Abstract
Taxonomic researchers have used multiple sources of evidence to support species hypotheses and delimitations. Grammostola Simon (Mygalomorphae: Theraphosidae) comprises 20 valid species endemic to South America, six occurring in Brazil. The classical morphological approach based mainly on genitalia may be misleading in recognizing species in this genus. Thus, we used morphology, geographical distribution, genetic distance, and phylogeny to support the redescription of Grammostola pulchra from southern Brazil, a species described a century ago. We also diagnosed and illustrated the species. Males have a developed apical keel at the apex of the embolus; for the first time, this type of structure has been reported in a species of Grammostola. The molecular analyses using the partial sequence of Cytochrome c oxidase subunit I showed 7% of genetic distance (p-distance) between G. pulchra and Grammostola anthracina. Distance and tree-based methods (ASAP and bPTP, respectively) assigned G. pulchra as a valid species. The gene-tree under Bayesian and Maximum-Likelihood recovered a similar topology, placing G. pulchra as closely related to Grammostola burzaquensis and G. anthracina. Morphological characters which could be important in the taxonomy of the genus are further discussed.
Keywords: Barcoding, COI, Integrative taxonomy, Neotropical region, Tarantula
BACKGROUND
In a broad sense, taxonomy is a scientific discipline of biology that consists of varied activities, e.g., comparing taxa intending to infer relational hypotheses, approaching intra-and interspecific variation, and constructing tools for accurate identification (Enghoff and Seberg 2006). Nevertheless, a core priority of taxonomic studies is to discover and delimitate taxa. Furthermore, since the species boundaries should be treated as a testable hypothesis, species concepts are built on various pieces of evidence and criteria to distinguish species lineages, and recognize taxonomic units (Yeates et al. 2011). Thus, taxonomic problems have been approached simultaneously by distinct techniques, using plural sources of evidence to elucidate species delimitation (Padial et al. 2010).
Morphological characters have been used to classify spiders, mainly those related to the copulatory organs (Pérez-Miles et al. 1996; Bertani 2000). However, some groups, like mygalomorphs, present very similar somatic morphology and less diverse genital structures compared to the sister group Araneomorphae (Hedin and Bond 2006). Because of this, the traditional morphological approach may be insufficient to recognize species accurately, bringing up taxonomic incongruences and inconsistencies (Raven 1990). For example, within the Theraphosidae, the species of Grammostola Simon, 1892, are difficult to sort out and identify due to the copulatory structures being extremely homogeneous among them (Bücherl 1957; Montes de Oca et al. 2016). In these situations, molecular data can provide promising evidence to refine the understanding of Mygalomorphae delimitations and species recognition (Starret and Hedin 2007; Bond and Stockman 2008; Hendrixon et al. 2013; Hamilton et al. 2014). Furthermore, multiple kinds of evidence (e.g., molecular data, geographic distribution, behavioral aspects) may generate a more robust hypothesis of species when added to morphological characters (Montes de Oca et al. 2016).
Grammostola is endemic to the subtropical region of South America (Ferretti et al. 2013). Currently, 20 valid species are distributed in Argentina, Bolivia, Brazil, Chile, Paraguay, and Uruguay (World Spider Catalog 2022). Although the identification of specimens and description of new taxa is challenging (Ferretti et al. 2011), studies related to taxonomy and natural history have been conducted in Argentina (Ferretti et al. 2011 2013) and Uruguay (Vol 2008; Montes de Oca et al. 2016). However, the specimens of Grammostola from Brazil have been scarcely explored since prominent research decades ago (Mello-Leitão 1921 1923; Bücherl 1951). The Brazilian species are distributed throughout the southern region of the country (Bücherl 1951), mainly in the state of Rio Grande do Sul, where six of them have been documented: Grammostola actaeon (Pocock, 1903), Grammostola anthracina (Koch, 1842), Grammostola iheringi (Keyserling, 1891), Grammostola pulchra Mello-Leitão, 1921, Grammostola pulchripes Simon, 1891, and Grammostola quirogai Montes de Oca, D’elía and Pérez-Miles, 2016 (Buckup et al. 2010; Malta-Borges et al. 2016).
The descriptions of Grammostola species from Brazil made in the 19th and early 20th centuries are short and superficial compared to the current taxonomic descriptions for the genus. Furthermore, identifying taxa may be a tangled task for non-specialists since understanding species limits and the capacity to refine descriptions and diagnoses have changed throughout history (De Queiroz 2007). A way to improve the taxonomic scenario is updating the species descriptions under the scrutiny of the current methods, homology hypotheses, and terminologies.
Grammostola pulchra was described a century ago (Mello-Leitão 1921). The original description is brief and vague, lacking essential features to the current taxonomy of the genus, such as the general aspect of spermathecae and morphology of palpal bulbs. Moreover, little additional information related to the taxonomic aspects of G. pulchra has been published. After a hundred years since the original description, we assessed type specimens and additional samples to update the taxonomic knowledge of G. pulchra. We used the somatic and genital morphology, geographical distribution, genetic distance, and phylogeny to build a robust redescription of G. pulchra. Diagnosis, illustrations of copulatory organs, distributional map, species delimitation based on a molecular marker, and phylogenetic discussion are provided.
MATERIALS AND METHODS
Morphological analyses and species redescription
Distinct sources of data were used to assess multiple lines of support for the redescription. We considered the accumulation of evidence from each method to recognize a more robust hypothesis for the species. Here, we assumed the unified species concept, understanding species as a lineage evolving separately from other lineages (De Queiroz 2007).
General character descriptions followed Montes de Oca et al. (2016) with some modifications— the spine classification method was made according to Petrunkevitch (1925), palpal bulb structures were classified according to Bertani (2000) and the classification of urticating hairs according to Cooke et al. (1972). All measurements are given in millimeters and were made with the Discovery V20 -Zeiss® stereomicroscope equipped with the Axiohome system. The pedipalp and leg measurements were taken from the dorsal aspect of the left side of the specimen. Photographs were taken with the stereomicroscope equipped with camera Leica® for microscopy, except photos of the live specimens and habitat that were made with Nikon P600 digital camera. Abbreviations: A = apical keel, ALE = anterior lateral eyes, AME = anterior median eyes, D = dorsal, P = prolateral, PI = prolateral inferior keel, PLE = posterior lateral eyes, PLS = posterior lateral spinnerets, PME = posterior median eyes, PMS = posterior median spinnerets, PS = prolateral superior keel, PV = proventral, R = retrolateral, RV = retroventral, V = ventral.
The examined specimens belong to the following collections (the acronyms following Evenhuis 2021): MCNZ = Brazil, Rio Grande do Sul, Porto Alegre, Museu de Ciências Naturais da Fundação Zoo-Botânica do Rio Grande do Sul; MCTP = Brazil, Rio Grande do Sul, Porto Alegre, Pontifícia Universidade, Museu de Ciências; MZSP = Brazil, São Paulo, São Paulo, Museu de Zoologia da Universidade de São Paulo. Therefore, the identifications were double-checked using the original descriptions.
The individuals studied were deposited into arachnological collections at the Museu de Ciências Naturais da Fundação Zoo-Botânica do Rio Grande do Sul (MCNZ) and Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul (MCTP) and preserved in ethanol 80%.
