ABSTRACT
Alternative delivery routes of the current Mycobacterium tuberculosis (Mtb) vaccine, intradermally (ID) delivered BCG, may provide better protection against tuberculosis, and be more easily administered. Here, we use rhesus macaques to compare the airway immunogenicity of BCG delivered via either ID or intragastric gavage vaccination. Ag-specific CD4 T cell responses in the blood were similar after BCG vaccination via gavage or ID injection. However, gavage BCG vaccination induced significantly lower T cell responses in the airways compared to intradermal BCG vaccination. Examining T cell responses in lymph node biopsies showed that ID vaccination induced T cell priming in skin-draining lymph nodes, while gavage vaccination induced priming in the gut-draining nodes, as expected. While both delivery routes induced highly functional Ag-specific CD4 T cells with a Th1* phenotype (CXCR3+CCR6+), gavage vaccination induced the co-expression of the gut-homing integrin α4β7 on Ag-specific Th1* cells, which was associated with reduced migration into the airways. Thus, in rhesus macaques, the airway immunogenicity of gavage BCG vaccination may be limited by the imprinting of gut-homing receptors on Ag-specific T cells primed in intestinal lymph nodes.
KEYWORDS: BCG, CD4 T cells, immunization, immunology, lung defense, rhesus macaques, tuberculosis vaccines
INTRODUCTION
Mycobacterium tuberculosis (Mtb) is a leading global cause of death due to infectious disease, and deaths from TB are now increasing due to the reduced access to TB diagnosis and treatment caused by the COVID-19 pandemic (1). The only available vaccine for Mtb is Bacillus Calmette-Guérin (BCG), which is given as an intradermal injection within the first 6 months of age. While a single administration of ID BCG is protective in children, it does little to prevent TB in adolescents and adults, and novel highly effective vaccine strategies for TB are sorely needed.
One approach to enhance vaccine-mediated protection against Mtb has been to develop alternate vaccine platforms, such as adjuvanted proteins, viral vectors, or other attenuated mycobacteria (2). Another has been to improve the use of BCG itself. It was recently shown that homologous boosting in adolescents, referred to as BCG re-vaccination, displayed protection based on the novel metric of sustained quantiferon conversion (3). There has also been interest in the ability of other routes of BCG administration to enhance protection against Mtb. This is perhaps best illustrated by recent reports that intravenous BCG vaccination induces extraordinarily high levels of protection against Mtb challenge in nonhuman primates (4, 5). Mucosal vaccination routes also display great promise. Administration of BCG into the lungs induces high levels of protection against subsequent challenge in nonhuman primates (5–7), and is being explored in humans (8).
Oral vaccination is another potentially important route of BCG vaccination. In fact, BCG was first deployed a century ago as an oral vaccine. Several studies in mice and guinea pigs have found that oral BCG vaccination induces immune responses similar to ID BCG vaccination, and is at least as protective against Mtb infection (9–12). One study even showed that rectal BCG vaccination of macaques induced IFNγ-spot forming cell responses in the spleen of similar magnitude as ID vaccination (13). A recent clinical trial testing several different combinations of oral and ID BCG vaccination showed that oral BCG vaccination not only generates Ag-specific T cells in the blood, but also induces higher responses in the airways compared to ID BCG (14). To better understand the generation of Mtb-specific T cell responses in the airways after oral vaccination, here we compare the magnitude, function, and phenotype of mycobacteria-specific CD4 T cells generated after either intradermal or gavage BCG vaccination in rhesus macaques.
RESULTS
Airway and circulating CD4 T cell responses after intradermal or gavage BCG vaccination in rhesus macaques.
Four rhesus macaques received an intradermal (ID) BCG injection, with 2 animals receiving a low dose of 5.3 × 104 CFU, and 2 receiving a high dose of 7.9 × 105 CFU. Alternatively, 4 animals received BCG directly into the stomach via gavage, with 2 receiving a lower dose of 6.7 × 108 CFU and 2 receiving a higher dose of 3.8 × 109 CFU (Fig. 1A). To identify Ag-specific CD4 T cells, bronchoalveolar lavage (BAL) cells, and peripheral blood mononuclear cells (PBMC) were restimulated in vitro with either Purified Protein Derivative (PPD) or a peptide megapool of Mtb-derived T cell epitopes (MTB300) (15). Responding cells detected by intracellular expression of TNF (Fig. 1B). Both ID and gavage BCG induced Ag-specific responses in the airways against PPD and MTB300 (Fig. 1C and D). However, ID vaccination induced a significantly larger response in the airways than gavage as calculated by area under the curve (AUC). In contrast, in the PBMCs, there was no statistical difference in the CD4 T cell responses to PPD and MTB300 between the two vaccination routes, as calculated by area under the curve (AUC) (Fig. 1E and F). There was no difference in the size of the response induced by the two doses of BCG. Not only were the responses to PPD larger than MTB300, PPD-specific T cells were preferentially skewed toward the BAL (i.e., PPD AUC/MTB300 AUC ratio in BAL > PBMC) (Fig. 1G). Nonetheless, both vaccination routes induce recruitment of CD4 T cells that are specific for Mtb into the airways, but ID BCG induced significantly larger airway responses.
FIG 1.
