Abstract
Astaxanthin (AX) is an antioxidant which may spare endogenous carbohydrates and improve fat oxidation rates, thus improving metabolic flexibility. To date, no studies have attempted to examine the impact of AX in an overweight cohort, whom often suffer from metabolic inflexibility. Nineteen subjects (mean ± SD: age: 27.5 ± 6.3 years; height: 169.7 ± 9.0 cm; body mass: 96.4 ± 17.9 kg; BF%: 37.9 ± 7.0%; BMI: 33.4 ± 5.6 kg/m2; VO2peak: 25.9 ± 6.7 ml·kg−1·min−1) were recruited and supplemented with either 12 mg of AX or placebo (PLA) for 4 weeks. Subjects completed a graded exercise test on a cycling ergometer to examine changes in substrate oxidation rates. A total of 5 stages, each lasting 5 min and resistance increased 15 W each stage, were completed to examine changes in levels of glucose and lactate, fat and carbohydrate (CHO) oxidation rates, heart rate, and rating of perceived exertion (RPE). Although there were no changes found in rates of fat oxidation, blood lactate or glucose, or RPE (all p > 0.05), a significant decrease was observed in CHO oxidation from pre to post supplementation in the AX group only. Further, the AX group demonstrated a 7% decrease in heart rate across the graded exercise test. These findings suggest that 4 weeks of AX supplementation may offer some cardiometabolic benefits to overweight individuals, and be a favorable supplement for these individuals beginning an exercise program.
Keywords: Substrate oxidation, lactate, supplement, metabolic flexibility
INTRODUCTION
According to a National Health and Nutrition Examination Survey in 2017–2018, 73.6% of adults in America were classified as overweight (i.e., body mass index [BMI] of ≥ 25 kg/m2; 14). Interestingly, no country has been successful in slowing the rates of weight-gain although the association of excess adipose tissue and cardiometabolic disease are becoming more well-established (24). High levels of body fat are associated with chronic low-grade inflammation which can increase markers of oxidative stress and result in mitochondrial dysfunction (31, 32).
Provided these reports, there is a growing interest in practical interventions which may improve the population’s health and mitigate the progression of weight-gain and the risks associated with it.
Astaxanthin (AX) is a carotenoid with a red-orange pigment, found primarily in marine species including, shrimp, lobster, salmon, and microalgae (6). Research has shown AX to be a strong antioxidant, capable of improving markers of inflammation (25) and oxidative stress (16). These favorable findings of AX are likely due to its molecular structure, which consists of two β-ionone ring systems linked by a polyene chain (33). Indeed, it is the polyene chain that enables AX to quench singlet oxygen and thus, mitigating the effects of oxidative stress which may hamper components of the mitochondria, such as the carnitine system (6, 8). However, studies elucidating the mechanisms of AX effects on oxidative stress and metabolic health have largely been conducted in animal models (2, 8, 11,17, 18, 22, 25, 26). Therefore, human trials examining the impact AX supplementation on markers of metabolic health are warranted.
A practical and non-invasive method for evaluating metabolic health is by assessing metabolic flexibility (15). Briefly, metabolic flexibility is an individual’s cellular capacity to modify substrate oxidation to substrate availability (12, 15). Arguably, metabolic flexibility is best observed during exercise as the manipulation of exercise intensity or duration modify the cell’s preferential fuel source for oxidation. An inability to modify fuel oxidation is known as metabolic inflexibility and is commonly observed in individuals suffering from cardiometabolic dysfunction such as insulin resistance or type 2 diabetes mellitus (4, 5, 20). These individuals often have a predominant reliance on carbohydrate (CHO) oxidation and a diminished capacity to oxidize fats for energy (15, 29). Interestingly, Aoi et al. (2) demonstrated that AX may improve markers of metabolic flexibility in exercising mice, as their data showed an increase in fat oxidation rates following 4 weeks of AX supplementation. These findings contrast a more recent study which found no significant differences in fat oxidation rates between AX and placebo (PLA) in well-trained cyclists (10, 27). However, well-trained endurance athletes are already metabolically flexible as demonstrated by a robust mitochondrial environment (3, 19). Therefore, it is unlikely that a dietary supplement, such as AX, would manifest significant shifts in oxidation rates in such a cohort, although one study found a small (+ 0.09 g·min−1) yet significant increase in fat oxidation rates in elite cyclists (7). Although Aoi et al. (2) observed changes in an animal model, these findings could have far reaching implications for individuals suffering from metabolic inflexibility. Provided that nutrient oxidation is largely dependent on mitochondrial health and function (21), a shift from CHO to fats and a subsequent reduction in lactate appearance would indirectly demonstrate more robust mitochondria and an overall healthier individual.
Therefore, in light of these findings, the purpose of the current study was to examine the impact AX supplementation might have on markers of metabolic flexibility in overweight individuals. It was hypothesized AX supplementation would increase rates of fat oxidation, while decreasing CHO oxidation and blood lactate accumulation.
