Abstract
Background:
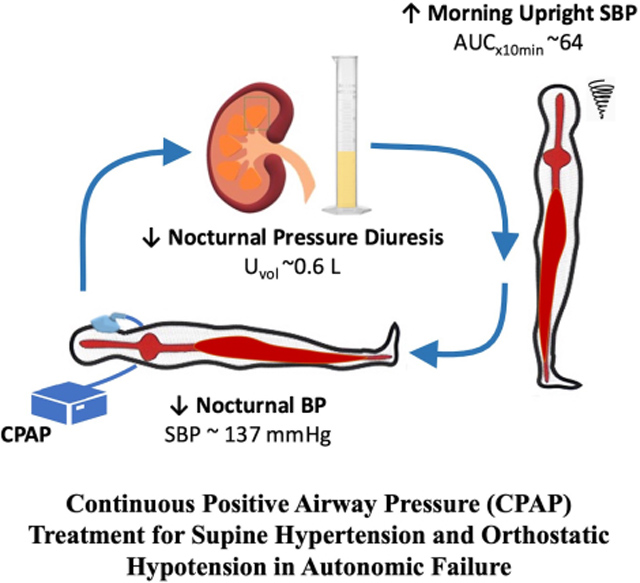
Supine hypertension affects most patients with orthostatic hypotension (OH) due to autonomic failure, but it is often untreated for fear of worsening OH. We hypothesized that increasing intrathoracic pressure with continuous positive airway pressure (CPAP) had a Valsalva-like blood-pressure-lowering effect that could be used to treat nocturnal supine hypertension in these patients, while reducing nocturnal pressure diuresis and improving daytime OH.
Methods:
In Protocol 1, we determined the acute hemodynamic effects of increasing levels of CPAP (0, 4, 8, 12, and 16 cm H2O, 3 minutes each) in 26 patients with autonomic failure and supine hypertension studied while awake and supine. In Protocol 2 (n=11), we compared the effects of overnight therapy with CPAP (8–12 cm H2O for 8 hours) versus placebo on nocturnal supine hypertension, nocturnal diuresis and daytime OH in a 2-night crossover study.
Results:
In Protocol 1, acute CPAP (4–16 cm H2O) decreased systolic blood pressure (SBP) in a dose-dependent manner (maximal drop 22±4 mmHg with CPAP 16) due to reductions in stroke volume (−16+3%) and cardiac output (−14±3%). Systemic vascular resistance and heart rate remained unchanged. In Protocol 2, overnight CPAP lowered nighttime SBP (maximal change −23±5 versus placebo −1±7 mmHg; P=0.023) and was associated with lower nighttime diuresis (609±84 versus placebo 1004±160 mL; P=0.004) and improved morning orthostatic tolerance (AUC upright SBP 642±121 versus placebo 410±109 mmHg*min; P=0.014).
Conclusions:
CPAP is a novel non-pharmacologic approach to treat the supine hypertension of autonomic failure while improving nocturia and daytime OH.
Clinical Trial Registration:
URL: https://clinicaltrials.gov/ct2/show/NCT03312556 Unique identifier: NCT03312556.
Keywords: supine hypertension, autonomic failure, continuous positive airway pressure, orthostatic hypotension
Graphical Abstract
Introduction
The clinical picture of patients with orthostatic hypotension (OH) due to autonomic failure (AF) is dominated by orthostatic intolerance. Most of these patients, however, also have supine hypertension, which is often overlooked because blood pressure (BP) is mostly measured in the seated posture when it is often normal. Even when recognized, supine hypertension often goes untreated because of concern that antihypertensive medications will worsen OH, and the belief that treatment of OH should be prioritized because it carries a higher short-term risk than supine hypertension.1
Supine hypertension, however, is associated with long-term cardiac and renal end-organ damage.2,3 It also induces nocturnal pressure natriuresis and nocturia, resulting in sleep disruption, increased risk for falls, and more importantly, a significant volume loss during the night. Severely affected patients lose on average 1.3 L of urine and 4 g of urinary sodium in one night,4 worsening OH in the morning, i.e., nocturnal supine hypertension begets daytime OH. The reluctance to treat supine hypertension would be overcome if there was a treatment that selectively reduces supine BPs without worsening OH, and that could even improve OH in the morning by reducing nocturnal pressure natriuresis. No antihypertensive medication tested so far has met all these criteria.5–9 Thus, there is an unmet need to develop an alternative approach that would effectively treat nocturnal hypertension while improving nocturia and daytime OH.
Sleeping in a head-up tilt (HUT) position is often recommended to reduce supine hypertension, by inducing splanchnic venous pooling and thus reducing venous return and cardiac output (CO). Tilt levels of ≥ 12° HUT have been shown to decrease nocturnal natriuresis and improve morning OH but are rarely tolerated by patients.10–12 Lesser degrees of HUT are better tolerated, but less effective, and may not suffice in treating the supine hypertension. Increasing intrathoracic pressure while recumbent, with the Valsalva maneuver for example, has similar hemodynamic effects; it induces splanchnic venous pooling, thus reducing venous return, CO, and thoracic blood volume.13 In patients with AF, these effects are amplified by the lack of autonomic compensatory responses and can result in profound drops in BP. We hypothesized, therefore, that increasing intrathoracic pressure could provide an alternative approach to control supine hypertension. We tested this hypothesis by using continuous positive airway pressure (CPAP). We initially determined the acute hemodynamic effects of CPAP in awake patients with supine hypertension due to primary forms of AF by applying increasingly higher positive airway pressures (Protocol 1). We then conducted a proof-of-concept study to determine the effect of nighttime CPAP therapy on BP, nocturia, and morning orthostatic tolerance in AF patients with nocturnal supine hypertension (Protocol 2).
Methods
The data that support the findings of this study will be made available by the corresponding author to any researcher upon reasonable request.
Subjects
Thirty-two patients with severe primary AF (17 with pure autonomic failure, 4 with Parkinson disease, 4 with probable multiple system atrophy, 2 with dementia with Lewy bodies, and 5 with autonomic failure of unknown pathogenesis) were recruited from referrals to Vanderbilt University Autonomic Dysfunction Center. All patients had neurogenic OH and comorbid supine hypertension of ≥150 mmHg in systolic BP (SBP) or ≥90 mmHg in diastolic BP (DBP).14 Fifteen patients participated in Protocol 1, six in Protocol 2 and eleven patients participated in both protocols. Patients were excluded if they had secondary causes of AF (e.g., diabetes mellitus or amyloidosis), if they were bedridden or if they had any significant cardiac or renal illness. The Vanderbilt University Institutional Review Board approved this study, and written informed consent was obtained from each patient before initiating the study.
