Abstract
The prognosis for patients with metastatic melanoma (MM) involving distant organs is grim, and treatment resistance is potentiated by tumor-initiating cells (TICs) that thrive under hypoxia. MM cells, including TICs, express a unique glycome featuring i-linear poly-N-acetyllactosamines through the loss of I-branching enzyme, β1,6 N-acetylglucosaminyltransferase 2. Whether hypoxia instructs MM TIC development by modulating the glycome signature remains unknown. In this study, we explored hypoxia-dependent alterations in MM glycome–associated genes and found that β1,6 N-acetylglucosaminyltransferase 2 was downregulated and a galectin (Gal)-8eligand axis, involving both extracellular and cell-intrinsic Gal-8, was induced. Low β1,6 N-acetylglucosaminyltransferase 2 levels correlated with poor patient outcomes, and patient serum samples were elevated for Gal-8. Depressed β1,6 N-acetylglucosaminyltransferase 2 in MM cells upregulated TIC marker, NGFR/CD271, whereas loss of MM cell–intrinsic Gal-8 markedly lowered NGFR and reduced TIC activity in vivo. Extracellular Gal-8 bound preferentially to i-linear poly-N-acetyllactosamines on N-glycans of the TIC marker and prometastatic molecule CD44, among other receptors, and activated prosurvival factor protein kinase B. This study reveals the importance of hypoxia governing the MM glycome by enforcing i-linear poly-N-acetyllactosamine and Gal-8 expression. This mechanistic investigation also uncovers glycome-dependent regulation of pro-MM factor, NGFR, implicating i-linear poly-N-acetyllactosamine and Gal-8 as biomarkers and therapeutic targets of MM.
INTRODUCTION
Although a diagnosis of metastatic melanoma (MM) portends a dismal prognosis (Balch et al., 2001; Sandru et al., 2014), recent advances in immune checkpoint inhibition therapy have led to promising and more durable responses. However, the 5-year survival rate remains ~37% (Larkin et al., 2019; Robert et al., 2019) owing to therapy resistance (Franklin et al., 2017; Jenkins et al., 2018; Jessurun et al., 2017; Larkin et al., 2015; Robert et al., 2015). Thus, there is a vital need for predictive biomarkers of therapeutic response and innovative therapeutic targets to augment current treatments. Although various genetic alterations and neoantigen evolution have dominated research investigations (Jiang et al., 2019), glycosylation alterations and the interaction of glycans with tumor microenvironmental factors have received little attention. Glycosylation, one of the most common modifications of proteins (Shental-Bechor and Levy, 2009), entails the addition of sugar chains, known as glycans. All cell surface membrane proteins contain N- and/or O-linked glycans built on asparagine and/or serine/threonine residues, respectively. Even intracellular proteins, membrane lipids, and ribonucleic acids have been reported to be glycosylated and deemed critical to their function (Flynn et al., 2021; Reily et al., 2019). Although glycans enforce molecular function, our knowledge of their roles as binding moieties to glycan-binding proteins or lectins in cancer progression pathways is incomplete.
β-Galactoside-binding lectins, known as galectins (Gals), have both microenvironmental and intracellular functions in normal immune, stromal, and parenchymal tissues and in cancer. There are 15 known Gals in humans, whose intracellular functions range from pre-mRNA splicing and inducing proapoptosis and antiapoptosis activity to regulating autophagy (Hong et al., 2021; Hsu et al., 2015). They lack a canonical signal secretion sequence and are routed to the extracellular surface in nonclassical secretion pathways (Popa et al., 2018). In the extracellular milieu, Gal-1, −3, and −9 bind cell surface glycans and alter cellular signaling activities that control proliferation, death, migration, and other effector functions (Farhadi and Hudalla, 2016; Giovannone et al., 2018) depending on cell type. Cancer-associated Gal-dependent activities are governed by Gal expression level and/or the glycan phenotype on the cancer cell surface (Feizi, 1985; Fuster and Esko, 2005) orchestrated by dysregulated expression of glycosyltransferases, such as MGAT5, MGAT3, ST6GalNAc-2, FUT8, and ST6Gal-I (Chakraborty et al., 2018; Stowell et al., 2015; Varki et al., 2015). Recent findings from our laboratory show that loss of I-branching β1,6 N-acetylglucosaminyltransferase 2 (GCNT2) with commensurate loss of I-branched poly-N-acetyllactosamines (poly-LacNAcs) yields elevated i-linear poly-LacNAcs and increased MM aggressiveness, prosurvival signaling, and growth in vivo (Dimitroff, 2019; Sweeney et al., 2018). The role of MM cell i-linear poly-LacNAcs, which are putative Gal-binding moieties, in metastatic progression and how the MM glycome is established are ill defined and the impetus for this investigation.
In MM, hypoxia is a prominent microenvironmental stressor attributing to the generation and persistence of tumor-initiating cells (TICs) (Alison et al., 2011; Fang et al., 2005). TICs help to confer immune evasion; self-renewal potential; and resistance to therapy, including immune checkpoint inhibition therapy (Zhou et al., 2021). Although research on hypoxia’s influence on metastasis has mainly focused on the genomic and proteomic pathways leading to HIF1α (Song et al., 2020) expression, few investigations delve into how hypoxia impacts the cancer glycome (Arriagada et al., 2019). Given that MMs feature a low tissue oxygen tension of only 1.5% (McKeown, 2014), exploring hypoxia-driven MM TIC generation in concert with the MM glycome signature is critical for understanding MM progression and therapy development.
In this study, we explored the influence of hypoxia on global glycome alterations in MM cells, including the signature MM glycome featuring loss of GCNT2/I-branching, and whether hypoxia-dependent glycome events altered TIC development. In MM cells subjected to hypoxia, global transcriptional and N-glycomic profiling revealed several dysregulated glycome-related genes and enhanced i-linear poly-LacNAc expression. Of these alterations, including downregulation of GCNT2 and I-branched poly-LacNAcs, there was significant upregulation of Gal-8 directly corresponding to the expression of a key TIC factor, NGFR/CD271, known to enhance MM progression and therapy resistance (Jiang et al., 2020). GCNT2 expression on patient melanomas was predictive of patient survival, and Gal-8 levels were elevated in the sera of patients with melanoma compared with those of healthy controls. Using GCNT2-enforced and -silenced MM cell variants, we found that low GCNT2 expression increased TIC marker levels and in vivo tumor-initiating potential. Importantly, MM cell NGFR expression inversely correlated with GCNT2 expression. Gal-8 incubation with MM cells elevated NGFR, whereas Gal-8 silencing dampened NGFR expression, even under hypoxia, and reduced tumor-forming activity in vivo. Furthermore, Gal-8 bound preferentially to MM cells with depressed GCNT2/I-branching and high i-linear poly-LacNAcs levels. Gal-8 affinity chromatography and proteomics analysis identified prometastatic and TIC marker CD44 as a major cell surface Gal-8 ligand, which was dependent on i-linear poly-LacNAc N-glycans for Gal-8 binding. Extracellular Gal-8 binding to i-linear poly-LacNAchi MM cells increased protein kinase B (Akt) phosphorylation, which is known to promote tumor cell survival and is a downstream target of various activating cell surface receptors, including CD44. Interestingly, NGFR loss in Gal-8–silenced MM cells was not rescued by exogenous Gal-8 binding, suggesting that extracellular and intracellular Gal-8 expression may both play key roles in promoting MM TIC potential. Taken together, this study shows the importance of hypoxia in governing the MM glycome to promote TIC formation and provides evidence for GCNT2/I-branching loss and elevated Gal-8 as biomarkers of MM.
RESULTS
Hypoxia globally altered MM glycobiology and Gal-8 expression
To assess hypoxia-dependent MM glycome alterations in glycan-binding lectins, glycan biosynthesis, and glycan degradation pathways, human A375, A2058, and SK-Mel-5 MM cells were cultured under normoxia or hypoxia and analyzed by RNA sequencing (Figure 1a). We observed upregulated and downregulated glycome genes shared among all (three) cell lines (Supplementary Table S1). Notably, among putative melanoma glycan-binding lectins, Gal-8 expression was significantly upregulated under hypoxia. Hypoxia induction was confirmed by observing elevated HIF1α expression (Figure 1b). In addition to significant Gal-8 upregulation, we observed concomitant downregulation of I-branching GCNT2, along with significant elevations in known TIC markers KLF4 and NGFR, by RT-qPCR (P < 0.01) (Figure 1c and d). Significant elevations in surface expression of Gal-8 and MM glycome i-linear poly-LacNAcs were also evidenced by flow cytometry with anti–i-linear poly-LacNAc mAb Osk28 of MM cells grown under hypoxia (P < 0.01) (Figure 1e–h). To confirm carbohydrate-dependent Gal-8-binding, lactose or sucrose was added to control groups before analysis by flow cytometry (Supplementary Figure S1).
Figure 1. Hypoxia-induced global alterations in glycosylation and glycome-associated genes.