METHODS
Subject
Approval from the Institutional Review Board (IRB) was granted before recruiting subjects. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (23). Each subject completed a Physical Activity Readiness Questionnaire (PAR-Q; 34), a medical questionnaire, and provided their written and verbal consent to voluntarily participate in the present study. Inclusion criteria for each subject included the following: 1) between the ages of 18–45; 2) free of any cardiometabolic disease medication; 3) classified as overweight with a bodyfat percent (BF%) of ≥ 20% for males and ≥ 25% for females (1); 4) and not currently pregnant or actively trying to become pregnant. Additionally, subjects eliminated all dietary supplements, including antioxidants, at least two weeks prior to the start of the study. Using G-Power software (v3.1, Düsseldorf, Germany) and changes to BF% as a primary dependent variable, we determined a priori that 20 subjects would be sufficient to achieve a desired power of 0.85, using a moderate-high effect size, and an alpha level set at 0.05. Although 46 individuals were recruited, only 21 fit the inclusion criteria and were randomized into either an AX or PLA group following their first visit. Due to two subjects withdrawing from the study as a result of health concerns not related to the study procedures, 19 subjects were included in statistical analysis (mean ± SD: age: 27.5 ± 6.3 years; height: 169.7 ± 9.0 cm; body mass: 96.4 ± 17.9 kg; BF%: 37.9 ± 7.0%; BMI: 33.4 ± 5.6; VO2peak: 25.9 ± 6.7 ml·kg−1·min−1).
Protocol
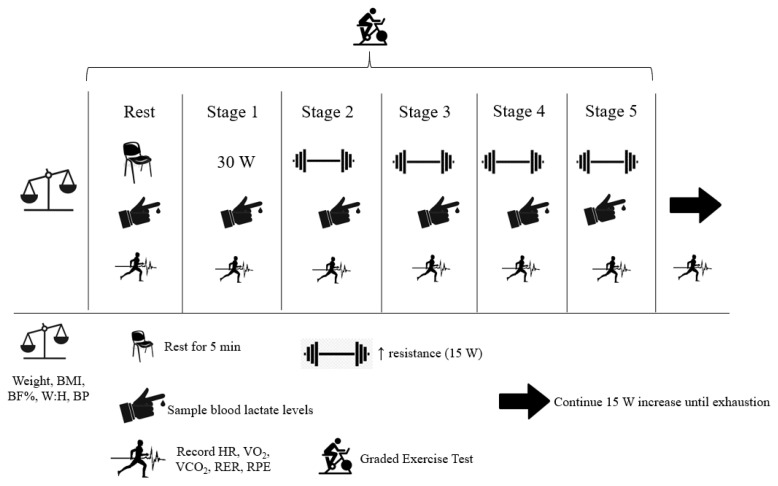
The current study implemented a double-blind, between-subject study design with dependent variables examined at multiple timepoints across each trial. To best observe changes in our selected dependent variables, subjects reported to the laboratory on two separate occasions (i.e. pre-post) following 4 weeks of AX supplementation (see Figure 1).
Figure 1.
Schematic overview of pre and post exercise trials.
During the first visit, subjects had their body composition assessed via bioelectrical impedance analysis (mBCA 514; Seca GmbH & Co., Hamburg, Germany) as well as height (Detecto, Webb City, MO, USA), mass (BWB-800, Tanita Inc. Tokyo, Japan) and blood pressure (BP) by the same, trained investigator pre-post intervention. Following the collection of anthropometric data, subjects who met inclusion and exclusion criteria were then asked to attempt to maintain their current dietary habits for the duration of the intervention. Dietary intake was then recorded pre-post intervention via 3-day food logs which consisted of 2 weekdays and 1 weekend day (i.e. Thursday, Friday, Saturday) to better reflect typical intakes. Subjects met with the primary investigator and were instructed how to complete the food logs, regarding quantity size and liquid amount estimations. Total energy intake, CHO, protein, and fats were subsequently analyzed for nutrient amounts using MyFitnessPal (MyFitnessPal, Inc., San Francisco, CA, USA, Version 21.18.5). Statistical analysis showed no significant differences in macronutrients or overall caloric load pre-post intervention for either group (p > 0.05; data not reported).
