Abstract
Severe maternal morbidity (SMM) has historically functioned as an umbrella term to define major, potentially-life threatening obstetric, medical, and surgical complications of pregnancy. There is no overarching or consensus definition of the constellation of conditions that have been used variably to define SMM, though it is clear that having a well-honed definition of SMM is important for research, quality improvement, and health policy purposes. While SMM may ultimately elude a single unifying definition, as different features may be relevant based on context and modality of data acquisition, it is valuable to explore the intellectual frameworks and various applications of SMM in current practice, and to consider the potential benefit of more consolidated terminology for maternal morbidity.
Keywords: Severe maternal morbidity, epidemiology, research methods, public health, quality improvement
Condensation
Severe maternal morbidity is a heterogenous umbrella term that may benefit from more unified definition to enhance research, quality improvement, and public policy efforts.
Introduction
Significant maternal pregnancy complications, often termed severe maternal morbidity (SMM), pose a significant challenge for healthcare providers, health systems, and, most of all, for patients. Confronting SMM involves averting preventable morbidity, preventing maternal mortality, and reckoning with the sequalae of obstetric morbidity for the patient and the health system, such as long-term medical complications, acute rehabilitation, psychosocial impact, hospital length of stay, and healthcare costs. On a global level, maternal health outcomes are considered a quality indicator of a country’s health system, with maternal mortality generally serving as the key marker. However, in its work addressing the maternal mortality pandemic, the World Health Organization (WHO) identified that maternal death cases constitute only a small fraction of patients who experience significant pregnancy complications that threaten maternal vitality.1 The entity of maternal morbidity impacts a much larger proportion of the population, but SMM has lacked a uniform definition and is therefore more challenging to surveil, prompting the establishment of the WHO Maternal Morbidity Working Group (MMWG) in 2012, tasked with scientifically describing maternal morbidity. This multidisciplinary, multinational group ultimately devised a global framework for SMM including specific metrics for hospitals to use in tracking maternal morbidity cases.
Around the same time, the Centers for Disease Control and Prevention (CDC) published a new metric for identification of SMM based on administrative indicators from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).2 Since that time, research endeavors investigating maternal morbidity as an outcome have proliferated substantially, and the CDC continues to track SMM, now using an updated algorithm based on ICD-10-CM codes. However, despite these robust scientific and public health surveillance efforts, there is no consensus definition of SMM used uniformly by clinicians, researchers and health departments. This presents a major challenge to interpreting and comparing research findings and epidemiologic trends using different classifications of SMM, as well as to potential collaborations across health systems and governmental organizations.
Frameworks for Defining Severe Maternal Morbidity
SMM can be conceived under several different intellectual frameworks (Figure 1). The first, and perhaps most obvious, defines SMM based on risk for maternal mortality. In this way, SMM can be thought of as “near miss” events for maternal death, and would constitute any medical complications that place a patient at significant risk for mortality. An SMM of metric of this nature should also consider including mortality, as it is intended to capture events on the same pathway. This is the ethos of the CDC definition of SMM, which includes 21 categories of diagnosis and procedural code indicators of severe conditions that may precipitate or be antecedent to mortality (chosen for their high statistical association with mortality). The advantage of this “near-miss” framework is the logical and direct association between SMM conceived as a risk factor for death and mortality itself. In addition, it creates a natural opportunity to consider the preventability of morbidity and mortality, which can prompt interventions to reduce both. The major shortcoming of this approach is that including only morbidity that constitutes a significant risk for mortality in the definition of SMM is a very narrow interpretation. These critical complications – such as myocardial infarction, cardiac arrest, and sepsis – are quite rare, and an SMM definition based on these alone will not capture important, but less perilous, maternal morbidities.
