Abstract
Introduction
Population‐based studies have rarely explored the associations of the triglyceride–glucose (TyG) index, a surrogate marker of insulin resistance, with dementia and plasma biomarkers for amyloid beta (Aβ) and neurodegeneration.
Methods
This population‐based study included 5199 participants (age ≥ 65 years); of these, plasma Aβ, total tau, and neurofilament light chain (NfL) were measured in 1287 persons. Dementia and subtypes were diagnosed following the international criteria. TyG index was calculated as ln(fasting triglyceride(mg/dL) × fasting glucose[mg/dL]/2). Data were analyzed using logistic and general linear regression models.
Results
Dementia, Alzheimer's disease (AD), and vascular dementia (VaD) were diagnosed in 301, 195, and 95 individuals, respectively. A high TyG index was significantly associated with increased likelihoods of dementia and AD; the significant association with dementia remained among participants without cardiovascular disease or diabetes. In the biomarker subsample, a high TyG index was correlated with elevated plasma Aβ, but not with total tau or NfL.
Discussion
High TyG index is associated with dementia, possibly via Aβ pathology.
Keywords: Alzheimer's disease, dementia, insulin resistance, plasma amyloid beta, population‐based study, triglyceride–glucose index
1. BACKGROUND
Type 2 diabetes is a well‐established risk factor for dementia and Alzheimer's disease (AD). 1 Insulin resistance represents a common pathophysiological feature shared by diabetes and AD. 2 Indeed, there is evidence that brain insulin resistance is increased progressively from normal cognition to mild cognitive impairment and AD independent of diabetes status. 3 In addition, epidemiological studies have shown that insulin resistance in middle‐aged and older adults is associated with brain amyloid beta (Aβ) deposition, brain atrophy, and poor cognitive function. 4 , 5 , 6 , 7 Experimental studies have suggested that insulin resistance could contribute to cognitive disorders by impairing synaptogenesis and neuronal function, causing glucose hypometabolism and mitochondrial dysfunction, increasing oxidative stress and neuroinflammation, and facilitating tau phosphorylation and Aβ deposition. 8 , 9
The hyperinsulinemic–euglycemic clamp technique is widely considered the gold standard method for quantifying insulin resistance. 10 However, this method rarely has been used in large‐scale epidemiological studies due to its time‐consuming and complex administration. The homeostasis model assessment of insulin resistance (HOMA‐IR) index, calculated as fasting insulin (µU/mL) × glucose (mmol/L)/22.5, is commonly used to evaluate the degree of insulin resistance. 11 Insulin quantification is relatively expensive and has limited value in people who are treated with insulin or who do not have functioning β cells. 12 Therefore, HOMA‐IR has limited utility in large‐scale population‐based studies. The triglyceride–glucose (TyG) index has recently emerged as a reliable surrogate marker for insulin resistance. 13 The TyG index closely mirrors the hyperinsulinemic–euglycemic clamp technique in the assessment of insulin sensitivity. 14 Clinical and population‐based studies show that the TyG index appears to be superior to HOMA‐IR in assessing insulin resistance 15 and predicting type 2 diabetes. 16 In addition, the TyG index is more widely available and less costly than HOMA‐IR index, which is crucial for clinical practice and large‐scale population studies.
Previous studies have shown that insulin resistance measured with HOMA‐IR is related to increased AD risk, 17 poor global cognition, 18 and accelerated cognitive decline. 19 The follow‐up data from the National Health Information Database in South Korea revealed a weak association between TyG index and risk of dementia, AD, and vascular dementia (VaD) independent of demographic, lifestyle, and metabolic factors. 20 However, it is unclear whether the association remains among people free of diabetes or cardiovascular disease (CVD). This is important because insulin resistance is closely correlated with diabetes and CVD, and both are well‐established risk factors for dementia and AD. In addition, no population‐based studies have investigated the relationships between the TyG index and plasma biomarkers for AD. Thus, epidemiological studies that integrate clinical and neuropsychological data with peripheral biomarkers may help clarify the underlying mechanisms linking the TyG index with dementia and subtypes.
Therefore, in this population‐based cross‐sectional study, we sought to investigate the associations of the TyG index with dementia, AD, VaD, and plasma AD biomarkers among rural‐dwelling older adults in China, and further explore whether the associations were present independent of overt CVDs or diabetes. We hypothesized that a high TyG index, as a surrogate marker of insulin resistance, is associated with dementia and plasma biomarkers for amyloid and neurodegeneration.
2. METHODS
2.1. Study design and participants
This population‐based cross‐sectional study used data from the Multimodal Interventions to Delay Dementia and Disability in rural China (MIND‐China), 21 a participating project of the World‐Wide FINGERS Network. 22 Eligible participants for MIND‐China included all registered residents who were aged ≥60 years by the end of 2017 and living in the 52 villages of Yanlou Town (n = 7698), Yanggu County, western Shandong Province. In March through September 2018, 5765 residents (74.89% of all eligible people) undertook the baseline examinations. 21 Because we aimed to investigate late‐onset dementia, we excluded 519 individuals who were 60 to 64 years of age. Of the 5246 participants who were aged ≥65 years, 47 were excluded due to missing diagnosis of dementia (n = 46) and fasting blood glucose (n = 1), leaving 5199 individuals for the analysis of the association between the TyG index and dementia. Of these, data on plasma biomarkers were available in 1287 individuals, which consisted of the analytical sample involving plasma biomarkers. Participants with plasma biomarkers (n = 1287) were slightly younger (mean age, 71.16 vs. 71.96 years, P = 0.003) and more likely to be female (60.84% vs. 55.90%, P = 0.002) than those without (n = 3912), but the two groups did not differ significantly in the distribution of education (P = 0.93). Figure 1 shows the flowchart of the study participants.
FIGURE 1.
Flowchart of the study participants. MIND‐China, Multimodal Interventions to Delay Dementia and Disability in rural China.