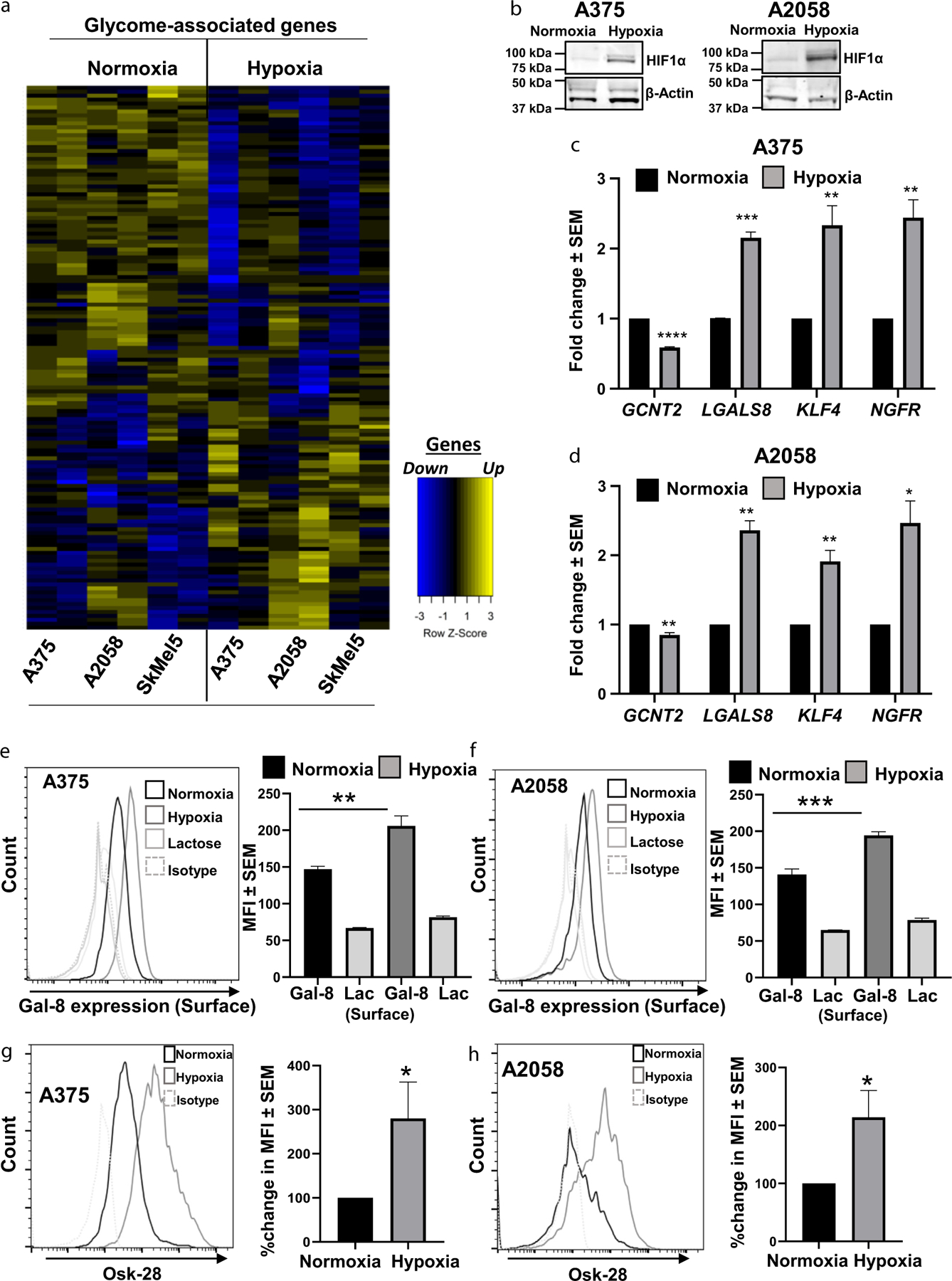
(a) RNA-sequencing analysis of glycosylation and glycome-associated genes of A375, A2058, and SkMel5 cells cultured under normoxia or hypoxia. (b) HIF1α immunoblot for validation of hypoxia induction in A375 and A2058 cells. Validation of GCNT2 downregulation and Gal-8, KLF4, and NGFR upregulation under hypoxia in (c) A375 and (d) A2058 by RT-qPCR. Flow cytometric analysis of Gal-8 expression on (e) A375 and (f) A2058 cells grown under normoxia or hypoxia. Flow cytometric analysis of i-linear poly-LacNAc expression using OSK-28 antibody on (g) A375 and (h) A2058 cells grown under normoxia or hypoxia. At least four biological replicates were performed. ***P < 0.001 **P < 0.01, and *P < 0.05. Gal-8, galectin 8; GCNT2, β1,6 N-acetylglucosaminyltransferase 2; poly-LacNAc, poly-N-acetyllactosamine.
To further assess glyco-structural elevations in i-linear poly-LacNAcs under hypoxia, N-glycans from MM cells cultured under normoxia or hypoxia were analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Glycans of human MM A375, A2058, and SK-MEL-5 cells grown under normoxia or hypoxia consisted of high mannose (data not shown) and complex N-glycan structures with extensive poly-LacNAc repeating units (reaching up to 11 total LacNAc units; m/z 8093) terminated mainly in N-acetylneuraminic acid residues and minor antennal fucosylation (Supplementary Figure S2). However, partial annotated matrix-assisted laser desorption ionization-time of flight mass spectrometry spectra of high-mass N-glycans from A375 and A2058 cells under normoxia revealed that A375 cells contained mainly i-linear poly-LacNAcs (Figure 2a), whereas A2058 cells displayed principally I-branched poly-LacNAcs (Figure 2b). These assignments were based on N-glycan molecular ion matrix-assisted laser-desorption/ionization time-of-flight/time-of-flight mass spectrometry, which helps to differentiate i-linear from I-branched poly-LacNAcs. The ratio of relative abundance of fragment ions corresponding to loss of two and three LacNAc repeats was characteristic of i-linear or I-branched poly-LacNAcs (Figure 2, gray inset, peaks b and c, respectively): a ratio of b/c >1 corresponded to i-linear poly-LacNAcs, whereas a ratio <1 corresponded to I-branched poly-LacNAcs. This relationship is based on the assumption that fragmentation of i-linear poly-LacNAcs and relative abundance of either two or three LacNAc units is relatively proportionate (Figure 2, gray inset, peaks b and c, upper panel), whereas fragmentation of I-branched poly-LacNAcs did not result in fragments containing two LacNAcs, and the relative abundance of the b fragment was much less than that of fragment c (Figure 2, gray inset, peaks b and c, lower panel). The molecular ion at m/z 6,384 from A375 cells exhibited a relative abundance of fragmented ions corresponding to the loss of two LacNAc repeats at m/z 5110 that was higher than those ions corresponding to three LacNAc repeats (m/z 4,661), which was indicative of i-linear poly-LacNAcs (Figure 2c). On the contrary, the same molecular ion from A2058 cells displayed a relative abundance of fragment ions at m/z 5,110 that was lower than the relative abundance of ions at m/z 4,661, suggesting that the ion at m/z 6,384 consisted mainly of I-branched poly-LacNAcs (Figure 2d). Under hypoxia, although LacNAc repeats in poly-LacNAcs per N-glycan antenna were not markedly changed (Supplementary Figure S2), there was a noted increase in the ratio of i-linear to I-branched poly-LacNAcs at m/z 6,384 of A375 cells (Figure 2e). Similarly, on A2058 cells grown under hypoxia, the ratio of relative abundance of fragment ions at m/z 5,110–4,661 was elevated compared with the levels on cells grown under normoxia (Figure 2d and f). These flattening of the V patterns indicative of increased i-linear poly-LacNAcs were also observed for other N-glycan species and were additionally observed on N-glycan spectra from human MM SK-MEL-5 cells (data not shown). Altogether, these results suggested that hypoxia helps to orchestrate signature i-linear poly-LacNAc structures on MM cells.
Figure 2. Mass spectrometry analysis confirmed increased i-linear poly-LacNAcs on MM cells cultured under hypoxia.
