Purpose of review
Melioidosis, caused by the soil-dwelling bacterium Burkholderia pseudomallei, is a tropical infection associated with high morbidity and mortality. This review summarizes current insights into melioidosis’ endemicity, focusing on epidemiological transitions, zoonosis, and climate change.
Recent findings
Estimates of the global burden of melioidosis affirm the significance of hot-spots in Australia and Thailand. However, it also highlights the paucity of systematic data from South Asia, The Americas, and Africa. Globally, the growing incidence of diabetes, chronic renal and (alcoholic) liver diseases further increase the susceptibility of individuals to B. pseudomallei infection. Recent outbreaks in nonendemic regions have exposed the hazard from the trade of animals and products as potential reservoirs for B. pseudomallei. Lastly, global warming will increase precipitation, severe weather events, soil salinity and anthrosol, all associated with the occurrence of B. pseudomallei.
Summary
Epidemiological transitions, zoonotic hazards, and climate change are all contributing to the emergence of novel melioidosis-endemic areas. The adoption of the One Health approach involving multidisciplinary collaboration is important in unraveling the real incidence of B. pseudomallei, as well as reducing the spread and associated mortality.
Keywords: Burkholderia pseudomallei, climate change, epidemiological transition, melioidosis, one health, zoonosis
INTRODUCTION
Melioidosis, an infectious disease with reported case fatality rates between 10 and 50% [1], is caused by the Gram-negative soil bacterium Burkholderia pseudomallei[2]. The disease is endemic in Southeast Asia and Northern Australia, although more regions of endemicity are recently uncovered in Africa [3,4], South Asia [5], the Pacific, and the Americas [2,6]. Its impact on healthcare across the globe is significant. A recent report estimates the global burden of melioidosis in terms of disability-adjusted life years to be 4.6 million outperforming many other tropical diseases, such as dengue and schistosomiasis [7,8,9▪]. Surprisingly, however, melioidosis is still not officially listed as a neglected tropical disease by the WHO.
Infection occurs through skin penetration, direct contact with contaminated water or soil, ingestion of contaminated water, and inhalation of dust or water droplets [10]. Disease presentation ranges from acute sepsis or pneumonia to chronic illness with abscess formation. The incubation period of melioidosis ranges from 1 to 21 days, and reports of latency go up to 29 years [11]. Melioidosis is mainly considered an opportunistic disease as individuals with an altered immune function because of comorbidities – such as diabetes, renal, and liver failure – are at risk for infection and may have impaired disease outcomes [12▪▪].
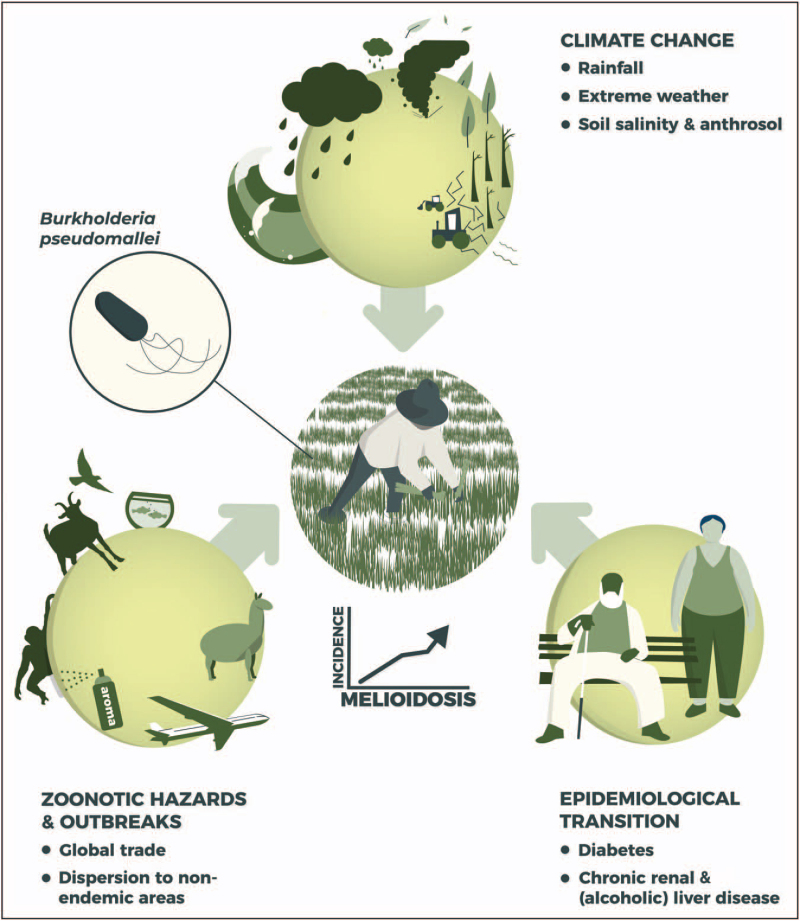
Human health has developed in unprecedented ways. As a consequence, individuals become older and suffer more frequently from comorbidities. Together with advances in mobility and globalization this might increase the susceptibility of individuals to emerging infectious diseases, such as melioidosis [13]. The global trade of animals and products, and global warming may further impact these changes. Here, we will review current insights into the increasing endemicity of melioidosis, focusing on epidemiological transitions, zoonotic hazards, and climate change (Fig. 1).
FIGURE 1.
The drivers behind the increasing endemicity of melioidosis. Several factors can be identified that are likely to contribute to an increasing endemicity of melioidosis. Epidemiological transitions leading to a significant rise of comorbidities, such as diabetes, chronic kidney, and (alcoholic) liver failure, will continue to increase the number of individuals at risk for melioidosis. Zoonotic hazards, such as importation of exotic animals and livestock from endemic areas, may spread melioidosis to nonendemic regions. Climate change causes increased precipitation, severe weather events, and soil salinity and anthrosol, which may lead to expansion of the geographic dissemination and incidence of Burkholderia pseudomallei.
