Abstract
Crocus sativus L. (saffron) is widely used as a traditional spice for flavoring, coloring and medicinal purposes. As a traditional Chinese herb, saffron promotes blood circulation, removes blood stasis, cools and detoxifies the blood, relieves depression and calms the mind. According to modern pharmacological studies, the active constituents of saffron, including crocetin, safranal and crocus aldehyde, exhibit antioxidant, anti-inflammatory, mitochondrial function-improving and antidepressant effects. Thus, saffron has the potential to treat neurodegenerative diseases (NDs) associated with oxidative stress, inflammation and impaired mitochondrial function, such as Alzheimer's disease, Parkinson's disease, multiple sclerosis and cerebral ischemia. The present article provides a review of the pharmacological effects of saffron and its constituents in terms of neuroprotective effects, including antioxidant and anti-inflammatory effects and the improvement of mitochondrial dysfunction, as well as their clinical application in treating NDs.
Keywords: neurodegenerative disease, Crocus sativus L, crocin, safranal, Alzheimer's disease, Parkinson's disease
1. Introduction
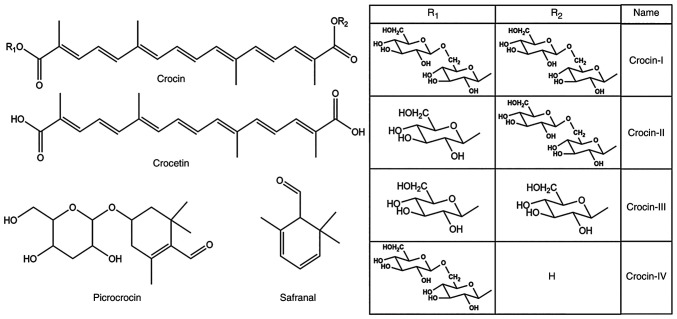
Saffron, the dried stigmas of the flowers of Crocus sativus L., is able to promote blood circulation and prevent blood stasis, cool and detoxify the blood, relieve depression and calm the mind (1). According to textual research, saffron was introduced to China during the Ming Dynasty (AC1368-1644) and had a long history of medicinal use (2). According to the Compendium of Materia Medica, saffron has been mainly used to treat palpitations, promote blood circulation and relieve depression or restlessness (3). In recent years, the constituents of saffron and pharmacological activities have been extensively studied in China and abroad. A total of >150 compounds have been identified in saffron (4), including terpenoids, anthraquinones, amino acids and alkaloids. Terpenoids, the most abundant compounds in saffron, include crocin (CR), safranal, picrocrocin and crocetin (structural formulas are provided in Fig. 1), of which the first three are responsible for the color, aroma and bitterness of saffron. Their contents are crucial indexes to measure the quality of saffron in various national quality standards, and the pharmacopoeias of numerous countries have clear regulations on the content of crocin and bitter picrocrocin (5). Saffron may be used to prevent and treat neurodegenerative diseases (NDs) due to its pharmacological effects, including the inhibition of atherosclerosis (6) and platelet aggregation (7), blood lipid reduction (8), antioxidant function (9), improvement of myocardial ischemia and hypoxia (10), anti-inflammatory (11,12), anticancer (13) and antidepressant functions (14). In addition to being used as a medicine, saffron is also sold as a food product. The application of saffron as a spice in the food industry is one of its numerous uses, as its unique fragrance stimulates the taste buds and increases appetite (15). Furthermore, numerous researchers have purified its active constituents and incorporated them into nutraceutical additives to prevent chronic diseases such as cancer and cardiovascular and neurodegenerative diseases (16,17). However, its high price has prevented its widespread use.
Figure 1.
Structural formula of main active components of saffron. Crocin-I: R1=R2=gentobiosyl-; crocin-II: R1=β-D-glucosyl-, R2=D-gentobiosyl-; crocin-III: R1=R2=β-D-glucosyl-; crocin-Ⅳ: R1=β-D-gentobiosyl-, R2=H.
ND is a type of disease in which cells and neurons of the brain and spinal cord are lost, which is caused by the loss of neurons or their myelin sheaths (18), eventually leading to dysfunction. NDs include chronic diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD) and multiple sclerosis (MS), as well as acute diseases, such as cerebral ischemia (CI) and brain injury (BI). NDs may be related to oxidative stress (OS), protein aggregate deposition, neuroinflammation, impaired mitochondrial function, apoptosis induction and autophagy changes (19,20). Extensive research on the mechanism and treatment of NDs has been conducted; however, the pathogenesis of these diseases remains to be fully elucidated. As there is currently no definite cure for NDs, most cases require conservative treatment, but conservative treatment with medications may be associated with gastrointestinal responses (such as nausea and vomiting), cardiovascular reactions (including tachycardia and arrhythmia) and mental disorders (such as anxiety and depression) (21,22).
Saffron and its active constituents (mainly CR, safranal and crocetin) have potent antioxidant and anti-inflammatory effects on brain cells, prevent amyloid β (Aβ) aggregation and regulate the steady-state concentration of metal ions in the brain (23,24). Thus, saffron has therapeutic potential for AD (25,26), PD (27) MS (28) and CI (29), and may reverse neurotoxicity caused by toxic substances, thereby protecting neurons (30,31). Furthermore, ND is usually accompanied by depressive symptoms. A previous study indicated that 36.23% of patients with PD suffer from depression, whereas 68.42% suffer from anxiety (32). Thus, due to its anti-depressant and anxiolytic properties, saffron may effectively treat depressive symptoms. Based on these pharmacological effects, saffron has a reasonable prospect as an auxiliary drug for ND treatment.
In the last decade, the pharmacological action of saffron for ND treatment has been extensively studied in animal models. However, the available reviews do not provide comprehensive summaries on the use and mechanism of action of saffron for the treatment of ND. The pathogenesis of ND is relatively complex (33,34). To achieve a better therapeutic effect, the pathway of saffron action must be further clarified. The present article reviewed the research progress on the use of saffron extract and its active constituents for ND treatment, particularly the pharmacological experiments performed in animal models of AD, PD, MS and CI, to comprehensively summarize the research results and administration methods of saffron in this field, focusing on its pharmacological effects during treatment. Up-to-date information on the potential mechanism by which saffron exerts neuroprotection through pharmacological activities and its therapeutic prospects in NDs is presented.
2. Methods
The literature was screened by WY and XQ. The Chinese National Knowledge Infrastructure, PubMed, ScienceDirect, ACS publication, Scopus and Medline databases, as well as Wiley Online Library, were searched for articles published in the Chinese and English languages, mainly referring to the literature from 1987 to 2022. The search terms mainly included crocin, safranal and other active constituents of saffron, as well as neurodegenerative disease,s such as AD and PD, which are terms that are commonly used in the pharmaceutical industry. The pharmacological research related to saffron was primarily distributed over the last decade. The final list of included studies was approved by MZ and JP.
3. Antioxidant effect of saffron and its active constituents
Aβ and Tau are associated with OS in AD
OS is caused by free radicals, atoms or groups with unpaired electrons, such as hydroxyl, superoxide and nitric monoxide (35). OS may cause damage to the cells and tissues of the body, such as the DNA, RNA, protein and lipid bilayer of nerve cells (36-38). Oxidative damage to nerve tissue has been found in NDs, such as AD, PD and amyotrophic lateral sclerosis. Studies have indicated a close association between Aβ, Tau protein and OS in neurons. For instance, Aβ aggregation may damage the mitochondria, leading to mitochondrial dysfunction and the release of numerous reactive oxygen species (ROS) and OS; the generation of ROS may also increase the production of Aβ (39,40). Free radicals, such as oxidized Fe3+, may promote the phosphorylation and aggregation of Tau (41). Furthermore, hyperphosphorylation and accumulation of Tau may also damage mitochondrial function, thereby generating a substantial amount of ROS. Chronic OS and the resultant peroxides, such as 4-hydroxynonenal, may also provoke Tau hyperphosphorylation, causing conformational changes and Tau aggregation (42). Aβ aggregation and Tau hyperphosphorylation have crucial roles in the pathogenesis of AD (43-46). In addition, OS is also associated with the free iron content of cells and elevated levels of iron ions may be found in the brains of patients with PD or AD (47). Therefore, it may be concluded that OS is intertwined with ND.
Inhibitory effects of saffron on Aβ aggregation and Tau abnormal phosphorylation
One of the main pathological features of AD is the formation of senile plaques by the accumulating Aβ outside the brain nerve cells. CR, the major active component of saffron, has been indicated to increase the tightness of a cell-based blood-brain barrier model, increase recombinant low-density lipoprotein receptor-related protein 1 and P-glycoprotein expression, improve Aβ clearance, reduce Aβ aggregation and inhibit the formation of senile plaques (48). Both CR and the extract of saffron (water/methanol, 50:50 v/v) was reported to inhibit the accumulation of Aβ in the human brain through antioxidant effects (49). Furthermore, saffron extract was able to antagonize aluminum oxide-induced neurotoxicity by elevating the activity of antioxidant enzymes, such as superoxide dismutase (SOD), catalase and glutathione peroxidase (GSH-Px) (50). Streptozotocin (STZ) may cause OS by increasing the production of oxygen-free radicals, thereby inducing cognitive impairment (51). In addition, CR may elevate GSH-Px activity and the total thiol content and reduce malondialdehyde (MDA) levels and OS damage, producing an antagonistic effect on STZ-induced cognitive impairment in rats (52-54). CR may also significantly decrease the Bax/Bcl-2 ratio and cleaved caspase-3 levels by reducing ROS production and inhibiting Aβ-induced apoptosis (55,56). Mitogen-activated protein kinases (MAPKs) are serine-threonine kinases that mediate intracellular signaling related to various cellular activities, including cell proliferation, differentiation and transformation (57). When OS is triggered, ROS activates downstream apoptosis pathways through the MAPK pathway, such as NF-κB and p53, triggering cell death (58-60). Safranal may reduce the ROS levels in cells, thereby reducing Aβ-induced apoptosis through the PI3K/AKT and MAPK/extracellular regulated protein kinase (ERK)1/2 pathways (61). Following treatment of AD rats with safranal, the hippocampal levels of MDA, ROS and protein carbonyl were observed to be reduced, while the activity of SOD and myeloperoxidase was increased in the hippocampal tissue (62).
