Abstract
The glucose homeostasis system ensures that the circulating glucose level is maintained within narrow physiological limits both in the fasting (or basal) state and following a nutrient challenge. Although glucose homeostasis is traditionally conceptualized as a single overarching system, evidence reviewed here suggests that basal glycemia and glucose tolerance are governed by distinct control systems. Specifically, whereas glucose tolerance appears to be determined largely by interactions between insulin secretion and insulin sensitivity, basal-state glucose homeostasis is predominated by insulin-independent mechanisms governed largely by the brain. In addition to a new perspective on how glucose homeostasis is achieved, this “dual control system” hypothesis offers a feasible and testable explanation for observations that are otherwise difficult to reconcile and sheds new light on the integration of central and peripheral metabolic control mechanisms. The implications of this model for the pathogenesis and treatment of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes are also discussed.
Introduction
Glucose homeostasis has long been viewed as a process governed primarily by the endocrine pancreas, with changes in the circulating glucose level eliciting adaptive changes in the secretion of insulin and other hormones. While these responses are of unquestioned importance, the list of observations that cannot be accounted for by this “islet-centered” model is long and growing. Among these are findings, reviewed here, that the fasting (or basal) glucose level and glucose tolerance are governed by distinct central and peripheral control mechanisms. This evidence is among several observations that challenge conventional wisdom and justify efforts to rethink how glucose homeostasis is achieved.
In this article, we draw upon both clinical and basic science research to develop this “dual control system” hypothesis of glucose homeostasis. Because the critical role of the endocrine pancreas in this process is well established (1), this review places greater emphasis on the role of the brain in glucose homeostasis, most of which stems from basic research in animal models. To counterbalance limitations inherent in the translation of such findings to human physiology (2), we highlight the complementary nature of human and rodent findings that support this dual control system hypothesis.
Insulin Action and Glucose Tolerance
The powerful glucose-lowering effects of insulin are mediated by simultaneously promoting tissue glucose uptake while reducing endogenous glucose production. These effects are mediated by activating insulin receptors expressed by insulin-sensitive tissues (e.g., muscle, adipose, liver, and heart). Because insulin secretion by pancreatic β-cells is stimulated by glucose, insulin levels increase when blood glucose levels rise and decrease as blood glucose levels fall. This effect of glucose on circulating insulin is made possible by glucose-sensing properties intrinsic to the β-cell (3), including expression of a specific glucose transporter molecule (GLUT2) that enables glucose entry into β-cells at a rate proportional to the circulating level. Glucose is then phosphorylated by a specialized hexokinase, glucokinase, which promotes its oxidation to generate ATP. The resultant increase in the intracellular ATP-to-ADP ratio closes ATP-sensitive potassium channels, leading to plasma membrane depolarization, increased intracellular calcium, fusion of insulin-containing secretory granules with the plasma membrane, and insulin release into the circulation (3).
While glucose is clearly the main driver of insulin secretion, the β-cell response to glucose is strongly influenced by a variety of extrinsic signals. Included among these are incretins generated in response to nutrient ingestion and circulating amino acids and other nonglucose nutrients absorbed from the gastrointestinal tract. In addition, glucagon secreted by islet α-cells is hypothesized to act in a paracrine manner to augment glucose-induced insulin secretion, and input from autonomic fibers innervating the pancreatic islet can potently impact the β-cell response to glucose (4–7). The key point in this glucose–insulin interaction is that the amount of insulin secreted in response to a glucose stimulus is adjusted on a moment-by-moment basis by numerous factors, including humoral, nutrient, and neural signals that reflect changes in nutritional status and fuel requirements.
Another important determinant of glucose-stimulated insulin secretion is the prevailing level of whole-body insulin sensitivity, with insulin secretion increasing in response to declining insulin sensitivity and vice versa. When insulin secretion is plotted as a function of insulin sensitivity, the relationship conforms to a hyperbola, such that for a given level of glucose tolerance, the product of the two remains relatively constant (8–10) (Fig. 1A). This product, termed the disposition index (DI), is the predominant determinant of glucose tolerance. So long as β-cell function adapts effectively to changing insulin sensitivity, glucose tolerance will remain stable, but if insulin secretion fails to compensate for insulin resistance, glucose intolerance will result.
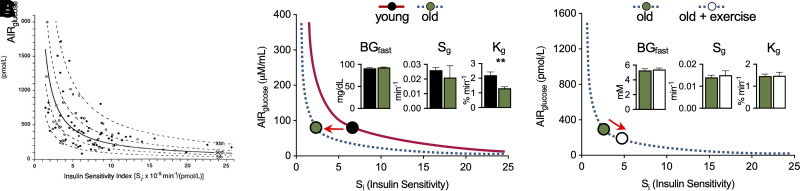
Figure 1.
Effects of aging and exercise on basal glucose, GE, and the relationship between insulin sensitivity and insulin secretion. A: DI in healthy individuals shows the hyperbolic relationship between the insulin sensitivity index (Si) and the acute insulin response to glucose (AIRglucose) (adapted from Kahn et al. [10], with permission). The DI is the primary determinant of glucose tolerance. B: Normal human aging produces a leftward shift in the DI (modeled from Chen et al. [12], with permission) that results from insulin resistance that is not compensated by increased insulin secretion. The inset shows that marked decreases of both the DI and glucose tolerance (Kg) induced by aging occurred despite no change of either fasting glucose (BGfast) or GE (Sg). C: Despite normalizing insulin sensitivity, exercise training does not reverse this aging-associated impairment of glucose tolerance (modeled from Kahn et al. [13], with permission). The inset shows that neither fasting glucose nor GE was altered by exercise training in older men.
