Abstract
An evaluation of the impact of vitamin E deficiency on expression of the alpha-tocopherol transfer protein (α-TTP) and related CRAL_TRIO genes was undertaken using livers from adult zebrafish based on the hypothesis that increased lipid peroxidation would modulate gene expression. Zebrafish were fed either a vitamin E sufficient (E+) or deficient (E−) diet for 9 months, then fish were euthanized, and livers were harvested. Livers from the E+ relative to E− fish contained 40-times more α-tocopherol (P<0.0001) and one fourth the malondialdehyde (P = 0.0153). RNA was extracted from E+ and E− livers, then subject to evaluation of gene expression of ttpa and other genes of the CRAL_TRIO family, genes of antioxidant markers, and genes related to lipid metabolism. Ttpa expression was not altered by vitamin E status. However, one member of the CRAL_TRIO family, tyrosine-protein phosphatase non-receptor type 9 gene (ptpn9a), showed a 2.4-fold increase (P=0.029) in E− relative to E+ livers. Further, we identified that the gene for choline kinase alpha (chka) showed a 3.0-fold increase (P=0.010) in E− livers. These outcomes are consistent with our previous findings that show vitamin E deficiency increased lipid peroxidation causing increases in phospholipid turnover.
Keywords: Alpha-tocopherol transfer protein (α-TTP), choline, CRAL_TRIO, ptpn9a, SEC14
Graphical Abstract
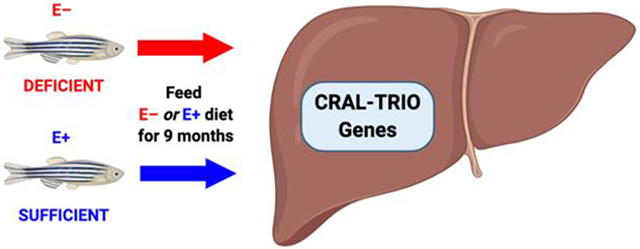
The Alpha-Tocopherol Transfer Protein (α-TTP) is a highly conserved protein that is characteristic of the CRAL_TRIO family. α-TTP is needed to maintain α-tocopherol concentrations in humans, as well as zebrafish. We hypothesized that fish consuming a vitamin E deficient diet would be stimulated to upregulate hepatic gene expression for CRAL_TRIO genes, if these were dependent on an adequate amount of α-tocopherol. Further, deficiency might also lead to increased expression of genes involved in responses to oxidized phospholipids.
INTRODUCTION
Alpha-tocopherol transfer protein (α-TTP) is a highly conserved protein among vertebrates that is needed to maintain α-tocopherol concentrations in humans and prevent vitamin E deficiency [1, 2]. Ataxia with isolated vitamin E deficiency (AVED) arises from a genetic disorder in which α-TTP has one of multiple known functional mutations [3]. These mutations restrict the α-TTP ability to preferentially bind α-tocopherol and impair α-tocopherol transfer to lipoproteins and thus cause human vitamin E deficiency [4]. AVED is characterized by degeneration of sensory neurons and progressive dying back of peripheral nerves, which causes a spinocerebellar ataxia [3, 5]. Progression of vitamin E deficiency symptoms can be halted by vitamin E supplementation [6].
The three-dimensional α-TTP structure, containing two separate lipid binding domains [7], is highly characteristic of its classification as a member of CRAL_TRIO (Pfam entry: PF00650). These two CRAL_TRIO domains are especially important because they allow α-TTP to bind RRR-α-tocopherol with high affinity and specificity compared with other tocols present in the diet [8, 9]. This protein structure is also critical for the discrimination between natural and synthetic vitamin E [10], which is defective in AVED [2].
CRAL_TRIO family proteins are cytosolic and contain the SEC14 carboxy-terminal domain [7, 9]. The CRAL_TRIO subgroup derives its name from the cellular retinaldehyde-binding protein (CRALBP) and the TRIO guanine exchange factor [11]. SEC14 was originally shown to be a cytosolic factor that promotes protein export from yeast Golgi [12]. The homologous domain within SEC14 proteins is believed to be essential to lipid binding, but multi-domain proteins containing a SEC14 domain may be involved in more complex processes including transport and signal transduction related to lipid metabolism [13]. A subgroup of the SEC14/CRAL_TRIO family is known as SEC14L. These latter proteins contain C-terminal Golgi dynamics (GOLD) domain, which is hypothesized to be involved in attaching specific proteins to the Golgi membrane for anchoring during protein-protein interactions [13, 14]. Before the link between SEC14 and CRALBP was established, α-TTP was recognized as the only protein that resembled CRALBP in structure, having ~58% sequence homology [15]. Perhaps not coincidentally, CRALBP’s function in visual organs resembles that of α-TTP; CRALBP discriminates between isoforms of retinaldehyde to preferentially bind and transport 11-cis-retinaldehyde [15]. The CRAL_TRIO family of proteins contains many coding genes in zebrafish, not all of which were evaluated in this study. Specifically, CRAL_TRIO genes were selected for this study based on the likelihood of expression in adult zebrafish hepatic tissue or their relationship to known lipid changes resulting from vitamin E deficiency.
Relatively little is known about α-TTP regulation. Further, the impact of vitamin E status on genes encoding other CRAL_TRIO family proteins [11] is unknown. Since α-tocopherol is a lipophilic antioxidant, the impacts of α-tocopherol availability or oxidant distress on regulation of the gene coding for α-TTP (ttpa) have gained attention. Etzl et al. [16] concluded, using an in vitro cell model, the human choriocarcinoma cell line BeWo, that chemically induced peroxidative stress is associated with increased α-TTP. However, Shaw and Huang [17] concluded that ttpa expression was not affected by vitamin E deficiency, but rather by protein insufficiency in the diet. Similarly, Fechner et al. [18] found that rats consuming a vitamin E deficient diet for 5 weeks did not have changed ttpa expression, but when the deficient rats were re-fed α-tocopherol or δ-tocopherol, hepatic ttpa expression increased nearly 7-fold.
Zebrafish are an excellent model for gene expression analysis, in large part due to their genome being highly similar to those of mammals [19]. We have studied vitamin E deficiency in zebrafish fed a defined diet lacking vitamin E and have shown that they have increased lipid peroxidation, dysregulated thiols and energy metabolism [20–22]. Dietary vitamin E deficiency in zebrafish adults causes impaired cognitive function, when tested with behavioral assays of learning and habituation [23]. Vitamin E deficient zebrafish brains exhibit increased lipid peroxidation, alterations in phospholipid and lysophospholipids [21] and disturbances to their energy metabolism, the likely mechanism preceding cognitive decline in cognitive function [23]. Long term vitamin E deficiency in adults exacerbates vitamin C deficiency and causes degenerative myopathy [24]. Additionally, their embryos display significantly higher levels of both mortality and morphological defects, especially in nervous system development [25, 26].
We hypothesized that livers from zebrafish consuming a vitamin E deficient diet would be stimulated to upregulate gene expression for α-TTP and other CRAL_TRIO genes, if these were dependent on an adequate amount of α-tocopherol for regulation. Further, vitamin E deficiency might also lead to increased expression of genes involved in antioxidant or metabolic responses to deficiency, based on studies in vitamin E deficient zebrafish and embryos [23, 26–29]. Thus, genes related to replacement of oxidized phospholipids, or to antioxidant responses were also examined, in addition to evaluating CRAL_TRIO genes.
