Highlights
-
•
MAb against Nile tilapia IgT was developed using a peptide-based strategy.
-
•
The mAb was specific for IgT and it did not cross-react with a purified serum IgM.
-
•
Different analytical tools were developed using the anti-IgT mAb.
Keywords: Monoclonal antibody, Igt, Nile tilapia, B-lymphocytes, TT-P0
Abstract
Teleost IgT/Z plays a principal role in the defense mechanisms against infectious agents in the mucosal compartments and in systemic immunity. Previously, Nile tilapia (Oreochromis niloticus) IgT was discovered and characterized at transcription level. In this work, we generated a monoclonal antibody (mAb) that specifically recognized the Nile tilapia IgT. BALB/c mice were immunized with three synthetic peptides conjugated to KLH. The sequences of these peptides derived from the constant region of the Nile tilapia IgT heavy chain. ELISA and Western blotting confirmed the specificity of the polyclonal sera and the culture supernatant from a positive hybridoma clone. We observed immunoreactivity against a recombinant IgT fragment and native IgT in skin mucus. The anti-IgT mAb did not cross-react with purified tilapia IgM. Direct ELISA analysis allowed the quantification of skin mucus IgM and IgT concentrations. Flow cytometry analysis revealed differences in the percentage of IgT+ B cell populations between juveniles and adults in peripheral blood, head kidney and spleen lymphocytes and among the tissues analyzed. For further validation of the anti-IgT mAb utility, a recombinant vaccine candidate against sea lice (TT-P0 Ls) was injected into juvenile tilapia. Direct ELISA results revealed a differential secretion of skin mucus IgT and IgM after immunostimulation. In addition, the percentages of IgT+ B cells were determined at 7 days after booster and ex-vivo stimulation by flow cytometry. This mAb constitutes an important immunological tool to study the biological function and structural characteristics of tilapia IgT.
1. Introduction
Monoclonal antibodies (mAbs) are powerful tools for a wide range of immunological methods due to their high specificity and affinity. Those methods include enzyme linked immunosorbent assay (ELISA) and immunoblotting procedures, flow cytometry (FCM) analysis, stereotyping and analysis of antigens in microbial pathogens in both, medical and veterinary sciences, as well as to diagnostic and therapeutic options for disease monitoring and treatment [1]. MAbs specifically reacting with immunoglobulins (Igs) provide effective tools for assessing antibody production following infection or vaccination, and for immunoblotting analysis of antigens recognized by the host immune system [2].
Most teleost species possess three immunoglobulin isotypes (i.e., IgM, IgD, and IgT/IgZ), which displays particular functions in disease resistance [3,4]. The IgM is the most abundant immunoglobulin isotype in the systemic compartments while IgT (also known as IgZ) is more specialized in the mucosal immune response [5,6]. However, the knowledge of IgT protein structure as well as its production and distribution by B cells in different species of fish, is still insufficient [7]. In the last decade, mAbs have been produced against serum immunoglobulins of a variety of marine and freshwater fish, including tilapia (Oreochromis sp.) species, mainly against IgM. However, there are few mAbs described against IgT [8], [9], [10], [11], [12] and only in rainbow trout (Oncorhynchus mykiss) is detailed a complete biochemical and functional characterization of this Ig class [4,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]].
Nile tilapia (Oreochromis niloticus) is one of the most extensively cultured fish species in the world. Additionally, tilapia is popular species in the study of teleost fish immunology [24]. The species possesses the three-immunoglobulin isotypes; it has B and T lymphocytes, and reacts to pathogens by inducing antigen-specific antibody responses [25]. Phuyindee et al. carried out a detailed molecular analysis of the immunoglobulin M heavy chain gene in Nile tilapia in 2015 [26]. Our research group first time identified the gene coding for the IgT heavy chain in Nile tilapia (O. niloticus) in 2018. The study demonstrated up regulation of IgT transcription in the skin after bacterial infection, pointing its predominant role in the mucosal immune response in epithelial tissue, similar to other reports in different teleost species [27].
The first production and characterization of mAbs specifically directed against Nile tilapia immunoglobulin was reported two decades ago [28]. The authors affirmed that all eight produced mAbs were specific to IgM heavy chain. These mAbs also recognized the immunoglobulin heavy chain of three other tilapia species/hybrids. Successful preparation of different polyclonal or monoclonal Abs against Nile tilapia IgM was also described further [29], [30], [31], [32].
Synthetic peptides are widely applied as antigens to generate custom antibodies, as advantageous approach to avoid cross-reaction with other immunoglobulins [33]. Recently, we produced and characterized a mAb against Nile tilapia IgM heavy chain using a peptide-based strategy [6]. Despite growing scientific production, knowledge about the structural and functional characteristics of IgT in Nile tilapia is limited, and commercial anti-IgT mAbs are not available in this species. Therefore, the development of mAbs against Nile tilapia IgT will be a major advance in the characterization of this Ig isotype. Additionally, the mAb specific for IgT will contribute to a better understanding of the mechanisms involved in systemic and mucosal immunity under different stimulation programs in this species.
The present work focused in the generation of a mAb that specifically recognized the Nile tilapia IgT heavy chain following a peptide-based strategy. For this reason, three synthetic peptides with immunogenic properties were designed from the CH2 fragment of the Nile tilapia IgT heavy chain. They were conjugated to Keyhole Limpet Hemocyanin (KLH) to use as antigens. The specificity and utility of the mAb produced was evaluated by Western blotting, ELISA and FCM. We also investigated the effect of a vaccine candidate against sea lice TT-P0 Ls [34] over IgT+ B cell populations and total Igs in skin mucus secretion in tilapia. The results suggest that this anti-IgT mAb could be useful tool to study the tilapia immune response and the protective effects of vaccines in this species.
2. Materials and methods
2.1. Animals
Fifteen female BALB/c mice of seven weeks-old with a body weight between 20 and 25 g were provided by the National Center for the Production of Laboratory Animals (CENPALAB), Havana, Cuba. Before starting the immunization schedule, blood samples were collected from each mouse to be used as pre-immunized control sera.
Nile tilapias (Oreochromis niloticus) were provided by the CIGB Aquaculture Station, Havana, Cuba, and were kept in aerated freshwater under natural photoperiod. Water temperature was maintained at 26 ± 2 °C. Fish were fed with a commercial pelleted feed, supplied by the CENPALAB. Prior any experimental procedure, fish were acclimatized to laboratory conditions for at least one week. Only the healthy fish were used for the studies, as determined by the general appearance and the level of activity. The Ethics Committee of the CIGB approved all animal experiments.
2.2. Antigens
Three synthetic and immunogenic peptides were selected with the prediction software for antibody epitope identification (http://tools.immuneepitope.org/bcell/). The amino acid sequence of the constant CH2 region of the Nile tilapia IgT (Oreochromis niloticus) (GenBank Accesion No.: AKN35078 and AKN35079) heavy chain was used as a template for the peptide search. The selected peptide sequences (CPQQQTDPYKPDE 1547.67 Da, CSLYTTSKANWDKKHPTK 2107.42 Da and CKAVSNAHGNSR 1242.38 Da) were blasted (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to ensure specificity. Peptides were synthesized by the Department of Peptide Synthesis of the CIGB and they were obtained with 99% purity. We added an N-terminal cysteine for conjugation purposes and were designated P62, P63 and P64, respectively. The peptides were conjugated to KLH (Sigma) for immunization and bovine serum albumin (BSA) (Sigma) for screening.
The recombinant fragment encoding the constant CH2 domain of Nile tilapia IgT (O. niloticus) was produced as hexahistidine-tagged protein in the Escherichia coli strain BL21(DE3) and purified under denaturing conditions employing IMAC Sepharose™ Fast Flow (GE Healthcare), according with the methodology described by Velázquez et al. [6]. The protein identity was confirmed by mass spectrometry (Supplementary Table 1).
Samples containing the native IgT were obtained from the skin mucus of Nile tilapia as described Xu et al., [15], with modifications. Nile tilapia skin mucus was gently scraped from the dorsolateral skin surface, transferred into an Eppendorf tube and diluted 1:2 with 20 mM Tris–HCl pH 8.0. It was vigorously shaken and centrifuged at 12,500 × g for 15 min at 4 °C to remove tilapia cells and skin bacteria from mucus. The clear supernatant was harvested, filtered with a 0.45 μm syringe filter (Millipore), and stored at -80 °C for further analysis by Western blot and ELISA.
The soluble IgM employed for cross-reactivity analysis was purified from Nile tilapia serum as described Velázquez et al. [6].
TT-P0 Ls protein used in tilapia immunization scheme was purified as described previously by Leal et al. [34]. This antigen is available in our laboratory from current vaccine research projects. The P0 peptide (amino acid sequence of GTKLEYLADPSKFASVAAAPAAGATKAAAAAPAKA) was synthesized by the Department of Peptide Synthesis of the CIGB and it was obtained with 99% purity.
2.3. Processing of samples by the filter-aided sample preparation (FASP) method for mass spectrometry analysis
Skin mucus samples extracted from 30 adults Nile tilapia with an average body weight of 300 gs were diluted with extraction solution (100 mM Tris/1.5% SDS/50 mM DTT, pH 8.8). Subsequently, they were heated at 95 °C for 10 min in a Thermomixer compact (Eppendorf, Germany). To block cysteines, the samples were treated with 2.5% Acrylamide for 1 h at 25 °C. The FASP procedure was performed according to Wisniewski et al. [35]. The samples were transferred to a 500 μl Vivacon filter equipped with a 30 kDa membrane and a collection tube, both supplied by Sartorius Stedim (Germany). The samples were diluted with a cleaning solution (100 mM Tris/8 M Urea/10 mM DTT, pH 8.8) and centrifuged at 11,200 × g for 30 min. This step was repeated five more times, centrifuging at 11,200 × g for 20 min. Subsequently, the samples were diluted with a digestion solution (50 mM Tris/10 mM DTT, pH 8.8) and were centrifuged at 11,200 × g for 20 min. This step was repeated two more times.