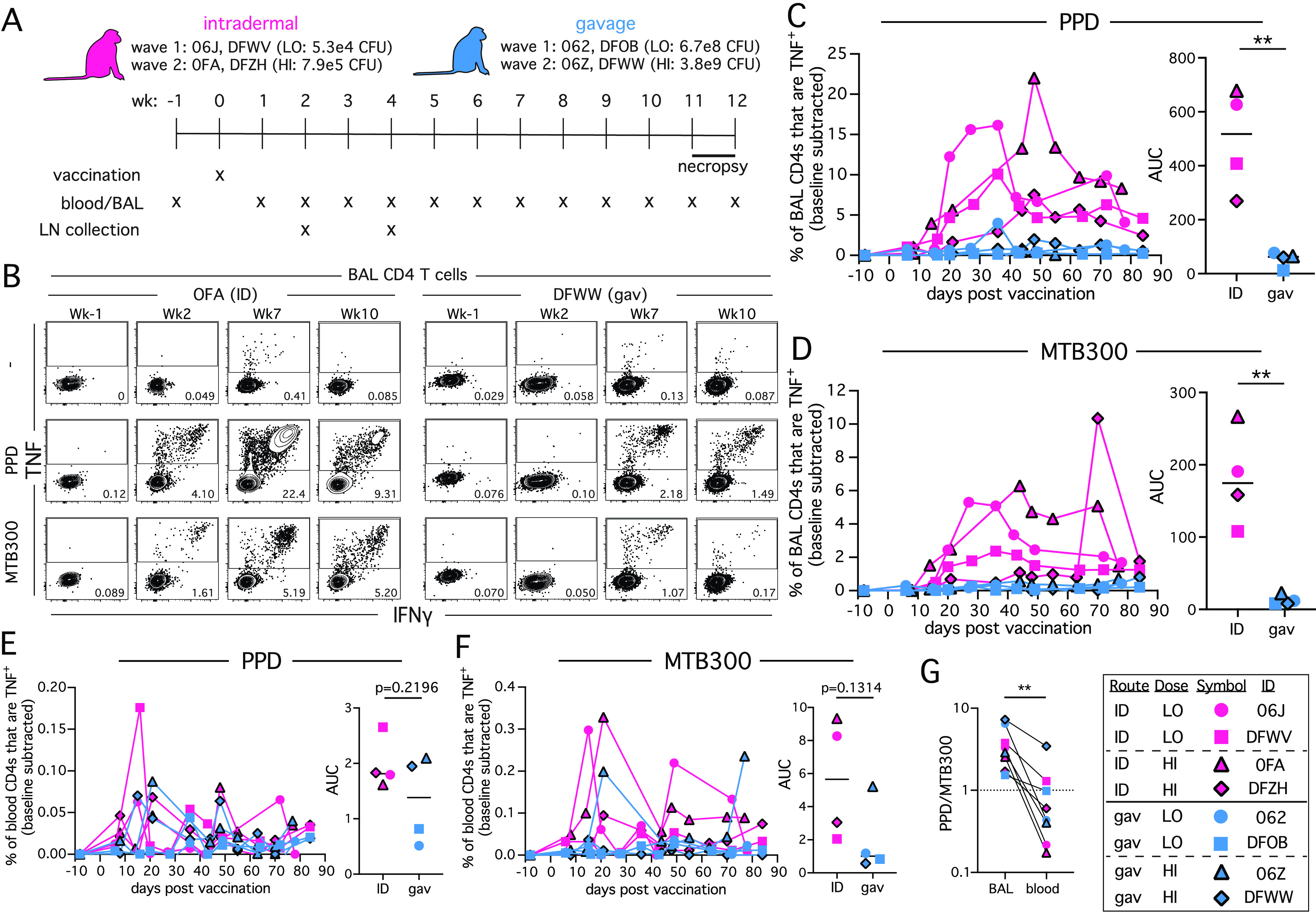
Intradermal BCG elicits greater mycobacteria-specific CD4 T cell responses in the airways compared to intragastric gavage BCG. (A) Four animals were intradermally vaccinated with BCG, 2 at a higher dose and 2 at a lower dose, and 4 animals were vaccinated by intragastric gavage, 2 at a higher dose and 2 at a lower dose. Animals were necropsied at 11 or 12 weeks postvaccination. (B) Example FACS plots of TNF and IFNγ staining on CD4 T cells from the BAL following in vitro stimulation with PPD or MTB300 MHC-II peptide megapool. (C) Graph showing percentage of BAL CD4 T cells that are TNF+ following in vitro PPD stimulation in each animal, and summary graph showing each animal’s area under the curve (AUC). (D) Graph showing percentage of BAL CD4 T cells that are TNF+ following in vitro MTB300 stimulation in each animal, and summary graph showing each animal’s area under the curve (AUC). (E) Graph showing percentage of CD4 T cells from PBMCs that are TNF+ following in vitro PPD stimulation in each animal, and summary graph showing each animal’s area under the curve (AUC). (F) Graph showing percentage of CD4 T cells from PBMCs that are TNF+ following in vitro MTB300 stimulation in each animal, and summary graph showing each animal’s area under the curve (AUC). (G) Ratio of PPD to MTB300-specific CD4 T cells from each animal in BAL and blood. **, P < 0.01.
We next examined CD8 T cell responses after MTB300 peptide pool restimulation. In the BAL, 2 of 4 ID vaccinated animals displayed detectable CD8 T cell responses at week 10 postinfection, while no MTB300-specific CD8 T cells were detected in the BAL of gavage BCG vaccinated animals (Fig. S1A). While this is consistent with the larger CD4 T cell responses following ID BCG compared to gavage BCG vaccination, the MTB300 peptide pool was designed for CD4 T cells and inefficiently stimulates CD8 T cells. Therefore, these data are likely an underrepresentation of the true Mtb-specific CD8 T cell response. We also measured Mtb-specific antibody responses. In the BAL fluid at week 10, 2 ID vaccinated animals showed low Mtb lysate-specific IgG responses, but none of the gavage vaccinated animals mounted a detectable response (Fig. S1B). This is consistent with previous results showing that Mtb-specific antibody responses in the BAL are generally very low after BCG vaccination (4).
