Abstract
Purpose:
Adolescents and young adults (AYAs) with cancer are in a unique situation due to their age and developmental stage in life and may have different symptoms and concerns than older patients. Patient-Reported Outcomes (PROs) questionnaires, routinely used in Alberta, can help identify the distinct needs of AYAs. We aimed to compare PROs data for AYAs and older adults (OAs) to better understand how the concerns of AYAs differ, which is key to providing individualized care and creating targeted programming and system-level change.
Methods:
Retrospective data were collected for two patient cohorts who completed at least one PROs questionnaire between October 1, 2019 and April 1, 2020. The AYA cohort was aged 18–39, and the OA cohort was aged 40 and older. Symptoms were compared using mean scores and multiple linear regression, and concerns were compared using counts and multivariate negative binomial regression.
Results:
AYAs had significantly higher mean scores on depression and anxiety, compared to OAs, and lower mean scores for most physical symptoms. They indicated significantly more concerns in the Emotional and Social/Family/Spiritual domains, and were over three times more likely to indicate Work/School as a concern.
Conclusion:
AYAs with cancer have distinct concerns that should be addressed to ensure comprehensive, quality cancer care for this population. PROs data are useful in identifying needs and facilitating evidence-based, data-driven change at all levels of the health care system.
Keywords: patient-reported outcomes, adolescents and young adults, young adults with cancer, AYA cancer, psychosocial, emotional concerns
Introduction
In Canada, ∼7600 adolescents and young adults (AYAs) are diagnosed with cancer each year,1 ranging in age from 15 to 39 years old. The survival rate for AYA cancers is over 80% and continues to increase, meaning that most will live for 50–60 years after their diagnosis.1 AYA cancer survivors may in turn have to manage the long-term effects of their diagnosis and its treatment(s) for many years and through different developmental stages of life. This can make an already challenging time as a cancer survivor even more complex to navigate as a younger person.
AYAs with cancer often have complex needs that differ from their older counterparts. A cancer diagnosis at this stage of life can have considerable impacts on mental health, as they may feel isolated from peers, and can create uncertainty about finances.2 As such, psychosocial and practical distress may be heightened in this population.2,3 Understanding, identifying, and addressing the needs of this population are essential to ensure that AYAs have optimal outcomes throughout their cancer journey.4,5 Patient-Reported Outcomes (PROs) are increasingly helping achieve this, by using standardized self-report tools that enable patients to identify symptoms and concerns they deem most relevant.6 Incorporating PROs into routine clinical workflows helps ensure provision of person-centered care that meets individuals' physical, emotional, and practical needs.7
In the province of Alberta, Canada, a publicly funded cancer program called Cancer Care Alberta (CCA) provides care through a network of 17 ambulatory cancer centers province-wide.8 This oncology program primarily treats adults, with patients younger than 18 being seen in the pediatric health system. Because of this, AYAs in CCA are typically between 18 and 39 years of age.
Since 2019, CCA has incorporated the routine clinical use of a PROs questionnaire with all patients and across all time points of a patient's cancer journey, including new consults, treatments, and follow-up. The questionnaire is completed at clinic appointments and contains two self-report tools.8 This not only helps clinicians focus their assessment on a patient's most severe symptoms and/or concerns, but PROs data can also be analyzed to understand the symptom burden for patient populations who share certain characteristics. By examining PROs data for AYAs specifically, their concerns can be better understood and, in turn, addressed. The purpose of this study was to use PROs data to identify the needs of AYA cancer patients and understand key differences from a cohort of older cancer patients in Alberta, Canada.
Methods
A retrospective study was conducted using several provincial data sources. The study utilized administrative data from the Alberta Cancer Registry (ACR) and clinical data from CCA's electronic medical record (EMR). Data linkage was accomplished using a unique provincial health care number assigned to each patient by the ACR. The dataset used was part of a larger study that received ethics approval from the Health Research Ethics Board of Alberta—Cancer Committee (approval number HREBA.CC-20-0022). This study did not involve any patient contact.
Sample
The study cohort consisted of cancer patients in Alberta aged 18 and older9,10 with any cancer diagnosis, across all 17 ambulatory cancer centers in the province. To be eligible for inclusion, patients had to have completed at least one PROs questionnaire between October 1, 2019 and April 1, 2020. If a patient completed multiple questionnaires within the study period, only the first was included in the analyses.11 Patients aged 18–39 comprised a subgroup identified as the AYA cohort9,10 while all other patients, aged 40 years and older,12 made up a control group to which the AYA population was compared. This older group was classified as the “Older Adult (OA)” cohort.
