120 EC-C18 Column, 100Å, 2.7 μm,2.1 mm×100 mm coupled to an Agilent UPLC system, which was operated at a flow rate of 400 μl/min. A gradient of buffer A (H20, 0.1% Formic acid) and buffer B (Acetonitrile, 0.1% Formic acid) were applied as: 0 min, 30% of buffer B; increase buffer B to 100% in 4 min; maintain B at 100% for 5 min. The column was then equilibrated for 3 min at 30% B between the injections with the MS detection is in negative mode. The MRM transitions of all targeted compounds include the precursor ions and the signature production ion. Unit resolution is used for both analyzers Q1 and Q3. The MS parameters such as declustering potential, collision energy and collision cell exit potential are optimized in order to achieve the optimal sensitivity. SCFAs are presented as individual SCFAs (μg/g), including: butyric acid, propionic acid, acetic acid and valeric acid, as well as total SCFAs (sum of 4).
METS data showed Ghanaians consumed the greatest amount of both soluble and insoluble fiber and had the lowest percentage energy from fat (42.5% of the Ghanaian cohort, dietary fiber intake: 24.9 g ±9.7g/day). The US has the highest proportion of energy from fat and the lowest fiber intake of the five sites (3.2% of the US cohort, dietary fiber intake: 14.2 g ± 7.1 g/day).
DNA extraction, Amplicon Sequencing.
Fecal samples were shipped on dry ice to the microbiome core sequencing facility, University of California, San Diego for 16S rRNA gene processing. Fecal samples were randomly sorted, transferred to 96-well extraction plates and DNA was extracted using MagAttract Power Microbiome kit. Blank controls and mock controls (ZymoBiomics) were included per extraction plate, which were carried through all downstream processing steps. Extracted DNA was used for amplification of the V4 region of the 16S rRNA gene with 515F-806R region-specific primers according to the Earth Microbiome Project (Thompson et al. 2017; Walters et al. 2016). Purified amplicon libraries were sequenced on the Illumina NovaSeq platform to produce 150 bp forward and reverse reads through the IGM Genomics Center, University of California San Diego. Full DNA extraction, amplification, quantification, and sequencing protocols and standards are available at http://www.earthmicrobiome.org/protocols-and-standards; (Thompson et al. 2017).
Bioinformatic analysis.
The generated raw sequence data were uploaded and processed in Qiita (Gonzalez et al. 2018) (Qiita ID 13512) an open-source, web-enabled microbiome analysis platform. Sequences were demultiplexed, quality filtered, trimmed, erroneous sequences were removed, and amplicon sequence variants (ASVs) were defined using Deblur (Amir et al. 2017). The deblur ASV table was exported to Qiime2 (Bolyen et al. 2019; Bokulich et al. 2018) and representative sequences of the ASVs were inserted into the Greengenes 13.8 99% identity tree with SATé-enabled phylogenetic placement (SEPP) using q2-fragment-insertion (Bolyen et al. 2019; Mirarab, Nguyen, and Warnow 2012) to generate an insertion tree for diversity computation. Additionally, the deblur ASV table was assigned taxonomic classification using the Qiime2 feature-classifier, with Naive Bayes classifiers trained on the SILVA database (version 138; (McLaren 2020)). A total of 463,258,036 reads, 154,952 ASVs and 1902 samples were obtained from the deblur table. The resulting ASV count table, taxonomy data, insertion tree, and sample metadata were exported and merged into a phyloseq (McMurdie and Holmes 2013) object in R (R Foundation for Statistical Computing, Vienna, Austria) for downstream analysis. Features with less than ten reads in the entire dataset and samples with fewer than 6,000 reads were removed from the phyloseq object. In addition, mitochondrial and chloroplast-derived sequences, non-bacterial sequences, as well as ASVs that were unassigned at phylum level were filtered prior to analyses. There were 433,364,873 reads and 13254 ASVs in the remaining 1873 fecal samples in the phyloseq object. The remaining samples after filtering were rarefied to a depth of 6,000 reads to avoid sequencing bias, before generating alpha diversity measures, leaving 9917 ASVs across 1873 samples.
Diversity and differential proportional analyses:
Alpha diversity measures based on Observed Amplicon Sequence Variants (ASVs), Faith’s Phylogenetic Diversity, and Shannon Index were conducted on rarified samples using phyloseq (McMurdie and Holmes 2013) and picante (Kembel et al. 2010) libraries. Beta diversity was determined using both weighted and unweighted UniFrac distance matrices (Lozupone and Knight 2005), generated in phyloseq. The Bacteroides Prevotella ratio was calculated by dividing the abundance of the genera Bacteroides by Prevotella. Participants were classified into Bacteroides enterotype (B-type) if the ratio was greater than 1, otherwise Prevotella enterotype (P-type). For differential abundance analysis, samples were processed to remove exceptionally rare taxa. First, the non-rarefied reads were filtered to remove samples with < 10,000 reads. Next, ASVs with fewer than 50 reads in total across all samples and/or were present in less than 2% of samples were excluded. This retained 2061 ASVs across 1694 samples. The retained ASVs were binned at genus level, and subsequently used in the analysis of compositions of microbiomes with bias correction (ANCOMBC; (H. Lin and Peddada 2020) to determine specific taxa differentially abundant across sites or obese phenotype. ANCOM-BC is a statistical approach that accounts for sampling fraction, normalizes the read counts by a process identical to log-ratio transformations while controlling for false discovery rates and increasing power. Site, age, sex, BMI were added as covariates in the ANCOM-BC formula to reduce the effect of confounders.
Random forest classifier:
Random Forest supervised learning models implemented in Qiime2 were used to estimate the predictive power of microbial community profiles for site and obese phenotype. The classifications were done with 500 trees based on 10-fold cross-validation using the QIIME “sample-classifier classify-samples” plugin (Bokulich et al. 2018). A randomly drawn 80% of samples were used for model training, whereas the remaining 20% were used for validation. Further, the 30 most important ASVs for differentiating between site or obese phenotype were predicted and annotated.