Mtb-specific CD8 T cell and IgG responses in the BAL. (A) BAL cells from week 10 postinfection were restimulated with MTB300, and the frequency of IFNγ+TNF+ CD8 T cells were measured by intracellular cytokine staining. (B) Mtb lysate-specific IgG responses in the BAL fluid were measured at baseline (black lines) and week 10 (ID: magenta and gavage: blue lines). Download FIG S1, PDF file, 0.4 MB (429.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
At necropsy, we also examined Ag-specific CD4 T cell responses in the lung tissue. However, only 5 of the 48 total lung specimens had Ag-specific CD4 T cell responses detectable above background after PPD restimulation. One of those samples was from an ID vaccinated animal (DFWV: 0.54% IFNγ+TNF+), and 4 samples were from gavage vaccinated animals (DFWW: 0.36%, 1.14% and 0.07%, and 06Z: 0.09% IFNγ+TNF+). Thus, CD4 T cell responses were very low in the lung tissue compared to BAL following both vaccination routes.
Vaccination route affects location of CD4 T cell priming.
To confirm that vaccination route impacts the site of T cell priming, T cell responses were measured in axillary and mesenteric lymph node biopsies obtained at weeks 2 and 4 postvaccination (Fig. 2A and B) and at necropsy (Fig. 2 C and D). We only detected Ag-specific T cell responses in the axillary LN biopsies after ID BCG, although the differences in the means of the responses did not reach statistical significance. In contrast, responses in the mesenteric node biopsies were higher after gavage compared to ID BCG (Fig. 2A and B). At necropsy, responses in the axillary LNs were higher in the ID BCG animals, while responses in the mesenteric nodes were now similar between the groups. (Fig. 2C, D).
FIG 2.
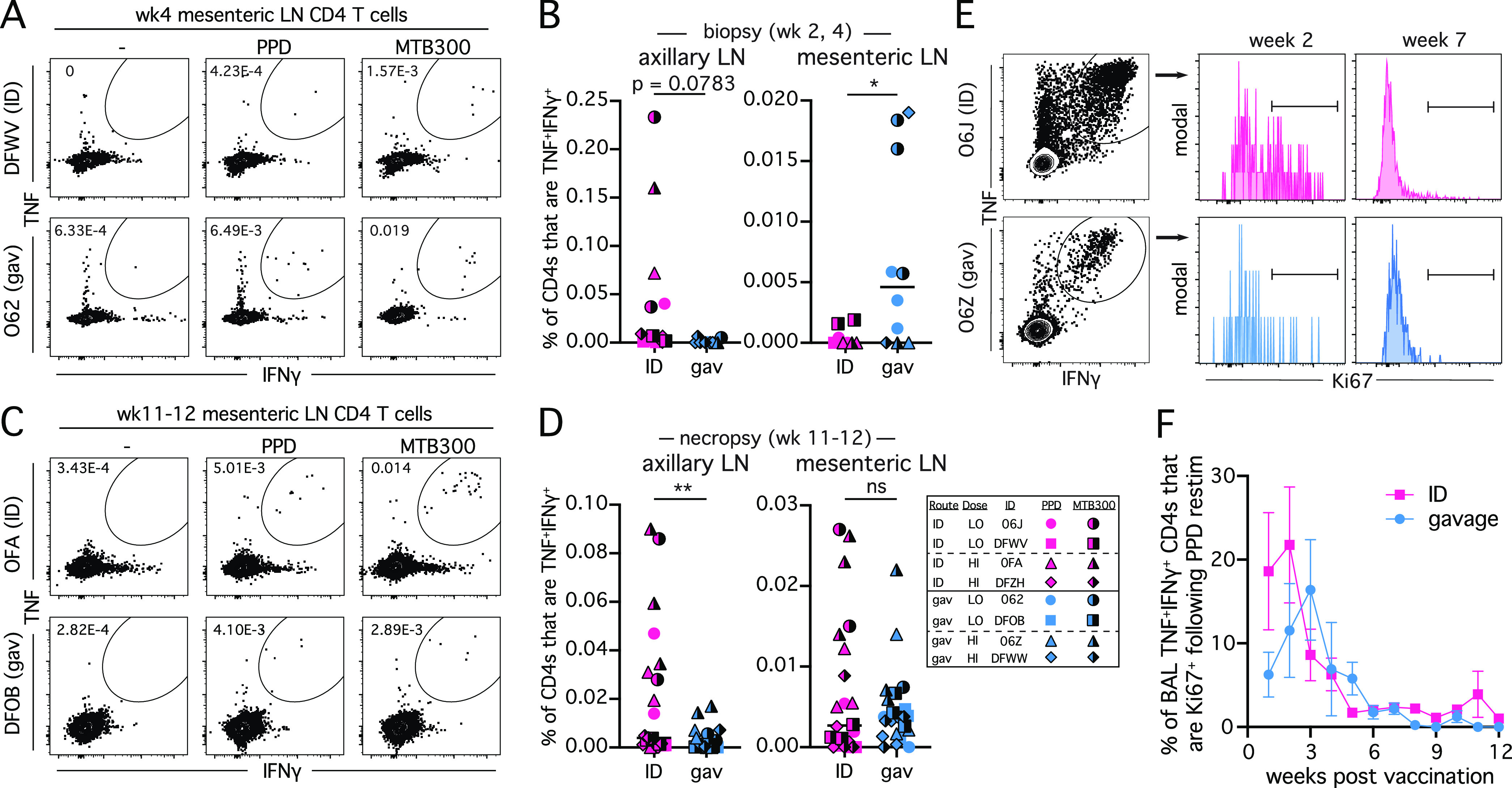
BCG vaccination route determines the site of Ag-specific T cell priming. (A) Example FACS plots of TNF and IFNγ staining on CD4 T cells in mesenteric lymph nodes at 4 weeks postvaccination. (B) Summary graphs showing the percentage of CD4 T cells that are TNF+IFNγ+ from all axillary and mesenteric lymph node biopsies between weeks 2 or 4 postvaccination. Full color symbols are PPD stimulation conditions and half-black symbols are MTB300 stimulation conditions. (C) Example FACS plots of TNF and IFNγ staining on CD4 T cells in mesenteric lymph nodes at necropsy. (D) Summary graphs showing the percentage of CD4 T cells that are TNF+IFNγ+ from all axillary and mesenteric lymph nodes collected at necropsy. Full color symbols are PPD stimulation conditions and half-black symbols are MTB300 stimulation conditions. (E) Example FACS plots of TNF and IFNγ staining on BAL CD4 T cells following PPD stimulation and Ki67 staining on TNF+IFNγ+ cells. (F) Summary graph of percentage of TNF+IFNγ+ CD4 T cells in the BAL that are Ki67+ following PPD stimulation. Graph depicts mean value for each group and error bars show standard error of the mean (SEM). *, P < 0.05 and **, P < 0.01.