Measures
Sociodemographic and disease-specific variables were extracted from the ACR to obtain information on patients' age, sex, rurality, tumor group, and Charlson Comorbidity Index (CCI),13–15 as well as whether patients visited a community, regional, or larger tertiary cancer center. Rurality for each patient was assigned based on the postal code of their most recent residence, using a seven-level rurality index created by Alberta Health Services (AHS).16 The seven levels were collapsed into three: Rural, Urban, and Metro. A modified version of the CCI was used, which excludes cancer and associated metastases as factors, and was calculated according to diagnoses coded in the 12 months before the patient's first PROs questionnaire in the study period.
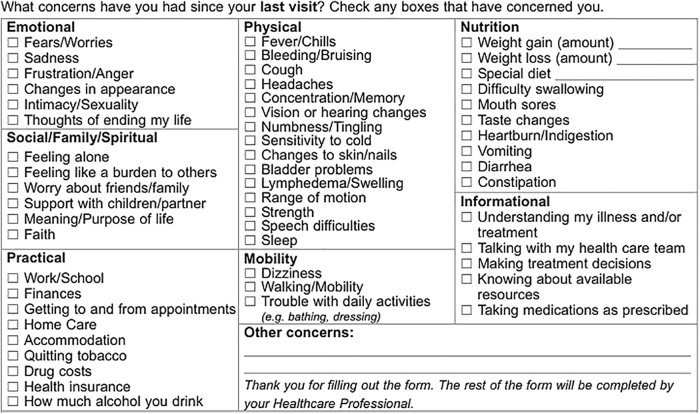
Outcome data, collected from the PROs questionnaires, were extracted from the cancer program's EMR. The questionnaire contains two components, the Edmonton System Assessment System-Revised (ESAS-r) and the Canadian Problem Checklist (CPC), both of which were included as outcomes.17 The ESAS-r is a validated PROs measure that assesses the severity of nine symptoms: pain, tiredness, drowsiness, nausea, lack of appetite, shortness of breath, depression, anxiety, and well-being.17–19 Patients rate each symptom on a severity scale from 0 to 10, with 10 indicating the highest severity (or the worst well-being, for that symptom). The CPC is an evidence-based self-report checklist that identifies common concerns that cancer patients experience.20,21 CCA previously modified the original CPC to include additional concerns relevant to patients in Alberta; the checklist on the current version of the PROs questionnaire has a total of 54 items (Fig. 1 presents the modified CPC).17
FIG. 1.
Modified 54-item version of the CPC. This version is included on the routinely used PROs questionnaire, which is used throughout CCA. CCA, Cancer Care Alberta; CPC, Canadian Problem Checklist; PROs, Patient-Reported Outcomes.
Data analysis
Demographic and clinical characteristics were cross-tabulated and compared by age cohort, using chi-squared tests for categorical variables and independent t-tests for continuous variables to assess significant differences. Prevalence of symptoms and concerns were presented by descriptive statistics, including mean, standard deviation, and frequency, when appropriate. To compare symptom severity between AYAs and OAs, multiple linear regressions were utilized, with each individual symptom constructed as a linear outcome. In each of the nine models, one symptom was selected as the dependent variable and age cohort was the primary association of interest, with the OA cohort serving as the reference group. Baseline demographic and clinical characteristics were controlled for in each model. Model fit was assessed by F value and the significance level.22,23
To assess the difference in counts of CPC concerns between the AYA and OA cohorts, multivariate negative binomial regressions were utilized for the seven CPC domains (constructed as the count outcome), controlling for baseline characteristics. Negative binomial regression was selected due to overdispersion, the count nature of the outcome data, and better goodness-of-fit statistics compared to Poisson regression.24,25 For each item on the CPC, we calculated the ratio for the AYA and OA cohorts by dividing the count of each item by the number of patients in that age group with at least one CPC concern. We then compared the ratios (AYAs to OAs) to identify differences between the cohorts. All data were exported into SPSS Version 25.0 (Chicago, IL) for analysis and statistical significance was set a priori at p < 0.05.