Figure 1.
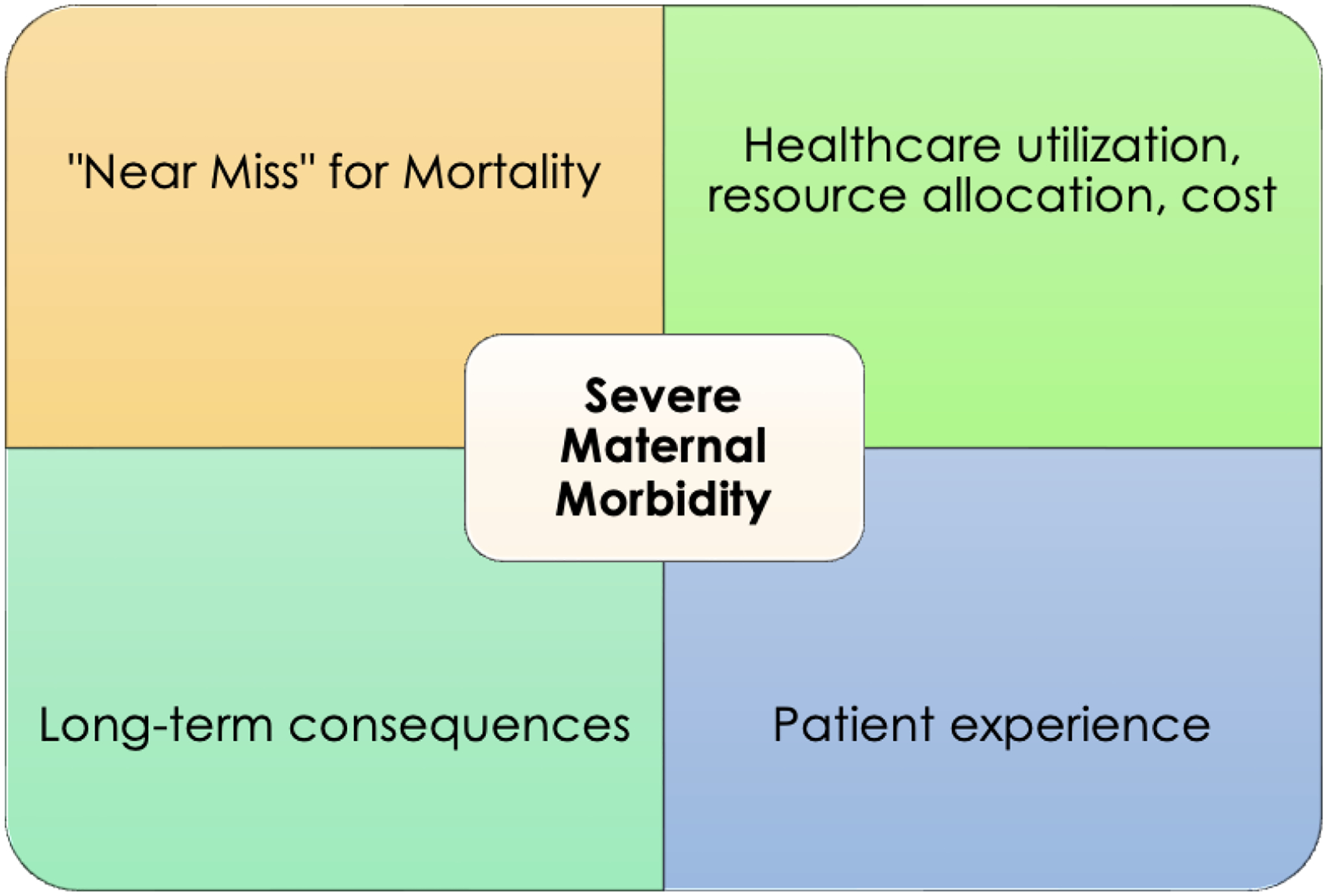
Intellectual Frameworks for Severe Maternal Morbidity
It is also useful to consider the methodology by which SMM is identified under this framework. One common approach is condition-based. This is the tactic utilized by the CDC and almost all operational composite definitions of SMM, creating a case-definition for SMM based on specific conditions or procedures that can be identified in the electronic medical record (EMR) or through administrative data. Strengths of this approach are that it is concrete and easy to replicate and generalize. Its major challenge is potentially missing cases of SMM that do not meet the specific criteria of the delineating conditions (ie: the CDC definition may miss a case of a critical disease process such as ketoacidosis or thyroid storm). Alternatively, cases with an ICD indicator that was liberally or inaccurately applied may be inappropriately flagged as SMM, such as an “acute renal failure” code for patients with a mild, transient elevation in serum creatinine. A more inclusive approach would be to identify patients with SMM based on critically abnormal vital signs, symptoms, or clinical tests such as lab work. Major drawbacks of this are likely insurmountable barriers in accessing and analyzing this type of patient-specific, detailed EMR data on a large scale, as well as inability to standardize data collection given hospital variability. In addition, it is difficult to extrapolate a patient’s clinical condition abstractly from vital signs or lab parameters alone.
A potentially more comprehensive and inclusive approach to describing SMM is through impact on healthcare utilization and resource allocation. Typical pregnancy care is completed entirely in the outpatient setting, and delivery admissions in the absence of significant complications should involve only a brief hospitalization. Patients who have experienced severe morbidity might be identified based on unanticipated intensive care unit (ICU) admission, extended postpartum hospitalizations, re-admissions, or hospital transfer, though these processes can also be protective against morbidity. Defining SMM based on level or intensity of inpatient care pairs well with endeavors to identify and streamline maternal level of care designations and to triage acutely ill obstetric patients to appropriate settings. In addition, this can allow SMM to easily translate into healthcare costs, which could better enable researchers and policymakers to understand the burden of SMM on the health system. However, inherent difficulties with this framework are multifold. First of all, it utilizes process measures as proxies for an outcome, which is a more indirect approach to measuring SMM. Second, a definition created this way may err in favor of being too broad, as some patients may have extended hospitalizations for social rather than medical reasons. And perhaps most importantly, it is difficult to generalize criteria and circumstances for inpatient care requirements across hospitals, as care delivery is highly subject to the protocols, culture, and resources of individual hospitals, thereby creating substantial heterogeneity in the definition of SMM.
SMM can also be conceptualized in terms of impact on long-term health outcomes for pregnant and postpartum patients. Acute obstetric morbidity can be short-lived if addressed swiftly in an otherwise healthy patient – for example, a significant hemorrhage managed effectively with uterotonic medications and blood product transfusion or a major infection treated with broad-spectrum antibiotics – but severe morbidity could be defined as that which casts a longer term rehabilitative or risk burden on the patient (ie: extended cardiovascular dysfunction, disability after a prolonged ICU admission, or long-term mental health disease). It is now well established that even routine pregnancy complications such as gestational diabetes and preeclampsia can impact a patient’s lifelong health. A longitudinal perspective on SMM has inherent challenges, as it is impossible to know the downstream impact of a pregnancy complication on a patient’s distal health in real-time, but it represents an important exercise in considering an optimal definition of SMM.