EXPERIMENTAL SECTION
Animal Use and Care
All animal care was done in accordance with Oregon State University Institutional Animal Care and Use Committee approved protocol #5068. Danio rerio, commonly known as tropical zebrafish, of the 5D strain were housed and raised at the Sinnhuber Aquatic Research Laboratory at Oregon State University. The facility was kept within conditions consistent with standard zebrafish breeding protocols, 28 °C and a 14h light/10h dark light cycle. Fish were housed in reverse osmosis water containing commercially available salt solution. After 55 days post-fertilization, the adult zebrafish were divided randomly into two experimental groups; one was fed a vitamin E deficient (E−) diet and the other a vitamin E sufficient (E+) diet. The E+ diet contained α-tocopherol (230 ± 11 mg/kg) and γ-tocopherol (16 mg/kg) and the E−diet contained substantially less of both (α-tocopherol = 6 mg/kg, γ-tocopherol = 2 mg/kg). The adult fish consumed their respective diets for 9-months before euthanasia by cold exposure in ice water, then livers were removed, frozen in liquid nitrogen, and the tissue was stored at −80 °C.
Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
RNA was extracted from zebrafish livers (n=7 per group) using a Direct-zol RNA Miniprep Kit per the manufacturer’s protocol (Zymo Research, Irvine CA). RNA concentration values and integrity were assessed using UV-Vis absorbance performed by spectrophotometry (BioTek, Winooski, VT). This data was used to calculate a 260/280 ratio for each sample and confirm that a minimum ratio of 1.75 was met. 260/280 ratios across both groups averaged 1.85 ± 0.05 (mean ± SD); a t-test indicated no significant differences between groups (P=0.396). cDNA was synthesized for use in reverse transcription using a High Capacity Superscript kit from Applied Biosystems.
Genes related to CRAL_TRIO, lipid metabolism, or antioxidant functions were selected based on likelihood of expression in zebrafish hepatic tissue or association with lipid metabolic pathways as hypothesized. Primers for these genes were designed using information from the NCBI site and the online NCBI PrimerBlast tool. Considering the duplicate genome of zebrafish [19] and the number of alias’ often used to refer to understudied genes, it was frequently necessary to use the NCBI website to identify zebrafish orthologs and confirm the conservation of function using the Zfin (https://zfin.org/) catalog of genes. NCBI Primer Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) was used to design primers that were limited to amplicons between 100-200 bp (Table 1). Primers were selected based on a high number of exon-spanning regions and low self-complimentary values. Sequences that fit criteria for selection were entered into Primer-BLAST using the manual forward and reverse sequence input option to ensure specificity. Selected primer sequences were synthesized (Integrated DNA Technologies), then the primer stock solution was diluted to 100 μmol/L and mixed 10:1 with DNase/RNase-free water to produce a working solution. PCR was performed with 1X SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules CA). In addition to genes of interest, three housekeeping genes (mitochondrial ribosomal protein S18A, mrps18; mitochondrial ribosomal protein L45, mrpl45; ornithine decarboxylase 1, odc1) previously determined to be unchanged by Vitamin E status [30], were analyzed. Bio-Rad 96-well PCR plates were used for thermocycling. Samples (n=7) from each group were analyzed in duplicate with a BioRad CFX96 instrument. Data are reported as fold change values relative to the E+ group (control). Any sample returning a Ct (cycle threshold) value above 35 was considered inconclusive and was removed from statistical analysis (see below).
Table 1 –
Primer Sequences
| NCBI gene | Abbreviationa | Forward Primer | Reverse Primer |
|---|---|---|---|
| 394197 | bnip2 | 5′-CACGAGAGCACCAGGAGTC-3′ | 5′-CCGTAAGACTGGGAAGCAGG-3′ |
| 558499 | chka | 5′-GATGAGCCAGACCAGCAGAC-3′ | 5′-CGTGGTTCATCTCCAAGGCT-3′ |
| 352928 | gpx4a | 5′-TACTGAAAGGCAGTCATGCGT-3′ | 5′-CGTGCATCTCTGCAAACTGAG-3′ |
| 352929 | gpx4b | 5′-CTGCAACCAGTTCGGAAAGC-3′ | 5′-CCCAGTGTTCCTCTGCCTTT-3′ |
| 564619 | gstr | 5′- GAAATGGCGCTCGTCTACCA-3′ | 5′- TCCTTCAGGCACAAGCCAAT-3′ |
| 564518 | nf1b | 5′-AGGAAGTGAGGAAAGCAGTCG-3′ | 5′-GTCAAGAATCCCAGCCTCGT-3′ |
| 360149 | nfe2l2a | 5′-GGCGATCCTCCTGTAAACCC-3′ | 5′-CGAAGGATCCGTCTTCGGTT-3′ |
| 393127 | pemt | 5′-GTTATTCGCTGGCCGTCCTA-3′ | 5′-TGACCAACAGAGAACCGACG-3′ |
| 100526653 | ptpn9a | 5′-GAGAGTGGATGATGGCCGAC-3′ | 5′-GACATCAAACTTGCGTGCCA-3′ |
| 562170 | ptpn9b | 5′-CATCCCAGTCAGCTCCCTTC-3′ | 5′-AGCAGACTCCCCAAGCAATC-3′ |
| 557037 | pparg | 5′-CATGTACAGCTTACAGGACACAGG-3′ | 5′-CTTCAGGAGTTTTGGAGAATGAAA-3′ |
| 387260 | scarb1 | 5′-TGAAGAGAGCGGCTACATCG-3′ | 5′-CGTTTTACTTTTTACCTTATCGGTG-3′ |
| 394073 | sec14l1 | 5′-GACTTCCTGCTGGACACATGG-3′ | 5′-CAGACCTTCCTCGTTGATGGA-3′ |
| 100333377 | slc44a1a | 5′- CACCATCTTTACTGTCCTTTTGCT-3′ | 5′- GTAGACCCAGAAGAGCATGAGTGT-3′ |
| 325906 | ttpa | 5′-CCTCATCCGTCATTGGACTT-3′ | 5′-TTTGGGGTTCCATTTACCAA-3′ |
| 321577 | ttpal | 5′-ACACGGAACTGATTGCGGAT-3′ | 5′-GGACCTGGGAAGCCTAAACC-3′ |
BCL2 interacting protein 2 (bnip2); choline kinase alpha (chka); glutathione peroxidase 4a (gpx4a); glutathione peroxidase 4b (gpx4b); glutathione S-transferase rho (gstr); neurofibromin 1b (nf1b); nuclear factor, erythroid 2-like 2a (nfe2l2a); phosphatidylethanolamine N-methyltransferase (pemt); protein tyrosine phosphatase non-receptor type 9a (ptpn9a); protein tyrosine phosphatase non-receptor type 9b (ptpn9b); peroxisome proliferator activated receptor gamma (pparg); scavenger receptor B1 (scarb1); SEC14-like lipid binding 1 (sec14l1); solute carrier family 44 member 1a (slc44a1a); tocopherol (alpha) transfer protein (ttpa); tocopherol (alpha) transfer protein-like (ttpal).