General Protocol
Patients were studied as inpatients in the Clinical Research Center at Vanderbilt University Medical Center. Medications affecting BP, blood volume, and the autonomic nervous system, including fludrocortisone, pressor drugs, or antihypertensive medications, were withheld for ≥5 half-lives before studies. All other medications were held constant during admission. The screening consisted of a medical history, physical examination, ECG, routine safety laboratory assessments, and standardized autonomic function tests (Valsalva maneuver, respiratory sinus arrhythmia, orthostatic stress test, and supine and upright plasma norepinephrine). All patients were also screened for nocturnal supine hypertension. See Data Supplement for a more detailed description of screening procedures.
Protocol 1: Acute Hemodynamic Effects of Graded Levels of CPAP
Studies were conducted during the daytime, on awake patients in the supine position, in a post-void state, and ≥2.5 hours after meals to avoid any confounding effects from postprandial hypotension. All patients were trained in the method of breathing with CPAP before the studies. BP and HR were monitored continuously with the finger volume clamp method (Nexfin, BMEYE) and ECG, and intermittently with an automated oscillometric sphygmomanometer (Vital-Guard 450C, Ivy Biomedical Systems, Inc, Brandford, CT). After 30 minutes of supine rest, CPAP was applied sequentially at 0, 4, 8, 12, and 16 cm H2O for 3 minutes at each level using a full-face mask connected to a CPAP titration system (REMstar Auto, Respironics Inc.). BP and HR were measured at 1 and 3 minutes on each CPAP level and after 5 minutes of recovery. A second set of CPAP levels was then applied using the same procedures.
In a subset of participants, we included measurements of hemodynamic parameters and relative changes in thoracic and abdominal volumes to assess the mechanisms underlying the BP effects of CPAP (secondary objective). Stroke volume (SV) was estimated using impedance cardiography and pulse contour analysis of the continuous BP curves (Modelflow algorithm), as described previously.15,16 CO was then calculated by multiplying SV by the HR obtained from oscillometric BP measurements. Systemic vascular resistance (SVR) was estimated by dividing oscillometric mean arterial pressure (MAP) by CO. Relative changes in thoracic and abdominal volumes were estimated by segmental impedance.17
Protocol 2: Proof-of-Concept Study to Assess the Efficacy of Overnight CPAP for the Treatment of Nocturnal Supine Hypertension
We compared the effects of overnight therapy with CPAP versus placebo pill in a 2-night crossover study. Studies were conducted from 8 PM to 8 AM, and ≥2.5 hours after the last meal. Patients were instructed to remain supine throughout the night. Fluid intake was restricted to avoid the pressor effect of water drinking.18 BP and HR were measured twice in a row at 2-hour intervals from 8 PM to 8 AM by an automated sphygmomanometer (Dinamap ProCare, GE Healthcare). Urine was collected throughout the night for determination of volume.
CPAP (REMstar Auto, Respironics Inc.) was applied with a full-face mask from 10 PM to 6 AM. The CPAP level was determined for each participant during an acute CPAP trial, similar to Protocol 1, as the highest tolerable CPAP level. The placebo pill was given at 8 PM with 50 mL of tap water. The following morning (8 AM), patients were asked to stand for ≤10 minutes. BP and HR were measured at 1, 3, 5, and 10 minutes of standing, or as tolerated, to assess morning orthostatic tolerance.
Statistical Analysis
In Protocol 1, the primary objective was to test the hypothesis that CPAP decreased supine BP in patients with supine hypertension. The primary outcome was the average supine SBP at each CPAP level. Mixed-effects models were used to examine whether the outcome differed from that at baseline (CPAP 0) and between CPAP levels. When significant, paired comparisons of outcome variables were performed using paired t-tests with Bonferroni correction as post hoc test. In the secondary objective, the changes from baseline in CO, SV, SVR, and thoracic and abdominal volumes were analyzed using the same approach. See Data Supplement for a more detailed description of the statistical analysis, sample size, and power calculations.
In Protocol 2, we hypothesized that overnight CPAP therapy would decrease nocturnal supine BP and urine volume and improve morning orthostatic tolerance compared to placebo. We chose an “antihypertensive” placebo pill, that would supposedly lower BP, as our control rather than sham CPAP, cognizant that patients would not be blinded by the latter approach. The primary outcome was the change from baseline (8 PM) in the supine SBP (ΔSBP) during the intervention period (10 PM – 6 AM). Differences between treatments were analyzed using the same approach described in Protocol 1. To summarize the overnight BP changes, the area under the curve (AUC) for the 7 measurements was calculated for each treatment and compared using Wilcoxon signed-rank tests. Baseline measurements, nocturnal diuresis, and morning orthostatic tolerance, defined as the AUC of standing SBP (AUCSBP) during the orthostatic test at 8 AM (mean upright SBP× standing time]),19 were compared between treatments using Wilcoxon signed-rank tests or paired t-tests depending on the data distribution. Data are presented as mean±SEM unless otherwise noted. All tests were 2-tailed, and a P value of <0.05 was considered significant. Analyses and graphing were performed with SPSS 28.0.1.1 (IBM Corp) and Prism 9 (GraphPad Software, LLC). LO and IB had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Patient Characteristics and Autonomic Testing
A total of 32 patients with severe AF and supine hypertension were enrolled (72±1 years, BMI 27±1 Kg/m2, supine BP 167±4/88±2 mm Hg, 24 men): 26 patients in Protocol 1 and 17 patients in Protocol 2. All participants enrolled in Protocol 1 completed the study. Six of the 17 patients enrolled in Protocol 2 were unable to complete the study because they did not have nocturnal supine hypertension (n=2) or did not tolerate the overnight CPAP therapy (n=4). The patient’s clinical and autonomic characteristics are shown in Tables 1 and 2. A history of essential hypertension preceded the diagnosis of AF in 46% of patients and supine hypertension started after the onset of AF in the remainder. Over one third of patients (36%) who completed the overnight CPAP therapy (Protocol 2) did not have a history of obstructive sleep apnea (OSA). All patients exhibited a profound decrease in BP on standing (OH) without an adequate compensatory increase in HR and had severe impairment of autonomic reflexes (Table 2). Respiratory sinus arrhythmia was blunted, suggesting parasympathetic dysfunction. Evidence of sympathetic dysfunction included an exaggerated decrease in SBP during phase II and the absence of BP overshoot during phase IV of the Valsalva maneuver.
Table 1.