For proteolytic digestion with Lysyl endopeptidase or Trypsin, 12 µg of the corresponding enzyme were dissolved in 100 µl of the digestion buffer were added to the samples (in a 1:50 enzyme: protein ratio) and the samples were incubated for 20 h at 37 °C. Peptide mixtures were centrifuged at 11,200 × g for 10 min at 20 °C. Then, 125 μl of the digestion buffer was added and centrifuged again. This step was repeated once again, but adding only 100 µl of the digestion buffer. Finally, the leptic/tryptic peptide samples were dried and stored at -20 °C.
Low-energy ESI-MS and MS/MS spectra were acquired using a QTOF-2™ mass spectrometer from Micromass (Manchester, UK). The multiply charged signals of highest intensity corresponding to leptic/tryptic peptides were further analyzed by ESI-MS/MS using appropriate collision energies to obtain either partial or complete amino acid sequences. The proteins identification based on the MS/MS spectra, was carried out using the MaxQuant program v1.6.2.10 against tilapia (Oreochromis sp.) genome annotated in the UniProtKB database. The search parameters included propionamido action of cysteines as a fixed modification. The methionine oxidation, the N-terminus acetylation and the asparagine deamidation were used as variable modifications. A mass tolerance error of 0.3 Da was allowed and a false discovery rate of 1% was set as statistical criterion of identification.
2.4. Immunization schedule and hybridoma generation
Synthetic peptides conjugated to KLH were used in the immunization scheme. Five mice with an average body weight of 20–25 gs were used per each peptide. They received three subcutaneous immunizations with an interval of 28 days among doses. In the first immunization, 60 µg of immunogen was injected with Freund's complete adjuvant (Sigma-Aldrich), while incomplete Freund's adjuvant (Sigma-Aldrich) was used with 30 µg of immunogen in the second and third immunizations. Four weeks after the third immunization, mice blood was obtained for serum titration of specific antibodies by ELISA, following the protocol described by Pérez-Bernal et al. [36] with some modifications. The 96 well Costar® 3590 high binding plates were coated with three different antigens: 10 µg/ml of each peptide conjugated to BSA, 10 µg/ml of the recombinant IgT fragment or three dilutions (1:2, 1:4 and 1:8) of a pooled sample from tilapia skin mucus containing native IgT (n = 10). The sera of the immunized animals were two-fold serially diluted from 1:250 to 1:128,000 for the ELISA using the conjugated peptides and from 1:50 to 1:3200 for the ELISA using the recombinant IgT fragment. For the ELISA using the skin mucus as coating antigen, it was applied a single serum dilution of 1:50. The mouse with highest serum titers against the most suitable peptide and against the target IgT (recombinant or native IgT) was selected for hybridoma production. Three days before the splenectomy, the mouse was injected intraperitoneally with 50 µg of the corresponding peptide conjugated to KLH (dissolved in sterile PBS (0.137 M NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4)).
Hybridomas were obtained by the method described by Köhler and Milstein [37]. Lymphocytes from the mouse spleen and exponentially growing mouse myeloma cells of the P3 × 63Ag8.653 line were used to perform cell fusion with 50% (w/v) polyethylene glycol solution (Sigma). The cells were distributed in 96-well culture plates, resuspended in RPMI-HAT selection medium (Sigma, Hybri-Max) with 20% of fetal bovine serum. The plates were incubated at 37 °C in a 5% CO2 atmosphere. After 10 days, the supernatant from wells with growing hybridomas were screened by the ELISA described previously using 10 µg/ml of the peptide conjugated to BSA or 10 µg/ml of the recombinant IgT fragment adsorbed to the solid phase, for the detection of anti-tilapia IgT antibodies. Wells with the highest absorbance values were cloned by a limiting dilution method [38] at a rate of one cell per well.
2.5. Identification of the mAb isotype
The class (IgM, IgA or IgG) and the IgG subclass (IgG1, IgG2a, IgG2b or IgG3) of the mAbs presents in the culture supernatant of the hybridoma clone selected were identified by ELISA with a commercial kit (Sigma-Aldrich), following the manufacturer's instructions.
2.6. Hybridoma culture for mAb stocks and purification of mAb
Cells of selected hybridoma were grown in 250 ml spinner flasks, from 3 × 105 cells/ml, in RPMI 1640 supplemented with 8% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, and 17 mM sodium hydrogen carbonate. Cells were maintained at 37 °C in an atmosphere of 5% CO2 and the medium was replaced every 48 h up to the maximum viable cell density (∼1 × 106 cells/ml) to produce working stocks of antibodies.
Anti-IgT peptide mAbs contained in the filtrated cell supernatants were purified by affinity chromatography using nProtein A Sepharose Fast Flow matrix (GE Healthcare Life Sciences) packed in a C10/10 column (GE Healthcare Life Sciences) with 8.5 cm of height with a linear flow rate of 0.5 ml/min. To this, 1.5 M Glycine/3 M sodium chloride, pH 8.9, and 100 mM citric acid pH 3.0 were used as adsorption and elution buffers, respectively. Extensive washings were performed with 1.5 M Glycine/3 M sodium chloride, pH 8.9, to remove contaminants. For buffer exchange, fraction purified by affinity chromatography was dialyzed against 20 mM Tris/150 mM sodium chloride, pH 7.0 overnight at 4 °C. Finally, mAbs were concentrated and filtered by 0.22 μm under sterile conditions. Each fraction was checked by 12.5% SDS-PAGE under reducing conditions. Protein concentration was determined with a BCA protein assay kit (Pierce) according to the manufacturer's instructions. The purity and concentration of mAbs was assayed by densitometry scanning of protein gels taking into account total protein concentration determined by BCA. The mAbs were conjugated to Horseradish Peroxidase (Sigma) and Fluorescein Isothiocyanate (Sigma) according to the manufacturer's instructions and were characterized by Western blotting, ELISA and FCM to test the specificity.
2.7. Specificity analysis of mouse anti-IgT mAb
2.7.1. Western blotting
Samples of tilapia skin mucus containing native IgT and purified IgM from tilapia serum or recombinant IgT fragment were processed under denaturing and reducing conditions in a Tris/Glycine/sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) at 12.5% and 15%, respectively. Samples were diluted in loading buffer (125 mM Tris–HCl, pH 6.8, 6% SDS, 20% glycerol, 10% β-mercaptoethanol, 0.05% bromophenol blue) and boiled for 5 min and processed 45 min in running buffer (0.25 M Tris–HCl, pH 8.8; 1.92 M glycine; 0.1% SDS, pH 8.3) at a constant voltage of 200 V. Following electrophoresis, the gels were stained with Coomassie Brilliant Blue or transferred to nitrocellulose membranes Hybond C (Amersham, USA) for Western blotting analysis. The membranes were blocked at room temperature (RT, approximately 25 °C) with 5% skimmed milk for an hour and then were washed with PBS-Tween 0.01% once and with PBS twice. After washing steps, the membranes only containing the tilapia skin mucus were incubated for 3 h at 25 °C with a 1:100 dilution of the hybridoma culture supernatant and then 1:2000 dilution of anti-mouse polyclonal antibody horseradish peroxidase (HRP) conjugate (Sigma) for 1 h at 25 °C as secondary antibody. The membranes containing the skin mucus and purified IgM or the recombinant IgT fragment were incubated with the mouse anti-IgT mAb-HRP (1.0 mg/ml, 1:500 dilution) or with the mouse anti-IgM mAb-HRP (1.2 mg/ml, 1:500 dilution) [6]. After washing steps, the immunoreactive bands were visualized using ECL detection system (Amersham, USA), according with the manufacturer's instructions.
2.7.2. ELISA
The 96 well Costar® 3590 high binding plates were coated different dilutions of individual samples (1:64, 1:128, 1:256 and 1:512, n = 9) of skin mucus from juvenile tilapias (average body weight of 32.9 ± 7.4 g) containing native IgT in 100 μl of carbonate-bicarbonate buffer and incubated overnight at 4 °C. The wells were washed three times with PBS containing 0.05% Tween-20 (PBS-T) and then blocked with 200 μl of 1% BSA in PBS-T for 2 h at 25 °C. The plates were washed as above and were incubated in blocking buffer with 100 μl of the mouse anti-IgT mAb-HRP (1.0 mg/ml, 1:2000 dilution) or the mouse anti-IgM mAb-HRP (1.2 mg/ml, 1:4000 dilution) [6] for 3 h at 25 °C. Positive controls were a standard curve of purified recombinant IgT fragment or serum tilapia IgM (starting from 1.0 to 0.002 μg/ml, respectively). In addition, negative controls (blocking buffer only) were used. After washing seven times, the chromogen TMB in substrate buffer was added and incubated for 10 min or until color development. After stop the reaction, color intensity was measured at 450 nm with a Multiskan GO Microplate Spectrophotometer and the program SkanIt v3.2 (Thermo Fisher Scientific). Each sample was assayed in duplicates and the experiment was repeated twice. Wells of the highest dilution those results in a value of twice above the negative control (blocking buffer only) for each animal were considered positive. For the determination of the native IgT and IgM concentration, the absorbance values of the two-fold serially diluted unknown mucus samples were assigned in relation to the standard curves of recombinant IgT and purified IgM, taking into account the dilution factor used. We selected the dilution factor of the unknown samples that had an absorbance value at least twice the value of the blank.