Vitamin E and Malondialdehyde Quantification
Livers from E+ (n=5) and E− (n=4) fish, which were from the same aged cohort as the fish used for gene expression, were homogenized in water. DC Protein Assay (Bio-Rad, Hercules, CA) was used to determine total cellular proteins, per kit instructions. Absorbance was read at 750 nm on the BioTek Synergy HTX (BioTek Instruments, Inc., Winooski, VT) with a 96-well flat bottom plate.
Liver α-tocopherol concentrations were measured in an aliquot of the liver homogenate using high-pressure liquid chromatography with electrochemical detection (HPLC-ECD), as described [31]. Briefly, livers were homogenized and then an aliquot saponified in alcoholic KOH with 1% ascorbic acid at 70° C for one hour. Using an isocratic method (99% methanol with 1% lithium perchlorate) samples were injected onto a Synergi™ 4 μm Hydro-RP 80 Å, LC Column 150 x 4.6 mm (Phenomenex, Torrance CA) with a Synergi™ SecurityGuard 4 x 3.0 mm guard column using a Shimadzu HPLC with a LC-10ADvp controller, and a SIL-10ADvp auto injector with a 50μl sample loop (Canby, OR) and measured by ECD (Bioanalytical Systems, BASi, West Lafayette, IN). The electrochemical detector was in the oxidizing mode, potential 500 mV, full recorder scale at 500 nA. Peak areas were integrated using Shimadzu Scientific 4.2 Class VP software package, and tocopherols were quantitated by comparison to authentic compounds.
Malondialdehyde (MDA) concentrations were measured in an aliquot of the liver homogenate according to the method by Hong et al. Briefly, an alkaline hydrolysis was performed in the presence of butylated hydroxy toluene (BHT) at 60°C for 30 min to release protein-bound MDA. Following a brief cooling, samples were acidified and thiobarbituric acid (TBA) reagent was added and the reaction mixture was heated at 95°C for 60 minutes. The MDA(TBA)2 adduct was then partitioned out of the mixture with n-butanol. An aliquot of the butanol phase was injected onto a Waters 2695 HPLC (Milford, MA) reverse phase system including a cooled autosampler and a Phenomenex Luna 5μ C18, 4.6 x 250 mm column. The MDA(TBA)2 adduct was eluted using an isocratic mobile phase consisting of 50% methanol and 50% 25 mM phosphate buffer at pH 6.5 at a flow rate of 1 mL/minute. Malondialdehyde was detected by fluorescence at excitation 532 nm and emission 533 nm. Quantitation was done using an external standard of 1, 1, 3, 3-tetraethoxypropane (Sigma, St Louis, MO) prepared using the same method.
Statistical Analyses
Vitamin E and MDA concentrations were logarithmically transformed to correct for unequal variances, then the Student’s t-test was used to compare data from the two groups. Pearson’s correlation was used evaluate the relationship between the two data sets.
Relative gene expression values were calculated using the 2−ΔΔCt method as described by Livak and Schmittgen [32]. In brief, sample averages for duplicate determinations of each gene were generated from raw Ct values. Next, ΔCt values were calculated by subtracting each sample’s reference value (calculated as the geometric mean of the three housekeeping genes) from each sample average. ΔΔCt values were then calculated for each gene and diet group by subtracting the E+ ΔCt group average from the E− ΔCt group average. Finally, fold change (FC) values, giving E− expression relative to E+, were calculated by 2−ΔΔCt. Statistical significance (P<0.05) was determined by performing a t-test comparing E+ sample average ΔCt values to E− (GraphPad Prism 6 for Mac).
RESULTS
Vitamin E Quantification
Livers from the E+ fish contained 40-times more α-tocopherol compared with E− fish (P<0.0001, Figure 1A). The E− vs E+ livers contained 4-times the malondialdehyde (MDA) concentrations, a measure of lipid peroxidation (P = 0.0153, Figure 1B). The α-tocopherol concentrations were negatively correlated (P = 0.0067) with the MDA concentrations, based on the Pearson’s correlation of the logarithmically transformed data from both groups
Figure 1: Liver Vitamin E Concentrations.
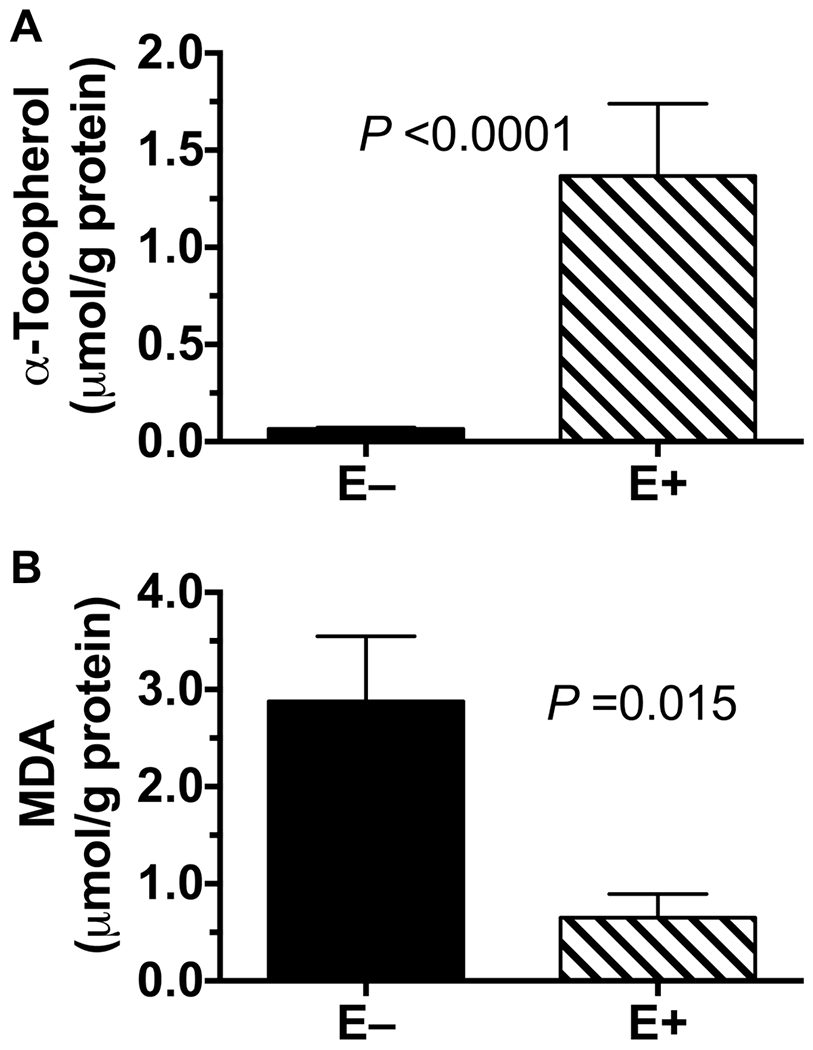
Livers from adult zebrafish fed in E+ (n=5) and E− (n=4) diets for 9 months were analyzed. α-Tocopherol (A) and malondialdehyde (MDA, B) are reported per protein (mean ± SEM, μmol/g). Statistical differences were evaluated using Student’s t-test and the logarithmic transformation of the data.