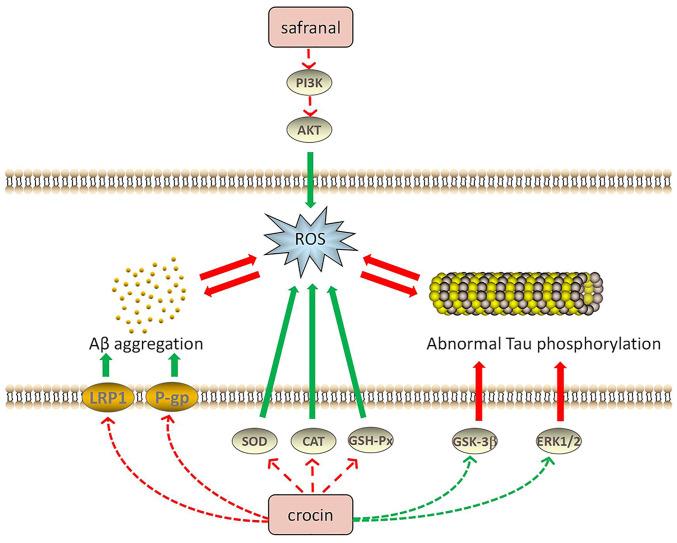
Abnormal Tau phosphorylation is also one of the leading causes of AD. In the brain of patients with AD, abnormally phosphorylated tau protein has been observed, which, unlike normal tau protein, does not bind to microtubule proteins; instead, its presence also prevents the latter from promoting the assembly of tubulin into microtubules, leading to neurofibrillary tangles (63). Therefore, decreasing Tau hyperphosphorylation is an effective way to treat AD. Trans-CR 4 and trans-crocetin were selected to treat two AD neuronal cell culture models and the results demonstrated that these two compounds did not affect the viability of neuron-like cells. Both trans-CR 4 and trans-crocetin exerted a crucial effect to inhibit amyloidogenic pathways and were influential in suppressing the active forms of ERK1/2 kinases and glycogen synthase kinase-3β, as well as markedly reducing Tau phosphorylation (64). Furthermore, CR was able to significantly decrease MDA, Aβ and phosphorylated Tau levels by modulating the MAPK signaling pathway (65). AD is also characterized by abnormal Tau aggregation, whereas CR is able to inhibit Tau aggregation and suppress the formation of Tau protein filaments (66). Fig. 2 illustrates the relationship between saffron components, Aβ aggregation and Tau abnormal phosphorylation in AD.
Figure 2.
Potential mechanisms of the antioxidant effects of saffron compounds in treating AD. Aβ and Tau proteins are closely associated with oxidative stress. Saffron compounds may reduce oxidative stress by regulating the activity of related proteins to inhibit Aβ aggregation, Tau phosphorylation or ROS production, thereby exerting therapeutic effects on neurodegenerative diseases. Red arrows indicate upregulation and green arrows indicate downregulation of the indicated pathway or factor. PI3K, phosphatidylinositol 3-kinase; AKT, protein kinases B; ROS, reactive oxygen species; AD, Alzheimer's disease; LRP1, recombinant low density lipoprotein receptor-related protein 1; P-gp, P-glycoprotein; SOD, superoxide dismutase; Aβ, amyloid β; CAT, catalase; GSH-Px, glutathione peroxidase; GSK3β, glycogen synthase kinase-3β; ERK, extracellular regulated protein kinase.
Antioxidant effects on PD
In addition to AD, saffron also improves PD symptoms through its antioxidant effects. CR has a protective effect in terms of reducing mitochondrial permeability transition pore-induced dopaminergic neuron damage and PD complications, in addition to ameliorating 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD complications and reducing substantia nigra cell death (67). It was reported that the antioxidant capacity of saffron contributes to PD treatment. After validating the neuroprotective efficacy of methanolic saffron extract and its active constituent, CR, in a drosophila model of Parkinson's disease, studies have proposed that saffron may be used as a complementary therapeutic agent for PD-mediated NDs (68,69). While common NDs, such as AD and PD, have been found to cause oxidative damage in neuronal tissues, saffron and its active compounds may reduce oxidative stress by inhibiting Aβ aggregation, tau protein phosphorylation or ROS production, proving that saffron has considerable therapeutic potential for NDs. Table I presents the relevant research progress regarding the use of saffron to treat NDs through its antioxidant effects.
Table I.
Possible mechanisms of action of saffron in the treatment of neurodegenerative diseases through antioxidant effects.
| Experimental animal | Model | Administration | Dose | Mechanism of action and target | (Refs.) |
|---|---|---|---|---|---|
| Mouse | Transgenic mice transfected with five FAD mutations | Feed with crocin and Crocus sativus extract-enriched diet | 10 mg/kg crocin; 50 mg/kg crocus sativus extract | Synaptic protein expression ↑, Aβ load ↓, P-gp, LRP1 ↑ | (48) |
| Rat | STZ-induced diabetes | Intraperitoneal injection of saffron extract | 200 mg/kg | SOD, CAT, GSH-Px activity↑ | (53) |
| Rat | STZ-induced cognitive impairment | Intracerebroven-tricular injection of crocin | 100 mg/kg | MDA level ↓, GSH-Px activity ↑, TSH content ↑ | (54) |
| Rat | Memory deficit | Peptide injection of crocin | 150, 300, 600 nmol/side via IH | Bax/Bcl-2 ratio ↓, cleave Caspase-3 level ↓ | (55,56) |
| Mouse | D-galactose and AlCl3-induced AD | Intraperitoneal injection of crocin | 5, 20 mg/kg | Rate of apoptosis ↓, mitochondrial dysfunction ↓, ROS ↓, Ca2+ overload ↓, Bax/Bcl-2 ratio, cleaved caspase-3 levels ↓, Aβ 1-42 deposition in the hippocampus ↓, GSH-Px, SOD, ChAT activity ↑ | (55,56) |
| PC12 cell | Aβ and H2O2 induced toxicity and oxidative damage | Culture in medium containing saffron | 2.5 µM | ROS ↓, apoptosis induced by the PI3K/AKT and MAPK/ERK1/2 pathways ↓ | (61) |
| Rat | Aβ1-40-induced AD | Microinjection of safranal | 0.2 ml/kg | MDA, protein base, ROS ↓, SOD, MPO ↑ | (62) |
| SH-SY5Y and PC12 neuronal cells | Overexpressing hyperphosphory- lated tau | Exposure to trans- crocin-4 or trans- crocetin concentrations | 0.1 µM-1 mM | β secretase (BACE1) ↓, γ secretase (PSEN1/PSEN2 complex) ↓, GSK3 and ERK1/2 kinase activity ↓, sAPPα, sAPPβ ↓ | (64) |
| Rat | Acrolein-induced neurotoxicity | Intraperitoneal injection of crocin | 12.5 mg/kg, 25 mg/kg, 50 mg/kg | MAPK signaling pathway, MDA, Aβ, phosphorylated Tau levels ↓ | (65) |
| Drosophila | ROT-induced PD | Maintained on SME or CR- enriched medium | SME: 0.05%, 0.1%; CR: 0, 25 µM | ROS, HP, NO, protein carbonyl ↓, SOD, GST, GSH, TSH ↑, TR, AchE ↓ | (68) |
↑ indicates elevation or promotion, while ↓ means downregulation or inhibition. AD, Alzheimer's disease; STZ, streptozotocin; Aβ, amyloid β; PD, Parkinson's disease; ROT, rotenone; CR, crocin; P-gp, P-glycoprotein; LRP1, recombinant low density lipoprotein receptor related protein 1; SOD, superoxide dismutase; CAT, catalase; LRP1, recombinant low density lipoprotein receptor related protein 1; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; TSH, thyroid stimulating hormone; Bax/Bcl-2, B-cell lymphoma 2; ROS, reactive oxygen species; ChAT, choline acetyltransferase; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinases B; ERK, extracellular regulated protein kinases; MPO, myeloperoxidase; GST, glutathione S-transferase; GSH, glutathione; sAPP, soluble amyloid precursor protein.
4. Anti-inflammatory capacity of saffron and its active constituents
Pathological process of neuroinflammation
OS has an essential role in the pathological changes of NDs, whereas neuroinflammation is also crucial for ND pathogenesis (70). Neuroinflammation is a defense mechanism that protects the central nervous system (CNS) from tissue damage or viral attack (71). However, a continuous inflammatory process in the CNS may inflict severe damage to the nervous system and eventually lead to CNS damage (72). Inflammation-derived ND is a specific CNS damage disease. The primary hallmark of inflammation in the brain is the activation of glial cells, particularly microglia, and ROS is involved in microglial activation (73). Microglia cells influence the NF-κB signaling pathway. Tumor necrosis factor-α (TNFα) and interleukin (IL)-1β are common cytokines secreted by activated glial cells (74).