2.2. Data collection and definitions
Data were collected by trained staff through face‐to‐face interviews, clinical examinations, neuropsychological testing, and laboratory tests. 21 The TyG index was calculated as ln(fasting triglycerides [mg/dL] × fasting glucose [mg/dL]/2). 13 We multiplied glucose in mmol/L by 18.0 to get the equivalent reading in mg/dL. Triglyceride in mmol/L was multiplied by 88.545 to get the equivalent reading in mg/dL. Text S1 in supporting information provides detailed descriptions of data collection and definitions.
RESEARCH IN CONTEXT
Systemic review: Epidemiological studies have linked insulin resistance, measured with the homeostasis model assessment of insulin resistance, to poor cognitive function and dementia. However, population‐based studies have rarely explored the associations of the triglyceride–glucose (TyG) index, a reliable surrogate marker of insulin resistance, with dementia and peripheral biomarkers for brain pathology.
Interpretations: High TyG index was associated all‐cause dementia, Alzheimer's disease (AD), and plasma amyloid beta (Aβ) in older adults. Aβ may represent the potential neuropathological mechanisms underlying the TyG index–dementia association.
Future directions: Population‐based longitudinal studies are warranted to elucidate the potential causal relationships of TyG index with cognitive phenotypes among older adults as well as the underlying neuropathological mechanisms and to further evaluate the role of TyG index as a clinical marker for dementia.
2.3. Clinical diagnosis of dementia, AD, and VaD
Dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria, 23 following a three‐step diagnostic procedure, as previously described. 21 , 24 Briefly, the trained clinicians and interviewers performed routine clinical examinations and neurocognitive assessments following structured questionnaires to collect data on medical history, cognitive function, and activities of daily living. Then, neurologists specialized in dementia diagnosis and care reviewed all records from the first step and made a preliminary diagnosis for participants who were suspected to have dementia. Finally, the neurologists conducted further face‐to‐face interviews with those who were suspected to have dementia or who had insufficient data for making a diagnosis of dementia status and informants, and a clinical diagnosis of dementia was made. We further categorized dementia into AD and VaD according to respective diagnostic criteria. AD was clinically diagnosed following the National Institute on Aging–Alzheimer's Association criteria for probable AD dementia. 25 Biological AD dementia was not defined due to lack of relevant AD biomarkers. VaD was clinically diagnosed following the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria for probable VaD. 26
2.4. Measurement of plasma biomarkers
As previously reported, 21 plasma biomarkers were measured using a single‐molecule array (Simoa) on the HD‐X platform (Quanterix). Plasma Aβ40, Aβ42, total tau (t‐tau), and neurofilament light chain (NfL) were detected with Human Neurology 3‐Plex A assay (N3PA) Kit and NF‐light advantage kit. Two quality control (QC) samples were run in duplicate on each plate for each analyte. The within‐ and between‐run coefficients of variation for both QC samples were controlled within 13%.
2.5. Statistical analysis
Characteristics of the study participants by dementia status were compared using the Wilcoxon rank‐sum test or Student t test for continuous variables, and the chi‐square test for categorical variables. Binary and multinomial logistic regression models were used to evaluate the associations of the TyG index with dementia, AD, and VaD. General linear regression models were used to analyze the associations of the TyG index with plasma biomarkers. We used restricted cubic splines with four knots at the 5th, 35th, 65th, and 95th percentiles to flexibly model the association of the TyG index with all‐cause dementia, AD, and VaD. The likelihood‐ratio test was used to test the potential non‐linearity by comparing the model with only a linear term against the model with linear and cubic spline terms. We reported the main results from two models: Model 1 was adjusted for age, sex, and education; Model 2 was additionally adjusted for apolipoprotein E (APOE) genotype, body mass index, smoking, alcohol consumption, hypertension, CVDs, hypercholesterolemia, and use of blood glucose–lowering drugs or insulin injection.
We used Stata Statistical Software, Release 14 (Stata Corp.), and R version 4.1.1 (R Foundation for Statistical Computing, https://www.R‐project.org) for Windows for all analyses. Two‐tailed P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of study participants
The mean age of the 5199 participants was 71.76 (standard deviation = 5.52) years, 57.13% were women, and 40.62% were illiterate. Of these, 301 (5.78%) were diagnosed with dementia, including 195 (3.75%) with AD, 95 (1.83%) with VaD, and 11 (0.21%) with other types of dementia (Table 1). Compared to dementia‐free participants, those with dementia were older; more likely to be female; less educated; more likely to have diabetes, CVDs, and higher levels of blood glucose, triglycerides, and TyG index; and less likely to be obese, drink alcohol, and smoke. The two groups did not differ significantly in the distribution of APOE genotype, hypertension, or hypercholesterolemia.
TABLE 1.
Characteristics of the study participants according to dementia status.
| Total sample | Dementia status | |||
|---|---|---|---|---|
| Characteristics a | (n = 5199) | No (n = 4898) | Yes (n = 301) | P‐value |
| Age, years | 71.76 (5.52) | 71.47 (5.28) | 76.55 (7.02) | <0.001 |
| Female sex | 2970 (57.13) | 2765 (56.45) | 205 (68.11) | <0.001 |
| Education | <0.001 | |||
| Illiteracy | 2112 (40.62) | 1909 (38.98) | 203 (67.44) | |
| Primary school | 2253 (43.34) | 2176 (44.43) | 77 (25.58) | |
| Middle school or above | 834 (16.04) | 813 (16.60) | 21 (6.98) | |
| APOE ɛ4 allele carriers | 799 (16.00) | 750 (15.90) | 49 (17.75) | 0.413 |
| Body mass index, kg/m2 | 24.82 (3.80) | 24.85 (3.78) | 24.29 (4.10) | 0.009 |
| Serum glucose, mmol/L | 5.58 (1.43) | 5.56 (1.37) | 5.97 (2.07) | 0.008 |
| Triglycerides, mmol/L | 1.43 (0.91) | 1.43 (0.92) | 1.50 (0.76) | 0.017 |
| TyG index | 8.62 (0.53) | 8.61 (0.53) | 8.73 (0.57) | 0.001 |
| Current smoking | 1059 (20.39) | 1026 (20.96) | 33 (11.00) | <0.001 |
| Current alcohol drinking | 1427 (27.75) | 1394 (28.77) | 33 (11.15) | <0.001 |
| Hypertension | 3469 (67.31) | 3267 (67.29) | 202 (67.56) | 0.924 |
| Hypercholesterolemia | 874 (16.81) | 814 (16.62) | 60 (19.93) | 0.136 |
| Diabetes | 739 (14.21) | 680 (13.88) | 59 (19.60) | 0.006 |
| Use of glucose‐lowering drugs or insulin injection | 246 (4.73) | 222 (4.53) | 24 (7.97) | 0.006 |
| Cardiovascular disease b | 1818 (34.97) | 1664 (33.97) | 154 (51.16) | <0.001 |
| Coronary heart disease | 1140 (21.93) | 1058 (21.60) | 82 (27.24) | 0.022 |
| Atrial fibrillation | 83 (1.60) | 74 (1.51) | 9 (2.99) | 0.047 |
| Heart failure | 152 (2.92) | 146 (2.98) | 6 (1.99) | 0.324 |
| Stroke | 827 (15.91) | 725 (14.80) | 102 (33.89) | <0.001 |
Note: Data are mean (SD) or n (%).
