Abstract
Hypertension in midlife contributes to cognitive decline and is a modifiable risk factor for dementia. The relationship between late-life hypertension and dementia is less clear. We have investigated the relationship of blood pressure and hypertensive status during late life (after 65 years) to post-mortem markers of Alzheimer’s disease (amyloid-β and tau loads); arteriolosclerosis and cerebral amyloid angiopathy; and to biochemical measures of ante-mortem cerebral oxygenation (the myelin-associated glycoprotein:proteolipid protein-1 ratio, which is reduced in chronically hypoperfused brain tissue, and the level of vascular endothelial growth factor-A, which is upregulated by tissue hypoxia); blood–brain barrier damage (indicated by an increase in parenchymal fibrinogen); and pericyte content (platelet-derived growth factor receptor β, which declines with pericyte loss), in Alzheimer’s disease (n = 75), vascular (n = 20) and mixed dementia (n = 31) cohorts. Systolic and diastolic blood pressure measurements were obtained retrospectively from clinical records. Non-amyloid small vessel disease and cerebral amyloid angiopathy were scored semiquantitatively. Amyloid-β and tau loads were assessed by field fraction measurement in immunolabelled sections of frontal and parietal lobes. Homogenates of frozen tissue from the contralateral frontal and parietal lobes (cortex and white matter) were used to measure markers of vascular function by enzyme-linked immunosorbent assay. Diastolic (but not systolic) blood pressure was associated with the preservation of cerebral oxygenation, correlating positively with the ratio of myelin-associated glycoprotein to proteolipid protein-1 and negatively with vascular endothelial growth factor-A in both the frontal and parietal cortices. Diastolic blood pressure correlated negatively with parenchymal amyloid-β in the parietal cortex. In dementia cases, elevated late-life diastolic blood pressure was associated with more severe arteriolosclerosis and cerebral amyloid angiopathy, and diastolic blood pressure correlated positively with parenchymal fibrinogen, indicating blood–brain barrier breakdown in both regions of the cortex. Systolic blood pressure was related to lower platelet-derived growth factor receptor β in controls in the frontal cortex and in dementia cases in the superficial white matter. We found no association between blood pressure and tau. Our findings demonstrate a complex relationship between late-life blood pressure, disease pathology and vascular function in dementia. We suggest that hypertension helps to reduce cerebral ischaemia (and may slow amyloid-β accumulation) in the face of increasing cerebral vascular resistance, but exacerbates vascular pathology.
Keywords: hypertension, Alzheimer’s disease, vascular dementia, cerebral ischaemia, blood–brain barrier
Tayler et al. report a complex relationship between high blood pressure in late life (≥65 years), vascular function and Alzheimer’s disease pathology. High blood pressure in late life may help to maintain brain perfusion and oxygenation and reduce amyloid-β plaque at the expense of increased arteriolosclerosis and blood–brain barrier damage.
See Magaki and Vinters (https://doi.org/10.1093/braincomms/fcad127) for a scientific commentary on this article.
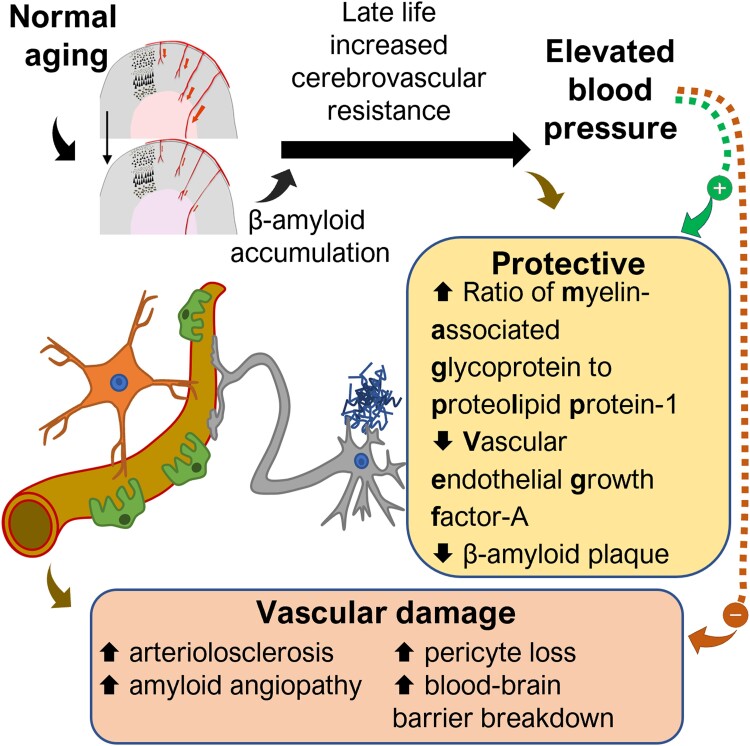
Graphical Abstract
Graphical Abstract.
See Magaki and Vinters (https://doi.org/10.1093/braincomms/fcad127) for a scientific commentary on this article.
Introduction
Hypertension in midlife contributes to cognitive decline and is a modifiable risk factor for dementia. The Honolulu-Asia study found a robust association between midlife hypertension (≥160/95 mmHg) at 45–68 years and clinical diagnosis of vascular dementia and Alzheimer’s disease in late life.1 The relative risk for dementia after midlife hypertension is 1.6 [95% confidence interval (CI) 1.2–2.2].2 Targeting raised blood pressure (BP) in midlife may protect against later cognitive decline and dementia, including Alzheimer’s disease.3–5
Hypertension is also a strong risk factor for stroke, which is itself responsible for cognitive impairment and dementia in 15–70% of survivors.6,7 Sustained hypertension in midlife is associated with increased small vessel disease, white matter (WM) hyperintensities,8 cerebral microinfarcts9 and reduced brain volume.10–12 The burden of cerebral small vessel disease increases with the duration of hypertension.13 Sustained high BP in midlife causes structural and functional cerebral vascular changes that, over time, increase the risk of inadequate perfusion of the brain, cognitive decline and dementia (reviewed14). Blood–brain barrier (BBB) leakiness, endothelial injury and impaired neurovascular coupling, due in part to pericyte damage, are probable early contributors to the onset of small vessel disease and cognitive decline.15
Elevated midlife BP may also be associated with increased amyloid-β (Aβ) and tau pathology in Alzheimer’s disease. In the Honolulu-Asia aging study, elevated systolic BP (SBP) (≥160 mmHg) was associated with increased cortical and hippocampal neuritic plaque load and elevated diastolic BP (DBP) (≥95 mmHg) with an increase in neurofibrillary tangles.1 Similar findings were reported in other studies.16,17 Elevated midlife DBP was associated with higher plasma Aβ in the Honolulu-Asia study.18 Another study found a U-shaped relationship between midlife SBP (but not DBP) and plaque density.19 We previously reported that Aβ plaque load was increased in hypertensive subjects.20 The Systolic Blood Pressure Intervention Trial - Memory and Cognition IN Decreased Hypertension (SPRINT MIND) clinical trial (n = 9361, mean age 67) found that intensive versus standard control of BP (SBP < 120 versus < 140 mmHg) did not significantly lower the rate of probable dementia but was associated with a reduced risk of mild cognitive impairment.21 However, that trial covered a wide age range, from 50 years upwards (28.2% of participants were ≥75 years).