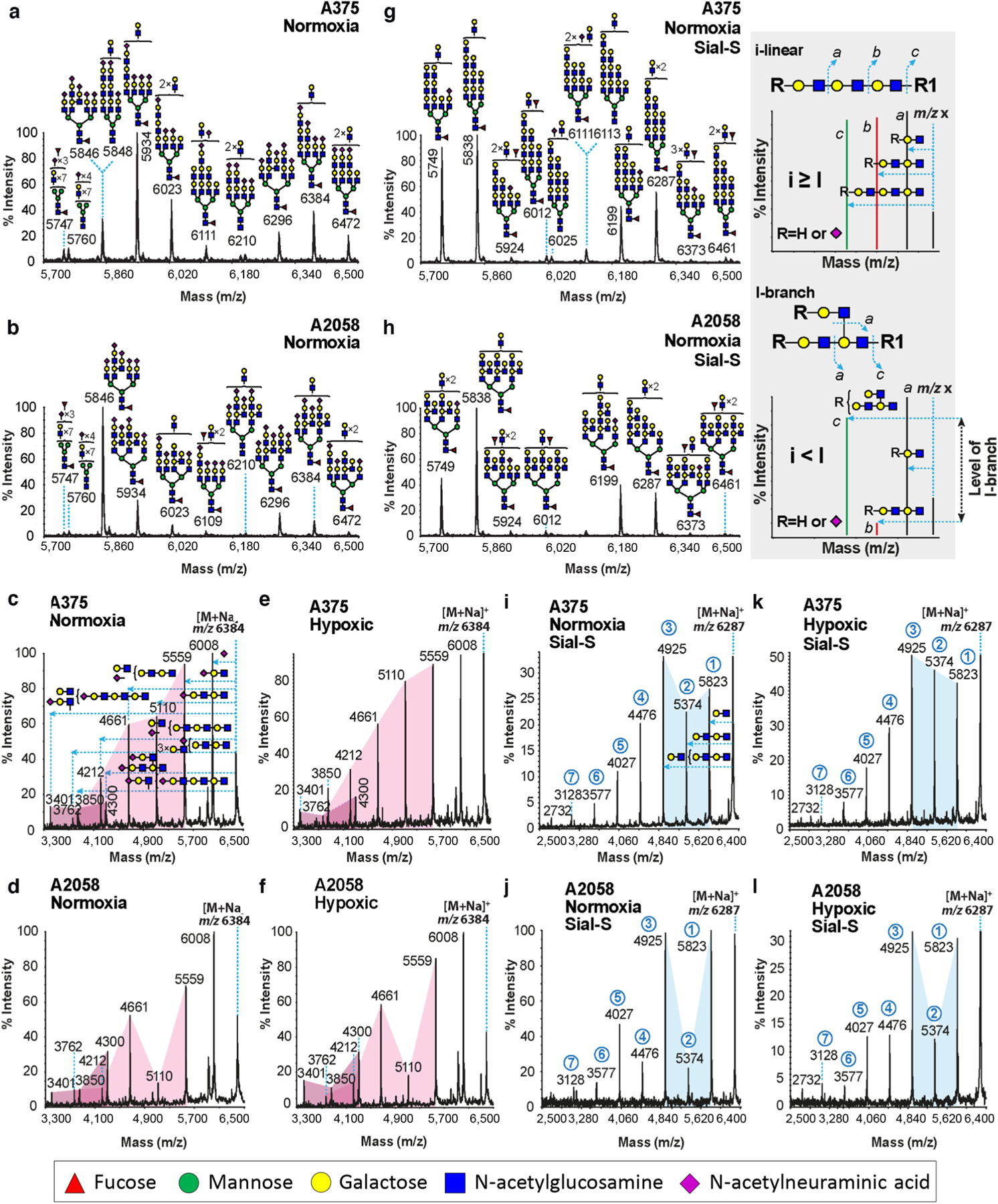
Partial MALDI-TOF MS spectra of permethylated N-glycans of (a) A375 and (b) A2058 under normal oxygen conditions. MALDI-TOF/TOF MS/MS spectra of the molecular ion at m/z 6,384 from (c) A375 and (d) A2058 grown under normal oxygen conditions and derived from a and b, respectively, or grown under hypoxic conditions (e and f, respectively). Pink highlighted areas were inserted to assist clarity. Partial MALDI-TOF MS spectra of permethylated N-glycans of (g) A375 and (h) A2058 after Sial-S (α2,3NeuAcs) digestion and under normal oxygen conditions. MALDI-TOF/TOF MS/MS spectra of the molecular ion at m/z 6,287 from (i) A375 and (j) A2058 grown under normal oxygen conditions and derived from g and h, respectively, or grown under hypoxic conditions (k and l, respectively). The Blue highlighted area was inserted to assist clarity. Circled numbers in blue correspond to the number of poly-LacNAc repeat losses from the [M+Na]+ molecular ion. (c) Horizontal blue dashed lines with an arrowhead correspond to the loss of the corresponding structure from the molecular ion. All fragment ions are sodium adducts. Gray inset: MALDI-TOF/TOF MS/MS of hypothetical ions detected at m/z x. The main fragment ion that differentiates the i-linear poly-LacNAcs N-glycan structures from the I-branched poly-LacNAcs is the fragment ion that corresponds to a loss of two LacNAc repeats, either undecorated or with a NeuAc residue on the nonreducing side (red peak b). When the relative abundance of b is compared with the relative abundance of the fragment ion that corresponds to three LacNAc repeats (green peak c), a relative characterization of whether the poly-LacNAcs are either in i-linear or I-branched format can be deduced on the basis of the ratio b to c. When this ratio (b/c) is >1, the poly-LacNAcs are principally in an i-linear format (upper panel), whereas when this ratio is <1, then the poly-LacNAcs are in I-branched format (lower panel). Intermediate values indicate intermediate states. MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry; MALDI-TOF/TOF MS/MS, matrix-assisted laser-desorption/ionization time-of-flight/time-of-flight mass spectrometry; MM, metastatic melanoma; NeuAc, N-acetylneuraminic acid; poly-LacNAc, poly-N-acetyllactosamine; Sial-S, α2,3 sialidase-S.
To determine whether hypoxia influenced sialylation on N-glycans, matrix-assisted laser desorption ionization-time of flight mass spectrometry and matrix-assisted laser-desorption/ionization time-of-flight/time-of-flight mass spectrometry were conducted on N-glycans digested with α2,3 sialidase-S from A375 and A2058 cells cultured under normoxia or hypoxia. Cumulative data indicated that there were no major differences in the abundance of α2,3-N-acetylneuraminic acid residues and that the majority of the N-acetylneuraminic acid residues at the termini of poly-LacNAcs were α2,3-linked N-acetylneuraminic acids (Supplementary Figure S3). Partial annotated high mass N-glycan spectra after sialidase-S digestion from A375 and A2058 cells did not show any alteration in the abundance of i-linear or I-branched poly-LacNAcs, respectively (Figure 2g and h). Furthermore, compared with sialidase-S–treated N-glycan at m/z 6,287 from cells grown in normoxia (Figure 2i and j), sialidase-S–treated N-glycans from cells grown under hypoxia also exhibited increases in the relative abundance of i-linear LacNAcs (Figure 2k and l). These data suggested that α2,3 sialylation of N-glycans was not affected by hypoxia and did not neutralize hypoxia-dependent induction of i-linear poly-LacNAcs.
Absence of GCNT2 correlated with poor outcomes in patients with MM and increased MM cell TIC characteristics, and Gal-8 was elevated in the serum of patients with melanoma
To assess the consequences of GCNT2 expression in patients with MM and disease outcomes, 64 samples from patients with MM who died from melanoma were stained for GCNT2 by immunohistochemistry. Stained slides were grouped into no, light, moderate, or strong staining categories. Patients with GCNT2 expression from no to light staining levels exhibited significantly decreased survival compared with patients with strong GCNT2 expression (P < 0.04) (Figure 3a and b). The clinical significance of Gal-8 in melanoma was elucidated by analyzing serum samples from patients with melanoma (n = 13) for Gal-8 levels compared with those from normal controls by ELISA. There was a significant increase in Gal-8 levels in patient sera (P < 0.05) (Figure 3c).
Figure 3. Patients with melanoma with a poor outcome had low GCNT2 levels, the sera from patients with melanoma were elevated for Gal-8, and GCNT2 loss increased the expression of TIC markers on MM cells.
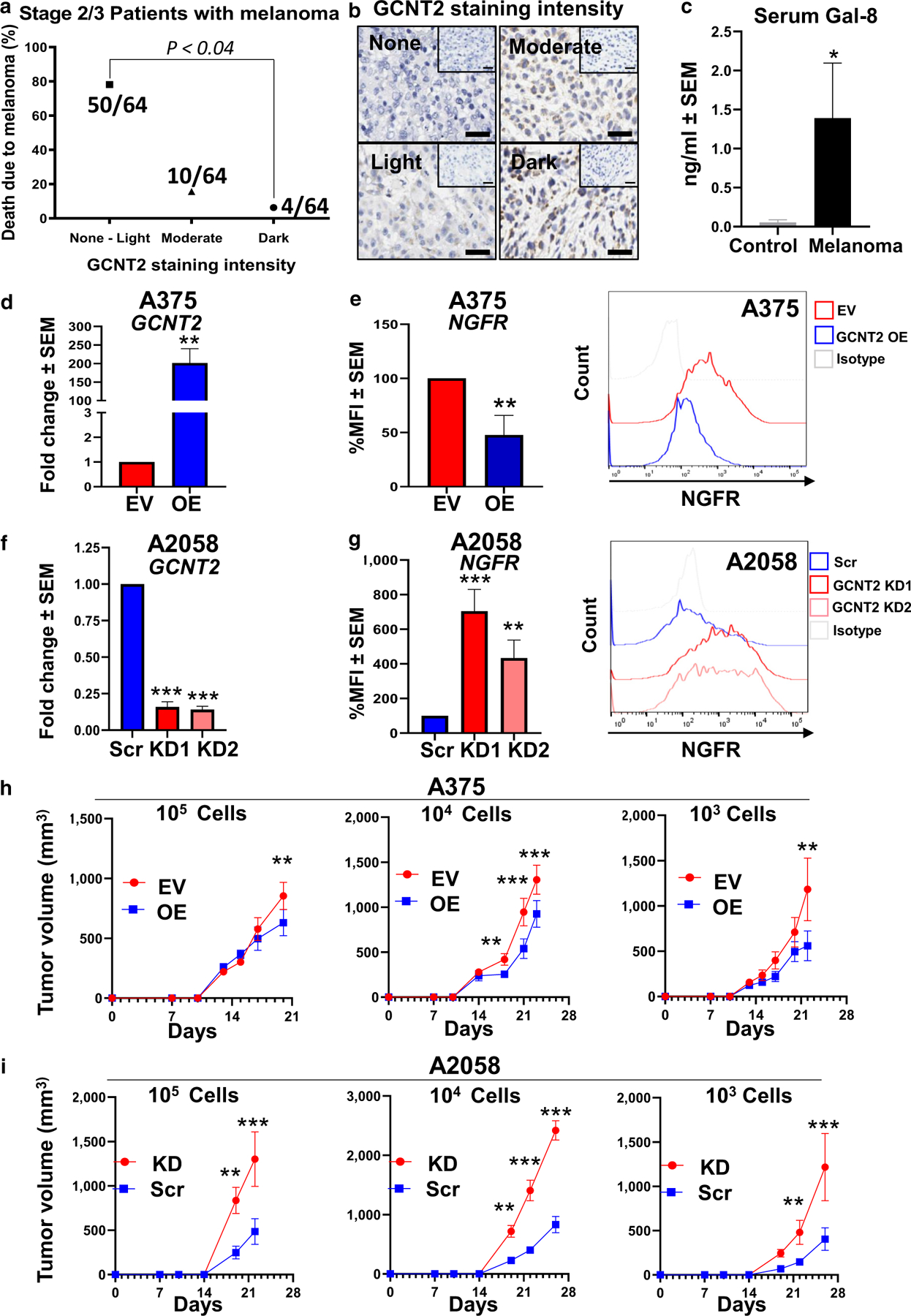
(a) IHC analysis of GCNT2 expression in patient melanomas. GCNT2 expression score on melanoma (n = 64) (range = 0–3: 0 = no staining, 1 = light staining, 2 = moderate staining, and 3 = dark staining) plotted against patient mortality outcome. (b) Representative IHC image. (c) ELISA of Gal-8 was performed on sera from normal healthy volunteers (n = 5) and patients with MM (n = 13). RT-qPCR analysis of GCNT2 in GCNT2-OE and -KD (d) A375 and (f) A2058 cells. Flow cytometry of NGFR on (e) GCNT2 EV and OE A375 cells and on (g) GCNT2 Scr and KD A2058 cells. In vivo limiting dilution assay of (h) A375 EV/OE cells and (i) A2058 Src/KD using cell numbers ranging from 105 to 103. At least four biological replicates were performed. ***P < 0.001, **P < 0.01, and *P < 0.05. EV, empty vector; Gal-8, galectin 8; GCNT2, β1,6 N-acetylglucosaminyltransferase 2; IHC, immunohistochemistry; KD, knockdown; MM, metastatic melanoma; OE, overexpressed; Scr, scrambled; TIC, tumor-initiating cell.
