Abstract
Cholangiocarcinoma (CCA) is an aggressive malignancy arising from the biliary epithelium. It may occur at any location along the biliary tree with the perihilar area being the most common. Prognosis is poor with 5-year overall survival at less than 10%, typically due to unresectable disease at presentation. Radical surgical resection with clear margins offers a chance of cure in patients with resectable tumours, but is frequently not possible due to locally advanced disease. On the other hand, orthotopic liver transplantation (LT) allows for a radical and potentially curative resection for these patients, but has been historically controversial due to the limited supply of donor grafts and previously poor outcomes. In patients with perihilar CCA, within specific criteria and following the implementation of a protocol combining neoadjuvant chemoradiation and LT, excellent results have been achieved in the last decades, resulting in its increasing acceptance as an indication for LT and the standard of care in several centres with significant experience. However, in intrahepatic CCA, the role of LT remains controversial and owing to dismal previous results it is not an accepted indication. Nevertheless, more recent studies have demonstrated favourable results with LT in early intrahepatic CCA, indicating that, under defined criteria, its role may increase in the future. This review highlights the history and contemporary advances of LT in CCA, with particular focus on the improving outcomes of LT in intrahepatic and perihilar CCA and future perspectives.
Keywords: Cholangiocarcinoma, Klatskin tumor, Liver transplantation, Liver cancer, Liver resection, Neoadjuvant therapy
Core Tip: Cholangiocarcinoma (CCA) is an aggressive malignancy with poor prognosis. Radical surgical resection with clear margins may offer a chance of cure but is frequently not possible due to locally advanced disease. In the last two decades, within specific criteria, a protocolised combination of neoadjuvant chemoradiation and orthotopic liver transplantation (LT) has produced excellent results in patients with perihilar CCA, while favourable results have been shown with LT in early intrahepatic CCA in recent years. We review the history and contemporary advances of LT in CCA and discuss future perspectives.
INTRODUCTION
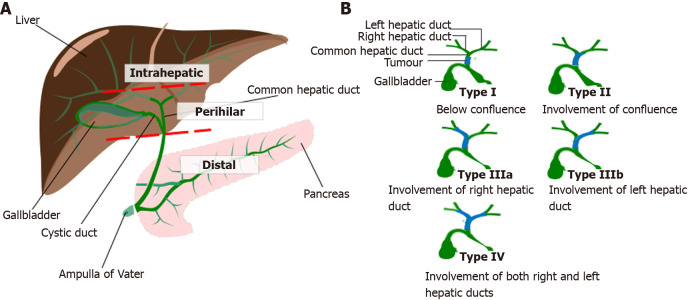
Cholangiocarcinoma (CCA) is a malignancy of the biliary epithelium. It is rare, affecting less than 6 persons per 100000 population[1,2]. CCA carries a poor prognosis with 5-year overall survival (OS) rates for all stages and subtypes at less than 10%[3-5]. CCA is further subdivided depending on the site of origin of the tumour in the biliary tract, with intrahepatic, perihilar (further subdivided by the Bismuth-Corlette classification) and distal variants recognised (Figure 1). These subtypes vary not only in their anatomical site, but also their biology with distinct phenotypes[6].
Figure 1.
Classification of cholangiocarcinoma[62,71]. A: Anatomical classification of cholangiocarcinoma: Intrahepatic cholangiocarcinoma-proximal to second order bile ducts; Perihilar cholangiocarcinomas-between second order branches of right and/or left hepatic ducts and cystic duct confluence; Distal cholangiocarcinoma-between cystic duct confluence and Ampulla of Vater; B: Bismuth Corlette Classification of perihilar cholangiocarcinoma, https://creativecommons.org/licenses/by-sa/4.0/deed.en. Citation: Borakati A, Froghi F, Bhogal RH, Mavroeidis VK. Stereotactic radiotherapy for intrahepatic cholangiocarcinoma. World J Gastrointest Oncol 2022; 14: 1478-1489 [PMID: 36160742 DOI: 10.4251/wjgo.v14.i8.1478] and Wikimedia Commons. File: Bismuth corlette classification for perihilar cholangiocarcinomas.svg. 2020 Oct 9 [visited 3 February 2023]. Available from: https://commons.wikimedia.org/wiki/File:Bismuth_corlette_classification_for_perihilar_cholangiocarcinomas.svg.
Complete surgical resection is the only curative therapy in CCA but is precluded in most individuals due to advanced disease at presentation. Unresectable disease has a median survival of between 6 mo to 1 year[7]. The type of surgical resection offered varies depending on the anatomic subtype of the CCA. Intrahepatic and perihilar CCAs mandate liver resections with or without excision of the extrahepatic bile ducts depending on the location and radiological[8] extent of invasion of the tumour. The risk of postoperative liver failure is largely dictated by the quality and volume of the future liver remnant which may prohibit resection in many patients. Techniques such as portal vein embolization and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) have been developed to extend the limits of resectability in liver surgery by inducing hypertrophy in the contralateral side of the liver (if/when disease-free), hence increasing the future liver remnant[9]. However, ALPPS is largely considered contraindicated in perihilar CCA (phCCA) owing to high perioperative mortality (48% at 90 d)[10]. For distal bile duct tumours, excision of the extrahepatic bile duct is combined with a pancreatoduodenectomy to achieve an adequate oncological margin.
With all of the above procedures, arteriovenous invasion of the tumour was historically seen as an absolute contraindication to resection with poor outcomes. More recently, both portovenous, caval and arterial reconstruction of hepatic, superior mesenteric and even the coeliac arteries have been described, with some series showing favourable long-term outcomes. Venous reconstruction can be performed with similar perioperative morbidity to conventional resection, whilst long-term survival is equivalent in some series[11]. Arterial reconstruction is associated with higher perioperative morbidity and mortality and significantly lower long-term disease-free and OS compared to standard resection, however, outcomes remain significantly superior to palliative care[12,13].
CCA may also involve both liver lobes either as a single mass at the hilum (Bismuth-Corlette type IV phCCA), as multicentric disease or by metastasis making disease unresectable without complete hepatectomy.
Despite this, most patients remain unresectable by virtue of locally advanced or distant metastatic disease, with a dismal median survival of 11.7 mo with palliative chemotherapy[7].
Liver transplantation (LT) has emerged as a potential solution to expand resectability of locally advanced CCA, with promising early patient outcomes. There is also new evidence that LT may confer superior patient outcomes even in technically resectable tumours[14]. This review aims to summarise the literature on the evolving role of LT in CCA.