To ask if the vaccination route impacted the duration of T cell clonal expansion, we measured Ki67 expression by Ag-specific (TNF+IFNγ+) CD4 T cells in the BAL (Fig. 2E). Both vaccination routes show an initial burst of Ki67+ Ag-specific T cells in the BAL which dissipated by week 6 postvaccination with similar kinetics between the groups (Fig. 2F). These results indicate that T cell priming occurs in the draining lymph nodes, as expected. Moreover, the reduced T cell responses in the BAL of gavage BCG vaccinated animals is not associated with a reduced proliferative phase as measured by Ki67.
Induction of gut-homing receptors on mycobacteria-specific Th1* cells after gavage BCG vaccination.
The magnitude of the Ag-specific T cell response in the airways was lower after gavage vaccination, which could reflect an overall lower T cell response or reduced ability to migrate into the lungs. We compared response in the BAL to PBMC by comparing the BAL AUC/PBMC AUC for each route. For both PPD and MTB300-specific CD4 T cells, the magnitude of responses in the BAL was much higher for ID versus gavage BCG, even after normalizing to PBMC responses (Fig. 2A and B). Thus, the defect in the BAL responses induced by gavage may be related to migratory ability of the T cells generated.
In humans and macaques, Mtb-specific CD4 T cells are primarily comprised of IFNγ-producing cells that express both Th1 and Th17 associated chemokine receptors, CXCR3 and CCR6, referred to as Th1* cells (16–19). We next asked, therefore, if gavage BCG vaccination induces Th1* cells (Fig. 3C). The majority of PPD and MTB300-specific IFNγ-producing CD4 T cells induced by both vaccination routes were CXCR3+CCR6+ in BAL (Fig. 3D and E). There was a slight, but significant, reduction of the PPD-specific Th1* cells in the gavage route (Fig. 3D). However, this was due to a single data point that was identified as an outlier by Grubb’s test, and after removal of this data point, there no longer was a statistically significant difference (P = 0.074). Overall, these data show that gavage BCG vaccination induces the expected phenotype of CD4 T cells, and the lower magnitude response is not associated with a lack of expression of these 2 major lung homing receptors. Given that priming in intestinal lymph nodes can induce the expression of gut-homing receptors, we also stained for the integrin α4β7 on Ag-specific Th1* cells. BCG vaccination induced much higher frequencies of α4β7 expressing Ag-specific CXCR3+CCR6+ IFNγ-producing CD4 T cells in both the blood and BAL (Fig. 3F). Importantly, when comparing within each vaccination route, there was consistently a higher percentage of α4β7+ CD4 T cells in blood compared to BAL (Fig. 3G and H). Thus, gavage BCG induces much higher frequencies of α4β7 expressing Th1* cells, which localize to the airways much less efficiently than their α4β7− counterparts.
FIG 3.