The species distribution map was made using the Arcgis 10.8.1 program, and the geographic coordinates were obtained using Google Earth (Lat/Long -WGS84).
DNA extraction, amplification, and sequencing
The DNA samples were obtained from ~80% ethanol-preserved species. The specimens had the metatarsus and tarsus removed containing muscle tissues from the third or fourth legs. The genomic DNAs were extracted using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, U.S.A.) according to the manufacturer’s instructions, but eluting to a final volume of 80 μL. The total genomic DNAs were stored at -20°C before amplification. The pair of primers used was designed by Folmer et al. (1994), targeting the “Folmer region” of the mitochondrial gene COI, LCOI 1490 (5'-GGTCAACAAATCATAAAGATATTGG-3') and HCOI 2198 (5'-TAAACTTCAGGGTGACCAA AAAATCA-3'). The PCR conditions were modified from Petersen et al. (2007), increasing the annealing temperature to 50°C/40 s, once amplified products using the recommended 47°C/1 m yielded non-specific bands when electrophoresed in the agarose gel. The PCR products were purified using Exonuclease I and shrimp alkaline phosphatase (Affymetrix, Inc. USB Products, Cleveland, OH, U.S.A.). Macrogen, Inc. (Seoul, South Korea) sequenced both DNA strands for all PCR products. Sequence chromatograms were visually inspected, verified, and manually edited using the Staden package (Staden et al. 2000). Sequences were verified using BLAST (http://blast.ncbi.nlm.nih. gov/Blast.cgi), confirming the high similarity of our submitted sequences to Grammostola species.
Alignment and Analyses
Additional COI sequences of Grammostola were obtained from GenBank, of which the access numbers are provided in table 1. The sample contained 29 terminal taxa, representing eight species of Grammostola, two of Aphonopelma Pocock, and one of Brachypelma Simon, besides our target species. Alignments of the sequences were performed using Mafft 7 (Katoh et al. 2017), online version (http://mafft. cbrc.jp/alignment/server/index.html), applying the strategy “Auto”. The appropriate substitution model was chosen using jModelTest v2.1.6 (Guindon and Gascuel 2003; Darriba et al. 2012) via the Bayesian information criterion (BIC) as suggested by Luo et al. (2010), also implemented in jModeltest 2.1.5. The GTR+I+G was selected for the COI matrix.
Table 1.
Species and individual GenBank access number of partial sequences of Cytochrome c oxidase subunit I of Theraphosidae species. G. pulchra* sensu Montes de Oca et al. 2016
Two probabilistic methods were used to build the COI gene tree, Maximum-Likelihood (ML) and Bayesian inference (BI). These analyses allow two simultaneous inferences: the reciprocal monophyly of the sampled taxa; and a phylogenetic hypothesis for the relationship of the species. The maximum-likelihood analysis was performed on the matrix using RAxML-HPC2 at CIPRES Science Gateway (Miller et al. 2011) (www.phylo.org/portal2/), applying the substitution model GTR-CAT. Nodal support was assessed with automatic Stop Bootstrapping Automatically with Majority Rule Criterion (autoMRE). Bayesian inference of the matrix was performed in the multithreading version of the program MrBayes 3.2.0 (Ronquist and Huelsenbeck 2003), setting nst = 6 rates = invgamma for the marker; 5 million of generation (nruns = 2 nchains = 4) with trees sampled every 1000 generations. Tracer v.1.6.0 (Rambaut et al. 2014) was used to inspect the convergence to the stationary distribution of the chains. The first 25% of the generations were discarded as “burn-in”, and then the chains were combined. The combined ESS values for each parameter were higher than 200. The posterior probability (PP) was estimated for the remaining generations. Phylogenetic trees were visualized and edited using FigTree v1.4.0 (Rambaut et al. 2014) (http://tree.bio.ed.ac.uk/software/figtree/). Brachypelma verdezi Schmidt was used to root the trees in both analyses.
For molecular species delimitation, two approaches were used: a distance-based approach called “assemble species by automatic partitioning” (ASAP) (Puillandre et al. 2021) available on the ASAP web (https://bioinfo.mnhn.fr/abi/public/asap/), setting Simple Distance (p-distances); and a tree-based approach, the Bayesian version of the Poisson Tree Processes model approach (bPTP) (Zhang et al. 2013) using Exelixis Lab’s web server (bPTP –http://species.h-its.org/ptp/) setting unrooted, 200000 MCMC generations, burn-in of 0.2.
RESULTS
(The taxonomy is based on the results of the integrative approach. See below)
TAXONOMY
Theraphosidae Thorell, 1869
Genus Grammostola Simon, 1892
Grammostola pulchra Mello-Leitão, 1921
(Figs. 1–21; Tables 2–3)
Grammostola pulchra Mello-Leitão, 1921: 298.
Grammostola pulchra Mello-Leitão, 1923: 198, figs. 66–68.
Grammostola pulchripes pulchra Bücherl, 1951: 118, pl. IV (reduced to subspecies).
Grammostola pulchripes pulchra Bücherl, 1957: 396, fig. 57.
Grammostola pulchra Schmidt, 1986: 52, fig. 70.
Grammostola pulchra Schmidt, 1993: 90, figs. 214, 221.
Grammostola pulchra Schmidt, 1997: 16, figs. 55, 62.
Grammostola pulchra Peters, 2000: 137, figs. 430–432.
Grammostola pulchra Peters, 2003: 195, figs. 785–786, 790.
Grammostola pulchra Schmidt, 2003: 167, figs. 382–383.
Grammostola pulchra Montes de Oca, D’Elía & Pérez-Miles, 2016: 328, fig. 4E.
Material examined
Type material: BRAZIL: Rio Grande do Sul: Uruguaiana, IX.1914, 1 male and 1 female, E. Garbe leg. (MZSP 122) (examined).
Additional material: 1 male, BRAZIL: Rio Grande do Sul: Capão do Leão, Campus Universitário da Universidade Federal de Pelotas [31°48'03.6"S, 52°25'18.7"W], 17.XI.2018, R. S. Pittella & P. G. Bassa leg. (MCTP 41831); 1 female, 20.XI.2018, R. S. Pittella leg. (MCTP 41833); 1 male, 01.XI.2018, R. S. Pittella leg. (MCN ARA-56829); 1 juvenile male, 20.XI.2018, R. S. Pittella leg. (MCTP 41832) matured in captivity; 1 female, 20.XI.2018, R. S. Pittella leg. (MCTP 43834); 1 male, 16.X.2018, R. S. Pittella & P. G. Bassa leg. (MCN ARA-56826); BRAZIL: Rio Grande do Sul: Pelotas, Road to Laranjal beach near Condomínio Veredas [31°45'27.5"S, 52°15'02.7"W], 1 male, 11.X.2018, R. S. Pittella leg. (MCN ARA-56827); Pelotas, Bairro Fragata [31°44'49.5"S, 52°22'51.7"W], 1 female, 20.X.2018, R. S. Pittella leg. (MCN ARA-56828); BRAZIL: Rio Grande do Sul: São Borja, Reserva Biológica São Donato, 1 male, 10.X.2012, M. Machado leg. (MCTP 36905).
