Abstract
The microbiota-gut-brain axis has been shown to influence human health and diseases, including depression. The interactions between drugs and intestinal microbiota are complex and highly relevant to treat diseases. Studies have shown an interaction between antidepressants and intestinal microbiota. Antidepressants may alter the abundance and composition of intestinal microbiota, which are closely related to the treatment outcomes of depression. Intestinal microbiota can influence the metabolism of antidepressants to change their availability (e.g., tryptophan can be metabolized to kynurenine by intestinal microbiota) and regulate their absorption by affecting intestinal permeability. In addition, the permeability of the blood–brain barrier can be altered by intestinal microbiota, influencing antidepressants to reach the central nervous system. Bioaccumulation is also a type of drug–microbiota interaction, which means bacteria accumulate drugs without biotransformation. These findings imply that it is important to consider intestinal microbiota when evaluating antidepressant therapy regimens and that intestinal microbiota can be a potential target for depression treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-023-01362-8.
Keywords: Depression, Antidepressants, Intestinal microbiota, Interaction, Metabolism
Introduction
Most bacteria in the human body are present in the intestine [1]. The microbiota-gut-brain axis is used to describe the bidirectional communication between the microbiota in the gut and brain [2]. This axis may function through mechanisms such as microbial metabolites, vagus nerve, enteric nervous system, immune signaling, serotonin, tryptophan, and tryptamine metabolism [3, 4]. In the human body, some live microorganisms are beneficial to human health and are called probiotics [5, 6].
Several studies have revealed a close relationship between intestinal microbiota and human diseases, including metabolic diseases [7], cancers [8, 9], and autoimmune diseases [10]. Moreover, intestinal microbiota composition is also associated with psychiatric diseases, such as Alzheimer’s disease, Parkinson’s disease, autism, and post-traumatic stress disorder [11–14].
The relationship between major depressive disorder (MDD) and gut microbiota has recently received extensive attention. Huang et al. [15] concluded that increased abundances of the phylum Actinobacteria, order Bacteroidales, family Enterobacteriaceae, genus Alistipes, and decreased abundances of the family Lachnospiraceae, genus Faecalibacterium, were associated with depression. The alterations in intestinal microbiota play an important role in the pathogenesis and treatment of depression.
The most commonly used antidepressants worldwide include monoamine-oxidase inhibitors (MAOI), tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and serotonin and norepinephrine reuptake inhibitors (SNRIs). The antidepressant effects of MAOI are generally related to the inhibition of MAO in the central nervous system to decrease the degradation of monoamine transmitters [16]. TCAs block serotonin and norepinephrine reuptake and maintain their levels in the synaptic cleft [17, 18]. SSRIs inhibit serotonin reuptake by the presynaptic membrane to maintain its levels in the synaptic cleft [19]. In recent years, ketamine has been used as a rapid-acting antidepressant for treating depression. The antidepressant effects of ketamine are associated with its blockade of the N-methyl d-aspartate receptor (NMDAR), an ionotropic glutamate receptor [20], and increased function of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors [21]. In addition to these primary mechanisms responsible for their antidepressant-like effects, some other potential mechanisms have also been investigated. For example, the anti-inflammatory effect may be one of the potential mechanisms of action of the SSRIs, SNRIs, and ketamine [22, 23]. Interestingly, the intestinal microbiota may participate in the processes mentioned above. Getachew at el. [24] showed that ketamine administration decreased the abundance of Ruminococcus and Mucispirillum in the stool samples of rats. Indeed, high levels of Ruminococcus may increase the severity of irritable bowel disease (IBD), while some species of Mucispirillum may lead to intestinal inflammation. Another study showed that ketamine could increase the levels of Lactobacillus johnsonii in LPS-induced depressive mice, which may play a role in improving depressive-like behaviors via the hypothalamic–pituitary–adrenal axis. The antidepressant effects of ketamine and its metabolites could also be related to improving the abundance of SCFAs-producing microbiota including Butyricimonas, Turicibacter, and Clostridiales [25]. Indeed, (R)-ketamine and lanicemine are both NMDAR antagonists but the former shows obvious antidepressant effects on treatment-resistant depressed patients, while the latter does not present antidepressant effects in such patients [26]. (R)-ketamine significantly attenuated the reduced levels of Mogibacteriaceae, Bacteroidales, and Clostridiales, as well as the increased levels of Ruminococcaceae and Clostridium in the chronic social defeat stress (CSDS) susceptible mice, while less potent effects of lanicemine on the intestinal microbiota were observed [26]. Taken together, the modulation of intestinal microbiota may partly mediate the antidepressant mechanism [27]. Notably, anxiety disorders often coexist with depression. Accumulating evidence indicates that SSRIs, dual SNRIs, and many TCAs can be used in improving lots of anxiety disorders [28, 29], regardless of the severity of mental status. IBD can lead to comorbidities of anxiety and depression by inducing neuroinflammation [30]. Given the close relationship between intestinal microbiota, anxiety symptoms, and the severity of mental status, antidepressants may not only affect the depression-related microbiota but also exert more complicated effects.
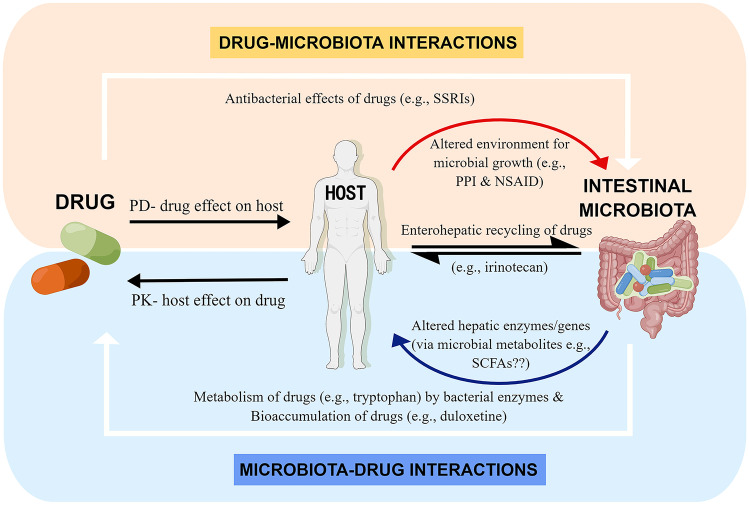
Research has shown that intestinal microbiota and various drugs have reciprocal interactions; i.e., the drugs can influence the ecology of the gut microbiome [31], while intestinal microbiota can directly participate in the chemical transformation and bioaccumulation of drugs [32, 33], as shown in Fig. 1. When metabolizing medications, intestinal microbiota mainly conducts hydrolytic and reductive reactions. For instance, the cardiovascular drug digoxin can be inactivated via biotransformation by intestinal microbiota, and the bacterial enzyme β-glucuronidase has been reported to be associated with the toxicity of the common colon cancer chemotherapeutic CPT-11 (also known as irinotecan) [34, 35]. These examples indicate that intestinal microbiota can affect the activity and toxicity of drugs [36–38]. Furthermore, it has also been found that intestinal microbiota is a significant factor affecting the efficacy of antidepressants [39].
Fig. 1.
