Summary
Dimetrodon is among the most recognizable fossil taxa, as well as the earliest terrestrial amniote apex predator. The neuroanatomy and auditory abilities of Dimetrodon has long been the subject of interest, but palaeoneurological analyses have been limited by the lack of three-dimensional endocast data. The first virtual endocasts reveal a strongly flexed brain with enlarged floccular fossae and a surprisingly well-ossified bony labyrinth clearly preserving the semicircular canals, along with an undifferentiated vestibule and putative perilymphatic duct. This first detailed palaeoneurological reconstruction reveals potential adaptations for a predatory lifestyle and suggests Dimetrodon was able to hear a wider range of frequencies than anticipated, potentially being sensitive to frequencies equal to or higher than many extant sauropsids, despite lacking an impedance matching ear. Ancestral state reconstructions support the long-standing view of Dimetrodon as representative of the ancestral state for therapsids, while underscoring the importance of validating reconstructive analyses with fossil data.
Subject areas: Biological sciences, Paleobiology, Palaeohistology
Graphical abstract
Highlights
-
•
The brain of Dimetrodon is strongly flexed with highly enlarged floccular fossae
-
•
The inner ear of Dimetrodon is very well ossified with large ampullae
-
•
ASR results confirm Dimetrodon as representing the ancestral therapsid condition
Biological sciences; Paleobiology; Palaeohistology
Introduction
Crown mammals have highly derived brain and ear morphologies that readily distinguish them from other tetrapods.1,2 The evolution of the mammalian brain and ear have been studied extensively,2,3,4,5 and that interest has extended beyond crown mammals to non-mammalian therapsids, the clade that includes the closest extinct relatives of mammals.6,7,8,9,10 However, the neuroanatomy of the pelycosaur-grade synapsids, the earliest members of the synapsid lineage, has received little attention; to date, the varanopids are the only non-therapsid synapsids whose neuroanatomy has been studied in comparable detail.11,12
The pelycosaur-grade synapsid Dimetrodon is one of the most iconic and best-known of all fossil taxa. Known primarily from the early Permian of North America,13,14 Dimetrodon has an extended fossil record and is one of the earliest terrestrial apex predators.14,15 As a sphenacodontid, Dimetrodon occupies an important position in synapsid phylogeny, being one of the closest relatives to Therapsida, yet still only approximately 20 million years from the beginning of the 316 million years long evolutionary history of the synapsids. Anatomical and paleobiological studies of Dimetrodon have included muscle reconstructions,16 dental anatomy,15 and osteohistology,17 but studies of its palaeoneurology have been limited, despite its importance for understanding its sensory abilities and paleoecology.
The auditory abilities of Dimetrodon, and of pelycosaur-grade synapsids in general, have long been debated,1,3,18,19 with the presence or absence of a postquadrate tympanum, and the impedance matching abilities implied therein, the subject of particular interest. The predominant view is that pelycosaur-grade synapsids lacked any form of distinct tympanic membrane, such that the mammalian tympanum and therefore, the impedance matching middle ear is not homologous to those of crown sauropsids19; however, little is known about the range of sounds that these earliest synapsids were able to hear. It has been suggested that if Dimetrodon could hear any form of airborne sound, it was only at very low frequencies, likely below 1000 Hz.1,19 Researchers are now able test these functional hypotheses,7,11 but this requires a thorough understanding of the palaeoneuroanatomy of the taxon in question, which in turn requires reconstruction of the relevant soft-tissue structures.
Previous neuroanatomical studies of Dimetrodon have been based primarily on the examination of osteological correlates,14,20 focusing chiefly on the overall shape of the brain, and the positioning of cranial nerve foramina. Cursory descriptions of the inner ear have been given1,14,20 but these descriptions have by necessity lacked detail. A physical cranial endocast of Dimetrodon limbatus has been described previously,20,21,22 but this endocast does not preserve the semicircular canals or the paths of any cranial innervations. Further, the vestibular portion of the endocast, which is the only portion of the inner ear preserved in that specimen, includes the canal that connects the fenestra vestibuli to the vestibule, obscuring much of the vestibular morphology.22 A wax endocast model of the inner ear of Dimetrodon that does include the labyrinth has been described previously,23 but this model has never been figured in detail and is a reconstruction from a serially sectioned skull that has been scaled up in size from the original size of the labyrinth,24 rendering it of limited utility for analyses that depend upon accurate measurements.
Here, we use micro-computed tomography to generate the first virtual endocast-based description of the brain and inner ear of any sphenacodontid and reveal more nuanced details of the neuroanatomy of Dimetrodon than were previously known. This new data allow estimation of the potential range of frequencies that Dimetrodon was able to hear, which has implications for the neurosensory function of this important early apex predator. We then conduct an ancestral state reconstruction to evaluate the evolution of four key neuroanatomical traits across synapsids.
Methods
Specimen
FMNH UC 423, isolated braincase referred to Dimetrodon loomisi Romer, 1937 by Romer and Price.14 Specimen preserves supraoccipital-opisthotic complex, both prootics, parasphenoid plate, basioccipital, and left exoccipital.
Occurrence
Craddock Bonebed, Baylor County, Texas, U.S.A. Arroyo Formation, Clear Fork Group, 272–279 Ma (late Cisuralian).
Taxonomic assignment
FMNH UC 423 was assigned to D. loomisi by Romer and Price14 based on the predominance of D. loomisi at the locality in which it was found; however, D. limbatus and Dimetrodon natalis are also known from the same locality.14 The specific diagnosis for D. loomisi does not include braincase characters, so the species assignment of FMNH UC 423 should be considered tentative. However, generic assignment to Dimetrodon is confident based on (i) the predominance of Dimetrodon among Craddock sphenacodontid material, and (ii) the morphology of the paroccipital processes that differ substantially across the major clades of pelycosaur-grade synapsids. The processes in Dimetrodon are long, slim, and posterolaterally directed, in contrast to Edaphosaurus (long, broad, horizontally directed), Ophiacodon (short, horizontally directed), or Casea (short, very broad, horizontally directed).14 Although the distal portions of both processes are missing in FMNH UC 423, the preserved proximal portions clearly indicate slim, posterolaterally directed structures, matching the condition described for Dimetrodon (Figure 1). Although Secodontosaurus is also known from the same locality, substantial differences in proportion easily distinguish between the two genera.25
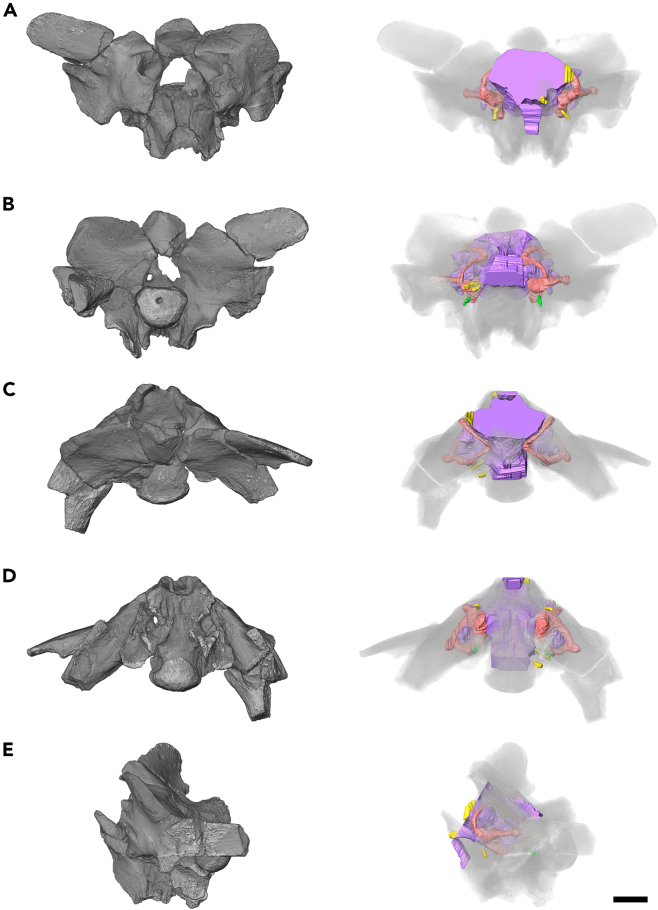
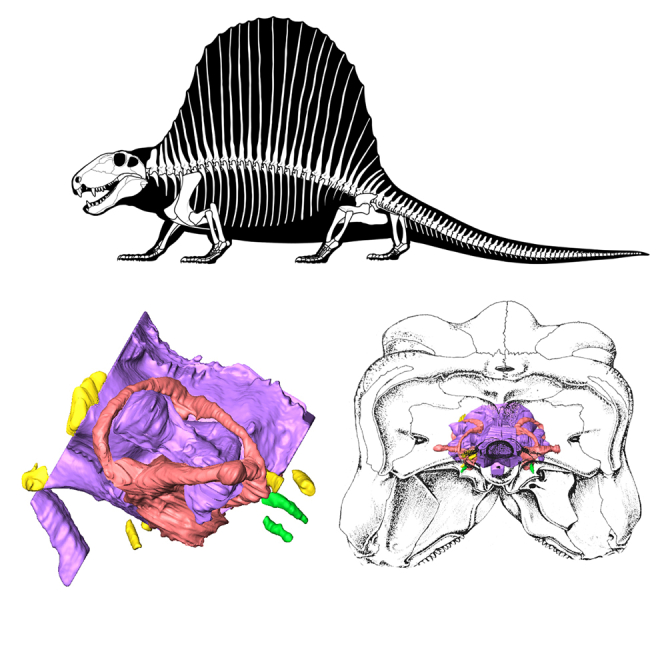
Figure 1.