To explore the pathobiological consequences of GCNT2 loss in MM, we utilized human MM A375 (low GCNT2 expression) and A2058 (moderate GCNT2 expression) cells engineered to overexpress or silence GCNT2. Corresponding overexpressed (OE) or short hairpin RNA knockdown (KD) GCNT2 variants in A375 and A2058 cells, including their empty vector and short hairpin RNA scrambled control (Scr) variants, respectively, were generated by lentiviral transduction, and GCNT2 expression was validated by RT-qPCR (Figure 3d and f). We first analyzed the expression of a common marker of MM-TICs, NGFR/CD271 (Boiko et al., 2010; Jiang et al., 2020). GCNT2-OE cells exhibited significantly decreased (P < 0.01) (Figure 3e), whereas GCNT2-KD1 and -KD2 cells expressed increased NGFR expression (P < 0.01) (Figure 3g). Furthermore, in vivo limiting dilution tumor-forming assays were performed in NOD-SCID IL-2Rγ–deficient mice using GCNT2-engineered MM cells. A375 GCNT2-OE and A2058 GCNT2-KD cells were injected subcutaneously at 103–105 cells/mouse along with their respective controls (empty vector and Scr). Lower GCNT2 expression led to increased growth potential even at 103, whereas increased GCNT2 expression hindered tumor-forming rate even at 105 cells/mouse (P < 0.01) (Figure 3h and i). Together, these results suggested that GCNT2 could regulate TIC potential in MM cells.
To explore the relationship between elevated Gal-8 levels and MM TIC generation, A375 and A2058 MM cell lines were incubated with recombinant human Gal-8, and NGFR expression was analyzed by immunoblotting. There was a significant elevation in NGFR in cells incubated with Gal-8 (P < 0.05) (Figure 4a and b). Furthermore, to determine whether intrinsic Gal-8 could alter NGFR expression, A375 and A2058 cells silenced for Gal-8 expression (KD) by short hairpin RNA technology were analyzed by RT-qPCR. Compared with that of short hairpin RNA Scr control cells, silencing of Gal-8 in KD cells was confirmed by RT-qPCR (Figure 4c and e). A375 and A2058 Scr and Gal-8 KD cells were cultured under normoxia or hypoxia and then assessed for NGFR induction. Under hypoxia, whereas NGFR was elevated in Scr control cells (Figure 4d, f, g, and h), Gal-8 KD cells did not upregulate NGFR. These data implicate both hypoxia-dependent and -independent roles of Gal-8 in NGFR regulation. In accordance with the downregulation of NGFR by silencing Gal-8, Gal-8 KD cells formed tumors at a significantly less rate than Scr cells in vivo (Figure 4i) (P < 0.01). Furthermore, whereas exogenous Gal-8 significantly upregulated NGFR in Scr cells, incubating Gal-8 with Gal-8 KD MM cells was not able to rescue NGFR expression (Figure 4j), indicating a dual role of Gal-8 as an extracellular (outside-in) and intracellular modulator of NGFR expression. In pan-Gal inhibitor lactose controls, we noted incomplete diminution that is often observed in signaling assessments (Chakraborty et al., 2021). Hence, although it is an appropriate control for short-term lectin-binding assays, lactose may not be the most ideal Gal neutralizer in signaling analyses. Altogether, our data present, to our knowledge, a previously unreported functional role of Gal-8 in regulating MM TIC marker, NGFR.
Figure 4. Gal-8 incubations increased NGFR expression on MM cells, whereas Gal-8 silencing in MM cells reduced NGFR expression and tumor formation in vivo.
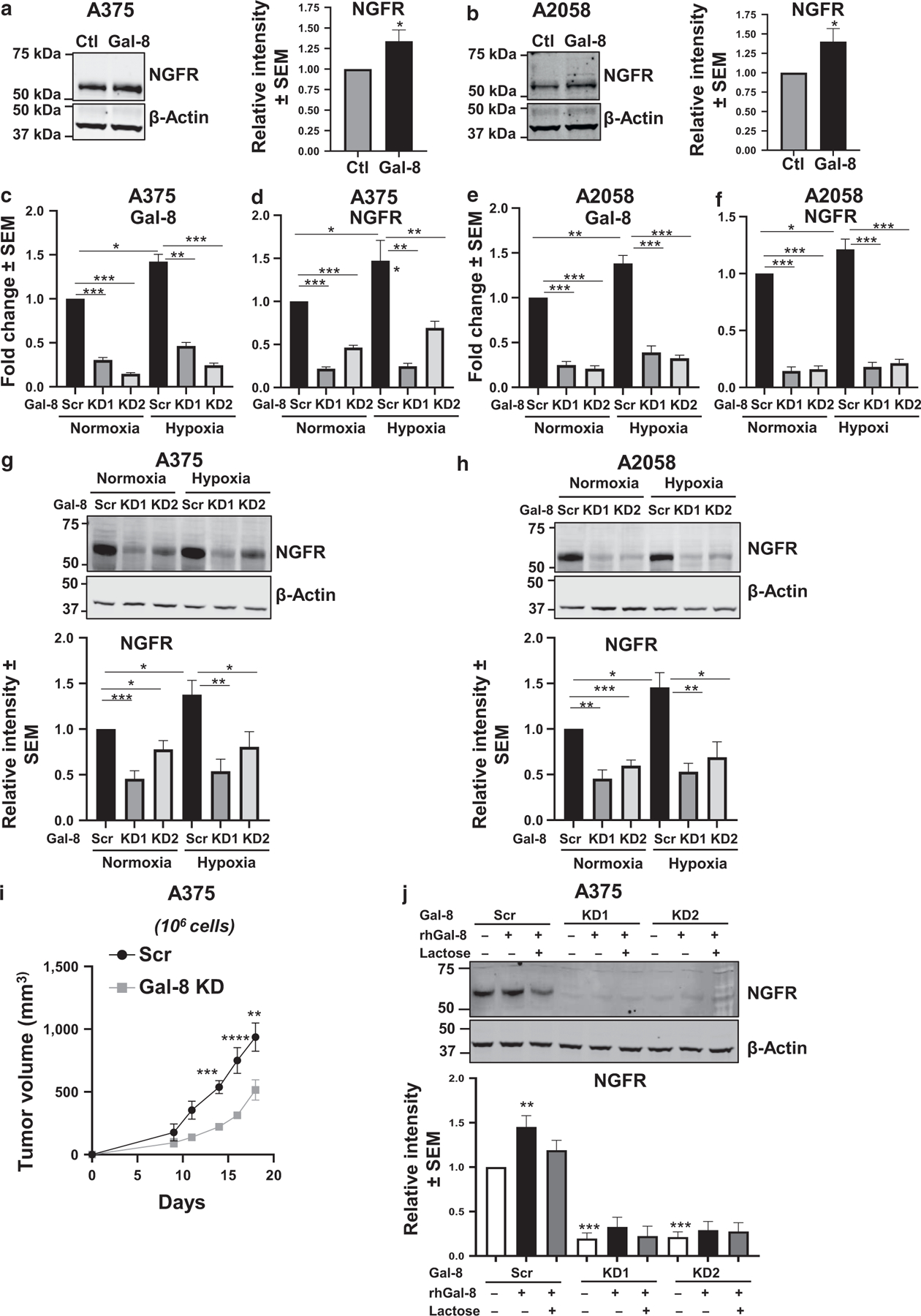
Immunoblot analysis of NGFR expression after 24-hr Gal-8 incubation (20 μg/ml) of (a) A375 and (b) A2058 cells. RT-qPCR analysis of Gal-8 and NGFR levels in (c, d) A375 Scr and Gal-8 KD cells and (e, f) A2058 Scr and Gal-8 KD cells under normoxia or hypoxia. Immunoblot analysis of NGFR expression in (g) A375 Scr and Gal-8 KD cells and (h) A2058 Scr and Gal-8 KD cells cultured under normoxia or hypoxia for 24 hr. (i) In vivo tumor formation assessment using A375 Gal-8 KD/Scr cells. Western blot analysis of NGFR expression in A375 Gal-8 KD/Scr cells after 24-hr Gal-8 incubation (20 μg/ml) with or without Gal-8 inhibitor 100 mM lactose (j). At least four biological replicates were performed. ***P < 0.001, **P < 0.01, and *P < 0.05. Ctl, control; Gal-8, galectin 8; hr, hour; KD, knockdown; MM, metastatic melanoma; rhGal-8, recombinant human galectin 8; Scr, scrambled.