Following the collection of preliminary data, subjects then completed a graded exercise test on a cycle ergometer (Velotron, RacerMate, Seattle, WA, USA). Prior to the start of exercise, subjects donned a heart rate (HR) monitor (T31 Transmitter, Polar Electro, Kempele, Finland), established seat height, and were then connected to a metabolic cart (Parvo Medics TrueOne 2400, Sandy, UT, USA) to record cardiorespiratory measures for the calculation of fat and CHO oxidation rates. Following connection to the metabolic cart, subjects sat quietly for 5 min to collect resting oxidation rates (‘rest’). Subjects then straddled the cycle ergometer and began pedaling at an initial 30 W (females) or 50 W (males) for 5 min (Stage 1). For each subsequent stage, resistance increased by 15 W and lasted for 5 min (Stages 2–5). Following completion of 5 stages (90 W total for females and 110 W total for males), resistance then increased by 15 W every minute, until volitional exhaustion was achieved and the subjects’ VO2peak was recorded. The present protocol was pilot tested (n = 6) prior to the initiation of the present study and determined by the investigative team as acceptable for non-exercising and overweight individuals to all complete with minimal fluctuation in HR (± 2 bpm) during the last min of each stage.
During the last 30 s of each stage, subjects had their finger pricked (detailed further below) for the measurement of capillary lactate and glucose. Furthermore, HR and overall ratings of perceived exertion (RPE; 28) were recorded at the end of each stage. Due to the collection of substrate oxidation rates, subjects were required to arrive 5 h fasted and avoid alcohol consumption for 48 h prior to each trial. Subjects were also required to abstain from caffeine on the day of testing and avoid strenuous lower body exercise 48 h prior to each experimental trial.
All cardiorespiratory measures collected during the graded exercise test were averaged from expired gas. The first 240 seconds of each stage were excluded and the remaining 60 seconds were recorded using breath-by-breath data and averaged in two, 30-second cycles. Stoichiometric equations were utilized when measuring total fat and CHO oxidation rates (g·min−1). These equations assumed protein oxidation rates were negligible (~5%) and therefore, were ignored (13).
At the end of each stage during the graded exercise test, a sample of capillary blood was collected from the subjects’ finger. The subjects had their finger wiped with an alcohol swab and then allowed to air dry prior to a prick with a self-withdrawing safety lancet. The first drop of blood was wiped away and the second drop of blood (~5μL) was analyzed using a Lactate Plus Meter Blood Analyzer (Nova Biomedical Corporation, Waltham, MA, USA). Additionally, capillary blood glucose concentrations were analyzed using a Precision Xtra Blood Glucose Analyzer (Abbott Diabetes Care, Alameda, CA, USA).
The supplementation intervention was scheduled following Trial 1 and took place for 4 weeks before subjects reported back to the laboratory for Trial 2. Subjects were stratified based on BF%, to supplement with either 12 mg of encapsulated AX (AX and sunflower oil; 6 mg per capsule) or PLA (sunflower oil only) daily in the form of two gel capsules. Both the AX and PLA pills were similar in appearance (red and oval shaped) and size and all supplements were provided by AstaReal (AstaReal Inc., Nacka, Sweden). Subjects were advised to ingest one capsule in the morning and one capsule at night. Subjects were provided an undisclosed, but previously counted number of capsules in each bottle and were asked to return the remaining capsules upon their return for post-testing. Compliance ([capsules ingested]/56) x 100) less than 90% was defined as “not acceptable”. All subjects were above the 90% compliance threshold upon their return for repeat testing. Further, there were no statistical differences in baseline BF% between either supplement group (p = 0.87, see Table 1).
