Abstract
There is a paucity of data to guide anterior nucleus of the thalamus (ANT) deep brain stimulation (DBS) with brain sensing. The clinical Medtronic Percept DBS device provides constrained brain sensing power within a frequency band (power-in-band [PIB]), recorded in 10-min averaged increments. Here, four patients with temporal lobe epilepsy were implanted with an investigational device providing full bandwidth chronic intracranial electroencephalogram (cEEG) from bilateral ANT and hippocampus (Hc). ANT PIB-based seizure detection was assessed. Detection parameters were cEEG PIB center frequency, bandwidth, and epoch duration. Performance was evaluated against epileptologist-confirmed Hc seizures, and assessed by area under the precision-recall curve (PR-AUC). Data included 99 days of cEEG, and 20, 278, 3, and 18 Hc seizures for Subjects 1–4. The best detector had 7-Hz center frequency, 5-Hz band width, and 10-s epoch duration (group PR-AUC = .90), with 75% sensitivity and .38 false alarms per day for Subject 1, and 100% and .0 for Subjects 3 and 4. Hc seizures in Subject 2 did not propagate to ANT. The relative change of ANT PIB was maximal ipsilateral to seizure onset for all detected seizures. Chronic ANT and Hc recordings provide direct guidance for ANT DBS with brain sensing.
Keywords: chronic brain recordings, deep brain stimulation, neuromodulation, seizure detection
1 |. INTRODUCTION
Epilepsy is a common neurological disorder (prevalence 621.5 per 100 000 people worldwide).1 Approximately one third of people with epilepsy have drug-resistant epilepsy (DRE).2 Many people with DRE are not candidates for epilepsy surgery or continue to have seizures despite surgery, and for these individuals electrical brain stimulation (EBS) is a viable treatment option.
Anterior nucleus of the thalamus (ANT) deep brain stimulation (DBS) has a CE mark and US Food and Drug Administration approval for the treatment of focal (partial) DRE.3 ANT DBS delivers open-loop electrical stimulation. Although ANT DBS is an effective therapy for focal DRE, ANT DBS, along with all forms of EBS, rarely results in seizure freedom.4 Furthermore, epilepsy therapies and optimization may be impacted by the poor reliability of patient-reported seizure diaries,5,6 with implications for clinical practice and formal clinical drug/device trials.7
The sensing capabilities of a new generation of DBS devices may address these challenges. The commercially available Medtronic Percept DBS device provides constrained chronic intracranial electroencephalographic (cEEG) data in the form of local field potential (LFP) power within a physician-specified frequency band saved in 10-min averaged increments. There is evidence for the use of these LFP power-in-band (PIB) trends in Parkinson disease8; however, little is known about thalamic PIB trends for epilepsy. Unresolved questions for ANT sensing for epilepsy include: (1) What are optimal sensing parameters for ANT PIB trend-based seizure detection? and (2) Can ANT PIB trends provide reliable seizure detections?
The investigational Medtronic Summit RC + S is a research implantable pulse generator (IPG) that provides flexible continuous full bandwidth (1–250, 500, or 1000 Hz) cEEG from channels that can be selected from the available 16 electrode contacts, four leads with four contacts per lead.9 In a first in human epilepsy investigational device exemption (IDE) study, we have implanted the device in four patients with DRE. Participants have mesial temporal lobe epilepsy, and leads were placed in bilateral ANT and hippocampus (Hc) for cEEG and EBS.
Here, we leverage the ANT and Hc cEEG data from four subjects implanted with the investigational device to optimize automated seizure detection using ANT PIB trend data. Optimization parameters were (1) PIB center frequency, (2) PIB center width, and (3) epoch length over which PIB trend data were averaged. These results provide timely guidance for ANT PIB-based seizure detection, which may enable adaptive DBS (combined open loop and responsive stimulation), improve therapy efficacy assessments with an automated electronic seizure diary, aid seizure lateralization, and facilitate seizure risk forecasting.10 More broadly, this study demonstrates how rich research device datasets can inform clinical devices and direct next generation device development.