2.8. Flow cytometry analysis for the quantification of IgT+ cells
2.8.1. Isolation of lymphocytes populations from peripheral blood (PBL), head kidney (HKL) and spleen (SpL)
Biological samples of peripheral blood, head kidney and spleen were obtained from 9 juvenile tilapias (52.2 ± 8.1 g) and from 10 tilapia adults (300–500 g). Nile tilapias were killed by benzocaine (Sigma) overdose. Blood was extracted from the caudal vein with a heparinized needle and diluted 5 times with Leibovitz medium (L-15, Invitrogen) supplemented with 100 IU/ml penicillin and 100 μg/ ml streptomycin (P/S, Life Technologies), 10 U/ml heparin (Sigma-Aldrich), and 5% fetal bovine serum (FBS, Life Technologies). Head kidney and spleen were extracted and single cell suspensions were obtained using 100 μm nylon cell strainers in 10 ml Leibovitz medium supplemented. Samples were placed onto Ficoll-Paque Plus (GE Healthcare) density gradients and leukocytes were isolated according to manufacturer's recommendations, with minor modifications. All suspensions were then centrifuged at 500 × g for 30 min at 4 °C. The interface cells were collected and washed twice with l-15 containing 5% FBS and P/S. Cell viability was determined by Trypan blue (Sigma-Aldrich) exclusion obtaining more than 95% of cell viability in all study cases.
2.8.2. Quantification of IgT+ cells
Total leukocyte populations from individual fish were dispensed into 96-well U bottom plates (Greiner bio-one, Germany) at a density of 1 × 106 cells/ml. Following two washing steps with ice-cold bovine serum albumin (BSA) 0.5% in phosphate-buffered saline (PBS), cells were incubated with 50 μl of FITC labeled mouse anti-IgT mAb (1.1 mg/ml, 1:10 dilution) for 1 h at 37 °C. After two washing steps with BSA-PBS 0.5%, cells were resuspended in 1% PBS. Two replicates per each fish were set. Just before the reading, 10 μl of propidium iodide (Sigma, working stock at 50 μg/ml) were added to each sample for the detection of viable cells from forward scatter (FSC) vs PI graphics. Positive IgT B cells were quantified by flow cytometry in a CyFlow Space Cytometer (Partec) equipped with FloMax® Software. Leukocytes without the FITC- mouse anti-IgT mAb conjugate were used as non-stained controls (NS) to optimize and standardize the system. Further analysis was performed with FlowJo v.10 (FlowJo LLC, Tree Star).
2.9. Fish immunization experiment with TT-P0 Ls antigen
2.9.1. Adjuvants
TT-P0 Ls antigen, unless specified, was formulated in Montanide ISA 50 V2 adjuvant (Seppic, France) at a ratio 50/50. Formulations were made in a Politron (Ultra-Turrax Homogenizer T25, IKA WERKE, Germany).
2.9.2. Fish immunization experiment to measure IgT immune response
A first immunization experiment was done to evaluate total IgT B cell immune response. Twenty-six tilapias per group (32.9 ± 7.4 g) were immunized by intraperitoneal (i.p.) injection on days 0 and 15. Two experimental groups were set. One group of tilapia was injected with the TT-P0 Ls antigen at the dose of 100 μg per fish. The control group received equal volume of buffer (150 mM NaCl, 10 mM Tris–HCl, pH 8.0).
Blood from the caudal vein and skin mucus samples were collected of all fish on days 0 (pre-immune sera) and 7 days after booster (day 22) and prepared for antibody detection. For confirmation that desirable positive stimulation was obtained with the antigen used, pP0-specific IgM antibodies in the serum of vaccinated fish were detected by indirect ELISA with anti-tilapia IgM F04 mAb (ADL Aquatic Diagnostics, UK), according to manufacturer's instructions. ELISA titers were expressed as the reciprocal of the highest dilution that results in a value of twice above the pre-immune serum for each animal.
Protein concentration of secreted total IgT and IgM in tilapia skin mucus samples were determined as described before. In this case, mucus samples (n = 13) were serially diluted from 1:16 to 1:512 in 100 μl of carbonate-bicarbonate buffer and were applied 100 μl of mouse anti-IgT mAb-HRP (1.0 mg/ml, 1:2000 dilution) or mouse anti-IgM mAb-HRP (1.2 mg/ml, 1:4000 dilution) [6], respectively.
Samples of peripheral blood, head kidney and spleen were obtained from 6 tilapias per group at 7 days after booster. They were used for lymphocyte isolation and flow cytometry analysis for the quantification of IgT+ cells as described before.
2.9.3. Specific B cell immune response
A second experiment was done to evaluate specific IgT+ B cell immune response. Six tilapias per group (50.0 ± 6.3 g) were used for the study of the specific B cell response against the synthetic P0 peptide. Two experimental groups were settled. One group of tilapia was immunized by intraperitoneal (i.p.) injection on days 0 and 15 with the TT-P0 Ls antigen at the dose of 100 μg per fish. The second group was the control non-immunized. Samples of blood from the caudal vein, head kidney and spleen were collected of all fish at 7 days after booster (day 22) and were used for lymphocyte isolation as described before. Total leukocyte populations from individual fish were dispensed into 96-well U bottom plates (Greiner bio-one, Germany), at a density of 2.5 × 105 cells/ml in 100 μl of l-15 containing 5% FBS and P/S. The cells were incubated with 100 μl of supplemented l-15 medium containing 10 μg/ml of synthetic P0 peptide or with supplemented medium only (negative controls), respectively. After 72 h of incubation at 25 °C, the leukocytes harvested and were analyzed based on side and forward light scatter parameters for the quantification of P0-specific IgT+ cells as described before.
2.10. Statistical analysis
The statistical analysis was carried out using GraphPad Prism software v8.0 (GraphPad Software). The normal distribution of data was analyzed with D'Agostino Pearson's test and the variance homogeneity with Shapiro-Wilk's test. All results are expressed as the mean ± SD, where a p value less than 0.05 was considered statistically significant.
3. Results
3.1. Mass spectrometry analysis of the secreted form of IgT in skin mucus of Nile tilapia
ESI-MS/MS analysis was performed from skin mucus samples of Nile tilapias. The fragmentation process generated 14,559 peptide sequences with an average MS/MS count of 2.62. The analysis confirmed the presence of the amino acid sequence corresponding to tilapia secreted IgT heavy chain (previously predicted from cDNA sequence) among the more than 1000 proteins identified in the tilapia skin mucus, with sequence coverage of 8.3%. Specifically, three tryptic peptides were sequenced (Fig. 1A). Among them, two first peptides are unique for IgT isotype in Nile tilapia and the third peptide is specific for IgT sequences in teleost fish (Fig. 1B).
Fig. 1.
Identification by mass spectrometry analysis of the native IgT in Nile tilapia (Oreochromis niloticus). (A) ESI-MS/MS mass spectra of the three IgT tryptic peptides obtained from skin mucus sample. The matched peaks are marked with “b” and “y”. B ions and Y ions are indicated below the corresponding mass spectra. (B) Amino acid sequence of the secreted form of tilapia IgT (GenBank Accession No.: AKN35078). The sequences of the positively identified tryptic peptides using the MaxQuant v1.6.2.10 program are indicated in bold and in blue, red and green, respectively.
3.2. Production and characterization of mAb against Nile tilapia immunoglobulin T
For production and characterization of a mAb against Nile tilapia (O. niloticus) immunoglobulin T, we selected three synthetic peptides with immunogenic characteristics in the CH2 domain of the secreted and membrane form of Nile tilapia immunoglobulin T (Fig. 2A). The peptide sequences were defined in the region with the lowest amino acid identity with secreted IgM isoforms in this species (Supplementary Fig.1). KLH-conjugated peptides were employed for BALB/c mice immunization. The serum titrations after the third immunization were evaluated by ELISA against BSA-conjugated peptides. The best result of the immunization schedule was obtained in mice immunized with P62-KLH (Supplementary Fig. 2). The ELISA results using a recombinant IgT fragment as coating antigen confirmed highest recognition by the antibodies present in sera from mice immunized with P62-KLH (Supplementary Fig. 3). Among them, mouse numbered as 5 exhibited the best reactivity against P62-BSA (Supplementary Fig. 2B), recombinant IgT (Supplementary Fig. 3) and tilapia skin mucus (Fig. 2B) which contains native IgT. This mouse was selected as source of splenocytes to obtain the IgT hybridomas producing clones. The fusion efficiency was 37.6% (Supplementary Table 2).
Fig. 2.
Production of a monoclonal antibody against Nile tilapia (Oreochromis niloticus) IgT heavy chain. (A) Three dimensional modeling of the tertiary structure of Nile tilapia IgT heavy chain according to I-Tasser and Raptor X prediction programs. All immunoglobulin domains are indicated, from up to down, as VH, CH1 and CH2. The white arrowhead and the yellow ribbons indicate the region of sequences corresponding to the selected immunogenic peptides. (B) Reactivity against the native form of Nile tilapia IgT. ELISA plates were coated with three dilutions (1:2, 1:4 and 1:8) of pooled skin mucus sample (n = 10). Mice sera with highest titers against the recombinant IgT fragment were applied at 1:50 dilution as primary antibody.