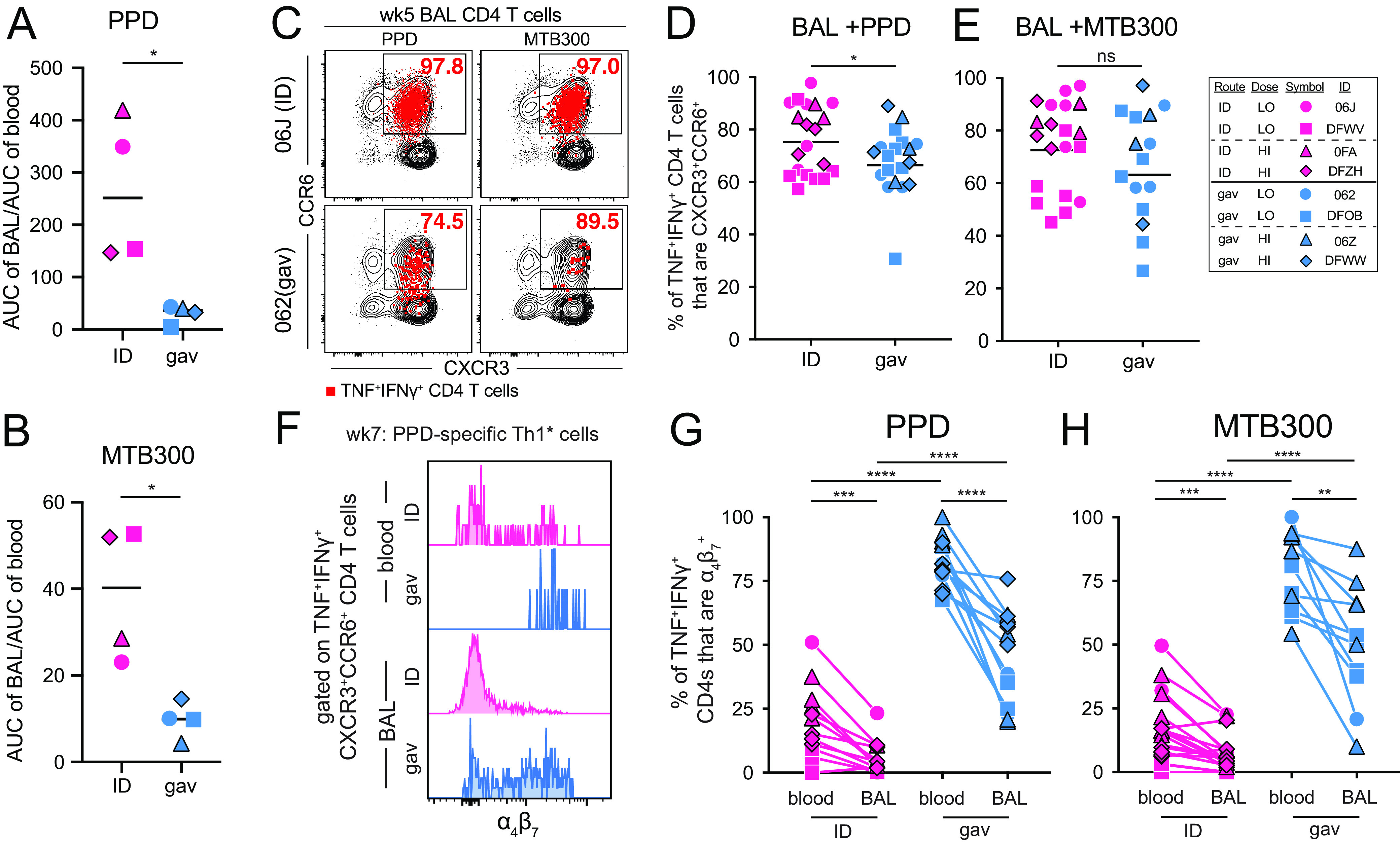
Gavage BCG vaccination induces gut-homing receptors on Ag-specific Th1* cells. (A and B) Ratio of the AUC of BAL TNF+ CD4 T cells to blood TNF+ CD4 T cells, as shown in Fig. 1C to F, following in vitro restimulation with either PPD (A) or MTB300 (B). (C) Example FACS plots showing CCR6 and CXCR3 staining in BAL CD4 T cells from week 5 postvaccination. Bulk CD4 T cells are shown in black, and red overlay depicts TNF+IFNγ+ CD4 T cells. (D and E) Graphs showing percentage of BAL TNF+IFNγ+ CD4 T cells that are CCR6+CXCR3+ from all time points postvaccination combined into 1 column, following either PPD (D) or MTB300 (E) stimulation. (F) Example histogram of α4β7 staining on CCR6+CXCR3+ BAL and blood CD4 T cells from week 7 postinfection. (G and H) Graphs showing percentage of TNF+IFNγ+ CD4 T cells that are α4β7+ following either PPD (G) or MTB300 (H) restimulation. Graphs show multiple time points combined into a single column with a line drawn to identical time points in the second tissue. A paired t test was used for this comparison, whereas an unpaired t test was used to compare identical tissues across vaccination groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001.
DISCUSSION
Although the route of BCG vaccination was changed from oral to intradermal in most countries after the Lübeck disaster in the early 1930s (20, 21), oral BCG was used in Brazil until the mid 1970s. Its eventual discontinuation in Brazil resulted from evidence of poor skin test conversion compared to ID vaccination (22), taken to mean that it induced inferior immunity. Hoft et al. later showed in a clinical trial that previous oral BCG vaccination inhibited the ability of subsequent ID BCG vaccination to induce PPD skin test reactivity (23), despite the presence of IFNγ-producing T cells in the blood. Moreover, subsequent studies by Hoft et al. further demonstrated that oral BCG vaccination, indeed, induces strong PPD-specific T cell and IgA responses in the bronchoalveolar lavage (14, 24). Therefore, in humans, the lack of a PPD skin test reaction after oral BCG vaccination indicates some form of oral tolerance to skin DTH responses, rather than lack of Ag-specific IFNγ-producing T cells. Similarly, we show here that vaccination by intragastric gavage of BCG results in a highly functional Ag-specific Th1* response in the airways of rhesus macaques.
One key difference between our results and those observed in humans relates to the relative magnitude of the Ag-specific T cell responses in the BAL and blood. In humans, oral BCG vaccination induced higher frequencies of Ag-specific T cells in the airways compared to ID vaccination. Here, we found the opposite: gavage vaccination in macaques resulted in lower magnitude airway responses compared to ID vaccination. This difference may be due to the way the bacteria were given. In the clinical trial, extremely high numbers of bacilli suspended in PBS were drank, potentially delivering some bacteria into the lymph nodes draining the mouth and esophagus or perhaps even a small number of bacteria into the airways. In this study, we introduced the bacteria with a tube directly into the stomach, bypassing the entire mucosal surface above. Thus, intragastric vaccination may be less airway immunogenic than oral vaccination.