2 |. MATERIALS AND METHODS
Four subjects were implanted with the investigational Medtronic Summit RC + S IPG with leads targeting bilateral ANT and Hc (3387 and 3391 leads, with electrode span of 10.5 and 24 mm, respectively). The IPG provided continuous cEEG time series data sampled at 250 Hz from four bipolar pairs selected from four leads. Time series data were remotely streamed to a physician-accessible cloud-based server (Figure 1).9 All analyses used RC + S ANT and Hc data acquired during the baseline stimulation-off period. This study was approved by the Food and Drug Administration (IDE G180224) and Mayo Clinic Institutional Review Board.
FIGURE 1.
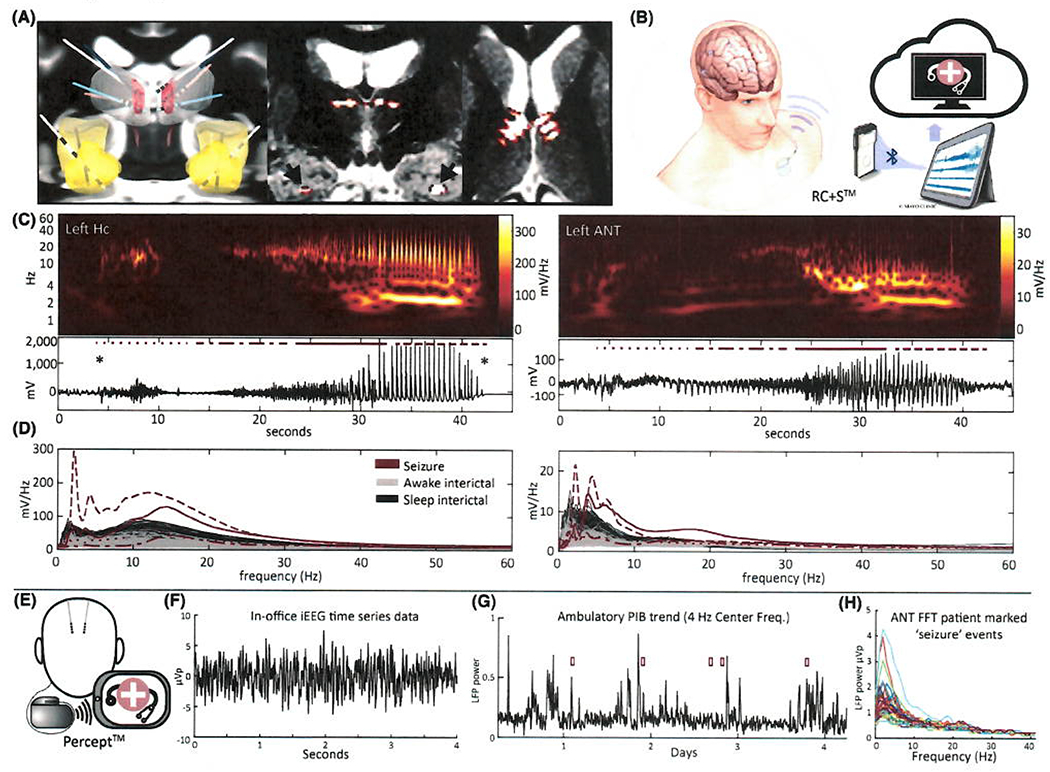
(A) Four lead implants for all subjects overlayed on Montreal Neurological Institute template brain (left panel), with anterior nucleus of the thalamus (ANT) and mammillothalamic tract colored red, thalamus white, and hippocampus (Hc) and amygdala yellow. Middle and right panels show Subject 1’s implant coregistered with preimplant fast gray matter acquisition T1 inversion recovery magnetic resonance imaging; electrode contacts are within the ANT and Hc bilaterally. (B) Illustration of the RC + S system: four implanted leads, implantable pulse generator (IPG), clinical telemetry module, epilepsy personal assistant device tablet, and physician-accessible cloud-based storage. (C) Representative seizure spectrogram and time series data, seen in Hc and ANT. Seizure onset is marked by asterisks. (D) Amplitude spectral density calculated over 10-s epochs corresponding to the seizure in C, overlayed on 30 min of awake and sleep interictal data. Dashed/dotted lines correspond to 10-s epochs marked in C. (E) Illustration of the Percept system: two implanted ANT leads, IPG, and clinician tablet that is required for power-in-band (PIB) trend and event data download and time series data streaming. Patients use a programmer in the ambulatory setting to mark “seizure” events, which triggers acquisition of the full Fast Fourier transform (FFT) spectrum (H). (F) In-office left ANT time series data. (G) Ninety-six hours of Percept ANT PIB trend data. Patient-marked “seizure” events indicated by red boxes did not have consistent correlates in the PIB trend data. The device provides unitless representation of local field potential (LFP) power. (H) FFT of 35 patient-marked “seizure” events. FFT was truncated at 40 Hz for visualization. Patient-triggered events prompt storage of the full FFT spectrum (1–100 Hz) from 30 s of data following event trigger. Of note, E–H show clinical data from a patient with the Percept DBS system (not a study subject) to demonstrate the Percept device capabilities, and are not intended to represent optimized detector performance. iEEG, intracranial electroencephalographic
ANT PIB measurements from the RC + S device were calculated with methods comparable to Percept PIB trend data. Fast Fourier transform (FFT) frequency domain data were generated from consecutive 1-s bins of ANT time series data. ANT time series data were recorded at 250 Hz (equivalent to Percept); frequency domain data spanned 2–98 Hz in 1-Hz steps. ANT PIB was calculated while varying (1) the center frequency (2–95 Hz in 1-Hz steps), (2) bandwidth of the ANT LFP (5, 10, and 20 Hz), and (3) epoch length over which PIB trend data were averaged (2, 5, 10, 20, 30, 60, 120, and 600 s).