Table 1.
Subject Characteristics for PLA and AX groups (mean ± SD).
| PLA | AX | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| VO2 Peak (ml·kg−1·min−1) | 26.7 ± 7.0 | 26.6 ± 7.6 | 25.6 ± 6.3 | 24.1 ± 6.4 |
| Age (y) | 25.6 ± 4.2 | 30.2 ± 7.3 | ||
| Height (cm) | 169.1 ± 8.4 | 170.8 ± 9.6 | ||
| Mass (kg) | 94.1 ± 20.9 | 94.7 ± 20.6 | 96.2 ± 17.6 | 96.6 ± 17.5 |
| BMI (kg/m2) | 32.7 ± 5.3 | 32.9 ± 5.3 | 33.2 ± 5.5 | 33.4 ± 5.6 |
| BF % | 38.3 ± 8.3 | 38.6 ± 3.7 | 38.0 ± 6.9 | 38.2 ± 7.4 |
| Systolic BP (mm Hg) | 118 ± 12 | 124 ± 12 | 122 ± 10 | 126 ± 10 |
| Diastolic BP (mm Hg) | 82 ± 10 | 74 ± 8 | 82 ± 8 | 84 ± 10 |
PLA = placebo treatment; AX = astaxanthin treatment; BF% = bodyfat %; BP = blood pressure; BMI = body mass index.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). An alpha (α) level was set at ≤ 0.05 to be considered significant. Separate 3-way mixed-factorial analysis of variance (ANOVA; 2 × 2 × 6; treatment [PLA vs. AX] × condition [pre-post] × timepoint [all stages]) were used for between and within comparisons of substrate oxidation rates, blood lactate and glucose concentrations, RPE, and HR. A dependent t-test (pre vs. post) was used to measure changes in BF%, BP (systolic and diastolic), mass, VO2peak, and each diet variable within each group. A Fisher’s least significant difference (LSD) post hoc analysis was used in the instance of a significant main effect. Where significant interactions occurred, partial eta-squared (η2p: 0.01 = small effect; η2p: 0.09 = moderate effect; η2p: 0.25 = large effect) are reported. Where significant treatment effects occurred, Cohen’s d (9; trivial < 0.2, small for ≥ 0.2 to < 0.6, moderate for ≥ 0.6 to < 1.2, large for ≥ 1.2 to < 2.0, and very large for ≥ 2.0) were calculated. Partial eta square and Cohen’s d were calculated to provide effect sizes. T-tests were conducted using Microsoft Excel (v. 2019) and all other statistical procedures were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
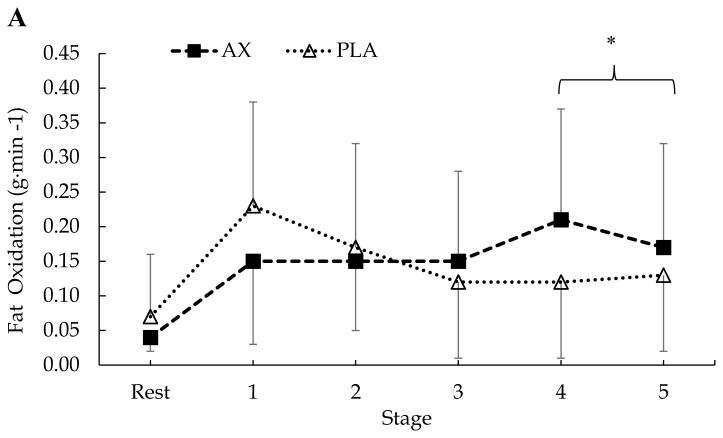
Regarding mean fat oxidation rates, AX supplementation had no effect as there was no significant treatment × condition × stage (p = 0.86), condition × stage (p = 0.86) or treatment × condition interaction (p = 0.89). There was a significant treatment × stage interaction (F = 4.13, p = 0.001, η2p = 0.10). Post hoc analysis indicated significantly higher fat oxidation rates overall in the AX group compared to PLA during stages 4 (p = 0.007, d = 0.75) and 5 (p = 0.001, d = 0.43; see Figure 2).
Figure 2.
Mean ± SD. Respiratory measures of (A) pre (AX vs. PLA) and (B) post (AX vs. PLA) fat oxidation rates. Differences were observed for treatment (AX vs. PLA; *p < 0.05). AX = astaxanthin; PLA = placebo.
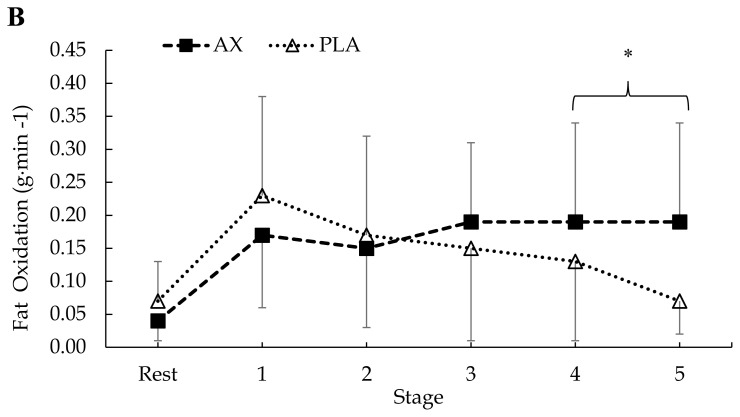
In terms of mean CHO oxidation, there was no treatment × condition × stage interaction (p = 0.79) or an interaction for condition × stage (p = 0.76). However, there was a significant treatment × condition interaction (F = 4.22, p = 0.04, η2p = 0.02). There was also a significant treatment × stage interaction (F = 2.72, p = 0.02, η2p = 0.07). Post hoc analysis indicated a significant overall decrease in CHO oxidation from pre to post supplementation in the AX group (p = 0.02, d = 0.23; see Figure 3). In addition, the AX group demonstrated significantly higher mean CHO oxidation rates during stage 3 compared to the PLA group (p = 0.04, d = 0.44).
Figure 3.
Mean ± SD. Respiratory measures of (A) pre (AX vs. PLA) and (B) post (AX vs. PLA) CHO oxidation rates. Differences were observed for condition (pre-post) with AX supplementation (*p < 0.05). AX = astaxanthin; PLA = placebo.