This coupling of insulin secretion to insulin sensitivity makes teleological sense in that homeostasis is promoted by adjusting glucose-induced insulin secretion in accord with the needs of the body. How this coupling is achieved, however, is a question that has eluded investigators for decades. When one considers that insulin sensitivity varies widely from one tissue to the next and can change dynamically in a tissue-specific manner, it is hard to grasp how this coupling is even possible. Stated differently, a major determinant of β-cell function amounts to a composite of innumerable individual, continuously changing components, and this regulation is superimposed on the variety of continuously changing inputs described above. A role for the autonomic nervous system in the mechanism linking changes of whole-body insulin sensitivity to adjustments of glucose-induced insulin secretion has been suggested (11).
Separating the Control of Glucose Tolerance From Basal Glycemia
The seamless control of glycemia across the transition between basal and postprandial states gives the impression that glucose homeostasis is achieved by a single, overarching control system. Strengthening this idea is evidence that both basal and postprandial glucose homeostasis rely on the same organ systems and hormones, and that a change in one tends to be paralleled by a similar change in the other. However, the underlying mechanisms are quite different: whereas glucose tolerance is governed primarily by the interaction between insulin secretion and insulin sensitivity, basal-state glucose disposal is largely insulin independent (as discussed further below). Although these two systems operate in parallel and appear to function as one, evidence compiled in this article points to their independent nature.
Early studies suggestive of this possibility were provided by Kahn et al. (8). These investigators performed a cross-sectional analysis of glucose and insulin data from a frequently sampled intravenous glucose tolerance test (FSIGT) performed in a large and diverse cohort of human subjects. The FSIGT method was selected for these studies because it yields quantitative estimates of variables important to glucose homeostasis, both insulin dependent and insulin independent. While some of these variables are measured directly, others can be estimated using the Bergman minimal model analysis of the dynamic relationship between changing glucose and insulin levels over time following a glucose bolus. Among direct measurements are the basal glucose level, glucose-induced insulin secretion (AIRglucose), and glucose tolerance (Kg).
Model-derived estimates include the insulin sensitivity index (Si), glucose effectiveness (GE; denoted Sg in the minimal model), and the DI) (Table 1).
Table 1.
Parameters identified by minimal model analysis of data from an FSIGT
| AIRglucose | Acute insulin response to glucose |
| DI | Disposition index; overall insulin effect |
| GE | Glucose effectiveness (denoted Sg in the minimal model); insulin-independent glucose disposal |
| FSIGT | Frequently sampled intravenous glucose tolerance test |
| Kg | Glucose disappearance constant, a measure of glucose tolerance |
| Si | Insulin sensitivity index |
This early article was among the first to show that in human subjects, the relationship between insulin secretion and insulin sensitivity conforms to a hyperbola (8). As expected, the DI was also shown to be strongly predictive of glucose tolerance. In comparison, while the basal glucose level was correlated with GE (a measure of insulin-independent glucose disposal), it was not associated with insulin secretion, insulin sensitivity, or glucose tolerance. Although correlative, these initial findings suggest that whereas insulin secretion and insulin sensitivity are both strongly linked to one another and important determinants of glucose tolerance, they are not closely associated with either GE or basal glycemia.
Can glucose tolerance and basal glycemia change independently of one another? A compelling answer to this question emerged unexpectedly from a study of human aging performed by Chen et al. (12). Here, the authors report that markedly impaired glucose tolerance is a feature of aging in normal men (an ∼40% decrease of glucose tolerance relative to young control individuals) and that this effect is entirely attributable to reduced insulin action. Specifically, aging was associated with a >60% reduction in insulin sensitivity index with no compensatory increase of glucose-induced insulin secretion, which in combination yields a 60% decrease of the DI. In contrast, neither basal glucose nor GE (measured as Sg in the minimal model) was altered (Fig. 1B). These findings therefore show that aging-associated glucose intolerance 1) results from the failure of insulin secretion to compensate for insulin resistance and 2) is not associated with changes of either basal glycemia or insulin-independent glucose disposal.
An interesting follow-up study was then undertaken by Kahn et al. (13) to determine whether aging-associated insulin resistance and glucose intolerance can be reversed by exercise training. This “exercise and aging” study showed that as expected, exercise training reverses aging-associated insulin resistance without affecting either basal glucose or GE. Unexpectedly, however, exercise training had no effect on aging-associated glucose intolerance; instead, the exercise-induced improvement of insulin sensitivity was offset by a proportionate decrease of insulin secretion, such that neither glucose tolerance nor the DI was affected (Fig. 1C).
These findings strengthen the conclusion that glucose tolerance, but not the basal glucose level, is determined primarily by the interaction between insulin secretion and insulin sensitivity. In addition, the observation that exercise training affected neither glucose tolerance nor the DI, despite reversing age-associated insulin resistance, raises the possibility that glucose tolerance is itself regulated and that the biologically defended level “resets” as part of the natural aging process.
Insulin-Independent Glucose Disposal
Although underlying mechanisms remain incompletely understood, the term insulin-independent glucose disposal was introduced to account for clearance of glucose from the bloodstream that cannot be accounted for by the action of insulin. Mechanistically, this phenomenon is assumed to reflect the passive entry of glucose down a concentration gradient from the extracellular compartment into cells via insulin-independent glucose transporters. In addition, however, a growing body of evidence has established that in animal models of diabetes, glucose lowering can be potently and rapidly activated by the brain with little or no change of either insulin secretion or insulin sensitivity (14,15). As such, mechanisms underlying brain-mediated reversal of diabetic hyperglycemia in these studies are largely insulin independent. Thus, the brain appears to have the inherent ability to activate insulin-independent glucose-lowering mechanisms.
Identifying these mechanisms has proven challenging, however, in part because insulin-independent glucose disposal is difficult to measure. Available tools include the Bergman minimal model estimate of GE (16,17), and brain-mediated stimulation of GE has been reported in a mouse model of type 2 diabetes (18). However, since GE is based on glucose clearance following an intravenous glucose bolus, it is not optimized for estimating basal-state glucose disposal (19).