Complex interplay between drugs and intestinal microbiota. The interactions between drugs and intestinal microbiota include microbiota-mediated alterations to drug pharmacokinetics and drug-mediated alterations to the function/composition of intestinal microbiota. “Drug–Microbiota Interactions”: drugs can have direct antibacterial effects on intestinal microbiota (e.g., SSRIs) and can also indirectly alter the environment for microbial growth by their pharmacodynamic effect on the host (e.g., proton pump inhibitor alters gastric acid production and pH, and non-steroidal anti-inflammatory drug changes mucosal integrity, illustrated by the curved-down line arrow). The interactions between the host and intestinal microbiota cause the enterohepatic recirculation of drugs, e.g., intestinal microbiota deconjugate the hepatic-glucuronidated irinotecan metabolite by β-glucuronidase enzymes. “Microbiota-Drug Interactions”: intestinal microbiota can directly metabolize drugs by bacterial enzymes (e.g., tryptophan), or bioaccumulate drugs (e.g., duloxetine). In addition, intestinal microbiota can alter hepatic enzymes/genes, which may influence the pharmacokinetic effect of the host on drugs, e.g., microbial-derived metabolites (e.g., SCFAs and secondary bile acids) may be potential mediators of this effect (illustrated by the curved-up line arrow). PK, pharmacokinetic; PD, pharmacodynamic. This figure was obtained from reference [39] with slight modification. By Figdraw
Although great progress has been made in treating depression, many issues remain unsolved. Despite administering sufficient doses and maintenance treatment, 30–40% of patients do not respond to the treatment [40–42]. The side effects of antidepressants are among the factors affecting treatment outcomes [43]. Headache, nausea, and insomnia are the three most common side effects of antidepressants, with incidence rates exceeding 10% [44]. In addition, tolerability, acceptability, pharmacokinetics, pharmacodynamics, and drug-drug interactions also affect antidepressant treatment outcomes [45]. Considering the possible role of intestinal microbiota in the treatment outcomes of antidepressants, we provide here a review of the recent discoveries on the possible interaction between antidepressants and intestinal microbiota, especially how intestinal microbiota can affect antidepressants and their efficacy, which might have reference value for investigating new pathways and factors influencing antidepressant effects. By describing the possible effects that the intestinal microbiota may have on antidepressants, we may provide a reference for better-applying antidepressants clinically considering intestinal microbiota. Moreover, this article also provides information about the possible therapeutic targets related to intestinal microbiota for the development of new antidepressants.
Effects of Intestinal Microbiota on Depression
Accumulating evidence demonstrates the role of the microbiota-gut-brain axis in psychiatric diseases, and more attention has been paid to the effect of intestinal microbiota on depression. Many studies have explored the relationship between intestinal microbiota and the changes in depressive phenotypes. In animal studies, fecal microbiota transplantation (FMT) of germ-free mice with “depression microbiota” derived from MDD patients, the absence of gut microbiota in germ-free mice, and antibiotic-induced microbiota perturbation all led to depression-like behaviors [46–48]. Specifically, FMT from patients with rheumatoid arthritis caused depression-like behaviors in antibiotic-treated mice via abnormal T cell differentiation [49]. These findings indicate that microbiota may have an important role in the pathogenesis of depression. Additionally, probiotic supplementation alleviates depression-like behaviors [50–53]. Meanwhile, another study showed that some probiotics improved cognitive function in patients with major depression [54]. Thus, probiotics may play a role in the treatment of depression. In contrast, another study showed that microbiota variation is related to antidepressant treatment resistance in patients with MDD [55]. Intestinal microbiota can affect the structure of the brain and regulate brain-derived neurotrophic factors [56]. Oscillibacter may have protective modulatory functions in the brain, thus increasing amygdala and hippocampal volumes, closely related to depression [57]. Furthermore, intestinal microbiota can serve as molecular markers for diagnosing MDD and general anxiety disorders [58].
The vagus nerve plays a key role in the microbiota-gut-brain axis. FMT from CSDS-susceptible mice and Chrna7 knock-out (KO) mice exhibited anhedonia-like behaviors, inflammation, and downregulation of synaptic proteins in the prefrontal cortex in antibiotic-treated mice [59–61]. Studies showed that abnormal composition of intestinal microbiota including F. rodentium, L. intestinalis, L. reuteri, and systemic inflammation may be responsible for these changes via the vagus nerve [60–62]. Subdiaphragmatic vagotomy (SDV) blocked the development of depression-like behaviors in Chrna7 KO mice [62] and antibiotic-treated mice [59–61]. Moreover, plasma levels of microbe-derived metabolites like 1,5-anhydro-d-sorbitol, l-citrulline, and taurocholic acid in the KO + SDV mice were higher than those of KO + sham-operated mice, suggesting their important role in the antidepressant-like effects of SDV in Chrna7 KO mice [62]. LPS administration caused depression-like behaviors, inflammation, and downregulation of synaptic proteins in the prefrontal cortex in the sham-operated mice but not in the SDV-operated mice [63]. Moreover, LPS significantly decreased α-diversity and relative abundances of intestinal microbiota in mice, and SDV blocked this change [62]. L. rhamnosus (JB-1), the nonpathogenic bacteria, can mediate the GABAergic system in mice, and therefore, improve depression and anxiety behaviors. Vagotomy blocked the anxiolytic and antidepressant effects of L. rhamnosus (JB-1) and the changes in the GABAergic system in the amygdala and the hippocampus [64]. To conclude, the vagus nerve is an important factor in the pathogenesis of depression through the microbiota-gut-brain axis.
SCFAs are important gut microbiome-derived metabolites within the microbiota-gut-brain axis, which are produced by bacteria fermenting dietary fiber in the gastrointestinal tract [65]. In the human body, acetate, propionate, and butyrate are the most abundant SCFAs [65]. Studies showed that SCFAs decreased significantly in depressed mice compared to control mice [66], while administration of SCFAs attenuated depression-like behaviors [67, 68]. Moreover, in depressed mice, some bacteria taxa showing low relative abundances significantly correlated with two major SCFAs with reduced levels (acetic acid and propionic acid) [66]. In a recent study focused on the depressive-like behaviors of high fructose-fed mice exposed to chronic stress, SCFA supplementation showed protective effects on hippocampal neurogenesis, ameliorated blood–brain barrier (BBB) damage, suppressed microglia activation, and neuroinflammation in these mice, which were related to antidepressant-like effects [69]. Lower butyrate levels may increase the gut barrier permeability, causing bacterial translocation into the systemic circulation and systemic inflammation [70]. The mechanisms of butyrate and other SCFAs in improving depression-like behaviors may correlate with their anti-inflammatory effects, inducing histone hyperacetylation and elevating BDNF levels [71]. These findings suggested that SCFAs may be essential mediators in depression.
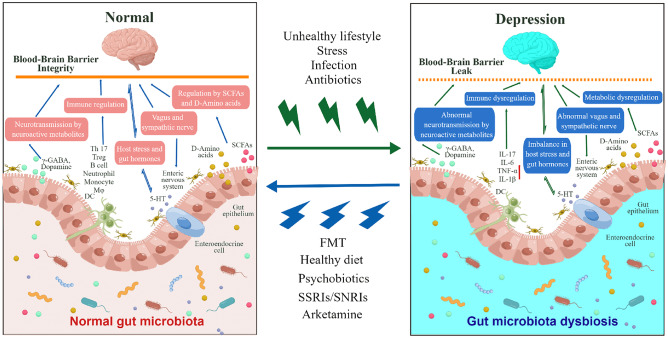
Based on these findings, it is apparent that investigating the relationship between intestinal microbiota and depression is important, as shown in Fig. 2.
Fig. 2.
Role of the microbiota–gut–brain axis in depression. An unhealthy lifestyle, increased and sustained stress, infection, antibiotics, or other factors can cause gut microbiota dysbiosis. Abnormal changes may occur in the body, which can be mediated by the microbiota–gut–brain axis via neural, immune, or chemical signals, thereby causing depression. Conversely, FMT, a healthy diet, psychobiotics, and antidepressants (e.g., SSRIs, SNRIs, and arketamine) can restore gut microbiota dysbiosis, abnormal brain function, and depressive symptoms via the microbiota-gut-brain axis. γ-GABA, γ-aminobutyric acid; CNS, central nervous system; DC, dendritic cell; 5-HT, 5-hydroxytryptamine; IL-6, interleukin 6; IL-17, interleukin 17; IL-1β, interleukin 1β; SCFA, short-chain fatty acid; TNF-α, tumor necrosis factor α. The figure is obtained from reference [72] with slight modification. By Figdraw
When drugs are consumed orally, they encounter a considerable abundance of intestinal microbiota, which can affect the ability of the drugs to treat depression. Fontana et al. [55] conducted a study to determine differences in the compositions of intestinal microbiota between patients with MDD and healthy controls (HCs) and between patients with treatment-resistant depression (TRD) and those responsive (R) to antidepressants. Several bacteria (Thaumarchaeota, Yersinia, and its species Yersinia pseudotuberculosis, Peptococcus, Fenollaria timonensis, Blautia spp. canine oral taxon 337, and Papillibacter cinnamivorans) were identified in the microbiota of TRD patients but not in that of the R patients. Compared to HC, Flavobacteriaceae, Hungatella, Yersinia, Citrobacter, Fenollaria, and Fenollaria timonensis were identified exclusively in TRD patients, whereas Elusimicrobia, Flavobacteriaceae, Fenollaria, and Robinsoniella sp. MCWD5 were found exclusively in treatment-responsive patients with MDD. This result indicated that intestinal microbiota was related to the pathogenesis of MDD and patients’ response to antidepressants.