HISTORY OF LIVER TRANSPLANTATION FOR MALIGNANCY
Initial indications for orthotopic liver transplants were focused on patients with malignancy[15]. Indeed, the second successful LT was performed for intrahepatic CCA (iCCA), by transplantation pioneer Thomas Starzl at the University of Colorado in 1963[15,16]. The recipient patient died on postoperative day 7 from respiratory failure and gastrointestinal bleeding. The early experience with LT unfortunately demonstrated prohibitively high perioperative mortality due to pulmonary emboli and haemorrhage, because of the veno-venous bypass and excessive anticoagulation used at the time. Coagulopathic complications were overcome with the routine use of thromboelastography to guide correction of clotting derangements in real-time. Veno-venous bypass was later used without anticoagulation and obviated by newer surgical techniques such as the ‘piggy-back’ method which retains the recipient inferior vena cava and does not necessitate complete clamping of this vessel and the resultant haemodynamic instabilities[17]. The other main driver of early postoperative mortality was biliary sepsis and obstruction. This was largely overcome by routinely performing biliary anastomoses over T-tubes or by using Roux-en-Y reconstructions.
The development of improved organ preservation solutions, most notably the University of Wisconsin solution in 1988[18], allowed greater cold ischaemic times with consequent reductions in vascular and biliary complications[19]. At present, liver malignancies indicated for LT in well selected patients include hepatocellular carcinoma (HCC), hepatic epitheloid hemangioendothelioma, hepatoblastoma and metastatic neuroendocrine tumours, while an increasing interest is developing about its role in CCA and metastatic colorectal cancer[20].
LIVER TRANSPLANTATION FOR CHOLANGIOCARCINOMA
These advances combined with use of newer cyclosporine and steroid immunosuppression allowed post-operative survival to increase significantly by the early 1980s and the Colorado group reported 1-year survival in excess of 70% during this time. This allowed meaningful evaluation of the oncological benefits of LT for the first time with postoperative recurrence rates and OS with cancer specific mortality. Of the 6 patients transplanted for unresectable CCA who survived more than 1 mo postoperatively, only 3 (50%) had recurrence free survival at 1 year. Those who did have recurrence all succumbed to disease at 1 year. The survival for those without recurrence at 1 year, ranged from 20 to 54 mo[21]. Poor survival for LT in CCA continued until the early 1990s, with 5-year overall, at less than 17% and recurrence greater than 50% in published series at the time[22-24].
The reasons for poor oncological outcomes despite optimisation of the LT procedure and immunosuppression protocols are in the main, twofold: Poor patient selection in early series, with presence of extrahepatic disease and utilisation for all anatomical subtypes of CCA, and absence of any neoadjuvant or adjuvant therapies to prevent recurrence.
This led to the development of neoadjuvant chemoradiotherapy and pre-transplant exploratory laparotomy protocols by the University of Nebraska[25] and Mayo Clinic in 1988 and 1993 respectively for phCCA only. The latter’s results, initially published in 2000, showed of the 8 patients transplanted with follow-up > 12 mo, all were alive at their last follow-up (median 44 mo, range 17 to 83 mo) and only one patient developed a recurrence[26]. A subsequent study with 28 patients showed a 5-year actuarial OS rate of 82% with only 4 (14.2%) developing recurrence at 23 to 63 mo post-LT[27].
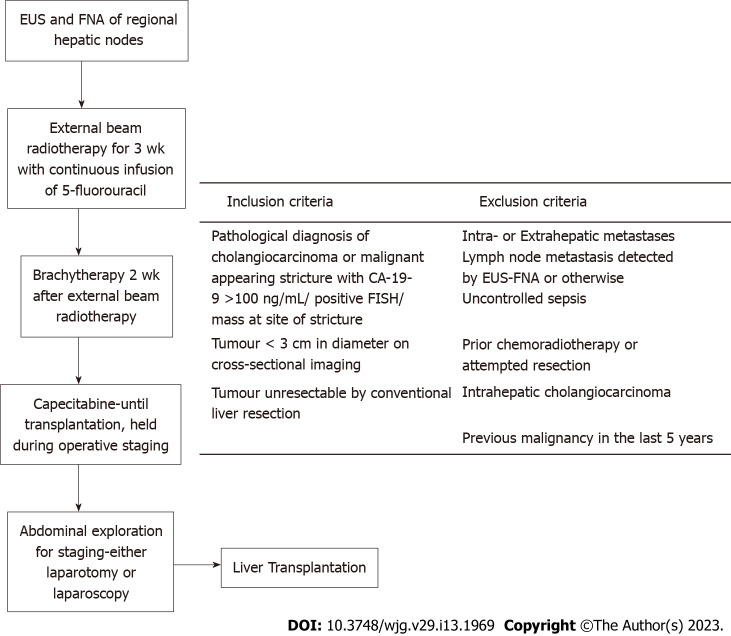
These impressive results have led to the adoption of the Mayo Clinic protocol more widely (Figure 2). In brief, patients with early stage hilar CCA on imaging (< 3 cm lesions, with no metastasis or lymph node involvement) who are technically unresectable undergo endoscopic ultrasound and fine needle aspiration of regional lymph nodes. Those with negative lymph nodes are given chemoradiotherapy for 5 wk and undergo laparoscopic or open abdominal exploration to exclude metastasis and examine regional lymph nodes prior to LT[27,28].
Figure 2.
Mayo clinic protocol for liver transplantation in hilar cholangiocarcinoma. EUS: Endoscopic ultrasound; FNA: Fine needle aspiration; CA-19-9: Carbohydrate antigen 19-9; FISH: Fluorescence in situ hybridization.
Current status of liver transplantation
There is a global discrepancy between donor livers availability and the number of patients on the liver transplant waiting list. Hence, there is understandably a clinical reticence in terms of utility to adopt LT for CCA given the historical poor prognosis when established benign and malignant indications for LT have proven significant benefit for this limited resource[29]. Established indications for LT include predominantly benign, chronic liver disease such as alcoholic and non-alcoholic fatty liver disease; autoimmune and metabolic pathologies such as primary sclerosing cholangitis (PSC) and Wilson’s disease respectively and viral hepatitis. Paracetamol and other overdoses remain important acute causes of liver failure in Western populations which are also indications for LT. Among liver malignancies, the most widely established indication for LT is HCC, in which LT is only performed for early stage disease as outlined in the Milan Criteria[29]. Contemporarily 1- and 5-year OS for these indications following LT approach 95% and 85%, respectively[30]. New indications for LT will have to show comparable outcomes to the established indications to justify investment and allocation of donor livers for these purposes.