Hybridoma clones were initially screened by ELISA to the BSA-conjugated peptide and the recombinant IgT fragment. Positive clones were subsequently cloned by limiting dilution method. After cloning, the hybridoma designated 4G9/1 was selected as the single secretory clone that reacted strongly and stably with the P62-BSA and with the recombinant IgT fragment. The class and subclass of the immunoglobulin produced by the 4G9/1 clone were determined using an ELISA-based mouse immunoglobulin´s isotyping kit, which revealed that the mAb belongs to IgG2b isotype. This clone was selected for a higher scale hybridoma production and purification for further studies. The antibody was obtained with purity higher than 95% after purification by affinity chromatography (Supplementary Fig. 4).
3.3. Specificity analysis of mAb against Nile tilapia immunoglobulin T
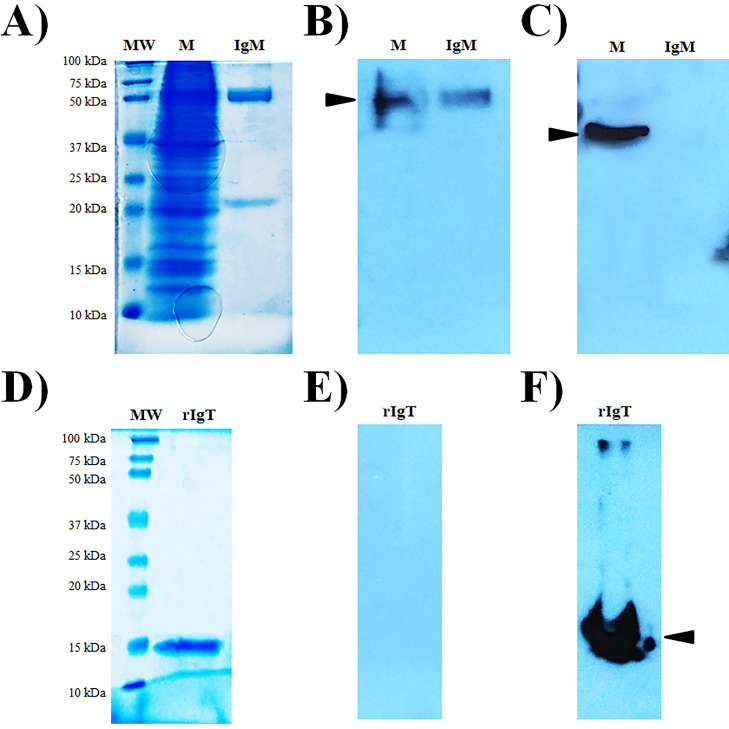
The specificity of the mAb secreted by 4G9/1 clone was investigated by SDS-PAGE and Western blotting (Fig. 3A and B, respectively). A single immunoreactive band under denaturing and reduce conditions appeared between 35 and 50 kDa, according with the predicted molecular weight of secreted Nile tilapia IgT, demonstrating positive immunoreaction of the generated mAb with this immunoglobulin in the skin mucus (Fig. 3B).
Fig. 3.
Specificity analysis of the monoclonal antibody 4G9/1 against the native IgT present in Nile tilapia (Oreochromis niloticus) skin mucus. (A) SDS-PAGE 12.5% under reducing conditions. (B) Western blotting using hybridoma culture supernatant from 4G9/1 clone (1:100 dilution) as primary antibody. MW: Molecular weight marker. M1, M2, and M3: skin mucus diluted 1:2, 1:4 and 1:8, respectively. Black arrowhead indicates positive signals corresponding with the putative soluble form of IgT from Nile tilapia.
The specificity of the mouse anti-IgT mAb was also assayed in Western blotting against tilapia IgM purified from serum, native IgT in skin mucus samples and recombinant IgT fragment. Samples were loaded under denaturing and reducing conditions (Fig. 4A and D), and after transfer, the membranes were incubated with the mouse anti-IgM mAb (Fig. 4B and E) and the mouse anti-IgT mAb (Fig. 4C and F). As a result, the membrane hybridized with the mouse anti-IgM mAb showed immunoreactive bands between 50 and 75 kDa in the samples corresponding with the skin mucus and purified IgM, according to the expected size of the IgM heavy chain (Fig. 4B). No reaction was obtained in the zone of the expected IgT molecular weight in skin mucus or with the recombinant IgT fragment (Fig. 4E). The membrane hybridized with the mouse anti-IgT mAb showed an immunogenic band between 37 and 50 kDa (Fig. 4C) in the lane corresponding to the skin mucus sample. Reactivity was also observed in the lane corresponding to recombinant IgT (Fig. 4F) where single band was detected with an electrophoretic migration near to 15 kDa, agreeing with the estimated size for the recombinant IgT fragment. However, the anti-IgT mAb did not react with the Nile tilapia IgM.
Fig. 4.
Cross-reactivity assay for assessing the specificity of anti-tilapia IgT monoclonal antibody. SDS-PAGE 12.5% (A) and 15% (D) under reducing conditions. (B and E) Western blotting using the mouse anti-IgM mAb HRP-conjugated (1.2 mg/ml, 1:500 dilution). (C and F) Western blotting using the mouse anti-IgT mAb HRP-conjugated (1.0 mg/ml, 1:500 dilution). MW: Molecular weight marker. M: pool of tilapia skin mucus (n = 10). IgM: IgM purified from tilapia serum. rIgT: recombinant IgT fragment. Black arrowheads indicate the expected positive signals corresponding to the specific mAbs used.
In the present work, the sensitivity of a direct ELISA method in the detection of native IgT and IgM molecules was evaluated using the tilapia anti-IgT and anti-IgM mAbs. Serial dilutions were used of recombinant IgT fragment, purified IgM and tilapia skin mucus samples. Both mAbs react against their target molecules. For the anti-IgT mAb, it was determined that the linear range against recombinant IgT is between 0.063 and 0.002 μg/ml (Fig. 5A) and for the anti-IgM mAb against purified IgM is between 0.125 and 0.002 μg/ml (Fig. 5B). These results allowed the quantification of native IgT and IgM molecules in juvenile tilapia. As a result, it was found a linear relationship between the absorbance ratio at 450 nm and the two-fold serial dilutions of the tested skin mucus samples at the dilutions tested. The coefficient of determination (R2) of this relationship was 0.9939 for IgT (Fig. 5C) and 0.9953 for IgM (Fig. 5D). Using this method, the secreted IgT concentration was 3.35 ± 1.75 μg/ml, while the secreted IgM concentration was 18.44 ± 11.25 μg/ml in the skin mucus of juvenile tilapias.
Fig. 5.
Direct ELISA assay using the mouse anti-IgT and anti-IgM mAbs HRP-conjugated. ELISA plates were coated with the recombinant IgT fragment (1.0 to 0.002 μg/ml) (A), the purified IgM (1.0 to 0.002 μg/ml) (B), skin mucus (C, D) (mucus dilutions 1:64, 1:128, 1:256 and 1:512). The mouse anti-IgT mAb HRP-conjugated (1.0 mg/ml, 1:2000 dilution) (A, C) and mouse anti-IgM mAb HRP-conjugated (1.2 mg/ml, 1:4000 dilution) (B, D) were applied. The graphs show the linear range of recombinant IgT, purified IgM and skin mucus dilutions after subtraction of the average zero standard optical density (O.D.), with the equation of the line and R2. Data represent mean ± SD (n = 9). Each sample was applied in duplicate wells and the experiment was repeated twice to ensure consistency of results.
3.4. Flow cytometry for the detection of IgT+ cells in juvenile and adult Nile tilapias
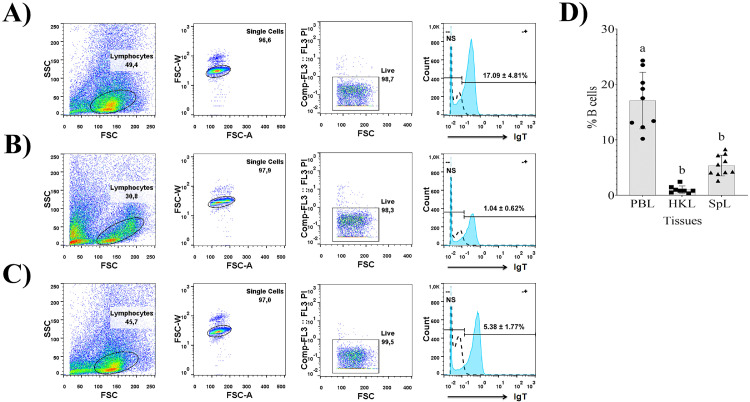
In the present study, the leukocyte populations of juvenile and adult Nile tilapias were analyzed to obtain the basal proportions of IgT+ B lymphocytes in different systemic compartments with a relevant role in fish immunity. These leukocytes were stained with anti-tilapia IgT mAb conjugated with FITC and analyzed by FCM. The forward scatter (FSC) and sideward scatter (SSC) parameters represented cell size and granularity, respectively. In the analysis, we obtained more than 97% of cell viability for all samples, which implies that the final identification of tilapia IgT+ B lymphocytes is not affected. In this study, based on the separation of the major cell lineages by lighter scatter characteristics, the lymphocytes of juvenile (Fig. 6) and adult (Fig. 7) Nile tilapia were gated and then the fractions of IgT+ B cells were determined in peripheral blood lymphocytes (PBL) (Figs. 6A and 7A), head kidney lymphocytes (HKL) (Figs. 6B and 7B) and spleen lymphocytes (SpL) (Figs. 6C and 7C).
Fig. 6.