Gene Expression Outcomes
Ttpa and CRAL_ TRIO Genes
Comparisons between livers from E+ and E− fish showed that ttpa gene expression was not significantly (P=0.36) different between the two diet groups (Figure 2). Expression levels for several other genes of the CRAL_TRIO family were also evaluated. The gene encoding alpha tocopherol transfer protein-like (ttpal) showed a non-significant (P = 0.46) 1.3 fold-change between E− and E+ livers. Both SEC14-like lipid binding 1 (sec14l1) and SEC14-like lipid binding 8 (sec14l8) are members of the SEC14L subgroup. Tocopherol associated protein (TAP) in humans is orthologous to zebrafish sec14l8, but this gene was in too low abundance for evaluation (data not shown). Vitamin E status had no significant effect on sec14l1 (P=0.29). BCL2 interacting protein 2 (bnip2) and neurofibromin 1b (nf1b) (orthologous to human NF1) were also not significantly different between groups (P=0.26 and P=0.64 respectively).
Figure 2: Relative gene expression of CRAL_TRIO genes.
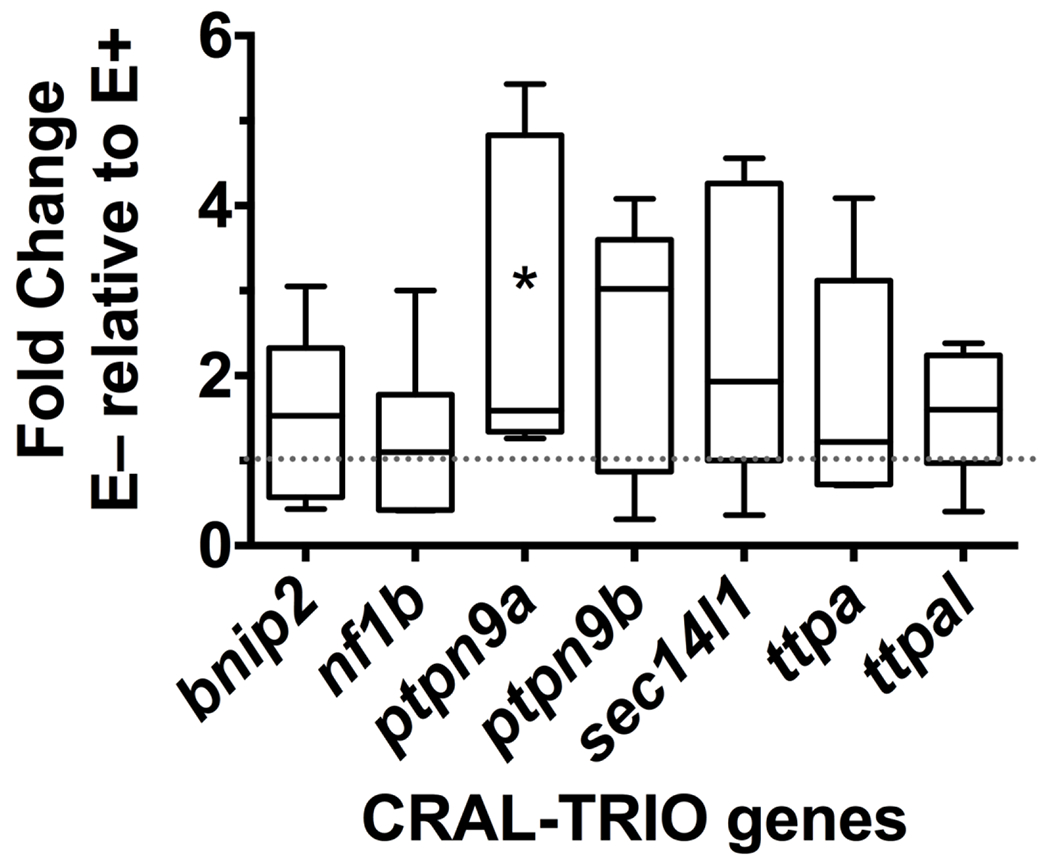
Relative mRNA expression is shown as E− fold change relative to E+ (control, dotted line at 1). Boxes extend from 25th to 75th percentile, the whiskers range between 10-90%, line shown is the median, n = 7 per group. Only ptpn9a (*) was significantly different between E+ and E− livers (P=0.029). Abbreviations: BCL2 interacting protein 2 (bnip2); neurofibromin 1b (nf1b); protein tyrosine phosphatase non-receptor type 9a (ptpn9a); protein tyrosine phosphatase non-receptor type 9b (ptpn9b); SEC14-like lipid binding 1 (sec14l1); tocopherol (alpha) transfer protein (ttpa); tocopherol (alpha) transfer protein-like (ttpal).
Both duplicates of the human tyrosine-protein phosphatase non-receptor type 9 gene present in the zebrafish genome, ptpn9a and ptpn9b, were evaluated. In E− fish livers, ptp9na showed a significant (P=0.01) 2.4 fold-increase relative to E+ livers. However, its gene duplicate, ptpn9b, was not different between groups (P=0.22). Overall, ptpn9a was the only CRAL_TRIO gene that demonstrated regulation by vitamin E status.
Antioxidant Related Genes
Given that α-tocopherol is a lipophilic, chain breaking antioxidant [33], genes related to antioxidant activity were also of interest (Figure 3). Zebrafish genes for glutathione peroxidase 4a (gpx4a) and glutathione peroxidase 4b (gpx4b) are both orthologues of human GPX4. Neither of the genes were significantly different between groups (P= 0.73 and 0.97, respectively). Glutathione S-transferase rho (gstr), zebrafish orthologue of human glutathione S-transferase theta 2B (GSTT2B), and nuclear factor, erythroid 2-like 2a (nfe2l2a, formerly known as nrf2), ortholog of human nuclear factor, erythroid 2 like 2 (NFE2L2), were not different between groups (P=0.49 and 0.35 respectively).
Figure 3: Relative expression of antioxidant related genes.