Diagnosis: Grammostola pulchra differs from other Grammostola species by the following combination of characteristics: brownish black coloration; body thickly covered by long hairs of the same color but with yellowish or greyish tips, more abundant in the ventral region (Figs. 1–3, 17, 20–21); short broad-based spiniform setae on the prolateral coxal faces of legs I–IV (Fig. 5); male’s tibial apophysis, with two branches originating from the same base; primary branch smaller, straight, with a group of subapical macrosetae and presence of a long black spine with acuminate apex on the inner side, shorter than the branch; secondary branch larger with slight distal curvature and presence of a short broad base apical conical process; and 1–2 retrolateral tibial spines (Figs. 6–8). Male’s palpal tarsi with small tuft of erected and rigid spiniform setae in the apical region (Fig. 4). Piriform bulb with inferior prolateral keel (PI) and superior prolateral keel (PS) developed, short embolus with developed apical keel (A), slightly curved, folded in the middle portion and slightly curved in the apical portion (Figs. 9–11). Females have spermathecae with two short and straight seminal receptacles with rounded apex (Fig. 12). Grammostola pulchra resembles G. quirogai by the coloration but differs from them by their smaller size, the slender palpal bulb with developed apical keel and the morphology and spine combination of the tibial apophysis (illustrated by Montes de Oca et al. 2016); also could resemble G. burzaquensis in the general appearance and size, but differs due to the presence of short broad-based spiniform setae on prolateral coxal faces of legs I–IV, the morphology and spine combination of the tibial apophysis and the shape of palpal bulb (illustrated by Ferretti et al. 2011 2016). Additionally, it can be distinguished from G. anthracina by its small size, morphology and spine combination of the tibial apophysis and by the absence of reddish hairs on the ventral face of all legs (illustrated by Montes de Oca et al. 2016).
Fig. 1.
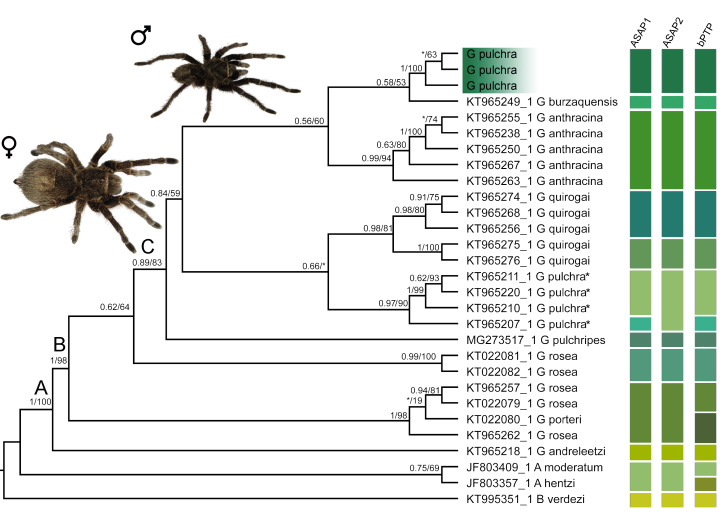
Maximum likelihood tree based on the partial sequence of Cytochrome c oxidase subunit I of Grammostola from South America and related Theraphosidae genus. Capital letters above nodes refer to lineages discussed in the text. Numbers close to nodes are the Bayesian posterior probabilities (PPs)/maximum likelihood bootstrap support (ML), respectively. Only nodal support above PP = 0.5 or ML = 50 is displayed (‘*’ indicates lower support values). Lineage assignments of distance-base (ASAP1 and ASAP2 means first and second best results of ASAP, respectively) and tree-based (bPTP) methods. Habitus photos of male and female of Grammostola pulchra. G. pulchra* means sensu Montes de Oca et al. 2016.
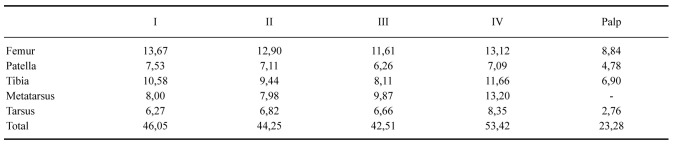
Redescription: Male (MCTP 41831): Total body length, excluding chelicerae and spinnerets: 38.36. Carapace length: 15.39, width: 14.18, with slightly elevated cephalic area and shallow thoracic striae (Fig. 2). Chelicerae length: 6.12, width: 4.54. Abdomen length: 21.86, width: 14.98. Anterior eyes row recurve, posterior procurve. Eyes size and interdistances: PME: 0.32; PLE: 0.49; AME: 0.40; ALE: 0.49; PME–PME: 1.26; PME–PLE: 0.13; AME–AME: 0.49; AME–ALE: 0.23; ALE–PLE: 0.25. Eye tubercle length 1.98, width 2.41; clypeus: 0.13. Fovea shape: transverse and straight (Fig. 2); width: 1.63. Labium length: 2.45, width: 3.27, with 125 cuspules (Fig. 3). Maxillae length 5.13, width: 3.15, with 232 cuspules arranged in a triangular group with the base at the proximal edge (Fig. 3). Sternum length 7.04, width 7.22; posterior angle does not separate the coxae IV (Fig. 3). Sigillae: anterior: 1 pair not much evident, medians: 1 pair; posterior: 1 pair; all ellipsoid, submarginal (Fig. 3). Chelicera with 8 promarginal and 4 retromarginal teeth. Setae -stridulatory: present on the retrolateral face of the palpal maxilla and on the opposite prolateral face of leg I, claviform, located both above and below the suture, the upper ones occupy the apical area and slightly longer and numerous, the lower ones shorter and less numerous occupying a basal area with interspersed spiniform setae; palpal tarsi setae: small tuft of erect and rigid spiniform setae in the tarsal apical region (Fig. 4); coxal setae: broad-base short spiniform on the prolateral face of the legs I–IV (Fig. 5). Tarsi I–IV densely scopulate, scopula entire; metatarsi: I completely scopulate, II scopula on apical two thirds, III scopula on apical third, IV minimally scopulate on apical third. Tibia I with paired distal proventral apophyses, composed of two branches originating from the same base; secondary branch larger with slight distal curvature and presence of a short broad-based apical conical process; primary branch smaller, straight, with a group of subapical macrosetae and presence of a long black spine with an acuminate apex on the inner side, shorter than the branch; and 1–2 retrolateral tibial spines (Figs. 6–8). Flexion of metatarsus I retrolateral in relation to tibial apophysis. Palpal organ pyriform with prolateral keels present, PI and PS developed, short embolus with developed apical keel (A), slightly curved, folded in the middle portion and slightly curved in the apical portion (Figs. 9–11). Length of leg and palpal segments in table 2; Legs formulla: IV–I–II–III. Spination: Femora: palp 0; I 1P; II 1–0–1 P; III 1R; IV 1R. Patella: palp and I–IV0.Tibia:palp1P,1–0–1PV;I1–0–1V,1PV,2R;II 1–0–1 P, 1–0–3–0–1 V; III 2–0–1 P, 1–0–1 R, 1–0–1 V, 1–0–1–0–2 PV, 1 RV; IV 1 P, 1–0–1 R, 1–0–2 V, 1 PV, 1 RV. Metatarsus: I 1 R, 1 PV, 2 RV; II 2–1 RV; III 1–1–1 P, 1–1–1 R, 1–1–1–1 PV, 1–1–1–2 V, 1–1 RV; IV 1–1–1 P, 1–1–1–1 R, 2–1–1–1 PV, 1–1–2 RV. Tarsus: palp and I–IV 0. Color (in vivo): brownish black body with long hairs of the same color with yellowish or greyish tips distributed over the cephalothorax, abdomen and legs, more abundant in the ventral region. Sternum, labium and coxae velvety blackish. Coxae of palp and chelicerae with reddish bristles. Presence of two slightly marked vertical striae on the patellae (Figs. 1–3, 20). In alcohol, the specimens have a dark brown color with yellowish bristles scattered throughout the body. Type III–IV urticating hairs present, gathered in the dorsal region of the abdomen, where they form a silver spot with semicircular shape. Spinnerets: PMS length: 1.97, monoarticulated; PLS length of articles: basal: 2.75, medial: 2.44, apical: 2.93, total: 8.12, triarticulated with apical segment digitiform.