Predicted metabolic gene pathway analysis:
The functional potential of microbial communities was inferred using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2) v2.5.1 with the ASV table processed to remove exceptionally rare taxa and the representative sequences as input files (Douglas et al. 2020). The metabolic pathway from the PICRUSt2 pipeline was annotated using the MetaCyc database (Caspi et al. 2016). The predicted MetaCyc abundances (unstratified pathway abundances) were analyzed with ANCOM-BC to determine differentially abundant pathway associations across sites and obese status. Site, age, sex, BMI were added as covariates in the ANCOM-BC formula to reduce the effect of confounders.
Statistical Analysis:
All statistical analyses and graphs were done with R software. Kruskal-Wallis test and Permutational Analysis of Variance (PERMANOVA) test with 999 permutations using the Adonis function in the vegan package (Oksanen et al. 2013) were performed to compare alpha and beta diversity measures respectively with multiple groups comparison correction. PERMANOVA models were adjusted for BMI, age, sex for country whereas age, sex and country were accounted for in obese groups. Variables that showed significant differences in the PERMANOVA analyses, PERMDISP test was performed to assess differences in dispersion or centroids. For differential abundance analysis, the false-discovery rate (FDR) method incorporated in the ANCOM-BC library was used to correct p-values for multiple testing. A cut-off of Padj < 0.05 was used to assess significance. Spearman correlations were performed between concentrations of short chain fatty acids, Shannon diversity or concentrations of short chain fatty acids and differentially abundant taxa that were identified either among study sites or in obese and non-obese individuals. The resulting p-values were adjusted for multiple testing using the false-discovery rate (FDR). P value < 0.05 was considered statistically significant. A mixed model was built using lme4 package to assess whether total SCFAs could be predicted by Shannon diversity, obesity, and country, setting obesity and Shannon diversity as fixed effects and random intercept by country.
Data availability:
All 16S rRNA gene sequence data are publicly available via the QIITA platform (https://qiita.ucsd.edu) under the study identifier (ID=13512) and will soon be deposited on the European Bioinformatics Institute (EBI) site. The SILVA 16 S rRNA database used for alignment is available at https://data.qiime2.org/2022.2/common/silva-138-99-515-806-nb-classifier.qza. The data and analyses generated in this study are available within the paper, Supplementary Information and Source data files provided with this paper.
Acknowledgements
We thank the METS participants who continue their ongoing participation in the METS studies, as well as the site-specific clinic staff in Ghana, South Africa, Jamaica, Seychelles and the US. The UCSD Microbiome Core performed sample extractions and library preparation utilizing protocols and primers published on the Earth Microbiome Project website (https://earthmicrobiome.org/). This publication includes data generated at the UC San Diego IGM Genomics Center utilizing an Illumina NovaSeq 6000 that was purchased with funding from a National Institutes of Health SIG grant (#S10 OD026929).
Funding
This work is supported by the National Institutes of Health grant R01-DK111848
Funding Statement
This work is supported by the National Institutes of Health grant R01-DK111848
Footnotes
Supplementary Files
This is a list of supplementary files associated with this preprint. Click to download.
METSMicrobiomeSourceData.xlsx
METSMicrobiomeSupplementaryInformation.docx
Contributor Information
Jack Gilbert, University of California San Diego.
Gertrude Ecklu-Mensah, University of California-San Diego.
Maria Gjerstad Maseng, University of Oslo.
Sonya Donato, University of California San Diego.
Candice Coo-Kang, Loyola University.
Lara Dugas, Loyola University Chicago.
Pascal Bovet, University Center for Primary Care and Public Health.
Kweku Bedu-Addo, Kwame Nkrumah University of Science and Technology.
Jacob Plange-Rhule, Kwame Nkrumah University of Science and Technology.
Terrence Forrester, University of West Indies.
Estelle Lambert, University of Cape Town.
Dale Rae, University of Cape Town.
Amy Luke, Loyola University School of Medicine.
Brian Layden, University of Illinois at Chicago.
References
- 1.Abdelaal Mahmoud, le Roux Carel W., and Docherty Neil G.. 2017. “Morbidity and Mortality Associated with Obesity.” Annals of Translational Medicine 5 (7): 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agyemang Charles, Boatemaa Sandra, Frempong Grace Agyemang, and de-Graft Aikins Ama. 2016. “Obesity in Sub-Saharan Africa.” In Metabolic Syndrome: A Comprehensive Textbook,edited by Ahima Rexford S, 41–53. Cham: Springer International Publishing. [Google Scholar]
- 3.Ajala O., Mold F., Boughton C., Cooke D., and Whyte M.. 2017. “Childhood Predictors of Cardiovascular Disease in Adulthood. A Systematic Review and Meta-Analysis.” Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 18 (9): 1061–70. [DOI] [PubMed] [Google Scholar]
