Abstract
Functional 1H magnetic resonance spectroscopy (fMRS) is a derivative of dynamic MRS imaging. This modality links physiologic metabolic responses with available activity and measures absolute or relative concentrations of various metabolites. According to clinical evidence, the mitochondrial glycolysis pathway is disrupted in many nervous system disorders, especially Alzheimer disease, resulting in the activation of anaerobic glycolysis and an increased rate of lactate production. Our study evaluates fMRS with J-editing as a cutting-edge technique to detect lactate in Alzheimer disease. In this modality, functional activation is highlighted by signal subtractions of lipids and macromolecules, which yields a much higher signal-to-noise ratio and enables better detection of trace levels of lactate compared with other modalities. However, until now, clinical evidence is not conclusive regarding the widespread use of this diagnostic method. The complex machinery of cellular and noncellular modulators in lactate metabolism has obscured the potential roles fMRS imaging can have in dementia diagnosis. Recent developments in MRI imaging such as the advent of 7 Tesla machines and new image reconstruction methods, coupled with a renewed interest in the molecular and cellular basis of Alzheimer disease, have reinvigorated the drive to establish new clinical options for the early detection of Alzheimer disease. Based on the latter, lactate has the potential to be investigated as a novel diagnostic and prognostic marker for Alzheimer disease.
Keywords: aerobic glycolysis, Alzheimer disease, functional proton magnetic resonance spectroscopy, lactate, metabolomics, neuroimaging
Functional 1H magnetic resonance spectroscopy (fMRS) is a derivative of dynamic MRS imaging. This modality links metabolic response with functional activity, showing spectra of resonances that measure absolute or relative concentrations of metabolites.1 When a specific functional area of the brain becomes engaged in a task, fMRS can identify the neuronal activity by measuring and recording metabolite alterations, which is a necessary component of effective neuronal firing.2
Alzheimer disease (AD) is an irreversible neurodegenerative brain disorder and is the primary cause of dementia worldwide. The central aspect of this disease is cognitive impairment, ranging from memory loss in early phases to distortion of executive functioning in later phases.3,4 Identifying metabolite changes in biochemical pathways involving different diseases will disclose how these alterations affect interconnected reactions. This will make it possible to follow changes as the disease progresses.5,6
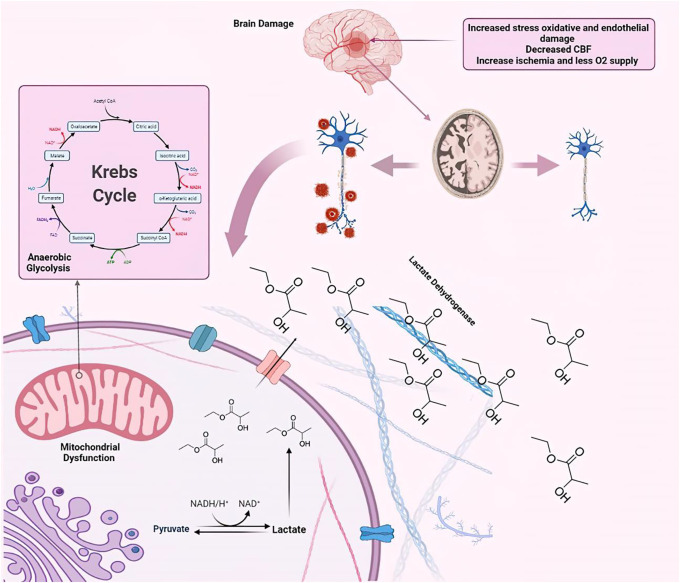
Evidence indicates that when glycolysis and mitochondrial metabolic pathways are disrupted in neurons or when the necessary substances for aerobic metabolism are not available, the anaerobic glycolysis cycle will be activated, generating lactate as the final product of the anaerobic pathway. Importantly, in pathological conditions and while the Krebs cycle is deficient, increased lactate levels are identified due to the reduction of oxygen (hypoxia or ischemia).7,8 This metabolite resonates in 1.32 ppm but shows a significant overlap with lipids/macromolecules, and this makes it challenging to differentiate. To overcome this issue, using echo time (TE) (135–144 msec) displayed to be helpful, so the lactate molecule (CH3COHCOOH) appears inverted and subtracted from lipids/macromolecules.8
Meanwhile, J-difference editing as a cutting-edge technique defines the “OFF” and “ON” spectra. ON acquisition includes a single-voxel Point Resolved Spectroscopy (PRESS) sequence.9 Mescher-Garwood scheme (MEGA)–PRESS is one of the popular protocols because it makes it possible to simultaneously edit the spectra and water suppression.10 These strategies can measure lactate metabolite in AD individuals. In this article, we discuss various facets of pathophysiologic conditions in AD (low blood flow and mitochondrial dysfunction) that directly or indirectly contribute to lactate imbalance. Furthermore, we will discuss the limitations and benefits of current imaging techniques while considering that the current literature surrounding fMRS imaging in AD is limited, we aim to emphasize on the potential role of lactate imaging in this disease.
PATHOGENESIS OF ALZHEIMER DISEASE: MORE THAN TANGLES
Cerebral Blood Flow and Microcirculation
Cerebral blood flow (CBF) is defined as the blood volume circulating through 100 grams of brain tissue per minute (50 ml/minute).11,12 The amount of blood gas and cerebral perfusion pressure are among the factors that determine the overall flow of blood through the brain tissue. CBF can change due to several factors; an increase in intracranial pressure due to the increased hydrostatic pressure results in high vessel outflow.12–15 Glial fibrillary acidic protein in astrocytes acts as another CBF regulator, which is believed to be reduced in both AD models and AD individuals. Alongside the CBF reduction because of the aging process, Aβ deposition, tauopathy, and reduced glial fibrillary acidic protein are expected, which intensify the deteriorating effect on the microcirculation of the central nervous system (CNS).16–18 This reduced blood flow can be used as an imaging marker in functional MR techniques, as it will be discussed further.
Energy, Metabolism, and Cell Signaling
Like other parts of the body, the function of neurons is highly dependent on glucose. After entering the brain cells, glucose is used in the Krebs cycle. Thirty-six to thirty-eight ATP molecules are expected to be produced throughout the Krebs cycle.19–21 This cycle requires oxygen for proper maintenance, and as oxygen supply drops, the anaerobic pathway is inevitably activated to provide the minimum amount of energy needed. In addition to the much lower amount of ATP produced, lactate will be present in the cytoplasm as the ultimate product of this cycle.22–24 After the increased lactate in neurons and astrocytes, there will be a decrease in pH and more potassium will be transported through the cell membranes, resulting in a disturbance of action potential.26
Dysregulation of Mitochondrial Function
Mitochondria have a crucial role in protein synthesis and ATP production in aerobic conditions (Krebs cycle), and mitochondrial dysfunction has been suggested as the main pathology of AD in some studies.26,27 Chou et al.26 observed mitochondrial dysfunctions as an early event in AD mouse models.
Hypoxia in various conditions such as decreased CBF as a secondary expected phenomenon in AD results in reduced ATP production, exacerbation of oxidative stress, and the formation of a defective cycle that leads to cell death due to an increase in reactive oxygen species.
Neurotransmitter Dysregulation (GABA/GLU—Lactate)
Neurotransmitters are chemical messengers in the central and peripheral nervous system and are made up of small amino acids or peptides and cause changes in the autonomic system regulation. Gamma-aminobutyric acid (GABA) and glutamate (GLU) are 2 neurotransmitters that are normally in equilibrium. This balance controls individual neurons and complexes of functional neurons in excited and nonexcited states. Any changes in the GABA/GLU balance lead to essential outcomes; in the case of excessive stimulation, GLU increases and GABA decreases, and in the cases of suppression, GABA increases in comparison with GLU, which causes parasympathetic overactivation.28–32 Acetylcholine levels are claimed to be reduced in AD individuals with consequent memory impairment.33,34 A higher ratio of GLU to GABA in AD individuals compared with the normal population is expected due to the effect of AD on GLU-GABA balance in the temporal lobe.28
Oxidative Stress and REDOX Damage
Oxidative stress is an unfortunate phenomenon that can occur in any cellular and subcellular components because of the imbalance between oxidants and pro-oxidants with antioxidants. In this phenomenon, superoxide is produced, which is highly reactive and can easily cause oxidative damage.35 It is now widely recognized that oxidative stress contributes to AD pathology. Accumulation of oxidizing cations such as iron and copper, Aβ deposits outside the cell, and hyperphosphorylated tau and mitochondrial dysfunction inside the cell are among the factors that have been known to cause oxidative stress and its exacerbation in AD individuals.36,37 Glutathione is an antioxidant that plays a vital role in the prevention of a defective cycle of oxidative stress in different parts of the body. Neuronal cells contain small quantities of glutathione, making the neurons susceptible to oxidative damage.37 Thus, it seems that low CBF in AD individuals result in lactate increase, which occurs because of the activation of the intracellular anaerobic cycle and neuronal cells fall into a defective cycle. This phenomenon leads to severe mitochondrial dysfunction.