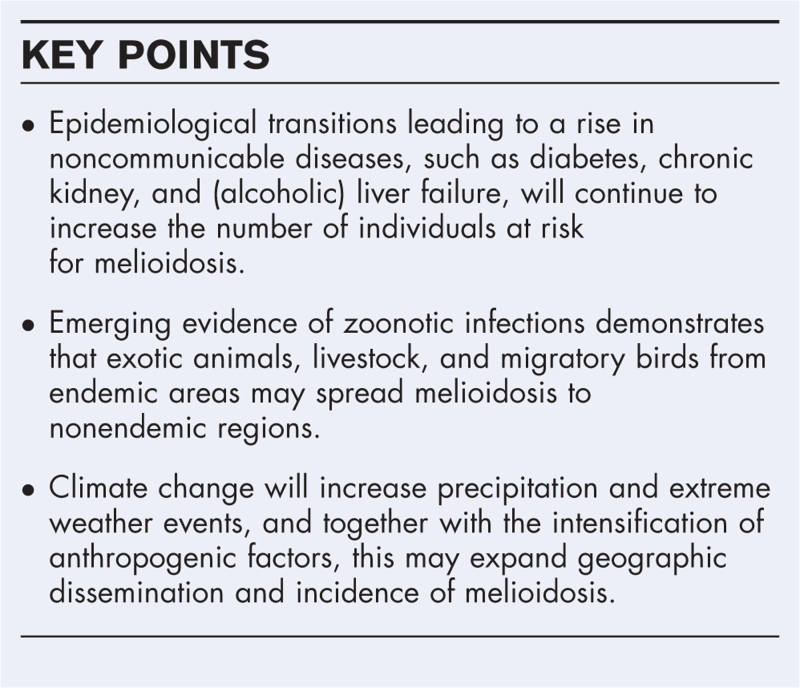
Box 1.
no caption available
CURRENT INSIGHTS INTO THE EPIDEMIOLOGY OF MELIOIDOSIS
Current estimates suggest that melioidosis accounts globally for approximately 165 000 (68 000–412 000) cases and 89 000 (36 000–227 000) deaths per year [7]. B. pseudomallei is predominantly present in subtropical and tropical regions with Northern Australia and South-East Asia known as endemic hotspots. An overview on the reported phylogenomic reconstruction of the dissemination of B. pseudomallei across the globe is provided in the Conclusion section.
In Northern Australia, the annual melioidosis incidence is 4.8–51.2 (median 20.5) per 100 000 people [12▪▪]. The Darwin Prospective Melioidosis Study, which recently reported data from 1148 patients, has provided key insights into the clinical and microbiology characteristics of the disease [12▪▪]. Although melioidosis patients can present with a wide range of symptoms, more than half presents with pneumonia [14,15]. This extensive registry highlights the notion that B. pseudomallei is predominantly an opportunistic pathogen as most patients have clinical risk factors, such as diabetes and hazardous alcohol use. Individuals with one or multiple risk factors are more than eight times as likely to die from melioidosis. Moreover, the absence of risk factors is strongly correlated with survival as only 3 of 186 patients without risk factors died from melioidosis [14,15]. Furthermore, the study indicates a relatively low proportion of melioidosis in children but a greater number of infections in indigenous Australians, explained by high comorbidity rates and environmental exposure to the pathogen [12▪▪]. The authors state that early diagnosis, availability of appropriate antimicrobial therapy, and state-of-the-art intensive care therapy, could reduce mortality rates to less than 10% [12▪▪].
The South-East Asian WHO region covers more than 60% of the estimated global burden, with the highest total burden in India, with 1.6 million DALYs [7,8]. A recent cohort study described the clinical manifestations and outcomes of 114 individuals with B. pseudomallei infection presented in a tertiary hospital in southern India [16]. More than 80% of individuals had diabetes, which is more than globally reported and a probable reflection of the emerging diabetes epidemic in India [8,12▪▪,16,17]. A large clinical epidemiology study in Thailand determined 7126 culture-confirmed melioidosis cases from 2012 to 2015 across the country [18]. The average calculated incidence was 4 per 100 000 and was highest in Northeast Thailand (8.7 per 100 000) [18]. Geographical regions within Thailand showed a variance in age, comorbidities, and disease presentation, possibly because of differences in the environmental distribution of B. pseudomallei, in occupation, and in risk factors of affected patients. The overall 30-day case fatality rate in this cohort was 39%. However, only 4% of deaths were reported to Thailand's national notifiable disease surveillance system [18]. This study shows that integrating information from available data sets will improve national statistics and could support priority setting for policymakers.
Currently, Brazil accounts for over half of reported melioidosis cases in South America [19]. The state of Ceará in northeastern Brazil, has the highest reported incidence in South America and is the only region on the continent where specific infection prevention and control measurements have been implemented [19]. A retrospective study in this region on childhood melioidosis reported a mortality rate of 45% in 20 children. Most children presented with sepsis, pneumonia, and/or septic shock, although underreporting of mild cases could be a possible explanation for these severe outcomes [20▪]. Growing awareness and improvement of diagnostic facilities could lead to increased recognition of melioidosis in areas where the disease was previously absent [2]. A recent retrospective survey highlights this finding and identified 12 cases in Panama between 2007 and 2017 [21▪]. Most patients came from rural areas, and all occurred during the rainy season. Two patients were previously misidentified as tuberculosis as most clinical symptoms of melioidosis are nonspecific [21▪].