In another study focusing on chronic unpredictable mild stress (CUMS) mice treated with escitalopram, the composition of intestinal microbiota differed between the responder and non-responder groups. The relative abundances of the genus Prevotellaceae_UCG-003 increased in the responder group, whereas the families Ruminococcaceae and Lactobacillaceae were depleted in the non-responder group [73].
Lee et al. [74] focused on the role of intestinal microbiota as a predictor of antidepressant treatment outcomes in geriatric depression. At the level of the individual taxa, a random forest classifier created using nine genera from the baseline microbiota accurately predicted remission. Of these, baseline enrichment of Faecalibacterium, Agathobacter, and Roseburia relative to the reference frame was associated with remission upon treatment. Differential abundance analysis revealed significant genus-level changes from baseline to post-treatment in remitters but not in non-remitters.
Dong et al. [75] found that among patients with MDD treated with antidepressants, intestinal microbiota composition at baseline differed significantly between responder and non-responder groups. The expression of 20 metabolites, mainly involved in lipid metabolism, differed significantly between the responder and non-responder groups. Therefore, alterations in intestinal microbiota and associated metabolites may affect the antidepressant treatment outcomes.
The rat model of adrenocorticotrophic hormone (ACTH) treatment has been widely accepted for TR depression. Chronic administration of ACTH leads to resistance to imipramine treatment in the forced swimming test, and resistance to other antidepressants [76–78]. Research has shown that ACTH-induced depression disturbs the gut microbiota composition, like Oscillospira, Ruminococcus, Akkermansia, Lactobacillus, and Klebsiella [79]. Changes in intestinal microbiota may be relevant to TR effects in ACTH-treated rats.
Based on the above studies, alterations in intestinal microbiota composition may be associated with the response to antidepressants and clinical treatment outcomes.
Antidepressants May Alter the Abundance and Composition of Intestinal Microbiota
Studies have shown that many intrinsic and extrinsic factors, such as diet, medication, smoking, lifestyle, host genetics, and diseases, affect intestinal microbiota in healthy individuals [80–82]. In recent years, numerous studies have revealed that many antidepressants may alter the abundance and composition of intestinal microbiota and that their antidepressant-like effects may also be related to these changes. The SSRIs fluoxetine (Flu) and escitalopram were found to reduce the abundance of intestinal microbiota, especially that of Ruminococcus, Adlercreutzia, and an undefined Alphaproteobacteria; the same was verified for two SNRIs, namely, venlafaxine and duloxetine. A decrease in intestinal microbiota richness may result in possible side effects. Further investigation showed that introducing a single Ruminococcus species (R. flavefaciens) can attenuate the effects of an antidepressant by inducing changes in synaptic and mitochondrial gene expression and alterations in monoamine neurotransmitter levels. It is also beneficial for alleviating antidepressant-induced constipation [83]. In addition, Zhang et al. [84] reported that the administrations of Flu and the tricyclic antidepressant amitriptyline (Ami) were associated with a low abundance of the phylum Firmicutes and a high abundance of the phylum Bacteroidetes, while a reduced ratio of Firmicutes/Bacteroidetes appears to be associated with an improvement in neurological conditions. At the genus level, the relative abundances of Bacteroides, Parabacteroides, and Butyricimonas were significantly increased in the feces of Ami- and Flu-treated rats compared to those in rats exposed to CUMS, suggesting that these microbes and their metabolites are related to brain health. Moreover, Ami and Flu treatments may also affect potentially harmful bacteria and intestinal microbiota metabolic functions, such as carbohydrate metabolism, membrane transport, and signal transduction. The mechanism of SSRIs’ antimicrobial action may be related to the inhibition of efflux pumps, as is observed in experiments, whereby SSRIs interact synergistically with antibiotics, thus decreasing the minimum inhibitory concentration for these antibiotics [85, 86]. TCAs present antiplasmid activity [87], possibly by targeting replicating plasmid DNA and the DNA gyrase enzymes, both crucial for DNA structural conformation [88].
Ketamine, a glutamate NMDAR blocker, has a rapid yet sustained antidepressant effect [21]. A study using male Wistar rats showed that chronic administration of ketamine significantly increased the levels of low-abundance bacterial genera (e.g., Lactobacillus, Turicibacter, and Sarcina) and significantly decreased opportunistic pathogens (e.g., Ruminococcus and Mucispirallum), which may partly contribute to its antidepressant and anti-inflammatory effects [24, 89]. Ketamine is a racemic mixture comprising equal parts of (R)-ketamine and (S)-ketamine. The (R)-ketamine (or arketamine) has superior and longer-lasting antidepressant effects and fewer side effects than (S)-ketamine in the animal models of depression. Study showed that (R)-ketamine changed the intestinal microbiota composition in the CSDS-susceptible mice [26]. However, because behavioral experiments using germ-free mice were not performed, these studies do not directly prove the effects of intestinal microbiota on the antidepressant actions of (R)-ketamine [26, 90].
Except for the former commonly used antidepressants, some potential novel antidepressants may have similar abilities to regulate intestinal microbiota. Inulin-type fructo-oligosaccharides purified from Morinda officinalis increased the abundance of Cyanobacteria in a rat stress model, producing metabolites such as hydrogen sulfide that have antidepressant-like effects [91]. A neuroprotectant, the C-terminal domain of the heavy chain of the tetanus toxin, may also exhibit antidepressant effects, as it has been reported to boost the abundance of probiotic bacteria (e.g., Lactobacillus, Bifidobacterium, and Butyrivibrio) and suppress the levels of bacteria associated with inflammation (e.g., Provettela and Mucispirallum) [92]. A dihydroquinoline analog of agomelatine, N-(2-(7methoxy-3,4-dihydroisoquinolin-1-yl)ethyl)acetamide hydrochloride, was found to alter the composition of the gut microbiota, reverse the dysbiosis caused by chronic stress, and regulate neuroinflammatory marker levels, thus attenuating depression-related behaviors [93]. The aqueous extract of Gastrodia elata Blume may prevent depression by regulating monoaminergic neurotransmission and intestinal microbiota composition and function [94]. Chlorogenic acid pretreatment improves depression-like behavior, with its effect likely related to serum proinflammatory cytokines and monoamine neurotransmitters; this treatment can modulate gut bacteria with certain phylotypes in rats with ACTH-induced depression [95].
In recent years, many studies have focused on traditional Chinese medicine for the treatment of depression, wherein some are used to treat depression or have potential antidepressant-like effects. These medicines include total iridoids of Valeriana jatamansi Jones (TIV), Semen Sojae Praeparatum, Puerarin, Xiaoyaosan, Jia Wei Xiao Yao San, Baihe Jizihuang Tang, Chaihu-Shugan-San, and Shugan Jieyu Capsule, and they can change the abundance and composition of intestinal microbiota [73, 96–102]. It is generally believed that the action of traditional Chinese medicine on intestinal microorganisms is an important mechanism for its antidepressant effects. Rosemary extracts, the crucial active constituents extracted from Rosmarinus officinalis, considerably alleviated depressive-like behaviors in mice subjected to chronic restraint stress by rebalancing intestinal microbiota [103]. In our previous study, we explored the antidepressant properties of neferine (Nef) in a mouse model of chronic stress–induced depression. Nef displayed an antidepressant-like effect and increased the relative abundance of Lactobacillus at the genus level. This result indicates that Nef may improve depression by regulating Lactobacillus levels, which can impact serotonin/norepinephrine/dopamine triple reuptake. Nef also mitigated depression by reducing hippocampal pyramidal cell necrosis and alleviating hippocampal lesions [104]. Some antidepressants and their effects on intestinal microbiota are included in Table 1. Conventional antidepressants may sometimes be ineffective and cause a series of side effects; therefore, new strategies to treat depression should be introduced to overcome this deficiency. Research on intestinal microbiota can help in the development of medication regimens.
Table 1.