For seizure detection scoring, the data were split into nonoverlapping 1-min segments. Each segment was labeled as “seizure” or “not seizure” by a board certified epileptologist using Hc cEEG (details below). The seizure classifier scored the same 1-min segments for 2–60-s epoch durations. For each 1-min segment, a label of “seizure” was recorded if the segment contained one or more epochs for which PIB surpassed the detection threshold (thresholds were varied for each subject to generate precision-recall [PR] and receiver operating characteristic [ROC] curves); otherwise, the segment was labeled “not seizure.” The same approach was used for 120- and 600-s epochs, with data split into 2-min or 10-min segments. A true positive detection was scored when a 1-min (or 2- or 10-min) segment was classified as “seizure” and the visually verified Hc cEEG label was “seizure.” A seizure was counted for at most one true positive detection.
cEEG Hc seizures were identified by a validated, high-sensitivity automated seizure classifier operating on full bandwidth Hc recordings11 and visually verified by a board certified epileptologist (G.A.W. or N.M.G.).
Seizure detection parameters were evaluated by area under the PR curve (PR-AUC) and ROC-AUC using all seizures from each subject. The PR curve is preferred for unbalanced datasets, such as for seizure detection.12 Electrodes were localized with the Lead-DBS toolbox.13 Analyses were performed using MATLAB (v2020b, MathWorks) and the Python programming language (Python Software Foundation, https://www.python.org/).
3 |. RESULTS
The investigational Medtronic Summit RC + S allows continuous 23/7 wireless streaming of time series data (1 h omitted daily during device charging) from four lead implants, whereas the Percept device stores PIB data from two leads on the implanted device (Figure 1). The investigational device data included 23.8, 22.6, 23.7, and 29.3 days of full bandwidth ANT and Hc cEEG, and 20, 278, 3, and 18 visually verified Hc seizures from Subjects 1–4, respectively. Subjects 1, 2, and 3 had left Hc onset seizures, whereas Subject 4 had independent bilateral Hc onset seizures. Detector parameter optimization was evaluated for all subjects (Figure 2A,B).
FIGURE 2.