Patient Characteristics
| Parameters, unit | Protocol 1 (n=26) | Protocol 2 (n=11) |
|---|---|---|
|
| ||
| Sex, male/female | 20/6 | 8/3 |
| Age, y | 72 ± 1 | 74 ± 2 |
| BMI, kg/m2 | 27 ± 1 | 27 ± 1 |
| Diagnosis, % (n) | ||
| PAF | 54 (14) | 73 (8) |
| MSA | 15 (4) | - |
| PD+AF | 12 (3) | 18 (2) |
| AF of unknown cause | 12 (3) | 9 (1) |
| DLB | 7 (2) | - |
| Medical history, % (n) | ||
| Essential HTN | 46 (12) | 46 (5) |
| OSA | 31 (8) | 64 (7) |
| Supine | ||
| Systolic BP, mmHg | 168 ± 5 | 179 ± 7 |
| Diastolic BP, mmHg | 88 ± 3 | 91 ± 4 |
| Heart rate, bpm | 66 ± 3 | 58 ± 2 |
| Plasma norepinephrine, pg/mL | 202 ± 39 | 153 ± 27 |
| Upright | ||
| Systolic BP, mmHg | 96 ± 5 | 85 ± 8 |
| Diastolic BP, mmHg | 61 ± 3 | 56 ± 4 |
| Heart rate, bpm | 74 ± 3 | 69 ± 3 |
| Plasma norepinephrine, pg/mL | 285 ± 43 | 249 ± 39 |
Data are presented as mean±SEM. Protocol 1, acute hemodynamic effects of graded levels of CPAP on awake patients with autonomic failure (AF). Protocol 2, overnight treatment with CPAP vs. placebo. AF, autonomic failure; BMI, body mass index; DLB, dementia with Lewy bodies; HTN, hypertension; MSA, multiple system atrophy; PAF, pure AF; PD+AF, Parkinson disease with AF; OSA, obstructive sleep apnea.
Table 2.
Autonomic Function Tests and Orthostatic Stress Test
| Parameters, unit | Protocol 1 (n=26) | Protocol 2 (n=11) | Normals* |
|---|---|---|---|
|
| |||
| Orthostatic change in systolic BP, mmHg | −70 ± 6 | −89 ± 10 | ≤ 20 |
| Orthostatic change in heart rate, bpm | 8 ± 2 | 12 ± 3 | 5 – 10 |
| Sinus arrhythmia ratio | 1.07 ± 0.02 | 1.09 ± 0 | 1.2 ± 0.1 |
| Depressor response to Valsalva in phase II, mmHg | −62 ± 5 | −72 ± 8 | ≤ 20 |
| BP response to Valsalva phase IV, mmHg † | −32 ± 4 | −39 ± 6 | >20 |
| Valsalva ratio | 1.18 ± 0.04 | 1.29 ± 0.1 | 1.5 ± 0.2 |
Values are expressed as mean±SEM. Pressor responses are given as changes in systolic BP.
Normal values are from the Autonomic Dysfunction Database at Vanderbilt University Medical Center.
A negative value for phase IV of the Valsalva maneuver indicates that the BP overshoot was absent.
Protocol 1: Acute Hemodynamic Effects of CPAP
Of the 26 patients studied, all but 4 tolerated all CPAP levels; one patient only tolerated CPAP 4, one patient tolerated ≤CPAP 8 and two patients tolerated ≤CPAP 12 cm H2O. At baseline (CPAP 0), the average supine SBP was 159±4 mm Hg, DBP 86±2 mm Hg, and MAP 110±3 mm Hg. Acute CPAP application significantly decreased supine SBP in a dose-dependent manner (P<0.001 by mixed-effects model; Figure 1). The effect was modest but significant even at CPAP 4 (SBP decreased by 5±1 mm Hg, to 154±4 mm Hg; P=0.001 versus baseline). CPAP 16 produced a SBP drop of 22±4 mmHg (to 139±5 mmHg; P<0.001versus baseline). Supine MAP and DBP followed a similar trend (P<0.001 by mixed-effects model for MAP and DBP; Supplemental Figure S1). The decrease in MAP was statistically significant at CPAP 8 (−4±1 mm Hg), 12 (−7±2 mm Hg), and 16 cm H2O (−10±3 mm Hg; P<0.05 versus baseline; Supplemental Figure S1A) whereas DBP decreased significantly only with CPAP 16 (−4±2 mm Hg; P=0.004 versus baseline; Supplemental Figure S1B). Baseline HR, on the other hand, did not differ significantly from that of any CPAP level (baseline 66±2 bpm versus 66±3, 66±3, 65±3, and 65±3 bpm at CPAP 4, 8, 12, and 16 cm H2O respectively; P=0.3451 by mixed-effects model). Within 5 minutes after removal of the CPAP (recovery), SBP, DBP, and MAP returned to baseline values (1±1, 2±1, and 2±1 mm Hg, respectively).
Figure 1. Acute BP effects of graded levels of CPAP in awake patients with autonomic failure and supine hypertension (Protocol 1).
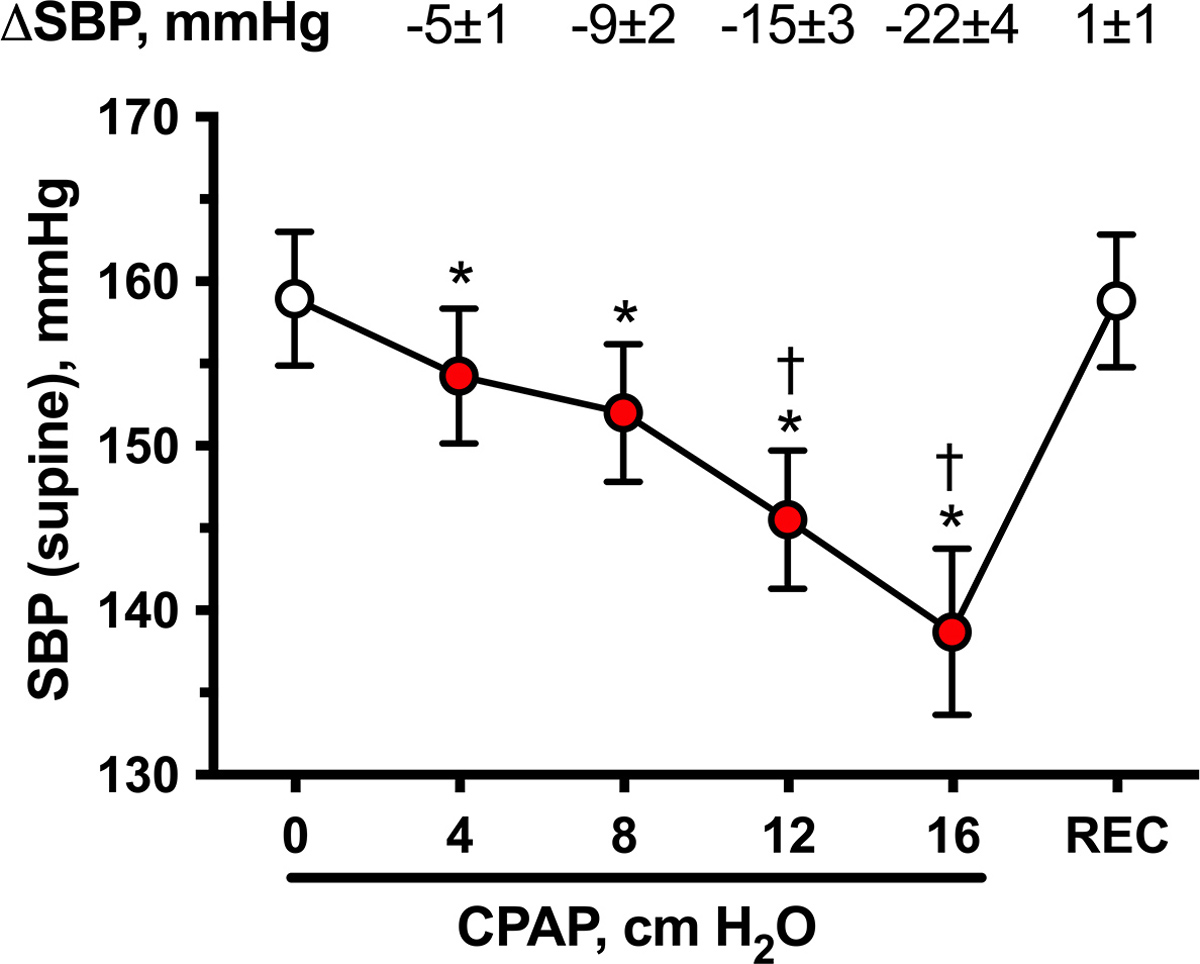
Supine systolic BP (SBP) and their changes from baseline (CPAP 0; ΔSBP) during increasing levels of CPAP (4, 8, 12, and 16 cm H2O for 3 minutes each) and immediately after recovery (REC). CPAP produced an acute dose-dependent decrease in SBP. Values are expressed as mean±SEM. Overall differences were analyzed by a mixed-effects model (P<0.001). *P<0.05 versus baseline (CPAP 0) and †P<0.05 versus the preceding CPAP level, adjusted for multiple comparisons using Bonferroni correction.