Antidepressants and their effects on intestinal microbiota
| Antidepressants | Effects on intestinal microbiota | Reference | |
|---|---|---|---|
| SSRI | Fluoxetine | Enhance the abundance of phylum Bacteroidetes, family Porphyromonadaceae, genus Parabacteroides, genus Butyricimonas, and genus Alistipes; reduce the abundance of phylum Firmicutes, Ruminococcus, Adlercreutzia, and an undefined Alphaproteobacteria | [83, 84] |
| Escitalopram | Reduce the abundance of Ruminococcus, Adlercreutzia, and an undefined Alphaproteobacteria | [83] | |
| SNRI | Venlafaxine, and duloxetine | Reduce the abundance of Ruminococcus, Adlercreutzia, and an undefined Alphaproteobacteria | [83] |
| Tricyclic antidepressant | Amitriptyline | Increase the abundance of phylum Bacteroidetes, family Porphyromonadaceae, family Bacteroidaceae, genus Parabacteroides, genus Butyricimonas, and genus Alistipes; reduce the abundance of phylum Firmicutes | [84] |
| N-Methyl-d-aspartate receptor (NMDAR) antagonist | Ketamine | Increase the abundance of bacteria genera (e.g., Lactobacillus, Turicibacter, and Sarcina); reduce the abundance of opportunistic pathogens (e.g., Ruminococcus and Mucispirallum) | [24] |
| (R)-ketamine (or arketamine) | Attenuated the reduced levels of Butyricimonas, Mollicutes, Mogibacteriaceae, Bacteroidales, and Clostridiales, as well as the increased levels of Deltaproteobacteria, Clostridium, and Ruminococcaceae in the CSDS susceptible mice | [26, 90] | |
| Extracts from traditional Chinese medicine | Neferine | Increase the relative abundances of species belonging to phylum Firmicutes; decrease those of species belonging to phylum Bacteroidetes | [103, 104] |
| Rosemary extracts | Enhance the sequences proportion of Lactobacillus and Firmicutes; reduce the sequences proportion of Bacteroidetes and Proteobacteria in feces | [103] | |
| Others | Inulin-type fructo-oligosaccharides | Increase the abundance of the phylum Cyanobacteria | [91] |
| C-terminal domain of the heavy chain of tetanus toxin | Increase the abundance of Lactobacillus, Bifidobacterium, and Butyrivibrio; reduce the abundance of Mucispirallum | [92] | |
| N-(2-(7methoxy-3,4-dihydroisoquinolin-1-yl)ethyl) acetamide hydrochloride | Reverse the phenomenon that CUMS increases the richness of the gut bacterial community, resulting in a return to a normal level of richness | [93] | |
Mechanisms of Intestinal Microbiota Influencing the Efficacy of Antidepressants
Intestinal Microbiota Influence Drug Metabolism
Intestinal microbiota can not only directly but also indirectly affect drug metabolism. For example, intestinal microbiota can chemically transform drugs and modulate host xenobiotic metabolism, including drug metabolism pathways [36]. With an increasing number of studies focusing on factors influencing the efficacy of antidepressants, many researchers have found that intestinal microbiota plays an important role in the metabolism of antidepressants.
Paeoniflorin, the main component of the Chinese traditional medicine Xiaoyaosan, displays antidepressant-like effects in rats treated with chronic unpredictable stress (CUS) [105]. It is difficult to be absorbed and to cross the BBB [106]. After oral administration of paeoniflorin in a rat model of CUS, the bioavailability was only 2.32% [107]; low permeability and metabolism of paeoniflorin may be one of the reasons for this result [108]. A study showed that the major metabolite of paeoniflorin in vivo may be paeoniflorgein [109]. Intestinal microbiota can convert paeoniflorin into benzoic acid using carboxylesterase [107]. Benzoic acid can cross the BBB and act as an inhibitor of D-amino acid oxidase in the brain, thus improving brain function and presenting antidepressant activity [110]. Therefore, when antibiotics reduce the abundance of microbiota in the intestine, the metabolic conversion of paeoniflorin to benzoic acid is also reduced, leading to low bioavailability [107].
Tryptophan (Trp) is an amino acid that cannot be produced by animal cells; therefore, humans must obtain it from the outside environment, mostly through diet. Trp is considered a supplementation for the treatment of depression and may be effective by increasing the precursor for 5-hydroxyindole (5-HT) synthesis and normalizing its release to recover serotonin deficiency. However, the availability of Trp is reduced in the mental disorders [111]. In the gut, Trp can be metabolized to kynurenine (Kyn) and its derivatives by the rate-limiting enzyme indoleamine 2,3-dioxygenase (IDO) 1. Intestinal microbiota plays a key role in stimulating IDO1 activity. In addition, specific intestinal microbiota can directly transfer Trp to Kyn and its derivatives, as they encode enzymes homologous to those of the eukaryotic kynurenine pathway [112, 113]. Therefore, it can be inferred that if the Kyn pathway overacts, Trp will mainly be diverted to Kyn instead of entering the brain to display an antidepressant effect, thus affecting its bioavailability [114].
5-Hydroxytryptophan (5-HTP) is used in some therapeutic regimens to treat depression [115]. It is converted to 5-HT via tryptophanase in various intestinal microbiome strains [116]. However, 5-HT cannot pass through the BBB [117]; therefore, 5-HTP must first cross the BBB, where it can be transformed to 5-HT, thus displaying an antidepressant-like effect [118–122]. Therefore, the metabolism of 5-HTP by intestinal microbiota may be the reason why 5-HTP itself only slightly elevates the brain’s extracellular 5-HT [123]. Interestingly, M. officinalis oligosaccharides, which are used to treat depression in China, can accelerate 5-HTP production from tryptophan and at the same time reduce 5-HT generation by alerting the activity of relevant enzymes in intestinal microbiota, thus accumulating 5-HTP. Then, 5-HTP from intestinal microbiota can be transported through the blood and cross the BBB to improve 5-HT levels in the brain [124].
Some intestinal microbiota is capable of N-demethylation, especially N-demethylating a tricyclic antidepressant, imipramine [125], causing fluctuations in the plasma concentration of this drug [126]. Cistanche tubulosa, a species of Cistanches Herba, has been confirmed to elicit antidepressant activity by regulating bacterial composition. Cistanche tubulosa extract (CTE) is metabolized to aglycones and the degradation products of phenylethanoid glycosides (PhGs) and iridoid glycosides by intestinal microbiota. The PhGs and iridoid glycosides in CTE were readily metabolized to secondary glycosides and aglycones in rats with CUS. These metabolites typically display high intestinal absorption and bioavailability, thereby exerting satisfactory biological activity [127–130].
Intestinal Microbiota Influence Drug Absorption
An altered microbiota state in patients with MDD is linked to increased gut permeability and regulation of intestinal drug transport and absorption [131]. Depression can modulate intestinal permeability and barrier function, which in turn may alter the drug absorption [132]. Acute stress has been reported to be associated with the expression of the tight junction proteins zonula occludens-1 (ZO-1) and occludin in the duodenal mucosa of rats subjected to water-immersion restraint stress [133], thus changing gut permeability. The bacterial enzyme tryptophanase produces indole and its derivatives from tryptophan. Indole also regulates the permeability of the intestinal barrier [134, 135]. Therefore, intestinal microbiota may affect gut permeability, thus influencing drug absorption.
Intestinal Microbiota Changes the Permeability of the BBB
Studies have shown that antibiotic-induced changes in gut microbial composition increase BBB permeability by intestinal microbiota-produced metabolites that change central nervous system functions. A study showed a consistent increase in BBB permeability in the hippocampus, which may explain why intestinal microbiota dysbiosis is strongly correlated with neurological and psychological diseases such as Alzheimer’s disease, autism spectrum disorder, and depression [136]. TIV may enhance the abundances of Firmicutes (e.g., Lactobacillus spp.) and Bacteroidetes to mediate the composition and function of intestinal microbiota and change the expression of ZO-1 and occludin, thus protecting the BBB to exert an antidepressant effect [137]. Yi et al. [138] reported that borneol can increase the permeability of the BBB and dose-dependently improve the distribution of puerarin in the brain. Puerarin is the main active ingredient in Puerariae Radix, a traditional Chinese medicinal herb. The self-microemulsifying drug delivery system co-loading borneol and puerarin resulted in the highest area under the curve (AUC)brain of all three oral formulations (nanocrystals suspension, inclusion compound solution, and self-microemulsifying drug delivery system) in the study, which was 10.27 times that of puerarin nanocrystals suspension without borneol. In addition, another study showed that the release of encapsulated 5′-(N-ethylcarboxamido)adenosine, an adenosine 2A receptor agonist, increased BBB permeability, thus amplifying the therapeutic efficacy of clinical drugs and immune checkpoint blockade antibodies in the treatment of glioblastoma [139]. Interestingly, puerarin can also alleviate CUMS-induced depression-like behaviors, possibly owing to the restoration of stress-induced disruptions of normal intestinal microflora [73]. Moreover, SCFAs derived from intestinal microbiota significantly increased the protein levels of ZO-1, claudin-5, and occludin in the brain vasculature of high fructose-fed mice exposed to chronic stress [69]. (R)-ketamine could ameliorate demyelination in cuprizone-treated mice possibly by normalizing the abnormal composition of intestinal microbiota, and could facilitate remyelination in the brain after cuprizone withdrawal possibly by improving the decreased levels of lactic acid [140]. Thus, it can be estimated that the permeability of the BBB greatly influences the treatment outcomes of drugs, potentially indicating the importance of intestinal microbiota in altering BBB permeability during depression treatment.