Following the publication of favourable results by the Mayo Clinic, with results similar to benign indications for LT, the United Network for Organ Sharing in United States, granted an exception to the Model for End Stage Liver Disease (MELD) used to prioritise those on the waiting list for LT. This exception allowed patients who had completed neoadjuvant therapy and had favourable operative staging to be added on the waiting list for LT with a MELD score equivalent to that of a patient with stage 1 HCC (as an established indication for LT)[31]. This improved access to LT for patients with phCCA and influenced the adoption of LT more widely with use of the MELD score and derivative scores internationally for LT allocations[29]. Further considerations of transplant allocation include the presence of underlying cirrhosis and greater degree of decompensation which increase the MELD score and may influence waiting time for transplantation but may also influence OS and recurrence rates when transplant is done for CCA.
The quality of the donor liver and whether it was from a live or deceased donor also has an impact on postoperative outcomes. Living donor LT may offer shorter waiting times, but is associated with higher rates of biliary complications (34% vs 17%, P < 0.001)[32]. Extended criteria donors with advanced age, steatosis or donors after circulatory death (DCD)[33] may increase the pool of organs but may be associated with higher graft failure rates of 27.3% at 3-year with DCD livers vs 18.2% with donation after brainstem death[34]. Normothermic machine perfusion is another new technique which allows evaluation of graft function preoperatively and may further expand the donor pool and improve outcomes by selecting grafts with more objectively proven function[35].
PERIHILAR CHOLANGIOCARCINOMA
The Mayo Clinic group published their series of LT for phCCA from the inception of their protocol in 1993 to 2019. Of 376 patients included into the protocol, 234 (62%) ultimately proceeded to LT. The reasons for not proceeding to LT were primarily due to progression of disease prior to LT or metastasis found on staging surgery. OS at 5 years [Kaplan-Meier (KM)] was 68% +/- 3% and at 10 years was 60% +/- 4%[36]. Other long-term follow-up and larger series have validated the Mayo Clinic protocol for LT in phCCA. A multi-centre retrospective study from the United States of patients undergoing LT for phCCA, with any neoadjuvant chemo- or radiotherapy reported on 287 patients. Two hundred and fourteen (74%) proceeded to LT. Actuarial OS and disease-free survival (DFS) were 78% and 80% respectively at a median follow-up of 2.5 years. KM 5-year DFS was 65% [95% confidence interval (CI) 57%-73%] with 5-year OS at 53% (95%CI: 46%-60%)[37]. Multicentre series in the United Kingdom[37], Spain[38] and Germany[39] showed poorer results, likely as neoadjuvant protocols were not adopted at the time. Hidalgo et al[37] in the United Kingdom reported 5-year actuarial OS of 20%, Robles and colleagues 5-year OS of 30% in Spain and 31% in Germany pre-1998. Post-1998, when neoadjuvant chemotherapy was adopted in German protocols, 5-year OS after LT increased to 48%. A recent meta-analysis of retrospective series published before 2019 reported on 428 patients who underwent LT for phCCA and demonstrated pooled 5-year OS without neoadjuvant therapy of 31.6% (95%CI: 23.1%-41.7%) whilst with neoadjuvant therapy OS was 51.7% (95%CI: 33.8%-69.4%). Three years recurrence rates were 51.7% (95%CI: 33.8%-69.4%) without neoadjuvant therapy vs 24.1% (95%CI: 17.9%-30.9%), highlighting the importance of neoadjuvant regimes[40].
Comparison of LT to resection with curative intent for phCCA has shown a trend in favour of LT, albeit with high heterogeneity between studies. Ethun et al[14] performed a registry-based study of patients with phCCA at 3 United States centres, who underwent either curative intent liver resection or LT. Patients undergoing LT had neoadjuvant regimens similar to the Mayo protocol. One hundred ninety-one patients underwent curative intent resection (without neoadjuvant therapies), whilst 46 underwent LT. Five-year KM OS was 64% in the LT vs 18% for the resection group (P < 0.001). There was no significant difference in actuarial recurrence at a median follow-up of 23 mo, with 24% vs 37% (P = 0.19) for LT and resection groups respectively. Twenty nine percent of the patients undergoing LT had no residual disease on explant, suggesting complete response to neoadjuvant therapy. The authors also conducted an intention-to-treat analysis of patients who were enrolled in the transplant protocol and patients who were planned for curative intent resection or who had attempted curative intent resection. Five-year OS was still higher in the LT group at 53% vs 17% (P < 0.001) for the resection group[14].
Moris and colleagues performed a meta-analysis of studies with patients with locally advanced phCCA comparing LT and resection with curative intent. Local staging and nodal involvement were similar in both groups. There was a higher rate of R0 resection in patients with LT, 92.2% vs 73.3% [Risk Ratio (RR) 1.17, 95%CI: 1.03-1.33, I2 = 46%]. They also reported a higher 3-year OS with a lower hazard ratio (HR) of death (HR 0.61, 95%CI: 0.4-0.93, I2 = 39%). At 5 years, there was a trend towards increased OS for LT, but this was not significant (HR 0.67, 95%CI: 0.44-1.02, I2 = 54%)[41].
PSC
PSC is an important subgroup of patients undergoing LT in CCA. PSC is a significant risk factor for development of CCA and is in itself an indication for LT due to progressive liver dysfunction. PSC is associated with a higher rate of multifocal CCA precluding oncological resection or necessitating more extensive resection. Further, the poor quality of the liver parenchyma due to the disease may mean that the regenerative capacity of the liver is reduced which in turn limits the extent of resection possible. These factors typically preclude curative liver resection, even for small, localised lesions[43]. This prompted consideration for the alternative approach of LT for CCA, in particular phCCA, to mitigate the limitations of standard liver resection, and is one of the accepted inclusion criteria for the Mayo Clinic protocol[27]. Current data are clearly in favour of a protocolised approach with neoadjuvant chemoradiation followed by LT in PSC-associated phCCA, as the optimal treatment strategy, with superior outcomes when compared with de novo phCCA[42-44]. The Mayo Clinic series found a significantly higher 5- and 10-year survival in patients with PSC after LT, 74% and 67% vs 58% and 47% respectively in patients with de novo CCA[35]. The meta-analysis by Cambridge et al[40] also supports that in unresectable phCCA, combined neoadjuvant chemoradiation followed by LT confers long-term survival in highly selected patients able to complete the protocol with the most favourable outcomes observed in PSC patients[43]. Despite the lack of direct evidence, it has been hypothesized that the significantly better outcomes in PSC patients could be at least in part explained by a possible higher responsiveness to radiation therapy for phCCA arising on a background of PSC[35,42,45].