Insulin Action in the Basal State
Because insulin levels are low in the basal state and increase markedly in response to a carbohydrate-rich meal (20,21) (Fig. 2A), logic dictates that insulin-mediated glucose disposal would play a greater role in the response to a glucose challenge than it does in basal-state glucose homeostasis (19) (Fig. 2B). Indeed, glucose disposal in the basal state is predominated by insulin-independent mechanisms (22–24). This assertion seems paradoxical, however, when one considers that fasting hyperglycemia is commonplace in patients with uncontrolled type 1 diabetes. How can insulin deficiency cause fasting hyperglycemia if glucose disposal in the basal state is largely insulin independent? The answer lies in distinguishing the indispensable, permissive role played by insulin in basal-state glucose homeostasis from its effects on glucose uptake.
Figure 2.
Contributions of insulin-dependent and insulin-independent mechanisms to glucose disposal. A: In healthy individuals, postprandial plasma glucose concentrations (red line) are maintained within a narrow range through meal-induced secretion of insulin (blue line) (from Owens et al. [21], with permission). B: GE accounts for 60–75% of glucose disposal in the presence of basal (10 µU/mL) plasma insulin. In comparison, the contribution made by GE is reduced to <30% when plasma insulin levels are high (120 µU/mL) (modeled from Best et al. [19], with permission).
Specifically, basal insulin signaling is required to constrain the activity of metabolic processes that are activated in response to fuel depletion, including lipolysis, ketogenesis, glycogenolysis, and gluconeogenesis. Consequently, each of these processes is activated in an unrestrained manner in the absence of an insulin signal (25,26). Left unchecked, this “runaway train” of metabolic impairment causes dehydration and ketoacid accumulation that can trigger a centrally driven stress response. The combination of increased sympathetic nervous system (SNS) outflow to liver, adipose, and other tissues with increased circulating glucocorticoids and catecholamines further activates these fuel mobilization pathways.
Another aggravating factor is severe leptin deficiency, which attends insulin deficiency since adipocyte leptin synthesis is also insulin dependent. The hyperphagia and centrally driven fuel mobilization driven by the combined effects of severe insulin and leptin deficiency exacerbate this unrestrained metabolic decompensation, effectively flooding the circulation with glucose, lactate, free fatty acids, ketones, and other substrates. If untreated, this pathogenic sequence can result in severe dehydration and electrolyte imbalance, cardiovascular collapse, and death.
It is therefore important to distinguish this indispensable, permissive role for insulin in the control of basal metabolic activity from its contribution to basal-state glucose disposal per se, with the latter process being largely insulin independent. Stated differently, basal-state glucose disposal is driven primarily by insulin-independent mechanisms that are overwhelmed by the cascade of events unleashed by the absence of baseline insulin signaling. At the same time, dysfunction of central glucoregulatory neurocircuits can impair both insulin secretion and insulin sensitivity (7,27). Thus, although glucose tolerance is governed primarily by interactions between insulin secretion and insulin sensitivity, these variables can be powerfully impacted by the central control mechanisms. Because these central and peripheral control systems are inextricably linked, dysfunction in one can predispose to dysregulation of the other, setting in motion a vicious cycle of progressive metabolic impairment.
Accordingly, correcting impairment of one metabolic system can be predicted to ameliorate impairment of the other, at least to some extent. In this context, it is noteworthy that the pathogenic sequence unleashed by peripheral insulin/leptin deficiency can be blunted or even reversed by interventions that target the brain of diabetic animals. As detailed below, these interventions engage insulin-independent mechanisms that can effectively reverse basal hyperglycemia while having little or no effect on glucose tolerance. Such basic research findings complement clinical evidence reviewed here that shows systems governing basal glycemia and glucose tolerance are separable from one another.
Targeting the Brain to Distinguish Control of Basal Glycemia From Glucose Tolerance
Evidence that the brain can be targeted to reverse basal hyperglycemia in rodent models of diabetes has forced reconsideration of how glucose homeostasis is achieved. As this brain effect is mediated largely by increasing insulin-independent glucose disposal in the basal state, it amounts to a mirror image of what occurs in human aging (Fig. 1B), where impaired glucose tolerance is not associated with impairment of basal glycemia and results exclusively from reduced insulin action.
The brain’s ability to reverse diabetic hyperglycemia was first observed during continuous central administration of the adipocyte hormone leptin. In these studies, leptin was administered for up to 2 weeks via intracerebroventricular infusion in rats or mice with severe, insulin-deficient diabetes induced by the pancreatic β-cell toxin streptozotocin (28–32). This work revealed that although the effect of leptin took several days to develop, markedly elevated blood glucose levels began to decline after ∼4 days of continuous intracerebroventricular infusion (30). By day 7, basal hyperglycemia had resolved and remained so until the intracerebroventricular leptin infusion was discontinued (Fig. 3A), at which point hyperglycemia comparable to that of vehicle-treated controls was reinstated.
Figure 3.
Targeting the brain to ameliorate diabetic hyperglycemia. A: Uncontrolled, insulin-deficient diabetes in streptozotocin-treated rats is corrected by continuous intracerebroventricular (icv) leptin administration (modeled from German et al. [30], with permission). B: Hyperglycemia in the obese ZDF rat model of type 2 diabetes is restored to normal in a sustained manner after a single intracerebroventricular FGF1 injection (modeled from Scarlett et al. [33], with permission). C: Evidence suggests that whereas the antidiabetic action of leptin is mediated in the hypothalamic ventromedial nucleus (VMH), FGF1 action involves the ARC. veh, vehicle.