Gal-8 preferentially bound i-linear poly-LacNAcs on MM cells and the MM cell surface receptor CD44, and removal of CD44–N-glycans ablated Gal-8 binding
We next assessed whether Gal-8 binding to i-linear poly-LacNAcs was favored over binding to I-branched poly-LacNAcs on MM cells. Gal-8 was incubated with A375 GCNT2-OE and empty vector cells and A2058 GCNT2-KD and -Scr cells. MM cells with low GCNT2 and high i-linear poly-LacNAcs bound Gal-8 at a significantly greater degree than cells with high GCNT2 expression and low i-linear poly-LacNAcs (P < 0.05 and P < 0.05) (Figure 5a and b). To identify specific glycoprotein ligands of Gal-8 on MM cell surfaces, we performed Gal-8 affinity chromatography followed by MS of eluates using both A375 and A2058 cell lines (Supplementary TableS2). After subtracting proteins found in lactose control eluates, CD44 was the top membrane glycoprotein identified in both MM cell lines. CD44 is a known metastasis-promoting factor that potentiates disease progression in multiple tumors, including MM (Dietrich et al., 1997; Samanna et al., 2006; Senbanjo and Chellaiah, 2017) ENREF 44. CD44 was subsequently confirmed as a Gal-8 ligand in Gal-8 eluates from A375 and A2058 cell lines (Figure 5c and e). Furthermore, treatment with PNGase, which cleaves N-glycans, ablated the capacity of Gal-8 to bind CD44 (Figure 5d and f) (P < 0.0001), implicating N-glycan dependency in Gal-8–CD44 binding interactions. To determine whether Gal-8–Gal-8 ligand interactions were affected by I-branched poly-LacNAc expression, A375 GCNT2-OE and A2058 GCNT2-KD cells and their respective controls were used for Gal-8 affinity chromatography. Gal-8 binding to CD44 was significantly greater on cells with low GCNT2 levels and high i-linear poly-LacNAcs than on cells with high GCNT2 levels and high I-branched poly-LacNAcs (P < 0.01) (Figure 5g and h). To assess whether Gal-8–Gal-8 ligand interactions triggered canonical intracellular signaling, we analyzed for activation of the prosurvival molecule, Akt, in MM cells with mainly i-linear poly-LacNAc expression (GCNT2 KD). Akt is a downstream effector of CD44 engagement (Herishanu et al., 2011). Gal-8 incubation with A2058 GCNT2-KD cells expressing i-linear poly-LacNAcs increased Akt activation compared with Scr control cells (Figure 5i) (P < 0.05). GCNT2-KD cells even had a higher basal level of Akt activation. These data indicate that Gal-8 modulated MM cell signaling, in part, through cell surface expression of i-linear poly-LacNAcs on the identified Gal-8 ligand, CD44.
Figure 5. Gal-8 bound i-linear poly-LacNAcs preferentially on MM cells, and the major Gal-8 ligand on MM cells was identified as CD44.
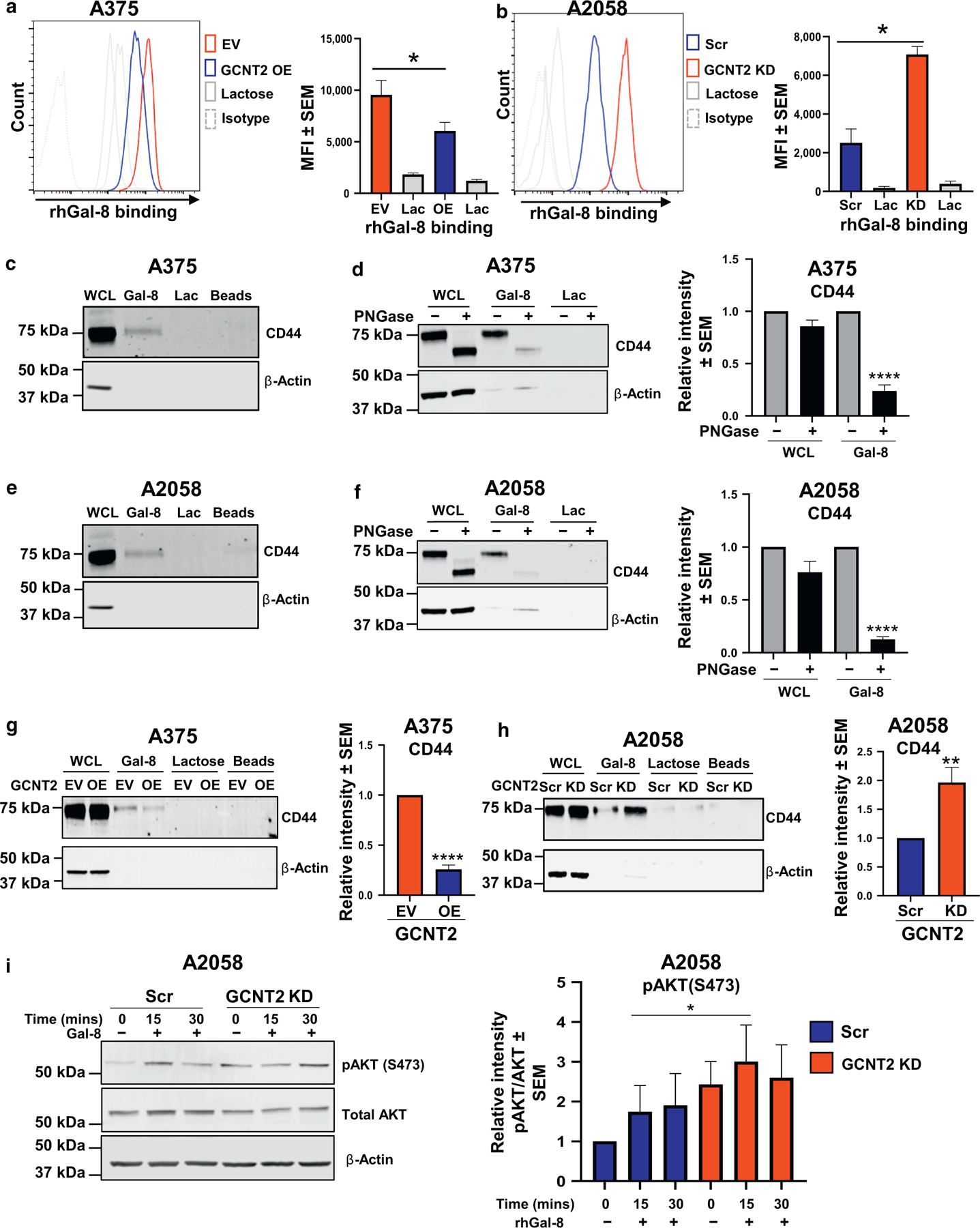
Flow cytometry analysis of rhGal-8 binding on (a) A375 GCNT2 EV/OE and (b) A2058 GCNT2 Scr/KD cells. GST-tagged Gal-8 affinity chromatography followed by immunoblotting for CD44 on parental (c) A375 and (e) A2058 cells, PNGase-treated and control parental (d) A375 and (f) A2058 cells, and (g) A375 GCNT2 EV/OE and (h) A2058 GCNT2 Scr/KD cells. Immunoblot analysis of Akt activation after Gal-8 treatment on (i) A2058 GCNT2 Scr/KD cells. At least four biological replicates were performed. ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05. Akt, protein kinase B; EV, empty vector; Gal-8, galectin 8; GCNT2, β1,6 N-acetylglucosaminyltransferase 2; GST, glutathione S-transferase; KD, knockdown; MM, metastatic melanoma; OE, overexpressed; poly-LacNAc, poly-N-acetyllactosamine; rhGal-8, recombinant human galectin 8; Scr, scrambled.
DISCUSSION
Recent developments in cancer immunotherapy have greatly improved the outcome of patients with MM; however, relapse and therapy resistance are still major clinical hurdles reducing durable response rates (Larkin et al., 2019). Recently, it has been reported that MM cells possess a cell surface glycosylation signature characterized by the loss of I-branching enzyme GCNT2 and the predominance of i-linear poly-LacNAcs on its N-glycan antennae (Sweeney et al., 2018). Although GCNT2 can attenuate malignant activities in other tumor types (Dimitroff, 2019; Perez et al., 2021), GCNT2/I-branching loss result in enhanced MM cell growth activities, prosurvival pathways, and antiapoptotic factors, thereby implicating loss of GCNT2/I-branching as a key player in melanoma progression (Sweeney et al., 2018). One of the other critical players in melanoma therapy resistance and disease relapse is microenvironmental hypoxia. Because MM exhibits a hypoxic intratumoral oxygen tension of only 1.5% (McKeown, 2014), we addressed the role of hypoxia in orchestrating the MM glycosylation signature and related malignancy-associated pathways. This investigation exposes how hypoxia preserves and even enhances signature features of the MM glycome by enforcing downregulation of GCNT2 and predominance of surface i-linear poly-LacNAcs and by regulating several other heretofore glycosylation constituents, notably Gal-8. Gal-8 favorably binds MM cell i-linear poly-LacNAcs and metastasis-associated glycoprotein CD44, among other cell surface receptors, and induces protumorigenic Akt cell signaling and the expression of MM TIC marker, NGFR. Gal-8 is not only induced in MM cells under hypoxia but is elevated in the serum of patients with melanoma, implicating its role in melanoma progression.
