Abstract
The IFN-γ/STAT1 immune signaling pathway impacts many homeostatic and pathological aspects of neurons, beyond its canonical role in controlling intracellular pathogens. Well known for its potent pro-inflammatory and anti-viral functions in the periphery, the IFN-γ/STAT1 pathway is rapidly activated then deactivated to prevent excessive inflammation; however, neurons utilize unique IFN-γ/STAT1 activation patterns, which may contribute to the non-canonical neuron-specific downstream effects. Though it is now well-established that the immune system interacts and supports the CNS in health and disease, many aspects regarding IFN-γ production in the CNS and how neurons respond to IFN-γ are unclear. Additionally, it is not well understood how the diversity of the IFN-γ/STAT1 pathway is regulated in neurons to control homeostatic functions, support immune surveillance, and prevent pathologies. In this review, we discuss the neuron-specific mechanisms and kinetics of IFN-γ/STAT1 activation, the potential sources and entry sites of IFN-γ in the CNS, and the diverse set of homeostatic and pathological effects IFN-γ/STAT1 signaling in neurons has on CNS health and disease. We will also highlight the different contexts and conditions under which IFN-γ-induced STAT1 activation has been studied in neurons, and how various factors might contribute to the vast array of downstream effects observed.
Keywords: cerebral spinal fluid, interferon-gamma, meninges, neurons, STAT1, T cells
1 ∣. INTRODUCTION
Cytokines have long been appreciated for their roles in immunity, but also exert many critical non-immune effects in the central nervous system (CNS), including influencing behaviors and maintaining CNS homeostasis.1-15 Because of their traditional role as immune mediators, much work has focused on cytokine signaling on immune cells in the CNS, like microglia, but cytokines can act directly on neurons and are required for important neuron-intrinsic functions. Because of the unique separation and protection of the CNS from the periphery, little is known about how cytokines are able to enter the CNS parenchyma and how they signal to neurons. Recent studies have identified unique pathways in which the immune system can interact with and support the CNS under physiological conditions.9,16-18 This discovery of immunological niches around the CNS and the glymphatic system has provided new insight into cytokine signaling on neurons, particularly the IFN-γ/STAT1 signaling pathway.
IFN-γ, the sole type II interferon, is a pro-inflammatory cytokine predominately produced by cells in the adaptive arm of the immune system,19 and is capable of stimulating neurons which express IFN-γ receptors (IFNGR).4,20-22 STAT1 is a transcription factor that facilitates IFN-γ-induced immune responses19 and has been gaining recognition for its non-canonical roles in both CNS homeostasis and pathology. The IFNGR is a heterodimer composed of two IFNGR1 and two IFNGR2 subunits. IFN-γ binds IFNGR1, causing a conformational change in IFNGR2 and autophosphorylation of Janus Kinase 2 (JAK2). JAK2 phosphorylates Janus Kinase 1 (JAK1), which phosphorylates IFNGR1. STAT1 binds IFNGR1 and is phosphorylated, likely by JAK2. Upon activation, STAT1 dimerizes and traffics to the nucleus, where phosphorylated STAT1 (pSTAT1) homodimers bind to chromatin at IFN-γ activation sites (GAS) and facilitate transcription of canonical pro-inflammatory, anti-viral, and anti-tumor IFN-γ stimulated genes (ISGs).19,23 In immune and non-immune cells in the periphery, this pathway is typically activated and deactivated quickly, in order to swiftly fight off the pathogen, without causing unnecessary and harmful inflammation. In neurons, however, current data suggest IFN-γ/STAT1 signaling does not strictly adhere to these classical signaling parameters, with muted but extended STAT1 signaling, as well as IFN-γ/STAT1-induced non-cytolytic viral clearance.24-26 This may be because neurons have unique constraints to work under, such as their irreplaceable nature and unique morphology.
IFN-γ production is greatest in the context of infection; however, it is also found at low physiological levels in the CNS in the absence of infections.27,28 Because the CNS is protected by the blood–brain barrier (BBB), it is unclear how IFN-γ enters the CNS and is able to directly stimulate neurons within the parenchyma. The glymphatic system and specialized immune compartments at the borders of the CNS offer promising potential as sources and sites of IFN-γ production and entry into the CNS, but to date there has been little work investigating the movement of cytokines in and out of the CNS.
Along with its role in controlling neurotropic infections, IFN-γ/STAT1 signaling also has homeostatic roles, including regulating proliferation and differentiation of neuronal stem cells (NSCs) and maintaining proper neuron excitability. Conversely, extraneous or aberrant IFN-γ/STAT1 signaling in neurons has been identified as a major contributing factor in neuronal pathology, including abnormal neural activity and gene expression impacting neurodevelopmental and neurodegenerative disorders. The underlying IFN-γ/STAT1 signaling mechanisms which lead to such drastically different outcomes in neurons are unknown, but they may be affected by the heterogeneous nature of neurons, as well as their complex morphology, and timing and strength of signaling.
While there has been increasing interest and work done in this area, there are not well-established and generally agreed upon working models that are consistently used across studies and groups. This has resulted in many studies using different neuronal cell types, varying by source and age, as well as different IFN-γ concentrations and timing, which make it difficult to interpret individual findings in the larger context. IFN-γ/STAT1 signaling has diverse outcomes in neurons, and it is important to consider many variables to reconcile seemingly conflicting findings. In this review, we will highlight different experimental variables, especially IFN-γ concentrations used in vitro, in works investigating IFN-γ/STAT1 signaling in neurons and will discuss how they contribute to a diverse array of outcomes in different conditions and contexts.
Overall, it has become clear that differential IFN-γ/STAT1 activation and signaling in neurons results in varying downstream effects, which likely depend on the context under which activation occurs. The emergence of non-canonical STAT1 activation and signaling in neurons warrants a comprehensive understanding of the underlying mechanisms leading to the different outcomes. It is crucial to identify the similarities and differences between IFN-γ/STAT1 activation in neurons in infection, homeostasis, and pathology to understand how the pathway functions and contributes to each context, and to identify and target steps in the pathway which may have therapeutic potential. This review will discuss IFN-γ/STAT1 signaling in neurons under various conditions, and how it contributes to CNS homeostasis and pathology. Our overarching goal is to uncover unifying patterns and models, and to better elucidate underlying mechanisms of IFN-γ/STAT1 signaling in neurons at steady state and in disease states.
2 ∣. NEURONAL STAT1 SIGNALING
The IFN-γ/STAT1 signaling pathway has been well characterized in many cell types. Once engaged with IFNGR, IFN-γ induces a signal transduction cascade that ultimately leads to STAT1 phosphorylation and translocation to the nucleus.19 In the nucleus, STAT1 classically upregulates genes to promote an anti-viral response or tumor surveillance.19 Despite the unique structure and cellularity between the CNS and the periphery, IFN-γ is required for control of viral infection in neurons29-34; however, in neurons the IFN-γ/STAT1 response regulates many aspects beyond controlling intracellular pathogens. Though the downstream signal transduction and kinetics have been well characterized in immune and peripheral cell types, little is known about the IFN-γ/STAT1 pathway in neurons. Relatively speaking, neural cells and their networks are more complex than many cell types in which STAT1 has been heavily studied, such as fibroblasts and immune cells. Structurally, neurons have intricate and extensive networks of processes, and range widely in size. Additionally, neurons are a highly heterogeneous population, with different neuronal subsets which both look and function very differently from each other. For example, the soma of a retinal bipolar cell is approximately 10μM in diameter, but its axon can extend at least 4–5 times that length.35 Retinal bipolar cells use graded potentials to pass signals from photoreceptors to retinal ganglion cells.36 On the contrary, Purkinje cells display enormous complexity and an intricate pattern work of dendrites, with the dendritic field reaching over 150μM in width.37 They receive inputs from hundreds of thousands of cells and inhibit excitatory neurons in the spinal cord to fine tune motor memory.38 In comparison, the diameter of a single T cell is approximately 5–7 μM and a dendritic cell is 10–15 μM.39 While we know many different neuronal subtypes can respond to IFN-γ, it is unclear whether each subtype responds in the same manner. STAT1 expression in the brain is highest in neurons in the olfactory bulb, hippocampus, basal ganglia, and granule and Purkinje neuron layers in the cerebellum.40 Some studies have reported site-specific IFN-γ-mediated viral clearance in the CNS, with the most substantial differences between spinal cord, brainstem, cerebellum, and the rest of the cortex.30 Mature neurons are relatively immobile within the brain parenchyma, and there are limited sources in which neurons may directly access IFN-γ (discussed below). Because of the unique morphology and size of neurons, a single neuron may span across different brain regions with varying degrees of access to IFN-γ in the CNS. This could confer specificity to the neural circuits responding to IFN-γ. Presumably, differential expression of the IFNGR, either on different neuronal populations or even at different subcellular sites, could further dictate how neurons access IFN-γ. Studies have shown that IFNGR are expressed on soma, dendrites, and synapses.21,22,41 Others have demonstrated that axons in the peripheral nervous system (PNS) treated with IFN-γ induced STAT1 translocation to the nucleus, suggesting that IFNGR are also present on axons.42 It is unclear if activating the IFN-γ/STAT1 pathway at different subcellular locations (i.e., on soma, axons, or dendrites) has different kinetics or downstream consequences. Considering the drastic distances different neuronal compartments are from the nucleus, activated STAT1 would need to travel much further if phosphorylated by JAKs bound to IFNGR on the axodendritic processes compared with IFNGR on the soma. We speculate that the subcellular location of IFN-γ/STAT1 activation in neurons may affect downstream signaling and function, though little work has been done to investigate this.
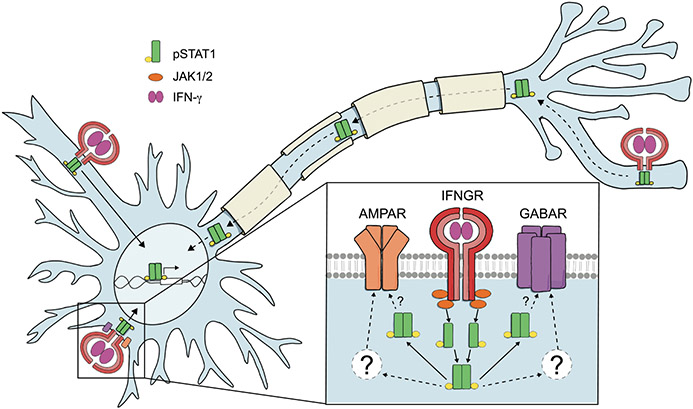
Recent reviews have pointed out that IFN-γ/STAT1 signaling is far more complex than the canonical pathway gives it credit for.43-45 For instance, IFN-γ can bind the intracellular region of IFNGR146 and has intracellular functions,47-49 but this “non-canonical” function has largely been underappreciated. Additionally, the common view that STAT1 is phosphorylated at the cell membrane and then disassociates from the IFNGR and translocates to the nucleus on its own is likely a vast simplification. IFN-γ, IFNGR1, JAK1 and JAK250-52 have all been observed in the nucleus and associated with GAS sites after IFN-γ treatment, suggesting all of these components may traffic to the nucleus together as a single complex.43 Additionally, STAT1 has been reported to have cytoplasmic non-transcriptional functions as well, in which it can act as a regulator of other signaling pathways by directly binding other cytoplasmic proteins.45,53-56 Non-transcriptional functions for STAT1 that could potentially occur at the synapse are an attractive model to contemplate, given the large and complex structure of neurons, along with their extremely quick responses to IFN-γ/STAT1, especially in the context of regulating neural activity (discussed further below). Interestingly, treating axons with IFN-β, a type-I IFN that also activates STAT1, resulted in local STAT1-mediated anti-viral effects within the axon, but did not result in nuclear translocation of pSTAT1 and was not inhibited by blocking transcription,42 suggesting STAT1 may have local transcription-independent roles restricted to neuronal processes. Though these non-canonical IFN-γ/STAT1 signaling pathways have not been well studied in neurons, we suspect that these and other neuron-specific non-canonical IFN-γ/STAT1 signaling pathways that have not been uncovered yet may contribute to the neuron-specific outcomes of IFN-γ/STAT1 (Figure 1). Here, we will review what is currently known about the activation and downstream steps of IFN-γ/STAT1 signaling in neurons.
FIGURE 1.