Variation (range (mean ± standard deviation)): Four adult males: total length 31.71–38.36 (34.93 ± 2.68); cephalothorax length 15–16.93 (15.66 ± 0.74), width 13–16.24 (14.19 ± 1.57); legs: I 46.05–51.72 (48.92 ± 2.43), II 44.25–49.24 (46.22 ± 1.91), III 42.51–45.28 (44.15) ± 1.14), IV 53.42–56.67 (55.26 ± 1.17), palp 22.82–28.55 (24.61 ± 2.29).
Fig. 2-8.
Morphological characters of Grammostola pulchra. Male (MTCP 41831), (2) carapace and chelicerae; (3) sternum, maxillae, labium and coxae; (4) palpal tarsi, prolateral view, arrow indicate the small tuft of spiniform setae; (5) prolateral face of coxa I; (6–8) Right leg I tibial apophysis, (6) ventral view, (7) prolateral view, (8) small branch with spine on the inner side and group of subapical macrosetae. Scale bars: 2–3 = 5 mm; 4–8 = 1 mm.
Table 2.
Length (mm) of legs and palpal segments of the male (MCTP 41831) of Grammostola pulchra
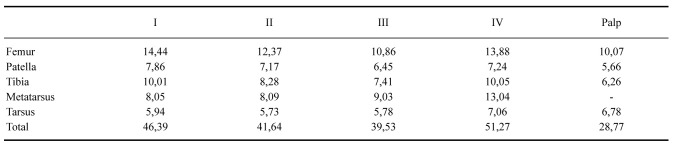
Female (MCTP 41833): Total body length, excluding chelicerae and spinnerets: 50.08. Carapace length 17.61, width 16.86, with slightly elevated cephalic area and shallow thoracic striae (Fig. 2). Chelicerae length 8.63, width 5.63. Abdomen length 29.96, width 25.79. Anterior eyes row recurve, posterior procurve. Eyes size and interdistances: PME: 0.29; PLE: 0.53; AME: 0.40; ALE: 0.57; PME–PME: 1.49; PME–PLE: 0.29; AME–AME: 0.70; AME–ALE: 0.47; ALE–PLE: 0.47. Eye tubercle length 1.87, width 2.82; clypeus: 0.18. Fovea shape: transverse and straight (Fig. 2); width 2.29. Labium length 2.46, width 4.41, with 210 cuspules. Maxillae length 6.44, width 4.44, with 233 cuspules arranged in a triangular group with the base at the proximal edge. Sternum length 8.28, width 8.29, posterior angle does not separate the coxae IV (Fig. 3). Sigillae: anterior: 1 pair not much evident, medians: 1 pair, posterior: 1 pair, all ellipsoid, submarginal (Fig. 3). Chelicera with 8 promarginal and 5 retromarginal teeth. Setae -stridulatory and coxal setae: as in male. Tarsi I–IV densely scopulate, scopula entire; metatarsi: I completely scopulate, II scopula on apical two thirds, III scopula on apical third, IV minimally scopulate on apical third. Length of leg and palpal segments in table 3; Legs formula: IV–I–II–III. Spination: Femora: palp 0;I1P;II1P;III1P;IV1R.Patella:palpandI–IV 0. Tibia: palp 1P, 1 R, 1 V, 1–0–2–1 PV, 1–0–2 RV; I 1 R, 1 PV, 1–0–1 RV; II 1 PV, 1–0–1–0–1 RV; III 1–1 P,1–1–1R,2V,IR1R,1V,1PV,1RV.Metatarsus:I 1–0–3V,1PV,2RV;II1P,3V,1PV,2–1RV;III1–1–1 P, 1–1 R, 1–1–0–5 V, 1–0–2–1–1 PV, 1–1 RV; IR 1–0–1 P, 1–2–1–1–2 R, 1–0–1–0–1 V, 1–0–1–0–2 PV, 2–2 RV. Tarsus: palp and I–IV 0. Color: as in male (Figs. 1, 17, 21). Type III-IV urticating hairs present, gathered in the dorsal region of the abdomen, where they form a silver spot and semicircular shape. Spinnerets: PMS length: 2.09; monoarticulated; PLS length of articles: basal: 3.72, medial: 2.58, apical: 2.91, triarticulated with apical segment digitiform. Spermathecae with two short and straight seminal receptacles with rounded apex (Fig. 12).
Table 3.
Length (mm) of legs and palpal segments of the female (MCTP 41833) of Grammostola pulchra
Distribution and natural history: G. pulchra is known for the western region (between the municipalities of Uruguaiana, São Borja and Maçambara) and for the southern region (which includes the municipalities of Capão do Leão and Pelotas) of the Pampa biome in the state of Rio Grande do Sul (Fig. 13), where occurs in rocky environments or near humid areas locally known as “banhados”. In the municipality of Pelotas, the individuals were found in burrows dug in the ground in open field areas with predominant herbaceous vegetation located between areas with anthropic modifications, such as the presence of a grove of Eucalyptus spp. and a native wetland region (Figs. 14–17). The burrows have circular entries and depths ranging from 50 to 80 cm (Fig. 16). Besides that, spiders were observed in remarkable agglomeration, with many shelters in a few square meters (Fig. 15). Additionally, G. pulchra occurs in sympatric distribution with other theraphosid genera, Eupalaestrus Pocock, 1901 and Catumiri Guadanucci, 2004 in their southern habitats, being also reasonable consider that it shares its habitat with G. quirogai by their western distribution in Rio Grande do Sul state.
Fig. 9-12.
Copulatory organs of Grammostola pulchra. (9–11): Male (MTCP 41831), (9) left palpal bulb retrolateral view, (10) left palpal bulb prolateral view, (11) embolus apex detail, arrow indicates the developed apical keel. (12) Female (MTCP 41833), spermathecae ventral view. Scale bars: 9, 10, 12 = 1 mm; 11 = 0.1 mm.
Fig. 13.