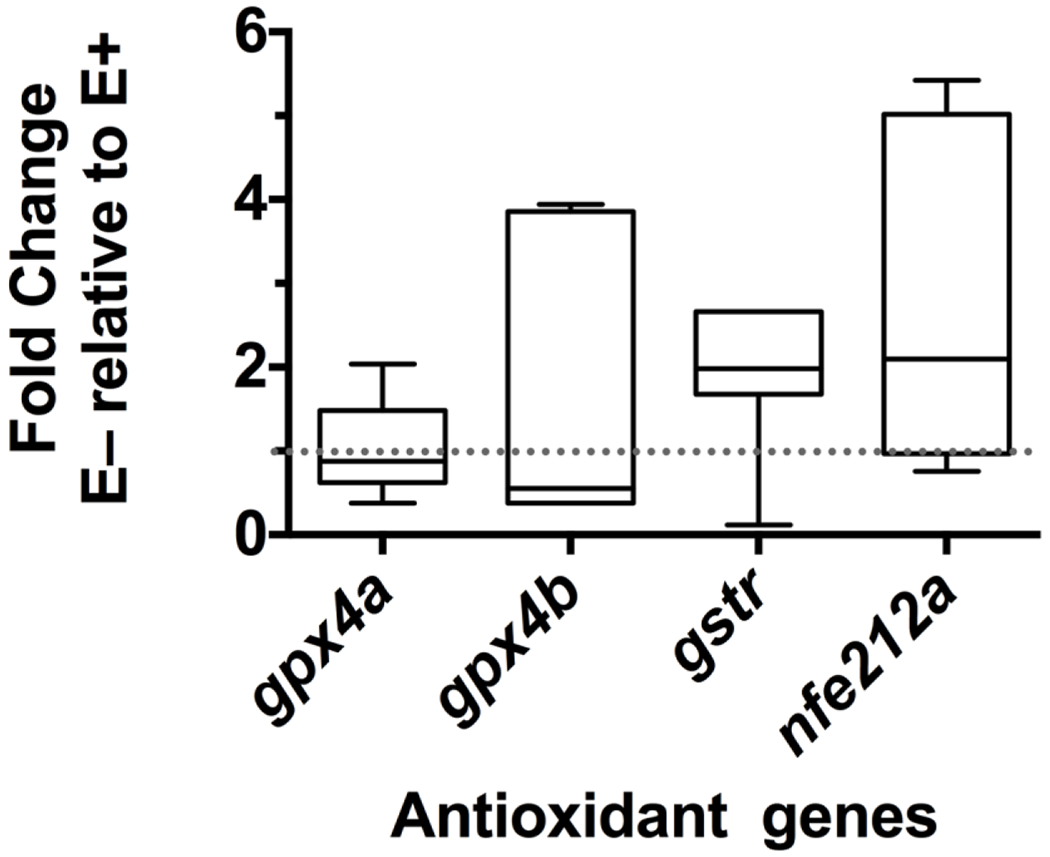
Relative mRNA expression is shown as E− fold change relative to E+ (control, dotted line at 1). Boxes extend from 25th to 75th percentile, the whiskers range between 10-90%, line shown is the median, n = 7 per group. No genes were significantly different between E+ and E− livers. Abbreviations: glutathione peroxidase 4a (gpx4a); glutathione peroxidase 4b (gpx4b); glutathione S-transferase rho (gstr); nuclear factor, erythroid 2-like 2a (nfe2l2a).
Lipid Metabolism Related Genes
Relative expression analysis of genes coding for the choline kinase alpha (chka), solute carrier family 44 member 1a (slc44a1a)—a choline transporter, and phosphatidylethanolamine N-methyltransferase (pemt) genes indicate that there is a disruption to normal choline metabolism in the E− livers (Figure 4). The 3.1-fold change in chka expression (P=0.01) suggests that choline metabolism is upregulated in E− fish livers. The PEMT pathway also tended towards up-regulation in the E− group; however, this finding lacked statistical significance (P=0.08).
Figure 4: Relative expression of lipid related genes.
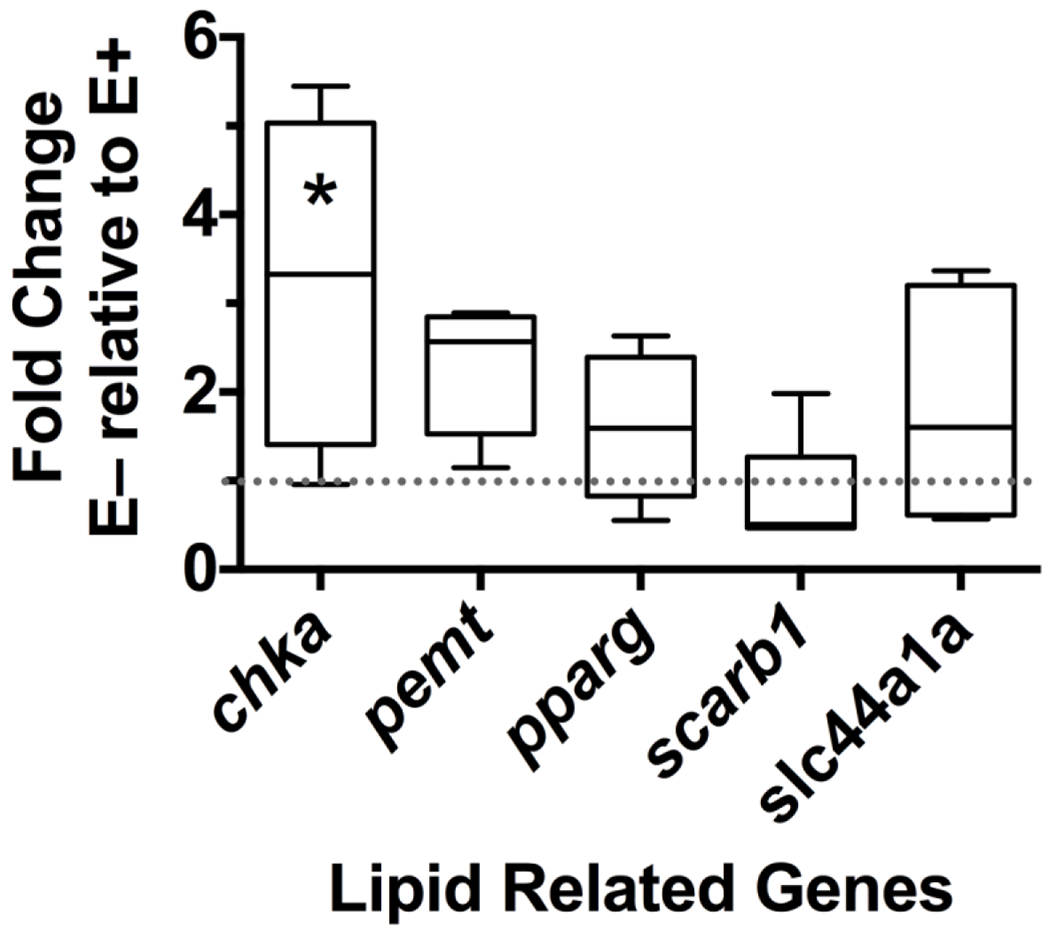
Relative mRNA expression is shown as E− fold change relative to E+ (control, dotted line at 1). Boxes extend from 25th to 75th percentile, the whiskers range between 10-90%, line shown is the median, n = 7 per group. Only chka (*) was significantly different between E+ and E− livers (P=0.010). Abbreviations: choline kinase alpha (chka); phosphatidylethanolamine N-methyltransferase (pemt); peroxisome proliferator activated receptor gamma (pparg); scavenger receptor B1 (scarb1); solute carrier family 44 member 1a (slc44a1a).
Neither peroxisome proliferator-activated receptor gamma (pparg) nor the scavenger receptor B1 (scrarb1) were regulated by vitamin E status (P= 0.22 and P= 0.99, respectively).