Systemic hemodynamics were measured in 19 patients and thoracic and abdominal volumes in 8 (Figure 2). The dose-dependent BP-lowering effect of CPAP was associated with a similar dose-dependent decrease in SV and CO (P<0.001 by mixed-effects model for both; Figure 2A and 2B). Baseline SV (89±5 mL) significantly decreased with CPAP 8, 12, and 16 cm H2O (−8±2, −11±3, and −16±3%, respectively; P<0.001versus baseline for all comparisons). CO followed a similar trend; baseline CO (4.5±0.2 L/min) decreased with CPAP 8, 12, and 16 cm H2O (−8±2, −11±3, and −14±3%, respectively; P<0.001versus baseline for all comparisons). Neither SVR nor HR changed significantly with any CPAP level (Figure 2D and 2E). Increasing CPAP levels induced a decrease in thoracic volume and an increase in the abdominal volume in a dose-dependent manner (P<0.001 by mixed-effects model for both (Figure 2C and 2F). CPAP 8, 12, and 16 cm H2O significantly decreased relative thoracic volumes (−88±36, −133±43, and −186±49 mL, respectively; P<0.050 versus baseline for all comparisons) and increased abdominal volumes (53±13, 71±20, and 92±27 mL; P<0.05 versus baseline for all comparisons).
Figure 2. Acute hemodynamic effects of graded levels of CPAP in awake patients with autonomic failure and supine hypertension (Protocol 1).
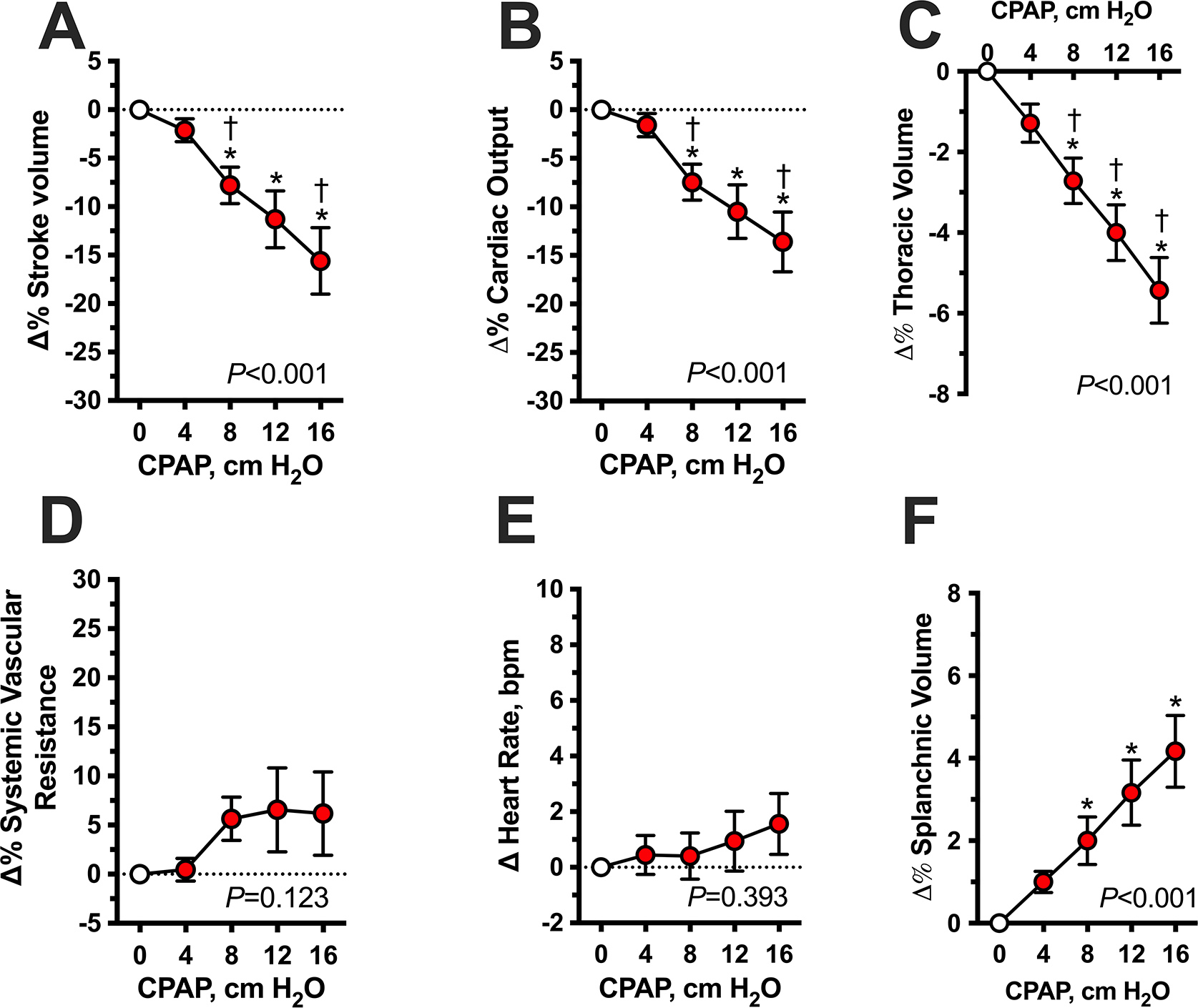
Hemodynamic parameters are expressed as percent changes (Δ%) from baseline (CPAP 0) during increasing levels of CPAP (4, 8, 12 and 16 cm H2O for 3 minutes each). CPAP produced an acute dose-dependent decrease in stroke volume (A), cardiac output (B) and thoracic volume (C) with a concomitant increase in abdominal volume (F). Systemic vascular resistance (D) and heart rate (E) did not change significantly. Values are expressed as mean±SEM. P values for overall differences were analyzed by mixed-effects model. *P<0.05 versus baseline (CPAP 0) and †P<0.05 versus the preceding CPAP level, adjusted for multiple comparisons using Bonferroni.