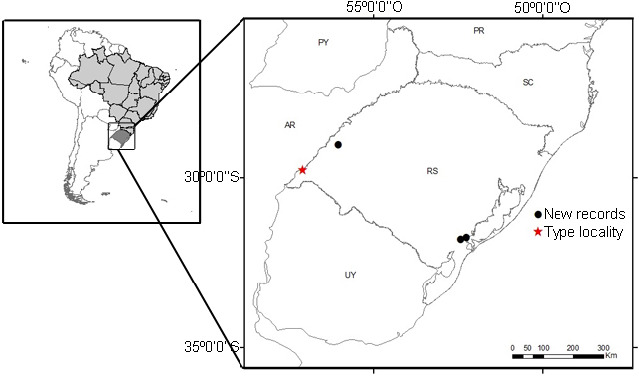
Distribution map of Grammostola pulchra.
Fig. 14-17.
Grammostola pulchra. Habitat in the municipality of Capão do Leão, State of Rio Grande do Sul, Brazil. (14) General view of native wetland area between groves of Eucalyptus spp.; (15) Burrows, arrows indicate the holes that compose entrances of different burrows; (16) Individual at the entrance of the burrow; (17) A female in natural habitat. Photographed by R. S. Pittella.
Remarks and affinities: Ferretti et al. (2013) mention a small bristle of erected and stiff setae on the palpal tarsi in males of Grammostola diminuta Ferretti, Pompozii, González & Pérez-Miles, 2013 as a diagnostic feature compared to all other species of the genus. However, a very similar structure was found in G. pulchra (Fig. 4), showing that this set of setae may not be unique to G. diminuta males. Thus, the analysis of this type of structure in other species may be relevant to understand the evolution of morphological characters and propose homologies between species of the genus.
Molecular analyses
The COI sequence was obtained for a total of three specimens of Grammostola pulchra, generating 650 bp sequences (see Material examined section: MCTP 41832, MCTP 41833, and MCN ARA-56826; Figs. S1–S3 for voucher pictures). The other specimens had low-quality DNA extraction, probably due to the conservation of the material. After the alignment, the analyzed matrix consisted of 565 bp, of which 183 were variable, and 155 were parsimoniously informative.
In all molecular analyses (see below), the G. pulchra specimens sequenced in this study were recovered as a distant lineage from the sequences labeled as G. pulchra in GenBank. To avoid misinterpretation in results and discussion sections, hereafter G. pulchra refers to the samples we sequenced. In contrast, G. pulchra (sensu Montes de Oca et al. 2016) refers to sequences deposited into GenBank by Montes de Oca et al. (2016).
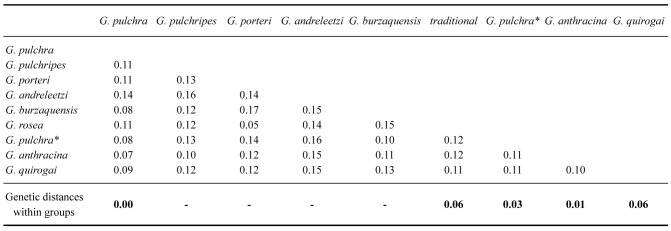
Within our Grammostola species sample, the genetic distance within groups ranged from 0% in G. pulchra to 6% in G. quirogai and G. rosea (Walckenaer, 1837) (Table 4). Unfortunately, some species only have a single sequence in GenBank, making their distance within groups impractical. The genetic distance between groups ranged from 7% to 17% (Table 4). The genetic distances were lower between G. pulchra and G. anthracina, and higher between G. porteri (Mello-Leitão, 1936) and G. andreleetzi Vol, 2008. The genetic distance between G. pulchra and G. pulchra (sensu Montes de Oca et al. 2016) was 8%, as was the distance between G. pulchra and G. burzaquensis Ibarra-Grasso, 1946 (Table 4).
Table 4.
Intra-and interspecific distances (uncorrected p-distance) of the partial fragment of Cytochrome c oxidase subunit I for species of Grammostola. G. pulchra* sensu Montes de Oca et al. 2016
The species delimitation DNA-based approaches (ASAP and bPTP) found similar results assigning G. pulchra as an independent lineage in relation to the congeneric sampled species (Fig. 1). The first (p = 0.0172) and second (p = 0.0014) best results of ASAP recovered 13 (threshold distance = 0.0616) and 12 (threshold distance = 0.06870) taxonomic units, respectively, for our dataset. The bPTP assigned 18 partitions with good support. Grammostola anthracina was recovered as a single taxon in our analyses, while results concerning the other species represented by more than one sample were incongruent among the analyses (Fig. 1). Grammostola pulchra (sensu Montes de Oca et al. 2016) was assigned as two lineages according to ASAP’s first best result and bPTP, and as a single lineage by ASAP’s second best result. ASAP analyses considered Aphonopelma moderatum (Chamberlin & Ivie, 1939) and Aphonopelma hentzi (Girard, 1852) as a single taxon (Fig. 1), as opposed to the bPTP result (Fig. 1).
The phylogenetic reconstructions under ML and BI based on COI recovered the same topology (Fig. 1). Grammostola pulchra was recovered as monophyletic with high support (1PP/100ML). The other Grammostola species represented by more than one sample were also recovered as monophyletic, but the samples of G. rosea were split into two lineages, including G. porteri. Within the Grammostola, only the nodes B and C presented high support (1PP/98ML and 0.89PP/83ML, respectively), limiting robust hypotheses of relationships (but see discussion). The node A (1PP/100ML) supports the monophyly of Grammostola; B supports G. andreleetzi as sister species of the other Grammostola sampled; C includes most of the sampled species: G. pulchripes, G. pulchra (sensu Montes de Oca et al. 2016), G. quirogai, G. pulchra, G. burzaquensis and G. anthracina. Although there was moderate/low branch support, G. pulchra was more related to G. burzaquensis and G. anthracina.
DISCUSSION
Here, the genetic distance, phylogeny, morphology, and geographical data were congruent to support G. pulchra as a valid species, allowing us to build a robust characterization for the redescription of this species. The homogeneity and subjective interpretation of the traditional morphological characters in Mygalomorphae (Ferretti et al. 2019), mainly Grammostola (Bücherl 1951), levered the use of multiple sources of data to support the description of G. quirogai (Montes de Oca et al. 2016). The use of different sources of evidence assists in hypotheses of lineage separation (De Queiroz 2007). The number of taxonomic papers associating morphological data to COI, mainly the DNA barcoding region, to describe species has increased in the past decade (DeSalle and Goldstein 2019), which is desirable to the taxonomy as a whole, as well as other areas of study.