Anti-inflammatory effects of saffron in treating AD and CI
Studies have demonstrated the therapeutic potential of saffron and its active constituents for neuroinflammation-mediated NDs. Saffron extracts were able to upregulate the synaptic proteins in the brains of 5XFAD mice, transgenic mice transfected with five Familial Alzheimer's disease mutations, and reduce Aβ pathology-related neuroinflammation (48). In AD mice, safranal not only reduced the expression of NF-κB and its downstream TNFα, IL-6, apoptosis markers and glial fibrillary acidic protein, but also elevated the mitochondrial membrane potential (∆ψm) (62). Safranal exerts anti-inflammatory effects by inhibiting the classic NF-κB inflammatory pathway, thereby improving AD (75). Safranal also reduces the hyperactivity of acetylcholinesterase (AChE) and inhibits cholinesterase overexpression. Furthermore, crocetin may suppress NF-κB activation and P53 expression in the hippocampus, significantly decreasing the pro-inflammatory cytokine secretion and increasing anti-inflammatory cytokine levels in plasma, while inhibiting apoptosis and decreasing Aβ in various brain areas (76).
Acute NDs, such as CI, are also affected by neuroinflammation, a crucial pathological process in the later stage of CI (77). It has been indicated that inflammation mediates CI-reperfusion injury. Ischemic stroke may cause depression, which is a severe disease inflicted by CI, and post-stroke depression (PSD) is a severe complication of stroke (78). It has been demonstrated that persistent CI leads to PSD (79). Thus, PSD is closely associated with inflammation. CR was able to inhibit the inflammatory response by inhibiting the activation of the Toll-like receptor 4/myeloid differentiation factor 88/NF-κB signaling pathway in the hippocampal tissue, thereby preventing the occurrence of PSD (80). Table II displays the relevant research progress regarding the use of saffron for treating NDs through its anti-inflammatory effects. Fig. 3 presents the anti-inflammatory mechanisms of saffron components in ND treatment.
Table II.
Possible mechanisms of action of saffron in the treatment of neurodegenerative diseases through anti-inflammatory effects.
| Experimental animal | Model | Administration | Dose | Mechanism of action and target | (Refs.) |
|---|---|---|---|---|---|
| Mouse | Transgenic mice transfected with five FAD mutations | Feed with Crocus sativus extract-enriched diet | 50 mg/kg | Synaptic protein expression ↑, Aβ load ↓, P-gp, LRP1 ↑ | (48) |
| Rat | Aβ1-40-induced AD | Microinjection of safranal | 0.2 ml/kg | IL-1β, 1L-6, TNFα, GFAP, MPO ↓, NF-κB ↓ | (62) |
| Mouse | APPsw transgenesis induced AD | Oral crocetin | 0, 10 and 30 mg/kg | Insoluble Aβs secretion ↓, TNFα, ΙL-1β, ΙL-8 and ΙL-6 ↓, IL-10 ↑, NF-κB activation and p53 expression ↓ | (76) |
| Rat | Cerebral stroke | Intraperitoneal injection of crocin | 50 mg/kg | Inhibition of the TLR4/MyD88/NF-κB signaling pathway in the brain tissue | (80) |
↑ indicates elevation or promotion, while ↓ means downregulation or inhibition. AD, Alzheimer's disease; Aβ, amyloid β; LRP1, recombinant low density lipoprotein receptor related protein 1; P-gp, P-glycoprotein; GFAP, glial fibrillary acidic protein; TNFα, tumor necrosis factor α; IL, interleukin; TLR4, toll-like receptor 4; IL, interleukin; MyD88, myeloid differentiation factor 88.
Figure 3.
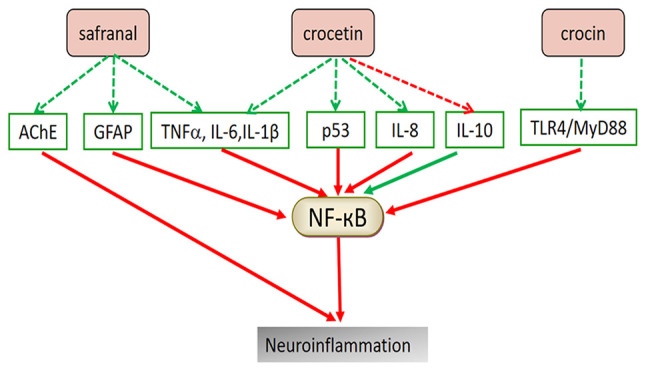
Potential mechanism of saffron compounds in treating neuroinflammation. The active components of saffron may inhibit the secretion of pro-inflammatory factors and increase the level of anti-inflammatory factors in serum, mainly through the NF-κB pathway. Red arrows indicate upregulation and green arrows indicate downregulation of the indicated pathway or factor. AChE, acetylcholinesterase; GFAP, glial fibrillary acidic protein; TNFα, tumor necrosis factor α; TLR4, toll-like receptor 4; IL, interleukin; MyD88, myeloid differentiation factor 88.
5. Improvement of mitochondrial dysfunction
Pathological process of mitochondrial dysfunction
Mitochondria are organelles with a double membrane structure found in the cytoplasm of eukaryotes containing extranuclear genetic material. Their internal membranes are the aggregation sites of respiratory chain complexes. Mitochondria, the main site of the body's energy metabolism, regulated the oxidative phosphorylation process and synthesize ATP, which may also be produced via glycolysis in microglial cells and astrocytes (81). Mitochondria generate energy and control the storage and release of Ca2+ to maintain the dynamic balance of the intracellular Ca2+ concentration. Furthermore, Ca2+ may participate in multiple cell activities, such as cell-matrix metabolism, cell apoptosis and initiation of signal transduction pathways (82). Mitochondrial dysfunction may prevent the aforementioned functions and is a major risk factor for neurodegeneration (83).
Treatment of PD with saffron
PD was the first ND associated with mitochondrial dysfunction. In the compact part of the substantia nigra, the nigrostriatal dopaminergic system and platelets of patients with PD, a 30% reduction in the activity of the mitochondrial respiratory chain complex I and a decrease in rate-limiting enzymes of the tricarboxylic acid cycle-ketoglutarate dehydrogenase complex were observed (84). In the rotenone-induced PD model in Drosophila, the level of mitochondrial enzyme activity in the Drosophila head was significantly reduced. Following treatment with saffron extract and CR, mitochondrial enzymes, succinate dehydrogenase and cytochrome c reductase returned to normal levels, indicating that saffron and its active constituents may improve mitochondrial dysfunction (68). Experiments using specific targeted small interfering RNA to knock down the expression of the C/EBP homologous protein (CHOP) revealed that CR-induced protection and inhibition of ER stress is mediated by inverting the 1-methyl-4-phenylpyridinium (ion)-induced decrease of Wnt protein through the CHOP pathway, thereby reducing cell damage and apoptosis, inhibiting mitochondrial dysfunction and maintaining ATP synthesis and ∆ψm (85).
Treatment of CI with saffron
Transient CI is responsible for sudden, temporary and reversible neurological dysfunction. It has been demonstrated that mitochondrial dysfunction may occur after CI reperfusion (86). After pre-treatment of BI rats with CR, it was observed that CR increased the mitochondrial membrane fluidity, membrane phospholipid content, ∆ψm, mitochondrial respiratory function, respiratory enzyme activity and ATP content. CR also reduced MPTP opening and the free Ca2+ concentration and protected the hippocampal mitochondrial structure and function in rats with ischemic BI by significantly ameliorating the hippocampal mitochondrial pathology (87). In addition, CR can improve the energy metabolism of cells after oxygen-glucose deprivation, restore ∆ψm, reduce the intracellular Ca2+ concentration, upregulate optic atrophy 1 (Opa1) expression, downregulate dynamin-related protein 1 (Drp1) expression and restore the normal mitochondrial fusion and fission (88). Given the effects of the active constituents of saffron on improving mitochondrial dysfunction in cells, saffron extract was used to treat rats with focal brain ischemia/reperfusion injury. The results suggested that saffron extract significantly inhibited rat neuronal necrosis and astrocyte proliferation, upregulated the expression of Opa1, downregulated the expression of Drp1 and restored normal mitochondrial fusion and fission (89). Table III presents the relevant research progress of studies using saffron in treating NDs by improving mitochondrial dysfunction. Fig. 4 illustrates the mechanisms of saffron components in improving mitochondrial dysfunction for ND treatment.
Table III.
Possible mechanisms of action of saffron in the treatment of neurodegenerative diseases by improving mitochondrial dysfunction.
| Experimental animal | Model | Administration | Dose | Mechanism of action and target | (Refs.) |
|---|---|---|---|---|---|
| Drosophila | ROT-induced PD | Maintained on SME or CR-enriched medium | SME: 0.05%, 0.1%; CR: 0, 25 µM | Mitochondrial enzyme activity, succinate dehydrogenase and NADH-cytochrome c reductase activity returned to normal | (68) |
| PC12 cell | MPP+-induced injury | Culture in medium containing crocin | 0.1, 1, 10, 100 µM | CHOP expression ↓, Wnt ↑, caspase-3 activity ↓, LDH ↓, ATP synthesis ↑, cell cleavage ↓, cellular proteolysis ↓ | (87) |
| SH-SY5Y cell | Oxygen-glucose deprivation | Culture in medium containing crocin | 25, 50 mg/ml | Δψm, Opa1 expression ↑, Ca2+ concentration, Drp1 expression ↓ | (88) |
| Rat | Middle cerebral artery occlusion induced CI | Duodenal injection | 3 mg/kg | Opa1 expression ↑, Drp1 expression ↓, inhibition of astrocyte proliferation | (89) |
↑ indicates elevation or promotion, while ↓ means downregulation or inhibition. PD, Parkinson's disease; ROT, rotenone; MPP+, 1-methyl-4-phenylpyridinium; CI, cerebral ischemia; SME, saffron methanolic extract; CHOP, C/EBP homologous protein; LDH, lactate dehydrogenase; Opa1, optic atrophy 1; Drp1, dynamin-related protein 1.