Protocol 2: Effects of Overnight CPAP Therapy on Nocturnal Supine Hypertension
Eleven patients completed both treatment arms; 7 patients had an overnight CPAP level of 10 cm H2O, 2 patients had CPAP 12 cm H2O and 2 patients had 8 cm H2O based on their CPAP tolerance during the acute CPAP trial. Baseline supine SBP (8 PM) was similar between treatment arms (CPAP: 160±8 versus placebo: 162±5 mmHg; P=1.000). Overnight CPAP therapy significantly decreased supine SBP compared with placebo (P=0.044 for the main treatment effect, mixed-effects model; Figure 3A), as was the AUC of SBP changes from baseline during CPAP (P=0.032 versus placebo, Figure 3B). The maximal reduction in SBP during overnight CPAP therapy was 23±5 mmHg at 4 hours (2 AM) post-intervention (versus −1±7 mmHg for placebo; P=0.023). DBP and MAP followed a similar trend but did not reach significance (P=0.875 and P=0.402 for treatment effect, respectively, mixed-effects model). HR had a small but statistically significant decrease with overnight CPAP at 10 PM (−7±2 bpm versus placebo 1±2 bpm; P=0.045).
Figure 3. Effect of overnight CPAP therapy on nighttime supine systolic BP (Protocol 2).
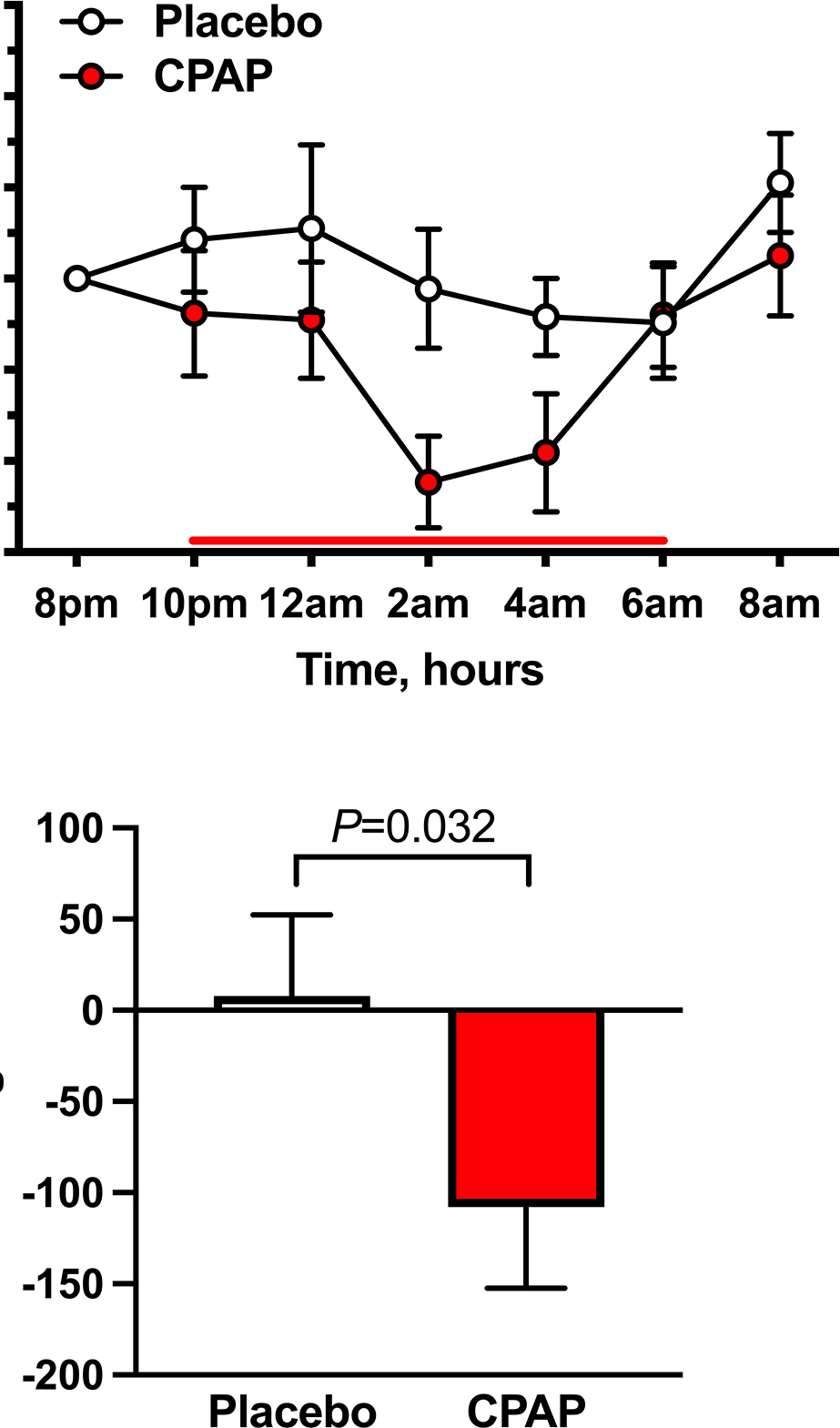
Overnight CPAP therapy was applied from 10 PM – 6 AM (bold line) to AF patients with nocturnal supine hypertension. Placebo pill was given at 8 PM. CPAP therapy decreased nighttime supine systolic BP (SBP, closed circles) compared with placebo (open circles), as shown by (A) the changes from baseline (8 PM) in SBP over time (P=0.044 for treatment effect, mixed-effects model) and (B) the area under the curve of supine SBP (AUCSBP). Data are expressed as mean±SEM. P values versus placebo using Wilcoxon signed-rank test.
Nocturnal urine volume could not be measured in 2 because of urinary incontinence. In the remaining 9 patients, overnight CPAP therapy had significantly lower nocturnal urine volumes compared to that of placebo (609±84 versus 1004±160 mL for placebo; P=0.004; Figure 4A). In the morning after overnight CPAP, BP at 1 minute of standing was 92±10 / 58±5 mmHg and 82± 7 / 53±4 mmHg with placebo. Morning orthostatic tolerance, estimated as the AUCSBP during a 10-minute test in the 11 patients, was significantly higher following the overnight CPAP therapy compared to placebo (642±121 versus 410±109 mmHg*min for placebo; P=0.014; Figure 4B). No significant differences in any of the outcomes were found between patients with and without history of OSA (7 vs 4 patients, respectively). However, we cannot exclude a type II error because of the small sample size.