The relationship of late-life hypertension to dementia and its associated pathologies is less clear-cut. Arvanitakis et al.22 found an association between mean late-life SBP (but not DBP) and tangle but not plaque pathology. In some studies, late-life hypertension did not influence the risk of cognitive decline and Alzheimer’s disease5,23–25; in others, higher SBP, lower DBP and higher BP variability were all associated with higher dementia risk.26–29 Verghese et al.30 reported that elevated BP was protective against dementia in those over 70. Several large longitudinal studies have documented a decline in BP in the years immediately preceding dementia. In middle-aged women over 37 years (n = 707), SBP declined more steeply over a 5-year period in those who developed dementia than in those who did not.31 Steep declines in BP preceding onset of dementia or clinical diagnosis of Alzheimer’s disease were also observed in the Kungsholmen project32,33 and in those over 78 years in the Adult Changes in Thought Study (n = 2356).34 Cheng et al.35 calculated that the risk of developing dementia was highest when hypertension was ‘stabilized’ and lowest in participants with normal BP who developed hypertension over the 6-year study period. In very late life, hypertension is protective against dementia.36,37 Although in several studies the treatment of hypertension in midlife reduced the long-term risk or progression of dementia,26,38,39 in frail or elderly patients, particularly if already cognitively impaired, lowering SBP below 130 mmHg was associated with increased mortality.40
In the present study, the objectives were to better understand the complex interactions between late-life BP and markers of Alzheimer’s disease and vascular pathology in a large pathological study. We have used a combination of histological and biochemical methods to analyse the relationships between late-life (after 65 years) SBP and DBP, Alzheimer’s disease pathology (Aβ and tau), non-amyloid small vessel disease (arteriolosclerosis) and cerebral amyloid angiopathy, and markers of cerebral vascular function: ante-mortem cerebral oxygenation [vascular endothelial growth factor-A (VEGF-A) and myelin-associated glycoprotein:proteolipid protein-1 (MAG:PLP1)], BBB integrity [fibrinogen (FGA)] and pericyte content [platelet-derived growth factor receptor β (PDGFRB)].
Materials and methods
Study cohort
Post-mortem human brain tissue was obtained from the South West Dementia Brain Bank (SWDBB), University of Bristol, UK. The study was approved by the management committee of the SWDBB (Human Tissue Authority licence number 12273) under the terms of Bristol Research Ethics Committee approval (18/SW/0029). The right cerebral hemisphere had previously been fixed in buffered formalin for 3 weeks and was used for pathological assessment. The left cerebral hemisphere had been sliced and frozen at −80°C. The frontal cortex (FC) (Brodmann area 6) and superficial underlying WM and parietal cortex (PC) (Brodmann area 7) and superficial underlying WM were used in this study. Most of the brains had been dissected within 72 h of death (Table 1).
Table 1.
Summary of cohort data
| Controls | Alzheimer’s disease | Mixed | Vascular dementia | |||||
|---|---|---|---|---|---|---|---|---|
| N = 100 | N = 75 | N = 31 | N = 20 | |||||
| Age (y) | 84 (78–90) | 80 (76–85) | 88 (84–92) | 83.5 (77.5–89) | ||||
| Sex (F:M) | 47:53 | 37:38 | 22:9 | 10:10 | ||||
| Post-mortem delay (h) | 43 (33–56) | 35 (22–53) | 36 (24–46) | 43 (29–58) | ||||
| Age of dementia onset (y) | — | 73 (66–80) | 79 (73–87) | 79 (71–86) | ||||
| Duration of dementia (y) | — | 7 (3–10) | 8 (5–11) | 4 (2–6) | ||||
| Braak tangle stage | ||||||||
| 0-II | 82 | 0 | 0 | 15 | ||||
| III-IV | 18 | 12 | 11 | 5 | ||||
| V-VI | 0 | 63 | 20 | 0 | ||||
| BP readings (n) | 19 (8–40) | 10 (4–16) | 23 (15–35) | 8 (5–16) | ||||
| Late-life DBP mean (mmHg) | 78 (73–83) | 80 (76–87) | 81 (74–85) | 80 (78–84) | ||||
| Late-life SBP mean (mmHg) | 140 (130–149) | 142 (130–150) | 148 (136–152) | 145 (133–157) | ||||
| Pre-dementia DBP mean (mmHg) | 81 (77–89) | 87 (80–91) | 85 (83–87) | |||||
| N = 32 | N = 26 | N = 5 | ||||||
| Pre-dementia SBP mean (mmHg) | 143 (127–151) | 148 (135–160) | 145 (144–150) | |||||
| N = 32 | N = 26 | N = 5 | ||||||
| Arteriolosclerosis score | Frontal | Parietal | Frontal | Parietal | Frontal | Parietal | Frontal | Parietal |
| 0 | 18 | 24 | 7 | 11 | 1 | 1 | 0 | 0 |
| 1 | 41 | 43 | 38 | 26 | 6 | 5 | 1 | 4 |
| 2 | 30 | 18 | 22 | 21 | 17 | 19 | 9 | 7 |
| 3 | 5 | 3 | 7 | 5 | 7 | 5 | 10 | 8 |
| Cerebral amyloid angiopathy score | Frontal | Parietal | Frontal | Parietal | Frontal | Parietal | Frontal | Parietal |
| 0 | 69 | 62 | 19 | 21 | 13 | 14 | 16 | 14 |
| 1 | 13 | 13 | 22 | 20 | 4 | 6 | 1 | 2 |
| 2 | 13 | 15 | 21 | 22 | 8 | 4 | 3 | 2 |
| 3 | 5 | 5 | 13 | 11 | 6 | 7 | 0 | 0 |
Age, post-mortem delay, BP readings and DBP and SBP means are presented as median and IQR. Arteriolosclerosis scores were not available for n = 6 controls and n = 1 Alzheimer’s disease case (frontal) and n = 12 controls, n = 12 Alzheimer’s disease, n = 1 mixed and n = 1 vascular dementia cases (parietal). Cerebral amyloid angiopathy scores were not available for n = 5 controls, n = 1 Alzheimer’s disease and n = 2 vascular dementia cases (parietal only). Mean late-life DBP and SBP were calculated from all available BP measurements after the age of 65 for every subject. Pre-dementia DBP and SBP means were calculated, where possible, from all available BP measurements between the age of 65 and 5 years prior to dementia diagnosis.
Abbreviations: DBP = diastolic BP; mixed = mixed Alzheimer’s/vascular dementia; SBP = systolic BP.
The age-matched control brains (n = 100) were from donors with no history of dementia, few or absent neuritic plaques, a Braak tangle stage of III or less and no other neuropathological abnormalities. The Alzheimer’s disease cases (n = 75) had a clinical diagnosis of dementia made during life and either intermediate or high Alzheimer’s disease neuropathological change according to the National Institute on Aging - Alzheimer’s Association (NIA-AA) guidelines.41 The vascular dementia cases (n = 20) had a clinical history of dementia with histopathological evidence of multiple infarcts/ischaemic lesions and moderate to severe atheroma and/or arteriosclerosis and only occasional neuritic plaques. The Alzheimer’s/vascular mixed dementia cases (n = 31) had multiple infarcts/ischaemic lesions and moderate to severe atheroma and/or arteriosclerosis but also fulfilled the criteria for Alzheimer’s disease.
Mean late-life DBP and SBP were calculated from all available BP measurements after the age of 65. The average number of BP readings was 18, and the minimum number of BP measurements for inclusion was 2. Mean pre-dementia DBP and SBP were calculated (where possible) from all available BP measurements between the age of 65 and 5 years prior to diagnosis. All brain donations to the SWDBB were considered at the time of case selection (2016) and included if (i) the neuropathological criteria were met; (ii) there were clinical data available from National Health Service (NHS) records obtained post-mortem (i.e. BP readings); and (iii) frozen tissue from the selected brain regions was available. The demographic and clinical features of the cohort are shown in Table 1.