Results
Sample characteristics
The total study cohort comprised 29,242 cancer patients who completed at least one PROs questionnaire within the 6-month study period. Two thousand eighty-nine patients were AYAs (7.1%), leaving 27,153 patients in the OA group (92.9%). The mean age was 32.5 for the AYAs and 65.4 for the OAs. Both cohorts included more females than males, with slightly different distributions (57.3% of AYAs were female, compared to 54.6% of OAs). The most common tumor groups were hematology (23.7% for AYAs, 20.8% for OAs) and breast (16.4% for AYAs, 20.4% for OAs). Only 4.4% of AYAs had a CCI of 1 or higher, compared to 14.4% of OAs. Cross-tabulations for all variables are presented in Table 1. Significantly different distributions were noted in all baseline characteristics between AYAs and OAs.
Table 1.
Patient Demographics Cross-Tabulated by Age Cohort (Adolescents and Young Adults and Older Adults)
| Age cohort |
p | ||
|---|---|---|---|
| AYA cohort (n = 2089) | OA cohort (n = 27,153) | ||
| Age (in years) | 0.000 | ||
| Mean (SD) | 32.5 (5.28) | 65.4 (11.7) | |
| Sex | 0.020 | ||
| Female | 1196 (57.3%) | 14,832 (54.6%) | |
| Male | 893 (42.7%) | 12,321 (45.4%) | |
| Rurality index | 0.000 | ||
| Metro | 1498 (71.7%) | 18,117 (66.7%) | |
| Urban | 228 (10.9%) | 3050 (11.2%) | |
| Rural | 302 (14.5%) | 5468 (20.1%) | |
| Unidentified | 61 (2.9%) | 518 (1.9%) | |
| Location of care | |||
| TCC 1 | 1129 (54.0%) | 13,359 (49.2%) | 0.000 |
| TCC 2 | 787 (37.7%) | 9818 (36.2%) | |
| RCCs | 163 (7.8%) | 3676 (13.5%) | |
| CCCs | 10 (0.5%) | 300 (1.1%) | |
| Tumor group | 0.000 | ||
| Breast | 342 (16.4%) | 5530 (20.4%) | |
| CNS | 143 (6.8%) | 533 (2.0%) | |
| Endocrine | 152 (7.3%) | 571 (2.1%) | |
| Gastrointestinal | 135 (6.5%) | 3456 (12.7%) | |
| Genitourinary | 283 (13.5%) | 3900 (14.4%) | |
| Gynecology | 197 (9.4%) | 2180 (8.0%) | |
| Head and neck | 36 (1.7%) | 940 (3.5%) | |
| Hematology | 496 (23.7%) | 5640 (20.8%) | |
| Intrathoracic | 19 (0.9%) | 2239 (8.2%) | |
| Melanoma | 72 (3.4%) | 853 (3.1%) | |
| Other malignant | 100 (4.8%) | 843 (3.1%) | |
| Sarcoma | 114 (5.5%) | 468 (1.7%) | |
| CCI | 0.000 | ||
| 0 | 1998 (95.6%) | 23,231 (85.6%) | |
| ≥1 | 91 (4.4%) | 3922 (14.4%) | |
AYA, adolescent and young adult; CCCs, community cancer centers; CCI, Charlson Comorbidity Index; CNS, central nervous system; OA, older adult; RCCs, regional cancer center; TCC, tertiary cancer center (1 and 2); SD, standard deviation.
Descriptive statistics of ESAS-r symptoms by age cohort
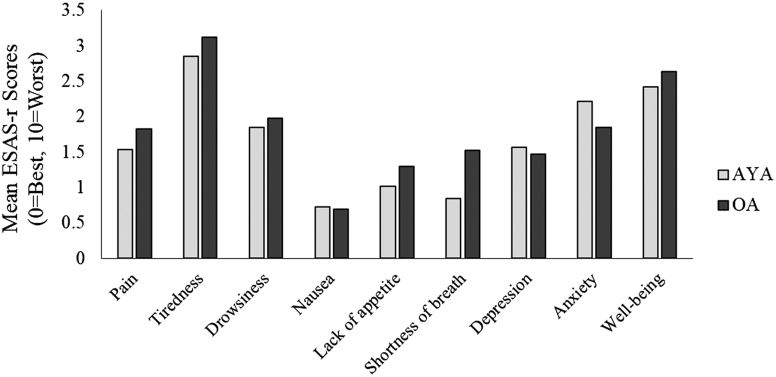
Mean scores for all nine symptoms, for both cohorts, are presented in Figure 2. The highest mean scores for AYAs were tiredness, well-being, and anxiety and the lowest were nausea, shortness of breath, and lack of appetite. Nausea was the lowest mean score for both cohorts. Compared to OAs, AYAs had higher mean scores for anxiety and depression, and slightly higher for nausea. The biggest observed difference in mean scores between cohorts was for shortness of breath (AYAs = 0.84 vs. OAs = 1.52).