Potential IFN-γ/STAT1 Signaling Mechanisms in Neurons. The exact mechanisms of IFN-γ/STAT1 signaling in neurons are unknown. In canonical IFN-γ/STAT1 signaling, IFN-γ binds the IFNGR, causing JAK1/2 to recruit and phosphorylate STAT1. STAT1 then disassociates from the IFNGR complex and translocates to the nucleus as a homodimer, where it can regulate transcription of ISGs. Neurons do upregulate ISGs in response to IFN-γ in a STAT1-dependent manner. However, the timing and kinetics of STAT1 activation differ from other cells, suggesting neurons may utilize non-canonical signaling mechanisms. The existence of non-canonical IFN-γ/STAT1 has been understudied, especially in neurons. Non-canonical signaling includes intracellular roles for IFN-γ, translocation of the entire IFN-γ/IFNGR1/JAK1/2/STAT1 complex to the nucleus, and non-transcriptional roles for STAT1 in the cytoplasm. STAT1 activation in neurons is delayed and extended, but it is unclear how or why this occurs. Though it has not been studied, we speculate that activation of the IFN-γ/STAT1 pathway in neurons at different subcellular locations may result in different outcomes, especially if activation occurs at a distal site such as on axons or dendrites versus on the soma. IFN-γ/STAT1 signaling also regulates neural activity, which occurs on the timescale of minutes, and JAK2/STAT1 activation regulates the elimination of specifically inactive axons and synapses. In these scenarios, we speculate that IFN-γ/STAT1 may utilize non-canonical mechanisms, which may allow for rapid and site-specific action. Non-transcriptional roles for cytoplasmic STAT1 could facilitate local effects at the synapse via direct interaction of STAT1 with neurotransmitter receptors, like GABAR and AMPAR, and/or other intermediates like PKC which have been implicated in IFN-γ-regulated neural activity
Perhaps unsurprisingly, the basic timing and kinetics of neuronal IFN-γ/STAT1 signaling are fundamentally different to that of other cell types. Rose et al. demonstrated that primary hippocampal neurons had a lower baseline level of STAT1 compared with mouse embryonic fibroblasts (MEFs). Continuous treatment with IFN-γ (100 U/mL) induced muted and delayed STAT1 activation in neurons compared with MEFs, with MEF pSTAT1 levels peaking within 30 min of treatment, and neuronal pSTAT1 not peaking until at least 24 h of treatment. After pulse treatment (30 min, then washout) of IFN-γ (100 U/mL), STAT1 activation in neurons continued to increase up to 48 h after IFN-γ removal, whereas in MEFs, pSTAT1 began to decrease by 3 hours post removal and reached near baseline levels by 48 h.25 This pattern of extended STAT1 activation was also observed in neural stem/progenitor cells (NSPCs) treated with IFN-γ (100 U/mL).57 Building on this work, Podolsky et al. demonstrated that pulse treatment with IFN-γ (100 U/mL) also caused extended activation of JAK1 and JAK2, the upstream kinases that phosphorylate STAT1, in neurons compared with MEFs. After pulsing with IFN-γ and then treating with JAK inhibitors, STAT1 dephosphorylation was delayed in neurons compared with MEFs. In MEFs, pSTAT1 levels returned to baseline by 24 h after JAK inhibition, whereas in neurons, pSTAT1 levels remained well above baseline for at least 48 h.24 Overall, neurons have delayed and extended STAT1 activation compared with non-neural cells. Factors that drive this unique STAT1 activation pattern in neurons are unclear but may include neuronal morphology and differential expression and/or function of regulators of STAT1 in neurons, though there has not been much work investigating these possibilities. IFN-γ/STAT1 signaling is regulated by many pathways, including a family of proteins called suppressor of cytokine signaling (SOCS) which negatively regulate JAK activity and protein tyrosine phosphatases (PTP) which dephosphorylate JAK and STAT1.58 SOCS1 and SOCS3, which are STAT1-regulated ISGs, are expressed at low levels at baseline, with expression increasing quickly after IFN-γ signaling.58 Interestingly, Rose et al. demonstrated that IFN-γ-induced expression of SOCS1 and SOCS3 was much lower in neurons compared with MEFs, with SOCS3 expression never rising above baseline.25 These data suggest neurons also have muted expression of JAK/STAT1 regulators, which may contribute to the extended STAT1 activation pattern observed. Regardless of the underlying mechanisms that perpetuate neuron-specific STAT1 activation, it is likely that this unique activation pattern contributes to the non-canonical downstream effects of IFN-γ/STAT1 signaling in neurons.
In most cell types, STAT1 activation is quickly attenuated, as this pathway could become pathological if not tightly regulated. For example, individuals with gain-of-function mutations in STAT1 often suffer from chronic mucocutaneous candidiasis, as well as autoimmune disorders including hyperthyroidism, type 1 diabetes, and systemic lupus erythematosus.59,60 Interestingly, patients also reported various neurological symptoms, including epilepsy, attention lapses, and multiple sclerosis. Patients were also diagnosed with cognitive disability at a slightly higher rate than the general population.60 This makes it even more intriguing that Rose et al. and Podolsky et al. observed extended STAT1 phosphorylation and delayed dephosphorylation in neurons after treatment with high (non-physiological) concentrations of IFN-γ (100 U/mL). It is unclear if this is a physiological activation pattern or if it mimics a gain-of-function like phenotype, and it is unclear whether the downstream effects of this extended STAT1 activation pattern are pathological. Perhaps the low baseline level of STAT1 observed in neurons prevents overly pathological consequences. Indeed, Rose et al. observed muted downstream STAT1-induced ISG expression in neurons compared with MEFs.25 But with a muted STAT1 response to protect neurons from inflammation induced death, how are they able to clear infections? Burdeinick-Kerr et al. demonstrated that rat neuronal cell lines (derived from either substantia nigra or olfactory bulb neurons), as well as primary neurons (derived from dorsal root ganglia) infected with Sindbis virus (SINV) were able to clear the infection in a non-cytolytic manner after IFN-γ (500 U/mL) treatment, as measured by decreased viral replication and increased cell viability.29 This work also demonstrated a similar IFN-γ-induced STAT1 activation pattern as observed in Rose et al..25 Treating cells with a JAK inhibitor reduced viral clearance, suggesting that STAT1 signaling is required for non-cytolytic viral clearance in neurons.29 In contrast, O'Donnell et al. (2012) demonstrated that STAT1 was dispensable for viral clearance in neurons.61 Here, they used a transgenic mouse model of neuron-restricted Measles Virus (MV) infection, by expressing the MV receptor on neurons using a neuron-specific promoter (NSE). They crossed this mouse to a Stat1−/− mouse and showed that Stat1−/− primary neurons in culture were able to restrict viral antigen expression with IFN-γ (100 U/mL) pretreatment, and to a lesser extent with IFN-γ treatment after infection. Additionally, they saw that 75% of Stat1−/− mice had similar survival as wildtype mice, while 25% died quickly (4–6 days post infection (dpi), before peak T cell infiltration into CNS) and also exhibited neurological damage and lethal seizures. When they analyzed viral mRNA present in the brain, Stat1−/− mice had significantly higher viral loads than wildtype at 4 dpi but were able to reduce viral loads to wildtype levels by 11 dpi.61 It is worth noting that type I interferons (IFN-α and IFN-β) can also activate STAT1. Similar to IFN-γ, type I interferons are important in controlling viral infection, and have also been implicated in the non-cytolitic viral response in the CNS.62 However, they activate STAT1 primarily as a heterodimer with STAT2 which acts on a similar, yet distinct set of genes compared with the genes regulated by IFN-γ.63 It is important to keep this in mind when interpreting results which utilize Stat1−/− mice, as there is potential for type I interferon signaling to be disrupted, which may contribute to the phenotype described here (and elsewhere in this review). This group later demonstrated that IFN-γ also induced a survival phenotype in neurons independent of STAT1, by activating the extracellular signal-regulated protein kinase (ERK1/2) pathway. In this work, they demonstrated that IFN-γ (100 U/mL) activated ERK1/2 immediately (as early as 5 min after IFN-γ exposure) in primary hippocampal neurons, in contrast to the previously discussed delayed IFN-γ-induced STAT1 activation kinetics. IFN-γ treatment increased neuron viability in an ERK1/2 dependent manner, as inhibiting ERK1/2 decreased viability in response to IFN-γ. In Stat1−/− neurons, inhibiting ERK1/2 during IFN-γ treatment caused no change in neuron viability, suggesting ERK1/2 confers survival by counteracting STAT1-induced cell death. IFN-γ (100 and 500 U/mL) prevented staurosporine induced apoptosis by reducing caspase-3 cleavage; this protection was abrogated in neurons pretreated with ERK1/2 inhibitors.26 This study did not specify how IFN-γ activates ERK1/2; however, others have demonstrated that IFN-γ-induced ERK1/2 activation is mediated by proline-rich tyrosine kinase 2 (PYK2)64 and MAP/ERK kinase (MEK1/2), and that IFN-γ/ERK1/2 signaling increased neurite outgrowth in Paju cells (a neuroblastoma cell line).65 The discrepancies in whether or not STAT1 is required for neurotropic viral clearance could be due to differences in responses to different viruses, as well as the timing of IFN-γ treatment and analysis of in vitro experiments. Taken together, these works suggest that the early activation of the ERK1/2 pathway by IFN-γ may confer a protective phenotype which is needed for neurons to survive the subsequent pro-inflammatory and anti-viral effects of IFN-γ-induced STAT1 activation, though STAT1 may not be necessary for clearance of all viruses. Overall, activation of the IFN-γ/STAT1 activation in neurons is muted, delayed, and extended, but it is unclear how or why this occurs. Neurons are able to take advantage of the canonical immune functions of the IFN-γ/STAT1 signaling pathway, while avoiding the typical cytolytic consequences.
2.1 ∣. The impact of the IFN-γ/STAT1 pathway on neural activity
The impact IFN-γ/STAT1 signaling has on regulating neural excitability is complex (Table 1). IFN-γ activates an acute response that is presumably independent of STAT1-induced transcription, given the effects take place over minutes, and a STAT1 transcription-dependent response that impacts neuronal physiology long-term (Figure 1). The STAT1-dependent transcriptional response likely alters the acute response to IFN-γ, adding to the complexity. Other variables to consider include how different specific populations of neurons (for example, excitatory versus inhibitory neurons) respond to IFN-γ, how activating IFNGRs in different subcellular locations affects signaling (soma versus axodendritic), the strength of the response (physiological versus pathological), and how glia cells (microglia and astrocytes) contribute to the overall neural response. Below we discuss these factors as we review the impact IFN-γ/STAT1 activation has on overall neural activity.
TABLE 1.