Gals have key extra and intracellular functions in cancer and normal cells (Popa et al., 2018). Extracellular Gal ligand binding is associated with various functions, including immunomodulation, cell differentiation, and cell survival (Thiemann and Baum, 2016). Intracellular Gals similarly modulate protein–protein interactions and RNA splicing to alter cell proliferation, apoptosis, and differentiation pathways (Liu et al., 2002; Nio-Kobayashi and Itabashi, 2021; Patterson et al., 2015; Vyakarnam et al., 1997). Although protumorigenic Gal-1, −3, and −9 have been extensively studied in melanoma (Braeuer et al., 2012a, 2012b; Cedeno-Laurent and Dimitroff, 2012; Cedeno-Laurent et al., 2012; Yang et al., 2021; Yazawa et al., 2015), Gal-8 has never been associated with melanoma progression, although it possesses protumorigenic properties in breast, prostate, glioblastoma, and colon cancers (Ferragut et al., 2019; Shatz-Azoulay et al., 2020). Identified as a tandem-repeat Gal, Gal-8 has three isoforms, Gal-8S, Gal-8M, and Gal-8L, of which Gal-8M is the most common, including in tumors (Bidon-Wagner and Le Pennec, 2002; Delgado et al., 2011; Gopalkrishnan et al., 2000; Troncoso et al., 2014).
Gal-8 plays a key role in lymphatic and vascular angiogenesis, which is implicated in the systemic dissemination of tumor cells (Troncoso et al., 2014). Cell surface Gal-8 ligands (Hadari et al., 2000; Pardo et al., 2019) include α3, α5, and β1 integrins; IL-2Rβ; TGFβ type I receptor (Sampson et al., 2016); podoplanin (Cueni and Detmar, 2009); CD166 (Delgado et al., 2011); and interestingly CD44 (Eshkar Sebban et al., 2007). As revealed in this report, Gal-8 has multiple binding partners on MM cells, including the prometastatic molecule CD44. Specifically, Gal-8 favors binding to N-glycans containing i-linear poly-LacNAcs on CD44. CD44 is a ubiquitous cell surface receptor with over 700 possible isoforms due to alternative splicing, which are associated with a number of pathologies, including tumor progression (Naor et al., 2002). In MM and other cancers, CD44 can potentiate metastasis (Dietrich et al., 1997; Gebhardt et al., 2009; Seiter et al., 1996; Senbanjo and Chellaiah, 2017; Wu et al., 2018). CD44 tumor-promoting activity can be transmitted from several key ligands, including hyaluronic acid (Senbanjo and Chellaiah, 2017), E- and L-selectin (Dimitroff et al., 2001a, 2000; Sackstein and Dimitroff, 2000) (a CD44 glycoform known as HCELL), Gal-9 (Wu et al., 2014), osteopontin (Senbanjo and Chellaiah, 2017), matrix metalloproteases (Senbanjo and Chellaiah, 2017), and now Gal-8 on MM cells. CD44 N- and O-glycans and their terminal sialylation play critical roles in promoting or preventing hyaluronic acid binding or HCELL activity on hematopoietic cells and human colorectal cancer cells (Burdick et al., 2006; Dimitroff et al., 2001a, 2001b, 2000; Faller and Guvench, 2014; Gee et al., 2003; Hanley et al., 2005; Sackstein and Dimitroff, 2000; Skelton et al., 1998; Thomas et al., 2008). The role of MM cell CD44 N-glycan–Gal-8 interactions through i-linear poly-LacNAcs posits another CD44 glycoform that can convey protumorigenic activity, further broadening the importance of post-translational modifications on CD44 in cancer. Although our efforts focused on CD44 owing to its known protumorigenic role, other Gal-8–binding targets identified in this study (Supplementary Table S2) may function in concert with Gal-8 to alter MM cell signaling.
Apart from our data on the glyco-structural mechanism of surface Gal-8 ligand binding to MM cells, results in this study underscore the complex roles of both extra and intracellular Gal-8 in MM TIC signaling. Exogenous binding of Gal-8 to MM cells increases the expression of the MM TIC marker NGFR, whereas silencing Gal-8 expression in MM cells inhibits NGFR expression and the capacity to form tumors in vivo. However, extracellular Gal-8 does not rescue NGFR downregulation in Gal-8–KD cells. Future studies exploring host-derived and MM cell–intrinsic Gal-8 may expose distinctions of Gal-8–dependent modulation of MM cell signaling by binding to surface-localized and/or cytosolic/nuclear-localized ligands.
In all, this report presents a human MM glycome–regulated pathway by which concomitant GCNT2 loss and i-linear poly-LacNAc and Gal-8 gain converge to boost melanoma cell malignancy (Figure 6). It is possible that this MM glycome pathway promotes MM progression in multiple ways by potentiating angiogenesis, mediating cell dissemination, and/or boosting antitumor immunity—all pathways functionally associated with Gal-8 (Bertelli et al., 2020; Obino et al., 2018; Pardo et al., 2017; Shatz-Azoulay et al., 2020; Tribulatti et al., 2020). Elucidating Gal-8’s role in these pathways will provide a rationale for therapeutic exploitation to complement the promise of immune checkpoint inhibition therapies. Moreover, further studies on whether stage-specific Gal-8 in patients with melanoma can predict clinical outcomes and/or guide therapeutic decisions would support strategic therapeutic targeting of the MM glycome.
Figure 6. Hypoxia enforces low GCNT2 expression and high Gal-8 levels that help to orchestrate promelanoma TIC marker NGFR expression and tumor-forming capacity.
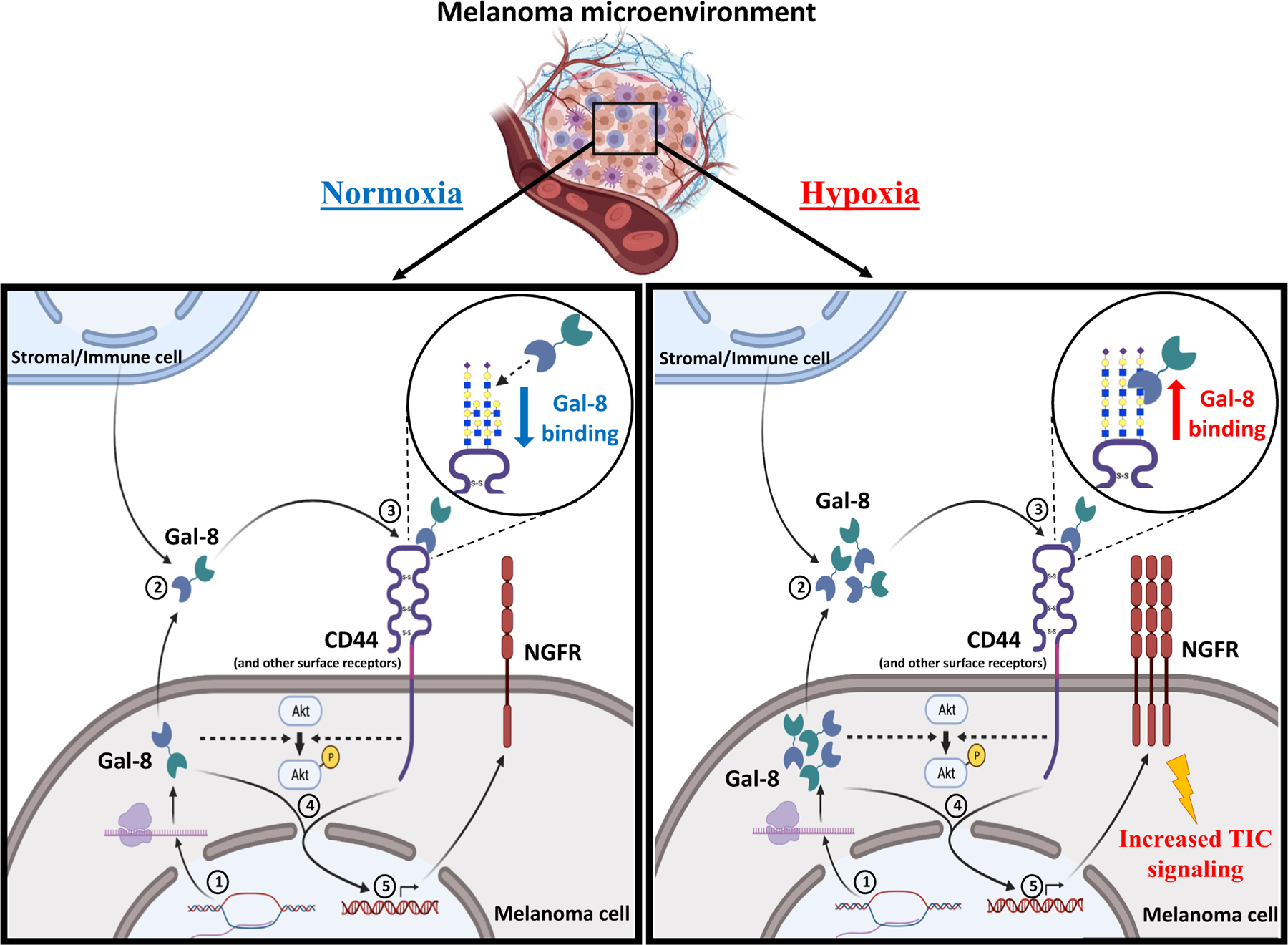
Hypoxia enforces Gal-8 gene expression in melanoma cells (1). Gal-8 produced by melanoma cells and/or stromal/immune cells is secreted into the tumor microenvironment (2) and binds preferentially to cell surface glycoproteins, namely CD44 and others, decorated predominantly with i-linear poly-LacNAcs (3) and to intracellular binding partners. These events collectively trigger the downstream signaling pathways (4) and expression of MM TIC marker, NGFR (5). Akt, protein kinase B; Gal-8, galectin 8; GCNT2, β1,6 N-acetylglucosaminyltransferase 2; MM, metastatic melanoma; poly-LacNAc, poly-N-acetyllactosamine; TIC, tumor-initiating cell.