- 4.Amir Amnon, Daniel McDonald Jose A. Navas-Molina, Kopylova Evguenia, Morton James T., Zhenjiang Zech Xu Eric P. Kightley, et al. 2017. “Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns.” MSystems 2 (2). 10.1128/mSystems.00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arumugam Manimozhiyan, Raes Jeroen, Pelletier Eric, Denis Le Paslier Takuji Yamada, Mende Daniel R., Fernandes Gabriel R., et al. 2011. “Enterotypes of the Human Gut Microbiome.” Nature 473 (7346): 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asnicar Francesco, Berry Sarah E., Valdes Ana M., Nguyen Long H., Piccinno Gianmarco, Drew David A., Leeming Emily, et al. 2021. “Microbiome Connections with Host Metabolism and Habitual Diet from 1,098 Deeply Phenotyped Individuals.” Nature Medicine 27 (2): 321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bäckhed Fredrik, Manchester Jill K., Semenkovich Clay F., and Gordon Jeffrey I.. 2007. “Mechanisms Underlying the Resistance to Diet-Induced Obesity in Germ-Free Mice.” Proceedings of the National Academy of Sciences of the United States of America 104 (3): 979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barengolts Elena, Green Stefan J., Chlipala George E., Layden Brian T., Eisenberg Yuval, Priyadarshini Medha, and Dugas Lara R.. 2019. “Predictors of Obesity among Gut Microbiota Biomarkers in African American Men with and without Diabetes.” Microorganisms 7 (9). 10.3390/microorganisms7090320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barone Monica, Garelli Silvia, Rampelli Simone, Agostini Alessandro, Matysik Silke, Federica D’Amico Sabrina Krautbauer, et al. 2022. “Multi-Omics Gut Microbiome Signatures in Obese Women: Role of Diet and Uncontrolled Eating Behavior.” BMC Medicine 20 (1): 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaumont Michelle, Goodrich Julia K., Jackson Matthew A., Yet Idil, Davenport Emily R., Sara Vieira-Silva Justine Debelius, et al. 2016. “Heritable Components of the Human Fecal Microbiome Are Associated with Visceral Fat.” Genome Biology 17 (1): 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokulich Nicholas A., Kaehler Benjamin D., Jai Ram Rideout Matthew Dillon, Bolyen Evan, Knight Rob, Huttley Gavin A., and Caporaso J. Gregory. 2018. “Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin.” Microbiome 6 (1): 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolyen Evan, Jai Ram Rideout Matthew R. Dillon, Bokulich Nicholas A., Abnet Christian C., Al-Ghalith Gabriel A., Alexander Harriet, et al. 2019. “Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2.” Nature Biotechnology 37 (8): 852–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonomo Raiza R., Cook Tyler M., Gavini Chaitanya K., White Chelsea R., Jones Jacob R., Bovo Elisa, Zima Aleksey V., et al. 2020. “Fecal Transplantation and Butyrate Improve Neuropathic Pain, Modify Immune Cell Profile, and Gene Expression in the PNS of Obese Mice.” Proceedings of the National Academy of Sciences of the United States of America 117 (42): 26482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulangé Claire L., Ana Luisa Neves Julien Chilloux, Nicholson Jeremy K., and Dumas Marc-Emmanuel. 2016. “Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease.” Genome Medicine 8 (1): 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canfora Emanuel E., Jocken Johan W., and Blaak Ellen E.. 2015. “Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity.” Nature Reviews. Endocrinology 11 (10): 577–91. [DOI] [PubMed] [Google Scholar]
- 16.Cani Patrice D., Amar Jacques, Miguel Angel Iglesias Marjorie Poggi, Knauf Claude, DelphineBastelica Audrey M. Neyrinck, et al. 2007. “Metabolic Endotoxemia Initiates Obesity and Insulin Resistance.” Diabetes 56 (7): 1761–72. [DOI] [PubMed] [Google Scholar]
- 17.Cani Patrice D., Bibiloni Rodrigo, Knauf Claude, Waget Aurélie, Neyrinck Audrey M., Delzenne Nathalie M., and Burcelin Rémy. 2008. “Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice.” Diabetes 57 (6): 1470–81. [DOI] [PubMed] [Google Scholar]
- 18.Cani Patrice D., and Delzenne Nathalie M.. 2009. “Interplay between Obesity and Associated Metabolic Disorders: New Insights into the Gut Microbiota.” Current Opinion in Pharmacology 9 (6): 737–43. [DOI] [PubMed] [Google Scholar]
- 19.Caspi Ron, Billington Richard, Ferrer Luciana, Foerster Hartmut, Fulcher Carol A., Keseler Ingrid M., Kothari Anamika, et al. 2016. “The MetaCyc Database of Metabolic Pathways and Enzymes and the BioCyc Collection of Pathway/Genome Databases.” Nucleic Acids Research 44 (D1): D471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers Edward S., Viardot Alexander, Psichas Arianna, Morrison Douglas J., Murphy Kevin G., Zac-Varghese Sagen E. K., MacDougall Kenneth, et al. 2015. “Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults.” Gut 64 (11): 1744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Nathan H., Djoko Karrera Y., Veyrier Frédéric J., and McEwan Alastair G.. 2016. “Formaldehyde Stress Responses in Bacterial Pathogens.” Frontiers in Microbiology 7 (March): 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Tingting, Long Wenmin, Zhang Chenhong, Liu Shuang, Zhao Liping, and Hamaker Bruce R.. 2017. “Fiber-Utilizing Capacity Varies in Prevotella-versus Bacteroides-Dominated Gut Microbiota.” Scientific Reports 7 (1): 2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clemente Jose C., Pehrsson Erica C., Blaser Martin J., Sandhu Kuldip, Gao Zhan, Wang Bin, Magris Magda, et al. 2015. “The Microbiome of Uncontacted Amerindians.” Science Advances 1 (3). 10.1126/sciadv.1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Companys Judit, Maria José Gosalbes Laura Pla-Pagà, Lorena Calderón-Pérez Elisabet Llauradó, Pedret Anna, Valls Rosa Maria, et al. 2021. “Gut Microbiota Profile and Its Association with Clinical Variables and Dietary Intake in Overweight/Obese and Lean Subjects: A Cross-Sectional Study.” Nutrients 13 (6). 10.3390/nu13062032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costello Elizabeth K., Stagaman Keaton, Dethlefsen Les, Bohannan Brendan J. M., and Relman David A.. 2012. “The Application of Ecological Theory toward an Understanding of the Human Microbiome.” Science 336 (6086): 1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuesta-Zuluaga Jacobo de la, Corrales-Agudelo Vanessa, Velásquez-Mejía Eliana P., Carmona Jenny A., Abad José M., and Escobar Juan S.. 2018. “Gut Microbiota Is Associated with Obesity and Cardiometabolic Disease in a Population in the Midst of Westernization.” Scientific Reports 8 (1): 11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuesta-Zuluaga Jacobo de la, Mueller Noel T., Álvarez-Quintero Rafael, Velásquez-Mejía Eliana P., Sierra Jelver A., Corrales-Agudelo Vanessa, Carmona Jenny A., Abad José M., and Escobar Juan S.. 2018. “Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors.” Nutrients 11 (1). 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahl Wendy J., and Stewart Maria L.. 2015. “Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber.” Journal of the Academy of Nutrition and Dietetics 115 (11): 1861–70. [DOI] [PubMed] [Google Scholar]
