Abstract
The recent pig heart transplant in a patient at the University of Maryland Medical Center has stimulated renewed interest in the xenotransplantation of organs from genetically engineered pigs. The barriers to the use of pigs as sources of organs have largely been overcome by 2 approaches – (1) the deletion of expression of the three known pig carbohydrate xenoantigens against which humans have preformed antibodies, and (2) the transgenic introduction of human ‘protective’ proteins, such as complement-regulatory proteins. These gene modifications, coupled with immunosuppressive therapy based on blockade of the CD40/CD154 costimulation pathway, have resulted in survival of baboons with life-supporting pig heart grafts for almost 9 months. The initial clinical success at the University of Maryland reinforces encouraging preclinical results. It suggests that pig hearts are likely to provide an effective bridge to an allotransplant, but their utility for destination therapy remains uncertain. Because of additional complex immunobiological problems, the same approach has been less successful in preclinical lung xenograft transplantation, where survival is still measured in days or weeks. The first formal clinical trials of pig heart transplantation may include patients who do not have access to an allotransplant, those with contraindications for mechanical circulatory support, those in need of retransplantation or with a high level of panel-reactive antibodies. Infants with complex congenital heart disease, should also be considered.
Keywords: clinical, heart, lung, nonhuman primate, pig, xenotransplantation
Xenotransplantation using organs from genetically modified nonprimate mammals offers a potentially unlimited supply of replacement organs. However, clinical translation has been impeded by vigorous innate and adaptive immune responses when organs from pigs – the species being developed for clinical xenotransplant indications – are transplanted into primates. Additional barriers include incompatibilities in thromboregulatory and ‘self-recognition’ molecular interactions between pigs and humans, and the risk of potentially contagious infection being transmitted from the pig to the xenograft recipient, and then to other humans.1,2 In response to the recent report of a clinical pig heart transplant in Maryland, we here review the current status of heart and lung xenotransplantation, and their prospects as viable therapies for end-stage cardiopulmonary diseases.
Thoracic organ xenotransplantation: brief history and recent experience
Even before the famous first human heart allotransplant by Barnard in 1967,3 in 1964 Hardy performed the world’s first clinical cardiac transplant using a chimpanzee heart that proved inadequate to support the circulation of the recipient.4 Subsequent attempts, involving nonhuman primate, sheep, and pig hearts, were also unsuccessful (Table 1). However, Bailey’s transplant of a baboon heart into an infant girl5 did much to stimulate the successful development of pediatric heart allotransplantation. Clinical lung xenotransplantation has not been reported.
Table 1.
World Experience in Clinical Heart Xenotransplantation93
| Year | Surgeon | Donor | type | Patient survival (days) |
|---|---|---|---|---|
| 1964 | Hardy | Chimpanzee | O | <1 |
| 1968 | Cooley | Sheep | O | <2 |
| 1968 | Ross | Pig | H | <3 |
| 1968 | Ross | Pig | Perfused with human blood but not transplanted | <4 |
| 1969 | Marion | Chimpanzee | ?O | <5 |
| 1977 | Barnard | Baboon | H | <6 |
| 1977 | Barnard | Chimpanzee | H | 4 |
| 1964 | Bailey | Baboon | O | 20 |
| 1992 | Religa | Pig | O | 1 |
| 1996 | Baruah | Pig | O | 7 |
| 2022 | Griffith | Pig | O | ~60 |
H heterotopic (auxiliary) heart transplantation, O orthotopic heart transplantation.
On January 7, 2022, the first clinical xenotransplant of a heart from a genetically-engineered pig (with 10 genetic modifications) was carried out at the University of Maryland, based to some extent on preclinical studies in Munich.6 The 57 years old recipient experienced good heart function for 2 months before succumbing in the context of graft dysfunction. This clinical experiment surprised and energized the thoracic transplant and xenotransplantation communities.
Developing ‘biocompatible’ pigs
Rationale for development
The historic use of organs from primate species raised ethical concerns,7 faced formidable logistical barriers, and posed potential risks of the transfer of infectious microorganisms.8 Focus therefore shifted to pigs as the organ source, which have advantages in (1) better size-matching with humans, (2) favorable breeding characteristics (faster sexual maturity, shorter pregnancy, greater number of offspring, and lower costs for husbandry and propagation), and (3) the feasibility of genetic modification.9 The short reproductive cycle of pigs and the development of in vitro fertilization and cloning technologies for this species enabled application of gene-editing to address the biologic obstacles of’ pig-to-human transplantation (summarized below).
Anti-pig antibodies
When wild-type (i.e., genetically unmodified) pig organs are exposed to human or NHP blood, antibody-mediated endothelial injury occurs within minutes, driven largely by binding of preformed ‘natural’ antibodies to the pig vascular endothelial cells, leading to complement activation and graft loss from hyperacute rejection.1 The majority of these natural human anti-pig antibodies are targeted at 3 carbohydrate antigens (Table 2), of which galactose-α 1,3-galactose (Gal)10 appears to be the most important. Work in the 1990s focused on elimination or neutralization of these antibodies utilizing plasmapheresis or immunoadsorption by immunoaffinity columns, which delayed, but did not prevent, rejection.11–13
Table 2.