B. pseudomallei is considered endemic in Africa with estimates suggesting the presence of large numbers of underreported infection across the continent [7,8]. Most reported cases are in travelers returning from the African continent, as exemplified by a recent case of melioidosis presenting as chronic femoral osteomyelitis in a Ghanaian man living in the United Kingdom [3,4,22▪]. A comprehensive Dutch retrospective surveillance study of 33 travelers with B. pseudomallei infection showed that imported melioidosis could additionally serve as sentinels for detecting the disease in areas not yet considered melioidosis-endemic, such as The Gambia [23]. A recent single surveillance study demonstrated a relatively low estimated incidence of 1.3–2 per 1 million person-years using blood cultures in Kilify Country, Kenya [24]. These incidence rates might be an underestimation since approximately 50–75% [2] of melioidosis patients present with bacteremia, and culture has only an estimated sensitivity of around 60% [25]. Additionally, B. pseudomallei is commonly misidentified by biochemical identification systems or remains unidentified, even in endemic regions [26].
EPIDEMIOLOGICAL TRANSITION
Epidemiological transition refers to changing population patterns in relation to life expectancy, mortality, fertility, and leading causes of death [27]. Accelerated mobility, developments in medical technology, and globalization have advanced the medical field in unprecedented ways. The world's population is ageing and individuals frequently suffer from comorbidities. These developments could make individuals more vulnerable to emerging infectious diseases, such as melioidosis. Given the rise in the numbers of immunocompromised travelers the incidence of imported melioidosis might also increase [23]. Most importantly, current epidemiological transitions in low-income and middle-income countries (LMICs) may lead to increased rates of individuals with risk factors for melioidosis.
Diabetes is present in 39–80% of cases depending on the cohort studied. Notably, people living with diabetes are 12 times as likely to get infected with B. pseudomallei[12▪▪,16,28,29]. As a comparison, in tuberculosis the presence of diabetes constitutes a three-fold increased risk to acquire the disease [30]. Numerous immunological studies demonstrate that diabetic patients have a reduced phagocytosis and killing capacity of B. pseudomallei as well as marked alterations in neutrophilic and monocytic cytokine responses [31–35]. Additionally, profound hyperglycemia is associated with higher infection rates and mortality because of bacterial infections [36]. Overall, the number of undiagnosed or poorly regulated diabetes patients in LMICs significantly exceeds numbers in high-income countries [35–37]. In 20 years, the total number of patients living with diabetes is estimated to increase by 55%, with more than 80% of diabetic patients living in LMICs [36].
Approximately 9–50% of melioidosis cases are reported to have underlying renal disease depending on the study location [8,12▪▪,38]. As with diabetes, chronic kidney disease is associated with impaired chemotaxis and reduced phagocytic capacity as demonstrated by experimental studies on other pathogens [39,40]. In 2017, the number of people receiving renal replacement therapy was estimated to be around 2.5 million worldwide, and this number will most likely have doubled in 2030 [41▪].
The earlier reported Darwin study describes hazardous alcohol use in 39% of cases, predominantly in disadvantaged communities [42]. Globally, chronic liver disease is present in approximately 7% of patients, according to a systematic review on the global burden of melioidosis [8]. Observed immune alterations in alcohol-related liver disease have overlapped with those seen in individuals with diabetes and renal impairment [43]. An experimental study showed that binge drinking impaired innate immune functions through increased barrier permeability during B. pseudomallei infection [44]. Notably, around 20–30% of mortality attributed to cirrhosis and liver cancer in the Asia-Pacific region are attributable to hazardous alcohol consumption [45,46▪]. Moreover, the prevalence of nonalcohol fatty liver disease will rise because of obesity and diabetes [47,48▪].
In short, epidemiological transitions further increase the susceptibility of individuals to B. pseudomallei infection in melioidosis-endemic regions (Fig. 1) [41▪]. LMICs will carry a double burden of disease with potential increasing numbers of both noncommunicable diseases and emerging infectious diseases like melioidosis [35,36].
OUTBREAKS AND ZOONOTIC HAZARDS
Although melioidosis is not always considered a zoonotic disease, animals can shed B. pseudomallei in the environment [49▪]. A wide range of animal species are susceptible for infection with B. pseudomallei, such as goats, sheep, camels, and alpacas [50]. Crocodiles, previously known as being highly resistant to the disease, also demonstrated susceptibility to the bacterium. A cluster of melioidosis infections in saltwater crocodiles of which two were fatal was recently described in Northern Australia [51]. Interestingly, several case studies reported that imported exotic animals, nonhuman primates, pet iguanas, and a canine transmitted B. pseudomallei to nonendemic regions, among others to the United States and Europe [52–55]. Although rare, infection outbreaks have been reported in temperate climatic regions and are generally attributable to a single origin in the environment. For example, several outbreaks have been reported in piggeries in Queensland, Australia [56], in primates imported from the Philippines to the United Kingdom [57], and in an donated panda from China to a zoo in Paris, France [58]. Additionally, following high rainfall, a melioidosis cluster in 23 alpacas (Vicugna pacos) and a parrot (Ara macao) was reported in southwest Australia [59▪]. More than 20 animals died on the alpaca farm. Genome sequencing indicated that B. pseudomallei can survive in nontropical environments in a latent state and activate later following favorable conditions [59▪]. Another example of possible zoonotic transmission is a recent report of a human case contracting melioidosis from her tropical fish aquarium in the United States [60▪▪]. Genetic analysis revealed clonal matches between the human isolate and those from her freshwater home aquarium [60▪▪]. A case of aquarium water contaminated by an imported tropical fish from Singapore has been earlier reported in France [61]. Overall, animals should be seriously considered as potential hazards in transmitting melioidosis to humans (Fig. 1).
