Full text
PDF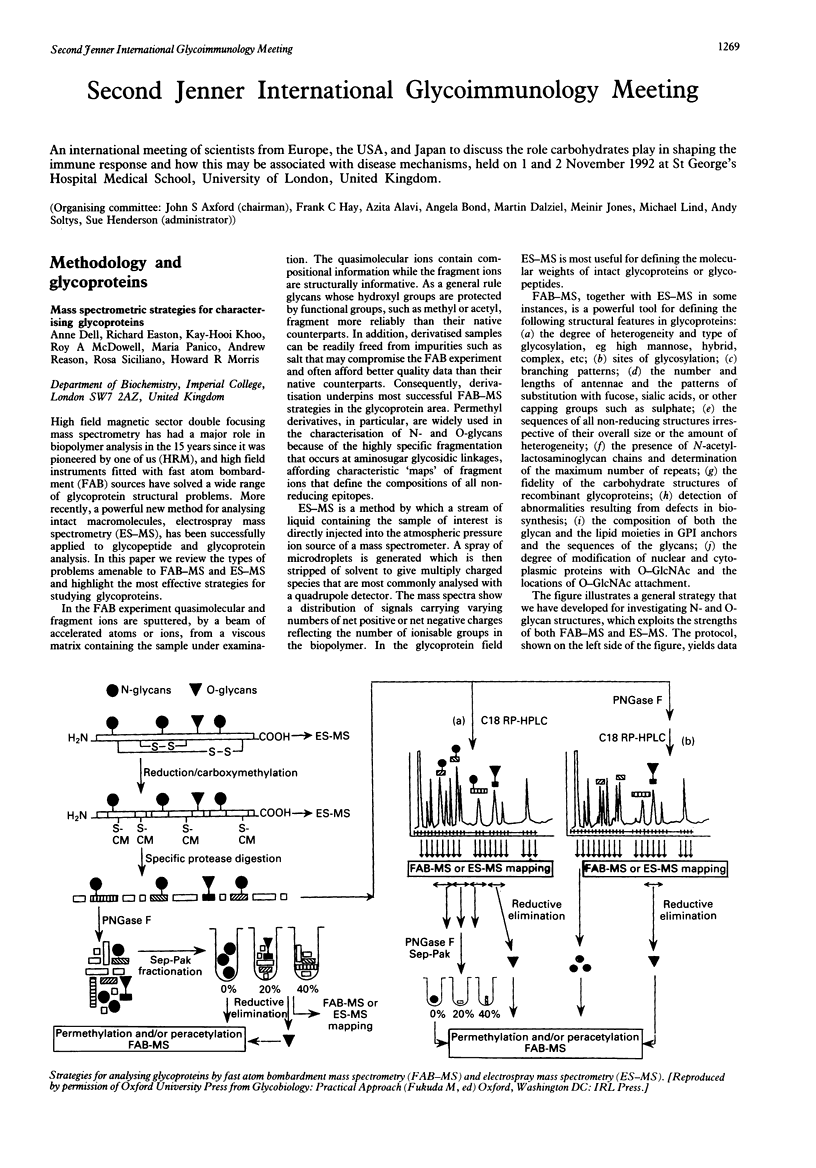


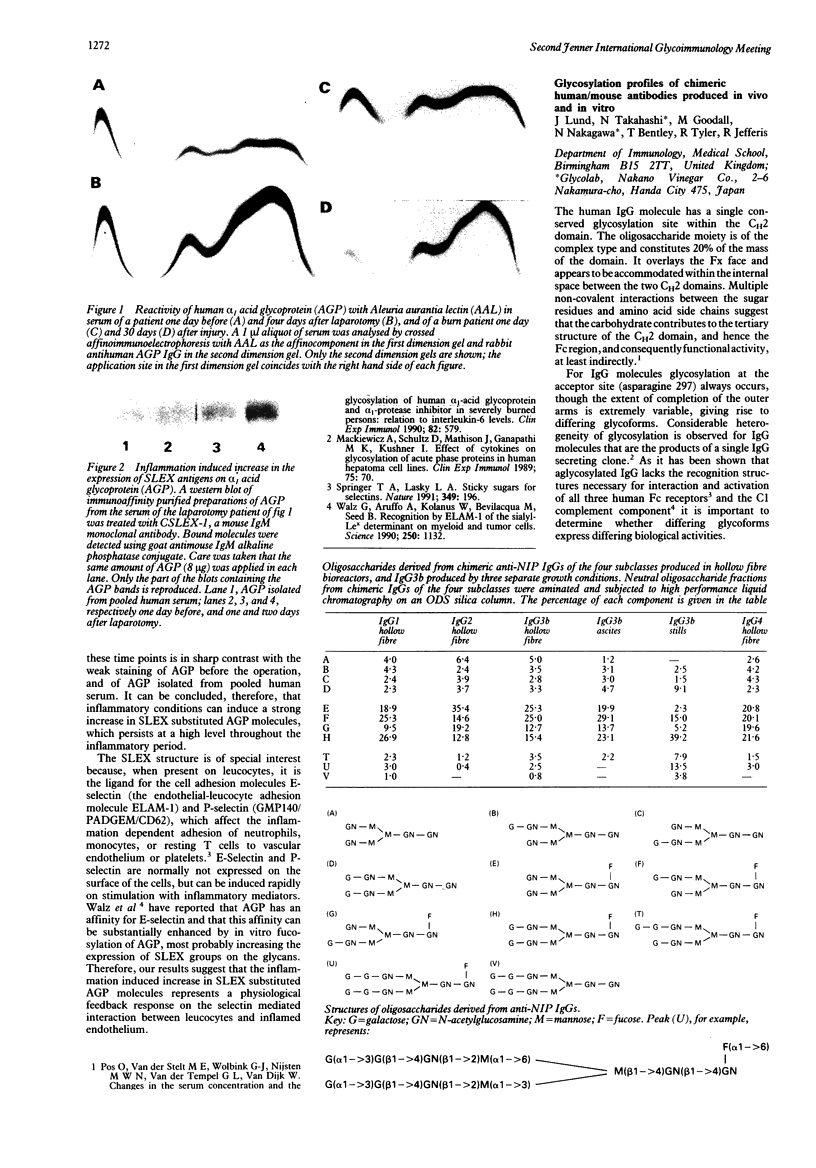




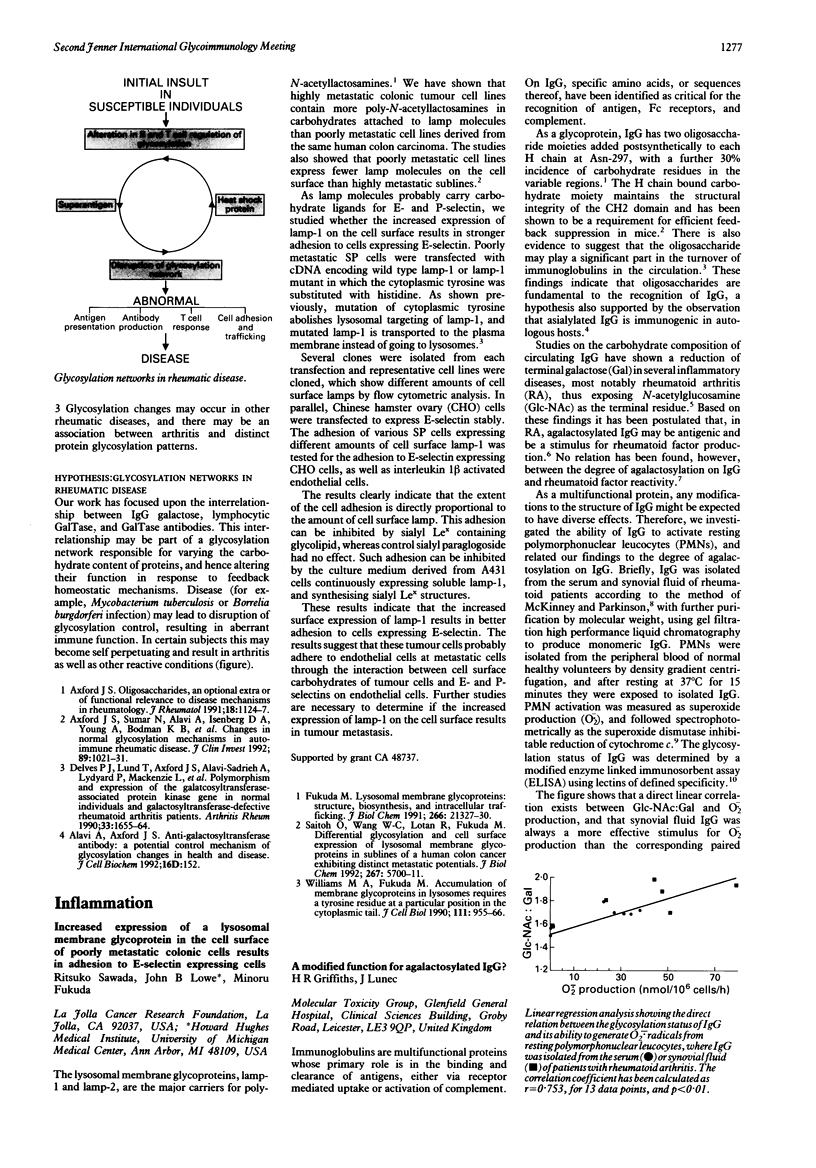






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. M., Hounsell E. F., Feizi T. Further studies of oligosaccharide recognition by the soluble 13 kDa lectin of bovine heart muscle. Ability to accommodate the blood-group-H and -B-related sequences. Biochem J. 1988 May 15;252(1):283–287. doi: 10.1042/bj2520283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axford J. S., Mackenzie L., Lydyard P. M., Hay F. C., Isenberg D. A., Roitt I. M. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet. 1987 Dec 26;2(8574):1486–1488. doi: 10.1016/s0140-6736(87)92621-3. [DOI] [PubMed] [Google Scholar]
- Axford J. S. Oligosaccharides: an optional extra or of relevance to disease mechanisms in rheumatology? J Rheumatol. 1991 Aug;18(8):1124–1127. [PubMed] [Google Scholar]
- Axford J. S., Sumar N., Alavi A., Isenberg D. A., Young A., Bodman K. B., Roitt I. M. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J Clin Invest. 1992 Mar;89(3):1021–1031. doi: 10.1172/JCI115643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axford J. S., Sumar N., Alavi A., Isenberg D. A., Young A., Bodman K. B., Roitt I. M. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J Clin Invest. 1992 Mar;89(3):1021–1031. doi: 10.1172/JCI115643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast B. J., Zhou L. J., Freeman G. J., Colley K. J., Ernst T. J., Munro J. M., Tedder T. F. The HB-6, CDw75, and CD76 differentiation antigens are unique cell-surface carbohydrate determinants generated by the beta-galactoside alpha 2,6-sialyltransferase. J Cell Biol. 1992 Jan;116(2):423–435. doi: 10.1083/jcb.116.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A., Cooke A., Hay F. C. Glycosylation of IgG, immune complexes and IgG subclasses in the MRL-lpr/lpr mouse model of rheumatoid arthritis. Eur J Immunol. 1990 Oct;20(10):2229–2233. doi: 10.1002/eji.1830201011. [DOI] [PubMed] [Google Scholar]
- Bunnell B. A., Adams D. E., Kidd V. J. Transient expression of a p58 protein kinase cDNA enhances mammalian glycosyltransferase activity. Biochem Biophys Res Commun. 1990 Aug 31;171(1):196–203. doi: 10.1016/0006-291x(90)91376-4. [DOI] [PubMed] [Google Scholar]
- Bunnell B. A., Adams D. E., Kidd V. J. Transient expression of a p58 protein kinase cDNA enhances mammalian glycosyltransferase activity. Biochem Biophys Res Commun. 1990 Aug 31;171(1):196–203. doi: 10.1016/0006-291x(90)91376-4. [DOI] [PubMed] [Google Scholar]
- Bunnell B. A., Heath L. S., Adams D. E., Lahti J. M., Kidd V. J. Increased expression of a 58-kDa protein kinase leads to changes in the CHO cell cycle. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7467–7471. doi: 10.1073/pnas.87.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
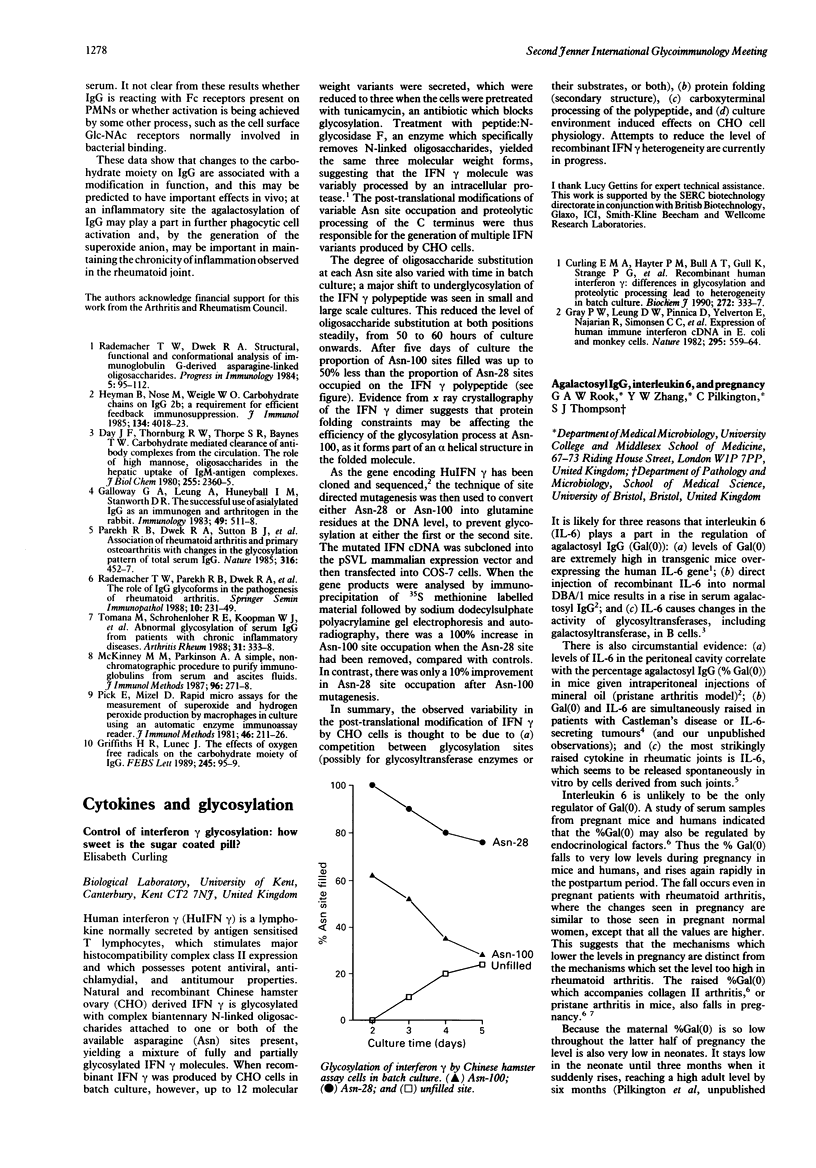