These observations are remarkable in several ways. First, blood glucose lowering induced by central leptin administration occurs in the face of ongoing, severe insulin deficiency, implying that the underlying mechanism is insulin independent. Second, basal glycemia appears to have been reset at a more normal level, based on the facts that glucose levels remain stable over time and that following perturbation (e.g., administration of glucose or insulin), glycemia returns to its newly reset level (despite persistently abnormal glucose tolerance) (30). Based on these observations, the new, lower blood glucose level induced by intracerebroventricular leptin administration behaves as if it is biologically defended.
A third remarkable finding is that leptin-mediated restoration of euglycemia occurs despite the near absence of an insulin signal even in response to a glucose challenge (30). This finding implies that leptin action in the brain is sufficient to reverse the runaway metabolic dysfunction characteristic of severe insulin deficiency alluded to above despite having no impact on the circulating insulin level. Consistent with this interpretation, the marked elevation of plasma free fatty acid and ketone body levels as well as the increase of hepatic glucose production characteristic of the streptozotocin–diabetes model are both reversed by intracerebroventricular leptin administration, although glucose intolerance in these animals is not reversed (30).
The mediobasal hypothalamus (MBH) has been identified as the brain area implicated in the antidiabetic action of intracerebroventricular leptin, since a similar glucose-lowering effect is induced by microinjection of a much lower leptin dose directly into this brain area (29). Thus, the metabolic consequences of severe insulin deficiency can be ameliorated by leptin receptor activation in the MBH. Implicit in these findings is 1) an indispensable role for responses initiated in the brain (e.g., increased SNS outflow) in the cascading metabolic derangements characteristic of uncontrolled diabetes and 2) these responses can be reversed by leptin. These findings collectively offer additional support for the hypothesis that basal glycemia and glucose tolerance are governed by distinct central and peripheral mechanisms.
A second body of work demonstrating that the brain can be targeted to restore normoglycemia to diabetic animals stems from studies involving central administration of fibroblast growth factor 1 (FGF1) (33–39). Like the response to intracerebroventricular leptin, the antidiabetic action of intracerebroventricular FGF1 is mediated by activating signal transduction mechanisms in the MBH (35). In addition, while both intracerebroventricular leptin and intracerebroventricular FGF1 injection cause transient anorexia and weight loss, these responses cannot explain their antidiabetic actions (30,33). This observation stands in contrast to the antidiabetic effects of incretin-mimetic drugs (discussed below), where weight loss and glucose-lowering effects are not easily separated from one another (40). A third similarity is that amelioration of hyperglycemia by intracerebroventricular FGF1 injection is limited to the basal state with no detectable effect on Kg (33). Lastly, the more normal blood glucose level induced by intracerebroventricular FGF1 injection appears to be biologically defended (Fig. 3B), and this effect cannot be attributed to increases of either insulin secretion or insulin sensitivity (34).
Despite these similarities, substantial differences also exist between the antidiabetic actions of FGF1 and leptin in the brain. Perhaps the most notable of these has to do with the duration of the blood glucose-lowering effect. Specifically, whereas normoglycemia induced by central leptin administration persists only so long as leptin is administered (30,33), remission of diabetes is sustained for weeks or even months following a single intracerebroventricular injection of FGF1 (33). In addition, whereas intracerebroventricular leptin inhibits hepatic glucose production and lowers elevated circulating levels of both cortisol and glucagon, the antidiabetic action of intracerebroventricular FGF1 is driven primarily by increased insulin-independent glucose uptake across multiple tissues, including the liver (34).
A third key difference is that whereas intracerebroventricular leptin is highly effective in animals with severe, insulin-deficient diabetes, the antidiabetic action of intracerebroventricular FGF1 is limited to animal models of obesity-associated type 2 diabetes and becomes completely ineffective in the absence of an insulin signal (33). This is a notable observation in that it suggests that while glucose lowering induced by intracerebroventricular FGF1 injection is largely insulin independent, the underlying mechanisms driving this FGF1 glucose homeostatic effect require a baseline insulin signal, which is not the case for the response to intracerebroventricular leptin infusion. One final distinction between the hypothalamic actions of leptin and FGF1 is that whereas the physiological role played by the former is well established, the question of whether endogenous FGF1 participates in central control of glucose homeostasis has yet to be addressed.
While the MBH is implicated in the antidiabetic actions of both leptin and FGF1, the specific brain areas involved appear to differ. Specifically, the ventromedial hypothalamic nuclei (VMN) and dorsomedial hypothalamic nuclei are implicated as key targets for the action of leptin (31,41), while FGF1 action appears to be mediated primarily in the adjacent arcuate nucleus (ARC) (Fig. 3C) (35). Each of these hypothalamic nuclei contains multiple neuronal and glial subpopulations implicated in both energy and glucose homeostasis, and studies to identify contributions made by both neuronal and nonneuronal cell types to reversal of diabetic hyperglycemia are underway.
Astrocytes, oligodendrocyte lineage cells, tanycytes, and perhaps other glial cells, rather than neurons, have emerged as primary targets of FGF1 action in the ARC (36). Although mechanisms linking FGF1-induced glial cell activation to diabetes remission remain uncertain, remodeling of extracellular matrix specializations, termed perineuronal nets, may contribute. These structures are composed of proteoglycans and hyaluronan, and they enmesh and thereby regulate the function and plasticity of distinct neuronal subsets, including some that are involved in glucose homeostasis (42,43). Indeed, a recent study showed that sustained FGF1-induced glucose lowering depends on reassembly of these MBH perineuronal nets (42,43). Taken together, these findings indicate that in rodent models of diabetes, 1) glucoregulatory neurocircuit dysfunction contributes to basal-state hyperglycemia, 2) the hypothalamic actions of FGF1 and leptin engage insulin-independent mechanisms that reverse basal-state hyperglycemia in diabetic animals, and 3) glucose tolerance is minimally impacted by this sequence of events. These collective observations are difficult to explain unless a brain control system that uses insulin-independent mechanisms to control basal glycemia can act independently of the islet-based system that controls glucose tolerance.