Abbreviations: APOE, apolipoprotein E; SD, standard deviation; TyG index, triglyceride–glucose index.
The number of participants with missing values was 205 for APOE genotype, 5 for smoking, 57 for alcohol drinking, 45 for hypertension, 30 for body mass index. As a covariate in the subsequent analyses, a dummy variable was created for each of the categorical variables to represent those with missing values. Continuous variables with missing values were replaced with the mean value.
Cardiovascular disease included coronary heart disease, atrial fibrillation, heart failure, and stroke.
3.2. Associations of the TyG index with dementia and subtypes (n = 5199)
We used restricted cubic splines to visualize the patterns of the relationships of the predicted TyG index with dementia and subtypes (Figure 2). The TyG index was significantly associated with elevated likelihoods of dementia and AD, even in the multivariable‐adjusted models (P for overall association <0.05; Figure 2A,B), whereas TyG index was significantly associated with an increased likelihood of VaD only in the model that was adjusted for the demographic factors, but not in the fully adjusted model (Figure 2C). The likelihoods of dementia and subtypes associated with the TyG index were relatively flat and stable until around the 50th (8.57) through 75th percentile (8.93) of the TyG index and started to increase thereafter (Figure 2). Thus, we initially categorized all participants according to percentiles of TyG index into <50th, 50th to 75th, and ≥75th percentile. Because the odds ratios of dementia, AD, and VaD associated with the 50th to 75th percentile (vs. <50th percentile) of the TyG index were all close to 1, we further dichotomized the TyG index into <75th versus ≥75th percentile in the final analysis. Compared to the <75th percentile, TyG index ≥75th percentile was significantly associated with an increased likelihood of dementia, AD, and VaD in Model 1; the associations with dementia and AD remained statistically significant in Model 2, but the association with VaD was diluted and became non‐significant (Table 2).
FIGURE 2.
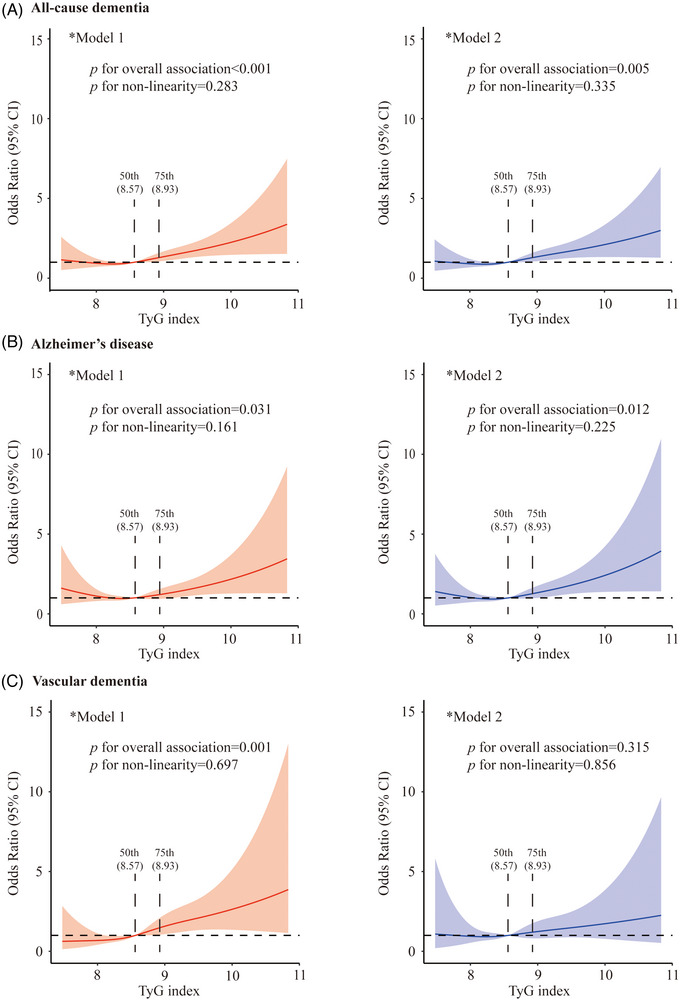
Associations of the triglyceride–glucose (TyG) index with all‐cause dementia (A), Alzheimer's disease (B), and vascular dementia (C). Solid lines represent adjusted odds ratios and shaded areas indicate 95% confidence intervals (CIs) derived from restricted cubic spline logistic regression models. In the analysis, the TyG index was used as an independent variable and dementia status was used as a dependent variable. *Model 1 was adjusted for age, sex, and education; *Model 2 was additionally adjusted for apolipoprotein E genotype, body mass index, current smoking, alcohol consumption, hypertension, hypercholesterolemia, cardiovascular diseases (coronary heart disease, heart failure, atrial fibrillation, and stroke), and use of glucose‐lowering drugs or insulin injection. P for non‐linearity <0.05 indicates a non‐linear association between the independent variable and the dependent variable. P for overall association <0.05 indicates an overall association between the independent variable and the dependent variable.