LACTATE AND NEURODEGENERATION
Lactate Metabolism and Significance in Glycolysis
Lactate is the conjugate base of lactic acid, a byproduct of energy metabolism in cells. Classically, glucose can be metabolized in 2 main ways. In low-oxygen environment, anaerobic glycolysis is the main route of energy production.38 Glucose is first phosphorylated to remain in the cytosol. Then, isomerase turns glucose-6-p to fructose-6-p, which itself is again phosphorylated to fructose 1,6-bisphosphate. Then, aldolase and subsequent machinery of enzymes lead to creating 2 pyruvate molecules from a single glucose molecule. Overall, the pathway produces 2 ATP and 2 nicotinamide adenine dinucleotide (NADH) molecules, which can be further used in energy production. NAD+ plays a vital role in various metabolic pathways, and its lack is inconsistent with prolonged cellular physiology. Thus, if NAD+ cannot be generated using the electron transport chain in the mitochondria, it is regenerated through the production of lactate from pyruvate, A step mediated by the lactate dehydrogenase.38–40
In oxygen sufficiency, pyruvate is turned into acetyl coenzyme A through pyruvate dehydrogenase, with the latter molecule entering the tricarboxylic acid cycle. For each Acetyl-CoA molecule, the cycle generates 3 NADH, a single guanosine triphosphate molecule, and a single flavin adenine dinucleotide (FADH2) molecule.41,42 These are then entered into the electron transport chain in the mitochondria and subsequently used to maintain an H+ gradient along with the spaces of the mitochondria. This gradient is used by an ATP synthesis complex to form ATP from adenosine diphosphate and inorganic phosphate. The completed aerobic pathway creates 36 ATP molecules, and the anaerobic way makes a scant 4 ATPs.43–45
Thus, many cells rely on the aerobic method to generate energy. Only a limited number of cells in the human body depended solely on anaerobic glycolysis to survive (namely red blood cells).46
Role of Lactate in Energy Metabolism: The Astrocyte-Neuron Lactate Shuttle
Energy metabolism in the CNS is highly dependent on aerobic glycolysis, especially in high neuronal activity. Much of the energy consumed in the neurons is bound to fuel Na/K pumps, which are essential for transmitting signals from one neuron to the other.47 Conventionally, it was assumed that because of the high rates of aerobic glycolysis, lactate would be scant in intercellular spaces of the CNS. This was refuted by in vivo studies suggesting that lactate had a more than expected presence, even when neuronal activity was not significant.48 Further light was shed on this issue when histopathologic studies highlighted the cellular heterogeneity of the CNS because cells derived from an embryonic mesodermal origin in the CNS were not as dependent on aerobic metabolism. Astrocytes and oligodendrocytes processed glycogen glycolytically, yielding lactate and pyruvate from glucose.49
Interestingly, astrocytes particularly have multiple mitochondria and would be expected to benefit from the more productive aerobic pathway. However, owing to enzymatic profiles (hyperphosphorylation of pyruvate dehydrogenase) and structural variants in the mitochondrial respiratory chain, complexes cause suboptimal mitochondrial respiration.50 This phenomenon is similar to the aerobic glycolysis seen in malignant tissue, otherwise known as the Warburg effect.51 Pellerin L et al. presented a unified model to link the drastically different metabolisms of astrocytes and neurons, further expanding through numerous animal studies.52 According to this theory (and based on the evidence presented by observational studies), increased neuronal activity leads to increased GLU release to the extracellular space through the excitatory amino acid transporter 3 (EAAT3) transporters, presumed to exist exclusively on neuronal cell membranes. Astrocytes then uptake GLU through EAAT1-2 transporters, driven by a Na gradient. This also causes an increased uptake of glucose, which is entered into the glycolytic pathway. The resulting lactate then trickles out of the astrocyte, into the extracellular fluid, and into neurons, where it is further processed using lactate dehydrogenase, turned into pyruvate, which then can create mediators entering the tricarboxylic acid cycle.53–56
Physiologic Role of Lactate in Neuronal Functioning
As mentioned, astrocytes may have more critical roles in neuronal physiology than previously thought. Recently, an increased focus has been put on the part of astrocytes in metabolically supporting neurons in instances of improved neuronal functioning. It is now proposed that gluconeogenesis dependent on astrocytic glycogenolysis may be critical in generating neurotransmitters such as GLU and GABA.57,58 It is postulated that the same could be true for lactate. Empiric evidence suggests that distribution of the lactate transfer from astrocytes to neurons (either by upstream metabolic distributions which reduce lactate synthesis or by downstream loss of function of lactate transfer molecules out of astrocytes) leads to the impairment of memory formation.
Moreover, loss of function of monocarboxylate transporter 2 channels, situated on neurons and responsible for entering lactate into them, leads to the distribution of memory formation even when an excess amount of lactate is present in the extracellular space.59–62 More studies suggest that lactate may be involved in various forms of learning and decision making, which relies on signal transduction among different anatomic structures such as cortex, amygdala, cingulate gyrus, and hippocampus.63,64 One interesting observation is that adding lactate to the hippocampus can mitigate the amnesic effects of anti-beta adrenergic such as propranolol.65,66 β-adrenergic signaling initiated by neurons located in locus coeruleus has a critical role in consolidating inhibitory avoidance memory and is a classic example of how lactate may affect neuronal signaling.67 Importantly, restoring or injecting excess glucose only marginally substitutes lactate, suggesting that lactate's memory-enhancing and proplasticity effects may be partially energy independent.68 These effects may be mediated by gene-amplifying characteristics of lactate or due to the shifts in redox balance (with the introduction of NADH after lactate formation). Whatever the cause, studies show that heightened aerobic glycolysis is correlated with increased neuronal plasticity, with direct genomic relations, as the expression of certain neotenic genes ensures plasticity in specific areas of the brain in mammals69,70 (Figure 1).
FIGURE 1.
Physiologic role of lactate in neuronal functioning. Note that the figure was created by using the website BioRender.