A Larger System for Metabolic Homeostasis
Evidence that glucose metabolism is tightly coupled to the control of both body temperature and body fat mass (44,45) can be observed by simply placing a mouse in a cool environment. To prevent hypothermia, heat production must increase. This response is driven by increased SNS outflow to thermogenic tissue (46), and it will deplete body fat stores unless energy intake increases to compensate. Indeed, “cold-induced hyperphagia” is detectable within minutes, well before any detectable loss of fat mass has occurred (47), with the amount consumed calibrated to precisely offset cold-induced energy costs. This set of responses ensures the stability of both body temperature and body fuel stores.
As this thermogenic response is dependent on increased glucose oxidation, adjustment by the glucose homeostasis system is required if hypoglycemia is to be averted. To achieve this goal, cold exposure elicits reciprocal changes of tissue insulin sensitivity and β-cell function. Specifically, cold exposure increases insulin sensitivity of thermogenic tissues in a manner that is offset by a proportionate decrease of glucose-induced insulin secretion mediated by increased SNS outflow (11). The net effect of these integrated SNS responses is that neither body temperature, body fat mass, nor blood glucose levels change substantially in response to cold exposure, even in the face of a profound increase in the rate of fuel utilization.
This set of adaptive responses is enabled by a process termed allostasis, wherein diverse interoceptive inputs are integrated with learned experience to form predictions regarding future need states. A key advantage of this type of regulation is that it enables adaptive responses to be mounted in anticipation of a change in the defended variable (e.g., blood glucose, body temperature, or body fuel stores) before the change occurs. For variables that can change rapidly and are vital to survival of the organism, this form of control is far more efficient than traditional negative feedback mechanisms, which are slower to respond because they require the defended variable to change before an adaptive response is mounted.
Once again, this point is elegantly illustrated by placing a mouse in a cold environment.
Simply sensing this environmental change is sufficient to engage adaptive responses that prevent internal temperature from changing. Had the system relied solely on negative feedback control, a change of body temperature would be required for adaptive responses to be mounted–an outcome incompatible with the goal of maintaining thermal stability. The mechanism that mediates the rapid increase of food intake during cold exposure (47) is anticipatory in the sense that it is mounted prior to a detectable change of fat stores and effectively prevents fat mass from changing. Similarly, the reciprocal changes of insulin secretion and insulin sensitivity that ensure that the body’s fuel needs are met with minimal impact on glycemia appear to be coordinated by cold-induced changes of SNS outflow (11). This interpretation is consonant with a rich literature describing control of insulin secretion by anticipatory and learned mechanisms in the service of optimal glucose homeostasis (48–51).
Relevance to Diabetes
Based on the dual control system hypothesis put forth here, we interpret the fact that both basal glycemia and glucose tolerance are abnormal in patients with type 2 diabetes as evidence of dysfunction that impacts both central and peripheral control systems. We can extend this logic to predict that therapeutic interventions that ameliorate both basal hyperglycemia and glucose intolerance involve effects in the brain as well as in the pancreas.
This hypothesis is supported by clinical evidence that while impaired fasting glucose (IFG) (which can be defined as dysfunction of basal-state glucose homeostasis) and impaired glucose tolerance (IGT) are both defining features of prediabetes, the two often occur independently of one another. In addition, when IFG occurs in the absence of IGT, recent evidence indicates that β-cell function (measured as the insulin secretion rate plotted as a function of the glucose level) is unaffected, whereas this is not true of patients with IGT (52). Finally, when both IFG and IGT are present, the risk for progression to type 2 diabetes is increased relative to that of individuals with just one of these defects (53,54). This finding that type 2 diabetes is much more likely to result when both systems are impaired is consistent with the dual control system hypothesis, which posits that central nervous system (CNS) and islet dysfunction feed upon one another to create a vicious cycle that progressively impairs both systems. We note that this pattern of progressive deterioration is characteristic of the natural history of type 2 diabetes.
Is diabetes in humans associated with impairment of central control mechanisms? While diabetes is clearly associated with various forms of brain dysfunction, separating cause from effect can be challenging. For example, diabetes is associated with both cognitive impairment and heightened risk of neurodegenerative disease, but this association is usually attributed to aggravating effects of hyperglycemia and associated metabolic impairment on underlying neuronal dysfunction (55–57). Similar considerations apply to the association between diabetes and central defects ranging from increased blood–brain barrier permeability (58,59) to impaired brain glucose uptake (60,61) and disrupted autoregulation of cerebral blood flow (56,62).
It is also possible, however, that CNS dysfunction predisposes to glucose metabolic impairment. This concept is supported by evidence that while diabetes can clearly predispose to the development and progression of Alzheimer disease, Alzheimer disease is associated with both systemic insulin resistance (63) and an increased risk of future type 2 diabetes (64). These observations support the notion that as neurodegeneration progresses, central control of glucose homeostasis can become impaired. The predicted outcome is a vicious cycle wherein neurodegeneration and glucose metabolic impairment progress in parallel, strengthening the association between them.
Additional evidence of a role for the brain in the pathogenesis of type 2 diabetes is provided by extensive evidence of increased SNS outflow in this disease (61,62,65,66). Since activation of SNS outflow to pancreas and liver inhibits glucose-induced insulin secretion while also stimulating hepatic glucose production, a causal role in type 2 diabetes pathogenesis can be considered. Consistent with this notion, increased SNS activity has been identified as a risk factor for future type 2 diabetes among at-risk individuals (66–69).
Glial cell activation localized to the MBH is a form of neuropathology unique to individuals with obesity and/or type 2 diabetes. Termed “reactive gliosis,” this process involves inflammatory activation of both astrocytes and microglia and is detected in the ARC of obese humans with or without type 2 diabetes, a finding recapitulated in animal models (70–73).