- Curling E. M., Hayter P. M., Baines A. J., Bull A. T., Gull K., Strange P. G., Jenkins N. Recombinant human interferon-gamma. Differences in glycosylation and proteolytic processing lead to heterogeneity in batch culture. Biochem J. 1990 Dec 1;272(2):333–337. doi: 10.1042/bj2720333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva R. P., Hall B. F., Joiner K. A., Sacks D. L. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989 Jul 15;143(2):617–622. [PubMed] [Google Scholar]
- Day J. F., Thornburg R. W., Thorpe S. R., Baynes J. W. Carbohydrate-mediated clearance of antibody . antigen complexes from the circulation. The role of high mannose oligosaccharides in the hepatic uptake of IgM . antigen complexes. J Biol Chem. 1980 Mar 25;255(6):2360–2365. [PubMed] [Google Scholar]
- Delves P. J., Lund T., Axford J. S., Alavi-Sadrieh A., Lydyard P. M., MacKenzie L., Smith M. D., Kidd V. J. Polymorphism and expression of the galactosyltransferase-associated protein kinase gene in normal individuals and galactosylation-defective rheumatoid arthritis patients. Arthritis Rheum. 1990 Nov;33(11):1655–1664. doi: 10.1002/art.1780331108. [DOI] [PubMed] [Google Scholar]
- Delves P. J., Lund T., Axford J. S., Alavi-Sadrieh A., Lydyard P. M., MacKenzie L., Smith M. D., Kidd V. J. Polymorphism and expression of the galactosyltransferase-associated protein kinase gene in normal individuals and galactosylation-defective rheumatoid arthritis patients. Arthritis Rheum. 1990 Nov;33(11):1655–1664. doi: 10.1002/art.1780331108. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Alhadeff B., Houghton A. N., Old L. J. Loss of heterozygosity at autosomal and X-linked loci during tumor progression in a patient with melanoma. Cancer Res. 1987 Aug 1;47(15):3995–4000. [PubMed] [Google Scholar]
- Duncan A. R., Winter G. The binding site for C1q on IgG. Nature. 1988 Apr 21;332(6166):738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- Eipers P. G., Barnoski B. L., Han J., Carroll A. J., Kidd V. J. Localization of the expressed human p58 protein kinase chromosomal gene to chromosome 1p36 and a highly related sequence to chromosome 15. Genomics. 1991 Nov;11(3):621–629. doi: 10.1016/0888-7543(91)90069-q. [DOI] [PubMed] [Google Scholar]
- Etienne-Decerf J., Malaise M., Mahieu P., Winand R. Elevated anti-alpha-galactosyl antibody titres. A marker of progression in autoimmune thyroid disorders and in endocrine ophthalmopathy? Acta Endocrinol (Copenh) 1987 May;115(1):67–74. doi: 10.1530/acta.0.1150067. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Filley E., Andreoli A., Steele J., Waters M., Wagner D., Nelson D., Tung K., Rademacher T., Dwek R., Rook G. A. A transient rise in agalactosyl IgG correlating with free interleukin 2 receptors, during episodes of erythema nodosum leprosum. Clin Exp Immunol. 1989 Jun;76(3):343–347. [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991 Nov 15;266(32):21327–21330. [PubMed] [Google Scholar]