Known Carbohydrate Xenoantigens Expressed on Pig Celts
| Carbohydrate antigen (abbreviation) | Responsible enzyme | Gene-knockout pig |
|---|---|---|
| Galactose-α1,3-galactose (Gal) | α1,3-galactosyltransferase | GTKO |
| N-glycolylneuraminic acid (Neu5Gc) | Cytidine monophosphate-N-acetylneuraminic acid hydroxylase | CMAH-KO |
| Sda | β-1,4N-acetylgalactosaminyl-transferase 2 | β4GalNT2-KO |
In 2003, the first pigs that did not express Gal were produced by knockout of the a1,3-galactosyltransferase gene (GTKO pigs)14 (Table 2). At the time, gene-editing techniques were less site-specific and very complex. Subsequent development of many gene editing technologies, like ZFNs, TALENS, and CRISPR-Cas9 (Table 3), enabled more precise and complex gene modification.15 In this regard, reengineering of Cas9 by Charpentier and Doudna made CRISPR-Cas9 a capable cost-effective technology and enabled high-precision targeting and editing of genomic loci, which has had a revolutionary impact on the life sciences. Those techniques facilitated additional deletion of expression of the remaining xenoantigens, yielding double16 and triple (TKO) knockout pigs.17
Table 3.
Timeline for Application of Evolving Techniques for Genetic Engineering of Pigs Employed in Xenotransplantation
| Year | Technique |
|---|---|
| 1992 | Microinjection of randomly-integrating transgenes |
| 2000 | Somatic cell nuclear transfer (SCNT) |
| 2002 | Homologous recombination |
| 2011 | Zinc finger nucleases (ZFNs) |
| 2013 | Transcription activator-like effector nucleases (TALENs) |
| 2014 | CRISPR-Cas9a |
CRISPR-Cas9 = clustered randomly interspaced short palindromic repeats and the associated protein 9.
Complicating preclinical assessment of TKO organs, the TKO modification has unveiled or activated a ‘fourth’ xenoantigen that may be associated with antibody-mediated rejection in NHPs, but not in humans.18
Complement activation
Complement activation, physiologically triggered in circumstances where the innate immune system senses ‘foreign invasion’ or ‘self-injury,’ is regulated by negative feedback mechanisms to avoid over-activation and collateral damage to healthy host tissues.19 This is achieved by the expression of several ‘protective’ complement pathway regulatory proteins (CPRPs) on the vascular endothelial cells,20 for example, decay accelerating factor (CD55); membrane cofactor protein (CD46), and membrane-attack-complex-inhibitory protein (CD59). CPRPs are relatively species-specific,21 and pig CPRPs are less effective in controlling human complement-mediated injury than are human CPRPs. The expression of human CPRPs on pig cells reduces complement-mediated cell injury,22 and has been associated with prolonged pig graft survival in a variety of NHP models.23–25
Coagulation pathway dysregulation
Even in the absence of detectable antipig antibodies, diffuse microvascular thrombosis in the graft (thrombotic microangiopathy) and consumptive coagulopathy in the recipient were observed after transplanting GTKO pig organs that expressed human CPRPs.26 This observation suggested that incompatibilities between pig and human thromboregulatory molecules might be physiologically consequential.27,28 Pig thrombomodulin (TBM) binds human thrombin, but is less potent in activating protein C. Its cofactor, pig endothelial protein C receptor (EPCR), is also less efficient in activating protein C, and hence in regulating the coagulation process.29 Pig tissue factor pathway inhibitor (TFPI) may be inefficient in regulating human tissue factor-initiated coagulation,30 although this view has been questioned.31
Coagulation dysfunction is more problematic in regard to pig lung transplantation. Relatedly, pig von Willebrand factor (vWF) aggregates and activates human platelets spontaneously through aberrant non-physiologic interaction with human glycoprotein (GP)Ib,32,33 a platelet surface membrane glycoprotein that functions as a receptor for vWF. Blocking the GPIb-binding site for vWF34 or ‘humanizing’ vWF reduces platelet sequestration during ex vivo perfusion of lungs with human blood, and after pig-to-baboon lung transplantation.35
Once activated, platelets propagate platelet and leukocyte aggregation through interactions mediated by GPIIb/IIIa, ADP- P2Y12, P- and E-selectins, integrins, and galectins, among others, leading to progressive thrombosis and necrotic destruction of the graft1,36–38 (Figure 1). As a consequence, each of these procoagulant and proadhesive interactions are logical targets to reduce physiologically inappropriate clotting and inflammation after cell and organ xenotransplantation.
Figure 1.