Lactate as a Cellular Messenger in Cell Signaling Pathways
Scholars have looked at the possible roles of lactate in neuronal signaling owing to the significant amounts of lactate in the extracellular spaces in the CNS and the demonstrated role of lactate in cell signaling outside of the CNS (such as in cancers).71,72
One peculiar association has been proposed to be the role of lactate in locus coeruleus. In vivo studies have found that injection of L-lactate in physiologic concentrations can increase the production of norepinephrine, such as excitatory neurotransmitters including L-GLU. Importantly, these effects are not dependent on neuronal associations of astrocytes, and lactate can exhibit the mentioned effects without ever having to enter neurons.73 More studies have shown that these effects are not mediated by lactate shunts and are exerted through 2 opposing lactate binders. One of these receptors is the orphan receptor coupled with Gi, G protein–coupled receptor 81, and the other is the G protein–coupled receptors (GPCRs), excitatory receptor, which increases the levels of cyclic adenosine monophosphate by activating protein kinase A.74
In vivo studies on mice have also suggested that lactate may have a role in maintaining the sleep-wake cycles. It has been shown that preventing intercellular trafficking of lactate by clocking connexin molecules in mice hippocampus inhibits the excitatory synaptic activity. Similar effects are also seen in other brain regions, such as subthalamic nuclei, pyramidal cells, and CA1 subregion of the hippocampus.75–77
Interestingly, other signaling cascades are also affected by lactate. Yang et al. in 2014 showed that lactate can promote neuronal plasticity by potentiating the effects of the NMDA GLU receptor.68,78 It also increased the intracellular NADH and calcium levels, leading to brain-derived neurotrophic factor signaling potentiation. Brain-derived neurotrophic factor is a mediating neuronal factor that has established a role in exercise-related neuronal plasticity. Many studies suggest that lactate modulates the exact mechanism involved in exercise-related neuronal plasticity by SIRT1 activation, which enhances PGC1α/FNDC5/BDNF signaling.70,79
IMAGING IN ALZHEIMER DISEASE
Currently, AD diagnosis is challenging because there are no precise paraclinical criteria, and it is usually made based on the clinical criteria, which themselves are combinations of clinical signs and symptoms and mental status examination results, while biomarkers (e.g., concentrations of Aβ peptides [Aβ1–42:Aβ1–40 ratio]) and neuroimaging modalities, such as MRI and positron emission tomography (PET) scan, are widely used as confirmatory for the primary diagnosis.80,81 The Food and Drug Administration has approved the florbetapir (Amyvid) PET scan to estimate beta-amyloid neurotic plaque density in AD individuals.82 Nonetheless, amyloid PET scan is an invasive modality with high radiation dosage, and the cost effectiveness of this modality is still questionable.83
MR imaging of the nervous system can facilitate the detection of early changes associated with dementia such as regional atrophy, and some advanced MR techniques have been investigated, mainly in research, for the early detection of AD disease. These techniques, such as functional magnetic resonance imaging (fMRI) and arterial spin labeling (ASL), facilitate the diagnosis by providing dynamic and static imaging, respectively. Nonetheless, some of these methods need further clinical evaluation and represent hypotheses during preclinical studies.84,85
MRI in Alzheimer Disease
The traditional MR imaging in AD is classically based on Braak staging.86 The levels of atrophy are evaluated by an expert neurologist, in some areas of the brain such as the medial temporal lobe including hippocampus and entorhinal cortex.87,88 In addition, the loss of limbic gray matter (thalamus, amygdala, and cingulate gyrus), which is associated with memory loss, is another finding in MR imaging.89,90
MR imaging could also determine signal intensities related to AD plaques which could be detected on T2-weighted images and fluid-attenuated inversion recovery (FLAIR) images.91 White matter hyperintensities are also shown to be associated with disease activity and even severity of clinical signs.84,92 In this regard, white matter high T2-weighted, T2*-weighted with susceptibility-weighted imaging sequence, coronal 2D T2-weighted turbo-FLAIR, coronal 2D T2 turbo/fast spin-echo, sagittal 3D T1 MPRAGE/IR-SPGR postcontrast, and axial spin-echo T1 postcontrast are strongly suggested.93 Undoubtedly, going through all the noted modalities is not realistic, and it seems that selecting the most suitable shall be according to the local equipment and facilities. Nonetheless, static MRI techniques cannot be used in assessing the proclivity of the condition to deteriorate and even pinpoint the most recent changes, and other advanced dynamic techniques are required for this purpose.
Advanced MR Techniques
Among advanced MR techniques, DTI, ASL, and fMRI are suggested. DTI is based on the anisotropic diffusion and Brownian motion of water molecules of the brain. In this modality, mean diffusivity (MD) and fractional anisotropy (FA), which relate to the average rate of water molecule diffusivity and the variability associated with diffusion, are respectively measured.84 Increased MD in some brain regions such as the frontal and occipital lobes is shown to be associated with AD. By contrast, FA is decreased in the occipital and parietal lobes. That is because this method is dependent on water motion. Accordingly, artifacts would be more common in this technique. In addition, initially, metabolic changes occur in these tissues and can be detected by DTI if these metabolic changes lead to structural changes.94,95
On the other hand, ASL is a noninvasive and noncontrast MR perfusion technique. ASL labels arterial blood water protons; therefore, it can measure fluctuations in cerebral blood flow as an AD marker. The CBF is reduced significantly in AD individuals in temporal, frontal, parietal, thalamus, hippocampus, and amygdala.96,97 Furthermore, lower CBF has been shown to be associated with faster cognitive decline in AD individuals.98 Although this modality is noninvasive and nonionizing, alterations in signal intensity and the low signal-to-noise ratio (SNR) reduce image quality.99,100 fMRI is a blood-oxygen-level-dependent or BOLD-contrast imaging technique.101 Individuals can be evaluated for brain activity during the task or resting state. Studies showed that hippocampal and medial temporal lobe have low activation rates in AD individuals.102
Working memory, semantic knowledge, attention, motor performance, and visuospatial ability are among the functions included in the task mode.103,104 The posterior cingulate cortex, entorhinal cortex, and hippocampus were evaluated in this mode, but the most engaged structure was medial temporal lobe.105,106 Nonetheless, fMRI is not used in routine clinical evaluation, possibly because of its low SNR for neuronal activity.107
As discussed, many dynamic modalities encounter quality limitations in clinical diagnostics. However, quantitative imaging techniques have shown promise in recent years. MRS as a quantitative imaging measures CNS metabolite concentrations. For example, N-acetyl aspartate (NAA)/creatine (Cr) and NAA/myo-inositol (mI) can be used as markers for AD.108 This modality, however, has limited sensitivity for diagnosing AD; therefore, the combination of different modalities could yield a better image in both quantity and quality. In this manner, fMRS has been developed to detect CNS abnormalities in mice and humans.2,8,108–115
fMRS PROTOCOL
Repetition Time, Echo Time, and T2* Relaxation
Repetition time (TR) and TE are measured in milliseconds (msec). The degree of longitudinal magnetization recovery between each pulse determines by TR between successive pulse sequences applied to the same slice.116 The time interval between radio frequency pulse transmissions and the reception of echo signals is called TE. Furthermore, TE regulates the quantity of T2* (transverse relaxation time),117 during which the gradient echo (GRE) loses signal strength.118 Magnetic field inhomogeneity can be macroscopic (constant across a voxel) or microscopic (variable across a voxel). The phase shift in a GRE image reflects the average magnetic field of protons in a voxel, which is affected by the tissues' local susceptibility.119
For the primary GRE sequence fast low-angle shot (FLASH), the greater flip angles give the image more T1 weighting and the lower flip angles give the image more T2* weighting while TE has increased.120 In this fast GRE sequence, the gradian is diminished semirandomly after every echo, eradicating transverse magnetization by changing the phase space. In addition, to avoid susceptibility artifact, the amount of TE is set to minimum.121
By contrast, there is no 180° refocusing pulse in GRE sequences; thus, dephasing effects are not reduced. As a result, transverse relaxation (i.e., T2* relaxation) in GRE sequences is a mix of “true” T2 relaxation and relaxation produced by magnetic field inhomogeneities. The 1/T2* = 1/T2 + γ ΔBinhom equation showed that T2* is shorter than T2; in this equation, ΔBinhom is the magnetic field inhomogeneity across a voxel and γ is the gyromagnetic ratio.122
There have been few studies evaluating lactate with 1H-MRS in AD individuals in recent years. In these studies, MRI equipment used 1.5 and 3 Tesla (T), short TE (11–35 msec), and long TR (500–2000 msec) with direct dimension bandwidth (2 and 2.5 kHz). These studies used the PRESS protocol (Table 1).127–130
Table 1.
Summarize Characteristics of MRS Protocol With Lactate for AD Individuals
| Study | MRI Equipment | Coil | MRS Protocol | MRS TE (msec) | MRS TR (msec) | DDB (kHz) |
| R Mullins et al., 2018123 | Philips 3T | 8‐channel SENSE head coil | J‐PRESS | 35 | 2000 | 2 |
| KE Weaver et al., 2015124 | Philips 3 T | 8-channel SENSE head coil | PRESS | 24 | 2000 | 2 |
| T Ernst et al., 1997125 | GE 1.5 T | NA | PRESS | 11 | 500 | 2.5 |
| W. Block et al., 1995126 | Philips 1.5 T | NA | NA | NA | 2000 | NA |
J‐PRESS, J‐modulated point‐resolved spectroscopy, DDB, direct dimension bandwidth.
However, the fMRS protocol for lactate in healthy volunteers was run with short and long TE (6–144 msec) and long TR (1500–7500 msec) and using MEGA, LASER, SPECIAL, PRESS, and STEAM protocols (Table 2).2,8,108–115
Table 2.
