Abstract
Epithelial tight junctions define the paracellular permeability of the intestinal barrier. Molecules can cross the tight junctions via two distinct size-selective and charge-selective paracellular pathways: the pore pathway and the leak pathway. These can be distinguished by their selectivities and differential regulation by immune cells. However, permeability increases measured in most studies are secondary to epithelial damage, which allows non-selective flux via the unrestricted pathway. Restoration of increased unrestricted pathway permeability requires mucosal healing. By contrast, tight junction barrier loss can be reversed by targeted interventions. Specific approaches are needed to restore pore pathway or leak pathway permeability increases. Recent studies have used preclinical disease models to demonstrate the potential of pore pathway or leak pathway barrier restoration in disease. In this Review, we focus on the two paracellular flux pathways that are dependent on the tight junction. We discuss the latest evidence that highlights tight junction components, structures and regulatory mechanisms, their impact on gut health and disease, and opportunities for therapeutic intervention.
Subject terms: Gastroenterology, Gastrointestinal system, Inflammatory bowel disease
Increased intestinal permeability owing to tight junction barrier loss could be targeted in gastrointestinal diseases associated with increased permeability. In this Review, the authors discuss the molecular components and regulation of the tight junction, and consider the relevance to gut diseases and therapeutic opportunities.
Key points
Increased intestinal permeability occurs in a wide range of disorders, including inflammatory bowel disease, coeliac disease and graft-versus-host disease, but the relative contributions of barrier dysfunction and immune responses are unclear.
Intestinal barrier loss can be a consequence of tight junction dysfunction or of epithelial damage; in most studies, these mechanisms are not distinguished.
Paracellular transport across the tight junction can occur via the pore pathway or the leak pathway, which have distinct size-selectivity and charge-selectivity and are differentially regulated by immune signalling.
Claudin-2 increases Na+ and water flux across the pore pathway but larger molecules are unable to traverse claudin-2 channels.
The leak pathway allows macromolecules to cross the epithelial barrier and is regulated by the cytoskeleton and epithelial long myosin light chain kinase splice variant 1 (MLCK1).
Blocking MLCK1 recruitment to the tight junction limits tight junction barrier loss without interfering with essential MLCK functions in epithelial cells and cells of other types.
Introduction
Epithelia separate the organism from the external environment and define individual compartments within tissues. At some sites, the epithelia form a nearly complete barrier, disruption of which is catastrophic. For example, massive disruption of the epidermal (skin) barrier by burn injury or mutagenesis in animal models can be fatal. However, at other sites, such as renal tubules and intestines, the balance between permeability and barrier function is nuanced, as selective permeability is essential for physiological processes but must also be precisely regulated.
Flux across the epithelial barrier occurs via transepithelial transport, which involves transcellular and paracellular pathways. Transcellular transport involves movement of molecules through cells, and is mediated by apical and basolateral transmembrane transporters with exquisite substrate specificity. Paracellular transport is less selective and can involve movement of molecules across the epithelial barrier via the pore pathway or the leak pathway. A third permeability route, the unrestricted pathway, is created by epithelial damage (Box 1). Flux across the pore and leak pathways reflects permeability of tight junctions, which seal the space between adjacent epithelial cells and are the rate-limiting components of paracellular transport. Both pore and leak pathways are size-selective, and the pore pathway is charge-selective. However, both pathways lack the structural specificity of transcellular transport. For example, l-glucose can be absorbed paracellularly but is not recognized by transmembrane transport proteins and, therefore, cannot be absorbed via the transcellular route.
Although barrier loss is considered most often in the context of disease, insufficient selective permeability — that is, barrier enhancement — can also contribute to disease. For example, the first monogenic tight junction disease to be discovered — familial hypomagnesaemia with hypercalciuria and nephrocalcinosis — is caused by mutation of claudin-16, which forms paracellular cation channels within the renal tubule. In the absence of claudin-16, paracellular Mg2+ and Ca2+ absorption in the thick ascending limb of the nephron fails1,2. Conversely, loss of claudin-14, which enhances paracellular barriers, in the organ of Corti causes deafness in mice and autosomal recessive nonsyndromic hearing loss 29 (DFNB29) in humans3–6.
In the intestines, the epithelial monolayer separates subepithelial immune cells from the luminal microbiome7. The balance between paracellular permeability and barrier function is, therefore, especially delicate because the epithelial monolayer must prevent unregulated paracellular flux of potentially pathogenic luminal materials8 while also allowing the selective paracellular permeability that is required for nutrient and water absorption9. This situation contrasts with that in the nephron, where both the lumen of the renal tubule and the interstitial space are sterile under normal conditions. In the gut lumen, which contains a diverse microbiome, disruption of the balance between selective permeability and barrier function is associated with a wide range of intestinal and systemic disorders10,11.
In this Review, we consider several disorders that involve intestinal barrier dysfunction, focusing on changes to paracellular permeability and the function of tight junctions. We delineate molecular mechanisms that alter paracellular permeability and cause intestinal barrier loss. We also endeavour to differentiate between barrier loss as a contributor to disease pathogenesis and barrier loss as a consequence of disease processes.
Box 1 The pore, leak and unrestricted permeability pathways.
Intestinal permeability can reflect contributions of three distinct pathways: the pore pathway, the leak pathway and the unrestricted pathway (see the figure). The pore and leak pathways reflect flux across tight junctions, whereas the unrestricted pathway is independent of tight junctions.
Pore pathway permeability is defined by claudin proteins, which form either channels or barriers at the tight junction291–294. Channels generated by pore-forming claudins are charge-selective and size-selective; the maximum diameter of solutes that can pass through them is 0.6 nm. Claudins form only cation-selective channels in the gastrointestinal tract, but anion-selective claudin channels are present at other sites, such as the nephron. Immune signals, including IL-13 and IL-22, lead to increased transcription and expression of intestinal epithelial claudin-2, which increases pore pathway permeability (see the figure, top)45,50,86.
The tight junction leak pathway allows molecules with diameters up to ~12.5 nm to traverse the epithelial barrier. The molecular structure of the leak pathway is poorly understood, but its permeability can be regulated by long myosin light chain kinase splice variant 1 (MLCK1)125,140,141,157. MLCK1 phosphorylates myosin regulatory light chain to trigger endocytosis of the tight junction protein occludin, leading to an increase in tight junction permeability (see the figure, bottom left). MLCK1 expression and enzymatic activity can be activated by cytokines that include IL-1β and tumour necrosis factor (TNF)124,125,135,140,158,295. Altered expression of other tight junction proteins, including tricellulin or angulin 1, might also modify leak pathway permeability55,146,147,296.
The unrestricted pathway refers to the diffusion of material across regions that lack a continuous epithelial barrier owing to epithelial cell damage or death (see the figure, bottom right). This route is independent of tight junctions, as they are either absent or severely damaged at these sites. The unrestricted pathway allows flux of very large molecules and even intact bacteria.
In summary, the pore pathway is a high-capacity pathway that is exquisitely charge-selective and size-selective, whereas the leak pathway is a low-capacity pathway, is not charge-selective, and, although size-selective, allows flux of molecules 20-fold larger than those accommodated by the pore pathway. Thus, the two tight-junction-dependent pathways are complementary. The unrestricted pathway is tight-junction-independent, high-capacity and non-selective.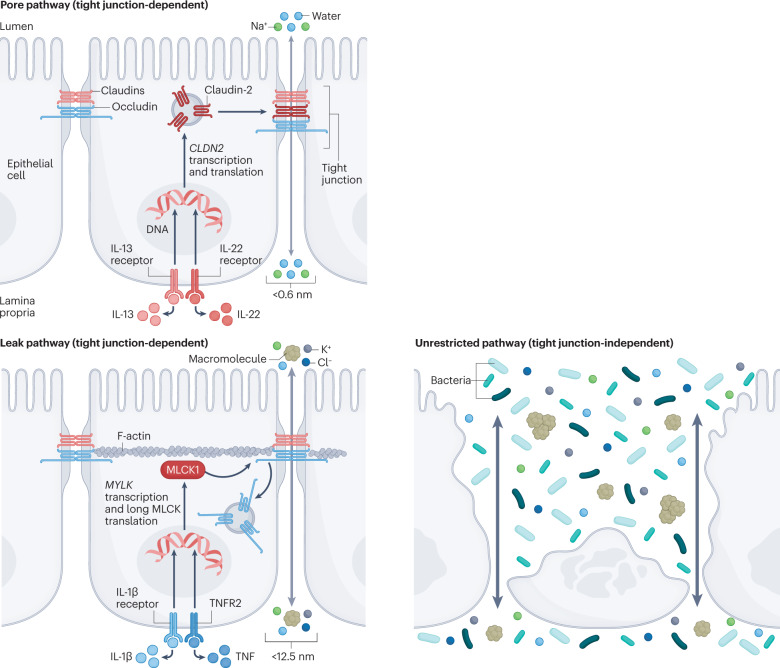
Adapted from ref. 297, Springer Nature Limited.
Intestinal permeability in disease
Intestinal permeability is increased — that is, barrier function is reduced — in many intestinal and systemic diseases (Table 1). Of these diseases, the most well studied intestinal disorders are inflammatory bowel disease (IBD) and coeliac disease. Although tight junction permeability is increased in IBD, the extensive barrier loss seen in advanced, active disease is more likely to reflect epithelial damage12–16. Similarly, increases in intestinal permeability in advanced graft-versus host disease (GVHD) can reflect tight junction regulation or immune-mediated epithelial damage17. Although discrimination between these disparate mechanisms of barrier loss is possible, most studies have relied on the use of only a single probe, such as fluorescein isothiocyanate–4-kDa dextran in mouse models, to measure permeability. As a result, the data are insufficent to differentiate between leak pathway and unrestricted pathway flux. Thus, correlations observed between the extent of barrier loss and the severity of disease in IBD, coeliac disease and GVHD are most likely to be secondary to epithelial damage. By contrast, increased intestinal permeability in Crohn’s disease during remission is more likely to reflect increased tight junction permeability18,19, particularly as increased permeability can occur up to 1 year before disease reactivation. Notably, psychological stress, which can increase intestinal permeability in rodents, is a risk factor for reactivation of Crohn’s disease in patients20,21.
Table 1.