TABLE 2.
Associations of the TyG index with all‐cause dementia, Alzheimer's disease, and vascular dementia in the total sample and by cardiovascular disease and diabetes (n = 5199).
| All‐cause dementia | Alzheimer's disease | Vascular dementia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TyG index | N | n | Model 1 a | Model 2 a | n | Model 1 a | Model 2 a | n | Model 1 a | Model 2 a |
| Total sample (n = 5199) | ||||||||||
| <75th percentile (7.28–8.93) | 3899 | 200 | 1.00 (reference) | 1.00 (reference) | 133 | 1.00 (reference) | 1.00 (reference) | 60 | 1.00 (reference) | 1.00 (reference) |
| ≥75th percentile (8.93–11.42) | 1300 | 101 | 1.74 (1.33–2.26)*** | 1.66 (1.24–2.20)** | 62 | 1.54 (1.11–2.14)** | 1.72 (1.21–2.45)** | 35 | 2.09 (1.36–3.23)** | 1.38 (0.85–2.24) |
| CVDs, no (n = 3381) | ||||||||||
| <75th percentile (7.28–8.93) | 2653 | 104 | 1.00 (reference) | 1.00 (reference) | 86 | 1.00 (reference) | 1.00 (reference) | 12 | 1.00 (reference) | 1.00 (reference) |
| ≥75th percentile (8.93–11.42) | 728 | 43 | 1.59 (1.08–2.35)* | 1.64 (1.07–2.50)* | 34 | 1.48 (0.96–2.28) | 1.57 (0.98–2.50) | 7 | 2.55 (0.97–6.71) | 2.09 (0.67–6.50) |
| CVDs, yes (n = 1818) | ||||||||||
| <75th percentile (7.28–8.93) | 1246 | 96 | 1.00 (reference) | 1.00 (reference) | 47 | 1.00 (reference) | 1.00 (reference) | 48 | 1.00 (reference) | 1.00 (reference) |
| ≥75th percentile (8.93–11.42) | 572 | 58 | 1.59 (1.11–2.27)* | 1.58 (1.07–2.33)* | 28 | 1.58 (0.95–2.62) | 1.90 (1.10–3.28)* | 28 | 1.50 (0.92–2.44) | 1.20 (0.70–2.06) |
| Diabetes, no (n = 4460) | ||||||||||
| <75th percentile (7.28–8.93) | 3609 | 181 | 1.00 (reference) | 1.00 (reference) | 124 | 1.00 (reference) | 1.00 (reference) | 50 | 1.00 (reference) | 1.00 (reference) |
| ≥75th percentile (8.93–11.42) | 851 | 61 | 1.52 (1.11–2.10)** | 1.60 (1.14–2.24)** | 41 | 1.42 (0.97–2.08) | 1.57 (1.05–2.35)* | 18 | 1.77 (1.01–3.09)* | 1.58 (0.86–2.87) |
| Diabetes, yes (n = 739) | ||||||||||
| <75th percentile (7.28–8.93) | 290 | 19 | 1.00 (reference) | 1.00 (reference) | 9 | 1.00 (reference) | 1.00 (reference) | 10 | 1.00 (reference) | 1.00 (reference) |
| ≥75th percentile (8.93–11.42) | 449 | 40 | 1.46 (0.80–2.65) | 1.55 (0.83–2.92) | 21 | 1.57 (0.68–3.61) | 2.45 (0.97–6.19) | 17 | 1.21 (0.54–2.74) | 0.89 (0.37–2.12) |
Abbreviations: APOE, apolipoprotein E; CVD, cardiovascular disease; TyG index, triglyceride–glucose index.
Data were odds ratios (95% confidence intervals) derived from logistic regression models, in which the TyG index was considered an independent variable and dementia status a dependent variable. Model 1 was adjusted for age, sex, and education; Model 2 was additionally adjusted for APOE genotype, body mass index, current smoking, alcohol consumption, hypertension, hypercholesterolemia, and if applicable, for CVDs (coronary heart disease, heart failure, atrial fibrillation, and stroke) and use of glucose‐lowering drugs or insulin injection.
P < 0.05, ** P < 0.01, *** P < 0.001.
We further examined whether the associations of the TyG index with dementia and subtypes differed between people without and with CVDs or diabetes. The analysis stratified by CVDs suggested that a high TyG index (≥75th vs. <75th percentile) was significantly associated with an increased likelihood of all‐cause dementia among participants without and with CVDs when controlling for all the examined potential confounders, whereas TyG index was not significantly associated with VaD in either group (Table 2). In addition, a high TyG index was significantly and marginally associated with an increased likelihood of AD among individuals with and without CVDs, respectively, after controlling for multiple confounders (Table 2). When the analysis was performed by diabetic status, a high TyG index was significantly associated with increased likelihoods of dementia and AD among participants without diabetes, but not in those with diabetes, while TyG index was not significantly associated with VaD in either stratum in Model 2 when controlling for all the examined potential confounders (Table 2). There were no statistical interactions of the TyG index with either CVDs or diabetes on dementia or subtypes (P for all interactions >0.05).
3.3. Associations of the TyG index with plasma biomarkers (n = 1287)
In the subsample of participants with plasma biomarker data (n = 1287), the TyG index was linearly correlated with elevated plasma Aβ40, Aβ42, and Aβ42/Aβ40 ratio, even in the multivariable‐adjusted models, but not significantly associated with plasma t‐tau or NfL (Table 3).
TABLE 3.