Among the many ways that this form of glial dysfunction can impair neuronal function is by interrupting the steady supply of glucose, lactate, and other substrates that neurons rely on (74).
Recent work from Schur and colleagues (73) using structural MRI shows that in humans with type 2 diabetes, the intensity of the MBH gliosis signal is strongly associated with the degree of glycemic impairment, even after controlling for degree of obesity. Given the key role played by the ARC in CNS control of glucose homeostasis, the hypothesis that this glial response contributes to the associated glucose metabolic impairment can be considered.
Hypothalamic neurocircuit dysfunction is also implicated in the pathogenesis of hyperglycemia in mouse models of type 1 diabetes. Specifically, Myers and colleagues (75) demonstrated that in mice with severe insulin-deficient diabetes, hyperglycemia is largely ameliorated by inactivation of a specific subset of neurons in the hypothalamic VMN. Interestingly, the same neuronal subset is implicated in CNS-driven stress responses, including the counterregulatory response to hypoglycemia (75).
We interpret this finding to suggest that hyperglycemia in this setting is driven in part by aberrant activation of a key subset of stress-responsive neurons in the VMN. As circulating levels of both leptin and insulin are extremely low in this setting, and as this brain area is implicated in leptin’s antidiabetic action, we hypothesize that diabetes-associated activation of these VMN neurons is triggered by effects of insulin and leptin deficiency, combined with the stress associated with cascading metabolic decompensation. We further speculate that hyperglycemia and associated systemic metabolic derangement are exacerbated by VMN neuron activation, creating a vicious cycle, and that leptin’s potent antidiabetic action involves reversal of this neuronal response.
Therapeutic Implications
A key unanswered question raised by these considerations is the extent to which brain control of glucose homeostasis can be targeted to treat diabetes. The recent emergence of long-acting glucagon-like peptide 1 (GLP-1) receptor agonists (also known as incretin mimetics) as one of the most effective type 2 diabetes treatment options is informative (40). These drugs clearly act both in the periphery and in the CNS, and in patients with type 2 diabetes they exert beneficial effects on both basal-state hyperglycemia and glucose intolerance (4,40). While these drugs clearly act on the β-cell to augment glucose-induced insulin secretion (by activating β-cell GLP-1 receptors), the brain and peripheral nervous system are responsible for reduced food intake and associated weight loss and may also contribute to the marked improvement of glucose metabolism mediated by these drugs. Support for the latter hypothesis includes evidence that 1) GLP-1 receptors are concentrated in brain areas important for glucose homeostasis (76), 2) activation of GLP-1 receptor–expressing neurons in a particular hypothalamic area (the dorsomedial nucleus) rapidly lowers the blood glucose level in mice (76), and 3) systemic GLP-1 administration to humans increases not only glucose-induced insulin secretion but also insulin-independent glucose disposal (77).
The recent introduction of tirzepatide (an incretin polyagonist drug that activates receptors for both GLP-1 and glucose-dependent insulinotropic polypeptide) builds on the unprecedented success of GLP-1–based therapeutics (78,79). While the potent antiobesity and antidiabetic effects of tirzepatide have been widely heralded (80), the fact that the brain and pancreas are both key targets for its metabolic restorative actions is less widely appreciated. With more of these dual agonist drugs under development (combinations of GLP-1 receptor, glucose-dependent insulinotropic polypeptide receptor, amylin receptor, and glucagon receptor agonists, among others), we anticipate that strategies for targeting the two distinct systems described here will emerge as an important adjunct to current diabetes treatment modalities.
Article Information
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, grants R01DK101997 and R01DK083042 (M.W.S.), American Diabetes Association grant 11-22-IBSPM-06, and Department of Defense grant AZ210089 (K.M.A.) and by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health–funded Nutrition Obesity Research Center (P30DK035816) and the Diabetes Research Center (P30DK017047) at the University of Washington.
Duality of Interest. M.W.S. has received research support from Novo Nordisk and is a consultant for NodThera. No other potential conflicts of interest relevant to this article were reported.
References
- 1. Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med 2016;48:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruce CR, Hamley S, Ang T, Howlett KF, Shaw CS, Kowalski GM. Translating glucose tolerance data from mice to humans: insights from stable isotope labelled glucose tolerance tests. Mol Metab 2021;53:101281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seino S, Iwanaga T, Nagashima K, Miki T. Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice. Diabetes 2000;49:311–318 [DOI] [PubMed] [Google Scholar]
- 4. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El K, Gray SM, Capozzi ME, et al. GIP mediates the incretin effect and glucose tolerance by dual actions on α cells and β cells. Sci Adv 2021;7:eabf1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capozzi ME, Wait JB, Koech J, et al. Glucagon lowers glycemia when β-cells are active. JCI Insight 2019;5:e129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faber CL, Deem JD, Campos CA, Taborsky GJ Jr, Morton GJ. CNS control of the endocrine pancreas. Diabetologia 2020;63:2086–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahn SE, Prigeon RL, Schwartz RS, et al. Obesity, body fat distribution, insulin sensitivity and islet beta-cell function as explanations for metabolic diversity. J Nutr 2001;131:354S–360S [DOI] [PubMed] [Google Scholar]
- 9. Porte D Jr. Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes 1991;40:166–180 [DOI] [PubMed] [Google Scholar]
- 10. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 11. Morton GJ, Muta K, Kaiyala KJ, et al. Evidence that the sympathetic nervous system elicits rapid, coordinated, and reciprocal adjustments of insulin secretion and insulin sensitivity during cold exposure. Diabetes 2017;66:823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen M, Bergman RN, Pacini G, Porte D Jr. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab 1985;60:13–20 [DOI] [PubMed] [Google Scholar]