FIG. 2.
Mean ESAS-r symptom scores for AYAs and OAs. AYAs, adolescents and young adults; ESAS-r, Edmonton System Assessment System-Revised; OAs, older adults.
Multiple linear regression of ESAS-r symptoms by age cohort
Multiple linear regression was used to examine the association between age cohort and ESAS-r scores, while controlling for sex, rurality, location of care, tumor group, and CCI. In the first model, pain was the dependent variable, with age cohort as the association of interest. A significant F-test [F(6) = 83.1, p < 0.01] indicated that the proposed association between the outcomes and the set of predictors was statistically reliable.21,22 Age group was found to be significantly associated with pain scores (p < 0.01), after controlling for other potentially confounding variables, with AYAs reporting significantly lower pain scores than OAs, on average (β = −0.024). Similar regression models of the same structure were performed to assess the association between age cohort and the eight other ESAS-r symptoms.
Compared with OAs, AYAs reported, on average, significantly lower mean scores for tiredness (β = −0.015, p < 0.05), lack of appetite (β = −0.022, p < 0.01), shortness of breath (β = −0.063, p < 0.01), and well-being (β = −0.014, p < 0.05). They also reported a lower mean score for drowsiness, however the difference was not significant (β = −0.002, p > 0.05). AYAs reported significantly higher mean scores for depression (β = 0.015, p < 0.05) and anxiety (β = 0.040, p < 0.01). AYAs also reported slightly higher mean scores for nausea, which approached significance (β = 0.010, p = 0.087). Details of the parameter estimates for each model, with OAs as the reference group, are presented in Table 2.
Table 2.
Regression Analysis Summary for Age Cohort (Adolescents and Young Adults vs. Older Adults) on Edmonton System Assessment System-Revised Symptoms
| ESAS-r symptoms | b | SE | 95% CI for B | β | t | p | |
|---|---|---|---|---|---|---|---|
| Pain | AYA | −0.222 | 0.056 | −0.332 to −0.113 | −0.024 | −4.00 | 0.000 |
| Tiredness | AYA | −0.159 | 0.063 | −0.283 to −0.036 | −0.015 | −2.53 | 0.012 |
| Drowsiness | AYA | −0.016 | 0.057 | −0.127 to 0.095 | −0.002 | −0.286 | 0.775 |
| Nausea | AYA | 0.065 | 0.038 | −0.010 to 0.140 | 0.010 | 1.71 | 0.087 |
| Lack of appetite | AYA | −0.192 | 0.053 | −0.296 to −0.089 | −0.022 | −3.65 | 0.000 |
| Shortness of breath | AYA | −0.568 | 0.053 | −0.672 to −0.464 | −0.063 | −10.7 | 0.000 |
| Depression | AYA | 0.132 | 0.053 | 0.028 to 0.235 | 0.015 | 2.50 | 0.013 |
| Anxiety | AYA | 0.387 | 0.057 | 0.276 to 0.499 | 0.040 | 6.80 | 0.000 |
| Well-being | AYA | −0.139 | 0.060 | −0.257 to −0.022 | −0.014 | −2.33 | 0.020 |
Note: reference group = OA cohort.
β, standardized coefficient; b, unstandardized coefficient; CI, confidence interval; ESAS-r, Edmonton System Assessment System-Revised; SE, standard error.
Canadian Problem Checklist
The CPC is located on the back page of the provincial PROs questionnaire and is often left blank. All patients who did not check at least one concern on the CPC were removed from this piece of the analysis, as it is impossible to determine if patients left the CPC blank by mistake or because they did not have concerns. This left 971 AYAs (46%) and 14,020 OAs (51.6%) with at least one CPC concern. There was no significant difference between cohorts in the number of concerns checked, with the mean being 5.49 concerns for AYAs and 5.43 for OAs.
Using the counts of CPC concerns (≥1) in each domain as outcomes in the multivariate negative binomial regressions, while controlling for the baseline characteristics listed in Table 1, we examined the seven domains and how they differed between cohorts. The results, presented in Table 3, showed that AYAs checked 23.5% more concerns in the emotional domain, compared to OAs (adjusted rate ratio [aRR] = 1.235, 95% CI: 1.014–1.383, p < 0.01). They also checked off 24.0% more concerns in the Social/Family/Spiritual domain (aRR = 1.240, 95% CI: 1.072–1.435, p < 0.01). The other five domains did not significantly differ between the two cohorts.