INTRAHEPATIC CHOLANGIOCARCINOMA
While phCCA is now increasingly accepted as an indication for LT using the Mayo Protocol, iCCA is still largely considered a contraindication[30]. Although early attempts at LT for CCA did not distinguish between subtypes in many cases and consequently iCCAs were transplanted[21] the outcomes remained poor until the advent of neoadjuvant protocols, such as that of the Mayo Clinic which are only applied to phCCA. Most literature on LT, therefore, focuses on incidentally found iCCA on explant, in patients who were transplanted for presumed HCC, which does not typically undergo neoadjuvant systemic therapy prior to transplant but rather only locoregional therapy such as radiofrequency ablation. There remains a lack of evidence to support such protocols for iCCA, partly because iCCA is the least common subtype of CCA (10% vs phCCA which represents 50%[46]) but also because of its distinct biological characteristics compared to other forms of CCA, with some being defined as mixed hepatocellular and intrahepatic cholangiocarcinoma[2].
Krasnodębski et al[47] provided one of the oldest series of 8 patients found on explant to have iCCA, having undergone LT without neoadjuvant therapies, from 1994-2019. Five-year OS and DFS were 25% and 28.6% respectively[47]. Lee et al[48] reported on their experience of LTs with pre-transplant diagnoses of HCC who were found to have iCCA on explant pathology. The majority of these patients also underwent neoadjuvant locoregional therapies such as radiofrequency ablation or transarterial chemoembolization. Seventeen cases of iCCA were identified from 1998-2018 and had 5-year actuarial OS of 51.9% and recurrence rate of 29.4%. This compared to the patients with HCC transplanted contemporaneously with OS of 71.5% and recurrence rate of 10.8%. Mixed hepatocellular and CCA LTs had a similar OS to iCCA at 55.0% but a significantly higher recurrence rate of 40.7%[48]. It was thought that perhaps the poor results for iCCA were due to poor patient selection as with phCCA and HCC. The latter is established as an indication for LT, but widely governed by the Milan criteria which restrict transplantation to early-stage tumours[49].
Sapisochin and colleagues performed a multicentre retrospective cohort study in Spain comparing incidentally found iCCA/mixed HCC/iCCAs on LT explant and LT for HCC. They found a significant decrease in survival in patients with pure iCCA compared to HCC (5-year actuarial OS 51% vs 93% respectively, P < 0.001), however mixed HCC/iCCAs had no significant difference in 5-year actuarial OS at 86% (P = 0.9). Subgroup analysis of patients with uninodular tumours < 2 cm in size, showed that of 12 patients with mixed HCC/iCCA (n = 5) and pure iCCA (n = 7), 5-year actuarial OS was 62% vs 80% in the HCC group (P = 0.4), implying no significant difference in survival. This finding may have been due to the low numbers of patients however[50].
A follow-up international multicentre retrospective series evaluating LT in early stage iCCA with unresectable unifocal tumours < 2 cm in size was conducted. Five-year actuarial OS in 14 patients with early stage iCCA was 65% vs 45% in those with advanced disease. KM recurrence rates were 18% at 5 years for early tumours compared to 61% for advanced tumours[51]. These figures compare favourably to curative intent liver resections with negative margins in iCCA with 5-year OS in the range of 30%-40% for all sizes of tumour. Early stage tumours < 2 cm in size can have near 100% survival at 5 years with negative margin resections. However, recurrence remains high with 5-year recurrence as high as 80% in the literature[52,53]. Hue et al[54] also did not find any significant differences in survival or recurrence with resection or LT for iCCA in their propensity matched registry-based study in the United States[54].
Studies which include neoadjuvant therapy prior to LT similar to the Mayo Protocol are limited to small case series. Lunsford et al[55] evaluated 12 patients with non-metastatic iCCA > 2 cm in size who were unresectable. They underwent neoadjuvant gemcitabine and platinum-based chemotherapy for 6 mo, and those who showed stable disease or regression proceeded to transplant. Six (50%) proceeded to LT and 5-year KM OS was 83.3% (95%CI: 27.3%-97.5%). Three patients developed recurrent disease at a median of 7.6 mo[56]. Wong et al[57] performed a similar study with the addition of transarterial chemoembolization and pre-transplant operative staging. Of 18 patients who started the neoadjuvant therapies only 5 (27.8) proceeded to transplant. Follow-up was limited to 1 year, actuarial OS was 80% (the single death was due to tumour recurrence) and recurrence developed in 2 patients (40%)[56].
A meta-analysis combining the above studies and others found a pooled 5-year OS of 42% (95%CI: 29%-55%) and 5-year DFS of 49% (95%CI: 41%-57%). However, the studies were all clinically heterogeneous with different preoperative protocols, different stages of tumours, degrees of cirrhosis in the background liver and used a variety of donor grafts. There was also statistically significant heterogeneity in all the meta-analyses with I2 values being significant at the < 0.01 Level[57].
Interpretation of all the above results is difficult given significant clinical differences in each study. But overall, it can be concluded that while LT can improve outcomes compared to palliative therapy for unresectable iCCA, the outcomes compared to transplant for other indications including phCCA are poorer at present, although evidence is limited. Very early stage unresectable tumours < 2 cm in size appear to have excellent prognosis with LT, with improved outcomes compared to even curative intent resection for iCCA. More evidence is needed, however, there is increasing opinion that unresectable iCCA may become an extended indication for LT in the same way as phCCA has. In 2020, the European Network for the Study of CCA endorsed the value of this modality as a potentially curative option for iCCA in a consensus statement, and recommended it should be considered especially in patients with very early stage unresectable tumours (≤ 2 cm) and concomitant cirrhosis[2].
A summary of key series of liver transplantation for phCCA and iCCA is presented in Table 1.
Table 1.