Other Effects of Intestinal Microbiota
A recent study showed that intestinal microbiota can also modulate the availability and efficacy of antidepressants in another way, bioaccumulation. Klunemann et al. [32] found that the efficacy of duloxetine on the behavior of Caenorhabditis elegans was reduced due to bioaccumulation by intestinal microbiota. During the experiment, the researchers confirmed that four selected strains (Streptococcus salivarius, Bacteroides uniformis, Escherichia coli IAI1, and E. coli ED1a) depleted duloxetine from the gut microbiome medium without biotransformation. This led to a direct reduction in drug availability. In addition, bioaccumulation can change metabolite secretion, which leads to changes in community composition, side effects, or even the mode of action of some drugs [141–143]. During the cultivation of S. salivarius in the presence of duloxetine, several metabolites were found to be accumulated, thus improving the growth of Eubacterium rectale. In terms of the drug duloxetine, gut bacterial interactions are involved in side effects such as weight gain, as well as in its mode of action [88, 144, 145]. Interestingly, another study indicated that R. flavefaciens, a type of intestinal microorganism, can reduce the antidepressant-like effect of duloxetine by impairing mitochondrial oxidative phosphorylation and neural plasticity in the medial prefrontal cortices [83].
Conclusions and Future Perspectives
There is a bidirectional relationship between antidepressants and intestinal microbiota. Antidepressants may alter the abundance and composition of intestinal microbiota, which is closely related to the treatment outcomes of depression. The mechanisms by which antidepressants mediate intestinal microbiota to alleviate depressive-like behaviors are unclear but some explanations have shed light on the microbial metabolites, neurotransmitters, and inflammatory factors in the brain-gut axis [3, 4, 146]. However, antidepressants may produce their effects through multiple mechanisms, and intestinal microbiota may just be one of them. Most recent studies have not performed behavioral experiments using germ-free mice, so these do not provide direct evidence of the effects of intestinal microbiota on the antidepressant actions of the drugs. Further investigations of the specific underlying mechanisms are needed, to help us fully understand the role of the brain-gut axis in treating depression.
Intestinal microbiota can determine the efficacy of antidepressants by influencing their metabolism, drug absorption, BBB permeability, and bioaccumulation. These findings imply that it is important to consider intestinal microbiota when considering antidepressant therapy regimens. However, studies on the interaction between antidepressants and intestinal microbiota are insufficient. Forslund et al. [147] showed that medication intake, including dosage, drug combination, and previous exposure to antibiotics, can cause variations in the microbiome and clinical phenotypes. As there has been an increase in polypharmacy involving antidepressants [148, 149], it is necessary to investigate the combinatorial effects and dosage on the microbiome and treatment outcomes and how intestinal microbiota affect the efficacy of these drugs, especially at the molecular level. After understanding the molecular mechanisms by which intestinal microbiota influence the efficacy of antidepressants, new medications targeting the corresponding molecular sites may be developed. Further studies should focus on the detailed mechanisms of how intestinal microbiota influence the efficacy of different antidepressants and how doctors can better utilize the advantages and bypass the disadvantages in treating depression. Some techniques have been developed to predict and identify pharmacokinetic changes mediated by the microbiome [150, 151], including tools that can map the ability of the human gut microbiome to metabolize small-molecule drugs [152]; these may be helpful for doctors to administer personalized medicine and appropriate therapy regimens. However, several factors, including diet, mental illness status, exercise, and other medications can also cause alterations in the intestinal microbiota [80, 153–155], while few studies have been conducted and concluded their confounding effects on the treatment of depression. Most antidepressants are effective in reducing anxiety disorders, too [29]. General anxiety disorder and MDD share many common features [58], and there is considerable comorbidity between them [28]. However, the gut-microbial compositions in patients with general anxiety disorder and MDD are different, and there is a correlation between the bacteria and clinical symptoms [58]. As such, antidepressants may alleviate depressive behaviors by mediating anxiety-related microbiota. However, most studies targeting MDD patients have not considered the effects of anxiety comorbidity, which may cause some bias. More studies should be conducted to demonstrate the combined effects of confounding factors on intestinal microbiota and the treatment of depression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage for the English language editing.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81621003 and 81801350) and the Key R&D Projects of the Science and Technology Department of Sichuan Province (Grant Nos. 2019YFS0217 and 2022YFS0350).
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cryan JF, O'Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 3.Long-Smith C, O'Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-gut-brain axis: new therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2020;60:477–502. doi: 10.1146/annurev-pharmtox-010919-023628. [DOI] [PubMed] [Google Scholar]
- 4.Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barichella M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. 2016;87:1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 6.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 8.Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6:92. doi: 10.1186/s40168-018-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195:74–85. doi: 10.1111/cei.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Liao J, Xia Y, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71:2233–2252. doi: 10.1136/gutjnl-2021-326269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klann EM, Dissanayake U, Gurrala A, et al. The gut-brain axis and its relation to Parkinson’s disease: a review. Front Aging Neurosci. 2022;13:782082. doi: 10.3389/fnagi.2021.782082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniya MA, Chung HJ, Al Mamun A, et al. Role of gut microbiome in autism spectrum disorder and its therapeutic regulation. Front Cell Infect Microbiol. 2022;12. [DOI] [PMC free article] [PubMed]
- 14.Hoke A, Chakraborty N, Gautam A, Hammamieh R, Jett M. Acute and delayed effects of stress eliciting post-traumatic stress-like disorder differentially alters fecal microbiota composition in a male mouse model. Front Cell Infect Microbiol. 2022;12. [DOI] [PMC free article] [PubMed]
- 15.Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet. 2019;10:98. doi: 10.3389/fgene.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27:789–797. doi: 10.1007/s40263-013-0097-3. [DOI] [PubMed] [Google Scholar]
- 17.Somogyi GT, Perel JM. Biphasic effect of tricyclic antidepressants on the release of norepinephrine from the adrenergic nerves of the rabbit heart. Psychopharmacology. 1991;104:237–243. doi: 10.1007/BF02244185. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz R, Blackmore R, Hazell P, Williams K, Woolfenden S. Tricyclic antidepressants for autism spectrum disorders (ASD) in children and adolescents. Cochrane Database Syst Rev. 2012. [DOI] [PMC free article] [PubMed]
- 19.Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry. 1999;60:4–13. [PubMed] [Google Scholar]
- 20.Hess EM, Riggs LM, Michaelides M, Gould TD. Mechanisms of ketamine and its metabolites as antidepressants. Biochem Pharmacol. 2022;197:114892. doi: 10.1016/j.bcp.2021.114892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez JP, Lucken MD, Brivio E, et al. Ketamine exerts its sustained antidepressant effects via cell-type-specific regulation of Kcnq2. Neuron. 2022;110:2283–2298. doi: 10.1016/j.neuron.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Gałecki P, Mossakowska-Wójcik J, Talarowska M. The anti-inflammatory mechanism of antidepressants - SSRIs, SNRIs. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:291–294. doi: 10.1016/j.pnpbp.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62:47–58. [PubMed] [Google Scholar]