DISCUSSION
Despite the significant differences between vitamin E quantities in the diets and liver concentrations, E− fish did not demonstrate significantly increased ttpa expression, consistent with our previous studies in vitamin E deficient zebrafish [25]. Our study is novel with respect to measuring ttpa expression in livers from adult fish that have become vitamin E deficient and remained on deficient diets for up to 9 months. However, Ulatowski et al [34], who used a mouse cell line to evaluate ttpa mRNA expression in vitro after oxidative stress was induced by hydrogen peroxide, suggest that there is transcriptional level regulation of α-TTP in response to oxidative stress. Further, Etzl et al. [16] concluded using BeWo cells induced with either 2,2 ′ -azobis (2-amidinopropane) dihydrochloride (a peroxyl radical generator) or with l-buthionine-(S,R)-sulfoximine (a glutathione synthesis inhibitor) that α-TTP expression was increased. Potentially, the level of oxidative distress in E− zebrafish livers may not be sufficient to significantly up-regulate ttpa mRNA expression, or fish may have additional mechanisms in place to relieve the stress since MDA concentrations were only 4-fold elevated in E− livers (Figure 1).
Only one gene belonging to the CRAL_TRIO family, ptpn9a, was found to show significantly increased expression in E− livers. Ptpn9a belongs to a family of proteins known as protein tyrosine phosphatases (PTPs), which can undergo reversible oxidation [35]. This class of enzymes is responsible for catalyzing the dephosphorylation of phosphotyrosyl in proteins, which can have wide ranging signaling effects including regulating cell growth and oncogenic transformation [36]. Additionally, human PTPN9 (also named PTP-MEG2 tyrosine phosphatase (PTP-MEG2)[37]) has been shown to be important in controlling vesicle fusion [38]. Critically, vitamin E has also been shown to have a key role in the promotion of membrane repair through vesicle formation [39]. Whether ptpn9a is playing such a role in zebrafish livers remains to be investigated. However, ptpn9b, the other zebrafish ortholog of human PTPN9, was not differentially expressed between groups.
TTPAL has recently emerged as a gene of interest, but its function and specific role in any biological process has not yet been extensively evaluated. According to its gene ontology (NCBI), it is predicted to have phosphatidylinositol bisphosphate binding activity. Gou et al. [40] found TTPAL to be preferentially amplified in their whole genome analysis of patients with colorectal cancer. They found that patients with increased TTPAL expression showed increased tumorigenicity and lower survival. The origin of the name tocopherol (alpha) transfer protein-like does not relate to its biologic activity, but perhaps to its common motif in the CRAL_TRIO family. For this reason, we hypothesized that it may be regulated in response to antioxidant activity or oxidative stress. However, our results indicate that ttpal expression is not regulated by vitamin E status in zebrafish livers.
Based on our previous work in vitamin E deficient zebrafish and embryos, we have found that phospholipids, especially phosphatidyl choline with docosahexaenoic acid (DHA-PC) is depleted by oxidative stress, leading to both decreased choline concentrations and increased phospholipid turnover [21, 23, 28, 41]. We, therefore, investigated liver genes that might be involved in phospholipid responses to vitamin E deficiency. Choline kinase is an enzyme that catalyzes the first irreversible step for phosphatidylcholine synthesis via the CDP-choline pathway [42]. The significantly increased chka expression in E− zebrafish livers (Figure 4) is likely a response to depleted PC resulting from dietary vitamin E inadequacy. The zebrafish gene coding for the phosphatidylethanolamine N-methyltransferase protein (pemt) was also evaluated because DHA-PC can be generated via the PEMT pathway using S-adenosylmethionine (SAM) as a methyl-donor. While pemt did not show significantly increased expression in this study, it had a fold change of 2.2 (P= 0.08), indicating a similar process may be occurring in livers from E− fish. Since PEMT activity is important for liver health [43], this pathway may be a critical response to inadequate vitamin E. By contrast, slc44a1a, a gene for a transporter that is involved in choline transport into the mitochondria for oxidation to betaine [44], was unchanged by vitamin E deficiency.
Previously, we also showed in vitamin E deficient zebrafish embryos that expression of antioxidant-responsive genes (including gst, gpx4a, and gpx4b) were not different between E- and E+ groups [25]. Similarly, we found in this study that these genes did not change in the adult livers in response to long-term vitamin E deficiency.
A limitation of this study is that not all Sec14-related genes were evaluated. To date 1551 Sec14 domains are annotated in the NCBI database (http://www.ncbi.nlm.nih.gov). Sec14-like phosphatidylinositol transfer proteins have also diversified and bind a variety of lipids [45]. In comparison, the purpose of our investigation was extremely limited, specifically genes changes in response to vitamin E deficiency. We evaluated genes that we thought might have some relationship both to vitamin E and Sec14, based on the suggestion by Mousley et al[46] that lipid metabolism might be sensed by these genes. Additionally, there are many genes annotated for the CRAL_TRIO domain in zebrafish, but we only chose genes likely to be expressed in the liver or modulated by vitamin E. Notably retinaldehyde binding protein 1a and 1b (rlbp1a, rlbp1b), genes that code for proteins involved in the retinol metabolic cycle, were excluded because these genes are expressed only in the eye [47]. ATCAY kinesin light chain-interacting caytaxin a and b (atcaya, atcayb), both of which are highly expressed neuron-specific genes [48], as well as Kalirin RhoGEF kinase a and b (kalrna, kalrnb), which code for a Rho GDP/GTP exchange factor, have been primarily studied for their role in the nervous system. Additional functions outside of the nervous system have been proposed for some kalrn isoforms; however, a study in mice found that Sec14p containing isoforms are notably absent in the liver [49]. The genes, Triple function domain (trioa, triob) and Prune homolog 2 (prune2) are also expressed specifically in the central nervous system [50, 51]. Thus, many genes with CRAL_TRIO domains were not evaluated for expression changes because they are unlikely modified by vitamin E status or found in zebrafish livers.
In summary, the major objective of this study was to further our understanding of the extent to which vitamin E deficiency impacts gene regulation in the liver. Our study showed vitamin E deficient zebrafish do not show significantly different levels of expression for the ttpa gene. With the exception of ptpn9a, no other genes of the CRAL_TRIO family showed changes in their expression as a result of vitamin E status. The ptpn9a gene regulation may be important for membrane repair and metabolism. Lastly, in building on the recent findings on zebrafish embryo lipid metabolism [28, 29, 52], we confirmed that a critical step in the CDP-choline pathway is indeed activated and upregulated in livers from E− zebrafish. This is an exciting insight that may provide answers to how vitamin E deficient animals are able to use other micronutrients to cope with their insufficient protection against lipid peroxidation. Therefore, a more comprehensive analysis of the impact of vitamin E deficiency on genes involved in the CDP-choline pathway is warranted.
HIGLIGHTS.
Alpha-tocopherol transfer protein (α-TTP) is a highly conserved protein
α-TTP is a member of CRAL_TRIO, a lipid binding protein family
Vitamin E deficiency causes lipid peroxidation
Gene expression responses show impacts to membrane and choline metabolism
ACKNOWLEDGEMENTS
This study was supported in part by the Linus Pauling Institute. MGT is supported in part by the Ava Helen Pauling endowment to the Linus Pauling Institute.