Summary of key contemporary series of liver transplantation for cholangiocarcinoma
|
Ref.
|
Country
|
Design
|
Tumour anatomical subtype
|
Treatments (s)
|
Total patients (n)
|
Median follow-up/yr (range)
|
Outcomes (5-year)1
|
||
|
Overall survival (%)
|
Disease free survival (%)
|
Graft survival (%)
|
|||||||
| Azad et al[28], 2020 | United States | Retrospective, single centre | Perihilar | Neoadjuvant chemo-radiotherapy and LT | De novo: 148, PSC: 228 | - | De novo: 58 KM, PSC: 74 KM | De novo: 55 KM, PSC: 78 KM | - |
| Darwish Murad et al[36], 2012 | United States | Retrospective, multi-centre | Perihilar | Neoadjuvant chemo-radiotherapy and LT | 287 | 2.5 (0.1-17.8) | 53 KM | 65 KM | 60 KM |
| Hidalgo et al[37], 2008 | United Kingdom | Retrospective, single centre | Perihilar | LT | 12 | 1.81 | 41 KM | - | - |
| Robles et al[38], 2004 | Spain | Retrospective, multi-centre | Perihilar | LT | 36 | - | 64 | 47 | - |
| Ethun et al[14], 2018 | United States | Retrospective, multi-centre | Perihilar | Neoadjuvant chemo-radiotherapy and LT | 70 | 4.83 (0.025-10.6) | 64 KM | 24 KM | - |
| Krasnodębski et al[47], 2020 | Poland | Retrospective, multi-centre | Intrahepatic | LT | 8 | 30 | 25 | 28.6 | - |
| Lee et al[48], 2018 | United States | Retrospective, single centre | Intrahepatic | Neoadjuvant TARE/TACE/RFA and LT | 17 | Mean 4.2 | 51.9 | 70.6 | - |
| Sapisochin et al[50], 2014 | Spain | Retrospective, multi-centre | Intrahepatic | Neoadjuvant ethanol injection/TACE/RFA and LT | 27 | 4.99 (0.97-11.9) | 51 | 36 | - |
| Sapisochin et al[51], 2016 | International | Retrospective, multi-centre | Intrahepatic- early1 and advanced | Neoadjuvant ethanol injection/TACE/RFA and LT | Early1-15, Advanced-33 | Early-4.78, Advanced-2.06 | Early-65, Advanced-45 | Early-86.7, Advanced-48.5 | - |
| Hue et al[54], 2021 | United States | Retrospective, multi-centre | Intrahepatic | Neoadjuvant chemo-radiation and LT | 74 | 3.9 | 33 KM | - | - |
| Lunsford et al[55], 2018 | United States | Prospective, single centre | Intrahepatic | Neoadjuvant chemotherapy and LT | 6 | 3 (2.42-4.25) | 83.3 | 50 | 100 |
| Wong et al[56], 2019 | United States | Prospective, single centre | Intrahepatic | Neoadjuvant chemotherapy and TACE and LT | 5 | 1.84 | 80 | 60 | - |
Early tumours defined as < 2 cm in size.
PSC: Primary sclerosing cholangitis; KM: Kaplan-Meier; LT: Liver transplant; TARE: Trans-arterial radioembolization; TACE: Trans-arterial chemoembolization; RFA: Radiofrequency ablation.
DISTAL CHOLANGIOCARCINOMA
phCCAs may extend down into the distal bile duct and it is established with the Mayo Protocol to potentially treat these with combined LT and pancreatoduodenectomy and many of the studies discussed above incorporate this either as a planned procedure or due to intraoperative findings of tumour extension[40]. Literature on LT in distal CCA (dCCA) is limited as these tumours are less likely to involve the liver and can be completely excised with pancreatoduodenectomy or bile duct excision with reconstruction depending on the site of the tumour. Extension into the liver or hilum would typically render the disease unresectable. Attempts at hepatopancreatoduodenectomy for these patients have been technically challenging with high rates of perioperative morbidity and mortality at up to 97.4% and 26% respectively in recent series. Five-year OS has ranged from 17.9%-49.2%[58]. Total hepatectomy, LT and pancreatoduodenectomy may have potential to be a better alternative therapy in this population. Case reports are sparse as dCCA is commonly an incidental finding in the context of LT for phCCA. Sutcliffe et al[59] report two patients at King’s College Hospital, United Kingdom who underwent LT for PSC but were found to incidentally have dCCA on the bile duct explant. They then underwent pancreatoduodenectomy 2 mo post-transplant. One patient died due to other causes with no evidence of recurrent disease over 5 years later. The other patient died 5 mo after pancreatoduodenectomy due to recurrence[59].
Patients with PSC may also develop dCCA and require resection for this and also LT for their underlying liver disease. Stauffer et al[61] reported on 6 patients with dCCA and PSC who underwent both LT and classical Whipple’s procedure. One patient survived to 58 mo of follow-up without any evidence of recurrence. Another patient, with an early T1 tumour survived to 52 mo of follow-up, with recurrence. All other patients developed recurrence and did not survive to 2 years[60].
DIRECTIONS FOR FUTURE RESEARCH
The literature at present consists mainly of retrospective case series of LT in CCA and predominantly in phCCA. The studies are heterogeneous in patient populations, in terms of staging of disease, preoperative neoadjuvant therapies, types of donor livers used and postoperative management and follow-up. Prospective multi-centre observational studies are needed for more robust evaluation of the Mayo Protocol and any modifications to the protocol should ideally be evaluated in a randomized trial. Unfortunately, due to the many confounding variables involved in LT and CCA and the rarity of suitable cases in general, recruitment to such trials with restrictive inclusion criteria to keep populations homogenous will no doubt be challenging.
The TRANSPHILL trial in France, has been ongoing since 2014 and aimed to recruit 54 patients with resectable phCCA for randomisation to either curative intent resection or LT. The primary outcome is 5-year OS, and 3-year recurrence is a secondary outcome[61]. Results are eagerly awaited from this trial as of writing, however, if the positive results of Ethun et al[14] are confirmed, this would represent a true paradigm shift away from resection to LT in phCCA.
The development of international registries may allow more generalisable and homogenous research beyond simple case series in future, by increasing the number of cases presented and capturing all relevant variables systematically.
Adjuvant and neoadjuvant protocols
Addition of newer neoadjuvant therapies such as stereotactic body radiation therapy (SBRT) have shown promise in preventing disease progression with lower toxicity than traditional external beam radiotherapy and may be beneficial prior to LT[62]. Oncological therapy has advanced in the management of CCA with improvement of survival with combination of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) as a second line chemotherapy agent[63] and with recent trial results showing a benefit for immunotherapies in CCA[64]. Both have potential as neoadjuvant or adjuvant therapies in CCA patients considered for LT. Finally, the BILCAP trial established capecitabine as an effective adjuvant agent after resection of CCA as the standard of care, by showing improved OS[65]. There have been no studies on adjuvant therapy after LT for CCA and this too may improve survival in this setting.