Scoring severity of cerebral amyloid angiopathy and arteriolosclerosis
Cerebral amyloid angiopathy scores were determined in pan-Aβ (4G8) antibody-labelled paraffin sections of frontal and parietal lobes (as for Aβ parenchymal load assessment). Cerebral amyloid angiopathy scores (0–3) represent the extent of amyloid deposition in the cerebral vessels, from none to severe, as previously reported42 and based on the method of Olichney et al.43
The severity of arteriolosclerosis was scored in haematoxylin- and eosin-stained paraffin sections of frontal and parietal lobes. The scores were based on the level of arteriolar vessel wall thickening and associated luminal narrowing in the subcortical WM, on a 0–3 scale representing none, mild, moderate and severe disease, as previously reported.42,44
Assessment of parenchymal Aβ and tau load by immunohistochemistry
Parenchymal Aβ and phospho-tau loads were determined in formalin-fixed paraffin-embedded sections of FC and PC, labelled by a standard 3,3′-diaminobenzidine (DAB) horseradish peroxidase method in a Ventana BenchMark ULTRA automated immunostainer (Roche Tissue Diagnostics, USA), with either a pan-Aβ 4G845 antibody (1:8000) or an AT8 phospho-tau46 antibody (1:500). Field fraction analysis of the area of section immunopositive for the relevant antigen was performed using image analysis software (Image-Pro Plus 7, Media Cybernetics, USA), as previously reported.42
Preparation of homogenates for biochemical assays
Brain tissue samples were diluted 20% w/v in 1% sodium dodecyl sulphate (SDS) lysis buffer [1% w/v SDS, 0.1 M sodium chloride (NaCl), 0.01 M Tris hydrochloride (Tris-HCl) (pH 7.6), 1 µg/ml of aprotinin and 1 µM phenylmethylsulphonyl fluoride (PMSF)] and homogenized with 5–10 silica beads (2.3 mm diameter) in a Precellys homogenizer (2 × 15 s at 6000 rpm). Homogenates were aliquoted and stored at −80°C prior to use. All subsequent biochemical assays were conducted blinded to the clinical and pathological variables.
Enzyme-linked immunosorbent assay measurement of Aβ40 and Aβ42
Insoluble (guanidine-extracted) tissue homogenates were prepared as previously described.42,47–50 Aβ40 and Aβ42 were measured by sandwich enzyme-linked immunosorbent assay (ELISA) (DAB140, DAB142; R&D Systems, USA) according to the manufacturer’s instructions. Assays on cortical brain homogenates were performed at a 1:4000 dilution and on WM homogenates at 1:2500.
Enzyme-linked immunosorbent assay measurement of myelin-associated glycoprotein and proteolipid protein-1
The concentration of MAG was determined by in-house direct ELISA and the concentration of PLP1 by sandwich ELISA (PLP1, SEA417Hu; Cloud-Clone Corp., USA/China), as previously described.42,44,47,48,51
Enzyme-linked immunosorbent assay measurement of vascular endothelial growth factor-A
The VEGF-A level was measured by sandwich ELISA (Human VEGF DuoSet, DY293B, R&D Systems, Oxford, UK) as in our previous studies.47–49
Enzyme-linked immunosorbent assay measurement of fibrinogen
FGA was measured by sandwich ELISA (EH3057; FineTest, China) according to the manufacturer’s instructions (and as in our previous studies42,49). To allow comparison of parenchymal FGA (i.e. to adjust for blood content), we also measured haemoglobin (Hb) levels by colorimetric assay (700540; Cayman Chemicals, USA), in a modified 384-well format, at a 1:10 dilution in triplicate, as previously described.42 Sample Hb content was interpolated from the linear standard (0.016–0.4 g/dl). The FGA level was adjusted for Hb in each sample relative to a mean adult blood Hb of 14 g/dl (e.g. if a sample contained 0.14 g/dl of Hb, 1% of the FGA was assumed to be of haematogenous origin), although in practice this had minimal impact on the data.
Enzyme-linked immunosorbent assay measurement of platelet-derived growth factor receptor β
PDGFRB was measured by sandwich ELISA (Human Total PDGFRB DuoSet DYC385, R&D systems, Oxford, UK), as previously.49
Statistical analysis
Statistical analyses and graphs were produced using either GraphPad Prism (version 9 GraphPad Software LLC, USA) or Stata (version MP 17.0, StataCorp LLC, USA). All data sets were assessed for normality of distribution and, if not normally distributed, were either log-transformed or analysed by a non-parametric test, as appropriate. The statistical tests used are indicated in the text.
Results
We studied 226 cases: 75 Alzheimer’s disease, 20 vascular dementia, 31 mixed Alzheimer’s disease/vascular dementia and 100 age-matched controls. The age at death differed slightly between the groups (Kruskal–Wallis test with Dunn’s post-test): Alzheimer’s disease cases were younger (P = 0.0006) than the controls. Gender distribution was evenly distributed across controls, Alzheimer’s disease and vascular dementia groups. The mixed dementia group included proportionately more females, but the difference was not statistically significant (χ2 (df 3) = 5.6697, P = 0.129). The groups were matched approximately for post-mortem delay. The age of dementia onset and duration of disease are shown in Table 1. The age of onset of dementia was youngest in Alzheimer’s disease cases (Dunn’s: Alzheimer’s disease versus mixed, P = 0.0055) and the duration of dementia shortest in vascular dementia cases (Dunn’s: Alzheimer’s disease versus vascular dementia, P = 0.0247; mixed versus vascular dementia, P = 0.0059).
The Alzheimer’s disease and vascular dementia cases had fewer recorded BP measurements; this may reflect the shorter duration of vascular dementia and the lower age of death of Alzheimer’s disease cases than controls.
Mean SBP and DBP and interquartile range (IQR) values for each group are presented in Table 1. DBP was higher in dementia cases than controls in an age- and sex-adjusted model (linear regression: F (3, 222) = 4.97, P = 0.0023 with R2 of 0.0529) (Supplementary Table 2: model 1). DBP was also higher in the Alzheimer’s disease and vascular dementia subtypes (F (5, 220) = 3.27, P = 0.0072; Supplementary Table 2: model 2). As BP may fall after dementia diagnosis, we also looked at the BP data for individuals to compare pre-dementia late-life DBP and SBP (i.e. BP measurements from after the age of 65 years but at least 5 years prior to diagnosis) to late-life BP in controls. The data available for this secondary analysis were limited because BP measurements were not always recorded between the age of 65 and dementia diagnosis, and in some cases, dementia commenced before or at 70 years. However, pre-dementia measurements of both DBP and SBP were higher in dementia cases overall (P < 0.001 and P = 0.021, respectively; n = 137; Supplementary Table 3: model 1), and DBP separately in Alzheimer’s disease (P = 0.047), mixed dementia (P < 0.001) and vascular dementia cases (P = 0.013) and SBP was also higher in vascular dementia cases (P = 0.037), although it should be noted that the small size of the ‘pure’ vascular dementia cohort limits the power of this last subgroup analysis (Supplementary Table 3: model 2).
Elevated late-life blood pressure was associated with more severe arteriolosclerosis and cerebral amyloid angiopathy scores
Donors with dementia and elevated late-life DBP had higher frontal and parietal arteriolosclerosis scores. The effect of higher DBP was significant in an ordered logistic regression model, adjusting for the effects of age and dementia subtype (adjusted OR per additional frontal arteriolosclerosis score point 1.07, 95% CI 1.03–1.11, P = 0.001; parietal arteriolosclerosis score point 1.05, 95% CI 1.02–1.09, P = 0.007; Table 2 and Supplementary Fig. 1). Similarly, donors with elevated late-life SBP had higher frontal and parietal arteriolosclerosis scores (adjusted OR per additional frontal arteriolosclerosis score point 1.03, 95% CI 1.01–1.05, P = 0.001; parietal arteriolosclerosis score point 1.02, 95% CI 1.01–1.04, P = 0.010; Supplementary Fig. 1 and Supplementary Table 4).
Table 2.
Ordered logistic regression for arteriolosclerosis score in relation to DBP by dementia subtype
| Variables | Unadjusted analysis | Adjusted analysis | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | ||
| Frontal arteriolosclerosis score | |||||||
| Cohort | Alzheimer’s disease | 1.38 | 0.78–2.44 | 0.275 | 1.31 | 0.71–2.40 | 0.382 |
| Mixed | 5.69 | 2.62–12.4 | <0.001 | 5.41 | 2.52–11.6 | <0.001 | |
| Vascular dementia | 19.1 | 7.53–48.2 | <0.001 | 17.0 | 6.39–45.5 | <0.001 | |
| Age (y) | 1.03 | 1.00–1.06 | 0.074 | 1.04 | 1.00–1.07 | 0.030 | |
| DBP (after 65 years) | 1.07 | 1.04–1.10 | <0.001 | 1.07 | 1.03–1.11 | 0.001 | |
| Parietal arteriolosclerosis score | |||||||
| Cohort | Alzheimer’s disease | 2.09 | 1.11–3.94 | 0.022 | 2.28 | 1.18–4.40 | 0.014 |
| Mixed | 8.79 | 4.19–18.4 | <0.001 | 8.19 | 3.91–17.2 | <0.001 | |
| Vascular dementia | 18.1 | 5.74–57.3 | <0.001 | 17.0 | 4.63–62.3 | <0.001 | |
| Age (y) | 1.04 | 1.01–1.07 | 0.022 | 1.05 | 1.01–1.08 | 0.005 | |
| DBP (after 65 years) | 1.06 | 1.03–1.09 | <0.001 | 1.05 | 1.01–1.09 | 0.007 | |
Frontal n = 219, parietal n = 200.