Most melioidosis cases reported in the United States are associated with travel to endemic areas. However, sporadic cases with no travel history suggest possible domestic exposure, for example, to contaminated products [6,62–64]. In some cases, genetic investigation of B. pseudomallei strains from human cases pointed towards South and East Asia as their probable origin. Recently, the CDC confirmed a multistate outbreak of melioidosis in four patients living in Georgia, Kansas, Minnesota, and Texas [65▪▪]. All four cases had no travel history to melioidosis-endemic areas. Only two of them were adults with comorbidities recognized as risk factors for melioidosis. The other two were children without a relevant medical history. Genetic analysis on the isolates indicated a genetic origin closely related to South Asia, suggesting a common source of infection. An extensive public health investigation on several environmental and consumer products revealed an aromatherapy spray as the source of infection in the Georgia's patient's house. The genetic fingerprint of the isolated strain genetically matched the B. pseudomallei strains in all four patients. This finding illustrates that the aromatherapy spray (or a component) was the source of infection in all four cases [65▪▪]. Earlier reports of irrigation fluids and hand detergents resulting in clusters of cutaneous melioidosis indicated the danger of contaminated products [66]. Therefore, B. pseudomallei infection should also be considered in the differential diagnosis in patients that matches the characteristics of melioidosis even in the absence of a travel history to endemic areas. Whole-genome sequencing can result in epidemiological insights and can provide knowledge about the transmission and environmental dispersal patterns.
CLIMATE CHANGE
Climate change could directly impact on the distribution of B. pseudomallei and the seasonal pattern of the disease. It is anticipated that global temperatures will continue to rise by 0.2 °C per decade even if greenhouse gas emissions are reduced drastically in the coming decades [67▪▪,68▪▪]. This ongoing temperature rise will increase precipitation, extreme weather events, and droughts [67▪▪,68▪▪,69]. Precipitation will decrease in some parts of the subtropics and tropics, while particularly the high latitudes, equatorial Pacific, and some monsoon regions will experience a heavy increase in the format of intense tropical storms [67▪▪,68▪▪]. Monsoon rainfall is expected to increase, particularly in South-Asia, East-Asia, South-East Asia, and West Africa [67▪▪,68▪▪].
For a long time, it has been recognized that rainfall leads to increased B. pseudomallei occurrence in the soil [70]. This correlation is most robust in melioidosis endemic hotspots, such as South-East Asia and Northern Australia, possibly because of the extreme precipitation experienced during the monsoon season. For instance, studies in Singapore and Malaysia indicated that the number of cases admitted to the hospital was directly related to the severity of rainfall and that melioidosis as such should be considered a seasonal disease [71,72]. The Darwin study demonstrated that for every 100 mm of rainfall, melioidosis incidence increased by 14% in Northern Australia [12▪▪]. In line, a study conducted in Laos and Cambodia showed that humidity and windy conditions significantly influence the seasonal burden of melioidosis [73]. During such periods of very high humidity, children appeared to have a three times higher risk of infection with B. pseudomallei compared with adults. Windy conditions were associated with pulmonary and disseminated infections [73]. Additionally, it has been shown that rivers can serve as potential carriers for B. pseudomallei and can facilitate the spread of the pathogen over long distances to nonendemic melioidosis areas [74].
Extreme weather events are frequently linked to higher melioidosis incidence rates. This is illustrated by the occurrence of clusters of B. pseudomallei infection after a typhoon and subsequent widespread flooding in Taiwan [75,76], a tsunami in Thailand [77], and tropical cyclones in Northern Australia [78]. The high-velocity wind that accompanies such events could lead to inhalation of aerosolized B. pseudomallei as indicated by earlier studies [75,79]. Recently, heavy rainfall and flooding in Sri Lanka caused a case cluster of 10 patients [80▪▪]. Moreover, extreme weather events can also activate B. pseudomallei in regions where the bacterium is latently present in the environment as has been demonstrated in central Australia [81]. The chances of flooding increase with sea-rise and intensification of anthropogenic activities in coastal areas, such as urban development, fisheries, and agricultural practices. As a consequence, protective ecosystems like mangroves and coral reefs are frequently lost. Subsequently, flooding can lead to higher salinization and anthrosol soil [7,82,83]. A prediction model found that high rainfall, soil types (e.g. anthrosol and acrisol), and salinity were all strongly correlated with the presence of B. pseudomallei in the environment [7]. Therefore, these factors were used to estimate the incidence of melioidosis globally. Of note, the model predicted that multiple regions suitable for B. pseudomallei establishment are present in Japan and the United States (e.g. Florida, Louisiana, and Texas) [7].
Continued global warming will also affect global migration patterns [82,84▪,85,86]. Food uncertainty because of heatwaves and droughts can become more frequent [82]. This may instigate the movement of people and agricultural practices from arid areas to more fertile and wet regions, consequently leading to a rise in the total number of at risk individuals living in melioidosis endemic areas [82,85]. Growing agricultural practices in these regions will increase anthrosol, promoting B. pseudomallei suitability. Overall, human-induced climate change increases favorable conditions for B. pseudomallei in and outside endemic areas worldwide [67▪▪,68▪▪] (Fig. 1).
THE ROAD AHEAD
As illustrated above, the epidemiology of melioidosis will be impacted by human, animal, and environmental developments. As a consequence, it is advisable to adopt the One Health approach as an integrated view recognizing this triad interconnection [87]. The One Health approach aims at better public health outcomes through monitoring and surveillance, and will be crucial in determining the actual incidence of B. pseudomallei in humans, animals, and the environment. Interdisciplinary, multidisciplinary, and transdisciplinary collaborations between human health, animal health, and environment sectors should be strengthened on a local, national, and global level to address the spread of melioidosis and decrease patient vulnerability [88]. By implementing the One Health approach, best practices can be exchanged by educating and engaging local communities, implementing preventive behavioral programs [89], and improving laboratories in resource-limited areas [2]. We recommended to use the current interactive tools and materials at www.melioidosis.info. Further, cooperating of the clinical field and basic scientists with veterinarians, ecologists, environmental experts, and agricultural professionals could bring innovative solutions to impact indirect drivers of melioidosis. These actions must take place not only in endemic areas but also in nonendemic susceptible regions.