Under molecular perspective
Using a fixed and arbitrary value for the distance thresholds between species for high taxonomic levels (e.g., 2% by Herbert et al. 2003) is considered a naive mistake for DNA barcoding uses (Collins and Cruickshank 2013). Plausible values may be obtained by accumulating data on a target taxon (e.g., Talavera et al. 2013; Gonçalves et al. 2021; Bianchi and Gonçalves 2021a), since the coalescent depths among species are variable in each lineage (Fujita et al. 2012). The 7% genetic distance between G. pulchra and G. anthracina seems acceptable to consider them different species since 6% has been considered an applicable threshold for Theraphosidae (Hamilton et al. 2011). The threshold values assigned by the two best results of the distance-based method were close to this value. Nevertheless, both G. quirogai and G. rosea presented a divergence within-group close to this threshold. Our analyses assigned them as more than one taxonomic unit. Despite the high genetic divergence, Montes de Oca et al. (2016) were parsimonious considering G. quirogai a single species, arguing that other evidence, such as morphology and distribution, did not support lineage separation within this species. On the other hand, our gene tree indicates G. rosea as non-reciprocally monophyletic, demonstrating the need for a more comprehensive review of this and allied species, such as G. porteri, with more extensive sampling and additional molecular markers.
Our hypothesis of the relationship within Grammostola must be interpreted carefully since most of the supports were moderated. The branch supports in the phylogenetic trees based on COI tend to find limited relevance due to variation within this gene (DeSalle and Goldstein 2019). However, the phylogenetic trees allow us to support the reciprocal monophyly of G. pulchra and hypothesize its relationship within the genus. Inferring the whole Grammostola as monophyletic is reckless since our sample design did not seek this aim. Thus, we reassert Montes de Oca et al. (2016), claiming the use of comprehensive taxa sampling and additional molecular markers, emphasizing nuclear DNA sequences to those researchers intending to elucidate the phylogenetic relationship of Grammostola.
The genetic incompatibility related to G. pulchra and G. pulchra (sensu Montes de Oca et al. 2016) may not be considered a rare situation. Sequences mislabeled may compose a significant amount of invertebrates deposited into public databases (Gonçalves et al. 2021; Bianchi and Gonçalves 2021a), mainly in taxa with cryptic diversity, ambiguous descriptions and diagnoses, or subjective interpretation of morphological features, such as within Theraphosidae genera (Ferretti et al. 2019; Candia-Ramírez and Francke 2021). The sequences of G. pulchra (sensu Montes de Oca et al. 2016) lack any online support information (i.e., collection site and voucher pictures) that allow these identifications to be contested. Providing meta-data related to specimen identification is a cheap, not time-consuming, and good practice that improves taxonomic verification (Bianchi and Gonçalves 2021b). The direct comparison of our samples with the type material of G. pulchra allowed us to have a reliable identification, matching the general appearance, morphology, and size (Figs. 18–21, but also see the original description in Mello-Leitão 1921). Our results indicate that the sequences from G. pulchra and G. pulchra (sensu Montes de Oca et al. 2016) belong to two distinct and phylogenetically distant lineages. Thus, probably G. pulchra (sensu Montes de Oca et al. 2016) is an undescribed species closely related to G. quirogai (see Montes de Oca et al. 2016). Future work should reassess the specimens used by the authors for an in-depth morphological evaluation.
Fig. 18-21.
Material comparison of Grammostola pulchra. (18) First illustration of the species since their original description, from Bücherl (1951, p. 199); (19) Type specimen; (20) Male habitus; (21) Female habitus. Scale bars: 10 mm. Photographed by P. G. Bassa.
Under morphological and geographical perspective
According to Ferretti et al. (2011), the morphology of spiniform setae on the pro-and retrolateral coxal faces of the legs and pedipalps could be an important feature of the Grammostola taxonomy. The spiniform setae found on the prolateral face of the legs I-IV of G. pulchra seems to correspond to the first type out of the three categories, which are short and strongly piriform setae with the surface completely covered by ridges. Together with other features (i.e., morphological and geographical), the type of coxal setae allowed us to distinguish G. pulchra from similar species (e.g., G. burzaquensis). Despite their importance, no other species were analyzed besides those sampled by Ferretti et al. (2011), and so a gap in the taxonomy regarding spiniform setae in Grammostola remains.
An apical keel below the apex of the palpal bulb embolus was found in G. pulchra (Fig. 11). Bücherl (1957) attributed only generic value to bulb morphology, while Schiapelli and Gerschman de Pikelin (1962) disagreed with Bücherl’s statement and accurately illustrated the palpal bulbs, often using the morphology of this structure to distinguish the species from Argentina. This type of keel has never been clearly shown in a species of Grammostola. Pérez-Miles (1989), studying the morphology of the bulb in G. anthracina [cited as Grammostola mollicoma (Ausserer, 1875)], mentioned a “conspicuous fin” at the basal region of the male embolus. It suggests the presence of a structure similar to the apical keel reported here to G. pulchra. However, Bertani (2000) characterized the palpal bulbs of the Theraphosinae and showed that Grammostola presented only prolateral superior and inferior keels developed. The author did not mention the presence of apical keel in G. pulchra and sampled only another four species of Grammostola, which lacked G. anthracina. Because of this, the morphology of the bulb may bring valuable evolutionary information about the relationship within Grammostola. Unfortunately, since many species still only have their original descriptions, they lack details concerning their bulb morphology. We emphasize the need for an in-depth investigation of the bulb morphology of the whole Grammostola genus as an essential tool for integrating these spiders’ classification. Also, it may contribute to the detection of other structures, bringing new insights into the evolution, phylogenetic, and natural history of the genus.
CONCLUSIONS
Grammostola is still a systematic and taxonomic challenge, as already indicated (Ferretti et al. 2011). The species delimitation and their relationships are broadly based on classical morphological features. However, these usual diagnostic characters are relatively homogeneous among the species of the genus (Bücherl 1951; Ferretti et al. 2013), and the differences may be present in the minute details. Once the morphology is complex and its interpretation may be subjective, using additional sources for evidence to support the species’ validity and delimitation is desirable. Besides, integrative approaches may provide more robust hypotheses about taxonomic units and improve phylogenies. In this sense, the redescription of G. pulchra and recent description of G. quirogai (see Montes de Oca et al. 2016) may be a basic blueprint for future species (re)descriptions and the review of Grammostola.
Supplementary materials
Voucher pictures and GenBank accession number of sequences sequenced in this study.
Acknowledgments
The authors would like to thank the curator of the scientific collections mentioned above: Dr. Ricardo Ott from MCNZ and Dr. Renato Augusto Teixeira from MCTP. Dr. Ricardo Pinto da Rocha from MZSP, and Mauro Cardoso Júnior for sending us pictures and the holotype of Grammostola pulchra (MZUSP122). Dr. Fernando Pérez-Miles, for his comments and opinions about general aspects of Grammostola taxonomy and for giving kindly his schemes, drawings, and photographs of various Grammostola species. We are also thankful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) Finance Code 001, for the fellowship of F. M. B.
Footnotes
Authors’ contributions: RSP and PGB collected the specimens in fieldwork; RSP made the morphological comparisons and redescribed the species; FMB performed the molecular data acquisition and analyses; RSP and EZ designed and drafted the manuscript. All authors contributed to writing and revising the manuscript. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no conflict of interests.
Availability of data and materials: All data are provided within the manuscript.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Ausserer A. 1875. Zweiter Beitrag zur Kenntniss der Arachniden-Familie der Territelariae Thorell (Mygalidae Autor). Verh Zool-Bot Ges Wien 25:125–206.