Summarized Characteristics and Technical Specifications of fMRI Imaging in Studies Aiming to Quantify Lactate Levels
| Study | MRI Equipment | Population | Evaluation | MRS Protocol | MRS TE (msec) | MRS TR (msec) | fMRI Paradigm | Outcomes |
| Y Koush et al., 2021120 | 3 T Siemens Prisma scanner using 64-channel head/neck coil | Twenty healthy volunteers | Cortical areas in the human brain | MEGA | 144 | 2700 | Visual stimulation/cognitive task | Lactate increased on activating the visual cortex but did not change on deactivating the posterior cingulate cortex. |
| CC Fernandes et al., 2020119 | 7 T Philips, 32‐channel receive head coil | Six healthy volunteers | Human visual cortex | MEGA (semi‐LASER) | 144 | 2000 | Visual stimulation | Lactate increases significantly (∼10%). |
| Ligneul et al., 2020117 | 9.4 T Bruker BioSpec scanner | 20 mice (C57Bl6 females, age 3–4 months) | Mouse superior colliculus activity | LASER | 28 | 1500 | Visual stimulation | NAAG, PCr, Cr, and GLU were increased measured in the superior colliculus |
| Y Koush et al., 2019116 | 4 T Bruker spectrometer using a 16-channel transmit-receive head coil | Ten healthy volunteers | Human motor cortex | MEGA | 144 | 3330 | Finger-to-thumb tapping | J-edited fMRS has high sensitivity and specificity for task-induced lactate modulation. |
| R Mekle et al., 2017127 | 7 T Siemens whole‐body system with a 60‐cm bore and a whole-body gradient coil | Twenty healthy volunteers | Human visual cortex | SPECIAL | 30 | 2000 | Visual stimulation | An increase in lactate of 0.04 mmol/L (7%) was the only significant effect. |
| P Bednařík et al., 2015118 | 7 T/90-cm magnet (Agilent/Magnex Scientific) | Fifteen healthy volunteers | Human visual cortex | Semi-LASER | 26 | 5000 | The block-designed paradigm of visual stimulation | BOLD-fMRI signals were a linear relationship positively correlated with lactate concentration changes. |
| B Schaller et al., 20142 | 7 T/68-cm scanner Siemens, 2-channel receive coil | Eleven healthy volunteers | Motor activation | SPECIAL | 12 | 7500 | Finger-to-thumb tapping | Increases in lactate during motor stimulation are small. The lactate changes may be a general manifestation of the increased neuronal activity. |
| B Schaller et al., 2013128 | 7 T/68-cm Siemens | Ten healthy volunteers | Human visual cortex | SPECIAL | 6 | 5000 | Visual stimulation | The rate of lactate concentration increases. |
| AL Lin et al., 2010121 | 3 T Trio MRI scanner (Siemens) | Twelve healthy volunteers | Human visual cortex | PRESS | 30 | 2000 | The reversing-checkerboard paradigm of visual stimulation | The energy demand of task-induced brain activation is approximately 15%. Increases in CBF positively correlated with lactate production. |
| S Mangia et al., 2007122 | 7 T/90-cm horizontal bore magnet (Magnex Scientific) | Twelve healthy volunteers | Human visual cortex | STEAM | 6 | 5000 | Visual stimulation | The lactate concentration reached the new steady-state level within the first minute of activation and returned to baseline only after the stimulus ended. |
Spin‐echo full‐intensity acquired localized (SPECIAL), Mescher-Garwood scheme (MEGA)
Metabolites such as lactate in pathologies could be recorded with edited 1H-MRS protocol if the expected levels are higher. In addition, long TE (144 msec) can differentiate the overlapping signals from lipids/macromolecules, which is required for exact evaluation of changes in lactate (or other metabolites) at low SNR.2,8
Signal-to-Noise Ratio
In imaging, the SNR is a measure of genuine signal (i.e., representing actual anatomy) to noise (e.g., random quantum mottle).131,132 The SNR is routinely evaluated in MRI by measuring the signal intensity difference between the region of interest (ROI) and the background.133
When MRI is used instead of 2D imaging, volume acquisition can increase the SNR. However, imaging time for spin-echo sequences is longer than GRE sequences.134 The use of surface coils increases the SNR, and increasing the number of excitations reduces the TE while increasing the TR.126,135
The main challenge of formulating a proper fMRS protocol with long TE is to reduce SNR while reaching a good SNR for measuring lactate. On the other hand, SNR can be reduced by decreasing bandwidth (BW) or increasing averaging. In addition, correcting the uniformity of the field automatically or manually could enhance SNR. Placing the MRS voxel in the correct position could also decrease noise (not including air and water inside the voxels and selecting voxels with dexterity, especially where the anatomical tissue changes are large, e.g., the base of the skull). Correctly placing the suppression bands around the voxel can also be effective. Increasing the TR also helps to some extent, although the amount of TR should not be less than 2000 msec.8,111
fMRS Examination
The fMRI technique measures brain activity and identifies minor changes in blood flow, showing stimuli or actions (most frequently BOLD imaging). This modality relies on minute changes in a low SNR environment, making it technically challenging.123,125
The individual's activity or stimulus is referred to as a paradigm, and each one is meant to extract a specific cerebral response. Many paradigms with varying levels of complexity have been developed. Four paradigms were assigned to different clinical responses (visual, motor, speech, memory, and clinical practice). It can look at the brain's functional architecture to see which regions oversee specific functions. It can be used to assess the impact of stroke, trauma, and degenerative diseases such as AD on brain function.114,116
Repeated paradigms are separated by blocks of inactivity or alternate action in a block design. In clinical fMRI, this is by far the most common design. Individual events, rather than blocks, are used in the event-related design, and they can be dispersed arbitrarily across the research. During an fMRI examination, tapping fingers or toes, pursing lips, moving tongue, reading, seeing images, listening to speech, and playing simple word games are possible ways to show the brain's activity.111,112
The fMRI is now a cornerstone of neuroimaging in clinical and fundamental brain research. However, fMRI would be an ideal diagnostic tool because of its noninvasive nature and significant spatial resolution while limited in temporal resolution. On the other hand, human studies were shown to cause a consistently detectable BOLD response, with even modest lactate alterations, creating plasma concentrations equivalent to moderate muscular activity.124,125,136 Similarly, the lactate level was significantly higher in the rodent somatosensory barrel cortex (S1bf) during stimulation or on the early visual cortex in animal experiments with the BOLD response.137,138 Nonetheless, fMRI shows functional activity, but brain metabolism is missed. There are some techniques such as autoradiography, positron emission tomography (PET), two-photon fluorescent confocal microscopy (TPEF or 2PEF), and MRS to analyze brain metabolism.139–141 Both autoradiography and PET are noninvasive methods and have low spatial resolution images.142 In addition, MRS reveals the number of metabolites but not the brain activity.135 As a result, functional MRS (fMRS) is a noninvasive and nonradioactive technique and may investigate any brain region.143