Diseases associated with intestinal barrier defects
| Disease | Findings in patients | Findings in animal models |
|---|---|---|
| IBD (Crohn’s disease and ulcerative colitis) | Increased permeability is a risk factor for IBD in healthy relatives of patients with Crohn’s disease and for relapse in patients with Crohn’s disease18,19,22,24,174,182,183 | Il10-knockout mice develop a permeability defect before disease onset; experimental IBD is more severe in genetically modified mice with increased intestinal permeability; genetic or pharmacological reduction of pore pathway or leak pathway function limits the severity of experimental IBD59,98,117,134,141,163,184,185 |
| Graft-versus-host disease | Positive correlation of pre-conditioning gastrointestinal toxicity (presumed to indicate degree of transient barrier loss) with disease activity17,186 | Gut damage is an essential driver of experimental disease; intestinal permeability is increased and tight junction protein organization is altered in experimental disease; disease progression and severity are attentuated in long MLCK-knockout mice137,187–191 |
| Type 1 diabetes mellitus | Permeability is increased in patients with pre-diabetes and diabetes mellitus192–194 | Permeability increases precede disease; barrier restoration can delay disease onset195,196 |
| Metabolic syndrome (including type 2 diabetes mellitus, obesity and nonalcoholic fatty liver disease) | Increased intestinal permeability is a risk factor for type 2 diabetes mellitus and is associated with obesity and nonalcoholic fatty liver disease194,197–201 | Hyperglycaemia, high-fat diet, nonalcoholic fatty liver disease and obesity are associated with increased intestinal permeability202–208 |
| HIV/AIDS | Increased permeability in HIV enteropathy; positive correlation with disease stage; increased in patients with untreated HIV infection90,209,210 | Increased intestinal permeability in simian immunodeficiency virus infection is associated with microbial translocation and systemic immune activation211,212 |
| Multiple organ dysfunction syndrome | Correlates with increased disease severity213 | Associated with shock; disease progression is limited in knockout mice that are protected from leak pathway permeability increases214–217 |
| IBS | Increased in diarrhoea-predominant, post-infectious and non-post-infectious IBS; unaltered in constipation-predominant IBS28,218–229 | Increased intestinal permeability is associated with and can cause changes in visceral sensitivity31,230,231 |
| Coeliac disease | Positive correlation between increased permeability and disease activity; increased permeability in patients and healthy relatives; gluten-free diet can lead to barrier restoration232–235 | Barrier loss is associated with disease in models of coeliac disease236–238 |
| Environmental enteric dysfunction | Increased permeability; altered expression of absorptive and barrier-enhancing proteins239–247 | Barrier loss is associated with malnutrition240,248,249 |
| Food allergy | Increased in people with food allergy250–252 | Permeability increased in mice after food antigen challenge253–257 |
| Sepsis | Increased permeability in sepsis; increased plasma zonulin258–261 | Permeability increased in experimental sepsis; relationship to disease progression is not defined262–266 |
| SARS-CoV-2 infection | Permeability is increased in severe systemic disease; some data suggest that barrier restoration using a zonulin antagonist is beneficial267–276 | No direct measures of intestinal permeability |
| Parkinson disease | Permeability increased in a subset of patients277,278 | No direct measures of intestinal permeability |
| Asthma | Permeability increased in people with asthma; IBD associated with increased risk of asthma279,280 | Correlation between disease and intestinal permeability in some models281,282 |
| Multiple sclerosis and amyotrophic lateral sclerosis | Increased intestinal permeability in a subset of patients283–285 | Increased intestinal permeability in experimental allergic encephalitis model286 |
| Rheumatic diseases (arthritis and ankylosing spondylitis) | Increased intestinal permeability in some patients287,288 | Increased permeability in mouse models; barrier restoration can limit disease287,289,290 |
IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; MLCK, myosin light chain kinase.
Reports that some healthy first-degree relatives of patients with Crohn’s disease have modest intestinal barrier dysfunction led to the hypothesis that loss of intestinal barrier function is a primary event in Crohn’s disease pathogenesis. The idea that loss of barrier function is an early pathogenic event is supported by evidence that increased intestinal permeability in healthy first-degree relatives of patients with Crohn’s disease is associated with the risk-associated NOD2 3020insC polymorphism22. Perhaps the most convincing evidence comes from another study of healthy relatives of patients with Crohn’s disease, in which the risk of developing disease was twofold to threefold higher among those with increased intestinal permeability than among those with normal permeability23.
In healthy relatives of patients with Crohn’s disease, increased intestinal permeability was also associated with reduced microbial diversity and alterations in specific genera and microbial metabolic pathways24. By contrast, faecal calprotectin — a marker of mucosal inflammation but not of barrier loss — was increased in some healthy relatives but was not an independent risk factor for development of Crohn’s disease25. Reports of increased intestinal permeability up to 3 years before onset of Crohn’s disease suggest that barrier loss is the initial trigger that activates intermediate events, such as mucosal immune activation, that culminate in disease. Together, these observations suggest that barrier loss is a primary event in Crohn’s disease pathogenesis.
Some data suggest that increased intestinal permeability is associated with poorly understood conditions, including irritable bowel syndrome and autism spectrum disorders26–30. The mechanistic underpinnings of these associations have not been defined and, in both patients and animal models, the question of whether barrier loss is a cause or consequence of disease remains. The observation that mice with genetically induced increases in intestinal permeability develop anxiety-like behaviours, hyporesponsiveness to rectal distension and activation of neurons within the thalamus, hypothalamus and hippocampus31 demonstrates that increased intestinal permeability can effect changes in behaviour, visceral sensation and neurological activity. These mice also have an altered microbiome composition.
Tight junctions and intestinal barrier function
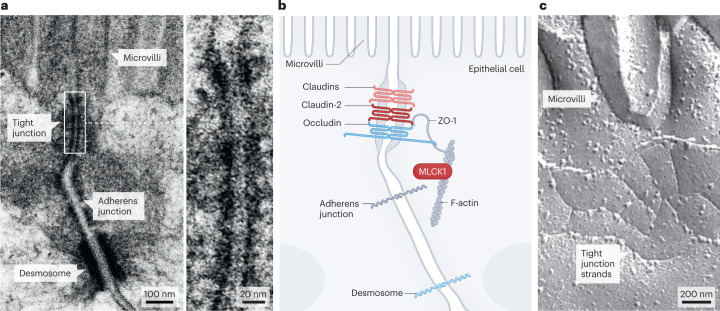
In the absence of epithelial damage, the tight junction is the rate-limiting determinant of passive paracellular transport. At the tight junction, the intercellular spaces between adjoining cells are eliminated and the outer leaflets of the plasma membrane lipid bilayer of adjacent epithelial cells are closely apposed and appear to fuse32 (Fig. 1). Subapical to the tight junction are the adherens junctions and desmosomes, which are linked to actin-based microfilaments and cytokeratin-based intermediate filaments, respectively. These cytoskeletal structures provide the tensile strength that supports tight junctions and maintains cell shape.
Fig. 1. Tight junction structure and morphology.
a, Transmission electron micrograph showing the tight junction, adherens junction and desmosome, which, together, comprise the apical junctional complex. The tight junction is located just below the base of the microvilli. The magnification shows the transition from the luminal space, between the microvilli, into the tight junction, where morphologically detectable paracellular space is obliterated. b, Schematic of the apical junctional complex shown in part a, showing the location of the tight junction proteins zonula occludens 1 (ZO-1), occludin, claudin-2 and other claudin family members. Long myosin light chain kinase 1 (MLCK1) is associated with perijunctional F-actin and is a key regulator of tight junction permeability. c, Freeze–fracture electron micrograph showing tight junction strands at the base of apical microvilli.
Tight junction pathways and proteins
The first tight junction protein to be discovered was zonula occludens 1 (ZO-1)33 (Fig. 1), followed by the two related proteins ZO-2 and ZO-3, and the unrelated protein cingulin34–37, though all of these proteins are intracellular peripheral membrane proteins. These discoveries were followed by the discovery of the tetraspan transmembrane tight junction proteins occludin38 and the claudins, which are encoded by 27 genes in mammals39,40.
When expressed in non-epithelial cells, claudins can self-assemble into large polymers to form structures that are reminiscent of tight junction strands seen by freeze–fracture electron microscopy41 (Fig. 1). Many claudins are critical for barrier function, but others form charge-selective and size-selective paracellular channels. The ensemble of expressed claudins dictates the size-selectivity and charge-selectivity of specific sites within tissues. Detailed characterization has demonstrated that the charge-selectivity of claudin channels is determined by specific residues within the first extracellular loop42,43. Regardless of whether they are cation-selective or anion-selective, all claudin channels studied to date are size-selective and allow paracellular flux of only molecules with a diameter of <0.6 nm (refs. 43–46). These channels define the pore pathway47 (Box 1) and are exemplified by claudin-2, which mediates paracellular flux of small cations, such as Na+, and water42,43,48,49. Claudin-2 cannot, however, accommodate the commonly used macromolecular probes lactulose, mannitol or 4 kDa dextran48,50,51, emphasizing the need to consider the physical characteristics of the solute being measured when assigning mechanisms of changes in paracellular permeability.
Although rejected by the pore pathway, molecules larger than 0.6 nm, including lactulose, mannitol and 4 kDa dextran, can cross tight junctions via a second paracellular flux route known as the leak pathway (Box 1), which can accommodate molecules with diameters of up to ~12.5 nm and is not charge-selective47,52. The specific molecular structure of the leak pathway has not been identified, but its permeability is regulated by occludin53,54, tricellulin (also known as MARVEL domain-containing protein 2, a member of the tight junction-associated MARVEL protein (TAMP) family)55, ZO-1 (refs. 52,56) and perijunctional actomyosin53,57–59.
Pore and leak pathway functions in health
The major pore-forming claudins expressed within the intestinal epithelium are claudin-2 and claudin-15 (refs. 9,60), both of which form cation-selective channels49,61,62. Mice that lack either claudin-2 or claudin-15 are viable, but mice that lack both claudins die before weaning9, consistent with the idea that these proteins are, at least partially, functionally redundant. Nevertheless, intestinal hypertrophy occurs in claudin-15-knockout, but not claudin-2-knockout, mice63,64.
Paracellular Na+ efflux via claudin-2 and claudin-15 channels is critical to transcellular nutrient transport. Brush border absorption is largely driven by Na+–nutrient cotransporters that rely on the Na+ gradient between the intestinal lumen and the cytoplasm of epithelial cells (Fig. 2). During this process, Na+ enters the cytoplasm and is exported across the basolateral membrane into the lamina propria by the Na+–K+ ATPase65. In the absence of paracellular Na+ transport, transcellular transport rapidly depletes luminal Na+ and nutrient cotransport across the apical brush border membrane stops. Flux across claudin-2 and claudin-15 channels allows Na+ efflux from the lamina propria to the lumen, where it can drive additional cycles of Na+–nutrient cotransport.
Fig. 2. Coordination of transcellular and paracellular transport.

The gradient of Na+ between the gut lumen and the cytoplasm of epithelial cells provides the driving force for nutrient absorption across the apical brush border membrane, such as glucose absorption via the intestinal epithelial Na+–glucose cotransporter SGLT1. Nutrients then exit the cell via facilitated exchange proteins, such as the glucose transporter GLUT2, and Na+ exits via the Na+–K+ ATPase. Na+–glucose cotransport also triggers signal transduction pathways that activate long myosin light chain kinase 1 (MLCK1) and increase tight junction permeability. The osmotic gradient generated by transcellular nutrient and Na+ transport draws water across the tight junction and, owing to the high concentration of nutrient monomers in the unstirred layer, nutrients are carried along with this fluid in a mechanism known as solvent drag71. This process would quickly exhaust luminal Na+ if not for claudin-2 and claudin-15, which form paracellular Na+ channels that enable efflux of absorbed Na+ in order to provide the driving force for continued transcellular nutrient absorption9,63.