- Gabrielli A., Leoni P., Danieli G., Herrmann K., Krieg T., Wieslander J. Antibodies against galactosyl (alpha 1----3) galactose in connective tissue diseases. Arthritis Rheum. 1991 Mar;34(3):375–376. doi: 10.1002/art.1780340321. [DOI] [PubMed] [Google Scholar]
- Galili U. Abnormal expression of alpha-galactosyl epitopes in man. A trigger for autoimmune processes? Lancet. 1989 Aug 12;2(8659):358–361. doi: 10.1016/s0140-6736(89)90539-4. [DOI] [PubMed] [Google Scholar]
- Galili U., Mandrell R. E., Hamadeh R. M., Shohet S. B., Griffiss J. M. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988 Jul;56(7):1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Shohet S. B., Kobrin E., Stults C. L., Macher B. A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988 Nov 25;263(33):17755–17762. [PubMed] [Google Scholar]
- Galloway G., Leung A. Y., Hunneyball I. M., Stanworth D. R. The successful use of asialylated IgG as an immunogen and arthritogen in the rabbit. Immunology. 1983 Jul;49(3):511–518. [PMC free article] [PubMed] [Google Scholar]
- Griffiths H. R., Lunec J. The effects of oxygen free radicals on the carbohydrate moiety of IgG. FEBS Lett. 1989 Mar 13;245(1-2):95–99. doi: 10.1016/0014-5793(89)80199-1. [DOI] [PubMed] [Google Scholar]
- Guy K., Andrew J. M. Expression of the CDw75 (beta-galactoside alpha 2,6-sialyltransferase) antigen on normal blood cells and in B-cell chronic lymphocytic leukaemia. Immunology. 1991 Oct;74(2):206–214. [PMC free article] [PubMed] [Google Scholar]
- Guy K., Green C. The influence of the In(Lu) gene on expression of CDw75 antigens on human red blood cells. Immunology. 1992 Apr;75(4):713–716. [PMC free article] [PubMed] [Google Scholar]
- Heyman B., Nose M., Weigle W. O. Carbohydrate chains on IgG2b: a requirement for efficient feedback immunosuppression. J Immunol. 1985 Jun;134(6):4018–4023. [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Hitsumoto Y., Thompson S. J., Zhang Y. W., Rook G. A., Elson C. J. Relationship between interleukin 6, agalactosyl IgG and pristane-induced arthritis. Autoimmunity. 1992;11(4):247–254. doi: 10.3109/08916939209035162. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Douglas J. G., Shaper N. L., Shaper J. H., Stafford-Hollis J. M., Evans R. J., Kirsch I. R. Genomic structure of murine beta-1,4-galactosyltransferase. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1069–1075. doi: 10.1016/0006-291x(89)90782-1. [DOI] [PubMed] [Google Scholar]
- Hounsell E. F., Davies M. J., Renouf D. V. Studies of oligosaccharide and glycoprotein conformation. Biochem Soc Trans. 1992 May;20(2):259–263. doi: 10.1042/bst0200259a. [DOI] [PubMed] [Google Scholar]
- Hounsell E. F., Gooi H. C., Feizi T. The monoclonal antibody anti-SSEA-1 discriminates between fucosylated type 1 and type 2 blood group chains. FEBS Lett. 1981 Aug 31;131(2):279–282. doi: 10.1016/0014-5793(81)80384-5. [DOI] [PubMed] [Google Scholar]