Abbreviations: CI = confidence interval; DBP = diastolic blood pressure; y = years.
The unadjusted ordered logistic regression analysis shows the effects of each of the separate variables (DBP, disease subtype, age) on frontal arteriolosclerosis score. The adjusted analysis is the same regression of DBP on frontal arteriolosclerosis score in the presence of the covariates, age and disease subtype. Significant P-values are denoted in bold.
SBP was associated with frontal cerebral amyloid angiopathy severity in an ordered logistic regression model, adjusting for the effects of age and dementia subtype (adjusted OR per additional frontal cerebral amyloid angiopathy score point 1.02, 95% CI 1.00–1.03, P = 0.038; Supplementary Table 5), but not parietal cerebral amyloid angiopathy severity. DBP was not significantly associated with cerebral amyloid angiopathy severity (not shown).
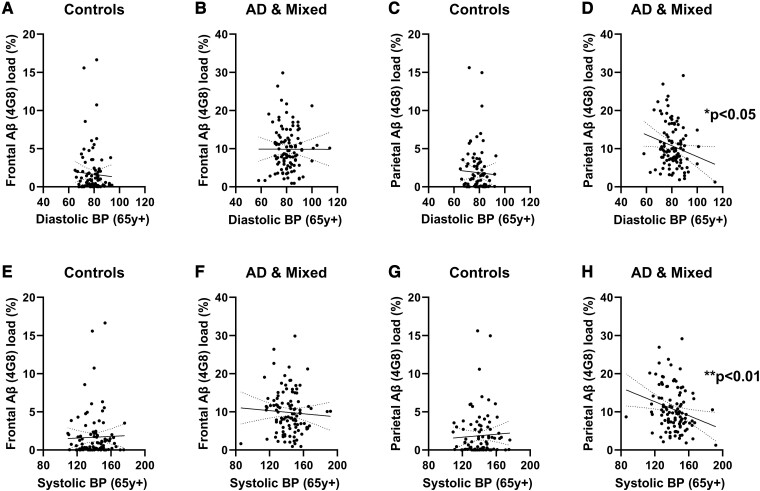
Elevated late-life diastolic blood pressure was associated with lower parenchymal Aβ load
In Alzheimer’s disease and mixed dementia, late-life DBP correlated negatively with parenchymal Aβ load in the PC (r = −0.2050, P = 0.0368; Fig. 1D) as did late-life SBP (r = −0.2570, P = 0.0084; Fig. 1H), but these relationships were not significant in controls (Fig. 1C and G) or in either group in the FC (Fig. 1A, B, D and E). We did not find associations between late-life BP and levels of insoluble Aβ40 or Aβ42 in tissue homogenates, or tau load in paraffin sections, in either brain region (data not shown).
Figure 1.
Elevated DBP and SBP in late life were associated with reduced parenchymal Aβ load in the PC in Alzheimer's disease and mixed dementia. No relationship was seen between DBP and frontal Aβ load in (A) controls (Spearman’s correlation, n = 97, r = −0.0043, ns) or in (B) Alzheimer’s disease and mixed cases (n = 106, Spearman’s r = −0.0398, ns). No relationship was seen between DBP and parietal Aβ load in (C) controls (n = 92, r = −0.02337, ns). However, late-life DBP was associated with lower parietal Aβ (4G8) load in (D) Alzheimer’s disease and mixed dementia cases (n = 104, r = −0.2050, P = 0.0368). Late-life SBP did not correlate with frontal Aβ load in either (E) controls (n = 97, r = 0.05707, ns) or (F) Alzheimer’s and mixed dementia cases (n = 106, r = −0.1189, ns). Late-life SBP also did not correlate with parietal Aβ load in (G) controls (n = 92, r = 0.0907, ns). However, in Alzheimer’s disease and mixed dementia cases, late-life SBP correlated with lower (H) parietal Aβ load (n = 104, r = − 0.2570, P = 0.0084). Each point represents a single case. The continuous and interrupted lines indicate the best-fit linear regression and 95% CIs. Abbreviations: Ad = Alzheimer’s disease; BP = blood pressure; FC = frontal cortex; PC = parietal cortex; y = years.
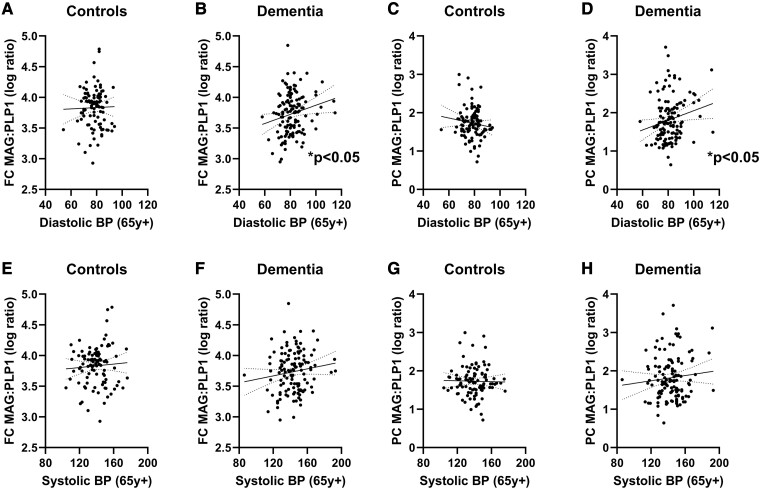
Elevated late-life diastolic blood pressure was associated with less biochemical evidence of reduced oxygenation
In all dementia cases (i.e. combining Alzheimer’s disease, mixed and vascular dementia) but not controls, MAG:PLP1 in the FC correlated positively with DBP (Pearson’s r = 0.1985, P = 0.0259, n = 126) and PC (r = 0.1971, P = 0.0282, n = 124) but not with SBP (Fig. 2). MAG:PLP1 in frontal WM or parietal WM was not related to DBP or SBP (Supplementary Fig. 2).
Figure 2.
Elevated DBP in late life was associated with higher cortical MAG:PLP1 in dementia. Late-life DBP was not significantly associated with FC MAG:PLP1 in (A) controls (Pearson’s correlation, ns, n = 99) but was associated with higher (B) FC MAG:PLP1 (Pearson’s r = 0.1985, P = 0.0259, n = 126) in dementia cases. Similarly, late-life DBP was not associated with (C) PC MAG:PLP1 ratios in controls (ns, n = 99) but correlated positively with (D) PC MAG:PLP1 (r = 0.1971, P = 0.0282, n = 124) ratios in dementia cases. Late-life SBP did not correlate with MAG:PLP1 in dementia cases or controls in either brain region (E–H; ns). Each point represents a single case. The continuous and interrupted lines indicate the best-fit linear regression and 95% CIs. Abbreviations: BP = blood pressure; FC = frontal cortex; MAG:PLP1 = myelin-associated glycoprotein:proteolipid protein-1; PC = parietal cortex; y = years.