Braincase of Dimetrodon
Opaque and semi-transparent volume renderings of FMNH UC 423 with endocast in (A) anterior, (B) posterior, (C) dorsal, (D) ventral, and (E) left lateral views. Scale bar equals 1 cm.
Scanning
FMNH UC 423 was micro-CT scanned at the University of Chicago’s PaleoCT facility at 150 kV and 400 μA, using a 1.0 mm Cu filter and a 0.5 mm Sn filter. A total of 1500 projections were taken over a total scan time of 1 h 40 min, producing a final voxel size of 60.07 × 60.07 × 60.07 μm.
Three-dimensional renderings of the cranial and otic endocasts were produced by manual segmentation in AVIZO Lite 9.7.0. Bony labyrinth measurements were taken following the protocol of Benoit et al.7 Ellipticity was calculated using the equation given by Goyens.26 Hearing range estimates were calculated using the equations of Walsh et al.27 Unprocessed 16-bit TIFF slices are available online through MorphoSource (https://www.morphosource.org/concern/media/000441468?locale=en).
Ancestral state reconstruction
Ancestral state reconstruction (ASR) analyses were conducted under both (i) a maximum likelihood (ML) framework in R (v.4.1.3) using the ace function from the package ape,28 which employs an equal rates model to estimate marginal ancestral states, and (ii) a maximum parsimony (MP) framework in Mesquite. Reconstructions were performed on a time-scaled phylogenetic tree of fossil synapsids,29 with a final sample of 32 taxa representing the majority of the major synapsid clades. All taxa were coded for the degree of brain flexure, flocculus size, presence and morphology of the secondary common crus, and the presence of a distinct cochlear recess.
Brain flexure was measured as the angle at the hypophyseal fossa, following the protocol of Macrini et al.30 Flocculus size was coded as (0) small/discrete bump that only just extends into the space defined by the anterior semicircular canal, or (1) large/pronounced protrusion extending past the anterior canal. Secondary common crus morphology was coded as (0) absent, (1) short and broad, or (2) elongate and narrow. The cochlear recess was coded as (0) absent or (1) present. The complete dataset used for the analyses is available in the supplemental information.
Institutional abbreviations
FMNH UC, Field Museum of Natural History, Chicago, U.S.A.
Anatomical abbreviations
aasc, ampulla of the anterior semicircular canal; alsc, ampulla of the lateral semicircular canal; asc, anterior semicircular canal; cc, common crus; CN.V, trigeminal nerve; CN.VI, abducens nerve; CN.VII, facial nerve; CN.IX-XI, metotic foramen; CN.XII, hypoglossal nerve; ff, floccular fossa; lsc, lateral semicircular canal; pd, perilymphatic duct; pf, pituitary fossa; psc, posterior semicircular canal; scc, secondary common crus; v, vestibule.
Results
Description
Brain
As it is only the posterior portion of the braincase that is preserved in FMNH UC 423, our reconstruction of the brain of Dimetrodon only extends as far anteriorly as the pituitary fossa. The contours of the different brain regions are ambiguous, indicating a relatively poor fit between the brain and the surrounding hard tissues, but the general shape is apparent (Figure 2). The medulla oblongata is mediolaterally broad and there is a pronounced pontine flexure of approximately 43°; the region of the cephalic flexure is not preserved.
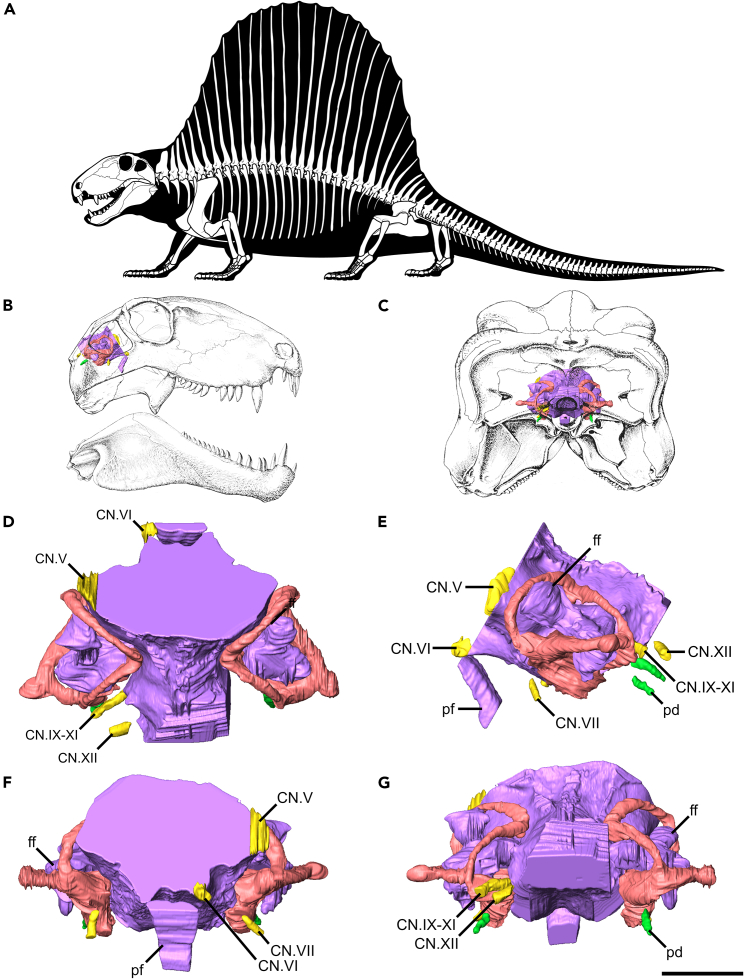
Figure 2.
Dimetrodon endocasts
(A) Skeletal reconstruction of Dimetrodon.
(B and C) Endocast in skull in (B) right, (C) occipital views.
(D–G) Endocast in (D) dorsal, (E) left, (F) anterior, (G) posterior views. Scale bar equals 1 cm. Views A-C not to scale.
A large recess, identified here as a floccular fossa, occupies much of the periotic region (Figure 2). The fossa extends anteroposteriorly to span the space between the anterior and posterior semicircular canals, ventrally to the dorsoventral midpoint of the vestibule, and almost completely fills the space defined by the lateral semicircular canal. The fossa identified here as housing the flocculus was suggested by Romer and Price14 to have been associated with the perilymphatic system, while Hopson22 identified a blunt swelling anterior to the inner ear as a putative flocculus. The identification of the structure described here as a floccular fossa is the most likely, as the fossa that houses the flocculus in modern tetrapods is always located within the space defined by the anterior semicircular canal,9,31,32,33 as is seen in Dimetrodon, and structures of similar shape within the same region have been identified as floccular fossae in other taxa.11,33,34 However, the possibility of a perilymphatic contribution to at least some portion of this fossa cannot be completely excluded; see discussion section for further detail.
The pituitary extends directly ventrally, terminating at the level of the ventral most extent of the vestibule (Figures 2E and 2F). Previous descriptions of the pituitary of Dimetrodon have disagreed on the morphology of the structure. Case20,21 describes the pituitary as extending far posteriorly into the body of the basisphenoid, while Hopson22 describes it as a vertical, narrow, elongated structure with no posterior extension. The large vacuity within the basisphenoid identified by Case20,21 is indeed present, but it is unlikely to represent a posterior expansion of the pituitary, as the space expands posterolaterally through the basisphenoid to seemingly open into the wide canal connecting the vestibule to the fenestra vestibuli.