The requirement for Na+ efflux in the intestine explains the critical roles of claudin-2 and claudin-15 in nutrient absorption but does not explain why expression of these claudins is so precisely regulated. At birth, intestinal epithelial claudin-2 expression is high throughout the crypt–villus axis of the intestinal epithelium60,63,66, but claudin-2 expression is markedly diminished and limited to crypt epithelium after weaning. Concurrently, claudin-15 expression increases throughout the crypt–villus axis. Although only subtle functional differences between these claudins have been detected in vitro48,62, this precise developmental regulation suggests that the in vivo properties of claudin-2 and claudin-15 channels must differ substantially. Hypothetically, claudin-2 might allow greater paracellular Na+ flux than claudin-15, which would be advantageous for the rapid growth that occurs during early postnatal development and depends on Na+–nutrient cotransport. Alternatively, channel open state probabilities or other subtle functional characteristics of claudin-2 and claudin-15 might differ. This question could be resolved by single-channel analysis but, unfortunately, such data have only been reported for claudin-2 (ref. 46).
Na+–nutrient cotransport triggers downstream signalling in the epithelial cell. These signalling events activate MLCK, which phosphorylates perijunctional myosin regulatory light chain (MLC) and increases leak pathway permeability67–69. This process amplifies paracellular nutrient transport via solvent drag (Fig. 2), whereby the osmotic gradient created by transcellular transport drives paracellular water absorption70. The absorbed water comes from the unstirred layer immediately adjacent to the epithelium, which is rich in small nutrient monomers owing to the activity of brush border digestive enzymes. This paracellular fluid absorption allows paracellular nutrient absorption to amplify transcellular transport71–74. Importantly, solvent drag only contributes substantially to nutrient absorption when luminal concentrations of nutrient monomers are high72. This subtlety probably explains why passive paracellular nutrient absorption via solvent drag has been detected in some studies and not others75–84. In contrast to transcellular transport, paracellular absorption via solvent drag is not stereospecific and can accommodate molecules for which there are no apical transporters, such as mannitol72,85.
Paracellular amplification of transcellular absorption, or solvent drag, probably explains why the rate of nutrient transport across the intestinal epithelium cannot be saturated72. Clinically, solvent drag contributes to the efficacy of simple Na+ and carbohydrate-containing oral rehydration solutions that have been used to treat countless individuals with potentially fatal, high-volume diarrhoeal diseases, such as cholera. By contrast, the glycosuria that occurs in diabetes mellitus suggests that no corresponding paracellular pathway for glucose resorption exists within the renal tubule.
The pore pathway in disease
Intestinal epithelial claudin-2 has been the subject of intense scrutiny owing to its markedly increased expression in a broad range of inflammatory disorders. Claudin-2 upregulation was first described in the contexts of ulcerative colitis and Crohn’s disease86,87. Subsequent work demonstrated that intestinal epithelial claudin-2 is also upregulated in coeliac disease88, irritable bowel syndrome89, HIV enteropathy90, enteric infection50, necrotizing enterocolitis91 and Whipple disease92. The factors that mediate claudin-2 upregulation are incompletely characterized, but in vitro and in vivo studies have implicated IL-1, IL-6, IL-13, IL-22 and tumour necrosis factor (TNF) as potential enhancers of claudin-2 expression50,86,93–96. By contrast, some in vitro studies suggest that butyrate can suppress claudin-2 expression and increase barrier function via an IL-10 receptor-dependent mechanism97. Together, these data indicate that the mucosal immune system can fine-tune claudin-2 expression.
Administration of recombinant IL-13 to mice increases claudin-2 expression and augments intestinal paracellular cation permeability98. Similarly, transgenic claudin-2 overexpression within the intestinal epithelium increases paracellular permeability to cations to levels similar to those in IL-13-treated wild-type mice98. By contrast, IL-13 has no effect on intestinal permeability in mice in which the Cldn2 gene, which encodes claudin-2, is knocked out. Thus, claudin-2 upregulation is both necessary and sufficient to increase paracellular cation permeability in vivo. The impact of this effect on disease is discussed in the following sections.
Claudin-2 attenuates diseases induced by luminal insults
A pair of studies of Cldn2-transgenic and Cldn2-knockout mice provided initial evidence that increased claudin-2 expression is beneficial in dextran sulfate sodium (DSS)-induced colitis98,99. However, claudin-2 overexpression also increases faecal water content50, suggesting that increased claudin-2 expression might reduce the severity of colitis simply by diluting DSS within the distal colon. An alternative possibility is that claudin-2 expression promotes epithelial growth and mucosal repair100–102, as discussed below (see ‘Claudin-2 and epithelial proliferation’).
Claudin-2 expression is increased during enteric infection in humans103. Similarly, the model pathogen Citrobacter rodentium triggers IL-22-dependent claudin-2 upregulation within 2 days of infection in mice50 (Fig. 3). Although IL-22 is pleiotropic, an increased number of mucosa-associated C. rodentium, delayed pathogen clearance and a greater severity of mucosal damage in Cldn2-knockout mice relative to wild-type mice demonstrate that IL-22-dependent claudin-2 upregulation contributes to host defence50. The observation that transgenic claudin-2 overexpression limits C. rodentium-induced colitis provides further support for this conclusion.
Fig. 3. Promotion of pathogen clearance by paracellular fluid efflux.
Citrobacter rodentium infection triggers an immune response that leads to IL-22 release within the lamina propria within 2 days of infection. IL-22 signalling activates claudin-2 transcription and increases claudin-2 channel-mediated Na+ and water efflux via the tight junction pore pathway, resulting in diarrhoea that promotes clearance of the infection. Adapted with permission from ref. 50, Elsevier.
The passage of either Na+ or water through claudin-2 channels104,105 could mediate the increased pathogen clearance and reduced disease severity associated with claudin-2 expression. In studies to dissect these mechanisms, wild-type, Cldn2-transgenic and Cldn2-knockout mice were infected with C. rodentium. Polyethylene glycol (PEG) was added to their drinking water 4 days later to create an osmotic gradient that increased fluid flow into the intestinal lumen. PEG treatment normalized the number of mucosa-associated C. rodentium, pathogen clearance and mucosal damage across genotypes, demonstrating that paracellular water efflux is the primary means by which claudin-2 promotes enteric pathogen clearance.
The effect of PEG was unlikely to have been a result of fluid efflux simply washing bacteria off the epithelial surface, as C. rodentium is an attaching and effacing pathogen that forms pedestals and is not easily displaced from intestinal epithelial cells. Moreover, intestinal epithelial cells turn over every few days during infection, so the cells present at the peak of disease (11 days after infection) are not the same cells that were initially colonized. Therefore, one possible explanation for the findings is that claudin-2-mediated paracellular water efflux reduces the efficiency with which new cells are infected. This hypothesis remains to be tested. Nevertheless, evidence indicates that the diarrhoea induced by claudin-2 upregulation is beneficial in the context of enteric infection. These results provide the first experimental data to show that diarrhoea promotes enteric pathogen clearance, an idea that has persisted for centuries despite a lack of supporting evidence106.
Claudin-2 exacerbates immune-mediated colitis
Claudin-2 transcription is exquisitely responsive to cytokine stimulation and is increased in a wide range of human and experimental disorders associated with mucosal inflammation. Given its protective role during enteric infection and DSS-induced injury, claudin-2 upregulation might also be expected to be beneficial in inflammatory disease. This hypothesis was tested by inducing immune-mediated colitis by T cell transfer in immunodeficient, claudin-2 wild-type, transgenic and knockout mice98. In contrast to the effects of claudin-2 in infectious colitis, its overexpression exacerbated immune-mediated colitis and was associated with severe weight loss, increased cytokine production, mucosal T cell infiltration and histopathological damage. Conversely, Cldn2 knockout attenuated all measures of colitis severity. Together with the effects of claudin-2 expression on pathogen clearance, these data suggest that claudin-2 upregulation triggers defence mechanisms that include immune activation and, therefore, exacerbates immune-mediated disease. Although the mechanisms by which claudin-2-mediated paracellular Na+ and water flux enhances immune activation is unknown, this phenomenon could explain the observed exacerbation of immune-mediated disease by high-Na+ diets107–114.
The findings in Cldn2-knockout mice also provide further support for the idea that substantial functional differences exist between claudin-2 and claudin-15 in vivo. Although claudin-15 expression is not altered in human IBD or experimental immune-mediated colitis, it was upregulated in colitic Cldn2-knockout mice98. However, Cldn2-knockout mice remained protected from immune-mediated colitis, indicating that claudin-15 cannot compensate for claudin-2 loss in this context.
Although disease severity was lower in Cldn2-knockout mice than in wild-type mice, survival was inferior98. The cause of death among Cldn2-knockout mice was intestinal obstruction. This observation could reflect an inability to increase luminal hydration, owing to lack of claudin-2-mediated water transport, that synergized with colitis-associated epithelial proliferation, mucosal expansion and luminal narrowing to allow formation of luminal faecaliths and intestinal obstruction. Consistent with this interpretation, induction of mild osmotic diarrhoea increased faecal water content, prevented intestinal obstruction and increased survival of the Cldn2-knockout mice50. Osmotic diarrhoea did not, however, affect overall disease severity in claudin-2 wild-type, transgenic or knockout mice98. Therefore, the increased survival due to osmotic diarrhoea does not reflect direct mitigation of immune activation or tissue damage.
The protection afforded by Cldn2 knockout suggests that pharmacological inhibition of claudin-2 function might be effective in immune-mediated disease. Of several reported approaches to claudin-2 channel inhibition115–118, only one has been assessed in vivo98. This approach relies on casein kinase 2 (CK2) inhibition, occludin dephosphorylation and assembly of a claudin-2–ZO-1–occludin complex that inactivates claudin-2 channels98,117. CK2 inhibition did not interfere with IL-13-induced increases in claudin-2 expression but did prevent IL-13-induced changes in paracellular Na+ permeability. CK2 inhibition markedly attenuated the severity of immune-mediated colitis in claudin-2 wild-type mice98. Although CK2 is a ubiquitously expressed, promiscuous kinase, CK2 inhibition did not affect disease severity in Cldn2-knockout mice, indicating that its therapeutic benefit is largely due to claudin-2 channel inactivation. Notably, CK2 inhibition did not cause intestinal obstruction in Cldn2 wild-type mice, probably owing to incomplete claudin-2 channel inactivation. Together with the fact that the intestinal lumen diameter is much greater in humans than in mice, this observation suggests that pharmacological claudin-2 channel inhibition is unlikely to cause intestinal obstruction in humans.