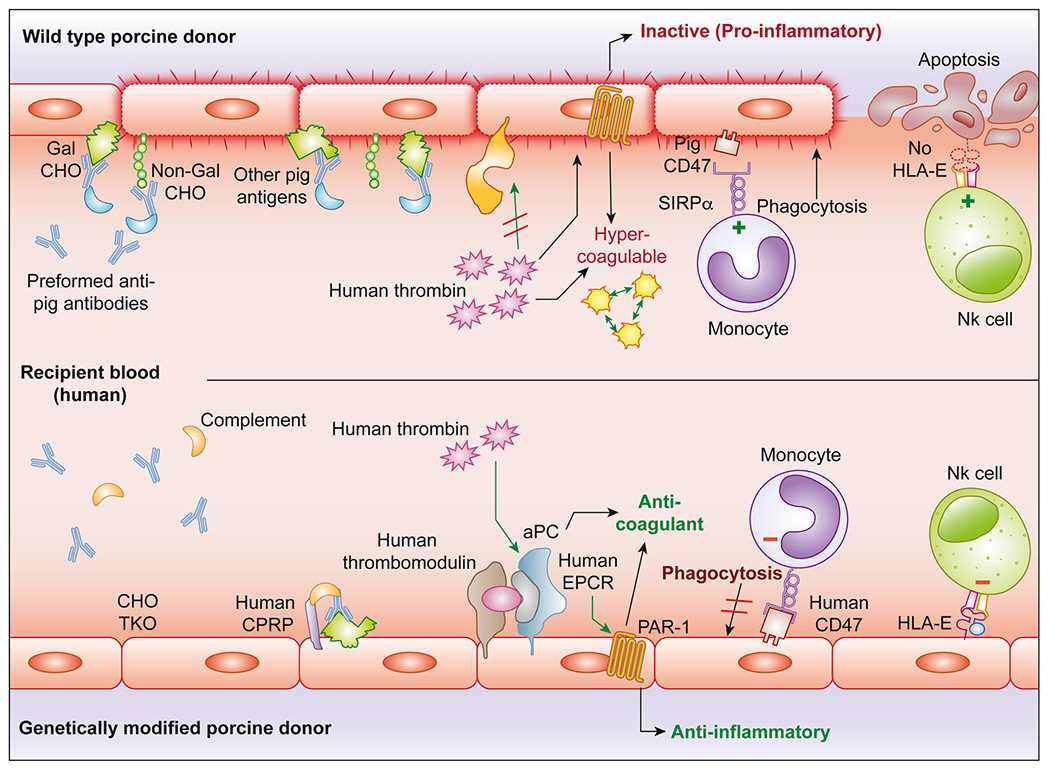
Genetic modifications designed to address mechanisms of xenograft injury: Examples of genetic modifications designed to prevent known xenograft injury (top) include Gal a 1-3Gal and 2 other carbohydrates (triple knockout, TKO) and expression of human complement pathway regulatory proteins (hCPRPs) and coagulation pathway regulatory proteins, eg, thrombomodulin and endothelial protein C receptor (bottom). Absence of carbohydrate antigens and expression of human complement and coagulation pathway regulatory molecules reduce endothelial activation and injury.
Coagulation dysfunction, together with an associated inflammatory response, has proved a major hurdle in achieving prolonged pig lung survival in NHPs.39
Self-identification
Cluster of differentiation molecule 47 (CD47) is a self-recognition marker that inhibits phagocytosis of CD47+ cells by macrophages and other innate immune ‘scavenging’ cell populations.40,41 When CD47 was introduced into GalT-KO pig cells by genetically engineering, bone marrow cells remained detectable in the circulation for days rather than minutes.42 Recent reports describe improved results associated with GTKO.hCPRP lungs and kidneys that additionally express CD47.43,44 While perhaps less essential than hCPRP or thromboregulatory genes, expression of human ‘self’ may prove advantageous for heart and lung xenografts.
Rapid pig organ growth after transplantation
Rapid growth and hypertrophy have been documented in pig hearts (and kidneys) implanted into NHPs, likely associated with differences in the rates of growth between pigs and NHPs.45,46 Cardiac xenograft hypertrophy can be reduced by hypotensive therapy and mTOR inhibition.46 It might be avoided by using pigs that grow slowly, for example, miniature swine, or have been genetically engineered to reach smaller size at maturity, for example, by knock-out of growth hormone receptors.47
Potential infectious concerns
Pigs could potentially transmit to humans a wide range of bacteria, parasites, and viruses.48,49 The FDA will require that the organ-source pigs be bred and housed in clean, biosecure conditions, and be certifiably free of pig organisms that are known pathogens for humans. Thus, the infections that are most likely to affect recipients of pig xenografts are the same as those that typically occur in immunosuppressed recipients of allografts (e.g., CMV reactivation, opportunistic bacterial infections), for which effective treatments are generally available.
In the 1990s, concerns were raised regarding the possibility of humans being infected by porcine endogenous retroviruses (PERVs) that are present within the genome of all pig cells.50 At that time, the pandemic spread of the human immunodeficiency virus (HIV), an exogenous retrovirus, helped feed these fears, appropriately raising concerns for regulatory authorities, and depressing public (and investor) enthusiasm for xenotransplantation.1,51
Reassuringly, PERV transmission to primates has never been observed in preclinical or clinical studies.52 In addition, (1) multiple innovative strategies have been developed to inhibit possible PERV transmission,53 (2) PERV-deleted pigs have been produced utilizing CRISPR-based technology,54,55 and (3) potent antiretroviral drugs that were developed to treat HIV are effective in vitro against PERV.56 Consequently, the risk of PERV infection for a given xenograft recipient appears to be very low; infection or disease, if either occurs, will likely be treatable; and subsequent transmission or pandemic infection seems improbable.
In summary, harvesting organs from pigs bred and raised under strictly controlled ‘designated pathogen-free’ (DPF) conditions57 could decrease the risk of infection to less than that seen in recipients of allografts, where the human donor might have been exposed to a variety of pathogens, and in whom the available screening strategies are time-limited. As long as a surveillance strategy designed to detect both known and ‘unknown unknown’ pathogens is consistently deployed, xenotransplantation may prove to be safer than contemporary allotransplantation with respect to the risk of donor-transmitted infection.
Preclinical progress
Cardiac xenotransplantation
Two valuable lessons have been learned from preclinical in vivo organ xenotransplantation studies. First, conventional immunosuppressive therapy (i.e., tacrolimus-based) is less effective in xenotransplantation than in allotransplantation.58 Rather, blocking the CD154/CD40 costimulatory pathway, first introduced into the pig-to-NHP model by Buhler et al in 2000,59 results in substantial improvement in graft survival. Blockade of the CD28/B7 costimulatory pathway is less successful.60,61 Second, pig hearts seem to be more susceptible to ischemia-reperfusion injury than human hearts.62 Längin et al. addressed this problem by using non-ischemic heart preservation by continuous perfusion with 8°C Steen solution.63,64 Consistent survival of 3 to 6 months was reported using GTKO/hCD46/hTBM hearts and blockade of the CD40/CD154 pathway.45
Encouraging with respect to long-term viability of pig heart xenografts, in a heterotopic (non-life-supporting) model (Figure 2), GTKO/hCD46/hTBM heart xenografts consistently survived for as long as CD40-blocking was given, in one case up to 945 days.65,66 In this context, the Langin and Brenner reports mark an important benchmark, reaching the prerequisite conditions for embarking on a clinical xenotransplantation trial that were proposed by the Advisory Committee to the International Society for Heart and Lung Transplantation (ISHLT) in 2000.67
Figure 2.