Innervations
The trigeminal nerve (CN.V) is the largest of the cranial nerves, exiting the brain directly medial to the ampulla of the anterior semicircular canal (Figure 2). The abducens nerve (CN.VI) passes directly anteriorly through the dorsum sellae, just lateral to the pituitary stalk. The facial nerve (CN.VII) originates anterior to the vestibule and passes posterolaterally to exit the braincase anterior to the fenestra vestibuli. An opening for an endolymphatic duct was identified by Romer and Price14 posterodorsal to the facial nerve, but no such path could be identified in FMNH UC 423. The metotic foramen (CN.IX-XI) is only slightly larger than the foramen for the hypoglossal nerve (CN.XII), which is relatively large. An anteroposteriorly directed canal, tentatively identified as the perilymphatic duct, exits the vestibule at the level of the secondary common crus and exits the braincase through the posterior surface of the opisthotic. The paths of the internal carotids could not be discerned in the CT data.
Endosseous labyrinth
The bony labyrinth of Dimetrodon is surprisingly well ossified with the paths of all canals, and the margins of the vestibule clearly traceable (Figures 1 and 2). The internal auditory meatus is widely open, but to a lesser degree than has been described previously,1 such that the medial wall of the vestibule and the ventral portion of the common crus is the only region of the inner ear that is not fully enclosed. The sutures between the supraoccipital, prootic, and opisthotic could not be discerned in the CT data, such that the relative contributions of each element to the housing of the bony labyrinth cannot be commented upon here.
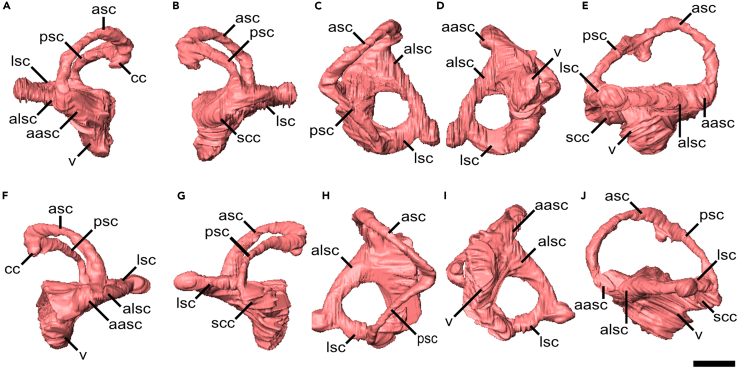
Only the dorsal most portion of the common crus is preserved, but the greater dorsoventral height of the anterior semicircular canal (ASC) relative to the posterior canal indicates that the common crus are slightly caudally tilted (Figure 3). The ASC is the longest and shows the largest radius of curvature. The posterior (PSC) and lateral (LSC) canals are sub equal in length and show roughly similar radii, with the radius of curvature of the PSC being marginally smaller than that of the LSC. The ASC is arcuate along its entire length, while the PSC is nearly straight in its midportion and is slightly anterodorsally bowed. The LSC is the most strongly curved of the canals and is only moderately elliptical, while the vertical canals are strongly elliptical, with the PSC showing slightly more ellipticity than the ASC. The angles between the ASC and PSC and between the ASC and LSC are nearly orthogonal at 85° and 88° respectively, while the angle between the LSC and PSC are more acute, although only by approximately 10°. All three canals are moderately to strongly elliptical, with the LSC being the least (ellipticity of 0.49) and the PSC is the most elliptical (ellipticity of 0.84). All measurements are given in Table 1.
Figure 3.
Virtual endocasts of the inner ear of Dimetrodon
(A–E) right labyrinth and (F–J) left labyrinth in (A and F) anterior, (B and G), posterior, (C and H) dorsal, (D and I) ventral, and (E and J) lateral views. Scale bar equals 5 mm.
Table 1.
Measurements of the bony labyrinth and estimation of the hearing range and mean best hearing frequency of Dimetrodon
| Left | Right | Average | |
|---|---|---|---|
| Basicranial length (mm) | – | – | 29.82 |
| Vestibule length (mm) | 5.48 | 5.44 | 5.46 |
| ASC height (mm) | 6.93 | 7.98 | 7.46 |
| ASC width (mm) | 9.63 | 9.21 | 9.42 |
| ASC length (mm) | 20.36 | 19.64 | 20.00 |
| ASC radius of curvature (mm) | 4.14 | 4.30 | 4.22 |
| ASC eccentricity | 0.80 | 0.79 | 0.79 |
| PSC height (mm) | 5.39 | 5.18 | 5.29 |
| PSC width (mm) | 8.03 | 7.95 | 7.99 |
| PSC length (mm) | 18.02 | 15.68 | 16.85 |
| PSC radius of curvature (mm) | 3.36 | 3.28 | 3.32 |
| PSC eccentricity | 0.84 | 0.84 | 0.84 |
| LSC height (mm) | 7.37 | 6.21 | 6.79 |
| LSC width (mm) | 7.51 | 7.50 | 7.51 |
| LSC length (mm) | 18.62 | 16.75 | 17.69 |
| LSC radius of curvature (mm) | 3.72 | 3.43 | 3.57 |
| LSC eccentricity | 0.47 | 0.52 | 0.49 |
| Hearing range (Hz) | 2484.10 | 2464.68 | 2474.39 |
| Mean best hearing frequency (Hz) | 1564.59 | 1554.05 | 1559.32 |
| ∠ ASC-PSC (o) | 83.2 | 88.6 | 85.90 |
| ∠ ASC-LSC (o) | 88.4 | 88.2 | 88.3 |
| ∠ PSC-LSC (o) | 76.6 | 79.9 | 78.25 |
Eccentricity values closer to 1 indicate greater ellipticity (more oval), while values closer to 0 indicate greater circularity.
All the ampullae are prominent (Figure 3). The PSC and LSC join in the secondary common crus; within this structure, the PSC passes posteroventrally to the LSC to enter the vestibule directly ventral to the entrance of the LSC. A relatively large, nubbin-like protrusion can be seen on the lateral surface of the LSC, just before it curves medially to meet with the PSC in the secondary common crus. It is unlikely that this structure has a functional role, as a genuine protrusion off or diversion of the path of any semicircular canal would impact the flow of endolymph and thus impede the sensitivity of that canal. This protrusion is most likely an artifact related to the ossification patterns of the endocranium.
The vestibule is short and rounded, forming an anteroposterior oval in ventral view (Figures 3D and 3I). No distinct cochlear recess could be identified. A relatively wide canal connects the vestibule to the fenestra vestibuli. The hearing frequency range and mean best hearing frequency were estimated from the length of the vestibule with estimated values of approximately 2500 Hz and 1500 Hz, respectively (Table 1).