Summary of experimental conditions investigating effects of IFN-γ on neural activity
| Category | Findings | Experimental model | Age | Species | Reported IFN-γ concentration or activity |
Timing of IFN-γ treatment |
Study |
|---|---|---|---|---|---|---|---|
| Excitatory current | acute IFN-γ: no effect; intermediate IFN-γ: increased sEPSC; long IFN-γ: decreased AMPAR clustering and sEPSC | Hippocampal cultures | Embryonic | Rat | 1000–100 KU/mL | acute: <10 m; intermediate: 48 h; long: 14 days | Vikman et al.73 |
| decreased GluR1 clusters on GAD+ neurons; increased sEPSC frequenct and amplitude; thought to be induced by disinhibition (tonic) | Spinal dorsal horn cultures | Embryonic | Rat | 1000 U/mL | 2 weeks DIV14-28 | Vikman et al.68 | |
| Inhibitory current | increased inhibitory current at DIV7 | Hippocampal cultures | Embryonic | Rat | 1000 U/mL | 5 days at DIV 3, 7, 14. analyzed at DIV30 | Brask et al.79 |
| increased sIPSC freqeuncy, not amplitude; increased tonic inhibition | Hippocampal slice—CA1 | Adult | Rat | 100 ng/mL | 3 h | Flood et al.74 | |
| Prolonged IFN-γ reduces inhibition in spinal cord, results in hypersensitivity and mechanical allodynia | Intrathecal injections into dorsal horn neurons (in vivo) | Adult | Rat | 1000 U | 4 injections over 8 days | Vikman et al.70 | |
| Failure to respond to bicuculin; ie IFN-γ caused disinhibition and reduced GABAergic tone | Intrathecal injections into dorsal horn neurons (in vivo) | Adult | Rat | 1000 U | 4 injections over 8 days | Vikman et al.69 | |
| IFNGR1 on layer V pyramidal neurons; increased amplitude sIPSC and mIPSC "augments inhibitory currents" | Slice culture - layer V pyramidal neurons | Adult | Rat | 1000 U/mL | 20 mins | Janach et al.41 | |
| increased mIPSC by increased surface GABAARg2 through PKC | Slice culture - layer V pyramidal neurons | Adult | Rat | 1000 U/mL | 30 mins | Janach et al.78 | |
| IFN-γ increased tonic inhibition | PFC Slices-Layer II/III neurons | Adult | Mouse | 20 pg/mL | 8.5 mins | Filiano et al.4 | |
| IFN-γ increased CA1 population spike only in the absence of bicuculin, suggesting IFN-γ mediates disinhibition | Hippocampal slice | Not reported | Mouse | 200 U/mL | 50 mins | Zhu et al.67 | |
| decreased freqeuncy of gamma oscilations | Hippocampal slice-CA1-CA3 | Postnatal | Rat | 100 ng/mL | 72 h | Papageorgiou et al.77 | |
| spontaneous action potential discharges | Hippocampal slice cultures - CA1 | Postnatal | Rat | 100 KU/mL | 12–60 mins | Muller et al.66 | |
| decreased threshold for spreading depolarization | Hippocampal slice | Postnatal | Rat | 500 U/mL | 24 h | Pusic et al.71 | |
| increased threshold for spreading depolarization | Hippocampal slice | Postnatal | Rat | 500 U/mL | 12 h x1 or x7 days | Pusic et al.72 |
In one of the first studies to investigate the effects of IFN-γ on neural activity, Muller et al., exposed rat hippocampal slice cultures to an extremely high level of mouse IFN-γ (up to 100 kU/mL) and measured both spontaneous and evoked responses in CA3 pyramidal neurons.66 After 10 min, most neurons produced spontaneous action potentials. This response was attributed to IFN-γ decreasing the amplitude of synaptic inhibition, thus inducing disinhibition. It was suggested that IFN-γ induces disinhibition by decreasing GABA release, although this was not directly measured. Similarly, lower concentrations of IFN-γ (200 U/mL, albeit still high) increased population spikes in CA1 within minutes, but only in the absence of the GABA receptor antagonist, bicuculine, again suggesting that IFN-γ mediates disinhibition in the hippocampus.67 IFN-γ mediated disinhibition was not specific to the hippocampus. Treating cultures of dorsal root ganglion neurons with IFN-γ (1000 U/mL) decreased the clustering of AMPA receptor subunit GluR1 on inhibitory neurons and increased the overall frequency and amplitude of spontaneous excitatory postsynaptic currents (EPSC).68 Using multiple intrathecal injections to model a chronic IFN-γ response in vivo, IFN-γ reduced GABAergic tone in the spinal cord and caused hypersensitivity to mechanical stimuli and allodynia.69,70 Although these studies suggest IFN-γ mediates disinhibition at multiple sites in the CNS, further work in the hippocampus demonstrated a more complex scenario. Exposing rat hippocampal slices to IFN-γ (500 U/mL) for 24 h decreased the threshold for inducing spreading depolarization suggesting IFN-γ may negatively impact neuronal inhibition.71 However, altering the treatment paradigm from 24 h to a phasic 12 h on, 12 h off for 7 days, completely reversed the outcome and IFN-γ then increased the threshold to induce spreading depolarization.72 These results demonstrated that a neural response to IFN-γ greatly depends on the timing, dose, and location. Unlike the Muller study above, which used hippocampal slices,66 Vikman et al., did not observe changes in spontaneous EPSCs when cultured primary hippocampal neurons were exposed to IFN-γ (1000 U/mL) for 10 min.73 It was not until the exposure was prolonged to 48 h where IFN-γ increased the frequency of spontaneous EPSCs. Although the discrepancy in timing is unclear, there is the potential for IFN-γ to activate glia in hippocampal slices unlike in pure neuronal cultures. Extending IFN-γ exposure to 4 weeks reduced AMPA clustering in cultured neurons and ultimately decreased activity, offering another example of how different variables can dramatically alter the neuronal response to IFN-γ. Using a lower concentration (100 U/mL) than the studies listed above, a 3 h exposure of IFN-γ increased the frequency of spontaneous postsynaptic currents (sIPSCs) and tonic inhibition in rat hippocampal CA1 pyramidal neurons.74 Tonic inhibition, unlike synaptic inhibition, is driven by extrasynaptic GABA receptors activated by the spill-over of GABA outside of the synaptic cleft. It is regulated by balancing GABA release and re-uptake as well as GABA receptor composition and trafficking.75 Tonic inhibition is extremely important for inhibiting neural circuits and can contribute as much as 75% of the total inhibitory conductance.76 The same concentration of IFN-γ likewise decreased gamma oscillations in hippocampal slices.77 In the cortex, IFN-γ activated layer I neurons and exposure of physiological levels (<1 U/mL) for 8 min boosted tonic inhibition in Layer II/III pyramidal cells.4 IFNGRs are also found on Layer V pyramidal neurons and exposing cortical slices to 20-min of IFN-γ (1000 U/mL) increased spontaneous and evoked IPSC.41 Unlike signaling through the canonical JAK/STAT1 pathway, IFN-γ increased inhibitory currents by increasing GABAA receptor trafficking to the surface via phosphorylation by PKC.78
IFNGRs are expressed on neurons and can be found both on the soma and axon-dendritic compartments, including at the synapse.21,22 Transiently treating primary hippocampal neurons in culture during synaptogenesis led to long-term changes with increased inhibitory currents 2 weeks after treatments.79 Whether these long-term changes in neural excitability are due to STAT1-dependent transcriptional processes are unclear; however, numerous studies suggest STAT1 can influence synapses. Lymphocytic choriomeningitis virus (LCMV) injected into the CNS of neonatal mice caused motor deficits through IFN-γ produced by CD8+ T cells.80 LCMV infects neurons and through STAT1 reduced the frequency and amplitude of IPSCs. Activation of STAT1 in neurons induced the expression and release of CCL2 to recruit microglia to eliminate synapses.81 Similar interactions were observed in autopsies of patients with Rasmussen's encephalitis where CNS infiltrating CD8+ T cells cluster in proximity to pSTAT1 and CCL2 and correlate with synaptic loss and recurrent seizures.81 STAT1 also regulates synaptic function during normal development. The JAK/STAT1 pathway is activated in inactive synapses to facilitate elimination and refinement of neural connections.82,83 Overall, the IFN-γ/STAT1 pathway plays a critical role in regulating neural activity in normal physiology and during pathology.
3 ∣. SOURCES OF IFN-γ IN THE CNS
IFN-γ has many roles in the CNS, but its source is not clear. IFN-γ is primarily produced by CD4+ Th1 cells, CD8+ T cells, NK cells, and ILC1s.23,84 Under homeostatic conditions, these cell types have limited access to the healthy CNS parenchyma,85 yet IFN-γ levels are maintained at low levels to support normal brain function.86,87 Studies have argued that CNS-resident cells can produce IFN-γ; however, these studies usually reflect experiments under severe pathological conditions, use relative expression values that are likely skewed due to extremely low or no expression level of IFN-γ at baseline in the cell of interest, or in vitro conditions.88,89 During pathology, such as infection or multiple sclerosis (MS) (and its mouse model, experimental autoimmune encephalomyelitis (EAE)), a more direct source of IFN-γ may be relevant as activated T cells, and other immune cells infiltrate into the CNS.9,90 Under homeostatic conditions, peripheral immune cells reside or traffic through the CNS borders (i.e., the meninges, choroid plexus, and perivascular spaces) and could serve as a potential source of IFN-γ in the CNS.91 At these sites, IFN-γ may be released into the cerebrospinal fluid (CSF), which connects to interstitial fluid (ISF) circulating throughout the brain parenchyma,17,92 allowing more direct access to neurons. Further, IFN-γ, like other blood–born components, may gain access to the parenchyma through the circumventricular organs (CVOs), which lack a canonical blood-brain barrier (BBB).93-95 Here, we will briefly introduce the different IFN-γ-producing cells in the CNS and discuss the various routes by which IFN-γ may enter the CNS under physiological conditions.
3.1 ∣. CNS borders as sites of IFN-γ entry
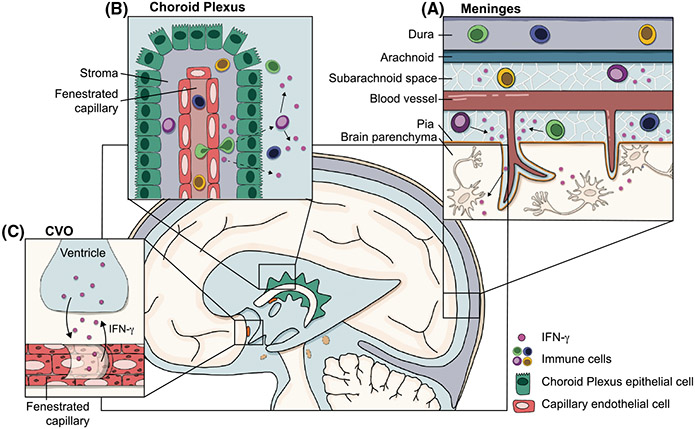
The CNS utilizes a specialized immune surveillance system that positions immune reactions to the CNS borders.9,96 In peripheral tissues, antigens within the extracellular fluid and migratory immune cells (like dendritic cells) enter initial lymphatic vessels then drain to local lymph nodes where they initiate an adaptive immune response. Once primed, T cells egress from lymph nodes back into circulation and home to target tissues for reactivation and effector function.97 Immune surveillance is extremely important to support tissue and prevent infection but can come at a cost.98,99 To maintain healthy neurons, the CNS parenchyma not only protects itself from peripheral pathogens, toxins, and other stressors using the BBB, but also maintains immune surveillance at the borders via the meninges. In the CNS, antigens flow into the CSF where they are either picked up by antigen-presenting cells in the meninges or drain to cervical lymph nodes through meningeal lymphatic vessels.16,18 After priming, CNS homing T cells traffic into the meningeal tissues, preferentially through the dural sinuses, where macrophages and other antigen-presenting cells are poised to present antigens to T cells.18 Under homeostatic conditions, CD4+ T cells take on a resident memory phenotype and are predominantly polarized to Th1.18 Up to 60% of T cells in the meninges are capable of producing IFN-γ, including both CD4+ and CD8+ T cells, and represent a cellular population that can produce IFN-γ in response to CNS-specific antigens4,100 (Figure 2A). Although CD4+ and CD8+ αβ T cells canonically function through T cell receptor stimulation, they can signal through bystander activation,101,102 independent of MHC-TCR interactions, to support CNS repair.103 The meninges also contain a population of γδ T cells, that although have predominately been shown to produce IL17 in mouse meninges,1,15 could be an antigen-independent source of IFN-γ that is released from thymic-derived γδ T cells in response to IL-2 or IL-15.104 Additionally, resident memory T cells can persist in the CNS, preferentially near anatomical barriers such as the meninges and choroid plexus (CP), after resolution of neurotropic viruses,105,106 and may provide another source of IFN-γ. Other potential sources include NK cells and ILC1s that are present in meninges and have the capacity to produce IFN-γ independent of antigens.107,108 Like the meninges, the CP functions as an interface between the CNS and the peripheral immune system and similarly houses IFN-γ producing T cells, NK cells, and ILC1s12,109 (Figure 2B). The CP is highly vascularized with fenestrated capillaries to form the blood–CSF barrier (BCSFB). The BCSFB seems to be more permeable to lymphocytes and cytokines than the BBB under steady state.110
FIGURE 2.
