Abstract
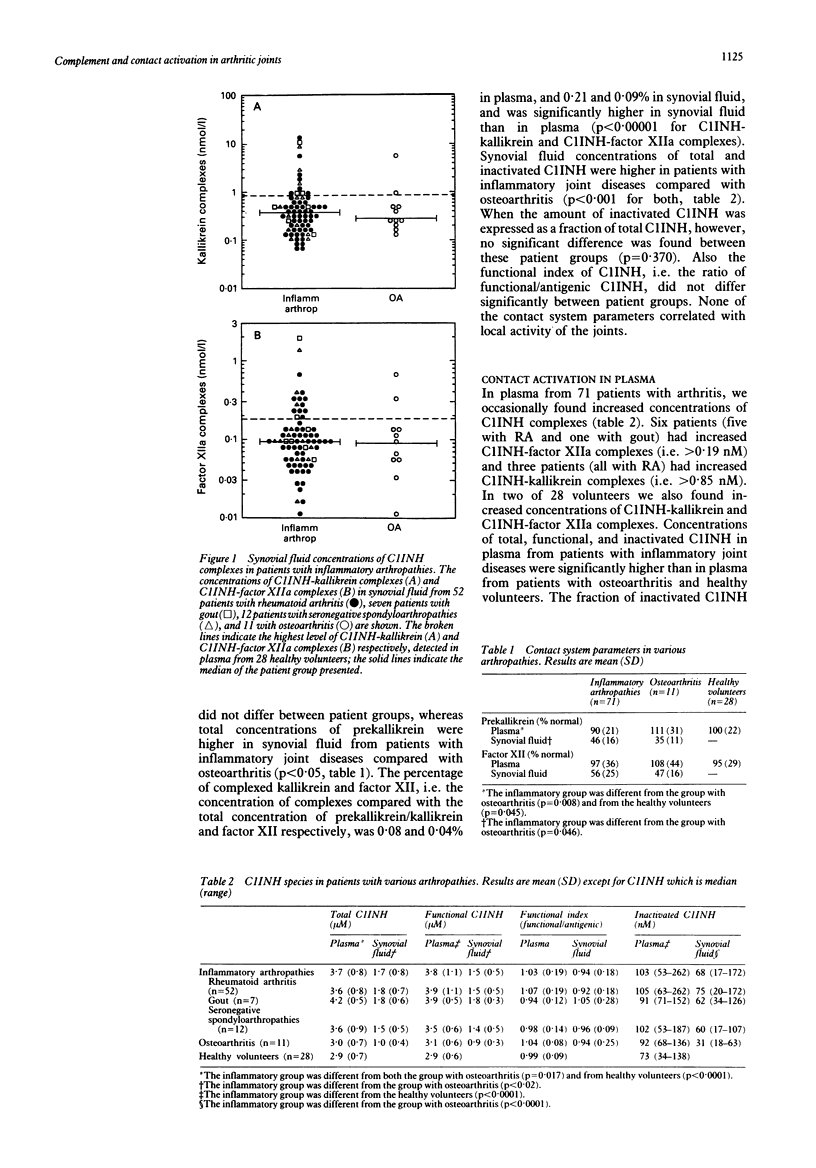
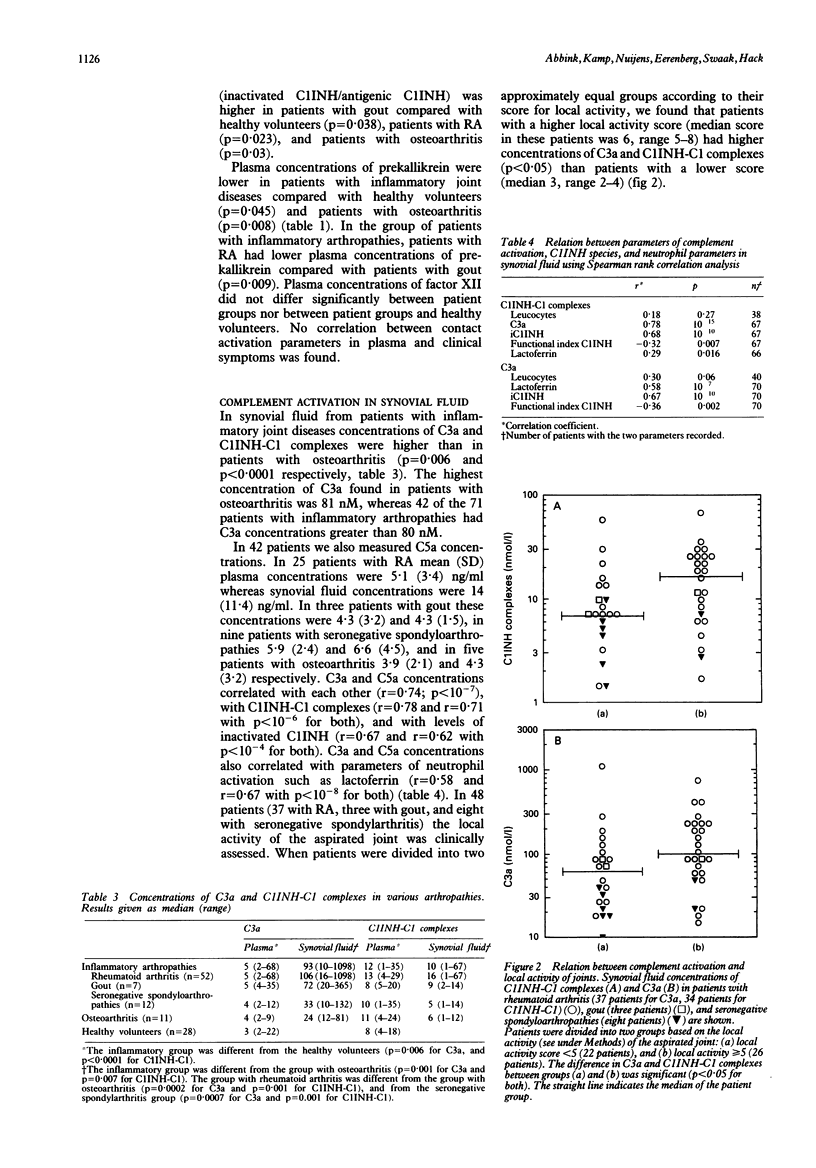
Although both the complement and contact system are thought to contribute to the inflammatory reaction in arthritic joints, only activation of complement has so far been well established, whereas contact activation and its contribution to arthritis has not been systematically explored. Complement and contact activation were assessed in 71 patients with inflammatory arthropathies and 11 with osteoarthritis using sensitive assays for C3a, and C1-inhibitor (C1INH)-kallikrein and C1INH-factor XIIa complexes respectively. Increased plasma concentrations of kallikrein-and factor XIIa-C1INH complexes were found in two and seven of the 71 patients with inflammatory arthropathies, respectively, and in none of the patients with osteoarthritis. Increased synovial fluid concentrations of kallikrein and factor XIIa complexes occurred in 13 and 15 patients with inflammatory joint diseases respectively, and in two patients with osteoarthritis. Contact system parameters did not correlate with clinical symptoms, local activity, or neutrophil activation. In contrast, synovial fluid concentrations of C3a and C1INH-C1 complexes were increased in all patients and in 20 patients with inflammatory arthropathies respectively, and were higher in patients with a higher local activity score. Synovial fluid C3a correlated with parameters of neutrophil activation such as lactoferrin. Increased plasma concentrations of C3a and C1INH-C1 complexes occurred in 13 and 11 patients with inflammatory joint diseases, and in one and two patients with osteoarthritis respectively. Plasma concentrations of C3a correlated with the number of painful joints. Thus contact activation occurs only sporadically in patients with arthritis and contributes little if anything to the local inflammatory reaction and neutrophil activation. These latter events are significantly related to the extent of complement activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Berkowicz A., Kappelgaard E., Petersen J., Nielsen H., Ingemann-Hansen T., Halkjaer-Kristensen J., Sørensen H. Complement C3c and C3d in plasma and synovial fluid in rheumatoid arthritis. Acta Pathol Microbiol Immunol Scand C. 1983 Dec;91(6):397–402. [PubMed] [Google Scholar]
- Brown K. A. The polymorphonuclear cell in rheumatoid arthritis. Br J Rheumatol. 1988 Apr;27(2):150–155. doi: 10.1093/rheumatology/27.2.150. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Ward P. A., Kellermeyer R. W., Naff G. B. Complement as a mediator of inflammation in acute gouty arthritis. II. Biological activities generated from complement by the interaction of serum complement and sodium urate crystals. J Lab Clin Med. 1973 May;81(5):761–769. [PubMed] [Google Scholar]