CONCLUSION
Estimates of the global burden of melioidosis not only affirm the significance of hot-spots in Australia and Thailand but also highlight the paucity of systematic data from South Asia, the Americas, and Africa. Many LMIC countries are going through an epidemiological transition, leading to a significant rise in individuals with risk factors of melioidosis, such as diabetes, chronic kidney, and (alcoholic) liver failure. Recent outbreaks of melioidosis in nonendemic regions demonstrate that zoonotic infections combined with global trade and animal migratory patterns can cause the dispersion of B. pseudomallei to nonendemic areas. Moreover, given that B. pseudomallei is a soil-dwelling bacterium that causes a seasonal disease, climate change will impact the melioidosis’ incidence and geographic dissemination. Melioidosis is often challenging to diagnose because of a wide range of disease presentations, low awareness, and a lack of good laboratory facilities. There is a need for better granularity of data on the worldwide distribution of B. pseudomallei to prevent and decrease mortality and morbidity from this disease. The adoption of the One Health approach involving multidisciplinary collaboration in all aspects of healthcare for humans, animals, and the environment could help to determine the real incidence and decrease the spread and morbidity associated with B. pseudomallei infection globally.
Phylogenomic reconstruction of the dissemination of B. pseudomallei across the globe; a genetic window to the past
The first case of melioidosis was identified in Myanmar in 1911 [90]. Chewapreecha et al.[91] Showed that B. pseudomallei most likely originated in Australia. By using whole-genome sequencing on 500 bacterial isolates from 30 countries, the authors could demonstrate that the movement of people and products has led to the dissemination of B. pseudomallei across the globe [91]. Approximately 16 000–225 000 years ago, during the last glacial period, B. pseudomallei was transferred from Australia to Southeast and East Asia [91,92]. Subsequently, historical trade routes, migration of populations, and migratory birds spread the bacterium throughout Asia [93–95]. For example, a study conducted in the melioidosis-endemic Darwin region in Australia identified B. pseudomallei in the beaks of wild birds, such as native finches and doves [93].
Melioidosis has probably been introduced in Madagascar 1500–2000 years ago. Phylogenomic reconstruction demonstrated that African isolates grouped together with Asian strains, indicating Asian origins [91,96]. The introduction of pigs, a known reservoir of melioidosis, and migratory birds could be a possible explanation of the dissemination of B. pseudomallei from Asia to Madagascar [97]. Next, anthropogenic factors, like human migration and trade routes, probably dispersed the disease to the African mainland [96]. Cargos transported during the transatlantic slave trade, such as contaminated food, water, plants, and animals, could have introduced B. pseudomallei into the Americas between 1650 and 1850. Strong genetic similarities between isolates from Africa and America supported this finding [96].
Acknowledgements
We would like to thank many members of the worldwide melioidosis community, embodied in the International Melioidosis Network, for all the insightful discussions over the last years leading to this work.
Financial support and sponsorship
This work was supported by a Research Grant (2018) from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID to E.B.) and the Netherlands Organization for Scientific Research (VIDI grant number 91716475 to W.J.W.).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 2005; 18:383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Virk HS, Torres AG, et al. Melioidosis. Nat Rev Dis Primers 2018; 4:17108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnie E, Wiersinga WJ, Limmathurotsakul D, Grobusch MP. Melioidosis in Africa: should we be looking more closely? Future Microbiol 2015; 10:273–281. [DOI] [PubMed] [Google Scholar]
- 4.Steinmetz I, Wagner GE, Kanyala E, et al. Melioidosis in Africa: time to uncover the true disease load. Trop Med Infect Dis 2018; 3:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay C, Shaw T, Varghese GM, Dance DAB. Melioidosis in South Asia (India, Nepal, Pakistan, Bhutan and Afghanistan). Trop Med Infect Dis 2018; 3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benoit TJ, Blaney DD, Doker TJ, Gee JE, et al. A review of melioidosis cases in the Americas. Am J Trop Med Hyg 2015; 93:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limmathurotsakul D, Golding N, Dance DA, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 2016; 1:15008. [DOI] [PubMed] [Google Scholar]
- 8.Birnie E, Virk HS, Savelkoel J, Spijker R, et al. Global burden of melioidosis in 2015: a systematic review and data synthesis. Lancet Infect Dis 2019; 19:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Savelkoel J, Dance DAB, Currie BJ, et al. A call to action: time to recognise melioidosis as a neglected tropical disease. Lancet Infect Dis 2021. [DOI] [PubMed] [Google Scholar]; This article provides evidence supporting the official recognition of melioidosis as a neglected tropical disease. This is relevant in order to control melioidosis and reduce the global burden.
- 10.Limmathurotsakul D, Kanoksil M, Wuthiekanun V, et al. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: a matched case-control study. PLoS Negl Trop Dis 2013; 7:e2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chodimella U, Hoppes WL, Whalen S, et al. Septicemia and suppuration in a Vietnam veteran. Hosp Pract (1995) 1997; 32:219–221. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Currie BJ, Mayo M, Ward LM, et al. The Darwin Prospective Melioidosis Study: a 30-year prospective, observational investigation. Lancet Infect Dis 2021; 21:1737–1746. [DOI] [PubMed] [Google Scholar]; This study documents all melioidosis cases and describes the epidemiology, clinical features, outcomes, and bacterial genomics from a 30-year period in Northern Australia. This article provides in-depth clinical and microbiology aspects of 1148 melioidosis patients in a high incidence region.