Figure 4. Effect of overnight CPAP therapy versus placebo on nocturnal diuresis and morning orthostatic tolerance.
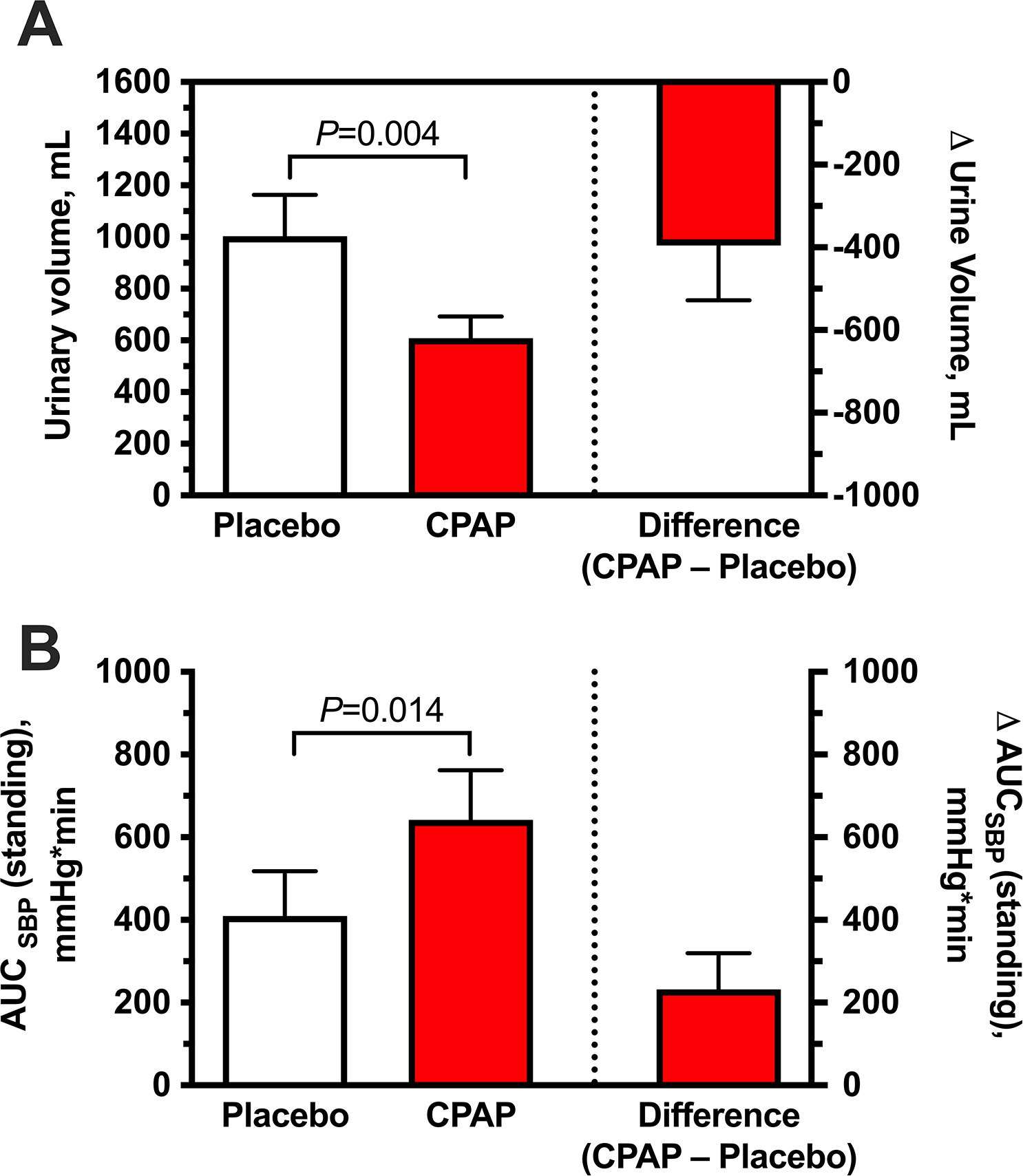
CPAP was associated with reduced nocturnal urine volume (A) and increased orthostatic tolerance in the morning (B, measured as the area under the curve of standing SBP [AUCSBP]) compared to placebo. Data are expressed as mean±SEM. P values versus placebo using Wilcoxon signed-rank test.
Discussion
The main findings of this study were that: 1) graded levels of CPAP, applied to awake AF patients with supine hypertension, acutely lowered supine BP in a dose-dependent manner, with an immediate onset of action and recovery; 2) this BP effect was associated with reductions in CO, SV and thoracic volume and increased abdominal volume, likely reflecting splanchnic venous pooling; 3) when applied for 8 hours overnight, CPAP lowered nocturnal BP, in a magnitude comparable to that of pharmacologic antihypertensive treatment we have previously studied; and, 4) overnight CPAP therapy reduced nocturnal urinary volume loss and improved morning orthostatic tolerance. Taken together, these results suggest that CPAP may offer a novel non-pharmacologic approach to effectively treat nocturnal supine hypertension in AF and may offer an advantage over pharmacologic therapies in improving daytime OH.
Supine hypertension is a significant management problem in most patients with OH but often goes untreated because of a legitimate concern that its treatment would worsen OH. The reluctance to treat nocturnal supine hypertension would be overcome if we had a treatment that would selectively lower nocturnal BP, reduce nighttime natriuresis, and thereby improve morning OH. Over the past 25 years, we have tested several drugs for their ability to meet these goals.5–9 We have been successful in reducing nighttime BP, but most medications failed to reduce nighttime diuresis, and none improved daytime OH, likely due to the lingering effects of these drugs.
Sleeping in a HUT position, on the other hand, has been shown to meet these goals but only in a handful of small observational studies using head of the bed elevations of ≥16 inches (≥ 12° HUT),10,11 levels that are not tolerated clinically by patients (and their spouses).12 Clinical guidelines, based on expert opinion, recommend that patients sleep with the head of the bed elevated by ~6–13 inches (5–10 degrees).12,20 These degrees of tilt are tolerated better but are also less effective. The only randomized clinical trial that tested the efficacy of increasing the head of the bed by 5° (~6 inches) failed to show an improvement in morning OH.21 A 10° of HUT (13-inch elevation of the head of the bed), the highest degree of tilt that patients can tolerate,12 produces only modest decreases in BP, and hypertension remains uncontrolled in most patients. Furthermore, getting out of a tilted bed during the night can increase the risk of falls, particularly for Parkinson disease and multiple system atrophy patients.
Increasing intrathoracic pressure with CPAP shares the hemodynamic mechanisms of HUT; it induces venous pooling into the splanchnic circulation, a large venous capacitance reservoir that can contain about one-third of the body’s total volume.22 Indeed, our studies show that CPAP, at levels used clinically for the treatment of OSA, induces an acute and reversible decrease in BP in AF patients due to a decrease in SV and thoracic volume associated with an increase in abdominal volume, likely reflecting splanchnic venous pooling. These dramatic hemodynamic effects are unmasked in AF patients because they lack the normal compensatory autonomic mechanisms, critical to maintaining normotension. This approach has the added benefit of an instantaneous onset and offset, which has a practical safety advantage over antihypertensive medications if patients get up at night because of nocturia.