Schematic representation of the flow cytometry analysis developed for detection of IgT+ B cells in peripheral blood lymphocytes (PBL), head kidney lymphocytes (HKL), and spleen lymphocytes (SpL) of juvenile (52.2 ± 8.1 g) Nile tilapia (Oreochromis niloticus). Representative pseudocolor plots show from left to right: FSC area (FSC—H)/SSC area (SSC—H) analysis where the black elliptical gate represents the lymphocyte populations, FSC-A/FSC-W pseudocolor plot represents single cell populations and FSC—H/FL3 pseudocolor plot where the black quadrant represents the viable cells detected with propidium iodide (0.5 μg/ml). Histograms are showing the staining of live IgT+ B cells in PBL (A), HKL (B) and SpL (C) using the mouse anti-IgT mAb-FITC (1.1 mg/ml, 1:10 dilution) (blue shaded histograms), with respect to the lymphocytes without antibody labeling (NS) (dashed lines). (D) Flow cytometry analysis of IgT+ B cells in PBL, HKL and SpL of juvenile Nile tilapia. Data represent mean ± SD of percentages of IgT+ B cells (n = 9, two replicates per fish). Differences of IgT+ B cells percentages among tissues were analyzed by an One-way ANOVA followed by Tukey's multiple comparisons test.
Fig. 7.
Schematic representation of the flow cytometry analysis developed for detection of IgT+ B cells in peripheral blood lymphocytes (PBL), head kidney lymphocytes (HKL), and spleen lymphocytes (SpL) of adult (300–500 g) Nile tilapia (Oreochromis niloticus). Representative pseudocolor plots show from left to right: FSC area (FSC—H)/SSC area (SSC—H) analysis where the black elliptical gate represents the lymphocyte populations, FSC-A/FSC-W pseudocolor plot represents single cell populations and FSC—H/FL3 pseudocolor plot where the black quadrant represents the viable cells detected with propidium iodide (0.5 μg/ml). Histograms are showing the staining of live IgT+ B cells in PBL (A), HKL (B) and SpL (C) using the mouse anti-IgT mAb-FITC (1.1 mg/ml, 1:10 dilution) (blue shaded histograms), with respect to the lymphocytes without antibody labeling (NS) (dashed lines). (D) Flow cytometry analysis of IgT+ B cells in PBL, HKL and SpL of adult Nile tilapia. Data represent mean ± SD of percentages of IgT+ B cells (n = 10, two replicates per fish). Differences of IgT+ B cells percentages among tissues were analyzed by an One-way ANOVA followed by Tukey's multiple comparisons test, where different letters represent statistically significant differences.
In juvenile tilapia, the percentage of IgT+ B lymphocytes in peripheral blood was 1.90 ± 0.74%, in head kidney was 0.94 ± 0.32% and in spleen was 1.06 ± 0.46% (Fig. 6D). There were no statistically significant differences in the percentages of IgT+ B lymphocytes among the different tissues studied (p>0.05). In tilapia adults, the percentage of IgT+ B lymphocytes in peripheral blood was 17.09 ± 4.81%, in head kidney was 1.04 ± 0.62% and in spleen was 5.38 ± 1.77% (Fig. 7D). The statistical analysis revealed that IgT+ B lymphocytes percentages in tilapia adults are statistically higher in peripheral blood than those obtained in head kidney (p < 0.001) and spleen (p < 0.01).
3.5. TT-P0 Ls vaccine candidate stimulates the production of total IgM and IgT in Nile tilapia
In vivo experiment with TT-P0 Ls vaccine candidate was performed in juvenile tilapias and fish from experimental groups were immunized twice with different antigens by intraperitoneal injection. Samples of blood and mucus were taken 7 days after booster. First, tilapia sera were assessed by indirect ELISA 7 days after booster to measure specific IgM Ab titers against the P0 peptide. The TT-P0 Ls protein adjuvanted in Montanide ISA 50 V2 induced a statistically significant specific IgM antibody response against pP0 after intraperitoneal immunization (p < 0.01) (Supplementary Fig. 5). Direct ELISA analysis on skin mucus samples obtained at 7 days after booster revealed that the total IgT concentration was significantly higher in TTP0+Montanide group than in the Control group (p < 0.05), while total expression levels of IgM were significantly downregulated (p < 0.0001) (Fig. 8).
Fig. 8.
Total concentration levels of IgT and IgM (μg/ml) determined by direct ELISA in skin mucus samples of Nile tilapia (Oreochromis niloticus) immunized with TT-P0 Ls antigen. Nile tilapia were injected twice intraperitoneally (days 0 and 15) with the antigen formulated in Montanide ISA 50 V2. Two experimental groups were settled: Buffer in adjuvant (Montanide) and injected with recombinant TT-P0 protein (TTP0+Montanide). Skin mucus samples were collected 7 days after booster and were used for direct ELISA assay. Data represent mean ± SD (n = 13). Differences of total IgM or IgT levels between groups were analyzed by an Unpaired t-test, * (p < 0.05) and **** (p < 0.0001).
3.6. TT-P0 Ls vaccine candidate stimulates the production of total IgT+ B lymphocyte populations in Nile tilapia
We evaluated the effect of TT-P0 Ls on total IgT+ B lymphocyte populations in systemic compartments of Nile tilapia (Fig. 9). In PBL, we did not detect statistically significant differences among the experimental groups analyzed for IgT+ B cells percentages (p > 0.05) (Fig. 9B). The percentages of IgT+ B lymphocytes were higher in TTP0+Montanide group compared to Control group in HKL (p < 0.01) (Fig. 9D) and SpL (p < 0.01) (Fig. 9F).
Fig. 9.
Activation of IgT+ B cell response in systemic lymphoid tissues of Nile tilapia (Oreochromis niloticus) immunized with TT-P0 Ls antigen. Nile tilapia were injected twice intraperitoneally (days 0 and 15) with the antigen formulated in Montanide ISA 50 V2. Two experimental groups were settled: Buffer in adjuvant (Montanide) and injected with recombinant TT-P0 protein (TTP0+Montanide). (A, B) Peripheral blood lymphocytes (PBL), (C, D) head kidney lymphocytes (HKL) and (E, F) spleen lymphocytes (SpL) were collected 7 days after booster and were used for lymphocyte isolation and flow cytometry analysis for the quantification of IgT+ B cells. (A, C, E) A representative example from six individuals is shown with anti-IgT staining (shaded histograms) or their respective non-stained control (dashed lines). (B, D, F) Data represents the mean ± SD (n = 6). Differences of IgT+ B cells percentages between groups were analyzed by a Mann Whitney test, ** (p < 0.01).
3.7. Determination of the specific B cell response against the P0 peptide in tilapia
To determine the tilapia specific B cell response against synthetic P0 peptide (Fig. 10), in vivo stimulation of juvenile tilapias was performed with TT-P0 Ls antigen and 7 days after booster samples of peripheral blood, head kidney and spleen were taken and processed for lymphocytes isolation. After ex-vivo stimulation with synthetic P0 peptide for 72 h, only PBL from TTP0+Montanide group showed a statistically significant increment in specific IgT+ B cell populations compared to the non-immunized control group (p < 0.05) (Fig. 10B). In HKL and SpL, no statistically significant differences were observed for the B IgT+ B cells among the experimental groups analyzed (p > 0.05) (Fig. 10D and 10F, respectively).
Fig. 10.
Specific immune response against the synthetic P0 peptide of IgT+ B cell response in systemic lymphoid tissues of Nile tilapia (Oreochromis niloticus) immunized with TT-P0 Ls antigen in comparison with the non-immunized control group. Two experimental groups were established: a non-immunized control group and a group vaccinated with the TT-P0 Ls antigen formulated in Montanide ISA 50 V2 (TTP0+Montanide). Nile tilapia were injected twice intraperitoneally (days 0 and 15) with the antigen. Peripheral blood lymphocytes (PBL), head kidney lymphocytes (HKL) and spleen lymphocytes (SpL) were collected 7 days after booster and were used for lymphocyte isolation. Synthetic P0 peptide (10 µg/ml) or medium alone was added to these populations. After 72 h of incubation, the cells were collected and analyzed by flow cytometry for the quantification of IgT+ B cells in: (A, B) PBL, (C, D) HKL and (E, F) SpL. (A, C, E) A representative example from six individuals is shown with anti-IgT staining (shaded histograms) or their respective non-stained control (dashed lines). (B, D, F) Data represents the mean ± SD (n = 6). Differences of specific IgT+ B cells percentages were analyzed by a Kruskall-Wallis test followed by Dunn's multiple comparisons test, where different letters represent statistically significant differences among the experimental groups.
4. Discussion
In a previous study, the cDNA sequences of the secreted and transmembrane forms of Nile tilapia immunoglobulin T (IgT) heavy chain were obtained from total RNA of head kidney lymphocytes by the RACE-PCR technique [27]. The transcriptional profiles of the IgT and IgM genes were analyzed by quantitative PCR at the larval stage and in different tissues of healthy or Edwardsiella tarda-infected adult tilapias. However, there was not confirmation of the tilapia IgT sequence at protein level, and therefore, no information is available about the structural features of this Ig isotype in Nile tilapia. MS is an extremely sensitive, highly versatile technique whose fields of application are experiencing rapid growth today. In this work, the identification of the IgT secreted form was carried out from the MS/MS spectra of the enzymatic digestion of the total proteins present in the Nile tilapia skin mucus. FASP method allowed the elimination by centrifugation of different metabolites, lipids and salts that may interfere in the positive detection of a target molecule in a complex mixture of proteins [35]. Among the 1000 proteins identified in the total skin mucus proteome, three tryptic peptides positively identified the sequence of the secreted form of tilapia IgT, validating the obtained cDNA sequences by the RACE-PCR technique [27].