Claudin-2 and epithelial proliferation
Some evidence suggests that claudin-2 promotes epithelial proliferation100,101,119,120. For example, the rate of intestinal epithelial proliferation was nearly doubled in one strain of mice with transgenic claudin-2 overexpression100. As mentioned above, this proliferation might contribute to the protection that claudin-2-transgenic mice have from DSS-induced colitis100. However, epithelial proliferation was not increased in a different Cldn2-transgenic mouse model50. The reasons for this discrepancy are unclear, as Cldn2 expression was under the control of the 9 kB villin promoter121 and pore pathway permeability was increased in both models. However, the transgenic mice differed in that one expressed human claudin-2 at high levels100, whereas the other expressed EGFP-tagged mouse claudin-2 at lower levels50. Although further study is needed, this difference could underlie the discrepancy between the two models and could also explain increases in leak pathway permeability that occurred in the first, but not the second, model.
Other data that suggest a role for claudin-2 in regulating epithelial proliferation include studies of SW480 and HCT116 human colon cancer cells, which demonstrated that claudin-2 overexpression increases proliferation in vitro, accelerates tumour growth in vivo and reduces apoptosis triggered by the chemotherapeutic agent 5-fluorouracil122. In a study in patients with colon cancer, high claudin-2 expression correlated with lower overall and disease-free survival, further supporting the notion that claudin-2 can promote epithelial proliferation123. Thus, although the mechanisms are not defined, claudin-2 overexpression might promote intestinal epithelial cell proliferation in some contexts.
The leak pathway in disease
Although the leak pathway is activated physiologically during Na+–nutrient cotransport, far greater increases in leak pathway permeability are induced by TNF124–126 (Box 1). The reasons for this difference between physiological and pathophysiological tight junction regulation are unclear because both depend on MLCK activation, but they might relate to the fact that occludin endocytosis occurs during TNF-induced barrier loss but occludin distribution is unaffected during Na+–nutrient cotransport-induced permeability increases53,68,125,127. Occludin is also internalized during MLCK-mediated barrier loss induced by the TNF-related cytokine LIGHT, IL-1β or lipopolysaccharide128–133.
In experimental, immune-mediated IBD, activation of intestinal epithelial MLCK accelerates disease progression, whereas genetic deletion of intestinal epithelial MLCK attenuates disease59,134. Interestingly, the claudin-2 upregulation that normally occurs in experimental, immune-mediated IBD is reduced in mice that lack intestinal epithelial MLCK134 and is restored by transgenic expression of constitutively active MLCK within intestinal epithelia134. Thus, the leak pathway and pore pathway are linked in disease. Notably, transgenic expression of constitutively active MLCK within intestinal epithelial cells, which modestly increases leak pathway permeability but does not induce disease, increases both mucosal IL-13 production and epithelial claudin-2 expression. Thus, increased leak pathway permeability can, via mucosal immune activation, trigger claudin-2 upregulation45. Conversely, as noted above, leak pathway permeability is increased in one of two claudin-2 overexpressing transgenic mice despite the absence of overt disease. Although the precise relationship between pore pathway and leak pathway regulation in the context of disease remains to be determined, the ability of distinct cytokines to specifically and independently regulate pore pathway or leak pathway permeability is striking.
TNF, LIGHT and IL-1β all trigger transcriptional and enzymatic activation of MLCK135–137, although reports differ as to whether the transcriptional activation is mediated by nuclear factor-κB, p38 mitogen-activated protein kinase (MAPK) or the transcription factor activator protein 1 (AP-1)135,138–140. Regardless of these discrepancies, MLCK activation clearly leads to perijunctional MLC phosphorylation and occludin endocytosis125,140,141. Despite ongoing debate regarding the functional significance of occludin, the consensus is that occludin, along with other proteins, is a critical regulator of leak pathway permeability. This role of occludin has been demonstrated in several in vitro studies52,142–144, but the strongest evidence comes from in vivo studies that have shown that blockade of occludin endocytosis or transgenic occludin overexpression limits TNF-induced leak pathway barrier loss54. This observation suggests that reduced occludin expression could explain the increased permeability of the leak pathway observed in human disease, including IBD86,145.
The tricellular tight junction proteins tricellulin and angulin 1 might also be important regulators of leak pathway permeability. In vitro studies have shown that deletion of either tricellulin or angulin-1 increases leak pathway permeability146–148. Similar to tricellulin, siRNA-mediated knockdown of the other TAMPs, MARVELD3 (refs. 146,149) or occludin142,143, also increased leak pathway permeability. Tricellulin redistribution from tricellular to bicellular tight junctions150 following occludin loss54,125 could, therefore, be an intermediate event that allows occludin to regulate leak pathway permeability. Thus, although a great deal has been learned about proteins that contribute to leak pathway barrier function and mechanisms of experimental and pathophysiological leak pathway regulation, the molecular structure of the leak pathway remains enigmatic.
Diverse occludin functions
Unexpectedly, intestinal epithelial specific occludin knockout protects mice from experimental colitis and epithelial injury driven by intrinsic and extrinsic TNF signalling pathways. This finding was ultimately explained by the observation that occludin enhances activity of the promoter for CASP3, which encodes caspase 3, through an undefined mechanism145. In cultured cell lines and mice, occludin downregulation led to a reduction of ~50% in caspase 3 expression that conferred protection from a diverse range of pro-apoptotic stimuli145,151. Analyses of biopsy samples suggests that this process also occurs in human disease, as occludin downregulation correlates with reduced epithelial caspase 3 expression in patients with ulcerative colitis or Crohn’s disease.
Thus, in addition to increasing leak pathway permeability, occludin downregulation can promote epithelial survival. However, this effect might not be entirely beneficial, as it could allow evolution of deleterious mutations that would have otherwise been eliminated by apoptosis. Consistent with this hypothesis, in vitro studies suggest that occludin functions as a tumour suppressor in some contexts151–155. Further exploration will, therefore, be required to fully understand extra-junctional functions of occludin.
Distinct functions of long MLCK splice variants
Epithelial MLCK is expressed from the same gene (MYLK) that encodes smooth muscle MLCK156. However, epithelial (long) MLCK is ~225 kDa (refs. 156,157), whereas smooth muscle (short) MLCK is only ~130 kDa (Fig. 4). Long MLCK transcription, which is activated by TNF, IL-1β and other stimuli135,158, generates mRNA transcripts that include additional 5′ exons that are not present in short MLCK transcripts156. This difference reflects the location of the short MLCK promoter within an intron of long MLCK. Nevertheless, the carboxy-terminal catalytic and calmodulin-binding regulatory domains are identical in long and short MLCK. The 5′ region that distinguishes long MLCK from short MLCK undergoes extensive alternative splicing156. Of the splice variants generated, only two — long MLCK1 and MLCK2 — are expressed in intestinal epithelial cells157 (Fig. 4). Although the underlying mechanisms have not been defined, splicing seems to be precisely regulated during differentiation, as MLCK2 is expressed throughout the crypt–villus axis but MLCK1 expression is limited to the upper villus157. Moreover, the increased MLC phosphorylation in active Crohn’s disease is specifically associated with perijunctional MLCK1 recruitment159,160 (Fig. 4).
Fig. 4. Epithelial and smooth muscle myosin light chain kinase.
a, The human MYLK gene encodes long (non-muscle) and short (smooth muscle) isoforms of myosin light chain kinase (MLCK) protein. Two long MLCK transcriptional start sites that result in expression of the same protein have been identified. However, extensive alternative splicing within the 5′ half of the transcript occurs, which, in intestinal epithelial cells, results in expression of two long MLCK splice variants, MLCK1 and MLCK2. These variants differ by a single exon (black), removal of which causes the third of the nine immunoglobulin-cell adhesion molecule (IgCAM) domains to be incomplete in long MLCK2. The short MLCK promoter is located within a long MLCK intron and drives transcription of smooth muscle MLCK, which lacks the six amino-terminal IgCAM domains that are present in long MLCK1. The kinase and calmodulin (CaM)-binding domains are encoded by sequences within the 3′ half of MYLK and are identical in long and short MLCK proteins. b, Inflammatory signals, such as tumour necrosis factor (TNF), trigger MLCK1 binding to FKBP8. This binding facilitates MLCK1 recruitment to the perijunctional actomyosin ring, where it phosphorylates MLC. This phosphorylation causes occludin internalization to increase leak pathway permeability. In contrast to MLCK1, MLCK2 distribution is not affected by TNF. c, MLCK1 expression and recruitment to the perijunctional actomyosin ring (arrows) are increased in Crohn’s disease. The insets show the boxed areas. MLCK1 and total MLCK are shown, as the absence of unique MLCK2 sequences prevents generation of MLCK2-specific antibodies. Nuclei appear yellow. Part a adapted from ref. 141, Springer Nature Limited.
The two intestinal epithelial long MLCK splice variants differ by a single exon that is present in MLCK1 but not in MLCK2 (ref. 157). The 69 amino acids encoded by this exon complete the third immunoglobulin–cell adhesion molecule domain (IgCAM3)157. This domain must, therefore, contribute to preferential perijunctional localization of MLCK1 relative to MLCK2, which is distributed more diffusely through the cytoplasm141,157. TNF triggers even greater recruitment of MLCK1 to the perijunctional actomyosin ring141. Similarly, MLCK1 is concentrated within the perijunctional actomyosin ring in intestinal biopsy samples from patients with active IBD141,160,161. This inflammation-inducible perijunctional MLCK1 recruitment, together with increased barrier function after MLCK1-specific knockdown157, suggests that this splice variant is central to tight junction regulation.
These findings prompted solution of the IgCAM3 crystal structure, which was then used for in silico screening of small drug-like molecules that were predicted to bind to IgCAM3 (ref. 141). In vitro testing identified one compound that diverts MLCK1 from the perijunctional actomyosin ring and reverses cytokine-induced MLCK1 recruitment in vivo and in excised human intestine141. This molecule, known as divertin, blocks MLCK1-mediated phosphorylation of perijunctional MLC, thereby preventing subsequent occludin endocytosis and increases in leak pathway permeability141. Divertin does not, however, interfere with other functions of long or short MLCK, including its involvement in epithelial cell migration and smooth muscle contraction, because IgCAM3 is not present in short MLCK and is distant from the MLCK1 catalytic and regulatory domains141. This functional selectivity is critical, as in vivo inhibition of MLCK activity leads to hypotension and visceral paralysis, including aperistalsis, thereby precluding therapeutic use of enzymatic inhibitors162.
Therapeutic targeting of MLCK1 recruitment
Divertin was remarkably effective in a mouse model of immune-mediated IBD — its effects were equal or superior to those of anti-TNF therapy by all measures, including survival141. This result supports the hypothesis that divertin-mediated interference with IgCAM3-mediated protein–protein interactions prevents perijunctional MLCK1 recruitment and, ultimately, disease progression.
A screen for potential MLCK1 binding partners was used to identify protein–protein interactions targeted by divertin. This process led to the discovery of tacrolimus binding protein FKBP8 as an MLCK1-interacting protein160 (Fig. 4). MLCK1–FKBP8 interactions were specifically increased in TNF-treated intestinal epithelial monolayer cultures160. These interactions tended to occur near the perijunctional actomyosin ring160. Similarly, increases in perijunctional MLCK1–FKBP8 interactions were detected in biopsy samples from patients with Crohn’s disease160. Tacrolimus also prevented TNF-induced perijunctional MLCK1 recruitment, MLCK1–FKBP8 interactions and perijunctional MLC phosphorylation in human intestinal organoids160. Finally, tacrolimus prevented MLCK1 recruitment, occludin internalization and barrier loss after acute T cell activation in mice160. Surprisingly, divertin did not interfere with FKBP8 binding to recombinant MLCK1 IgCAM domains one to four in vitro. Thus, despite the efficacy of divertin in experimental colitis, agents that prevent MLCK1 interactions with FKBP8 or other MLCK1 binding partners should also be sought as potential therapeutics.