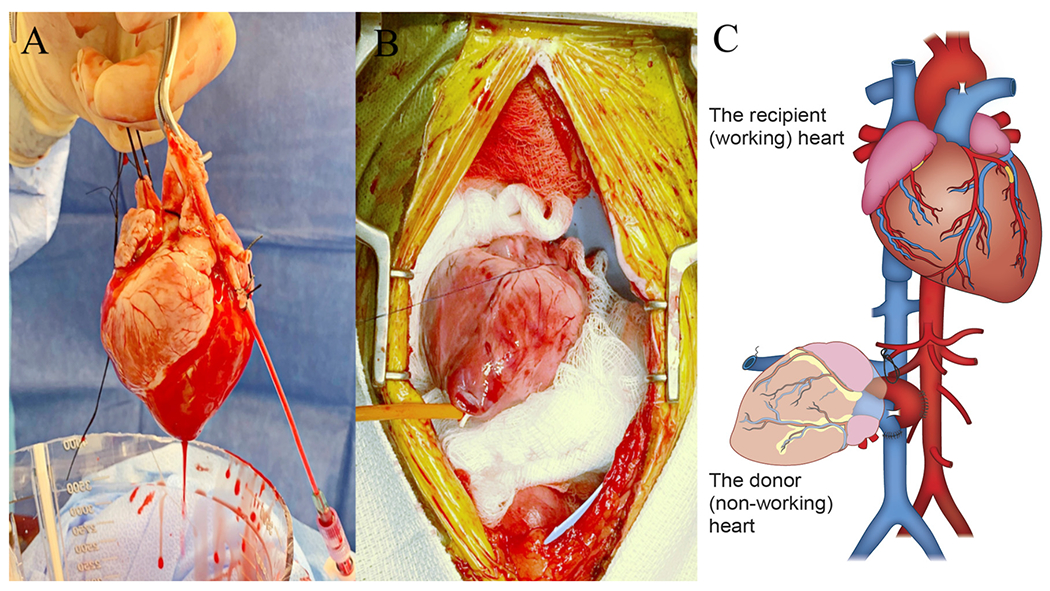
Experimental heterotopic non-working heart transplantation: After harvesting the pig heart and perfusing it for 30 minutes in ex vivo (A), it is implanted in heterotopic position in baboon abdomen (B), by anastomosing the donor aorta to the recipient abdominal aorta and the donor pulmonary artery to the recipient inferior caval vein, after legating both donor caval veins and closing the left atrium (C).
Nevertheless, it should be noted that to date the longest period of life support by a pig heart in a NHP has been limited to <9 months.68 Based on this data and the recent Maryland clinical experience, we suggest that at present pig heart xenotransplantation might most appropriately be considered as a bridge to allotransplantation. In our estimation, to justify heart xenotransplantation trials as ‘destination therapy,’ consistent success in preclinical models, similar to that recently reported6,45 or demonstration of more durable success in a ‘compassionate use’ clinical application – will be necessary.
Lung xenotransplantation
Lung xenotransplantation research to date has primarily employed ex vivo models incorporating human blood perfusion to evaluate the effect of genetic modification of the pig and/or drug treatment on the rise in pulmonary vascular resistance and loss of vascular barrier function characteristic of pig lung injury.69 The knowledge gained, however, has translated into relatively limited improvement in life-supporting lung function and recipient survival in the in vivo pig-to-NHP lung xenograft model.39,69 Probably because of the more extensive endothelial surface area, pro-inflammatory resident immune cells, and anatomic vulnerability to even localized injury flooding adjacent airways, lung injury is more difficult to prevent, and thus in vivo lung xenograft survival remains limited to days or weeks.39,70
Increasingly sophisticated genetically-engineered pigs have been produced with 10 or even 15 gene edits,1–71 including those designed to address some of the mechanisms of lung injury identified in the ex vivo lung perfusion model and partially validated in vivo.39 We speculate that adding a humanized vWF modification35 in the context of these mechanism-directed gene edits will be necessary, and may be sufficient, to enable prolonged lung graft survival in our non-human primate model, so as to eventually justify an initial clinical trial.
Prospects for clinical heart or lung xenotransplantation
What additional evidence is needed to justify regulatory approval for a definitive, ‘qualifying’ trial of clinical heart xenotransplantation? The pig sources of hearts in the Munich studies (with 3 genetic modifications) were suitable for transplantation into NHPs, but may not prove to be optimal for transplantation into human patients.72 The University of Maryland team provided encouraging results following the transplantation in baboons of hearts from pigs with 10 genetic manipulations, designed to be fully ‘biocompatible’ with humans, thus enabling the US Food and Drug Administration (FDA) to approve a single initial ‘compassionate use’ clinical study.