MATERIALS AND METHODS
Complete detailed protocols are available in Supplementary Materials and Methods, and reagent details are available in Supplementary Table S3.
Cells
Human A375, A2058, and SK-MEL-5 MM cell lines were obtained from ATCC (Manassas, VA) and grown in DMEM media with 10% fetal bovine serum (Atlanta, Flowery Branch, GA) and 1% antibiotic antimitotic (Gibco, Waltham, MA).
Murine melanoma growth model
NOD-SCID IL-2Rγ–deficient mice were used for in vivo tumorigenicity experiments.
Immunohistochemistry of GCNT2
Archival formalin-fixed, paraffin-embedded human normal skin, nevi, and melanoma tissue microarray sections from patients were obtained after written informed consent and Ethics approval and kindly provided by Richard Scolyer and colleagues (Melanoma Institute of Australia, Wollstonecraft, Australia).
ELISA of Gal-8 in the serum of patients with melanoma
Samples of patients with melanoma were provided after written informed consent requested by the Biospecimen Repository Facility at Baptist Health-South Florida/Miami Cancer Institute (Miami, FL) and used for Gal-8 expression analysis by ELISA (Sigma-Aldrich, Burlington, MA).
Glycome gene expression analysis
RNA was isolated for sequencing from A375, A2058, and SkMel5 cells grown under chronic hypoxia (1% oxygen) and normoxia conditions and distributed to the Genomics Core Facility at the University of Miami Miller School of Medicine for RNA-sequencing analysis.
RT-qPCR analysis
RNA samples from MM cells grown under normoxia or hypoxia were used to assess gene expression of TIC markers and candidate glycome factors identified in the RNA-sequencing analysis.
Immunoblotting
Protein expression was assessed in lysates from MM cells grown under normoxia or hypoxia by immunoblot analysis.
Gal-8 affinity chromatography and proteomics analysis
A375, A2058, A375 empty vector, A375 GCNT2-OE, A2058-Scr, and A2058 GCNT2-KD cells were used for Gal-8 affinity chromatography assay. Glutathione S-transferase–tagged Gal-8 (SinoBiologicals, Beijing, China) was used for affinity chromatography of Gal-8 ligands using glutathione beads. Eluates were then evaluated by proteolysis and nanoscale liquid chromatography coupled to tandem mass spectrometry analyses.
Flow cytometry
To analyze surface and intracellular expression of TIC markers and glycome structures, flow cytometry was performed using validated antibodies and methods as we routinely describe.
Glycomic analysis
A375, A2058, and SkMel5 cells were grown under normoxia (~20%) or hypoxia (1% oxygen) until cells were able to proliferate with no visible signs of cell death. Cells were harvested using 1 mM EDTA (Invitrogen, Waltham, MA) for 5 minutes at room temperature, washed in PBS (two times), and stored at −80 °C until analysis.
Statistical analysis
Prism 8.0 software (GraphPad Software, San Diego, CA) was used for statistical analysis. For tests involving two groups, unpaired two-tailed Student’s t-test was used. For patient samples, appropriate tests were chosen for assumptions of normality. Throughout, error bars depict SEM. For analysis of two groups with repeated measures, two-way ANOVA was used followed by Sidak’s multiple comparison analysis (in vivo assay). P < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kyle Martin for providing us with normal human serum samples. We also thank Zasha Pou, Baptist Health-South Florida/Miami Cancer Institute (Miami, FL), for helping us with the procurement of serum samples from patients with metastatic melanoma. We thank Angela Bernasconi for working with Miami Cancer Institute in the procurement of patient samples. This work was supported by the following grants: National Institutes of Health/National Institute of Allergy and Infectious Diseases R21AI146368 (CJD), National Institutes of Health/National Cancer Institute Alliance of Glycobiologists for Cancer Research U01CA225644 (CJD), PhRMA Foundation Postdoctoral Fellowship in Translational Medicine Award (AC), National Institutes of Health/National Cancer Institute Alliance of Glycobiologists for Cancer Research U01CA225730 (RS), National Institutes of Health/National Heart, Lung, and Blood Institute K12HL141953 (RS), Wellcome Trust 082098 (SMH), and Biotechnology and Biological Sciences Research Council BBF0083091 (AD and SMH). This work was also supported by a National Health and Medical Research Council of Australia Program Grant (APP1093017) (RAS and JFT). RAS is also supported by a National Health and Medical Research Council of Australia Practitioner Fellowship (APP1141295), Deborah McMurtrie and John McMurtrie AM, The Ainsworth Foundation, CLEARbridge Foundation, and The Cameron Family. RAS gratefully acknowledges colleagues at Melanoma Institute Australia and Royal Prince Alfred Hospital.
Abbreviations:
- Akt
protein kinase B
- Gal
galectin
- GCNT2
β1,6 N-acetyl-glucosaminyltransferase 2
- KD
knockdown
- MM
metastatic melanoma
- OE
overexpressed
- poly-LacNAc
poly-N-acetyllactosamine
- Scr
scrambled control
- TIC
tumor-initiating cell
Footnotes
CONFLICT OF INTEREST
JFT has received honoraria for advisory board participation from BMS Australia, MSD Australia, GSK, and Provectus and travel and conference support from GSK, Provectus, and Novartis. RAS has received fees for professional services from F. Hoffmann-La Roche, Evaxion, Provectus Bio-pharmaceuticals Australia, Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN, Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2022.07.033
Data availability statement
Raw FASTQ and gene count matrix are available in the Gene Expression Omnibus GSE188986 and Supplementary Table S1. A list of the top 10 galectin-8 ligands identified is included in Supplementary Table S2. Datasets related to this article can be found at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE188986, hosted at the National Library of Science and the National Center for Biotechnology Information.
REFERENCES
- Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? [published correction appears in J Pathol 2012;226:e1] J Pathol 2011;223: 147–61. [DOI] [PubMed] [Google Scholar]
- Arriagada C, Silva P, Torres VA. Role of glycosylation in hypoxia-driven cell migration and invasion. Cell Adh Migr 2019;13:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635–48. [DOI] [PubMed] [Google Scholar]
- Bertelli A, Sanmarco LM, Pascuale CA, Postan M, Aoki MP, Leguizamón MS. Anti-inflammatory role of galectin-8 during Trypanosoma cruzi chronic infection. Front Cell Infect Microbiol 2020;10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidon-Wagner N, Le Pennec JP. Human galectin-8 isoforms and cancer. Glycoconj J 2002;19:557–63. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271 [published correction appears in Nature 2011;470: 424] Nature 2010;466:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeuer RR, Shoshan E, Kamiya T, Bar-Eli M. The sweet and bitter sides of galectins in melanoma progression. Pigment Cell Melanoma Res 2012a;25: 592–601. [DOI] [PubMed] [Google Scholar]
- Braeuer RR, Zigler M, Kamiya T, Dobroff AS, Huang L, Choi W, et al. Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res 2012b;72:5757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick MM, Chu JT, Godar S, Sackstein R. HCELL is the major E- and L-selectin ligand expressed on LS174T colon carcinoma cells. J Biol Chem 2006;281:13899–905. [DOI] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Dimitroff CJ. Galectins and their ligands: negative regulators of anti-tumor immunity. Glycoconj J 2012;29:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedeno-Laurent F, Opperman MJ, Barthel SR, Hays D, Schatton T, Zhan Q, et al. Metabolic inhibition of galectin-1-binding carbohydrates accentuates antitumor immunity. J Invest Dermatol 2012;132:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Dorsett KA, Trummell HQ, Yang ES, Oliver PG, Bonner JA, et al. ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. J Biol Chem 2018;293:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Staudinger C, King SL, Erickson FC, Lau LS, Bernasconi A, et al. Galectin-9 bridges human B cells to vascular endothelium while programming regulatory pathways. J Autoimmun 2021;117:102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni LN, Detmar M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp Cell Res 2009;315: 1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado VMC, Nugnes LG, Colombo LL, Troncoso MF, Fernández MM, Malchiodi EL, et al. Modulation of endothelial cell migration and angiogenesis: a novel function for the “tandem-repeat” lectin galectin-8. FASEB J 2011;25:242–54. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Tanczos E, Vanscheidt W, Schöpf E, Simon JC. High CD44 surface expression on primary tumours of malignant melanoma correlates with increased metastatic risk and reduced survival. Eur J Cancer 1997;33: 926–30. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ. I-branched carbohydrates as emerging effectors of malignant progression. Proc Natl Acad Sci USA 2019;116:13729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Fuhlbrigge RC, Sackstein R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc Natl Acad Sci USA 2000;97:13841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol 2001a;153:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Schor KS, Sandmaier BM, Sackstein R. Differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J Biol Chem 2001b;276:47623–31. [DOI] [PubMed] [Google Scholar]
- Eshkar Sebban L, Ronen D, Levartovsky D, Elkayam O, Caspi D, Aamar S, et al. The involvement of CD44 and its novel ligand galectin-8 in apoptotic regulation of autoimmune inflammation. J Immunol 2007;179: 1225–35. [DOI] [PubMed] [Google Scholar]
- Faller CE, Guvench O. Terminal sialic acids on CD44 N-glycans can block hyaluronan binding by forming competing intramolecular contacts with arginine sidechains. Proteins 2014;82:3079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 2005;65:9328–37. [DOI] [PubMed] [Google Scholar]
- Farhadi SA, Hudalla GA. Engineering galectin-glycan interactions for immunotherapy and immunomodulation. Exp Biol Med (Maywood) 2016;241: 1074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 1985;314:53–7. [DOI] [PubMed] [Google Scholar]