- 24.Getachew B, Aubee JI, Schottenfeld RS, Csoka AB, Thompson KM, Tizabi Y. Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018;18:1–10. doi: 10.1186/s12866-018-1373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua H, Huang C, Liu H, et al. Depression and antidepressant effects of ketamine and its metabolites: the pivotal role of gut microbiota. Neuropharmacology. 2022;220:109272. doi: 10.1016/j.neuropharm.2022.109272. [DOI] [PubMed] [Google Scholar]
- 26.Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto KJBRB. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-16060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ait Chait Y, Mottawea W, Tompkins TA, Hammami R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-74934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 29.Tiller JW. Depression and anxiety. Med J Aust. 2013;199:S28–S31. doi: 10.5694/mja12.10628. [DOI] [PubMed] [Google Scholar]
- 30.Ge L, Liu S, Li S, et al. Psychological stress in inflammatory bowel disease: psychoneuroimmunological insights into bidirectional gut-brain communications. Front Immunol. 2022;13. [DOI] [PMC free article] [PubMed]
- 31.Nagata N, Nishijima S, Miyoshi-Akiyama T, et al. Population-level metagenomics uncovers distinct effects of multiple medications on the human gut microbiome. Gastroenterology. 2022;163:1038–1052. doi: 10.1053/j.gastro.2022.06.070. [DOI] [PubMed] [Google Scholar]
- 32.Klunemann M, Andrejev S, Blasche S, et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature. 2021;597:533–538. doi: 10.1038/s41586-021-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindell AE, Zimmermann-Kogadeeva M, Patil KR. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat Rev Microbiol. 2022;20:431–443. doi: 10.1038/s41579-022-00681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doestzada M, Vila AV, Zhernakova A, et al. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell. 2018;9:432–445. doi: 10.1007/s13238-018-0547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol. 2010;2:51–63. doi: 10.1177/1758834009355164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017;179:204–222. doi: 10.1016/j.trsl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott TA, Quintaneiro LM, Norvaisas P, et al. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 2017;169:442–456. doi: 10.1016/j.cell.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364. [DOI] [PMC free article] [PubMed]
- 39.Walsh J, Griffin BT, Clarke G, Hyland NP. Drug-gut microbiota interactions: implications for neuropharmacology. Br J Pharmacol. 2018;175:4415–4429. doi: 10.1111/bph.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maalouf FT, Brent DA. Child and adolescent depression intervention overview: what works, for whom and how well? Child Adolesc Psychiatr Clin. 2012;21:299–312. doi: 10.1016/j.chc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Thomas SJ, Shin M, McInnis MG, Bostwick JR. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35:433–449. doi: 10.1002/phar.1576. [DOI] [PubMed] [Google Scholar]
- 42.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85:270–288. doi: 10.1159/000447034. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Z, Chen Z, Xue M, Zhang J, Leng L. Application of antidepressants in depression: a systematic review and meta-analysis. J Clin Neurosci. 2020;80:169–181. doi: 10.1016/j.jocn.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Bayes A, Parker G. How to choose an antidepressant medication. Acta Psychiatr Scand. 2019;139:280–291. doi: 10.1111/acps.13001. [DOI] [PubMed] [Google Scholar]
- 46.Kelly JR, Borre Y, O’Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 48.Guida F, Turco F, Iannotta M, et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Pu Y, Zhang Q, Tang Z, et al. Fecal microbiota transplantation from patients with rheumatoid arthritis causes depression-like behaviors in mice through abnormal T cells activation. Transl Psychiatry. 2022;12:223. doi: 10.1038/s41398-022-01993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aygun H, Akin AT, Kizilaslan N, Sumbul O, Karabulut D. Probiotic supplementation alleviates absence seizures and anxiety- and depression-like behavior in WAG/Rij rat by increasing neurotrophic factors and decreasing proinflammatory cytokines. Epilepsy Behav. 2022;128:108588. doi: 10.1016/j.yebeh.2022.108588. [DOI] [PubMed] [Google Scholar]
- 51.Ding Y, Bu F, Chen T, et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. 2021;105:8411–8426. doi: 10.1007/s00253-021-11622-2. [DOI] [PubMed] [Google Scholar]
- 52.Chudzik A, Orzylowska A, Rola R, Stanisz GJ. Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: modulation of the brain-gut-microbiome axis. Biomolecules. 2021;11:1000. doi: 10.3390/biom11071000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyagi P, Tasleem M, Prakash S, Chouhan G. Intermingling of gut microbiota with brain: Exploring the role of probiotics in battle against depressive disorders. Food Res Int. 2020;137:109489. doi: 10.1016/j.foodres.2020.109489. [DOI] [PubMed] [Google Scholar]
- 54.Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Fontana A, Manchia M, Panebianco C, et al. Exploring the role of gut microbiota in major depressive disorder and in treatment resistance to antidepressants. Biomedicines. 2020;8:311. doi: 10.3390/biomedicines8090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dehghani F, Abdollahi S, Shidfar F, Clark CCT, Soltani S. Probiotics supplementation and brain-derived neurotrophic factor (BDNF): a systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci. 2022:1–11. [DOI] [PubMed]
- 57.Lee SM, Milillo MM, Krause-Sorio B, et al. Gut microbiome diversity and abundance correlate with gray matter volume (GMV) in older adults with depression. Int J Environ Res Public Health. 2022;19:2405. doi: 10.3390/ijerph19042405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong Z, Shen X, Hao Y, et al. Gut microbiome: a potential indicator for differential diagnosis of major depressive disorder and general anxiety disorder. Front Psych. 2021;12:651536. doi: 10.3389/fpsyt.2021.651536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pu Y, Tan Y, Qu Y, et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun. 2021;94:318–326. doi: 10.1016/j.bbi.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Wang S, Ishima T, Qu Y, et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: a role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. J Affect Disord. 2021;292:565–573. doi: 10.1016/j.jad.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S, Ishima T, Zhang J, et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflammation. 2020;17:241. doi: 10.1186/s12974-020-01916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Eguchi A, Wan X, et al. A role of gut-microbiota-brain axis via subdiaphragmatic vagus nerve in depression-like phenotypes in Chrna7 knock-out mice. Prog Neuropsychopharmacol Biol Psychiatry. 2023;120:110652. doi: 10.1016/j.pnpbp.2022.110652. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020;10:186. doi: 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 66.Wu M, Tian T, Mao Q, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. 2020;10:350. doi: 10.1038/s41398-020-01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian P, Zhu H, Qian X, et al. Consumption of butylated starch alleviates the chronic restraint stress-induced neurobehavioral and gut barrier deficits through reshaping the gut microbiota. Front Immunol. 2021;12:755481. doi: 10.3389/fimmu.2021.755481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z, Li L, Ma S, et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J Agric Food Chem. 2020;68:13697–13710. doi: 10.1021/acs.jafc.0c04290. [DOI] [PubMed] [Google Scholar]
- 69.Tang C-F, Wang C-Y, Wang J-H, et al. Short-chain fatty acids ameliorate depressive-like behaviors of high fructose-fed mice by rescuing hippocampal neurogenesis decline and blood-brain barrier damage. Nutrients. 2022;14:1882. doi: 10.3390/nu14091882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palepu MSK, Dandekar MP. Remodeling of microbiota gut-brain axis using psychobiotics in depression. Eur J Pharmacol. 2022;931:175171. doi: 10.1016/j.ejphar.2022.175171. [DOI] [PubMed] [Google Scholar]
- 71.Chang L, Wei Y, Hashimoto KJBRB. Brain Research Bulletin: Special Issue: Brain–body communication in health and diseases, Brain–gut–microbiota axis in depression: A historical overview and future directions. Brain Res Bull. 2022. [DOI] [PubMed]