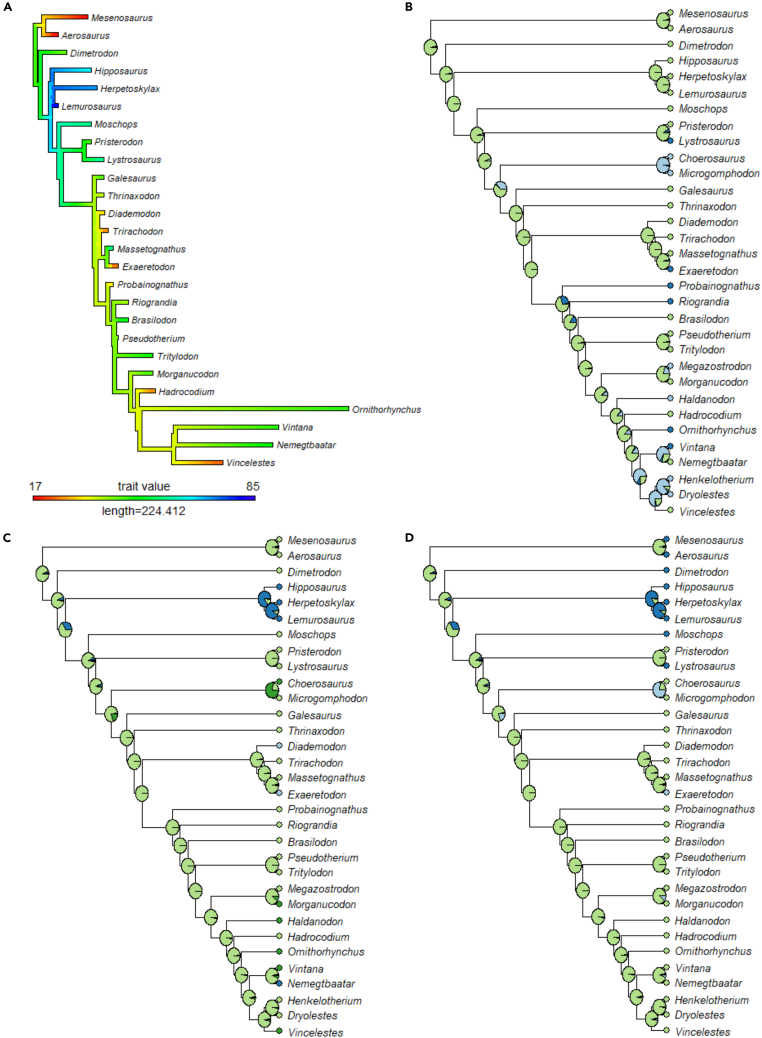
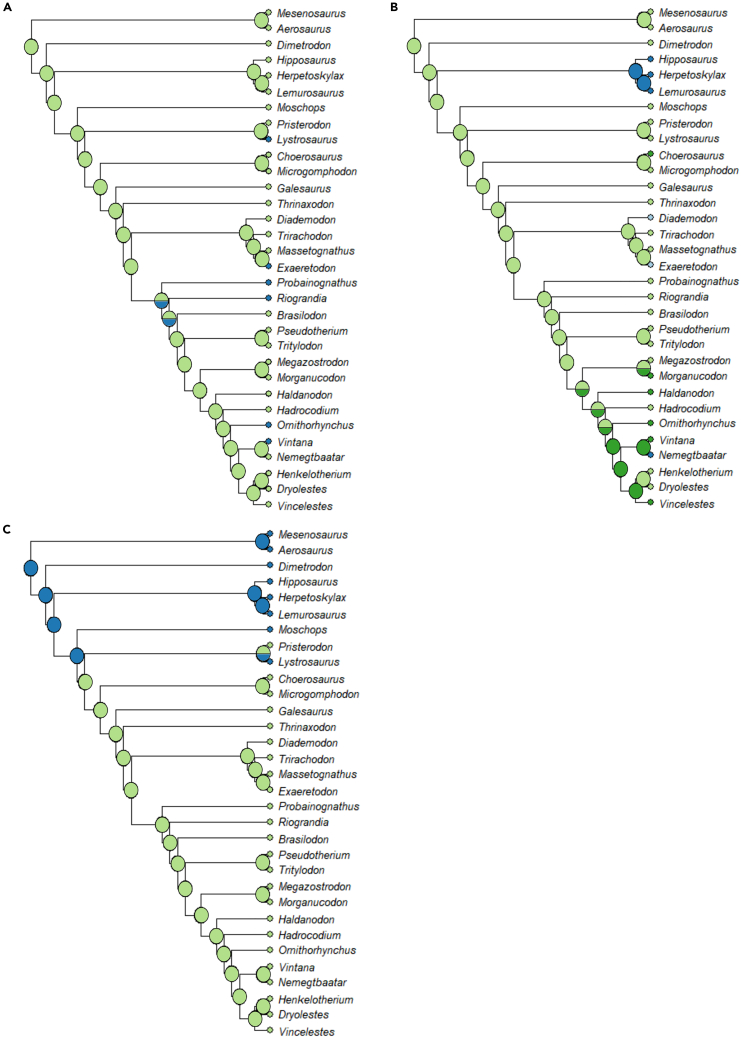
Ancestral state reconstructions
A moderate brain flexure of approximately 50° is reconstructed as ancestral both for Therapsida, aligning with the condition seen in Dimetrodon, and for Synapsida as a whole (Figure 4A). Both the ML and MP analyses overwhelmingly support the presence of large floccular fossae (Figures 4B and 5B) and short, broad secondary common crus (Figures 4C and 5C) at the root of the synapsid clade. A distinct cochlear recess is reconstructed by the ML analysis at the ancestral condition throughout synapsids (Figure 4D). In contrast, the MP analysis reconstructs the absence of a cochlear recess as ancestral below dicynodonts (Figure 5D).
Figure 4.
Ancestral state reconstructions for four key neuroanatomical traits conducted via maximum likelihood
(A) Brain flexure, with character states ranging from 17° to 85°.
(B) Flocculus size, with character states of either a small bump extending just into the area bounded by the ASC (state 0; dark blue) or a large protrusion extending far past the ASC (state 1; light green).
(C) Secondary common crus, with character states of absent (state 0; dark blue), short and broad (state 1; light green), or elongate and narrow (state 2; dark green).
(D) Cochlea, with character states of absent (state 0; dark blue) or present (state 1; light green). In all reconstructions, light blue represents taxa for which a given trait could not be coded.
Figure 5.
Ancestral state reconstructions for flocculus size, secondary common crus morphology, and cochlear recess under a maximum parsimony framework
(A) Flocculus size, with character states of either a small bump extending just into the area bounded by the ASC (state 0; light green) or a large protrusion extending far past the ASC (state 1; dark blue).
(B) Secondary common crus, with character states of absent (state 0; dark blue), short and broad (state 1; light green), or elongate and narrow (state 2; dark green).
(C) Cochlea, with character states of absent (state 0; dark blue) or present (state 1; light green).
Discussion
Comparisons with other Paleozoic amniotes
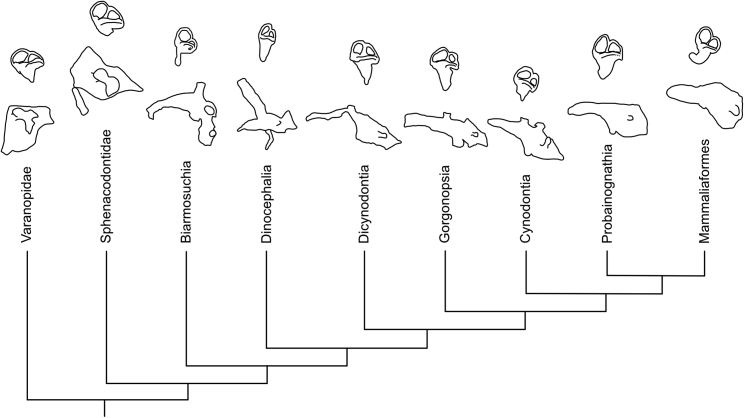
Comparative interpretations of our findings with other pelycosaur-grade synapsids are restricted by a general lack of endocast data and are by necessity limited to varanopids and therapsids, but nonetheless provide considerable new insights into the paleobiology of this early apex predator. A simplified phylogeny showing the brain and inner ear endocasts of the major clades of synapsids is given in Figure 6.
Figure 6.
Simplified phylogeny of some of the major clades of synapsids, with representative endocasts for each clade
Clades are represented as follows: Varanopidae by an indeterminate mycterosaurine11; Sphenacodontidae by Dimetrodon; Biarmosuchia by Lemurosaurus6; Dinocephalia by Moschops6; Dicynodontia by Niassodon28; Gorgonopsia by an indeterminate gorgonopsian27; Cynodontia by Galesaurus35; Probainognathia by Riograndia36,37 (cranial endocast) and Pseudotherium38 (inner ear endocast); Mammaliaformes by Morganucodon.2,39 Endocasts are not to scale.
Endocast
Overall, the endocast of Dimetrodon is largely plesiomorphic than that of more derived synapsids, with no expansion of the cerebellum and no direct imprint of the brain or surrounding organs on the endocast, suggesting a relatively poor fit between the brain and the braincase. The strong pontine flexure is similar to that described in the biarmosuchians and dicynodonts,6,32 differing from the more linear morphology seen in the varanopids and most other non-mammalian therapsids.8,9,11,31,40 Brain flexures are known to vary substantially across taxa with an apparent trend toward stronger flexures in smaller taxa.41,42 The relationship between overall body size and degree of brain flexure results from the negative allometric relationship between brain and body size, wherein smaller taxa tend to possess relatively larger brains that are thus more constrained by the small size of the surrounding skull and the additional spatial constraints imposed by the other structures that must be contained within a skull, including the eyes.42 This trend is not universal; however, as differences in overall skull shape can override the influence of size.42 As FMNH UC 423 likely represents a relatively small taxon of Dimetrodon, it is possible that the flexure seen here relates to the small size of the animal; however, due to the absence of other associated materials from the specimen, and the lack of endocast data for other species of Dimetrodon, the presence or nature of any relationship between body size and cranial flexure in this genus is uncertain.
The floccular fossa in Dimetrodon is similar in shape and relative size to that of the varanopids;11 in more derived synapsids the structure is typically more rounded and lacks the posterior and ventral extensions seen in the varanopids and Dimetrodon.9,31,32 The Early Triassic cynodont Galesaurus also displays a strongly protruding floccular fossa more similar to that in Dimetrodon but, like other therapsids, lacks the aforementioned extensions.35 Romer and Price14 noted the recess identified here as a floccular fossa and suggested that its unusual morphology indicates a perilymphatic function. The structure described here is most likely at least partly floccular, as only the flocculus is known to extend into the space defined by the anterior semicircular canal,9,31,32,33 and structures within the same region are almost universally identified as floccular fossae in other taxa.11,33,34 However, given the dramatic difference in morphology shown here compared to that of therapsids, a perilymphatic function for some portion of the large fossa described in both Dimetrodon and the varanopids cannot be excluded. If part of this region is perilymphatic, the significance of its expansion in the region of the floccular fossa would have no equivalent in modern tetrapods.