Figure 4.
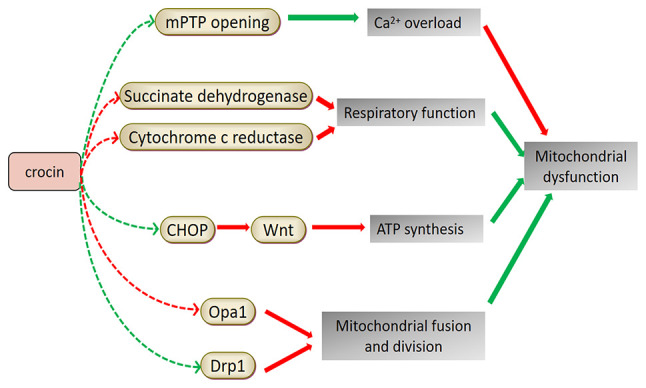
Potential mechanism of saffron compounds in improving mitochondrial dysfunction. Cell apoptosis and calcium overload may lead to mitochondrial dysfunction. Crocin is able to control calcium storage and release, prevent cell apoptosis, restore respiratory function and induce the synthesis of ATP, thereby protecting the mitochondria. It may also restore normal mitochondrial fusion and fission by regulating related proteins. Red arrows indicate upregulation and green arrows indicate downregulation of the indicated pathway or factor. mPTP, mitochondrial permeability transition pore; CHOP, C/EBP homologous protein; Opa1, optic atrophy 1; Drp1, dynamin-related protein 1.
6. Improvement of cognition and learning ability
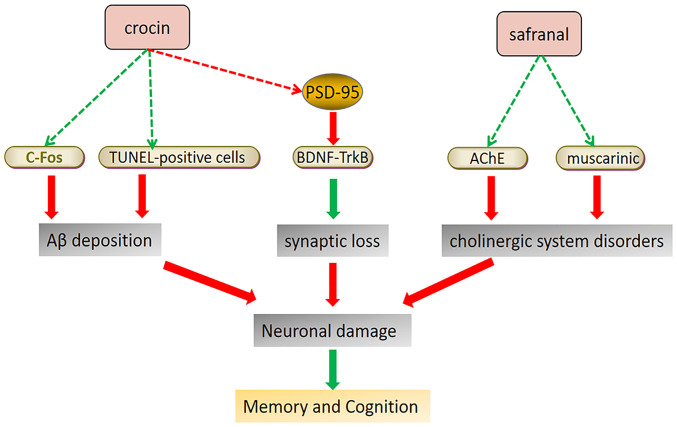
Although the pathophysiological mechanisms remain to be fully elucidated, patients with AD frequently exhibit symptoms of reduced cognitive and memory functions, indicating that AD is closely related to memory impairment. Aβ deposition, synaptic loss, Tau phosphorylation and cholinergic system disorders are all possible factors responsible for neuronal damage (3,90). It has been reported that CR may increase the expression of brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB) in the prefrontal cortex, thereby activating the BDNF-TrkB signaling pathway and increasing the expression of the memory-related protein postsynaptic density-95, which improves the learning and memory ability in AD rats (91). A bilateral frontal-cortex Aβ injection trial in rats demonstrated that CR significantly reduced the number of TUNEL-positive cells in the cortical area 1 and decreased c-Fos expression in the hippocampus, thereby alleviating memory impairment due to Aβ deposition (92). Furthermore, electric shock experiments conducted in mice demonstrated that saffron extract prevented and improved the memory impairment of morphine-treated mice (93). Similarly, pentylenetetrazol-induced learning and memory deficits in rats were significantly alleviated by CR (94), although the mechanism of action requires further investigation.
Important neurotransmitters in the CNS, including dopamine, norepinephrine, acetylcholine and serotonin, act on the corresponding neurons and participate in short- and long-term memory (95). Acetylcholine-decomposing inhibitors of AChE are considered the primary treatment for AD because of their ability to improve cognitive impairment and the learning disabilities of AD. Studies have reported that the loss of cholinergic neurons in PD is higher than that in AD (96,97). Certain cholinergic fibers come from basal forebrain cholinergic neurons. Patients with non-dementia PD lose 30% of those neurons, whereas patients with PD dementia lose 54-70% (98). Saffron was found to be a source of novel AChE inhibitors for treating AD using in vitro enzymology and molecular docking methods (99). Furthermore, saffron extract and CR may act on muscarinic choline receptors to improve learning and memory ability (100,101). The accumulation and aggregation of lead (Pb) in the food chain may poison the nervous system. Experiments have demonstrated that Pb exposure may cause PD, resulting in memory and cognitive impairment, symptoms similar to dementia in AD (102,103). Saffron extract was able to improve Pb-induced dopamine and noradrenergic injuries by restoring tyrosine hydroxylase levels within the substantia nigra compacta, ventral tegmental area, locus coeruleus, dorsal striatum and medial forebrain bundle (104,105). Patients with MS usually suffer from cognitive impairment (106), with memory impairment and spatial perception disorders being the most common cognitive deficits (107,108). Saffron extract has a positive effect in improving learning and memory impairment and alleviate impaired hippocampal stress parameters in rats with ethidium bromide-induced MS (109). Table IV presents the relevant research progress on the use of saffron for treating NDs by improving cognition and learning ability. Fig. 5 illustrates the mechanisms of saffron components to improve cognition and learning ability for ND treatment.
Table IV.
Possible mechanism of saffron in the treatment of neurodegenerative diseases by improving cognitive and learning ability.
| Experimental animal | Model | Administration | Dose | Mechanism of action and target | (Refs.) |
|---|---|---|---|---|---|
| Rat | Aβ1-40-induced AD | Microinjection of safranal | 0.2 ml/kg | AChE ↓ | (62) |
| Rat | Aβ25-35-induced AD | Intracerebroventricular injection of crocin | 40 mg/kg | Activation of the BDNF/TrkB signaling pathway, PSD-95, BDNF, TrkB expression ↑ | (91) |
| Rat | Aβ1-42-induced AD | Intraperitoneal injection of crocin | 30 mg/kg | c-Fos expression ↓, neuronal apoptosis ↓ | (92) |
| Rat | Ethidium bromide -induced MS | Microinjection of saffron extract | 5, 10 µg | Amelioration of disturbances in oxidative stress parameters in the hippocampus | (109) |
↑ indicates elevation or promotion, while ↓ means downregulation or inhibition. AD, Alzheimer's disease; AChE, acetylcholinesterase; MS, multiple sclerosis; AChE, acetylcholinesterase; BDNF, brain-derived neurotrophic factor; PSD-95, postsynaptic density-95.
Figure 5.
Potential mechanism of saffron compounds in protecting brain nerves and improving learning and cognitive ability. Neuronal damage to hippocampal tissue can affect cognition and learning ability. Saffron has a mitigating effect on the triggers of neuronal damage, such as Aβ deposition, synaptic loss and disorders of the cholinergic system. Red arrows indicate upregulation and green arrows indicate downregulation of the indicated pathway or factor. Aβ, amyloid β; BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor kinase B; AChE, acetylcholinesterase; PSD-95, postsynaptic density-95.
7. Other effects of saffron and its active constituents
Relief of depression
Depression is one of the psychological symptoms of NDs. Studies have demonstrated that patients with NDs, including AD and PD, exhibit depressive symptoms. Furthermore, elevated levels of pro-inflammatory factors, such as IL-1β, IL-6 and TNFα, are frequently found in the cerebrospinal fluid of patients with depression, indicating the relationship between depression and the occurrence of neuroinflammation (110-115). The antidepressant mechanism of saffron has only been studied in the last decades. CR had a significant antidepressant effect in a chronic corticosterone-induced depression model in rats, as evidenced by a substantial reduction in IL-1β and SOD levels in the hippocampus, suggesting that the inhibition of inflammation and OS is associated with the antidepressant effect (116). Patients with depression usually have higher plasma corticosterone than normal individuals, it has been demonstrated that saffron water extract and CR were able to reduce the plasma levels of corticosterone in a rat model of depression (117,118). Increasing the transcriptional level of BDNF in the hippocampus may also have an antidepressant effect (119,120), demonstrating that saffron may alleviate the depressive symptoms of NDs. Therefore, saffron may not only relieve the main symptoms of NDs, such as nervous disorders and limb and cognitive dysfunction, but also have an antidepressant effect treating its potential complications, which is an advantage that other drugs do not possess.
Anti-epileptic effects
Epilepsy is a chronic disease characterized by sudden abnormal discharges of nerve cells in the brain, leading to temporary brain dysfunction. NDs are characterized by cell death and destruction of brain structures, which may increase the risk of epileptic seizures (121). The appearance and increase in the levels of inflammatory cytokines, such as TNF, IL-1 and IL-6, is closely linked to the onset of epilepsy (122). CR and crocetin, the active constituents of saffron, inhibit the increase in the inflammatory cytokines to varying degrees (75). This finding suggests that the anti-epileptic action of saffron may be mediated by its anti-inflammatory properties.