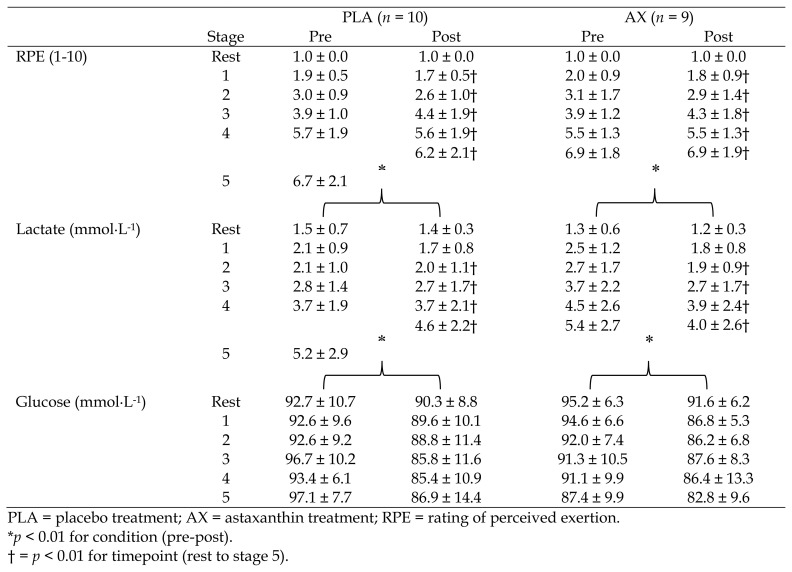
In terms of mean RPE data, there was no significant treatment × condition × stage (p = 0.99), treatment × stage (p = 0.90), condition × stage (p = 0.80) or treatment × condition interaction (p = 0.81). There was no main effect for condition (p = 0.61) or treatment (p = 0.23). However, there was a main effect for stage (F = 163.07, p < 0.001, η2p = 0.82). Post hoc analysis indicated a significant increase in RPE during each stage of exercise (p < 0.001; see Table 2).
Table 2.
Graded Exercise Test (mean ± SD).
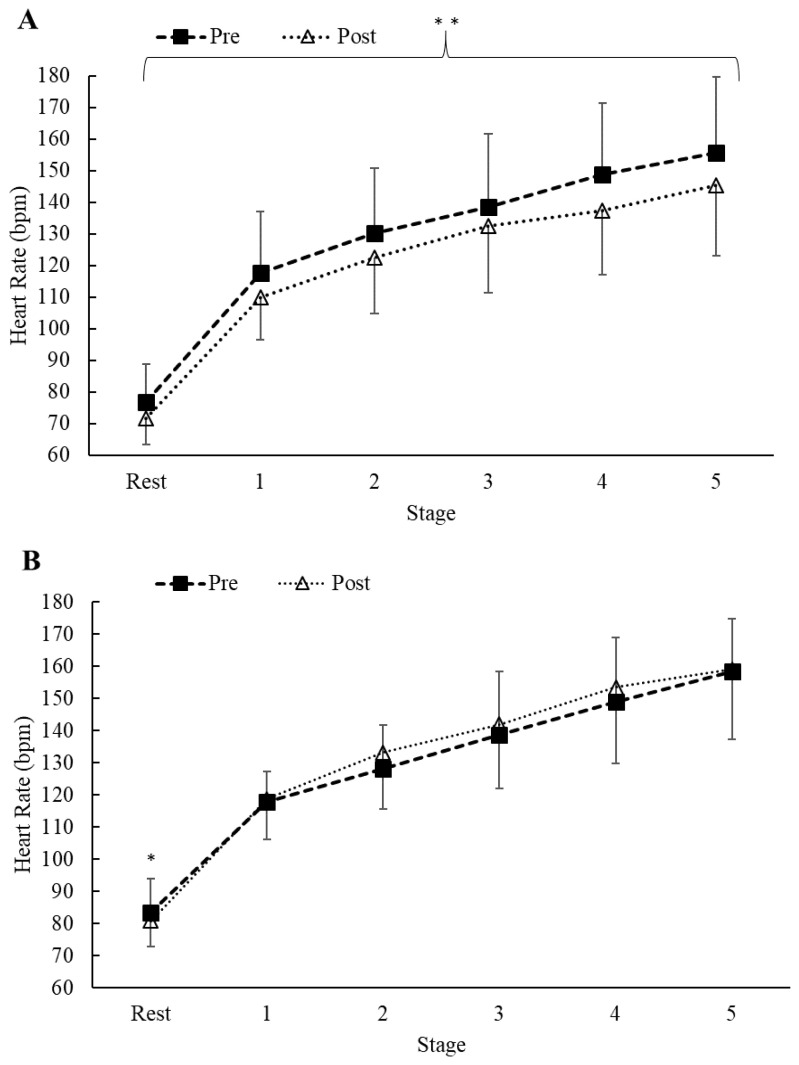
Regarding mean HR, there was no treatment × condition × stage interaction (p = 0.78) and no treatment × stage (p = 0.89) or condition × stage interaction (p = 0.98). However, a significant treatment × condition interaction was found (F = 16.23, p < 0.001, η2p = 0.08). Post hoc analysis indicated a significant decrease in mean HR from pre to post supplementation in the AX treatment (p < 0.001, d = 0.46; see Figure 4) which was not found in the PLA treatment (p = 0.26).