- Hounsell E. F., Jones N. J., Gooi H. C., Feizi T., Donald A. S., Feeney J. 500-MHz 1H-n.m.r. and conformational studies of fucosyloligosaccharides recognised by monoclonal antibodies with specificities related to Le(a), Le(b), and SSEA-1. Carbohydr Res. 1988 Jul 15;178:67–78. doi: 10.1016/0008-6215(88)80102-2. [DOI] [PubMed] [Google Scholar]
- Hounsell E. F., Renouf D. V., Liney D., Dalgleish A. G., Habeshaw J. A proposed molecular model for the carboxy terminus of HIV-1 gp120 showing structural features consistent with the presence of a T-cell alloepitope. Mol Aspects Med. 1991;12(4):283–296. doi: 10.1016/0098-2997(91)90021-d. [DOI] [PubMed] [Google Scholar]
- Humphreys-Beher M. G., Schneyer C. A., Kidd V. J., Marchase R. B. Isoproterenol-mediated parotid gland hypertrophy is inhibited by effectors of 4 beta-galactosyltransferase. J Biol Chem. 1987 Aug 25;262(24):11706–11713. [PubMed] [Google Scholar]
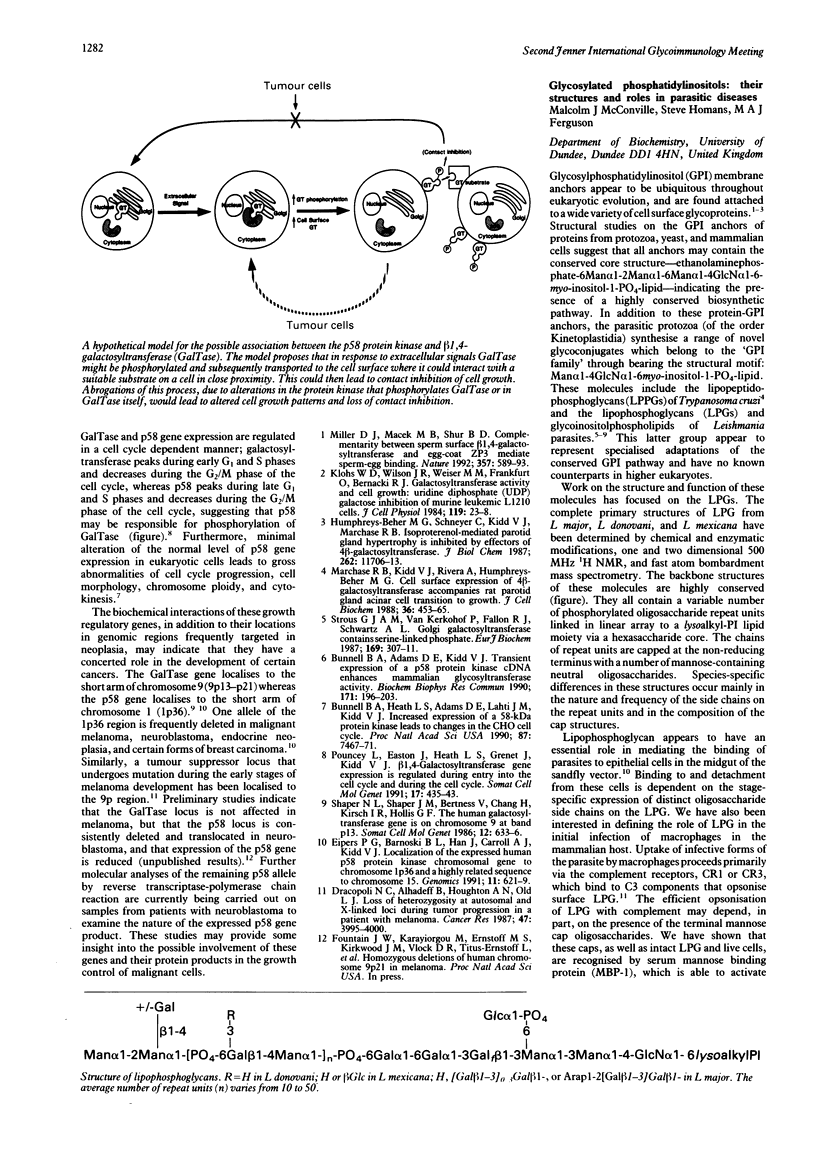
- Ilg T., Etges R., Overath P., McConville M. J., Thomas-Oates J., Thomas J., Homans S. W., Ferguson M. A. Structure of Leishmania mexicana lipophosphoglycan. J Biol Chem. 1992 Apr 5;267(10):6834–6840. [PubMed] [Google Scholar]
- Jackson P. Polyacrylamide gel electrophoresis of reducing saccharides labeled with the fluorophore 2-aminoacridone: subpicomolar detection using an imaging system based on a cooled charge-coupled device. Anal Biochem. 1991 Aug 1;196(2):238–244. doi: 10.1016/0003-2697(91)90460-b. [DOI] [PubMed] [Google Scholar]
- Jackson P. The use of polyacrylamide-gel electrophoresis for the high-resolution separation of reducing saccharides labelled with the fluorophore 8-aminonaphthalene-1,3,6-trisulphonic acid. Detection of picomolar quantities by an imaging system based on a cooled charge-coupled device. Biochem J. 1990 Sep 15;270(3):705–713. doi: 10.1042/bj2700705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P., Williams G. R. Polyacrylamide gel electrophoresis of reducing saccharides labeled with the fluorophore 8-aminonaphthalene-1,3,6-trisulphonic acid: application to the enzymological structural analysis of oligosaccharides. Electrophoresis. 1991 Jan;12(1):94–96. doi: 10.1002/elps.1150120118. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Lund J., Mizutani H., Nakagawa H., Kawazoe Y., Arata Y., Takahashi N. A comparative study of the N-linked oligosaccharide structures of human IgG subclass proteins. Biochem J. 1990 Jun 15;268(3):529–537. doi: 10.1042/bj2680529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R., Lund J., Pound J. Molecular definition of interaction sites on human IgG for Fc receptors (huFc gamma R). Mol Immunol. 1990 Dec;27(12):1237–1240. doi: 10.1016/0161-5890(90)90027-w. [DOI] [PubMed] [Google Scholar]
- Joziasse D. H., Shaper J. H., Jabs E. W., Shaper N. L. Characterization of an alpha 1----3-galactosyltransferase homologue on human chromosome 12 that is organized as a processed pseudogene. J Biol Chem. 1991 Apr 15;266(11):6991–6998. [PubMed] [Google Scholar]