- Cochrane C. G., Griffin J. H. The biochemistry and pathophysiology of the contact system of plasma. Adv Immunol. 1982;33:241–306. doi: 10.1016/s0065-2776(08)60837-8. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Wachtfogel Y. T., Kucich U., Weinbaum G., Hahn S., Pixley R. A., Scott C. F., de Agostini A., Burger D., Schapira M. Effect of cleavage of the heavy chain of human plasma kallikrein on its functional properties. Blood. 1985 Feb;65(2):311–318. [PubMed] [Google Scholar]
- Doherty M., Richards N., Hornby J., Powell R. Relation between synovial fluid C3 degradation products and local joint inflammation in rheumatoid arthritis, osteoarthritis, and crystal associated arthropathy. Ann Rheum Dis. 1988 Mar;47(3):190–197. doi: 10.1136/ard.47.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M., Whicher J. T., Dieppe P. A. Activation of the alternative pathway of complement by monosodium urate monohydrate crystals and other inflammatory particles. Ann Rheum Dis. 1983 Jun;42(3):285–291. doi: 10.1136/ard.42.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Jaques B., Cochrane C. G., Griffin J. H. Urate crystal--dependent cleavage of Hageman factor in human plasma and synovial fluid. J Lab Clin Med. 1980 Apr;95(4):497–506. [PubMed] [Google Scholar]
- Hack C. E., Hannema A. J., Eerenberg-Belmer A. J., Out T. A., Aalberse R. C. A C1-inhibitor-complex assay (INCA): a method to detect C1 activation in vitro and in vivo. J Immunol. 1981 Oct;127(4):1450–1453. [PubMed] [Google Scholar]
- Hack C. E., Paardekooper J., Eerenberg A. J., Navis G. O., Nijsten M. W., Thijs L. G., Nuijens J. H. A modified competitive inhibition radioimmunoassay for the detection of C3a. Use of 125I-C3 instead of 125I-C3a. J Immunol Methods. 1988 Apr 6;108(1-2):77–84. doi: 10.1016/0022-1759(88)90405-x. [DOI] [PubMed] [Google Scholar]
- Hadler N. M., Spitznagel J. K., Quinet R. J. Lysosomal enzymes in inflammatory synovial effusions. J Immunol. 1979 Aug;123(2):572–577. [PubMed] [Google Scholar]
- Hasselbacher P. C3 activation by monosodium urate monohydrate and other crystalline material. Arthritis Rheum. 1979 Jun;22(6):571–578. doi: 10.1002/art.1780220603. [DOI] [PubMed] [Google Scholar]
- Hunder G. G., McDuffie F. C., Mullen B. J. Activation of complement components C3 and factor B in synovial fluids. J Lab Clin Med. 1977 Jan;89(1):160–171. [PubMed] [Google Scholar]
- Inman R. D., Harpel P. C. C1 inactivator-C1s complexes in inflammatory joint disease. Clin Exp Immunol. 1983 Sep;53(3):521–528. [PMC free article] [PubMed] [Google Scholar]
- KELLERMEYER R. W., BRECKENRIDGE R. T. THE INFLAMMATORY PROCESS IN ACUTE GOUTY ARTHRITIS. I. ACTIVATION OF HAGEMAN FACTOR BY SODIUM URATE CRYSTALS. J Lab Clin Med. 1965 Feb;65:307–315. [PubMed] [Google Scholar]