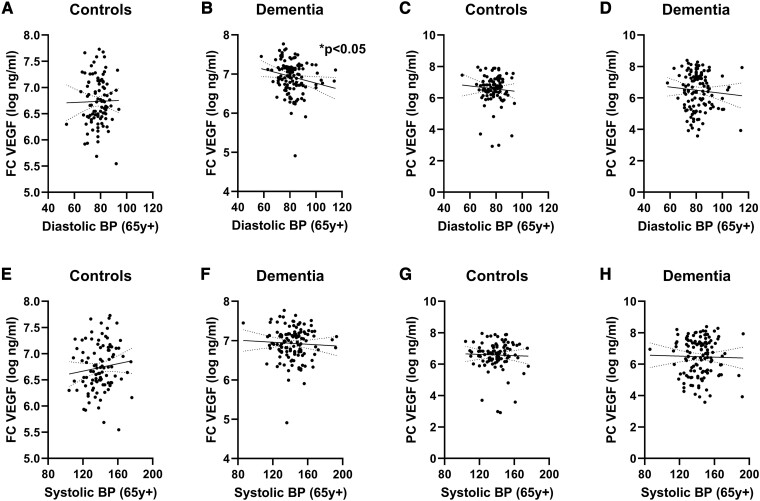
In the FC (Pearson’s r = −0.1909, P = 0.0322; Fig. 3B) but not the PC (ns; Fig. 3D), the VEGF level, which increases in response to recent brain ischaemia, correlated negatively with late-life DBP in dementia cases. The VEGF level did not correlate in either brain region with late-life DBP in controls (both ns; Fig. 3A and C). VEGF levels did not correlate with late-life SBP in controls or dementia cases in either brain region (ns; Fig. 3E–H).
Figure 3.
Elevated DBP in late life was associated with lower VEGF in the FC in dementia. No relationship was seen between DBP and (A) FC VEGF in controls (Pearson’s correlation, ns, n = 99). Late-life DBP was associated with lower (B) FC VEGF (Pearson’s r = −0.1909, P = 0.0322, n = 126) in dementia cases. No relationship was seen between DBP and PC VEGF in (C) controls (ns, n = 97) or in (D) dementia cases (ns, n = 125). Late-life SBP did not correlate with VEGF in controls or dementia cases in either brain region (E–H; ns). Each point represents a single case. The continuous and interrupted lines indicate the best-fit linear regression and 95% CIs. Abbreviations: BP = blood pressure; FC = frontal cortex; PC = parietal cortex; VEGF = vascular endothelial growth factor; y = years.
The VEGF level in the frontal WM also correlated negatively with DBP in dementia cases (r = −0.2267, P = 0.0107; Supplementary Fig. 3) but not controls. Again, the relationships with SBP were not significant. VEGF in the parietal WM did not vary with respect to DBP or SBP in dementia or control cases.
Elevated late-life diastolic and systolic blood pressure was associated with blood–brain barrier leakiness in dementia
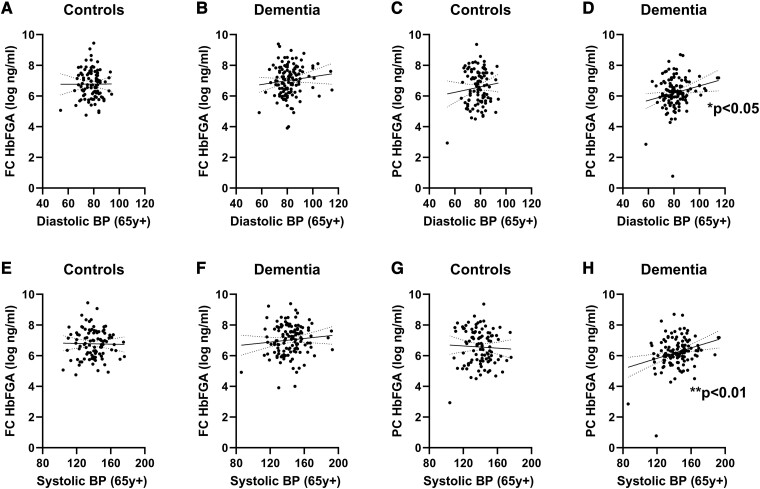
In the PC, the FGA level (adjusted for Hb content) correlated positively with late-life DBP in the combined dementia cohort (Pearson’s r = 0.2119, P = 0.0186). This correlation was not seen in the FC in dementia cases (ns) or in controls in either brain region (both ns) (Fig. 4). Late-life SBP also correlated with Hb-adjusted FGA levels in the PC in dementia cases (r = 0.2719, P = 0.0023) but not controls (ns). There was no correlation in the FC in either dementia cases or controls (both ns).
Figure 4.
Elevated DBP and SBP in late life were associated with higher parietal cortical Hb-adjusted FGA levels in dementia. No relationship was seen between DBP and FC HbFGA in (A) controls (Pearson’s correlation, ns, n = 99) or in (B) dementia cases (ns, n = 126). Similarly, no significant relationship was found between DBP and PC HbFGA in (C) controls (ns, n = 99). Late-life DBP was associated with lower (D) PC HbFGA (Pearson’s r = 0.2119, P = 0.0186, n = 123). There was no relationship between late-life SBP and FC HbFGA in either (E) controls (ns) or (F) dementia cases (ns). There was no relationship between late-life SBP and PC HbFGA in (G) controls (ns). However, late-life SBP correlated positively with PC HbFGA in (H) dementia cases (Pearson’s r = 0.2719, P = 0.0023). Each point represents a single case. The continuous and interrupted lines indicate the best-fit linear regression and 95% CIs. Abbreviations: BP = blood pressure; FC = frontal cortex; HbFGA = haemoglobin-adjusted fibrinogen; PC = parietal cortex; y = years.
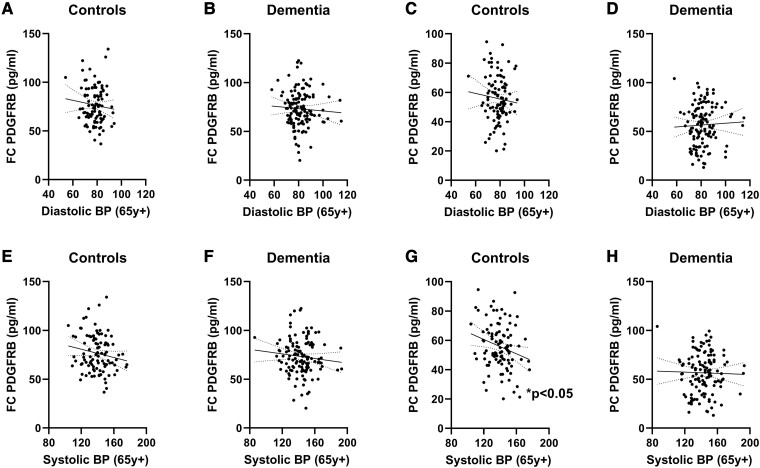
Lower late-life systolic blood pressure was associated with pericyte damage in dementia in frontal white matter
In the FC and PC, PDGFRB did not correlate with DBP in either controls or dementia cases (all ns; Fig. 5A–D). Late-life SBP correlated negatively with PC PDGFRB in controls (r = −0.2290, P = 0.0219; Fig. 5G) but not dementia cases (ns; Fig. 5H). Late-life SBP did not correlate with FC PDGFRB in either controls or dementia cases (both ns; Fig. 5E and F).
Figure 5.
Elevated SBP in late life was associated with higher PC PDGFRB levels in controls. Late-life DBP did not correlate (Pearson’s correlation) with (A) FC PDGFRB in controls or (B) dementia cases or with (C) PC PDGFRB in controls or (D) dementia cases. There were no significant correlations between late-life SBP and FC PDGFRB in either (E) controls or (F) dementia cases (both ns). Late-life SBP negatively correlated with PC PDGFRB in (G) controls (Pearson’s r = −0.2290, P = 0.0219, n = 100) but not (H) dementia cases (ns, n = 126). Abbreviations: BP = blood pressure; FC = frontal cortex; PC = parietal cortex; PDGFRB = platelet-derived growth factor receptor β; y = years.
In the frontal and parietal WM, PDGFRB did not correlate with DBP in controls or dementia cases (all ns; Supplementary Fig. 4A–D). Late-life SBP correlated negatively with frontal WM PDGFRB in dementia cases (r = −0.1821, P = 0.0413; Supplementary Fig. 4F) but not controls (ns; Supplementary Fig. 4E) and not in the parietal WM in either group (both ns; Supplementary Fig. 4G–H).