Finally, another potential framework for defining SMM that has thus far been neglected is the impact on the patient’s birth experience. Patient-centered outcomes, the output of healthcare oriented to a patient’s values and priorities, is a subject of increasing study and attention at a national level. Little is known at this time about patient perspectives on SMM and how a patient’s experiences in the healthcare system relate to experienced morbidity. However, pregnancy care is unique in that there is an important experiential, non-medical component of birth. Conceiving of SMM in terms of deviations that complicate and detract from a patient’s birth experience is yet another way to reckon with the broad potential scope of SMM. While impossible to implement data collection of this type at the level of every individual patient, garnering patient perspectives to hone a workable definition of SMM may complement other strategies favorably. And although these latter three frameworks do not currently play a significant role in SMM data collection, and each may be problematic to capture reliably on a large-scale, conceptualizing SMM in these alternative ways can help elucidate and essentialize key components of SMM data collection going forward.
Established Models of SMM for Research and Healthcare Quality
On an international level, prior to 2012, the WHO used a maternal mortality near-miss framework as an operational definition of maternal morbidity, which included clinical, laboratory, and management criteria.3 Near-miss events, sequalae of maternal life-threatening conditions, were divided into categories of hemorrhagic disorders, hypertensive disorders, “severe management indicators” (blood transfusion, central access, hysterectomy, ICU admission, prolonged postpartum stay > 7 days, emergent intubation, re-operation, unplanned surgical procedure), and other systemic disorders (endometritis, pulmonary edema, respiratory failure, seizure, sepsis, shock, thrombocytopenia < 100,000, thyroid crisis). The MMWG’s work then culminated in a definition of maternal morbidity as “any health condition attributed to and/or complicating pregnancy and childbirth that has a negative impact on the woman’s wellbeing and/or functioning” and a maternal morbidity measurement (MMM) framework that included the following continuum of outcomes: full recovery, non-severe maternal morbidity, potentially life-threatening conditions, maternal near-miss, and maternal death.1 In developing their framework, they stressed six key principles (Box 1).4 These groupings form overall categories of obstetric morbidities, medical morbidities, and injuries, including 121 total conditions identified by 58 symptoms, 29 signs, 44 “investigations” (blood work and imaging), and 35 management strategies (specific treatments). While this definition is both granular and comprehensive, it is ultimately designed as a tool for institution-level maternal morbidity surveillance, would be difficult to use for retrospective data collection, and is impossible to apply to administrative data.
Box 1. World Health Organization Maternal Morbidity Measurement Framework: Key Principles4.
| (1) A “woman-centered” approach in prioritizing outcomes that are important to patients |
| (2) The cyclical nature of maternal morbidity risks in the setting of subsequent pregnancies |
| (3) Longitudinal consequences of maternal morbidity throughout the life span |
| (4) Social and economic implications of maternal morbidity |
| (5) The impact of context and environment on the experience of lived morbidity |
| (6) Meaningful groupings of types of morbidity |
Numerous other specific SMM definitions are employed across the globe as well (Table 1). Each of these criteria utilizes standard ICD-10 codes as well as country-specific birth and discharge registry information and procedure coding schemes. They have significant overlap with the U.S.-based CDC definition, though with some notable differences, such as including acute psychosis, acute abdomen, and status asthmaticus. These varying methods for characterizing SMM, while similar, introduce challenges in comparing SMM incidence in different countries and developing unified mitigation strategies.
Table 1.
Comparison of Administrative Severe Maternal Morbidity (SMM) Definitions in Use Internationally
| SMM Definition | Diagnoses | Procedures |
|---|---|---|
|
Centers for Disease Control and Prevention (CDC) Severe Maternal Morbidity (SMM)2 [Country of origin = U.S.; Data source = ICD-10-CM codes] |
-Acute myocardial infarction -Aneurysm -Acute renal failure -Adult respiratory distress syndrome -Amniotic fluid embolism -Cardiac arrest/ventricular fibrillation -Disseminated intravascular coagulation (DIC) -Eclampsia -Heart failure/arrest during surgery or procedure -Puerperal cerebrovascular disorders -Pulmonary edema/ acute heart failure -Severe anesthesia complications -Sepsis -Shock -Sickle cell disease with crisis -Air and thrombotic embolism |
-Conversion of cardiac rhythm -Blood products transfusion -Hysterectomy -Temporary tracheostomy -Ventilation |
|
Maternal Morbidity Outcome Indicator (MMOI)14 [Country of origin = Australia; Data source = ICD-10-AM (Australian modification) codes and Australian Classification of Health Intervention codes] |