Associations of the TyG index with plasma biomarkers in the total sample and by cardiovascular disease and diabetes (n = 1287).
| TyG index | No. of subjects | β coefficient (95% confidence interval), plasma biomarkers | |
|---|---|---|---|
| Model 1 a | Model 2 a | ||
| Aβ40, pg/mL, log‐transformed | |||
| Total sample | 1287 | 0.015 (0.003–0.027)* | 0.022 (0.009–0.035)** |
| CVDs, no | 873 | 0.015 (−0.000 to 0.030) | 0.029 (0.013–0.046)*** |
| CVDs, yes | 414 | 0.013 (−0.007 to 0.032) | 0.010 (−0.011 to 0.031) |
| Diabetes, no | 1088 | 0.014 (−0.001 to 0.028) | 0.021 (0.006–0.037)** |
| Diabetes, yes | 199 | 0.005 (−0.026 to 0.036) | 0.008 (−0.023 to 0.039) |
| Aβ42, pg/mL | |||
| Total sample | 1287 | 0.774 (0.453–1.096)*** | 0.905 (0.560–1.251)*** |
| CVDs, no | 873 | 0.718 (0.308–1.128)** | 1.005 (0.565–1.446)*** |
| CVDs, yes | 414 | 0.762 (0.239–1.284)** | 0.751 (0.190–1.312)** |
| Diabetes, no | 1088 | 0.807 (0.426–1.188)*** | 0.960 (0.555–1.364)*** |
| Diabetes, yes | 199 | 0.996 (0.177–1.816)* | 0.916 (0.074–1.760)* |
| Aβ42/Aβ40 ratio (×1000) | |||
| Total sample | 1287 | 2.150 (0.371–3.929)* | 2.016 (0.104–3.928)* |
| CVDs, no | 873 | 1.888 (−0.418 to 4.193) | 1.609 (−0.885 to 4.103) |
| CVDs, yes | 414 | 2.530 (−0.252 to 5.311) | 2.876 (−0.090 to 5.843) |
| Diabetes, no | 1088 | 2.785 (0.666–4.905)* | 2.545 (0.292–4.797)* |
| Diabetes, yes | 199 | 4.562 (0.263–8.861)* | 3.852 (−0.619 to 8.323) |
| Total tau, pg/mL | |||
| Total sample | 1287 | −0.102 (−0.208 to 0.003) | −0.074 (−0.188 to 0.039) |
| CVDs, no | 873 | −0.069 (−0.201 to 0.063) | −0.038 (−0.181 to 0.105) |
| CVDs, yes | 414 | −0.156 (−0.332 to 0.021) | −0.141 (−0.330 to 0.048) |
| Diabetes, no | 1088 | −0.141 (−0.266 to −0.015)* | −0.111 (−0.245 to 0.023) |
| Diabetes, yes | 199 | −0.002 (−0.258 to 0.255) | 0.078 (−0.180 to 0.337) |
| NfL, pg/mL, log‐transformed | |||
| Total sample | 1287 | 0.007 (−0.017 to 0.031) | 0.020 (−0.006 to 0.045) |
| CVDs, no | 873 | 0.003 (−0.026 to 0.033) | 0.015 (−0.017 to 0.046) |
| CVDs, yes | 414 | 0.009 (−0.034 to 0.052) | 0.024 (−0.021 to 0.069) |
| Diabetes, no | 1088 | −0.020 (−0.048 to 0.008) | −0.002 (−0.032 to 0.027) |
| Diabetes, yes | 199 | 0.047 (−0.014 to 0.108) | 0.060 (−0.002 to 0.122) |
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; CVD, cardiovascular disease; NfL, neurofilament light chain; TyG index, triglyceride–glucose index.
Data were β coefficients (95% confidence intervals) derived from the general linear regression models, in which the TyG index was considered an independent variable and plasma biomarkers a dependent variable. Model 1 was adjusted for age, sex, and education; Model 2 was additionally adjusted for APOE genotype, body mass index, current smoking, alcohol consumption, hypertension, hypercholesterolemia, and if applicable, for CVDs (coronary heart disease, heart failure, atrial fibrillation, and stroke) and use of glucose‐lowering drugs or insulin injection.
P < 0.05, ** P < 0.01, *** P < 0.001.
Stratified analysis by CVDs showed that controlling for all the potential confounders, the TyG index was significantly associated with an elevated plasma Aβ42, but not with plasma Aβ42/Aβ40 ratio, t‐tau, or NfL in both groups of people without and with CVDs. The association of high TyG index with increased plasma Aβ40 was significant among individuals without CVDs, but not among those with CVDs (Table 3).
The analysis stratified by diabetic status yielded significant associations of high TyG index with elevated plasma Aβ42 in both non‐diabetic and diabetic groups. A high TyG index was significantly associated with increased plasma Aβ40 and Aβ42/Aβ40 among diabetes‐free participants, but not among those with diabetes, after controlling for all the examined potential confounders. The TyG index was not significantly associated with plasma t‐tau or NfL regardless of diabetic status in Model 2 controlling for multiple potential confounders (Table 3).
When the analysis was performed by dementia status, a high TyG index was significantly associated with elevated plasma Aβ40 and Aβ42 only among dementia‐free participants, but not among those with dementia (Table 4).
TABLE 4.
Associations of the TyG index with plasma biomarkers by dementia status (n = 1287).
| TyG index | No. of subjects | β coefficient (95% confidence interval), plasma biomarkers | |
|---|---|---|---|
| Model 1 a | Model 2 a | ||
| Aβ40, pg/mL, log‐transformed | |||
| Dementia, no | 1145 | 0.016 (0.003–0.029)* | 0.021 (0.008–0.035)** |
| Dementia, yes | 142 | 0.007 (−0.029 to 0.043) | 0.029 (−0.011 to 0.069) |
| Aβ42, pg/mL | |||
| Dementia, no | 1145 | 0.748 (0.419–1.077)*** | 0.844 (0.490–1.197)*** |
| Dementia, yes | 142 | 0.864 (−0.374 to 2.102) | 1.324 (−0.029 to 2.676) |
| Aβ42/Aβ40 ratio (×1000) | |||
| Dementia, no | 1145 | 1.898 (−0.009 to 3.806) | 1.812 (−0.237 to 3.862) |
| Dementia, yes | 142 | 3.792 (−1.236 to 8.821) | 3.190 (−2.309 to 8.689) |
| Total tau, pg/mL | |||
| Dementia, no | 1145 | −0.091 (−0.201 to 0.019) | −0.061 (−0.180 to 0.058) |
| Dementia, yes | 142 | −0.224 (−0.577 to 0.130) | −0.180 (−0.565 to 0.204) |
| NfL, pg/mL, log‐transformed | |||
| Dementia, no | 1145 | 0.003 (−0.022 to 0.028) | 0.014 (−0.013 to 0.040) |
| Dementia, yes | 142 | 0.018 (−0.061 to 0.098) | 0.029 (−0.058 to 0.117) |
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; NfL, neurofilament light chain; TyG index, triglyceride–glucose index.