Although the predictive value of the NHP model has never been tested and may introduce confounding immune barriers,73 several important remaining questions can potentially be answered in preclinical models: (1) define a ‘necessary and sufficient’ pig phenotype, (2) validate a consistently effective method of heart preservation, (3) identify a consistently effective, safe immunosuppressive regimen, and (4) test candidate strategies to prevent or manage graft hypertrophy, when evident. Cautious clinical experimentation is justifiable in the hands of experienced investigators, not least because information gained will determine whether or not NHP preclinical studies are informative.
We gauge that additional advances in understanding and modulating the mechanisms of lung xenograft injury will be required before pig lungs can emerge as a viable clinical option. Hopefully, with further experience in the pig-to-NHP lung transplantation model and from experience with clinical xenotransplantation of the heart and other cells and organs, the remaining barriers to clinical pig lung transplantation will also be resolved.
Patient selection for clinical trials of pig heart transplantation
In our estimation, selection of patients for initial xenotransplantation trials should adhere to the general guidelines for heart allotransplant candidacy.74 Until the actual risks and complications associated with receipt of a life-supporting pig heart are defined by clinical experience, basic ethical principles75 will dictate first enrolling patients who are unlikely to have access to, or to benefit from, currently-available therapeutic alternatives. These patients include those with:
Relative or absolute contraindications to mechanical circulatory support
Patients with restrictive or hypertrophic cardiomyopathies or severe right ventricular dysfunction experience a high morbidity and mortality after implantation of a left ventricular assist device (LVAD). Furthermore, the presence of a dysfunctional mechanical valve, or a degenerated bioprosthesis (e.g., associated with mitral or aortic stenosis, or aortic insufficiency), or an atrial or ventricular septal defect, greatly complicate bridging or destination therapy with any form of mechanical circulatory support (LVAD or BiVAD). These patients often exhibit rapid and unpredictable deterioration, and might benefit from timely access to a pig heart as a bridge to allotransplantation.
High titers of broadly panel-reactive anti-HLA antibodies (PRA)
High PRA candidates (1) experience long waiting times for allotransplantation and a high wait-list mortality, (2) may require potentially high-risk and incompletely effective treatment to reduce anti-HLA titers, and (3) experience higher rates of rejection and early graft vasculopathy even after receipt of a ‘cross-match negative’ heart allograft. We caution to initially avoid patients with a high PRA who exhibit cross-reactivity with swine leukocyte antigens (SLAs) unless they are demonstrated to have a negative flow cytometric cross-match with cells from the ‘donor’ pig.76
Chronic rejection after cardiac allotransplantation
Cardiac allograft recipients with graft vasculopathy have (1) a high risk of sudden death, (2) a low priority on the waiting list for a second deceased human donor organ, and (3) suboptimal support by a mechanical device. The risks of acute and/or chronic immune injury to a second or subsequent heart allograft are high.77 Elective access to a crossmatch-negative pig heart (see above) might be attractive for patients with rapidly progressive graft vasculopathy or for those at high risk for fatal arrhythmia.
Infants and children with complex congenital heart disease
Infants with complex congenital heart disease, especially those with single ventricle physiology, have limited access to allotransplantation due to the scarcity of size-matched deceased donor organs in that age group.78–80 The results of mechanical support in these patients are poor,81–82 and the results of multiple staged surgical reconstructive procedures for palliation remain mixed.81 A genetically-engineered pig heart might be life-supporting as a bridge until a heart from a deceased human donor can be obtained. The relatively immature and ‘flexible’ immune system of infants may enable greater resistance to rejection than in older patients.83 Theoretically, a pig heart might grow proportionately with the infant, or could be electively replaced later if a size-mismatch develops.
Future considerations
Traditional methods of monitoring for rejection (e.g., echocardiography, measurement of troponin and CK-MB levels84 have been found to be helpful in determining whether graft atherosclerosis is developing. The utility of endomyocardial biopsy in patients with a pig heart transplant remains to be determined, but we are mindful that routine histologic surveillance of the graft was arguably pivotal to the current success of heart allotransplantation. Several novel, but largely unproven, methods of detecting rejection are worthy of exploration, for example, donor-derived cell-free DNA,85–87 species-specific gene-expressing profiling,84,88 or circulating organ-specific miRNAs.89,90
Success of pig heart transplantation in NHPs has been achieved to date only when experimental immunomodulatory drugs (not yet approved by US FDA or European Medicines Agency - EMA) have been used.91 Fortunately, in published guidance92 and by its approval on compassionate grounds of the Maryland patient, the FDA has demonstrated its willingness to consider an experimental drug as part of a clinical protocol when that approach is supported by preclinical data.
Maintaining ‘designated pathogen-free’ pigs in isolation will be costly, and, when and if clinically approved, cost may become a significant barrier to access. On the other hand, prolonged costly pre-transplant stays in the intensive care unit will become unnecessary, and high-cost, high-risk alternative medical or surgical therapies will become obsolete as the availability of a suitable pig organ will enable transplantation to be carried out timely when indicated and optimal for the recipient. Based on the initial success achieved in the University of Maryland case, this idealized vision for cardiac xenotransplantation now seems within reach.
In summary, in the initial clinical trials, we caution against including patients with poor prognosis based on medical or surgical risk factors not directly related to their heart pathology (e.g., advanced frailty, widespread peripheral atherosclerotic disease, a ‘hostile’ mediastinum), as this approach is likely to yield poor outcomes and undermine public and peer support for xenotransplantation. Similarly, we believe that enrolling subjects under emergency circumstances that do not allow thorough assessment of patient suitability should be avoided. Current or recently treated advanced malignancy or an active infection are likely to cause life-limiting complications under immunosuppressive therapy. Finally, adequate psychosocial support, a robust process of preprocedure education, and thorough informed consent for both the patient and his/her caregivers are important to encourage compliance with post-transplant treatment, monitoring, and public safety protocols.