Even if the posterior and ventral extensions of the fossa are in fact perilymphatic, the definitively floccular portion of the fossa remains quite large. The large size of the floccular fossa in Dimetrodon is potentially indicative of adaptations for rapid head movements;34 as the flocculus in extant taxa is heavily involved in gaze stabilization and reflex control of the neck, enlarged floccular fossae have often been interpreted as indicative of a high degree of head-eye coordination.33,43,44,45 However, the relationship of floccular fossa size with ecological or behavioral factors is not universally agreed upon. Work by Ferreira-Cardoso et al.46 found no significant relationships between floccular fossa size and agility, locomotor style, feeding strategy, or activity patterns in either extant mammals or birds. Earlier work by Walsh et al.44 similarly found no relationship between the size of the floccular fossa and flying ability in birds. Further, identification of fossae such as these in fossil taxa as floccular fossae is problematic, as this fossa is known to house different neural tissues in various extant clades and the size relationship between the flocculus itself and the fossa in which it resides is not precise, thus hindering analyses of floccular size in extinct vertebrates.34,46 Conclusions regarding the function of the flocculus in Dimetrodon would be aided by comparison with closely related herbivorous taxa such as the edaphosaurs or caseasaurs, but such comparisons are precluded by a lack of published data; Case47 provided a description of the inner ear of Edaphosaurus but neither described nor figured the floccular recess, and no description of the recess could be found for any caseasaur.
Canals and vestibule
The bony labyrinth of Dimetrodon is very similar in overall shape to that of the varanopids.11 While the common crus could not be extracted in its entirety in Dimetrodon, the paths of all three canals are nearly identical to the varanopids in all views.11 The dorsal expansion of the anterior canal relative to the posterior canal is much less pronounced in Dimetrodon than in the gorgonopsians, dinocephalians, or the dicynodont Pristerodon,8,9,31 being more similar to the condition in the varanopids and the dicynodont Niassodon.11,32 The strong ellipticity is also most similar to that of the varanopids and dicynodonts, contrasting strongly with the circular canals of mammals.11,26,48
Along with the near-orthogonality of the canal system in Dimetrodon, the high ellipticity may indicate specialization for rapid head movements, aligning with the hypothesized active predatory lifestyle of Dimetrodon; however, the degree of relationship between ellipticity, orthogonality, and canal sensitivity is debated.48,49 If the ellipticity seen in Dimetrodon is indeed related to the rapid head movements involved in predation, herbivorous taxa such as Edaphosaurus would be expected to display less ellipticity. Case47 provides a description of the inner ear of Edaphosaurus; in contrast to the expected low degree of ellipticity, the semicircular canals figured by Case appear to be at least moderately elliptical, but the lack of any measurements of the canal system in Edaphosaurus preclude a more precise determination of ellipticity.
The short, rounded vestibule seen in Dimetrodon is similar to that of the varanopids,11 contrasting with the more conical condition seen in most of the more derived synapsids.7,8,9,31,32,40 Unlike the varanopids, in which the fenestra vestibuli directly contacts the posteroventral most portion of the vestibule,11 the fenestra vestibuli in Dimetrodon is more similar to some biarmosuchians in being situated at the end of a long canal.7 However, while this canal is thin and extends through the basicranium in the biarmosuchians,7 in Dimetrodon it is widely open.
Functional implications
Vestibule length has been shown to correlate with hearing sensitivity in a wide array of tetrapods, with relatively longer vestibules (scaled to basicranial axis length) being associated with sensitivity to higher frequencies.27 Sensitivity estimates for a range of non-mammalian therapsids were provided by Benoit et al.7; however, these initial estimates were calculated using data that were not log-transformed, resulting in estimated sensitivities in the range of extant mammals and birds. Re-calculated estimates for selected taxa using log-transformed data, as per the method of Walsh et al.,27 are provided in Table 2. The estimated hearing range and mean best hearing frequency (2500 Hz and 1500 Hz, respectively) in Dimetrodon are similar to those for varanopids and most other non-mammalian synapsids.7,11
Table 2.
Estimates of hearing range and mean best hearing frequency of selected synapsid taxa
| Taxon | Clade | Hearing range (Hz) | Best hearing frequency (Hz) |
|---|---|---|---|
| Lemurosaurus | Biarmosuchia | 2580.68 | 1616.98 |
| Hipposaurus | Biarmosuchia | 1745.68 | 1164.03 |
| Moschops | Dinocephalia | 2776.94 | 1723.44 |
| Patronomodon | Anomodontia | 3786.09 | 2270.86 |
| Eodicynodon | Dicynodontia | 2868.19 | 1772.94 |
| Pristerodon | Dicynodontia | 3606.42 | 2173.39 |
| Scylacocephalus | Gorgonopsia | 3003.67 | 1846.43 |
| Choerosaurus | Therocephalia | 1441.31 | 998.92 |
| Euchambersia | Therocephalia | 2354.92 | 1494.51 |
| Cynosaurus | Cynodontia | 1550.49 | 1058.15 |
| Galesaurus | Cynodontia | 2041.34 | 1324.41 |
| Trirachodon | Cynognathia | 2483.85 | 1564.45 |
| Lumkuia | Probainognathia | 1556.92 | 1061.63 |
| Megazostrodon | Mammaliaformes | 4879.48 | 2863.97 |
Estimates re-calculated using the vestibular length and basicranial axis length measurements provided by Benoit et al. (2017b).7
Previous estimates of the auditory abilities of Dimetrodon, based solely on the massive size of the stapes and the lack of a tympanic membrane, suggested sensitivity to only very low frequencies, below 1000 Hz.1 While the estimates given here for Dimetrodon are indeed very low relative to extant synapsids, they are slightly higher than the original estimate by Allin and Hopson1 and are similar to or higher than those of many extant sauropsids27,50,51,52,53 excluding birds.54,55 Interestingly, the estimates for Dimetrodon are higher than some taxa for whom some degree of air-borne hearing is known, including some geckos and skinks,50 suggesting strongly that Dimetrodon was sensitive to a greater range of frequencies beyond the ultra-low-frequency ground-borne sounds previously anticipated, and may have been important in prey acquisition. The sphenacodontids are the first synapsids to possess a reflected lamina of the angular, which would come to play a critical role in the evolution of the mammalian ear3; thus, clarifying the auditory capabilities of Dimetrodon is essential for accurately contextualizing the evolutionary changes leading to hearing in crown mammals.
The presence of a probable perilymphatic duct further complicates the question of sphenacodontid audition; perilymphatic ducts plesiomorphically serve to drain the perilymph from the inner ear in amniotes but are often co-opted to function within impedance matching hearing systems in reptiles.56 Such systems are typically associated with gracile moving stapes that are actuated by a relatively larger tympanic membrane, and with stapedial footplates57 that are smaller than the fenestra vestibuli where they are attached. Due to the forces created within the inner ear by the actuation of the fenestra vestibuli by movement of the stapes, impedance matching hearing systems require a pressure-release mechanism.58 This pressure release can be achieved by a fenestra rotunda as in crown mammals or a perilymphatic sac or duct as in many diapsids.39,56,59 Ducts similar or identical to that found in Dimetrodon have been identified as perilymphatic ducts across amniotes, including archosaurs and archosauromorphs,42,60 squamates,61 and mammaliaforms.39 In Dimetrodon; however, the stapes is massive, contacts the rim of the fenestra vestibuli, and attaches to the paroccipital process of the opisthotic via a large dorsal process, and thus likely served a bracing function to support the braincase within the skull.14 Given the clearly non-tympanic role of the stapes in Dimetrodon and the probable absence of an air-filled middle ear, it is currently unclear what functions a pressure release mechanism such as a perilymphatic duct would serve.