Based on these acute effects, we designed a proof-of-concept study to assess the efficacy of overnight CPAP therapy for the treatment of supine hypertension. We found that overnight CPAP therapy was effective in controlling nocturnal hypertension, converting patients from a non-dipping to a dipping pattern.23 The magnitude of this effect, with a trough decrease in supine SBP of 23±5 mmHg, is equivalent to many of the antihypertensive medications we have previously tested, including nitroglycerin, sildenafil, clonidine, nebivolol, losartan, nifedipine, and eplerenone.5–9 Furthermore, the use of CPAP was associated with a reduction in nocturia and an improvement in early morning orthostatic tolerance. We are particularly encouraged by these preliminary results because we have not been able to produce these effects with any of the various antihypertensive drugs we have tried in previous studies.5–9 Of the medications tested previously, only clonidine and losartan reduced nocturnal sodium excretion, and none improved morning OH.5,8
Nocturnal hypertension and OSA are both prevalent in patients with essential hypertension. OSA can contribute to hypertension because nighttime apnea/hypopnea events are thought to induce sympathetic activation that increases BP.24–27 Disappointedly, CPAP produces only small decreases in nocturnal hypertension (2–3 mmHg in SBP) in patients with essential hypertension despite effectively suppressing OSA.28–30 It is possible that the antihypertensive effects of treating OSA with CPAP may be blunted in patients with essential hypertension because of the reflex sympathetic activation induced by increasing intrathoracic pressure with CPAP, as seen acutely during the Valsalva maneuver. Our findings in the acute hemodynamic studies in AF patients (Protocol 1) support this notion; CPAP at therapeutic levels for OSA produced significant reductions in BP, SV, and thoracic volume in our patients that would normally trigger a compensatory sympathetic activation. Thus, AF is a unique condition that allows us to characterize the direct hemodynamic effects of CPAP in the absence of autonomic reflexes.
OSA is also prevalent in patients with primary AF,31,32 and it could be argued that the improvement in supine hypertension was due to correction of underlying OSA in our patients. We did not systematically rule out the presence of OSA in our cohort, or the efficacy of our CPAP intervention in suppressing sleep apnea. However, the observation that CPAP acutely lowered BP in awake patients suggests that the mechanical hemodynamic effects were sufficient to explain our results even if we cannot rule out a contribution of sleep apnea suppression. Therefore, CPAP could be indicated in these patients even in the absence of sleep apnea and because the BP-lowering effect of CPAP was observed at therapeutic levels for OSA, it is likely that the safety profile of CPAP in autonomic failure patients would be similar to that of CPAP for the general OSA population.
Study Limitations
Our study has some limitations that should be considered before recommending CPAP as a therapeutic option but at the same time opens opportunities for further research. The overnight study was designed as a proof-of-concept study in a small number of patients studied under controlled conditions using acute interventions that were not randomized. Subjects received placebo on the first study night. Thus, we cannot rule out a contribution of the treatment sequence to the BP responses observed with overnight CPAP. However, the observation that the maximal systolic BP drop with overnight CPAP was similar to that of acute CPAP during the daytime studies (23±5 vs. 22±4 mmHg, respectively) suggests that the BP changes observed during the CPAP study night were driven by increased intrathoracic pressure. Larger, randomized, sham-controlled studies in a home setting would be preferable to assess the long-term efficacy, and importantly, patient compliance with this intervention. Compliance with CPAP is notoriously poor, but improvement of nocturia could provide an incentive for its use in AF patients. Likewise, a side-by-side comparison with HUT is not available; it is possible and likely that the effects of these interventions are additive, and their use could be combined. Finally, pressure-diuresis is thought to be largely due to pressure-natriuresis. However, urinary sodium excretion was not assessed in these proof-of-concept studies. 33
Perspectives
Our results suggest that CPAP can offer a novel approach for the treatment of nocturnal hypertension in AF patients. If confirmed, this approach could resolve the controversy of whether to treat or not to treat supine hypertension.1 Furthermore, it offers the potential to significantly improve the quality of life of our patients, not only by improving daytime orthostatic tolerance while controlling supine hypertension but also by reducing nocturia. Nocturia is not only a common symptomatic complaint, but it also exposes patients to the risk of falls during the night.12 The traditional approach of using antihypertensives at bedtime does not offer these advantages.4
Supplementary Material
Novelty and Relevance:
1. What is New:
In autonomic failure patients with supine hypertension, increasing intrathoracic pressure with CPAP, at levels used for the treatment of obstructive sleep apnea, acutely lowered BP by reducing stroke and thoracic volumes and increasing abdominal volume.
Overnight therapy with CPAP lowered nocturnal BP, nocturnal urine output and improved orthostatic tolerance in the following morning.
2. What is Relevant:
Supine hypertension complicates the management of OH in autonomic failure; it is associated with end-organ damage, nocturia, and worse daytime OH, but there is a reluctance to use antihypertensives for fear of worsening OH.
CPAP acutely lowers BP in autonomic failure patients through a Valsalva-like effect. Thus, it has a rapid onset and offset of action, and when applied overnight, it can improve nocturia and daytime OH.
3. Clinical/Pathophysiological Implications:
Overnight CPAP therapy may offer a novel non-pharmacologic approach to treating the nocturnal supine hypertension of AF.
Acknowledgments
The authors thank the patients who volunteered for these studies and the CRC nurses who made this study possible.
Sources of Funding
This work was supported by the National Institutes of Health (NIH) grants R01 HL144568, R01 HL161095, PO1 HL056693, U54 NS065736, R01 HL122847, and UL1 TR000445 (National Center for Advancing Translational Sciences). Additional support was provided by the American Heart Association grant 14CRP20380211 (L.O.), the Overton and Jeannette Smith Fund and NIH R01 HL142583 (A.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the NIH.
Non-standard Abbreviations and Acronyms:
- AF
autonomic failure
- AUC
area under the curve
- BP
blood pressure
- CO
cardiac output
- CPAP
continuous positive airway pressure
- DBP
diastolic blood pressure
- HR
heart rate
- HUT
head-up tilt
- MAP
mean arterial pressure
- OH
orthostatic hypotension
- OSA
obstructive sleep apnea
- SBP
systolic blood pressure
- SV
stroke volume
- SVR
systemic vascular resistance
Footnotes
Disclosures
IB has received consultant fees and research support from Lundbeck and Theravance Biopharma, Inc for the development of therapies for orthostatic hypotension. A.D, I.B., and L.O. are patent holders for the use of an automated binder in the treatment of orthostatic hypotension. The remaining authors have no discloses.