Regulation of Ca2+ homeostasis
As the second messenger in neurons (123), Ca2+ regulates nerve synapses and has a vital role in cell growth, apoptosis, neuronal development and the regulation of various metabolic channels (124). An abnormal Ca2+ concentration may lead to cell damage and even apoptosis. The entry of Ca2+ from the external cell environment, a central characteristic associated with cell death, leads to Ca2+ imbalance and has an essential role in Ca2+ overload in NDs, such as PD and AD (125). For instance, the significant reduction of Ca2+ in the platelets of patients with early AD (detected as Aβ oligomer and associated with hypomnesia) increases the secretion of parathyroid hormone. This essential hormone maintains Ca2+ homeostasis and promotes calcium influx, leading to increased Ca2+ in brain cells, which causes dementia by damaging mitochondrial function and reducing cellular energy metabolism (126). Studies have reported that saffron and its active constituents may maintain Ca2+ homeostasis in neurons, but the specific mechanism of action remains to be elucidated (10,127). Therefore, the mechanism by which saffron regulates Ca2+ homeostasis requires further investigation.
8. Conclusions and prospects
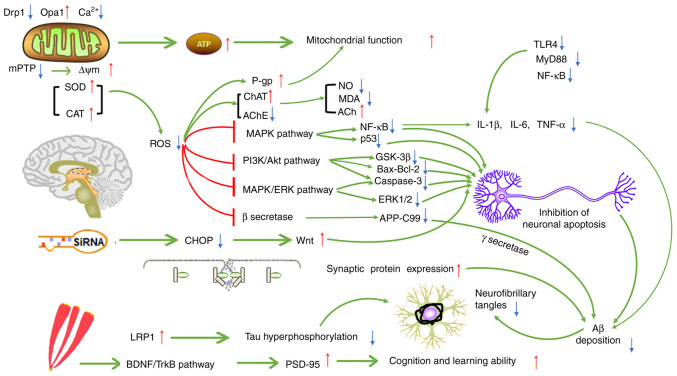
Saffron has always been widely used for food coloring and flavoring. There has been a growing interest in using special diets with saffron, and scientists have been paying increasing attention to its safety while maintaining its taste (15-17). Furthermore, researchers have widely explored its nutritional quality and medicinal effect. Numerous phytochemicals in Crocus sativus have been proven to be the bioactive, therapeutic constituents of saffron. Saffron has been used in the clinical setting to treat cardiovascular and cerebrovascular diseases, mental disorders and abnormal blood lipid and glucose levels (128,129). Researchers have recently paid considerable attention to treating NDs, focusing mainly on diseases such as AD, PD, MS and CI (130). CR and crocetin, the active constituents of saffron with antioxidant effects, inhibit free radical formation and excitotoxic damage, thereby protecting neurons. These constituents may also reduce Aβ deposition, inhibit the abnormal aggregation of Tau protein, reduce the secretion of inflammatory factors, improve cognition and memory, improve mitochondrial dysfunction and regulate the homeostasis of metal ions in ND models. Furthermore, the pathways of the anti-inflammatory and antioxidant effects of saffron and its capacity to improve mitochondrial function and cognitive impairment are not independent; the interaction with one another may affect ND pathogenesis, thereby protecting nerve cells and preventing the further development of NDs. Fig. 6 illustrates the possible mechanisms of saffron for ND treatment.
Figure 6.
Possible mechanism of saffron in the treatment of ND. Through their antioxidant effects, the active constituents of saffron may reduce Aβ aggregation and the abnormal phosphorylation of Tau, thus alleviating the neurotoxicity caused by ROS. ROS may also lead to inflammation and damage to mitochondrial function. The active constituents of saffron may also act on different pathways to inhibit neuroinflammation and improve mitochondrial function. Different pathways may be connected and interact with each other, and may also represent the potential mechanisms of action of saffron in treating ND. T-type arrows indicate inhibition, pointed arrows indicate promotion, ↑ indicates upregulation and ↓ indicates downregulation. ND, neurodegenerative diseases; ROS, reactive oxygen species; siRNA, small inhibitory RNA; Aβ, amyloid β; BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor kinase B; AChE, acetylcholinesterase; LRP1, recombinant low density lipoprotein receptor-related protein 1; mPTP, mitochondrial permeability transition pore; SOD, superoxide dismutase; CHOP, C/EBP homologous protein; CI, cerebral ischemia; TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88; MDA, malondialdehyde; NO, nitric oxide; CAT, catalase; ChAT, choline acetyltransferase; PSD-95, postsynaptic density-95; P-gp, P-glycoprotein; GSK3β, glycogen synthase kinase-3β; Opa1, optic atrophy 1; Drp1, dynamin-related protein 1; ERK, extracellular regulated protein kinase; TNFα, tumor necrosis factor α; IL, interleukin; MAPK, mitogen-activated protein kinase; ∆ψm, mitochondrial membrane potential; APP-C99, amyloid precursor protein C-terminal fragment 99.
The therapeutic potential of saffron has been proved in ND models. Its action on classic signaling pathways, such as NF-κB and MAPK, and its antioxidant and anti-inflammatory mechanisms, are also well known (65,76). Several beneficial pharmacological effects of saffron or its constituents have been confirmed in animal studies, but these effects have rarely been demonstrated in clinical trials. The results of a clinical trial suggested that saffron had similar effects to improve cognitive function of patients with AD as donepezil, while AChE levels were similar to those in the donepezil-treated group, indicating that saffron may inhibit the accumulation of senile plaques through its antioxidant effects, which is consistent with the results of a preclinical pharmacological study (131). Furthermore, the therapeutic effect of saffron on cerebral ischemia is similar to that of statins. Another clinical trial involving patients with AD demonstrated that oral saffron extract increased serum BDNF levels, inhibited inflammation and prevented neuronal apoptosis (132). Saffron has been clinically available to treat moderate AD, and antioxidant, anti-inflammatory and other mechanisms of its active compounds have been observed (133,134); it is thought that saffron has excellent potential for future application in treating NDs.
Furthermore, saffron has only been used as a traditional Chinese medicine to treat ND with limited efficacy. Its use in treating NDs is still experimental and further clinical studies are still required. The specific safety and effective dose for the human body are unknown and numerous factors are involved in determining its clinical efficacy. Accordingly, the following may be suggested: i) The use of saffron in the treatment of AD and CI may be investigated in subsequent clinical trials or drug developmental stages, whereas the pharmacological effect for treating other NDs requires further assessment in clinical trials; ii) saffron may be used to prevent the side effects (including anxiety, among others) of commonly used ND drugs, such as levodopa and memantine; and iii) clinical trials and safety evaluation should be conducted to assess the clinical value and effective dose of saffron and to elucidate the dose-response and dose-toxicity relationships of the active constituents of saffron.
Acknowledgements
Not applicable.
Funding Statement
Funding: The preparation of the article was supported by the National Interdisciplinary Innovation Team of Traditional Chinese Medicine (grant no. ZYYCXTD-D-202209) and Xinglin Talent Program of Chengdu University of Traditional Chinese Medicine (grant no. BSH2020019).
Availability of data and materials
Not applicable.