- 72.Chang L, Wei Y, Hashimoto K. Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res Bull. 2022;182:44–56. doi: 10.1016/j.brainresbull.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Duan J, Huang Y, Tan X, et al. Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl Psychiatry. 2021;11:303. doi: 10.1038/s41398-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SM, Dong TS, Krause-Sorio B, et al. The intestinal microbiota as a predictor for antidepressant treatment outcome in geriatric depression: a prospective pilot study. Int Psychogeriatr. 2022;34:33–45. doi: 10.1017/S1041610221000120. [DOI] [PubMed] [Google Scholar]
- 75.Dong Z, Shen X, Hao Y, et al. Gut microbiome: a potential indicator for predicting treatment outcomes in major depressive disorder. Front Neurosci. 2022;16:813075. doi: 10.3389/fnins.2022.813075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitamura Y, Araki H, Gomita Y. Influence of ACTH on the effects of imipramine, desipramine and lithium on duration of immobility of rats in the forced swim test. Pharmacol Biochem Behav. 2002;71:63–69. doi: 10.1016/S0091-3057(01)00625-6. [DOI] [PubMed] [Google Scholar]
- 77.Iwai T, Ohnuki T, Sasaki-Hamada S, Saitoh A, Sugiyama A, Oka J. Glucagon-like peptide-2 but not imipramine exhibits antidepressant-like effects in ACTH-treated mice. Behav Brain Res. 2013;243:153–157. doi: 10.1016/j.bbr.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 78.Walker AJ, Burnett SA, Hasebe K, et al. Chronic adrenocorticotrophic hormone treatment alters tricyclic antidepressant efficacy and prefrontal monoamine tissue levels. Behav Brain Res. 2013;242:76–83. doi: 10.1016/j.bbr.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 79.Song J, Ma W, Gu X, et al. Metabolomic signatures and microbial community profiling of depressive rat model induced by adrenocorticotrophic hormone. J Transl Med. 2019;17:1–12. doi: 10.1186/s12967-019-1970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bonder MJ, Tigchelaar EF, Cai X, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8:1–11. doi: 10.1186/s13073-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 83.Lukic I, Getselter D, Ziv O, et al. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry. 2019;9:133. doi: 10.1038/s41398-019-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang W, Qu W, Wang H, Yan H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl Psychiatry. 2021;11:131. doi: 10.1038/s41398-021-01254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L, Kromann S, Olsen JE, Svenningsen SW, Olsen RH. Insight into synergetic mechanisms of tetracycline and the selective serotonin reuptake inhibitor, sertraline, in a tetracycline-resistant strain of Escherichia coli. J Antibiot. 2017;70:944–953. doi: 10.1038/ja.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bohnert JA, Szymaniak-Vits M, Schuster S, Kern WV. Efflux inhibition by selective serotonin reuptake inhibitors in Escherichia coli. J Antimicrob Chemother. 2011;66:2057–2060. doi: 10.1093/jac/dkr258. [DOI] [PubMed] [Google Scholar]
- 87.Molnár J. Antiplasmid activity of tricyclic compounds. Methods Find Exp Clin Pharmacol. 1988;10:467–474. [PubMed] [Google Scholar]
- 88.Macedo D, Filho A, Soares de Sousa CN, et al. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22–32. doi: 10.1016/j.jad.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Huang N, Hua D, Zhan G, et al. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav. 2019;176:93–100. doi: 10.1016/j.pbb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Yang C, Qu Y, Fujita Y, et al. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. 2017;7:1294. doi: 10.1038/s41398-017-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chi L, Khan I, Lin Z, et al. Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine. 2020;67:153157. doi: 10.1016/j.phymed.2019.153157. [DOI] [PubMed] [Google Scholar]
- 92.Getachew B, Tizabi Y. Effects of C-terminal domain of the heavy chain of tetanus toxin on gut microbiota in a rat model of depression. Clin Pharmacol Transl Med. 2019;3:152–159. [PMC free article] [PubMed] [Google Scholar]
- 93.An Q, Li C, Chen Y, et al. Scaffold hopping of agomelatine leads to enhanced antidepressant effects by modulation of gut microbiota and host immune responses. Pharmacol Biochem Behav. 2020;192:172910. doi: 10.1016/j.pbb.2020.172910. [DOI] [PubMed] [Google Scholar]
- 94.Huang YJ, Choong LC, Panyod S, et al. Gastrodia elata Blume water extract modulates neurotransmitters and alters the gut microbiota in a mild social defeat stress-induced depression mouse model. Phytother Res. 2021;35:5133–5142. doi: 10.1002/ptr.7091. [DOI] [PubMed] [Google Scholar]
- 95.Song J, Zhou N, Ma W, et al. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019;10:2947–2957. doi: 10.1039/C8FO02599A. [DOI] [PubMed] [Google Scholar]
- 96.Ji S, Han S, Yu L, et al. Jia Wei Xiao Yao San ameliorates chronic stress-induced depression-like behaviors in mice by regulating the gut microbiome and brain metabolome in relation to purine metabolism. Phytomedicine. 2022;98:153940. doi: 10.1016/j.phymed.2022.153940. [DOI] [PubMed] [Google Scholar]
- 97.Wang L, Sun Y, Zhao T, et al. Antidepressant effects and mechanisms of the total iridoids of Valeriana jatamansi on the brain-gut axis. Planta Med. 2020;86:172–179. doi: 10.1055/a-1068-9686. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y, Xiao N, Chen Y, et al. Semen Sojae Praeparatum alters depression-like behaviors in chronic unpredictable mild stress rats via intestinal microbiota. Food Res Int. 2021;150:110808. doi: 10.1016/j.foodres.2021.110808. [DOI] [PubMed] [Google Scholar]
- 99.Hao W, Wu J, Yuan N, et al. Xiaoyaosan improves antibiotic-induced depressive-like and anxiety-like behavior in mice through modulating the gut microbiota and regulating the NLRP3 inflammasome in the colon. Front Pharmacol. 2021;12:619103. doi: 10.3389/fphar.2021.619103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu JP, Wu HY, Zi Y, Xia XB, Xie MZ, Yuan ZY. Baihe Jizihuang Tang ameliorates chronic unpredictable mild stress-induced depression-like behavior: integrating network pharmacology and brain-gut axis evaluation. Evid Based Complement Alternat Med. 2021. [DOI] [PMC free article] [PubMed]
- 101.Han SK, Kim JK, Park HS, Shin YJ, Kim DH. Chaihu-Shugan-San (Shihosogansan) alleviates restraint stress-generated anxiety and depression in mice by regulating NF-kappaB-mediated BDNF expression through the modulation of gut microbiota. Chin Med. 2021;16:1–13. doi: 10.1186/s13020-021-00492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan J, Li X, Zhu Y, et al. Antidepressant Shugan Jieyu Capsule alters gut microbiota and intestinal microbiome function in rats with chronic unpredictable mild stress -induced depression. Front Pharmacol. 2022;13:828595. doi: 10.3389/fphar.2022.828595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo Y, Xie J, Li X, et al. Antidepressant effects of rosemary extracts associate with anti-inflammatory effect and rebalance of gut microbiota. Front Pharmacol. 2018;9:1126. doi: 10.3389/fphar.2018.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong Z, Xie Q, Xu F, et al. Neferine alleviates chronic stress-induced depression by regulating monoamine neurotransmitter secretion and gut microbiota structure. Front Pharmacol. 2022;13:974949. doi: 10.3389/fphar.2022.974949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiu FM, Zhong XM, Mao QQ, Huang Z. Antidepressant-like effects of paeoniflorin on the behavioural, biochemical, and neurochemical patterns of rats exposed to chronic unpredictable stress. Neurosci Lett. 2013;541:209–213. doi: 10.1016/j.neulet.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 106.Huang X, Su S, Cui W, et al. Simultaneous determination of paeoniflorin, albiflorin, ferulic acid, tetrahydropalmatine, protopine, typhaneoside, senkyunolide I in Beagle dogs plasma by UPLC-MS/MS and its application to a pharmacokinetic study after Oral Administration of Shaofu Zhuyu Decoction. J Chromatogr B. 2014;962:75–81. doi: 10.1016/j.jchromb.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 107.Yu JB, Zhao ZX, Peng R, et al. Gut microbiota-based pharmacokinetics and the antidepressant mechanism of paeoniflorin. Front Pharmacol. 2019;10:268. doi: 10.3389/fphar.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou YX, Gong XH, Zhang H, Peng C. A review on the pharmacokinetics of paeoniflorin and its anti-inflammatory and immunomodulatory effects. Biomed Pharmacother. 2020;130:110505. doi: 10.1016/j.biopha.2020.110505. [DOI] [PubMed] [Google Scholar]