Sources of IFN-γ in the CNS. Physiological levels of IFN-γ, as well as immune cells which can produce IFN-γ, are present in the CSF during steady state. Because it is hypothesized that the CSF connects with the interstitial fluid (ISF), which circulates throughout the brain parenchyma, we focus on the CSF as a potential carrier which may grant IFN-γ direct access to neurons. However, it is still unclear how IFN-γ gets into the CSF and ultimately into the parenchyma. A) The meninges contain an immunologically rich niche, including T cells, NK cells, and ILC1s, all of which can produce IFN-γ. Immune cells in the subarachnoid space and perivascular spaces may act as a source of physiological IFN-γ found in the CSF. B) The choroid plexus sits outside of the blood–brain barrier and instead acts as a blood–CSF barrier, where proteins and solutes can exit the fenestrated capillaries and enter the CSF. Immune cells (including IFN-γ producing T cells, NK cells, and ILC1s) have been identified in the choroid plexus stroma, but it is unclear whether they (or their secreted cytokines) can cross the epithelial layer of the choroid plexus to enter the CSF. C) Circumventricular organs (CVOs) are often referred to as the “windows of the brain,” as they also lack a classical blood–brain barrier. Early studies using HRP have demonstrated that HRP injected intraventricularly or intravenously is able to permeate the parenchyma, suggesting solutes can access the parenchyma from both the CSF and the blood. Though this has not been directly tested with IFN-γ, it may be a potential route for IFN-γ to gain access to the parenchyma. Additionally, studies have demonstrated that cytokines can exit the fenestrated capillaries and act directly on the cells of the CVOs. However, it is still unclear whether cytokines can enter the CSF through CVOs, or whether cytokines have direct access to neurons within CVOs
Although the CSF is relatively acellular, in a healthy brain it contains up to 500,000 lymphocytes, the majority of which are central memory T cells.110 T cells can access CSF-filled perivascular spaces between blood vessels in the brain and leptomeningeal connective tissue surrounding the parenchyma.111 T cells can exit the fenestrated blood vessels of the CP, a site of major CSF production and lymphocyte accumulation, into the choroid stroma, but it is less clear if and how they can cross the epithelial layer to access the CSF.91 Kivisakk et al. demonstrated that the vasculature of the subarachnoid space and the CP express cell adhesion markers that could facilitate T cell entry into the CSF, and T cells were found in the CP stroma of individuals without neurological disorders,110 suggesting a physiological role for T cell presence at the CP. Whether IFN-γ arises from lymphocytes in the meninges, the CP or the CSF, it can be measured in the CSF at low concentrations (pg/mL) under physiological conditions.87 There is a need for better tools to study cell-specific IFN-γ production. For example, the lack of an IFN-γ flox mouse model makes it difficult to manipulate and study specific tissues and cells that produce IFN-γ. Ultimately, more work needs to be done to dissect out cell type-specific production and release of IFN-γ under homeostatic and pathological conditions in the CNS. Identifying the cellular source of IFN-γ is an important first step to understanding IFN-γ/STAT1 signaling in the CNS, but it is also critical to understand how IFN-γ reaches its target to activate STAT1.
The CSF reservoir is thought to integrate with ISF as CSF is driven through the periarteriolar spaces of penetrating vessels. Early studies injecting horseradish peroxidase into lateral ventricles of anesthetized dogs and cats first demonstrated that proteins flow from the CSF of the subarachnoid space via the perivascular spaces surrounding penetrating arterioles and reach the capillary beds of the basal laminae within minutes of injection.112,113 More sophisticated in vivo imaging studies demonstrated that once CSF flows into perivascular spaces, solutes can exchange with ISF between openings in astrocyte end feet via the glymphatic system.33 This system has access to most regions of the CNS and is a likely candidate in bringing IFN-γ into the brain parenchyma to facilitate direct contact between IFN-γ and neurons.92 Whether solutes enter the parenchyma at specific sites or if openings between astrocytes are dynamic and somehow regulated are unknown. Previous studies have also demonstrated that cytokines injected into the CSF become distributed throughout the brain parenchyma. IL-1β and IL-1ra injected into the lateral ventricle of rats was found in areas such as the CP, hypothalamus, hippocampus, and along fiber bundles in the corpus callosum, presumably via “volume transmission”114 through perivascular spaces.115 Similarly, IFN-γ injected into the lateral ventricles of rats resulted in upregulated MHC expression by microglia in periventricular parenchyma, cerebral cortex, cerebellum, major fiber tracts, and brainstem superficial parenchyma.116 Additionally, IFN-γ injected intrathecally into the sacral subarachnoid space of rats resulted in increased MHC expression in monocytes in the meninges and perivascular space, and in microglia in the spinal cord parenchyma.117,118 These studies suggest that IFN-γ in the CSF is able to act on cells within perivascular spaces as well as deep within the parenchyma. It is unclear if all or only subgroups of neurons express IFNGR or if there is varying expression among different brain regions. Assuming IFN-γ is also able to diffuse from the CSF to the brain parenchyma, it is unclear which brain regions would be most responsive. That being said, we demonstrated that a single injection of IFN-γ directly into the CSF via the cisterna magna led to increase activation of cortical neurons; however, whether this is due to IFN-γ directly activating neurons or due to an indirect secondary response is unknown.4
There is also potential for IFN-γ to reach the CNS through highly vascularized CVOs (Figure 2C). There are 7 CVOs within the brain, including the subfornical organ (SFO), median eminence, organum vasculosum of the lamina terminallus (OVLT), area postrema, neurohypophysis, pineal gland, and subcommissural organ.93,95 Like the CP, CVOs lack a canonical BBB, with fenestrated capillaries separated from the CSF by a lining of ependymal cells with tight junctions.119 Unlike the CP, CVOs are regions within the brain parenchyma, and therefore, may provide circulating cytokines direct access to neurons. Indeed, these regions have been reported to be important for physiology and the response to cytokines, such as IL-1 to induce fever.93,120,121 In the context of fever and IL-1, many studies suggest IL-1 activates neurons indirectly by inducing the cells of the CVOs to produce other signals, such as prostaglandins, which can act on neurons.93,120,121 Additionally, others have demonstrated that TNF and IFN-α applied to the OVLT in brain slice preparations from guinea pigs resulted in increased firing rates in some neurons, further suggesting neurons within and near CVOs are responsive to cytokines122 (Figure 2C). Few studies have focused on IFN-γ signaling within CVOs; however, IFNGR has been visualized in the median eminence and area postrema.123 CVOs may also provide IFN-γ access to the CSF. Despite the presence of tight junctions, early studies which injected HRP intravenously and intracerebroventricularly demonstrated that HRP was able to fill the median eminence, by flowing bidirectionally through select extracellular spaces between ependymal cells, which presumably lacked tight junctions,119 suggesting that proteins are able to access the CSF from the blood, and vice versa, via CVOs. Though there are no reports directly studying the ability of IFN-γ to permeate through CVOs into the CSF, the molecular weight of IFN-γ as a functional homodimer is about 45 kDa, and the molecular weight of HRP is around 40 kDa. Therefore, it seems reasonable to speculate that CVOs may provide a route of entry for IFN-γ into the CSF.
4 ∣. NEURONAL IFN-γ/STAT1 SIGNALING IN CNS HEALTH AND DISEASE
IFN-γ/STAT1 signaling in neurons has many functions beyond its classical immunological functions but it is unclear how IFN-γ/STAT1 signaling facilitates so many different outcomes. Here, we will review the downstream effects of IFN-γ/STAT1 signaling in neurons, with a focus on various factors which may contribute to the specificity and varying outcomes of IFN-γ/STAT1 signaling in neurons (summarized in Table 2).
TABLE 2.
Summary of experimental conditions investigating in vitro effects of IFN-γ
| Category | Findings | Experimental model | Age | Species | Reported IFN-γ concentration or activity |
Timing of IFN-γ treatment | Study |
|---|---|---|---|---|---|---|---|
| Proliferation | decreased neurosphere formation | NSC/neurospheres | Adult | Mouse | 1–100 ng/mL | 7–10 days | Li et al.124 |
| decreased proliferation | Primary neurons | Postnatal | Rat | 50 ng/mL | Added at time of plating | Gomez et al.129 | |
| decreased proliferation | NSCs | Embryonic | Mouse | 100–1000 U/mL | 48 h | Walter et al.125 | |
| decreased proliferation | NSC/neurospheres | Adult | Mouse | 100 U/mL | Added at time of plating and every 2 days after; analyzed at DIV 4-6 | Wong et al.126 | |
| increased neurosphere formation | NSC/neurospheres | Embryonic | Mouse | 1–50 ng/mL | 7–10 days | Li et al.124 | |
| inhibited neurosphere growth | Primary NSPC | Embryonic | Mouse | 1–1000 U/mL | 3–7 days (added at time of plating) | Kulkarni et al.57 | |
| no effect on proliferation | NSCs | Embryonic | Mouse | 1–10 U/mL | 48 h | Walter et al.125 | |
| Differentiation | decrease differentiation (only in differentiation media) | NPCs | Embryonic | Mouse | 5–50 ng/mL | 2 days | Ahn et al.127 |
| differentiation into abnormal phenotype | NSCs | Embryonic | Mouse | 1000 U/mL | 3 days | Walter et al.125 | |
| increased differtiation and neurogenesis | NSC/neurospheres | Adult | Mouse | 100 U/mL | 24–72 h (added at time of differentiation) | Wong et al.126 | |
| Neurotoxicity | dendritic beading | Primary cortical neurons; slice cultures | Embryonic; newborn | Mouse | 100 ng/mL | 48 h (added on DIV13) | Mizuno et al.22 |
| dendritic beading | Cultured spinal motor neurons | Embryonic | Mouse | 100 ng/mL | 12 h (added on DIV13) | Sengupta et al.147 | |
| increased apoptosis; restricted cell cycle | Primary NSPC | Embryonic | Mouse | 100–1000 U/mL | 72 h | Kulkarni et al.57 | |
| not protective against glutamate neurotoxicity | Hippocampal cultures | Embryonic | Rat | 1–100 U/mL | 48 h (prior to glutamate treatment) | Lee et al.148 | |
| protective against glutamate neurotoxicity | Hippocampal cultures | Embryonic | Rat | 10 U/mL | 48 h (prior to glutamate treatment) | Lee et al.148 | |
| Neurite Outgrowth | increased neurite outgrowth | NSC/neurospheres | Adult | Mouse | 100 U/mL | 3 days (added at time of plating) | Wong et al.126 |
| increased neurite outgrowth | hIPSC-derived NPC | Adult | Human | 25 ng/mL | Daily for 4 days (DIV 17-20), then plated terminally, analyzed DIV26-40 | Warre-Cornish et al.139 | |
| Gene Regulation | ASD/SZ-like gene dysregulation and transcriptional changes | hIPSC-derived NPC/neurons | Adult | Human | 25 ng/mL | Once on DIV18 or DIV30; daily for 5 days (DIV17-21); daily for 5 days (DIV17-21) plus on DIV30 | Warre-Cornish et al.139 |
4.1 ∣. Neurogenesis
Many studies have demonstrated a role for IFN-γ/STAT1 signaling in regulating the proliferation and differentiation of NSCs, though with sometimes conflicting results. Many studies use different sources of NSCs, including embryonic stem cell lines and primary cells derived from different regions of the brain at different developmental timepoints. Additionally, many studies utilize varying ranges of IFN-γ, reported as either concentration or specific activity, which further complicates the interpretation of conflicting results. Here, we will focus on factors such as cell source, developmental period, and concentration of IFN-γ utilized to gain a better understanding of the effects of IFN-γ/STAT1 signaling on neurogenesis.
Neurospheres are a commonly used in vitro culture system used to study neurogenesis in a physiologically relevant manner. Briefly, NSCs are isolated and expanded 3-dimensionally to form neurospheres. Li et al. demonstrated that IFN-γ treatment (1–100 ng/mL) of neurospheres inhibited proliferation and reduced the size of primary neurospheres cultured from the SVZ of postnatal (P2) and adult mice, by acting directly on precursor cells.124 Walter et al. demonstrated that NSCs differentiated from an undifferentiated embryonic stem cell line (SV-129) and treated with 100 or 1000U/mL IFN-γ for 48 hours caused decreased proliferation and increased caspase 3/7 activity, while lower concentrations of IFN-γ (1 or 10U/mL) had no effect.125 Additionally, IFN-γ treatment (100 U/mL over 6 days) of NSCs derived from adult SVZ decreased neurosphere proliferation and size.126
Conversely, Li et al. demonstrated that primary neurospheres derived from the mesencephalon and telencephalon of embryonic mice (E12) had increased formation after IFN-γ treatment (1–50 ng/mL),124 suggesting NSCs may respond differentially depending on the stage in development when IFN-γ is applied. They attributed this effect to sonic hedgehog (SHH), as inhibiting SHH activity in embryonic neurospheres prevented IFN-γ-induced proliferation and treating neurospheres with SHH in the absence of IFN-γ also resulted in increased proliferation. This suggests that IFN-γ can regulate SHH to control proliferation of embryonic neurospheres.124
On the contrary, Kulkarni et al. contradicted this finding by demonstrating that higher concentrations of IFN-γ (1–1000 U/mL, over 7 days) inhibited growth of neurospheres derived from cortical tissue of embryonic mice (E12.5) in a dose-dependent manner.57 They also demonstrated that IFN-γ restricted embryonic neurosphere proliferation at the G1/S checkpoint, by regulating positive growth regulators and cell cycle checkpoint proteins. All these IFN-γ-induced effects were mediated by STAT1 and abrogated in cells without functional STAT1 (STAT1 mutant which cannot bind GAS). Interestingly, the lack of functional STAT1 did not affect cells treated with 1000 U/mL of IFN-γ, further suggesting the possibility that IFN-γ has dose-dependent functions.57 Another factor that may explain the contradicting results is the brain region from which the neurosphere cultures were derived. Li et al. derived postnatal neurosphere cultures from SVZ or olfactory bulbs and embryonic neurosphere cultures from mesencephalon and telencephalon,124 which are primarily made up of the brainstem and cerebrum, whereas Kulkarni et al. utilized cortical tissue for embryonic neurosphere cultures,57 suggesting that cells from different brain regions may respond differently to IFN-γ as well.