- Bianchi FM, Gonçalves LT. 2021a. Borrowing the Pentatomomorpha tome from the DNA barcode library: Scanning the overall performance of cox1 as a tool. J Zool Syst Evol Res 59:992–1012. doi:10.1111/jzs.12476.
- Bianchi FM, Gonçalves LT. 2021b. Getting science priorities straight: how to increase the reliability of specimen identification? Biol Lett 17:20200874. doi:10.6084/m9.figshare.c.5393787. . [DOI] [PMC free article] [PubMed]
- Bertani R. 2000. Male palpal bubs and homologous features in Theraphosinae (Araneae: Theraphosidae). Jo A 28:29–42. doi:10.1636/0161-8202(2000)028[0029:MPBAHF]2.0.CO;2.
- Bond JE, Stockman AK. 2008. An integrative method for delimiting cohesion species: finding the populationp -species interface in a group of Californian trapdoor spiders with extreme genetic divergence and geographic structuring. Syst Biol 57:628–646. doi:10.1080/10635150802302443. . [DOI] [PubMed]
- Bücherl W. 1951. Estudos sobre a biologia e a sistemática do género Grammostola Simon, 1892. Instituto Butantan, São Paulo, Brazil.
- Bücherl W. 1957. Sobre a importância dos bulbos copuladores e das apófises tibiais dos machos na sistemática das aranhas caranguejeiras (Orthognatha). An Acad Bras Cienc 29:377–416.
- Buckup EH, Marques MAL, Rodrigues ENL, Ott R. 2010. Lista das espécies de aranhas (Arachnida, Araneae) do estado do Rio Grande do Sul, Brasil. Ihering Ser Zool 100:483–518. doi:10.1590/S0073-47212010000400021.
- Candia-Ramírez DT, Francke OF. 2021. Another stripe on the tiger makes no difference? Unexpected diversity in the widespread tiger tarantula Davus pentaloris (Araneae: Theraphosidae: Theraphosinae). Zool J Linn Soc 192:75–104. doi:10.1093/zoolinnean/zlaa107.
- Chamberlin RV, Ivie W. 1939. New tarantulas from the southwestern states. Bulletin of the University of Utah 29(11):1–17.
- Cooke JAL, Roth VD, Miller FH. 1972. The urticating hairs of theraphosid spiders. Am Mus Novit 2498:1–43.
- Collins RA, Cruickshank RH. 2013. The seven deadly sins of DNA barcoding. Mol Ecol Resour, 13:969–975. doi:10.1111/1755-0998.12046. [DOI] [PubMed]
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat methods 9:772. doi:10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed]
- De Queiroz K. 2007. Species concepts and species delimitation. Syst Boil 56:879–886. doi:10.1080/10635150701701083. [DOI] [PubMed]
- DeSalle R, Goldstein P. 2019. Review and Interpretation of Trends in DNA Barcoding. Front Ecol Evol 7:302. doi:10.3389/fevo.2019.00302.
- Enghoff H, Seberg O. 2006. A taxonomy of taxonomy and taxonomists. Systematist 27:13–15.
- Evenhuis NL. 2021. The insect and spider collections of the world website. Available at: http://hbs.bishopmuseum.org/codens./Accessed 11 Mar. 2021.
- Ferretti N, Pompozzi G, Pérez-Miles F. 2011. The species of Grammostola (Araneae: Theraphosidae) from Central Argentina: taxonomy, distribution, and surface ultrastructure of coxal setae. Zootaxa 2828:1–18. doi:10.11646/zootaxa.2828.1.1.
- Ferretti N, Pompozzi G, González A, Pérez-Miles F. 2013. The genus Grammostola Simon 1892 (Araneae: Theraphosidae): a new species from western Argentina, new synonymy and distributional data. J Nat Hist 47:2961–2977. doi:10.1080/00222 933.2013.791945.
- Ferretti N, Schwerdt L, Peralta L, Farina J, Pompozzi G. 2016. Nuevos datos de distribución de Grammostola burzaquensis Ibarra-Grasso, 1946 (Araneae, Theraphosidae) en el sistema serrano de Tandilia. Hist Natur 6(1):75–82.
- Ferretti N, Soresi DS, González A, Arnedo M. 2019. An integrative approach unveils speciation within the threatened spider Calathotarsus simoni (Araneae: Mygalomorphae: Migidae). Syst Biodiv 17:439–457. doi:10.1080/14772000.2019.1643423.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechn 3:294–299. . [PubMed]
- Fujita MK, Leaché AD, Burbrink FT, McGuire JA, Moritz C. 2012. Coalescent-based species delimitation in an integrative taxonomy. Trends Ecol Evol 27:480–488. doi:10.1016/j.tree.2012.04.012. . [DOI] [PubMed]
- Girard C. 1853. Arachnidians. In: Marcy R. (ed) Natural History of the Red River of Louisiana. Washington, 32nd Congress, 2nd Session, Senate Appendix F (Zoology), pp. 262–271.
- Gonçalves LT, Bianchi FM, Deprá M, Calegaro-Marques C. 2021. Barcoding a can of worms: testing cox1 performance as a DNA barcode of Nematoda. Genome 64(7):705–717. doi:10.1139/gen-2020-0140. . [DOI] [PubMed]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi:10.1080/10635150390235520. . [DOI] [PubMed]
- Hamilton CA, Formanowicz DR, Bond JE. 2011. Species delimitation and phylogeography of Aphonopelma hentzi (Araneae, Mygalomorphae, Theraphosidae): cryptic diversity in North American Tarantulas. PLoS ONE 6:e26207. doi:10.1371/journal. pone.0026207. . [DOI] [PMC free article] [PubMed]
- Hamilton CA, Hendrixon BE, Brewer AS, Bond JE. 2014. An evaluation of sampling effects on multiple DNA barcoding methods leads to an integrative approach for delimiting species: a case study of the North American tarantula genus Aphonopelma (Araneae, Mygalomorphae, Theraphosidae). Mol Phylogenetics Evol 71:79–93. doi:10.1016/j.ympev.2013.11.007. . [DOI] [PubMed]
- Hedin M, Bond JE. 2006. Molecular phylogenetics of the spider infraorder Mygalomorphae using nuclear rRNA genes (18S and 28S): Conflict and agreement with the current system of classification. Mol Phylogenetics Evol 41:454–471. doi:10.1016/j.ympev.2006.05.017. . [DOI] [PubMed]
- Hendrixon BE, DeRussy BM, Hamilton CA, Bond JE. 2013. An exploration of species boundaries in turret-building tarantulas of the Mojave Desert (Araneae, Mygalomorphae, Theraphosidae, Aphonopelma). Mol Phylogenetics Evol 66:327–340. doi:10.1016/j.ympev.2012.10.004. . [DOI] [PubMed]
- Herbert PDN, Cywinska A, Ball SL, deWaard J. 2003. Biological identifications through DNA barcodes. Proc Biol Sci 270:313–321. doi:10.1098/rspb.2002.2218. . [DOI] [PMC free article] [PubMed]
- Ibarra-Grasso A. 1946. Arañas y araneismo (Las arañas peligrosas en la República Argentina). Sem Med 50:763–793.