Table 3.
Multivariate Negative Binomial Regression Model for Age Cohort (Adolescents and Young Adults vs. Older Adults) on Canadian Problem Checklist Domains
| CPC domains | Wald | p | aRR (95%) | |
|---|---|---|---|---|
| Emotional | AYA | 13.5 | 0.000 | 1.235 (1.104–1.383) |
| Social/family/spiritual | AYA | 8.34 | 0.004 | 1.240 (1.072–1.435) |
| Practical | AYA | 1.65 | 0.199 | 1.098 (0.952–1.265) |
| Physical | AYA | 1.69 | 0.194 | 0.938 (0.821–1.033) |
| Mobility | AYA | 0.20 | 0.658 | 1.053 (0.838–1.322) |
| Nutrition | AYA | 0.14 | 0.713 | 0.974 (0.844–1.123) |
| Informational | AYA | 2.00 | 0.157 | 1.128 (0.955–1.334) |
Note: reference group = OA cohort.
aRR, adjusted rate ratio; CPC, Canadian Problem Checklist.
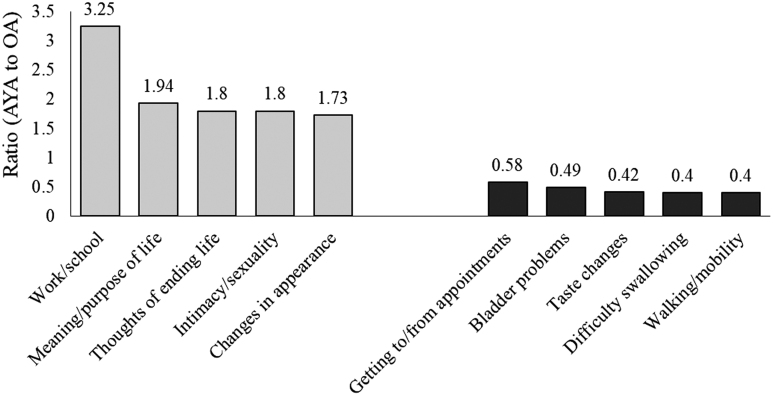
To assess differences in the number of times specific concerns were checked, ratios were generated to compare AYAs to OAs on all 54 concerns. Figure 3 presents the ratios for the 10 concerns with the largest differences between the cohorts. AYAs checked “Work/School” more than three times as much as OAs did, while they were less than half as likely to check physical concerns such as “Difficulty Swallowing” and “Walking/Mobility.”
FIG. 3.
Largest and smallest ratios of AYA to OA for CPC concerns. Orange represents the five concerns with the largest ratios (where AYAs outnumbered OAs), and blue represents the five concerns with the smallest ratios (where OAs outnumbered AYAs).
Discussion
It is clear that AYAs with cancer have distinct symptoms and/or concerns and may require tailored care responses. The Royal College of Physicians and Surgeons of Canada developed a specialized certification in AYA oncology in 2016.26,27 More recently, in 2019, the Canadian Partnership Against Cancer (CPAC) released the Canadian Framework for the Care and Support of AYAs with Cancer.1 These resources emphasize the importance of addressing the specific needs and challenges of this younger patient population. By utilizing PROs, efforts can be taken at the individual, program and organization levels of the health care system to ensure AYAs receive adequate resources and timely support to enable their optimal participation in life and contribution to society as cancer survivors.1
Using PROs to provide individualized AYAs cancer care
Routine use of PROs can help ensure that providers are aware of the specific needs of the AYA population, making them better equipped to provide individualized and AYA-specific patient care, along with optimal symptom management and appropriate resource recommendations and referrals. While the PROs questionnaire used in CCA is not specific to AYAs, the data collected from this tool are highly informative if analyzed based on age.