The peak of macromolecule is unknown; variations in their quantities have been linked to stroke, multiple sclerosis, and malignancies. Several peaks are at 0.9, 2.05, and 3.0 ppm.144 Acetate and macromolecular proteins are among the lipids with multiple vast peaks between 0.8 and 1.3 ppm. Lipid levels, lactate, and alanine increase in various cancers.145 The methine proton is J-coupled to the 3 methyl group protons in lactate through the C-C connection. In addition, ±3.47 Hz (J=6.93 Hz) of precession frequency shift is caused by coupling between 2 groups of protons.146
As proton MR spectroscopy shows, anaerobic glycolysis ends with the lactate resonance. Stroke, high-grade malignancies, and abscesses are examples of diseases compatible with our understanding of the biochemical processes taking place in the body. Long-echo proton MRS investigations use J-coupling to distinguish lactate methyl and methine proton peaks (which occur at 7 Hertz).10
If lengthy echo durations (144 and 288 msec) (multiples of 1/7 Hz) are used, it can differentiate lactate signals from lipid signals in the blood. Spin or J-coupling inverts the resonance when sampling with a 144 ms echo period.143,147 As a result, the lactate doublet peak may be distinguished from macromolecules and lipids. Still, the signal strength is reduced, particularly troublesome at low concentrations. At 288 msec, the doublet peak appears above the baseline.8,110 The lactate peak seen at the doublet may be unbalanced due to transmission and reception problems. In addition, the signal should physically match the high-fat sign-on imaging at a time interval of 288 ms if it originates from lipids. Thus, anaerobic glycolysis is the cause of lactate development which is considered pathological. Depending on the situation, the lactate signal may potentially represent mitochondrial dysfunction.148
A greater B0 field demands different methods to B1-related abnormalities in J-difference MRS in vivo, although the MEGA-PRESS sequence is the most frequent lactate detection by 1H-MRS with a long TE J-difference, which naturally eliminates overlapping signals. The MEGA-PRESS is a J-difference edited MRS pulse sequence consisting of ‘ON’ and ‘OFF’ subexperiments.149 In contrast to the ‘OFF' experiment, the' ON' experiment uses frequency-selective radio frequency editing pulses to edit the lactate molecule's 1.32 ppm resonance.8
Lactate is separated from overlapping resonances and macromolecules using spectral editing with J-modulation, which uses the quantum mechanical characteristics of specific molecules to “edit” them out of the overall 1H-MRS spectrum.8,111 On the other hand, J-editing is prone to subtraction mistakes caused by motion, which can hide tiny changes in the lactate by using quantum mechanical characteristics of lactate to remove it from the overall spectrum; J-edited 1H-MRS can provide direct insights into the overlapped resonances that occur from numerous molecules such as fatty acids or macromolecules.110 Because of considerable suppression of the relatively short T2 for the lipid and macromolecule background signals, 1H-MRS has demonstrated that physiological modulations of lactate in the visual cortex may be seen even at 1.5 T using long TE.143
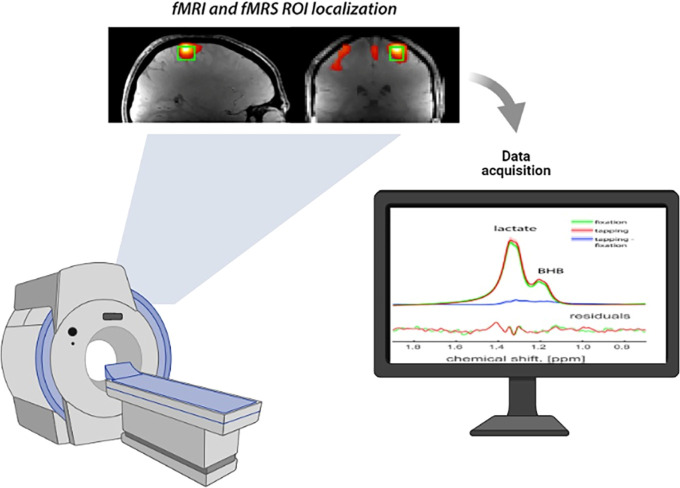
Overall, J-edited fMRS has high sensitivity and specificity for task-induced lactate modulation8 and shows the rate of increase in lactate concentration.2 Compared with the fixation condition, a significant increase was detected in lactate levels.8,108,111 The rate of lactate increases significantly between 7 and 15 percent.110,112,114 In addition, the result shows that lactate increased on activating the visual cortex but did not change on deactivating the posterior cingulate cortex111; however, increases in lactate during motor stimulation are less significant2 (Figure 2, Figure 3).150
FIGURE 2.
fMRS experimental design and results. Note that the figure was created by using the website BioRender.
FIGURE 3.
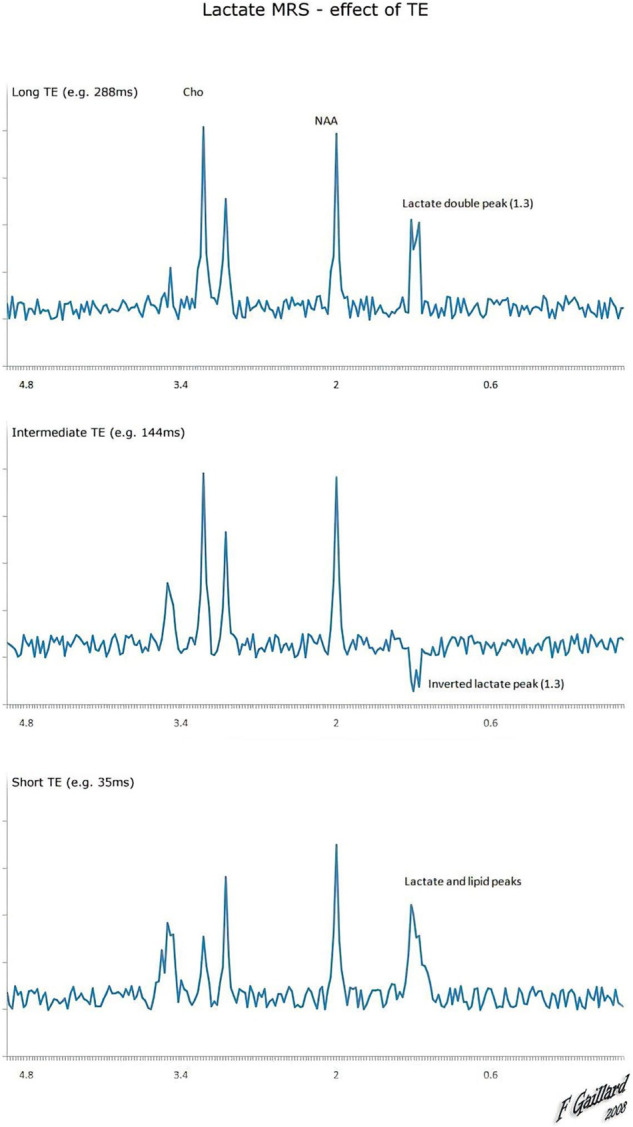
Long TE can differentiate the overlapping signals from lipids/macromolecules; the main challenge of formulating a proper fMRS protocol with long TE is to reduce SNR while reaching a good SNR for measuring lactate.
LIMITATIONS AND FUTURE PROSPECTS
Although the prospect of different derivatives of MRI imaging in AD is promising, several issues limit the possible beneficence of these techniques in routine clinical consideration. One problem is the low specificity of certain imaging signs and quantitative outputs (such as those of MRS) in differentiating dementia from other common clinical pathologies, such as stroke, benign senile changes, and mild to moderate mental decline.151 Furthermore, there is no consensus on the criteria for patient selection for specific derivatives of MR imaging in dementia, and simply changing the study population could have significant effects on the diagnostic profile of the studied modality (most notably the positive and negative predictive values). Importantly, the relative importance of quantifiable findings (such as those of MRS) is not known compared with anatomical studies, which are the major yield in conventional MR imaging.152 Note that, in contrary to Cholin and NAA, specifically NAA can demonstrate relationship to disease severity,153–155 clinical results for this association for lactate levels are limited. A recent clinical study on AD and mild cognitively impaired adults showed that CSF lactate levels were associated with age and blood-brain barrier integrity but not with clinical severity or CSF biomarkers of AD.156 This hypothesis should be investigated through clinical studies, and future research could focus on the possible role of lactate imaging in AD diagnosis by focusing on the combined efficacy of novel quantitative MR methods and the anatomic preciseness offered by conventional MR imaging.
CONCLUSION
This comprehensive review concludes that fMRS with J-editing is a cutting-edge technique to detect lactate in ADs. This modality during functional activation by omitting overlapping lipid and macromolecule signals provides high SNR than other modalities to measure lactate. However, this method is still not indicated for diagnostic purposes based on the clinical evidence. Still, it seems that lactate evaluation with high B0 fMRS equipment increase 3–7 Tesla provides more accurate results.
Footnotes
The authors declare no conflict of interest
All authors contributed equally to the study.
Contributor Information
Kiarash Shirbandi, Email: Shirbandi.k@gmail.com.
Reza Rikhtegar, Email: reza_rikhtegar@yahoo.com.
Mohammad Khalafi, Email: mohammadkhalafi4287@gmail.com.
Mohammad Mirza Aghazadeh Attari, Email: mohammadkhalafi4287@gmail.com.
Farzaneh Rahmani, Email: farzaneh.rahmani.usern@gmail.com.
Pouya Javanmardi, Email: Pjavanmardi98@gmail.com.
Sajjad Iraji, Email: sajjad.irj@gmail.com.
Zahra Babaei Aghdam, Email: zahraba@gmail.com.
Amir Mohammad Rezaei Rashnoudi, Email: Rezaeirashnoudi@gmail.com.