Authors' contributions
WY and XQ performed the literature search and wrote the first draft of the manuscript. QW, TZ, MZ and JP obtained funding, designed and conceived the study, supervised the preparation of the article and revised the manuscript. FC contributed to translation and data collection as part of the manuscript preparation. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1. China Pharmacopoeia Commission: Pharmacopoeia of the People's Republic of China. 2020 edition, Part 1. Chinese medicines and Technology Press, Beijing, 2020. [Google Scholar]
- 2.Dai RC, Nabil WNN, Xu HX. The history of saffron in China: From its origin to applications. Chin Med Cult. 2021;4:228–234. [Google Scholar]
- 3.Huang WJ, Long CL. The medicinal history and recent study of Crocus sativus. J Minzu Univ China (Natural Sciences Edition) 2015;24:55–58. [Google Scholar]
- 4.Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle García-Rodríguez M, Serrano-Díaz J, Tarantilis PA, López-Córcoles H, Carmona M, Alonso GL. Determination of saffron quality by high-performance liquid chromatography. J Agric Food Chem. 2014;62:8068–8074. doi: 10.1021/jf5019356. [DOI] [PubMed] [Google Scholar]
- 6.Hatziagapiou K, Lambrou GI. The protective role of Crocus sativus L. (saffron) against ischemia-reperfusion injury, hyperlipidemia and atherosclerosis: Nature opposing cardiovascular diseases. Curr Cardiol Rev. 2018;14:272–289. doi: 10.2174/1573403X14666180628095918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thushara RM, Hemshekhar M, Santhosh MS, Jnaneshwari S, Nayaka SC, Naveen S, Kemparaju K, Girish KS. Crocin, a dietary additive protects platelets from oxidative stress-induced apoptosis and inhibits platelet aggregation. Mol Cell Biochem. 2013;373:73–83. doi: 10.1007/s11010-012-1476-7. [DOI] [PubMed] [Google Scholar]
- 8.Rahmani J, Manzari N, Thompson J, Clark CCT, Villanueva G, Varkaneh HK, Mirmiran P. The effect of saffron on weight and lipid profile: A systematic review, meta-analysis, and dose-response of randomized clinical trials. Phytother Res. 2019;33:2244–2255. doi: 10.1002/ptr.6420. [DOI] [PubMed] [Google Scholar]
- 9.Zhang A, Shen Y, Cen M, Hong X, Shao QS, Chen Y, Zheng B. Polysaccharide and crocin contents, and antioxidant activity of saffron from different origins. Ind Crop Prod. 2019;133:111–117. [Google Scholar]
- 10.Xue Y, Jin W, Xue Y, Zhang Y, Wang H, Zhang Y, Guan S, Chu X, Zhang J. Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca2+ homeostasis. J Pharmacol Sci. 2020;143:156–164. doi: 10.1016/j.jphs.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Poma A, Fontecchio G, Carlucci G, Chichiriccò G. Anti-inflammatory properties of drugs from saffron crocus. Antiinflamm Antiallergy Agents Med Chem. 2012;11:37–51. doi: 10.2174/187152312803476282. [DOI] [PubMed] [Google Scholar]
- 12.Singh G, Haileselassie Y, Ji AR, Maecker HT, Sinha SR, Brim H, Habtezion A, Ashktorab H. Protective effect of saffron in mouse colitis models through immune modulation. Digest Dis Sci. 2022;67:2922–2935. doi: 10.1007/s10620-021-07163-3. [DOI] [PubMed] [Google Scholar]
- 13.Lambrianidou A, Koutsougianni F, Papapostolou I, Dimas K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules. 2020;26(86) doi: 10.3390/molecules26010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui SA, Ali Redha A, Snoeck ER, Singh S, Simal-Gandara J, Ibrahim SA, Jafari SM. Anti-depressant properties of crocin molecules in saffron. Molecules. 2022;27(2076) doi: 10.3390/molecules27072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.José Bagur M, Alonso Salinas GL, Jiménez-Monreal AM, Chaouqi S, Llorens S, Martínez-Tomé M, Alonso GL. Saffron: An old medicinal plant and a potential novel functional food. Molecules. 2017;23(30) doi: 10.3390/molecules23010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howes MR, Perry NSL, Vásquez-Londoño C, Perry EK. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br J Pharmacol. 2020;177:1294–1315. doi: 10.1111/bph.14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bathaie SZ. Saffron as a functional food and a nutraceutical using saffron and its constituents as the nutraceutics to protect against chronic diseases. Acta Hortic. 2018;1200:201–204. [Google Scholar]
- 18.Hardy J. Pathways to primary neurodegenerative disease. Ann N Y Acad Sci. 2000;924:29–34. doi: 10.1111/j.1749-6632.2000.tb05556.x. [DOI] [PubMed] [Google Scholar]
- 19.Höglund K, Salter H. Molecular biomarkers of neurodegeneration. Expert Rev Mol Diagn. 2013;13:845–861. doi: 10.1586/14737159.2013.850033. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh S, Safia Haque E, Mir SS. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J Neurodegener Dis. 2013;2013(563481) doi: 10.1155/2013/563481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Stefano A, Sozio P, Cerasa LS. Antiparkinson prodrugs. Molecules. 2008;13:46–68. doi: 10.3390/molecules13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klietz M, Greten S, Wegner F, Höglinger GU. Safety and tolerability of pharmacotherapies for Parkinson's disease in geriatric patients. Drugs Aging. 2019;36:511–530. doi: 10.1007/s40266-019-00654-z. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 24.Salem M, Shaheen M, Tabbara A, Borjac J. Saffron extract and crocin exert anti-inflammatory and anti-oxidative effects in a repetitive mild traumatic brain injury mouse model. Sci Rep. 2022;12(5004) doi: 10.1038/s41598-022-09109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeedi M, Rashidy-Pour A. Association between chronic stress and Alzheimer's disease: Therapeutic effects of Saffron. Biomed Pharmacother. 2021;133(110995) doi: 10.1016/j.biopha.2020.110995. [DOI] [PubMed] [Google Scholar]
- 26.Gregory J, Vengalasetti YV, Bredesen DE, Rao RV. Neuroprotective herbs for the management of Alzheimer's disease. Biomolecules. 2021;11(543) doi: 10.3390/biom11040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatziagapiou K, Kakouri E, Lambrou GI, Bethanis K, Tarantilis PA. Antioxidant properties of Crocus Sativus L. and its constituents and relevance to neurodegenerative diseases; Focus on Alzheimer's and Parkinson's disease. Curr Neuropharmacol. 2019;17:377–402. doi: 10.2174/1570159X16666180321095705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghasemi Sakha F, Azimi Saeen A, Moazzeni SM, Etesam F, Vaezi G. A randomized, triple-blind placebo-controlled trial to determine the effect of saffron on the serum levels of MMP-9 and TIMP-1 in patients with multiple sclerosis. Iran J Allergy Asthma Immunol. 2020;19:297–304. doi: 10.18502/ijaai.v19i3.3457. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Y, Shan X, Men W, Zhai H, Qiao X, Geng L, Li C. The effect of crocin on memory, hippocampal acetylcholine level, and apoptosis in a rat model of cerebral ischemia. Biomed Pharmacother. 2020;130(110543) doi: 10.1016/j.biopha.2020.110543. [DOI] [PubMed] [Google Scholar]
- 30.Jahromi GP, Khodadadi H, Fasihi-Ramandi M, Esmaeili M, Shahriary A. Neuroprotective and antiapoptotic effects of N-acetylcystein and Crocus sativus aqueous extract on arsenic-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. Indian J Pharm Educ Res. 2019;53:695–702. [Google Scholar]
- 31.Razavi BM, Hosseinzadeh H. Saffron as an antidote or a protective agent against natural or chemical toxicities. Daru. 2015;23(31) doi: 10.1186/s40199-015-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuquilín-Arista F, Álvarez-Avellón T, Menéndez-González M. Prevalence of depression and anxiety in Parkinson disease and impact on quality of life: A community-based study in Spain. J Geriatr Psychiatry Neurol. 2020;33:207–213. doi: 10.1177/0891988719874130. [DOI] [PubMed] [Google Scholar]
- 33.Farkhondeh T, Samarghandian S, Shaterzadeh Yazdi H, Samini F. The protective effects of crocin in the management of neurodegenerative diseases: A review. Am J Neurodegener Dis. 2018;7:1–10. [PMC free article] [PubMed] [Google Scholar]
- 34.Bian Y, Zhao C, Lee SMY. Neuroprotective potency of saffron against neuropsychiatric diseases, neurodegenerative diseases, and other brain disorders: From bench to bedside. Front Pharmacol. 2020;11(579052) doi: 10.3389/fphar.2020.579052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 37.Praticò D. Oxidative stress hypothesis in Alzheimer's disease: A reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- 39.Wojsiat J, Zoltowska KM, Laskowska-Kaszub K, Wojda U. Oxidant/antioxidant imbalance in Alzheimer's disease: Therapeutic and diagnostic prospects. Oxid Med Cell Longev. 2018;2018(6435861) doi: 10.1155/2018/6435861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danielson SR, Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radical Bio Med. 2008;44:1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: Implications in the formation of neurofibrillary tangles of Alzheimer's disease. J Neurochem. 2002;82:1137–1147. doi: 10.1046/j.1471-4159.2002.t01-1-01061.x. [DOI] [PubMed] [Google Scholar]
- 42.Su B, Wang X, Lee HG, Tabaton M, Perry G, Smith MA, Zhu X. Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neurosci Lett. 2010;468:267–271. doi: 10.1016/j.neulet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Kumar V, Gill KD. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology. 2014;41:154–166. doi: 10.1016/j.neuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Lim J, Yue Z. Neuronal aggregates: Formation, clearance, and spreading. Dev Cell. 2015;32:491–501. doi: 10.1016/j.devcel.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabayan B, Namazi MR, Mowla A, Moniri SA. Are patients with Darier and Haily-Haily diseases susceptible to Alzheimer's disease? A theory based on abnormal intraneuronal Ca2+ homeostasis. J Alzheimers Dis. 2009;16:521–523. doi: 10.3233/JAD-2009-0956. [DOI] [PubMed] [Google Scholar]