Discussion
In this study, we have investigated the relationship between late-life BP and markers of disease and cerebral vascular pathology and dysfunction in people with dementia. We have found late-life BP to be associated with lower parenchymal Aβ load and with biochemical markers of lower ischaemic damage of the cerebral cortex, i.e. may be protective against cerebral hypoperfusion and Aβ accumulation. The relationship between BP and dementia risk is complex. A recent Mendelian randomization study using UK Biobank data found that high BP was associated with reduced late-life risk of developing Alzheimer’s disease.52 Our pathological observations support recent studies showing that elevated BP in late life may be protective against Alzheimer’s disease.26–30
Our study has weaknesses. The repeated BP measurements were obtained retrospectively from clinical records. Fewer BP measurements were recorded in dementia cases. In addition, changes in the diagnosis and management of hypertension between 1985 and 2019, the period during which the brains for this study were donated, are likely to have impacted the frequency of BP recordings in some cases. A strength of this study is that we have collected all available data from clinical records and used the average of many late-life BP measurements for each individual in our analyses. Generally, in our cohort, BP was monitored more regularly, and hypertension was treated more often for the more recent donations. Some of the regression modelling for this study was limited by the small number of cases in each group, particularly since there were few pure vascular dementia cases, which limited the statistical power of our analyses. We also noted some moderate differences in the average age of the groups. We included age and disease subtype as covariates in our regression modelling partly because of the known associations with arteriolosclerosis and cerebral vascular function.
We found that elevated DBP was associated with more severe arteriolosclerosis in dementia cases. This is in keeping with evidence of a relationship between the severity of arteriolosclerosis and DBP.22 Previous studies demonstrated that longer-term exposure to hypertension strengthened the association with cerebral small vessel disease burden.13 Higher late-life BP was also associated with an increased number of brain infarcts, and exposure to vascular risk factors, including hypertension, adversely affected WM integrity.53 Muller et al.54 found an association between higher late-life BP and increased risk of WM lesions and cerebral microbleeds in older participants; the effect was most pronounced in those without a history of midlife hypertension.
In the frontal lobe, the severity of cerebral amyloid angiopathy correlated with SBP. Other neuropathological studies did not find SBP to be associated with cerebral amyloid angiopathy severity.55,56 However, arterial hypertension is a frequent comorbidity in people with clinically diagnosed cerebral amyloid angiopathy57 and has been shown to interact with severe cerebral amyloid angiopathy to increase the risk of infarction.58 In Tg2576 mice, Aβ pathology promoted hypertension-induced intracerebral haemorrhage,59 and BP-lowering strategies were suggested as a means to reduce the risk of recurrent cerebral amyloid angiopathy-associated intracerebral haemorrhage.60,61 However, another study found that hypertensive cerebral amyloid angiopathy patients had lower mortality than normotensive patients.62 In hypertensive rats, severe small vessel disease promoted the development of cerebral amyloid angiopathy.63
Observational studies have shown that elevated SBP may be protective in older individuals and may be induced as a response to a decline in brain vascular health.64,65 Cerebroventricular infusion of Aβ40 in rats significantly elevated BP and exacerbated existing hypertension, probably via modulation of autonomic activity.66 In cognitively normal elderly individuals, Aβ deposition (measured by florbetapir-Aβ PET) and WM hyperintensity volume both affected rates of neurodegeneration, but these effects were independent with no evidence of interaction.67 Both arteriolosclerosis and cerebral amyloid angiopathy lead to luminal narrowing and increased cerebral vascular resistance, which puts the brain at risk of hypoperfusion. An elevation in BP in response to these vascular alterations may be a physiological adaptation that allows the brain to maintain cerebral perfusion (and maintain clearance of Aβ).68 The late-life decline in BP that often precedes the onset of dementia may reflect the decompensation of these adaptive mechanisms for maintaining cerebral perfusion (perhaps contributing to Aβ accumulation).
Damage and leakiness of the BBB are associated with cognitive decline in the early stages of Alzheimer’s disease69 and vascular mild-cognitive impairment70 and underpin the development of small vessel disease.71 We recently found that BBB breakdown was elevated in controls and dementia cases with terminal systemic infection72 and that BBB damage was more severe in mixed vascular/Alzheimer’s dementia than in pure Alzheimer’s or vascular dementia cases.42 In the present study, FGA levels correlated positively with DBP, in keeping with other evidence of a link between hypertension and BBB damage.73–75 Dysfunction of the BBB is likely to be but one manifestation of hypertensive vessel wall damage that impairs the clearance of metabolites and impacts Aβ accumulation in Alzheimer’s disease. Damage to pericytes probably contributes to BBB damage in the early stages of Alzheimer’s disease.69,76 Here, we show that elevated SBP was inversely related to PDGFRB content in the FC in controls and in the superficial WM in Alzheimer’s disease.
In the present study, we have shown that elevated DBP in individuals aged 65 years and over correlated negatively with parenchymal Aβ load, specifically in the PC. This contrasts with previous reports of a positive relationship between hypertension and elevated parenchymal Aβ and tau; however, participants in those studies had midlife hypertension.1 Our findings also suggest that high BP in late life may protect against some other Alzheimer’s disease-associated changes, including chronic cerebral hypoperfusion. This is evidenced by the higher MAG:PLP1 ratio (see Tayler et al.,42 Barker et al.44, 51 and Miners et al.48,49 and reviewed in Love and Miners77,78) in brain donors with a history of elevated late-life DBP and the lower levels of VEGF-A.47 We previously showed that cerebral hypoperfusion in Alzheimer’s disease is due in part to elevated expression of potent vasoconstrictors, including endothelin-148,79 and angiotensin-II.80,81 Aβ peptides themselves have direct and indirect vasoconstrictor actions.82–85 In contrast, we found no evidence of a relationship between DBP and tangle pathology. This supports previous data.22
Conclusion
In conclusion, there is a complex relationship between late-life BP and markers of vascular and Alzheimer’s disease pathology in dementia. Elevated late-life DBP is associated with biochemical markers, which indicate lower ischaemia and less accumulation of Aβ, but also with more severe vascular damage, including arteriolosclerosis, cerebral amyloid angiopathy, BBB breakdown and pericyte loss. Late-life elevated BP may be a physiological response to maintain cerebral oxygenation in response to increased cerebral vascular resistance—an adaptation that maintains cerebral blood flow and Aβ homeostasis but, when sustained over a longer period, exacerbates vascular pathology until eventually BP declines, preceding the onset of clinical disease.
Supplementary Material
Acknowledgements
We thank the SWDBB for providing brain tissue for this study. The SWDBB is part of the Brains for Dementia Research programme, jointly funded by Alzheimer’s Research UK and Alzheimer’s Society, and is supported by BRACE (Bristol Research into Alzheimer’s and Care of the Elderly) and the Medical Research Council. We sincerely thank undergraduate placement student Bethany Reichelt and master’s student Amina Chabach for their contributions to support the work of this project.
Abbreviations
- Aβ =
amyloid-β
- BBB =
blood–brain barrier
- BP =
blood pressure
- CI =
confidence interval
- DAB =
diaminobenzidine
- DBP =
diastolic blood pressure
- FC =
frontal cortex
- FGA =
fibrinogen
- Hb =
haemoglobin
- MAG =
myelin-associated glycoprotein
- MAG:PLP1 =
myelin-associated glycoprotein:proteolipid protein-1
- mixed =
mixed dementia
- NaCl =
sodium chloride
- NHS =
National Health Service (UK)
- NIA-AA =
National Institute on Aging - Alzheimer’s Association
- PC =
parietal cortex
- PDGFRB =
platelet-derived growth factor receptor β
- PMSF =
phenylmethylsulphonyl fluoride
- SBP =
systolic blood pressure
- SDS =
sodium dodecyl sulphate
- SPRINT MIND =
Systolic Blood Pressure Intervention Trial - Memory and Cognition IN Decreased Hypertension
- SWDBB =
South West Dementia Brain Bank
- Tris-HCl =
Tris hydrochloride
- VEGF-A =
vascular endothelial growth factor-A
- WM =
white matter
Contributor Information
Hannah M Tayler, Dementia Research Group, Institute of Clinical Neurosciences, Bristol Medical School, University of Bristol, Bristol, BS10 5NB, UK.