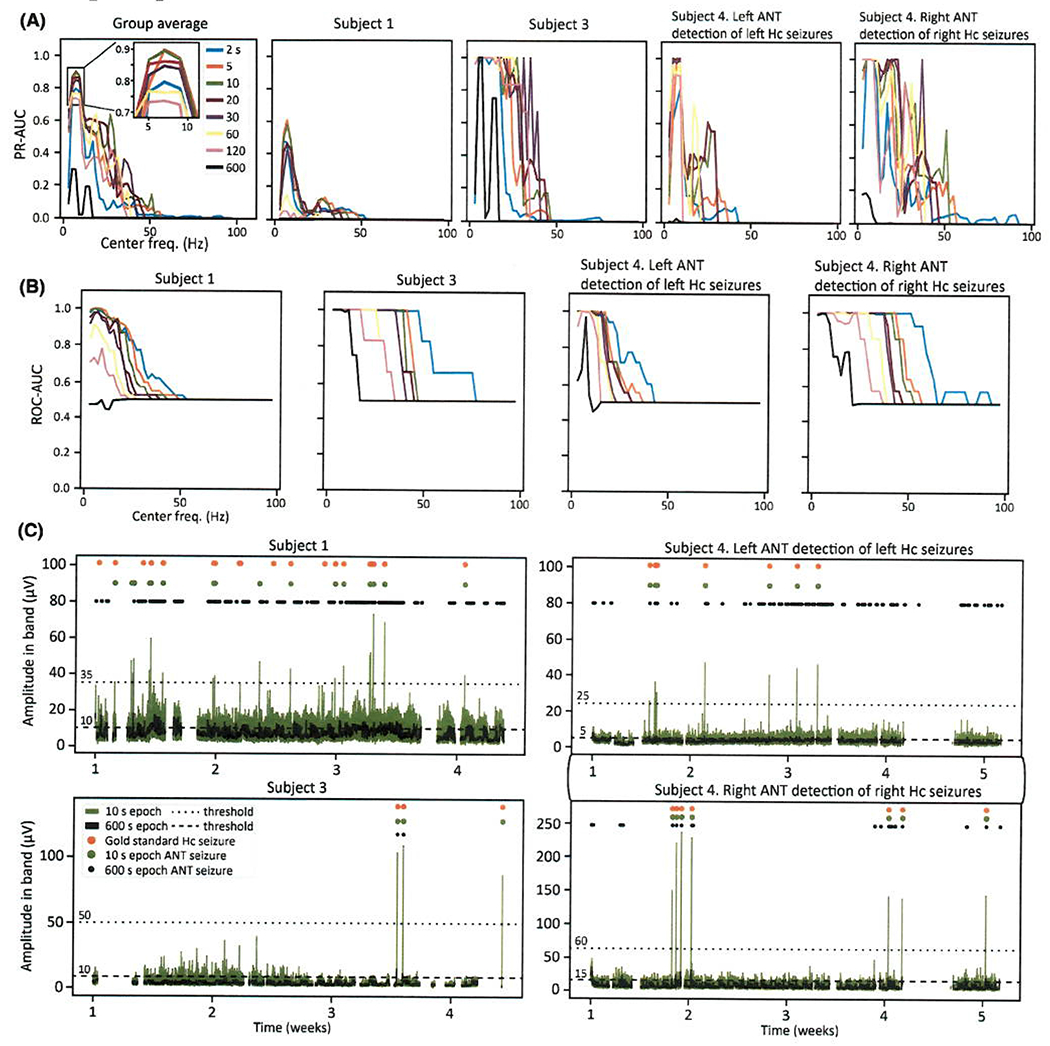
(A) Anterior nucleus of the thalamus (ANT) power-in-band (PIB) trend-based seizure detection area under the precision-recall curve (PR-AUC) across all tested center frequencies and epoch durations. The group average is the average PR-AUC value across Subjects 1, 3, and 4 shown in the right four panels. A random classifier has PR-AUC < .001 for all subjects (random classifier PR-AUC = P/[P + N], where P = the total number of “positives” [gold standard seizures] and N = the total number of “negatives” [the number of interictal segments]12). Five-hertz bandwidth was used for all plots in Figure 2. (B) Receiver operating characteristic (ROC)-AUC plots corresponding to A. A random classifier has ROC-AUC = .50. (C) RC + S-derived ANT PIB trend data using optimized seizure detection parameters (7-Hz center frequency, 5-Hz bandwidth, 10-s epoch) and optimized parameters with 10-min epoch duration. Visually confirmed hippocampus (Hc) seizure detections and ANT PIB trend-based seizure detections are marked. Detection thresholds are marked by dashed lines
The best seizure detection performance was achieved with 7-Hz center frequency (Figure 2A,B). The best performing epoch durations were 5–20 s (10 s was used for subsequent analyses), with a gradual decline through durations up to 120 s, and poor performance for 600 s epochs (Figure 2A,B). Seizure detection was optimal with a narrow frequency band (5 Hz), with a gradual drop in performance as bandwidth increased from 5 through 20 Hz (data not shown). Subject 1 and Subject 4 left ANT had a narrow range of parameters that provided optimal performance, whereas Subject 3 and Subject 4 right ANT had a broader range of parameters that achieved perfect detector performance (Figure 2A,B).