The findings reveal the heightened psychosocial distress that AYAs with cancer often experience. AYAs had higher mean scores than OAs on depression and anxiety, and identified over 20% more concerns in the emotional domain on the CPC. This demonstrates the importance of ensuring that AYAs are well connected to psychosocial supports throughout their cancer journey. Literature suggests that AYA patients often feel isolated from their healthy peers, as their life path diverges from others their age3; therefore, connecting them to peer support programs or other AYA patients who are navigating similar challenges may be beneficial.1 Peer connection has been demonstrated to be critical for many young patients, perhaps even more so than disease-specific supports.1,4,5
This study demonstrates the benefit of using the ESAS-r and CPC in tandem, as the CPC captures concerns beyond those related to the physical and emotional domains. A clear example is that “Work/School” was identified as a concern by AYAs over three times more than by OAs, with research demonstrating that this younger population is often actively working or in school during this phase of their life.1 Many AYAs are diverted from their educational or career paths by a cancer diagnosis. Even after cancer treatments are complete, patients may experience residual symptoms or late effects such as fatigue and tiredness, further complicating a return to work or school.
While it is important to recognize that AYAs and OAs had several differences, many symptoms and concerns will be common to all or most patients with cancer. Tiredness was the highest rated symptom for both cohorts, although AYAs were significantly lower than OAs. This highlights the importance of not only focusing on AYAs' distinct symptoms and concerns but on all of their issues to provide comprehensive symptom management and developmentally appropriate, quality cancer care.
How PROs can inform program-level specialized supports
Given that AYAs may have multiple distinct needs, dedicated programming and resources could benefit this young population. An AYA-specific navigation program, for example, is a specialized support that can have considerable benefits for AYAs with cancer. Navigation programs are intended to meet the needs of underserved patient populations,28 and literature suggests that AYA patients have been chronically underserved in the health care system.4 CCA currently has an AYA navigation program available,29 to provide support to younger patients and families by coordinating care across the health care system and reducing barriers to care.28 The navigator can also act as a liaison between patients and providers to help improve experiences by promoting access to information and services and aiding in seamless transitions, all in keeping with a person-centered model of care.29–31
How PROs can contribute to system-level improvements
One of the key strategies identified in the 2019 AYA Framework from CPAC is the recommendation of using routinely collected PROs data to drive evidence-based improvements across entire health care systems.1 However, there are gaps that prevent system-level changes from taking place, specifically in the area of oncofertility and fertility preservation. There are few data quantifying how well-informed AYA patients are on these issues, although literature suggests that the majority may not receive fertility counseling even though many feel that maintaining their options for having biological children is a high priority.32,33 Efforts should be taken to ensure that this topic is discussed in detail so that fertility preservation is considered a viable option for all AYAs with cancer.34–37
The current CCA PROs questionnaire does not include a question on fertility preservation, however, work is underway to incorporate an additional screening question on this topic, which would be given to patients aged 45 and younger.38 To align with this new question, education is being developed to help support staff in discussing fertility preservation and taking appropriate actions.
Limitations
While this study contributes interesting findings to the AYA literature, certain limitations must be acknowledged. As this study relied on PROs data, only patients who completed a PROs questionnaire within the study time period could be included. We have no visibility of the symptoms and concerns of AYA cancer patients who did not complete a questionnaire. The nature of this study was cross-sectional to include as many patients as possible, as the majority only completed one PROs questionnaire within the timeframe. Because of this, only the first questionnaire from each patient with multiple questionnaires was included. While a cross-sectional method has been utilized in similar studies, it prevents a longitudinal analysis of how AYAs' symptoms and concerns change over time. As AYAs are at a stage of life where circumstances and needs can change quickly and frequently, a longitudinal analysis could reveal important findings,9,10 and will be considered in the future.
It is important to note that, although all cancer patients aged 40 and older were grouped as a cohort for this study, this was done only to enable comparisons to the AYA group. We are not suggesting that all patients in this older cohort share the same concerns; on the contrary, literature suggests that cancer patients who are truly “older adults,” meaning patients aged 65 and older, have unique needs that impact their experience with cancer.39,40
Conclusion
The needs of AYAs with cancer can be distinct and may create unique challenges throughout their cancer journey. The survival rate for this group continues to increase, but impacts on long-term psychosocial, physical, and practical outcomes cannot be ignored. AYAs are likely to live many years beyond their diagnosis, and inadequate supports or unmet needs can result in years of distress and disability. Providers must be able to identify and respond to these needs and help AYAs in managing their concerns and accessing appropriate resources. Using PROs is an important step to help tailor individualized care for AYAs, given their rapidly changing and sometimes vulnerable stage of life. Analyzing PROs can also help inform and guide the creation and development of larger AYA-specific programming and services.
Acknowledgments
We acknowledge CPAC for their allotment of resources and support to this work, and CCA's Surveillance and Reporting team for their work to provide the dataset.