Conclusions
The first tight junction protein, ZO-1 (ref. 33), was discovered nearly 40 years ago, and numerous other tight junction proteins have been identified since37–39,149,163–167, leading to substantial data describing the molecular interactions responsible for selective permeability and barrier regulation144,146,147,168–172. This work has led to conceptual advances, including the pore and leak pathway model of paracellular permeability47,173, and foundational understanding of tight junction cell biology, physiology and pathobiology.
In the same year that ZO-1 was discovered33, increased intestinal permeability was identified in a subset of first-degree relatives of people with Crohn’s disease174. More recently, these modest leak pathway permeability increases were validated as an independent risk factor for IBD24. However, all human studies to date have relied on probes, such as lactulose and mannitol, that are too large to cross the pore pathway. Thus, despite increased claudin-2 expression in human disease64,86,87,92,175,176 and experimental data showing that claudin-2-dependent pore pathway permeability increases exacerbate disease in mice50,98,100, the relevance of claudin-2 to human intestinal disease remains to be determined. Our understanding of how barrier function and disease are affected by polymorphisms in barrier-related genes associated with IBD, including INAVA177–179 and CDH1 (refs. 180,181), which encode innate immune activator and E-cadherin, respectively, is even more limited.
In conclusion, our understanding of how permeability of the pore and leak pathways contributes to health and disease remains relatively rudimentary. Thus, although much has been accomplished, much more remains to be discovered. Remaining challenges include identification of the sites and molecular structure of the leak pathway, elucidation of the differences that must exist between seemingly redundant claudins, and the definition of non-canonical tight junction protein functions. Nevertheless, the promise of tight junction-targeted therapeutics remains compelling, and implementation of such therapeutic approaches is growing progressively closer.
Acknowledgements
The authors are supported by National Institute of Diabetes and Digestive and Kidney Disease grants R01DK61931 and R01DK68271 (J.R.T.), U.S. Department of Defense award PR181271 (J.R.T.), and the Belgian American Educational Foundation (X.H.). The authors also greatly appreciate the contributions of S. Hagen, N. Shashikanth, L. Zuo, S. Abtahi, L.-S. Beier and G. Marsischky, as well as H. Marlatt (Nationwide Histology) and T. S. Davanzo (Slaybaugh Studios). The freeze–fracture electron micrograph in Fig. 1 was provided by the late E. E. Schneeberger (Harvard Medical School), an exceptional friend, pathologist and scientist.
Author contributions
A.H. and J.R.T. wrote the manuscript. All authors researched data for the article, made substantial contributions to discussion of content and reviewed and edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks A. Keshavarzian, R. Rao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
J.R.T. is a consultant for Entrinsic and Kallyope. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simon DB, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 2.Kausalya PJ, et al. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J. Clin. Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilcox ER, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/S0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Yosef T, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, et al. Multiple claudin–claudin cis interfaces are required for tight junction strand formation and inherent flexibility. Commun. Biol. 2018;1:50. doi: 10.1038/s42003-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wattenhofer M, et al. Different mechanisms preclude mutant CLDN14 proteins from forming tight junctions in vitro. Hum. Mutat. 2005;25:543–549. doi: 10.1002/humu.20172. [DOI] [PubMed] [Google Scholar]
- 7.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018;10:a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada M, Tamura A, Takahashi N, Tsukita S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology. 2013;144:369–380. doi: 10.1053/j.gastro.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin. Gastroenterol. Hepatol. 2013;11:1075–1083. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brien TG, O’Hagan R, Muldowney FP. Chromium-51-EDTA in the determination of glomerular filtration rate. Acta Radiol. Ther. Phys. Biol. 1969;8:523–529. doi: 10.3109/02841866909134478. [DOI] [PubMed] [Google Scholar]
- 14.Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn’s disease of the terminal ileum and colon. Digestion. 1983;27:70–74. doi: 10.1159/000198932. [DOI] [PubMed] [Google Scholar]
- 15.Howden CW, Robertson C, Duncan A, Morris AJ, Russell RI. Comparison of different measurements of intestinal permeability in inflammatory bowel disease. Am. J. Gastroenterol. 1991;86:1445–1449. [PubMed] [Google Scholar]
- 16.Peeters M, et al. Increased permeability of macroscopically normal small bowel in Crohn’s disease. Dig. Dis. Sci. 1994;39:2170–2176. doi: 10.1007/BF02090367. [DOI] [PubMed] [Google Scholar]
- 17.Johansson JE, Ekman T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig. Dis. Sci. 2007;52:2340–2345. doi: 10.1007/s10620-006-9404-x. [DOI] [PubMed] [Google Scholar]
- 18.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-H. [DOI] [PubMed] [Google Scholar]
- 19.D’Inca R, et al. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am. J. Gastroenterol. 1999;94:2956–2960. doi: 10.1111/j.1572-0241.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- 20.Bitton A, et al. Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut. 2008;57:1386–1392. doi: 10.1136/gut.2007.134817. [DOI] [PubMed] [Google Scholar]
- 21.Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- 22.Buhner S, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres J, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology. 2020;159:96–104. doi: 10.1053/j.gastro.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Turpin W, et al. Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology. 2020;159:2092–2100.e5. doi: 10.1053/j.gastro.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, et al. Anti-microbial antibody response is associated with future onset of Crohn’s disease independent of biomarkers of altered gut barrier function, subclinical inflammation, and genetic risk. Gastroenterology. 2021;161:1540–1551. doi: 10.1053/j.gastro.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Edwinson AL, et al. Gut microbial β-glucuronidases regulate host luminal proteases and are depleted in irritable bowel syndrome. Nat. Microbiol. 2022;7:680–694. doi: 10.1038/s41564-022-01103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol. Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 28.Edogawa S, et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut. 2020;69:62–73. doi: 10.1136/gutjnl-2018-317416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Magistris L, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010;51:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inczefi O, et al. Targeted intestinal tight junction hyperpermeability alters the microbiome, behavior, and visceromotor responses. Cell Mol. Gastroenterol. Hepatol. 2020;10:206–208.e3. doi: 10.1016/j.jcmgh.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farquhar M, Palade G. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson BR, Heintzelman MB, Anderson JM, Citi S, Mooseker MS. ZO-1 and cingulin: tight junction proteins with distinct identities and localizations. Am. J. Physiol. 1989;257:C621–C628. doi: 10.1152/ajpcell.1989.257.4.C621. [DOI] [PubMed] [Google Scholar]
- 35.Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J. Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 38.Furuse M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mineta K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–612. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki H, et al. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc. Natl Acad. Sci. USA. 2003;100:3971–3976. doi: 10.1073/pnas.0630649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angelow S, Yu AS. Structure–function studies of claudin extracellular domains by cysteine-scanning mutagenesis. J. Biol. Chem. 2009;284:29205–29217. doi: 10.1074/jbc.M109.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu AS, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J. Gen. Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Itallie CM, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 45.Weber CR, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber CR, et al. Claudin-2-dependent paracellular channels are dynamically gated. eLife. 2015;4:e09906. doi: 10.7554/eLife.09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner JR. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal R, et al. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017;219:521–536. doi: 10.1111/apha.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amasheh S, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 50.Tsai PY, et al. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe. 2017;21:671–681.e4. doi: 10.1016/j.chom.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenthal R, Fromm M. Significant water absorption goes paracellular in kidney proximal tubules. Am. J. Physiol. Renal Physiol. 2014;306:F51–F52. doi: 10.1152/ajprenal.00545.2013. [DOI] [PubMed] [Google Scholar]
- 52.Buschmann MM, et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell. 2013;24:3056–3068. doi: 10.1091/mbc.e12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim. Biophys. Acta. 2008;1778:709–716. doi: 10.1016/j.bbamem.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krug SM, et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell. 2009;20:3713–3724. doi: 10.1091/mbc.e09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell. 2009;20:3930–3940. doi: 10.1091/mbc.e09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell. 2005;16:3919–3936. doi: 10.1091/mbc.e04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen L, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 59.Su L, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 62.Rosenthal R, et al. Claudin-15 forms a water channel through the tight junction with distinct function compared to claudin-2. Acta Physiol. 2020;228:e13334. doi: 10.1111/apha.13334. [DOI] [PubMed] [Google Scholar]
- 63.Tamura A, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Tamura A, et al. Megaintestine in claudin-15–deficient mice. Gastroenterology. 2008;134:523–534. doi: 10.1053/j.gastro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 65.Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 2011;73:261–281. doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ong M, Yeruva S, Sailer A, Nilsen SP, Turner JR. Differential regulation of claudin-2 and claudin-15 expression in children and adults with malabsorptive disease. Lab. Invest. 2020;100:483–490. doi: 10.1038/s41374-019-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner JR, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 68.Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1487–G1493. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- 69.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 70.Pappenheimer JR. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J. Membr. Biol. 1987;100:137–148. doi: 10.1007/BF02209146. [DOI] [PubMed] [Google Scholar]
- 71.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J. Membr. Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 72.Meddings JB, Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin. Sci. 1989;76:403–413. doi: 10.1042/cs0760403. [DOI] [PubMed] [Google Scholar]
- 73.Turner JR, Cohen DE, Mrsny RJ, Madara JL. Noninvasive in vivo analysis of human small intestinal paracellular absorption: regulation by Na+-glucose cotransport. Dig. Dis. Sci. 2000;45:2122–2126. doi: 10.1023/A:1026682900586. [DOI] [PubMed] [Google Scholar]
- 74.Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology. 2000;119:1029–1036. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 75.Lane JS, et al. Paracellular glucose transport plays a minor role in the unanesthetized dog. Am. J. Physiol. 1999;276:G789–G794. doi: 10.1152/ajpgi.1999.276.3.G789. [DOI] [PubMed] [Google Scholar]
- 76.Uhing MR, Arango V. Intestinal absorption of proline and leucine in chronically catheterized rats. Gastroenterology. 1997;113:865–874. doi: 10.1016/S0016-5085(97)70181-1. [DOI] [PubMed] [Google Scholar]
- 77.Uhing MR, Kimura RE. The effect of surgical bowel manipulation and anesthesia on intestinal glucose absorption in rats. J. Clin. Invest. 1995;95:2790–2798. doi: 10.1172/JCI117983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner JR, Madara JL. Physiological regulation of intestinal epithelial tight junctions as a consequence of Na+-coupled nutrient transport. Gastroenterology. 1995;109:1391–1396. doi: 10.1016/0016-5085(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 79.Fine KD, Ana CAS, Porter JL, Fordtran JS. Mechanism by which glucose stimulates the passive absorption of small solutes by the human jejunum in-vivo. Gastroenterology. 1994;107:389–395. doi: 10.1016/0016-5085(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 80.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of D-glucose on intestinal permeability and its passive absorption in human small intestine in vivo. Gastroenterology. 1993;105:1117–1125. doi: 10.1016/0016-5085(93)90957-E. [DOI] [PubMed] [Google Scholar]