REFERENCES
- 1.Hyder F, Rothman DL. Advances in Imaging Brain Metabolism. Annu Rev Biomed Eng. 2017;19:485–515. [DOI] [PubMed] [Google Scholar]
- 2.Schaller B, Xin L, O'Brien K, et al. Are glutamate and lactate increases ubiquitous to physiological activation? A (1)H functional MR spectroscopy study during motor activation in human brain at 7Tesla. NeuroImage. 2014;93:138–145. [DOI] [PubMed] [Google Scholar]
- 3.Shirbandi K, Khalafi M, Mirza-Aghazadeh-Attari M, et al. Accuracy of deep learning model-assisted amyloid positron emission tomography scan in predicting Alzheimer's disease: a systematic review and meta-analysis. Inform Med Unlocked. 2021;25:100710. [Google Scholar]
- 4.Pimplikar SW, Nixon RA, Robakis NK, et al. Amyloid-independent mechanisms in Alzheimer's disease pathogenesis. J Neurosc. 2010;30(45):14946–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toledo JB, Arnold M, Kastenmuller G, et al. Metabolic network failures in Alzheimer's disease: A biochemical road map. Alzheimer's Demen. 2017;13(9):965–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkins JM, Trushina E. Application of metabolomics in Alzheimer's disease. Front Neurol. 2017;8:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liguori C, Chiaravalloti A, Sancesario G, et al. Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2016;43(11):2040–2049. [DOI] [PubMed] [Google Scholar]
- 8.Koush Y, de Graaf RA, Jiang L, et al. Functional MRS with J-edited lactate in human motor cortex at 4T. NeuroImage. 2019;184:101–108. [DOI] [PubMed] [Google Scholar]
- 9.Wijnen JP, Haarsma J, Boer VO, et al. Detection of lactate in the striatum without contamination of macromolecules by J-difference editing MRS at 7T. NMR Biomed. 2015;28(4):514–522. [DOI] [PubMed] [Google Scholar]
- 10.Mescher M, Merkle H, Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. [DOI] [PubMed] [Google Scholar]
- 11.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18(7):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korte N, Nortley R, Attwell D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer's disease. Acta Neuropathologica. 140(6), 2020:793–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polinder-Bos HA, García DV, Kuipers J, et al. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol. 2018;29(4):1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slupe AM, Kirsch JR. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J Cereb Blood Flow Metab. 2018;38(12):2192–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol. 2017;102(11):1356–1371. [DOI] [PubMed] [Google Scholar]
- 16.Brenner M. Role of GFAP in CNS injuries. Neurosci Lett. 2014;565:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villapol S, Byrnes KR, Symes AJ. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte–vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front Neurol. 2014;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirbandi K, Khalafi M, Bevelacqua JJ, et al. Exposure to low levels of radiofrequency electromagnetic fields emitted from cell-phones as a promising treatment of Alzheimer’s Disease: A scoping review study. J Biomed Phys Eng. 2023;13:3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004:19:169–175. [DOI] [PubMed] [Google Scholar]
- 20.Ryan DG, O'Neill LA. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38(1):289–313. [DOI] [PubMed] [Google Scholar]
- 21.Scaini G, Santos PM, Benedet J, et al. Evaluation of Krebs cycle enzymes in the brain of rats after chronic administration of antidepressants. Brain Res Bull. 2010;82(3-4):224–227. [DOI] [PubMed] [Google Scholar]
- 22.Chan TS, Cassim S, Raymond V-A, et al. Upregulation of Krebs cycle and anaerobic glycolysis activity early after onset of liver ischemia. PLoS One. 2018;13(6):e0199177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evangelisti S, Lettieri P, Borello D, et al. Life cycle assessment of energy from waste via anaerobic digestion: a UK case study. Waste Manag. 2014;34(1):226–237. [DOI] [PubMed] [Google Scholar]
- 24.Müller M, Mentel M, van Hellemond JJ, et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol Mol Biol Rev. 2012;76(2):444–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcinek DJ, Kushmerick MJ, Conley KE. Lactic acidosis in vivo: testing the link between lactate generation and H+ accumulation in ischemic mouse muscle. J Appl Physiol. 2010;108(6):1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou JL, Shenoy DV, Thomas N, et al. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer's disease. J Proteomics. 2011;74(4):466–479. [DOI] [PubMed] [Google Scholar]
- 27.Dai W, Jiang L. Dysregulated mitochondrial dynamics and metabolism in obesity, diabetes, and cancer. Front Endocrinol. 2019;10:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Alloza M, Tsang SW, Gil-Bea FJ, et al. Involvement of the GABAergic system in depressive symptoms of Alzheimer's disease. Neurobiol Aging. 2006;27(8):1110–1117. [DOI] [PubMed] [Google Scholar]
- 29.Huang D, Liu D, Yin J, et al. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol. 2017;27(7):2698–2705. [DOI] [PubMed] [Google Scholar]
- 30.Manyevitch R, Protas M, Scarpiello S, et al. Evaluation of metabolic and synaptic dysfunction hypotheses of Alzheimer's disease (AD): A meta-analysis of CSF markers. Curr Alzheimer Res. 2018;15(2):164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naylor DE. Glutamate and GABA in the balance: convergent pathways sustain seizures during status epilepticus. Epilepsia. 2010;51:106–109. [DOI] [PubMed] [Google Scholar]
- 32.Yin Y, Zhao Y, Han S, et al. Autophagy-ERK1/2-Involved Disinhibition of Hippocampal Neurons Contributes to the Pre-Synaptic Toxicity Induced by Aβ 42 Exposure. J Alzheimer's Dis. 2017;59(3):851–869. [DOI] [PubMed] [Google Scholar]
- 33.Aykac A, Ozbeyli D, Uncu M, et al. Evaluation of the protective effect of Myrtus communis in scopolamine-induced Alzheimer model through cholinergic receptors. Gene. 2019;689:194–201. [DOI] [PubMed] [Google Scholar]
- 34.Giacobini E, Sugaya K, Elble RJ. Markers of cholinergic dysfunction in Alzheimer disease. In: Alzheimer Disease. Boca Raton, FL: CRC Press; 2020:137–156. [Google Scholar]
- 35.Sies H. On the history of oxidative stress: Concept and some aspects of current development. Curr Opin Toxicol. 2018;7:122–126. [Google Scholar]
- 36.Cheignon C, Tomas M, Bonnefont-Rousselot D, et al. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Zhong C. Oxidative stress in Alzheimer's disease. Neurosci Bull. 2014;30(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melkonian EA, Schury MP. Biochemistry, Anaerobic Glycolysis. Treasure Island, FL: StatPearls Publishing. 2019. [PubMed] [Google Scholar]
- 39.Farhana A, Lappin SL. Biochemistry, Lactate Dehydrogenase. Treasure Island, FL: StatPearls Publishing. 2020. [PubMed] [Google Scholar]
- 40.Khan AA, Allemailem KS, Alhumaydhi FA, et al. The biochemical and clinical perspectives of lactate dehydrogenase: an enzyme of active metabolism. Endocr Metab Immune Disorders-Drug Targets. 2020;20(6):855–868. [DOI] [PubMed] [Google Scholar]
- 41.Jones W, Bianchi K. Aerobic glycolysis: beyond proliferation. Front Immunol. 2015;6:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naifeh J, Dimri M, Varacallo M. Biochemistry, Aerobic Glycolysis. Treasure Island, FL: StatPearls Publishing. 2017. [PubMed] [Google Scholar]
- 43.Frazier AE, Thorburn DR. Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methods Mol Biol. 2012;837:49–62. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad M, Wolberg A, Kahwaji CI. Biochemistry, Electron Transport Chain. Treasure Island, FL: StatPearls Publishing. 2020. [PubMed] [Google Scholar]
- 45.Zhao RZ, Jiang S, Zhang L, et al. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019;44(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol BioSystems. 2013;9(6):1196–1209. [DOI] [PubMed] [Google Scholar]
- 47.Rao J, Oz G, Seaquist ER. Regulation of cerebral glucose metabolism. Minerva Endocrinologica. 2006;31(2):149–158. [PubMed] [Google Scholar]
- 48.Riske L, Thomas RK, Baker GB, et al. Lactate in the brain: an update on its relevance to brain energy, neurons, glia and panic disorder. Ther Adv Psychopharmacol. 2017;7(2):85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fünfschilling U, Supplie LM, Mahad D, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Fabuel I, Le Douce J, Logan A, et al. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci. 2016;113(46):13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32(7):1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pellerin L, Bouzier-Sore AK, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55(12):1251–1262. [DOI] [PubMed] [Google Scholar]
- 54.Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. The Neuroscientist. 2004;10(1):53–62. [DOI] [PubMed] [Google Scholar]