One possibility deserving of further exploration is the potential for the auditory system of Dimetrodon to represent a very early stage in the evolution of the mammalian impedance-matching ear, with the reflected lamina of the angular acting as an incipient tympanum in coordination with the overlying musculature. This possible function was initially proposed in therapsids by Allin3 and, should the lamina have served a sound-receiving function in these more derived synapsids, it is not unreasonable to expect a similar function in Dimetrodon. The mechanisms by which the lamina could have performed such a function in therapsids have been discussed by Olroyd and Sidor,62 with the deepening of the angular cleft allowing the lamina to vibrate more freely and the topography of the lamina serving to modify its vibrational qualities. Alternatively, the reflected lamina may have served to support a true tympanic membrane, if one was present (see Kemp19 for a review of the arguments for and against the presence of a postquadrate tympanum in sphenacodontids). However, under either hypothesized function for the reflected lamina, we would expect to see an increase, however slight, in sensitivity to higher frequency sounds relative to taxa lacking this structure. Comparison of the estimated hearing sensitivity values for Dimetrodon and several therapsid taxa (Tables 1 and 2) with those of varanopids, the only synapsids without a reflected lamina for which hearing sensitivity estimates are currently available, shows no apparent difference in hearing range or mean best hearing frequency.11 The lack of sensitivity estimates for other pelycosaur-grade synapsids that do not possess reflected laminae precludes any definitive determination of the impact of the reflected lamina on hearing sensitivity, but the data currently available do not support the hypothesis of a tympanic function for the reflected lamina of the angular.
A perilymphatic duct exiting the otic capsule via a distinct foramen and invading the metotic foramen has been suggested to be the plesiomorphic condition for amniotes.63 This is supported by the presence of perilymphatic ducts in an array of extinct and extant members of both the sauropsid and synapsid lineages.39,56,59,64 However, there are a number of taxa for which there are no indications of a distinct perilymphatic duct, including captorhinids65 and the basal diapsid Youngina.56 If the presence of a perilymphatic duct is in fact plesiomorphic for amniotes, two issues are raised. First, perilymphatic ducts must have initially served other functions unrelated to hearing and were only later co-opted for pressure release as impedance matching hearing systems evolved in amniotes. Second, the criteria for identifying perilymphatic ducts may be inadequate, as current definitions must be failing to capture the ducts present in amniotes such as the captorhinids and Youngina.
Ancestral state reconstruction
In six of the seven reconstructions, the reconstructed ancestral state for therapsids aligns with the condition seen here in Dimetrodon, thereby supporting the long-standing practice of viewing Dimetrodon as broadly representative of the ancestral therapsid condition. A moderate brain flexure, similar to that seen in the crown mammals Vintana, Nemegtbaatar, and Ornithorhynchus is reconstructed as ancestral for both Synapsida and Therapsida and appears throughout the phylogeny. This suggests that, contrary to previous assumptions that a tubular, unflexed brain represented the plesiomorphic state for synapsids,11,31,40 both the strongly flexed brains seen in biarmosuchians and the more tubular brains of varanopids and Vincelestes may instead represent derived conditions relative to an ancestrally moderately flexed state. The analyses of floccular fossa size and secondary common crus morphology overwhelmingly support a large flocculus and short, broad secondary common crus as ancestral for synapsids under both the ML and MP frameworks, aligning with the morphologies described here in Dimetrodon and by Bazzana et al.11 in the varanopids.
The only significant disagreement between the results of the ML and MP analyses are in the cochlear recess reconstructions, with the MP analysis supporting the absence of a recess stemward of dicynodonts while the ML strongly supports a distinct recess as ancestrally present even for those taxa for which no cochlear recess exists. This result underscores the importance of using fossil data to validate the results of backward-projecting analyses like ASR. Given the presence of distinct cochlear recesses in the vast majority of fossil synapsids, if we did not have endocast data for cochlea-lacking taxa such as the pelycosaurs or biarmosuchians, we would erroneously assume that cochleae were present in the ancestor of all synapsids. While incorrectly attributing a character state to a taxon may seem superficially to be a minor issue, an error such as this could have significant ramifications for our understanding of inner ear evolution in amniotes as a whole. Taken in isolation, the reconstruction provided by the ML analysis would suggest that distinct cochlear recesses could have been present in the very earliest amniotes, or even in the ancestor of amniotes, which would in turn imply a homology between the expanded cochleae of synapsids and sauropsids, something which is known to be incorrect from examination of early members of both lineages.11,66,67 It is only with the examination of the fossils themselves that the results of any reconstruction can be validated or accurately contextualized.
This first computed tomographic study of the brain and bony labyrinth of the iconic synapsid Dimetrodon reveals previously unrecognized aspects of sphenacodontid neuroanatomy that shed new light on its palaeobiology and the evolution of the mammalian inner ear. Our findings extend our understanding of synapsid palaeoneuroanatomy to one of the most widely recognizable early synapsids and early terrestrial apex predators, and indicate a much greater diversity in the sensory function of pelycosaur-grade synapsids than previously anticipated. As the sister group to Therapsida, the sphenacodontid neuroanatomy and sensory function revealed here help to define the plesiomorphic condition for therapsids as a whole and clarify the foundation upon which the defining features of mammals eventually arose. ASR indicate that the neuroanatomy of Dimetrodon is generally representative of the ancestral condition for therapsids, and likely for the broader synapsid condition, while also underscoring the importance of validating any reconstructive analysis with empirical fossil data.
Limitations of the study
Our results are based on a single endocast virtually extracted from an isolated braincase; as a result, our interpretations lack the broader context provided by larger sample sizes, and it is unclear whether the features identified in Dimetrodon are shared by other sphenacodontids. In addition, our ancestral state reconstruction is limited by the relative paucity of endocast data on fossil synapsids. While the number of non-mammalian synapsids to receive neuroanatomical descriptions has greatly increased in recent years, the endocasts of the vast majority of fossil synapsids remain to be studied, and the information gained from such taxa could change the reconstructions and the resultant interpretations of synapsid neuroevolution.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Raw CT data | This paper | https://www.morphosource.org/concern/media/000441468?locale=en |
| Software and algorithms | ||
| phytools v.1.0-3 | R v.4.1.3 | |
| ape v.5.6-2 | R v.4.1.3 | |
| castor v.1.7.6 | R v.4.1.3 | |
Resource availability
Lead contact
Further information and requests should be directed to the lead contact, Kayla Bazzana-Adams (kayla.bazzana@mail.utoronto.ca).
Materials availability
Unprocessed 16-bit TIFF slices are available online through MorphoSource (https://www.morphosource.org/concern/media/000441468?locale=en).
Experimental model and subject details
CT scanning
We scanned an isolated braincase of Dimetrodon cf. loomisi (FMNH UC 423) from the Craddock Bonebeds of Texas. Details regarding the scanning procedure used in this study are provided in the “scanning” sub-section of the methods section.
Method details
Details regarding the segmentation method used in this study are detailed in the “scanning” sub-section of the methods section. The height and width of each canal were measured with the inner ear oriented such that the plane of the canal being measured was flat relative to the viewer, with height measured as the distance from the distalmost point of the canal arc from the vestibule, to the vestibule wall directly below that point (see Figure 2 in Benoit et al.7 for illustration of these measurements). The radius of curvature for each canal was then calculated as r = 0.5∗((H+W)/2). Eccentricity was calculated as e = (1-(minor axis/major axis)2) (see Goyens26 for visual illustration of the major vs minor axes). Canal length was calculated by summing the distances of a spline taken along the centre of each canal; detailed protocol for length calculations, along with visual aids, are available from the lead contact. Brain flexure was measured with endocasts in lateral view.
Quantification and statistical analysis
Ancestral state reconstructions were performed in R Studio using the function ace for the maximum likelihood analyses, and the function hsp_parsimony for the parsimony analyses.
Acknowledgments
Thanks to Ken Angielczyk for data access, Danielle Dufault for the skeletal reconstruction in Figure 2, and Mary Silcox for discussions. Thanks to the comments of the anonymous reviewers of an earlier version of this manuscript, whose feedback greatly improved the overall work. This research was supported by NSERC grants to D.C.E.(RGPIN-2018-06788) and R.R.R.(RGPIN-2020-04959) and an NSERC scholarship to K.B.A.
Author contributions
K.B.A., D.C.E., and R.R.R. conceived the study; K.B.A. collected and analyzed the data; K.B.A. wrote the paper with the input of D.C.E. and R.R.R. All authors approved the submitted version.
Declaration of interests
The authors declare no competing interests.
Published: March 21, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106473.
Supplemental information
Data and code availability
-
•
Data used in the ancestral state reconstruction analyses are available in Data S1 the supplemental information for this paper, or from the lead contact.