- 81.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of changing intestinal flow rate on a measurement of intestinal permeability. Gastroenterology. 1995;108:983–989. doi: 10.1016/0016-5085(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 82.Fordtran JS. Stimulation of active and passive sodium absorption by sugars in the human jejunum. J. Clin. Invest. 1975;55:728–737. doi: 10.1172/JCI107983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner JR. Show me the pathway! Regulation of paracellular permeability by Na+-glucose cotransport. Adv. Drug. Deliv. Rev. 2000;41:265–281. doi: 10.1016/S0169-409X(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 84.Kellett GL. The facilitated component of intestinal glucose absorption. J. Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am. J. Physiol. 1990;258:C77–C85. doi: 10.1152/ajpcell.1990.258.1.C77. [DOI] [PubMed] [Google Scholar]
- 86.Heller F, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Zeissig S, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schumann M, et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–228. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- 89.Martinez C, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 90.Epple HJ, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58:220–227. doi: 10.1136/gut.2008.150425. [DOI] [PubMed] [Google Scholar]
- 91.Bergmann KR, et al. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am. J. Pathol. 2013;182:1595–1606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Epple HJ, et al. Architectural and functional alterations of the small intestinal mucosa in classical Whipple’s disease. Mucosal Immunol. 2017;10:1542–1552. doi: 10.1038/mi.2017.6. [DOI] [PubMed] [Google Scholar]
- 93.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/S0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamamoto T, et al. IL-1β regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Exp. Cell Res. 2004;299:427–441. doi: 10.1016/j.yexcr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 96.Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann. NY Acad. Sci. USA. 2009;1165:267–273. doi: 10.1111/j.1749-6632.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- 97.Zheng L, et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J. Immunol. 2017;199:2976–2984. doi: 10.4049/jimmunol.1700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raju P, et al. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J. Clin. Invest. 2020;130:5197–5208. doi: 10.1172/JCI138697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curry JN, et al. Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease. J. Clin. Invest. 2020;130:1948–1960. doi: 10.1172/JCI127750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmad R, et al. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol. 2014;7:1340–1353. doi: 10.1038/mi.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buchert M, et al. Symplekin promotes tumorigenicity by up-regulating claudin-2 expression. Proc. Natl Acad. Sci. USA. 2010;107:2628–2633. doi: 10.1073/pnas.0903747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dhawan P, et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene. 2011;30:3234–3247. doi: 10.1038/onc.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Denizot J, et al. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn’s disease patients. Inflamm. Bowel Dis. 2012;18:294–304. doi: 10.1002/ibd.21787. [DOI] [PubMed] [Google Scholar]
- 104.Muto S, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl Acad. Sci. USA. 2010;107:8011–8016. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosenthal R, et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 106.Davison WC. A bacteriological and clinical consideration of bacillary dysentery in adults and children. Medicine. 1922;1:389–510. doi: 10.1097/00005792-192211000-00001. [DOI] [Google Scholar]
- 107.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Safa K, et al. Salt accelerates allograft rejection through serum- and glucocorticoid-regulated kinase-1-dependent inhibition of regulatory T cells. J. Am. Soc. Nephrol. 2015;26:2341–2347. doi: 10.1681/ASN.2014090914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguiar SLF, et al. High-salt diet induces IL-17-dependent gut inflammation and exacerbates colitis in mice. Front. Immunol. 2017;8:1969. doi: 10.3389/fimmu.2017.01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monteleone I, et al. Sodium chloride-enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J. Crohns Colitis. 2017;11:237–245. doi: 10.1093/ecco-jcc/jjw139. [DOI] [PubMed] [Google Scholar]
- 111.Wei Y, et al. High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice. Oncotarget. 2017;8:70–82. doi: 10.18632/oncotarget.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toussirot E, Bereau M, Vauchy C, Saas P. Could sodium chloride be an environmental trigger for immune-mediated diseases? An overview of the experimental and clinical evidence. Front. Physiol. 2018;9:440. doi: 10.3389/fphys.2018.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matthias J, et al. Salt generates antiinflammatory Th17 cells but amplifies their pathogenicity in proinflammatory cytokine microenvironments. J. Clin. Invest. 2020;130:4587–4600. doi: 10.1172/JCI137786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuang R, O’Keefe SJD, Ramos Del Aguila de Rivers C, Koutroumpakis F, Binion DG. Is salt at fault? Dietary salt consumption and inflammatory bowel disease. Inflamm. Bowel Dis. 2023;29:140–150. doi: 10.1093/ibd/izac058. [DOI] [PubMed] [Google Scholar]
- 115.Koch S, et al. Protein kinase CK2 is a critical regulator of epithelial homeostasis in chronic intestinal inflammation. Mucosal Immunol. 2013;6:136–145. doi: 10.1038/mi.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma J, et al. Identification of selective claudin cation channel blockers [abstract] Gastroenterology. 2021;160(Suppl. 3):S33–S34. doi: 10.1053/j.gastro.2021.01.100. [DOI] [Google Scholar]
- 117.Raleigh DR, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takigawa M, et al. Creation of a claudin-2 binder and its tight junction-modulating activity in a human intestinal model. J. Pharmacol. Exp. Ther. 2017;363:444–451. doi: 10.1124/jpet.117.242214. [DOI] [PubMed] [Google Scholar]
- 119.Amoozadeh Y, et al. Cell confluence regulates claudin-2 expression: possible role for ZO-1 and Rac. Am. J. Physiol. Cell Physiol. 2018;314:C366–C378. doi: 10.1152/ajpcell.00234.2017. [DOI] [PubMed] [Google Scholar]
- 120.Ikari A, et al. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim. Biophys. Acta. 2014;1843:2079–2088. doi: 10.1016/j.bbamcr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 121.Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J. Biol. Chem. 1999;274:6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 122.Buhrmann C, et al. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015;98:51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]
- 123.Abu-Farsakh S, Wu T, Lalonde A, Sun J, Zhou Z. High expression of claudin-2 in esophageal carcinoma and precancerous lesions is significantly associated with the bile salt receptors VDR and TGR5. BMC Gastroenterol. 2017;17:33. doi: 10.1186/s12876-017-0590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zolotarevsky Y, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 125.Clayburgh DR, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology. 2002;123:1179–1190. doi: 10.1053/gast.2002.36002. [DOI] [PubMed] [Google Scholar]
- 127.Yu D, et al. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl Acad. Sci. USA. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaminsky LW, Al-Sadi R, Ma TY. IL-1β and the intestinal epithelial tight junction barrier. Front. Immunol. 2021;12:767456. doi: 10.3389/fimmu.2021.767456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwarz BT, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nighot M, et al. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by Toll-like receptor 4/myeloid differentiation primary response 88 (Myd88) activation of myosin light chain kinase expression. Am. J. Pathol. 2017;187:2698–2710. doi: 10.1016/j.ajpath.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nighot M, et al. Lipopolysaccharide-induced increase in intestinal permeability is mediated by TAK-1 activation of IKK and MLCK/MYLK gene. Am. J. Pathol. 2019;189:797–812. doi: 10.1016/j.ajpath.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang F, et al. IFN-γ-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J, et al. The critical role of LIGHT in promoting intestinal inflammation and Crohn’s disease. J. Immunol. 2005;174:8173–8182. doi: 10.4049/jimmunol.174.12.8173. [DOI] [PubMed] [Google Scholar]
- 134.Su L, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Graham WV, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J. Biol. Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 136.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 137.Nalle SC, et al. Graft-versus-host disease propagation depends on increased intestinal epithelial tight junction permeability. J. Clin. Invest. 2019;129:902–914. doi: 10.1172/JCI98554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Al-Sadi R, Ye D, Said HM, Ma TY. IL-1β-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-κB pathway. Am. J. Pathol. 2010;177:2310–2322. doi: 10.2353/ajpath.2010.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Al-Sadi R, et al. Mechanism of IL-1β modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J. Immunol. 2013;190:6596–6606. doi: 10.4049/jimmunol.1201876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang F, et al. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005;166:409–419. doi: 10.1016/S0002-9440(10)62264-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Graham WV, et al. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat. Med. 2019;25:690–700. doi: 10.1038/s41591-019-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu AS, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 143.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J. Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nusrat A, et al. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J. Biol. Chem. 2000;275:29816–29822. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- 145.Kuo WT, et al. Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterology. 2019;157:1323–1337. doi: 10.1053/j.gastro.2019.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saito AC, et al. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell. 2021;32:722–738. doi: 10.1091/mbc.E20-07-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sugawara T, Furuse K, Otani T, Wakayama T, Furuse M. Angulin-1 seals tricellular contacts independently of tricellulin and claudins. J. Cell Biol. 2021;220:e202005062. doi: 10.1083/jcb.202005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shashikanth N, Rizzo HE, Pongkorpsakol P, Heneghan JF, Turner JR. Electrophysiologic analysis of tight junction size and charge selectivity. Curr. Protoc. 2021;1:e143. doi: 10.1002/cpz1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Raleigh DR, et al. Tight junction-associated MARVEL proteins marvelD3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell. 2010;21:1200–1213. doi: 10.1091/mbc.e09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S. Loss of occludin affects tricellular localization of tricellulin. Mol. Biol. Cell. 2008;19:4687–4693. doi: 10.1091/mbc.e08-05-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beeman N, Webb PG, Baumgartner HK. Occludin is required for apoptosis when claudin–claudin interactions are disrupted. Cell death Dis. 2012;3:e273. doi: 10.1038/cddis.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Z, et al. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2007;26:1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- 153.Wang Z, Mandell KJ, Parkos CA, Mrsny RJ, Nusrat A. The second loop of occludin is required for suppression of Raf1-induced tumor growth. Oncogene. 2005;24:4412–4420. doi: 10.1038/sj.onc.1208634. [DOI] [PubMed] [Google Scholar]
- 154.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J. Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Osanai M, et al. Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes. Cancer Res. 2006;66:9125–9133. doi: 10.1158/0008-5472.CAN-06-1864. [DOI] [PubMed] [Google Scholar]
- 156.Lazar V, Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK) Genomics. 1999;57:256–267. doi: 10.1006/geno.1999.5774. [DOI] [PubMed] [Google Scholar]
- 157.Clayburgh DR, et al. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J. Biol. Chem. 2004;279:55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-α modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 159.Chanez-Paredes, S. D. et al. Distinct steady-state properties and TNF responses of epithelial long myosin light chain kinase (MLCK) splice variants. Preprint at bioRxiv10.1101/2022.10.13.512159 (2023).