- 29.Dalile Boushra, Lukas Van Oudenhove Bram Vervliet, and Verbeke Kristin. 2019. “The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication.” Nature Reviews. Gastroenterology & Hepatology 16 (8): 461–78. [DOI] [PubMed] [Google Scholar]
- 30.Filippis De, Francesca Nicoletta Pellegrini, Vannini Lucia, Jeffery Ian B., Antonietta La Storia Luca Laghi, Serrazanetti Diana I., et al. 2016. “High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome.” Gut 65 (11): 1812–21. [DOI] [PubMed] [Google Scholar]
- 31.Filippo De, Carlotta Duccio Cavalieri, Monica Di Paola Matteo Ramazzotti, Jean Baptiste Poullet Sebastien Massart, Collini Silvia, Pieraccini Giuseppe, and Lionetti Paolo. 2010. “Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa.” Proceedings of the National Academy of Sciences of the United States of America 107 (33): 14691–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filippo De, Carlotta Monica Di Paola, Ramazzotti Matteo, Albanese Davide, Pieraccini Giuseppe, Banci Elena, Miglietta Franco, Cavalieri Duccio, and Lionetti Paolo. 2017. “Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy.” Frontiers in Microbiology 8 (October): 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiBaise John K., Zhang Husen, Crowell Michael D., Krajmalnik-Brown Rosa, Decker G. Anton, and Rittmann Bruce E.. 2008. “Gut Microbiota and Its Possible Relationship with Obesity.” Mayo Clinic Proceedings. Mayo Clinic 83 (4): 460–69. [DOI] [PubMed] [Google Scholar]
- 34.Douglas Gavin M., Maffei Vincent J., Zaneveld Jesse R., Yurgel Svetlana N., Brown James R., Taylor Christopher M., Huttenhower Curtis, and Langille Morgan G. I.. 2020. “PICRUSt2 for Prediction of Metagenome Functions.” Nature Biotechnology 38 (6): 685–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugas Lara R., Beatriz Peñalver Bernabé Medha Priyadarshini, Fei Na, Seo Jin Park Laquita Brown, Plange-Rhule Jacob, et al. 2018. “Decreased Microbial Co-Occurrence Network Stability and SCFA Receptor Level Correlates with Obesity in African-Origin Women.” Scientific Reports 8 (1): 17135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dugas Lara R., Lie Louise, Jacob Plange-Rhule Kweku Bedu-Addo, Bovet Pascal, Lambert Estelle V., Forrester Terrence E., Luke Amy, Gilbert Jack A., and Layden Brian T.. 2018. “Gut Microbiota, Short Chain Fatty Acids, and Obesity across the Epidemiologic Transition: The METS-Microbiome Study Protocol.” BMC Public Health 18 (1): 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ecklu-Mensah Gertrude, Gilbert Jack, and Devkota Suzanne. 2022. “Dietary Selection Pressures and Their Impact on the Gut Microbiome.” Cellular and Molecular Gastroenterology and Hepatology 13(1): 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esposito Katherine, Kastorini Christina-Maria, Panagiotakos Demosthenes B., and Giugliano Dario. 2011. “Mediterranean Diet and Weight Loss: Meta-Analysis of Randomized Controlled Trials.” Metabolic Syndrome and Related Disorders 9 (1): 1–12. [DOI] [PubMed] [Google Scholar]
- 39.Falony Gwen, Joossens Marie, Sara Vieira-Silva Jun Wang, Darzi Youssef, Faust Karoline, Kurilshikov Alexander, et al. 2016. “Population-Level Analysis of Gut Microbiome Variation.” Science 352 (6285): 560–64. [DOI] [PubMed] [Google Scholar]
- 40.Fei Na, Beatriz Peñalver Bernabé Louise Lie, Baghdan Danny, Kweku Bedu-Addo Jacob Plange-Rhule, Forrester Terrence E., et al. 2019. “The Human Microbiota Is Associated with Cardiometabolic Risk across the Epidemiologic Transition.” PloS One 14 (7): e0215262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fei Na, Candice Choo-Kang Sirimon Reutrakul, Crowley Stephanie J., Rae Dale, Kweku Bedu-Addo Jacob Plange-Rhule, et al. 2021. “Gut Microbiota Alterations in Response to Sleep Length among African-Origin Adults.” PloS One 16 (9): e0255323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fei Na, and Zhao Liping. 2013. “An Opportunistic Pathogen Isolated from the Gut of an Obese Human Causes Obesity in Germfree Mice.” The ISME Journal 7 (4): 880–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandes J., Su W., Rahat-Rozenbloom S., Wolever T. M. S., and Comelli E. M.. 2014. “Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans.” Nutrition & Diabetes 4(6): e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finucane Mariel M., Sharpton Thomas J., Laurent Timothy J., and Pollard Katherine S.. 2014. “A Taxonomic Signature of Obesity in the Microbiome? Getting to the Guts of the Matter.” PloS One 9(1): e84689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Zhanguo, Yin Jun, Zhang Jin, Ward Robert E., Martin Roy J., Lefevre Michael, Cefalu William T., and Ye Jianping. 2009. “Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice.” Diabetes 58 (7): 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geng Jiafeng, Ni Qingqiang, Sun Wei, Li Liangge, and Feng Xiujing. 2022. “The Links between GutMicrobiota and Obesity and Obesity Related Diseases.” Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 147 (March): 112678. [DOI] [PubMed] [Google Scholar]