Robert MacLachlan, Dementia Research Group, Institute of Clinical Neurosciences, Bristol Medical School, University of Bristol, Bristol, BS10 5NB, UK.
Özge Güzel, Dementia Research Group, Institute of Clinical Neurosciences, Bristol Medical School, University of Bristol, Bristol, BS10 5NB, UK.
J Scott Miners, Dementia Research Group, Institute of Clinical Neurosciences, Bristol Medical School, University of Bristol, Bristol, BS10 5NB, UK.
Seth Love, Dementia Research Group, Institute of Clinical Neurosciences, Bristol Medical School, University of Bristol, Bristol, BS10 5NB, UK.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This study was funded by Alzheimer’s Research UK (ARUK-PG2015-11).
Competing interests
The authors report no competing interests.
Data availability
The UK Brain Banks Network identifiers of the brains that were used in this study are listed in (Supplementary Table 1). The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Launer LJ, Ross GW, Petrovitch H, et al. . Midlife blood pressure and dementia: The Honolulu-Asia aging study. Neurobiol Aging. 2000;21(1):49–55. [DOI] [PubMed] [Google Scholar]
- 2. Livingston G, Huntley J, Sommerlad A, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottesman RF, Schneider AL, Zhou Y, et al. . Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skoog I, Lernfelt B, Landahl S, et al. . 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–1145. [DOI] [PubMed] [Google Scholar]
- 5. Yu JT, Xu W, Tan CC, et al. . Evidence-based prevention of Alzheimer's disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91(11):1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rost NS, Meschia JF, Gottesman R, et al. . Cognitive impairment and dementia after stroke: Design and rationale for the DISCOVERY study. Stroke. 2021;52(8):e499–e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubow J, Fink ME. Impact of hypertension on stroke. Curr Atheroscler Rep. 2011;13(4):298–305. [DOI] [PubMed] [Google Scholar]
- 8. Wartolowska KA, Webb AJS. Midlife blood pressure is associated with the severity of white matter hyperintensities: Analysis of the UK Biobank cohort study. Eur Heart J. 2021;42(7):750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. Cerebral small vessel disease and stage 1 hypertension defined by the 2017 American College of Cardiology/American Heart Association Guidelines. Hypertension. 2019;73(6):1210–1216. [DOI] [PubMed] [Google Scholar]
- 10. Lane CA, Barnes J, Nicholas JM, et al. . Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): An epidemiological study. Lancet Neurol. 2019;18(10):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S, Choi SH, Lee YM, et al. . Periventricular white matter hyperintensities and the risk of dementia: A CREDOS study. Int Psychogeriatr. 2015;27(12):2069–2077. [DOI] [PubMed] [Google Scholar]
- 12. Salvado G, Brugulat-Serrat A, Sudre CH, et al. . Spatial patterns of white matter hyperintensities associated with Alzheimer's disease risk factors in a cognitively healthy middle-aged cohort. Alzheimers Res Ther. 2019;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petrea RE, O'Donnell A, Beiser AS, et al. . Mid to late life hypertension trends and cerebral small vessel disease in the Framingham Heart Study. Hypertension. 2020;76(3):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ungvari Z, Toth P, Tarantini S, et al. . Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat Rev Nephrol. 2021;17(10):639–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ozkan E, Cetin-Tas Y, Sekerdag E, et al. . Blood-brain barrier leakage and perivascular collagen accumulation precede microvessel rarefaction and memory impairment in a chronic hypertension animal model. Metab Brain Dis. 2021;36(8):2553–2566. [DOI] [PubMed] [Google Scholar]
- 16. Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC III. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131(2):162–169. [DOI] [PubMed] [Google Scholar]
- 17. Hoffman LB, Schmeidler J, Lesser GT, et al. . Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72(20):1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah NS, Vidal JS, Masaki K, et al. . Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: The Honolulu Asia aging study. Hypertension. 2012;59(4):780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrovitch H, White LR, Izmirilian G, et al. . Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Honolulu-Asia aging study. Neurobiol Aging. 2000;21(1):57–62. [DOI] [PubMed] [Google Scholar]
- 20. Ashby EL, Miners JS, Kehoe PG, Love S. Effects of hypertension and anti-hypertensive treatment on amyloid-beta (Abeta) plaque load and Abeta-synthesizing and Abeta-degrading enzymes in frontal cortex. J Alzheimers Dis. 2016;50(4):1191–1203. [DOI] [PubMed] [Google Scholar]
- 21. Williamson JD, Pajewski NM, Auchus AP, et al. . Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA. 2019;321(6):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arvanitakis Z, Capuano AW, Lamar M, et al. . Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018;91(6):e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scherr PA, Hebert LE, Smith LA, Evans DA. Relation of blood pressure to cognitive function in the elderly. Am J Epidemiol. 1991;134(11):1303–1315. [DOI] [PubMed] [Google Scholar]
- 24. Andre-Petersson L, Hagberg B, Janzon L, Steen G. A comparison of cognitive ability in normotensive and hypertensive 68-year-old men: Results from population study “men born in 1914,” in Malmo, Sweden. Exp Aging Res. 2001;27(4):319–340. [DOI] [PubMed] [Google Scholar]
- 25. Di Carlo A, Baldereschi M, Amaducci L, et al. . Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2000;48(7):775–782. [DOI] [PubMed] [Google Scholar]
- 26. Ou YN, Tan CC, Shen XN, et al. . Blood pressure and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020;76(1):217–225. [DOI] [PubMed] [Google Scholar]
- 27. Cacciatore F, Abete P, Ferrara N, et al. . The role of blood pressure in cognitive impairment in an elderly population. Osservatorio Geriatrico Campano Group. J Hypertens. 1997;15(2):135–142. [DOI] [PubMed] [Google Scholar]
- 28. Vinyoles E, De la Figuera M, Gonzalez-Segura D. Cognitive function and blood pressure control in hypertensive patients over 60 years of age: COGNIPRES study. Curr Med Res Opin. 2008;24(12):3331–3339. [DOI] [PubMed] [Google Scholar]
- 29. Tsivgoulis G, Alexandrov AV, Wadley VG, et al. . Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73(8):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61(12):1667–1672. [DOI] [PubMed] [Google Scholar]
- 31. Joas E, Bäckman K, Gustafson D, et al. . Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59(4):796–801. [DOI] [PubMed] [Google Scholar]
- 32. Qiu C, von Strauss E, Winblad B, Fratiglioni L. Decline in blood pressure over time and risk of dementia: A longitudinal study from the Kungsholmen project. Stroke. 2004;35(8):1810–1815. [DOI] [PubMed] [Google Scholar]
- 33. Qiu C, Winblad B, Fratiglioni L. Low diastolic pressure and risk of dementia in very old people: A longitudinal study. Dement Geriatr Cogn Disord. 2009;28(3):213–219. [DOI] [PubMed] [Google Scholar]
- 34. Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. 2001;58(10):1640–1646. [DOI] [PubMed] [Google Scholar]
- 35. Cheng G, He S, He Q, et al. . Trajectory patterns of blood pressure change up to six years and the risk of dementia: A nationwide cohort study. Aging (Albany NY). 2021;13(13):17380–17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corrada MM, Hayden KM, Paganini-Hill A, et al. . Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimers Dement. 2017;13(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: Longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res. 2007;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 38. Hughes D, Judge C, Murphy R, et al. . Association of blood pressure lowering with incident dementia or cognitive impairment: A systematic review and meta-analysis. JAMA. 2020;323(19):1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rouch L, Cestac P, Hanon O, et al. . Antihypertensive drugs, prevention of cognitive decline and dementia: A systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs. 2015;29(2):113–130. [DOI] [PubMed] [Google Scholar]
- 40. Mossello E. Hypertension, hypotension, longevity and dementia. Monaldi Arch Chest Dis. 2020;90(4). [DOI] [PubMed] [Google Scholar]