-Severe preeclampsia/HELLP/Eclampsia/ Convulsions -DIC -Thrombocytopenia -Shock -Cardiac Failure -Conduction disorders -Diabetic acidosis -Acute liver failure -Acute renal failure -Anuria -Intestinal obstruction -Trauma to abdominal organs -Complications of procedures -Acute psychosis -Pneumonia -Sepsis -Acute abdomen -HIV -Venous thrombosis -Uterine rupture -Placenta accreta -Maternal admission > 10 days |
-Blood transfusion -Platelets/ coagulation factor transfusion -Exploration of uterus under general anesthesia -D&C under general anesthesia -Manual removal of placenta -Repair of ruptured uterus -Repair of disrupted abdominal wound -Laparotomy -Hysterectomy -Procedures to control bleeding -Intra-myometrial prostaglandin -Bimanual compression of uterus -Repair of cervix with general anesthesia -Repair of vaginal with general anesthesia -Evacuation of hematoma -Repair of bladder -Cystotomy -Repair of large intestine -Repair of anus or rectum -Dialysis -Procedure on the aorta -Mechanical ventilation -Central line -Arterial line -Epidural blood patch -Emergency classical cesarean section -General anesthesia -ICU admission -Unplanned return to operating room -Maternal transfer |
|
Canadian Perinatal Surveillance System (CPSS)15 [Country of origin = Canada; Data source = ICD-10CA (Canadian modification) codes, Canadian Classification of Health Interventions (CCI) codes] |
-Severe preeclampsia/ HELLP/eclampsia -Severe hemorrhage -Surgical complications -Sepsis -Embolism/shock/DIC -Cardiac conditions -Acute renal failure -Severe uterine rupture -Cerebrovascular accidents -Pulmonary/cardiac/CNS complications of anesthesia -Status asthmaticus -Adult respiratory distress syndrome -Acute abdomen -Sickle cell anemia with crisis -Acute psychosis -Status epilepticus -HIV |
-Maternal ICU admission -Hysterectomy -Assisted ventilation -Surgical or manual correction of inverted uterus |
|
EURO-PERISTAT16 [Country of origin = European Union; Data source = ICD-10 codes, country-specific birth and discharge registries] |
-Eclampsia | -Hysterectomy -Embolization -Blood transfusion -ICU admission |
|
English Maternal Morbidity Outcome Indicator (EMMOI)17 [Country of origin = United Kingdom; Data source = ICD-10 codes, OPCS Classification of Interventions and Procedures used by the NHS hospitals in the UK] |
-Acute abdomen -Acute renal failure -Acute psychosis -Cardiac arrest/failure or infarction -Cerebral edema or coma -DIC -Cerebrovascular accident -Major complication of anesthesia -Obstetric embolism -Shock -Sickle cell anemia with crisis -Status asthmaticus -Status epilepticus -Uterine rupture -Eclampsia -Sepsis -Cerebral venous thrombosis |
-Assisted ventilation including tracheostomy -Curettage in combination with general anesthetic -Dialysis -Evacuation of hematoma -Hysterectomy -Procedures to reduce blood flow to uterus -Re-closure of disrupted cesarean section wound -Repair of bladder or cystotomy -Repair of intestine |
In the U.S., the CDC’s SMM composite has informed and defined research exploring the epidemic of maternal morbidity for the past decade.2 It is designed to be utilized in administrative data sets and comprises a mixture of severe conditions and procedures, as enumerated in Table 1. One notable feature of this composite is that it incorporates components that are both indicators of severity as well as interventions to prevent morbidity and mortality, such as blood transfusion and hysterectomy. In addition, including transfusion in the composite altogether introduces significant heterogeneity in the disease severity of the overall SMM definition. Blood transfusion is by far the most prevalent element of the composite, and arguably does not represent true severe morbidity for many patients, such as those who receive one or two units of blood for postpartum symptomatic anemia, or a prophylactic transfusion at the time of delivery for low hemoglobin or platelet count. The CDC’s criteria for SMM (ICD-9 iteration) have been extensively evaluated for their correlation with clinician-identified cases of severe morbidity. Specifically, they were validated compared to a clinical gold standard determined by four rounds of a Delphi process and found to have a sensitivity of 0.77, positive predictive value (PPV) of 0.44, and c-statistic of 0.87, demonstrating strong discrimination.5
While the CDC’s definition of SMM was initially intended for use in database research and passive disease surveillance (when public health bodies review incident cases of an outcome based on reports submitted post facto by hospitals), an updated version of the CDC indicators (involving careful ICD-10 translation) is now a Title V National Performance Measure that the Agency for Healthcare Research and Quality (AHRQ) will report annually by state.6 Another set of outcome measures was devised to assess obstetric healthcare quality and termed the Adverse Outcome Index.7 Recognizing the absence of unified perinatal quality metrics, experts convened over a series of conferences to develop a ten-item index inclusive of both maternal (death, uterine rupture, ICU admission, re-operation, blood transfusion, third/fourth degree perineal laceration) and neonatal (death, NICU admission, birth trauma, five-minute Apgar < 7) morbidities. This index has significant drawbacks as a quality metric, however, as it does not reliably assess appropriate escalations in care, mixes maternal and neonatal outcomes, uses a scoring system that may lack face validity, and does not provide a comprehensive picture of maternal morbidities that do not require intensive care or repeat surgery.