- 47.Gomez Andres, Petrzelkova Klara J., Burns Michael B., Yeoman Carl J., Amato Katherine R., Vlckova Klara, Modry David, et al. 2016. “Gut Microbiome of Coexisting BaAka Pygmies and Bantu Reflects Gradients of Traditional Subsistence Patterns.” Cell Reports 14 (9): 2142–53. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez Antonio, Navas-Molina Jose A., Kosciolek Tomasz, McDonald Daniel, Vázquez-Baeza Yoshiki, Ackermann Gail, DeReus Jeff, et al. 2018. “Qiita: Rapid, Web-Enabled Microbiome Meta-Analysis.” Nature Methods 15 (10): 796–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gophna Uri, Konikoff Tom, and Nielsen Henrik Bjørn. 2017. “Oscillospira and Related Bacteria - From Metagenomic Species to Metabolic Features.” Environmental Microbiology 19 (3): 835–41. [DOI] [PubMed] [Google Scholar]
- 50.Gouda Hebe N., Charlson Fiona, Sorsdahl Katherine, Ahmadzada Sanam, Ferrari Alize J., Erskine Holly, Leung Janni, et al. 2019. “Burden of Non-Communicable Diseases in Sub-Saharan Africa, 1990–2017: Results from the Global Burden of Disease Study 2017.” The Lancet. Global Health 7(10): e1375–87. [DOI] [PubMed] [Google Scholar]
- 51.Greenblum Sharon, Turnbaugh Peter J., and Borenstein Elhanan. 2012. “Metagenomic Systems Biology of the Human Gut Microbiome Reveals Topological Shifts Associated with Obesity and Inflammatory Bowel Disease.” Proceedings of the National Academy of Sciences of the United States of America 109 (2): 594–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta Vinod K., Paul Sandip, and Dutta Chitra. 2017. “Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity.” Frontiers in Microbiology 8 (June): 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hales Craig M., Carroll Margaret D., Fryar Cheryl D., and Ogden Cynthia L.. 2020. “Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018.” NCHS Data Brief, no. 360 (February): 1–8. [PubMed] [Google Scholar]
- 54.He Yan, Wu Wei, Zheng Hui-Min, Li Pan, Daniel McDonald Hua-Fang Sheng, Chen Mu-Xuan, et al. 2018. “Regional Variation Limits Applications of Healthy Gut Microbiome Reference Ranges and Disease Models.” Nature Medicine 24 (10): 1532–35. [DOI] [PubMed] [Google Scholar]
- 55.Bart van der Hee, and Wells Jerry M.. 2021. “Microbial Regulation of Host Physiology by Short-Chain Fatty Acids.” Trends in Microbiology 29 (8): 700–712. [DOI] [PubMed] [Google Scholar]
- 56.Henagan Tara M., Stefanska Barbara, Fang Zhide, Navard Alexandra M., Ye Jianping, Lenard Natalie R., and Devarshi Prasad P.. 2015. “Sodium Butyrate Epigenetically Modulates High-Fat Diet-Induced Skeletal Muscle Mitochondrial Adaptation, Obesity and Insulin Resistance through Nucleosome Positioning.” British Journal of Pharmacology 172 (11): 2782–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu Xiaojie, Gao Jinlong, Zhang Qianyuan, Fu Yuanqing, Li Kelei, Zhu Shankuan, and Li Duo. 2013. “Soy Fiber Improves Weight Loss and Lipid Profile in Overweight and Obese Adults: A Randomized Controlled Trial.” Molecular Nutrition & Food Research 57 (12): 2147–54. [DOI] [PubMed] [Google Scholar]
- 58.Jumpertz Reiner, Duc Son Le Peter J. Turnbaugh, Trinidad Cathy, Bogardus Clifton, Gordon Jeffrey I., and Krakoff Jonathan. 2011. “Energy-Balance Studies Reveal Associations between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans.” The American Journal of Clinical Nutrition 94 (1): 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kao Christina C., Cope Julia L., Hsu Jean W., Dwarkanath Pratibha, Karnes Jeffrey M., Luna Ruth A., Hollister Emily B., Thame Minerva M., Kurpad Anura V., and Jahoor Farook. 2015. “The Microbiome, Intestinal Function, and Arginine Metabolism of Healthy Indian Women Are Different from Those of American and Jamaican Women.” The Journal of Nutrition 146 (4): 706–13. [DOI] [PubMed] [Google Scholar]
- 60.Karlsson Fredrik H., Frida Fåk Intawat Nookaew, Tremaroli Valentina, Fagerberg Björn, Petranovic Dina, Bäckhed Fredrik, and Nielsen Jens. 2012. “Symptomatic Atherosclerosis Is Associated with an Altered Gut Metagenome.” Nature Communications 3: 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kembel Steven W., Cowan Peter D., Helmus Matthew R., Cornwell William K., Morlon Helene, Ackerly David D., Blomberg Simon P., and Webb Campbell O.. 2010. “Picante: R Tools for Integrating Phylogenies and Ecology.” Bioinformatics 26 (11): 1463–64. [DOI] [PubMed] [Google Scholar]
- 62.Kim Mi-Hyun, Kyung Eun Yun Jimin Kim, Park Eunkyo, Chang Yoosoo, Ryu Seungho, Kim Hyung-Lae, and Kim Han-Na. 2020. “Gut Microbiota and Metabolic Health among Overweight and Obese Individuals.” Scientific Reports 10 (1): 19417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Sun-Young, Park Eunkyo, Lim Weon-Jeong, Soo In Kim Sang Won Jeon, Chang Yoosoo, Ryu Seungho, Kim Hyung-Lae, and Kim Han-Na. 2022. “Association Between Gut Microbiota and Depressive Symptoms: A Cross-Sectional Population-Based Study in South Korea.” Psychosomatic Medicine 84 (7): 757–65. [DOI] [PubMed] [Google Scholar]
- 64.Koh Ara, Filipe De Vadder Petia Kovatcheva-Datchary, and Bäckhed Fredrik. 2016. “From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites.” Cell 165 (6): 1332–45. [DOI] [PubMed] [Google Scholar]
- 65.Kolodziejczyk Aleksandra A., Zheng Danping, and Elinav Eran. 2019. “Diet-Microbiota Interactions and Personalized Nutrition.” Nature Reviews. Microbiology 17 (12): 742–53. [DOI] [PubMed] [Google Scholar]
- 66.Konikoff Tom, and Gophna Uri. 2016. “Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota.” Trends in Microbiology 24 (7): 523–24. [DOI] [PubMed] [Google Scholar]
- 67.Chatelier Le, Emmanuelle Trine Nielsen, Qin Junjie, Prifti Edi, Hildebrand Falk, Falony Gwen, Almeida Mathieu, et al. 2013. “Richness of Human Gut Microbiome Correlates with Metabolic Markers.” Nature 500 (7464): 541–46. [DOI] [PubMed] [Google Scholar]