- Joziasse D. H., Shaper J. H., Van den Eijnden D. H., Van Tunen A. J., Shaper N. L. Bovine alpha 1----3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J Biol Chem. 1989 Aug 25;264(24):14290–14297. [PubMed] [Google Scholar]
- Klohs W. D., Wilson J. R., Weiser M. M., Frankfurt O., Bernacki R. J. Galactosyltransferase activity and cell growth: uridine diphosphate (UDP)galactose inhibition of murine leukemic L1210 cells. J Cell Physiol. 1984 Apr;119(1):23–28. doi: 10.1002/jcp.1041190105. [DOI] [PubMed] [Google Scholar]
- Larsen R. D., Rivera-Marrero C. A., Ernst L. K., Cummings R. D., Lowe J. B. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:beta-D-Gal(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase cDNA. J Biol Chem. 1990 Apr 25;265(12):7055–7061. [PubMed] [Google Scholar]
- Lawson A. M., Hounsell E. F., Stoll M. S., Feeney J., Chai W. G., Rosankiewicz J. R., Feizi T. Characterisation of minor tetra- to hepta-saccharides O-linked to human meconium glycoproteins by t.l.c.-m.s. microsequencing of neoglycolipid derivatives in conjunction with conventional m.s. and 1H-n.m.r. spectroscopy. Carbohydr Res. 1991 Dec 16;221:191–208. doi: 10.1016/0008-6215(91)80056-s. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Rice K. G., Rao N. B., Ichikawa Y., Barthel T., Piskarev V., Lee Y. C. Binding characteristics of N-acetylglucosamine-specific lectin of the isolated chicken hepatocytes: similarities to mammalian hepatic galactose/N-acetylgalactosamine-specific lectin. Biochemistry. 1989 Oct 17;28(21):8351–8358. doi: 10.1021/bi00447a013. [DOI] [PubMed] [Google Scholar]
- Lowe J. B., Stoolman L. M., Nair R. P., Larsen R. D., Berhend T. L., Marks R. M. ELAM-1--dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990 Nov 2;63(3):475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Schultz D., Mathison J., Ganapathi M., Kushner I. Effect of cytokines on glycosylation of acute phase proteins in human hepatoma cell lines. Clin Exp Immunol. 1989 Jan;75(1):70–75. [PMC free article] [PubMed] [Google Scholar]
- Marchase R. B., Kidd V. J., Rivera A. A., Humphreys-Beher M. G. Cell surface expression of 4 beta-galactosyltransferase accompanies rat parotid gland acinar cell transition to growth. J Cell Biochem. 1988 Apr;36(4):453–465. doi: 10.1002/jcb.240360413. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Blackwell J. M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991 Aug 15;266(23):15170–15179. [PubMed] [Google Scholar]
- McConville M. J., Homans S. W., Thomas-Oates J. E., Dell A., Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990 May 5;265(13):7385–7394. [PubMed] [Google Scholar]
- McConville M. J., Thomas-Oates J. E., Ferguson M. A., Homans S. W. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem. 1990 Nov 15;265(32):19611–19623. [PubMed] [Google Scholar]