- 55.Pellerin L. Lactate as a pivotal element in neuron–glia metabolic cooperation. Neurochem Int. 2003;43(4-5):331–338. [DOI] [PubMed] [Google Scholar]
- 56.Pellerin L. Food for thought: the importance of glucose and other energy substrates for sustaining brain function under varying levels of activity. Diabetes Metab. 2010;36:S59–S63. [DOI] [PubMed] [Google Scholar]
- 57.Schousboe A, Scafidi S, Bak LK, et al. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anlauf E, Derouiche A. Glutamine synthetase as an astrocytic marker: its cell type and vesicle localization. Front Endocrinol. 2013;4:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouzier-Sore A-K, Pellerin L. Unraveling the complex metabolic nature of astrocytes. Front Cell Neurosci. 2013;7:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54(3):214–222. [DOI] [PubMed] [Google Scholar]
- 61.Tadi M, Allaman I, Lengacher S, et al. Learning-induced gene expression in the hippocampus reveals a role of neuron-astrocyte metabolic coupling in long term memory. PLoS One. 2015;10:e0141568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16(1):547–563. [DOI] [PubMed] [Google Scholar]
- 64.Müller N, Knight R. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139(1):51–58. [DOI] [PubMed] [Google Scholar]
- 65.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63(3):844–914. [DOI] [PubMed] [Google Scholar]
- 66.Magistretti P, Morrison J. Noradrenaline-and vasoactive intestinal peptide-containing neuronal systems in neocortex: functional convergence with contrasting morphology. Neuroscience. 1988;24(2):367–378. [DOI] [PubMed] [Google Scholar]
- 67.Atucha E, Roozendaal B. The inhibitory avoidance discrimination task to investigate accuracy of memory. Front Behav Neurosci. 2015;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, Ruchti E, Petit J-M, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci. 2014;111(33):12228–12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goyal MS, Vlassenko AG, Blazey TM, et al. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 2017;26(2):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Müller P, Duderstadt Y, Lessmann V, et al. BDNF: Key mediators of exercise induced neuroplasticity? J Clin Med. 2020;9(4):1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, et al. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, Wang D, Han S, et al. Bioactivity-guided identification and cell signaling technology to delineate the lactate dehydrogenase A inhibition effects of Spatholobus suberectus on breast cancer. PLoS One. 2013;8(2):e56631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang F, Lane S, Korsak A, et al. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5(1):3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Q, Hu Y, Wan J, et al. Lactate: a novel signaling molecule in synaptic plasticity and drug addiction. BioEssays. 2019;41(8):1900008. [DOI] [PubMed] [Google Scholar]
- 75.Sada N, Lee S, Katsu T, et al. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347(6228):1362–1367. [DOI] [PubMed] [Google Scholar]
- 76.Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–1555. [DOI] [PubMed] [Google Scholar]
- 77.Clasadonte J, Scemes E, Wang Z, et al. Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron. 2017;95(6):1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leßmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res. 2009;65(1):11–22. [DOI] [PubMed] [Google Scholar]
- 79.El Hayek L, Khalifeh M, Zibara V, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiwari S, Atluri V, Kaushik A, et al. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 2019;14:5541–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graff-Radford J, Yong KXX, Apostolova LG, et al. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang L, Rieves D, Ganley C. Brain amyloid imaging—FDA approval of Florbetapir F18 injection. New Eng J Med. 2012;367(10):885–887. [DOI] [PubMed] [Google Scholar]
- 83.Lameka K, Farwell MD, Ichise M. Positron emission tomography. Handb Clin Neurol. 2016;135:209–227. [DOI] [PubMed] [Google Scholar]
- 84.Chandra A, Dervenoulas G, Politis M, Magnetic resonance imaging in Alzheimer’s disease and mild cognitive impairment. J Neurol. 2019;266(6):1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Politis M, Piccini P. Positron emission tomography imaging in neurological disorders. J Neurol. 2012;259(9):1769–1780. [DOI] [PubMed] [Google Scholar]
- 86.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 87.Du AT, Schuff N, Kramer JH, et al. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology. 2004;62(3):422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pennanen C, Kivipelto M, Tuomainen S, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD Neurobiol Aging. 2004;25(3):303–310. [DOI] [PubMed] [Google Scholar]
- 89.Cavedo E, Boccardi M, Ganzola R, et al. Local amygdala structural differences with 3T MRI in patients with Alzheimer disease. Neurology 2011;76(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomann PA, Dos Santos V, Toro P, et al. Reduced olfactory bulb and tract volume in early Alzheimer's disease–a MRI study. Neurobiol Aging. 2009;30(5):838–841. [DOI] [PubMed] [Google Scholar]
- 91.Bocti C, Swartz RH, Gao FQ, et al. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke. 2005;36(10):2126–2131. [DOI] [PubMed] [Google Scholar]
- 92.Nasrabady SE, Rizvi B, Goldman JE, et al. White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun. 2018;6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chamberlain R, Reyes D, Curran GL, et al. Comparison of amyloid plaque contrast generated by T2-weighted, T2*-weighted, and susceptibility-weighted imaging methods in transgenic mouse models of Alzheimer's disease. Magn Reson Med. 2009;61(5):1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Schuff N, Du AT, et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain. 2009;132(9):2579–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alexopoulos P, Sorg C, Förschler A, et al. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer's disease measured by pulsed arterial spin labeling MRI. Eur Arch Psychiatry Clin Neurosci. 2012;262(1):69–77. [DOI] [PubMed] [Google Scholar]
- 97.Mak HKF, Qian W, Ng KS, et al. Combination of MRI hippocampal volumetry and arterial spin labeling MR perfusion at 3-Tesla improves the efficacy in discriminating Alzheimer's disease from cognitively normal elderly adults. J Alzheimers Dis. 2014;41(3):749–758. [DOI] [PubMed] [Google Scholar]
- 98.Benedictus MR, Leeuwis AE, Binnewijzend MAA, et al. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur Radiol. 2017;27(3):1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alsaedi A, Thomas D, Bisdas S, et al. Overview and critical appraisal of arterial spin labelling technique in brain perfusion imaging. Contrast Media Mol Imaging. 2018;2018:5360375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshiura T, Hiwatashi A, Noguchi T, et al. Arterial spin labelling at 3-T MR imaging for detection of individuals with Alzheimer’s disease. Eur Radiol. 2009;19(12):2819–2825. [DOI] [PubMed] [Google Scholar]
- 101.Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. [DOI] [PubMed] [Google Scholar]
- 102.Vidoni ED, Thomas GP, Honea RA, et al. Evidence of altered corticomotor system connectivity in early-stage Alzheimer's disease. J Neurol Phys Ther. 2012;36(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yetkin FZ, Rosenberg RN, Weiner MF, et al. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer's disease. Eur Radiol. 2006;16(1):193–206. [DOI] [PubMed] [Google Scholar]
- 104.Parra MA, Pattan V, Wong D, et al. Medial temporal lobe function during emotional memory in early Alzheimer's disease, mild cognitive impairment and healthy ageing: an fMRI study. Psychiatry. 2013;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Small SA, Perera GM, DeLaPaz R, et al. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol. 1999;45(4):466–472. [DOI] [PubMed] [Google Scholar]
- 106.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGuire PK, Robertson D, Thacker A, et al. Neural correlates of thinking in sign language. Neuroreport. 1997;8(3):695–698. [DOI] [PubMed] [Google Scholar]
- 108.Ligneul C, Fernandes FF, Shemesh N. Functional Magnetic Resonance Spectroscopy in the mouse. arXiv:200108505. 2020; [DOI] [PubMed] [Google Scholar]