- 46.Singh N, Haldar S, Tripathi AK, McElwee MK, Horback K, Beserra A. Iron in neurodegenerative disorders of protein misfolding: A case of prion disorders and Parkinson's disease. Antioxid Redox Signal. 2014;21:471–484. doi: 10.1089/ars.2014.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hare DJ, Double KL. Iron and dopamine: A toxic couple. Brain. 2016;139:1026–1035. doi: 10.1093/brain/aww022. [DOI] [PubMed] [Google Scholar]
- 48.Batarseh YS, Bharate SS, Kumar V, Kumar A, Vishwakarma RA, Bharate SB, Kaddoumi A. Crocus sativus extract tightens the blood-brain barrier, reduces Amyloid β load and related toxicity in 5XFAD mice. ACS Chem Neurosci. 2017;8:1756–1766. doi: 10.1021/acschemneuro.7b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, Lamari FN. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54:8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 50.Shati AA, Elsaid FG, Hafez EE. Biochemical and molecular aspects of aluminium chloride-induced neurotoxicity in mice and the protective role of Crocus sativus L. extraction and honey syrup. Neuroscience. 2011;175:66–74. doi: 10.1016/j.neuroscience.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 51.Reeta KH, Singh D, Gupta YK. Chronic treatment with taurine after intracerebroventricular streptozotocin injection improves cognitive dysfunction in rats by modulating oxidative stress, cholinergic functions and neuroinflammation. Neurochem Int. 2017;108:146–156. doi: 10.1016/j.neuint.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Bharate SS, Kumar V, Singh G, Singh A, Gupta M, Singh D, Kumar A, Vishwakarma RA, Bharate SB. Preclinical development of Crocus sativus-based botanical lead IIIM-141 for Alzheimer's disease: Chemical standardization, efficacy, formulation development, pharmacokinetics, and safety pharmacology. ACS Omega. 2018;3:9572–9585. doi: 10.1021/acsomega.8b00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapucu A. Crocin ameliorates oxidative stress and suppresses renal damage in streptozotocin induced diabetic male rats. Biotech Histochem. 2021;96:153–160. doi: 10.1080/10520295.2020.1808702. [DOI] [PubMed] [Google Scholar]
- 54.Naghizadeh B, Mansouri MT, Ghorbanzadeh B, Farbood Y, Sarkaki A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013;20:537–542. doi: 10.1016/j.phymed.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 55.Asadi F, Jamshidi AH, Khodagholi F, Yans A, Azimi L, Faizi M, Vali L, Abdollahi M, Ghahremani MH. Reversal effects of crocin on amyloid β-induced memory deficit: Modification of autophagy or apoptosis markers. Pharmacol Biochem Behav. 2015;139:47–58. doi: 10.1016/j.pbb.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Wang C, Cai X, Hu W, Li Z, Kong F, Chen X, Wang D. Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer's disease. Int J Mol Med. 2019;43:956–966. doi: 10.3892/ijmm.2018.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osaki LH, Gama P. MAPKs and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int J Mol Sci. 2013;14:10143–10161. doi: 10.3390/ijms140510143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: Signal transduction. Biofactors. 2013;39:27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 60.Vriz S, Reiter S, Galliot B. Cell death: A program to regenerate. Curr Top Dev Biol. 2014;108:121–151. doi: 10.1016/B978-0-12-391498-9.00002-4. [DOI] [PubMed] [Google Scholar]
- 61.Rafieipour F, Hadipour E, Emami SA, Asili J, Tayarani-Najaran Z. Safranal protects against beta-amyloid peptide-induced cell toxicity in PC12 cells via MAPK and PI3K pathways. Metab Brain Dis. 2019;34:165–172. doi: 10.1007/s11011-018-0329-9. [DOI] [PubMed] [Google Scholar]
- 62.Baluchnejadmojarad T, Mohamadi-Zarch SM, Roghani M. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid β-induced rat model of Alzheimer's disease: Underlying mechanisms. Metab Brain Dis. 2019;34:1747–1759. doi: 10.1007/s11011-019-00481-6. [DOI] [PubMed] [Google Scholar]
- 63.Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chalatsa I, Arvanitis DA, Koulakiotis NS, Giagini A, Skaltsounis AL, Papadopoulou-Daifoti Z, Tsarbopoulos A, Sanoudou D. The Crocus sativus compounds trans-crocin 4 and trans-crocetin modulate the amyloidogenic pathway and tau misprocessing in Alzheimer disease neuronal cell culture models. Front Neurosci. 2019;13(249) doi: 10.3389/fnins.2019.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rashedinia M, Lari P, Abnous K, Hosseinzadeh H. Protective effect of crocin on acrolein-induced tau phosphorylation in the rat brain. Acta Neurobiol Exp (Wars) 2015;75:208–219. [PubMed] [Google Scholar]
- 66.Koulakiotis NS, Purhonen P, Gikas E, Hebert H, Tsarbopoulos A. Crocus-derived compounds alter the aggregation pathway of Alzheimer's disease: Associated beta amyloid protein. Sci Rep. 2020;10(18150) doi: 10.1038/s41598-020-74770-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haeri P, Mohammadipour A, Heidari Z, Ebrahimzadeh-Bideskan A. Neuroprotective effect of crocin on substantia nigra in MPTP-induced Parkinson's disease model of mice. Anat Sci Int. 2019;94:119–127. doi: 10.1007/s12565-018-0457-7. [DOI] [PubMed] [Google Scholar]
- 68.Rao SV, Muralidhara Yenisetti SC, Rajini PS. Evidence of neuroprotective effects of saffron and crocin in a Drosophila model of parkinsonism. Neurotoxicology. 2016;52:230–242. doi: 10.1016/j.neuro.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Finley JW, Gao S. A Perspective on Crocus sativus L. (saffron) constituent crocin: A potent water-soluble antioxidant and potential therapy for Alzheimer's disease. J Agric Food Chem. 2017;65:1005–1020. doi: 10.1021/acs.jafc.6b04398. [DOI] [PubMed] [Google Scholar]
- 70.Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review) Mol Med Rep. 2016;13:3391–3396. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 72.McManus RM, Heneka MT. Role of neuroinflammation in neurodegeneration: New insights. Alzheimers Res Ther. 2017;9(14) doi: 10.1186/s13195-017-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simpson DSA, Oliver PL. ROS Generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel) 2020;9(743) doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Korani S, Korani M, Sathyapalan T, Sahebkar A. Therapeutic effects of crocin in autoimmune diseases: A review. Biofactors. 2019;45:835–843. doi: 10.1002/biof.1557. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Wang Y, Dong X, Liu J. Crocetin attenuates inflammation and amyloid-β accumulation in APPsw transgenic mice. Immun Ageing. 2018;15(24) doi: 10.1186/s12979-018-0132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: From pathophysiology to therapeutic strategies. Int J Mol Sci. 2021;23(14) doi: 10.3390/ijms23010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das J, G K R. Post stroke depression: The sequelae of cerebral stroke. Neurosci Biobehav Rev. 2018;90:104–114. doi: 10.1016/j.neubiorev.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Wang SH, Zhang ZJ, Guo YJ, Sui YX, Sun Y. Involvement of serotonin neurotransmission in hippocampal neurogenesis and behavioral responses in a rat model of post-stroke depression. Pharmacol Biochem Behav. 2010;95:129–137. doi: 10.1016/j.pbb.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 80.Xu QQ, Qian XD, Sun F, Liu H, Dou ZJ, Gong J, Zhang XR. Effects of crocin on inflammatory response and TLR4/MyD88/NF-κB signaling pathway in post-stroke depression rats. Chin J Immunol. 2021;37:179–185. [Google Scholar]
- 81.Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9:780–786. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hernández-Reséndiz S, Buelna-Chontal M, Correa F, Zazueta C. Targeting mitochondria for cardiac protection. Curr Drug Targets. 2013;14:586–600. doi: 10.2174/1389450111314050008. [DOI] [PubMed] [Google Scholar]
- 83.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 84.Shults CW. Mitochondrial dysfunction and possible treatments in Parkinson's disease-a review. Mitochondrion. 2004;4:641–648. doi: 10.1016/j.mito.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 85.Zhang GF, Zhang Y, Zhao G. Crocin protects PC12 cells against MPP(+)-induced injury through inhibition of mitochondrial dysfunction and ER stress. Neurochem Int. 2015;89:101–110. doi: 10.1016/j.neuint.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 86.Vongsfak J, Pratchayasakul W, Apaijai N, Vaniyapong T, Chattipakorn N, Chattipakorn SC. The alterations in mitochondrial dynamics following cerebral ischemia/reperfusion injury. Antioxidants (Basel) 2021;10(1384) doi: 10.3390/antiox10091384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wen B. Study on the protective effect of crocin pretreatment on hippocampal mitochondria in the rats with ischemic brain injury. Mod J Integr Tradit Chin West Med. 2020;29(6) [Google Scholar]
- 88.Zhang YH, Cong WH, Liu JX. Effect of crocin on mitochondrial dynamics in SH-SY5Y cells against injury induced by oxygen-glucose deprivation. Chin Pharmacol Bull. 2016;32:986–990. [Google Scholar]
- 89.Zhang YH, Yao MJ, Cong WH, Liu JX. Effect of extraction of saffron crocus on mitochondrial dynamics in ischemia/reperfusion rats. Chin Pharmacol Bull. 2018;34:770–775. [Google Scholar]
- 90.Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer's disease. Brain Res. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin L, Liu G, Yang L. Crocin improves cognitive behavior in rats with Alzheimer's disease by regulating endoplasmic reticulum stress and apoptosis. Biomed Res Int. 2019;2019(9454913) doi: 10.1155/2019/9454913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hadipour M, Kaka G, Bahrami F, Meftahi GH, Pirzad Jahromi G, Mohammadi A, Sahraei H. Crocin improved amyloid beta induced long-term potentiation and memory deficits in the hippocampal CA1 neurons in freely moving rats. Synapse. 2018;72(e22026) doi: 10.1002/syn.22026. [DOI] [PubMed] [Google Scholar]