- McKinney M. M., Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987 Feb 11;96(2):271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Macek M. B., Shur B. D. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature. 1992 Jun 18;357(6379):589–593. doi: 10.1038/357589a0. [DOI] [PubMed] [Google Scholar]
- Nakao H., Nishikawa A., Karasuno T., Nishiura T., Iida M., Kanayama Y., Yonezawa T., Tarui S., Taniguchi N. Modulation of N-acetylglucosaminyltransferase III, IV and V activities and alteration of the surface oligosaccharide structure of a myeloma cell line by interleukin 6. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1260–1266. doi: 10.1016/0006-291x(90)91585-g. [DOI] [PubMed] [Google Scholar]
- Nakao H., Nishikawa A., Nishiura T., Kanayama Y., Tarui S., Taniguchi N. Hypogalactosylation of immunoglobulin G sugar chains and elevated serum interleukin 6 in Castleman's disease. Clin Chim Acta. 1991 Mar 29;197(3):221–228. doi: 10.1016/0009-8981(91)90142-y. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Roitt I. M., Isenberg D. A., Dwek R. A., Ansell B. M., Rademacher T. W. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988 Apr 30;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Roitt I. M., Isenberg D. A., Dwek R. A., Ansell B. M., Rademacher T. W. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988 Apr 30;1(8592):966–969. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- Parekh R., Isenberg D., Rook G., Roitt I., Dwek R., Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun. 1989 Apr;2(2):101–114. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Parekh R., Roitt I., Isenberg D., Dwek R., Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988 May 1;167(5):1731–1736. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Plesnila N., Lipford G. B., Kromer S., Deusch K., Wagner H. A lectin-binding, protease-resistant mycobacterial ligand specifically activates V gamma 9+ human gamma delta T cells. J Immunol. 1992 Jan 15;148(2):575–583. [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Pimenta P. F., Turco S. J., McConville M. J., Lawyer P. G., Perkins P. V., Sacks D. L. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992 Jun 26;256(5065):1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Pos O., van der Stelt M. E., Wolbink G. J., Nijsten M. W., van der Tempel G. L., van Dijk W. Changes in the serum concentration and the glycosylation of human alpha 1-acid glycoprotein and alpha 1-protease inhibitor in severely burned persons: relation to interleukin-6 levels. Clin Exp Immunol. 1990 Dec;82(3):579–582. doi: 10.1111/j.1365-2249.1990.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S., Gordon S. Differential expression of membrane sialoglycoproteins in exudate and resident mouse peritoneal macrophages. J Cell Sci. 1989 Aug;93(Pt 4):623–630. doi: 10.1242/jcs.93.4.623. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Rice K. G., Lee Y. C. Modification of triantennary glycopeptide into probes for the asialoglycoprotein receptor of hepatocytes. J Biol Chem. 1990 Oct 25;265(30):18423–18428. [PubMed] [Google Scholar]