Abbreviations
- α-TTP
Alpha-tocopherol transfer protein
- AVED
Ataxia with isolated vitamin E deficiency
- bnip2
BCL2 interacting protein 2
- chka
choline kinase alpha
- CRALBP
cellular retinaldehyde-binding protein
- gpx4a
glutathione peroxidase 4a
- gpx4b
glutathione peroxidase 4b
- gstr
glutathione S-transferase rho
- HPLC-ECD
high-pressure liquid chromatography with electrochemical detection
- nf1b
neurofibromin 1b
- nfe2l2a
nuclear factor, erythroid 2-like 2a
- pemt
phosphatidylethanolamine N-methyltransferase
- ptpn9a
protein tyrosine phosphatase non-receptor type 9a
- ptpn9b
protein tyrosine phosphatase non-receptor type 9b
- pparg
peroxisome proliferator activated receptor gamma
- scarb1
scavenger receptor B1
- sec14l1
SEC14-like lipid binding 1
- ttpa
tocopherol (alpha) transfer protein
- ttpal
tocopherol (alpha) transfer protein-like
- TRIO
guanine exchange factor
- E−
vitamin E deficient
- E+
vitamin E sufficient
Footnotes
The authors state that they have no conflicts of interest with regards to this work. Alexander T. Watt wrote a thesis which was submitted to the University Honors College of Oregon State University in partial fulfillment of the requirements for the degree of Honors Baccalaureate of Science in Biology.
REFERENCES
- [1].Traber MG, Sokol RJ, Burton GW, Ingold KU, Papas AM, Huffaker JE, et al. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J Clin Invest. 1990;85:397–407. doi: 10.1172/JCI114452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Traber MG, Sokol RJ, Kohlschutter A, Yokota T, Muller DP, Dufour R, et al. Impaired discrimination between stereoisomers of alpha-tocopherol in patients with familial isolated vitamin E deficiency. J Lipid Res. 1993;34:201–10. doi: [PubMed] [Google Scholar]
- [3].Schuelke M Ataxia with Vitamin E Deficiency. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 2005. May 20 [Updated 2016 Oct 13]. [Google Scholar]
- [4].Traber MG, Arai H. Molecular mechanisms of vitamin E transport. Annu Rev Nutr. 1999;19:343–55. doi: 10.1146/annurev.nutr.19.1.343 [DOI] [PubMed] [Google Scholar]
- [5].Di Donato I, Bianchi S, Federico A. Ataxia with vitamin E deficiency: update of molecular diagnosis. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2010;31:511–5. doi: 10.1007/s10072-010-0261-1 [DOI] [PubMed] [Google Scholar]
- [6].Kohlschutter A, Finckh B, Nickel M, Bley A, Hubner C. First Recognized Patient with Genetic Vitamin E Deficiency Stable after 36 Years of Controlled Supplement Therapy. Neurodegener Dis. 2020;20:35–8. doi: 10.1159/000508080 [DOI] [PubMed] [Google Scholar]
- [7].Min KC, Kovall RA, Hendrickson WA. Crystal structure of human alpha-tocopherol transfer protein bound to its ligand: implications for ataxia with vitamin E deficiency. Proc Natl Acad Sci U S A. 2003;100:14713–8. doi: 10.1073/pnas.2136684100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, et al. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–8. doi: 10.1016/s0014-5793(97)00499-7 [DOI] [PubMed] [Google Scholar]
- [9].Panagabko C, Morley S, Hernandez M, Cassolato P, Gordon H, Parsons R, et al. Ligand specificity in the CRAL-TRIO protein family. Biochem. 2003;42:6467–74. doi: 10.1021/bi034086v [DOI] [PubMed] [Google Scholar]
- [10].Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, et al. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–84. doi: 10.1093/ajcn/67.4.669 [DOI] [PubMed] [Google Scholar]
- [11].CRAL-TRIO lipid binding domain (IPR001251). InterPro. http://www.ebi.ac.uk/interpro/entry/InterPro/IPR001251/:EMBL-EBI; 2020. [Google Scholar]
- [12].Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108:1271–81. doi: 10.1083/jcb.108.4.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saito K, Tautz L, Mustelin T. The lipid-binding SEC14 domain. Biochim Biophys Acta. 2007;1771:719–26. doi: 10.1016/j.bbalip.2007.02.010 [DOI] [PubMed] [Google Scholar]
- [14].Anantharaman V, Aravind L. The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol. 2002;3:research0023. doi: 10.1186/gb-2002-3-5-research0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sato Y, Arai H, Miyata A, Tokita S, Yamamoto K, Tanabe T, et al. Primary structure of alpha-tocopherol transfer protein from rat liver. Homology with cellular retinaldehyde-binding protein. J Biol Chem. 1993;268:17705–10. doi: [PubMed] [Google Scholar]
- [16].Etzl RP, Vrekoussis T, Kuhn C, Schulze S, Poschl JM, Makrigiannakis A, et al. Oxidative stress stimulates alpha-tocopherol transfer protein in human trophoblast tumor cells BeWo. J Perinat Med. 2012;40:373–8. doi: 10.1515/jpm-2011-0307 [DOI] [PubMed] [Google Scholar]
- [17].Shaw HM, Huang C. Liver alpha-tocopherol transfer protein and its mRNA are differentially altered by dietary vitamin E deficiency and protein insufficiency in rats. J Nutr. 1998;128:2348–54. doi: 10.1093/jn/128.12.2348 [DOI] [PubMed] [Google Scholar]
- [18].Fechner H, Schlame M, Guthmann F, Stevens PA, Rustow B. alpha- and delta-tocopherol induce expression of hepatic alpha-tocopherol-transfer-protein mRNA. Biochem J. 1998;331 (Pt 2):577–81. doi: 10.1042/bj3310577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bradford YM, Toro S, Ramachandran S, Ruzicka L, Howe DG, Eagle A, et al. Zebrafish Models of Human Disease: Gaining Insight into Human Disease at ZFIN. ILAR J. 2017;58:4–16. doi: 10.1093/ilar/ilw040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lebold KM, Jump DB, Miller GW, Wright CL, Labut EM, Barton CL, et al. Vitamin E deficiency decreases long-chain PUFA in zebrafish (Danio rerio). J Nutr. 2011;141:2113–8. doi: 10.3945/jn.111.144279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Choi J, Leonard SW, Kasper K, McDougall M, Stevens JF, Tanguay RL, et al. Novel function of vitamin E in regulation of zebrafish (Danio rerio) brain lysophospholipids discovered using lipidomics. J Lipid Res. 2015;56:1182–90. doi: 10.1194/jlr.M058941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kirkwood JS, Lebold KM, Miranda CL, Wright CL, Miller GW, Tanguay RL, et al. Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J Biol Chem. 2012;287:3833–41. doi: 10.1074/jbc.M111.316018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McDougall M, Choi J, Magnusson K, Truong L, Tanguay R, Traber MG. Chronic vitamin E deficiency impairs cognitive function in adult zebrafish via dysregulation of brain lipids and energy metabolism. Free Radic Biol Med. 2017;112:308–17. doi: 10.1016/j.freeradbiomed.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lebold KM, Lohr CV, Barton CL, Miller GW, Labut EM, Tanguay RL, et al. Chronic vitamin E deficiency promotes vitamin C deficiency in zebrafish leading to degenerative myopathy and impaired swimming behavior. Comp Biochem Physiol C Toxicol Pharmacol. 