- Kalter E. S., Daha M. R., ten Cate J. W., Verhoef J., Bouma B. N. Activation and inhibition of Hageman factor-dependent pathways and the complement system in uncomplicated bacteremia or bacterial shock. J Infect Dis. 1985 Jun;151(6):1019–1027. doi: 10.1093/infdis/151.6.1019. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Kay A. B., Austen K. F. A prealbumin activator of prekallikrein. 3. Appearance of chemotactic activity for human neutrophils by the conversion of human prekallikrein to kallikrein. J Exp Med. 1972 Jan;135(1):81–97. doi: 10.1084/jem.135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleesiek K., Reinards R., Brackertz D., Neumann S., Lang H., Greiling H. Granulocyte elastase as a new biochemical marker in the diagnosis of chronic joint diseases. Rheumatol Int. 1986;6(4):161–169. doi: 10.1007/BF00541283. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Webster M. E., Goldfinger S. E., Seegmiller J. E. The presence of a kinin in inflammatory synovial effusion from arthritides of varying etiologies. Arthritis Rheum. 1967 Feb;10(1):13–20. doi: 10.1002/art.1780100103. [DOI] [PubMed] [Google Scholar]
- Moll J. M., Wright V. New York clinical criteria for ankylosing spondylitis. A statistical evaluation. Ann Rheum Dis. 1973 Jul;32(4):354–363. doi: 10.1136/ard.32.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnes T. E., Paus A. Complement activation in synovial fluid and tissue from patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1986 Nov;29(11):1359–1364. doi: 10.1002/art.1780291108. [DOI] [PubMed] [Google Scholar]
- Moskowitz R. W., Schwartz H. J., Michel B., Ratnoff O. D., Astrup T. Generation of kinin-like agents by chondroitin sulfate, heparin, chitin sulfate, and human articular cartilage: possible pathophysiologic implications. J Lab Clin Med. 1970 Nov;76(5):790–798. [PubMed] [Google Scholar]
- Moxley G., Ruddy S. Elevated C3 anaphylatoxin levels in synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 1985 Oct;28(10):1089–1095. doi: 10.1002/art.1780281003. [DOI] [PubMed] [Google Scholar]
- Nuijens J. H., Eerenberg-Belmer A. J., Huijbregts C. C., Schreuder W. O., Felt-Bersma R. J., Abbink J. J., Thijs L. G., Hack C. E. Proteolytic inactivation of plasma C1- inhibitor in sepsis. J Clin Invest. 1989 Aug;84(2):443–450. doi: 10.1172/JCI114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijens J. H., Huijbregts C. C., Eerenberg-Belmer A. J., Abbink J. J., Strack van Schijndel R. J., Felt-Bersma R. J., Thijs L. G., Hack C. E. Quantification of plasma factor XIIa-Cl(-)-inhibitor and kallikrein-Cl(-)-inhibitor complexes in sepsis. Blood. 1988 Dec;72(6):1841–1848. [PubMed] [Google Scholar]