References
- 1.Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurology. 2016;15:954–966. [DOI] [PubMed] [Google Scholar]
- 2.Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL, Biaggioni I, Robertson D. Renal Impairment of Pure Autonomic Failure. Hypertension. 2009;54:1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;355:725–726. [DOI] [PubMed] [Google Scholar]
- 4.Park J-W, Okamoto LE, Biaggioni I. Advances in the Pathophysiology and Management of Supine Hypertension in Patients with Neurogenic Orthostatic Hypotension. Curr Hypertens Rep. 2022;24:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. Clonidine for the Treatment of Supine Hypertension and Pressure Natriuresis in Autonomic Failure. Hypertension. 2006;47:522–526. [DOI] [PubMed] [Google Scholar]
- 6.JORDAN J, SHANNON JR, POHAR B, PARANJAPE SY, ROBERTSON D, ROBERTSON R-M, BIAGGIONI I. Contrasting Effects of Vasodilators on Blood Pressure and Sodium Balance in the Hypertension of Autonomic Failure. J Am Soc Nephrol. 1999;10:35–42. [DOI] [PubMed] [Google Scholar]
- 7.Arnold AC, Okamoto LE, Gamboa A, Black BK, Raj SR, Elijovich F, Robertson D, Shibao CA, Biaggioni I. Mineralocorticoid Receptor Activation Contributes to the Supine Hypertension of Autonomic Failure. Hypertension. 2016;67:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. Angiotensin II, Independent of Plasma Renin Activity, Contributes to the Hypertension of Autonomic Failure. Hypertension. 2018;61:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto LE, Gamboa A, Shibao CA, Arnold AC, Choi L, Black BK, Raj SR, Robertson D, Biaggioni I. Nebivolol, But Not Metoprolol, Lowers Blood Pressure in Nitric Oxide–Sensitive Human Hypertension. Hypertension. 2018;64:1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HARKEL ADJT, LIESHOUT JJ, WIELING W. Treatment of orthostatic hypotension with sleeping in the head-up tilt position, alone and in combination with fludrocortisone. J Intern Med. 1992;232:139–145. [DOI] [PubMed] [Google Scholar]
- 11.MacLean AR, Allen EV. Orthostatic hypotension and orthostatic tachycardia - Treatment with the “head-up” bed. Journal of the American Medical Association. 1940;115:2162–2167. [Google Scholar]
- 12.Jordan J, Fanciulli A, Tank J, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, Lahrmann H, Mancia G, Mayer G, Norcliffe-Kaufmann L, Traon AP-L, Raj SR, Robertson D, Rocha I, Reuter H, Struhal W, Thijs RD, Tsioufis KP, Dijk JG van, Wenning GK, Biaggioni I. Management of supine hypertension in patients with neurogenic orthostatic hypotension: scientific statement of the American Autonomic Society, European Federation of Autonomic Societies, and the European Society of Hypertension. J Hypertens. 2019;37:1541–1546. [DOI] [PubMed] [Google Scholar]
- 13.Stewart JM, Montgomery LD. Reciprocal splanchnic-thoracic blood volume changes during the Valsalva maneuver. Am J Physiol-heart C. 2004;288:H752–H758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062–7. [DOI] [PubMed] [Google Scholar]
- 15.Thomas SH. Impedance cardiography using the Sramek-Bernstein method: accuracy and variability at rest and during exercise. British journal of clinical pharmacology. 1992;34:467–476. [PMC free article] [PubMed] [Google Scholar]
- 16.Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. [DOI] [PubMed] [Google Scholar]
- 17.Diedrich A, Biaggioni I. Segmental orthostatic fluid shifts. Clin Auton Res. 2004;14:146–147. [DOI] [PubMed] [Google Scholar]
- 18.Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water. Lancet. 1999;353:723. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto LE, Shibao C, Gamboa A, Choi L, Diedrich A, Raj SR, Black BK, Robertson D, Biaggioni I. Synergistic Effect of Norepinephrine Transporter Blockade and α-2 Antagonism on Blood Pressure in Autonomic Failure. Hypertension. 2012;59:650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. In: J Neurol. 2017. p. 1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan CW, Walsh C, Cunningham CJ. The effect of sleeping with the head of the bed elevated six inches on elderly patients with orthostatic hypotension: an open randomised controlled trial. Age and Ageing. 2011;40:187–192. [DOI] [PubMed] [Google Scholar]
- 22.Gelman S Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–748. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR, Robertson D, Biaggioni I. Nocturnal Blood Pressure Dipping in the Hypertension of Autonomic Failure. Hypertension. 2009;53:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. Journal of Clinical Investigation. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamisier R, Tan CO, Pepin J-L, Levy P, Taylor JA. Blood Pressure Increases in OSA due to Maintained Neurovascular Sympathetic Transduction: Impact of CPAP. Sleep. 2015;38:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA : the journal of the American Medical Association. 2012;307:2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. Journal of applied physiology (Bethesda, Md : 1985). 1989;67:2101–2106. [DOI] [PubMed] [Google Scholar]
- 28.Fava C, Dorigoni S, Vedove FD, Danese E, Montagnana M, Guidi GC, Narkiewicz K, Minuz P. Effect of CPAP on Blood Pressure in Patients With OSA/Hypopnea. Chest. 2014;145:762–771. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-García M-A, Capote F, Campos-Rodríguez F, Lloberes P, Atauri MJD de, Somoza M, Masa JF, González M, Sacristán L, Barbe F, Duran-Cantolla J, Aizpuru F, Mañas E, Barreiro B, Mosteiro M, Cebrián JJ, Peña M de la, García-Río F, Maimó A, Zapater J, Hernández C, SanMarti NG, Montserrat JM. Effect of CPAP on Blood Pressure in Patients With Obstructive Sleep Apnea and Resistant Hypertension. Jama. 2013;310:2407–9. [DOI] [PubMed] [Google Scholar]
- 30.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 31.Freilich S, Goff EA, Malaweera ASN, Twigg GL, Simonds AK, Mathias CJ, Morrell MJ. Sleep architecture and attenuated heart rate response to arousal from sleep in patients with autonomic failure. Sleep Medicine. 2010;11:87–92. [DOI] [PubMed] [Google Scholar]
- 32.Cock VCD. Sleep Abnormalities in MultipleSystem Atrophy. Current Treatment Options in Neurology. 2018;20:16. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto LE, Celedonio JE, Smith EC, Gamboa A, Shibao CA, Diedrich A, Paranjape SY, Black BK, Muldowney JAS, Peltier AC, Habermann R, Crandall CG, Biaggioni I. Local Passive Heat for the Treatment of Hypertension in Autonomic Failure. J Am Hear Assoc Cardiovasc Cerebrovasc Dis. 2021;10:e018979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosqueda-Garcia R Evaluation of autonomic failure. Disorders of the Autonomic Nervous System Luxembourg: Harwood Academic Publishers GmbH. 1995;25:59. [Google Scholar]
- 35.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J, Committee for the NHBPEPC. Primary Prevention of Hypertension: Clinical and Public Health Advisory From the National High Blood Pressure Education Program. Jama. 2002;288:1882–1888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