-
•
R code used to perform the ancestral state reconstruction analyses is available on Zenodo: https://doi.org/10.5281/zenodo.7745782, or from the lead contact.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Allin E.F., Hopson J.A. In: The Evolutionary Biology of Hearing. Webster D.B., Fay R.R., AN P., editors. Springer; New York: 1992. Evolution of the auditory system in Synapsida (“mammal-like reptiles” and primitive mammals) as seen in the fossil record; pp. 587–614. [DOI] [Google Scholar]
- 2.Rowe T.B., Macrini T.E., Luo Z.-X. Fossil evidence on origin of the mammalian brain. Science. 2011;332:955–957. doi: 10.1126/science.1203117. [DOI] [PubMed] [Google Scholar]
- 3.Allin E.F. Evolution of the mammalian middle ear. J. Morphol. 1975;147:403–437. doi: 10.1002/jmor.1051470404. [DOI] [PubMed] [Google Scholar]
- 4.Barton R.A., Harvey P.H. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- 5.Luo Z.-X. The inner ear and its bony housing in tritylodontids and implications for evolution of the mammalian ear. Bull. Mus. Comp. Zool. 2001;156:81–97. [Google Scholar]
- 6.Benoit J., Fernandez V., Manger P.R., Rubidge B.S. Endocranial casts of pre-mammalian therapsids reveal an unexpected neurological diversity at the deep evolutionary root of mammals. Brain Behav. Evol. 2017;90:311–333. doi: 10.1159/000481525. [DOI] [PubMed] [Google Scholar]
- 7.Benoit J., Manger P.R., Fernandez V., Rubidge B.S. The bony labyrinth of late Permian Biarmosuchia: palaeobiology and diversity in non-mammalian Therapsida. Palaeontol. Afr. 2017;52:58–77. [Google Scholar]
- 8.Benoit J., Manger P.R., Norton L., Fernandez V., Rubidge B.S. Synchrotron scanning reveals the palaeoneurology of the head-butting Moschops capensis (Therapsida, Dinocephalia) PeerJ. 2017;5:e3496. doi: 10.7717/peerj.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laaß M. Virtual reconstruction and description of the cranial endocast of Pristerodon mackayi (Therapsida, Anomodontia) J. Morphol. 2015;276:1089–1099. doi: 10.1002/jmor.20397. [DOI] [PubMed] [Google Scholar]
- 10.Laaß M. Bone-conduction hearing and seismic sensitivity of the late Permian anomodont Kawingasaurus fossilis. J. Morphol. 2015;276:121–143. doi: 10.1002/jmor.20325. [DOI] [PubMed] [Google Scholar]
- 11.Bazzana K.D., Evans D.C., Bevitt J.J., Reisz R.R. Neurosensory anatomy of Varanopidae and its implications for early synapsid evolution. J. Anat. 2022;240:833–849. doi: 10.1111/joa.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benoit J., Ford D., Miyamae J., Ruf I. Can maxillary canal morphology inform varanopid phylogenetic affinities? Acta Palaeontol. Pol. 2021;66:389–393. doi: 10.4202/app.00816.202. [DOI] [Google Scholar]
- 13.Reisz R.R. 1986. Pelycosauria. Handbuch der Paläoherpetologie, Teil 17A. [Google Scholar]
- 14.Romer A.S., Price L.I. Geological Society of America; 1940. Review of the Pelycosauria. [Google Scholar]
- 15.Brink K.S., Reisz R.R. Hidden dental diversity in the oldest terrestrial apex predator Dimetrodon. Nat. Commun. 2014;5:3269. doi: 10.1038/ncomms4269. [DOI] [PubMed] [Google Scholar]
- 16.Barghusen H.R. The adductor jaw musculature of Dimetrodon (Reptilia, Pelycosauria) J. Paleontol. 1973;47:823–834. [Google Scholar]
- 17.Huttenlocker A.K., Rega E., Sumida S.S. Comparative anatomy and osteohistology of hyperelongate neural spines in the sphenacodontids Sphenacodon and Dimetrodon (Amniota: Synapsida) J. Morphol. 2010;271:1407–1421. doi: 10.1002/jmor.10876. [DOI] [PubMed] [Google Scholar]
- 18.Hopson J.A. The origin of the mammalian middle ear. Am. Zool. 1966;6:437–450. doi: 10.1093/icb/6.3.437. [DOI] [PubMed] [Google Scholar]
- 19.Kemp T.S. In: Evolution of the Vertebrate Ear – Evidence from the Fossil Record. Clack J.A., Fay R.R., Popper A.N., editors. Springer; New York: 2016. Non-mammalian synapsids: the beginning of the mammal line; pp. 107–137. [Google Scholar]
- 20.Case E.C. Vol. 55. Carnegie Institution of Washington publication; 1907. pp. 1–176. (Revision of the pelycosauria of North America). [DOI] [Google Scholar]
- 21.Case E.C. On the foramina perforating the cranial region of a Permian reptile and on a cast of its brain cavity. Am. J. Sci. 1897;s4–3:321–326. doi: 10.2475/ajs.s4-3.16.321. [DOI] [Google Scholar]
- 22.Hopson J.A. In: Biology of the Reptilia 9-A. Gans C., Northcutt R.G., Ulinsky P., editors. Academic Press; London: 1979. Paleoneurology; pp. 39–146. [Google Scholar]
- 23.Olson E.C. The occipital, otic, basicranial and pterygoid regions of the Gorgonopsia. J. Morphol. 1938;62:141–175. doi: 10.1002/jmor.1050620202. [DOI] [Google Scholar]
- 24.Araújo R., David R., Benoit J., Lungmus J., Spoor F., Stoessel A., Barrett P., Maisano J., Ekdale E., Orliac M., et al. Inner ear biomechanics reveals Late Triassic origin of mammalian endothermy. Nature. 2022;607:726–731. doi: 10.1038/s41586-022-04963-z. [DOI] [PubMed] [Google Scholar]
- 25.Reisz R.R., Berman D.S., Scott D. The cranial anatomy and relationships of Secodontosaurus, an unusual mammal-like reptile (Synapsida: Sphenacodontidae) from the early Permian of Texas. Zool. J. Linn. Soc. 1992;104:127–184. doi: 10.1111/j.1096-3642.1992.tb00920.x. [DOI] [Google Scholar]
- 26.Goyens J. High ellipticity reduces semi-circular canal sensitivity in squamates compared to mammals. Sci. Rep. 2019;9:16428. doi: 10.1038/s41598-019-52828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh S.A., Barrett P.M., Milner A.C., Manley G., Witmer L.M. Inner ear anatomy is a proxy for deducing auditory capability and behaviour in reptiles and birds. Proc. Biol. Sci. 2009;276:1355–1360. doi: 10.1098/rspb.2008.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paradis E., Claude J., Strimmer K. Ape : analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 29.Hellert, S., Grossnickle, D., Lloyd, G., Kammerer, C., and Angielczyk, K. Derived faunivores are the forerunners of major synapsid radiations. Preprint at Res. Sq., 10.21203/rs.3.rs-1582761/v1 [DOI] [PubMed]
- 30.Macrini T.E., De Muizon C., Cifelli R.L., Rowe T. Digital cranial endocast of Pucadelphys andinus, a Paleocene metatherian. J. Vertebr. Paleontol. 2007;27:99–107. doi: 10.1671/0272-4634(2007)27. [DOI] [Google Scholar]
- 31.Araújo R., Fernandez V., Polcyn M.J., Fröbisch J., Martins R.M.S. Aspects of gorgonopsian paleobiology and evolution: insights from the basicranium, occiput, osseous labyrinth, vasculature, and neuroanatomy. PeerJ. 2017;5:e3119. doi: 10.7717/peerj.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castanhinha R., Araújo R., Júnior L.C., Angielczyk K.D., Martins G.G., Martins R.M.S., Chaouiya C., Beckmann F., Wilde F. Bringing dicynodonts back to life: paleobiology and anatomy of a new emydopoid genus from the Upper Permian of Mozambique. PLoS One. 2013;8:e80974. doi: 10.1371/journal.pone.0080974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witmer L.M., Chatterjee S., Franzosa J., Rowe T. Neuroanatomy of flying reptiles and implications for flight, posture and behavior. Nature. 2003;425:950–953. doi: 10.1038/nature02048. [DOI] [PubMed] [Google Scholar]
- 34.Schade M., Rauhut O.W.M., Evers S.W. Neuroanatomy of the spinosaurid Irritator hallenger (Dinosauria: Theropoda) indicates potential adaptations for piscivory. Sci. Rep. 2020;10:9259. doi: 10.1038/s41598-020-66261-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pusch L.C., Kammerer C.F., Fröbisch J. Cranial anatomy of the early cynodont Galesaurus planiceps and the origin of mammalian endocranial characters. J. Anat. 2019;234:592–621. doi: 10.1111/joa.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues P.G., Martinelli A.G., Schultz C.L., Corfe I.J., Gill P.G., Soares M.B., Rayfield E.J. Digital cranial endocast of Riograndia guaibensis (Late Triassic, Brazil) sheds light on the evolution of the brain in non-mammalian cynodonts. Hist. Biol. 2018;31:1195–1212. doi: 10.1080/08912963.2018.1427742. [DOI] [Google Scholar]