- 41. Montine TJ, Monsell SE, Beach TG, et al. . Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer's disease. Alzheimers Dement. 2016;12(2):164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tayler H, Miners JS, Guzel O, MacLachlan R, Love S. Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer's disease, vascular dementia and mixed dementia. Brain Pathol. 2021;31(4):e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olichney JM, Hansen LA, Hofstetter CR, Lee JH, Katzman R, Thal LJ. Association between severe cerebral amyloid angiopathy and cerebrovascular lesions in Alzheimer disease is not a spurious one attributable to apolipoprotein E4. Arch Neurol. 2000;57(6):869–874. [DOI] [PubMed] [Google Scholar]
- 44. Barker R, Wellington D, Esiri MM, Love S. Assessing white matter ischemic damage in dementia patients by measurement of myelin proteins. J Cereb Blood Flow Metab. 2013;33(7):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alafuzoff I, Pikkarainen M, Arzberger T, et al. . Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: A study of the BrainNet Europe consortium. Acta Neuropathol. 2008;115(5):533–546. [DOI] [PubMed] [Google Scholar]
- 46. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas T, Miners S, Love S. Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer's disease and vascular dementia. Brain. 2015;138(Pt 4):1059–1069. [DOI] [PubMed] [Google Scholar]
- 48. Miners JS, Palmer JC, Love S. Pathophysiology of hypoperfusion of the precuneus in early Alzheimer's disease. Brain Pathol. 2016;26(4):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miners JS, Schulz I, Love S. Differing associations between Abeta accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer's disease. J Cereb Blood Flow Metab. 2018;38(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miners JS, Jones R, Love S. Differential changes in Abeta42 and Abeta40 with age. J Alzheimers Dis. 2014;40(3):727–735. [DOI] [PubMed] [Google Scholar]
- 51. Barker R, Ashby EL, Wellington D, et al. . Pathophysiology of white matter perfusion in Alzheimer's disease and vascular dementia. Brain. 2014;137(Pt 5):1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sproviero W, Winchester L, Newby D, et al. . High blood pressure and risk of dementia: A two-sample Mendelian randomization study in the UK Biobank. Biol Psychiatry. 2021;89(8):817–824. [DOI] [PubMed] [Google Scholar]
- 53. Maillard P, Carmichael OT, Reed B, Mungas D, DeCarli C. Cooccurrence of vascular risk factors and late-life white-matter integrity changes. Neurobiol Aging. 2015;36(4):1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muller M, Sigurdsson S, Kjartansson O, et al. . Joint effect of mid- and late-life blood pressure on the brain: The AGES-Reykjavik study. Neurology. 2014;82(24):2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Esiri M, Chance S, Joachim C, et al. . Cerebral amyloid angiopathy, subcortical white matter disease and dementia: Literature review and study in OPTIMA. Brain Pathol. 2015;25(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamada M, Tsukagoshi H, Otomo E, Hayakawa M. Cerebral amyloid angiopathy in the aged. J Neurol. 1987;234(6):371–376. [DOI] [PubMed] [Google Scholar]
- 57. Wagner A, Maderer J, Wilfling S, et al. . Cerebrovascular risk factors in possible or probable cerebral amyloid angiopathy, modifier or bystander? Original research. Front Neurol. 2021;12:676931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Olichney JM, Hansen LA, Hofstetter CR, Grundman M, Katzman R, Thal LJ. Cerebral infarction in Alzheimer's disease is associated with severe amyloid angiopathy and hypertension. Arch Neurol. 1995;52(7):702–708. [DOI] [PubMed] [Google Scholar]
- 59. Passos GF, Kilday K, Gillen DL, Cribbs DH, Vasilevko V. Experimental hypertension increases spontaneous intracerebral hemorrhages in a mouse model of cerebral amyloidosis. J Cereb Blood Flow Metab. 2016;36(2):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biffi A, Anderson CD, Battey TW, et al. . Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015;314(9):904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arima H, Tzourio C, Anderson C, et al. . Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: The PROGRESS trial. Stroke. 2010;41(2):394–396. [DOI] [PubMed] [Google Scholar]
- 62. Zhang S, Wang Z, Zheng A, et al. . Blood pressure and outcomes in patients with different etiologies of intracerebral hemorrhage: A multicenter cohort study. J Am Heart Assoc. 2020;9(19):e016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jandke S, Garz C, Schwanke D, et al. . The association between hypertensive arteriopathy and cerebral amyloid angiopathy in spontaneously hypertensive stroke-prone rats. Brain Pathol. 2018;28(6):844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sabayan B, Wijsman LW, Foster-Dingley JC, et al. . Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ. 2013;347:f4600. [DOI] [PubMed] [Google Scholar]
- 65. van Dalen JW, Brayne C, Crane PK, et al. . Association of systolic blood pressure with dementia risk and the role of age, U-shaped associations, and mortality. JAMA Intern Med. 2022;182(2):142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tayler HM, Palmer JC, Thomas TL, Kehoe PG, Paton JF, Love S. Cerebral Abeta40 and systemic hypertension. J Cereb Blood Flow Metab. 2018;38(11):1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Keuss SE, Coath W, Nicholas JM, et al. . Associations of beta-amyloid and vascular burden with rates of neurodegeneration in cognitively normal members of the 1946 British birth cohort. Neurology. 2022;99(2):e129–e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Warnert EA, Rodrigues JC, Burchell AE, et al. . Is high blood pressure self-protection for the brain? Circ Res. 2016;119(12):e140–e151. [DOI] [PubMed] [Google Scholar]
- 69. Nation DA, Sweeney MD, Montagne A, et al. . Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li M, Li Y, Zuo L, Hu W, Jiang T. Increase of blood-brain barrier leakage is related to cognitive decline in vascular mild cognitive impairment. BMC Neurol. 2021;21(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wardlaw JM, Chappell FM, Valdes Hernandez MDC, et al. . White matter hyperintensity reduction and outcomes after minor stroke. Neurology. 2017;89(10):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Asby D, Boche D, Allan S, Love S, Miners JS. Systemic infection exacerbates cerebrovascular dysfunction in Alzheimer's disease. Brain. 2021;144(6):1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mueller SM, Heistad DD. Effect of chronic hypertension on the blood-brain barrier. Hypertension. 1980;2(6):809–812. [DOI] [PubMed] [Google Scholar]
- 74. Setiadi A, Korim WS, Elsaafien K, Yao ST. The role of the blood-brain barrier in hypertension. Exp Physiol. 2018;103(3):337–342. [DOI] [PubMed] [Google Scholar]
- 75. Katsi V, Marketou M, Maragkoudakis S, et al. . Blood-brain barrier dysfunction: The undervalued frontier of hypertension. J Hum Hypertens. 2020;34(10):682–691. [DOI] [PubMed] [Google Scholar]
- 76. Montagne A, Barnes SR, Sweeney MD, et al. . Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Love S, Miners JS. Cerebral hypoperfusion and the energy deficit in Alzheimer's disease. Brain Pathol. 2016;26(5):607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2016;131(5):645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Palmer JC, Barker R, Kehoe PG, Love S. Endothelin-1 is elevated in Alzheimer's disease and upregulated by amyloid-beta. J Alzheimers Dis. 2012;29(4):853–861. [DOI] [PubMed] [Google Scholar]
- 80. Miners JS, Ashby E, Van Helmond Z, et al. . Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer's disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2008;34(2):181–193. [DOI] [PubMed] [Google Scholar]
- 81. Kehoe PG, Hibbs E, Palmer LE, Miners JS. Angiotensin-III is increased in Alzheimer's disease in association with amyloid-beta and tau pathology. J Alzheimers Dis. 2017;58(1):203–214. [DOI] [PubMed] [Google Scholar]
- 82. Niwa K, Younkin L, Ebeling C, et al. . Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97(17):9735–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281(6):H2417–H2424. [DOI] [PubMed] [Google Scholar]
- 84. Niwa K, Carlson GA, Iadecola C. Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20(12):1659–1668. [DOI] [PubMed] [Google Scholar]
- 85. Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002;9(1):61–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UK Brain Banks Network identifiers of the brains that were used in this study are listed in (Supplementary Table 1). The data that support the findings of this study are available from the corresponding author upon reasonable request.