- 109.Hsiu SL, Lin YT, Wen KC, Hou YC, Chao PD. A deglucosylated metabolite of paeoniflorin of the root of Paeonia lactiflora and its pharmacokinetics in rats. Planta Med. 2003;69:1113–1118. doi: 10.1055/s-2003-45192. [DOI] [PubMed] [Google Scholar]
- 110.Zhao ZX, Fu J, Ma SR, et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics. 2018;8:5945–5959. doi: 10.7150/thno.28068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaluzna-Czaplinska J, Gatarek P, Chirumbolo S, Chartrand MS, Bjorklund G. How important is tryptophan in human health? Crit Rev Food Sci Nutr. 2019;59:72–88. doi: 10.1080/10408398.2017.1357534. [DOI] [PubMed] [Google Scholar]
- 112.Wu L, Ran L, Wu Y, et al. Oral administration of 5-hydroxytryptophan restores gut microbiota dysbiosis in a mouse model of depression. Front Microbiol. 2022;13:864571. doi: 10.3389/fmicb.2022.864571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 114.Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maffei ME. 5-Hydroxytryptophan (5-HTP): Natural occurrence, analysis, biosynthesis, biotechnology, physiology and toxicology. Int J Mol Sci. 2020;22:181. doi: 10.3390/ijms22010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Waclawikova B, Bullock A, Schwalbe M, et al. Gut bacteria-derived 5-hydroxyindole is a potent stimulant of intestinal motility via its action on L-type calcium channels. PLoS Biol. 2021;19:e3001070. doi: 10.1371/journal.pbio.3001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bouchaud C. Démonstration par radioautographie de l'existence d'une barrière hématoencéphalique pour la 5-hydroxytryptamine. 1972. [PubMed]
- 118.Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc B. 2013;368:20120536. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Denoyer M, Kitahama K, Sallanon M, Touret M, Jouvet M. 5-Hydroxytryptophan uptake and decarboxylating neurons in the cat hypothalamus. Neuroscience. 1989;31:203–211. doi: 10.1016/0306-4522(89)90042-0. [DOI] [PubMed] [Google Scholar]
- 120.Bogdanski DF, Weissbach H, Udenfriend S. Pharmacological studies with the serotonin precursor, 5-hydroxytryptophan. J Pharmacol Exp Ther. 1958;122:182–194. [PubMed] [Google Scholar]
- 121.Arai R, Karasawa N, Nagatsu T, Nagatsu I. Exogenousl-5-hydroxytryptophan is decarboxylated in neurons of the substantia nigra pars compacta and locus coeruleus of the rat. Brain Res. 1995;669:145–149. doi: 10.1016/0006-8993(94)01259-K. [DOI] [PubMed] [Google Scholar]
- 122.Kitahama K, Jouvet A, Fujimiya M, Nagatsu I, Arai R. 5-Hydroxytryptophan (5-HTP) uptake and decarboxylation in the kitten brain. J Neural Transm. 2002;109:683–689. doi: 10.1007/s007020200057. [DOI] [PubMed] [Google Scholar]
- 123.Jacobsen JPR, Krystal AD, Krishnan KRR, Caron MG. Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: clinical and preclinical rationale. Trends Pharmacol Sci. 2016;37:933–944. doi: 10.1016/j.tips.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang ZW, Gao CS, Zhang H, et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharmaceutica Sinica B. 2022;12:3298–3312. doi: 10.1016/j.apsb.2022.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clark AM, Clinton RT, Baker JK, Hufford CD. Demethylation of imipramine by enteric bacteria. J Pharm Sci. 1983;72:1288–1290. doi: 10.1002/jps.2600721113. [DOI] [PubMed] [Google Scholar]
- 126.Lindenbaum J, Rund DG, Butler VP, Jr, Tse-Eng D, Saha JR. Inactivation of digoxin by the gut flora: reversal by antibiotic therapy. N Engl J Med. 1981;305:789–794. doi: 10.1056/NEJM198110013051403. [DOI] [PubMed] [Google Scholar]
- 127.Li Y, Peng Y, Ma P, et al. In vitro and in vivo metabolism of Cistanche tubulosa extract in normal and chronic unpredictable stress-induced depressive rats. J Chromatogr B. 2019;1125:121728. doi: 10.1016/j.jchromb.2019.121728. [DOI] [PubMed] [Google Scholar]
- 128.Xu J, Chen HB, Li SL. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med Res Rev. 2017;37:1140–1185. doi: 10.1002/med.21431. [DOI] [PubMed] [Google Scholar]
- 129.Liu H, Yang J, Du F, et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 130.Laparra JM, Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res. 2010;61:219–225. doi: 10.1016/j.phrs.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 131.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 132.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee HS, Kim DK, Kim YB, Lee KJ. Effect of acute stress on immune cell counts and the expression of tight junction proteins in the duodenal mucosa of rats. Gut and Liver. 2013;7:190–196. doi: 10.5009/gnl.2013.7.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Trzeciak P, Herbet M. Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients. 2021;13:927. doi: 10.3390/nu13030927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huc T, Konop M, Onyszkiewicz M, et al. Colonic indole, gut bacteria metabolite of tryptophan, increases portal blood pressure in rats. Am J Physiol Reg Integr Comp Physiol. 2018;315:R646–R655. doi: 10.1152/ajpregu.00111.2018. [DOI] [PubMed] [Google Scholar]
- 136.Wu Q, Zhang Y, Zhang Y, et al. Potential effects of antibiotic-induced gut microbiome alteration on blood-brain barrier permeability compromise in rhesus monkeys. Ann N Y Acad Sci. 2020;1470:14–24. doi: 10.1111/nyas.14312. [DOI] [PubMed] [Google Scholar]
- 137.Zhang L, Wang L, Huang L, et al. Antidepressant effects of total iridoids of Valeriana jatamansi via the intestinal flora-blood-brain barrier pathway. Pharm Biol. 2021;59:910–919. doi: 10.1080/13880209.2021.1944222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yi T, Tang D, Wang F, et al. Enhancing both oral bioavailability and brain penetration of puerarin using borneol in combination with preparation technologies. Drug Delivery. 2017;24:422–429. doi: 10.1080/10717544.2016.1259372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meng L, Wang C, Lu Y, et al. Targeted regulation of blood-brain barrier for enhanced therapeutic efficiency of hypoxia-modifier nanoparticles and immune checkpoint blockade antibodies for glioblastoma. ACS Appl Mater Interfaces. 2021;13:11657–11671. doi: 10.1021/acsami.1c00347. [DOI] [PubMed] [Google Scholar]
- 140.Wang X, Chang L, Wan X, et al. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: a role of gut–microbiota–brain axis. Neurobiol Dis. 2022;165:105635. doi: 10.1016/j.nbd.2022.105635. [DOI] [PubMed] [Google Scholar]
- 141.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 143.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dent R, Blackmore A, Peterson J, et al. Changes in body weight and psychotropic drugs: a systematic synthesis of the literature. PLoS ONE. 2012;7:e36889. doi: 10.1371/journal.pone.0036889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Evrensel A, Ceylan ME. The gut-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci. 2015;13:239–244. doi: 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Forslund SK, Chakaroun R, Zimmermann-Kogadeeva M, et al. Combinatorial, additive and dose-dependent drug-microbiome associations. Nature. 2021;600:500–505. doi: 10.1038/s41586-021-04177-9. [DOI] [PubMed] [Google Scholar]
- 148.Diaz-Caneja CM, Espliego A, Parellada M, Arango C, Moreno C. Polypharmacy with antidepressants in children and adolescents. Int J Neuropsychopharmacol. 2014;17:1063–1082. doi: 10.1017/S1461145712001265. [DOI] [PubMed] [Google Scholar]
- 149.Zito JM, Zhu Y, Safer DJ. Psychotropic polypharmacy in the US pediatric population: a methodologic critique and commentary. Front Psych. 2021;12:644741. doi: 10.3389/fpsyt.2021.644741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hitchings R, Kelly L. Predicting and understanding the human microbiome's impact on pharmacology. Trends Pharmacol Sci. 2019;40:495–505. doi: 10.1016/j.tips.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klunemann M, Schmid M, Patil KR. Computational tools for modeling xenometabolism of the human gut microbiota. Trends Biotechnol. 2014;32:157–165. doi: 10.1016/j.tibtech.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 152.Javdan B, Lopez JG, Chankhamjon P, et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020;181:1661–1679.e22. doi: 10.1016/j.cell.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 154.Codella R, Luzi L, Terruzzi I. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis. 2018;50:331–341. doi: 10.1016/j.dld.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 155.Nikolova VL, Hall MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and Meta-analysis. JAMA Psychiat. 2021;78:1343–1354. doi: 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.