The best performing parameters (7-Hz center frequency, 5-Hz bandwidth, and 10-s epoch duration), with optimal patient-specific thresholds, provided 75% sensitivity, .38 daily false alarm rate (FAR), 64% positive predictive value, and .58 PR-AUC for Subject 1, and 100% sensitivity and .0 daily FAR for Subjects 3 and 4. Subject 2 had previously undergone left anterior temporal lobectomy and amygdalohippocampectomy (ATL; Figure S1), and remnant Hc tail onset seizures had no ANT involvement by visual inspection or spectral analysis (Figure S2). Figure 2C show RC + S ANT PIB trend data with optimized parameters and with 10-min epoch durations for Subjects 1, 3, and 4. The relative change in ictal versus interictal ANT PIB was maximal ipsilateral to seizure onset for all detected seizures (Figure 2C; Figure S3); the ipsilateral to contralateral ratio was 1.5, 6.5, 9.0, and 5.2 for Subjects 1, 3, and 4 left Hc seizures and Subject 4 right Hc seizures, respectively.
4 |. DISCUSSION
Using a unique dataset of chronic full bandwidth ANT and Hc recordings from four ambulatory subjects with mesial temporal lobe epilepsy monitored in their natural environments, this work provides the first direct evidence that ANT PIB-based seizure detection is feasible, and can lateralize seizure onset. Application of these insights to a new generation of ANT DBS devices with brain sensing may facilitate adaptive DBS, objective assessments of device and drug trials with automated seizure diaries, and potentially seizure risk forecasting.10
This work suggests that for some individuals with temporal lobe epilepsy, reliable ANT PIB-based seizure detection can be achieved by theta band center frequency (7 Hz), narrow band width (5 Hz), and relatively short epoch duration (5–20 s). The time over which LFP PIB data are averaged is not synchronized with seizure onset; short epoch durations allow for an entire epoch to reflect the ictal signal of interest and avoid dilution by interictal noise. Subject-specific differences in ictal activity impact detector performance (Figure 2); however, these results provide evidence for initial detector parameter selection, and demonstrate that ANT PIB-based seizure detection is feasible.
Patient 1 had a markedly active interictal record (hourly spike rate = 1900), which reduced the discriminability of ictal versus interictal PIB trend data. The optimized detector had seizure sensitivity of 75% and daily FAR of .38. Patients 3 and 4 had greater contrast between ictal and interictal ANT PIB, with perfect detector performance (sensitivity = 100%, FAR = .0). Perfect detector performance was achieved over a range of parameters for Subject 3 and Subject 4 right He onset seizures (Figure 2), with a preference for relatively low PIB center frequencies (theta to beta frequency range). Larger cohorts will determine how representative these thalamic seizure patterns are for the general population treated with ANT DBS. Although short epoch durations were superior to long epoch durations, some seizures were perceptible with 10-min epoch durations for Subjects 3 and 4 (Figure 2C). For all ANT detected seizures across all subjects, there was a greater relative change in ANT PIB ipsilateral to seizure onset, supporting seizure lateralization based on ANT PIB.
Subject 2, after left ATL, had no apparent seizure propagation from the remnant left Hc tail to the ANT. The reduced propensity for cortical recruitment with activation of the posterior Hc,14 and the atrophic Papez circuit (fornix atrophy on magnetic resonance imaging) may have suppressed Hc seizure propagation. Although these findings highlight subject-specific variability in performance, ANT PIB-based seizure detection was possible for all subjects with ANT seizure involvement. Recent work with thalamus stereo EEG (ANT involved in 75% of seizures across 11 patients)15 and ANT responsive neurostimulation from the RNS System16 provides further support for ANT seizure detection.
Clinicians and patients may want confidence in an individual’s seizure detection performance. There are several options: (1) correlate patient-marked “seizure” events with changes in ANT trend data, (2) identify an ictal signature from full spectrum FFT data acquired in the seizure versus interictal states (patient-triggered events record full spectrum FFT from available DBS devices with brain sensing), and (3) record seizures with concurrent video EEG. Approach 2 may require “seizure” events to reflect the ictal rather than postictal state, which would benefit from storage of buffered data, not available with current devices.