- 160.Zuo, L. et al. Tacrolimus-binding protein FKBP8 directs myosin light chain kinase-dependent barrier regulation and is a potential therapeutic target in Crohn’s disease. Gut10.1136/gutjnl-2021-326534 (2022). [DOI] [PMC free article] [PubMed]
- 161.Chanez-Paredes SD, Abtahi S, Kuo WT, Turner JR. Differentiating between tight junction-dependent and tight junction-independent intestinal barrier loss in vivo. Methods Mol. Biol. 2021;2367:249–271. doi: 10.1007/7651_2021_389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.He WQ, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Laukoetter MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vetrano S, et al. Unique role of junctional adhesion molecule-A in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–184. doi: 10.1053/j.gastro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 165.Ikenouchi J, et al. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Masuda S, et al. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J. Cell Sci. 2011;124:548–555. doi: 10.1242/jcs.072058. [DOI] [PubMed] [Google Scholar]
- 167.Higashi T, Katsuno T, Kitajiri S, Furuse M. Deficiency of angulin-2/ILDR1, a tricellular tight junction-associated membrane protein, causes deafness with cochlear hair cell degeneration in mice. PLoS ONE. 2015;10:e0120674. doi: 10.1371/journal.pone.0120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Srivastava AK, et al. Serine 408 phosphorylation is a molecular switch that regulates structure and function of the occludin α-helical bundle. Proc. Natl Acad. Sci. USA. 2022;119:e2204618119. doi: 10.1073/pnas.2204618119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Otani T, et al. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J. Cell Biol. 2019;218:3372–3396. doi: 10.1083/jcb.201812157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hempstock W, et al. Angulin-2/ILDR1, a tricellular tight junction protein, does not affect water transport in the mouse large intestine. Sci. Rep. 2020;10:10374. doi: 10.1038/s41598-020-67319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Otani T, Furuse M. Tight junction structure and function revisited. Trends Cell Biol. 2020;30:805–817. doi: 10.1016/j.tcb.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 172.Beutel O, Maraspini R, Pombo-Garcia K, Martin-Lemaitre C, Honigmann A. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell. 2019;179:923–936.e11. doi: 10.1016/j.cell.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 173.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hollander D, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 175.Kuo WT, et al. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology. 2021;161:1924–1939. doi: 10.1053/j.gastro.2021.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schumann M, et al. Defective tight junctions in refractory celiac disease. Ann. NY Acad. Sci. USA. 2012;1258:43–51. doi: 10.1111/j.1749-6632.2012.06565.x. [DOI] [PubMed] [Google Scholar]
- 177.Chang D, et al. Small-molecule modulators of INAVA cytosolic condensate and cell–cell junction assemblies. J. Cell Biol. 2021;220:e202007177. doi: 10.1083/jcb.202007177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Luong P, et al. INAVA-ARNO complexes bridge mucosal barrier function with inflammatory signaling. eLife. 2018;7:e38539. doi: 10.7554/eLife.38539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mohanan V, et al. C1orf106 is a colitis risk gene that regulates stability of epithelial adherens junctions. Science. 2018;359:1161–1166. doi: 10.1126/science.aan0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kline KT, et al. Neonatal injury increases gut permeability by epigenetically suppressing E-cadherin in adulthood. J. Immunol. 2020;204:980–989. doi: 10.4049/jimmunol.1900639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Muise AM, et al. Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn’s disease. Gut. 2009;58:1121–1127. doi: 10.1136/gut.2008.175117. [DOI] [PubMed] [Google Scholar]
- 182.Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand. J. Gastroenterol. 2000;35:1163–1169. doi: 10.1080/003655200750056637. [DOI] [PubMed] [Google Scholar]
- 183.Turpin W, et al. Analysis of genetic association of intestinal permeability in healthy first-degree relatives of patients with Crohn’s disease. Inflamm. Bowel Dis. 2019;25:1796–1804. doi: 10.1093/ibd/izz116. [DOI] [PubMed] [Google Scholar]
- 184.Madsen KL, et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 185.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lounder DT, et al. Lower levels of vitamin A are associated with increased gastrointestinal graft-versus-host disease in children. Blood. 2017;129:2801–2807. doi: 10.1182/blood-2017-02-765826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brown GR, Lee E, Thiele DL. TNF-TNFR2 interactions are critical for the development of intestinal graft-versus-host disease in MHC class II-disparate (C57BL/6J–C57BL/6J→bm12)F1 mice. J. Immunol. 2002;168:3065–3071. doi: 10.4049/jimmunol.168.6.3065. [DOI] [PubMed] [Google Scholar]
- 188.Noth R, et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol. 2011;11:109. doi: 10.1186/1471-230X-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nalle SC, et al. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Sci. Transl. Med. 2014;6:243ra287. doi: 10.1126/scitranslmed.3008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hanash AM, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takashima S, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J. Exp. Med. 2011;208:285–294. doi: 10.1084/jem.20101559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bosi E, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 193.Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51Cr)-EDTA in human type 2 diabetes. Diabet. Med. 2014;31:559–563. doi: 10.1111/dme.12360. [DOI] [PubMed] [Google Scholar]
- 194.Genser L, et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018;246:217–230. doi: 10.1002/path.5134. [DOI] [PubMed] [Google Scholar]
- 195.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am. J. Physiol. 1999;276:G951–G957. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- 196.Watts T, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc. Natl Acad. Sci. USA. 2005;102:2916–2921. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Damms-Machado A, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017;105:127–135. doi: 10.3945/ajcn.116.131110. [DOI] [PubMed] [Google Scholar]
- 198.Cox AJ, et al. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017;43:163–166. doi: 10.1016/j.diabet.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 199.Wilbrink J, et al. Intestinal barrier function in morbid obesity: results of a prospective study on the effect of sleeve gastrectomy. Int. J. Obes. 2020;44:368–376. doi: 10.1038/s41366-019-0492-z. [DOI] [PubMed] [Google Scholar]
- 200.Wigg AJ, et al. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor α in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kaushal K, et al. Demonstration of gut-barrier dysfunction in early stages of non-alcoholic fatty liver disease: a proof-of-concept study. J. Clin. Exp. Hepatol. 2022;12:1102–1113. doi: 10.1016/j.jceh.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thaiss CA, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 203.Nascimento JC, Matheus VA, Oliveira RB, Tada SFS, Collares-Buzato CB. High-fat diet induces disruption of the tight junction-mediated paracellular barrier in the proximal small intestine before the onset of type 2 diabetes and endotoxemia. Dig. Dis. Sci. 2021;66:3359–3374. doi: 10.1007/s10620-020-06664-x. [DOI] [PubMed] [Google Scholar]
- 204.Bashir M, et al. Enhanced gastrointestinal passive paracellular permeability contributes to the obesity-associated hyperoxaluria. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G1–G14. doi: 10.1152/ajpgi.00266.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee JC, et al. Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PLoS ONE. 2017;12:e0187515. doi: 10.1371/journal.pone.0187515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lam YY, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE. 2012;7:e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cheng C, Tan J, Qian W, Zhang L, Hou X. Gut inflammation exacerbates hepatic injury in the high-fat diet induced NAFLD mouse: attention to the gut-vascular barrier dysfunction. Life Sci. 2018;209:157–166. doi: 10.1016/j.lfs.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Z, Li M, Cui B, Chen X. Antibiotic disruption of the gut microbiota enhances the murine hepatic dysfunction associated with a high-salt diet. Front. Pharmacol. 2022;13:829686. doi: 10.3389/fphar.2022.829686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sharpstone D, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45:70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Keating J, et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995;37:623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 212.Maingat F, et al. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB J. 2011;25:2211–2220. doi: 10.1096/fj.10-175992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Doig CJ, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am. J. Respir. Crit. Care Med. 1998;158:444–451. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- 214.Fink MP, Antonsson JB, Wang HL, Rothschild HR. Increased intestinal permeability in endotoxic pigs. Mesenteric hypoperfusion as an etiologic factor. Arch. Surg. 1991;126:211–218. doi: 10.1001/archsurg.1991.01410260101014. [DOI] [PubMed] [Google Scholar]
- 215.Deitch EA, Morrison J, Berg R, Specian RD. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit. Care Med. 1990;18:529–536. doi: 10.1097/00003246-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 216.Novosad VL, Richards JL, Phillips NA, King MA, Clanton TL. Regional susceptibility to stress-induced intestinal injury in the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G418–G426. doi: 10.1152/ajpgi.00166.2013. [DOI] [PubMed] [Google Scholar]
- 217.Zahs A, et al. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G705–G712. doi: 10.1152/ajpgi.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dunlop SP, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 219.Marshall JK, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 220.Magnus Y, et al. Bile acid diarrhea is associated with increased intestinal permeability compared with irritable bowel syndrome-diarrhea. Gastroenterology. 2022;162:1343–1345.e1. doi: 10.1053/j.gastro.2021.12.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Saffouri GB, et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat. Commun. 2019;10:2012. doi: 10.1038/s41467-019-09964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rao AS, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G919–G928. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wu RL, et al. Gluten-induced symptoms in diarrhea-predominant irritable bowel syndrome are associated with increased myosin light chain kinase activity and claudin-15 expression. Lab. Invest. 2017;97:14–23. doi: 10.1038/labinvest.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Peters SA, et al. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am. J. Gastroenterol. 2017;112:913–923. doi: 10.1038/ajg.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.McOmber M, et al. Increased gut permeability in first-degree relatives of children with irritable bowel syndrome or functional abdominal pain. Clin. Gastroenterol. Hepatol. 2020;18:375–384.e1. doi: 10.1016/j.cgh.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Prospero L, et al. Psychological and gastrointestinal symptoms of patients with irritable bowel syndrome undergoing a low-FODMAP diet: the role of the intestinal barrier. Nutrients. 2021;13:2469. doi: 10.3390/nu13072469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Witt ST, et al. Interactions between gut permeability and brain structure and function in health and irritable bowel syndrome. Neuroimage Clin. 2019;21:101602. doi: 10.1016/j.nicl.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Awad K, et al. Impaired intestinal permeability of tricellular tight junctions in patients with irritable bowel syndrome with mixed bowel habits (IBS-M) Cells. 2023;12:236. doi: 10.3390/cells12020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Li L, et al. Increased small intestinal permeability and RNA expression profiles of mucosa from terminal ileum in patients with diarrhoea-predominant irritable bowel syndrome. Dig. Liver Dis. 2016;48:880–887. doi: 10.1016/j.dld.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 230.Long Y, et al. MLCK-mediated intestinal permeability promotes immune activation and visceral hypersensitivity in PI-IBS mice. Neurogastroenterol. Motil. 2018;30:e13348. doi: 10.1111/nmo.13348. [DOI] [PubMed] [Google Scholar]