- 68.Leone Vanessa, Gibbons Sean M., Martinez Kristina, Hutchison Alan L., Huang Edmond Y., Cham CandaceM., Pierre Joseph F., et al. 2015. “Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism.” Cell Host & Microbe 17 (5): 681–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewandowski Cutler T., Md Wasim Khan Manel BenAissa, Dubrovskyi Oleksii, Martha Ackerman-Berrier Mary Jo LaDu, Layden Brian T., and Thatcher Gregory R. J.. 2021. “Metabolomic Analysis of a Selective ABCA1 Inducer in Obesogenic Challenge Provides a Rationale for Therapeutic Development.” EBioMedicine 66 (April): 103287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ley Ruth E. 2010. “Obesity and the Human Microbiome.” Current Opinion in Gastroenterology 26 (1): 5–11. [DOI] [PubMed] [Google Scholar]
- 71.Ley Ruth E., Fredrik Bäckhed Peter Turnbaugh, Lozupone Catherine A., Knight Robin D., and Gordon Jeffrey I.. 2005. “Obesity Alters Gut Microbial Ecology.” Proceedings of the National Academy of Sciences of the United States of America 102 (31): 11070–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ley Ruth E., Turnbaugh Peter J., Klein Samuel, and Gordon Jeffrey I.. 2006. “Microbial Ecology: Human Gut Microbes Associated with Obesity.” Nature 444 (7122): 1022–23. [DOI] [PubMed] [Google Scholar]
- 73.Lin Hua V., Frassetto Andrea, Kowalik Edward J. Jr, Nawrocki Andrea R., Lu Mofei M., Kosinski Jennifer R., Hubert James A., et al. 2012. “Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms.” PloS One 7(4): e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Huang, and Peddada Shyamal Das. 2020. “Analysis of Compositions of Microbiomes with Bias Correction.” Nature Communications 11 (1): 3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lippert K., Kedenko L., Antonielli L., Kedenko I., Gemeier C., Leitner M., Kautzky-Willer A., Paulweber B., and Hackl E.. 2017. “Gut Microbiota Dysbiosis Associated with Glucose Metabolism Disorders and the Metabolic Syndrome in Older Adults.” Beneficial Microbes 8 (4): 545–56. [DOI] [PubMed] [Google Scholar]
- 76.Liu Xiaoxia, Zhao Ke, Jing Nana, Kong Qingjun, and Yang Xingbin. 2021. “Epigallocatechin Gallate(EGCG) Promotes the Immune Function of Ileum in High Fat Diet Fed Mice by Regulating Gut Microbiome Profiling and Immunoglobulin Production.” Frontiers in Nutrition 8 (September): 720439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lozupone Catherine, and Knight Rob. 2005. “UniFrac: A New Phylogenetic Method for Comparing Microbial Communities.” Applied and Environmental Microbiology 71 (12): 8228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu Yuanyuan, Fan Chaonan, Li Ping, Lu Yanfei, Chang Xuelian, and Qi Kemin. 2016. “Short ChainFatty Acids Prevent High-Fat-Diet-Induced Obesity in Mice by Regulating G Protein-Coupled Receptors and Gut Microbiota.” Scientific Reports 6 (November): 37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luke Amy, Bovet Pascal, Forrester Terrence E., Lambert Estelle V., Jacob Plange-Rhule Dale A. Schoeller, Dugas Lara R., et al. 2011. “Protocol for the Modeling the Epidemiologic Transition Study: A Longitudinal Observational Study of Energy Balance and Change in Body Weight, Diabetes and Cardiovascular Disease Risk.” BMC Public Health 11 (December): 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mancabelli Leonardo, Milani Christian, Gabriele Andrea Lugli Francesca Turroni, Ferrario Chiara, van Sinderen Douwe, and Ventura Marco. 2017. “Meta-Analysis of the Human Gut Microbiome from Urbanized and Pre-Agricultural Populations.” Environmental Microbiology 19 (4): 1379–90. [DOI] [PubMed] [Google Scholar]
- 81.Marchesi Julian R., Adams David H., Fava Francesca, Hermes Gerben D. A., Hirschfield Gideon M., Hold Georgina, Quraishi Mohammed Nabil, et al. 2016. “The Gut Microbiota and Host Health: A New Clinical Frontier.” Gut 65 (2): 330–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McLaren Michael R. 2020. Silva SSU Taxonomic Training Data Formatted for DADA2 (Silva Version 138). 10.5281/zenodo.3731176. [DOI] [Google Scholar]
- 83.McMurdie Paul J., and Holmes Susan. 2013. “Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data.” PloS One 8 (4): e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meehan Conor J., and Beiko Robert G.. 2014. “A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria.” Genome Biology and Evolution 6 (3): 703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta Supal, Lara Ruth Dugas Candice Choo-Kang, Bovet Pascal, Forrester Terrence, Kweku Bedu-Addo Estelle Vicki Lambert, et al. 2021. “Consumption of Monounsaturated Fatty Acids Is Associated with Improved Cardiometabolic Outcomes in Four African-Origin Populations Spanning the Epidemiologic Transition.” Nutrients 13 (7). 10.3390/nu13072442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mirarab S., Nguyen N., and Warnow T.. 2012. “SEPP: SATé-Enabled Phylogenetic Placement.” Pacific Symposium on Biocomputing. Pacific Symposium on Biocomputing, 247–58. [DOI] [PubMed] [Google Scholar]
- 87.Moreau N. M., Goupry S. M., Antignac J. P., Monteau F. J., Le Bizec B. J., Champ M. M., Martin L. J., and Dumon H. J.. 2003. “Simultaneous Measurement of Plasma Concentrations and 13C-Enrichment of Short-Chain Fatty Acids, Lactic Acid and Ketone Bodies by Gas Chromatography Coupled to Mass Spectrometry.” Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 784 (2): 395–403. [DOI] [PubMed] [Google Scholar]
- 88.Morotomi Masami, Nagai Fumiko, and Watanabe Yohei. 2012. “Description of Christensenella Minuta Gen. Nov., Sp. Nov., Isolated from Human Faeces, Which Forms a Distinct Branch in the Order Clostridiales, and Proposal of Christensenellaceae Fam. Nov.” International Journal of Systematic and Evolutionary Microbiology 62 (Pt 1): 144–49. [DOI] [PubMed] [Google Scholar]
- 89.“National Diabetes Statistics Report.” 2022. June 29, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html.