- 37.Kerber L., Ferreira J.D., Fonseca P.H.M., Franco A., Martinelli A.G., Soares M.B., Ribeiro A.M. An additional brain endocast of the ictidosaur Riograndia guaibensis (Eucynodontia: Probainognathia): intraspecific variation of endocranial traits. An. Acad. Bras. Cienc. 2021;93:e20200084. doi: 10.1590/0001-3765202120200084. [DOI] [PubMed] [Google Scholar]
- 38.Wallace R.V.S., Martínez R., Rowe T. First record of a basal mammaliamorph from the early late Triassic ischigualasto formation of Argentina. PLoS One. 2019;14:e0218791. doi: 10.1371/journal.pone.0218791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Z.-X., Schultz J.A., Ekdale E. In: Evolution of the Vertebrate Ear – Evidence from the Fossil Record. Clack J.A., Fay R.R., Popper A.N., editors. Springer New York; 2016. Evolution of the middle and inner ears of mammaliaforms: the approach to mammals; pp. 139–174. [Google Scholar]
- 40.Bendel E.-M., Kammerer C.F., Kardjilov N., Fernandez V., Fröbisch J. Cranial anatomy of the gorgonopsian Cynariops robustus based on CT-reconstruction. PLoS One. 2018;13:e0207367. doi: 10.1371/journal.pone.0207367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giffin E.B. Pachycephalosaur paleoneurology (Archosauria: Ornithischia) J. Vertebr. Paleontol. 1989;9:67–77. doi: 10.1080/02724634.1989.10011739. [DOI] [Google Scholar]
- 42.Dudgeon T.W., Maddin H.C., Evans D.C., Mallon J.C. The internal cranial anatomy of Champsosaurus (Choristodera: Champsosauridae): implications for neurosensory function. Sci. Rep. 2020;10:7122. doi: 10.1038/s41598-020-63956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spoor F., Garland T., Krovitz G., Ryan T.M., Silcox M.T., Walker A. The primate semicircular canal system and locomotion. Proc. Natl. Acad. Sci. USA. 2007;104:10808–10812. doi: 10.1073/pnas.0704250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh S.A., Iwaniuk A.N., Knoll M.A., Bourdon E., Barrett P.M., Milner A.C., Nudds R.L., Abel R.L., Sterpaio P.D. Avian cerebellar floccular fossa size is not a proxy for flying ability in birds. PLoS One. 2013;8:e67176. doi: 10.1371/journal.pone.0067176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Zeeuw C.I., Koekkoek S.K. Signal processing in the C2 module of the flocculus and its role in head movement control. Prog. Brain Res. 1997;114:299–320. doi: 10.1016/S0079-6123(08)63371-3. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira-Cardoso S., Araújo R., Martins N.E., Martins G.G., Walsh S., Martins R.M.S., Kardjilov N., Manke I., Hilger A., Castanhinha R. Floccular fossa size is not a reliable proxy of ecology and behaviour in vertebrates. Sci. Rep. 2017;7:2005. doi: 10.1038/s41598-017-01981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Case E.C. On the structure of the inner ear in two primitive reptiles. Biol. Bull. 1914;27:213–216. doi: 10.2307/1535976. [DOI] [Google Scholar]
- 48.Araújo R., Fernandez V., Rabbitt R.D., Ekdale E.G., Antunes M.T., Castanhinha R., Fröbisch J., Martins R.M.S. Endothiodon cf. bathystoma (Synapsida: Dicynodontia) bony labyrinth anatomy, variation and body mass estimates. PLoS One. 2018;13:e0189883. doi: 10.1371/journal.pone.0189883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekdale E.G. Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS One. 2013;8:e66624. doi: 10.1371/journal.pone.0066624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wever E.G. Princeton University Press; 1978. The Reptile Ear: Its Structure and Function. [Google Scholar]
- 51.Manley G.A. Springer Berlin; 1990. Peripheral Hearing Mechanisms in Reptiles and Birds. [Google Scholar]
- 52.Manley G.A. In: Comparative Hearing: Birds and Reptiles. Dooling R.J., Fay R.R., Popper A.N., editors. Springer New York; 2000. The hearing organs of lizards; pp. 139–196. [Google Scholar]
- 53.Dooling R.J., Lohr B., Dent M.L. In: Comparative Hearing: Birds and Reptiles. Dooling R.J., Fay R.R., Popper A.N., editors. Springer New York; 2000. Hearing in birds and reptiles; pp. 308–359. [Google Scholar]
- 54.Wever E.G., Herman P.N., Simmons J.A., Hertzler D.R. Hearing in the blackfooted penguin, Spheniscus demersus, as represented by the cochlear potentials. Proc. Natl. Acad. Sci. USA. 1969;63:676–680. doi: 10.1073/pnas.63.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manley G.A., Köppl C., Yates G.K. Activity of primary auditory neurons in the cochlear ganglion of the emu Dromaius novaehollandiae: spontaneous discharge, frequency tuning, and phase locking. J. Acoust. Soc. Am. 1997;101:1560–1573. doi: 10.1121/1.418273. [DOI] [PubMed] [Google Scholar]
- 56.Sobral G., Reisz R.R., Neenan J.M., Müller J., Scheyer T.M. In: Evolution of the Vertebrate Ear – Evidence from the Fossil Record. Clack J.A., Fay R.R., Popper A.N., editors. Springer New York; 2016. Basal reptilians, marine diapsids, and turtles: the flowering of reptile diversity; pp. 207–243. [Google Scholar]
- 57.Kemp T.S. Acoustic transformer function of the postdentary bones and quadrate of a nonmammalian cynodont. J. Vertebr. Paleontol. 2007;27:431–441. doi: 10.1671/0272-4634(2007)27. [DOI] [Google Scholar]
- 58.Ekdale E.G. In: Evolution of the Vertebrate Ear – Evidence from the Fossil Record. Clack J.A., Fay R.R., Popper A.N., editors. Springer New York; 2016. The ear of mammals: from monotremes to humans; pp. 175–206. [Google Scholar]
- 59.Sobral G., Müller J. In: Evolution of the Vertebrate Ear – Evidence from the Fossil Record. Clack J.A., Fay R.R., Popper A.N., editors. Springer New York; 2016. Archosaurs and their kin: the ruling reptiles; pp. 285–326. [Google Scholar]
- 60.Gower D.J., Walker A.D. New data on the braincase of the aetosaurian archosaur (Reptilia: Diapsida) Stagonolepis robertsoni Agassiz. Zool. J. Linn. Soc. 2002;136:7–23. doi: 10.1046/j.1096-3642.2002.00023.x. [DOI] [Google Scholar]
- 61.Bever G.S., Bell C.J., Maisano J.A. The ossified braincase and cephalic osteoderms of Shinisaurus crocodilurus (Squamata, Shinisauridae) Palaeontol. Electron. 2005;8:4A. [Google Scholar]
- 62.Olroyd S.L., Sidor C.A. Nomenclature, comparative anatomy, and evolution of the reflected lamina of the angular in non-mammalian synapsids. J. Vertebr. Paleontol. 2022;42:e2101923. doi: 10.1080/02724634.2022.2101923. [DOI] [Google Scholar]
- 63.Gower D.J., Weber E. The braincase of Euparkeria, and the evolutionary relationships of birds and crocodiles. Biol. Rev. Camb. Philos. Soc. 1998;73:367–411. doi: 10.1017/S0006323198005222. [DOI] [Google Scholar]
- 64.Evans S.E. In: Evolution of the Vertebrate Ear: Evidence from the Fossil Record. Clack J.A., Fay R.R., Popper A.N., editors. Springer New York; 2016. The lepidosaurian ear: variations on a theme; pp. 245–284. [Google Scholar]
- 65.Heaton M.J. Cranial anatomy of primitive reptiles from the late pennsylvanian and early permian Oklahoma and Texas. OGS (Obstet. Gynecol. Surv.) 1979;127:1–84. [Google Scholar]
- 66.Gardner N.M., Holliday C.M., O’Keefe F.R. The braincase of Youngina capensis (Reptilia, Diapsida): new insights from high-resolution CT scanning of the holotype. Palaeontol. Electron. 2010;13:19A. [Google Scholar]
- 67.Bazzana K.D., Evans D.C., Bevitt J.J., Reisz R.R. Endocasts of the basal sauropsid Captorhinus reveal unexpected neurological diversity in early reptiles. Anat. Rec. 2023;306:552–563. doi: 10.1002/ar.25100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data used in the ancestral state reconstruction analyses are available in Data S1 the supplemental information for this paper, or from the lead contact.
-
•
R code used to perform the ancestral state reconstruction analyses is available on Zenodo: https://doi.org/10.5281/zenodo.7745782, or from the lead contact.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.