Data were β coefficients (95% confidence intervals) derived from the general linear regression models, in which the TyG index was considered an independent variable and plasma biomarkers a dependent variable. Model 1 was adjusted for age, sex, and education; Model 2 was additionally adjusted for APOE genotype, body mass index, current smoking, alcohol consumption, hypertension, hypercholesterolemia, cardiovascular diseases (i.e., coronary heart disease, heart failure, atrial fibrillation, and stroke) and use of glucose‐lowering drugs or insulin injection.
P < 0.05, ** P < 0.01, *** P < 0.001.
Finally, we did not detect any statistical interactions of the TyG index with CVDs, diabetes, or dementia on any of the examined plasma biomarkers (P for all interactions >0.05).
4. DISCUSSION
This large‐scale population‐based study of rural‐dwelling older adults in China suggested that a high TyG index was notably associated with an increased likelihood of dementia and AD as well as with elevated plasma Aβ independent of a range of potential confounders. We found no independent associations of TyG index with plasma t‐tau and NfL. These results indicate that insulin resistance, assessed with TyG index, is cross‐sectionally associated with dementia and AD in older adults, possibly via Aβ pathologies.
To the best of our knowledge, this was the first population‐based study that investigated the associations of the TyG index with dementia and peripheral biomarkers for amyloid and neurodegeneration among older adults. Previously, population‐based studies have examined insulin resistance measured with HOMA‐IR in association with dementia and AD. For instance, the cross‐sectional data from the Australian Imaging Biomarker and Lifestyle study showed that HOMA‐IR index was higher in persons with AD compared to cognitively normal adults. 7 Population‐based studies also suggested that a higher HOMA‐IR index was associated with poorer cognitive function. 7 , 27 , 28 In addition, a few prospective cohort studies showed that a high HOMA‐IR index was related to AD and cognitive decline. 17 , 18 , 19 The results from our study are in line with those from the studies using HOMA‐IR index as a marker for insulin resistance. Coincidently, the retrospective cohort study from South Korea that used data derived from the National Health Information Database (2009–2015) engaging people aged ≥40 years showed weak associations of the TyG index with dementia, AD, and VaD. 20 In that study, dementia and subtypes were defined according to the ICD‐10 codes and prescription of anti‐dementia medications. Our population‐based study engaged rural‐dwelling older adults, and dementia and subtypes of dementia were clinically diagnosed via comprehensive in‐person assessments following a standard diagnostic procedure. Of note, our study revealed a threshold for the TyG index that corresponded to approximately the 75th percentile of the TyG index, above which the TyG index was notably associated with elevated likelihoods of all‐cause dementia and AD independent of a range of potential confounders. We further revealed that the significant association of high TyG index with dementia remained among individuals who were free of either CVDs or diabetes. Taken together, our study provides additional evidence supporting the association of a high TyG index with an increased likelihood of dementia and AD in older adults.
Several pathogenic mechanisms might underlie the associations between insulin resistance and dementia. First, insulin resistance results in brain glucose hypometabolism, 29 which may further cause energetic deficits and neurotoxic protein accumulation, thus contributing to cognitive deterioration. 30 Second, insulin is a vasoactive hormone that regulates peripheral and cerebral blood flow. Insulin resistance‐mediated vasodilation could potentially lead to low cerebral perfusion and ischemic lesions, which may contribute to dementia. 31 Third, individuals with insulin resistance usually have endothelial dysfunction and are prone to hyperinsulinemia, hyperglycemia, hypertension, and dyslipidemia; all these factors have been linked with dementia. 32 Finally, insulin resistance reduces synaptic plasticity, promotes neuronal apoptosis, and facilitates tau phosphorylation and Aβ deposition. 8 , 9
To further understand the potential mechanisms linking the TyG index with dementia, we examined the relations of the TyG index with plasma AD biomarkers. We found that a high TyG index was significantly associated with increased peripheral Aβ (i.e., Aβ40, Aβ42, and Aβ42/Aβ40), but not with plasma t‐tau or NfL. To the best of our knowledge, this was the first population‐based study that linked insulin resistance, as indicated by a high TyG index, with peripheral AD biomarkers. Previous studies that examine the associations of HOMA‐IR with AD biomarkers in the central nervous system have yielded mixed results. 4 , 7 , 33 , 34 Differences in the study design, characteristics of the study sample, and detection methods of biomarkers may partially contribute to the discrepant results. The TyG index is a composite measure that integrates fasting blood glucose and triglycerides. Data from the Health and Aging Brain study among Latino Elders (HABLE) showed that both high fasting blood glucose and high triglycerides were associated with elevated plasma Aβ42 and Aβ40. 35 The cross‐sectional data from the Chinese Alzheimer's Biomarker and Lifestyle (CABLE) study showed that fasting blood glucose was positively associated with Aβ42 and Aβ42/Aβ40 ratio in cerebrospinal fluid among cognitively normal non‐diabetic elders. 36 In addition, data from the population‐based HABLE and Mayo Clinic Study of Aging did show that diabetes and dyslipidemia were associated with elevated plasma Aβ42 and Aβ40. 35 , 37 A case‐control study from Australia found that diabetes was associated with higher plasma Aβ42/Aβ40 ratio. 38 Our results appeared to be in good agreement with reports from these studies.