In 2015, in an effort to design an improved definition of SMM for real-time clinical use, the Joint Commission crafted an expanded sentinel event definition for intensive case review inclusive of events that result in severe temporary harm to a patient, analogous to SMM in the context of obstetrics, which they defined as a transfusion of four or more units of packed red blood cells and/or unanticipated ICU admission.8 The transfusion criterion is intended to capture a patient with a major, potentially life-threatening hemorrhage, but exclude patients with less critical blood product needs. The ICU criterion is designed to encompass all obstetric patients with severe complications atypical of usual pregnancy experiences and requiring a higher level of care than a typical obstetric unit. While decision-making and resource availability regarding transfusion volume and inpatient level of care may vary by hospital, these screening criteria for SMM can be applied widely and used actively by obstetric departments to review SMM cases, as has been recommended by the Council on Patient Safety in Women’s Health Care.9 On a more theoretical level, the National Quality Foundation also developed a framework for measuring maternal morbidity along the temporal domains of an obstetric patient’s healthcare – preconception, prenatal care, intrapartum, and postpartum – and including sub-domains of patient co-morbidities, health behaviors, access to care, risk-appropriate care, racism, and mental health, among others, in order to provide enhanced context to SMM as an outcome.10
The use of SMM as a healthcare quality indicator invites the fundamental question of whether SMM should be risk-adjusted as an outcome. The rationale behind this is that risk-adjustment for patient- and hospital-level factors allows SMM to be more directly compared across institutions, for research and for hospital evaluation and healthcare quality purposes. To this end, the Centers for Medicare & Medicaid Services commissioned the development of an electronic health record (EHR)-based, risk-adjusted SMM metric, called the Severe Obstetric Complications electronic clinical quality measure (eQCM).11 This eQCM uses an outcome definition inclusive of the CDC-defined indicators for SMM, maternal mortality, and a risk adjustment framework incorporating maternal co-morbidities, admission laboratory tests and vital signs, and socioeconomic instability. It is designed to provide a hospital-specific risk standardized obstetric complications rate (RSOCR), accounting for hospital-level differences in patient mix and for the assumption that underling quality of care differences between hospitals contribute to differential SMM rates. Ultimately, this approach constitutes a novel implementation of the CDC’s SMM definition with clinically and statistically informed risk-adjustment to leverage SMM as a potentially meaningful hospital quality metric.
Heterogeneity in Contemporary Research
In addition to these established criteria for maternal morbidity, there are also a plethora of institution-specific and study-specific definitions of SMM. Researchers may designate outcome criteria for SMM based on availability in a given database or specific types of morbidity most relevant to a specific inquiry. To illustrate this, Table 2 demonstrates all of the full-length research articles (excluding abstracts, systematic reviews, and research letters) published in a prominent journal of obstetrics and gynecology in calendar year 2020 that investigate a maternal morbidity composite as a primary or secondary outcome. The two most common outcome definitions, as evident in the table, are the CDC composite and a composite of ICU admission, blood transfusion, uterine rupture, unplanned hysterectomy, re-operation, and, in some cases, eclampsia. This latter definition represents maternal outcome variables collected as part of U.S. birth certificate data. Numerous other articles employ study-specific definitions. This generates difficulty in synthesizing data from different studies investigating similar risk factors for SMM, and in comparing relative risks of different patient characteristics, practices, or interventions. Furthermore, there may be differential ascertainment bias based on the SMM definition and type of data used. For example, the maternal morbidities reported on U.S. birth certificates have demonstrated low sensitivity and PPV compared to hospital discharge data [ie: blood transfusion sensitivity 0.12, PPV 0.73; uterine rupture sensitivity 0.26, PPV 0.18].12
Table 2.
Catalog of Severe Maternal Morbidity (SMM) Outcomes Reported in Full-Length Research Articles* Published in a Prominent Journal of Obstetrics and Gynecology in 2020
| Article Title | SMM Definition |
|---|---|
| Adverse Infant and Maternal Outcomes Among Low-Risk Term Pregnancies Stratified by Race and Ethnicity18 | Blood transfusion, ICU admission, uterine rupture, unplanned hysterectomy |
| An Expanded Obstetric Comorbidity Scoring System for Predicting Severe Maternal Morbidity19 | CDC SMM |
| Association of Obstetric and Neonatal Outcomes With Deviation From Guidelines for Gestational Carriers20 | Eclampsia, blood transfusion, unplanned hysterectomy, ICU admission, death |
| Association of Prior Cesarean Delivery With Early Term Delivery and Neonatal Morbidity21 | Blood transfusion, hysterectomy, ruptured uterus, ICU admission |
| Association of Time of Delivery With Composite Adverse Outcomes in Low-Risk Pregnancies22 | Blood transfusion, ICU admission, uterine rupture, unplanned hysterectomy |
| Associations Between Comorbidities and Severe Maternal Morbidity23 | CDC SMM with either (a) in-hospital mortality, (b) cesarean delivery with a length of stay 5 days or longer, or (c) a vaginal delivery with a length of stay 3 days or longer |
| Cephalic Elevation Device for Second-Stage Cesarean Delivery: A Randomized Controlled Trial24 | Fever, disseminated intravascular coagulation, ICU admission; also length of stay as a continuous variable |
| Early Pregnancy Blood Pressure Elevations and Risk for Maternal and Neonatal Morbidity25 | CDC SMM, ICU admission, postpartum length of stay (>3 standard deviations above the mean postpartum length of stay for the study period, stratified by mode of delivery) |
| Maternal and Neonatal Morbidity After 4 and 6 Hours of Protracted Active Labor in Nulliparous Term Pregnancies26 | Maternal fever (chorioamnionitis or endo-metritis), postpartum hemorrhage, any blood transfusion, postpartum length of hospital stay 5 days or more |
| Neonatal Seizures Among Low-Risk Pregnancies at Term: Risk Factors and Adverse Outcomes27 | Blood transfusion, ICU admission, uterine rupture, unplanned hysterectomy |
| Outcomes of Subsequent Births After Placenta Accreta Spectrum28 | Maternal Morbidity Outcome Indicator (MMOI)14 |
| Placenta Accreta Spectrum Without Placenta Previa29 | Transfusion of 8 or more units of red blood cells, re-operation, acute kidney injury (absolute postpartum creatinine level 2.0 or greater or doubling from baseline), pulmonary edema, deep venous thrombosis or venous thromboembolism, maternal death |