2013;157:382–9. doi: 10.1016/j.cbpc.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miller GW, Labut EM, Lebold KM, Floeter A, Tanguay RL, Traber MG. Zebrafish (Danio rerio) fed vitamin E-deficient diets produce embryos with increased morphologic abnormalities and mortality. J Nutr Biochem. 2012;23:478–86. doi: 10.1016/j.jnutbio.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Head B, La Du J, Tanguay RL, Kioussi C, Traber MG. Vitamin E is necessary for zebrafish nervous system development. Sci Rep. 2020;10:15028. doi: 10.1038/s41598-020-71760-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Head B, Ramsey SA, Kioussi C, Tanguay RL, Traber MG. Vitamin E Deficiency disrupts gene expression networks during zebrafish development. Nutrients. 2021;13:468. doi: 10.3390/nu13020468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McDougall M, Choi J, Kim HK, Bobe G, Stevens JF, Cadenas E, et al. Lethal dysregulation of energy metabolism during embryonic vitamin E deficiency. Free Radic Biol Med. 2017;104:324–32. doi: 10.1016/j.freeradbiomed.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McDougall M, Choi J, Truong L, Tanguay R, Traber MG. Vitamin E deficiency during embryogenesis in zebrafish causes lasting metabolic and cognitive impairments despite refeeding adequate diets. Free Radic Biol Med. 2017;110:250–60. doi: 10.1016/j.freeradbiomed.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miller GW, Ulatowski L, Labut EM, Lebold KM, Manor D, Atkinson J, et al. The alpha-tocopherol transfer protein is essential for vertebrate embryogenesis. PLoS One. 2012;7:e47402. doi: 10.1371/journal.pone.0047402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37:893–901. doi: [PubMed] [Google Scholar]
- [32].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- [33].Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ulatowski L, Dreussi C, Noy N, Barnholtz-Sloan J, Klein E, Manor D. Expression of the alpha-tocopherol transfer protein gene is regulated by oxidative stress and common single-nucleotide polymorphisms. Free Radic Biol Med. 2012;53:2318–26. doi: 10.1016/j.freeradbiomed.2012.10.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Karisch R, Fernandez M, Taylor P, Virtanen C, St-Germain JR, Jin LL, et al. Global proteomic assessment of the classical protein-tyrosine phosphatome and “Redoxome”. Cell. 2011;146:826–40. doi: 10.1016/j.cell.2011.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhao S, Sedwick D, Wang Z. Genetic alterations of protein tyrosine phosphatases in human cancers. Oncogene. 2015;34:3885–94. doi: 10.1038/onc.2014.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kruger JM, Fukushima T, Cherepanov V, Borregaard N, Loeve C, Shek C, et al. Protein-tyrosine phosphatase MEG2 is expressed by human neutrophils. Localization to the phagosome and activation by polyphosphoinositides. J Biol Chem. 2002;277:2620–8. doi: 10.1074/jbc.M104550200 [DOI] [PubMed] [Google Scholar]
- [38].Huynh H, Bottini N, Williams S, Cherepanov V, Musumeci L, Saito K, et al. Control of vesicle fusion by a tyrosine phosphatase. Nat Cell Biol. 2004;6:831–9. doi: 10.1038/ncb1164 [DOI] [PubMed] [Google Scholar]
- [39].Howard AC, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. Nat Commun. 2011;2:597. doi: 10.1038/ncomms1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gou H, Liang JQ, Zhang L, Chen H, Zhang Y, Li R, et al. TTPAL Promotes Colorectal Tumorigenesis by Stabilizing TRIP6 to Activate Wnt/beta-Catenin Signaling. Cancer Res. 2019;79:3332–46. doi: 10.1158/0008-5472.CAN-18-2986 [DOI] [PubMed] [Google Scholar]
- [41].McDougall MQ, Choi J, Stevens JF, Truong L, Tanguay RL, Traber MG. Lipidomics and H2(18)O labeling techniques reveal increased remodeling of DHA-containing membrane phospholipids associated with abnormal locomotor responses in alpha-tocopherol deficient zebrafish (danio rerio) embryos. Redox Biol. 2016;8:165–74. doi: 10.1016/j.redox.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fagone P, Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta. 2013;1831:523–32. doi: 10.1016/j.bbalip.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wan S, van der Veen JN, Bakala N’Goma JC, Nelson RC, Vance DE, Jacobs RL. Hepatic PEMT activity mediates liver health, weight gain, and insulin resistance. FASEB J. 2019;33:10986–95. doi: 10.1096/fj.201900679R [DOI] [PubMed] [Google Scholar]
- [44].Hedtke V, Bakovic M. Choline transport for phospholipid synthesis: An emerging role of choline transporter-like protein 1. Exp Biol Med (Maywood). 2019;244:655–62. doi: 10.1177/1535370219830997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tripathi A, Martinez E, Obaidullah AJ, Lete MG, Lonnfors M, Khan D, et al. Functional diversification of the chemical landscapes of yeast Sec14-like phosphatidylinositol transfer protein lipid-binding cavities. J Biol Chem. 2019;294:19081–98. doi: 10.1074/jbc.RA119.011153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mousley CJ, Davison JM, Bankaitis VA. Sec14 like PITPs couple lipid metabolism with phosphoinositide synthesis to regulate Golgi functionality. Subcell Biochem. 2012;59:271–87. doi: 10.1007/978-94-007-3015-1_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Collery R, McLoughlin S, Vendrell V, Finnegan J, Crabb JW, Saari JC, et al. Duplication and divergence of zebrafish CRALBP genes uncovers novel role for RPE- and Muller-CRALBP in cone vision. Invest Ophthalmol Vis Sci. 2008;49:3812–20. doi: 10.1167/iovs.08-1957 [DOI] [PubMed] [Google Scholar]
- [48].Bomar JM, Benke PJ, Slattery EL, Puttagunta R, Taylor LP, Seong E, et al. Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nat Genet. 2003;35:264–9. doi: 10.1038/ng1255 [DOI] [PubMed] [Google Scholar]
- [49].Mandela P, Yankova M, Conti LH, Ma XM, Grady J, Eipper BA, et al. Kalrn plays key roles within and outside of the nervous system. BMC Neurosci. 2012;13:136. doi: 10.1186/1471-2202-13-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schmidt S, Debant A. Function and regulation of the Rho guanine nucleotide exchange factor Trio. Small GTPases. 2014;5:e29769. doi: 10.4161/sgtp.29769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Anuppalle M, Maddirevula S, Kumar A, Huh TL, Choe J, Rhee M. Expression patterns of prune2 is regulated by Notch and retinoic acid signaling pathways in the zebrafish embryogenesis. Gene Expr Patterns. 2017;23-24:45–51. doi: 10.1016/j.gep.2017.03.002 [DOI] [PubMed] [Google Scholar]
- [52].Zhang J, Head B, Leonard SW, Choi J, Tanguay RL, Traber MG. Vitamin E deficiency dysregulates thiols, amino acids and related molecules during zebrafish embryogenesis. Redox Biol. 2021;38:101784. doi: 10.1016/j.redox.2020.101784 [DOI] [PMC free article] [PubMed] [Google Scholar]