The mAbs are usually used for monitoring and diagnosing diseases during aquaculture production and possess the advantage of their unlimited generation [39]. For mAb validation, this must be specific, selective and reproducible in the context that will be used [40]. In teleost fish, different authors described that IgT and IgM heavy chains have common gene segments in the constant CH1-CH2 domains [41], [42], [43], [44]. Dixon et al. [45] propose two suitable ways to minimize the possibilities of cross-reactivity. In the first place, they suggest generating mAbs against the Ig of interest, with the aim of purifying it by affinity chromatography using immobilized Abs to obtain the pure Ig fraction. However, the low levels of secreted IgT, both in serum and in mucus, constitute a challenge for the purification of this molecule. The other option is to generate the mAbs against fragments of the Ig molecules produced in recombinant eukaryotic systems, to enhance specificity, recognition and decrease the chances of cross-reactivity. Recent studies affirm that the use of synthetic peptides for antibody generation prevents cross-reaction with other immunoglobulins, because the epitope recognized is well defined [33]. Peptide sequences that mimic Ig epitopes are used to increase sensitivity and specificity in diagnostic systems [46].
In the present study, we selected three immunogenic peptides derived from the CH2 fragment of the heavy chain of Nile tilapia IgT for mice immunization. This domain has the lowest amino acid identity percent with the IgM sequence from this specie [27]. In this way, the possibility that anti-IgT mAb has cross-reactivity with the IgM was ruled out. The efficacy of the application of synthetic peptide-KLH conjugates in the immunization to obtain murine mAbs has been proved previously [6,47,48]. KLH is a suitable carrier protein because of its adjuvant properties intrinsic to its large size, many available groups for coupling and the fact that is genetically distant from the proteins of the animal to be used for immunization [49]. BSA is a more water-soluble molecule than KLH because it is smaller; therefore, it is used more commonly in immunoassays for screening of specific antibody titers [50].
After pAbs preparation, ELISA analysis revealed that the three synthetic immunogenic peptides were capable of generating high Ab titers in the immunized mice when the reactivity was measured against them, but the P62-KLH peptide was shown to be the most immunogenic. This peptide induced high Ab titers against the BSA-conjugated peptide, the recombinant IgT fragment and the native IgT form in tilapia skin mucus. The fusion efficiency obtained felt into the range between 35 and 60%, which is considered as satisfactory [51]. During mAb preparation, we demonstrated by ELISA and Western blotting analysis that selected 4G9/1 clone was able to detect the native Nile tilapia secreted IgT in skin mucus samples. Lower absorbance values were obtained with the hybridoma culture supernatant compared to the mice polyclonal antisera, probably due to lower cellular density. However, after higher scale hybridoma production, antibody purification and HRP-conjugation, the anti-IgT mAb displayed enhanced sensitivity against native IgT. The ELISA results showed IgT concentration in tilapia skin mucus samples (3.35 ± 1.75 µg/ml), which is about 10-fold higher than skin IgT concentration in rainbow trout [52]. These differences may be due to interspecific variations, because one of these species is from warm waters and the other is a cold-water species, respectively, or due to the immunological status of the species.
Western blotting analysis revealed strong positive signals at the predicted size of the constant heavy chain of native Nile tilapia secreted IgT, which is predicted to have a theoretical molecular mass of 42 kDa [27]. Polyclonal antisera prepared in mice against specific polypeptide located on the IgT heavy chain of Nile tilapia also confirmed the presence of IgT in the total skin protein, with a single band between 40 and 50 kDa in the Western blotting assay [53]. The molecular size of IgT heavy chain can vary among teleost species. The IgT heavy chain of Megalabrama amblycephala possesses 62.1 kDa [54], in flounder fish (Paralichthys olivaceus) possesses 66.6 kDa [43] and in turbot fish (Scophthalmus maximus) possesses 61.96 kDa [44]. In rainbow trout, IgT has an electrophoretic migration under denaturing/reducing conditions of approximately 75 kDa [13], while IgT from the large yellow croaker (Larimichthys crocea) possesses 45 kDa [11]. These differences are because IgT, like IgD, exhibits structural differences among species, not the case with IgM, which remains evolutionarily more conserved [44]. There was no evidence of cross-reactivity between the generated anti-IgT mAb and the Nile tilapia IgM, which suggested that anti IgT mAb can discriminate between IgT and IgM in Nile tilapia. The peptide-based design used to obtain the anti-IgT mAb was effective to achieve its specific binding to the Nile tilapia IgT, by avoiding the recognition of conserved regions in the amino acid sequences of the IgT and IgM heavy chains.
Until now, no commercial antibodies are available to detect IgT from teleost fish. The only successfully characterized anti-IgT mAb is the one developed by Zhang et al. [13], which allowed the detection and purification of IgT from different fluids of rainbow trout. More recently, Xu et al. [23] applied this mAb for the study of pathogen and microbiota control at different mucosal sites in this species, using a new teleost fish model with selective IgT depletion, revealing the great utility of this approach for validate the biological function of IgT. Thus, the anti-IgT mAb developed in this work is a useful tool for future differential studies between tilapia Ig isotypes.
The IgT+ B cells comprise a separate lineage of fish B cells that appears to have a main role in mucosal surfaces such as the gut, the skin and the gills [13,15,19], and only has being extensively characterized in rainbow trout and zebrafish (Danio rerio) [4,10,18,21]. Other studies conducted in sea bass (Dicentrarchus labrax) [8,9], Atlantic salmon [55,56], large yellow croaker (Larimichthys crocea) [11] and grouper (Epinephelus coioides) [12] shed light over some aspects of the structural and functional characteristics of this Ig isotype. The present study describes for the first time the use of a monoclonal antibody against Nile tilapia IgT in the identification of IgT+ B lymphocyte populations by flow cytometry. Despite possible need of further standardization, the IgT+ B cell population was characterized. We observed that IgT+ B cells percentages followed a tissue distribution pattern of peripheral blood lymphocytes > spleen lymphocytes > head kidney lymphocytes, in contrast to other fish species with marked prevalence of IgT+ B lymphocytes in spleen followed by head kidney and then peripheral blood [10,12]. A recent study in systemic tissues of healthy large yellow croakers (body weight 400 ± 50 g) revealed a rate of IgT+ B cells of approximately 13.1%, 5.6% and 2.6% in SpL, HKL and PBL, respectively, with the lowest levels observed in PBL [11].
Upon activation, B cells start a differentiation process that leads them to a plasmablast state, and they eventually to become plasma cells, with a greater capacity to secrete immunoglobulins (Igs) [57]. The IgT has been described as an Ig specialized in mucosal immunity [13], but some studies reported the presence and activation of IgT+ B cells in systemic compartments after viral, bacterial or parasitic challenge [3,10,56,58]. The effect of the enteric myxozoan parasite Enteromyxum leei on circulating Igs in gilthead sea bream (Sparus aurata) from a long-term infection revealed a significant increase in IgT levels in serum, which was significantly higher than IgM levels in challenged fish. In addition, IgT relative expression was significantly upregulated in the head kidney at 64 days post-exposure [3]. These data suggest that IgT response is not only limited to mucosal compartments, but also possesses important role in systemic defenses in fish. There is also recent evidence indicating the maternal transfer of IgZ/IgT pointing out the contribution of this Ig isotype to the early defense of fish against pathogen infection. The IgZ from zebrafish is highly expressed in oocytes in ovarian tissues and from unfertilized eggs to early stages of larval development. The authors demonstrated the IgZ bacteriostatic activity against different bacterial strains with potential high risk to the offspring [59].
In this work, we used TT-P0 Ls protein as a model antigen to study the utility of the Mabs generated in the evaluation of IgT immune response by FCM. Previously, it was demonstrated that TT-P0 Ls antigen produced a strong humoral immune response through specific IgM response against P0 compared to the synthetic P0 peptide in different teleost species, only when the recombinant TT-P0 was injected in “water in oil” formulation with Montanide ISA 50 V2 [34]. In addition, a significant increase in the relative expression levels of IgM and IgT transcripts was observed in spleen, head kidney and skin in TT-P0 Ls vaccinated groups compared to the placebo group in Atlantic salmon [60]. The present study revealed that the TT-P0 Ls fusion protein stimulated a specific IgM Ab response against P0 peptide in tilapia serum, similar to that described before [34]. Until now, the effect of TT-P0 Ls vaccine candidate on fish B cells immune response is unclear. Herein we observed a significant effect of TT-P0 Ls by promoting the proliferation of total IgT+ B cell populations in head kidney and spleen 7 days after the booster dose.
Furthermore, the production of total IgT in the skin mucus samples was stimulated, determining a high correlation between the IgT+ B cells percentage in spleen with the total expression levels of IgT produced in the tilapia skin mucosa. Our results suggested that the addition of promiscuous TCE's on P0 peptide enhanced the humoral immune response by the activation of IgT+ B cells at systemic level. Some teleost species shown prevalence of IgT+ B lymphocytes in spleen, which may result in the infiltration of these cells at mucosal sites, where they can proliferate and generate the secreted specific IgT [10,12,15,19]. These elements reinforce our premise that activation of IgT+ B cells in primary lymphoid tissues can lead to the production of an IgT-specific antibody response in both systemic and mucosal compartments. In vitro mitogenesis assays are based on polyclonal stimulation of lymphocyte populations and are generally accepted as a way to assess lymphocyte function [61,62]. Polyclonal B cell activators (PBCAs) are substances such as polysaccharides, lipopolysaccharides and proteins, which are able to increased proliferation and the complete differentiation of B cells to antibody secretion. Therefore, PBCAs have been used to elucidate the various mechanisms of B cell differentiation activated simply by antigen specific stimulation [63]. In this work, we obtained the first evidences that ex-vivo stimulation with the synthetic P0 peptide after vaccination with the TT-P0 Ls antigen is capable to enhance the specific immune responses through the proliferation of IgT+ B cells in PBL. This result suggests that the elicited specific proliferative responses may correlate with the observed presence of high antibody titers against the P0 peptide.