- Katoh K, Rozewicki J, Yamada KD. 2017. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. doi:10.1093/bib/bbx108. . [DOI] [PMC free article] [PubMed]
- Keyserling E. 1891. Die Spinnen Amerikas. Brasilianische Spinnen. Nürnberg 3:1–278.
- Koch CL. 1842. Die Arachniden. Nürnberg, Neunter Band 9:57–108.
- Luo A,Qiao H,Zhang Y,Shi W,Ho SY,Xu W,Zhang A,Zhu C. 2010. Performance of criteria for selecting evolutionary models in phylogenetics: a comprehensive study based on simulated datasets. BMC Evol Biol 10:242. doi:10.1186/1471-2148-10-242. . [DOI] [PMC free article] [PubMed]
- Malta-Borges L, Da Rosa CM, Dri GF, Bertani R. 2016. Predation of the snake Erythrolamprus almadensis (Wagler, 1824) by the tarantula Grammostola quirogai Montes de Oca, D’Elía & Pérez-Miles, 2016. Herpet Notes 9:321–322.
- Mello-Leitão CF. 1921. On the genus Grammostola, Simon. Nat Hist 7:293–305.
- Mello-Leitão CF. 1923. Theraphosoideas do Brasil. Rev Mus Paulista 13:1–438.
- Mello-Leitão CF. 1936. Etude sur les arachnides de Papudo et Constitucion (Chili), recueillis par le prof. Dr Carlos E. Porter. Rev Chil Hist Nat 40:112–129.
- Miller MA, Pfeiffer W, Schwartz T. 2011. The CIPRES science gateway: a community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery (TG '11). Association for Computing Machinery, New York, NY, USA, Article 41, pp. 1–8. doi:10.1145/2016741.2016785.
- Montes de Oca L, D’Elía G, Pérez-Miles F. 2016. An integrative approach for species delimitation in the spider genus Grammostola (Theraphosidae, Mygalomorphae). Zool Scr 45:322–333. doi:10.1111/zsc.12152.
- Padial JM, Miralles A, De la Riva I, Vences M. 2010. The integrative future of taxonomy. Front Zool 7:2–14. doi:10.1186/1742-9994-7-16. . [DOI] [PMC free article] [PubMed]
- Pérez-Miles F. 1989. Variación relativa de caracteres somáticos y genitales en Grammostola mollicoma (Araneae, Theraphosidae). Jo A 17:263–274.
- Pérez-Miles F, Lucas SM, da Silva Jr PI, Bertani R. 1996. Systematic revision and cladistic analysis of Theraphosinae (Araneae: Theraphosidae). Mygalomorph 1:33–68.
- Peters HJ. 2000. Tarantulas of the world: Kleiner Atlas der Vogelspinnen -Band 1. Published by the author.
- Peters HJ. 2003. Tarantulas of the world: Amerika’s Vogelspinnen. Published by the author, Wegberg, Germany.
- Petersen SD, Mason T, Akber S, West R, White B, Wilson P. 2007. Species identification of tarantulas using exuviae for international wildlife law enforcement. Conserv 8:497–502. doi:10.1007/s10592-006-9173-2.
- Petrunkevitch A. 1925. Arachnida from Panama. Trans Conn Acad Arts Sci 27:51–248.
- Pocock RI. 1903. On some genera and species of South-American Aviculariidae. Nat Hist 11:81–115. doi:10.1080/0022293030867 8729.
- Puillandre N, Brouillet S, Achaz G. 2021. ASAP: assemble species by automatic partitioning. Mol Ecol Resour 21:609–620. doi:10.1111/1755-0998.13281. . [DOI] [PubMed]
- Rambaut A, Suchard MA, Xie D, Drummond AJ. 2014. Computer Program and Documentation Distributed by the Author [WWW document]. Available at: http://beast.bio.ed.ac.uk/Tracer. Accessed 17 Jan. 2021.
- Raven RJ. 1990. Comments on the proposed precedence of Aphonopelma Pocock 1901 (Arachnida, Araneae) over Rechostica Simon 1892. Bull Zool Nomen 47:126. doi:10.5962/bhl.part.2678.
- Ronquist F, Huelsenbeck JP. 2003. MrBayes3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180. . [DOI] [PubMed]
- Schmidt G. 1986. Vogelspinnen: Lebensweise, Bestimmungsschlüssel, Haltung und Zucht. Albrecht Philler, Minden.
- Schmidt G. 1993. Vogelspinnen: Vorkommen, Lebensweise, Haltung und Zucht, mit Bestimmungsschlüsseln für alle Gattungen, Vierte Auflage. Landbuch, Hannover.
- Schmidt G. 1997. Bestimmungsschlüssel für die Gattungen der Unterfamilie Theraphosinae (Araneae: Theraphosidae). Arachnol Mag 3:1–27.
- Schmidt G. 2003. Die Vogelspinnen: Eine weltweite Übersicht. Neue Brehm-Bücherei, Hohenwarsleben. (Araneae: Theraphosidae: Theraphosinae). Tarantulas World 88:12–16.
- Schiapelli RD, Gerschman de Pikelín BS. 1962. Importancia de las espermatecas en la sistemática de las arañas del suborden Mygalomorphae (Araneae). Physis 23:69–75.
- Simon E. 1892. Histoire naturelle des araignées. Paris 1:1–256.
- Staden R, Beal KF, Bonfeld JK. 2000. The Staden package, 1998. Methods Mol Biol 32:115–130. doi:10.1385/1-59259-192-2:115. . [DOI] [PubMed]
- Starret J. Hedin M. 2007. Multilocus genealogies reveal multiple cryptic species and biogeographical complexity in the California turret spider Antrodiateus riversi (Mygalomorphae, Antrodiaetidae). Mol Ecol 16:583–604. doi:10.1111/j.1365-294X.2006.03164.x. [DOI] [PubMed]
- Talavera G, Dincǎ V, Vila R. 2013. Factors affecting species delimitations with the GMYC model: insights from a butterfly survey. Methods Ecol Evol 4:1101–1110. doi:10.1111/2041-210X.12107.
- Thorell T. 1869. On European spiders. Part I. Review of the European genera of spiders, preceded by some observations on zoological nomenclature. Nova Acta Reg Soc Sci Upsal 7:1–108.
- Vol F. 2008. Description d’une espèce naine de Grammostola Simon, 1892 (Araneae, Theraphosidae, Theraphosinae) provenant de l’Uruguay et notes sur sa biologie. L’arachnologiste 1:22–37.
- Yeates DK, Seago A, Nelson L, Cameron SL, Joseph L, Trueman JW. 2011. Integrative taxonomy, or iterative taxonomy? Syst Entomol 36:209–217. doi:10.1111/j.1365-3113.2010.00558.x.
- Walckenaer CA. 1837. Histoire naturelle des insectes. Aptères. Paris 1:1–682. doi:10.5962/bhl.title.61095.
- World Spider Catalog. 2022. Natural History Museum Bern. Available at: http://wsc.nmbe.ch, version 20.5. Accessed 10 Feb. 2022.
- Zhang J, Kapli P, Pavlidis P, Stamatakis A. 2013. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29:2869–2876. doi:10.1093/bioinformatics/btt499. . [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Voucher pictures and GenBank accession number of sequences sequenced in this study.