Acknowledgments
RC is supported by the Benjamin Research Fellowship from the German Research Foundation (DFG). DC and RP receive grant support from the NIH (NIH NIAID U19 grant AI090959, and UO1 grant AI153612), and the Department of Defense (grant W81XWH2010559; DKCC), and previously received research funding from Revivicor, a subsidiary of United Therapeutics. RP has received research support from eGenesis and Tonix.
Abbreviations:
- CRISPR
clustered regularly interspaced short palindromic repeats
- CPRPs
complement pathway regulatory proteins
- EPCR
endothelial protein C receptor
- GTKO
α1,3-galactosyltransferase knockout
- hTBM
human thrombomodulin
- LVAD
left ventricular assist device
- mTOR
mammalian target of rapamycin
- NHP
nonhuman primates
- PERV
porcine endogenous retrovirus
- PRA
panel-reactive anti-HLA antibodies
- TFPI
tissue factor pathway inhibitor
- TKO
triple knockout
Footnotes
Conflicts of interest statement
DC is a consultant to eGenesis Bio, Cambridge, MA, USA. No other author declares a conflict of interest.
References
- 1.Pierson RN 3rd, Fishman JA, Lewis GD, et al. Progress toward cardiac xenotransplantation. Circulation 2020;142:1389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DK, Satyananda V, Ekser B, et al. Progress in pig-to-non-human primate transplantation models (1998-2013): a comprehensive review of the literature. Xenotransplantation 2014;21:397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J 1967;41:1271–4. [PubMed] [Google Scholar]
- 4.Hardy JD, Kurrus FD, Chavez CM, et al. Heart transplantation in man: developmental studies and report of a case. JAMA 1964;188:1132–40. [PubMed] [Google Scholar]
- 5.Bailey LL, Nehlsen-Cannarella SL, Concepcion W, Jolley WB. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA 1985;254:3321–9. [PubMed] [Google Scholar]
- 6.Reichart B, Längin M, Radan J, et al. Pig-to-non-human primate heart transplantation: the final step toward clinical xenotransplantation? J Heart Lung Transplant 2020;39:751–7. [DOI] [PubMed] [Google Scholar]
- 7.Prescott MJ. Ethics of primate use. Adv Sci Res 2010;5:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan JS. The risk of using baboons as transplant donors. Exogenous andendogenous viruses. Ann N Y Acad Sci 1998;862:87–99. [DOI] [PubMed] [Google Scholar]
- 9.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature 1997;385:810–3. [DOI] [PubMed] [Google Scholar]
- 10.Cooper DK, Good AH, Koren E, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol 1993;1:198–205. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DK, Human PA, Lexer G, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant 1988;7:238–46. [PubMed] [Google Scholar]
- 12.Ye Y, Neethling FA, Niekrasz M, et al. Evidence that intravenously administered alpha-galactosyl carbohydrates reduce baboon serum cytotoxicity to pig kidney cells (PK15) and transplanted pig hearts. Transplantation 1994;58:330–7. [PubMed] [Google Scholar]
- 13.Rieben R, von Allmen E, Korchagina EY, et al. Detection, immunoabsorption, and inhibition of cytotoxic activity of anti-αGal antibodies using newly developed substances with synthetic Gal α1–3Gal disaccharide epitopes. Xenotransplantation 1995;2:98–106. [Google Scholar]
- 14.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003;299:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014;346 (6213):1258096. [DOI] [PubMed] [Google Scholar]
- 16.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013;20:27–35. [DOI] [PubMed] [Google Scholar]
- 17.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto T, Iwase H, Patel D, et al. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci Rep 2020;10:9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leventhal JR, Dalmasso AP, Cromwell JW, et al. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation 1993;55:857–65. [DOI] [PubMed] [Google Scholar]
- 20.Lachmann PJ. The control of homologous lysis. Immunol Today 1991;12:312–5. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson JP, Oglesby TJ, White D, Adams EA, Liszewski MK. Separation of self from non-self in the complement system: a role for membrane cofactor protein and decay accelerating factor. Clin Exp Immunol 1991;86:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med 1995;1:964–6. [DOI] [PubMed] [Google Scholar]
- 23.Schmoeckel M, Nollert G, Shahmohammadi M, et al. Prevention of hyperacute rejection by human decay accelerating factor in xenogeneic perfused working hearts. Transplantation 1996;62:729–34. [DOI] [PubMed] [Google Scholar]
- 24.Kroshus TJ, Bolman RM 3rd, Dalmasso AP, et al. Expression of human CD59 in transgenic pig organs enhances organ survival in an ex vivo xenogeneic perfusion model. Transplantation 1996;61:1513–21. [DOI] [PubMed] [Google Scholar]
- 25.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing humanCD59 and decay-accelerating factor produce an intrinsic barrier to complementmediated damage. Transplantation 1997;63:149–55. [DOI] [PubMed] [Google Scholar]
- 26.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med 2005;11:29–31. [DOI] [PubMed] [Google Scholar]
- 27.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation 2014;21:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol 2009;21:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopp CW, Grey ST, Siegel JB, et al. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation 1998;66:244–51. [DOI] [PubMed] [Google Scholar]
- 30.Schulte am Esch J 2nd, Rogiers X, Robson SC. Molecular incompatibilities in hemostasis between swine and men–impact on xenografting. Ann Transplant 2001;6:12–6. [PubMed] [Google Scholar]