Along with proliferation, IFN-γ/STAT1 signaling also regulates NSC differentiation and has typically been understood to have a pro-neurogenic affect. Wong et al. demonstrated that in NSCs from adult SVZ treatment with IFN-γ (100 U/mL over 6 days) increased the differentiation of neurospheres into βIII-tubulin+ mature neurons. They also observed increased neurite outgrowth, measured as increased length and number of neurites.126 Similarly, Walter et al. demonstrated that treating NSCs derived from embryonic (E14) neurospheres with IFN-γ (1000 U/mL, 7 days) also caused upregulation of βIII-tubulin, as well as microtubule associated protein (MAP2), another marker of post-mitotic neurons.125 However, they also observed an increase in expression of the astrocyte marker, GFAP. Treating NSCs with 100–1000 U/mL IFN-γ for 3 days caused co-expression of βIII-tubulin and GFAP, and downregulation of proneural genes. βIII-tubulin+GFAP+ cells were functionally distinct from mature neurons and astrocytes, exhibiting abnormal electrophysiological properties and impaired formation of functional neural networks in vitro. Additionally, IFN-γ treatment of these cells resulted in increased expression of Stat1 mRNA, which is expected as Stat1 is a downstream target of IFN-γ/STAT1 signaling. Surprisingly, IFN-γ treatment upregulated Ifngr1/2 mRNA expression, which is not typically regulated by IFN-γ/STAT1, and downregulated iNOS expression, which is typically upregulated in response to IFN-γ.125 These data suggest that high concentrations of IFN-γ may skew NSCs to differentiate towards an abnormal phenotype. In another study, Ahn et al. demonstrated that primary neural progenitor cells (NPCs) prepared from embryonic mice (E14.5) responded differently to IFN-γ depending on whether they were in proliferation or differentiation media.127 NPCs cultured under differentiation conditions and treated with 5–50 ng/mL IFN-γ for 2 days had inhibited differentiation in a dose-dependent manner, as measured by decreased TUJ1 and increased Nestin expression. These effects were not observed in NPCs treated with IFN-γ while cultured under proliferation conditions. NPCs treated under differentiation conditions also had decreased NEUROG2 expression, which was mediated by JAK signaling, as Ruxolitinib (a JAK inhibitor) abrogated this effect. IFN-γ-induced inhibition of differentiation was also mediated by JAK/STAT1 signaling, as both treatment with Ruxolitinib and transduction of NPCs with Stat1 shRNA prevented inhibition of differentiation.127
Most in vitro studies utilize very high concentrations of IFN-γ, which likely resemble levels seen during infection in vivo.128 However, it is known that even in the absence of infection, there are low physiological levels of IFN-γ present in the CNS. Li et al. demonstrated a role for endogenous IFN-γ in the non-inflammatory brain in regulating neural precursor proliferation and differentiation in the SVZ. Ifng−/− mice had an increased number of proliferating cells in the SVZ, which when cultured in vitro from P2 mice, also exhibited increased formation of neurospheres which were larger than those from wildtype mice. Neurospheres from Ifng−/− mice also formed an increased number of secondary neurospheres and were able to be passaged much longer than neurospheres from wildtype mice, demonstrating an increased capacity for self-renewal, which was reversed when treated with IFN-γ. Primary neurospheres from Ifng−/− mice had increased neuronal and oligodendrocyte differentiation and decreased astrocyte differentiation compared with neurospheres from wildtype mice and Ifng−/− neurospheres treated with IFN-γ. Interestingly, no differences were observed in neurospheres cultured from E12 wildtype or Ifng−/− mice.124 IFN-γ (50 ng/mL) activated JAK2 and STAT1 in Nestin+ progenitors in SVZ-dissociated cultures from postnatal rats (P7-9).129 In SVZ explants, IFN-γ treatment increased the area of migrating cells moving from the core of the explant, which was not seen in explants from Stat1−/− mice. Infusing IFN-γ directly into the third ventricle (via an osmotic pump, 50 ng/mL, 0.5 μL/h over 7 days) of adult mice decreased proliferating and cycling cells, as well as Nestin+ cells in the SVZ, and increased the proportion of committed neural cells. However, less fully differentiated neural cells were observed in the olfactory bulb of the mice infused with IFN-γ. STAT1 was required for these effects, as IFN-γ infused Stat1−/− mice had normal proliferation, differentiation, and neurogenesis. However, the lack of STAT1 in untreated mice had no effects on proliferation, differentiation, or neurogenesis, suggesting the effects of endogenous IFN-γ may not be STAT1-dependent.129 Similarly, Ahn et al. retrovirally expressed IFN-γ in the brains of embryonic mice (injected into lateral ventricles at E9.5) and observed most transduced cells still in the ventricular zone, with less transduced cells migrated into the TUJ1+ region at E14.5 compared with control mice127 (at peak neurogenesis130). These studies highlight the different outcomes and degree of severity IFN-γ has depending on concentration, brain region, and timing/stage of development when signaling occurs (summarized in Table 2). In addition to the different effects it has at the cellular level, IFN-γ also causes various outcomes at the systemic level depending on developmental stage, which we will discuss in the following sections.
4.2 ∣. Neurodevelopment
Studies investigating the effects of IFN-γ/STAT1 signaling on neurogenesis suggest IFN-γ/STAT1 may have different roles in different stages of development. Here, we will review studies focusing on the physiological and pathological roles of IFN-γ/STAT1 signaling in neurons during neurodevelopment.
Ectopic IFN-γ expression in the CNS (driven by the Gfap promotor; transgene injected into single cell embryos) resulted in ataxia, early death, abnormal brain cytoarchitecture (especially in cerebellum and hippocampus), regional hypomyelination, and increased the number of cells in the external granular layer (EGL).131 Lin et al. also drove ectopic IFN-γ expression under the Gfap promoter using a different transgenic mouse but observed similar results.132 In this mouse model, IFN-γ expression was regulated by a tet-OFF system driven under the Gfap promoter. When IFN-γ expression was induced at E16 via removing doxycycline from a pregnant dam, the offspring had similar neurological symptoms as the mice studied by LaFerla et al. (growth retardation, tremor, ataxia),131 as well as decreased survival.132 Additionally, these mice had increased cell proliferation in the molecular layer/EGL.132 Using the same mouse model, Wang et al. demonstrated abnormal development of the cerebellum, with increased proliferative activity in the EGL.133 These studies suggest the non-physiological presence of IFN-γ in the CNS during development can be detrimental to neurodevelopment.
STAT1 expression during critical periods of neurodevelopment has also been implicated in the homeostatic regulation of visual cortical plasticity.83 STAT1 protein levels increased in the visual cortex postnatally after eye opening and remained high during the critical period for optical dominance and into adulthood. STAT1 expression increased after a short period of monocular deprivation (MD; 4 days) versus a longer period (7 days). Wildtype mice subjected to MD increased open-eye responses after 7 days of MD, whereas in Stat1−/− mice, open-eye responses increased by 4 days of MD, suggesting the absence of STAT1 causes enhanced optical dominance (OD) plasticity. Conversely, wildtype mice treated with IFN-γ (830 U/g intraperitoneal injection; once a day during 7 days of MD) had decreased OD plasticity, with open-eye responses after 7 days of MD resembling those of mice without MD. This effect was not observed in Stat1−/− mice treated with IFN-γ, suggesting that IFN-γ/STAT1 signaling negatively regulates OD plasticity. Stat1−/− mice also had increased expression of the AMPAR subunit GluA1 after 4 days of MD compared with wildtype mice. These data suggest that IFN-γ/STAT1 signaling can regulate the expression of neuron-specific receptors and is important in regulating homeostatic visual cortical plasticity.83
JAK2 and STAT1 activation have been implicated in synapse refinement. Yasuda et al. utilized a system which inhibits neurotransmitter release in neurons expressing tetanus toxin light chain (TTLC) to eliminate inactive axons and decreased axon density.82 They demonstrated that overexpressing a dominant-negative mutant of JAK2 or STAT1, as well as overexpressing SOCS3 (a negative regulator of JAK2), inhibited axon elimination. Additionally, JAK2 was activated in TTLC+ neurons, but only when surrounding active neurons were present, as JAK2 was not activated when all neuron activity was globally suppressed (via tetrodotoxin (TTX)). Expressing a constitutively active JAK2 mutant or dominant-negative SOCS3 mutant was sufficient to enable axon elimination. They also demonstrated that mice expressing a dominant-negative JAK2 mutant decreased the number of synapses eliminated. In wildtype neurons, inactive synapses had increased JAK2 activation compared to more active synapses. This work suggests that JAK2 is activated in inactive synapses by signals from neighboring active synapses and acts as an “elimination signal” by signaling through STAT1.82 It is unclear how STAT1 mediates synaptic elimination; however, others have demonstrated that STAT1 induced CCL2 expression in neurons recruited microglia, which may execute the elimination of inactive synapses.81 However, more work is needed to determine how this mechanism works at a synapse-specific level.
The IFN response has recently been implicated in many neurodevelopmental disorders. In a mouse model of the developmental disorder Cornelia de Lange Syndrome (CdLS) with impaired synapse maturation and anxiety-related behavior, RNA-seq and cluster/GO analysis revealed an enrichment in the IFN pathway, and increased STAT1 expression in the cortex. Stat1 knockdown in cortical neurons partially rescued synapse formation in this model.134 The IFN response has also been heavily implicated in autism spectrum disorder (ASD) and schizophrenia.135-137 Cristino et al. analyzed multiple previously published autism and schizophrenia genome-wide datasets which looked at whole brain tissue. From these datasets, they generated a database of unique ASD- and SZ-associated SNPs and found that the most over-represented loci mapped back to STAT1 binding sites, suggesting STAT1-regulated genes may be critical for typical neurodevelopment.138 However, the cell type specificity of these SNPs was not considered, so it is unclear if these STAT1-regulated genes are expressed in neurons or other CNS cell types. A recent study from Warre-Cornish et al. used human IPSC-derived (hIPSC) NPCs and neurons to demonstrate that neuron-specific IFN-γ signaling resulted in dysregulated transcriptomes as seen in ASD and SZ. hIPSC-derived NPCs treated with IFN-γ (25 ng/mL; daily for 5 days) had increased neurite length, branch points, and number of neurites per cell compared with untreated cells. hIPSCs treated with IFN-γ at the NPC stage and then allowed to differentiate into mature neurons had lasting transcriptional changes 9 days after IFN-γ removal, with upregulation of MHCI antigen presentation related genes, and downregulation of the GABAergic transcription factor LHX6. hIPSC-derived neurons pretreated with IFN-γ at the NPC stage and then treated again at the neuron stage also downregulated synaptic genes, with the downregulated genes highly enriched for the GO term “synapse.” Furthermore, differentially expressed genes (DEGs) from the brains of individuals with SZ and ASD were highly enriched among the genes upregulated and downregulated by both NPCs and neurons treated with IFN-γ.139 Interestingly, some of the ASD-associated genes downregulated in NPCs treated with IFN-γ, such as SHANK2, were also represented in ChIP-seq datasets examining STAT1 regulated genes in HeLa cells.140 The authors attributed these IFN-γ-induced effects to the increased expression of promyelocytic leukemia (PML) bodies and MHCI expression. They demonstrated that IFN-γ treatment increased the number of PML bodies in NPCs, and that disrupting PML bodies, as well as inhibiting MHCI expression by silencing β2 microglubulin (B2M) expression, prevented IFN-γ-induced neurite outgrowth.139 Although these studies focus on the diverse roles of IFN-γ/STAT1 signaling in neurodevelopment at the synaptic and transcriptional levels, IFN-γ/STAT1 signaling in neurons is also implied in regulating behavior which is affected by many neurodevelopmental disorders.