- Rice K. G., Wu R. G., Brand L., Lee Y. C. Interterminal distance and flexibility of a triantennary glycopeptide as measured by resonance energy transfer. Biochemistry. 1991 Jul 9;30(27):6646–6655. doi: 10.1021/bi00241a003. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Stanford J. L. Slow bacterial infections or autoimmunity? Immunol Today. 1992 May;13(5):160–164. doi: 10.1016/0167-5699(92)90119-R. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Brealey R., Whyte A., Isenberg D., Sumar N., Nelson J. L., Bodman K. B., Young A., Roitt I. M. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun. 1991 Oct;4(5):779–794. doi: 10.1016/0896-8411(91)90173-a. [DOI] [PubMed] [Google Scholar]
- Rook G., Thompson S., Buckley M., Elson C., Brealey R., Lambert C., White T., Rademacher T. The role of oil and agalactosyl IgG in the induction of arthritis in rodent models. Eur J Immunol. 1991 Apr;21(4):1027–1032. doi: 10.1002/eji.1830210425. [DOI] [PubMed] [Google Scholar]
- Saitoh O., Wang W. C., Lotan R., Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992 Mar 15;267(8):5700–5711. [PubMed] [Google Scholar]
- Shaper N. L., Shaper J. H., Bertness V., Chang H., Kirsch I. R., Hollis G. F. The human galactosyltransferase gene is on chromosome 9 at band p13. Somat Cell Mol Genet. 1986 Nov;12(6):633–636. doi: 10.1007/BF01671948. [DOI] [PubMed] [Google Scholar]
- Skacel P. O., Edwards A. J., Harrison C. T., Watkins W. M. Enzymic control of the expression of the X determinant (CD15) in human myeloid cells during maturation: the regulatory role of 6-sialytransferase. Blood. 1991 Sep 15;78(6):1452–1460. [PubMed] [Google Scholar]
- Smith K. D., Harbin A. M., Carruthers R. A., Lawson A. M., Hounsell E. F. Enzyme degradation, high performance liquid chromatography and liquid secondary ion mass spectrometry in the analysis of glycoproteins. Biomed Chromatogr. 1990 Nov;4(6):261–266. doi: 10.1002/bmc.1130040612. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Koch G. L. Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes. J Cell Sci. 1987 Feb;87(Pt 1):113–119. doi: 10.1242/jcs.87.1.113. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Sgroi D., Aruffo A., Sy M. S., Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991 Sep 20;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- Sumar N., Colaço C. B., Bodman K. B., Parekh R., Williams P., Dwek R., Rademacher T., Isenberg D. A., Soltys A., Hay F. C. Abnormalities in the glycosylation of IgG in spouses of patients with rheumatoid arthritis. A family study. J Autoimmun. 1991 Dec;4(6):907–914. doi: 10.1016/0896-8411(91)90053-f. [DOI] [PubMed] [Google Scholar]
- Sutton P., Stoddart R. W., Hutchinson I. V. Altered patterns of glycosylation on rat lymphocytes associated with activation. Immunology. 1992 Jul;76(3):472–477. [PMC free article] [PubMed] [Google Scholar]