- 31.Lee KF, Salvaris EJ, Roussel JC, Robson SC, d’Apice AJ, Cowan PJ. Recombinant pig TFPI efficiently regulates human tissue factor pathways. Xenotransplantation 2008;15:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulte Am Esch J 2nd, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation 2005;12:30–7. [DOI] [PubMed] [Google Scholar]
- 33.Pareti FI, Mazzucato M, Bottini E, Mannucci PM. Interaction of porcine von Willebrand factor with the platelet glycoproteins Ib and IIb/IIIa complex. Br J Haematol 1992;82:81. [DOI] [PubMed] [Google Scholar]
- 34.Burdorf L, Riner A, Rybak E, et al. Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor. Xenotransplantation 2016;23:222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly MR, Kuravi K, Burdorf L, et al. Humanized von Willebrand factor reduces platelet sequestration in ex vivo and in vivo xenotransplant models. Xenotransplantation 2021;28:e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.French BM, Sendil S, Pierson RN 3rd, Azimzadeh AM. The role of sialic acids in the immune recognition of xenografts. Xenotransplantation 2017: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laird CT, Hassanein W, O’Neill NA, et al. P- and E-selectin receptor antagonism prevents human leukocyte adhesion to activated porcine endothelial monolayers and attenuates porcine endothelial damage. Xenotransplantation 2018;25:e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.French BM, Sendil S, Sepuru KM, et al. Interleukin-8 mediates neutrophil-endothelial interactions in pig-to-human xenogeneic models. Xenotransplantation 2018;25:e12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdorf L, Laird CT, Harris DG, et al. Pig-to-baboon lung xenotransplantation: extended survival with targeted genetic modifications and pharmacologic treatments. Am J Transplant 2022;22:28–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Sanz P, Hoogendijk AJ, Verkuijlen PJJH, et al. CD47-SIRPα checkpoint inhibition enhances neutrophil-mediated killing of dinutuximab-opsonized neuroblastoma cells. Cancers 2021;13:4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci 2012;109:6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tena AA, Sachs DH, Mallard C, et al. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation 2017;101:316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe H, Sahara H, Nomura S, et al. GalT-KO pig lungs are highly susceptible to acute vascular rejection in baboons, which may be mitigated by transgenic expression of hCD47 on porcine blood vessels. Xenotransplantation 2018;25:e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeuchi K, Ariyoshi Y, Shimizu A, et al. Expression of human CD47 in pig glomeruli prevents proteinuria and prolongs graft survival following pig-to-baboon xenotransplantation. Xenotransplantation 2021;28:e12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018;564:430–3. [DOI] [PubMed] [Google Scholar]
- 46.Tanabe T, Watanabe H, Shah JA, et al. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant 2017;17:1778–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinrichs A, Kessler B, Kurome M, et al. Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver. Mol Metab 2018;11:113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss RA. Infection hazards of xenotransplantation: Retrospect and prospect. Xenotransplantation 2018;25:e12401. [DOI] [PubMed] [Google Scholar]
- 49.Fishman JA. Infection in xenotransplantation: opportunities and challenges. Curr Opin Organ Transplant 2019;24:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bach FH, Fishman JA, Daniels N, et al. Uncertainty in xenotransplantation: individual benefit versus collective risk. Nat Med 1998;4:141–4. [DOI] [PubMed] [Google Scholar]
- 51.Martin U, Steinhoff G. Porcine endogenous retroviruses (PERV): in vitro artifact or a big problem for xenotransplantation? Dtsch Tierarztl Wochenschr 1999;106:146–9. German. [PubMed] [Google Scholar]
- 52.Denner J Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 2018;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denner J, Tönjes RR. Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin Microbiol Rev 2012;25:318–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu D, Wei HJ, Lin L, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 2017;357:1303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L, Güell M, Niu D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 2015;350:1101–4. [DOI] [PubMed] [Google Scholar]
- 56.Denner J Can antiretroviral drugs be used to treat porcine endogenous retrovirus (PERV) infection after xenotransplantation? Viruses 2017;9:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fishman JA. Prevention of infection in xenotransplantation: designated pathogen-free swine in the safety equation. Xenotransplantation 2020;27:e12595. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T, Hara H, Foote J, et al. Life-supporting kidney xenotransplantation from genetically engineered pigs in baboons: a comparison of two immunosuppressive regimens. Transplantation 2019;103:2090–104. [DOI] [PubMed] [Google Scholar]
- 59.Bühler L, Awwad M, Basker M, et al. A nonmyeloablative regimen with CD40L blockade leads to humoral and cellular hyporesponsiveness to pig hematopoietic cells in baboons. Transplant Proc 2000;32:1100. [DOI] [PubMed] [Google Scholar]
- 60.Wu G, Pfeiffer S, Schraöder C, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation 2005;12:197–208. [DOI] [PubMed] [Google Scholar]
- 61.Iwase H, Kobayashi T. Current status of pig kidney xenotransplantation. Int J Surg 2015;23:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrne GW, McGregor CG. Cardiac xenotransplantation: progress and challenges. Curr Opin Organ Transplant 2012;17:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steen S, Paskevicius A, Liao Q, Sjöberg T. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand Cardiovasc J 2016;50:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Längin M, Reichart B, Steen S, et al. Cold non-ischemic heart preservation with continuous perfusion prevents early graft failure in orthotopic pig-to-baboon xenotransplantation. Xenotransplantation 2021;28:e12636. [DOI] [PubMed] [Google Scholar]