4.3 ∣. Behavior
T cells are required for the maintenance of CNS homeostasis1,2,5,6 and T cell-derived IFN-γ specifically is required for supporting normal behavior, cognition, and neuronal inhibitory tone.4 We demonstrated that SCID mice (which lack lymphocytes) exhibited decreased social preference, which could be rescued by reconstituting the mice with lymphocytes. Treating wildtype mice with anti-VLA4 antibody also produced a social deficit, suggesting T cells need to be able to reach tissues, such as the meninges, in order to have this effect on social behavior. Mice lacking IFN-γ, lacking IFNGR1 in all neurons, or lacking STAT1 in inhibitory neurons also had social deficits, suggesting that T cell-derived IFN-γ affects social behavior by acting directly on neurons. Additionally, we performed gene set enrichment analysis (GSEA) to determine if the IFN-γ pathway was enriched in brains of mice and rats under different biological states. Using 41 transcriptomes from mouse and rat brain cortices, we determined that the IFN-γ pathway was enriched in the brains of animals exposed to social stimuli. Additionally, the IFN-γ transcriptional signature was over-represented in the brain transcriptomes from group-housed mice and rats, as well as zebrafish, while it was lost in transcriptomes from isolated rodents. Flies do not have T cells or express IFN-γ but do express orthologs to genes in the JAK/STAT pathway.141 Interestingly, the JAK/STAT1 pathway was enriched in the head transcriptomes of flies selected for low aggressiveness traits, which correlates with social experience.142 Furthermore, when the promoters of the highly enriched social genes were analyzed, they were enriched for STAT1 binding motifs.4 It is interesting to contemplate how and why a canonical immune signaling pathway would be involved in regulating something so seemingly unrelated as behavior, but we have previously hypothesized that the role of IFN-γ in pro-social behavior may be the result of an evolutionary “arms race” between virus and host.3
Elements involved in regulating the IFN-γ/STAT1 pathway have also been implicated in regulating spatial learning.143 In the Morris water maze, which measures spatial learning, rats identified as “fast swimmers” had increased levels of protein inhibitor of activated STAT1 (PIAS1) mRNA in the hippocampus. PIAS1, an E3 SUMO ligase, facilitates the sumoylation of STAT1, which decreases its ability to bind DNA and negatively regulates STAT1-mediated transcription.58 Overexpressing PIAS1 in the hippocampus of rats enhanced their spatial learning in the Morris water maze. PIAS1 overexpression also increased STAT1 sumolyation, decreased STAT1 phosphorylation, and decreased STAT1-DNA binding in the CA1 region. Transfection of the CA1 region with Pias1 siRNA reversed all these effects and impaired spatial learning. Co-transfection of Pias1 siRNA and mutant pSTAT1 (which decreased STAT1 phosphorylation) rescued learning, while enhanced learning was seen with transfection of mutant pSTAT1 alone. Transfection of mutant STAT1 which cannot undergo sumoylation impaired spatial learning. PIAS1 levels and STAT1 activity were similar in naive mice, suggesting that increased PIAS1 and decreased STAT1 activity are a result of training and learning.143 Similarly, Hsu et al. demonstrated that STAT1 protein and DNA binding were decreased in the CA1 region during spatial learning in the Morris water maze.144 Stat1−/− mice exhibited enhanced spatial learning and memory, while STAT1 overexpression in CA1 impaired learning and performance. This effect was facilitated by increased STAT1 binding of the GAS element in the promoter and expression of laminin β1 (LB1), an extracellular matrix protein associated with memory and cognitive impairment.144 Overall, this study suggests that STAT1 activation in neurons needs to be tightly regulated to promote optimal cognition.
4.4 ∣. Neurodegeneration
As discussed above, the IFN-γ/STAT1 pathway often results in cytotoxicity in the context of infection, yet neurons have adapted different ways to prevent excessive neuronal death. However, IFN-γ/STAT1 signaling can still have neurotoxic effects, especially in the context of injury. After ischemic brain injury, STAT1 activation was increased and colocalized with TUNEL positive neurons. Injury in Stat1−/− mice caused less severe injury and less TUNEL+ neurons, suggesting STAT1 activation may contribute to cell death. Stat1−/− mice had increased phosphorylation of AKT, a kinase which has been associated with neurotoxicity,145 and decreased caspase 3 activation after injury.146 High concentrations of IFN-γ also seem to be associated with neurotoxicity (Table 2). Mizuno et al. demonstrated that IFNGR formed a complex with AMPAR subunit GluR1 in mouse primary cortical neurons, and IFN-γ (100 ng/mL) resulted in GluR1 phosphorylation through JAK/STAT1 and PKA activation.22 IFN-γ also resulted in neurotoxicity and dendritic beading through increased calcium flux through the IFNGR/AMPAR complex, increased nitric oxide (NO) production, and depletion of ATP.22 Others have also demonstrated the ability of IFN-γ (100 ng/mL) to cause dendritic beading in cultured embryonic spinal motor neurons.147 In contrast, Lee et al. demonstrated that a lower concentration of IFN-γ (10 U/mL) was protective against glutamate induced neurotoxicity in rat hippocampal neuron cultures, while higher concentrations (100U/mL) did not have a protective effect. This mechanism also appeared to be driven by a rapid increase in calcium levels, but only after glutamate treatment.148 These seemingly opposing results emphasize the differential effects of IFN-γ depending on concentration.
STAT1 signaling in neurons has also been implicated in neurodegenerative diseases such as Alzheimer's Disease (AD). AD is primarily thought to be caused by the aggregation of proteins in neurons, including amyloid beta (Aβ) and Tau.149 Aβ, which is formed when the enzyme BACE1 cleaves amyloid precursor protein (APP), can aggregate into plaques which contribute to the pathogenesis of AD. Cho et al. demonstrated that STAT1 was constitutively bound to the Bace1 promoter in the SH-SY5Y neuronal cell line.150 BACE1 protein levels and promoter activity were decreased with inhibition of JAK2/STAT1 (using AG490), expression of a dominant-negative STAT1, and overexpression of SOCS1 and SOCS3. Inhibiting JAK2/STAT1 also decreased BACE1 expression and Aβ levels in primary cultured neurons and in vivo. In SH-SY5Y cells, BACE1 expression, activity, and STAT1-Bace1 promoter binding were not affected by IFN-γ treatment, suggesting STAT1 regulates BACE expression independently of IFN-γ.150 Another protein involved in AD is Tau, which can accumulate and form neurofibrillary tangles. Li et al. overexpressed human Tau (hTau) in HEK293 cells and observed increased STAT1 expression, phosphorylation (in total lysate and nuclear fraction), activity, nuclear translocation, dimerization, and STAT1-DNA binding.151 Overexpressing hTau in vivo by injecting AAV-hTau into the hippocampus of mice also resulted in increased STAT1 phosphorylation and expression, which colocalized with NeuN+ cells. Increased pSTAT1 and STAT1 levels were also observed in the cortex of AD patients in this study. Overexpressing hTau in the hippocampus of mice resulted in memory deficits, as measured by Morris water maze and contextual fear conditioning, which were rescued by Stat1 knockdown in the hippocampus. Overexpressing hTau also resulted in suppression of long-term potentiation (LTP) and NMDAR-mediated currents, both of which were attenuated in Stat1-knockdown mice. Overexpression of either hTau or STAT1 decreased the expression of NMDAR subunits, while Stat1-knockdown in hTau mice increased their expression. STAT1 was able to directly bind the promoters of the NMDAR subunits, and hTau overexpression increased this binding, which inhibited their transcription. Inhibition of JAK2 in HEK293 cells overexpressing hTau prevented STAT1-activation. All of these effects were attenuated by expressing a dominant-negative mutant STAT1 which cannot be phosphorylated.151 While STAT1 may not be the original instigator in AD, these studies suggest that JAK2/STAT1 signaling does contribute to and may perpetuate the pathogenesis and symptoms of AD, and therefore, may be a potential target in treating AD.
5 ∣. CONCLUSION
Neurons utilize unique IFN-γ/STAT1 signaling which regulates many aspects beyond canonical immunological functions. We summarized the neuron-specific mechanisms of IFN-γ/STAT1 activation, the potential sources and entry sites of IFN-γ in the CNS, and the diverse set of effects IFN-γ/STAT1 signaling in neurons has on CNS health and disease. IFN-γ-induced STAT1 activation in neurons is muted and extended, with delayed deactivation of the pathway. The IFN-γ/STAT1 pathway is unique in neurons in that it facilitates non-cytolytic viral clearance and regulates neuronal excitability. The source of physiological IFN-γ in the CNS has been understudied, likely due to the lack of good tools to study cell- and tissue-specific production of IFN-γ. Immunological niches including the meninges and the CP may serve as sites for IFN-γ production in the CNS, while CVOs and CSF flux via the glymphatic system may provide entry sites for IFN-γ to access the parenchyma and gain direct access to neurons. In addition to its immune role, IFN-γ/STAT1 signaling in neurons contributes to a wide array of physiological and pathological outcomes, including the regulation of neurogenesis and behavior, and has been implicated in neurodevelopmental and neurodegenerative disorders. It is still unclear what underlying mechanisms cause IFN-γ/STAT1 signaling to have such diverse consequences, but we speculate that factors such as neuronal subtype, and subcellular location and strength of signaling may affect the conditions under which the pathway is activated and its downstream outcomes. It is important to note that many studies that have investigated the role of IFN-γ/STAT1 signaling in neurons, especially in vitro, utilize varying concentrations and timing of IFN-γ application in primary cultures derived from different sources, brain regions, and developmental timepoints, and sex is not consistently taken into consideration. More work is required to elucidate the specific mechanisms and factors which facilitate the unique set of IFN-γ/STAT1 regulated pathways in neurons. Understanding the complex underlying mechanisms of IFN-γ/STAT1 signaling in neurons in the context of infection, homeostasis, and pathology will not only offer us insight on how the immune system can influence neuronal function but also will be crucial to identify and target steps in the pathway which may have therapeutic potential for disorders such as ASD, SZ, epilepsy, multiple sclerosis, AD, and pathological aging.
ACKNOWLEDGEMENTS
The authors would like to thank all the members of the Filiano lab and others in the Marcus Center for Cellular Cures for fruitful discussion about several aspects included in this review. This work was supported by grants from the NIH R01NS123084 and the Marcus Foundation.
Funding information
The Marcus Foundation, Grant/Award Number: 2704; National Institute of Neurological Disorders and Stroke, Grant/Award Number: R01NS123084
Footnotes
CONFLICT OF INTEREST
AJF is an inventor on intellectual property licensed to CryoCell.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed for this review.