- 13.Amuasi JH, Lucas T, Horton R, Winkler AS. Reconnecting for our future: the Lancet One Health Commission. Lancet 2020; 395:1469–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo TJ, Ang LW, James L, Goh KT. Melioidosis in a tropical city state, Singapore. Emerg Infect Dis 2009; 15:1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaowagul W, White NJ, Dance DA, et al. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis 1989; 159:890–899. [DOI] [PubMed] [Google Scholar]
- 16.Koshy M, Jagannati M, Ralph R, et al. Clinical manifestations, antimicrobial drug susceptibility patterns, and outcomes in melioidosis cases, India. Emerg Infect Dis 2019; 25:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suputtamongkol Y, Chaowagul W, Chetchotisakd P, et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis 1999; 29:408–413. [DOI] [PubMed] [Google Scholar]
- 18.Hantrakun V, Kongyu S, Klaytong P, et al. Clinical epidemiology of 7126 melioidosis patients in Thailand and the implications for a national notifiable diseases surveillance system. Open Forum Infect Dis 2019; 6:ofz498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolim DB, Lima RXR, Ribeiro AKC, et al. Melioidosis in South America. Trop Med Infect Dis 2018; 3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Lima RXR, Rolim DB. Melioidosis in children, Brazil. Emerg Infect Dis 2021; 27:1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the demographical characteristics of 20 melioidosis cases in children in Ceara, Brazil, between 1989 and 2019. The authors report a high mortality rate in children, possibly because of underreporting of melioidosis.
- 21▪.Arauz AB, Castillo K, Santiago E, et al. Geographic distribution and incidence of melioidosis, Panama(1). Emerg Infect Dis 2020; 26:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]; This retrospective study identified and described the clinical characteristics of 12 melioidosis cases in Panama during 2007–2017.
- 22▪.Mabayoje DA, Kenna DTD, Dance DAB, NicFhogartaigh C. Melioidosis manifesting as chronic femoral osteomyelitis in patient from Ghana. Emerg Infect Dis 2022; 28:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a case of imported melioidosis manifesting as chronic femoral osteomyelitis in a patient from Ghana. Due to possible underreporting in the African continent, most cases are reported in returning travelers.
- 23.Birnie E, Savelkoel J, Reubsaet F, et al. Dutch Melioidosis Study Group. Melioidosis in travelers: an analysis of Dutch melioidosis registry data 1985–2018. Travel Med Infect Dis 2019:101461. [DOI] [PubMed] [Google Scholar]
- 24.Muthumbi EM, Gordon NC, Mochamah G, et al. Population-based estimate of melioidosis, Kenya. Emerg Infect Dis 2019; 25:984–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limmathurotsakul D, Jamsen K, Arayawichanont A, et al. Defining the true sensitivity of culture for the diagnosis of melioidosis using Bayesian latent class models. PLoS One 2010; 5:e12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greer RC, Wangrangsimakul T, Amornchai P, et al. Misidentification of Burkholderia pseudomallei as Acinetobacter species in northern Thailand. Trans R Soc Trop Med Hyg 2019; 113:48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Q 2005; 83:731–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg 2010; 82:1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limmathurotsakul D, Chaowagul W, Chierakul W, et al. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis 2006; 43:979–986. [DOI] [PubMed] [Google Scholar]
- 30.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kronsteiner B, Chaichana P, Sumonwiriya M, et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol 2019; 49:1092–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis 2012; 31:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easton A, Haque A, Chu K, et al. A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J Infect Dis 2007; 195:99–107. [DOI] [PubMed] [Google Scholar]
- 34.Chanchamroen S, Kewcharoenwong C, Susaengrat W, et al. Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect Immun 2009; 77:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunachie S, Chamnan P. The double burden of diabetes and global infection in low and middle-income countries. Trans R Soc Trop Med Hyg 2019; 113:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Crevel R, van de Vijver S, Moore DAJ. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol 2017; 5:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flood D, Seiglie JA, Dunn M, et al. The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680 102 adults. Lancet Healthy Longevity 2021; 2:e340–e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zueter A, Yean CY, Abumarzouq M, et al. The epidemiology and clinical spectrum of melioidosis in a teaching hospital in a North-Eastern state of Malaysia: a fifteen-year review. BMC Infect Dis 2016; 16:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis 1999; 34:384–399. [DOI] [PubMed] [Google Scholar]
- 40.Iida T, Umezawa K, Tanaka K, et al. Polymorphonuclear cells in chronic hemodialysis patients have intact phagocytotic and impaired bactericidal activities. Nephron 1997; 75:41–47. [DOI] [PubMed] [Google Scholar]
- 41▪.Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study. Lancet 2020; 395:709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports a comprehensive analysis of the global prevalence and burden of chronic kidney diseases. These findings are significant as chronic renal disease is a considerable risk factor for melioidosis.
- 42.Jimenez VM, Jr, Settles EW, Currie BJ, et al. Persistence of Burkholderia thailandensis E264 in lung tissue after a single binge alcohol episode. PLoS One 2019; 14:e0218147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustot T, Fernandez J, Szabo G, et al. Sepsis in alcohol-related liver disease. J Hepatol 2017; 67:1031–1050. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez V, Jr, Moreno R, Settles E, et al. A mouse model of binge alcohol consumption and Burkholderia infection. PLoS One 2018; 13:e0208061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The global, regional, and national burden of cirrhosis by cause in 195 countries and territories 1990–2017: a systematic analysis for the Global Burden of Disease Study. Lancet Gastroenterol Hepatol 2020; 5:245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Bhadoria AS, Mohapatra A. Alcohol-related cirrhosis and liver cancer: a call for inclusion in noncommunicable disease action plans. Lancet Gastroenterol Hepatol 2021; 6:982. [DOI] [PubMed] [Google Scholar]; The authors state that alcohol is an important factor in cirrhosis and suggest prioritizing alcohol-related liver disease in the Asia-Pacific region. This is relevant as alcohol-related liver disease is a significant risk factor for melioidosis.