Further, immunosuppression is known to be a risk factor for the development of malignancy and recurrence. There has been little research into the optimum suppressive regimen to balance the risks of graft rejection vs risk of recurrence after LT for CCA. In LT for HCC, there has been some evidence that reduction of immunosuppression[66] and use of mechanistic target of rapamycin (mTOR) inhibitors of suppression has a beneficial effect on OS after recurrence[67]. A recent series of patients undergoing LT for iCCA and phCCA showed that a reduced immunosuppressive regimen after recurrence was significantly associated with survival with an increased odds ratio of survival at 4.2 (95%CI: 1.3-13.6; P = 0.02)[68].
Further advances in LT and perioperative care along with novel chemotherapeutic and biological agents may lead to further improved outcomes[69].
Establishment of LT as a treatment modality for CCA
For LT to become uniformly established as a treatment modality for CCA, in both iCCA and phCCA, consistent reports with 5-year survival exceeding 50% and in the range of established indications of LT are needed in each group. The supply of donor livers will need to be improved to match increased demand from this population of patients and technologies such as normothermic machine perfusion and policy changes such as opt-out organ donation[70] being more widely enacted are expected to increase this supply.
CONCLUSION
LT has emerged as an effective treatment option for CCA in suitably selected patients. Treatment of unresectable phCCA has been transformed by LT with all modern series approaching parity with outcomes with other indications for transplant. Early evidence shows that LT may offer even better survival than curative intent resection for early resectable hilar tumours and may render transplant as the treatment of choice for the disease. Evidence for LT in iCCA is limited, but has considerable potential, with comparable outcomes to perihilar tumours in early disease and might become an established treatment option for suitable patients. Further improvements in LT and postoperative management along with novel chemotherapeutic and biological agents may further improve the current outcomes. Larger, high quality studies are needed in each group of tumours.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: General Medical Council (United Kingdom), 7451513; Royal College of Surgeons of England, 9092145; International College of Surgeons, M21313; American College of Surgeons, 03340060; Faculty of Surgical Trainers of Edinburgh, Royal College of Surgeons of Edinburgh, 188646.
Peer-review started: December 6, 2022
First decision: February 1, 2023
Article in press: March 21, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li HL, China; Zhang JW, China S-Editor: Li L L-Editor: A P-Editor: Li L
Contributor Information
Aditya Borakati, Department of HPB and Liver Transplantation Surgery, Royal Free Hospital NHS Foundation Trust, London NW3 2QG, United Kingdom.
Farid Froghi, Department of HPB and Liver Transplantation Surgery, Royal Free Hospital NHS Foundation Trust, London NW3 2QG, United Kingdom.
Ricky H Bhogal, Department of Academic Surgery, The Royal Marsden NHS Foundation Trust, London SW3 6JJ, United Kingdom.
Vasileios K Mavroeidis, Department of Academic Surgery, The Royal Marsden NHS Foundation Trust, London SW3 6JJ, United Kingdom. vasileios.mavroeidis@nhs.net.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M, Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 4.Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol. 2013;5:171–176. doi: 10.4251/wjgo.v5.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bile Duct Cancer Survival Rates. Cholangiocarcinoma Survival Rates. [cited 3 February 2023]. Available from: https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html .
- 6.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, Haj Mohammad N, de Reuver PR, Verheij J, de Vos-Geelen J, Wilmink JW, Groot Koerkamp B, Klümpen HJ, Besselink MG Dutch Pancreatic Cancer Group. Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol. 2019;58:1048–1055. doi: 10.1080/0284186X.2019.1590634. [DOI] [PubMed] [Google Scholar]
- 9.Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, Man N, Poon R, Lo CM. ALPPS Versus Portal Vein Embolization for Hepatitis-related Hepatocellular Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant Before Major Hepatectomy. Ann Surg. 2021;273:957–965. doi: 10.1097/SLA.0000000000003433. [DOI] [PubMed] [Google Scholar]
- 10.Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R, Topp SA, Vivarelli M, Aldrighetti LA, Robles Campos R, Oldhafer KJ, Jarnagin WR, van Gulik TM. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017;19:381–387. doi: 10.1016/j.hpb.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reames BN, Ejaz A, Koerkamp BG, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Martel G, Marsh JW, Pawlik TM. Impact of major vascular resection on outcomes and survival in patients with intrahepatic cholangiocarcinoma: A multi-institutional analysis. J Surg Oncol. 2017;116:133–139. doi: 10.1002/jso.24633. [DOI] [PubMed] [Google Scholar]
- 12.Alikhanov R, Dudareva A, Trigo MÁ, Serrablo A. Vascular Resection for Intrahepatic Cholangiocarcinoma: Current Considerations. J Clin Med. 2021;10 doi: 10.3390/jcm10173829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiura T, Uesaka K, Okamura Y, Ito T, Yamamoto Y, Ashida R, Ohgi K, Otsuka S, Nakagawa M, Aramaki T, Asakura K. Major hepatectomy with combined vascular resection for perihilar cholangiocarcinoma. BJS Open. 2021;5 doi: 10.1093/bjsopen/zrab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ethun CG, Lopez-Aguiar AG, Anderson DJ, Adams AB, Fields RC, Doyle MB, Chapman WC, Krasnick BA, Weber SM, Mezrich JD, Salem A, Pawlik TM, Poultsides G, Tran TB, Idrees K, Isom CA, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Cardona K, Maithel SK. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann Surg. 2018;267:797–805. doi: 10.1097/SLA.0000000000002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE. History of Liver and Other Splanchnic Organ Transplantation. In: Doria, C. (eds) Contemporary Liver Transplantation. Switzerland: Springer, Cham, 2016. [Google Scholar]
- 16.Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 17.Gurusamy KS, Pamecha V, Davidson BR. Piggy-back graft for liver transplantation. Cochrane Database Syst Rev. 2011:CD008258. doi: 10.1002/14651858.CD008258.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Kalayoglu M, Sollinger HW, Stratta RJ, D'Alessandro AM, Hoffmann RM, Pirsch JD, Belzer FO. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617–619. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 19.Belzer FO, D'Alessandro AM, Hoffmann RM, Knechtle SJ, Reed A, Pirsch JD, Kalayoglu M, Sollinger HW. The use of UW solution in clinical transplantation. A 4-year experience. Ann Surg. 1992;215:579–583; discussion 584. doi: 10.1097/00000658-199206000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talakić E, Janek E, Mikalauskas S, Schemmer P. Liver Transplantation in Malignancies: A Comprehensive and Systematic Review on Oncological Outcome. Visc Med. 2021;37:302–314. doi: 10.1159/000517328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwatsuki S, Gordon RD, Shaw BW Jr, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–407. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726–734; discussion 734. [PubMed] [Google Scholar]
- 23.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 24.Castaldo ET, Pinson CW. Liver transplantation for non-hepatocellular carcinoma malignancy. HPB (Oxford) 2007;9:98–103. doi: 10.1080/13651820601156090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B Jr, McCashland T, Sorrell M, Tempero M, Langnas A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 26.De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, Burgart L, Gores GJ. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 27.Heimbach JK, Haddock MG, Alberts SR, Nyberg SL, Ishitani MB, Rosen CB, Gores GJ. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10:S65–S68. doi: 10.1002/lt.20266. [DOI] [PubMed] [Google Scholar]
- 28.Azad AI, Rosen CB, Taner T, Heimbach JK, Gores GJ. Selected Patients with Unresectable Perihilar Cholangiocarcinoma (pCCA) Derive Long-Term Benefit from Liver Transplantation. Cancers (Basel) 2020;12 doi: 10.3390/cancers12113157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polyak A, Kuo A, Sundaram V. Evolution of liver transplant organ allocation policy: Current limitations and future directions. World J Hepatol. 2021;13:830–839. doi: 10.4254/wjh.v13.i8.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NHS Blood and Transplant. Annual Report on Liver Transplantation - 2020/2021. Sep 2021. [cited 3 February 2023]. Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/24593/nhsbt-liver-transplant-report-2021-final.pdf .