REFERENCES
- 1.Alves de Lima K, Rustenhoven J, Da Mesquita S, et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21(11):1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derecki NC, Cardani AN, Yang CH, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filiano AJ, Gadani SP, Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci. 2017;18(6):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filiano AJ, Xu Y, Tustison NJ, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535(7612):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22(6):861–869. [DOI] [PubMed] [Google Scholar]
- 6.Ellwardt E, Walsh JT, Kipnis J, Zipp F. Understanding the Role of T Cells in CNS Homeostasis. Trends Immunol. 2016;37(2):154–165. [DOI] [PubMed] [Google Scholar]
- 7.Herz J, Fu Z, Kim K, et al. GABAergic neuronal IL-4R mediates T cell effect on memory. Neuron. 2021;109(22):3609–3618.e3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12(9):663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol. 2020;38(1):597–620. [DOI] [PubMed] [Google Scholar]
- 10.Ziv Y, Ron N, Butovsky O, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–275. [DOI] [PubMed] [Google Scholar]
- 11.Reed MD, Yim YS, Wimmer RD, et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577(7789):249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunis G, Baruch K, Rosenzweig N, et al. IFN-γ-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain. 2013;136(11):3427–3440. [DOI] [PubMed] [Google Scholar]
- 13.Sukoff Rizzo SJ, Neal SJ, Hughes ZA, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiat. 2012;2(12):e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei H, Chadman KK, McCloskey DP, et al. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2012;1822(6):831–842. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro M, Brigas HC, Temido-Ferreira M, et al. Meningeal gammadelta T cell derived IL-17 controls synaptic plasticity and shortterm memory. Sci Immunol. 2019;4(40):5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogensen FL-H, Delle C, Nedergaard M. The Glymphatic System (En)during Inflammation. Int J Mol Sci. 2021;22(14):7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rustenhoven J, Drieu A, Mamuladze T, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. 2021;184(4):1000–1016.e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-γ-dependent signaling. Trends Immunol. 2002;23(2):96–101. [DOI] [PubMed] [Google Scholar]
- 20.Janach GMS, Reetz O, Döhne N, et al. Interferon-γ acutely augments inhibition of neocortical layer 5 pyramidal neurons. J Neuroinflammation. 2020;17(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vikman K, Robertson B, Grant G, Liljeborg A, Kristensson K. Interferon-γ receptors are expressed at synapses in the rat superficial dorsal horn and lateral spinal nucleus. J Neurocytol. 1998;27(10):749–760. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno T, Zhang G, Takeuchi H, et al. Interferon-γ directly induces neurotoxicity through a neuron specific, calcium-permeable complex of IFN-γ receptor and AMPA GluR1 receptor. FASEB J. 2008;22(6):1797–1806. [DOI] [PubMed] [Google Scholar]
- 23.Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67(1):227–264. [DOI] [PubMed] [Google Scholar]
- 24.Podolsky MA, Solomos AC, Durso LC, Evans SM, Rall GF, Rose RW. Extended JAK activation and delayed STAT1 dephosphorylation contribute to the distinct signaling profile of CNS neurons exposed to interferon-gamma. J Neuroimmunol. 2012;251(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose RW, Vorobyeva AG, Skipworth JD, Nicolas E, Rall GF. Altered levels of STAT1 and STAT3 influence the neuronal response to interferon gamma. J Neuroimmunol. 2007;192(1):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Donnell LA, Henkins KM, Kulkarni A, et al. Interferon gamma induces protective non-canonical signaling pathways in primary neurons. J Neurochem. 2015;135(2):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deczkowska A, Baruch K, Schwartz M. Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends Immunol. 2016;37(3):181–192. [DOI] [PubMed] [Google Scholar]
- 28.Baruch K, Deczkowska A, David E, et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346(6205):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burdeinick-Kerr R, Govindarajan D, Griffin DE. Noncytolytic clearance of sindbis virus infection from neurons by gamma interferon is dependent on Jak/Stat signaling. J Virol. 2009;83(8):3429–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binder GK, Griffin DE. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293(5528):303–306. [DOI] [PubMed] [Google Scholar]
- 31.Burdeinick-Kerr R, Wind J, Griffin DE. Synergistic roles of antibody and interferon in noncytolytic clearance of sindbis virus from different regions of the central nervous system. J Virol. 2007;81(11):5628–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson CE, Lawrence DMP, Echols LA, Rall GF. Immunemediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76(9):4497–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu T, Bi Z, Reiss CS. Interferon-γ induced type I nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68(1):101–108. [DOI] [PubMed] [Google Scholar]
- 34.Larena M, Regner M, Lobigs M. Cytolytic effector pathways and IFN-γ help protect against Japanese encephalitis. Eur J Immunol. 2013;43(7):1789–1798. [DOI] [PubMed] [Google Scholar]
- 35.Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci. 2014;15(8):507–519. [DOI] [PubMed] [Google Scholar]
- 36.Juusola M, French AS, Uusitalo RO, Weckström M. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19(7):292–297. [DOI] [PubMed] [Google Scholar]
- 37.Takeda T, Ishikawa A, Ohtomo K, Kobayashi Y, Matsuoka T. Fractal dimension of dendritic tree of cerebellar Purkinje cell during onto- and phylogenetic development. Neurosci Res. 1992;13(1):19–31. [DOI] [PubMed] [Google Scholar]
- 38.Jang DC, Shim HG, Kim SJ. Intrinsic plasticity of cerebellar Purkinje cells contributes to motor memory consolidation. J Neurosci. 2020;40(21):4145–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tasnim H, Fricke GM, Byrum JR, Sotiris JO, Cannon JL, Moses ME. Quantitative measurement of naïve T cell association with dendritic cells, FRCs, and blood vessels in lymph nodes. Front Immunol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell IL. Cytokine-mediated inflammation, tumorigenesis, and disease-associated JAK/STAT/SOCS signaling circuits in the CNS. Brain Res Rev. 2005;48(2):166–177. [DOI] [PubMed] [Google Scholar]
- 41.Janach GMS, Reetz O, Dohne N, et al. Interferon-gamma acutely augments inhibition of neocortical layer 5 pyramidal neurons. J Neuroinflammation. 2020;17(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song R, Koyuncu OO, Greco TM, et al. Two modes of the axonal interferon response limit alphaherpesvirus neuroinvasion. MBio. 2016;7(1):e02145–02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson HM, Ahmed CM. Noncanonical IFN signaling: mechanistic linkage of genetic and epigenetic events. Mediators Inflamm. 2016;2016:9564814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson HM, Noon-Song E, Ahmed CM. Noncanonical IFN signaling, steroids, and STATs: a probable role of V-ATPase. Mediators Inflamm. 2019;2019:4143604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majoros A, Platanitis E, Kernbauer-Hölzl E, Rosebrock F, Müller M, Decker T. Canonical and non-canonical aspects of JAK–STAT signaling: lessons from interferons for cytokine responses. Front Immunol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szente BE, Johnson HM. Binding of IFNγ and Its C-terminal peptide to a cytoplasmic domain of its receptor that is essential for function. Biochem Biophys Res Commun. 1994;201(1):215–221. [DOI] [PubMed] [Google Scholar]
- 47.Fidler IJ, Fogler WE, Kleinerman ES, Saiki I. Abrogation of species specificity for activation of tumoricidal properties in macrophages by recombinant mouse or human interferon-gamma encapsulated in liposomes. J Immunol. 1985;135(6):4289–4296. [PubMed] [Google Scholar]
- 48.Sancéau J, Sondermeyer P, Béranger F, Falcoff R, Vaquero C. Intracellular human gamma-interferon triggers an antiviral state in transformed murine L cells. Proc Natl Acad Sci USA. 1987;84(9):2906–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith M, Muegge K, Keller J, Kung H, Young H, Durum S. Direct evidence for an intracellular role for IFN-gamma. Microinjection of human IFN-gamma induces Ia expression on murine macrophages. J Immunol. 1990;144(5):1777–1782. [PubMed] [Google Scholar]
- 50.Subramaniam PS, Mujtaba MG, Paddy MR, Johnson HM. The carboxyl terminus of interferon-γ contains a functional polybasic nuclear localization sequence*. J Biol Chem. 1999;274(1):403–407. [DOI] [PubMed] [Google Scholar]
- 51.Dawson MA, Bannister AJ, Göttgens B, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature. 2009;461(7265):819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noon-Song EN, Ahmed CM, Dabelic R, Canton J, Johnson HM. Controlling nuclear JAKs and STATs for specific gene activation by IFNγ. Biochem Biophys Res Commun. 2011;410(3):648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Koga T, Isobe M, et al. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 2003;17(16):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Wu TR, Cai S, Welte T, Chin YE. Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation. Mol Cell Biol. 2000;20(13):4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesemann DR, Benveniste EN. STAT-1α and IFN-γ as modulators of TNF-α signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1α complex. J Immunol. 2003;171(10):5313. [DOI] [PubMed] [Google Scholar]
- 56.Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A. STAT-1 Interacts with p53 to Enhance DNA Damage-induced Apoptosis. J Biol Chem. 2004;279(7):5811–5820. [DOI] [PubMed] [Google Scholar]
- 57.Kulkarni A, Scully TJ, O'Donnell LA. The antiviral cytokine interferon-gamma restricts neural stem/progenitor cell proliferation through activation of STAT1 and modulation of retinoblastoma protein phosphorylation. J Neurosci Res. 2017;95(8):1582–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shuai K, Liu B. Regulation of JAK–STAT signalling in the immune system. Nat Rev Immunol. 2003;3(11):900–911. [DOI] [PubMed] [Google Scholar]
- 59.Sampaio EP, Hsu AP, Pechacek J, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013;131(6):1624–1634.e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toubiana J, Okada S, Hiller J, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127(25):3154–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Donnell LA, Conway S, Rose RW, et al. STAT1-independent control of a neurotropic measles virus challenge in primary neurons and infected mice. J Immunol. 2012;188(4):1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moseman EA, Blanchard AC, Nayak D, McGavern DB. T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci Immunol. 2020;5(48):1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murira A, Lamarre A. Type-I interferon responses: from friend to foe in the battle against chronic viral infection. Front Immunol. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takaoka A, Tanaka N, Mitani Y, et al. Protein tyrosine kinase Pyk2 mediates the Jak-dependent activation of MAPK and Stat1 in IFN-γ, but not IFN-α, signaling. EMBO J. 1999;18(9):2480–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song JH, Wang CX, Song DK, Wang P, Shuaib A, Hao C. Interferon gamma induces neurite outgrowth by up-regulation of p35 neuron-specific cyclin-dependent kinase 5 activator via activation of ERK1/2 pathway. J Biol Chem. 2005;280(13):12896–12901. [DOI] [PubMed] [Google Scholar]
- 66.Muller M, Fontana A, Zbinden G, Gahwiler BH. Effects of interferons and hydrogen peroxide on CA3 pyramidal cells in rat hippocampal slice cultures. Brain Res. 1993;619(1–2):157–162. [DOI] [PubMed] [Google Scholar]
- 67.Zhu PJ, Huang W, Kalikulov D, et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell. 2011;147(6):1384–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vikman KS, Hill RH, Backström E, Robertson B, Kristensson K. Interferon-γ induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. Pain. 2003;106(3):241–251. [DOI] [PubMed] [Google Scholar]
- 69.Vikman KS, Duggan AW, Siddall PJ. Interferon-gamma induced disruption of GABAergic inhibition in the spinal dorsal horn in vivo. Pain. 2007;133(1–3):18–28. [DOI] [PubMed] [Google Scholar]
- 70.Vikman KS, Siddall PJ, Duggan AW. Increased responsiveness of rat dorsal horn neurons in vivo following prolonged intrathecal exposure to interferon-gamma. Neuroscience. 2005;135(3):969–977. [DOI] [PubMed] [Google Scholar]
- 71.Pusic AD, Mitchell HM, Kunkler PE, Klauer N, Kraig RP. Spreading depression transiently disrupts myelin via interferon-gamma signaling. Exp Neurol. 2015;264:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pusic AD, Kraig RP. Phasic treatment with interferon gamma stimulates release of exosomes that protect against spreading depression. J Interferon Cytokine Res. 2015;35(10):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vikman KS, Owe-Larsson B, Brask J, Kristensson KS, Hill RH. Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Res. 2001;896(1-2):18–29. [DOI] [PubMed] [Google Scholar]
- 74.Flood L, Korol SV, Ekselius L, Birnir B, Jin Z. Interferon-gamma potentiates GABAA receptor-mediated inhibitory currents in rat hippocampal CA1 pyramidal neurons. J Neuroimmunol. 2019;337: 577050. [DOI] [PubMed] [Google Scholar]