- 13. Kahn SE, Larson VG, Beard JC, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol 1990;258:E937–E943 [DOI] [PubMed] [Google Scholar]
- 14. Mirzadeh Z, Faber CL, Schwartz MW. Central nervous system control of glucose homeostasis: a therapeutic target for type 2 diabetes? Annu Rev Pharmacol Toxicol 2022;62:55–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myers MG Jr, Affinati AH, Richardson N, Schwartz MW. Central nervous system regulation of organismal energy and glucose homeostasis. Nat Metab 2021;3:737–750 [DOI] [PubMed] [Google Scholar]
- 16. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 17. Bergman RN. Origins and history of the minimal model of glucose regulation. Front Endocrinol (Lausanne) 2021;11:583016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morton GJ, Matsen ME, Bracy DP, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 2013;123:4799–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996;19:1018–1030 [DOI] [PubMed] [Google Scholar]
- 20. Kipnis DM. Insulin secretion in normal and diabetic individuals. Adv Intern Med 1970;16:103–134 [PubMed] [Google Scholar]
- 21. Owens DR, Zinman B, Bolli GB. Insulins today and beyond. Lancet 2001;358:739–746 [DOI] [PubMed] [Google Scholar]
- 22. Kahn SE, Prigeon RL, McCulloch DK, et al. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes 1994;43:587–592 [DOI] [PubMed] [Google Scholar]
- 23. Edelman SV, Laakso M, Wallace P, Brechtel G, Olefsky JM, Baron AD. Kinetics of insulin-mediated and non-insulin-mediated glucose uptake in humans. Diabetes 1990;39:955–964 [DOI] [PubMed] [Google Scholar]
- 24. Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol 1988;255:E769–E774 [DOI] [PubMed] [Google Scholar]
- 25. McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 1980;49:395–420 [DOI] [PubMed] [Google Scholar]
- 26. Miles JM, Gerich JE. Glucose and ketone body kinetics in diabetic ketoacidosis. Clin Endocrinol Metab 1983;12:303–319 [DOI] [PubMed] [Google Scholar]
- 27. Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci 2011;1243:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meek TH, Morton GJ. The role of leptin in diabetes: metabolic effects. Diabetologia 2016;59:928–932 [DOI] [PubMed] [Google Scholar]
- 29. Meek TH, Matsen ME, Dorfman MD, et al. Leptin action in the ventromedial hypothalamic nucleus is sufficient, but not necessary, to normalize diabetic hyperglycemia. Endocrinology 2013;154:3067–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. German JP, Thaler JP, Wisse BE, et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology 2011;152:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. da Silva AA, Hall JE, Moak SP, et al. Role of autonomic nervous system in chronic CNS-mediated antidiabetic action of leptin. Am J Physiol Endocrinol Metab 2017;312:E420–E428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fujikawa T, Berglund ED, Patel VR, et al. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab 2013;18:431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scarlett JM, Rojas JM, Matsen ME, et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med 2016;22:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scarlett JM, Muta K, Brown JM, et al. Peripheral mechanisms mediating the sustained antidiabetic action of FGF1 in the brain. Diabetes 2019;68:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown JM, Scarlett JM, Matsen ME, et al. The hypothalamic arcuate nucleus-median eminence is a target for sustained diabetes remission induced by fibroblast growth factor 1. Diabetes 2019;68:1054–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bentsen MA, Rausch DM, Mirzadeh Z, et al. Transcriptomic analysis links diverse hypothalamic cell types to fibroblast growth factor 1-induced sustained diabetes remission. Nat Commun 2020;11:4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown JM, Bentsen MA, Rausch DM, et al. Role of hypothalamic MAPK/ERK signaling and central action of FGF1 in diabetes remission. iScience 2021;24:102944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hwang E, Scarlett JM, Baquero AF, et al. Sustained inhibition of NPY/AgRP neuronal activity by FGF1. JCI Insight 2022;7:e160891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roberts BL, Kim EJ, Lindsley SR, Tennant KG, Kievit P. Fibroblast growth factor-1 activates neurons in the arcuate nucleus and dorsal vagal complex. Front Endocrinol (Lausanne) 2021;12:772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mingrone G, Castagneto-Gissey L, Bornstein SR. New horizons: emerging anti-diabetic medications. J Clin Endocrinol Metab 2022;107:e4333–e4340 [DOI] [PubMed] [Google Scholar]
- 41. Rupp AC, Tomlinson AJ, Affinati AH, et al. Leptin-mediated suppression of food intake by conserved Glp1r-expressing neurons prevents obesity. bioRxiv. 11 December 2021 [preprint]. DOI: 10.1101/2021.12.10.472115 [DOI] [Google Scholar]
- 42. Mirzadeh Z, Alonge KM, Cabrales E, et al. Perineuronal net formation during the critical period for neuronal maturation in the hypothalamic arcuate nucleus. Nat Metab 2019;1:212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alonge KM, Mirzadeh Z, Scarlett JM, et al. Hypothalamic perineuronal net assembly is required for sustained diabetes remission induced by fibroblast growth factor 1 in rats. Nat Metab 2020;2:1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bentsen MA, Mirzadeh Z, Schwartz MW. Revisiting how the brain senses glucose-and why. Cell Metab 2019;29:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brown JM, Scarlett JM, Schwartz MW. Rethinking the role of the brain in glucose homeostasis and diabetes pathogenesis. J Clin Invest 2019;129:3035–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gasparetti AL, de Souza CT, Pereira-da-Silva M, et al. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. J Physiol 2003;552:149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deem JD, Faber CL, Pedersen C, et al. Cold-induced hyperphagia requires AgRP neuron activation in mice. eLife 2020;9:e58764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Langhans W, Watts AG, Spector AC. The elusive cephalic phase insulin response: triggers, mechanisms, and functions. Physiol Rev 2022;103:1423–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vahl TP, Aulinger BA, Smith EP, et al. Meal feeding improves oral glucose tolerance in male rats and causes adaptations in postprandial islet hormone secretion that are independent of plasma incretins or glycemia. Am J Physiol Endocrinol Metab 2014;307:E784–E792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Begg DP, Woods SC. Interactions between the central nervous system and pancreatic islet secretions: a historical perspective. Adv Physiol Educ 2013;37:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramsay DS, Woods SC. Physiological regulation: how it really works. Cell Metab 2016;24:361–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kohlenberg JD, Laurenti MC, Egan AM, et al. Differential contribution of alpha and beta cell dysfunction to impaired fasting glucose and impaired glucose tolerance. Diabetologia 2022;66:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Y, Li J, Wu Y, et al. Evidence from a systematic review and meta-analysis: classical impaired glucose tolerance should be divided into subgroups of isolated impaired glucose tolerance and impaired glucose tolerance combined with impaired fasting glucose, according to the risk of progression to diabetes. Front Endocrinol (Lausanne) 2022;13:835460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yip WCY, Sequeira IR, Plank LD, Poppitt SD. Prevalence of pre-diabetes across ethnicities: a review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycaemia. Nutrients 2017;9:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cooke S, Pennington K, Jones A, Bridle C, Smith MF, Curtis F. Effects of exercise, cognitive, and dual-task interventions on cognition in type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS One 2020;15:e0232958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chau ACM, Cheung EYW, Chan KH, et al. Impaired cerebral blood flow in type 2 diabetes mellitus—a comparative study with subjective cognitive decline, vascular dementia and Alzheimer’s disease subjects. Neuroimage Clin 2020;27:102302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X, Jiang X, Han S, Liu Q, Zhou J. Type 2 diabetes mellitus is associated with the risk of cognitive impairment: a meta-analysis. J Mol Neurosci 2019;68:251–260 [DOI] [PubMed] [Google Scholar]
- 58. Banks WA. The blood-brain barrier interface in diabetes mellitus: dysfunctions, mechanisms and approaches to treatment. Curr Pharm Des 2020;26:1438–1447 [DOI] [PubMed] [Google Scholar]
- 59. Rom S, Zuluaga-Ramirez V, Gajghate S, et al. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol 2019;56:1883–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boersma GJ, Johansson E, Pereira MJ, et al. Altered glucose uptake in muscle, visceral adipose tissue, and brain predict whole-body insulin resistance and may contribute to the development of type 2 diabetes: a combined PET/MR study. Horm Metab Res 2018;50:627–639 [DOI] [PubMed] [Google Scholar]
- 61. Hwang JJ, Jiang L, Hamza M, et al. Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JCI Insight 2017;2:e95913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barloese MCJ, Bauer C, Petersen ET, Hansen CS, Madsbad S, Siebner HR. Neurovascular coupling in type 2 diabetes with cognitive decline. A narrative review of neuroimaging findings and their pathophysiological implications. Front Endocrinol (Lausanne) 2022;13:874007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kellar D, Craft S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol 2020;19:758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004;53:474–481 [DOI] [PubMed] [Google Scholar]
- 65. Frank CJ, McNay EC. Breakdown of the blood-brain barrier: a mediator of increased Alzheimer’s risk in patients with metabolic disorders? J Neuroendocrinol 2022;34:e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwartz RS, Jaeger LF, Veith RC, Lakshminarayan S. The effect of diet or exercise on plasma norepinephrine kinetics in moderately obese young men. Int J Obes 1990;14:1–11 [PubMed] [Google Scholar]
- 67. Lips MA, de Groot GH, De Kam M, et al. Autonomic nervous system activity in diabetic and healthy obese female subjects and the effect of distinct weight loss strategies. Eur J Endocrinol 2013;169:383–390 [DOI] [PubMed] [Google Scholar]
- 68. Lee DY, Lee MY, Cho JH, et al. Decreased vagal activity and deviation in sympathetic activity precedes development of diabetes. Diabetes Care 2020;43:1336–1343 [DOI] [PubMed] [Google Scholar]
- 69. Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 2003;108:3097–3101 [DOI] [PubMed] [Google Scholar]
- 70. Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dorfman MD, Thaler JP. Hypothalamic inflammation and gliosis in obesity. Curr Opin Endocrinol Diabetes Obes 2015;22:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rosenbaum JL, Melhorn SJ, Schoen S, et al. Evidence that hypothalamic gliosis is related to impaired glucose homeostasis in adults with obesity. Diabetes Care 2022;45:416–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sewaybricker LE, Kee S, Melhorn SJ, Schur EA. Greater radiologic evidence of hypothalamic gliosis predicts adiposity gain in children at risk for obesity. Obesity (Silver Spring) 2021;29:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Henn RE, Noureldein MH, Elzinga SE, Kim B, Savelieff MG, Feldman EL. Glial-neuron crosstalk in health and disease: a focus on metabolism, obesity, and cognitive impairment. Neurobiol Dis 2022;170:105766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Flak JN, Goforth PB, Dell’Orco J, et al. Ventromedial hypothalamic nucleus neuronal subset regulates blood glucose independently of insulin. J Clin Invest 2020;130:2943–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Huang Z, Liu L, Zhang J, et al. Glucose-sensing glucagon-like peptide-1 receptor neurons in the dorsomedial hypothalamus regulate glucose metabolism. Sci Adv 2022;8:eabn5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. D’Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 1994;93:2263–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jastreboff AM, Aronne LJ, Ahmad NN, et al.; SURMOUNT-1 Investigators . Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387:205–216 [DOI] [PubMed] [Google Scholar]
- 79. Frías JP, Davies MJ, Rosenstock J, et al.; SURPASS-2 Investigators . Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–515 [DOI] [PubMed] [Google Scholar]
- 80. Chipkin SR. Tirzepatide for patients with type 2 diabetes. JAMA 2022;327:529–530 [DOI] [PubMed] [Google Scholar]