| Postpartum Readmissions Among Women With Diabetes30 | CDC SMM |
| Race and Ethnicity, Medical Insurance, and Within-Hospital Severe Maternal Morbidity Disparities31 | CDC SMM |
| Racial and Ethnic Disparities in Maternal and Neonatal Adverse Outcomes in College-Educated Women32 | ICU admission, transfusion of blood products, ruptured uterus, unplanned hysterectomy, or unplanned operating room procedure after delivery |
| Risk Factors Associated With Cesarean Delivery After Induction of Labor in Women With Class III Obesity33 | Postpartum hemorrhage (1000 mL estimated blood loss or greater), transfusion of blood products (packed red blood cells, fresh frozen plasma or platelets), operative injury (uterine extension, uterine artery laceration requiring O’Leary or compression stitch), bladder or bowel injury requiring repair, and hysterectomy, postpartum endometritis (defined as temperature greater than 38°C with fundal tenderness), culture-proven urinary tract infection with more than 100,000 colony forming units, wound cellulitis treated with antibiotics and culture-proven sepsis, postpartum ileus or bowel obstruction, wound complication (breakdown or dehiscence), venous thromboembolism diagnosed on imaging, ICU admission, death |
| Severe Maternal Morbidity and Mortality Among Indigenous Women in the United States34 | CDC SMM |
| Trial of Labor After Two Prior Cesarean Deliveries: Patient and Hospital Characteristics and Birth Outcomes35 | CDC SMM |
| Use of Uterine Tamponade and Interventional Radiology Procedures During Delivery Hospitalizations36 | CDC SMM |
Only full-length original articles were included (systematic reviews, abstracts, and research letters excluded); these manuscripts were identified by using the search term “maternal morbidity” in the online search function of Obstetrics and Gynecology with date restriction between January 1, 2020 and December 31, 2020, followed by individual review of all results
Current Approach of Professional Organizations
The leading obstetric professional organizations in the U.S. – the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) – have identified a process for institutional maternal morbidity review.13 They recommend using the Joint Commission’s criteria – transfusion of four or more units of blood or ICU admission – to screen for potential cases of SMM, followed by departmental review to determine whether the case represents true SMM and identify potential preventability. These criteria, specifically ICU admission, have been found to have a high PPV for SMM confirmed by clinical experts and chart review.5 An accompanying categorized list of examples of SMM was also developed to assist with the identification of true SMM cases, though this list is not intended to be exhaustive or to represent a single, comprehensive definition of SMM (Figure 2).
Figure 2.

Severe Maternal Morbidity Categories Proposed by ACOG/SMFM13
Benefits and Challenges of a Composite Metric
Mortality as a research endpoint has been replaced in many fields by morbidity composites, as these can highlight more proximal and more common adverse outcomes. These composites are fundamentally challenging as they may comprise a mix of severity levels and cannot illuminate a single pathway from a risk factor to a specific complication. In the context of SMM, composite variables are further complicated by containing procedures that are both indicators of severity as well as interventions to reduce morbidity (such as transfusion, hysterectomy, and ICU admission). A singular SMM definition is impractical in some ways as SMM requires identification for different purposes and contexts – research, hospital-level quality improvement, and public health and policy – and may require risk-adjustment to serve as a meaningful quality index. However, evaluating each potential component of SMM individually can obscure data patterns as each distinct outcome is relatively rare, and the ability to study and communicate about the phenomenon of SMM in aggregate remains important to improve care. Furthermore, given established significant disparities in maternal obstetric outcomes by race and ethnicity, unifying SMM data collection can potentially facilitate more precise identification of patterns, trends, and opportunities for intervention.
The Way Forward
While a single, absolute definition of SMM is likely unfeasible, it may be beneficial to establish a standard for research purposes. This definition could then also serve as a guide, as suggested by ACOG and SMFM, for real-time case identification at the clinical level, as well as a quality metric. A process to establish such a definition might include first creating a consensus on broad SMM categories based on end-organ system compromise that could then be mapped to specific clinical diagnoses and ICD-10-CM codes (Figure 3). Certain categories of morbidity, which may not reflect the same level of severity as other components – such as hemorrhage or surgical complications – should possibly be considered as distinct metrics. An additional care utilization metric (including elements such as extended postpartum length of stay, hospital transfer, ICU admission, and re-admission) could also be devised as a corollary to SMM and to serve as a proxy for identifying morbidity when appropriate. Within a composite SMM definition, it is also reasonable to consider a weighting scheme based on the severity or potential preventability of individual components. This could be established either by consensus or based on the individual association of each component condition with maternal mortality. The purpose and data source of a particular study could then determine which subsets of the broader SMM composite comprise the study’s outcome.
Figure 3.

Proposed Strategy for SMM Data Collection and Categorization
-A potential multifaceted scheme for defining SMM based on weighted organ system complications (examples included not intended to be comprehensive), with distinct categories for hemorrhage and surgical complications and a healthcare utilization component
-Depending on the research question and data source, some or all elements may be included in a composite outcome, with an underlying conceptual structure translated into precise diagnosis/procedure codes and discrete utilization endpoints serving as common language for data collection and outcome comparison
Though the specific definition of SMM used in a given study will ultimately depend on the context and parameters of the research question, the entire process of evaluating maternal morbidity as an outcome measure would benefit from increased uniformity. These steps can potentially help hone and optimize existing SMM frameworks into a better standard for maternal population health research. As public health and policy decisions are often predicated on epidemiologic data, this would create a more unified language for researchers, healthcare providers, healthcare organizations, payers, and policymakers to communicate about maternal outcomes and risk reduction, as well as to evaluate hospitals and trends in maternal health equity.