This work relies on data from a small cohort of subjects with mesial temporal lobe epilepsy. Future efforts should evaluate larger cohorts that include individuals with extratemporal limbic epilepsy and extralimbic epilepsy, the latter of which may have reduced ANT seizure detection performance based on brain connectomics. These findings should be validated with clinical DBS devices with brain sensing. Although the cohort is small, the dataset is sizeable (99 days; 41 seizures with ANT involvement), and ambulatory cEEG recordings are uniquely positioned to inform clinical ANT DBS with brain sensing. This analysis was optimized for reliable seizure detection, which was delayed from seizure onset (Figure 1C,D), and early detection may be achieved with other parameters at the expense of seizure specificity. Lead placement and selection of sensing contacts may impact ANT seizure detection; these recordings were from the baseline stimulation-off time period, as we did not use sensing-friendly stimulation configurations.
To date, there is a striking scarcity of data from patients with epilepsy to inform ANT DBS with brain sensing. To the best of our knowledge, this work provides the first direct evidence for seizure detection and lateralization with available clinical ANT DBS devices with brain sensing. The ability to reliably identity ANT seizure activity could lead to new opportunities for adaptive DBS and seizure risk forecasting, and improve the accuracy of seizure outcome metrics in therapeutic trials.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the American Epilepsy Society Research & Training Fellowship for Clinicians (N.M.G.), National Institutes of Health (NIH; U01-NS073557, R01-NS92882, UH2&3-NS95495), and Epilepsy Foundation Epilepsy Innovation Institute My Seizure Gauge. V.K. was partially supported by institutional support from Czech Technical University in Prague. We thank CertiCon a.s. for the use of the Cyber PSG tool.
Funding information
American Epilepsy Society Research & Training Fellowship for Clinicians; National Institutes of Health, Grant/Award Number: U01-NS073557, R01-NS92882 and UH2&3-NS95495; Epilepsy Foundation
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTEREST
G.A.W., B.N.L., J.J.V.V., and B.H.B. declare intellectual property licensed to Cadence Neuroscience. N.M.G., G.A.W., and B.N.L. are investigators for the Medtronic Deep Brain Stimulation Therapy for Epilepsy Post-Approval Study. V.S.M. is a paid summer intern at Medtronic. V.K. consults for CertiCon. The remaining authors declare that they have no competing interests. The Medtronic Summit RC+S devices used in this research were provided free of charge as part of NIH Brain Initiative UH2/UH3- NS95495. This work benefited from the community expertise and resources made available by the NIH Open Mind Consortium (NIH U24-NS113637; https://openmind-consortium.github.io/). We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Beghi E, Giussani G, Nichols E, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9. [DOI] [PubMed] [Google Scholar]
- 3.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. [DOI] [PubMed] [Google Scholar]
- 4.Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9):e1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook MJ, O’Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12(6):563–71. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64(11):1595–9. [DOI] [PubMed] [Google Scholar]
- 7.Blachut B, Hoppe C, Surges R, Elger C, Helmstaedter C. Subjective seizure counts by epilepsy clinical drug trial participants are not reliable. Epilepsy Behav. 2017;67:122–7. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127(Pt 4):735–46. [DOI] [PubMed] [Google Scholar]
- 9.Kremen V, Brinkmann BH, Kim I, Guragain H, Nasseri M, Magee AL, et al. Integrating brain implants with local and distributed computing devices: a next generation epilepsy management system. IEEE J Transl Eng Health Med. 2018;6:2500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karoly PJ, Rao VR, Gregg NM, Worrell GA, Bernard C, Cook MJ, et al. Cycles in epilepsy. Nat Rev Neurol. 2021;17(5):267–84. [DOI] [PubMed] [Google Scholar]
- 11.Baldassano SN, Brinkmann BH, Ung H, Blevins T, Conrad EC, Leyde K, et al. Crowdsourcing seizure detection: algorithm development and validation on human implanted device recordings. Brain. 2017;140(6):1680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One. 2015;10(3):e0118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn A, Kuhn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–35. [DOI] [PubMed] [Google Scholar]
- 14.Weitz AJ, Fang Z, Lee HJ, Fisher RS, Smith WC, Choy M, et al. Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage. 2015;107:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizarro D, Ilyas A, Chaitanya G, Toth E, Irannejad A, Romeo A, et al. Spectral organization of focal seizures within the thalamo-temporal network. Ann Clin Transl Neurol. 2019;6(9):1836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elder C, Friedman D, Devinsky O, Doyle W, Dugan P. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open. 2019;4(1):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