Figure 4.
Mean ± SD. Measures of (A) AX (pre vs. post) and (B) PLA (pre vs. post) HR. Differences were observed for treatment (AX vs. PLA) and condition (pre-post) with AX supplementation (**p < 0.05). HR = heart rate; AX = astaxanthin; PLA = placebo.
In terms of mean lactate concentrations, there was no treatment × condition × stage interaction (p = 0.67) and no treatment × stage (p = 0.45), condition × stage (p = 0.97), or treatment × condition interaction (p = 0.19). However, there was a main effect for stage (F = 29.26, p < 0.001, η2p = 0.45), and condition (F = 7.22, p = 0.005, η2p = 0.02). There was no change in lactate from resting to stage 1 during exercise, however, each subsequent stage demonstrated a significant increase (p < 0.001). In addition, there was a significant decrease in mean lactate concentrations in both groups from pre to post supplementation (p = 0.01; see Table 2).
With respect to glucose concentrations, there was no treatment × condition × stage interaction (p = 0.60) and no treatment × stage (p = 0.34), condition × stage (p = 0.89), or treatment × condition interaction (p = 0.68), however there was a main effect for condition (F = 23.53, p < 0.001, η2p = 0.12). Post hoc analysis indicated a significant decrease in glucose concentrations during exercise from pre to post supplementation in both groups (p < 0.001; see Table 2).
In terms of mean VO2peak, there was no condition × treatment interaction (p = 0.80), or main effect for treatment (p = 0.05) or time (p = 0.85; see Table 1). Regarding mean mass, no differences were noted for either AX (p = 0.36) or PLA (p = 0.25) following supplementation. As it relates to BF%, there were no differences found for either AX (p = 0.75) or PLA (p = 0.90; see Table 1) following supplementation. Regarding mean systolic BP, no differences were found for either AX (p = 0.07) or PLA (p = 0.36). Additionally, no differences were found for diastolic BP in either the AX (p = 0.88) or PLA (p = 0.86) groups (see Table 1).
DISCUSSION
The primary objective of this study was to investigate the impact of AX supplementation on multiple markers of metabolic flexibility, including changes in fat oxidation rates and blood lactate concentrations during a graded exercise test in overweight individuals. In agreement with previous investigations (10, 27), our study found no statistical differences in fat oxidation rates, blood lactate or glucose levels, body mass, BF%, RPE, or BP (all p > 0.05) as a result of AX supplementation. However, we did observe a significant decrease (~7%) in exercising HR, along with a small but significant decrease in CHO oxidation rates from AX supplementation only. These findings may suggest that AX supplementation offers an overweight cohort some cardiometabolic benefits during exercise.
While several studies in animal models have observed increases to rates of fat oxidation following AX supplementation (2, 18, 22, 26), the current study found no significant changes between AX and PLA (Figure 2B). Although AX is known to upregulate lipolytic enzymes (2, 7, 22), the lack of change to fat oxidation rates may explain why BF% and mass remained unchanged. To date, the effects of AX on fat oxidation rates appear mixed. Our findings are consistent with some previous human trials, which found no differences in rates of fat oxidation following AX supplementation in well-trained male cyclists (10, 27). However, in contrast, Brown et al. (7), who tested a recreationally trained population with AX for 7 days, found a significant increase (p = 0.03; ~0.1 g·min−1) in fat oxidation rates during the last km of a 40 km cycling trial. These discrepancies in fat oxidation may be partially explained by the varying fitness levels of the subjects. The current authors postulate that for highly trained endurance athletes nearing their maximal capacity to oxidize fat, supplementation with AX or any dietary supplement would likely not impact highly robust mitochondria. This would then explain the lack of changes to fat oxidation rates in well-trained subjects (10, 27), and support the changes observed in fat oxidation rates among recreationally trained subjects where there is a greater relative ceiling for extracting and oxidizing endogenous fat sources (7). Based on these findings, our team speculated that AX would therefore impact fat oxidation rates to an even greater extent in subjects who expressed some characteristics of metabolic inflexibility (e.g. overweight). Interestingly, our hypothesis was not supported by our data. Upon further evaluation, the lack of statistical changes in fat oxidation rates is likely due to our adoption of a parallel group design compared to a within design. Among the various previous investigations examining changes to substrate oxidation rates following AX supplementation, only Brown et al. (7) observed differences in similar markers and their study is also the only one to incorporate a within design. Provided the high interindividual variability that exists among substrate oxidation rates even when fitness status is comparable, these findings suggest that future studies attempting to examine changes to fat oxidation rates following AX supplementation should incorporate similar within-crossover designs to protect the statistical ‘sensitivity’ of these measures.