- 75.Egawa K, Fukuda A. Pathophysiological power of improper tonic GABA(A) conductances in mature and immature models. Front Neural Circuits. 2013;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27(9):569–575. [DOI] [PubMed] [Google Scholar]
- 77.Papageorgiou IE, Lewen A, Galow LV, et al. TLR4-activated microglia require IFN-gamma to induce severe neuronal dysfunction and death in situ. Proc Natl Acad Sci USA. 2016;113(1):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janach GMS, Bohm M, Dohne N, Kim HR, Rosario M, Strauss U. Interferon-gamma enhances neocortical synaptic inhibition by promoting membrane association and phosphorylation of GABAA receptors in a protein kinase C-dependent manner. Brain Behav Immun. 2022;101:153–164. [DOI] [PubMed] [Google Scholar]
- 79.Brask J, Kristensson K, Hill RH. Exposure to interferon-gamma during synaptogenesis increases inhibitory activity after a latent period in cultured rat hippocampal neurons. Eur J Neurosci. 2004;19(12):3193–3201. [DOI] [PubMed] [Google Scholar]
- 80.Kreutzfeldt M, Bergthaler A, Fernandez M, et al. Neuroprotective intervention by interferon-gamma blockade prevents CD8+ T cell-mediated dendrite and synapse loss. J Exp Med. 2013;210(10):2087–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Liberto G, Pantelyushin S, Kreutzfeldt M, et al. Neurons under T cell attack coordinate phagocyte-mediated synaptic stripping. Cell. 2018;175(2):458–471 e419. [DOI] [PubMed] [Google Scholar]
- 82.Yasuda M, Nagappan-Chettiar S, Johnson-Venkatesh EM, Umemori H. An activity-dependent determinant of synapse elimination in the mammalian brain. Neuron. 2021;109(8):1333–1349.e1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagakura I, Van Wart A, Petravicz J, Tropea D, Sur M. STAT1 Regulates the homeostatic component of visual cortical plasticity via an AMPA receptor-mediated mechanism. J Neurosci. 2014;34(31):10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim AI, Di Santo JP. ILC-poiesis: ensuring tissue ILC differentiation at the right place and time. Eur J Immunol. 2019;49(1):11–18. [DOI] [PubMed] [Google Scholar]
- 85.Banks WA, Erickson MA. The blood–brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. [DOI] [PubMed] [Google Scholar]
- 86.Li X, Chauhan A, Sheikh AM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Gao L, Zang D. Elevated levels of IFN-γ in CSF and serum of patients with amyotrophic lateral sclerosis. PLoS One. 2015;10(9):e0136937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lau LT, Yu AC-H. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18(3):351–359. [DOI] [PubMed] [Google Scholar]
- 89.Kawanokuchi J, Mizuno T, Takeuchi H, et al. Production of interferon-γ by microglia. Multi Scler J. 2006;12(5):558–564. [DOI] [PubMed] [Google Scholar]
- 90.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11(2):125–137. [DOI] [PubMed] [Google Scholar]
- 91.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26(9):485–495. [DOI] [PubMed] [Google Scholar]
- 92.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buller KM. Circumventricular organs: gateways to the brain role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic– pituitary-adrenal axis. Clin Exp Pharmacol Physiol. 2001;28(7):581–589. [DOI] [PubMed] [Google Scholar]
- 94.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7(8):678–686. [DOI] [PubMed] [Google Scholar]
- 95.Sisó S, Jeffrey M, González L. Sensory circumventricular organs in health and disease. Acta Neuropathol. 2010;120(6):689–705. [DOI] [PubMed] [Google Scholar]
- 96.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12(9):623–635. [DOI] [PubMed] [Google Scholar]
- 97.Förster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012;33(6):271–280. [DOI] [PubMed] [Google Scholar]
- 98.Gao H-M, Hong J-S. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: The role and consequences. Neurosci Res. 2014;79:1–12. [DOI] [PubMed] [Google Scholar]
- 100.Abromson-Leeman S, Bronson RT, Dorf ME. Encephalitogenic T cells that stably express both T-bet and RORγt consistently produce IFNγ but have a spectrum of IL-17 profiles. J Neuroimmunol. 2009;215(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee HG, Cho MZ, Choi JM. Bystander CD4(+) T cells: crossroads between innate and adaptive immunity. Exp Mol Med. 2020;52(8):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salerno F, Guislain A, Cansever D, Wolkers MC. TLR-mediated innate production of IFN-gamma by CD8+ T cells is independent of glycolysis. J Immunol. 2016;196(9):3695–3705. [DOI] [PubMed] [Google Scholar]
- 103.Walsh JT, Hendrix S, Boato F, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125(2):699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ribot JC, Ribeiro ST, Correia DV, Sousa AE, Silva-Santos B. Human gammadelta thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J Immunol. 2014;192(5):2237–2243. [DOI] [PubMed] [Google Scholar]
- 105.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci. 2010;107(42):17872–17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steinbach K, Vincenti I, Kreutzfeldt M, et al. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med. 2016;213(8):1571–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26(4):221–226. [DOI] [PubMed] [Google Scholar]
- 108.Seillet C, Belz GT, Huntington ND. Development, homeostasis, and heterogeneity of NK cells and ILC1. In: Vivier E, Di Santo J, Moretta A, eds. Natural Killer Cells. Springer International Publishing; 2016:37–61. [DOI] [PubMed] [Google Scholar]
- 109.Van Hove H, Martens L, Scheyltjens I, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021–1035. [DOI] [PubMed] [Google Scholar]
- 110.Kivisäkk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100(14):8389–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67(12):1113–1121. [DOI] [PubMed] [Google Scholar]
- 112.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘Paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326(1):47–63. [DOI] [PubMed] [Google Scholar]
- 113.Banks WA, Broadwell RD. Blood to brain and brain to blood passage of native horseradish peroxidase, wheat germ agglutinin, and albumin: pharmacokinetic and morphological assessments. J Neurochem. 1994;62(6):2404–2419. [DOI] [PubMed] [Google Scholar]
- 114.Agnati LF, Zoli M, Strömberg I, Fuxe K. Intercellular communication in the brain: Wiring versus volume transmission. Neuroscience. 1995;69(3):711–726. [DOI] [PubMed] [Google Scholar]
- 115.Konsman JP, Tridon V, Dantzer R. Diffusion and action of intracerebroventricularly injected interleukin-1 in the CNS. Neuroscience. 2000;101(4):957–967. [DOI] [PubMed] [Google Scholar]
- 116.Peng Z-C, Kristensson K, Bentivoglio M. Distribution and temporal regulation of the immune response in the rat brain to intracerebroventricular injection of interferon-γ. Exp Neurol. 1998;154(2):403–417. [DOI] [PubMed] [Google Scholar]
- 117.Voorthuis JAC, Uitdehaag BMJ, De Groot CJA, Goede PH, Meide PHVD, Dijkstra CD. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin Exp Immunol. 1990;81(2):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vass K, Lassmann H. Intrathecal application of interferon gamma. Progressive appearance of MHC antigens within the rat nervous system. Am J Pathol. 1990;137(4):789. [PMC free article] [PubMed] [Google Scholar]
- 119.Broadwell RD, Balin BJ, Salcman M, Kaplan RS. Brain-blood barrier? Yes and no. Proc Natl Acad Sci USA. 1983;80(23):7352–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin JH, Lin MT. Inhibition of nitric oxide synthase or cyclooxygenase pathways in organum vasculosum laminae terminalis attenuates interleukin-1β fever in rabbits. Neurosci Lett. 1996;208(3):155–158. [DOI] [PubMed] [Google Scholar]
- 121.Elmquist JK, Scammell HE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20(12):565–570. [DOI] [PubMed] [Google Scholar]
- 122.Shibata M, Blatteis CM. Human recombinant tumor necrosis factor and interferon affect the activity of neurons in the organum vasculosum laminae terminalis. Brain Res. 1991;562(2):323–326. [DOI] [PubMed] [Google Scholar]
- 123.Robertson B, Kong G-Y, Peng Z-C, Bentivoglio M, Kristensson K. Interferon-γ-responsive neuronal sites in the normal rat brain: receptor protein distribution and cell activation revealed by Fos induction. Brain Res Bull. 2000;52(1):61–74. [DOI] [PubMed] [Google Scholar]
- 124.Li L, Walker TL, Zhang Y, Mackay EW, Bartlett PF. Endogenous interferon γ directly regulates neural precursors in the non-inflammatory brain. J Neurosci. 2010;30(27):9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Walter J, Honsek SD, Illes S, et al. A new role for interferon gamma in neural stem/precursor cell dysregulation. Mol Neurodegener. 2011;6(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong G, Goldshmit Y, Turnley AM. Interferon-γ but not TNFα promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187(1):171–177. [DOI] [PubMed] [Google Scholar]
- 127.Ahn J, Lee J, Kim S. Interferon-gamma inhibits the neuronal differentiation of neural progenitor cells by inhibiting the expression of Neurogenin2 via the JAK/STAT1 pathway. Biochem Biophys Res Commun. 2015;466(1):52–59. [DOI] [PubMed] [Google Scholar]
- 128.Frei K, Leist TP, Meager A, et al. Production of B cell stimulatory factor-2 and interferon gamma in the central nervous system during viral meningitis and encephalitis. Evaluation in a murine model infection and in patients. J Exp Med. 1988;168(1):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gómez L, Medina R, Baena M, Planas A, Pozas E. IFN gamma regulates proliferation and neuronal differentiation by STAT1 in adult SVZ niche. Front Cell Neurosci. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bayer SA, Altman J. Development of the endopiriform nucleus and the claustrum in the rat brain. Neuroscience. 1991;45(2):391–412. [DOI] [PubMed] [Google Scholar]
- 131.LaFerla FM, Sugarman MC, Lane TE, Leissring MA. Regional hypomyelination and dysplasia in transgenic mice with astrocyte-directed expression of interferon-γ. J Mol Neurosci. 2000;15(1):45–59. [DOI] [PubMed] [Google Scholar]
- 132.Lin W, Kemper A, McCarthy KD, et al. Interferon-γ induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24(45):10074–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J, Lin W, Popko B, Campbell IL. Inducible production of interferon-γ in the developing brain causes cerebellar dysplasia with activation of the Sonic hedgehog pathway. Mol Cell Neurosci. 2004;27(4):489–496. [DOI] [PubMed] [Google Scholar]
- 134.Fujita Y, Masuda K, Bando M, et al. Decreased cohesin in the brain leads to defective synapse development and anxiety-related behavior. J Exp Med. 2017;214(5):1431–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gandal MJ, Zhang P, Hadjimichael E, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362(6420):8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gupta S, Ellis SE, Ashar FN, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5(1):5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cristino AS, Williams SM, Hawi Z, et al. Neurodevelopmental and neuropsychiatric disorders represent an interconnected molecular system. Mol Psychiatry. 2014;19(3):294–301. [DOI] [PubMed] [Google Scholar]
- 139.Warre-Cornish K, Perfect L, Nagy R, et al. Interferon-γ signaling in human iPSC–derived neurons recapitulates neurodevelopmental disorder phenotypes. Science Advances. 2020;6(34):9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Satoh J-I, Tabunoki H. A Comprehensive profile of ChIP-Seq-based STAT1 target genes suggests the complexity of STAT1-mediated gene regulatory mechanisms. Gene Regul Syst Bio. 2013;7:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198(1):72–82. [DOI] [PubMed] [Google Scholar]
- 142.Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci. 2008;105(15):5657–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tai DJC, Hsu WL, Liu YC, Ma YL, Lee EHY. Novel role and mechanism of protein inhibitor of activated STAT1 in spatial learning. EMBO J. 2011;30(1):205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hsu W-L, Ma Y-L, Hsieh D-Y, Liu Y-C, Lee EH. STAT1 negatively regulates spatial memory formation and mediates the memory-impairing effect of Aβ. Neuropsychopharmacology. 2014;39(3):746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo HR, Hattori H, Hossain MA, et al. Akt as a mediator of cell death. Proc Natl Acad Sci. 2003;100(20):11712–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takagi Y, Harada J, Chiarugi A, Moskowitz MA. STAT1 is Activated in Neurons after Ischemia and Contributes to Ischemic Brain Injury. J Cereb Blood Flow Metab. 2002;22(11):1311–1318. [DOI] [PubMed] [Google Scholar]
- 147.Sengupta S, Le TT, Adam A, et al. Interferon-γ Receptor 1 and GluR1 upregulated in motor neurons of symptomatic hSOD1G93A mice. Eur J Neurosci. 2019;49(1):62–78. [DOI] [PubMed] [Google Scholar]
- 148.Lee J, Kim SJ, Son TG, Chan SL, Mattson MP. Interferon-γ is upregulated in the hippocampus in response to intermittent fasting and protects hippocampal neurons against excitotoxicity. J Neurosci Res. 2006;83(8):1552–1557. [DOI] [PubMed] [Google Scholar]
- 149.Goedert M, Spillantini MG. A Century of Alzheimer's Disease. Science. 2006;314(5800):777–781. [DOI] [PubMed] [Google Scholar]
- 150.Cho HJ, Jin SM, Son SM, et al. Constitutive JAK2/STAT1 activation regulates endogenous BACE1 expression in neurons. Biochem Biophys Res Commun. 2009;386(1):175–180. [DOI] [PubMed] [Google Scholar]
- 151.Li X-G, Hong X-Y, Wang Y-L, et al. Tau accumulation triggers STAT1-dependent memory deficits by suppressing NMDA receptor expression. EMBO Rep. 2019;20(6):e47202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed for this review.