In conclusion, this study reported for the first time the generation and purification of a mAb that specifically recognize the recombinant and native form of the Nile tilapia IgT. The mAb is capable of discriminate between IgM and IgT isotypes and it is useful in Western blotting, ELISA and FCM assays. In this study, we determined the concentration of IgT in skin mucus samples and successfully detected the IgT+ B cells in different systemic compartments both in juvenile and adult tilapia. We were also able to find a positive stimulation of IgT+ B cells in tilapia head kidney and spleen and a higher production of total IgT in the skin mucus after vaccination with the TT-P0 Ls antigen designed to control sea lice infestation in salmonids. In addition, a specific proliferation of IgT+ B cells was evidenced for the first time against P0 peptide in PBL. This anti-IgT mAb specifically react with both soluble and membrane-bound IgT molecules and could be a suitable tool in infection and vaccination studies on Nile tilapia, with important implications for the aquaculture progress. Future research will focus on the differential modulation of IgT vs IgM immune response in systemic and mucosal compartments after different stimulation programs.
Funding
This work was supported by the Center for Genetic Engineering and Biotechnology, Havana, Cuba.
Declaration of Competing Interest
The authors have no conflicting commercial or financial interest in publishing this paper.
Acknowledgments
The authors would like to thanks Ricardo U Martínez-Rosales from Biomedical Research Direction, CIGB, for the technical support in the FCM assay.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fsirep.2023.100093.
Contributor Information
Mario Pablo Estrada, Email: mario.pablo@cigb.edu.cu.
Yamila Carpio, Email: yamila.carpio@cigb.edu.cu.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- 1.Holzlöhner P., Hanack K. Generation of murine monoclonal antibodies by hybridoma technology. JoVE J. Vis. Exp. 2017;(119):e54832. doi: 10.3791/54832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohanty S., Makesh M., Rajendran K.V., Suresh Babu P.P., Anand D., Kumar S.…Sarma K. Production and characterization of monoclonal antibodies against immunoglobulins of Cirrhinus mrigala (Hamilton 1822) Indian J. Fish. 2020;67(2):55–61. [Google Scholar]
- 3.Piazzon M.C., Galindo-Villegas J., Pereiro P., Estensoro I., Calduch-Giner J.A., Gómez-Casado E.…Pérez-Sánchez J. Differential modulation of IgT and IgM upon parasitic, bacterial, viral, and dietary challenges in a perciform fish. Front. Immunol. 2016;7:637. doi: 10.3389/fimmu.2016.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salinas I., Fernández-Montero Á., Ding Y., Sunyer J.O. Mucosal immunoglobulins of teleost fish: a decade of advances. Dev. Comp. Immunol. 2021;121 doi: 10.1016/j.dci.2021.104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilal S., Etayo A., Hordvik I. Immunoglobulins in teleosts. Immunogenetics. 2021;73(1):65–77. doi: 10.1007/s00251-020-01195-1. [DOI] [PubMed] [Google Scholar]
- 6.Velázquez J., Rodríguez A., Aragón H., Haidar A., González M., Valdés R., Carpio Y. Monoclonal antibody against Nile tilapia (Oreochromis niloticus) IgM heavy chain: a valuable tool for detection and quantification of IgM and IgM+ cells. Fish Shellfish Immunol. 2021;110:44–54. doi: 10.1016/j.fsi.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Awatif E., Brugman S., Wiegertjes G., Forlenza M. Proceedings of the WIAS Annual Conference 2020: Frontiers in Animal Sciences. WIAS; 2020. Preliminary characterization of IgT1 and IgT2 immunoglobulin isotypes in systemic and mucosal tissues in carp; p. 56. [Google Scholar]
- 8.Picchietti S., Nuñez-Ortiz N., Stocchi V., Randelli E., Buonocore F., Guerra L., Scapigliati G. Evolution of lymphocytes. Immunoglobulin T of the teleost sea bass (Dicentrarchus labrax): quantitation of gene expressing and immunoreactive cells. Fish Shellfish Immunol. 2017;63:40–52. doi: 10.1016/j.fsi.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Buonocore F., Stocchi V., Nunez-Ortiz N., Randelli E., Gerdol M., Pallavicini A.…Picchietti S. Immunoglobulin T from sea bass (Dicentrarchus labrax L.): molecular characterization, tissue localization and expression after nodavirus infection. BMC Mol. Biol. 2017;18(1):1–14. doi: 10.1186/s12867-017-0085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji J.F., Hu C.B., Shao T., Fan D.D., Zhang N., Lin A.F.…Shao J.Z. Differential immune responses of immunoglobulin Z subclass members in antibacterial immunity in a zebrafish model. Immunology. 2021;162(1):105–120. doi: 10.1111/imm.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Q., Wei Z., Chen Y., Xie J., Zhang X., He T., Chen X. Development of monoclonal antibody against IgT of a perciform fish, large yellow croaker (Larimichthys crocea) and characterization of IgT+ B cells. Dev. Comp. Immunol. 2021;119 doi: 10.1016/j.dci.2021.104027. [DOI] [PubMed] [Google Scholar]
- 12.Han Q., Hu Y., Lu Z., Wang J., Chen H., Mo Z.…Li Y. Study on the characterization of grouper (Epinephelus coioides) immunoglobulin T and its positive cells. Fish Shellfish Immunol. 2021;118:102–110. doi: 10.1016/j.fsi.2021.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y.A., Salinas I., Li J., Parra D., Bjork S., Xu Z.…Sunyer J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010;11(9):827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salinas I., Zhang Y.A., Sunyer J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011;35(12):1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Parra D., Gómez D., Salinas I., Zhang Y.A., von Gersdorff Jørgensen L.…Sunyer J.O. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. 2013;110(32):13097–13102. doi: 10.1073/pnas.1304319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X.T., Yu Y.Y., Xu H.Y., Huang Z.Y., Liu X., Cao J.F.…Xu Z. Prevailing role of mucosal Igs and B cells in teleost skin immune responses to bacterial infection. J. Immunol. 2021;206(5):1088–1101. doi: 10.4049/jimmunol.2001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tongsri P., Meng K., Liu X., Wu Z., Yin G., Wang Q.…Xu Z. The predominant role of mucosal immunoglobulin IgT in the gills of rainbow trout (Oncorhynchus mykiss) after infection with Flavobacterium columnare. Fish Shellfish Immunol. 2020;99:654–662. doi: 10.1016/j.fsi.2020.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Parra D., Korytář T., Takizawa F., Sunyer J.O. B cells and their role in the teleost gut. Dev. Comp. Immunol. 2016;64:150–166. doi: 10.1016/j.dci.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z., Takizawa F., Parra D., Gómez D., von Gersdorff Jørgensen L., LaPatra S.E., Sunyer J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016;7(1):1–14. doi: 10.1038/ncomms10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N., Zhang X.J., Chen D.D., Sunyer J.O., Zhang Y.A. Molecular characterization and expression analysis of three subclasses of IgT in rainbow trout (Oncorhynchus mykiss) Dev. Comp. Immunol. 2017;70:94–105. doi: 10.1016/j.dci.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong W.G., Yu Y.Y., Dong S., Huang Z.Y., Ding L.G., Cao J.F.…Xu Z. Pharyngeal immunity in early vertebrates provides functional and evolutionary insight into mucosal homeostasis. J. Immunol. 2019;203(11):3054–3067. doi: 10.4049/jimmunol.1900863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y.Y., Kong W., Yin Y.X., Dong F., Huang Z.Y., Yin G.M.…Xu Z. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLoS Pathog. 2018;14(11) doi: 10.1371/journal.ppat.1007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z., Takizawa F., Casadei E., Shibasaki Y., Ding Y., Sauters T.J.…Sunyer J.O. Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 2020;5(44):eaay3254. doi: 10.1126/sciimmunol.aay3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo G., Gao Q., Wang C., Liu W., Sun D., Li L., Tan H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture. 2014;422:1–7. [Google Scholar]
- 25.Flajnik M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018;18(7):438–453. doi: 10.1038/s41577-018-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phuyindee C., Unajak S., Srisapoome P. Diversity analysis of the immunoglobulin M heavy chain gene in Nile tilapia, Oreochromis niloticus (Linnaeus) Afr. J. Biotechnol. 2015;14(29):2282–2299. [Google Scholar]
- 27.Velázquez J., Acosta J., Lugo J.M., Reyes E., Herrera F., González O.…Estrada M.P. Discovery of immunoglobulin T in Nile tilapia (Oreochromis niloticus): a potential molecular marker to understand mucosal immunity in this species. Dev. Comp. Immunol. 2018;88:124–136. doi: 10.1016/j.dci.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Al-Harbi A.H., Truax R., Thune R.L. Production and characterization of monoclonal antibodies against tilapia Oreochromis niloticus immunoglobulin. Aquaculture. 2000;188(3–4):219–227. [Google Scholar]
- 29.Shelby R.A., Shoemaker C.A., Klesius P.H. Detection of humoral response to Streptococcus iniae infection of Nile tilapia, Oreochromis niloticus, by a monoclonal antibody-based ELISA. J. Appl. Aquacult. 2002;12(3):23–31. [Google Scholar]