- 231.Zhao L, et al. Changes in intestinal barrier protein expression and intestinal flora in a rat model of visceral hypersensitivity. Neurogastroenterol. Motil. 2022;34:e14299. doi: 10.1111/nmo.14299. [DOI] [PubMed] [Google Scholar]
- 232.van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut. 1993;34:354–357. doi: 10.1136/gut.34.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Duerksen DR, Wilhelm-Boyles C, Parry DM. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig. Dis. Sci. 2005;50:785–790. doi: 10.1007/s10620-005-2574-0. [DOI] [PubMed] [Google Scholar]
- 234.Smecuol E, et al. Gastrointestinal permeability in celiac disease. Gastroenterology. 1997;112:1129–1136. doi: 10.1016/S0016-5085(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 235.Leffler DA, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 2012;107:1554–1562. doi: 10.1038/ajg.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Natividad JM, et al. Host responses to intestinal microbial antigens in gluten-sensitive mice. PLoS ONE. 2009;4:e6472. doi: 10.1371/journal.pone.0006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Verdu EF, et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G217–G225. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]
- 238.Lamas B, et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med. 2020;12:eaba0624. doi: 10.1126/scitranslmed.aba0624. [DOI] [PubMed] [Google Scholar]
- 239.Abtahi S, et al. Intestinal epithelial digestive, transport, and barrier protein expression is increased in environmental enteric dysfunction. Lab. Invest. 2023;103:100036. doi: 10.1016/j.labinv.2022.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen RY, et al. Duodenal microbiota in stunted undernourished children with enteropathy. N. Engl. J. Med. 2020;383:321–333. doi: 10.1056/NEJMoa1916004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Semba RD, et al. Metabolic alterations in children with environmental enteric dysfunction. Sci. Rep. 2016;6:28009. doi: 10.1038/srep28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Singh A, et al. Biomarkers of environmental enteric dysfunction are differently associated with recovery and growth among children with moderate acute malnutrition in Sierra Leone. Am. J. Clin. Nutr. 2021;113:1556–1564. doi: 10.1093/ajcn/nqaa434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lauer JM, et al. Markers of environmental enteric dysfunction are associated with poor growth and iron status in rural Ugandan infants. J. Nutr. 2020;150:2175–2182. doi: 10.1093/jn/nxaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.McCormick BJJ, et al. Intestinal permeability and inflammation mediate the association between nutrient density of complementary foods and biochemical measures of micronutrient status in young children: results from the MAL-ED study. Am. J. Clin. Nutr. 2019;110:1015–1025. doi: 10.1093/ajcn/nqz151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yu J, et al. Environmental enteric dysfunction includes a broad spectrum of inflammatory responses and epithelial repair processes. Cell Mol. Gastroenterol. Hepatol. 2016;2:158–174.e1. doi: 10.1016/j.jcmgh.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Amadi B, et al. Impaired barrier function and autoantibody generation in malnutrition enteropathy in Zambia. EBioMedicine. 2017;22:191–199. doi: 10.1016/j.ebiom.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lima AA, et al. Mucosal injury and disruption of intestinal barrier function in HIV-infected individuals with and without diarrhea and cryptosporidiosis in northeast Brazil. Am. J. Gastroenterol. 1997;92:1861–1866. [PubMed] [Google Scholar]
- 248.Bhattacharjee A, et al. Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy. Immunity. 2021;54:1745–1757.e7. doi: 10.1016/j.immuni.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Salameh E, et al. Modeling undernutrition with enteropathy in mice. Sci. Rep. 2020;10:15581. doi: 10.1038/s41598-020-72705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ventura MT, et al. Intestinal permeability in patients with adverse reactions to food. Dig. Liver Dis. 2006;38:732–736. doi: 10.1016/j.dld.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 251.Jackson PG, Lessof MH, Baker RW, Ferrett J, MacDonald DM. Intestinal permeability in patients with eczema and food allergy. Lancet. 1981;1:1285–1286. doi: 10.1016/S0140-6736(81)92459-4. [DOI] [PubMed] [Google Scholar]
- 252.Rath T, Dieterich W, Kätscher-Murad C, Neurath MF, Zopf Y. Cross-sectional imaging of intestinal barrier dysfunction by confocal laser endomicroscopy can identify patients with food allergy in vivo with high sensitivity. Sci. Rep. 2021;11:12777. doi: 10.1038/s41598-021-92262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen T, et al. Food allergens affect the intestinal tight junction permeability in inducing intestinal food allergy in rats. Asian Pac. J. Allergy Immunol. 2014;32:345–353. doi: 10.12932/AP0443.32.4.2014. [DOI] [PubMed] [Google Scholar]
- 254.Forbes EE, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Leyva-Castillo JM, et al. Mechanical skin injury promotes food anaphylaxis by driving intestinal mast cell expansion. Immunity. 2019;50:1262–1275.e4. doi: 10.1016/j.immuni.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mathias CB, et al. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J. Allergy Clin. Immunol. 2011;127:795–805.e6. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Stefka AT, et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl Acad. Sci. USA. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Johnston JD, Harvey CJ, Menzies IS, Treacher DF. Gastrointestinal permeability and absorptive capacity in sepsis. Crit. Care Med. 1996;24:1144–1149. doi: 10.1097/00003246-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 259.Sipola S, et al. Colon epithelial injury in critically ill colectomized patients: aberration of tight junction proteins and Toll-like receptors. Minerva Anestesiol. 2017;83:1017–1025. doi: 10.23736/S0375-9393.17.11715-3. [DOI] [PubMed] [Google Scholar]
- 260.Klaus DA, et al. Increased plasma zonulin in patients with sepsis. Biochem. Med. 2013;23:107–111. doi: 10.11613/BM.2013.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tatucu-Babet OA, et al. Serum zonulin measured by enzyme-linked immunosorbent assay may not be a reliable marker of small intestinal permeability in healthy adults. Nutr. Res. 2020;78:82–92. doi: 10.1016/j.nutres.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 262.Yoseph BP, et al. Mechanisms of intestinal barrier dysfunction in sepsis. Shock. 2016;46:52–59. doi: 10.1097/SHK.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Banerjee S, et al. C/EBPδ protects from radiation-induced intestinal injury and sepsis by suppression of inflammatory and nitrosative stress. Sci. Rep. 2019;9:13953. doi: 10.1038/s41598-019-49437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fredenburgh LE, et al. Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J. Immunol. 2011;187:5255–5267. doi: 10.4049/jimmunol.1101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hu Q, et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine. 2019;41:497–508. doi: 10.1016/j.ebiom.2019.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Klingensmith NJ, et al. Junctional adhesion molecule-A deletion increases phagocytosis and improves survival in a murine model of sepsis. JCI Insight. 2022;7:e156255. doi: 10.1172/jci.insight.156255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jin X, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zang R, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020;5:eabc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ziegler CGK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e9. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Livanos AE, et al. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160:2435–2450.e4. doi: 10.1053/j.gastro.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Suarez-Farinas M, et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2-related disease. Gastroenterology. 2021;160:287–301.e20. doi: 10.1053/j.gastro.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ungaro RC, et al. Impact of medications on COVID-19 outcomes in inflammatory bowel disease: analysis of more than 6000 patients from an international registry. Gastroenterology. 2022;162:316–319.e5. doi: 10.1053/j.gastro.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yantiss RK, et al. Intestinal abnormalities in patients with SARS-CoV-2 infection: histopathologic changes reflect mechanisms of disease. Am. J. Surg. Pathol. 2022;46:89–96. doi: 10.1097/PAS.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 274.Yonker LM, et al. Zonulin antagonist, larazotide (AT1001), as an adjuvant treatment for multisystem inflammatory syndrome in children: a case series. Crit. Care Explor. 2022;10:e0641. doi: 10.1097/CCE.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yonker LM, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Invest. 2021;131:e149633. doi: 10.1172/JCI149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Giron LB, et al. Plasma markers of disrupted gut permeability in severe COVID-19 patients. Front. Immunol. 2021;12:686240. doi: 10.3389/fimmu.2021.686240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Davies KN, King D, Billington D, Barrett JA. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad. Med. J. 1996;72:164–167. doi: 10.1136/pgmj.72.845.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Perez-Pardo P, et al. Role of TLR4 in the gut–brain axis in Parkinson’s disease: a translational study from men to mice. Gut. 2019;68:829–843. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- 279.Hijazi Z, et al. Intestinal permeability is increased in bronchial asthma. Arch. Dis. Child. 2004;89:227–229. doi: 10.1136/adc.2003.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Barbiellini Amidei C, et al. Acid-suppressive medications in the first year of life and risk of childhood asthma: a population-based birth cohort study. Eur. Respir. J. 2020;56:2000197. doi: 10.1183/13993003.00197-2020. [DOI] [PubMed] [Google Scholar]
- 281.D’Costa S, et al. Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability. J. Allergy Clin. Immunol. 2019;143:1865–1877.e4. doi: 10.1016/j.jaci.2018.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hong Y, et al. IL-17A aggravates asthma-induced intestinal immune injury by promoting neutrophil trafficking. J. Leukoc. Biol. 2022;112:425–435. doi: 10.1002/JLB.3MA0622-426RR. [DOI] [PubMed] [Google Scholar]
- 283.Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig. Dis. Sci. 1996;41:2493–2498. doi: 10.1007/BF02100148. [DOI] [PubMed] [Google Scholar]
- 284.Sjöström B, et al. Increased intestinal permeability in primary Sjögren’s syndrome and multiple sclerosis. J. Transl. Autoimmun. 2021;4:100082. doi: 10.1016/j.jtauto.2021.100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Buscarinu MC, et al. Altered intestinal permeability in patients with relapsing–remitting multiple sclerosis: a pilot study. Mult. Scler. 2017;23:442–446. doi: 10.1177/1352458516652498. [DOI] [PubMed] [Google Scholar]
- 286.Wu S, Yi J, Zhang YG, Zhou J, Sun J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 2015;3:e12356. doi: 10.14814/phy2.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Matei DE, et al. Intestinal barrier dysfunction plays an integral role in arthritis pathology and can be targeted to ameliorate disease. Med. 2021;2:864–883.e9. doi: 10.1016/j.medj.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tajik N, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020;11:1995. doi: 10.1038/s41467-020-15831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Liu B, et al. Anti-TNF-α therapy alters the gut microbiota in proteoglycan-induced ankylosing spondylitis in mice. Microbiologyopen. 2019;8:e927. doi: 10.1002/mbo3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wen J, et al. Epithelial HIF2α expression induces intestinal barrier dysfunction and exacerbation of arthritis. Ann. Rheum. Dis. 2022;81:1119–1130. doi: 10.1136/annrheumdis-2021-222035. [DOI] [PubMed] [Google Scholar]
- 291.Gunzel D, Fromm M. Claudins and other tight junction proteins. Compr. Physiol. 2012;2:1819–1852. doi: 10.1002/cphy.c110045. [DOI] [PubMed] [Google Scholar]
- 292.Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tsukita S, Tanaka H, Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem. Sci. 2019;44:141–152. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 294.Gunzel D. Claudins: vital partners in transcellular and paracellular transport coupling. Pflugers Arch. 2017;469:35–44. doi: 10.1007/s00424-016-1909-3. [DOI] [PubMed] [Google Scholar]
- 295.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Krug SM, et al. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018;11:345–356. doi: 10.1038/mi.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sweat, Y. Y. et al. in Tight Junctions (ed. González-Mariscal, L.) 85–107 (Springer, 2022).