Our data highlight a possible explanation for the relatively reduced Ag-specific T cell response in the airways following gavage BCG vaccination. CXCR3+CCR6+ Th1* cells were generated after priming in either skin or gut-draining lymph nodes, indicating that gavage vaccination was equally able to drive mycobacteria-specific T cells to this polarization state. Moreover, we noted no difference on the cytokine producing function of T cells generated after either route of vaccination. Thus, despite the potential impact of oral tolerance mechanisms, gavage vaccination generated the expected type of effector cells with no apparent defect in function. However, the Th1* cells generated after gavage vaccination co-expressed α4β7, a gut-homing integrin that binds MADCAM1 on intestinal venules (25). Indeed, priming in gut-draining lymph nodes is known to induce α4β7 expression by responding T cells (26–28). Thus, gavage BCG vaccination imprints a gut-homing phenotype onto Th1* polarizing mycobacteria-specific CD4 T cells. When compared to their α4β7− counterparts, α4β7+ Ag-specific T cells are greatly underrepresented in the airways, indicating that these cells are less able to enter the BAL. Therefore, it is possible that the induction of gut-homing receptors on Ag-specific CD4 T cells limits the airway immunogenicity of orally delivered BCG. We did not challenge the animals in this experiment to test protection, so it remains unclear to what extent this represents a barrier to generating protective immunity against pulmonary Mtb infection with this vaccination route.
Airway immunity generated by oral BCG vaccination might be improved by several different approaches, including alternate formulations or by combining oral vaccination with other vaccination routes. Our data suggest that, if oral BCG could be adjuvanted to prevent the induction of gut-homing receptors, the population of Mtb-specific T cells generated in the airways may be increased. Future studies are needed to explore the mechanisms controlling the homing potential of T cells generated after different routes of vaccination with BCG.
MATERIALS AND METHODS
Rhesus macaques.
Four male and 4 female rhesus macaques (Macaca mulatta) were housed in an AAALAC-approved Biosafety Level 2 animal vivarium in accordance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and all other applicable laws and regulations. All animals were between 4 and 5 years old, and were confirmed PPD skin test negative prior to use. All procedures, including anesthesia and euthanasia, were done in accordance with the AVMA Guidelines on Euthanasia and approved under the animal study proposal LPD-25E.
Anesthesia procedures.
All procedures were performed with the animals anesthetized following an overnight fast. For short procedures, such as blood draws, bronchioalveolar lavages, ETC, animals were sedated with either ketamine (10 mg/kg, IM) or Telazol (3-6 mg/kg, IM). For procedures that required longer anesthetic time, the animals were sedated with the sedatives previous mentioned, and then intubated and started on an inhalant anesthetic, isoflurane (1 to 3%).
Vaccinations.
BCG-Pasteur used in this study was provided by Aeras (Rockville, MD). For intradermal vaccination, animals were anesthetized and 100 μL of diluted bacteria was instilled between the epidermis and dermis. Animals 06J and DFWV received 5.3 × 104 CFU (CFU while 0FA and DFZH received 7.9 × 105) CFU For gavage vaccination, animals were anesthetized, and a gavage tube was inserted through the pharynx and into the stomach. A total of 15mls of 1M sodium bicarbonate was administered to neutralize stomach acid. After 5 min, 10mls of diluted BCG were administered and the tube withdrawn. Animals were monitored to ensure they did not regurgitate the inoculum. Animals 062 and DFOB received 6.7 × 108cfu, while animals 06Z and DFWW received 3.8x109 CFU.
Lymph node biopsy collection.
The right or left axillary region was palpated to identify the presence of a lymph node. Surgical preparation of the area was performed, which included clipping the hair around the axillary region and 3 alternating scrubs with chlorhexidine scrub and chlorhexidine solution. A #10 surgical blade was used to incise the skin, and blunt dissection was performed to isolate the lymph node. The node was excised and placed in transport media. The biopsy site was closed with absorbable suture material and bupivacaine was instilled at the site to reduce any pain or discomfort secondary to the procedures. Three axillary lymph node biopsies were performed at 2 weeks, 4 weeks, and 12 weeks postinoculation with BCG.
The mesenteric lymph nodes were collected by laparoscopy. The animal was intubated and placed in a surgical plane of anesthesia using isoflurane. The ventral abdomen is surgically prepared by clipping the hair from the xyphoid to the pubis, and then 3 alternating surgical scrubs were performed using Chlorhexidine scrub and Chlorhexidine solution. Two access ports were created in the ventral abdomen to insert the laparoscopic camera and biopsy forceps. Once the mesenteric lymph node was identified, the node is grasped with the forceps and exteriorized through the port. The vessels attached to the mesenteric lymph node was ligated, and the node was excised. After 2 mesenteric lymph nodes were collected, the access ports were removed and the abdominal wall was closed in a 2-layer suture pattern using absorbable suture material. Bupivacaine was instilled into the surgical site to alleviate any pain or discomfort, and a long-acting buprenorphine (Buprenex SR 0.2 mg/kg) was given subcutaneously. Mesenteric lymph nodes were collected at 2 weeks, 4 weeks, and 12 weeks post BCG inoculation.
BAL.
Animals were provided 100% oxygen via a pediatric nasal cannula. Silicone tubing was advanced into the trachea to the level of the bronchiole bifurcation. A total of 35-50 mLs of PBS was instilled in the lungs at 15 mL increments. After each 15 mLs PBS instillation, aspiration was gently performed to collect fluid from the lungs. Once the fluid had been recovered, the silicone tubing was removed, and the animal was monitored until full recovery. BAL samples were collected weekly during the 12-week study.