- Szarfman A., Terranova V. P., Rennard S. I., Foidart J. M., de Fatima Lima M., Scheinman J. I., Martin G. R. Antibodies to laminin in Chagas' disease. J Exp Med. 1982 Apr 1;155(4):1161–1171. doi: 10.1084/jem.155.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. R., Dwek R. A., Rademacher T. W. Structure, biosynthesis, and function of glycosylphosphatidylinositols. Biochemistry. 1990 Jun 12;29(23):5413–5422. doi: 10.1021/bi00475a001. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., McConville M. J., Thomas-Oates J. E., Homans S. W., Ferguson M. A., Gorin P. A., Greis K. D., Turco S. J. Refined structure of the lipophosphoglycan of Leishmania donovani. J Biol Chem. 1992 Apr 5;267(10):6829–6833. [PubMed] [Google Scholar]
- Tomana M., Schrohenloher R. E., Koopman W. J., Alarcón G. S., Paul W. A. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 1988 Mar;31(3):333–338. doi: 10.1002/art.1780310304. [DOI] [PubMed] [Google Scholar]
- Walker M. R., Lund J., Thompson K. M., Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989 Apr 15;259(2):347–353. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Watkins W. M. Biochemical genetics of blood group antigens: retrospect and prospect. Biochem Soc Trans. 1987 Aug;15(4):620–624. doi: 10.1042/bst0150620. [DOI] [PubMed] [Google Scholar]
- Watkins W. M. Blood-group substances. Science. 1966 Apr 8;152(3719):172–181. doi: 10.1126/science.152.3719.172. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Fukuda M. Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol. 1990 Sep;111(3):955–966. doi: 10.1083/jcb.111.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F., Hakomori S. Sugar-nucleotide donor specificity of histo-blood group A and B transferases is based on amino acid substitutions. J Biol Chem. 1990 Nov 5;265(31):19257–19262. [PubMed] [Google Scholar]
- Yamamoto F., McNeill P. D., Hakomori S. Identification in human genomic DNA of the sequence homologous but not identical to either the histo-blood group ABH genes or alpha 1----3 galactosyltransferase pseudogene. Biochem Biophys Res Commun. 1991 Mar 29;175(3):986–994. doi: 10.1016/0006-291x(91)91662-v. [DOI] [PubMed] [Google Scholar]
- Young A., Sumar N., Bodman K., Goyal S., Sinclair H., Roitt I., Isenberg D. Agalactosyl IgG: an aid to differential diagnosis in early synovitis. Arthritis Rheum. 1991 Nov;34(11):1425–1429. doi: 10.1002/art.1780341113. [DOI] [PubMed] [Google Scholar]
- Young A., Sumar N., Bodman K., Goyal S., Sinclair H., Roitt I., Isenberg D. Agalactosyl IgG: an aid to differential diagnosis in early synovitis. Arthritis Rheum. 1991 Nov;34(11):1425–1429. doi: 10.1002/art.1780341113. [DOI] [PubMed] [Google Scholar]
- de Lederkremer R. M., Lima C., Ramirez M. I., Ferguson M. A., Homans S. W., Thomas-Oates J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi Epimastigotes. J Biol Chem. 1991 Dec 15;266(35):23670–23675. [PubMed] [Google Scholar]