In addition to changes in fat oxidation rates, we hypothesized that blood lactate levels and CHO oxidation rates would decrease following AX supplementation. Although we observed decreases to circulating lactate and glucose levels in both AX and PLA following the intervention, we also observed a decrease in CHO oxidation rates only in the AX group (Figure 3B). These findings are interesting as it is unusual to observe a decrease in CHO oxidation rates without a subsequent increase in fat oxidation. This strengthens our earlier speculation suggesting that if there were subsequent changes to fat oxidation rates then the incorporation of a between design likely prevented statistical changes, if any, from being observed. Provided only the AX group experienced a significant decrease in CHO oxidation rates, it is reasonable to assume AX may have impacted fat oxidation rates, but the interindividual variance underpowered any differences and likely reduced any statistical sensitivity of this outcome. As for the decreases observed in lactate and glucose concentrations among both groups following the supplementation period, these simply appear to reflect increases in daily levels of physical activity (e.g. improved exercise economy). Although subjects were told to continue their normal daily exercise routines and dietary patterns, our team did not collect any activity logs across the intervention. It is quite likely that subjects increased their activity levels during the 4-week intervention and thus, presents a limitation to the current study.
Although AX did not impact several of our chosen markers, we did find evidence showing possible cardiovascular effects. Similar to Talbott et al. (30) who found a 10% decrease in average HR during submaximal running in recreational runners, the present study also found a decrease in average exercising HR by ~7% (Figure 4A). Specifically, the AX group had a lower exercising HR post treatment compared to the PLA group (p < 0.001). Although our team did not collect mechanistic data, a supporting argument for our findings may be due to the strong antioxidant properties of AX. During exercise, AX counteracts the superoxide induced nitric oxide degradation by donating an electron and therefore, stabilizing nitric oxide (17). Hence, the restoration of the nitric oxide-induced vasodilation could result in a decreased HR during exercise as a result of improving vascular elastin. One additional explanation may come from AX capabilities to modulate the autonomic function by increasing vagus nerve innervation and thus, hyperpolarizing cardiac cells which would result in a lower exercising HR (11). Regardless of the mechanisms, due to the strong antioxidant and anti-inflammatory properties of AX and its lack of interactions with prescription drugs, AX may serve as a natural pharmacological agent to treat some markers of heart disease while mitigating the risk of possible drug interactions that typical beta-blocker users may experience.
Some limitations exist within the present study that should be addressed. Although our power analysis required 20 subjects to adequately detect statistical changes, our study finished with only 19 subjects. Additionally, a potential increase in physical activity levels by our subjects, along with the adoption of a between design may have masked further changes to our primary dependent variables.
In conclusion, AX supplementation did not significantly impact fat oxidation rates in an overweight population. However, a decrease in CHO oxidation rates as well as a decrease in exercising HR was observed in the AX group only. These data are the first to suggest there may be cardioprotective properties offered to overweight individuals supplementing with AX and may be of interest to clinicians as an alternative to prescription medication when prescribing exercise to overweight individuals.
ACKNOWLEDGEMENTS
The authors would like to thank AstaReal Inc. and Dr. Karen Hecht for providing the astaxanthin and placebo supplements, as well as suggesting the adopted supplement dose (~12 mg/d) for the present study. We would also like to thank the Center for Student Grants through the University of North Alabama for financial assistance with this study.
REFERENCES
- 1.ACSM’s Guidelines for exercise testing and prescription. 11th ed . Lippincott Williams & Wilkins; Philadelphia, Pa USA: 2021. pp. 64–67. [Google Scholar]
- 2.Aoi W, Naito Y, Takanami Y, Ishii T, Kawai Y, Akagiri S, Kato Y, Osawa T, Yoshikawa T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem Biophys Res Commun. 2008;366(4):892–897. doi: 10.1016/j.bbrc.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol Endocrinol Metab. 1999;276(1):106–117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- 4.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. J Diabetes. 2000;49(12):2102–2107. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- 5.Blaak EE, Wagenmakers AJM, Glatz JFC, Wolffenbuttel BHR, Kemerink GJ, Langenberg CJM, Heidendal GAK, Saris WHM. Plasma free fatty acid utilization and fatty acid binding protein content are diminished in forearm skeletal muscle of type 2 diabetic subjects. Am J Physiol. 2000;279(1):146–154. doi: 10.1152/ajpendo.2000.279.1.E146. [DOI] [PubMed] [Google Scholar]