Cell isolations and stimulations.
Blood was collected in EDTA tubes, and peripheral blood mononuclear cells (PBMCs) were isolated using 90% ficoll-paque density centrifugation (GE Life Sciences). BAL samples were collected as stated above and samples were passed through a 100 μm cell strainer, and counted for analysis. Lymph nodes were dissociated using an OctoMacs cell dissociator (Miltenyi Biotech). Cells were stimulated for 6 h at 37°C in X-Vivo 15 media supplemented with 10% fetal calf serum (FCS) in either media alone, media with PPD (50 μg/mL), or media with the MTB300 MHC-II peptide megapool (2 μg/mL), all in the presence of brefeldin A and monensin (eBioscience).
Flow cytometry.
Cells were stained with fluorochrome-labeled monoclonal antibodies, following stimulation, with the exception of chemokine receptors which were added during stimulation. Surface antigens were stained in PBS + 1% FCS + 0.1% sodium azide for 20 min at 4°C, followed by live/dead staining with a fixable viability dye. Cells were then fixed/permeabilized with the Foxp3 Transcription Factor Staining Buffer Kit (eBioscience), and then stained for intracellular antigens for 30 min at 4°C. Samples were acquired on an LSRFortessa (BD Biosciences), and data were analyzed using FlowJo 9 and 10. Lymphocytes were gated on by excluding dead cells and doublets, and were CD3+.
Antibody enzyme-linked immunosorbent assay.
Half-well 96-well plates were coated with M. tuberculosis H37Rv lysate at 10 μg/mL for 1 h at 37°C. Plates were washed five times with PBS with 0.05% Tween 20 (PBSt), and blocked with 3% nonfat milk overnight at 4°C. Plates were washed with PBSt five times. BAL fluid was diluted in 1% milk in 2-fold serial dilutions and added to the plates, and incubated for 1 h at 37°C. Following incubation, plates were washed five times in PBSt and secondary antibody (Goat anti-monkey IgG-HRP [H&L] from Novus Biologicals) was diluted in 1% milk and added at 0.33 μg/mL, and incubated for 1 h at 37°C. Following incubation, plates were washed 5 times with PBSt, and One-step Ultra TMB enzyme-linked immunosorbent assay (ELISA) substrate Solution (Thermo scientific) was added for development and the reaction was stopped with 0.5N sulfuric acid. OD was then measured at 450 nm and analyzed using GraphPad Prism.
ACKNOWLEDGMENTS
We thank Dominick Laddy for providing the BCG used in this study. We thank members of the NIAID DIR flow cytometry core facility, and all animal husbandry and technical staff at the NIH Animal Center in Poolesville, MD for their help and contributions to this study.
This work was supported by the Intramural Research Program of the NIAID/National Institutes of Health (NIH). D.L.B., S.G.H., D.F.H., and K.D.K. conceived and designed the study. S.G.H., K.D.K., and S.S. performed experiments. D.J.L. provided critical reagents. R.H. provided veterinary support and animal technical oversight. D.F.H. provided critical insight into protocol for gavage infections. K.D.K., R.H., and D.L.B. wrote the manuscript.
Contributor Information
Daniel L. Barber, Email: barberd@niaid.nih.gov.
Stefan H.E. Kaufmann, Max Planck Institute for Infection Biology
REFERENCES
- 1.Organization WH. 2022. Global tuberculosis report 2022. https://www.who.int/publications/i/item/9789240061729. Accessed December 1 2022.
- 2.Dockrell HM, McShane H. 2022. Tuberculosis vaccines in the era of Covid-19 - what is taking us so long? EBioMedicine 79:103993. doi: 10.1016/j.ebiom.2022.103993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, Mabwe S, Makhethe L, Erasmus M, Toefy A, Mulenga H, Hanekom WA, Self SG, Bekker LG, Ryall R, Gurunathan S, DiazGranados CA, Andersen P, Kromann I, Evans T, Ellis RD, Landry B, Hokey DA, Hopkins R, Ginsberg AM, Scriba TJ, Hatherill M, Team CS, C-040–404 Study Team . 2018. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, Pokkali S, Swanson PA, Grant NL, Rodgers MA, Kamath M, Causgrove CM, Laddy DJ, Bonavia A, Casimiro D, Lin PL, Klein E, White AG, Scanga CA, Shalek AK, Roederer M, Flynn JL, Seder RA. 2020. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A, Basaraba R, Clark S, Hall G, Rayner E, Williams A, Marsh PD, Dennis M. 2016. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 101:174–190. doi: 10.1016/j.tube.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White AD, Sarfas C, West K, Sibley LS, Wareham AS, Clark S, Dennis MJ, Williams A, Marsh PD, Sharpe SA. 2015. Evaluation of the immunogenicity of Mycobacterium bovis BCG delivered by aerosol to the lungs of macaques. Clin Vaccine Immunol 22:992–1003. doi: 10.1128/CVI.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, Kocken CHM, Ottenhoff THM, Kondova I, Khayum MA, Haanstra KG, Vierboom MPM, Verreck FAW. 2019. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med 25:255–262. doi: 10.1038/s41591-018-0319-9. [DOI] [PubMed] [Google Scholar]
- 8.Manjaly Thomas ZR, McShane H. 2015. Aerosol immunisation for TB: matching route of vaccination to route of infection. Trans R Soc Trop Med Hyg 109:175–181. doi: 10.1093/trstmh/tru206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagranderie M, Chavarot P, Balazuc AM, Marchal G. 2000. Immunogenicity and protective capacity of Mycobacterium bovis BCG after oral or intragastric administration in mice. Vaccine 18:1186–1195. doi: 10.1016/s0264-410x(99)00386-2. [DOI] [PubMed] [Google Scholar]