- Ferragut F, Cagnoni AJ, Colombo LL, Sánchez Terrero C, Wolfenstein-Todel C, Troncoso MF, et al. Dual knockdown of galectin-8 and its glycosylated ligand, the activated leukocyte cell adhesion molecule (ALCAM/CD166), synergistically delays in vivo breast cancer growth. Biochim Biophys Acta Mol Cell Res 2019;1866:1338–52. [DOI] [PubMed] [Google Scholar]
- Flynn RA, Pedram K, Malaker SA, Batista PJ, Smith BAH, Johnson AG, et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 2021;184:3109–24.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: recent advances and future directions. Eur J Surg Oncol 2017;43:604–11. [DOI] [PubMed] [Google Scholar]
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 2005;5:526–42. [DOI] [PubMed] [Google Scholar]
- Gebhardt C, Averbeck M, Anderegg U, Simon JC. CHAPTER 17.Role of hyaluronan and CD44. In: In: Melanoma Progression Stern R, editor. Hyaluronan in cancer biology San Diego: Academic Press; 2009. p. 329–39. [Google Scholar]
- Gee K, Kozlowski M, Kumar A. Tumor necrosis factor-α induces functionally active hyaluronan-adhesive CD44 by activating sialidase through p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated human monocytic cells. J Biol Chem 2003;278:37275–87. [DOI] [PubMed] [Google Scholar]
- Giovannone N, Smith LK, Treanor B, Dimitroff CJ. Galectin-glycan interactions as regulators of B cell immunity. Front Immunol 2018;9:2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalkrishnan RV, Roberts T, Tuli S, Kang D, Christiansen KA, Fisher PB. Molecular characterization of prostate carcinoma tumor antigen-1, PCTA-1, a human galectin-8 related gene. Oncogene 2000;19:4405–16. [DOI] [PubMed] [Google Scholar]
- Hadari YR, Arbel-Goren R, Levy Y, Amsterdam A, Alon R, Zakut R, et al. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J Cell Sci 2000;113:2385–97. [DOI] [PubMed] [Google Scholar]
- Hanley WD, Burdick MM, Konstantopoulos K, Sackstein R. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res 2005;65:5812–7. [DOI] [PubMed] [Google Scholar]
- Herishanu Y, Gibellini F, Njuguna N, Hazan-Halevy I, Farooqui M, Bern S, et al. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk Lymphoma 2011;52:1758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MH, Weng IC, Li FY, Lin WH, Liu FT. Intracellular galectins sense cytosolically exposed glycans as danger and mediate cellular responses. J Biomed Sci 2021;28:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Saegusa J, Liu FT. Analysis of the intracellular role of galectins in cell growth and apoptosis. Methods Mol Biol 2015;1207: 451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018;118:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessurun CAC, Vos JAM, Limpens J, Luiten RM. Biomarkers for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Front Oncol 2017;7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Huang S, Wang J, Zhang Y, Xiong Y, Zeng SX, et al. Inactivating p53 is essential for nerve growth factor receptor to promote melanoma-initiating cell-stemmed tumorigenesis. Cell Death Dis 2020;11:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, et al. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol 2019;12:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma [published correction appears in N Engl J Med 2018;379:2185] N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. [DOI] [PubMed] [Google Scholar]
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta 2002;1572:263–73. [DOI] [PubMed] [Google Scholar]
- McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol 2014;87:20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci 2002;39:527–79. [DOI] [PubMed] [Google Scholar]
- Nio-Kobayashi J, Itabashi T. Galectins and their ligand glycoconjugates in the central nervous system under physiological and pathological conditions. Front Neuroanat 2021;15:767330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obino D, Fetler L, Soza A, Malbec O, Saez JJ, Labarca M, et al. Galectin-8 favors the presentation of surface-tethered antigens by stabilizing the B cell immune synapse. Cell Rep 2018;25:3110–22.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo E, Barake F, Godoy JA, Oyanadel C, Espinoza S, Metz C, et al. Galectin-8 is a neuroprotective factor in the brain that can be neutralized by human autoantibodies. Mol Neurobiol 2019;56:7774–88. [DOI] [PubMed] [Google Scholar]
- Pardo E, Cárcamo C, Uribe-San Martín R, Ciampi E, Segovia-Miranda F, Curkovic-Peña C, et al. Galectin-8 as an immunosuppressor in experimental autoimmune encephalomyelitis and a target of human early prognostic antibodies in multiple sclerosis. PLoS One 2017;12:e0177472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RJ, Haudek KC, Voss PG, Wang JL. Examination of the role of galectins in pre-mRNA splicing. Methods Mol Biol 2015;1207:431–49. [DOI] [PubMed] [Google Scholar]
- Perez M, Chakraborty A, Lau LS, Mohammed NBB, Dimitroff CJ. Melanoma-associated glycosyltransferase GCNT2 as an emerging biomarker and therapeutic target. Br J Dermatol 2021;185:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa SJ, Stewart SE, Moreau K. Unconventional secretion of annexins and galectins. Semin Cell Dev Biol 2018;83:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol 2019;15:346–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;381:626–36. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- Sackstein R, Dimitroff CJ. A hematopoietic cell L-selectin ligand that is distinct from PSGL-1 and displays N-glycan-dependent binding activity. Blood 2000;96:2765–74. [PubMed] [Google Scholar]
- Samanna V, Wei H, Ego-Osuala D, Chellaiah MA. Alpha-V-dependent outside-in signaling is required for the regulation of CD44 surface expression, MMP-2 secretion, and cell migration by osteopontin in human melanoma cells. Exp Cell Res 2006;312:2214–30. [DOI] [PubMed] [Google Scholar]
- Sampson JF, Suryawanshi A, Chen WS, Rabinovich GA, Panjwani N. Galectin-8 promotes regulatory T-cell differentiation by modulating IL-2 and TGFb signaling [published correction appears in Immunol Cell Biol 2016;94:220] Immunol Cell Biol 2016;94:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandru A, Voinea S, Panaitescu E, Blidaru A. Survival rates of patients with metastatic malignant melanoma. J Med Life 2014;7:572–6. [PMC free article] [PubMed] [Google Scholar]
- Seiter S, Schadendorf D, Herrmann K, Schneider M, Rösel M, Arch R, et al. Expression of CD44 variant isoforms in malignant melanoma. Clin Cancer Res 1996;2:447–56. [PubMed] [Google Scholar]
- Senbanjo LT, Chellaiah MA. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol 2017;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz-Azoulay H, Vinik Y, Isaac R, Kohler U, Lev S, Zick Y. The animal lectin galectin-8 promotes cytokine expression and metastatic tumor growth in mice. Sci Rep 2020;10:7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shental-Bechor D, Levy Y. Folding of glycoproteins: toward understanding the biophysics of the glycosylation code. Curr Opin Struct Biol 2009;19:524–33. [DOI] [PubMed] [Google Scholar]
- Skelton TP, Zeng C, Nocks A, Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol 1998;140:431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Pearce MC, Jiang Y, Yang L, Goodall C, Miranda CL, et al. Delineation of hypoxia-induced proteome shifts in osteosarcoma cells with different metastatic propensities. Sci Rep 2020;10:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol 2015;10:473–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JG, Liang J, Antonopoulos A, Giovannone N, Kang S, Mondala TS, et al. Loss of GCNT2/I-branched glycans enhances melanoma growth and survival. Nat Commun 2018;9:3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann S, Baum LG. Galectins and immune responses-just how do they do those things they do? Annu Rev Immunol 2016;34:243–64. [DOI] [PubMed] [Google Scholar]
- Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E-and L-selectin in shear flow. J Biol Chem 2008;283:15647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulatti MV, Carabelli J, Prato CA, Campetella O. Galectin-8 in the onset of the immune response and inflammation. Glycobiology 2020;30: 134–42. [DOI] [PubMed] [Google Scholar]
- Troncoso MF, Ferragut F, Bacigalupo ML, Cárdenas Delgado VM, Nugnes LG, Gentilini L, et al. Galectin-8: A matricellular lectin with key roles in angiogenesis. Glycobiology 2014;24:907–14. [DOI] [PubMed] [Google Scholar]
- Varki A, Kannagi R, Toole B, Stanley P. Glycosylation changes in cancer. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. , editors. Essentials of glycobiology Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; Copyright 2015–2017 by The Consortium of Glycobiology Editors, La Jolla, California. All rights reserved; 2015. p. 597–609. [Google Scholar]
- Vyakarnam A, Dagher SF, Wang JL, Patterson RJ. Evidence for a role for galectin-1 in pre-mRNA splicing. Mol Cell Biol 1997;17:4730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014;41:270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RL, Sedlmeier G, Kyjacova L, Schmaus A, Philipp J, Thiele W, et al. Hyaluronic acid-CD44 interactions promote BMP4/7-dependent Id1/3 expression in melanoma cells. Sci Rep 2018;8:14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun 2021;12:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa EM, Geddes-Sweeney JE, Cedeno-Laurent F, Walley KC, Barthel SR, Opperman MJ, et al. Melanoma cell galectin-1 ligands functionally correlate with malignant potential. J Invest Dermatol 2015;135: 1849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HM, Zhang JG, Zhang X, Li Q. Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct Target Ther 2021;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw FASTQ and gene count matrix are available in the Gene Expression Omnibus GSE188986 and Supplementary Table S1. A list of the top 10 galectin-8 ligands identified is included in Supplementary Table S2. Datasets related to this article can be found at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE188986, hosted at the National Library of Science and the National Center for Biotechnology Information.