- 90.Navarro Guadalupe, Sharma Anukriti, Dugas Lara R., Forrester Terrence, Gilbert Jack A., and Layden Brian T.. 2018. “Gut Microbial Features Can Predict Host Phenotype Response to Protein Deficiency.” Physiological Reports 6 (23): e13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newton Gerald L., Buchmeier Nancy, and Fahey Robert C.. 2008. “Biosynthesis and Functions of Mycothiol, the Unique Protective Thiol of Actinobacteria.” Microbiology and Molecular Biology Reviews: MMBR 72 (3): 471–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nooromid Michael, Chen Edmund B., Xiong Liqun, Shapiro Katherine, Jiang Qun, Demsas Falen, Eskandari Maeve, et al. 2020. “Microbe-Derived Butyrate and Its Receptor, Free Fatty Acid Receptor 3, But Not Free Fatty Acid Receptor 2, Mitigate Neointimal Hyperplasia Susceptibility After Arterial Injury.” Journal of the American Heart Association 9 (13): e016235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nordmo Morten, Danielsen Yngvild Sørebø, and Nordmo Magnus. 2020. “The Challenge of Keeping It off, a Descriptive Systematic Review of High-Quality, Follow-up Studies of Obesity Treatments.” Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 21 (1): e12949. [DOI] [PubMed] [Google Scholar]
- 94.“Obesity and Overweight.” n.d. Accessed February 25, 2023. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 95.Oksanen J., Blanchet F. G., Kindt R., Legendre P., and Wagner H.. 2013. “Vegan: Community Ecology Package. R Package Version. 2.0–10,” January. [Google Scholar]
- 96.Pasolli Edoardo, Asnicar Francesco, Manara Serena, Zolfo Moreno, Karcher Nicolai, FedericaArmanini Francesco Beghini, et al. 2019. “Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle.” Cell 176 (3): 649–662.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peters Brandilyn A., Shapiro Jean A., Church Timothy R., Miller George, Chau Trinh-Shevrin Elizabeth Yuen, Friedlander Charles, Hayes Richard B., and Ahn Jiyoung. 2018. “A Taxonomic Signature of Obesity in a Large Study of American Adults.” Scientific Reports 8 (1): 9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qin Junjie, Li Yingrui, Cai Zhiming, Li Shenghui, Zhu Jianfeng, Zhang Fan, Liang Suisha, et al. 2012. “A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes.” Nature 490 (7418): 55–60. [DOI] [PubMed] [Google Scholar]
- 99.Rahat-Rozenbloom S., Fernandes J., Gloor G. B., and Wolever T. M. S.. 2014. “Evidence for Greater Production of Colonic Short-Chain Fatty Acids in Overweight than Lean Humans.” International Journal of Obesity 38 (12): 1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rampelli Simone, Schnorr Stephanie L., Consolandi Clarissa, Turroni Silvia, Severgnini Marco, CleliaPeano Patrizia Brigidi, Crittenden Alyssa N., Henry Amanda G., and Candela Marco. 2015. “Metagenome Sequencing of the Hadza Hunter-Gatherer Gut Microbiota.” Current Biology: CB 25(13): 1682–93. [DOI] [PubMed] [Google Scholar]
- 101.Reiman Derek, Layden Brian T., and Dai Yang. 2021. “MiMeNet: Exploring Microbiome-Metabolome Relationships Using Neural Networks.” PLoS Computational Biology 17 (5): e1009021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reingruber Hernán, and Pontel Lucas Blas. 2018. “Formaldehyde Metabolism and Its Impact on Human Health.” Current Opinion in Toxicology 9 (June): 28–34. [Google Scholar]
- 103.Richardson A. J., Calder A. G., Stewart C. S., and Smith A.. 1989. “Simultaneous Determination of Volatile and Non-volatile Acidic Fermentation Products of Anaerobes by Capillary Gas Chromatography.” Letters in Applied Microbiology 9 (1): 5–8. [Google Scholar]
- 104.Ridaura Vanessa K., Faith Jeremiah J., Rey Federico E., Cheng Jiye, Duncan Alexis E., Kau Andrew L.,Griffin Nicholas W., et al. 2013. “Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice.” Science 341 (6150): 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riva Alessandra, Borgo Francesca, Lassandro Carlotta, Verduci Elvira, Morace Giulia, Borghi Elisa, and Berry David. 2017. “Pediatric Obesity Is Associated with an Altered Gut Microbiota and Discordant Shifts in Firmicutes Populations.” Environmental Microbiology 19 (1): 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roth Gregory A., Mensah George A., Johnson Catherine O., Addolorato Giovanni, Ammirati Enrico,Baddour Larry M., Barengo Noël C., et al. 2020. “Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study.” Journal of the American College of Cardiology 76 (25): 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanna Serena, van Zuydam Natalie R., Mahajan Anubha, Kurilshikov Alexander, Vich Vila Arnau, Võsa Urmo, Mujagic Zlatan, et al. 2019. “Causal Relationships among the Gut Microbiome, Short-Chain Fatty Acids and Metabolic Diseases.” Nature Genetics 51 (4): 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schnorr Stephanie L., Candela Marco, Rampelli Simone, Centanni Manuela, Consolandi Clarissa,Basaglia Giulia, Turroni Silvia, et al. 2014. “Gut Microbiome of the Hadza Hunter-Gatherers.” Nature Communications 5 (April): 3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwiertz Andreas, Taras David, Klaus Schäfer Silvia Beijer, Bos Nicolaas A., Donus Christiane, and Hardt Philip D.. 2010. “Microbiota and SCFA in Lean and Overweight Healthy Subjects.” Obesity 18(1): 190–95. [DOI] [PubMed] [Google Scholar]
- 110.Sonnenburg Erica D., and Sonnenburg Justin L.. 2019. “The Ancestral and Industrialized Gut Microbiota and Implications for Human Health.” Nature Reviews. Microbiology 17 (6): 383–90. [DOI] [PubMed] [Google Scholar]