Authors' Contributions
All authors contributed substantially to this article. All authors were involved in the conception, draft/revisions, and final approval of the work, and all agree to be accountable for all aspects of the work, in keeping with the ICMJE authorship guidelines. Conceptualization, L.W., S.Q., C.L., A.D., S.T., and S.M.; methodology, S.Q. and C.L.; software, S.Q.; validation, L.W. and S.Q.; formal analysis, S.Q. and C.L.; investigation, L.W., S.Q., C.L., and A.D.; resources, L.W.; data curation, S.Q. and C.L.; writing—original draft preparation, S.Q., C.L., S.T., and A.D.; writing—review and editing, C.L., A.D., L.W., and S.M.; visualization, S.Q. and C.L.; supervision, L.W.; project administration, A.D.; funding acquisition, L.W.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki. The protocol was part of a larger study using the same dataset, and was approved by the Health Research Ethics Board of Alberta's Cancer Committee (HREBA-CC-20-0022). This study did not include any patient contact.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Canadian Partnership Against Cancer. Canadian framework for the care and support of adolescents and young adults with cancer. Toronto, ON: Canadian Partnership Against Cancer; September 2019. Accessed October 25, 2021 from: https://s22457.pcdn.co/wp-content/uploads/2019/10/AYA-Framework-2019-EN.pdf
- 2. Burkart M, Sanford S, Dinner S, et al. Future health of AYA survivors. Pediatr Blood Cancer. 2019;66(2):e27516. [DOI] [PubMed] [Google Scholar]
- 3. Schulte FSM, Chalifour K, Eaton G, Garland SN. Quality of life among survivors of adolescent and young adult cancer in Canada: a Young Adults With Cancer in Their Prime (YACPRIME) study. Cancer. 2021;127(8):1325–33. [DOI] [PubMed] [Google Scholar]
- 4. Barakat LP, Galtieri LR, Szalda D, Schwartz LA. Assessing the psychosocial needs and program preferences of adolescents and young adults with cancer. SCC. 2016;24(2):823–32. [DOI] [PubMed] [Google Scholar]
- 5. Reed D, Block RG, Johnson R. Creating an adolescent and young adult cancer program: lessons learned from pediatric and adult oncology practice bases. JNCCN. 2014;12(10):1409–15. [DOI] [PubMed] [Google Scholar]
- 6. Warsame R, D'Souza A. Patient Reported Outcomes have arrived: a practical overview for clinicians in using Patient Reported Outcomes in oncology. Mayo Clin Proc. 2019;94(11):2291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuthbert CA, Watson L, Xu Y, et al. Patient-reported outcomes in Alberta: rationale, scope, and design of a database initiative. Curr Oncol. 2019;26(4):e503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson L, Qi S, DeIure A, et al. Using autoregressive integrated moving average (ARIMA) modelling to forecast symptom complexity in an ambulatory oncology clinic: harnessing predictive analytics and patient-reported outcomes. IJERPH. 2021;18:8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leuteritz K, Friedrich M, Nowe E, et al. Life situation and psychosocial care of adolescent and young adult (AYA) cancer patients – Study protocol of a 12-month prospective longitudinal study. BMC Cancer. 2017;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Husson O, Prins J, Kaal S, et al. Adolescent and young adult (AYA) lymphoma survivors report lower health-related quality of life compared to a normative population: results from the PROFILES registry. Acta Oncol. 2017;56:288–94. [DOI] [PubMed] [Google Scholar]
- 11. Barbera L, Atzema C, Sutradhar R, et al. Do patient-reported symptoms predict emergency department visits in cancer patients? A population-based analysis. Ann Emerg Med. 2013;61:427–37. [DOI] [PubMed] [Google Scholar]
- 12. Lopez G, Liu W, Madden K, et al. Adolescent-young adults (AYA) with cancer seeking integrative oncology consultations: demographics, characteristics, and self-reported outcomes. SCC. 2018;26:1611–7. [DOI] [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 14. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 16. Alberta Health Services. Official standard geographic areas. Alberta, Canada: Alberta Health Services and Alberta Health; 2017. Accessed October 25, 2021 from: internal document (accessible upon request).