- 109.Bednařík P, Tkáč I, Giove F, et al. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. 2015;35(4):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandes CC, Lanz B, Chen C, et al. Measurement of brain lactate during visual stimulation using a long TE semi-LASER sequence at 7 T. NMR Biomed. 2020;33(4):e4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koush Y, de Graaf RA, Kupers R, et al. Metabolic underpinnings of activated and deactivated cortical areas in human brain. J Cereb Blood Flow Metab. 2021;41(5):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin A-L, Fox PT, Hardies J, et al. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci. 2010;107(18):8446–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mangia S, Tkác I, Gruetter R, et al. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27(5):1055–1063. [DOI] [PubMed] [Google Scholar]
- 114.Mekle R, Kühn S, Pfeiffer H, et al. Detection of metabolite changes in response to a varying visual stimulation paradigm using short-TE (1) H MRS at 7 T. NMR Biomed. 2017; 30:e3672. [DOI] [PubMed] [Google Scholar]
- 115.Schaller B, Mekle R, Xin L, et al. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. Research. 2013;91(8):1076–1083. [DOI] [PubMed] [Google Scholar]
- 116.Constable RT, Spencer DD. Repetition time in echo planar functional MRI. Magn Reson Med. 2001;46(4):748–755. [DOI] [PubMed] [Google Scholar]
- 117.Liu G, Sobering G, Olson AW, et al. Fast echo-shifted gradient-recalled MRI: combining a short repetition time with variable T2* weighting. Magn Reson Med. 1993;30(1):68–75. [DOI] [PubMed] [Google Scholar]
- 118.Wang X, Hernando D, Reeder SB. Phase-based T(2) mapping with gradient echo imaging. Magn Reson Med. 2020;84(2):609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ngo GC, Bilgic B, Gagoski BA, et al. Correction of magnetic field inhomogeneity effects for fast quantitative susceptibility mapping. Magn Reson Med. 2019;81(3):1645–1658. [DOI] [PubMed] [Google Scholar]
- 120.Boyle GE, Ahern M, Cooke J, et al. An interactive taxonomy of MR imaging sequences. Radiographics. 2006;26(6):e24. [DOI] [PubMed] [Google Scholar]
- 121.Franconi F, Mowat P, Lemaire L, et al. Single-scan quantitative T2* methods with susceptibility artifact reduction. NMR Biomed. 2006;19(5):527–534. [DOI] [PubMed] [Google Scholar]
- 122.Chavhan GB, Babyn PS, Thomas B, et al. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29(5):1433–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matthews PM, Jezzard P. Functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2004;75(1):6–12. [PMC free article] [PubMed] [Google Scholar]
- 124.Freund H, Oyono-Enguéllé S, Heitz A, et al. Comparative lactate kinetics after short and prolonged submaximal exercise. Int J Sports Med. 1990;11(04):284–288. [DOI] [PubMed] [Google Scholar]
- 125.Herman P, Sanganahalli BG, Blumenfeld H, et al. Quantitative basis for neuroimaging of cortical laminae with calibrated functional MRI. Proc Natl Acad Sci. 2013;110(37):15115–15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Godlewska BR, Clare S, Cowen PJ, et al. Ultra-high-field magnetic resonance spectroscopy in psychiatry. Front Psychiatry. 2017;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Block W, Träber F, Kuhl CK, et al. 1H-MR spectroscopic imaging in patients with clinically diagnosed Alzheimer's disease. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 1995;163(3):230–237. [DOI] [PubMed] [Google Scholar]
- 128.Ernst T, Chang L, Melchor R, et al. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203(3):829–836. [DOI] [PubMed] [Google Scholar]
- 129.Mullins R, Reiter D, Kapogiannis D. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer's brain. Ann Clin Transl Neurol. 2018;5(3):262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weaver KE, Richards TL, Logsdon RG, et al. Posterior cingulate lactate as a metabolic biomarker in amnestic mild cognitive impairment. Biomed Res Int. 2015;2015:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Godenschweger F, Kägebein U, Stucht D, et al. Motion correction in MRI of the brain. Phys Med Biol. 2016;61(5):R32–R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marques JP, Simonis FFJ, Webb AG., et al. : Low‐field MRI: An MR physics perspective. J Magn Reson Imaging. 2019;49(6):1528–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoshida T, Shirata K, Urikura A, et al. Signal-to-noise ratio and parallel imaging performance of commercially available phased array coils in 3.0 T brain magnetic resonance imaging. Radiol Phys Technol. 2015;8(2):305–311. [DOI] [PubMed] [Google Scholar]
- 134.Komlosi P, Altes TA, Qing K, et al. Signal-to-noise ratio, T(2), and T2* for hyperpolarized helium-3 MRI of the human lung at three magnetic field strengths. Magn Reson Med. 2017;78(4):1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fujima N, Carlota Andreu-Arasa V, Barest GD, et al. Magnetic resonance spectroscopy of the head and neck: principles, applications, and challenges. Neuroimaging Clin N Am. 2020;30(3):283–293. [DOI] [PubMed] [Google Scholar]
- 136.Purnell JQ, Klopfenstein BA, Stevens AA, et al. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab. 2011;13(3):229–234. [DOI] [PubMed] [Google Scholar]
- 137.Sonnay S, Duarte JMN, Just N. Lactate and glutamate dynamics during prolonged stimulation of the rat barrel cortex suggest adaptation of cerebral glucose and oxygen metabolism. Neuroscience. 2017;346:337–348. [DOI] [PubMed] [Google Scholar]
- 138.von Pföstl V, Li J, Zaldivar D, et al. Effects of lactate on the early visual cortex of non-human primates, investigated by pharmaco-MRI and neurochemical analysis. NeuroImage 2012;61(1):98–105. [DOI] [PubMed] [Google Scholar]
- 139.Cholet N, Pellerin L, Welker E, et al. Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. J Cereb Blood Flow Metab. 2001;21(4):404–412. [DOI] [PubMed] [Google Scholar]
- 140.Voutsinos-Porche B, Bonvento G, Tanaka K, et al. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37(2):275–286. [DOI] [PubMed] [Google Scholar]
- 141.Zimmer ER, Parent MJ, Souza DG, et al. [(18)F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci. 2017;20(3):393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haiss F, Jolivet R, Wyss MT, et al. Improved in vivo two-photon imaging after blood replacement by perfluorocarbon. J Physiol. 2009;58713:3153–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blanc J, Roumes H, Mazuel L, et al. Functional magnetic resonance spectroscopy at 7 T in the rat barrel cortex during whisker activation. J Vis Exp. 2019;144:58912. [DOI] [PubMed] [Google Scholar]
- 144.Petroff OA, Pleban LA, Spencer DD. Symbiosis between in vivo and in vitro NMR spectroscopy: the creatine, N-acetylaspartate, glutamate, and GABA content of the epileptic human brain. Magn Reson Imaging. 1995;13(8):1197–1211. [DOI] [PubMed] [Google Scholar]
- 145.Cecil KM. Proton magnetic resonance spectroscopy: technique for the neuroradiologist. Neuroimaging Clin N Am. 2013;23(3):381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buonocore MH, Maddock RJ. Magnetic resonance spectroscopy of the brain: a review of physical principles and technical methods. Rev Neurosci. 2015;26(6):609–632. [DOI] [PubMed] [Google Scholar]
- 147.Bak LK, Walls AB. CrossTalk opposing view: lack of evidence supporting an astrocyte-to-neuron lactate shuttle coupling neuronal activity to glucose utilisation in the brain. J Physiol. 2018;596(3):351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roumes H, Jollé C, Blanc J, et al. Lactate transporters in the rat barrel cortex sustain whisker-dependent BOLD fMRI signal and behavioral performance. Proc Natl Acad Sci. 2021;47:e2112466118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Terpstra M, Henry PG, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med. 2003;50(1):19–23. [DOI] [PubMed] [Google Scholar]
- 150.Gaillard F. Lactate MRS Effect of TE. Case study. 2015. Available at: https://radiopaedia.org/cases/36021. Accessed 02 Jan 2023. [Google Scholar]
- 151.Kantarci K. Magnetic resonance spectroscopy in common dementias. Neuroimaging Clin N Am. 2013;23(3):393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maul S, Giegling I, Rujescu D. Proton magnetic resonance spectroscopy in common dementias—current status and perspectives. Front Psychiatry. 2020:11:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wilcock GK, Esiri MM, Bowen DM, et al. Alzheimer's disease: Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci. 1982;57:407–417. [DOI] [PubMed] [Google Scholar]
- 154.Liu H, Zhang D, Lin H, et al. Meta-Analysis of Neurochemical Changes Estimated via Magnetic Resonance Spectroscopy in Mild Cognitive Impairment and Alzheimer's Disease. Front Aging Neurosci. 2021;13:738971, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sheikh-Bahaei N. MR spectroscopy in Alzheimer’s disease. Biomed Spectrosc Imaging. 2020;9(1-2):13-21, [Google Scholar]
- 156.Zebhauser PT, Berthele A, Goldhardt O, et al. Cerebrospinal fluid lactate levels along the Alzheimer’s disease continuum and associations with blood-brain barrier integrity, age, cognition, and biomarkers. Alzheimer's Res Ther. 2022;14(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]