- 47.Paik JM, Golabi P, Younossi Y, et al. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 2020; 72:1605–1616. [DOI] [PubMed] [Google Scholar]
- 48▪.Sarin SK, Kumar M, Eslam M, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2020; 5:167–228. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the burden of liver disease in the Asia-Pacific region and makes recommendations. This is relevant as liver disease is a significant risk factor for melioidosis.
- 49▪.Rees EM, Minter A, Edmunds WJ, et al. Transmission modelling of environmentally persistent zoonotic diseases: a systematic review. Lancet Planet Health 2021; 5:e466–e478. [DOI] [PubMed] [Google Scholar]; The authors systematically review dynamic transmission models of zoonotic diseases, amongst others melioidosis. The study highlights the urgency for a holistic One Health approach for data-driven modelling of pathogens, such as B. pseudomallei.
- 50.Sprague LD, Neubauer H. Melioidosis in animals: a review on epizootiology, diagnosis and clinical presentation. J Vet Med B Infect Dis Vet Public Health 2004; 51:305–320. [DOI] [PubMed] [Google Scholar]
- 51.Rachlin A, Kleinecke M, Kaestli M, et al. A cluster of melioidosis infections in hatchling saltwater crocodiles (Crocodylus porosus) resolved using genome-wide comparison of a common north Australian strain of Burkholderia pseudomallei. Microb Genom 2019; 5:e000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan CW, Bishop K, Blaney DD, et al. Public health response to an imported case of canine melioidosis. Zoonoses Public Health 2018; 65:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zehnder AM, Hawkins MG, Koski MA, et al. Burkholderia pseudomallei isolates in 2 pet iguanas, California, USA. Emerg Infect Dis 2014; 20:304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hellebuyck T, Wattiau P, Boyen F, et al. Isolation of Burkholderia pseudomallei from a Pet Green Iguana, Belgium. Emerg Infect Dis 2018; 24:2331–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elschner MC, Hnizdo J, Stamm I, et al. Isolation of the highly pathogenic and zoonotic agent Burkholderia pseudomallei from a pet green Iguana in Prague, Czech Republic. BMC Vet Res 2014; 10:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ketterer PJ, Webster WR, Shield J, et al. Melioidosis in intensive piggeries in south eastern Queensland. Aust Vet J 1986; 63:146–149. [DOI] [PubMed] [Google Scholar]
- 57.Dance DA, King C, Aucken H, et al. An outbreak of melioidosis in imported primates in Britain. Vet Rec 1992; 130:525–529. [DOI] [PubMed] [Google Scholar]
- 58.Mollaret HH. L’affaire du jardin des plantesou comment la mélioïdose fit son apparition en France. Médecine et Maladies Infectieuses 1988; 18:643–654. [Google Scholar]
- 59▪.Webb JR, Buller N, Rachlin A, et al. A persisting nontropical focus of Burkholderia pseudomallei with limited genome evolution over five decades. mSystems 2020; 5:e00726-20. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports a new melioidosis cluster in animals following high rainfall in southwest Western Australia. The authors demonstrate that B. pseudomallei can survive in nontropical environments in a latent state an activate later following favorable condittions.
- 60▪▪.Dawson P, Duwell MM, Elrod MG, et al. Human melioidosis caused by novel transmission of Burkholderia pseudomallei from Freshwater Home Aquarium, United States. Emerg Infect Dis 2021; 27:3030–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study suggests a novel route of transmission and the possible importation of melioidosis to a nonendemic area, the United States. The authors highlight that animals should be seriously considered as a potential hazard in transmitting melioidosis to humans.
- 61.Galimand M, Dodin EG. The health risks of importing tropical fish [in French]. A Revue Française d’Aquariologie Herpétologie 1981; 8:19–22. [Google Scholar]
- 62.Gee JE, Gulvik CA, Elrod MG, et al. Phylogeography of Burkholderia pseudomallei Isolates, Western Hemisphere. Emerg Infect Dis 2017; 23:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engelthaler DM, Bowers J, Schupp JA, et al. Molecular investigations of a locally acquired case of melioidosis in Southern AZ, USA. PLoS Negl Trop Dis 2011; 5:e1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benoit TJ, Blaney DD, Gee JE, et al. Melioidosis cases and selected reports of occupational exposures to Burkholderia pseudomallei--United States. MMWR Surveill Summ 2015; 64:1–9. [PubMed] [Google Scholar]
- 65▪▪.Gee JE, Bower WA, Kunkel A, et al. Multistate outbreak of melioidosis associated with imported aromatherapy spray. N Engl J Med 2022; 386:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report confirms a multistate outbreak of melioidosis in four different states in the United States. This observation shows that imported products could introduce B. pseudomallei to nonendemic areas and may cause severe disease.
- 66.Merritt AJ, Peck M, Gayle D, et al. Cutaneous Melioidosis cluster caused by contaminated wound irrigation fluid. Emerg Infect Dis 2016; 22:1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪▪. IPCC, 2022: Summary for Policymakers [Pörtner HO, Roberts DC, Poloczanska ES, et al (eds.)]. In: Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. In Press. [Accessed 1 April 2022] [Google Scholar]; This report demonstrates the scientific evidence of the interactions of climate, ecosystems and biodiversity, and human societies and integrates knowledge across the natural, ecological, social and economic sciences. This is essential for understanding the influence of climate change on the epidemiology of melioidosis.
- 68▪▪. IPCC Summary for Policymakers. In: Global warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above preindustrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Masson-Delmotte V, Zhai P, Portner HO, et al. [Accessed 22 December 2022] [in press] [Google Scholar]; The Intergovernmental Panel on Climate Change (IPCC) describes the scientific knowledge on climate change. This is essential for understanding the influence of climate change on the epidemiology of melioidosis.