- 17. Cuthbert CA, Boyne DJ, Yuan X, et al. Patient-reported symptom burden and supportive care needs at cancer diagnosis: a retrospective cohort study. SCC. 2020;28:5889–99. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psycho-Oncology. 2012;21:977–85. [DOI] [PubMed] [Google Scholar]
- 19. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. JPSM. 2017;53:630–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naik H, Leung B, Laskin J, et al. Emotional distress and psychosocial needs in patients with breast cancer in British Columbia: younger versus older adults. Breast Cancer Res Treat. 2020;179:471–7. [DOI] [PubMed] [Google Scholar]
- 21. Jammu A, Chasen M, van Heest R, et al. Effects of a cancer survivorship clinic—preliminary results. SCC. 2020;28:2381–8. [DOI] [PubMed] [Google Scholar]
- 22. UCLA Statistical Consulting Group. Regression analysis. Los Angeles, CA: UCLA Institute for Digital Research & Education; 2021. Accessed October 5, 2021 from: https://stats.idre.ucla.edu/spss/output/regression-analysis/
- 23. Grace-Martin K. Assessing the fit of regression models. 2021. Ithaca, NY: The Analysis Factor; 2013. Accessed October 5, 2021 from: https://www.theanalysisfactor.com/assessing-the-fit-of-regression-models/
- 24. Rodríguez G. Generalized linear models: models for over-dispersed count data. Princeton, NJ: Princeton University, Statistics and Population; 2021. Accessed October 5, 2021 from: https://data.princeton.edu/wws509/r/overdispersion
- 25. Mooney K, Beck S, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrari A, Barr RD. International evolution in AYA oncology: current status and future expectations: Ferrari and Barr. Pediatr Blood Cancer. 2017;64(9):e26528. [DOI] [PubMed] [Google Scholar]
- 27. Patterson P, McDonald FEJ, Zebrack B, Medlow S. Emerging issues among adolescent and young adult cancer survivors. Semin Oncol Nurs. 2015;31(1):53–9. [DOI] [PubMed] [Google Scholar]
- 28. Canadian Association of Nurses in Oncology. Navigation position statement. Vancouver, BC: Canadian Association of Nurses in Oncology; April 2020. Accessed November 8, 2021 from: https://www.cano-acio.ca/page/position_statements
- 29. Alberta Health Services. Adolescent & young adult (AYA) patient navigation. Alberta, Canada: Alberta Health Services; 2020. Accessed November 8, 2021 from: https://www.albertahealthservices.ca/assets/info/cca/if-cca-adolescent-young-adult-patient-navigation.pdf
- 30. Watson LC, Vimy K, Anderson J, et al. Developing a provincial cancer patient navigation program utilizing a quality improvement approach part three: evaluation and outcomes. CONJ. 2016;26(4):276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pannier ST, Warner EL, Fowler B, et al. Age-specific patient navigation preferences among adolescents and young adults with cancer. JCE. 2019;34(2):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yee S, Buckett W, Campbell S, et al. A national study of the provision of oncofertility services to female patients in Canada. J Obstet Gynaecol Can. 2012;34(9):849–58. [DOI] [PubMed] [Google Scholar]
- 33. Yee S, Buckett W, Campbell S, et al. A national study of the provision of oncology sperm banking services among Canadian fertility clinics. Eur J Cancer Care. 2013;22(4):440–9. [DOI] [PubMed] [Google Scholar]
- 34. Anazodo A, Laws P, Logan S, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. 2019;25(2):159–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Canadian Partnership Against Cancer. Business case for oncofertility screening in the cancer system. Toronto, ON: Canadian Partnership Against Cancer; 2021. Accessed October 5, 2021 from: internal document (accessible upon request).
- 36. Dornisch A, Yang EH, Gruspe J, et al. Theory-guided development of fertility care implementation strategies for adolescent and young adult cancer survivors. JAYAO. 2021;10(5):512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sena LA, Sedhom R, Scott S, et al. Trainee-led quality improvement project to omprove fertility preservation counseling for patients with cancer. JCO Oncol Pract. 2022;18(3):e403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watson, L. Project Proposal: Improving the patient experience through the integration of oncofertility supports for adolescent and young adult cancer patients in Alberta and Northwest Territories. Calgary, AB, Canada: Alberta Health Services, Cancer Care Alberta; 2020. Internal Document: Available Upon Request. [Google Scholar]
- 39. Presley CJ, Krok-Schoen JL, Wall SA, et al. Implementing a multidisciplinary approach for older adults with cancer: geriatric oncology in practice. BMC Geriatrics. 2020;20:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guerard EJ, Nightingale G, Bellizzi K, et al. Survivorship care for older adults with cancer: U13 conference report. J Geriatr Oncol. 2016;7:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]