Current evidence supports that plasma Aβ42/Aβ40 ratio is a more reliable biomarker for brain amyloidosis than plasma Aβ42. 39 However, studies have suggested that the patterns of dynamic changes in peripheral Aβ might vary across the AD clinical continuum, with plasma Aβ being increased at the early pre‐clinical phase, followed by a decrease in the late stage. 40 , 41 In addition, a recent study showed that increased plasma Aβ42 and Aβ40 were correlated with Aβ accumulation in the brain at the early pre‐pathological stage. 41 In line with these findings, we found that a high TyG index was associated with elevated plasma Aβ42 and Aβ40 among dementia‐free participants, indicating that a high TyG index might be associated with brain Aβ pathology at the very early preclinical stage. Therefore, the relationships of the TyG index with plasma AD biomarkers deserve further investigation while taking into account the AD clinical spectrum.
Several potential mechanisms may explain the associations between the TyG index and plasma Aβ. First, insulin resistance causes defective Aβ clearance. Insulin and Aβ both are substrates of insulin‐degrading enzyme (IDE). Because IDE is more selective for insulin than Aβ, under the insulin‐resistant condition, Aβ degradation is impaired. 42 Second, peripheral organs (e.g., kidneys) are involved in Aβ catabolism and constitute potential Aβ clearance pathways. 43 However, insulin resistance could greatly contribute to chronic kidney disease, 44 which may compromise the efficiency of Aβ clearance and result in an elevation of circulating Aβ42 and Aβ40. 35 , 37 Finally, insulin resistance may promote Aβ production. Aβ is derived from the proteolytic cleavage of amyloid precursor protein (APP), which is expressed not only in brain cells but also in peripheral cells, such as platelets. 43 In fact, platelets are the primary source (≈90%) of Aβ peptides in human blood. 45 Insulin resistance can cause platelet hyperactivity, 46 which contributes to Aβ overproduction. 43 , 45
Our community‐based study engaged rural‐dwelling older adults in China, a demographic group that has been substantially underrepresented in dementia and AD research. In addition, the interdisciplinary MIND‐China database that integrated the TyG index, a surrogate marker of insulin resistance, with comprehensive clinical data and plasma AD biomarkers assessed with the state‐of‐the‐art Simoa technology provides the unique opportunity to investigate the associations of insulin resistance with cognitive outcomes and the potential neuropathological mechanisms underlying their associations. However, our study also has limitations. First, the cross‐sectional design does not allow us to infer a causal relationship for any of the observed associations, and the observed cross‐sectional associations may be subject to selective survival bias. Second, although the TyG index is considered a credible surrogate marker for insulin resistance, 13 , 14 it may be affected by dietary habits. Third, plasma Aβ levels might reflect only to a certain extent the Aβ aggregation in the brain due to its peripheral generation, degradation by circulating enzymes, and metabolism in peripheral organs (e.g., liver and kidneys), and we could not explore the potential associations of the TyG index with tau pathology due to lack of relevant plasma biomarkers (e.g., phosphorylated tau [p‐tau]181, p‐tau217, and p‐tau231). Fourth, we had limited statistical power to detect the weak‐to‐moderately strong associations of the TyG index with dementia and plasma biomarkers among some subgroups. Fifth, due to the lack of AD biomarkers in the central nervous system, we could not define biological AD dementia or examine the associations of theh TyG index with plasma Aβ according to the absence and presence of Aβ pathology in the brain. Finally, our study sample was derived only from one rural region in western Shandong province, which should be kept in mind when generalizing our research findings to other populations.
In conclusion, this population‐based study of rural‐dwelling older adults in China showed evidence supporting the associations of insulin resistance, as indicated by high TyG index, with all‐cause dementia and AD as well as with plasma biomarkers for amyloid (Aβ40, Aβ42, and Aβ42/Aβ40 ratio), but not for neurodegeneration (t‐tau and NfL). These results suggest that the TyG index in older adults may be a readily available clinical marker for dementia and AD and that amyloid pathology may underlie their association. Future longitudinal studies are warranted to elucidate the potential causal relationships of the TyG index with cognitive phenotypes as well as the underlying mechanisms.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
The MIND‐CHINA protocol was approved by the ethics committee at Shandong Provincial Hospital affiliated to Shandong University in Jinan, Shandong, China. Written informed consent was obtained from all participants, or in the case of cognitively impaired persons, from a proxy (usually a guardian or a family member).
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank all participants of the MIND‐China project as well as our research staff at the Yanlou Town Hospital and the Department of Neurology, Shandong Provincial Hospital for their collaboration in data collection and management. This work was supported by the Brain Science and Brain‐like Intelligence Technology Research and Development Projects of China (2021ZD0201808 and 2021ZD0201801), the National Natural Science Foundation of China (82200980, 82011530139, 81861138008, 81772448), the Shandong Provincial Natural Science Foundation of China (ZR2021QH240 and ZR2021MH392), the National Key R&D Program of China Ministry of Sciences and Technology (2017YFC1310100), the Alzheimer's Association Grant (AACSFD‐22‐922844), the Integrated Traditional Chinese and Western Medicine Program in Shandong Province (YXH2019ZXY008), and the Academic Promotion Program of Shandong First Medical University (2019QL020). L.J. Launer is supported by the Intramural Research Program, National Institute on Aging, Maryland, USA. C. Qiu received grants from the Swedish Research Council (2017‐05819 and 2020‐01574); the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) CH2019‐8320 for the Joint China‐Sweden Mobility program; and the Karolinska Institutet (2018‐01854 and 2020‐01456), Stockholm, Sweden. The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Tian N, Fa W, Dong Y, et al. Triglyceride–glucose index, Alzheimer's disease plasma biomarkers, and dementia in older adults: The MIND‐China study. Alzheimer's Dement. 2023;15:e12426. 10.1002/dad2.12426
Contributor Information
Tingting Hou, Email: houtingting@sdfmu.edu.cn.
Yifeng Du, Email: du-yifeng@hotmail.com.