- 69.Raymond C, Horton RM, Zscheischler J, et al. Understanding and managing connected extreme events. Nat Climate Change 2020; 10:611–621. [Google Scholar]
- 70.Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis 2003; 9:1538–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X, Pang L, Sim SH, Goh KT, et al. Association of melioidosis incidence with rainfall and humidity, Singapore, 2003–2012. Emerg Infect Dis 2015; 21:159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sam IC, Puthucheary SD. Melioidosis and rainfall in Kuala Lumpur, Malaysia. J Infect 2007; 54:519–520. [DOI] [PubMed] [Google Scholar]
- 73.Bulterys PL, Bulterys MA, Phommasone K, et al. Climatic drivers of melioidosis in Laos and Cambodia: a 16-year case series analysis. Lancet Planet Health 2018; 2:e334–e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmermann RE, Ribolzi O, Pierret A, et al. Rivers as carriers and potential sentinels for Burkholderia pseudomallei in Laos. Sci Rep 2018; 8:8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ko WC, Cheung BM, Tang HJ, et al. Melioidosis outbreak after typhoon, southern Taiwan. Emerg Infect Dis 2007; 13:896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mu JJ, Cheng PY, Chen YS, et al. The occurrence of melioidosis is related to different climatic conditions in distinct topographical areas of Taiwan. Epidemiol Infect 2014; 142:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chierakul W, Winothai W, Wattanawaitunechai C, et al. Melioidosis in 6 tsunami survivors in southern Thailand. Clin Infect Dis 2005; 41:982–990. [DOI] [PubMed] [Google Scholar]
- 78.Kaestli M, Grist EPM, Ward L, et al. The association of melioidosis with climatic factors in Darwin, Australia: a 23-year time-series analysis. J Infect 2016; 72:687–697. [DOI] [PubMed] [Google Scholar]
- 79.Chen PS, Chen YS, Lin HH, et al. Airborne transmission of melioidosis to humans from environmental aerosols contaminated with B. pseudomallei. PLoS Negl Trop Dis 2015; 9:e0003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80▪▪.Jayasinghearachchi HS, Francis VR, Sathkumara HD, et al. Nonclonal Burkholderia pseudomallei Population in Melioidosis Case Cluster, Sri Lanka. Emerg Infect Dis 2021; 27:2955–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrated a melioidosis case cluster in 10 patients after a flooding (extreme weather event) in eastern Sri Lanka. Climate change could directly impact flooding and influence the epidemiology of melioidosis.
- 81.Yip TW, Hewagama S, Mayo M, et al. Endemic melioidosis in residents of desert region after atypically intense rainfall in central Australia. Emerg Infect Dis 2015; 21:1038–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Black R, Adger WN, Arnell NW, et al. The effect of environmental change on human migration. Glob Environ Change 2011; 21:S3–S11. [Google Scholar]
- 83.Ribolzi O, Rochelle-Newall E, Dittrich S, et al. Land use and soil type determine the presence of the pathogen Burkholderia pseudomallei in tropical rivers. Environ Sci Pollut Res Int 2016; 23:7828–7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84▪.Hoffmann R, Dimitrova A, Muttarak R, et al. A meta-analysis of country-level studies on environmental change and migration. Nat Climate Change 2020; 10:904–912. [Google Scholar]; This meta-analysis elucidates the relation between climate change and migration, two factors possibly contributing to an increased incidence of melioidosis.
- 85.Merritt AJ, Inglis TJJ. The role of climate in the epidemiology of melioidosis. Curr Trop Med Rep 2017; 4:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McMichael C. Climate change-related migration and infectious disease. Virulence 2015; 6:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gibbs P. Origins of One Health and One Medicine. Vet Rec 2014; 174:152. [DOI] [PubMed] [Google Scholar]
- 88.Zinsstag J, Mackenzie JS, Jeggo M, et al. Mainstreaming one health. Ecohealth 2012; 9:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suntornsut P, Chaowagul W, Thongklang W, et al. Feasibility and initial outcomes of a multifaceted prevention programme of melioidosis in diabetic patients in Ubon Ratchathani, northeast Thailand. PLoS Negl Trop Dis 2018; 12:e0006765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whitmore A. On the bacteriology of an infective disease occurring in Rangoon. Br Med J 1912; 1306–1308. [Google Scholar]
- 91.Chewapreecha C, Holden MT, Vehkala M, et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat Microbiol 2017; 2:16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baker AL, Pearson T, Sahl JW, et al. Burkholderia pseudomallei distribution in Australasia is linked to paleogeographic and anthropogenic history. PLoS One 2018; 13:e0206845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hampton V, Kaestli M, Mayo M, et al. Melioidosis in Birds and Burkholderia pseudomallei Dispersal, Australia. Emerg Infect Dis 2011; 17:1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamthan A, Shaw T, Mukhopadhyay C, Kumar S. Molecular analysis of clinical Burkholderia pseudomallei isolates from southwestern coastal region of India, using multilocus sequence typing. PLoS Negl Trop Dis 2018; 12:e0006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webb JR, Win MM, Zin KN, et al. Myanmar Burkholderia pseudomallei strains are genetically diverse and originate from Asia with phylogenetic evidence of reintroductions from neighbouring countries. Sci Rep 2020; 10:16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarovich DS, Garin B, De Smet B, et al. Phylogenomic analysis reveals an Asian origin for African Burkholderia pseudomallei and further supports melioidosis endemicity in Africa. MSphere 2016; 1:e00089–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferry R, Poutrel B, Bruneau F. Isolation of Whitmore's Bacillus from lesions found in pigs from the Niamey slaughterhouse in Niger. Bull Soc Pathol Exot Filiales 1973; 66:42–45. [PubMed] [Google Scholar]