- 93.Naghibi SM, Hosseini M, Khani F, Rahimi M, Vafaee F, Rakhshandeh H, Aghaie A. Effect of aqueous extract of Crocus sativus L. on morphine-induced memory impairment. Adv Pharmacol Sci. 2012;2012(494367) doi: 10.1155/2012/494367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazumder AG, Sharma P, Patial V, Singh D. Crocin attenuates kindling development and associated cognitive impairments in mice via inhibiting reactive oxygen species-mediated NF-κB activation. Basic Clin Pharmacol Toxicol. 2017;120:426–433. doi: 10.1111/bcpt.12694. [DOI] [PubMed] [Google Scholar]
- 95.Canal CE, Chang Q, Gold PE. Amnesia produced by altered release of neurotransmitters after intraamygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci USA. 2007;104:12500–12505. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D'Amato RJ, Zweig RM, Whitehouse PJ, Wenk GL, Singer HS, Mayeux R, Price DL, Snyder SH. Aminergic systems in Alzheimer's disease and Parkinson's disease. Ann Neurol. 1987;22:229–236. doi: 10.1002/ana.410220207. [DOI] [PubMed] [Google Scholar]
- 97.Müller ML, Bohnen NI. Cholinergic dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep. 2013;13(377) doi: 10.1007/s11910-013-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain. 2014;137:2493–2508. doi: 10.1093/brain/awu193. [DOI] [PubMed] [Google Scholar]
- 99.Geromichalos GD, Lamari FN, Papandreou MA, Trafalis DT, Margarity M, Papageorgiou A, Sinakos Z. Saffron as a source of novel acetylcholinesterase inhibitors: Molecular docking and in vitro enzymatic studies. J Agric Food Chem. 2012;60:6131–6138. doi: 10.1021/jf300589c. [DOI] [PubMed] [Google Scholar]
- 100.Pitsikas N, Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain Res. 2006;173:112–115. doi: 10.1016/j.bbr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 101.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats' memory. Behav Brain Res. 2007;183:141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 102.White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Bakulski KM, Seo YA, Hickman RC, Brandt D, Vadari HS, Hu H, Park SK. Heavy metals exposure and Alzheimer's disease and related dementias. J Alzheimers Dis. 2020;76:1215–1242. doi: 10.3233/JAD-200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Butnariu M, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Singh L, Aborehab NM, Bouyahya A, Venditti A, Sen S, Acharya K, et al. The pharmacological activities of Crocus sativus L.: A review based on the mechanisms and therapeutic opportunities of its phytoconstituents. Oxid Med Cell Longev. 2022;2022(8214821) doi: 10.1155/2022/8214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tamegart L, Abbaoui A, Makbal R, Zroudi M, Bouizgarne B, Bouyatas MM, Gamrani H. Crocus sativus restores dopaminergic and noradrenergic damages induced by lead in Meriones shawi: A possible link with Parkinson's disease. Acta Histochem. 2019;121:171–181. doi: 10.1016/j.acthis.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Sartori E, Edan G. Assessment of cognitive dysfunction in multiple sclerosis. J Neurol Sci. 2006;245:169–175. doi: 10.1016/j.jns.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 107.Glanz BI, Healy BC, Hviid LE, Chitnis T, Weiner HL. Cognitive deterioration in patients with early multiple sclerosis: A 5-year study. J Neurol Neurosurg Psychiatry. 2012;83:38–43. doi: 10.1136/jnnp.2010.237834. [DOI] [PubMed] [Google Scholar]
- 108.Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghaffari S, Hatami H, Dehghan G. Saffron ethanolic extract attenuates oxidative stress, spatial learning, and memory impairments induced by local injection of ethidium bromide. Res Pharm Sci. 2015;10:222–232. [PMC free article] [PubMed] [Google Scholar]
- 110.Bonuccelli U, Meco G, Fabbrini G, Tessitore A, Pierantozzi M, Stocchi F, Ceravolo R, Caltagirone C, Silvestrini M, Morgante F, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson's disease. Expert Opin Pharmacother. 2012;13:2269–2280. doi: 10.1517/14656566.2012.736490. [DOI] [PubMed] [Google Scholar]
- 111.Dobkin RD, Menza M, Bienfait KL, Gara M, Marin H, Mark MH, Dicke A, Friedman J. Depression in Parkinson's disease: Symptom improvement and residual symptoms after acute pharmacologic management. Am J Geriatr Psychiatry. 2011;19:222–229. doi: 10.1097/JGP.0b013e3181e448f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lozupone M, La Montagna M, D'Urso F, Piccininni C, Sardone R, Dibello V, Giannelli G, Solfrizzi V, Greco A, Daniele A, et al. Pharmacotherapy for the treatment of depression in patients with alzheimer's disease: A treatment-resistant depressive disorder. Expert Opin Pharmacother. 2018;19:823–842. doi: 10.1080/14656566.2018.1471136. [DOI] [PubMed] [Google Scholar]
- 113.Novais F, Starkstein S. Phenomenology of depression in Alzheimer's disease. J Alzheimers Dis. 2015;47:845–855. doi: 10.3233/JAD-148004. [DOI] [PubMed] [Google Scholar]
- 114.Pålhagen S, Qi H, Mårtensson B, Wålinder J, Granérus AK, Svenningsson P. Monoamines, BDNF, IL-6 and corticosterone in CSF in patients with Parkinson's disease and major depression. J Neurol. 2010;257:524–532. doi: 10.1007/s00415-009-5353-6. [DOI] [PubMed] [Google Scholar]
- 115.Schrag A, Taddei RN. Depression and anxiety in Parkinson's disease. Int Rev Neurobiol. 2017;133:623–655. doi: 10.1016/bs.irn.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 116.Xiao Q, Xiong Z, Yu C, Zhou J, Shen Q, Wang L, Xie X, Fu Z. Antidepressant activity of crocin-I is associated with amelioration of neuroinflammation and attenuates oxidative damage induced by corticosterone in mice. Physiol Behav. 2019;212(112699) doi: 10.1016/j.physbeh.2019.112699. [DOI] [PubMed] [Google Scholar]
- 117.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR, Pahlvan S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 118.Halataei BAS, Khosravi M, Arbabian S, Sahraei H, Golmanesh L, Zardooz H, Jalili C, Ghoshooni H. Saffron (Crocus sativus) aqueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother Res. 2011;25:1833–1838. doi: 10.1002/ptr.3495. [DOI] [PubMed] [Google Scholar]
- 119.Ghasemi T, Abnous K, Vahdati F, Mehri S, Razavi BM, Hosseinzadeh H. Antidepressant effect of Crocus sativus aqueous extract and its effect on CREB, BDNF, and VGF transcript and protein levels in rat hippocampus. Drug Res (Stuttg) 2015;65:337–343. doi: 10.1055/s-0034-1371876. [DOI] [PubMed] [Google Scholar]
- 120.Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22(16) doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cano A, Fonseca E, Ettcheto M, Sánchez-López E, de Rojas I, Alonso-Lana S, Morató X, Souto EB, Toledo M, Boada M, et al. Epilepsy in neurodegenerative diseases: Related drugs and molecular pathways. Pharmaceuticals (Basel) 2021;14(1057) doi: 10.3390/ph14101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Filippini A, D'Amore A, D'Alessio A. Calcium mobilization in endothelial cell functions. Int J Mol Sci. 2019;20(4525) doi: 10.3390/ijms20184525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maklad A, Sharma A, Azimi I. Calcium signaling in brain cancers: Roles and therapeutic targeting. Cancers (Basel) 2019;11(145) doi: 10.3390/cancers11020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schrank S, Barrington N, Stutzmann GE. Calcium-handling defects and neurodegenerative disease. Cold Spring Harb Perspect Biol. 2020;12(a035212) doi: 10.1101/cshperspect.a035212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xing B, Li S, Yang J, Lin D, Feng Y, Lu J, Shao Q. Phytochemistry, pharmacology, and potential clinical applications of saffron: A review. J Ethnopharmacol. 2021;281(114555) doi: 10.1016/j.jep.2021.114555. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Z, Zheng B, Li J, Wei Z, Chu S, Han X, Chu L, Wang H, Chu X. Influence of crocetin, a natural carotenoid dicarboxylic acid in saffron, on L-type Ca2+ current, intracellular Ca2+ handling and contraction of isolated rat cardiomyocytes. Biol Pharm Bull. 2020;43:1367–1374. doi: 10.1248/bpb.b20-00298. [DOI] [PubMed] [Google Scholar]
- 128.Moshiri M, Vahabzadeh M, Hosseinzadeh H. Clinical Applications of saffron (Crocus sativus) and its constituents: A review. Drug Res (Stuttg) 2015;65:287–295. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- 129.Omidkhoda SF, Hosseinzadeh H. Saffron and its active ingredients against human disorders: A literature review on existing clinical evidence. Iran J Basic Med Sci. 2022;25:913–933. doi: 10.22038/IJBMS.2022.63378.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Foster ER. Themes from the special issue on neurodegenerative diseases: What have we learned, and where can we go from here? Am J Occup Ther. 2014;68:6–8. doi: 10.5014/ajot.2014.009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi SS, Yousefi MH, Alimardani R, Jamshidi A, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology (Berl) 2010;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 132.Asadollahi M, Nikdokht P, Hatef B, Sadr SS, Sahraei H, Assarzadegan F, Pirzad Jahromi G. Protective properties of the aqueous extract of saffron (Crocus sativus L.) in ischemic stroke, randomized clinical trial. J Ethnopharmacol. 2019;238(111833) doi: 10.1016/j.jep.2019.111833. [DOI] [PubMed] [Google Scholar]
- 133.Zandi N, Pazoki B, Momeni Roudsari N, Lashgari NA, Jamshidi V, Momtaz S, Abdolghaffari AH, Akhondzadeh S. Prospects of saffron and its derivatives in Alzheimer's disease. Arch Iran Med. 2021;24:233–252. doi: 10.34172/aim.2021.35. [DOI] [PubMed] [Google Scholar]
- 134.Marzabadi LR, Fazljou SMB, Araj-Khodaei M, Sadigh-Eteghad S, Naseri A, Talebi M. Saffron reduces some inflammation and oxidative stress markers in donepezil-treated mild-to-moderate Alzheimer's disease patients: A randomized double-blind placebo-control trial. J Herb Med. 2022;34(100574) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.