Disclosures
The authors report no conflict of interest. Adina Kern-Goldberger is supported by a National Institutes of Health T32 Training Grant in Perinatal Epidemiology at the University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Filippi V, Chou D, Barreix M, Say L. A new conceptual framework for maternal morbidity. International Journal of Gynecology & Obstetrics. 2018;141:4–9. doi: 10.1002/ijgo.12463 [DOI] [Google Scholar]
- 2.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. Nov 2012;120(5):1029–36. doi:http://10.1097/AOG.0b013e31826d60c5 [DOI] [PubMed] [Google Scholar]
- 3.Say L, Souza JP, Pattinson RC. Maternal near miss – towards a standard tool for monitoring quality of maternal health care. Best Practice & Research Clinical Obstetrics & Gynaecology. 2009;23(3):287–296. doi: 10.1016/j.bpobgyn.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Chou D, Tunçalp Ö, Firoz T, et al. Constructing maternal morbidity – towards a standard tool to measure and monitor maternal health beyond mortality. BMC Pregnancy and Childbirth. 2016;16(1)doi: 10.1186/s12884-015-0789-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol. May 2016;214(5):643 e1–643 e10. doi: 10.1016/j.ajog.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Maternal and Child Health Bureau HRaSA. Federally Available Data (FAD) resource document. 2022. 12/1/2022. https://mchb.tvisdata.hrsa.gov/Admin/FileUpload/DownloadContent?fileName=FadResourceDocument.pdf&isForDownload=False
- 7.Mann S, Pratt S, Gluck P, et al. Assessing quality obstetrical care: development of standardized measures. Jt Comm J Qual Patient Saf. Sep 2006;32(9):497–505. doi: 10.1016/s1553-7250(06)32065-x [DOI] [PubMed] [Google Scholar]
- 8.Changes to the Joint Commission Sentinel Events Chapter – Focus on Severe Maternal Morbidity. Council on Patient Safety in Women’s Healthcare. Updated 2015. Accessed 2/9/2022, https://safehealthcareforeverywoman.org/event/changes-to-the-joint-commission-sentinel-events-chapter-focus-on-severe-maternal-morbidity/
- 9.SEVERE MATERNAL MORBIDITY REVIEW FORM. Council on Patient Safety in Women’s Healthcare. Updated 2020. Accessed 2/9/2022, https://safehealthcareforeverywoman.org/council/patient-safety-tools/severe-maternal-morbidity-forms/
- 10.NQF. Perinatal and Women’s Health, Spring 2021 Cycle: CDP Report. 2022. https://www.qualityforum.org/Publications/2021/08/Maternal_Morbidity_and_Mortality_Measurement_Recommendations_Final_Report.aspx?utm_source=nqf+website&utm_medium=final+report&utm_campaign=abstract+page
- 11.Severe Obstetric Complications Electronic Clinical Quality Measure (eCQM) Methodology Report. October 2021. https://www.cms.gov/files/document/measure-methodology-report.pdf
- 12.Luke B, Brown MB, Liu CL, Diop H, Stern JE. Validation of Severe Maternal Morbidity on the US Certificate of Live Birth. Epidemiology. Jul 2018;29(4):e31–e32. doi: 10.1097/ede.0000000000000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of O, Gynecologists, the Society for Maternal-Fetal M, Kilpatrick SK, Ecker JL. Severe maternal morbidity: screening and review. Am J Obstet Gynecol. Sep 2016;215(3):B17–22. doi: 10.1016/j.ajog.2016.07.050 [DOI] [PubMed] [Google Scholar]
- 14.Roberts CL, Cameron CA, Bell JC, Algert CS, Morris JM. Measuring maternal morbidity in routinely collected health data: development and validation of a maternal morbidity outcome indicator. Med Care. Aug 2008;46(8):786–94. doi: 10.1097/MLR.0b013e318178eae4 [DOI] [PubMed] [Google Scholar]
- 15.Dzakpasu S, Deb-Rinker P, Arbour L, et al. Severe maternal morbidity surveillance: Monitoring pregnant women at high risk for prolonged hospitalisation and death. Paediatr Perinat Epidemiol. Jul 2020;34(4):427–439. doi: 10.1111/ppe.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouvier-Colle MH, Mohangoo AD, Gissler M, et al. What about the mothers? An analysis of maternal mortality and morbidity in perinatal health surveillance systems in Europe. Bjog. Jun 2012;119(7):880–9; discussion 890. doi: 10.1111/j.1471-0528.2012.03330.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair M, Kurinczuk JJ, Knight M. Establishing a National Maternal Morbidity Outcome Indicator in England: A Population-Based Study Using Routine Hospital Data. PLoS One. 2016;11(4):e0153370. doi: 10.1371/journal.pone.0153370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parchem JG, Gupta M, Chen HY, Wagner S, Mendez-Figueroa H, Chauhan SP. Adverse Infant and Maternal Outcomes Among Low-Risk Term Pregnancies Stratified by Race and Ethnicity. Obstet Gynecol. Apr 2020;135(4):925–934. doi: 10.1097/aog.0000000000003730 [DOI] [PubMed] [Google Scholar]