- Nydegger U. E., Zubler R. H., Gabay R., Joliat G., Karagevrekis C. H., Lambert P. H., Miescher P. A. Circulating complement breakdown products in patients with rheumatoid arthritis. Correlation between plasma C3d, circulating immune complexes, and clinical activity. J Clin Invest. 1977 May;59(5):862–868. doi: 10.1172/JCI108708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Nydegger U. E., Zubler R. H., Lambert P. H., Miescher P. A. Correlation between levels of breakdown products of C3, C4, and properdin factor B in synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 1977 Mar;20(2):647–652. doi: 10.1002/art.1780200202. [DOI] [PubMed] [Google Scholar]
- Pixley R. A., Schapira M., Colman R. W. The regulation of human factor XIIa by plasma proteinase inhibitors. J Biol Chem. 1985 Feb 10;260(3):1723–1729. [PubMed] [Google Scholar]
- Prakash S., Mehra N. K., Bhargava S., Malaviya A. N. HLA B27 related 'unclassifiable' seronegative spondyloarthropathies. Ann Rheum Dis. 1983 Dec;42(6):640–643. doi: 10.1136/ard.42.6.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Despland E., Scott C. F., Boxer L. A., Colman R. W. Purified human plasma kallikrein aggregates human blood neutrophils. J Clin Invest. 1982 May;69(5):1199–1202. doi: 10.1172/JCI110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Reboul A., Arlaud G. J., Villiers C. L., Colomb M. G. Interaction of 125I-labelled complement subcomponents C-1r and C-1s with protease inhibitors in plasma. FEBS Lett. 1979 Jan 1;97(1):111–115. doi: 10.1016/0014-5793(79)80063-0. [DOI] [PubMed] [Google Scholar]
- Vogt W. Anaphylatoxins: possible roles in disease. Complement. 1986;3(3):177–188. doi: 10.1159/000467894. [DOI] [PubMed] [Google Scholar]
- Wagner J. L., Hugli T. E. Radioimmunoassay for anaphylatoxins: a sensitive method for determining complement activation products in biological fluids. Anal Biochem. 1984 Jan;136(1):75–88. doi: 10.1016/0003-2697(84)90308-7. [DOI] [PubMed] [Google Scholar]
- Wallace S. L., Robinson H., Masi A. T., Decker J. L., McCarty D. J., Yü T. F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977 Apr;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Activation of neutrophils and the lesions of rheumatoid arthritis. J Lab Clin Med. 1982 Sep;100(3):322–333. [PubMed] [Google Scholar]
- Woo P., Lachmann P. J., Harrison R. A., Amos N., Cooper C., Rosen F. S. Simultaneous turnover of normal and dysfunctional C1 inhibitor as a probe of in vivo activation of C1 and contact activatable proteases. Clin Exp Immunol. 1985 Jul;61(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- van der Graaf F., Koedam J. A., Bouma B. N. Inactivation of kallikrein in human plasma. J Clin Invest. 1983 Jan;71(1):149–158. doi: 10.1172/JCI110743. [DOI] [PMC free article] [PubMed] [Google Scholar]