- 30.Kuendee N., Klaynongsruang S., Bunyatratchata W., Tengjaroenkul B., Ngamcharoen K., Daduang J.…Daduang S. Ontogeny of Nile tilapia (Oreochromis niloticus) immunoglobulin type M antibody response. Israeli J. Aquacult. Bamidgeh. 2015 [Google Scholar]
- 31.Soonthonsrima T., Wangman P., Chaivisuthangkura P., Pengsuk C., Sithigorngul P., Longyant S. Generation of mouse monoclonal antibodies specific to tilapia immunoglobulin using fish immunoglobulin/BSA complex for monitoring of the immune response in Nile tilapia Oreochromis niloticus. Aquac. Res. 2019;50(1):277–283. [Google Scholar]
- 32.Yin X., Mu L., Fu S., Wu L., Han K., Wu H.…Ye J. Expression and characterization of Nile tilapia (Oreochromis niloticus) secretory and membrane-bound IgM in response to bacterial infection. Aquaculture. 2019;508:214–222. [Google Scholar]
- 33.Jirapongpairoj W., Hirono I., Kondo H. Development and evaluation of polyclonal antisera for detection of the IgM heavy chain of multiple fish species. J. Immunol. Methods. 2017;449:71–75. doi: 10.1016/j.jim.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Leal Y., Velazquez J., Hernandez L., Swain J.K., Rodríguez A.R., Martínez R., Carpio Y. Promiscuous T cell epitopes boosts specific IgM immune response against a P0 peptide antigen from sea lice in different teleost species. Fish Shellfish Immunol. 2019;92:322–330. doi: 10.1016/j.fsi.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Wiśniewski J.R. Quantitative evaluation of filter aided sample preparation (FASP) and multienzyme digestion FASP protocols. Anal. Chem. 2016;88(10):5438–5443. doi: 10.1021/acs.analchem.6b00859. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Bernal M., Valdivia O., Blanco R., Pérez J., Domínguez A., Basabe L., Cabrera Y. Murine monoclonal antibodies against the antimicrobial peptide oreoch-2 from tilapia (Oreochromis niloticus) Int. J. Sci. Res. Biol. Sci. 2021;8(1) [Google Scholar]
- 37.Kohler G., Milstein C. Continuous cultures of fused cells secreting antibody–antigen interactions. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 38.Quiroz J., Tsao Y.S. Statistical analysis of data from limiting dilution cloning to assess monoclonality in generating manufacturing cell lines. Biotechnol. Prog. 2016;32(4):1061–1068. doi: 10.1002/btpr.2290. [DOI] [PubMed] [Google Scholar]
- 39.Rathore G., Kumar G., Sood N., Kapoor D., Lakra W.S. Development of monoclonal antibodies to rohu [Labeo rohita] immunoglobulins for use in immunoassays. Fish Shellfish Immunol. 2008;25(6):761–774. doi: 10.1016/j.fsi.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Bordeaux J., Welsh A.W., Agarwal S., Killiam E., Baquero M.T., Hanna J.A., Rimm D.L. Antibody validation. BioTechniques. 2010;48(3):197–209. doi: 10.2144/000113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mashoof S., Pohlenz C., Chen P.L., Deiss T.C., Gatlin D., Buentello A., Criscitiello M.F. Expressed IgH μ and τ transcripts share diversity segment in ranched Thunnus orientalis. Dev. Comp. Immunol. 2014;43(1):76–86. doi: 10.1016/j.dci.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giacomelli S., Buonocore F., Albanese F., Scapigliati G., Gerdol M., Oreste U., Coscia M.R. New insights into evolution of IgT genes coming from Antarctic teleosts. Mar. Genom. 2015;24:55–68. doi: 10.1016/j.margen.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Du Y., Tang X., Zhan W., Xing J., Sheng X. Immunoglobulin tau heavy chain (IgT) in flounder, Paralichthys olivaceus: molecular cloning, characterization, and expression analyses. Int. J. Mol. Sci. 2016;17(9):1571. doi: 10.3390/ijms17091571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang X., Du Y., Sheng X., Xing J., Zhan W. Molecular cloning and expression analyses of immunoglobulin tau heavy chain (IgT) in turbot, Scophthalmus maximus. Vet. Immunol. Immunopathol. 2018;203:1–12. doi: 10.1016/j.vetimm.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Dixon B., Barreda D.R., Sunyer J.O. Perspective on the development and validation of Ab reagents to fish immune proteins for the correct assessment of immune function. Front. Immunol. 2018:2957. doi: 10.3389/fimmu.2018.02957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreau V., Fleury C., Piquer D., Nguyen C., Novali N., Villard S., Molina F. PEPOP: computational design of immunogenic peptides. BMC Bioinform. 2008;9(1):1–15. doi: 10.1186/1471-2105-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato Y., Jin G., Kuan C.T., McLendon R.E., Yan H., Bigner D.D. A monoclonal antibody IMab-1 specifically recognizes IDH1R132H, the most common glioma-derived mutation. Biochem. Biophys. Res. Commun. 2009;390(3):547–551. doi: 10.1016/j.bbrc.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hadavi R., Zarnani A.H., Ahmadvand N., Mahmoudi A.R., Bayat A.A., Mahmoudian J., Rabbani H. Production of monoclonal antibody against human nestin. Avicenna J Med Biotechnol. 2010;2(2):69. [PMC free article] [PubMed] [Google Scholar]
- 49.Swaminathan A., Lucas R.M., Dear K., McMichael A.J. Keyhole limpet haemocyanin–a model antigen for human immunotoxicological studies. Br. J. Clin. Pharmacol. 2014;78(5):1135–1142. doi: 10.1111/bcp.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gathuru J.K., Koide F., Ragupathi G., Adams J.L., Kerns R.T., Coleman T.P., Livingston P.O. Identification of DHBcAg as a potent carrier protein comparable to KLH for augmenting MUC1 antigenicity. Vaccine. 2005;23(39):4727–4733. doi: 10.1016/j.vaccine.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Amin-Blanco N., Reyes-López F., Camacho-Casanova F., Otero-Alfaro O., Cuello-Pérez M., Núñez-Martínez D., García-Imía L.G. Generation of a murine monoclonal antibody to capsular polysaccharide Vi from Salmonella Typhi. Vaccimonitor. 2015;24(2):57–63. [Google Scholar]
- 52.Yu Y., Wang Q., Huang Z., Ding L., Xu Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020:2597. doi: 10.3389/fimmu.2020.567941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhi T., Huang C., Sun R., Zheng Y., Chen J., Xu X., Yang T. Mucosal immune response of Nile tilapia Oreochromis niloticus during Gyrodactylus cichlidarum infection. Fish Shellfish Immunol. 2020;106:21–27. doi: 10.1016/j.fsi.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 54.Xia H., Liu W., Wu K., Wang W., Zhang X. sIgZ exhibited maternal transmission in embryonic development and played a prominent role in mucosal immune response of Megalabrama amblycephala. Fish Shellfish Immunol. 2016;54:107–117. doi: 10.1016/j.fsi.2016.03.165. [DOI] [PubMed] [Google Scholar]
- 55.Peñaranda M., Michelle D., Jensen I., Tollersrud L.G., Bruun J.A., Jørgensen J.B. Profiling the atlantic salmon IgM+ B cell surface proteome: novel information on teleost fish B cell protein repertoire and identification of potential B cell markers. Front Immunol. 2019;10:37. doi: 10.3389/fimmu.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakke A.F., Bjørgen H., Koppang E.O., Frost P., Afanasyev S., Boysen P., Lund H. IgM+ and IgT+ B cell traffic to the heart during sav infection in atlantic salmon. Vaccines. 2020;8(3):493. doi: 10.3390/vaccines8030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muñoz-Atienza E., Díaz-Rosales P., Tafalla C. Systemic and mucosal B and T cell responses upon mucosal vaccination of teleost fish. Front. Immunol. 2021:3901. doi: 10.3389/fimmu.2020.622377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castro R., Jouneau L., Pham H.P., Bouchez O., Giudicelli V., Lefranc M.P., Boudinot P. Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PLoS Pathog. 2013;9(1) doi: 10.1371/journal.ppat.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji J.F., Hu C.B., Zhang N., Huang X., Shao T., Fan D.D., Shao J.Z. New insights into IgZ as a maternal transfer ig contributing to the early defense of fish against pathogen infection. J. Immunol. 2021;206(9):2001–2014. doi: 10.4049/jimmunol.2001197. [DOI] [PubMed] [Google Scholar]
- 60.Swain J.K., Carpio Y., Johansen L.H., Velazquez J., Hernandez L., Leal Y., Estrada M.P. Impact of a candidate vaccine on the dynamics of salmon lice (Lepeophtheirus salmonis) infestation and immune response in Atlantic salmon (Salmo salar L.) PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Swart R.L., Kluten R.M., Huizing C.J., Vedder L.J., Reijnders P.J., Visser I.K., Osterhaus A.D. Mitogen and antigen induced B and T cell responses of peripheral blood mononuclear cells from the harbour seal (Phoca vitulina) Vet. Immunol. Immunopathol. 1993;37(3–4):217–230. doi: 10.1016/0165-2427(93)90195-a. [DOI] [PubMed] [Google Scholar]
- 62.Coutinho A., Gronowicz E., Bullock W.W., Möller G. Mechanism of thymus-independent immunocyte triggering: mitogenic activation of B cells results in specific immune responses. J. Exp. Med. 1974;139(1):74–92. doi: 10.1084/jem.139.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaattari S.L., Yui M.A. Polyclonal activation of salmonid B lymphocytes. Dev. Comp. Immunol. 1987;11(1):155–165. doi: 10.1016/0145-305x(87)90017-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.