REFERENCES
- 1. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tumminia A, Vinciguerra F, Parisi M, Frittitta L. Type 2 diabetes mellitus and Alzheimer's disease: role of insulin signalling and therapeutic implications. Int J Mol Sci. 2018;19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF‐1 resistance, IRS‐1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle‐aged adults. Alzheimers Dement. 2015;11(5):504–510 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan ZS, Beiser AS, Fox CS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle‐aged adults: the Framingham Offspring Study. Diabetes Care. 2011;34(8):1766‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willette AA, Xu G, Johnson SC, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle‐aged adults. Diabetes Care. 2013;36(2):443‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laws SM, Gaskin S, Woodfield A, et al. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci Rep. 2017;7(1):9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63(7):2232‐2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sedzikowska A, Szablewski L. Insulin and insulin resistance in Alzheimer's disease. Int J Mol Sci. 2021;22(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214‐23. [DOI] [PubMed] [Google Scholar]
- 11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. [DOI] [PubMed] [Google Scholar]
- 12. Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride‐glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simental‐Mendia LE, Rodriguez‐Moran M, Guerrero‐Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299‐304. [DOI] [PubMed] [Google Scholar]
- 14. Guerrero‐Romero F, Simental‐Mendia LE, Gonzalez‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347‐3351. [DOI] [PubMed] [Google Scholar]
- 15. Vasques AC, Novaes FS, de Oliveira Mda S, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98‐e100. [DOI] [PubMed] [Google Scholar]
- 16. Park HM, Lee HS, Lee YJ, Lee JH. The triglyceride‐glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. 2021;180:109042. [DOI] [PubMed] [Google Scholar]
- 17. Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75(22):1982‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hooshmand B, Rusanen M, Ngandu T, et al. Serum insulin and cognitive performance in older adults: a longitudinal study. Am J Med. 2019;132(3):367‐373. [DOI] [PubMed] [Google Scholar]
- 19. Ennis GE, Koscik RL, Ma Y, et al. Insulin resistance is related to cognitive decline but not change in CSF biomarkers of Alzheimer's disease in non‐demented adults. Alzheimers Dement (Amst). 2021;13(1):e12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong S, Han K, Park CY. The insulin resistance by triglyceride glucose index and risk for dementia: population‐based study. Alzheimers Res Ther. 2021;13(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Han X, Zhang X, et al. Health status and risk profiles for brain aging of rural‐dwelling older adults: data from the interdisciplinary baseline assessments in MIND‐China. Alzheimers Dement (N Y). 2022;8(1):e12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM‐IV). American Psychiatric Association; 1994. [Google Scholar]
- 24. Liu R, Ren Y, Hou T, et al. Associations of sleep timing and time in bed with dementia and cognitive decline among Chinese older adults: a cohort study. J Am Geriatr Soc. 2022;70(11):3138‐3151. [DOI] [PubMed] [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43(2):250‐260. [DOI] [PubMed] [Google Scholar]
- 27. Ekblad LL, Rinne JO, Puukka PJ, et al. Insulin resistance is associated with poorer verbal fluency performance in women. Diabetologia. 2015;58(11):2545‐2553. [DOI] [PubMed] [Google Scholar]
- 28. Benedict C, Brooks SJ, Kullberg J, et al. Impaired insulin sensitivity as indexed by the HOMA score is associated with deficits in verbal fluency and temporal lobe gray matter volume in the elderly. Diabetes Care. 2012;35(3):488‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle‐aged adults at risk for Alzheimer disease. JAMA Neurol. 2015;72(9):1013‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cunnane SC, Trushina E, Morland C, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19(9):609‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hughes TM, Craft S. The role of insulin in the vascular contributions to age‐related dementia. Biochim Biophys Acta. 2016;1862(5):983‐991. [DOI] [PubMed] [Google Scholar]
- 32. Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. The Lancet Neurology. 2020;19(9):758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoscheidt SM, Starks EJ, Oh JM, et al. Insulin resistance is associated with increased levels of cerebrospinal fluid biomarkers of Alzheimer's disease and reduced memory function in at‐risk healthy middle‐aged adults. J Alzheimers Dis. 2016;52(4):1373‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thambisetty M, Jeffrey Metter E, Yang A, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70(9):1167‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Bryant SE, Petersen M, Hall J, Johnson LA. Medical comorbidities and ethnicity impact plasma Alzheimer's disease biomarkers: important considerations for clinical trials and practice. Alzheimers Dement. 2023;19(1):36‐43. [DOI] [PubMed] [Google Scholar]
- 36. Ou YN, Shen XN, Hu HY, et al. Fasting blood glucose and cerebrospinal fluid Alzheimer's biomarkers in non‐diabetic cognitively normal elders: the CABLE study. Aging (Albany NY). 2020;12(6):4945‐4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Syrjanen JA, Campbell MR, Algeciras‐Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18(6):1128‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peters KE, Davis WA, Taddei K, et al. Plasma amyloid‐β peptides in type 2 diabetes: a matched case‐control study. J Alzheimers Dis. 2017;56(3):1127‐1133. [DOI] [PubMed] [Google Scholar]
- 39. Brand AL, Lawler PE, Bollinger JG, et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer's disease: a literature review. Alzheimers Res Ther. 2022;14(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ritchie K, Carrière I, Berr C, et al. The clinical picture of Alzheimer's disease in the decade before diagnosis: clinical and biomarker trajectories. J Clin Psychiatry. 2016;77(3):e305‐11. [DOI] [PubMed] [Google Scholar]
- 41. Botella Lucena P, Vanherle S, Lodder C, et al. Blood‐based Aβ42 increases in the earliest pre‐pathological stage before decreasing with progressive amyloid pathology in preclinical models and human subjects: opening new avenues for prevention. Acta Neuropathol. 2022;144(3):489‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boccardi V, Murasecco I, Mecocci P. Diabetes drugs in the fight against Alzheimer's disease. Ageing Res Rev. 2019;54:100936. [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease ‐ insights from amyloid‐β metabolism beyond the brain. Nat Rev Neurol. 2017;13(10):612‐623. [DOI] [PubMed] [Google Scholar]
- 44. Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Haring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721‐737. [DOI] [PubMed] [Google Scholar]
- 45. Carbone MG, Pagni G, Tagliarini C, Imbimbo BP, Pomara N. Can platelet activation result in increased plasma Abeta levels and contribute to the pathogenesis of Alzheimer's disease? Ageing Res Rev. 2021;71:101420. [DOI] [PubMed] [Google Scholar]
- 46. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