- 10.Clark SO, Kelly DL, Badell E, Castello-Branco LR, Aldwell F, Winter N, Lewis DJ, Marsh PD. 2010. Oral delivery of BCG Moreau Rio de Janeiro gives equivalent protection against tuberculosis but with reduced pathology compared to parenteral BCG Danish vaccination. Vaccine 28:7109–7116. doi: 10.1016/j.vaccine.2010.07.087. [DOI] [PubMed] [Google Scholar]
- 11.Ancelet LR, Aldwell FE, Rich FJ, Kirman JR. 2012. Oral vaccination with lipid-formulated BCG induces a long-lived, multifunctional CD4(+) T cell memory immune response. PLoS One 7:e45888. doi: 10.1371/journal.pone.0045888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldwell FE, Tucker IG, de Lisle GW, Buddle BM. 2003. Oral delivery of Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in mice. Infect Immun 71:101–108. doi: 10.1128/IAI.71.1.101-108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abolhassani M, Lagranderie M, Chavarot P, Balazuc AM, Marchal G. 2000. Mycobacterium bovis BCG induces similar immune responses and protection by rectal and parenteral immunization routes. Infect Immun 68:5657–5662. doi: 10.1128/IAI.68.10.5657-5662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoft DF, Xia M, Zhang GL, Blazevic A, Tennant J, Kaplan C, Matuschak G, Dube TJ, Hill H, Schlesinger LS, Andersen PL, Brusic V. 2018. PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4(+) T cell transcriptomal molecular signatures. Mucosal Immunol 11:486–495. doi: 10.1038/mi.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindestam Arlehamn CS, McKinney DM, Carpenter C, Paul S, Rozot V, Makgotlho E, Gregg Y, van Rooyen M, Ernst JD, Hatherill M, Hanekom WA, Peters B, Scriba TJ, Sette A. 2016. A quantitative analysis of complexity of human pathogen-specific CD4 T cell responses in healthy M. tuberculosis infected South Africans. PLoS Pathog 12:e1005760. doi: 10.1371/journal.ppat.1005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singhania A, Dubelko P, Kuan R, Chronister WD, Muskat K, Das J, Phillips EJ, Mallal SA, Seumois G, Vijayanand P, Sette A, Lerm M, Peters B, Lindestam Arlehamn C. 2021. CD4+CCR6+ T cells dominate the BCG-induced transcriptional signature. EBioMedicine 74:103746. doi: 10.1016/j.ebiom.2021.103746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, Kim Y, Sidney J, James EA, Taplitz R, McKinney DM, Kwok WW, Grey H, Sallusto F, Peters B, Sette A. 2013. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog 9:e1003130. doi: 10.1371/journal.ppat.1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burel JG, Lindestam Arlehamn CS, Khan N, Seumois G, Greenbaum JA, Taplitz R, Gilman RH, Saito M, Vijayanand P, Sette A, Peters B. 2018. Transcriptomic analysis of CD4(+) T cells reveals novel immune signatures of latent tuberculosis. J Immunol 200:3283–3290. doi: 10.4049/jimmunol.1800118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 20.Donald P, Kaufmann S, Thee S, Mandalakas AM, Lange C. 2022. Pathogenesis of tuberculosis: the 1930 Lübeck disaster revisited. Eur Respir Rev 31:1–13. doi: 10.1183/16000617.0046-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox GJ, Orlova M, Schurr E. 2016. Tuberculosis in newborns: the lessons of the “Lübeck Disaster” (1929–1933). PLoS Pathog 12:e1005271. doi: 10.1371/journal.ppat.1005271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benevolo-de-Andrade TC, Monteiro-Maia R, Cosgrove C, Castello-Branco LR. 2005. BCG Moreau Rio de Janeiro: an oral vaccine against tuberculosis–review. Mem Inst Oswaldo Cruz 100:459–465. doi: 10.1590/s0074-02762005000500002. [DOI] [PubMed] [Google Scholar]
- 23.Hoft DF, Brown RM, Belshe RB. 2000. Mucosal bacille Calmette-Guérin vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-gamma responses. Clin Infect Dis 30 Suppl 3:S217–S222. doi: 10.1086/313864. [DOI] [PubMed] [Google Scholar]
- 24.Brown RM, Cruz O, Brennan M, Gennaro ML, Schlesinger L, Skeiky YA, Hoft DF. 2003. Lipoarabinomannan-reactive human secretory immunoglobulin A responses induced by mucosal bacille Calmette-Guérin vaccination. J Infect Dis 187:513–517. doi: 10.1086/368096. [DOI] [PubMed] [Google Scholar]
- 25.Habtezion A, Nguyen LP, Hadeiba H, Butcher EC. 2016. Leukocyte trafficking to the small intestine and colon. Gastroenterology 150:340–354. doi: 10.1053/j.gastro.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora JR, von Andrian UH. 2006. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol 27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Campbell DJ, Butcher EC. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med 195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med 201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mtb-specific CD8 T cell and IgG responses in the BAL. (A) BAL cells from week 10 postinfection were restimulated with MTB300, and the frequency of IFNγ+TNF+ CD8 T cells were measured by intracellular cytokine staining. (B) Mtb lysate-specific IgG responses in the BAL fluid were measured at baseline (black lines) and week 10 (ID: magenta and gavage: blue lines). Download FIG S1, PDF file, 0.4 MB (429.7KB, pdf) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.