- 65.Chan JL, Mohiuddin MM. Heart xenotransplantation. Curr Opin Organ Transplant 2017;22:549–54. [DOI] [PubMed] [Google Scholar]
- 66.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooper DK, Keogh AM, Brink J, et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J Heart Lung Transplant 2000;19:1125–65. [DOI] [PubMed] [Google Scholar]
- 68.Cleveland DC, Jagdale A, Carlo WF, et al. The genetically engineered heart as a bridge to allotransplantation in infants just around the corner? [e-pub ahead of print]. Ann Thorac Surg 2021. S0003-4975(21) 00982-6. 10.1016/j.athoracsur.2021.05.025. accessed May 31, 2022. [DOI] [PubMed] [Google Scholar]
- 69.Burdorf L, Azimzadeh AM, Pierson RN 3rd. Xenogeneic lung transplantation models. Methods Mol Biol 2020;2110:173–96. [DOI] [PubMed] [Google Scholar]
- 70.Lu T, Yang B, Wang R, Qin C. Xenotransplantation: current status in preclinical research. Front Immunol 2020;10:3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yue Y, Kan Y, Xu W, et al. Extensive mammalian germline genome engineering. BioRxiv. 10.1101/2019.12.17.876862. [DOI] [Google Scholar]
- 72.Iwase H, Jagdale A, Yamamoto T, et al. Evidence suggesting that deletion of expression of N-glycolylneuraminic acid (Neu5Gc) in the organ-source pig is associated with increased antibody-mediated rejection of kidney transplants in baboons. Xenotransplantation 2021;28:e12700. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto T, Hara H, Ayares D, Cooper DKC. The problem of the “4th xenoantigen” after pig organ transplantation in non-human primates may be overcome by expression of human “protective” proteins. Xenotransplantation. 2021;28:e12658. [DOI] [PubMed] [Google Scholar]
- 74.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 75.Department of Health, Education, and Welfare e Research, National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research. Available at: https://videocast.nih.gov/pdf/ohrp_belmont_report.pdf. [PubMed]
- 76.Byrne GW. Does human leukocyte antigens sensitization matter for xenotransplantation? Xenotransplantation 2018;25:e12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colvin MM, Cook JL, Chang PP, et al. Sensitization in heart transplantation: emerging knowledge: a scientific statement from the American Heart Association. Circulation. 2019;139:e553–78. [DOI] [PubMed] [Google Scholar]
- 78.Donné M, De Pauw M, Vandekerckhove K, Bové T, Panzer J. Ethical and practical dilemmas in cardiac transplantation in infants: a literature review. Eur J Pediatr 2021;180:2359–65. [DOI] [PubMed] [Google Scholar]
- 79.Almond CSD, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation 2009;119:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Denfield SW, Azeka E, Das B, et al. Pediatric cardiac waitlist mortality-Still too high. Pediatr Transplant 2020;24:e13671. [DOI] [PubMed] [Google Scholar]
- 81.Cleveland D, Adam Banks C, Hara H, Carlo WF, Mauchley DC, Cooper DKC. The case for cardiac xenotransplantation in nonates: is now the time to reconsider xenotransplantation for hypoplastic left heart syndrome? Pediatr Cardiol 2019;40:437–44. [DOI] [PubMed] [Google Scholar]
- 82.Morales DLS, Rossano JW, VanderPluym C, et al. Third annual pediatric interagency registry for mechanical circulatory support (Pedimacs) report: preimplant characteristics and outcomes. Ann Thorac Surg 2019;107:993–1004. [DOI] [PubMed] [Google Scholar]
- 83.Bikhet M, Morsi M, Hara H, et al. The immune system in infants: relevance to xenotransplantation. Pediatr Transplant 2020;24:e13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shah KS, Kittleson MM, Kobashigawa JA. Updates on heart transplantation. Curr Heart Fail Rep 2019;16:150–6. [DOI] [PubMed] [Google Scholar]
- 85.Keller M, Sun J, Mutebi C, et al. Donor-derived cell-free DNA as a composite marker of acute lung allograft dysfunction in clinical care. J Heart Lung Transplant 2021;41:458–66. [DOI] [PubMed] [Google Scholar]
- 86.Keller M, Agbor-Enoh S. Donor-derived cell-free DNA for acute rejection monitoring in heart and lung transplantation. Curr Transplant Rep 2021;8:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khush KK. Clinical utility of donor-derived cell-free DNA testing in cardiac transplantation. J Heart Lung Transplant 2021;40:397–404. [DOI] [PubMed] [Google Scholar]
- 88.Crespo-Leiro MG, Stypmann J, Schulz U, et al. Clinical usefulness of gene-expression profile to rule out acute rejection after heart transplantation: CARGO II. Eur Heart J 2016;37:2591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou M, Hara H, Dai Y, et al. Circulating organ-specific micrornas serve as biomarkers in organ-specific diseases: implications for organ allo- and xeno-transplantation. Int J Mol Sci 2016;17:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim J, Cho IS, Hong JS, Choi YK, Kim H, Lee YS. Identification and characterization of new microRNAs from pig. Mamm Genome 2008;19:570–80. [DOI] [PubMed] [Google Scholar]
- 91.Cooper DKC. Experimental pig heart xenotransplantation-recent progress and remaining problems. Ann Thorac Surg 2019;107:989–92. [DOI] [PubMed] [Google Scholar]
- 92.Cooper DKC, Cowan P, Fishman JA, et al. Joint FDA-IXA symposium, September 20, 2017. Xenotransplantation 2017: 24. [DOI] [PubMed] [Google Scholar]
- 93.Taniguchi S, Cooper DK. Clinical xenotransplantation: past, present and future. Ann R Coll Surg Engl 1997;79:13–9. [PMC free article] [PubMed] [Google Scholar]