- 31.Gores GJ, Gish RG, Sudan D, Rosen CB MELD Exception Study Group. Model for end-stage liver disease (MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 2006;12:S95–S97. doi: 10.1002/lt.20965. [DOI] [PubMed] [Google Scholar]
- 32.Reichman TW, Katchman H, Tanaka T, Greig PD, McGilvray ID, Cattral MS, Renner EL, Selzner M, Ghanekar A, Levy G, Grant DR. Living donor versus deceased donor liver transplantation: a surgeon-matched comparison of recipient morbidity and outcomes. Transpl Int. 2013;26:780–787. doi: 10.1111/tri.12127. [DOI] [PubMed] [Google Scholar]
- 33.Vodkin I, Kuo A. Extended Criteria Donors in Liver Transplantation. Clin Liver Dis. 2017;21:289–301. doi: 10.1016/j.cld.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Callaghan CJ, Charman SC, Muiesan P, Powell JJ, Gimson AE, van der Meulen JH UK Liver Transplant Audit. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: a cohort study. BMJ Open. 2013;3:e003287. doi: 10.1136/bmjopen-2013-003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 36.Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98.e3; quiz e14. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidalgo E, Asthana S, Nishio H, Wyatt J, Toogood GJ, Prasad KR, Lodge JP. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol. 2008;34:787–794. doi: 10.1016/j.ejso.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, López P, Gómez M, de Vicente E, Loinaz C, Santoyo J, Fleitas M, Bernardos A, Lladó L, Ramírez P, Bueno FS, Jaurrieta E, Parrilla P. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaiser GM, Sotiropoulos GC, Jauch KW, Löhe F, Hirner A, Kalff JC, Königsrainer A, Steurer W, Senninger N, Brockmann JG, Schlitt HJ, Zülke C, Büchler MW, Schemmer P, Settmacher U, Hauss J, Lippert H, Hopt UT, Otto G, Heiss MM, Bechstein WO, Timm S, Klar E, Hölscher AH, Rogiers X, Stangl M, Hohenberger W, Müller V, Molmenti EP, Fouzas I, Erhard J, Malagó M, Paul A, Broelsch CE, Lang H. Liver transplantation for hilar cholangiocarcinoma: a German survey. Transplant Proc. 2008;40:3191–3193. doi: 10.1016/j.transproceed.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 40.Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Søreide K, Wigmore SJ, Guest RV. Meta-analysis and Meta-regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann Surg. 2021;273:240–250. doi: 10.1097/SLA.0000000000003801. [DOI] [PubMed] [Google Scholar]
- 41.Moris D, Kostakis ID, Machairas N, Prodromidou A, Tsilimigras DI, Ravindra KV, Sudan DL, Knechtle SJ, Barbas AS. Comparison between liver transplantation and resection for hilar cholangiocarcinoma: A systematic review and meta-analysis. PLoS One. 2019;14:e0220527. doi: 10.1371/journal.pone.0220527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saffioti F, Mavroeidis VK. Review of incidence and outcomes of treatment of cholangiocarcinoma in patients with primary sclerosing cholangitis. World J Gastrointest Oncol. 2021;13:1336–1366. doi: 10.4251/wjgo.v13.i10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hand F, Hoti E. Contemporary role of liver transplantation for the treatment of cholangiocarcinoma. Expert Rev Gastroenterol Hepatol. 2020;14:475–481. doi: 10.1080/17474124.2020.1765771. [DOI] [PubMed] [Google Scholar]
- 44.Kitajima T, Hibi T, Moonka D, Sapisochin G, Abouljoud MS, Nagai S. Center Experience Affects Liver Transplant Outcomes in Patients with Hilar Cholangiocarcinoma. Ann Surg Oncol. 2020;27:5209–5221. doi: 10.1245/s10434-020-08682-5. [DOI] [PubMed] [Google Scholar]
- 45.Tan EK, Rosen CB, Heimbach JK, Gores GJ, Zamora-Valdes D, Taner T. Living Donor Liver Transplantation for Perihilar Cholangiocarcinoma: Outcomes and Complications. J Am Coll Surg. 2020;231:98–110. doi: 10.1016/j.jamcollsurg.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 46.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krasnodębski M, Grąt M, Jastrzębski M, Szczęśniak M, Morawski M, Zając K, Patkowski W, Zieniewicz K. Unsatisfactory Long-term Results of Liver Transplant in Patients With Intrahepatic Cholangiocarcinoma. Transplant Proc. 2020;52:2463–2467. doi: 10.1016/j.transproceed.2020.02.095. [DOI] [PubMed] [Google Scholar]
- 48.Lee DD, Croome KP, Musto KR, Melendez J, Tranesh G, Nakhleh R, Taner CB, Nguyen JH, Patel T, Harnois DM. Liver transplantation for intrahepatic cholangiocarcinoma. Liver Transpl. 2018;24:634–644. doi: 10.1002/lt.25052. [DOI] [PubMed] [Google Scholar]
- 49.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 50.Sapisochin G, de Lope CR, Gastaca M, de Urbina JO, López-Andujar R, Palacios F, Ramos E, Fabregat J, Castroagudín JF, Varo E, Pons JA, Parrilla P, González-Diéguez ML, Rodriguez M, Otero A, Vazquez MA, Zozaya G, Herrero JI, Antolin GS, Perez B, Ciria R, Rufian S, Fundora Y, Ferron JA, Guiberteau A, Blanco G, Varona MA, Barrera MA, Suarez MA, Santoyo J, Bruix J, Charco R. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259:944–952. doi: 10.1097/SLA.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 51.Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178–1188. doi: 10.1002/hep.28744. [DOI] [PubMed] [Google Scholar]