- 111.Sonnenburg Justin L., and Bäckhed Fredrik. 2016. “Diet-Microbiota Interactions as Moderators of Human Metabolism.” Nature 535 (7610): 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stanislawski Maggie A., Dabelea Dana, Wagner Brandie D., Sontag Marci K., Lozupone Catherine A., and Eggesbø Merete. 2017. “Pre-Pregnancy Weight, Gestational Weight Gain, and the Gut Microbiota of Mothers and Their Infants.” Microbiome 5 (1): 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Teixeira Tatiana F. S., Łukasz Grześkowiak Sylvia C. C. Franceschini, Bressan Josefina, Ferreira Célia L. L. F., and Peluzio Maria C. G.. 2013. “Higher Level of Faecal SCFA in Women Correlates with Metabolic Syndrome Risk Factors.” The British Journal of Nutrition 109 (5): 914–19. [DOI] [PubMed] [Google Scholar]
- 114.Thompson L. R., Sanders J. G., McDonald D., and Amir A.. 2017. “A Communal Catalogue RevealsEarth’s Multiscale Microbial Diversity.” Nature. https://www.nature.com/articles/nature24621?report=reader. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tseng Ching-Hung, and Wu Chun-Ying. 2019. “The Gut Microbiome in Obesity.” Journal of the Formosan Medical Association = Taiwan Yi Zhi 118 Suppl 1 (March): S3–9. [DOI] [PubMed] [Google Scholar]
- 116.Turnbaugh Peter J., Fredrik Bäckhed Lucinda Fulton, and Gordon Jeffrey I.. 2008. “Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome.” Cell Host & Microbe 3 (4): 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turnbaugh Peter J., Hamady Micah, Yatsunenko Tanya, Cantarel Brandi L., Duncan Alexis, Ley Ruth E., Sogin Mitchell L., et al. 2009. “A Core Gut Microbiome in Obese and Lean Twins.” Nature 457 (7228): 480–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turnbaugh Peter J., Ley Ruth E., Mahowald Michael A., Magrini Vincent, Mardis Elaine R., and Gordon Jeffrey I.. 2006. “An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest.” Nature 444 (7122): 1027–31. [DOI] [PubMed] [Google Scholar]
- 119.Valdes Ana M., Walter Jens, Segal Eran, and Spector Tim D.. 2018. “Role of the Gut Microbiota in Nutrition and Health.” BMJ 361 (June): k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vinolo Marco A. R., Rodrigues Hosana G., Nachbar Renato T., and Curi Rui. 2011. “Regulation of Inflammation by Short Chain Fatty Acids.” Nutrients 3 (10): 858–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vrieze Anne, Els Van Nood Frits Holleman, Jarkko Salojärvi Ruud S. Kootte, Bartelsman Joep F. W. M., Dallinga-Thie Geesje M., et al. 2012. “Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome.” Gastroenterology 143 (4): 913–6.e7. [DOI] [PubMed] [Google Scholar]
- 122.Walters William, Hyde Embriette R., Donna Berg-Lyons Gail Ackermann, Humphrey Greg, Parada Alma, Gilbert Jack A., et al. 2016. “Improved Bacterial 16S RRNA Gene (V4 and V4–5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys.” MSystems 1(1). 10.1128/mSystems.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Gary D., Chen Jun, Hoffmann Christian, Bittinger Kyle, Chen Ying-Yu, Keilbaugh Sue A., Bewtra Meenakshi, et al. 2011. “Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes.” Science 334 (6052): 105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yan Hang, Qin Qian, Chen Jengfeng, Yan Su, Li Tiantian, Gao Xinxin, Yang Yang, Li Ang, and Ding Suying. 2021. “Gut Microbiome Alterations in Patients With Visceral Obesity Based on Quantitative Computed Tomography.” Frontiers in Cellular and Infection Microbiology 11: 823262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Teng, Tedersoo Leho, Soltis Pamela S., Soltis Douglas E., Sun Miao, Ma Yuying, Ni Yingying, et al. 2022. “Plant and Fungal Species Interactions Differ between Aboveground and Belowground Habitats in Mountain Forests of Eastern China.” Science China. Life Sciences, December. 10.1007/s11427-022-2174-3. [DOI] [PubMed] [Google Scholar]
- 126.Yatsunenko Tanya, Rey Federico E., Manary Mark J., Trehan Indi, Maria Gloria Dominguez-Bello Monica Contreras, Magris Magda, et al. 2012. “Human Gut Microbiome Viewed across Age and Geography.” Nature 486 (7402): 222–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Qiang, Zou Rong, Guo Min, Duan Mengmeng, Li Quan, and Zheng Huajun. 2021.“Comparison of Gut Microbiota between Adults with Autism Spectrum Disorder and Obese Adults.” PeerJ 9 (March): e10946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao Liping. 2013. “The Gut Microbiota and Obesity: From Correlation to Causality.” Nature Reviews. Microbiology 11 (9): 639–47. [DOI] [PubMed] [Google Scholar]
- 129.Zhernakova Alexandra, Kurilshikov Alexander, Marc Jan Bonder Ettje F. Tigchelaar, Schirmer Melanie, Vatanen Tommi, Mujagic Zlatan, et al. 2016. “Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity.” Science 352 (6285): 565–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All 16S rRNA gene sequence data are publicly available via the QIITA platform (https://qiita.ucsd.edu) under the study identifier (ID=13512) and will soon be deposited on the European Bioinformatics Institute (EBI) site. The SILVA 16 S rRNA database used for alignment is available at https://data.qiime2.org/2022.2/common/silva-138-99-515-806-nb-classifier.qza. The data and analyses generated in this study are available within the paper, Supplementary Information and Source data files provided with this paper.