- 6.Brown DR, Gough LA, Deb SK, Sparks SA, McNaughton LR. Astaxanthin in exercise metabolism, performance and recovery: a review. Front Nutr. 2018;76(4):1–9. doi: 10.3389/fnut.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DR, Warner AR, Deb SK, Gough LA, Sparks SA, McNaughton LR. The effect of astaxanthin supplementation on performance and fat oxidation during a 40 km cycling time trial. J Sci Med Sport. 2021;24(1):92–97. doi: 10.1016/j.jsams.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Chien YH, Pan CH, Hunter B. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquac Res. 2003;216(1–4):177–191. [Google Scholar]
- 9.Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. pp. 19–30. [Google Scholar]
- 10.Earnest CP, Lupo M, White KM, Church TS. Effect of astaxanthin on cycling time trial performance. Int J Sports Med. 2011;32(11):882–888. doi: 10.1055/s-0031-1280779. [DOI] [PubMed] [Google Scholar]
- 11.Escutia-Reyes D, Oros-Pantoja R, Reyes-Lagos JJ, Pliego-Carrillo AC, Ledesma-Ramírez CI, Rodríguez-Muñoz D, Torres-García E. Effects of astaxanthin on pulse rate variability of mice under chronic stress. Latin Amer Conference on Biomed Engineering. 2019;(75):718–723. [Google Scholar]
- 12.Fernández-Verdejo R, Bajpeyi S, Ravussin E, Galgani JE. Metabolic flexibility to lipid availability during exercise is enhanced in individuals with high insulin sensitivity. Am J Physiol Endocrinol Metab. 2018;315(4):715–722. doi: 10.1152/ajpendo.00126.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 14.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, trends 1960–1962 through 2017–2018. NCHS Health E-Stats. 2020 Web. [Google Scholar]
- 15.Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;25(5):1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewlings SJ. Human health benefits of astaxanthin derived from Haematococcus Pluvialis: a review. EC Nutr. 2019;14(10):902–916. [Google Scholar]
- 17.Hussein G, Nakamura M, Zhao Q, Iguchi T, Goto H, Sankawa U, Watanabe H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol Pharm Bull. 2005;28(1):47–52. doi: 10.1248/bpb.28.47. [DOI] [PubMed] [Google Scholar]
- 18.Ikeuchi M, Koyama T, Takahashi J, Yazawa K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol Pharm Bull. 2006;29(10):2106–2110. doi: 10.1248/bpb.29.2106. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol. 2013;114(3):344–350. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab. 1999;277(6):1130–1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 21.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu PH, Aoi W, Takami M, Terajima H, Tanimura Y, Naito Y, Itoh Y, Yoshikawa T. The astaxanthin-induced improvement in lipid metabolism during exercise is mediated by a PGC-1α increase in skeletal muscle. J Clin Biochem Nutr. 2014;54(2):13–110. doi: 10.3164/jcbn.13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013 a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohgami K, Shiratori K, Kotake S, Nishida T, Mizuki N, Yazawa K, Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. IOVS. 2003;44(6):2694–2701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- 26.Polotow TG, Vardaris CV, Mihaliuc AR, Gonçalves MS, Pereira B, Ganini D, Barros MP. Astaxanthin supplementation delays physical exhaustion and prevents redox imbalances in plasma and soleus muscles of Wistar rats. Nutrients. 2014;6(12):5819–38. doi: 10.3390/nu6125819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Res PT, Cermak NM, Stinkens R, Tollakson TJ, Haenen GR, Bast A, Van Loon LJ. Astaxanthin supplementation does not augment fat use or improve endurance performance. Med Sci Sports Exerc. 2013;45(6):1158–1165. doi: 10.1249/MSS.0b013e31827fddc4. [DOI] [PubMed] [Google Scholar]
- 28.Robertson RJ, Goss FL, Dube J, Rutkowski J, Dupain M, Brennan C, Andreacci J. Validation of the adult OMNI scale of perceived exertion for cycle ergometer exercise. Med Sci Sports Exerc. 2004;36(1):102–108. doi: 10.1249/01.MSS.0000106169.35222.8B. [DOI] [PubMed] [Google Scholar]
- 29.San-Millán I, Brooks GA. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sports Med. 2018;48(2):467–479. doi: 10.1007/s40279-017-0751-x. [DOI] [PubMed] [Google Scholar]
- 30.Talbott S, Hantla D, Capelli B, Ding L, Li Y, Artaria C. Effect of astaxanthin supplementation on cardiorespiratory function in runners. EC Nutr. 2016;11(6):253–259. [Google Scholar]
- 31.Vahdat K, Azizi F, Zandi K, Assadi M, Nabipour I. Chronic inflammation is correlated with percentage of body fat independent of the burden of infection. Inf. 2012;35(4):1322–1329. doi: 10.1007/s10753-012-9445-6. [DOI] [PubMed] [Google Scholar]
- 32.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J of Obes. 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 33.Visioli F, Artaria C. Astaxanthin in cardiovascular health and disease: mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017;8(1):39–63. doi: 10.1039/c6fo01721e. [DOI] [PubMed] [Google Scholar]
- 34.Warburton DER, Jamnik VK, Bredin SSD, Gledhill N. The physical activity readiness questionnaire (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+) HFJC. 2014;4(2):3–23. [Google Scholar]