- 19.Leonard SA, Kennedy CJ, Carmichael SL, Lyell DJ, Main EK. An Expanded Obstetric Comorbidity Scoring System for Predicting Severe Maternal Morbidity. Obstet Gynecol. Sep 2020;136(3):440–449. doi: 10.1097/AOG.0000000000004022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson K, Letourneau JM, Kuppermann M, Einerson BD. Association of Obstetric and Neonatal Outcomes With Deviation From Guidelines for Gestational Carriers. Obstet Gynecol. Aug 2020;136(2):387–393. doi: 10.1097/aog.0000000000003918 [DOI] [PubMed] [Google Scholar]
- 21.Forde B, DeFranco EA. Association of Prior Cesarean Delivery With Early Term Delivery and Neonatal Morbidity. Obstet Gynecol. Jun 2020;135(6):1367–1376. doi: 10.1097/aog.0000000000003878 [DOI] [PubMed] [Google Scholar]
- 22.Wagner SM, Chen HY, Gupta M, Chauhan SP. Association of Time of Delivery With Composite Adverse Outcomes in Low-Risk Pregnancies. Obstet Gynecol. Mar 2020;135(3):527–534. doi: 10.1097/aog.0000000000003675 [DOI] [PubMed] [Google Scholar]
- 23.Brown CC, Adams CE, George KE, Moore JE. Associations Between Comorbidities and Severe Maternal Morbidity. Obstet Gynecol. Nov 2020;136(5):892–901. doi: 10.1097/aog.0000000000004057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lassey SC, Little SE, Saadeh M, et al. Cephalic Elevation Device for Second-Stage Cesarean Delivery: A Randomized Controlled Trial. Obstet Gynecol. Apr 2020;135(4):879–884. doi: 10.1097/aog.0000000000003746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton EF, Rogan SC, Lopa S, Sharbaugh D, Muldoon MF, Catov JM. Early Pregnancy Blood Pressure Elevations and Risk for Maternal and Neonatal Morbidity. Obstet Gynecol. Jul 2020;136(1):129–139. doi: 10.1097/aog.0000000000003885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindappagari S, Greene N, Burwick R, Wong MS, Gregory KD. Maternal and Neonatal Morbidity After 4 and 6 Hours of Protracted Active Labor in Nulliparous Term Pregnancies. Obstet Gynecol. Jan 2020;135(1):185–193. doi: 10.1097/aog.0000000000003587 [DOI] [PubMed] [Google Scholar]
- 27.Doty MS, Chen HY, Chauhan SP. Neonatal Seizures Among Low-Risk Pregnancies at Term: Risk Factors and Adverse Outcomes. Obstet Gynecol. Jun 2020;135(6):1417–1425. doi: 10.1097/aog.0000000000003866 [DOI] [PubMed] [Google Scholar]
- 28.Baldwin HJ, Nippita TA, Torvaldsen S, Ibiebele I, Ford JB, Patterson JA. Outcomes of Subsequent Births After Placenta Accreta Spectrum. Obstet Gynecol. Oct 2020;136(4):745–755. doi: 10.1097/aog.0000000000004051 [DOI] [PubMed] [Google Scholar]
- 29.Carusi DA, Fox KA, Lyell DJ, et al. Placenta Accreta Spectrum Without Placenta Previa. Obstet Gynecol. Sep 2020;136(3):458–465. doi: 10.1097/aog.0000000000003970 [DOI] [PubMed] [Google Scholar]
- 30.Mourad M, Wen T, Friedman AM, Lonier JY, D’Alton ME, Zork N. Postpartum Readmissions Among Women With Diabetes. Obstet Gynecol. Jan 2020;135(1):80–89. doi: 10.1097/aog.0000000000003551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howell EA, Egorova NN, Janevic T, et al. Race and Ethnicity, Medical Insurance, and Within-Hospital Severe Maternal Morbidity Disparities. Obstet Gynecol. Feb 2020;135(2):285–293. doi: 10.1097/aog.0000000000003667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanner LD, Chen HY, Sibai BM, Chauhan SP. Racial and Ethnic Disparities in Maternal and Neonatal Adverse Outcomes in College-Educated Women. Obstet Gynecol. Jul 2020;136(1):146–153. doi: 10.1097/aog.0000000000003887 [DOI] [PubMed] [Google Scholar]
- 33.Paidas Teefey C, Reforma L, Koelper NC, et al. Risk Factors Associated With Cesarean Delivery After Induction of Labor in Women With Class III Obesity. Obstet Gynecol. Mar 2020;135(3):542–549. doi: 10.1097/aog.0000000000003703 [DOI] [PubMed] [Google Scholar]
- 34.Kozhimannil KB, Interrante JD, Tofte AN, Admon LK. Severe Maternal Morbidity and Mortality Among Indigenous Women in the United States. Obstet Gynecol. Feb 2020;135(2):294–300. doi: 10.1097/aog.0000000000003647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dombrowski M, Illuzzi JL, Reddy UM, et al. Trial of Labor After Two Prior Cesarean Deliveries: Patient and Hospital Characteristics and Birth Outcomes. Obstet Gynecol. Jul 2020;136(1):109–117. doi: 10.1097/aog.0000000000003845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merriam AA, Huang Y, Wright JD, Goffman D, D’Alton ME, Friedman AM. Use of Uterine Tamponade and Interventional Radiology Procedures During Delivery Hospitalizations. Obstet Gynecol. Mar 2020;135(3):674–684. doi: 10.1097/aog.0000000000003722 [DOI] [PMC free article] [PubMed] [Google Scholar]