- 52.Sapisochin G, Ivanics T, Heimbach J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: Ready for Prime Time? Hepatology. 2022;75:455–472. doi: 10.1002/hep.32258. [DOI] [PubMed] [Google Scholar]
- 53.Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Groeschl R, Gamblin TC, Marsh JW, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Choti MA, Gigot JF, Mentha G, Pawlik TM. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–818. doi: 10.1016/j.surg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hue JJ, Rocha FG, Ammori JB, Hardacre JM, Rothermel LD, Chavin KD, Winter JM, Ocuin LM. A comparison of surgical resection and liver transplantation in the treatment of intrahepatic cholangiocarcinoma in the era of modern chemotherapy: An analysis of the National Cancer Database. J Surg Oncol. 2021;123:949–956. doi: 10.1002/jso.26370. [DOI] [PubMed] [Google Scholar]
- 55.Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC) Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337–348. doi: 10.1016/S2468-1253(18)30045-1. [DOI] [PubMed] [Google Scholar]
- 56.Wong M, Kim J, George B, Eriksen C, Pearson T, Robbins J, Zimmerman MA, Hong JC. Downstaging Locally Advanced Cholangiocarcinoma Pre-Liver Transplantation: A Prospective Pilot Study. J Surg Res. 2019;242:23–30. doi: 10.1016/j.jss.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 57.Ziogas IA, Giannis D, Economopoulos KP, Hayat MH, Montenovo MI, Matsuoka LK, Alexopoulos SP. Liver Transplantation for Intrahepatic Cholangiocarcinoma: A Meta-analysis and Meta-regression of Survival Rates. Transplantation. 2021;105:2263–2271. doi: 10.1097/TP.0000000000003539. [DOI] [PubMed] [Google Scholar]
- 58.Fancellu A, Sanna V, Deiana G, Ninniri C, Turilli D, Perra T, Porcu A. Current role of hepatopancreatoduodenectomy for the management of gallbladder cancer and extrahepatic cholangiocarcinoma: A systematic review. World J Gastrointest Oncol. 2021;13:625–637. doi: 10.4251/wjgo.v13.i6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutcliffe RP, Lam W, O'Sullivan A, Prachalias A, Rela M, Heaton N. Pancreaticoduodenectomy after liver transplantation in patients with primary sclerosing cholangitis complicated by distal pancreatobiliary malignancy. World J Surg. 2010;34:2128–2132. doi: 10.1007/s00268-010-0624-z. [DOI] [PubMed] [Google Scholar]
- 60.Stauffer JA, Steers JL, Bonatti H, Dougherty MK, Aranda-Michel J, Dickson RC, Harnois DM, Nguyen JH. Liver transplantation and pancreatic resection: a single-center experience and a review of the literature. Liver Transpl. 2009;15:1728–1737. doi: 10.1002/lt.21932. [DOI] [PubMed] [Google Scholar]
- 61.ClinicalTrials.gov Liver Resection Versus Radio-chemotherapy-Transplantation for Hilar Cholangiocarcinoma. Nov 3, 2021. [sited 3 February 2023]. Available from: https://clinicaltrials.gov/ct2/show/NCT02232932 .
- 62.Borakati A, Froghi F, Bhogal RH, Mavroeidis VK. Stereotactic radiotherapy for intrahepatic cholangiocarcinoma. World J Gastrointest Oncol. 2022;14:1478–1489. doi: 10.4251/wjgo.v14.i8.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, Nam AR, Oh KS, Kim JM, Lee Y, Guthrie V, McCoon P, Li W, Wu S, Zhang Q, Rebelatto MC, Kim JW. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7:522–532. doi: 10.1016/S2468-1253(22)00043-7. [DOI] [PubMed] [Google Scholar]
- 65.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 66.Vivarelli M, Dazzi A, Zanello M, Cucchetti A, Cescon M, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation. 2010;89:227–231. doi: 10.1097/TP.0b013e3181c3c540. [DOI] [PubMed] [Google Scholar]
- 67.Grigg SE, Sarri GL, Gow PJ, Yeomans ND. Systematic review with meta-analysis: sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2019;49:1260–1273. doi: 10.1111/apt.15253. [DOI] [PubMed] [Google Scholar]
- 68.Gül-Klein S, Schmitz P, Schöning W, Öllinger R, Lurje G, Jonas S, Uluk D, Pelzer U, Tacke F, Schmelzle M, Pratschke J, Ossami Saidy RR, Eurich D. The Role of Immunosuppression for Recurrent Cholangiocellular Carcinoma after Liver Transplantation. Cancers (Basel) 2022;14 doi: 10.3390/cancers14122890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Resch T, Esser H, Cardini B, Schaefer B, Zoller H, Schneeberger S. Liver transplantation for hilar cholangiocarcinoma (h-CCA): is it the right time? Transl Gastroenterol Hepatol. 2018;3:38. doi: 10.21037/tgh.2018.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmad MU, Hanna A, Mohamed AZ, Schlindwein A, Pley C, Bahner I, Mhaskar R, Pettigrew GJ, Jarmi T. A Systematic Review of Opt-out Versus Opt-in Consent on Deceased Organ Donation and Transplantation (2006-2016) World J Surg. 2019;43:3161–3171. doi: 10.1007/s00268-019-05118-4. [DOI] [PubMed] [Google Scholar]
- 71.Wikimedia Commons. File:Bismuth corlette classification for perihilar cholangiocarcinomas.svg. 2020 Oct 9 [visited 3 February 2023]. Available from: https://commons.wikimedia.org/wiki/File:Bismuth_corlette_classification_for_perihilar_cholangiocarcinomas.svg .